
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 12 April 2018
Sec. Immunological Tolerance and Regulation
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.00542
This article is part of the Research TopicAutoantibodiesView all 89 articles
 Yosuke Mai1
Yosuke Mai1 Wataru Nishie1*
Wataru Nishie1* Kazumasa Sato2
Kazumasa Sato2 Moeko Hotta1
Moeko Hotta1 Kentaro Izumi1
Kentaro Izumi1 Kei Ito2
Kei Ito2 Kazuyoshi Hosokawa3
Kazuyoshi Hosokawa3 Hiroshi Shimizu1
Hiroshi Shimizu1
Bullous pemphigoid (BP) is a common autoimmune blistering disease in which autoantibodies mainly target the hemidesmosomal component BP180 (also known as type XVII collagen) in basal keratinocytes. Various triggering factors are known to induce BP onset, including radiotherapy, burns, ultraviolet exposure, surgery, and the use of dipeptidyl peptidase-IV inhibitors (DPP4i), which are widely used antihyperglycemic drugs. Here, we present a case of BP triggered by a thermal burn under medication with DPP4i. A 60-year-old man with type II diabetes had been treated with the DPP4i linagliptin for 1 year. After the right forearm experienced a thermal burn, blisters developed around the burned area and gradually spread over the whole body with the production of autoantibodies targeting the non-NC16A domain of BP180. The diagnosis of BP was confirmed by immunohistopathological examination. Upon withdrawal of linagliptin and treatment with topical steroid and minocycline, complete remission was achieved after 4 months. Previously, 13 cases of BP that developed after thermal burns have been reported, and our case shared some of the clinical features of these thermal burn-induced BP cases. Interestingly, the present case also showed the typical clinical, histopathological, and immunological features of the non-inflammatory type of DPP4i-associated BP (DPP4i-BP). Although the pathogenesis of BP remains uncertain, the present case suggests that DPP4i may trigger the onset of BP similarly to a thermal burn. In addition, the clinical and histopathological features of DPP4i-BP may be distinct from other types of BP.
A 60-year-old man with type II diabetes was referred to our department due to blisters and erosions over the whole body. He had been treated with linagliptin, a dipeptidyl peptidase-IV inhibitor (DPP4i), for 1 year. Two months before the referral, he had suffered a deep, 7-cm-long dermal burn on the right forearm from a kitchen accident. Within 2 days after the burn, blisters had appeared on the right forearm, which were treated with a topical antibiotic ointment. However, multiple blisters and erosions gradually developed over the body over the course of 2 months. Physical examination revealed tense blisters and erosions of 5 mm to 2 cm in diameter with circumscribed erythematous lesions predominantly on the right forearm (Figure 1A). Blisters and erosions less than 5 mm in diameter without erythema were found on the face, the trunk, and the left leg (Figure 1B). Although BP180 NC16A chemiluminescent enzyme immunoassay (CLEIA) was negative (5.9 U/mL; normal, <9.0 U/mL), histopathological examination of the blister showed sub-epidermal blister formation with some eosinophilic infiltration in the dermis (Figure 1C). Direct immunofluorescence showed linear deposition of IgG autoantibodies along the dermal–epidermal junction (Figure 1D), and 1 M NaCl-split skin indirect immunofluorescence revealed circulating IgG autoantibodies reacting with the epidermal side of the artificial blisters (not shown). Notably, enzyme-linked immunosorbent assay (ELISA) using full-length recombinant BP180 was positive (index value, 41.8; normal, <4.64) (1). Based on these findings, the diagnosis of bullous pemphigoid (BP) was made. Linagliptin was withdrawn 2 days after the referral, and treatment with a topical steroid and minocycline at 100 mg/day was started. His skin lesions gradually improved, and ELISA with full-length recombinant BP180 became negative (index value, 1.03). Complete remission off therapy was achieved 4 months later (Figure 2). Although no DPP4i was re-administered, blisters appeared on the left forearm 16 months later, when he developed cellulitis on the right leg. The histopathology of the blister showed sub-epidermal blistering with scant eosinophilic infiltration in the dermis (not shown), which was consistent with BP. BP180 NC16A CLEIA was still negative (<3 U/mL). Topical steroid, minocycline at 200 mg/day, and nicotinamide at 1,500 mg/day were initiated, and the lesions completely resolved 2 months later. At 2 months after the cessation of treatment, no recurrence was observed (Figure 2).
