- 1Viral Pathogenesis Laboratory, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
- 2Department of Microbiology and Immunology, Georgetown University Medical Center, Washington, DC, United States
- 3Department of Biochemistry and Molecular Genetics, Israel Institute for Biological Research, Ness-Ziona, Israel
- 4Institute for Immunology and Infectious Diseases, Murdoch University, Perth, WA, Australia
- 5Division of Infection and Immunity, Cardiff University School of Medicine, Cardiff, United Kingdom
- 6Human Immunology Section, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
Tissue-resident memory T (TRM) cells provide first-line defense against invading pathogens encountered at barrier sites. In the lungs, TRM cells protect against respiratory infections, but wane more quickly than TRM cells in other tissues. This lack of a sustained TRM population in the lung parenchyma explains, at least in part, why infections with some pathogens, such as influenza virus and respiratory syncytial virus (RSV), recur throughout life. Intranasal (IN) vaccination with a murine cytomegalovirus (MCMV) vector expressing the M protein of RSV (MCMV-M) has been shown to elicit robust populations of CD8+ TRM cells that accumulate over time and mediate early viral clearance. To extend this finding, we compared the inflationary CD8+ T cell population elicited by MCMV-M vaccination with a conventional CD8+ T cell population elicited by an MCMV vector expressing the M2 protein of RSV (MCMV-M2). Vaccination with MCMV-M2 induced a population of M2-specific CD8+ TRM cells that waned rapidly, akin to the M2-specific CD8+ TRM cell population elicited by infection with RSV. In contrast to the natural immunodominance profile, however, coadministration of MCMV-M and MCMV-M2 did not suppress the M-specific CD8+ T cell response, suggesting that progressive expansion was driven by continuous antigen presentation, irrespective of the competitive or regulatory effects of M2-specific CD8+ T cells. Moreover, effective viral clearance mediated by M-specific CD8+ TRM cells was not affected by the coinduction of M2-specific CD8+ T cells. These data show that memory inflation is required for the maintenance of CD8+ TRM cells in the lungs after IN vaccination with MCMV.
Introduction
Tissue-resident memory T (TRM) cells protect against invading pathogens in barrier tissues by direct killing of infected cells and by recruitment of other immune effector cell populations into the tissue. Much work has been done in recent years to characterize the migration pattern, function, and phenotype of TRM cells in various anatomical locations (1–4). It has become clear that TRM cells are heterogeneous, and that the requirements for localization and maintenance differ across tissues (4–9). In the lungs, TRM cells have been shown to mediate immune protection against respiratory syncytial virus (RSV) (10–12) and heterosubtypic cross-protection against influenza virus (13–16). TRM cells are also important for immune protection against cancer (17–23). In particular, TRM cells have been shown to enhance the efficacy of intranasally administered cancer vaccines in mouse orthotopic head and neck tumor models (23). The abundance of TRM cells in malignant lung tumors further correlated with survival in humans (23). However, lung-resident TRM cells tend to wane over time, potentially reflecting a harsher and more dynamic environment compared with other barrier tissues (13, 14, 16, 24, 25). This progressive loss of TRM cells likely explains why recurrent infections with RSV and influenza virus occur throughout life. Vaccination strategies aimed at maintaining high levels of TRM cells in the lungs may therefore enhance immunity against respiratory pathogens and cancers.
Cytomegalovirus (CMV) has been shown to elicit robust populations of TRM cells in some tissues (26, 27). The persistent nature of CMV leads to a unique phenomenon among memory CD8+ T cells, which has been well characterized in mouse models using murine cytomegalovirus (MCMV). Specifically, MCMV infection generates two distinct populations of memory CD8+ T cells, termed conventional and inflationary (28–32). Conventional CD8+ T cell populations expand during acute infection and then contract, whereas inflationary CD8+ T cell populations, which may not predominate in the early phase, continue to accumulate over time within the effector memory (EM) compartment. The ability to drive memory inflation may explain why CMV vectors have shown promise as vaccine candidates, protecting against various cancers and infectious agents and providing effective immunocontraception (33–41).
Several factors determine whether a particular epitope will elicit conventional or inflationary CD8+ T cell populations. For inflationary memory responses, the source protein must be transcribed during latency, a feature that depends primarily on location within the genome (42). In addition, the derived epitope may require processing by constitutive proteasomes, because antigen presentation occurs predominantly on the surface of non-hematopoietic cells, which lack immunoproteasomes (43, 44). Interclonal competition may also play a role, given the observation that high-avidity clonotypes are preferentially selected for inflation during MCMV infection (45–47). Similar findings have been reported in the setting of human CMV infection (41, 48–50). Other potential contributors include epitope-dependent requirements for co-stimulation and CD4+ T cell help (51–55). Memory inflation is therefore difficult to predict, even in well-defined mouse models, yet a detailed understanding of this phenomenon is critical for the design of effective vaccines that deliver protective antigens vectored by CMV.
Infection of CB6F1 mice with RSV elicits CD8+ T cell responses that reproducibly target an immunodominant epitope from the M2 protein (Kd/M282–90) and a subdominant epitope from the M protein (Db/M187–195) (56). The M-specific CD8+ T cell population typically incorporates high-avidity clonotypes expressing private T cell receptors with characteristic sequence motifs, leading to greater levels of cytokine production and more effective killing of virus-infected targets in side-by-side comparisons with the M2-specific CD8+ T cell population (57–59). In addition, M-specific CD8+ T cells regulate the magnitude of the otherwise numerically dominant M2-specific CD8+ T cell population, an effect that mitigates the immunopathology associated with acute RSV infection (57).
Intranasal (IN) vaccination with an MCMV vector expressing the M protein of RSV (MCMV-M) has been shown to generate a robust population of M-specific CD8+ TRM cells with an effector/EM phenotype and augment early viral control relative to vaccination with MCMV alone or MCMV-M inoculated via the intraperitoneal (IP) route (60). In this study, we characterized the M2-specific CD8+ T cell response to IN vaccination with an MCMV vector expressing the M2 protein of RSV (MCMV-M2). Vaccination with MCMV-M2 induced a population of M2-specific CD8+ TRM cells in the lungs that subsequently waned over time, whereas vaccination with MCMV-M induced a population of M-specific CD8+ TRM cells in the lungs that subsequently inflated over time. Coadministration of both vaccines diminished the M2-specific CD8+ T cell response, but not the M-specific CD8+ T cell response, during the acute phase of infection, but had no impact on the magnitude of the conventional M2-specific CD8+ T cell population or the inflationary M-specific CD8+ T cell population during the chronic phase of infection. Moreover, the inclusion of MCMV-M2 neither enhanced nor impaired the protective effects of vaccination with MCMV-M alone in challenge experiments with RSV.
Materials and Methods
Mice
All experiments were conducted with age-matched (6–10 weeks) female CB6F1/J mice (Jackson Laboratories, Bar Harbor, ME, USA). Mice were maintained under specific-pathogen-free conditions on standard rodent chow and water supplied ad libitum in the Animal Care Facility at the National Institute of Allergy and Infectious Diseases. This study was carried out in accordance with the recommendations and guidelines of the NIH Guide to the Care and Use of Laboratory Animals. The protocol was approved by the Animal Care and Use Committee of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Mice were housed in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal procedures were conducted in strict accordance with all relevant federal and National Institutes of Health guidelines and regulations.
Cell Lines
CB6F1 mouse embryonic fibroblasts (MEFs) were isolated as described previously (60). MEFs were cultured in Advanced Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 10 U/ml penicillin G, 10 µg/ml streptomycin sulfate, and 0.1 M HEPES (DMEM-10). Human epithelial type 2 (HEp-2) cells were cultured in Eagle’s Minimal Essential Medium (MEM; Invitrogen) containing 10% FBS, 2 mM glutamine, 10 U/ml penicillin G, 10 µg/ml streptomycin sulfate, and 0.1 M HEPES (MEM-10).
Viruses and Infection
Recombinant MCMVs were made using a bacterial artificial chromosome (BAC) system as described previously (35). Briefly, the M and M2 proteins from RSV were inserted into the IE2 gene of the K181Δm157 strain of MCMV using two-step allele replacement. BACs were extracted from E. coli using a NucleoBond Xtra Maxi Prep Kit (Clontech, Mountain View, CA, USA). MEFs were transfected with recombinant BACs by calcium phosphate precipitation (Clontech) as described previously (35). Single plaques were isolated by serial dilution after viral passage and selected based on excision of the BAC cassette determined by loss of GFP and confirmed by PCR. Viral stocks were made by sonication of infected MEFs, and plaque assays were performed in triplicate on CB6F1 MEFs. Mice were vaccinated IN with 3 × 105 PFU of recombinant MCMV-M and/or MCMV-M2 in 100 µl of DMEM-10 under isoflurane anesthesia (3%). For RSV challenge, stocks were generated from the A2 strain by sonication of infected HEp-2 monolayers as described previously (61). Mice were challenged IN with 2 × 106 PFU of RSV in 100 µl of MEM-10 under isoflurane anesthesia (3%). All mice were euthanized via the administration of pentobarbital (250 mg/kg).
