- 1Department of Food Science and Technology, University of California, Davis, Davis, CA, United States
- 2Foods for Health Institute, University of California, Davis, Davis, CA, United States
- 3McMaster Immunology Research Centre, Department of Pathology and Molecular Medicine, Michael G. DeGroote Institute for Infectious Disease Research, McMaster University, Hamilton, ON, Canada
The majority of infants’ breastfeeding from their HIV-infected mothers do not acquire HIV-1 infection despite exposure to cell-free virus and cell-associated virus in HIV-infected breast milk. Paradoxically, exclusive breastfeeding regardless of the HIV status of the mother has led to a significant decrease in mother-to-child transmission (MTCT) compared with non-exclusive breastfeeding. Although it remains unclear how these HIV-exposed infants remain uninfected despite repeated and prolonged exposure to HIV-1, the low rate of transmission is suggestive of a multitude of protective, short-lived bioactive innate immune factors in breast milk. Indeed, recent studies of soluble factors in breast milk shed new light on mechanisms of neonatal HIV-1 protection. This review highlights the role and significance of innate immune factors in HIV-1 susceptibility and infection. Prevention of MTCT of HIV-1 is likely due to multiple factors, including innate immune factors such as lactoferrin and elafin among many others. In pursuing this field, our lab was the first to show that soluble toll-like receptor 2 (sTLR2) directly inhibits HIV infection, integration, and inflammation. More recently, we demonstrated that sTLR2 directly binds to selective HIV-1 proteins, including p17, gp41, and p24, leading to significantly reduced NFκB activation, interleukin-8 production, CCR5 expression, and HIV infection in a dose-dependent manner. Thus, a clearer understanding of soluble milk-derived innate factors with known antiviral functions may provide new therapeutic insights to reduce vertical HIV-1 transmission and will have important implications for protection against HIV-1 infection at other mucosal sites. Furthermore, innate bioactive factors identified in human milk may serve not only in protecting infants against infections and inflammation but also the elderly; thus, opening the door for novel innate immune therapeutics to protect newborns, infants, adults, and the elderly.
Introduction
Although it had been recognized for centuries that breastfeeding and infant health were associated, one of the earliest systematic studies to demonstrate this was conducted by Grulee et al. in 1935 (1). They studied over 20,000 mother–infant dyads and showed that compared with breastfed infants, non-breastfed infants had 3.1-fold higher morbidity and 7.1-fold higher mortality due to gastrointestinal disease, 1.4-fold higher morbidity and 1.9-fold higher mortality due to respiratory disease, and 2.5-fold higher morbidity and 4.3-fold higher mortality from other diseases (1). These differences clearly indicate that breastfeeding and breast milk have protective activities. Indeed, it has been established that human milk contains a growing list of bioactive molecules, including components of the innate and adaptive immune system of the mother, primarily secretory immunoglobulin A (SIgA) (2–4). The title of this review is taken, in part, from Dr. David S. Newburg who coined the term “innate immune system of human milk” (2).
Newborns and infants bear the greatest burden of infectious disease. The World Health Organization estimates 10.6 million children under the age of 5 die every year with the highest mortality rates occurring in the first month of life (5, 6). Importantly, 95% of infant morbidity and mortality occur in low and middle-income countries, such as sub-Saharan Africa where HIV and TB are endemic. Universal breastfeeding could prevent annual deaths of 823,000 children under the age of 5 years as well as 20,000 breast cancer deaths each year (7). Economic losses close to three billion dollars per year are associated with not breastfeeding (8).
Breastfeeding unquestionably protects against death and disease. Studies conducted in low and middle-income countries have clearly demonstrated that exclusively breastfed infants are protected against morbidity and mortality with only 12% of the risk of death compared with those who were not breastfed (9). Non-breastfed infants younger than 6 months had 3.5 times (boys) and 4.1 times (girls) increased mortality compared with infants receiving breast milk (10). In high-income countries, breastfeeding has been shown to be associated with a 36% reduction in sudden infant death and a 58% decrease in necrotizing enterocolitis (11). Furthermore, breastfeeding protects infants against 50% of all diarrhea episodes and a third of respiratory infections in infants who are not breastfed (12).
These protective effects of breast milk undoubtedly can be attributed to the multitude of bioactive molecules that have been shown protective against infections, reducing inflammation, facilitating immune system and organ development, and beneficially influencing the infant microbiome. Since the majority of bioactive factors in milk have not yet been identified, characterization of novel factors in milk will open the door for the development of novel antimicrobial immunotherapeutics.
Breastfeeding Behaviors and HIV-1
The benefits of breastfeeding for infants arise from the unique composition of breast milk. Breast milk completely nourishes the infant while establishing and promoting a healthy microbiome, and providing passive protection through maternal innate and adaptive immunological factors. Breastfeeding is well recognized to protect infants against gastrointestinal and respiratory infections, diarrheal diseases, and provides long-term health benefits (13–16). Additional socioeconomic benefits extend to the mother and family since breastfeeding promotes child spacing, social acceptance of the nursing woman, and is cost effective (17, 18).
It became clear in the 1980s that breast milk serves as a medium for HIV-1 transmission. The exact mechanism(s) of postnatal mother-to-child transmission (MTCT) of HIV remains unclear. In addition to postnatal transmission via breast milk, infants can become infected from their HIV-infected mothers in utero or following exposure to maternal fluids during parturition (19). However, this risk is significantly attenuated if the mother is given antiretroviral (ARV) therapy pre- and post-cesarean delivery (19). Without proper intervention strategies, an estimated 11–42% of infants will become infected from their HIV-infected mothers (19, 20) depending on factors such as maternal viral load and CD4 count, breast milk composition including innate immune factor levels, ARV treatment, breast pathology (particularly mastitis that can increase milk HIV RNA up to 10-fold in the affected breast), duration of breastfeeding, weaning practices and breastfeeding behavior (exclusive versus mixed) (21–24).
The past decade has shown significant progress in the prevention of mother-to-child transmission (PMTCT) of HIV globally through improved access to ARV therapies for women and infants, as well as the universal promotion of exclusive breastfeeding (EBF) when safe and sustainable alternatives are not readily available. As a result of these prevention strategies, for the first time the elimination of MTCT of HIV is considered a realistic public health goal (25). For example, ARV therapies such as single-dose nevirapine, given to the mother during delivery and the infant within 72 h postpartum has proven effective and has undoubtedly played an important role in the drastic decrease of approximately 800,000 cases of MTCT of HIV in 2002 to 300,000 cases in 2011 (26). Recently, an infant born infected with HIV was immediately treated with ARVs and cleared the infection (27). However, follow-up studies revealed that the HIV infection reappeared. Despite the effectiveness of these therapies, in 2011 only an estimated 57% of pregnant or lactating HIV-infected women globally were receiving any ARV therapy (25), largely due to the cost, lack of health-care support workers and inconsistent supply of ARVs (28). In many resource-limited areas where HIV-infected mothers have inadequate access to ARV therapies, mothers are encouraged to exclusively breastfeed their infants as an alternative preventive intervention (29).
Given that HIV-infected breast milk can contain high levels of cell-free virus (CFV) and cell-associated virus (CAV), it seems paradoxical that with prolonged and repeated exposure to HIV during EBF can significantly decrease postnatal MTCT of HIV compared with mixed feeding or non-exclusive breastfeeding (nEBF) (21–24). Thus, EBF infants of HIV-positive mothers who regularly consume HIV containing breast milk have increased protection against infection compared with mixed fed infants who are less frequently exposed to the virus. In four large cohort studies, EBF reduced HIV MTCT by 4- to 10-fold compared with nEBF or mixed feeding. Kuhn et al. (21) showed that nEBF more than doubled the risk of postnatal HIV transmission, while Iliff et al. (23) showed transmission rates as low as 1.3% in women who were EBF up to 6 months. Furthermore, there were no significant differences in viral load, CD4+ T cell levels or co-infections between the women who EBF and those that nEBF their infants that could account for the difference in transmission rates (22, 23). This preventative method is so effective in the PMTCT of HIV and in protection against enteric infections that the WHO recommends EBF despite the HIV status of the mother when safe and sustainable alternative feeding is unavailable (30). While the reasons underlying this phenomenon remain unanswered, they have been closely linked with innate immune factors in breast milk (31, 32). In addition, intestinal permeability is significantly increased during nEBF, replacement-fed (formula) and weaned infants which is associated with MTCT of HIV-1 (33). This strongly suggests that breast milk factors facilitate the nursing infant’s intestinal maturation and help maintain integrity of the intestinal barrier. We have hypothesized that short-lived innate factors in milk would have to be consistently provided to the nursing infant via EBF to sustain a protective innate immune threshold to prevent HIV transmission via milk. Although a number of comprehensive reviews of breastfeeding and HIV transmission have been published (34, 35), this article will highlight some of the current insights into biological and immunological factors in breast milk that are associated with protection from HIV infection via breastfeeding.