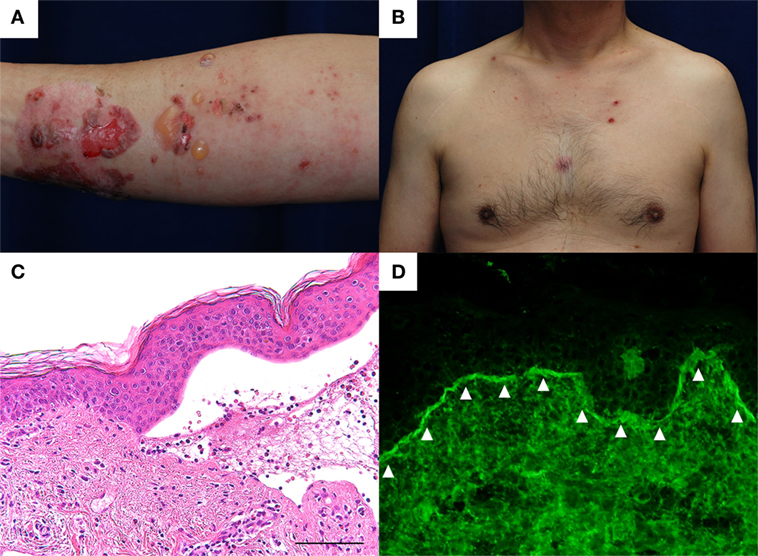
Figure 1. (A) Tense blisters and erosions of less than 2 cm in diameter developed over the thermal burn scar on the right forearm. Circumscribed erythematous lesions were also found. (B) Small erosions of less than 5 mm in diameter were found on the trunk without erythema. (C) Histopathological examination of the blister shows sub-epidermal blistering with some eosinophilic infiltration in the dermis. Scale bar: 100 µm. (D) Direct immunofluorescence shows linear IgG autoantibody deposits at the basement membrane zone (arrowheads).
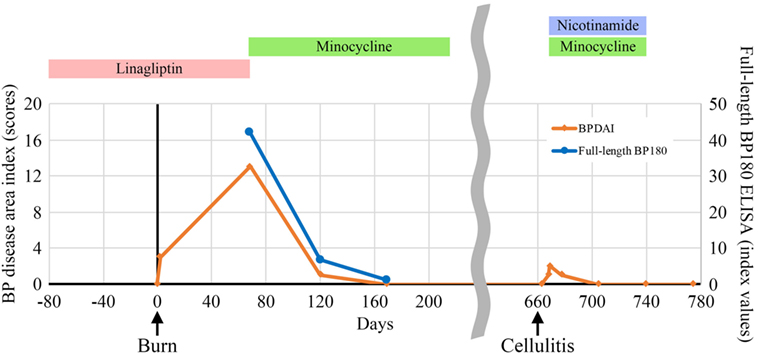
Figure 2. Indices of the bullous pemphigoid (BP) disease area index scores (2) and enzyme-linked immunosorbent assay (ELISA) using full-length recombinant BP180 during the clinical course.
Bullous pemphigoid is a common autoimmune blistering disease in which autoantibodies target the hemidesmosomal components BP180 (also known as type XVII collagen) and/or BP230 at the dermal–epidermal junction (3). Clinically, tense blister formation associated with itchy urticarial erythema is typically observed in BP patients, and sub-epidermal blister formation with eosinophilic infiltration is histopathologically observed (3). Although the etiology of BP remains unclear, various factors, including radiotherapy, burns, ultraviolet (UV) exposure, trauma, surgical procedures, topical medications, and infections are known to trigger the onset of the disease (4, 5). Recently, emerging evidence reports that the use of DPP4i, which are widely used to treat patients with diabetes mellitus, increases the risk of BP onset (6–9).
Herein, we report a case of BP in which a thermal burn triggered the onset of the disease. As shown in Table 1, various BP-inducing physical factors, including radiation (5, 10–18), UV radiation (5, 19–30), surgical wounds (5, 31–45), ostomy (31, 46–51), burns (5, 52–62), skin grafts (59, 63–67), and other trauma (5, 68–72) have been reported in the English literature. Regarding thermal burns, 13 cases have been reported (Table 2) (5, 52–61). Notably, in contrast to the present case, none of those 13 cases relapsed (5, 52–61) suggesting that the present case had a unique clinical course.