Intravascular Staining and Flow Cytometry
Mice were injected intravenously (IV) with 3 µg of anti-CD45 (BD Biosciences, San Jose, CA, USA). Five minutes after intravascular staining, mice were euthanized with pentobarbital, and the lungs were harvested at various time points. Lymphocytes were isolated by physical disruption of tissue using a GentleMACs Machine (Miltenyi Biotec, San Diego, CA, USA) and separated using density gradient centrifugation with Fico-LITE (Thermo Fisher Scientific, Waltham, MA, USA). Isolated mononuclear cells were washed with phosphate-buffered saline (PBS) and resuspended in fluorescence-activated cell sorting buffer (PBS supplemented with 1% FBS and 0.05% sodium azide). Cells were stained with directly conjugated antibodies specific for the lineage markers CD3 (145-2C11) and CD8 (53-6.7) (BD Biosciences) and the memory markers CD44 (IM7), CD62L (MEL-14), CD127 (A7R34), KLRG1 (2F1/KLRG1), CD69 (H1.2F3), and CD103 (M290) (BD Biosciences or BioLegend, San Diego, CA, USA). Dead cells were excluded from the analysis using LIVE/DEAD Fixable Aqua (Invitrogen). Antigen-specific CD8+ T cells were identified using Db/M187–195 (RSV M) or Kd/M282–90 (RSV M2) tetramers (MBL, Woburn, MA, USA). For validation of intravascular staining, cells were labeled with directly conjugated antibodies specific for CD3 (145-2C11), CD11c (N418), CD64 (X54-5/7.1), SiglecF (E50-2440), and CD11b (M1/70) (BD Biosciences or BioLegend). Data were acquired using an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software version 9.9.6 (TreeStar, Ashland, OR, USA). Memory phenotypes were further analyzed using Pestle version 1.6.2 and SPICE version 6.0 (http://exon.niaid.nih.gov/spice/).
ONX-0914 Inhibition Study
Mice were treated subcutaneously on days 0, 2, 4, and 6 with 2, 6, or 10 mg/kg of ONX-0914 (PR-957; Selleck Chemical, Houston, TX, USA) or vehicle control (10% captisol in 10 mM sodium citrate). On day 0, mice were infected IN as described above with 2 × 106 PFU of RSV. On day 7, mice were euthanized with pentobarbital, and the lungs were harvested and processed as described above.
Plaque Assay
Lungs were weighed and quick-frozen in 10% MEM-10, and plaque assays were performed as described previously (62). Briefly, thawed lung tissue was dissociated using a GentleMACs Machine (Miltenyi Biotec). Cell suspensions were pelleted to remove cellular debris, and clarified supernatants were serially diluted and inoculated in triplicate on 80% confluent HEp-2 cell monolayers. After rocking for 1 h at room temperature, monolayers were overlaid with 0.75% methyl cellulose in MEM-10 and incubated at 37°C. Cells were fixed with 10% buffered formalin and stained with hematoxylin and eosin on day 4. Plaques were counted and expressed as Log10 PFU/g of lung tissue. The limit of detection was 1.8 Log10 PFU/g.
Statistical Analysis
Statistical analyses were performed using a one-way or two-way ANOVA as appropriate for multiple comparisons (GraphPad Prism, San Diego, CA, USA). Memory phenotypes were compared using a permutation test (10,000 rounds) in SPICE version 6.0 (http://exon.niaid.nih.gov/spice/).
Results
IN Vaccination With MCMV-M2 Elicits More Lung-Resident M2-Specific CD8+ T Cells Than IP Vaccination
We and others have demonstrated that IN vaccination is necessary to elicit TRM cells in the lungs (19, 23, 60). In particular, our earlier work showed that IN vaccination with MCMV-M elicited more M-specific CD8+ T cells in the lung parenchyma than IP vaccination with MCMV-M (Figure 1A) (60). To extend this finding, we vaccinated mice with MCMV-M2 via the IN or IP route and used intravascular staining in conjunction with Kd/M282–90 tetramers to analyze M2-specific CD8+ T cell responses in the blood and the lung parenchyma after 1 week. The intravascular staining protocol was validated in the context of IN vaccination to ensure that direct infection of the lungs did not lead to increased permeability due to inflammation (Figure S1 in Supplementary Material). Akin to the differences observed after vaccination with MCMV-M (Figures 1B,C), we found that IN vaccination with MCMV-M2 induced significantly more lung-resident M2-specific CD8+ T cells than IP vaccination with MCMV-M2, both in terms of frequency (P < 0.01; Figure 1D) and number (P < 0.05; Figure 1E). By contrast, IP vaccination with MCMV-M2 elicited higher frequencies of M2-specific CD8+ T cells in the blood (P < 0.0001, Figure 1D) and in total (P < 0.05), but similar numbers of M2-specific CD8+ T cells in the blood and in total. We therefore focused on IN vaccination in our efforts to induce and maintain lung-resident CD8+ T cells.
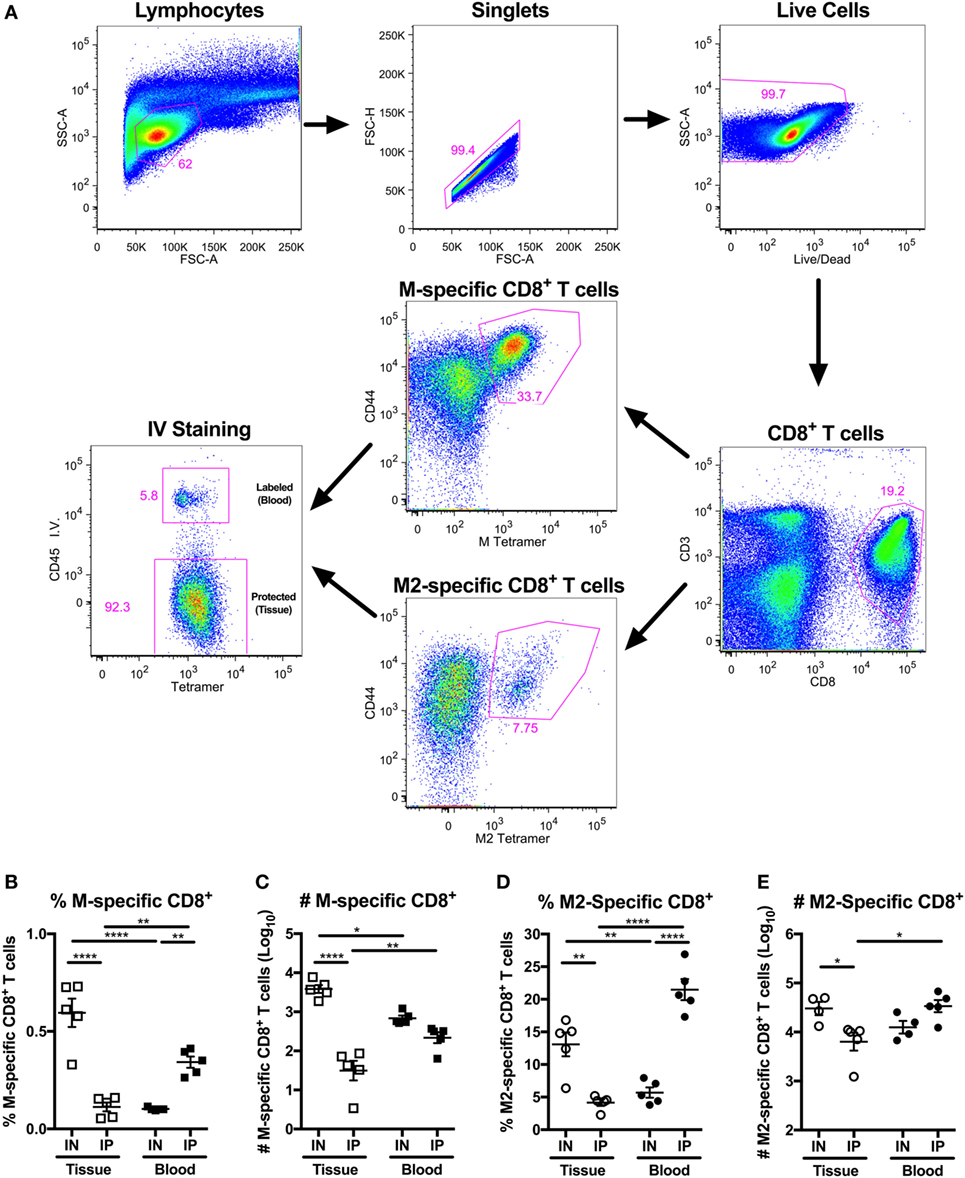
Figure 1. Intranasal (IN) vaccination with murine cytomegalovirus (MCMV)-M2 elicits more lung-resident M2-specific CD8+ T cells than intraperitoneal (IP) vaccination. (A–E) Mice were vaccinated with MCMV-M or MCMV-M2 via the IN or IP route. Intravascular staining was used in conjunction with Db/M187–195 and Kd/M282–90 tetramers to quantify epitope-specific CD8+ T cells in the lung tissue and blood after 1 week. (A) Gating strategy used to identify M-specific and M2-specific CD8+ T cells in the tissue and blood of the lungs. (B) Frequency and (C) number of M-specific CD8+ T cells in the tissue and blood of lungs 1 week after MCMV-M vaccination. (D) Frequency and (E) number of M2-specific CD8+ T cells in the tissue and blood of the lungs 1 week after MCMV-M2 vaccination. Bars indicate mean ± SEM (n = 5 mice/group). ****P < 0.0001, **P < 0.01, *P < 0.05 by two-way ANOVA. Data are shown from one experiment and representative of two independent experiments.
