
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 August 2017
Sec. Microbial Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00919
This article is part of the Research Topic Dampness and Mold Hypersensitivity Syndrome (DMHS) View all 5 articles
Long-term exposure to dampness microbiota induces multi-organ morbidity. One of the symptoms related to this disorder is non-thyroidal illness syndrome (NTIS). A retrospective study was carried out in nine patients with a history of mold exposure, experiencing chronic fatigue, cognitive disorder, and different kinds of hypothyroid symptoms despite provision of levothyroxine (3,5,3′,5′-tetraiodothyronine, LT4) monotherapy. Exposure to volatile organic compounds present in water-damaged buildings including metabolic products of toxigenic fungi and mold-derived inflammatory agents can lead to a deficiency or imbalance of many hormones, such as active T3 hormone. Since the 1970s, the synthetic prohormone, levothyroxine (LT4), has been the most commonly prescribed thyroid hormone in replacement monotherapy. It has been presumed that the peripheral conversion of T4 (3,5,3′,5′-tetraiodothyronine) into T3 (3,5,3′-triiodothyronine) is sufficient to satisfy the overall tissue requirements. However, evidence is presented that this not the case for all patients, especially those exposed to indoor air molds. This retrospective study describes the successful treatment of nine patients in whom NTIS was treated with T3-based thyroid hormone. The treatment was based on careful interview, clinical monitoring, and laboratory analysis of serum free T3 (FT3), reverse T3 (rT3) and thyroid-stimulating hormone, free T4, cortisol, and dehydroepiandrosterone (DHEA) values. The ratio of FT3/rT3 was calculated. In addition, some patients received adrenal support with hydrocortisone and DHEA. All patients received nutritional supplementation and dietary instructions. During the therapy, all nine patients reported improvements in all of the symptom groups. Those who had residual symptoms during T3-based therapy remained exposed to indoor air molds in their work places. Four patients were unable to work and had been on disability leave for a long time during LT4 monotherapy. However, during the T3-based and supportive therapy, all patients returned to work in so-called “healthy” buildings. The importance of avoiding mycotoxin exposure via the diet is underlined as DIO2 genetic polymorphism and dysfunction of DIO2 play an important role in the development of symptoms that can be treated successfully with T3 therapy.
Long-term exposure to molds in water-damaged buildings (WDB) has been associated with numerous health problems including allergic airway symptoms (1–3), fungal sinusitis (1, 2, 4), abnormalities in T and B cells (5–7), infection sensitivity (6, 8), asthma (9–11), respiratory infections (3, 11, 12), central and peripheral neuropathy and polyendocrinopathy (8), neurologic symptoms (1, 4, 13), neuropsychological cognitive dysfunction (CD) (14–16), neuropsychiatric symptoms (3, 14, 17), and chronic fatigue (CF) (6, 14, 18). It is now well established that mold and mycotoxins are important constituents of the milieu in WDB and that they can provoke a huge spectrum of illnesses (3, 8, 12, 15, 18–37).
Exposure to volatile organic compounds including metabolites produced by toxigenic fungi, some of which are inflammatory agents, can lead to a deficiency or imbalance of many hormones, such as insufficient amounts of the active form of thyroid hormone, commonly abbreviated to T3 hormone (38, 39).
Thyroid hormones play a very important role in development, growth, and glucose–fat–protein metabolic homeostasis in all tissues by affecting the expression of many genes. It has been shown that the most important factors in thyroid hormone regulation are the activities of the three deiodinase enzymes (DIO 1, 2, 3). In particular, DIO2 regulates the activities of thyroid hormone action by metabolizing the precursor molecule thyroxine (T4) that is secreted by the thyroid gland into the biologically active molecule, T3. Two of the deiodinases (DIO1, DIO2) contain selenium and are responsible for transforming T4 either into its active metabolite, i.e., T3 or to an inhibitory reversed T3 form, rT3 (DIO3) (40–43). The importance of DIO enzymes in thyroid hormone homeostasis has become increasingly clear by experiments conducted in DIO knockout mice (44). DIO enzymes affect the thyroid hormone regulation by controlling thyroid hormone homeostasis at the cellular level, such as in the case of symptoms in mold exposure or in other situations in which there is a lack of active T3 hormone in the peripheral tissues or brain (45). A deficiency of active cellular T3 hormone has been described as a non-thyroidal illness syndrome (NTIS) (46). The patients with this disease presents with normal function of thyroid or with required exogenous T4 with normal thyroid-stimulating hormone (TSH), free T4 (FT4), and free T3 (FT3) values in the blood, but still with symptoms of hypothyroidism. Importantly, the major part of T3 is generated locally from T4 by DIO2 in most tissues of the body and in the brain, especially at the hypothalamus–pituitary level (47).
