- 1Key Laboratory of Laboratory Medical Diagnostics Designated by the Ministry of Education, School of Laboratory Medicine, Chongqing Medical University, Chongqing, China
- 2Department of Clinical Laboratory, Chongqing Hospital for Women and Children, Chongqing, China
Streptococcus pneumoniae is a major pathogen leading to substantial morbidity and mortality in children under 5 years of age. Vaccination is an effective strategy to prevent S. pneumoniae infection. SPY1 is a pneumococcal vaccine candidate strain obtained in our previous study. To improve its stability and immunogencity, in this study, we constructed the SPY1ΔlytA strain that lacks autolysin activity and was coated with an artificial exterior surface calcium phosphate shell by in situ mineralization. The resulting strain SPY1ΔlytACaPi displayed enhanced thermal stability enabling storage at 37°C for 1 week. Furthermore, mucosal and subcutaneous immunization with the SPY1ΔlytACaPi strain induced better protective effects than SPY1ΔlytA in anti-colonization after challenging with 19F and anti-invasion by D39 in mice. Subcutaneous immunization with SPY1ΔlytACaPi elicited higher IgG level while mucosal immunization primarily elicited an immune response which is supposed to be related to Th17 cells. Taken together, the mineralized strain may be a promising candidate for an attenuated S. pneumoniae vaccine.
Introduction
Streptococcus pneumoniae is a major cause of pneumonia, otitis media, sepsis, and meningitis in children and the elderly (1). Vaccination is an effective strategy to prevent S. pneumoniae infection, the 23-valent pneumococcal polysaccharide vaccine and protein-conjugate vaccines (PCV) are currently in use including PCV7, PCV9, PCV10, and PCV13 (2–4). However, these vaccines have some disadvantages including weak immunogenicity, limited serotype coverage, serotype replacement, and high cost (5, 6). Therefore, developing a new and more ideal pneumococcal vaccine has become an important goal.
Streptococcus pneumoniae vaccine development includes whole-cell, polysaccharide, polysaccharide conjugate, as well as subunit protein vaccines. S. pneumoniae is highly diverse genetically and can rapidly change itself by recombination. A protein that is not essential for viability but significant in immunological recognition by the host can be altered or eliminated (pspA and psrP) (7). Candidate vaccines combining several common proteins may reduce the possibility that pneumococcus would readily evolve to evade immunity induced by vaccination. Generally, soluble antigen vaccines tend to lead host immune tolerance while bacteria-like particles including diversity antigens and agonists of toll-like receptors could activate multiple Toll-like receptors to induce stronger immune responses (8). Therefore, an attenuated live vaccine would be an ideal candidate to protect against pneumococcal infection.
SPY1 is a live attenuated strain of S. pneumoniae with significantly reduced amounts of capsular polysaccharide and pneumolysin (9). SPY1 can induce Th1/Th2/Th17 immune responses and protect mice against pneumococcal infection (10). SPY1 also offer better protection effect than the commercial 23-valent polysaccharide vaccine and therefore is a candidate vaccine strain (9). However, because of the weak immunogenicity of SPY1, it must be given with an adjuvant such as cholera toxin (CT) that is toxic for humans (11).
For vaccine, maintaining the cold chain is critical for adequate bioactivity. Despite preservation at low temperature, nearly half of the amount of vaccine was estimated to be abandoned around the world due to heat inactivation. And the cost of refrigeration contributes to about 80% of the cost of vaccine (12). Biological mineralization technology has been successfully used to improve thermal stability of living organisms (13–19). This technique involves the use of calcium phosphate to form an eggshell-like exterior that has improved the thermal stability and immunogenicity of viruses and yeast (17–19). A vaccine virus treated in this manner can be stored at 26°C for more than 9 days and at 37°C for 1 week (19). However, there are no reports of biomineralization used for live bacterial vaccines. In order to improve the thermal stability of SPY1 and further enhance its immunogenicity, this study focused on the in situ mineralization of SPY1 and evaluated its immune protection effects.
Materials and Methods
Mice
6- to 8-week-old C57BL/6 female mice were obtained from the animal center of Chongqing Medical University. Mice were kept under specific pathogen-free conditions at the animal centers of Chongqing Medical University during the time of the experiments.
Bacteria Strains
Streptococcus pneumoniae strain NCTC 7466 (D39, serotype 2) was obtained from the National Collection of Type Cultures (NCTC; London, United Kingdom). S. pneumoniae clinical isolates CMCC 31693 (serotype 19F) were obtained from the National Center for Medical Culture Collections (CMCC; Beijing, China). The S. pneumoniae SPY1 strain used in this study was obtained previously and stored in 20% glycerol at −80°C (10). All S. pneumoniae strains were grown on Columbia sheep blood agar plates or in casein-based medium with yeast extract (C+Y medium) at 37°C under 5% CO2 atmosphere. Zeta potential were measured by Malvern Zetasizer when SPY1 and SPY1ΔlytA were suspended in sterility phosphate-buffered saline (PBS) at pH 7.2.
SPY1ΔlytA Strain Construction
We constructed an attenuated S. pneumoniae strain SPY1ΔlytA by insertional inactivation. The plasmid pEVP3 were constructed with a lytA homologous arm was transformed into SPY1 and intergrated into the lytA gene by homologous recombination (20). Primers lytA-ins F and lytA-ins R were designed by Primer Premiers 5 according to the sequence of the S. pneumoniae D39 lytA gene (Table 1). The lytA-ins gene was integrated into the plasmid pEVP3 (21) and pEVP3-lytA-ins was inserted into the SPY1 lytA gene using insertional inactivation (20). The SPY1ΔlytA mutant strain was screened on blood plates containing erythromycin (0.25 µg/ml) and chloramphenicol (2.5 µg/ml). PCR were used to confirm the mutation in chloramphenicol resistant isolates (Table 1). To evaluate the production of LytA protein in SPY1ΔlytA mutant strain, the expression of LytA was analyzed by Western blotting with anti-LytA sera.