Interestingly, linagliptin, which is a DPP4i, was adminis-tered to our case until the onset of BP. DPP4i-associated BP (DPP4i-BP) is a unique, recently reported subtype of BP that develops with the administration of DPP4i medications such as vildagliptin, sitagliptin, and linagliptin (6–9). DPP4i-BP tends to show scant erythema and low levels of autoantibodies targeting the NC16A domain of BP180 or the absence of such autoantibodies (1, 73). In our case, BP developed after 1 year of DPP4i administration, in which erythema was scantly observed and autoantibodies specifically targeted the non-NC16A domain of BP180. In addition, complete remission was achieved after the with-drawal of DPP4i and the initiation of treatment with topical steroid and systemic minocycline. Histopathologically, eosinophilic infiltration was not evident. These characteristics closely resembled those of the non-inflammatory type of DPP4i-BP (1, 73); therefore, DPP4i use may also be involved in the development of the disease. It should be noted that a recent study reported that 18% of DPP4i-BP cases relapsed even after the withdrawal of DPP4i (7). Although the immunohistopathological results were uncertain, the present case developed BP-like blisters when he suffered from cellulitis 16 months after complete remission, indicating that DPP4i was also associated with the BP onset in the present case.
The pathogenic mechanisms of the physical trigger for BP onset remain elusive. The physical factors cause tissue destruction that activates the inflammatory process, which may result in the observed auto-reactivity to basement membrane proteins, including BP180 (5). Alternatively, basement membrane proteins may be altered as a result of physical factors (74) resulting in immunogenicity with increased affinity to certain human leukocyte antigen (HLA) alleles (75). Notably, 86% of cases of the non-inflammatory type of DPP4i-BP carried HLA-DQB1*03:01 (73). Although the HLA allele was not examined, it is likely that the present case had a genetic propensity to the breakdown of self-tolerance to BP180.
In conclusion, we reported a case of BP induced by a thermal burn. Interestingly, the patient received DPP4i and showed the typical clinical, histopathological, and immunological characteristics of the non-inflammatory type of DPP4i-BP. Although the pathogenesis of thermal burn-induced BP and DPP4i-BP remains uncertain, BP may occur due to various triggering factors.
This report on a single patient complies with the Declaration of Helsinki. The patient gave written informed consent for the publication of this report.
YM, WN, and KIzumi drafted the paper. KS, MH, KIto, and KH were involved in treating the patient and collecting the clinical data. HS supervised the writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
The authors thank the patient and express their appreciation to Hiroko Azuma for her technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (#24390274 to WN) and Challenging Exploratory Research (#15K15409 to WN).
BP, bullous pemphigoid; DPP4i, dipeptidyl peptidase-IV inhibitor; DPP4i-BP, dipeptidyl peptidase-IV inhibitor-associated bullous pemphigoid; CLEIA, chemiluminescent enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; HLA, human leukocyte antigen.