The toxins released by the microbes living in damp buildings can induce oxidative stress (OS). OS has been proposed to be one of the most important mechanisms behind the adverse health outcomes associated with living in a damp indoor environment. One of the putative consequences of mycotoxin-induced OS is a blockade of crucial mitochondrial functions (47). OS may cause cytotoxic, genotoxic, and inflammatory responses by increasing the production of reactive oxygen species (18, 48).
Oxidative stress also impacts negatively on various hormonal influences, e.g., causing antioxidant imbalance and impairing the functions of the deiodinase enzymes. For example, OS reduces the capacity of DIO2 to convert thyroxine (T4) into its biologically active form of T3. Different defense mechanisms that protect against the free radical damage have been characterized in various cellular localizations, including the endoplasmic reticulum, mitochondria, plasma membrane, peroxisomes, and cytosol.
There are several enzymes such as superoxide dismutase, catalase and glutathione peroxidase, and transition-metal binding proteins, which rapidly inactivate free radicals (49).
Thus, OS can be defined as a failure of the antioxidant system to cope with the excess of free radicals. One putative hypothesis is that OS facilitates the development of hypothyroidism or rather a lack of availability of the T3 hormone at the tissue level, the so-called NTIS (38). In the 1970s, much of the basic biochemistry of thyroid metabolism was clarified (46, 50). It has been postulated that the level of rT3 and the FT3/rT3 ratio correlate with tissue DIO activities and reflect the peripheral metabolism of thyroid hormones. In a normal physiological situation in the human body, the amount of FT3 should be about 2–2.5 times higher than that of rT3; this represents the optimal level of active T3 in peripheral and brain tissues. In the normal physiological situation, rT3 is metabolized 2.5 times more rapidly than T3, and therefore, the FT3/rT3 calculated ratio should be at least around 2–2.5 (50) (or 20–25, depending on the form or expression).
In insulin-resistant patients, the T3/rT3 ratio is significantly increased in comparison to the corresponding value in insulin-sensitive controls (51). In the treatment of obesity, it has been suggested that attention should be paid to correcting the uncoupling of the mitochondrial respiratory chain (52). For example, in an experimental diet-induced obesity model, higher rT3 concentrations were detected in obese animals, one consequence of which might be reduced by oxygen consumption (53).
Patients who have been exposed for a prolonged time to indoor air molds have high serum levels of rT3 (unpublished observation). This indicates an imbalance between rT3 and FT3 and decreased tissue metabolism of T4 to be converted to T3, in other words NTIS. In these patients, DIO2 does not function properly (45), therefore T3 therapy is indicated. The rationale for T3 therapy is: T3 is biologically active hormone that does not require activity of the DIO2 which is needed for conversion from endogenous prohormone T4 to active T3 hormone, or when exogenous levothyroxine (LT4) monotherapy is administered. I will describe the treatment of patients with diagnosed NTIS due to long-term exposure to dampness microbiota.
Nine female patients, aged 31–49 years, with a history of mold exposure and a variety of symptoms compatible with NTIS were enrolled into this retrospective study. The patients received LT4 monotherapy without success. Data have been collected since 2012 in a private medical clinic. Informed written consent was obtained from all of the patients.
