
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 13 April 2018
Sec. Immunological Tolerance and Regulation
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00622
This article is part of the Research Topic Autoantibodies View all 89 articles
 Philippe Musette1*
Philippe Musette1* Jean David Bouaziz2*
Jean David Bouaziz2*
B cells are major effector cells in autoimmunity through antibody production, T cell help and pro-inflammatory cytokine production. Major advances have been made in human B cell biology knowledge using rituximab and type II new anti-CD20 antibodies, anti-CD19 antibodies, anti-CD22 antibodies, autoantigen specific B cell depleting therapy (chimeric antigen receptor T cells), and B cell receptor signaling inhibition (Bruton’s tyrosine kinase inhibitors). However, in certain circumstances B cell depleting therapy may lead to the worsening of the autoimmune disease which is in accordance with the existence of a regulatory B cell population. Current concepts and future directions for B cell modulating therapies in autoimmune diseases with a special focus on pemphigus are discussed.
B cells were primary identified for their key role as enhancers of the immune response in autoimmunity, because they give rise to autoantibody producing plasma cells and promote CD4+ T cell responses by antigen presentation. The B cells bearing these functions are usually considered as effector B cells. Recently published studies indicate that B cells can also act as negative sensors of the immune response in autoimmunity, these regulatory properties are mainly attributed to the recently identified interleukin 10 (IL-10) regulatory B cell compartment (Breg) (1–3). New therapies target these B cell populations with drugs directed against B cell surface markers (CD20, CD22), activating factors (BAFF, TACI), or cytokines (IL-6, TNFα, IFNα) (4, 5). The most focused strategy to target B cells in autoimmune diseases would be to specifically remove autoreactive effector B cells, and amplify autoantigen driven Bregs, while maintaining immune surveillance. Such a strategy is difficult to achieve especially because antigen specific targeting is challenging and, the relative contribution of B cells to the pathogenesis of autoimmune disease might differ considerably from one disease to another.
Autoreactive B cells give rise to autoreactive plasma cells whose pathogenicity might be direct through production of IgG+ autoantibodies that bind to specific target molecules (e.g., acetylcholine receptor on the motor end plate in myasthenia gravis; desmoglein 1 and 3 on keratinocyte in pemphigus) or through the formation of immune complexes in tissues that locally activate the complement cascade. B cells are also important effector cells in autoimmune diseases because they regulate lymphoid tissue structure, contribute to antigen presentation and costimulation (6), regulate dendritic cell function and pathways of T helper cell differentiation, and release inflammatory cytokines including IL-8, IL-6, LT-α, and TNF-α (7–10). Our review will focus on B cell therapies in various autoimmune disorders with a special focus on pemphigus (an autoimmune blistering skin disease). Table 1 summarizes recent studies in pemphigus that target B cells or their pathogenic antibodies.
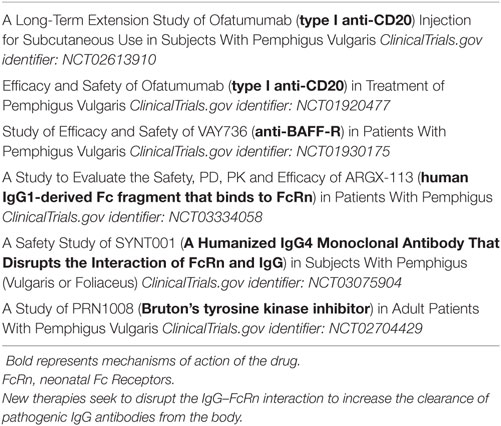
Table 1. Current ongoing studies in pemphigus that target B cells or their pathogenic antibodies (excluding studies with rituximab).
B cell depletion using type 1 anti-CD20 monoclonal antibodies (rituximab, ofatumumab, ocrelizumab) has shown varying degrees of efficacy in some human autoimmune diseases ranging from dramatic efficacy to sometimes worsening of symptoms (4, 11). Rituximab has been proved to be highly efficient in rheumatoid arthritis, pemphigus (12, 13), granulomatosis with polyangiitis, and microscopic polyangiitis (14). Ocrelizumab was recently proved to be efficient and FDA approved in relapsing multiple sclerosis (15, 16).