Biomineralization of SPY1ΔlytA
The SPY1ΔlytA mutant was cultured in C + Y liquid medium to mid-exponential growth phase (OD620 = 0.4–0.5, 1.5 × 108 CFU/mL) and washed twice with 0.9% NaCl by centrifuging at 12,000 rpm for 5 min. The cell density was then adjusted to 1 × 109 CFU/mL in normal saline. The bacterial suspension was added to 5 ml of a 10 mM CaCl2 solution and placed at 4°C for 1 h. The mixture was then centrifuged 10–15 min at 2,000 rpm and the supernatant was removed. The pellets were suspended with equal volumes of 4, 6, and 10 mM Na2HPO4 (pH 6.8) and centrifuged 5 min at 3,000 rpm. The pellets were washed and suspended in sterilize saline. Bacteria were visualized using scanning electron microscope (SEM, Model S-3000N, Hitachi, Japan).
Immunization and Challenge
SPY1 and SPY1ΔlytA were grown at 37°C in 5% CO2 in C+Y medium to approximately 1.5 × 108 CFU/ml. The cells were collected by centrifugation, washed twice, and suspended in sterile PBS. For mucosal immunization, the mice were randomly divided into six groups: PBS, CT (Sigma-Aldrich), SPY1, SPY1ΔlytA, SPY1ΔlytA + CT, and SPY1ΔlytACaPi. Similarly, we divided the mice into six groups for subcutaneous immunization using: PBS, alum adjuvant (alum; Thermo Fisher Scientific), SPY1, SPY1ΔlytA, SPY1ΔlytA + alum, and SPY1ΔlytACaPi. The experimental groups were vaccinated either subcutaneously with 100 µl SPY1 (l × 108 CFU) mixed with equal volume of alum adjuvant or via the mucosa with SPY1 (l × 108 CFU) plus 1 µg CT in 30 µl PBS. For subcutaneous immunization, mice were vaccinated three times over 2 weeks, whereas for mucosal immunization, mice were immunized once per week for four consecutive weeks. Serum samples were collected 1 week after the final immunization. Blood samples were collected from tails. All samples were stored at −80°C.
For the colonization model, 2 weeks after the last vaccination, mice were anesthetized and challenged with l × 108 CFU of pneumococcal strain CMCC 31693 (serotype 19F) and sacrificed 72 h later. CFU counts in nasal washes and lung homogenates were determined as previously described (22). For the lethal intranasal challenge model, C57BL/6 mice were anesthetized and then inoculated intranasally with pneumococcal strain NCTC 7466 (D39, serotype 2; l × 108 CFU) in 30 µl of PBS. Survival was monitored for 21 days.
Antibody Titers and IgG Isotype Determinations
Protein concentrations were determined by BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China) and adjusted to equal protein concentrations. For measurement of antigen specific IgG titers, antibody levels were determined by using 96-well plates coated with SPY1 as previously described (22, 23).
Cytokine Measurements
One week after the final immunization, mouse splenocytes were isolated (24) and transferred into 24-well tissue culture plates (2 × 106 cells/well) in 1 ml of DMEM with 10% fetal calf serum (HyClone). Then, cells were treated for 72 h with Concanavalin A (5 mg/ml, Sigma, St. Louis, MO, USA) or 70% ethanol-killed SPY1 (equivalent to 107 CFU/ml). Cytokines IFN, IL-4, IL-10, and IL-17A in the culture supernatants were determined using commercial kits (Biolegend, CA, USA) according to the manufacturer’s recommendations.
Statistical Analysis
Data were compared using Student’s t test or a Mann–Whitney U test. Survival rates were analyzed with a log-rank test. Differences at a P value of <0.05 were considered significant. Statistical analysis was performed using GraphPad Prism, version 5.
Results
SPY1ΔlytA Strain Construction
An interesting characteristic of Streptococcus pneumoniae, autolysis, is highly related to LytA protein (25). In order to avoid bacterial autolysis during mineralization, non-autolysis SPY1 strain (SPY1ΔlytA) was constructed by insertional inactivation method.
Due to the erythromycin resistance of SPY1 and CAT gene from the introduced pEVP3 plasmid, blood agar plates supplemented with appropriate antibiotics were used to screen out the correctly constructed mutant strain SPY1ΔlytA (Figure 1A). Besides, SPY1ΔlytA strain was authenticated with PCR. The amplified fragment lytA-ins (lane 3) refers to the correct insertion of lytA homologous arm into the SPY1 strain. No lytA fragments were amplified (lane 4) and amplified pEVP3 fragment (lane 5) indicated that the pEVP3 vector was integrated into SPY1 chromosome and disrupted lytA gene (Figure 1B). Western blot revealed that LytA was knocked out in SPY1ΔlytA (Figure 1C). In conclusion, LytA has been silenced in the SPY1ΔlytA strain.
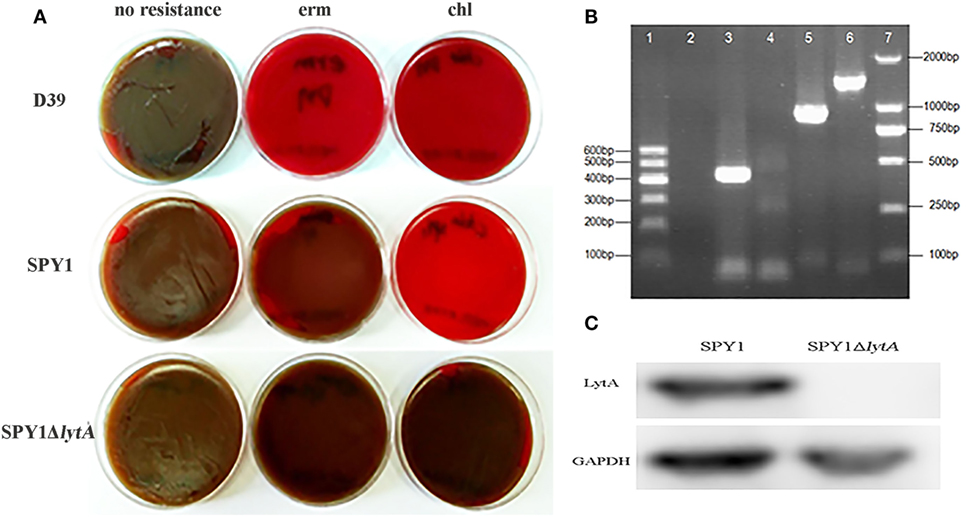
Figure 1. Construction of SPY1ΔlytA. (A) The antibiotic-resistance of constructed strains. Streptococcus pneumoniae strain D39, SPY1, and SPY1ΔlytA was seeded on blood agar plates supplemented erythromycin (erm) and chloramphenicol (chl) followed by 16 h incubation at 37°C under 5% CO2 atmosphere. (B) PCR products gel electrophoresis determination of plasmid integration. From left to right: 600 bp DNA marker (lane 1); Negative control, no template DNA (lane 2); lytA-ins (lane 3); lytA (lane 4); pEVP3 vector fragment (lane 5); ply (lane 6); 2,000 bp DNA marker (lane 7). (C) Detection of LytA protein by western blotting.
