- 1World Health Organization, Geneva, Switzerland
- 2University of Tokyo, Tokyo, Japan
- 3Centers for Disease Control and Prevention, Atlanta, GA, United States
- 4Taskforce for Child Health, Atlanta, GA, United States
Individuals with primary immunodeficiency disorder may excrete poliovirus for extended periods and will constitute the only remaining reservoir of virus after eradication and withdrawal of oral poliovirus vaccine. Here, we analyzed the epidemiology of prolonged and chronic immunodeficiency-related vaccine-derived poliovirus cases in a registry maintained by the World Health Organization, to identify risk factors and determine the length of excretion. Between 1962 and 2016, there were 101 cases, with 94/101 (93%) prolonged excretors and 7/101 (7%) chronic excretors. We documented an increase in incidence in recent decades, with a shift toward middle-income countries, and a predominance of poliovirus type 2 in 73/101 (72%) cases. The median length of excretion was 1.3 years (95% confidence interval: 1.0, 1.4) and 90% of individuals stopped excreting after 3.7 years. Common variable immunodeficiency syndrome and residence in high-income countries were risk factors for long-term excretion. The changing epidemiology of cases, manifested by the greater incidence in recent decades and a shift to from high- to middle-income countries, highlights the expanding risk of poliovirus transmission after oral poliovirus vaccine cessation. To better quantify and reduce this risk, more sensitive surveillance and effective antiviral therapies are needed.
Introduction
Since the launch of the Global Polio Eradication Initiative in 1988, there has been substantial progress toward eradication, documented by a decline in the incidence of cases from >350,000 in 1988 to 37 in 2016, and a decrease in the number of endemic countries from 125 to 3 (1). Although wild poliovirus type 1 continues to circulate in Afghanistan, Nigeria, and Pakistan, no wild poliovirus type 2 or wild poliovirus type 3 have been detected globally since October 1999 (2) and November 2012 (3), periods of 17 and 4 years, respectively.
These achievements have been accomplished through the extensive use of oral poliovirus vaccines (OPV), which are easy to administer, suitable for mass campaigns, and able to induce both humoral and mucosal immunity (4). However, in rare instances, live Sabin strains contained in OPV can cause vaccine-associated paralytic poliomyelitis, with paralytic manifestations indistinguishable from those caused by wild poliovirus (4). Furthermore, these viruses have the potential to revert to neurovirulence and re-acquire the transmissibility characteristics of wild poliovirus, resulting in outbreaks of circulating vaccine-derived poliovirus (VDPV) (5). In the presence of high population immunity, VDPVs rarely emerge and cause outbreaks. However, in areas with low population immunity, these viruses could potentially re-establish endemic transmission (6).
Immunodeficiency-related vaccine-derived poliovirus (iVDPV) is a type of VDPV in which individuals with a primary immunodeficiency disorder (PID) excrete Sabin polioviruses; in some cases, for substantially longer periods than immunocompetent individuals (4). After exposure to OPV, immunocompetent individuals usually excrete the vaccine virus for 4–8 weeks (7). However, in immunodeficient individuals, an inability to mount an adequate immune response can result in persistence of the intestinal infection with poliovirus and prolonged viral shedding (8). In the process, the virus can mutate to re-acquire the neurovirulence and transmissibility characteristics of wild poliovirus (9–12).
To address the risks associated with OPV use, the Strategic Plan of Action 2013–2018 of the Global Polio Eradication Initiative calls for a sequential global withdrawal of OPV, starting with the Sabin type 2 component that was removed in April 2016 and followed by Sabin type 1 and Sabin type 3 after certification of wild type 1 and 3 eradication, respectively (13). Although this strategy may minimize the long-term risk of circulating VDPV outbreaks, iVDPV cases will remain a potential source of live poliovirus in communities after the withdrawal of OPV and in the post eradication era, and could pose a substantial risk of poliovirus reintroduction in the population. The post-OPV cessation risks of long-term excretors have been modeled previously (14–16).
A number of case reports and case series of iVDPV excretors have been published with a systematic review of published cases undertaken (17), and more recently, studies have assessed iVDPV excretion among persons with PIDs (18–20). However, many of these works focus on only a subset of iVDPV cases, with case reports often limited to upper-middle income and high-income countries and patients with paralysis.
We present analysis of iVDPV cases known to the World Health Organization (WHO), from the inception of widespread OPV use (1962–2016). The main objectives of this study are to conduct demographic, risk factor, and survival analysis of reported iVDPV cases to determine the populations most at risk and to better understand the threat posed by iVDPV cases to global poliovirus eradication.
Materials and Methods
Study Population
This study analyzed cases in the iVDPV case registry maintained by WHO, containing reported cases from 1962 to 2016, irrespective of reporting source or paralysis status (see Table S2 in Supplementary Table). The main sources contributing cases to the registry include: (1) the acute flaccid paralysis (AFP) surveillance system, which is the routine way countries report cases; (2) the regional polio laboratory network; (3) specific studies and pilot poliovirus surveillance projects targeting PID patients; and (4) regularly conducted systematic literature reviews of scientific journals reporting on iVDPV cases, where key search terms include VDPV, iVDPV, immunodeficiency, immunocomprised, excretion, polio, and “vaccine-associated paralytic poliomyelitis.” In order to obtain the most recent information on ongoing cases, corresponding authors of case reports and/or relevant scientific and medical personnel, including regional laboratory coordinators, were contacted.