The M-Specific CD8+ T Cell Population Inflates, Whereas the M2-Specific CD8+ T Cell Population Contracts, After Vaccination With MCMV
Next, we used a similar approach to evaluate CD8+ T cell responses at weeks 1, 8, and 16 after vaccination with MCMV-M or MCMV-M2 alone or a combination of MCMV-M and MCMV-M2. Intravascular staining was used as above in conjunction with Db/M187–195 and Kd/M282–90 tetramers to quantify epitope-specific CD8+ T cells in the blood and lung parenchyma. MCMV-M administered either alone or together with MCMV-M2 generated an M-specific CD8+ T cell population that inflated between weeks 1 and 8 (P < 0.0001) and remained stable through week 16 (Figure 2A). This trend was observed in the lung tissue and blood (P < 0.0001; Figures 2B,C). By contrast, M2-specific CD8+ T cells in the lung tissue and blood contracted over time (P < 0.0001; Figures 2D–F), irrespective of coadministration with MCMV-M. After RSV infection, which generates only conventional memory responses as a consequence of self-limited antigen production, the M-specific and M2-specific CD8+ T cell populations both contracted dramatically between weeks 1 and 8 in the lung tissue and blood (P < 0.001; Figures 2A–F). Similar epitope-specific patterns were observed when assessing T cell frequency in the lung and spleen (Figure S2 in Supplementary Material). In addition, the MCMV-encoded M38-specific CD8+ T cell response was largely equivalent among experimental groups, suggesting that the observed loss of M2-specific CD8+ T cells over time was not attributable to clearance of MCMV-M2. Thus, the M-specific CD8+ T cell population is inflationary, whereas the M2-specific CD8+ T cell population is not inflationary, after vaccination with MCMV.
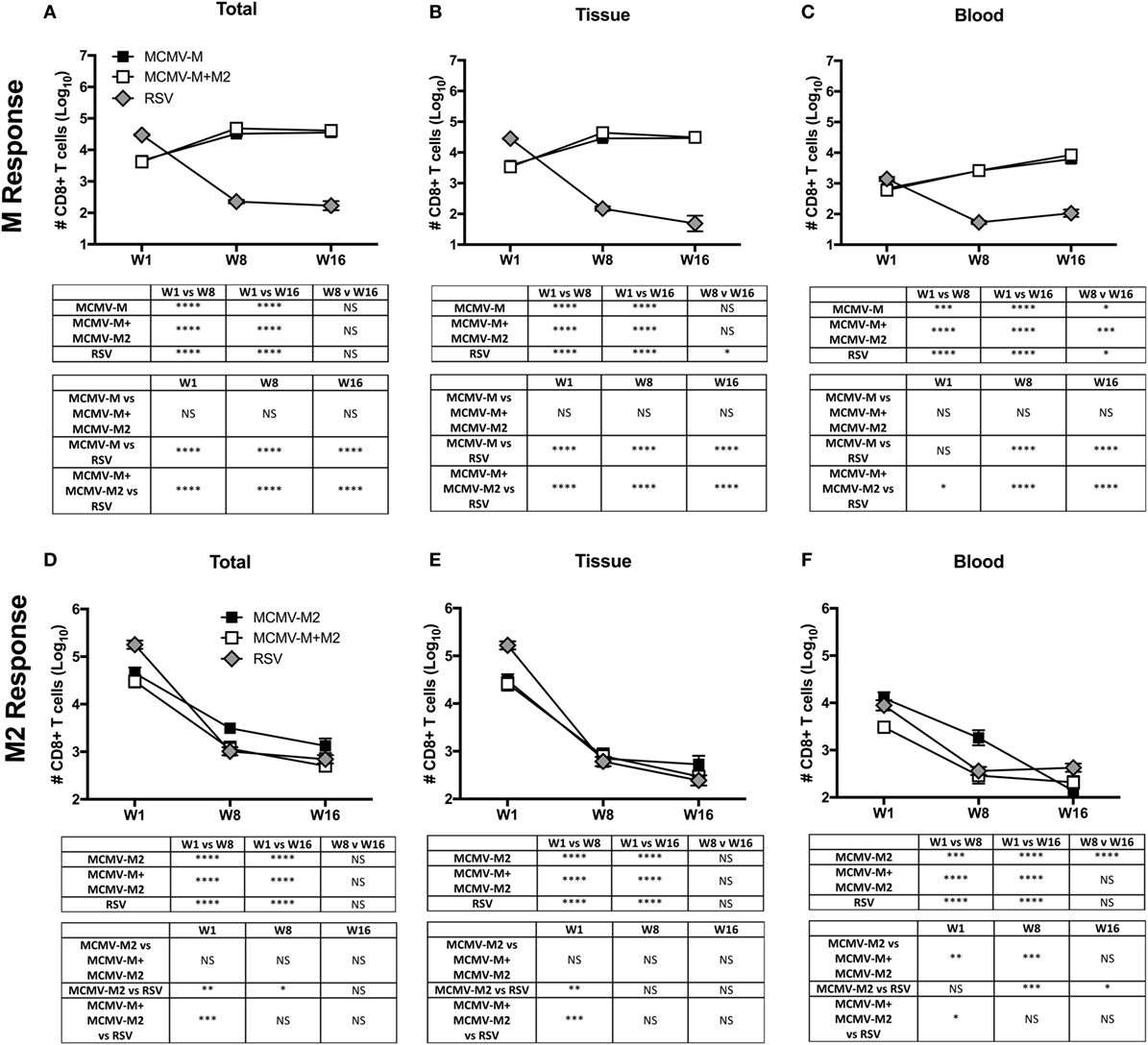
Figure 2. The M-specific CD8+ T cell population inflates, whereas the M2-specific CD8+ T cell population contracts, after vaccination with murine cytomegalovirus (MCMV). (A–F) Mice were infected with respiratory syncytial virus (RSV) or vaccinated with MCMV-M or MCMV-M2 alone or a combination of MCMV-M and MCMV-M2 via the intranasal route. Intravascular staining was used in conjunction with Db/M187–195 and Kd/M282–90 tetramers to quantify M-specific (A–C) and M2-specific (D–F) CD8+ T cells in the lung tissue and blood at weeks 1 (W1), 8 (W8), and 16 (W16). Total (A,D) denotes all tetramer+ CD8+ T cells regardless of location. Bars indicate mean ± SEM (n = 5 mice/group). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by two-way ANOVA. Data are shown from one experiment and representative of two independent experiments.