The limited efficacy or adverse effects of B cell depleting therapies in some diseases may be partially explained by the fact that rituximab also depletes the Breg compartment. IL-10-producing Bregs were first identified in mice and shown to downregulate immune response and inflammation, making them probably instrumental for maintenance of self tolerance. Numerous recent studies have also characterized IL-10-producing Bregs in humans and have started to decipher their phenotype and mode of suppression. The cell surface phenotype of human Bregs is mainly composed of CD24highCD27+ B cell subpopulation (17, 18) and CD24highCD38high transitional B cell subpopulation (19). Mechanisms of suppression include inhibition of CD4+ T proliferation, associated with induction and expansion of regulatory T cells, inhibition of Th1 differentiation, and also suppression of monocyte activation. The decreased frequency and/or decreased suppressive activity of Bregs have been recently shown in patients with lupus (19), immune thrombocytopenia (20), rheumatoid arthritis (21), ANCA-associated vasculitis (22), pemphigus (23), and systemic sclerosis (24). In addition an increase in Bregs is associated with better prognosis or complete remission in certain autoimmune diseases (25, 26). B cell depletion therapy using rituximab in systemic lupus erythematosus (SLE) (26, 27) and pemphigus (25) patients may also promote the emergence of a functional transitional Breg pool upon B cell reconstitution which may contribute to the long-term efficacy of rituximab in these diseases. Indeed rituximab induces a prolonged and continuous repopulation with naive B cells with a new repertoire (25, 28) after the initial B-cell depletion, whereas the reappearance of memory B cells is markedly delayed (25, 26). This blockage of B cell maturation is associated with a blockage of the auto-reactive IgM to IgG class switching process (25). Finally, B cell depletion induces a two step mechanism of immunosupression by eliminating the autoreactive B cells involved in the production of pathogenic IgG+ autoantibodies (28, 29) and by promoting the appearance of Bregs (25) (Figure 1). Rituximab therapy may, however, decrease the humoral immune response to recall antigens (30) which may increase the risk of patient infection including hepatitis B reactivation and progressive multifocal leukoencephalopathy (polyomavirus JC), whereas long-term anti tetanus and anti-pneumococcal antibody response is maintained (25). It also seems conceivable that, given the role of B cells in generating antitumor responses (31), B cell depletion using rituximab may contribute to long-term expansion of uncontrolled tumor cells (32). B cell depletion therapy using rituximab may be particularly efficient in pemphigus because it depletes memory B cells that give rise to short-lived plasma cells that produce pathogenic autoantibodies. Rituximab preserves long-lived plasma cells that may be more important for “natural” protection against infection. Pathogenic autoantibodies may be the result of a temporary breakdown of immune tolerance giving rise to pathogenic B cells clones that are abrogated using rituximab (33). B cell-activating factor (BAFF) and a proliferation-inducing ligand are important B cell differentiation and survival factors. Their targeting could also be interesting for pemphigus treatment.
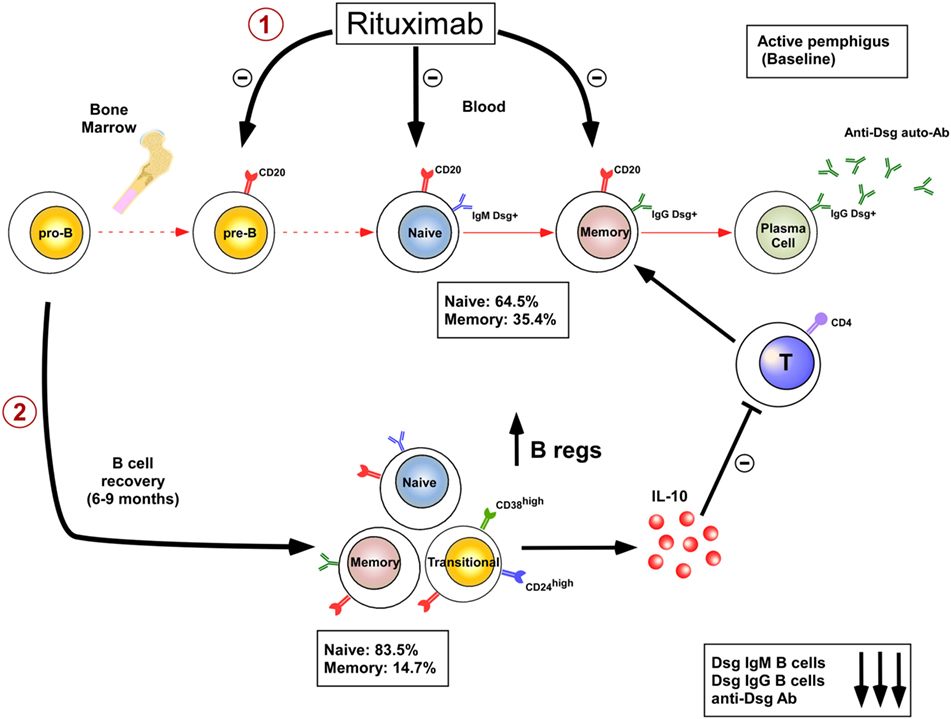
Figure 1. Dual mechanisms of B cell depletion: 1: elimination of autoreactive B cells; 2: induction of regulatory B cells.