SPY1ΔlytA Strain Lacks Autolysis Ability
Autolysis is an important feature of S. pneumoniae and LytA protein is essential to it (25). After lytA inactivation, the autolysis ability was measured. During culturing in C + Y medium, the OD620 of SPY1 increased gradually to about 1.0 and then decreased rapidly, while the OD620 of SPY1ΔlytA increased to about 1.0 and could maintain for 36 h (Figure 2A). The SPY1 bacterial suspension became clear at 12 h, while the SPY1ΔlytA bacterial suspension was still cloudy (Figure 2B).
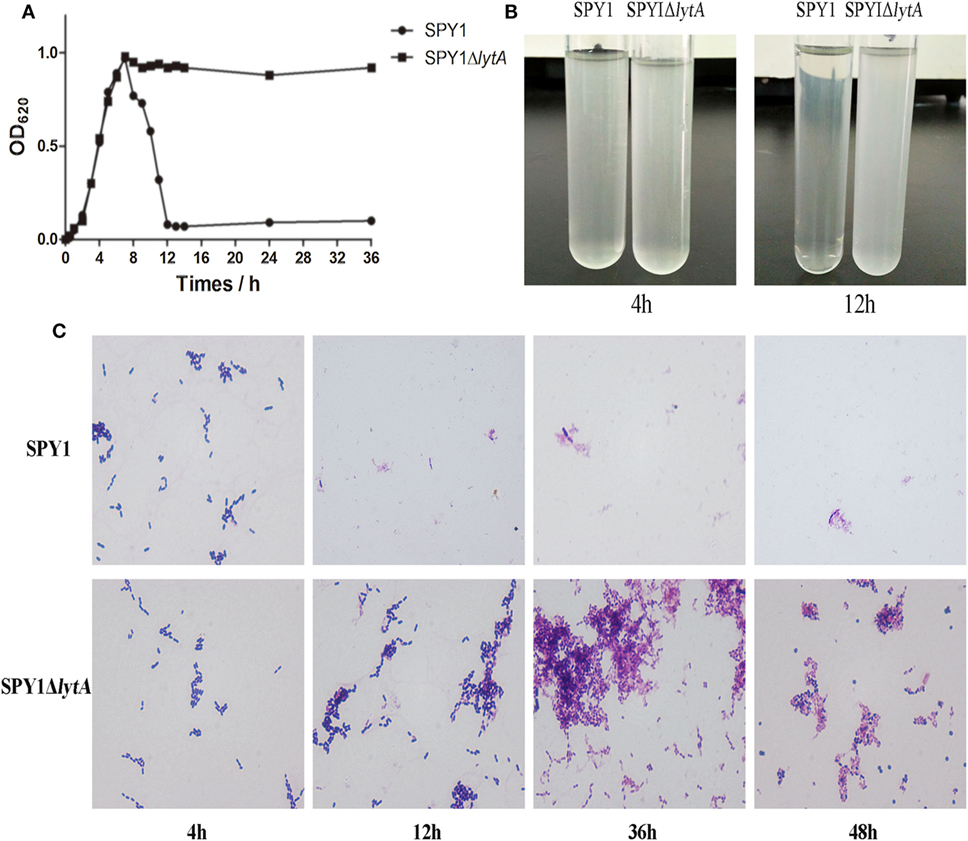
Figure 2. Growth and autolytic characteristics of strain SPY1ΔlytA. (A) Growth curve of SPY1 and SPY1ΔlytA in C + Y medium at 37°C. (B) Appearance of SPY1 and SPY1ΔlytA in C + Y medium at 4 and 12 h. (C) Morphology of Gram stained SPY1 and SPY1ΔlytA after incubation at 37°C for the indicated times.
Gram stains of SPY1 and SPY1ΔlytA cultured in C + Y liquid medium for 4 h indicated the morphology of these two bacteria was similar: Gram-positive, spearhead-like and in pairs or short chain-like arrangements. After 12 and up to 36 h, SPY1 was not visible in stained preparations of culture medium. However, SPY1ΔlytA cultures were similar to the 4 h samples at these later times. Cultures of SPY1ΔlytA for 24–36 h varied to round and abnormally form (Figure 2C). Together, these data indicated that we had successfully inactivated the autolysin function of SPY1.
In Situ Mineralization of SPY1ΔlytA
The bacterial strain SPY1 lacks capsule and therefore exposes other surface components such as teichoic acids. Zeta potential showed that SPY1 and SPY1ΔlytA present a net negative surface charge (−14.97 ± 0.75 and −15.2 ± 0.82, respectively), which enabled coating with calcium phosphate by in situ mineralization. The SPY1ΔlytA strain was treated with calcium-rich physiological saline to obtain a calcium-rich surface layer that acted as a nucleation site. Disodium hydrogen phosphate was then titrated into the calcium-rich SPY1ΔlytA suspension to obtain a CaPi mineralized surface layer (Figure 3A). SEM indicated that the optimum phosphate concentration for SPY1ΔlytACaPi was 6 mM (Figure 3B). Tetracycline hydrochloride was used to characterize the calcium phosphate shell (Figure 3C), for it being able to bind with calcium ions to induce a golden-yellow fluorescence under UV light (360–370 nm). These results confirmed that SPY1ΔlytACaPi was successfully constructed.