Inclusion criteria comprised: excretion of poliovirus for greater than 6 months; confirmed PID; and laboratory-confirmed VDPV. A prolonged excretor was defined as a person excreting virus for ≥6 months and ≤5 years, and a chronic excretor was defined as excreting for >5 years (14).
Primary immunodeficiency disorder in the registry includes individuals with congenital onset of B-cell deficiency, T-cell deficiency, major histocompatibility complex deficiency, or a combination of the above. For the purposes of these analyses, immunodeficiency disorders were divided into the following broad categories:
• Antibody disorder, including hypogammaglobulinemia, agammaglobulinemia, X-linked agammaglobulinemia, and other antibody deficiencies.
• Severe combined immunodeficiency disorder and other combined humoral/T-cell deficiencies.
• Common variable immunodeficiency disorder (CVID).
• Other, including major histocompatibility complex deficiencies, the immunodeficiency, centromere instability, and facial anomalies syndrome, as well as other unknown or undiagnosed causes of immunodeficiency.
Laboratory Processing of Poliovirus Isolates
Until the establishment of sensitive surveillance for polio eradication, viruses were mainly isolated in a few specialized laboratories. With implementation of surveillance for polio eradication, poliovirus was increasingly isolated from patient stool samples by laboratories participating in the WHO Global Polio Laboratory Network. Isolates were identified by several steps: first, specimens were grown in tissue culture; second, molecular methods were used to identify the serotype; and third, intratypic differentiation to determine vaccine or wild strains was done either by enzyme-linked immunosorbent assay using highly specific cross-absorbed antisera and/or by diagnostic reverse transcriptase-polymerase chain reaction (21). If the enzyme-linked immunosorbent assay and molecular intratypic differentiation methods yielded discordant results, the ~900-nucleotide interval encoding the major capsid protein, the viral protein 1 (VP1), was sequenced. Poliovirus genomes appear to evolve at a rate of ~1.1% mutations in VP1 region per year (22) and VDPVs are defined as having a VP1 nucleotide divergence >1% for type 1 and 3, and >0.6% for type 2, from the corresponding parenteral OPV strain (23), consistent with prolonged replication or transmission.
Demographic and Clinical Variables
Cases were recorded with the following demographic and clinical parameters: year of detection; country of residence and its income classification [based on the 2016 World Bank classifications (24)]; date of birth; date of onset; age at onset; gender; PID; presence or absence of paralysis; OPV vaccination history; clinical outcome (alive, alive and stopped excreting, dead or unknown); and first and most recent positive specimen dates, serotype of virus, percentage VP1 divergence from the parenteral Sabin strain, and recombination with other non-polio enteroviruses.
Statistical Analysis
Descriptive analysis was conducted to identify demographic and clinical characteristics of iVDPV cases. According to available information, duration of excretion was estimated following the algorithm provided in Table S1 in Supplementary Material. The algorithm bases the length of excretion on either the time period between OPV administration (or exposure) and the last iVDPV isolate, the time period calculated by VP1 divergence from parental Sabin strain based on an evolution rate of ~1.1% divergence per year (22), or where this information was not available, the excretion time under observation. To model the length of excretion, Kaplan–Meier survival analysis was employed and log-rank tests used to determine difference in survival times between groups. To allow for multiple comparisons, the P-value for significance was set at P < 0.01. All analyses were conducted using the statistical software R 3.3.1 (25) and Stata MP 13 (26).
Results
Demographics
As of July 2016, the WHO registry included 110 suspected iVDPV cases with onset of excretion or paralytic symptoms during 1962–2016. Of these, 101 (91.8%) met the definition of prolonged or chronic iVDPV excretion and were included in the analysis. Figure 1 shows the number of reported iVDPV cases over time since 1962. There has been an increase in the number of reported cases since the year 2000, with the highest number the period since 2010 (56/101). Within this, there are two divergent trends: an increase in cases reported from low- and middle-income countries and a decrease in the number of cases reported from high-income countries (Figure 1).
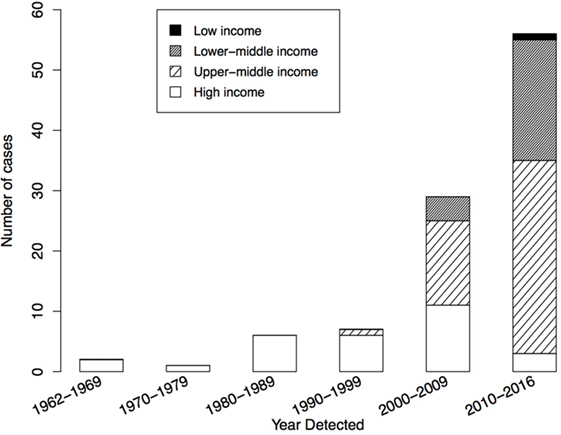
Figure 1. Year of detection of 101 reported chronic and prolonged immunodeficiency-related vaccine-derived poliovirus cases from 1962 to 2016, by income classification of country of residence: low income (n = 1), lower-middle income (n = 24), upper-middle income (n = 47), and high-income (n = 29). Income classification based on 2016 World Bank Classification.