Innate and Adaptive Immune Factors and Cells in Human Milk that Inhibit HIV
Humans are mammals because we have mammary glands which many believe evolved as part of the innate immune system (36). Innate and adaptive immune factors in breast milk have been shown to play critical evolutionary roles in protecting newborn infants against a wide variety of infections. Recently, of about 415 proteins identified in a pooled milk sample, 261 were novel (37). Importantly, many of these factors have not been well characterized, but many have immunomodulatory and/or antimicrobial activities critical to protecting the immunologically naïve infant and promoting intestinal development and microbiome. Consequently, identification of these novel factors in milk and elucidation of their functions could inform the development of novel therapeutics or vaccines.
Non-Cellular Components in Human Milk
Non-cellular factors in breast milk that have been attributed to the protection from HIV infection observed in breastfed infants include innate factors, cytokines, and oligosaccharides (31, 32, 38) (Table 1). For other factors such as HIV-specific antibodies, the data are less clear (39, 40). The levels of many breast milk factors correlate with decreased MTCT of HIV and/or have direct anti-HIV activity in vitro, including lactoferrin, secretory leukocyte protease inhibitor (SLPI), mucin, and soluble toll-like receptor 2 (sTLR2) (41–44) (Figure 1). Specifically, lactoferrin, whose levels have been shown to correlate with reduced MTCT of HIV (45), has been shown in vitro to bind to the V3 loop of gp120, thus inhibiting gp120 interaction with host CD4 receptor (46). Lactoferrin has also been shown to inhibit bacterial-induced inflammation (47, 48). Similarly, SLPI levels correlated with decreased MTCT HIV transmission through breast milk (49), which is supported by in vitro studies indicating that it interacts with target cells to inhibit viral entry (50). Another component abundant in breast milk, mucin-1, can inhibit HIV infection in vitro by preventing dendritic cell (DC)-SIGN-mediated transmission of HIV from DCs to activated CD4+ T cells (41).
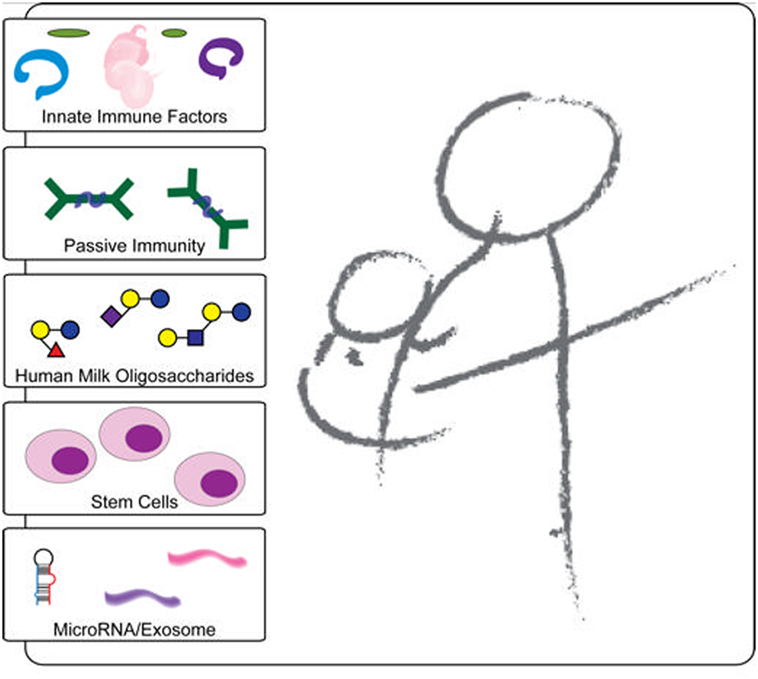
Figure 1. Schematic representation of the protective components in breast milk. Breast milk innate factors that have anti-inflammatory and antimicrobial properties are likely to affect the passage of HIV through the breastfeeding infant’s intestine by modulating the integrity of the intestinal mucosa and directly inactivating the virus. In contrast to exclusively breastfed infants, non-exclusively breastfed infants may be exposed to contaminated water and/or food antigens without a protective threshold level of innate factors in breast milk, which may lead to increased intestinal permeability and microbial translocation. In addition, the foreign antigens likely increase inflammation and recruit increased target cells leading to increased HIV transmission.
Another recently identified innate factor in breast milk is Tenascin-C (TNC), which has the capability to neutralize HIV-1 virions by binding to the chemokine co-receptor binding site on the HIV-1 envelope (51). Like lactoferrin, TNC binds to an epitope on the V3 loop of the HIV-1 envelope protein, blocking the virus’s interaction with mucosal epithelial cells due to this electrostatic interaction (51). These investigators concluded that due to TNC’s broad spectrum HIV-neutralizing activity it may provide a prophylactic agent to be orally administered to infants before breastfeeding (51). TNC is also an extracellular matrix protein previously shown to be involved in wound healing and fetal brain development (52, 53). Unfortunately, it was recently shown that the amount of TNC correlated only weakly with overall innate HIV-neutralizing activity of breast milk of uninfected women, indicating that the amount of TNC in mucosal fluids was inadequate to impede HIV transmission (54). Further, polyclonal IgG from HIV-infected breast milk blocked neutralizing activity of TNC (54).
We were the first to demonstrate that breast milk sTLR2 directly interacts with specific HIV-1 structural proteins, namely, p17, p24, and gp41, thus inhibiting cellular activation and cell-free HIV infection in vitro (44, 55). Furthermore, sTLR2 has known antimicrobial properties that significantly inhibit pro-inflammatory cytokine production in human intestinal epithelial cells (IECs), as well as reducing bacterial-associated inflammation in mice without impairing microbial clearance (44, 55, 56), and therefore, if developed, may provide a novel prophylactic anti-inflammatory agent in breastfeeding infants (57).
Recently, trappin-2/elafin was shown to be a biomarker of resistance to HIV infection in cervico-vaginal lavage of highly exposed seronegative commercial sex workers in Kenya (58). Our lab went on to demonstrate that elafin is 130 times more potent against HIV compared with its precursor, trappin-2, and part of the antiviral activity of this antiprotease was due to modulation of innate sensing (59–61). Hence, it is anticipated that breast milk trappin-2/elafin along with other antiproteases might serve as natural host-based immunomodulatory molecules. Further, these factors function concomitantly to control aberrant microbial-induced inflammation, have direct antimicrobial effects, as was most recently shown by Pfaender et al. (62), and inhibit HIV–host interaction thus are protective against postnatal HIV transmission to infants.
Breast milk contains a range of cytokines, some of which could potentially influence immune function and directly correlate with MTCT HIV transmission. Specifically, the pro-inflammatory chemokine ligand 5 (RANTES/CCL5) indirectly inhibits HIV infection in vitro by binding to its ligand CCR5, thus inhibiting gp120 binding to its co-receptor which is integral to host–viral attachment (63). However, RANTES levels in breast milk positively correlated with increased MTCT of HIV (64). Conversely, levels of breast milk interleukin (IL) 15, a pleotropic cytokine involved in activating CD8+ T and NK cells, positively correlated with protection from MTCT of HIV (65). Furthermore, we showed a positive correlation of sTLR2 and IL-15 levels in breast milk (55), thus indicating that these protective factors can function in concert to reduce MTCT of HIV.
Studies on African HIV-Exposed Infants and Breastfeeding
Over the past decade, more than two million HIV-exposed uninfected (HEU) infants were born each year. In sub-Saharan Africa, HEU infants suffer up to four times increased risk of dying in the first 2 years of life and increased risk for infectious morbidity (66). The etiology of increased susceptibility of HEU infants to infectious disease remains undetermined but is likely due to having their immune systems’ compromised.
A number of studies have been conducted on HEU infants relating breastfeeding to their health. One particular study conducted by John-Stewart et al. concluded that EBF of HIV-exposed infants decreased the likelihood of them falling ill to pneumonia (67). Specifically, of the 388 HEU infants in Kenya followed from birth to 12 months of age, the breastfed infants had a 47% lower chance of getting pneumonia with a 74% lower chance of being hospitalized due to pneumonia (67). It can be concluded from these studies that breastfeeding HEU infants has many benefits including lower risk of HIV infection, pneumonia, and infectious diseases.
In another study, Bork (68) discovered that HEU infants in Kesho Bora who were not breastfed had an increased risk of infection between birth and 2.9 months. HIV-infected pregnant women from five locations in Burkina Faso, Kenya, and South Africa involved in a study on MTCT in the Kesho Bora trail were asked to either exclusively breastfeed or formula feed their infants. In total, 751 infants were investigated for 2 years to see the health implications (particularly fever, diarrhea, vomiting, and other serious infections) in relation to feeding method. The results of this study further support that breastfeeding HEU infant’s has many positive implications on the health of the infants when compared with HEU infants who are not breastfed.