A new class of “type II” anti-CD20 antibodies (obinutuzumab) that induce little complement-dependent cytotoxicity but induce signaling-dependent B cell death is under development (34). This type II class is particularly interesting because, contrary to rituximab, it may be more efficient at depleting organ resident B cells.
New strategies will require specific targeting of the pathogenic effector functions of B cells and if possible the promotion of their regulatory function without modifying B cell dependent immune surveillance.
Anti-CD20 mAbs efficiently deplete mature naive and memory B cells, inhibit the development of short-lived plasma cells, but spare long-lived plasma cells and previously produced protective antibodies acquired during infection or vaccination. Contrary to CD20, CD19 is expressed from early B cell development (pro-B stage) to the last differentiation stage including plasma cells. These features make CD19 an attractive target for B cell depletion. MEDI-551 (inebilizumab) is a human IgG1 anti-CD19 mAb that was developed for the treatment of multiple sclerosis (35). Interestingly, bispecific antibodies (blinatumomab) that redirect T cells to CD19 have been developed and have shown efficacy for the treatment of acute lymphoblastic leukemia (36). Such bispecific antibodies that target leukemic and normal B cells could be an interesting approach for the treatment of autoimmune diseases in the future.
Epratuzumab is a humanized monoclonal antibody that binds to the glycoprotein CD22 (an inhibitory C-type lectin) expressed on mature and malignant B cells. Epratuzumab has completed phase III trials for the treatment of SLE (37). Although endpoints were not reached in this study, the drug was well tolerated. Epratuzumab diminishes B-cell receptor activation (38) and induces only partial B-cell depletion (39).
Targeting antigen specific immune response represents a very interesting strategy. In this respect a promising strategy has been developed for the treatment of pemphigus vulgaris, a blistering autoimmune skin disease, in which autoimmune B cells produce autoantibodies against the desmoglein 1 and 3 antigens present in the skin. Until recently, different approaches including immunoabsorption of Dsg autoantibodies ex vivo (40), or anti idiotype antibodies directed against Dsg3 specific autoantibodies (41) were developed without clinical impact. Recently, alive T cells that express a chimeric antigen receptor (CAR) composed of an antibody structure fused to the CD3 signaling domain engineered to recognize tumor-associated antigens have shown remarkable efficacy in B cell leukemia (42). Moreover CAR T cells are able to proliferate and expand in vivo. A new approach is now being developed with a CAAR including truncated fragments of the Dsg3 extracellular domain fused to CD137/CD3 signaling domains expressed on T cells. This CAAR is able to recognize autoantibodies against Dsg3 fixed on the B cell membrane (Figure 2). CAAR T cells have shown efficacy in eliminating anti-Dsg3 specific B cells in vitro and in a pemphigus mice model (43).
Activation of the B cell receptor induces downstream signaling of multiple kinases crucial for B cell activation. Among them, Lyn, Syk, PI3K, and Bruton’ tyrosine kinase (BTK) are potential therapeutic targets for autoreactive B cell silencing and depletion. Among the potential drugs, ibrutinib, a pharmacological BTK inhibitor has been developed and licensed for the treatment of chronic lymphocytic leukemia. Targeting BTK using ibrutinib may be an interesting approach to treat autoimmune diseases. Indeed, transgenic mice overexpressing BTK in B cells manifested SLE-like autoimmune disease involving kidneys, lungs, and salivary glands (44). Recently, ibrutinib has shown efficacy in chronic graft versus host disease the physiopathology of which involves allo-reactive B and T cells. Based on these results, ibrutinib was approved in the US for treatment of adult patients with GVHD after failure of one or more lines of systemic therapy (45). A new BTK inhibitor known as PRN1008 is under evaluation for the treatment of pemphigus in humans (ClinicalTrials.gov Identifier: NCT02704429).