Figure 2 provides the geographic location of these cases by associated serotype, illustrating a large concentration of cases around the Middle East. The characteristics of the 101 prolonged and chronic iVDPV cases are presented in Table 1. The residence of cases by worldwide region shows the highest proportion of cases 43/101 (43%) occurred in the Eastern Mediterranean Region. There were 24/101 (24%) cases in lower-middle-income countries, 47/101 (47%) in upper-middle income countries, and 29/101 (29%) in high-income countries, with all seven chronic excretor cases resident in high-income countries. Only one case was reported from a low-income country, which was in Afghanistan in 2013.
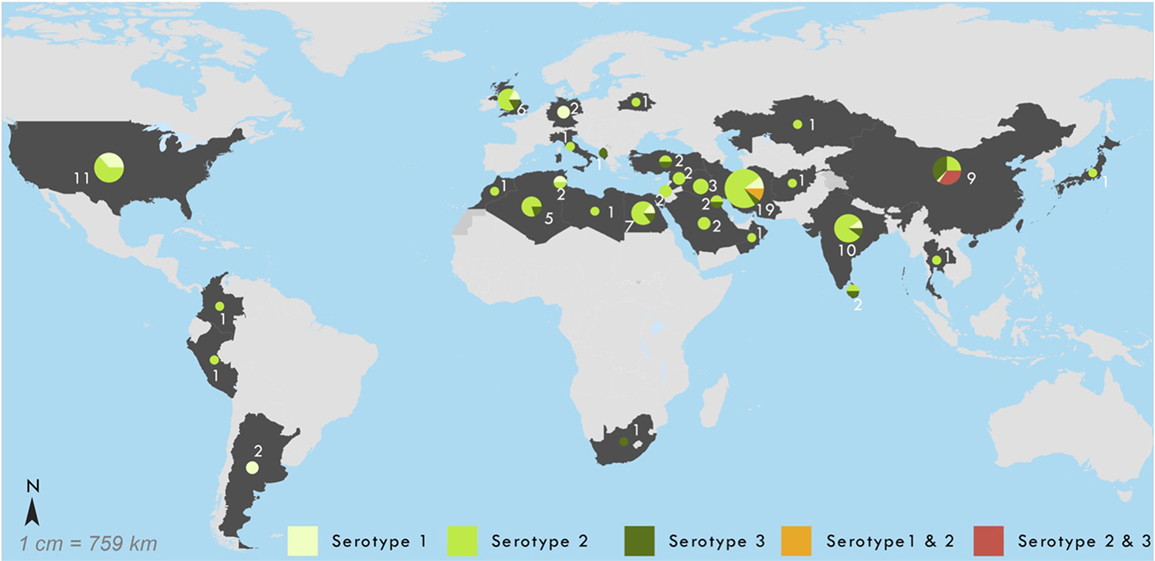
Figure 2. Geographic location of 101 reported chronic and prolonged immunodeficiency-related vaccine-derived poliovirus cases, 1962–2016. Shown by serotype of virus in most recent specimen available: 1 (n = 15), 2 (n = 68), 3 (n = 13), 1 + 2 (n = 2), and 2 + 3 (n = 3).
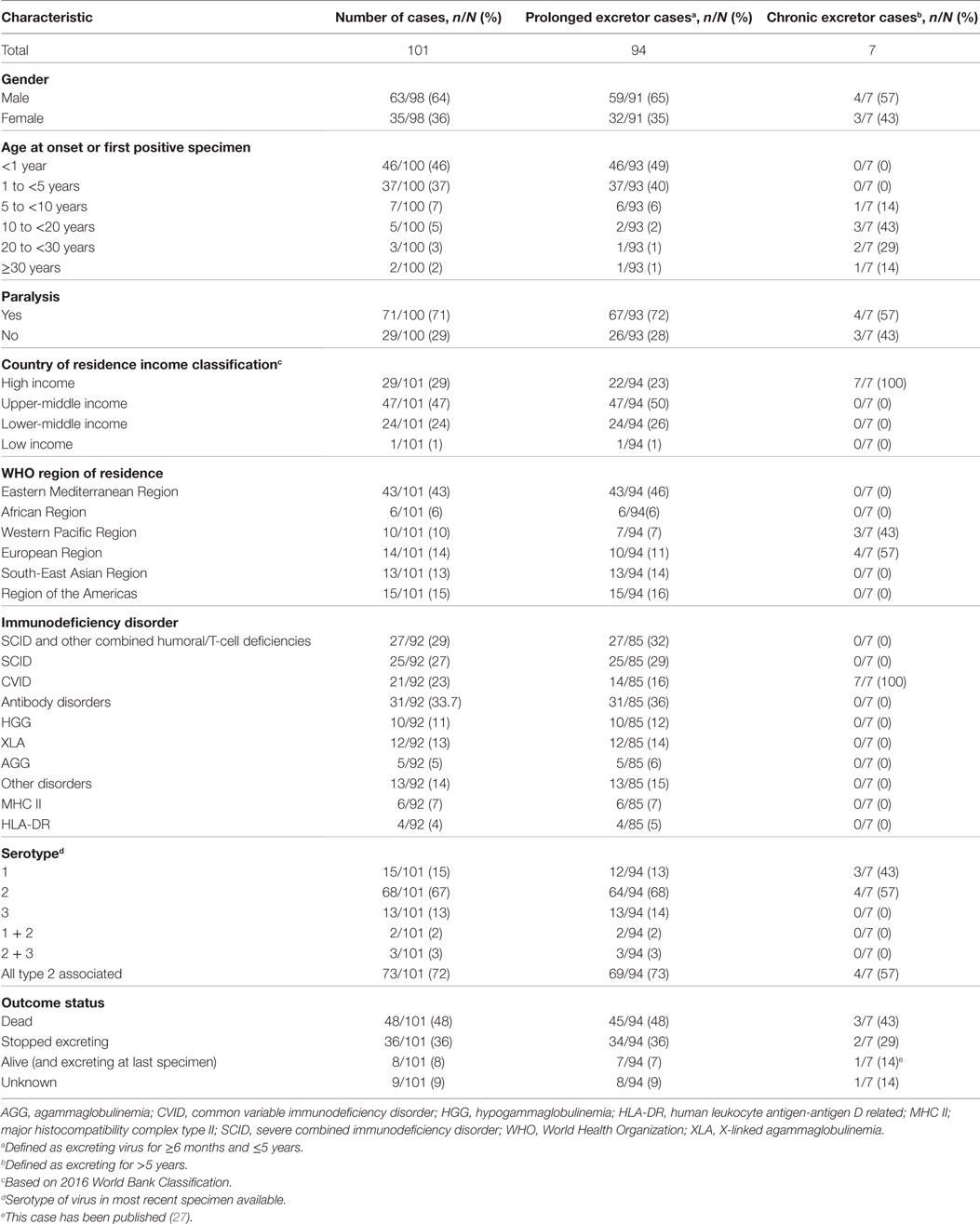
Table 1. Baseline characteristics of 101 reported chronic and prolonged immunodeficiency-related vaccine-derived poliovirus cases at time of detection, 1962–2016.