Beneficial Effects of Human Milk Oligosaccharides (HMOs)
Human milk oligosaccharides consist of a family of structurally diverse, unconjugated sugars that are found at very high abundance (5–23 g/l) and high complexity in human milk. More than 200 different HMOs have been identified and are unique to human milk (69–71). Together, HMOs make up the third largest component of breast milk and are completely indigestible to infants. Although once believed to have no biological significance, it is now clear that they can function as pathogen blocking agents or decoy receptors for pathogenic microbes (72), have direct signaling ability (73, 74), and play a major role in establishing a healthy microbiome (75–78).
To cause intestinal infection, bacterial and viral pathogens often adhere to lectin–glycan structures on mucosal surfaces to initiate colonization. For example, a common cause of bacterial diarrhea and infant mortality is due to Campylobacter jejuni, which binds to α1,2-fucosylatd glycan. Although recently contested (79), previous manuscripts have shown that HMOs directly reduce microbial infections by serving as soluble decoy receptors, which prevent pathogen binding to these glycans (80, 81). Addition of soluble α1,2-fucosylated HMO blocks binding of C. jejuni to human intestinal mucosa and reduces colonization in mice (82). The beneficial effects of this HMO on reducing C. jejuni-associated diarrhea were confirmed in a prospective study on 100 mother–infant pairs in Mexico City (83). In addition, HMOs have antiviral properties. Most recently, specific HMOs, namely, 2′ and 3′ fucosylactose (2′FL and 3′FL, respectively) have been shown to structurally mimic histo-blood group aantigens, and thus block norovirus from binding to this surrogate (84). Interestingly, HIV gp120 envelope glycoprotein binds to DC-specific ICAM3-grabbing non-integrin (DC-SIGN) on human DCs, which is important for mother-to-child HIV transmission via breastfeeding. DC-SIGN, though, has higher affinity for the Lewis (Le) blood group antigens (85, 86). HMOs containing Le blood group antigens compete with gp120 for binding to DC-SIGN in vitro (87). In the breastfed infant, mucosal surfaces are covered with high levels of HMOs that may block HIV entry via DC-SIGN, which may contribute to the relatively inefficient MTCT of HIV via breastfeeding (88).
Human milk oligosaccharides may also directly modulate IEC and immune cell responses. In vitro studies of human ECs incubated with the HMO 3′-sialyllactose decreases expression of sialyltransferases resulting in reduced binding of enteropathogenic Escherichia coli to intestinal ECs (89–91) and can directly alter growth-related cell cycle gene expression in intestinal ECs (92). It has also been proposed that sialylated HMOs may affect T lymphocyte maturation and promote a more balanced Th1/Th2 cytokine response (93). Indeed, exposure to sialylated HMOs was shown to reduce IL-4 production in lymphocytes from adult patients with peanut allergy (94) and, more recently, ingestion of 2′FL and 6′sialyllactose was shown to reduce food allergy through induction of IL-10 (+) T regulatory cells and indirect stabilization of mast cells in an animal model (95). Together, these data suggest HMOs may contribute to allergy prevention.
Arguably most importantly, our group, as well as others, have extensively elucidated how oligosaccharides, originally identified as “bifidus factor” (96), help promote and establish a healthy microbiome of breast feeding infants. HMOs, as a complex mixture of free glycans, provide a perfect growth advantage to Bifidobacterium infantis (97), which has evolved to contain all glycosyl hydrolases necessary to utilize HMOs internally (98) resulting in beneficial short-chain fatty acids and other metabolite production. This favors the growth of commensals in addition to lowering luminal pH (99).
Milk Components have Temporal and Spatial Specificity
There are various different protective factors in milk. Defense factors include maternal-derived SIgA and SIgM, oligosaccharides, anti-inflammatory factors, antioxidants, epithelial growth factors, and the aforementioned leukocytes and cytokines (100). One of the most prevalent antibodies present in breast milk, SIgA, is produced by plasma cells in the mammary gland (101). It is argued that SIgA B cells migrate from the pregnant mother’s gut to her breast before delivery, although migration of maternal IgA B cells is not well characterized. In mucosal tissues, SIgA is produced as a dimer in which two immunoglobulin monomers are linked by the J chain. Upward of 75,000 IgA-producing plasma cells are present in the normal human intestine with 3–4 g of IgA secreted daily. This significantly exceeds the production of all other immunoglobulin classes. Continuous production of large amounts of SIgA occurs in the absence of pathogen invasion and is driven by recognition of resident microbiota. IgA secreted in the gut lumen binds to mucus coating epithelial surfaces where it is involved in preventing attachment to the epithelium and invasion by pathogens. IgA can also neutralize microbial toxins, bacterial lipopolysaccharide, and viruses it encounters. The formation of IgA:antigen complexes can enhance the uptake and transcytosis of luminal antigen by M or microfold cells and facilitate its uptake by Peyer’s patch DCs (101).
Full-term neonates are deficient in SIgA especially at the colostral stage of lactation. Breastfeeding provides specific maternal-derived SIgA antibodies to protect the infant (102). Similarly, SIgM is also transferred via breast milk and is key in combating neonatal enteric antigens such as microorganisms and food proteins (13).
Cellular Components
The biological relevance of breast milk cells in MTCT of HIV remains unclear. Indeed, there are arguments that infected cells both facilitate and protect against HIV transmission in breastfeeding infants (103–104). Depending on the stage of lactation, the predominant cells types in milk consist of various leukocytes, in colostrum (4 × 106/ml) and mature breast milk (105–106/ml), and mammary epithelial cells (MECs). The majority of leukocytes in breast milk have an activated phenotype (105) and are comprised of macrophages (55–60%) and neutrophils (30–40%), while 5–10% are lymphocytes (~65% CD8+ T cells, 15% CD4+ T cells, and 20% B cells) (106–108).
Stem Cells in Human Breast Milk
Although most studies of cells in breast milk have focused on leukocytes and their immunological activities, particularly postpartum, more recent exciting studies have identified pluripotent stem cells in breast milk. Using a well-known stem cell marker found in neural, bone marrow, pancreatic and epithelial stem cells, nestin (109–111), Cregan et al. were the first to identify human breast milk stem cells (hBSCs) in milk from full-term mothers (112). These dynamic cells, which account for an estim ated 10–15% of all breast milk cells, were later shown to be pluripotent and successfully differentiate breast milk stem cells into adipogenic, chondrogenic, and osteogenic lineages (113, 114). Moreover, some investigators hypothesize that these cells may promote growth and development of the infant (115). Indeed, in mouse models, ingested milk stem cells were shown to survive in the gastrointestinal tract (116, 117). Given these insightful studies, it is clear that additional investigation of hBSCs will not only further our understanding of how hBSCs ingestion in early life may reduce disease burden in later life but may also provide novel regenerative medicine to replenish and restore damaged tissues.
Cell-Free Versus Cell-Associated HIV: Which is Responsible for MTCT of HIV?
Despite the increasing knowledge of breast milk virus, it remains unclear whether CFV or CAV is responsible for HIV-1 acquisition in the infant. Indeed, both CFV HIV RNA and CAV proviral DNA can be found in HIV-infected breast milk (when the mother is not receiving ARV therapy), and both levels correlate with postpartum MTCT of HIV (118, 119). Importantly, it has been shown that multiple innate immune factors that are endogenous to breast milk, including mucin, SLPI, sTLR2, lactoferrin, lysozyme, and oligosaccharides can effectively inactivate CFV infection in vitro (31, 32, 44, 55, 58, 88, 120–123), whereas innate immune factors seemingly have little to no effect on CAV infection in vitro (32, 124). CAV HIV-1 infection has been shown to be more efficient compared with CFV infection (125) and is significantly more difficult to neutralize (126), thus indicating that CAV might be responsible for postnatal MTCT transmission. Conversely, ARV therapy significantly decreases HIV RNA and correlates with reduction in breast milk transmission rates (127, 128), while proviral DNA levels remain largely unaltered (129, 130). These observations suggest that CFV likely plays an important role in breast milk transmission. Given these contradictory studies, it could be argued that multiple factors including overall maternal viral load, breast health (i.e., mastitis), innate immune factor levels, as well as feeding practices all contribute to the founding viral infection in the infant.
Based on a collection of studies examining cell-free and cell-associated HIV-1, there is a clear trend suggesting that CFV is more predominantly associated with viral load in the later stages postpartum. Ndirangu et al. showed that at 6 months, CFV was more heavily associated with HIV transmission. Up until 6 months, however, the viral load levels were analogous (131). Similarly, Koulinska et al. (130) showed that cell-free HIV-1 is a significant predictor of transmission after 9 months postpartum. In the earlier stages postpartum in both of the aforementioned studies, a 10-fold increase in both viral levels corresponded to a 3-fold increase in viral transmission (35, 131).