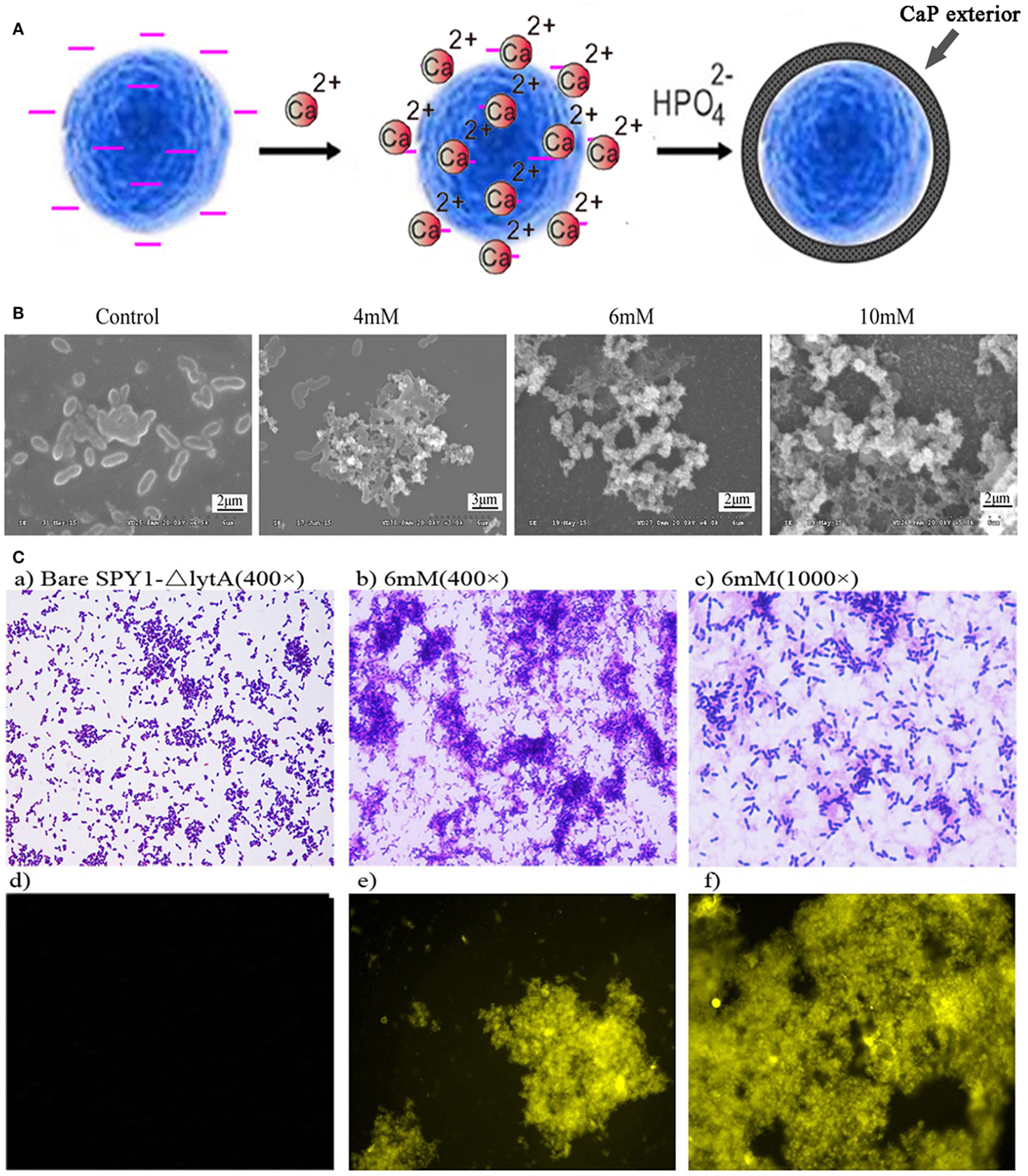
Figure 3. Construction of SPY1ΔlytACaPi. (A) Schematic diagram of the in situ mineralization process of SPY1ΔlytA. (B) Scanning electron microscope images of CaP-mineralized SPY1ΔlytA after titration with phosphate solution in different concentrations. The native SPY1ΔlytA strain was used as control. (C) Mineral shells surrounding SPY1ΔlytA. (a–c) SPY1ΔlytA was stained with Gram staining. (d–f) the mineral shells was stained with tetracycline hydrochloride and observed under UV light (360–370 nm).
The Mineralized Layer Improved the Thermal Stability of SPY1ΔlytA at 37°C
Thermal stability was determined by detecting bacterial viability at different time points after incubation at 37°C. The viability of SPY1ΔlytACaPi was significantly greater than both SPY1 and SPY1ΔlytA (Figure 4).
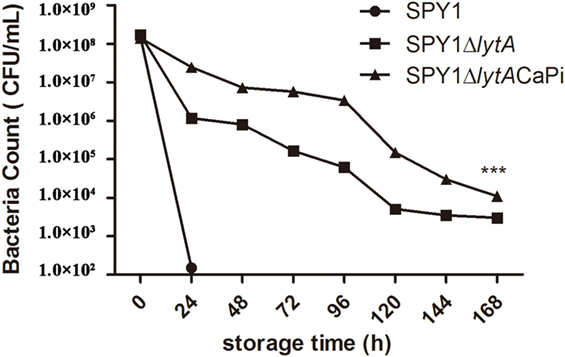
Figure 4. Thermal stability of SPY1ΔlytACaPi. SPY1, SPY1ΔlytA, and SPY1ΔlytACaPi were incubated at 37°C for 7 days, the concentration of bacteria was measured by CFU counting every 24 h. *P < 0.05, **P < 0.01, ***P < 0.001.