One week after vaccination, coadministration of MCMV-M and MCMV-M2 elicited an epitope-specific hierarchy equivalent to that observed after RSV infection, with a dominant CD8+ T cell response to Kd/M282–90 and a subdominant CD8+ T cell response to Db/M187–195 (Figures 2A,D). At weeks 8 and 16, this hierarchy was inverted as a consequence of M-specific CD8+ T cell inflation and M2-specific CD8+ T cell contraction (Figures 2A,D). Coadministration of MCMV-M and MCMV-M2 did not alter the number or frequency of M-specific CD8+ T cells in the blood or the tissue at any time point relative to vaccination with MCMV-M alone (Figures 2B,C; Figure S2B,C in Supplementary Material). By contrast, coadministration of MCMV-M and MCMV-M2 dampened the frequency, but not the overall magnitude, of the M2-specific CD8+ T cell response at week 1 (P < 0.01), but not at weeks 8 and 16 (Figure 2D; Figure S2D in Supplementary Material). This effect was anatomically discrepant. Specifically, coadministration of MCMV-M and MCMV-M2 did not significantly reduce the number or frequency of M2-specific CD8+ T cells in the lung tissue (Figure 2E; Figure S2E in Supplementary Material), but did significantly reduce the number and frequency of M2-specific CD8+ T cells in the blood at weeks 1 and 8 relative to vaccination with MCMV-M2 alone (P < 0.01; Figure 2F; Figure S2F in Supplementary Material). No significant differences in the frequency of M2-specific CD8+ T cells were observed after contraction of the response at week 16 (Figures 2D–F). The reduction of M2-specific CD8+ T cells at the acute time point after coadministration of MCMV-M and MCMV-M2 was not unexpected, because competition between the M-specific and M2-specific CD8+ T cell populations has been demonstrated after RSV infection of CB6F1 mice (57).
The M2 Epitope Is Preferentially Generated by the Immunoproteasome
Memory inflation likely requires epitope generation via the constitutive proteasome, because antigen processing and presentation are thought to occur predominantly by non-hematopoietic cells, which lack immunoproteasomes (43, 44). To determine if proteasomal processing impacted the M-specific or M2-specific CD8+ T cell responses, we infected mice with RSV and treated them with the immunoproteasome inhibitor ONX-0914 on days 0, 2, 4, and 6 at doses of 2, 6, or 10 mg/kg. On day 7, we evaluated M-specific and M2-specific CD8+ T cells in the lungs. Treatment with ONX-0914 significantly reduced the frequency and number of M2-specific CD8+ T cells, but not M-specific CD8+ T cells, in a dose-dependent manner (Figures 3A,B). These data suggest that the M2 peptide is preferentially generated by the immunoproteasome, whereas the M peptide is preferentially generated by the constitutive proteasome, which is unaffected by ONX-0914.
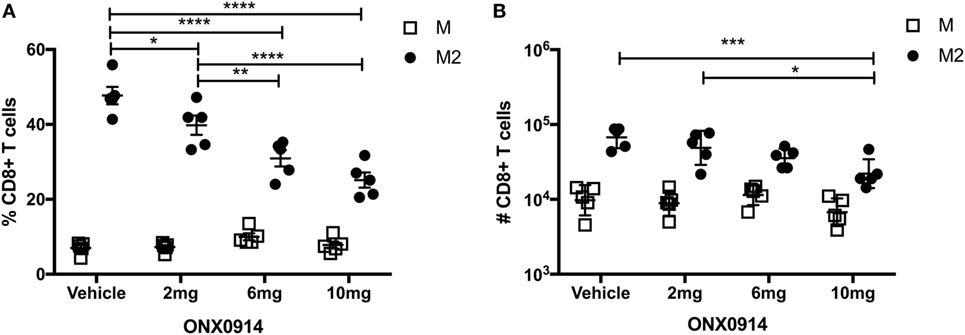
Figure 3. The M2 epitope is preferentially generated by the immunoproteasome. (A,B) Mice were infected with respiratory syncytial virus (RSV) and treated with the immunoproteasome inhibitor ONX-0914 or vehicle control on days 0, 2, 4, and 6 at doses of 2, 6, or 10 mg/kg. Db/M187–195 and Kd/M282–90 tetramers were used to quantify the frequency (A) and number (B) of M-specific and M2-specific CD8+ T cells in the lungs on day 7. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by two-way ANOVA. Bars indicate mean ± SEM (n = 5 mice/group). Data are shown from one experiment and representative of two independent experiments.
IN Vaccination With MCMV Elicits CD8+ TRM Cells
A previous study demonstrated that IN vaccination with MCMV-M generated a robust population of TRM cells, identified by expression of CD103 (60). However, it has also been shown that not all TRM cells express CD103 (7). We therefore used intravascular staining to quantify M-specific and M2-specific TRM cells in the lung parenchyma based on expression of CD69 and CD103 after infection with RSV or vaccination with MCMV-M or MCMV-M2 alone or a combination of MCMV-M and MCMV-M2. The administration of MCMV-M, either alone or together with MCMV-M2, generated a substantial population of CD69+ TRM cells that was largely maintained between weeks 8 and 16, and significantly outnumbered the corresponding population of CD69+ TRM cells induced by RSV infection at both time points (P < 0.0001; Figures 4A,B,D). By contrast, the M2-specific CD69+ TRM population significantly decreased between weeks 8 and 16, irrespective of M2 protein expression via MCMV or RSV (P < 0.01; Figures 4A,C, E). There was no difference in the number of M2-specific TRM cells elicited by vaccination with MCMV-M2 or infection with RSV. As the M-specific TRM population induced by MCMV was maintained in the lungs and the M2-specific TRM population induced by MCMV waned in the lungs, there were significantly more M-specific TRM cells than M2-specific TRM cells in the lung parenchyma at weeks 8 and 16 (P < 0.0001; Figures 4B–E). In the context of RSV infection, however, there were significantly more M2-specific TRM cells than M-specific TRM cells in the lung parenchyma at both time points (P < 0.05; Figures 4B–E). Coadministration of MCMV-M and MCMV-M2 did not affect the number of M-specific or M2-specific TRM cells at either time point compared with the administration of MCMV-M or MCMV-M2 alone (Figures 4B–E).
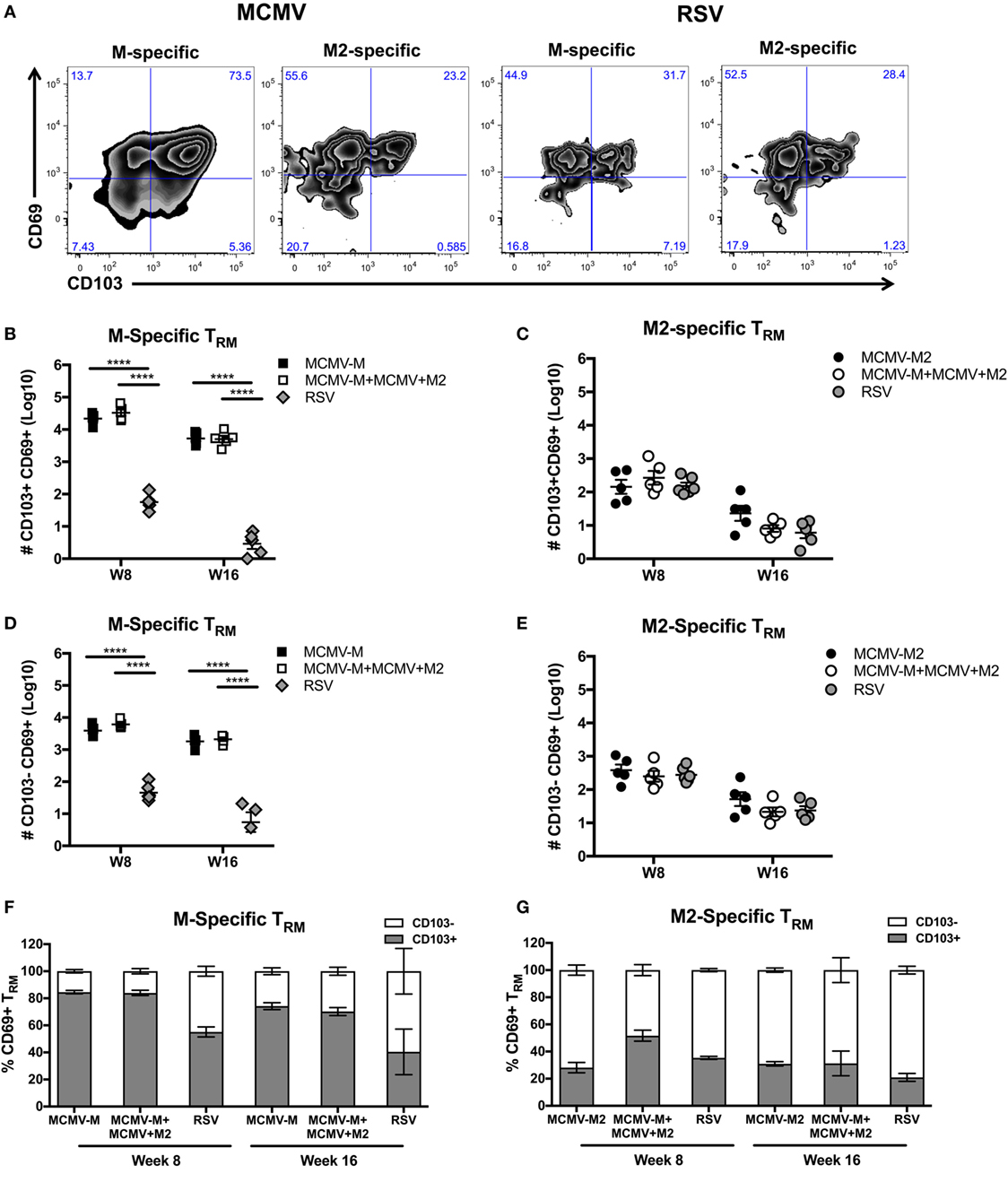
Figure 4. Intranasal (IN) vaccination with murine cytomegalovirus (MCMV) elicits CD8+ tissue-resident memory T (TRM) cells. (A–E) Mice were infected with respiratory syncytial virus (RSV) or vaccinated with MCMV-M or MCMV-M2 alone or a combination of MCMV-M and MCMV-M2 via the IN route. Intravascular staining was used in conjunction with Db/M187–195 and Kd/M282–90 tetramers to quantify M-specific (A,B,D,F) and M2-specific (A,C,E,G) CD8+ T cells in the lung tissue at weeks 8 (W8) and 16 (W16). (A) Representative flow cytometry plots showing expression of CD69 and CD103 on epitope-specific CD8+ T cells in the lung parenchyma at week 8. (B,D) The number of M-specific CD103+CD69+ TRM cells (B) and CD103−CD69+ TRM cells (D) elicited by infection with RSV or vaccination with MCMV-M alone or together with MCMV-M2. (C,E) The number of M2-specific CD103+CD69+ TRM cells (C) and CD103−CD69+ TRM cells (E) elicited by infection with RSV or vaccination with MCMV-M2 alone or together with MCMV-M. (F,G) Percentage of CD103+ and CD103− M-specific CD69+ TRM cells (F) and M2-specific CD69+ TRM cells (G). ****P < 0.0001 by two-way ANOVA. Bars indicate mean ± SEM (n = 5 mice/group). Data are shown from one experiment and representative of two independent experiments.