Much progress has been made in depleting circulating and resident B cells present in inflamed tissue and secondary lymphoid organs. An ideal strategy in the future will require specific targeting of the pathogenic effector functions of B cells and if possible the promotion of their regulatory function without modifying B cell-dependent immune surveillance. More targeted therapies on specific B cell populations and functions will allow better patient management.
PM and JDB contributed equally to the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to Nikki Sabourin-Gibbs (Rouen University Hospital) for her help in editing the manuscript.
The paper was funded by INSERM.
CAR, chimeric antigen receptor; CAAR, chimeric autoantibody receptor; BTK, Bruton’s tyrosine kinase.
1. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3:944–50. doi:10.1038/ni833
2. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol (2012) 30:221–41. doi:10.1146/annurev-immunol-020711-074934
3. Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev (2008) 224:201–14. doi:10.1111/j.1600-065X.2008.00661.x
4. Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev (2010) 237:264–83. doi:10.1111/j.1600-065X.2010.00945.x
5. Jordan N, Lutalo PMK, D’Cruz DP. Novel therapeutic agents in clinical development for systemic lupus erythematosus. BMC Med (2013) 11:120. doi:10.1186/1741-7015-11-120
6. Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med (1993) 177:679–90. doi:10.1084/jem.177.3.679
7. Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol (2000) 1(6):475–82. doi:10.1038/82717
8. Defuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T cell function and inflammatory cytokine profile. Proc Natl Acad Sci U S A (2013) 110:5133–8. doi:10.1073/pnas.1215840110
9. Hamze M, Desmetz C, Guglielmi P. B cell-derived cytokines in diseases. Eur Cytokine Netw (2013) 24:20–6. doi:10.1684/ecn.2013.0327
10. Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med (2012) 209:1001–10. doi:10.1084/jem.20111675
11. Thaunat O, Morelon E, Defrance T. Am“B”valent: anti CD20 antibodies unravel the dual role of B cells in immunopathogenesis. Blood (2010) 116:515–21. doi:10.1182/blood-2010-01-266668
12. Cohen MD, Keystone E. Rituximab for rheumatoid arthritis. Rheumatol Ther (2015) 2:99–111. doi:10.1007/s40744-015-0016-9
13. Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet (2017) 389:2031–40. doi:10.1016/S0140-6736(17)30070-3
14. Guillevin L, Pagnoux C, Karras A. Rituximab versus azathioprine for maintenance in ANCA associated vasculitis. N Engl J Med (2014) 371:1771–80. doi:10.1056/NEJMoa1404231
15. Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med (2017) 376:221–34. doi:10.1056/NEJMoa1601277
16. Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med (2017) 376:209–20. doi:10.1056/NEJMoa1606468
17. Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol (2010) 40:2686–91. doi:10.1002/eji.201040673
18. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011) 117:530–41. doi:10.1182/blood-2010-07-294249
19. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity (2010) 32:129–40. doi:10.1016/j.immuni.2009.11.009
20. Li X, Zhong H, Bao W, Boulad N, Evangelista J, Haider MA, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood (2012) 120(16):3318–25. doi:10.1182/blood-2012-05-432575
21. Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH2 differentiation. Sci Transl Med (2013) 5:173ra23. doi:10.1126/scitranslmed.3005407
22. Wilde B, Thewissen M, Damoiseaux J, Knippenberg S, Hilhorst M, van Paassen P, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis (2013) 72:1416–9. doi:10.1136/annrheumdis-2012-202986
23. Zhu HQ, Xu RC, Chen YY, Yuan HJ, Cao H, Zhao XQ, et al. Impaired function of CD19(+) CD24(hi) CD38(hi) regulatory B cells in patients with pemphigus. Br J Dermatol (2015) 172(1):101–10. doi:10.1111/bjd.13192
24. Matsushita T, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. Decreased levels of regulatory B cells in patients with systemic sclerosis: association with autoantibody production and disease activity. Rheumatology (Oxford) (2016) 55(2):263–7. doi:10.1093/rheumatology/kev331
25. Colliou N, Picard D, Caillot F, Calbo S, Le Corre S, Lim A, et al. Long-Term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med (2013) 5(175):175ra30. doi:10.1126/scitranslmed.3005166
26. Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum (2007) 56:3044–56. doi:10.1002/art.22810
27. Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol (2009) 182:5982–93. doi:10.4049/jimmunol.0801859
28. Mouquet H, Musette P, Gougeon ML, Jacquot S, Lemercier B, Lim A, et al. B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J Invest Dermatol (2008) 128:2859–69. doi:10.1038/jid.2008.178
29. Joly P, Mouquet H, Roujeau JC, D’Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med (2007) 357:545–52. doi:10.1056/NEJMoa067752
30. van der Kolk LE, Baars JW, Prins MH, Van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood (2002) 100:2257–9.
31. Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol (2009) 183(5):3195–203. doi:10.4049/jimmunol.0803773
32. Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, et al. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol (2011) 29(7):814–24. doi:10.1200/JCO.2010.28.9777
33. Hammers CM, Chen J, Lin C, Kacir S, Siegel DL, Payne AS, et al. Persistence of anti-desmoglein 3 IgG(+) B-cell clones in pemphigus patients over years. J Invest Dermatol (2015) 135(3):742–9. doi:10.1038/jid.2014.291
34. Niederfellner G, Lammens A, Mundigl O, Georges GJ, Schaefer W, Schwaiger M, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of anti CD20 antibodies. Blood (2011) 118:358–67. doi:10.1182/blood-2010-09-305847
35. Agius MA, Klodowska-Duda G, Maciejowski M, Potemkowski A, Li J, Patra K, et al. Safety and tolerability of inebilizumab (MEDI-551) an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler (2017). doi:10.1177/1352458517740641
36. Velasquez MP, Bonifant C, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood (2018) 131:30–8. doi:10.1182/blood-2017-06-741058
37. Wallace DJ, Gordon C, Strand V, Hobbs K, Petri M, Kalunian K, et al. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus from two randomized, double-blind, placebo-controlled multicentre studies (ALLEVIATE) and follow-up. Rheumatology (2013) 52:1313–22. doi:10.1093/rheumatology/ket129
38. Sieger N, Fleischer SJ, Mei HE. CD22 ligation inhibits downstream B cell receptor signaling and downstream Ca2+ flux upon activation. Arthritis Rheum (2013) 65:770–9. doi:10.1002/art.37818
39. Wallace DJ, Kalunian K, Petri MA. Efficacy and safety of eprazumab in patients with moderate/severe active systemic lupus erythematous: results from EMBLEM, a phase IIb, randomized, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis (2014) 73:183–90. doi:10.1136/annrheumdis-2012-202760
40. Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest (1994) 94:919–26. doi:10.1172/JCI117349
41. Payne AS, Siegel DL, Stanley JR. Targeting pemphigus autoantibodies through their heavy-chain variable region genes. J Invest Dermatol (2007) 127:1681–91. doi:10.1038/sj.jid.5700790
42. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med (2011) 365:725–33. doi:10.1056/NEJMoa1103849
43. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science (2016) 353:179–84. doi:10.1126/science.aaf6756
44. Kil LP, de Bruijn MJ, Van Nimwegen M, Corneth OB, van Hamburg JP, Dingjan GM, et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood (2012) 119:3744–56. doi:10.1182/blood-2011-12-397919
Keywords: autoimmunity, autoantibodies, B cell, B cell depletion, regulatory B cell
Citation: Musette P and Bouaziz JD (2018) B Cell Modulation Strategies in Autoimmune Diseases: New Concepts. Front. Immunol. 9:622. doi: 10.3389/fimmu.2018.00622
Received: 12 January 2018; Accepted: 13 March 2018;
Published: 13 April 2018
Edited by:
Ralf J. Ludwig, University of Lübeck, GermanyReviewed by:
John Stanley, University of Pennsylvania, United StatesCopyright: © 2018 Musette and Bouaziz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philippe Musette, cGhpbGlwcGUubXVzZXR0ZUBjaHUtcm91ZW4uZnI=;
Jean David Bouaziz, amVhbi1kYXZpZC5ib3Vheml6QGFwaHAuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.