Vaccination with SPY1ΔlytACaPi Induced Strong Immune Response in Mice
We examined the humoral and cellular immune responses of mice immunized mucosally and subcutaneously with SPY1ΔlytACaPi. In subcutaneous-vaccinated mice, antigen-specific IgG levels of SPY1ΔlytACaPi group were significantly higher than those of other groups. However, in mucosally vaccinated mice, IgG levels of SPY1ΔlytACaPi group were similar to controls (Figure 5A).
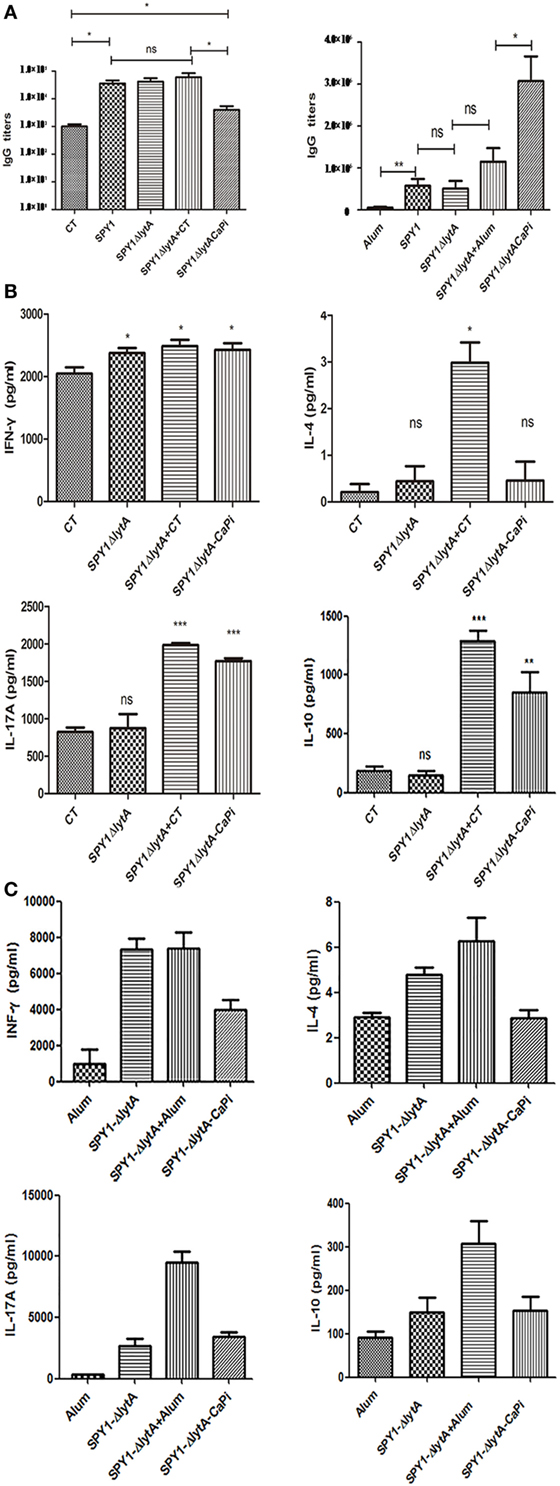
Figure 5. Humoral and cellular immune responses in mice induced by SPY1ΔlytACaPi vaccine. For mucosal immunization, C57BL/6 mice were immunized with cholera toxin (CT), SPY1, SPY1ΔlytA, SPY1ΔlytA + CT, and SPY1ΔlytACaPi, respectively. For subcutaneous immunization, C57BL/6 mice was immunized with alum adjuvant, SPY1, SPY1ΔlytA, SPY1ΔlytA + alum, and SPY1ΔlytACaPi. (A) IgG titers in serum were measured 1 week after mucosal (left) or subcutaneous (right) final immunization. One week after the last immunization, suspensions of splenocytes (1 × 105 cells/well) were cultured and exposed to 70% ethanol-killed SPY1 (equivalent to 107 CFU/ml) for 72 h at 37°C. The levels of cytokine of splenocytes from (B) mucosal and (C) subcutaneous immunized mice were determined by ELISA kits. *P < 0.05, **P < 0.01, ***P < 0.001.
In mucosally vaccinated mice, levels of IL-17A and IL-10 produced by splenocytes of mice immunized with SPY1ΔlytACaPi were significantly higher than SPY1ΔlytA (Figure 5B). The elevation of cytokine in SPY1ΔlytACaPi equals to which in SPY1ΔlytACaPi + CT group, suggesting the mineralized vaccine strain had an adjuvant affect. However, the subcutaneous immunization of the mineralized vaccine strain SPY1ΔlytACaPi did not result in elevated levels of IFN-γ, IL-4, IL-17A, and IL-10 (Figure 5C). These results suggested the immune responses induced by SPY1ΔlytACaPi mucosal vaccination may be mediated by Th17 and Treg cells, while subcutaneous immunization stimulated humoral immune responses.
Immunization with SPY1ΔlytACaPi Reduced Pneumococcal Colonization
Nasopharyngeal colonization represents the initial step of invasive pneumococcal infection (26). The bacteria loads of mice mucosally or subcutaneously immunization challenged intranasally with S. pneumoniae strain 19F were measured by CFU counting. Nasal CFU counts in the SPY1 and SPY1ΔlytA vaccinated mice groups were not significantly different, which indicated that the deletion of the LytA protein did not affect the immunogenicity of SPY1. The bacterial loads for the mineralized vaccine SPY1ΔlytACaPi group were significantly lower than the non-mineralized vaccine SPY1ΔlytA group, while it is similar to which of non-mineralized vaccine groups in the presence of adjuvants (SPY1ΔlytA + CT or SPY1ΔlytA + alum) (Figures 6A,B). These results suggested that the mineralized shells had adjuvant effects both in mucosal and subcutaneous vaccinations and that mineralized strain vaccination reduced pneumococcal colonization.