Next, we assessed the expression of CD103 on CD69+ TRM cells. After vaccination with single MCMV vectors, a higher proportion of M-specific CD8+ T cells coexpressed CD69 and CD103 compared with M2-specific cells at week 8 (84.6 vs. 28.1%; P < 0.0001) and week 16 (74.2 vs. 30.9%; P < 0.05) (Figures 4F,G). A similar trend was observed after coadministration of MCMV-M and MCMV-M2. At week 8, the vaccine-induced M-specific TRM population also contained a significantly higher proportion of cells expressing CD103 than the M-specific TRM population elicited by RSV infection (84.6% for MCMV-M and 83.9% for MCMV-M + MCMV-M2 vs. 55% for RSV; P < 0.0001).
These data show that inflation of the M-specific CD8+ T cell population elicited by vaccination with MCMV enhances the frequency and number of TRM cells relative to acute infection with RSV. By contrast, M2-specific CD8+ TRM cells were induced at similar levels irrespective of M2 protein expression via MCMV or RSV. It is also notable that a larger fraction of M-specific CD69+ TRM cells elicited by vaccination with MCMV coexpressed CD103 compared with either M2-specific CD69+ TRM cells elicited by vaccination with MCMV or TRM cells of either specificity elicited by infection with RSV.
M-Specific and M2-Specific CD8+ T Cells Are Phenotypically Distinct in the Lung Tissue and Blood After Vaccination With MCMV
Inflationary and conventional epitope-specific CD8+ T cell populations have previously been shown to differ phenotypically after IP infection with MCMV (63). In this context, inflationary memory cells are predominantly CD127−KLRG1+ effectors, while conventional memory cells display a more CD127+CD62L+ central memory (CM)-like phenotype. This pattern is recapitulated after IP vaccination with MCMV-M. However IN vaccination with MCMV-M induces a CD8+ T cell population with predominantly effector and EM phenotypes (60). We therefore analyzed the phenotype of antigen-specific CD8+ T cells elicited by MCMV-M and/or MCMV-M2 vaccination at 8 weeks post-vaccination. We categorized the RSV-specific CD8+ T cell populations as CM, EM, effector, or KLRG1+ effectors (KLRG1+) (Figure 5). Populations were defined as follows: all: tetramer+ CD44+; CM: CD127+KLRG1−CD62L+; EM: CD127+KLRG1−CD62L−; effector: CD127−KLRG1−CD62L−; KLRG1+ effector: CD62L−KLRG1+. Overall, there were no obvious phenotypic differences when the MCMV vectors were administered alone or in combination (Figure 5B). By contrast, distinct phenotypes were observed across anatomical compartments for both the M-specific and M2-specific CD8+ T cell populations, with higher frequencies of KLRG1+ effectors (yellow) and CM cells (blue) and lower frequencies of EM cells (green) in the blood compared with the tissue (P < 0.05). A comparison of M-specific and M2-specific CD8+ T cells in the blood and tissue also showed that these antigen-specific populations were comprised of different proportions of memory subsets (Figure 5, P < 0.05). In the blood, the M2-specific CD8+ T cell population incorporated larger fractions of CM (blue) and KLRG1+ effectors (yellow) and smaller fractions of effector (orange) and EM (green) cells than the M-specific CD8+ T cell population. Although statistically significant, the differences between the M-specific and M2-specific CD8+ T cell population were more subtle in the tissue. Interestingly, we observed higher levels of CD44 expression on CD8+ T cells in the lung tissue compared with CD8+ T cells in the blood, irrespective of antigen specificity and vaccination modality (Figure 5C). When parsed out by location, expression of CD44 by M-specific and M2-specific CD8+ T cells was relatively high compared with the corresponding bulk CD8+ T cell populations in the blood and tissue of the lungs (Figure S3 in Supplementary Material).