The first demonstration of selective transmission of HIV variants was conducted by Wolinsky et al. in 1992 (132) which showed that a minor subset of maternal virus was transmitted to the infant. However, the understanding of transmitted/founder viruses in breast milk is still not clearly defined (133, 134). Similar to other mucosally transmitted founder viruses, postnatal acquisition is primarily CCR5 tropic (134). Phylogenetic comparison of milk and plasma envelope gene sequences revealed that monotypic viruses are significantly more common in milk as compared with plasma from the same mother (133, 134), thus suggesting that the majority of breast milk viruses are produced by infected cells of the mammary gland. In addition, infant and maternal HIV variants did not differ in their sensitivity to broadly neutralizing antibodies; however, the viruses from transmitting mothers tended to be less sensitive to antibody-dependent neutralization (135). By contrast, other studies suggest that viral species found in the breast milk and plasma of infected mothers were genetically similar (136). Taken together, the role of breast milk cells in MTCT of HIV remains vague, and to explain these divergent observations, one possibility may be that the breast is continuously replenished with systemic CFV or CAV that can readily be transmitted to the breastfeeding infant and/or undergoes local replication in the mammary compartment (134). It is important to note that although MECs can endocytose cell-free HIV (137); whether or not the virus integrates into the host genome remains controversial (137, 138).
Cells Involved in MTCT
The biological relevance of breast milk cells in MTCT of HIV also remains unclear. Indeed, there are arguments that infected cells both facilitate and protect against HIV-1 transmission in breastfeeding infants (103, 104, 139).
Macrophages and MECs are thought to facilitate MTCT of HIV-1 for multiple reasons. First, macrophages make up the majority of leukocytes in breast milk (106), are readily infected with HIV-1, and express DC-SIGN, a DC-specific receptor for HIV-1 that facilitates HIV-1 infection in vitro (103). In addition, oral administration of macrophages to newborn mice survived several hours and were found in the neonatal intestine (104). Second, MECs make up a substantial portion of the cells in breast milk (108). These cells express several canonical HIV receptors (including CD4 and CCR5), readily endocytose cell-free HIV-1, and can act as a viral reservoir (137, 138). Furthermore, our laboratory recently found that MECs exposed to cell-free R5 HIV-1 on the basolateral surface readily transcytosed virus through the monolayer without damaging tight junctions but did not integrate virus into the genome (data not shown). Moreover, HIV-1 basolateral exposure significantly altered TLR expression, led to significantly elevated pro-inflammatory cytokine production in both apical and basolateral compartments, and increased CCR5 expression. Viral production in MECs, CD4+ cells, and breast milk macrophages has previously been shown (103, 138, 140). In fact, the HIV-infected CD4+ T cells in breast milk are 17 times more effective than in blood (35).
Multiple target sites in the nursing infant’s gastrointestinal tract, including oral, esophageal, and intestinal mucosal epithelium have been proposed. Specifically, the oral epithelium has been shown to be permissive to both CFV and CAV in vitro (141). Yet, the oral environment also has effective anti-HIV properties (31) making MTCT HIV transmission possible, albeit at a very low incidence (141). A recently published in vivo infection model demonstrated that humanized mice were readily infected with HIV through the oral cavity; however, breast milk had strong inhibitory effects on both CFV and CAV (142). A persuasive argument has been made for MTCT HIV transmission through the infant’s intestine through multiple studies showing that mixed feeding doubled the risk of an infant acquiring disease compared with EBF (21–23, 143). In addition, a previous publication reported significantly increased lipopolysaccharide (LPS) levels in infants that were nEBF or weaned, indicating a disruption in the intestinal mucosa (33). Notably, this seminal publication showed that systemic LPS levels were a significant predictor of MTCT through breast milk, thus indicating that tight junctions were disrupted in the infant’s intestinal mucosa. Previously, Nazli et al. (144) showed that HIV-dependent production of pro-inflammatory cytokines disrupted tight junctions in IECs.
Next Steps in Research on Innate Immune Factors: sTLR2
We showed that sTLR2 inhibited bacterial and viral-induced cellular activation in intestinal cells (44, 55, 57) and HIV virions induced cellular activation through a TLR-mediated pathway leading to increased infection in vitro (145). This indicates that sTLR2 likely plays a role in inhibiting HIV and/or bacterial-induced cellular activation directly at the infant’s intestinal mucosa.
Toll-like receptors are evolutionarily conserved transmembrane pattern recognition receptors (PRRs) that recognize highly conserved pathogen-associated molecular patterns (PAMPs). They are part of the first line of defense against pathogen invasion and trigger innate immune responses and subsequent antigen-specific adaptive immunity. The 10 TLRs in humans recognize highly conserved molecules broadly shared and also expressed by pathogens, but not found in mammals, such as dsRNA, ssRNA, flagellin, CpG DNA, and LPS either intracellularly or extracellularly.
Historically, TLRs have not been extensively evaluated for their role in MTCT of HIV, despite the fact that soluble TLRs provide the most direct attenuation of inflammation and innate immune responses to pathogens by binding PAMPs, thus effectively inhibiting PAMP–PRR engagement (146). LeBouder et al. (147) were the first to identify forms of sTLR2 in breast milk and plasma and through computational molecular docking revealed a cylindrical arrangement between sTLR2 and soluble CD14 that encapsulates synthetic bacterial lipoprotein Pam3CSK4 preventing bacterial-induced cellular activation through membrane-bound TLR2. Additional publications have highlighted sTLR2’s role in significantly inhibiting bacterially induced pro-inflammatory cytokine production in vitro in oral epithelial cells, placental tissue explants, and human IECs (44, 55, 148, 149).
Although sTLR2 is important in regulating bacteria-induced cellular activation, sTLR2-dependent regulation of immune activation during viral infection remains poorly understood. Accruing evidence indicates that a range of soluble molecules, including defensins, interferons, antiproteases, and chemokines suppress and control viral infections (59, 150). In addition, our investigation showed that sTLR2 directly interacts with HIV PAMPs, including p17, p24, and gp41, which leads to significantly reduced IL-8 production, CCR5 expression, NFκB activation, and HIV infection in a dose-dependent manner (55).
In proceeding with this research to solidify the evidence, the next step is to establish the mechanism by which this interaction takes place. In support of this hypothesis, we also showed that sTLR2 has a very short half-life at physiological temperatures (44). Interestingly, sTLR2 levels were significantly increased in HIV-infected compared with uninfected breast milk samples, and significantly correlated with p24 [a marker of viremia (151)] and IL-15 concentrations (55). The correlation between sTLR2 and IL-15 might have important implications since IL-15 in breast milk has been shown to correlate with decreased MTCT of HIV (65). These findings not only have important implications for our fundamental understanding of HIV infection and pathogenesis but also have the potential to inform novel therapeutic approaches to prevent mucosal transmission of HIV, including MTCT. We envision that sTLR2 alone or combined with other innate antiviral factors, e.g., IL-15, could be directly added to expressed breast milk and orally administered to infants to augment prevention of HIV mucosal transmission. This would, however, be limited to addition to breast milk, as sTLR2 would not be stable in acidic environments such as the gastrointestinal tract and vaginal mucosa according to our observations. Importantly, host innate factors, including soluble TLR immunotherapeutics, are unlikely to be toxic. Similarly, sTLR2, alone or in combination with other innate factors, might be useful as a natural immunomodulatory molecule to prevent sexual transmission of HIV-1.
Breast Milk, MicroRNAs (miRNAs) and Exosomes: Regulators of Growth, Development, and Immune Protection
Human milk contains short, non-coding single RNA molecules called miRNAs that are approximately 22 nucleotides in length. Compared with other body fluids, milk is one of the richest sources of miRNAs, which are present in all three fractions of human milk, including cells, lipids, and skim milk (152). miRNAs serve as key regulators of gene expression at the posttranscriptional level by binding to an mRNA target to either inhibit translation of mRNA into protein or promote its degradation (153–155). miRNAs are first transcribed into primary microRNA (pri-microRNA) from specific genes on DNA by RNA polymerase II, and then are converted into hairpin precursor microRNA (pre-microRNA) by the Drosha–DGCR8 complex. The enzyme Dicer then produces mature miRNA from pre-microRNA in the cytoplasm (156, 157). A single mature miRNA can bind and regulate multiple mRNAs (158). Studies indicate that miRNAs can regulate up to 50% of protein synthesis (154).
Importantly, miRNAs play a key role in regulating the immune system, including T and B cell development (159, 160), release of inflammatory mediators (161), proliferation of monocytes and neutrophils (162), and differentiation of macrophages and DCs (163).
Breast milk miRNAs are frequently packaged in vesicles such as exosomes which play an important role in their survival under harsh conditions (164, 165). Indeed, since the nursing infant’s gut is less acidic and more permeable compared with adults, this provides strong support for survival, adsorption, and integration of milk miRNAs in the infant and facilitate early growth, protection, and development. Thus, it is important to characterize miRNAs in human milk and examine factors that might influence it.