1. Izumi K, Nishie W, Mai Y, Wada M, Natsuga K, Ujiie H, et al. Autoantibody profile differentiates between inflammatory and noninflammatory bullous pemphigoid. J Invest Dermatol (2016) 136:2201–10. doi:10.1016/j.jid.2016.06.622
2. Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol (2012) 66:479–85. doi:10.1016/j.jaad.2011.06.032
3. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet (2013) 26:320–32. doi:10.1016/S0140-6736(12)61140-4
4. Lo SA, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol (2013) 31:391–9. doi:10.1016/j.clindermatol.2013.01.006
5. Dănescu S, Chiorean R, Macovei V, Sitaru C, Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol (2016) 43:134–40. doi:10.1111/1346-8138.13031
6. Skandalis K, Spirova M, Gaitanis G, Tsartsarakis A, Bassukas ID. Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol (2012) 26:249–53. doi:10.1111/j.1468-3083.2011.04062.x
7. Attaway A, Mersfelder TL, Vaishnav S, Baker JK. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors. A case report and review of literature. J Dermatol Case Rep (2014) 8:24–8. doi:10.3315/jdcr.2014.1166
8. Béné J, Moulis G, Bennani I, Auffret M, Coupe P, Babai S, et al. Bullous pemphigoid and dipeptidyl peptidase IV inhibitors: a case-noncase study in the French pharmacovigilance database. Br J Dermatol (2016) 175:296–301. doi:10.1111/bjd.14601
9. Sakai A, Shimomura Y, Ansai O, Saito Y, Tomii K, Tsuchida Y, et al. Linagliptin-associated bullous pemphigoid that was most likely caused by IgG autoantibodies against the midportion of BP180. Br J Dermatol (2017) 176:541–3. doi:10.1111/bjd.15111
10. Mul VE, van Geest AJ, Pijls-Johannesma MC, Theys J, Verschueren TA, Jager JJ, et al. Radiation-induced bullous pemphigoid: a systematic review of an unusual radiation side effect. Radiother Oncol (2007) 82:5–9. doi:10.1016/j.radonc.2006.11.014
11. Cabrera-Rodríguez JJ, Muñoz-García JL, Quirós Rivero J, Ropero Carmona F, Ríos Kavadoy Y. Radio-induced bullous pemphigoid. Clin Transl Oncol (2010) 12:66–8. doi:10.1007/s12094-010-0469-9
12. Olsha O, Lijoretzky G, Grenader T. Bullous pemphigoid following adjuvant radiotherapy for breast cancer. Breast J (2011) 17:204–5. doi:10.1111/j.1524-4741.2010.01060.x
13. Zhou X, Velez NF, Farsani T, Tsiaras W, Laga AC, Werchniak AE, et al. Multiple tense bullae localized to the right breast in a woman in her seventies. JAMA Dermatol (2013) 149:1427–8. doi:10.1001/jamadermatol.2013.4704
14. Nieder C, Al-Shibli K, Tollåli T. Nontargeted effect after radiotherapy in a patient with non-small cell lung cancer and bullous pemphigoid. Case Rep Oncol Med (2015) 2015:964687. doi:10.1155/2015/964687
15. Campa M, Mansouri B, Wilcox B, Griffin JR. Radiation-induced localized bul-lous pemphigoid in a patient with breast carcinoma. Dermatol Online J (2016) 22.
16. Shon W, Wada DA, Kalaaji AN. Radiation-induced pemphigus or pemphigoid disease in 3 patients with distinct underlying malignancies. Cutis (2016) 97:219–22.
17. Hirotsu K, Chiou AS, Chiang A, Kim J, Kwong BY, Pugliese S. Localized bullous pemphigoid in a melanoma patient with dual exposure to PD-1 checkpoint inhibition and radiation therapy. JAAD Case Rep (2017) 3:404–6. doi:10.1016/j.jdcr.2017.06.004
18. Kluger N, Mandelin J, Santti K, Jeskanen L, Nuutinen P. Bullous pemphigoid triggered by radiotherapy for breast cancer. Presse Med (2017) 46:128–30. doi:10.1016/j.lpm.2016.09.019
19. George PM. Bullous pemphigoid possibly induced by psoralen plus ultraviolet A therapy. Photodermatol Photoimmunol Photomed (1996) 11:185–7. doi:10.1111/j.1600-0781.1995.tb00166.x
20. Roeder C, Driesch PV. Psoriatic erythroderma and bullous pemphigoid treated successfully with acitretin and azathioprine. Eur J Dermatol (1999) 9:537–9.