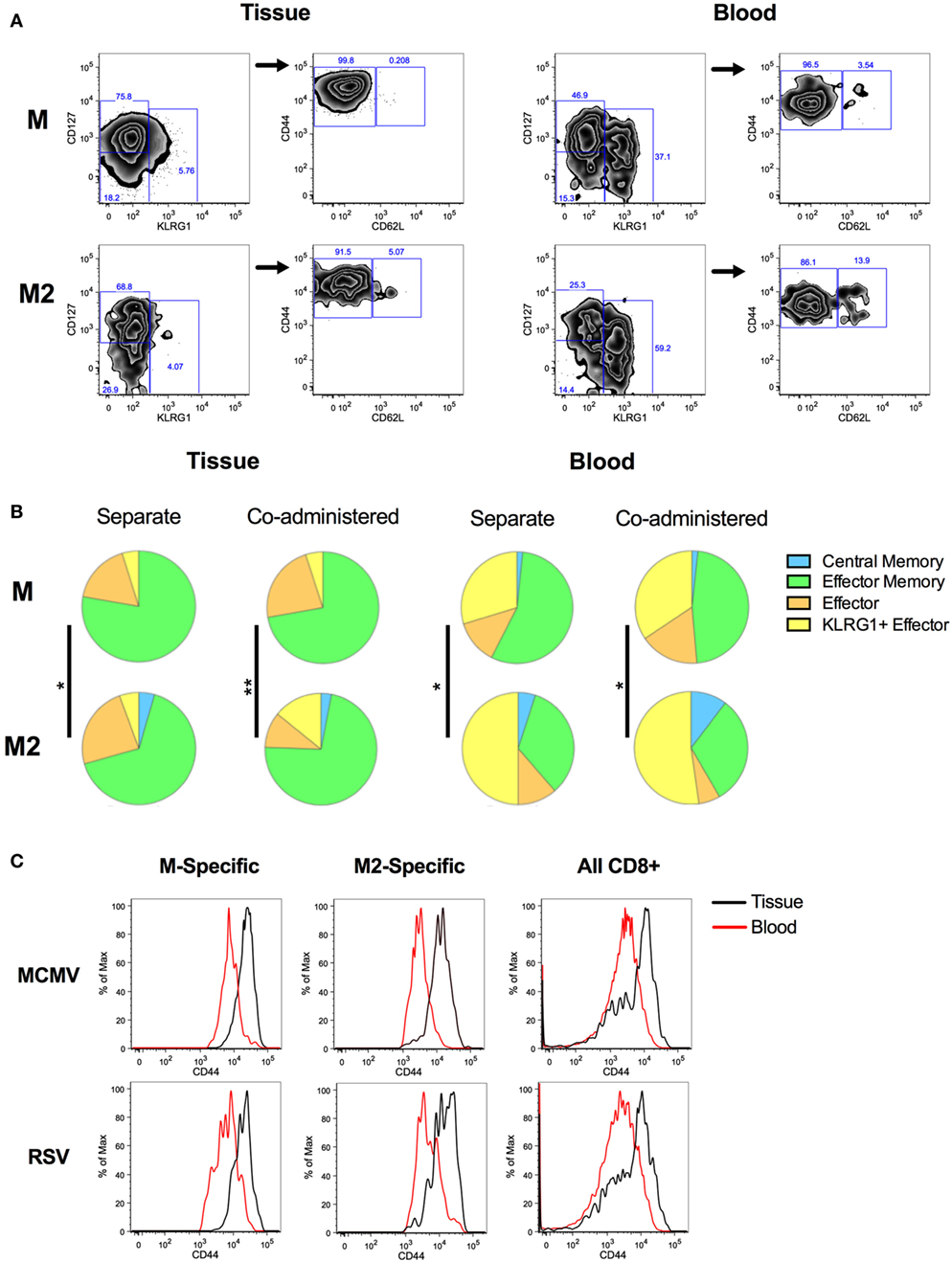
Figure 5. Phenotype of M-specific and M2-specific CD8+ T cells elicited by murine cytomegalovirus (MCMV) vaccination. Mice were vaccinated with MCMV-M or MCMV-M2 alone or a combination of MCMV-M and MCMV-M2 via the IN route. Intravascular staining was used in conjunction with Db/M187–195 and Kd/M282–90 tetramers to identify M-specific and M2-specific CD8+ T cells in the blood and tissue of the lungs at week 8. (A) Gating strategy for phenotypic analysis. Populations were defined as follows: CD127+KLRG1−CD62L+ [central memory (CM)]; CD127+KLRG1−CD62L− [effector memory (EM)]; CD127−KLRG1−CD62L− (effector); and KLRG1+CD62L− (KLRG1+ effector). (B) The proportions of CM cells (blue), EM cells (green), effectors (orange), and KLRG1+ effectors (yellow) in the lungs are shown for each specificity. (C) CD44 expression on M-specific, M2-specific, and all CD8+ T cells in the tissue and blood of the lungs. *P ≤ 0.05, **P < 0.01 by permutation test (SPICE). Data are shown from one experiment (n = 5/group) and representative of two independent experiments.
MCMV-Elicited TRM Cells Expedite Viral Clearance After Infection With RSV
To evaluate the biological relevance of these observations, we challenged mice with 2 × 106 PFU of RSV delivered via the IN route 16 weeks after vaccination with MCMV-M, MCMV-M2, or a combination of MCMV-M and MCMV-M2. Viral loads were measured on days 3 and 5 after infection with RSV. On day 3, mice vaccinated with MCMV-M or MCMV-M together with MCMV-M2 exhibited significantly lower viral loads in the lungs compared with mice vaccinated with the MCMV vector alone (P < 0.01 and P < 0.05, respectively; Figure 6A). By contrast, vaccination with MCMV-M2 did not lead to a significant reduction in viral load on day 3. All vaccination regimens significantly reduced viral loads on day 5 relative to the MCMV vector alone (P < 0.0001 for MCMV-M, P < 0.01 for MCMV-M2, P < 0.0001 for MCMV-M + MCMV-M2; Figure 6B). However, simultaneous vaccination with MCMV-M and MCMV-M2 did not enhance viral clearance relative to vaccination with MCMV-M alone (Figure 6B).
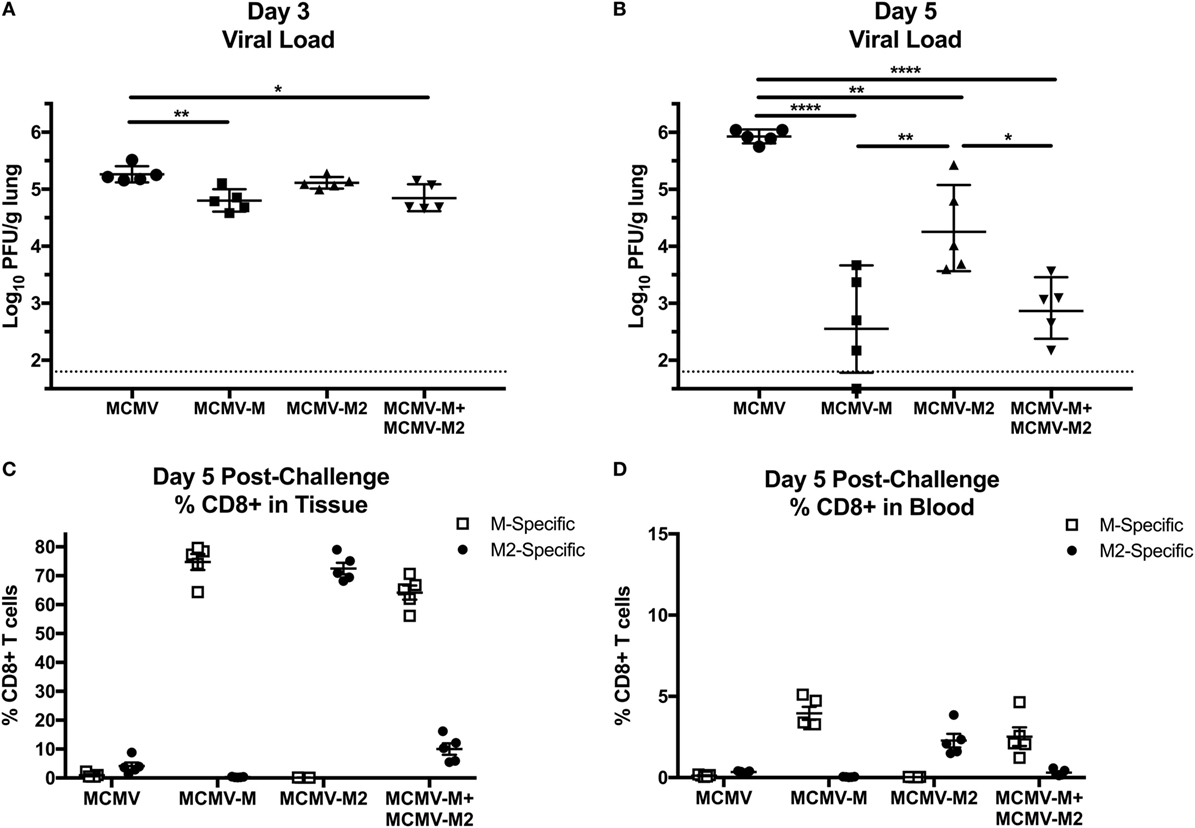
Figure 6. Murine cytomegalovirus (MCMV)-elicited tissue-resident memory T cells expedite viral clearance after infection with respiratory syncytial virus (RSV). (A–D) Mice were vaccinated with MCMV vector, MCMV-M or MCMV-M2 alone, or a combination of MCMV-M and MCMV-M2 via the intranasal route and challenged with RSV at week 16. Viral titers in the lungs were measured by plaque assay on days 3 (A) and 5 (B). ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by one-way ANOVA. (C,D) Intravascular staining was used in conjunction with Db/M187–195 and Kd/M282–90 tetramers to quantify M-specific and M2-specific CD8+ T cells in the lungs (C) and the blood (D). ****P < 0.0001, **P < 0.01, *P < 0.05 by two-way ANOVA. Data shown from one experiment and representative of two independent experiments.
Inflation of the M-Specific CD8+ T Cell Population Alters Immunodominance After Challenge With RSV
In further experiments, we assessed the frequency of antigen-specific CD8+ T cells in the lung parenchyma on day 5 after challenge with RSV (Figures 6C,D). Mice vaccinated with the MCMV vector alone harbored relatively few M-specific or M2-specific CD8+ T cells in the lungs, but the M2-specific population was immunodominant, as typically observed in unvaccinated mice after infection with RSV. As expected, mice vaccinated with MCMV-M or MCMV-M2 alone mounted immunodominant CD8+ T cell responses to the corresponding vaccine antigens, whereas mice vaccinated with both MCMV-M and MCMV-M2 displayed very high frequencies of M-specific CD8+ T cells relative to M2-specific CD8+ T cells, inverting the natural immunodominance hierarchy observed after infection with RSV. This finding may explain why the addition of MCMV-M2 did not enhance the protective effects of vaccination with MCMV-M alone in response to challenge with RSV.