Of 12 body fluids examined, breast milk contained vastly more miRNAs than any other fluid tested, including greater than 80-fold the concentration found in amniotic fluid and colostrum (152). miRNAs are resistant to acidic conditions, digestion by RNAse, incubation at room temperature and various freeze thaw cycles (166–169). In breast milk, this resistance is due to the fact that miRNAs are contained in exosomes or microvesicles. Treatment with detergent, Triton-X, that disrupts lipid membranes, results in degradation of miRNAs by RNAse (170). Resistance to acidic conditions ensures passage through the stomach and adsorption into the bloodstream, which in turn allows the exchange of genetic information between mother and offspring. Valadi et al. were the first to demonstrate that exosome-mediated transfer of mRNAs and miRNAs is a novel mechanism of genetic exchange between cells (171). Secreted miRNAs represent a newly recognized layer of gene regulation and intercellular communication (172–174), while exosomal miRNAs play a pivotal role in horizontal miRNA transfer (174) as was originally shown by Raposo et al. who provided the first evidence for exosome-mediated immune cell communication (175).
MicroRNAs were shown to play a critical role in innate antiviral defense (176–178). This was demonstrated by Triboulet et al. who knocked down two important miRNA processing proteins, Drosha and Dicer, resulting in significant enhancement of viral replication in peripheral blood mononuclear cells (PBMCs) from HIV-infected patients and in latently infected cells (179). Interestingly, they also demonstrated that knockdown of some of these effectors led to virus reactivation in PBMCs isolated from HIV-infected patients undergoing suppressive combination ART (180). These studies highlight the importance of miRNs in modulating HIV-1 infection. In a ground-breaking study, Huang et al. showed that a selected group of miRNAs, including miR-28, miR-125b, miR-150, miR223, and miR-382, bound to the 3′ UTR of viral mRNAs and showed that activation of resting CD4+ T cells resulted in downregulation of these miRNAs, which correlated with enhanced HIV-1 susceptibility (181). Further, experiments in which all five of these miRNAs were inhibited in resting CD4+ T cells from cART-treated individuals (with undetectable viremia) displayed enhanced HIV-1 production indicating that “anti-HIV-1 miRNAs” contribute to HIV-1 latency in resting CD4+ T cells. Thus, these studies suggest these novel anti-HIV-1 miRNAs could play a role in controlling HIV latency and their manipulation could potentially contribute to purging of viral reservoirs (181).
Other modulators of anti-HIV-1 miRNAs include cytokines and TLR ligands. For example, stimulation of TLR3 was shown to induce an anti-HIV effect in primary macrophages, partially through upregulation of miR-28, miR-125b, miR-150, and miR-382 (182). More recently, activation of TLR3 in primary human macrophages resulted in significantly enhanced expression of miR-155 that correlated with decreased HIV-1 infectivity (183). These investigators also showed that miR-155 inhibited HIV-1 at a postentry, pre-integration step (183). Together, these findings indicate interplay between miRNAs, TLRs, and HIV-1 that have important implications for HIV-1 infection, replication, and chronic immune activation (184).
Conclusion and Future Directions
The majority of infants’ breastfeeding from their HIV-infected mothers do not acquire HIV. Indeed, EBF has been one of the most successful interventions in protecting infants in resource-limited countries from a broad range of infectious diseases, including HIV. Although the reason for this remains unclear, coordination of a number of innate immune factors in breast milk seem crucial for providing protection when infants are most vulnerable. Identification and characterization of natural immune factors that protect susceptible individuals from acquiring HIV might facilitate the production of novel innate immunotherapeutics in the near future. Given that a number of innate factors have demonstrated anti-HIV activity, and ensuing decreased MTCT, it can be concluded that innate factors are indeed a viable option to pursue as protective therapies. In addition, other factors such as sTLR2 and IL-15 have shown promising results and should be pursued to further understand their mechanisms of binding and blocking HIV-1 MTCT.
Human milk is a gold mine of uncharacterized, natural innate bioactive factors that have great promise to be developed and utilized for therapy or treatment of infections and inflammatory conditions in infants as well as adults and the elderly.
Author Contributions
KR initiated the review article, contributed to the design and conductance of the study, collection of relevant literature, and writing and editing of the manuscript. BH played a key role in design and conductance of the study. She performed experiments noted in the review, analysis of the data as well as contributing to the writing and editing of the review. Similarly, X-DY carried out much of the experimentation and bench research. She also made major contributions to analysis of the data and writing of the manuscript. Both BH and X-DY spent substantial time working on our projects in Kenya and South Africa. LN and AR were students who contributed to collecting, reading, and identifying relevant literature for the review. All authors contributed to editing the review. LN is now a resident physician at McMaster University Health Sciences Centre.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors are grateful for the support from the Canadian Institutes of Health Research (CIHR) and the Canadian HIV Vaccine Initiative (CHVI) and the Innovation Institute for Food and Health (IIFH) University of California, Davis. This study was conducted, in part, as a Large Team Grant in collaboration with investigators from the University of Cape Town and Stellenbosch University in South Africa, the Institute of Human Virology in Nigeria, the University of Ottawa, the University of Manitoba, the Public Health Agency of Canada (PHAC), and McMaster University.
References
1. Grulee CG, Sanford HN, Schwartz H. Breast and artificially fed infants. JAMA (1935) 104:1986–88. doi:10.1001/jama.1935.02760220032011
3. Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci (2008) 87:26–34. doi:10.2527/jas.2008-1347
4. Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res (2007) 61:2–8. doi:10.1203/01.pdr.0000250274.68571.18
5. Bryce J, Victora CG, Black RE. The unfinished agenda in child survival. Lancet (2013) 382:1049–59. doi:10.1016/S0140-6736(13)61753-5
7. Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet (2016) 387:475–90. doi:10.1016/S0140-6736(15)01024-7
8. Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet (2016) 387:491–504. doi:10.1016/S0140-6736(15)01044-2
9. Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr (2015) 104:3–13. doi:10.1111/apa.13147
10. Victora CG, Barros AJD, Fuchs SC, Francisco A, Morris J, Hall AJ, et al. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet (2000) 355:451–5. doi:10.1016/S0140-6736(00)82011-5
11. Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) (2007) (153):1–186.
12. Horta BL, Victora CG. Short-term Effects of Breastfeeding. A Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality. (2013).
13. Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine (2003) 21:3382–8. doi:10.1016/S0264-410X(03)00338-4
14. Brandtzaeg P. The gut as communicator between environment and host: immunological consequences. Eur J Pharmacol (2011) 668(Suppl 1):S16–32. doi:10.1016/j.ejphar.2011.07.006
15. Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr (2010) 156:S8–15. doi:10.1016/j.jpeds.2009.11.014
16. Hanson LA, Korotkova M, Telemo ER. Breast-feeding, infant formulas, and the immune system. Ann Allergy Asthma Immunol (2003) 90:59–63. doi:10.1016/S1081-1206(10)61662-6
17. Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of breastfeeding intervention trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA (2001) 285:413–20. doi:10.1001/jama.285.4.413
18. Thapa S, Short RV, Potts M. Breast feeding, birth spacing and their effects on child survival. Nature (1988) 335:679–82. doi:10.1038/335679a0
19. Breastfeeding and HIV International Transmission Study Group, Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis (2004) 189:2154–66. doi:10.1086/420834
20. Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis (2006) 6:726–32. doi:10.1016/S1473-3099(06)70629-6
21. Kuhn L, Sinkala M, Kankasa C, Semrau K, Kasonde P, Scott N, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS One (2007) 2:e1363. doi:10.1371/journal.pone.0001363
22. Coutsoudis A, Kuhn L, Pillay K, Coovadia HM. Exclusive breast-feeding and HIV transmission. AIDS (2002) 16:498–9. doi:10.1097/00002030-200202150-00028
23. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS (2005) 19:699–708. doi:10.1097/01.aids.0000166093.16446.c9
24. Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet (2007) 369:1107–16. doi:10.1016/S0140-6736(07)60283-9
25. World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva: WHO (2011). p. 1–117.
26. UNAIDS. Report on the Global AIDS Epidemic. (2012). p. 1–110. Available from: http://www.unaids.org/sites/default/files/media_asset/20121120_UNAIDS_Global_Report_2012_with_annexes_en_1.pdf
27. Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Chun T-W, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med (2013) 369:1828–35. doi:10.1056/NEJMoa1302976
28. Schramm DB, Kuhn L, Gray GE, Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr (2006) 42:545–53. doi:10.1097/01.qai.0000225009.30698.ce
29. WHO Organization. HIV and Infant Feeding. (2010). p. 1–58. Available from: http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf
30. World Health Organization. Global HIV/AIDS Response. World Health Organization Progress Report 2011. Geneva: WHO (2011). p. 1–233.