The patients’ symptoms were categorized as follows: allergic symptoms (AG), airway symptoms (AW), CD, CF, edema/swelling (E), gastrointestinal symptoms (GI), high body temperature/feeling cold and feverish (HBF), heart/vascular disorder (HV), infection sensitivity (Inf), low body temperature/feeling cold, freezing (LBF), multiple chemical sensitivity, muscle and joint symptoms (MJ), neurological symptoms (Neu), psychological symptoms (Psy), imbalance in sex hormones (Sex), stress intolerance (SI: physical, psychological, or social stress) (Figure 1). Evaluation of symptoms was done on the basis of careful interview and clinical status.
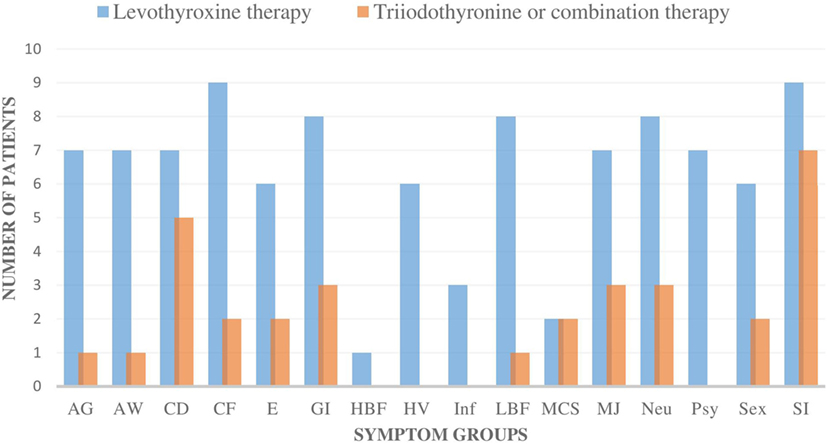
Figure 1. The number of all the individual symptom groups during levothyroxine monotherapy and during triiodothyronine monotherapy (T3) or combination therapy (T3 + T4) in all nine patients. All of the nine patients had fewer symptoms in all of the symptom groups during the T3-based therapy. In addition, all of the symptoms became either milder or were totally eliminated during T3-based therapy. Symptoms were categorized as follows: allergic symptoms (AG), airway symptoms (AW), cognitive dysfunction (CD), chronic fatigue (CF), edema/swelling (E), gastrointestinal symptoms (GI), high body temperature/feeling cold and feverish (HBF), heart/vascular disorder (HV), infection sensitivity (Inf), low body temperature/feeling cold, freezing (LBF), multiple chemical sensitivity (MCS), muscle and joint symptoms (MJ), neurological symptoms (Neu), psychological symptoms (Psy), imbalance in sex hormones (Sex), stress intolerance (SI: physical, psychological, or social stress).
Patients were instructed about aspects of nutritional therapy: adherence to a gluten-free diet, regular use of nutritional supplements, low-dose hydrocortisone, and/or dehydroepiandrosterone (DHEA) when required, and adrenal supportive therapy for a few months. If the above described treatment was not effective enough, T3-based therapy was initiated.
Serum TSH, FT4, FT3, TPOAb, TyglAb, cortisol, and DHEA were assayed by Genova Diagnostics (Asheville, NC, USA) and the United Medix Laboratories Ltd. (Helsinki, Finland). The level of reverse T3 was assayed by Mayo Clinic (Scottsdale, AZ, USA). DIO2 was measured in the ZRT Laboratory (Beaverton, OR, USA) and in the United Medix Laboratories Ltd. (Helsinki, Finland). Saliva concentrations of cortisol and DHEA were assayed by Genova Diagnostics (Asheville, NC, USA) and ZRT Laboratory (Beaverton, OR, USA). The FT3/rT3 ratio was calculated according to the formula (FT3 × 6.51/rT3), where FT3 is picomoles per litre and rT3 is nanograms per deciliter.
All patients reported an exposure to indoor air molds. The duration of exposure to molds ranged from 5 to 27 years (average 11.4 years). It is worth stressing that all of the patients reported that their disease started during a long exposure to a moldy environment and that their health condition deteriorated significantly during periods of re-exposure. In all cases, there was a correlation of symptoms with the growth of dampness microbiota in the buildings as well as visible evidence of water damage. Mold growth and indoor air investigations were done by accredited laboratories using accepted culture techniques with conventional isolation media and quantitation of colony forming units per, e.g., cubic meter (54). The majority of the damaged buildings were dismantled or renovated afterward.