Discussion
Vaccination with MCMV-M via the IN route has been shown to generate a robust population of M-specific CD8+ TRM cells in the lungs that subsequently inflates over time (60). To extend this finding, we evaluated MCMV vaccine-induced CD8+ T cell responses to the immunodominant M2 epitope. We found that IN vaccination with MCMV-M2 induced a conventional memory response, but failed to establish a stable population of lung-resident M2-specific CD8+ TRM cells. Moreover, coadministration of MCMV-M and MCMV-M2 inverted the natural immunodominance hierarchy, but did not significantly impact the generation of M-specific or M2-specific CD8+ TRM cells. As a consequence, the protective effects of vaccination with MCMV-M were neither impeded nor enhanced by the addition of MCMV-M2.
Memory inflation is essential for the maintenance of lung-resident CD8+ TRM cell populations. In the setting of self-limiting viral infections of the respiratory tract, conventional epitopes induce populations of CD8+ TRM cells in the lung parenchyma that wane over time (14). Our data further show that persistent antigen expression is insufficient to overcome this decline, consistent with the findings of Smith et al., who demonstrated that TRM cells are maintained in the salivary glands via continuous production rather than via long-term survival after infection with MCMV (26). In our previous work, we demonstrated that the robust population of M-specific CD8+ TRM cells induced by IN vaccination with MCMV-M contributed to early clearance of RSV (60). This effect was maintained after treatment with a sphingosine 1-phosphate receptor modulator, suggesting that protection was independent of recirculation via the lymph nodes. These data concur with the observation herein that IN vaccination with MCMV-M2 failed to mediate early immune control of RSV. Together, these studies highlight the importance of lung-tropic TRM cells in protection against respiratory infection. Accordingly, immunization with a persistent vector offers no immediate advantages over traditional vaccine platforms for conventional epitopes like M2. By contrast, the induction and maintenance of inflationary epitope-specific CD8+ TRM cells in the lungs after vaccination with MCMV may enhance immune protection against respiratory pathogens, which typically induce only transient memory responses at the site of infection (14–16, 24).
Several factors determine the immunogenicity and memory characteristics of any given epitope. In this study, the M and M2 sequences were inserted into the IE2 gene, which naturally encodes inflationary epitopes, and the proteins were under the control of the constitutive promoter IE1 (30, 31, 45). Despite identical genomic locations, the M2 epitope failed to elicit inflationary CD8+ T cell responses. This lack of inflation may reflect greater dependence on the immunoproteasome compared with the M-specific CD8+ T cell response, consistent with previous studies that postulated a key role for antigen processing as a determinant of immunodominance patterns in the context of infection with MCMV (43, 44). In addition, M-specific CD8+ T cells operate with higher composite avidities than M2-specific CD8+ T cells after infection with RSV (57). However, this factor alone may not preclude M2-driven memory inflation, because recent work has demonstrated the existence of low-avidity inflationary CD8+ T cell populations (41). It is also difficult to exclude other possible influences, such as competition between CD8+ T cells with different antigen specificities and variable requirements for co-stimulation and CD4+ T cell help, which are more difficult to assess directly. Any or all of these factors may contribute to the lack of inflation among M2-specific CD8+ T cells. In vivo testing is therefore required to assess the true inflationary potential of any given epitope, a process that will become more difficult as vaccines advance from inbred animal models to human populations with diverse genetic backgrounds. A better understanding of the factors that govern memory inflation and how they can be manipulated will be important for the development of CMV vaccines.
As memory inflation is difficult to predict, it is important to study the effect of both inflationary and conventional epitopes in vaccine settings. Coadministration of MCMV-M and MCMV-M2 reduced the overall magnitude of the conventional M2-specific CD8+ T cell response acutely after vaccination but did not impact the inflationary M-specific CD8+ T cell response at any stage after vaccination. Moreover, dual immunization was equivalent to vaccination with MCMV-M alone in terms of protective efficacy after challenge with RSV. These data suggest that both conventional and inflationary epitopes can be included in a persistent vaccine without detrimental effects. However, it should be noted that competition for antigen can occur if inflationary epitopes are delivered by the same vector (45). Individual epitopes are therefore probably best expressed separately if polyvalency is required to prevent immune escape.
In summary, we have shown that memory inflation is required for the maintenance of CD8+ TRM cells in the lungs after IN vaccination with MCMV. These findings highlight an important consideration in the development of persistent vectors and suggest that epitope selection will be a central determinant of efficacy in the setting of vaccines that deliver antigens on a continuous basis.
Ethics Statement
This study was carried out in accordance with the recommendations and guidelines of the NIH Guide to the Care and Use of Laboratory Animals. The protocol was approved by the Animal Care and Use Committee of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Mice were housed in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal procedures were conducted in strict accordance with all relevant federal and National Institutes of Health guidelines and regulations.
Author Contributions
KM, TR, DP, and BG conceived and designed studies; KM, TR, EB-H, and DN performed animal studies and analyzed data; SM and AR designed and generated recombinant BACs; KM, TR, DP, and BG wrote the manuscript. All authors provided critical feedback and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health. DP is a Wellcome Trust Senior Investigator (100326/Z/12/Z).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01861/full#supplementary-material.
References
1. Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291:2413–7. doi:10.1126/science.1058867
2. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol (2009) 10:524–30. doi:10.1038/ni.1718
3. Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science (2014) 346:101–5. doi:10.1126/science.1254803
4. Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol (2014) 5:320. doi:10.3389/fimmu.2014.00320
5. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity (2014) 41:886–97. doi:10.1016/j.immuni.2014.12.007
6. Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol (2015) 16:406–14. doi:10.1038/ni.3108
7. Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell (2015) 161:737–49. doi:10.1016/j.cell.2015.03.031
8. Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol (2016) 16:79–89. doi:10.1038/nri.2015.3
9. Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep (2017) 20:2921–34. doi:10.1016/j.celrep.2017.08.078
10. Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun (2015) 6:10224. doi:10.1038/ncomms10224
11. Li H, Callahan C, Citron M, Wen Z, Touch S, Monslow MA, et al. Respiratory syncytial virus elicits enriched CD8+ T lymphocyte responses in lung compared with blood in African green monkeys. PLoS One (2017) 12:e0187642. doi:10.1371/journal.pone.0187642
12. Kinnear E, Lambert L, Mcdonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol (2018) 11:290. doi:10.1038/mi.2017.79
13. Anderson KG, Masopust D. Editorial: Pulmonary resident memory CD8 T cells: here today, gone tomorrow. J Leukoc Biol (2014) 95:199–201. doi:10.1189/jlb.0913493
14. Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol (2014) 95:215–24. doi:10.1189/jlb.0313180
15. Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol (2017) 2:eaam6970. doi:10.1126/sciimmunol.aam6970
16. Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol (2017) 2:eaag2031. doi:10.1126/sciimmunol.aag2031
17. Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest (2012) 122:4606–20. doi:10.1172/JCI63287
18. Decrausaz L, Pythoud C, Domingos-Pereira S, Derre L, Jichlinski P, Nardelli-Haefliger D. Intravaginal live attenuated Salmonella increase local antitumor vaccine-specific CD8(+) T cells. Oncoimmunology (2013) 2:e22944. doi:10.4161/onci.22944
19. Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, et al. Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med (2013) 5:172ra120. doi:10.1126/scitranslmed.3004888