Out of the 101 cases, 63/98 (64%) were males, 71/100 (71%) presented with paralytic manifestations, as defined by AFP, and the most common age of onset was <1 year in 46/100 (46%) cases, followed by 1 to <5 years old in 37/100 (37%) (Table 1).
Underlying PID
The underlying immunodeficiency disorders of cases are shown in Table 1. Antibody disorders were the most frequent, accounting for 31/91 (34%) of the cases, primarily composed of hypogammaglobulinemia, agammaglobulinemia, and X-linked agammaglobulinemia. Severe combined immunodeficiency disorder and CVID were also common, present in 25/92 (27%) and 21/92 (23%) of cases, respectively. All the seven chronic excretors had CVID.
Virological Factors
The serotype of excreted virus was predominantly type 2 poliovirus, in 68/101 cases (67%), followed by type 1 in 15/101 cases (15%) and type 3 in 13/101 cases (13%) (Table 1). Among the six cases with multi-serotype infections, two were type 1 and 2 co-infections and three were type 2 and 3 co-infections, resulting in 73/101 (72%) cases in the database associated with type 2 poliovirus.
Survival Analysis
The length of excretion was calculated for all 101 iVDPV cases, of which 94 (93%) were prolonged excretors and 7 (7%) were chronic excretors. At the time of analysis, 48/101 (48%) individuals had died and 36/101 (36%) had stopped excreting. There were 8/101 (8%) individuals who were still alive and excreting at last specimen and 9/101 (9%) were lost to follow-up (Table 1).
The median length of excretion for all 101 cases was 1.3 years [95% confidence interval (CI): 1.0–1.4], with a range of 0.5–28.6 years. Figure 3 shows the Kaplan–Meier survival curves for time to cessation of poliovirus excretion, stratified by PID and country income classification. After 2.4 and 3.7 years, 80 and 90% of individuals had stopped excreting, respectively (Figure 3A). There was a significant difference in the Kaplan–Meier curves for length of excretion between different PIDs (P < 0.001), with individuals with CVID having the longest length of excretion, a median of 3.0 years (95% CI: 1.6–6.7) (Figure 3B). There was also a highly significant difference between different income classification of countries (P < 0.001), with a longer median length of excretion for high-income countries (2.5 years, 95% CI: 1.3–3.7) than upper-middle income countries (1.0 year, 95% CI: 0.9–1.3), lower-middle income countries (1.0 years, 95% CI: 0.8–1.5), and low-income countries (0.8 years) (Figure 3C). No statistical significance was found in the Kaplan–Meier curves for sex (P = 0.68) or poliovirus serotype (P = 0.21) (results not shown).