21. Denli YG, Uslular C, Acar MA. Bullous pemphigoid in a psoriatic patient. J Eur Acad Dermatol Venereol (2000) 14:316–7. doi:10.1046/j.1468-3083.2000.00059-4.x
22. Salmhofer W, Soyer HP, Wolf P, Födinger D, Hödl S, Kerl H. UV light-induced linear IgA dermatosis. J Am Acad Dermatol (2004) 50:109–15. doi:10.1016/S0190-9622(03)02120-0
23. Washio H, Hara H, Suzuki H, YoshidA M, Hashimoto T. Bullous pemphigoid on psoriasis lesions after UVA radiation. Acta Derm Venereol (2005) 85:561–3. doi:10.1080/00015550510035677
24. Barnadas MA, Gilaberte M, Pujol R, Agustí M, Gelpí C, Alomar A. Bullous pemphigoid in a patient with psoriasis during the course of PUVA therapy: study by ELISA test. Int J Dermatol (2006) 45:1089–92. doi:10.1111/j.1365-4632.2004.02517.x
25. Sugita K, Kabashima K, Nishio D, Hashimoto T, Tokura Y. Th2 cell fluctuation in association with reciprocal occurrence of bullous pemphigoid and psoriasis vulgaris. J Eur Acad Dermatol Venereol (2007) 21:569–70. doi:10.1111/j.1468-3083.2006.01966.x
26. Saraceno R, Citarella L, Spallone G, Chimenti S. A biological approach in a patient with psoriasis and bullous pemphigoid associated with losartan therapy. Clin Exp Dermatol (2008) 33:154–5. doi:10.1111/j.1365-2230.2007.02603.x
27. Kao CL, Krathen RA, Wolf JE Jr, Fuerst JF, Hsu S. Psoralen plus ultraviolet A-induced bullous pemphigoid. J Drugs Dermatol (2008) 7:695–6.
28. Takeichi S, Kubbo Y, Arase S, Hashimoto T, Ansai S. Brunsting-Perry type localized bullous pemphigoid, possibly induced by furosemide administration and sun exposure. Eur J Dermatol (2009) 19:500–3. doi:10.1684/ejd.2009.0715
29. Riyaz N, Nasir N, Bindu V, Sasidharanpillai S. Bullous pemphigoid induced by topical PUVASOL. Indian J Dermatol Venereol Leprol (2014) 80:363–4. doi:10.4103/0378-6323.136936
30. Özkesici B, Koç S, Akman-Karakaş A, Yılmaz E, Başsorgun İC, Uzun S. PUVA induced bullous pemphigoid in a patient with mycosis fungoides. Case Rep Dermatol Med (2017) 2017:6134752. doi:10.1155/2017/6134752
31. Asbrink E, Hovmark A. Clinical variations in bullous pemphigoid with respect to early symptoms. Acta Derm Venereol (1981) 61:417–21.
33. Massa MC, Freeark RJ, Kang JS. Localized bullous pemphigoid occurring in a surgical wound. Dermatol Nurs (1996) 8:101–3.
34. Freeman BD, Rubin BG. Bullous pemphigoid after prosthetic vascular graft placement. Surgery (1998) 124:112–3. doi:10.1016/S0039-6060(98)70085-6
35. Honl BA, Elston DM. Autoimmune bullous eruption localized to a breast reconstruction site: response to niacinamide. Cutis (1998) 62:85–6.
36. Anderson CK, Mowad CM, Goff ME, Pelle MT. Bullous pemphigoid arising in surgical wounds. Br J Dermatol (2001) 145:670–2. doi:10.1046/j.1365-2133.2001.04427.x
37. Yesudian PD, Dobson CM, Ahmad R, Azurdia RM. Trauma-induced bullous pemphigoid around venous access site in a haemodialysis patient. Clin Exp Dermatol (2002) 27:70–2. doi:10.1046/j.0307-6938.2001.00938.x
38. Korfitis C, Gregoriou S, Georgala S, Christofidou E, Danopoulou I. Trauma-induced bullous pemphigoid. Indian J Dermatol Venereol Leprol (2009) 75:617–9. doi:10.4103/0378-6323.57732
39. Sen BB, Ekiz Ö, Rifaioglu EN, Sen T, Atik E, Dogramaci AÇ. Localized bullous pemphigoid occurring on surgical scars. Indian J Dermatol Venereol Leprol (2013) 79:554. doi:10.4103/0378-6323.113111
40. Lo Schiavo A, Caccavale S, Alfano R, Gambardella A, Cozzi R. Bullous pemphigoid initially localized around the surgical wound of an arthroprothesis for coxarthrosis. Int J Dermatol (2014) 53:e289–90. doi:10.1111/ijd.12222
41. Zeng R, Chen H, Jiang Y, Li M. Bullous pemphigoid after femur fracture surgery: a mere coincidence? Indian J Dermatol Venereol Leprol (2014) 80:195. doi:10.4103/0378-6323.129438