31. Kazmi SH, Naglik JR, Sweet SP, Evans RW, O’Shea S, Banatvala JE, et al. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin Vaccine Immunol (2006) 13:1111–8. doi:10.1128/CDLI.00426-05
32. Lyimo MA, Howell AL, Balandya E, Eszterhas SK, Connor RI. Innate factors in human breast milk inhibit cell-free HIV-1 but not cell-associated HIV-1 infection of CD4+ cells. J Acquir Immune Defic Syndr (2009) 51:117–24. doi:10.1097/QAI.0b013e3181a3908d
33. Kourtis AP, Wiener J, Kayira D, Chasela C, Ellington SR, Hyde L, et al. Health outcomes of HIV-exposed uninfected African infants. AIDS (2013) 27:749–59. doi:10.1097/QAD.0b013e32835ca29f
34. Kourtis AP, Bulterys M. Mother-to-child transmission of HIV: pathogenesis, mechanisms and pathways. Clin Perinatol (2010) 37:721–37, vii. doi:10.1016/j.clp.2010.08.004
35. Van de Perre P, Rubbo P-A, Viljoen J, Nagot N, Tylleskär T, Lepage P, et al. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci Transl Med (2012) 4:143sr3. doi:10.1126/scitranslmed.3003327
36. Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system? Bioessays (2006) 28:606–16. doi:10.1002/bies.20423
37. Molinari CE, Casadio YS, Hartmann BT, Livk A, Bringans S, Arthur PG, et al. Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res (2012) 11:1696–714. doi:10.1021/pr2008797
38. Aldrovandi GM, Kuhn L. What infants and breasts can teach us about natural protection from HIV infection. J Infect Dis (2010) 202(Suppl 3):S366–70. doi:10.1086/655972
39. Kuhn L, Trabattoni D, Kankasa C, Sinkala M, Lissoni F, Ghosh M, et al. HIV-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. J Pediatr (2006) 149:611–6. doi:10.1016/j.jpeds.2006.06.017
40. Hocini H, Belec L, Iscaki S, Garin B, Pillot J, Becquart P, et al. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res Hum Retroviruses (1997) 13:1179–85. doi:10.1089/aid.1997.13.1179
41. Saeland E, de Jong MAWP, Nabatov AA, Kalay H, Geijtenbeek TBH, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol (2009) 46:2309–16. doi:10.1016/j.molimm.2009.03.025
42. Groot F, Geijtenbeek TBH, Sanders RW, Baldwin CE, Sanchez-Hernandez M, Floris R, et al. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN – gp120 interaction. J Virol (2005) 79:3009–15. doi:10.1128/JVI.79.5.3009-3015.2005
43. McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest (1995) 96:456–64. doi:10.1172/JCI118056
44. Henrick BM, Nag K, Yao X-D, Drannik AG, Aldrovandi GM, Rosenthal KL. Milk matters: soluble toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PLoS One (2012) 7:e40138. doi:10.1371/journal.pone.0040138.t001
45. Semba RD, Miotti PG, Lan Y, Chiphangwi JD, Hoover DR, Dallabetta GA, et al. Maternal serum lactoferrin and vertical transmission of HIV. AIDS (1998) 12:331–2.
46. van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK. Antiviral activities of lactoferrin. Antiviral Res (2001) 52:225–39. doi:10.1016/S0166-3542(01)00195-4
47. Bernt KM, Walker WA. Human milk as a carrier of biochemical messages. Acta Paediatr Scand (1999) 88:27–41. doi:10.1111/j.1651-2227.1999.tb01298.x
48. Gomez HF, Ochoa TJ, Carlin LG, Cleary TG. Human lactoferrin impairs virulence of Shigella flexneri. J Infect Dis (2003) 187:87–95. doi:10.1086/345875
49. Farquhar C, VanCott TC, Mbori-Ngacha DA, Horani L, Bosire RK, Kreiss JK, et al. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J Infect Dis (2002) 186:1173–6. doi:10.1086/343805
50. McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood (1997) 90:1141–9.
51. Fouda GG, Jaeger FH, Amos JD, Ho C, Kunz EL, Anasti K, et al. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc Natl Acad Sci U S A (2013) 110:18220–5. doi:10.1073/pnas.1307336110
52. Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal (2009) 3:287–310. doi:10.1007/s12079-009-0075-1
53. Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett (2006) 244:143–63. doi:10.1016/j.canlet.2006.02.017
54. Mansour RG, Stamper L, Jaeger F, McGuire E, Fouda G, Amos J, et al. The presence and anti-HIV-1 function of tenascin C in breast milk and genital fluids. PLoS One (2016) 11:e0155261. doi:10.1371/journal.pone.0155261
55. Henrick BM, Yao X-D, Drannik AG, Abimiku A, Rosenthal KL; INFANT Study Team. Soluble toll-like receptor 2 is significantly elevated in HIV-1 infected breast milk and inhibits HIV-1 induced cellular activation, inflammation and infection. AIDS (2014) 28:2023–32. doi:10.1097/QAD.0000000000000381
56. Raby A-C, Le Bouder E, Colmont C, Davies J, Richards P, Coles B, et al. Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J Immunol (2009) 183:506–17. doi:10.4049/jimmunol.0802909
57. Henrick BM, Yao X-D, Taha AY, German JB, Rosenthal KL. Insights into soluble toll-like receptor 2 as a downregulator of virally induced inflammation. Front Immunol (2016) 7:157–9. doi:10.3389/fimmu.2016.00291
58. Drannik AG, Nag K, Yao X-D, Henrick BM, Jain S, Ball TB, et al. Anti-HIV-1 activity of elafin is more potent than its precursor’s, trappin-2, in genital epithelial cells. J Virol (2012) 86:4599–610. doi:10.1128/JVI.06561-11
59. Drannik AG, Henrick BM, Rosenthal KL. War and peace between WAP and HIV: role of SLPI, trappin-2, elafin and ps20 in susceptibility to HIV infection. Biochem Soc Trans (2011) 39:1427–32. doi:10.1042/BST0391427
60. Drannik AG, Nag K, Yao X-D, Henrick BM, Ball TB, Plummer FA, et al. Anti-HIV-1 activity of elafin depends on its nuclear localization and altered innate immune activation in female genital epithelial cells. PLoS One (2012) 7:e52738. doi:10.1371/journal.pone.0052738.g006
61. Drannik AG, Nag K, Yao X-D, Henrick BM, Sallenave J-M, Rosenthal KL. Trappin-2/elafin modulate innate immune responses of human endometrial epithelial cells to PolyI:C. PLoS One (2012) 7:e35866. doi:10.1371/journal.pone.0035866.g008
62. Pfaender S, Heyden J, Friesland M, Ciesek S, Ejaz A, Steinmann J, et al. Inactivation of hepatitis C virus infectivity by human breast milk. J Infect Dis (2013) 208:1943–52. doi:10.1093/infdis/jit519
63. Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature (1996) 381:667–73. doi:10.1038/381667a0
64. Bosire R, Guthrie BL, Lohman-Payne B, Mabuka J, Majiwa M, Wariua G, et al. Longitudinal comparison of chemokines in breastmilk early postpartum among HIV-1-infected and uninfected Kenyan women. Breastfeed Med (2007) 2:129–38. doi:10.1089/bfm.2007.0009
65. Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M, Kankasa C, et al. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis (2009) 200:1498–502. doi:10.1086/644603
66. Evans C, Humphrey JH, Ntozini R, Prendergast AJ. HIV-exposed uninfected infants in Zimbabwe: insights into health outcomes in the pre-antiretroviral therapy era. Front Immunol (2016) 7:190. doi:10.3389/fimmu.2016.00190
67. Ásbjörnsdóttir KH, Slyker JA, Weiss NS, Mbori-Ngacha D, Maleche-Obimbo E, Wamalwa D, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS (2013) 27:2809–15. doi:10.1097/01.aids.0000432540.59786.6d
68. Bork KA, Cournil A, Read JS, Newell ML, Cames C, Meda N, et al. Morbidity in relation to feeding mode in African HIV-exposed, uninfected infants during the first 6 mo of life: the Kesho Bora study. Am J Clin Nutr (2014) 100:1559–68. doi:10.3945/ajcn.113.082149
69. Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics (1993) 91:637–41.
70. Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr (2000) 20:699–722. doi:10.1146/annurev.nutr.20.1.699
71. Niñonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, et al. A strategy for annotating the human milk glycome. J Agric Food Chem (2006) 54:7471–80. doi:10.1021/jf0615810
72. Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr (2005) 25:37–58. doi:10.1146/annurev.nutr.25.050304.092553
73. Newburg DS. Glycobiology of human milk. Biochemistry (Mosc) (2013) 78:771–85. doi:10.1134/S0006297913070092
74. He Y, Liu S, Kling DE, Leone S, Lawlor NT, Huang Y, et al. The human milk oligosaccharide 2’-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut (2016) 65:33–46. doi:10.1136/gutjnl-2014-307544
75. Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr (2006) 136:2127–30.