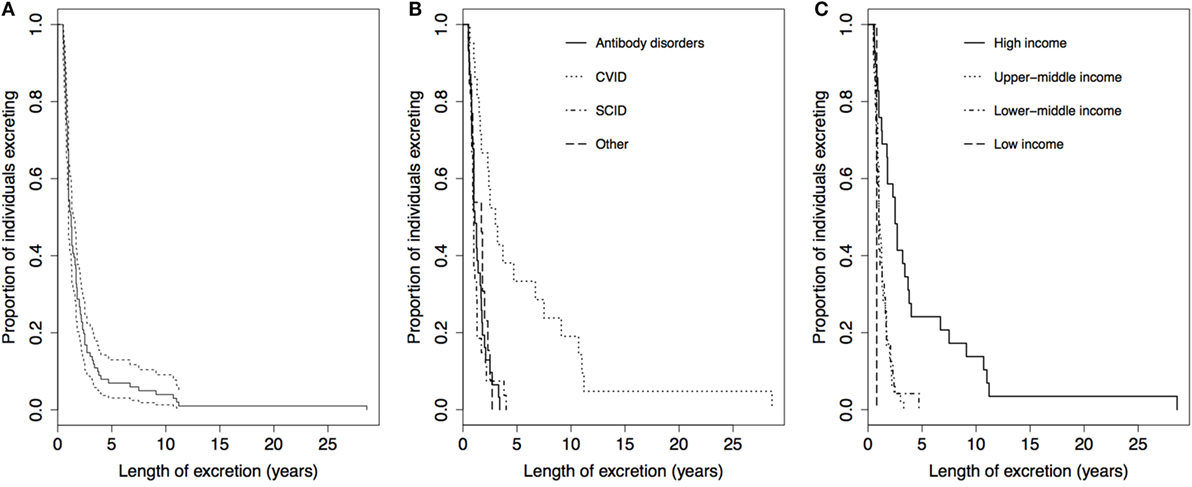
Figure 3. Kaplan–Meier curves for the length of poliovirus excretion in reported chronic and prolonged immunodeficiency-related vaccine-derived poliovirus cases, 1962–2016. (A) For all reported cases (n = 101, dotted lines: 95% Confidence Limits). (B) Comparison by income classification of country of residence: low income (n = 1), lower-middle income (n = 24), upper-middle income (n = 47), and high-income (n = 29). Two-tailed P < 0.001 for log-rank test for equality of Kaplan–Meier curves. (C) Comparison by primary immunodeficiency disorder: antibody disorders (n = 31), CVID (n = 21), SCID and other combined humoral T-cell deficiencies (n = 27), and other disorders (n = 13). Two-tailed P < 0.001 for log-rank test for equality of Kaplan–Meier curves. Abbreviation: CVID, common variable immunodeficiency disorder; SCID, severe combined immunodeficiency disorder.
Evolution of Length of Excretion
To understand the risk of iVDPV2 to the communities, we stratified the length of excretion by the date of onset (Table 2). Only those cases that were known to have stopped excreting (outcome alive and stopped excreting or dead) were included in this analysis to ensure comparability of the length of excretion between the two time periods 1962–2010 and 2011–2016. There was a decrease in the median length of excretion from cases with onset in 1962–2010 to 2011–2016: 1.75 years (95% CI: 1.3–2.5) and 1.0 years (95% CI: 0.8–1.4), respectively. The difference in length of excretion for the two time periods was found to be highly statistically significant when all serotypes (P < 0.001) were considered and specifically for type 2 (P < 0.001).
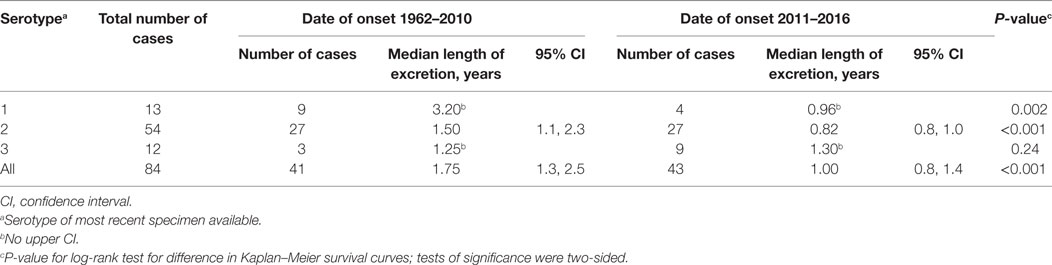
Table 2. Kaplan–Meier Estimates of the length of excretion for 84 prolonged and chronic immunodeficiency-related vaccine-derived poliovirus cases with outcome dead or stopped excreting, stratified by date of onset, 1962–2016.
Discussion
Our analyses document the changing epidemiology of iVDPV cases since the turn of the millenium, when the incidence of reported cases dramatically increased. The cases reside primarily from middle-income countries and are concentrated in the Middle East and North Africa. We confirm that the preponderance of cases are excreting poliovirus type 2, and demonstrate that the length of virus excretion has decreased in recent years. We also estimated that the last reported chronic excretor (greater than 5 years of excretion) had onset in 1998 and, finally, identified CVID as the major risk factor for chronic excretion.
The documented increase in number of reported iVDPV cases since the year 2000 was consistent with previous reports (17) and could be attributed to better case ascertainment, improved health care allowing immunodeficient patients to survive longer, or a combination of these factors. The surveillance sensitivity for AFP has risen in parallel with case incidence since 2000; however, this system is designed to capture cases with paralytic manifestations only (28), including those with PID. During the same period, capabilities of PID diagnostics have been enhanced and may now be available in many low- and middle-income countries. Therefore, it is possible that the increase in iVDPV cases is a surveillance artifact.
The shift in iVDPV cases from high-income to middle-income countries likely reflects two different trends: (1) high-income countries have changed their immunization schedules and switched to exclusive inactivated poliovirus vaccine use (thus preventing new iVDPVs from being generated); (2) middle-income countries have established increasingly sensitive surveillance for AFP, which better captures PIDs with paralysis; and (3) health systems in middle-income countries provide better medical care for PIDs, allowing these cases to be diagnosed and treated. However, this shift in case distribution causes new challenges to polio eradication.
The striking concentration of iVDPV cases in the Middle East and North Africa may be due to: (1) an increased risk of PID due to co-sanguinity (29) and (2) an interest of immunologists in these Regions to strengthen surveillance for PID and create immunodeficiency registries. In particular, iVDPV surveillance projects have taken place in Egypt (30), Iran (31), and Tunisia (20).