42. Neville JA, Yosipovitch G. Flare of bullous pemphigoid in surgically treated skin. Cutis (2015) 75:169–70.
43. Garrido Colmenero C, García Durá E, Aneiros Fernández J, Arias Santiago S. Bullous pemphigoid on surgical scar. Med Clin (Barc) (2015) 144:e1. doi:10.1016/j.medcli.2014.05.011
44. Singh D, Swann A. Bullous pemphigoid after bilateral forefoot surgery. Foot Ankle Spec (2015) 8:68–72. doi:10.1177/1938640014546865
45. Murphy B, Walsh M, McKenna K. Bullous pemphigoid arising in lower leg vein graft incision site. J Card Surg (2016) 31:57–9. doi:10.1111/jocs.12668
46. Cecchi R, Paoli S, Giomi A. Peristomal bullous pemphigoid. J Eur Acad Dermatol Venereol (2004) 18:515–6. doi:10.1111/j.1468-3083.2004.00957.x
47. Torchia D, Caproni M, Ketabchi S, Antiga E, Fabbri P. Bullous pemphigoid initially localized around a urostomy. Int J Dermatol (2006) 45:1387–9. doi:10.1111/j.1365-4632.2006.03118.x
48. Nozu T, Mita H. Bullous pemphigoid and percutaneous endoscopic gastrostomy. Intern Med (2010) 49:971–5. doi:10.2169/internalmedicine.49.3346
49. Marzano AV, Vezzoli P, Colombo A, Serini SM, Crosti C, Berti E. Peristomal bullous pemphigoid. J Dermatol (2010) 37:840–2. doi:10.1111/j.1346-8138.2010.00851.x
50. Batalla A, Peón G, De la Torre C. Localized bullous pemphigoid at urostomy site. Indian J Dermatol Venereol Leprol (2011) 77:625. doi:10.4103/0378-6323.84067
51. Felton S, Al-Niaimi F, Lyon C. Peristomal and generalized bullous pemphigoid in patients with underlying inflammatory bowel disease: is plectin the missing link? Ostomy Wound Manage (2012) 58:34–8.
52. Jevtic A, Grigoris I. Bullous pemphigoid induced by a burn. Australas J Dermatol (1991) 32:69–70. doi:10.1111/j.1440-0960.1991.tb00065.x
53. Quartey-Papafio CM, Hudson PM. Bullous pemphigoid initially localized to sites of burns (scalds) in a patient on sulphasalazine for ulcerative colitis. Clin Exp Dermatol (1994) 19:281. doi:10.1111/j.1365-2230.1994.tb01192.x
54. Balato N, Ayala F, Patruno C, Ruocco V. Bullous pemphigoid induced by a thermal burn. Int J Dermatol (1994) 33:55–6. doi:10.1111/j.1365-4362.1994.tb01498.x
55. Vassileva S, Mateev G, Balabanova M, Tsankov N. Burn-induced bullous pemphigoid. J Am Acad Dermatol (1994) 30:1027–8. doi:10.1016/S0190-9622(09)80149-7
56. Wagner GH, Ive FA, Paraskevopoulos S. Bullous pemphigoid and burns: the unveiling of the attachment plaque? Australas J Dermatol (1995) 36:17–20. doi:10.1111/j.1440-0960.1995.tb00918.x
57. Xu HH, Xiao T, He CD, Jin GY, Wang YK, Gao XH, et al. Bullous pemphigoid triggered by a boiling water burn. Eur J Dermatol (2008) 18:466–7. doi:10.1684/ejd.2008.0449
58. Bachmeyer C, Cabanne-Hamy A, Moguelet P, Doizi S, Callard P. Bullous pemphigoid after boiling water burn. South Med J (2010) 103:1175–7. doi:10.1097/SMJ.0b013e3181efb58c
59. Neri I, Antonucci VA, Balestri R, Tengattini V, Iozzo I, Bardazzi F. Bullous pemphigoid appearing both on thermal burn scars and split-thickness skin graft donor sites. J Dtsch Dermatol Ges (2013) 11:675–6. doi:10.1111/ddg.12094
60. Damevska K, Gocev G, Nikolovska S. A case of burn-induced bullous pemphigoid. J Burn Care Res (2014) 35:e281–2. doi:10.1097/BCR.0b013e3182901124
61. Morita R, Oiso N, Ishii N, Tatebayashi M, Matsuda H, Hashimoto T, et al. Case of burn-associated bullous pemphigoid caused by anti-BP230 immunoglobulin G autoantibodies. J Dermatol (2015) 42:657–8. doi:10.1111/1346-8138.12848
62. Chen DM, Fairley JA. A bullous pemphigoid-like skin eruption after a chemical burn. J Am Acad Dermatol (1998) 38:337–40. doi:10.1016/S0190-9622(98)70578-X
63. McGrath J, Black M. Split skin grafting and bullous pemphigoid. Clin Exp Dermatol (1991) 16:72–3. doi:10.1111/j.1365-2230.1991.tb00306.x
64. Levin DL, Sadhwani A. Blistering on a squamous cell carcinoma graft site in a patient with bullous pemphigoid. Cutis (1994) 54:40.