76. Newburg DS. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr (2000) 30(Suppl 2):S8–17. doi:10.1097/00005176-200000002-00003
77. German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program (2008) 62:205–18; discussion 218–22. doi:10.1159/000146322
78. Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr (2014) 34:143–69. doi:10.1146/annurev-nutr-071813-105721
79. Cilieborg MS, Sangild PT, Jensen ML, Østergaard MV, Christensen L, Rasmussen SO, et al. α1,2-fucosyllactose does not improve intestinal function or prevent Escherichia coli F18 diarrhea in newborn pigs. J Pediatr Gastroenterol Nutr (2017) 64:310–8. doi:10.1097/MPG.0000000000001276
80. Simon PM, Goode PL, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun (1997) 65:750–7.
81. Gustafsson A, Hultberg A, Sjöström R, Kacskovics I, Breimer ME, Borén T, et al. Carbohydrate-dependent inhibition of Helicobacter pylori colonization using porcine milk. Glycobiology (2006) 16:1–10. doi:10.1093/glycob/cwj031
82. Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem (2003) 278:14112–20. doi:10.1074/jbc.M207744200
83. Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr (2004) 145:297–303. doi:10.1016/j.jpeds.2004.04.054
84. Weichert S, Koromyslova A, Singh BK, Hansman S, Jennewein S, Schroten H, et al. Structural basis for norovirus inhibition by human milk oligosaccharides. J Virol (2016) 90:4843–8. doi:10.1128/JVI.03223-15
85. Naarding MA, Ludwig IS, Groot F, Berkhout B, Geijtenbeek TBH, Pollakis G, et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest (2005) 115:3256–64. doi:10.1172/JCI25105
86. van Liempt E, Bank CMC, Mehta P, Garciá-Vallejo JJ, Kawar ZS, Geyer R, et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett (2006) 580:6123–31. doi:10.1016/j.febslet.2006.10.009
87. Hong P, Niñonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br J Nutr (2009) 101:482–6. doi:10.1017/S0007114508025804
88. Bode L, Kuhn L, Kim H-Y, Hsiao L, Nissan C, Sinkala M, et al. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr (2012) 96:831–9. doi:10.3945/ajcn.112.039503
89. Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, et al. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology (2005) 15:31–41. doi:10.1093/glycob/cwh143
90. Kuntz S, Rudloff S, Kunz C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br J Nutr (2008) 99:462–71. doi:10.1017/S0007114507824068
91. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev (2015) 91:619–22. doi:10.1016/j.earlhumdev.2015.09.001
92. Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One (2013) 8:e76236. doi:10.1371/journal.pone.0076236
93. Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, et al. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res (2004) 56:536–40.
94. Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, et al. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol (2010) 21:1179–88. doi:10.1111/j.1399-3038.2010.01062.x
95. Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy (2015) 70:1091–102. doi:10.1111/all.12650
96. Gyorgy P, Norris RF, Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys (1954) 48:193–201. doi:10.1016/0003-9861(54)90323-9
97. Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem (2010) 58:5334–40. doi:10.1021/jf9044205
98. LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol (2010) 76:7373–81. doi:10.1128/AEM.00675-10
99. Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, et al. Persistence of supplemented bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere (2017) 2:e00501–17. doi:10.1128/mSphere.00501-17
100. Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J (1993) 12:664–71. doi:10.1097/00006454-199308000-00008
101. Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol (2004) 4:565–72. doi:10.1038/nri1393
102. Liu B, Newburg DS. Human milk glycoproteins protect infants against human pathogens. Breastfeed Med (2013) 8:354–62. doi:10.1089/bfm.2013.0016
103. Satomi M, Shimizu M, Shinya E, Watari E, Owaki A, Hidaka C, et al. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J Infect Dis (2005) 191:174–81. doi:10.1086/426829
104. Hughes A, Brock JH, Parrott DM, Cockburn F. The interaction of infant formula with macrophages: effect on phagocytic activity, relationship to expression of class II MHC antigen and survival of orally administered macrophages in the neonatal gut. Immunology (1988) 64:213–8.
105. Michie CA, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw (1998) 9:123–9.
106. Goldman AS. Human milk, leukocytes, and immunity. J Pediatr (1977) 90:167–8. doi:10.1016/S0022-3476(77)80805-6
107. Jain N, Mathur NB, Sharma VK, Dwarkadas AM. Cellular composition including lymphocyte subsets in preterm and full term human colostrum and milk. Acta Paediatr Scand (1991) 80:395–9. doi:10.1111/j.1651-2227.1991.tb11872.x
108. Hassiotou F, Geddes DT, Hartmann PE. Cells in human milk: state of the science. J Hum Lact (2013) 29:171–82. doi:10.1177/0890334413477242
109. Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci (1992) 103(Pt 2):589–97.
110. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell (1990) 60:585–95. doi:10.1016/0092-8674(90)90662-X
111. Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol (2002) 178:288–93. doi:10.1006/exnr.2002.8039
112. Cregan MD, Fan Y, Appelbee A, Brown ML, Klopcic B, Koppen J, et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res (2007) 329:129–36. doi:10.1007/s00441-007-0390-x
113. Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger A-J, Metzger P, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells (2012) 30:2164–74. doi:10.1002/stem.1188
114. Patki S, Kadam S, Chandra V, Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell (2010) 23:35–40. doi:10.1111/j.1749-0774.2010.00083.x
115. Twigger AJ, Hodgetts S, Filgueira L, Hartmann PE, Hassiotou F. From breast milk to brains: the potential of stem cells in human milk. J Hum Lact (2013) 29:136–9. doi:10.1177/0890334413475528
116. Hassiotou F, Hartmann PE. At the dawn of a new discovery: the potential of breast milk stem cells. Adv Nutr (2014) 5:770–8. doi:10.3945/an.114.006924
117. Dutta P, Burlingham WJ. Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism (2010) 1:2–10. doi:10.4161/chim.1.1.12667
118. Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis (2004) 190:1880–8. doi:10.1086/425076
119. Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis (2003) 187:741–7. doi:10.1086/374273
120. Habte HH, de Beer C, Lotz ZE, Tyler MG, Schoeman L, Kahn D, et al. The inhibition of the human immunodeficiency virus type 1 activity by crude and purified human pregnancy plug mucus and mucins in an inhibition assay. Virol J (2008) 5:59. doi:10.1186/1743-422X-5-59
121. Habte HH, de Beer C, Lotz ZE, Tyler MG, Kahn D, Mall AS. Inhibition of human immunodeficiency virus type 1 activity by purified human breast milk mucin (MUC1) in an inhibition assay. Neonatology (2008) 93:162–70. doi:10.1159/000108414
122. Ma G, Greenwell-Wild T, Lei K, Jin W, Swisher J, Hardegen N, et al. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J Exp Med (2004) 200:1337–46. doi:10.1084/jem.20041115
123. Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, Hershow RC, et al. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol (2007) 14:1102–7. doi:10.1128/CVI.00386-06
124. Yamaguchi K, Sugiyama T, Takizawa M, Yamamoto N, Honda M, Natori M. Viability of infectious viral particles of HIV and BMCs in breast milk. J Clin Virol (2007) 39:222–5. doi:10.1016/j.jcv.2007.04.011
125. Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood (2008) 111:4660–3. doi:10.1182/blood-2007-12-130070
126. Chen P, Hübner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol (2007) 81:12582–95. doi:10.1128/JVI.00381-07
127. Kilewo C, Karlsson K, Massawe A, Lyamuya E, Swai A, Mhalu F, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr (2008) 48:315–23. doi:10.1097/QAI.0b013e31816e395c
128. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med (2010) 362:2282–94. doi:10.1056/NEJMoa0907736
129. Kantarci S, Koulinska IN, Aboud S, Fawzi WW, Villamor E. Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J Acquir Immune Defic Syndr (2007) 46:651–4. doi:10.1097/QAI.0b013e31815b2db2
130. Koulinska IN, Villamor E, Chaplin B, Msamanga G, Fawzi W, Renjifo B, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr (2006) 41:93–9. doi:10.1097/01.qai.0000179424.19413.24
131. Ndirangu J, Viljoen J, Bland RM, Danaviah S, Thorne C, Van de Perre P, et al. Cell-free (RNA) and cell-associated (DNA) HIV-1 and postnatal transmission through breastfeeding. PLoS One (2012) 7:e51493. doi:10.1371/journal.pone.0051493
132. Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, et al. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science (1992) 255:1134–7. doi:10.1126/science.1546316
133. Gantt S, Carlsson J, Heath L, Bull ME, Shetty AK, Mutsvangwa J, et al. Genetic analyses of HIV-1 env sequences demonstrate limited compartmentalization in breast milk and suggest viral replication within the breast that increases with mastitis. J Virol (2010) 84:10812–9. doi:10.1128/JVI.00543-10