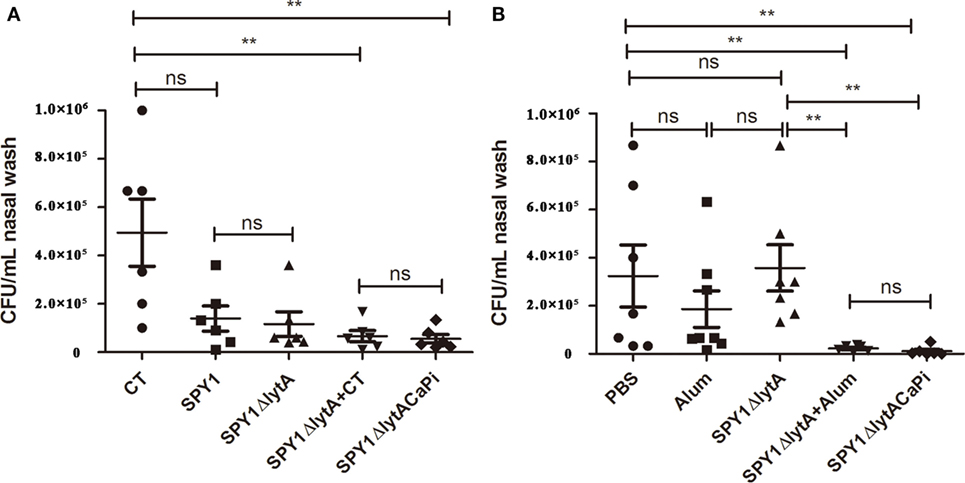
Figure 6. Immunization with SPY1ΔlytACaPi reduced pneumococcal colonization. Mice mucosally (A) or subcutaneously (B) immunized with indicated antigens (mucosal with cholera toxin (CT), SPY1, SPY1ΔlytA, SPY1ΔlytA + CT, and SPY1ΔlytACaPi, and subcutaneous with alum adjuvant, phosphate-buffered saline (PBS), SPY1ΔlytA, SPY1ΔlytA + alum, and SPY1ΔlytACaPi) were intranasally challenged with Streptococcus pneumoniae serotype 19F (CMCC 31693, 1.5 × 107). Bacterial loads in nasal washes were determined three days postinfection. *P < 0.05; **P < 0.01.
Immunization with SPY1ΔlytACaPi Provided Protection against Pneumococcal Lethal Infections
To evaluate the protective effects of SPY1ΔlytACaPi vaccination against lethal infection of pneumococci, mice were challenged intranasally with S. pneumoniae strain D39 at 1 × 108 CFU/mouse 2 weeks after last immunization. The mice survival rates for mucosal vaccination using SPY1ΔlytACaPi were significantly higher than that of SPY1ΔlytA (Figure 7A). Similarly, the mice survival rates for subcutaneously vaccination using SPY1ΔlytACaPi were higher than that of SPY1ΔlytA, but did not reach statistical significance (Figure 7B). In short, the mineralized strain vaccination could provide a better protective effect against pneumococcal infections than the non-mineralized strain.
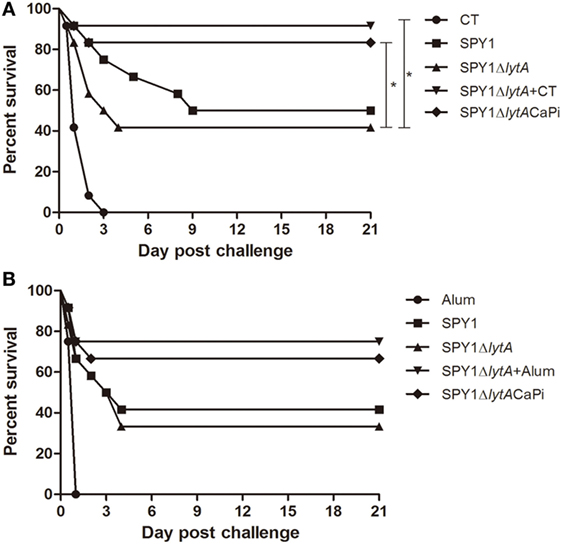
Figure 7. Protection against bacterial lethal infections by SPY1ΔlytACaPi strain. C57BL/6 mice were mucosally (A) or subcutaneously (B) immunized with indicated antigens (mucosal with CT, SPY1, SPY1ΔlytA, SPY1ΔlytA + CT, and SPY1ΔlytACaPi, and subcutaneous with alum adjuvant, SPY1, SPY1ΔlytA, SPY1ΔlytA + alum, and SPY1ΔlytACaPi) and intranasally challenged with 1 × 108 CFU of the D39 strain 2 weeks after last immunization. Survival was monitored for 21 consecutive days. The results were analyzed by log-rank test. *P < 0.05.
Discussion
SPY1 is a live attenuated S. pneumoniae strain and a vaccine candidate strain because it can elicit strong protective effects against S. pneumoniae challenges in mice (9, 10). However, the autolysin of the strain prevents its growth to the end of the logarithmic growth phase. This property limits the potential of SPY1 as a vaccine strain. We therefore inactivated lytA and the strain SPY1ΔlytA maintained unbroken cell shapes for an extended period indicating the autolysis property was eliminated. This made the strain more stable for storage and transport than the parent SPY1.
The instability of the vaccine products and inactivation of vaccines during storage and transportation have seriously hampered their effectiveness. The strain SPY1ΔlytA also needed to be cryopreserved. Mineralized vaccines can be stored at 37°C for more than 7 days and can stimulate higher antibody production after immunization (19). Therefore, we intended to improve the thermal stability and immunogenicity of SPY1ΔlytA by mineralization treatment.
The surface of SPY1 lacks a capsule and surface components such as teichoic acid are exposed. When SPY1ΔlytA is cultured in an alkaline environment, it has a net negative surface charge. We initially treated SPY1ΔlytA with a calcium-rich physiological saline to obtain a calcium-rich surface layer that could act as a nucleation site. We then determined the optimal phosphate to add to the suspension to obtain a CaPi mineralized surface layer. This resulted in a greater thermal stability for the mineralized vaccine than the non-mineralized strain. In addition, this procedure improved the immunogenicity of SPY1ΔlytA and mineralization induced high titers of IgG indicating the CaPi mineralized shell had also a good adjuvant effect. This means additional adjuvant such as CT is no longer needed. CT is toxic for humans and can not be used in human body, while CaPi is a component of human teeth and bones and had good biocompatibility and is easily absorbed (27, 28), and these are qualities for a good adjuvant.