Our analysis is consistent with previous studies documenting that iVDPV type 2 constitutes more than 70% of all iVDPV cases (17), including a case that has excreted poliovirus type 2 for almost 30 years (27). Since the removal of Sabin type 2 from OPV was implemented in April 2016, no further Sabin type 2 are being introduced into populations, except for outbreak control (13, 32), which should prevent the generation of almost all new iVDPV type 2 cases.
On average, the duration of excretion of poliovirus among iVDPV cases is relatively short (approximately 1 year) and has further declined in recent years. This declining length of poliovirus excretion for all serotypes, but especially type 2, is likely to be associated with the shift in cases from high to middle income countries. This may be caused by shorter survival of iVDPV cases in middle-income countries or a higher likelihood of spontaneous cessation of excretion. However, we have little evidence to support the latter assumption.
We also document that chronic excretors are all associated with CVID in our case series. This is not surprising since CVID consists of a variety of underlying pathologies associated with later diagnosis and longer survival than some of the other PIDs diagnoses (15, 33). What is more surprising is that all chronic excretors originated from high-income countries, and that no new chronic cases have been detected since 2009. Whether this is a reflection of the quality of the health systems in developing countries to both detect and care for PID patients remains an open question.
In our case series, only eight iVDPV cases were documented to be alive and excreting. The rest either had a fatal outcome or spontaneously stopped excretion. An additional nine cases were lost to follow-up or had no information on excretion. However, if we apply the observed mortality to these cases, very few would be expected to be alive and excreting. Therefore, the known prevalence of actively excreting iVDPV cases (i.e., those captured by current surveillance systems) is small.
In terms of limitations, our analyses could only focus on those iVDPV cases reflected in the WHO registry. Our study does not address the unknown number of PIDs who excrete poliovirus but have not been captured by the AFP surveillance system because they are not paralyzed and did not come to the attention of immunologists because of either a lack of suspicion or specialist immunological services are not available. This reflects the broader challenge of tracking iVDPV cases and limitations of registries in the absence of routine screening of PID patients for poliovirus infection and excretion. Furthermore, the information available in the registry is limited by the quality of reported data. In the calculation for length of excretion, cases that were still excreting or lost to follow-up were censored at the time of last positive sample and may continue to excrete for longer time periods. Furthermore, the analysis assumed a standard evolution rate of 1.1% mutations in VP1 region of the poliovirus genome per year.
Our results have important implications for the polio endgame. The withdrawal of Sabin type 2 poliovirus in April 2016 went well (32), and future immunity to type 2 poliovirus will be induced solely by inactivated poliovirus vaccine. Our duration of excretion analysis suggests that the highest risk period for iVDPV cases re-seeding communities is in the next 2–3 years.
However, there are ways in which the Global Polio Eradication Initiative can actively decrease the risk of iVDPV cases. Generating effective therapeutic options for clearing poliovirus infections is critical. Progress has been made in this field over the last decade or more, and antiviral drugs are nearing practical applicability (34). The first drug, a capsid inhibitor, is now ready for deployment under an Investigational New Drug protocol (35). Because of high levels of drug resistance, a second drug is needed for a combination treatment (35) and related studies with a protease inhibitor are in progress (36). These drugs, as well as specific high-titer monoclonal antibodies, should provide effective treatment for iVDPV excretors. In this context, identifying PIDs with poliovirus to enable treatment with these new drugs becomes an urgent priority, not only for public health but also because these patients are at risk of developing paralytic poliomyelitis, which has a high mortality burden.
In parallel, we need to establish sensitive surveillance systems to identify and report iVDPV cases with or without paralytic symptoms, which constitute the last remaining sources of poliovirus type 2. Recent modeling by Tebbens et al. has shown that treating iVDPVs with antiviral drugs alone will have limited impact and requires integration with expanded surveillance (15). This surveillance system would be complementary to the AFP surveillance system that captures children with paralytic manifestations. The new system would also target children with PIDs (without paralytic manifestations) and would use the 10 Warning Signs of the Jeffrey Modell Foundation as a screening case definition (37). Children meeting at least two signs would then be included in the AFP surveillance system and followed up accordingly (with two stool samples collected and related laboratory investigations). The Strategic Advisory Group of Experts on Immunization Polio Working Group has endorsed this approach, and pilot country studies are being established (38). Furthermore, analysis suggests that extended surveillance could save between US$0.7 to 1.5 billion and identify 25–90% of asymptomatic iVDPV excretors (16).
This dual approach of developing therapeutic options to clear poliovirus infection and establishing sensitive surveillance for PIDs should further quantify the risks and would allow the Global Polio Eradication Initiative to actively mitigate these risks, so that global polio eradication cannot be undone by few iVDPV cases that may inadvertently re-seed communities with poliovirus, potentially leading to re-establishment of endemic or epidemic transmission. With these enhancements to the surveillance and treatment system for PIDs, we can make the final steps to a world free of polio.