134. Salazar-Gonzalez JF, Salazar MG, Learn GH, Fouda GG, Kang HH, Mahlokozera T, et al. Origin and evolution of HIV-1 in breast milk determined by single-genome amplification and sequencing. J Virol (2011) 85:2751–63. doi:10.1128/JVI.02316-10
135. Mabuka J, Goo L, Omenda MM, Nduati R, Overbaugh J. HIV-1 maternal and infant variants show similar sensitivity to broadly neutralizing antibodies, but sensitivity varies by subtype. AIDS (2013) 27:1535–44. doi:10.1097/QAD.0b013e32835faba5
136. Henderson GJ, Hoffman NG, Ping LH, Fiscus SA, Hoffman IF, Kitrinos KM, et al. HIV-1 populations in blood and breast milk are similar. Virology (2004) 330:295–303. doi:10.1016/j.virol.2004.09.004
137. Dorosko SM, Connor RI. Primary human mammary epithelial cells endocytose HIV-1 and facilitate viral infection of CD4+ T lymphocytes. J Virol (2010) 84:10533–42. doi:10.1128/JVI.01263-10
138. Toniolo A, Serra C, Conaldi PG, Basolo F, Falcone V, Dolei A. Productive HIV-1 infection of normal human mammary epithelial cells. AIDS (1995) 9:859–66. doi:10.1097/00002030-199508000-00005
139. Lohman-Payne B, Slyker JA, Moore S, Maleche-Obimbo E, Wamalwa DC, Richardson BA, et al. Breast milk cellular HIV-specific interferon γ responses are associated with protection from peripartum HIV transmission. AIDS (2012) 26:2007–16. doi:10.1097/QAD.0b013e328359b7e0
140. Petitjean G, Becquart P, Tuaillon E, Tabaa Al Y, Valea D, Huguet M-F, et al. Isolation and characterization of HIV-1-infected resting CD4+ T lymphocytes in breast milk. J Clin Virol (2007) 39:1–8. doi:10.1016/j.jcv.2007.02.004
141. Moore JS, Rahemtulla F, Kent LW, Hall SD, Ikizler MR, Wright PF, et al. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology (2003) 313:343–53. doi:10.1016/S0042-6822(03)00283-6
142. Wahl A, Swanson MD, Nochi T, Olesen R, Denton PW, Chateau M, et al. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog (2012) 8:e1002732. doi:10.1371/journal.ppat.1002732.s001
143. Coovadia H, Kindra G. Breastfeeding to prevent HIV transmission in infants: balancing pros and cons. Curr Opin Infect Dis (2008) 21:11–5. doi:10.1097/QCO.0b013e3282f40689
144. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog (2010) 6:e1000852. doi:10.1371/journal.ppat.1000852
145. Henrick BM, Yao X-D, Rosenthal KL; INFANT Study Team. HIV-1 structural proteins serve as PAMPs for TLR2 heterodimers significantly increasing infection and innate immune activation. Front Immunol (2015) 6:426. doi:10.3389/fimmu.2015.00426
146. Liew FY, Xu D, Brint EK, O’Neill LAJ. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol (2005) 5:446–58. doi:10.1038/nri1630
147. LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, et al. Soluble forms of toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol (2003) 171:6680–9. doi:10.4049/jimmunol.171.12.6680
148. Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, et al. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol (2009) 182:7244–53. doi:10.4049/jimmunol.0803517
149. Kuroishi T, Tanaka Y, Sakai A, Sugawara Y, Komine K-I, Sugawara S. Human parotid saliva contains soluble toll-like receptor (TLR) 2 and modulates TLR2-mediated interleukin-8 production by monocytic cells. Mol Immunol (2007) 44:1969–76. doi:10.1016/j.molimm.2006.09.028
150. DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol (2004) 2:401–13. doi:10.1038/nrmicro878
151. Ledergerber B, Flepp M, Böni J, Tomasik Z, Cone RW, Lüthy R, et al. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J Infect Dis (2000) 181:1280–8. doi:10.1086/315366
152. Weber JA, Baxter DH, Zhang S, Huang DY, How Huang K, Jen Lee M, et al. The microRNA spectrum in 12 body fluids. Clin Chem (2010) 56:1733–41. doi:10.1373/clinchem.2010.147405
153. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet (2004) 5:522–31. doi:10.1038/nrg1379
154. Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet (2012) 13:358–69. doi:10.1038/nrg3198
155. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem (2010) 79:351–79. doi:10.1146/annurev-biochem-060308-103103
156. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol (2009) 11:228–34. doi:10.1038/ncb0309-228
157. Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development (2005) 132:4645–52. doi:10.1242/dev.02070
158. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet (2010) 11:597–610. doi:10.1038/nrg2843
159. Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell (2008) 132:875–86. doi:10.1016/j.cell.2008.02.019
160. Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol (2008) 9:405–14. doi:10.1038/ni1575
161. Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell (2005) 120:623–34. doi:10.1016/j.cell.2004.12.038
162. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell (2007) 129:1401–14. doi:10.1016/j.cell.2007.04.040
163. O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A (2007) 104:1604–9. doi:10.1073/pnas.0610731104
164. Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, et al. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr (2017) 147:3–10. doi:10.3945/jn.116.238949
165. Alsaweed M, Lai C, Hartmann P, Geddes D, Kakulas F. Human milk cells contain numerous miRNAs that may change with milk removal and regulate multiple physiological processes. Int J Mol Sci (2016) 17:E956. doi:10.3390/ijms17060956
166. Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci (2012) 8:118–23. doi:10.7150/ijbs.8.118
167. Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X, et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One (2012) 7:e43691. doi:10.1371/journal.pone.0043691
168. Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun (2010) 396:528–33. doi:10.1016/j.bbrc.2010.04.135
169. Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci (2012) 95:4831–41. doi:10.3168/jds.2012-5489
170. Gigli I, Maizon DO. MicroRNAs and the mammary gland: a new understanding of gene expression. Genet Mol Biol (2013) 36:465–74. doi:10.1590/S1415-47572013005000040
171. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9:654–9. doi:10.1038/ncb1596
172. Liang H, Huang L, Cao J, Zen K, Chen X, Zhang C-Y. Regulation of mammalian gene expression by exogenous microRNAs. Wiley Interdiscip Rev RNA (2012) 3:733–42. doi:10.1002/wrna.1127
173. Ludwig A-K, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol (2012) 44:11–5. doi:10.1016/j.biocel.2011.10.005
174. Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol (2012) 22:125–32. doi:10.1016/j.tcb.2011.12.001
175. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med (1996) 183:1161–72. doi:10.1084/jem.183.3.1161
176. Kumar A. RNA interference: a multifaceted innate antiviral defense. Retrovirology (2008) 5:17. doi:10.1186/1742-4690-5-17
177. Müller S, Imler J-L. Dicing with viruses: microRNAs as antiviral factors. Immunity (2007) 27:1–3. doi:10.1016/j.immuni.2007.07.003
178. Schütz S, Sarnow P. Interaction of viruses with the mammalian RNA interference pathway. Virology (2006) 344:151–7. doi:10.1016/j.virol.2005.09.034
179. Triboulet R, Mari B, Lin Y-L, Chable-Bessia C, Bennasser Y, Lebrigand K, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science (2007) 315:1579–82. doi:10.1126/science.1136319
180. Chable-Bessia C, Meziane O, Latreille D, Robinson R, Zamborlini A, Wagschal A, et al. Suppression of HIV-1 replication by microRNA effectors. Retrovirology (2009) 6:26. doi:10.1186/1742-4690-6-26
181. Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med (2007) 13:1241–7. doi:10.1038/nm1639
182. Zhou Y, Wang X, Liu M, Hu Q, Song L, Ye L, et al. A critical function of toll-like receptor-3 in the induction of anti-human immunodeficiency virus activities in macrophages. Immunology (2010) 131:40–9. doi:10.1111/j.1365-2567.2010.03270.x
183. Swaminathan G, Rossi F, Sierra L-J, Gupta A, Navas-Martín S, Martín-García J. A role for microRNA-155 modulation in the anti-HIV-1 Effects of toll-like receptor 3 stimulation in macrophages. PLoS Pathog (2012) 8:e1002937. doi:10.1371/journal.ppat.1002937
Keywords: breast milk, breastfeeding behaviors, HIV-1, human breast milk stem cells, human milk oligosaccharides, innate immune bioactive factors, mother-to-child HIV transmission, soluble toll-like receptor 2
Citation: Henrick BM, Yao X-D, Nasser L, Roozrogousheh A and Rosenthal KL (2017) Breastfeeding Behaviors and the Innate Immune System of Human Milk: Working Together to Protect Infants against Inflammation, HIV-1, and Other Infections. Front. Immunol. 8:1631. doi: 10.3389/fimmu.2017.01631
Received: 27 July 2017; Accepted: 09 November 2017;
Published: 29 November 2017
Edited by:
Shokrollah Elahi, University of Alberta, CanadaReviewed by:
François J. M. A. Meurens, Agroalimentaire et de l’alimentation de Nantes-Atlantique, FranceLijun Xin, Cincinnati Children’s Hospital Medical Center, United States
Copyright: © 2017 Henrick, Yao, Nasser, Roozrogousheh and Rosenthal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth L. Rosenthal, cm9zZW50aGxAbWNtYXN0ZXIuY2E=
 Bethany M. Henrick
Bethany M. Henrick Xiao-Dan Yao
Xiao-Dan Yao Laila Nasser
Laila Nasser Ava Roozrogousheh3
Ava Roozrogousheh3 Kenneth L. Rosenthal
Kenneth L. Rosenthal