We evaluated the immune protective mechanism of the SPY1ΔlytACaPi mineralization vaccine in terms of humoral and cellular immunity. In mice vaccinated subcutaneously, IgG titers in the SPY1ΔlytACaPi group were higher than those of the SPY1ΔlytA + alum group. This suggested that SPY1ΔlytACaPi not only induces an effective humoral immune response in subcutaneous-vaccinated mice, but the mineralized components of the mineralized strain have an adjuvant effect. However, our experimental results in mucosal vaccinated mice showed that SPY1ΔlytACaPi did not increase IgG level, this most likely due to the different modes of immunization.
In addition to humoral immunity, cellular immunity also plays an important role in antimicrobial infection. Proinflammatory cytokines such as IFN-γ, IL-4, IL-17A, and IL-10 play important roles in the immunization process against S. pneumonia (29–34). Our results indicated that SPY1ΔlytACaPi significantly promoted the secretion of IL-17A and IL-10, compared with SPY1ΔlytA in mucosally vaccinated mice, suggesting that it could induce Th17 and Treg cell immune responses. However, IL-4 was not significantly induced in mice after immunization with mineralized strains, but IL-10 was elevated significantly. Because IL-10 is also a Th2-type cytokine as is IL-4, this indicated that the mineralized vaccine might also induce Th2 cell immune responses. However, there was no significant difference between SPY1ΔlytACaPi and SPY1ΔlytA + CT group suggesting the mineralized layer creates an adjuvant effect and can activate Th17 and Treg cell immune responses.
We have also studied the protective effect of SPY1ΔlytACaPi on nasal colonization of S. pneumoniae. Th17 cellular immune response plays an important role in this type of colonization by S. pneumoniae (34–36) and IL-4 is not involved (34). The results of our experiments showed that the bacterial loads in the nasal cavities of mice immunized with SPY1ΔlytACaPi were significantly lower than that of the non-mineralized vaccine group (P < 0.01). Mechanistically this may be due to a greater Th17 cell immune response after mucosal immunization. Secretion of IL-17A into the nasal cavity recruited neutrophils that effectively removed the bacterial infection (37).
In our study, we found that the colonization effect of SPY1ΔlytACaPi group bears comparison with non-mineralized vaccine groups in the presence of adjuvants (SPY1ΔlytA + CT or SPY1ΔlytA + alum). Familiar phenomenon was also observed in survival rates evaluation. These results suggested that the mineralized shell had an adjuvant effect. In addition, in mice vaccinated mucosally, the survival rates of the SPY1ΔlytACaPi group were significantly higher than those of SPY1ΔlytA group, which were consistent with the results of cytokines. This indicated that mucosally vaccinated the mineralized strain could provide better protection against pneumococcal infections than the non-mineralized strain in mice.
In summary, we constructed a mineralized bacterial strain SPY1ΔlytACaPi and evaluated its thermal stability, immunoprotective effects, and immune protection mechanism. The mineralized shell increased bacterial stability. The protect effects of mineralized vaccine were superior to non-mineralized vaccine both in mucosal and subcutaneous vaccinations. The former induced mainly Th17 response while the later increased IgG levels. The results of this study lay the foundation for further research and development of S. pneumoniae vaccines.
Ethics Statement
All the animal experiments were done in accordance with the guidelines of the Institutional Animal Care and Use Committee of Chongqing Medical University.
Author Contributions
WX and XZ designed the studies and wrote the paper. XZ and JC performed experiments about SPY1ΔlytA mutant strain construction and mineralization. YW, HW, and YQ performed animal experiments. JW and YM analyzed data. YH, XZ, and YY provided advice in experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the Natural Science Foundation of China (No. 81671639 to WX and 31270984 to WX).
References
1. O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet (2009) 374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6
2. Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Infect (2014) 69(4):309–25. doi:10.1016/j.jinf.2014.06.006
3. World Health Organization. Meeting of the Immunization Strategic Advisory Group of Experts, November 2007 – conclusions and recommendations. Wkly Epidemiol Rec (2008) 83(1):1–15.