Author Contributions
Participated in research design: GM, YL, MT, OM, OK, and RS. Collected data: GM, YL, MT, KD, OM, and RS. Performed data analysis: GM, MT, YL, and SG. Wrote or contributed to drafting of early manuscripts: GM, YL, MT, KD, and RS. Critically revised manuscript: GM, YL, MT, KD, SG, OM, OK, and RS.
iVDPV Working Group
Ousmane Diop (WHO, Switzerland), Nicksy Gumede Moeletsi (WHO Regional Office for Africa, Congo), Raffaella Williams (National Institute for Communicable Diseases, South Africa), Mohamed Seghier (Institut Pasteur d’Algérie, Algeria), Francis Delpeyroux (Institut Pasteur, France), Gloria Rey Benito (WHO Regional Office for America, USA), Maria Cecilia Freire (Instituto Nacional de Endemedades Infecciosos, Argentina), Cara Burns (Centers for Disease Control, USA), Humayun Asghar (WHO Organization Regional Office for Eastern-Mediterranean, Egypt), Salman Sharif (National Institute of Health, Pakistan), Jagadish Deshpande (Enterovirus Research Center, India), Shohreh Shahmahmoodi (Tehran University of Medical Sciences, Iran), Henda Triki (Institut Pasteur de Tunis, Tunisia), Laila E Bassioni (Egyptian Organization for Biological and Vaccine Production (VACSERA), Egypt), Amina Al-Jardani (Central Public Health Laboratory, Oman), Eugene Gavrilin (WHO Regional Office for Europe, Denmark), Merav Weil (Central Virology Laboratory, Israel), Javier Martín (National Institute for Biological Standards and Control, UK), Sirima Pattamadilok (WHO Regional Office, India), Sunethra Gunasena (Medical Research Institute, Sri Lanka), Yan Zhang (WHO Regional Office for Western Pacific, Philippines), Wenbo Xu (Chinese Center for Disease Control and Prevention, China).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.01103/full#supplementary-material.
Abbreviations
AFP, acute flaccid paralysis; CVID, common variable immunodeficiency; iVDPV, immunodeficiency-related vaccine-derived poliovirus; OPV, oral poliovirus vaccines; PID, primary immunodeficiency disorder; VDPV, vaccine-derived poliovirus; WHO, World Health Organization.
References
1. World Health Organisation. Acute Flaccid Paralysis (AFP) Surveillance Data and Polio Case Count. Geneva: World Health Organisation (2017). Available from: https://extranet.who.int/polis/public/CaseCount.aspx
2. Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morb Mortal Wkly Rep (2001) 50(12):222–4.
3. Kew OM, Cochi SL, Jafari HS, Wassilak SG, Mast EE, Diop OM, et al. Possible eradication of wild poliovirus type 3 – worldwide, 2012. MMWR Morb Mortal Wkly Rep (2014) 63(45):1031–3.
4. Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol (2005) 59:587–635. doi:10.1146/annurev.micro.58.030603.123625
5. Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine – live. 6th ed. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: Saunders Elsevier (2013). p. 599–645.
6. Kew OM, Wright PF, Agol VI, Delpeyroux F, Shimizu H, Nathanson N, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ (2004) 82(1):16–23.
7. Alexander JP Jr, Gary HE Jr, Pallansch MA. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J Infect Dis (1997) 175(Suppl 1):S176–82. doi:10.1093/infdis/175.Supplement_1.S176
8. Martin J. Vaccine-derived poliovirus from long term excretors and the end game of polio eradication. Biologicals (2006) 34(2):117–22. doi:10.1016/j.biologicals.2006.02.005
9. Kew OM, Sutter RW, Nottay BK, McDonough MJ, Prevots DR, Quick L, et al. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J Clin Microbiol (1998) 36(10):2893–9.
10. Martín J, Dunn G, Hull R, Patel V, Minor PD. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J Virol (2000) 74(7):3001–10. doi:10.1128/JVI.74.7.3001-3010.2000
11. Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology (1999) 265(2):178–84. doi:10.1006/viro.1999.0003
12. Buttinelli G, Donati V, Fiore S, Marturano J, Plebani A, Balestri P, et al. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J Gen Virol (2003) 84(Pt 5):1215–21. doi:10.1099/vir.0.18974-0
13. Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013-2018. Geneva: World Health Organisation (2013). (WHO/POLIO/13.02).
14. Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal (2006) 26(6):1471–505. doi:10.1111/j.1539-6924.2006.00827.x
15. Duintjer Tebbens RJ, Pallansch MA, Thompson KM. Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infect Dis (2015) 15:379. doi:10.1186/s12879-015-1115-5
16. Duintjer Tebbens RJ, Thompson KM. Comprehensive screening for immunodeficiency-associated vaccine-derived poliovirus: an essential oral poliovirus vaccine cessation risk management strategy. Epidemiol Infect (2017) 145(2):217–26. doi:10.1017/S0950268816002302
17. Guo J, Bolivar-Wagers S, Srinivas N, Holubar M, Maldonado Y. Immunodeficiency-related vaccine-derived poliovirus (iVDPV) cases: a systematic review and implications for polio eradication. Vaccine (2015) 33(10):1235–42. doi:10.1016/j.vaccine.2015.01.018
18. Li L, Ivanova O, Driss N, Tiongco-Recto M, da Silva R, Shahmahmoodi S, et al. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis (2014) 210(Suppl 1):S368–72. doi:10.1093/infdis/jiu065
19. de Silva R, Gunasena S, Ratnayake D, Wickremesinghe GD, Kumarasiri CD, Pushpakumara BA, et al. Prevalence of prolonged and chronic poliovirus excretion among persons with primary immune deficiency disorders in Sri Lanka. Vaccine (2012) 30(52):7561–5. doi:10.1016/j.vaccine.2012.10.035
20. Driss N, Ben-Mustapha I, Mellouli F, Ben Yahia A, Touzi H, Bejaoui M, et al. High susceptibility for enterovirus infection and virus excretion features in Tunisian patients with primary immunodeficiencies. Clin Vaccine Immunol (2012) 19(10):1684–9. doi:10.1128/CVI.00293-12
21. World Health Organisation. Polio Laboratory Manual. 4th ed. Geneva: World Health Organisation (2004). (WHO/IVB/04.10).
22. Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol (2008) 82(9):4429–40. doi:10.1128/JVI.02354-07
23. World Health Organization. Update on vaccine-derived polioviruses detected worldwide, April 2011–June 2012. Wkly Epidemiol Rec (2012) 87(38):358–68.
24. The World Bank. Country and Lending Groups. (2016). Available from: http://data.worldbank.org/about/country-and-lending-groups
25. Core Team R. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2016).
26. Stata Corporation. Stata Statistical Software: Release 13. College Station, TX: Stata Corporation (2013).
27. Dunn G, Klapsa D, Wilton T, Stone L, Minor PD, Martin J. Twenty-eight years of poliovirus replication in an immunodeficient individual: impact on the global polio eradication initiative. PLoS Pathog (2015) 11(8):e1005114. doi:10.1371/journal.ppat.1005114
28. World Health Organisation. WHO–Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases. Geneva: World Health Organisation (2003). (WHO/V&B/03.01).
29. Rezaei N, Pourpak Z, Aghamohammadi A, Farhoudi A, Movahedi M, Gharagozlou M, et al. Consanguinity in primary immunodeficiency disorders; the report from Iranian Primary Immunodeficiency Registry. Am J Reprod Immunol (2006) 56(2):145–51. doi:10.1111/j.1600-0897.2006.00409.x
30. El-Sayed ZA, Mach O, Hossny EM, Galal NM, El-Sawy I, Elmarsafy A, et al. Poliovirus excretion among persons with primary immune deficiency disorders: summary of data from enhanced poliovirus surveillance in Egypt, 2011–2014. J Vaccines Vaccin (2016) 7(4):1000331. doi:10.4172/2157-7560.1000331
31. Shaghaghi M, Shahmahmoodi S, Abolhassani H, Soleyman-Jahi S, Parvaneh L, Mahmoudi S, et al. Vaccine-derived polioviruses and children with primary immunodeficiency, Iran, 1995-2014. Emerg Infect Dis (2016) 22(10):1712–9. doi:10.3201/eid2210.151071
32. Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine – worldwide, 2016. MMWR Morb Mortal Wkly Rep (2016) 65(35):934–8. doi:10.15585/mmwr.mm6535a3
33. Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol (2007) 27(3):308–16. doi:10.1007/s10875-007-9075-1
34. Couzin J. Infectious diseases. Report concludes polio drugs are needed – after disease is eradicated. Science (2006) 311(5767):1539. doi:10.1126/science.311.5767.1539a
35. Collett MS, Hincks JR, Benschop K, Duizer E, van der Avoort H, Rhoden E, et al. Antiviral activity of pocapavir in a randomized, blinded, placebo-controlled human oral poliovirus vaccine challenge model. J Infect Dis (2017) 215(3):335–43.
36. McKinlay MA, Collett MS, Hincks JR, Oberste MS, Pallansch MA, Okayasu H, et al. Progress in the development of poliovirus antiviral agents and their essential role in reducing risks that threaten eradication. J Infect Dis (2014) 210(Suppl 1):S447–53. doi:10.1093/infdis/jiu043
37. Jeffrey Modell Foundation. 10 Warning Signs of Primary Immunodeficiency. (2017). Available from: http://www.info4pi.org/library/educational-materials/10-warning-signs
38. World Health Organisation. 12th Meeting of the SAGE Polio Working Group. Geneva: World Health Organisation (2016). Available from: http://www.who.int/immunization/sage/meetings/2016/october/1_12th_meeting_of_the_SAGE_polio_WG_Note_for_the_Record_final_August_2016.pdf?ua=1
Keywords: polio eradication, primary immunodeficiency, vaccine-derived poliovirus, oral poliovirus vaccine, immunodeficiency-related vaccine-derived poliovirus
Citation: Macklin G, Liao Y, Takane M, Dooling K, Gilmour S, Mach O, Kew OM, Sutter RW and The iVDPV Working Group (2017) Prolonged Excretion of Poliovirus among Individuals with Primary Immunodeficiency Disorder: An Analysis of the World Health Organization Registry. Front. Immunol. 8:1103. doi: 10.3389/fimmu.2017.01103
Received: 20 April 2017; Accepted: 23 August 2017;
Published: 25 September 2017
Edited by:
Menno C. van Zelm, Monash University, AustraliaReviewed by:
Kimberly Thompson, Kid Risk Inc., United StatesFrancis Delpeyroux, Institut Pasteur, France
Copyright: © 2017 Macklin, Liao, Takane, Dooling, Gilmour, Mach, Kew, Sutter and The iVDPV Working Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grace Macklin, Z3JtYWNrbGluMUBnbWFpbC5jb20=
 Grace Macklin
Grace Macklin Yi Liao1,2
Yi Liao1,2 Kathleen Dooling
Kathleen Dooling Roland W. Sutter
Roland W. Sutter