65. Ghura HS, Johnston GA, Milligan A. Development of a bullous pemphigoid after split-skin grafting. Br J Plast Surg (2001) 54:447–9. doi:10.1054/bjps.2001.3601
66. Hafejee A, Coulson IH. Localized bullous pemphigoid 20 years after split skin grafting. Clin Exp Dermatol (2005) 30:187–8. doi:10.1111/j.1365-2230.2004.01689.x
67. Orvis AK, Ihnatsenka V, Hatch RL. Bullous lesions on a skin graft donor site. J Am Board Fam Med (2009) 22:89–92. doi:10.3122/jabfm.2009.01.080036
69. Dahl MG, Cook LJ. Lesions induced by trauma in pemphigoid. Br J Dermatol (1979) 101:469–73. doi:10.1111/j.1365-2133.1979.tb00029.x
70. Macfarlane AW, Verbov JL. Trauma-induced bullous pemphigoid. Clin Exp Dermatol (1989) 14:245–9. doi:10.1111/j.1365-2230.1989.tb00944.x
71. Twine CP, Malik G, Street S, Williams IM. Bullous pemphigoid presenting as dry gangrene in a revascularized limb. J Vasc Surg (2010) 51:732–4. doi:10.1016/j.jvs.2009.10.110
72. Rakvit P, Kerr AC, Ibbotson SH. Localized bullous pemphigoid induced by photodynamic therapy. Photodermatol Photoimmunol Photomed (2011) 27:251–3. doi:10.1111/j.1600-0781.2011.00609.x
73. Ujiie H, Muramatsu K, Mushiroda T, Ozeki T, Miyoshi H, Iwata H, et al. HLA-DQB1*03:01 as a biomarker for genetic susceptibility to bullous pemphigoid induced by DPP-4 inhibitors. J Invest Dermatol (2017) (in press). doi:10.1016/j.jid.2017.11.023
74. Danno K, Takigawa M, Horio T. The alterations of keratinocyte surface and basement membrane markers by treatment with 8-methoxypsoralen plus long-wave ultraviolet light. J Invest Dermatol (1983) 80:172–4. doi:10.1111/1523-1747.ep12533415
Keywords: bullous pemphigoid, dipeptidyl peptidase-IV inhibitor, dipeptidyl peptidase-IV inhibitor-associated bullous pemphigoid, burn, cellulitis, autoimmune disease, autoantibodies, physical factors
Citation: Mai Y, Nishie W, Sato K, Hotta M, Izumi K, Ito K, Hosokawa K and Shimizu H (2018) Bullous Pemphigoid Triggered by Thermal Burn Under Medication With a Dipeptidyl Peptidase-IV Inhibitor: A Case Report and Review of the Literature. Front. Immunol. 9:542. doi: 10.3389/fimmu.2018.00542
Received: 15 November 2017; Accepted: 02 March 2018;
Published: 12 April 2018
Edited by:
Ralf J. Ludwig, University of Lübeck, GermanyReviewed by:
Jean Kanitakis, Hospices Civils de Lyon, FranceCopyright: © 2018 Mai, Nishie, Sato, Hotta, Izumi, Ito, Hosokawa and Shimizu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wataru Nishie, bmlzaGllQG1lZC5ob2t1ZGFpLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.