4. Word Health Orgnization. Pneumococcal vaccines. WHO positionpaper-2012. Wkly Epidemiol Rec (2012) 14:129–44.
5. Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med (1991) 325(21):1453–60. doi:10.1056/NEJM199111213252101
6. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet (2011) 378:1962–73. doi:10.1016/S0140-6736(10)62225-8
7. Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, et al. Rapid pneumococcal evolution in response to clinical interventions. Science (2011) 331(6016):430–4. doi:10.1126/science.1198545
8. Malley R, Anderson PW. Serotype-independent pneumococcal experimental vaccines that induce cellular as well as humoral immunity. Proc Natl Acad Sci U S A (2012) 109(10):3623–7. doi:10.1073/pnas.1121383109
9. Wu KF, Yao R, Wang H, Pang D, Liu YS, Xu HM, et al. Mucosal and systemic immunization with a novel attenuated pneumococcal vaccine candidate confers serotype independent protection against Streptococcus pneumoniae in mice. Vaccine (2014) 32:4179–88. doi:10.1016/j.vaccine.2014.05.019
10. Xu X, Wang H, Liu Y, Wang Y, Zeng L, Wu K, et al. Mucosal immunization with the live attenuated vaccine SPY1 induces humoral and Th2-Th17-regulatory T cell cellular immunity and protects against pneumococcal infection. Infect Immun (2015) 83(1):90–100. doi:10.1128/IAI.02334-14
11. Zeng L, Liu Y, Wang H, Liao P, Song Z, Gao S, et al. Compound 48/80 acts as a potent mucosal adjuvant for vaccination against Streptococcus pneumoniae infection in young mice. Vaccine (2015) 33(8):1008–16. doi:10.1016/j.vaccine.2015.01.013
12. Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Fiorenzo G, et al. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc Natl Acad Sci U S A (2012) 109(30):11981–6. doi:10.1073/pnas.1206210109
13. Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science (2001) 294(5547):1684–8. doi:10.1126/science.1063187
14. Roy MD, Stanley SK, Amis EJ, Becker ML. Identification of a highly specific hydroxyapatite-binding peptide using phage display. Adv Mater (2008) 20(10):610–7. doi:10.1002/adma.200702322
15. Yao H, Dao M, Imholt T, Huang J, Wheeler K, Bonilla A, et al. Protection mechanisms of the iron-plated armor of a deep sea hydrothermal vent gastropod. Proc Natl Acad Sci U S A (2010) 107(3):987–92. doi:10.1073/pnas.0912988107
16. Hamm CE, Merkel R, Springer O, Jurkojc P, Maier C, Prechtel K, et al. Architecture and material properties of diatom shells provide effective mechanical protection. Nature (2003) 421(6925):841–3. doi:10.1038/nature01416
17. Wang G, Li X, Mo L, Chen W, Deng Y, Zhao H, et al. Eggshell-inspired biomineralization generates vaccines that do not require refrigeration. Angew Chem Int Ed Engl (2012) 51(42):10576–9. doi:10.1002/anie.201206154
18. Wang B, Liu P, Jiang W, Pan H, Xu X, Tang R. Yeast cells with an artificial mineral shell: protection and modification of living cells by biomimetic mineralization. Angew Chem Int Ed Engl (2008) 47(19):3560–4. doi:10.1002/anie.200704718
19. Wang G, Cao RY, Chen R, Mo L, Han JF, Wang X, et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc Natl Acad Sci U S A (2013) 110(19):7619–24. doi:10.1073/pnas.1300233110
20. Thanassi JA, Hartman-Neumann SL, Dougherty TJ, Dougherty BA, Pucci MJ. Identification of 113 conserved essential gene using a high-throughput gene disruption system in Streptococcus pneumonia. Nucleic Acids Res (2002) 30(14):3152–62. doi:10.1093/nar/gkf418
21. Claverys JP, Dintilhac A, Pestova EV, Martin B, Morrison DA. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene (1995) 164(1):123–8. doi:10.1016/0378-1119(95)00485-O
22. Roche AM, King SJ, Weiser JN. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun (2007) 75:2469–75. doi:10.1128/IAI.01972-06
23. Cao J, Chen DP, Xu WC, Chen TM, Xu SX, Luo JY, et al. Enhanced protection against pneumococcal infection elicited by immunization with the combination of PspA, PspC, and ClpP. Vaccine (2007) 25:4996–5005. doi:10.1016/j.vaccine.2007.04.069
24. Ferreira DM, Darrieux M, Oliveira MLS, Leite LCC, Miyaji EN. Optimized immune response elicited by a DNA vaccine expressing pneumococcal surface protein A is characterized by a balanced immunoglobulin G1 (IgG1)/IgG2a Ratio and proinflammatory cytokine production. Clin Vaccine Immunol (2008) 15:499–505. doi:10.1128/CVI.00400-07
25. Mellroth P, Daniels R, Eberhardt A, Rönnlund D, Blom H, Widengren J, et al. LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan. J Biol Chem (2012) 287:11018–29. doi:10.1074/jbc.M111.31858
26. Bogaert D, de Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis (2004) 4:144–54. doi:10.1016/S1473-3099(04)00938-7
27. Tan ACL, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, et al. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm (2012) 9:2710–8. doi:10.1021/mp300257x
28. He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ. Calcium phosphate nanoparticle adjuvant. Clin Diagn Lab Immunol (2000) 7(6):899–903.
29. He Q, Mitchell A, Morcol T, Bell SJ. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin Diagn Lab Immunol (2002) 9(5):1021–4.
30. Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, et al. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol (2003) 170(1):438–44. doi:10.4049/jimmunol.170.1.438
31. Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol (2005) 7(11):1603–15. doi:10.1111/j.1462-5822.2005.00578.x
32. Van Rossum AMC, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun (2005) 73(11):7718–26. doi:10.1128/IAI.73.11.7718-7726.2005
33. Malaviya R, Ikeda T, Ross E, Abroham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature (1996) 381(6577):77–80. doi:10.1038/381077a0
34. Cruse G, Fernandes VE, de Salort J, Pankhania D, Marinas MS, Brewin H, et al. Human lung mast cells mediate pneumococcal response to activation by pneumolysin. J Immunol (2010) 184(12):7108–15. doi:10.4049/jimmunol.0900802
35. Jackson DC, Lau YF, Le T, Sugrbier A, Deliyannis G, Cheers C, et al. A totally synthetic vaccine of generic structure that targets toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A (2004) 101(43):15440–5. doi:10.1073/pnas.0406740101
36. Cheng C, Jain P, Bettahi I, Pal S, Tifrea D, De la Maza LM. A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine (2011) 29(38):6641–9. doi:10.1016/j.vaccine.2011.06.105
Keywords: Streptococcus pneumoniae, live attenuated vaccine, biomineralization, thermal stability, immunogenicity
Citation: Zhang X, Cui J, Wu Y, Wang H, Wang J, Qiu Y, Mo Y, He Y, Zhang X, Yin Y and Xu W (2018) Streptococcus pneumoniae Attenuated Strain SPY1 with an Artificial Mineral Shell Induces Humoral and Th17 Cellular Immunity and Protects Mice against Pneumococcal Infection. Front. Immunol. 8:1983. doi: 10.3389/fimmu.2017.01983
Received: 20 October 2017; Accepted: 20 December 2017;
Published: 11 January 2018
Edited by:
Hao Shen, University of Pennsylvania, United StatesCopyright: © 2018 Zhang, Cui, Wu, Wang, Wang, Qiu, Mo, He, Zhang, Yin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenchun Xu, MTAwOTc3QGNxbXUuZWR1LmNu
†These authors have contributed equally to this work.
 Xinyuan Zhang
Xinyuan Zhang Jingjing Cui2†
Jingjing Cui2† Xuemei Zhang
Xuemei Zhang Yibing Yin
Yibing Yin Wenchun Xu
Wenchun Xu