20. Nizard M, Diniz MO, Roussel H, Tran T, Ferreira LC, Badoual C, et al. Mucosal vaccines: novel strategies and applications for the control of pathogens and tumors at mucosal sites. Hum Vaccin Immunother (2014) 10:2175–87. doi:10.4161/hv.29269
21. Nizard M, Roussel H, Tartour E. Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clin Cancer Res (2016) 22:530–2. doi:10.1158/1078-0432.CCR-15-2364
22. Sun YY, Peng S, Han L, Qiu J, Song L, Tsai Y, et al. Local HPV recombinant vaccinia boost following priming with an HPV DNA vaccine enhances local HPV-specific CD8+ T-cell-mediated tumor control in the genital tract. Clin Cancer Res (2016) 22:657–69. doi:10.1158/1078-0432.CCR-15-0234
23. Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun (2017) 8:15221. doi:10.1038/ncomms15221
24. Takamura S, Yagi H, Hakata Y, Motozono C, Mcmaster SR, Masumoto T, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med (2016) 213:3057–73. doi:10.1084/jem.20160938
25. Takamura S. Persistence in temporary lung niches: a survival strategy of lung-resident memory CD8(+) T cells. Viral Immunol (2017) 30:438–50. doi:10.1089/vim.2017.0016
26. Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep (2015) 13:1137–48. doi:10.1016/j.celrep.2015.09.076
27. Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8(+) T Cells, facilitating protection from local cytomegalovirus infection. Cell Rep (2015) 13:1125–36. doi:10.1016/j.celrep.2015.09.082
28. Holtappels R, Thomas D, Podlech J, Reddehase MJ. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J Virol (2002) 76:151–64. doi:10.1128/JVI.76.1.151-164.2002
29. Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol (2003) 170:2022–9. doi:10.4049/jimmunol.170.4.2022
30. Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, et al. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol (2004) 78:2255–64. doi:10.1128/JVI.78.5.2255-2264.2004
31. Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol (2005) 35:1113–23. doi:10.1002/eji.200425534
32. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med (2005) 202:673–85. doi:10.1084/jem.20050882
33. Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol Reprod (2003) 68:2024–32. doi:10.1095/biolreprod.102.012880
34. Wang X, Messerle M, Sapinoro R, Santos K, Hocknell PK, Jin X, et al. Murine cytomegalovirus abortively infects human dendritic cells, leading to expression and presentation of virally vectored genes. J Virol (2003) 77:7182–92. doi:10.1128/JVI.77.13.7182-7192.2003
35. Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, et al. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. J Virol (2005) 79:2998–3008. doi:10.1128/JVI.79.5.2998-3008.2005
36. Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature (2011) 473:523–7. doi:10.1038/nature10003
37. Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis (2011) 5:e1275. doi:10.1371/journal.pntd.0001275
38. Tierney R, Nakai T, Parkins CJ, Caposio P, Fairweather NF, Sesardic D, et al. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine (2012) 30:3047–52. doi:10.1016/j.vaccine.2012.02.043
39. Beverley PC, Ruzsics Z, Hey A, Hutchings C, Boos S, Bolinger B, et al. A novel murine cytomegalovirus vaccine vector protects against Mycobacterium tuberculosis. J Immunol (2014) 193:2306–16. doi:10.4049/jimmunol.1302523
40. Tsuda Y, Parkins CJ, Caposio P, Feldmann F, Botto S, Ball S, et al. A cytomegalovirus-based vaccine provides long-lasting protection against lethal Ebola virus challenge after a single dose. Vaccine (2015) 33:2261–6. doi:10.1016/j.vaccine.2015.03.029
41. Borkner L, Sitnik KM, Dekhtiarenko I, Pulm AK, Tao R, Drexler I, et al. Immune protection by a cytomegalovirus vaccine vector expressing a single low-avidity epitope. J Immunol (2017) 199:1737–47. doi:10.4049/jimmunol.1602115
42. Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin-Sain L. The context of gene expression defines the immunodominance hierarchy of cytomegalovirus antigens. J Immunol (2013) 190:3399–409. doi:10.4049/jimmunol.1203173
43. Hutchinson S, Sims S, O’hara G, Silk J, Gileadi U, Cerundolo V, et al. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PLoS One (2011) 6:e14646. doi:10.1371/journal.pone.0014646
44. Seckert CK, Griessl M, Buttner JK, Scheller S, Simon CO, Kropp KA, et al. Viral latency drives ‘memory inflation’: a unifying hypothesis linking two hallmarks of cytomegalovirus infection. Med Microbiol Immunol (2012) 201:551–66. doi:10.1007/s00430-012-0273-y
45. Farrington LA, Smith TA, Grey F, Hill AB, Snyder CM. Competition for antigen at the level of the APC is a major determinant of immunodominance during memory inflation in murine cytomegalovirus infection. J Immunol (2013) 190:3410–6. doi:10.4049/jimmunol.1203151
46. Turula H, Smith CJ, Grey F, Zurbach KA, Snyder CM. Competition between T cells maintains clonal dominance during memory inflation induced by MCMV. Eur J Immunol (2013) 43:1252–63. doi:10.1002/eji.201242940
47. Smith CJ, Turula H, Snyder CM. Systemic hematogenous maintenance of memory inflation by MCMV infection. PLoS Pathog (2014) 10:e1004233. doi:10.1371/journal.ppat.1004233
48. Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med (2005) 202:1349–61. doi:10.1084/jem.20051357
49. Trautmann L, Rimbert M, Echasserieau K, Saulquin X, Neveu B, Dechanet J, et al. Selection of T cell clones expressing high-affinity public TCRs within human cytomegalovirus-specific CD8 T cell responses. J Immunol (2005) 175:6123–32. doi:10.4049/jimmunol.175.9.6123
50. Day EK, Carmichael AJ, Ten Berge IJ, Waller EC, Sissons JG, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J Immunol (2007) 179:3203–13. doi:10.4049/jimmunol.179.5.3203
51. Humphreys IR, Loewendorf A, De Trez C, Schneider K, Benedict CA, Munks MW, et al. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: a CD4-dependent mechanism. J Immunol (2007) 179:2195–202. doi:10.4049/jimmunol.179.4.2195
52. Snyder CM, Loewendorf A, Bonnett EL, Croft M, Benedict CA, Hill AB. CD4+ T cell help has an epitope-dependent impact on CD8+ T cell memory inflation during murine cytomegalovirus infection. J Immunol (2009) 183:3932–41. doi:10.4049/jimmunol.0900227
53. Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, et al. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol (2010) 40:2762–8. doi:10.1002/eji.200940256
54. Walton SM, Torti N, Mandaric S, Oxenius A. T-cell help permits memory CD8(+) T-cell inflation during cytomegalovirus latency. Eur J Immunol (2011) 41:2248–59. doi:10.1002/eji.201141575
55. O’Hara GA, Welten SP, Klenerman P, Arens R. Memory T cell inflation: understanding cause and effect. Trends Immunol (2012) 33:84–90. doi:10.1016/j.it.2011.11.005
56. Rutigliano JA, Ruckwardt TJ, Martin JE, Graham BS. Relative dominance of epitope-specific CD8+ T cell responses in an F1 hybrid mouse model of respiratory syncytial virus infection. Virology (2007) 362:314–9. doi:10.1016/j.virol.2006.12.023
57. Ruckwardt TJ, Luongo C, Malloy AM, Liu J, Chen M, Collins PL, et al. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J Immunol (2010) 185:4673–80. doi:10.4049/jimmunol.1001606
58. Billam P, Bonaparte KL, Liu J, Ruckwardt TJ, Chen M, Ryder AB, et al. T cell receptor clonotype influences epitope hierarchy in the CD8+ T cell response to respiratory syncytial virus infection. J Biol Chem (2011) 286:4829–41. doi:10.1074/jbc.M110.191437
59. Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, Mcclaren JL, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog (2011) 7:e1002377. doi:10.1371/journal.ppat.1002377
60. Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol (2017) 10:545–54. doi:10.1038/mi.2016.48
61. Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol (1988) 26:153–62. doi:10.1002/jmv.1890260207
62. Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog (2014) 10:e1003934. doi:10.1371/journal.ppat.1003934
Keywords: CD8+ T cells, cytomegalovirus, memory inflation, respiratory syncytial virus, tissue-resident memory, vaccine
Citation: Morabito KM, Ruckwardt TJ, Bar-Haim E, Nair D, Moin SM, Redwood AJ, Price DA and Graham BS (2018) Memory Inflation Drives Tissue-Resident Memory CD8+ T Cell Maintenance in the Lung After Intranasal Vaccination With Murine Cytomegalovirus. Front. Immunol. 9:1861. doi: 10.3389/fimmu.2018.01861
Received: 26 March 2018; Accepted: 27 July 2018;
Published: 14 August 2018
Edited by:
Fathia Mami-Chouaib, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Steven Varga, University of Iowa, United StatesGeorg Gasteiger, Julius-Maximilians-Universität, Germany
Copyright: © 2018 Morabito, Ruckwardt, Bar-Haim, Nair, Moin, Redwood, Price and Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barney S. Graham, YmdyYWhhbUBuaWguZ292
 Kaitlyn M. Morabito
Kaitlyn M. Morabito Tracy J. Ruckwardt1
Tracy J. Ruckwardt1 Syed M. Moin
Syed M. Moin Alec J. Redwood
Alec J. Redwood Barney S. Graham
Barney S. Graham