Seven patients had been already diagnosed by hypothyreosis with clear or borderline levels of TSH and FT4; two patients had normal TSH and FT4 concentrations before initiating LT4 monotherapy. Four patients were not able to increase the prescribed dose of LT4 to the required level because of side effects (Table 3). Two patients had Hashimoto’s thyroiditis, one patient had goiter, and one had been semi-thyroidectomized because of goiter. Two patients had been diagnosed with newly onset asthma during the time when they were exposed to indoor air molds. Three of the nine patients had a deficiency or borderline levels of cortisol. Two patients had low or borderline cortisol and DHEA levels. Only three patients had normal cortisol and DHEA levels in serum, although they had symptoms consistent with an adrenal insufficiency (SI) (Table 3). While on LT4 monotherapy, six patients presented with very severe/severe imbalance in the FT3 and rT3 levels with FT3/rT3 ratios ranging from 0.75 to 1.3. Two patients had a moderate imbalance (range 1.43–1.61) and only one patient had a normal ratio (2.5) while consuming a low dose (50 µg) of LT4 monotherapy (Table 1).
The symptoms of all nine patients were relieved in all of the symptom groups when they were administered T3-based therapy (Figures 1 and 2). Those patients, who had residual symptoms during T3-based therapy, were continually exposed to molds at their work places (Figure 2). Four patients were unable to work and had been on a disability leave during LT4 monotherapy. However, during the T3-based and supportive therapy, all of these patients returned to work in so-called “healthy” buildings and all of these patients returned to work in so-called “healthy” buildings and were able to tolerate small amounts of mold and toxins.
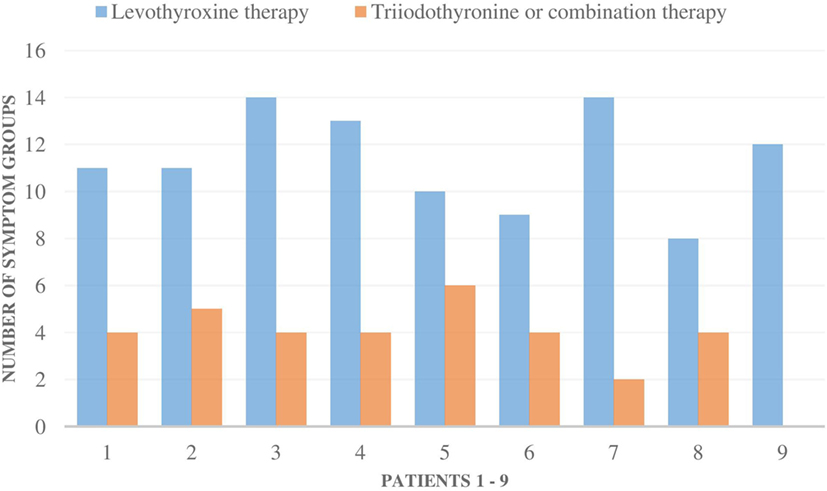
Figure 2. The number of all 16 symptom groups [allergic symptoms (AG) to stress intolerance] in each of the nine patients during levothyroxine monotherapy and during triiodothyronine monotherapy (T3) or combination therapy (T3 + T4). Special remarks: patient 1–5, 8: all the symptoms were milder during T3-based therapy; patient 1: still a mild mold exposure at work, patient 2: strong symptoms if mold exposure, patient 3: need less asthma medication, no airway symptoms (AW) during T3 therapy, patient 4: strong mold exposure period just recently, gets AW and AG symptoms immediately if mold exposure, patient 9: if not mold exposure, all the symptoms stay away.
The transition from LT4 monotherapy to T3-based therapy was conducted over a 2-week period when the patient’s symptoms were monitored to adjust to an individually appropriate T3 dosage. T3 monotherapy was prescribed to four patients in a dosage range from 30 to 55 μg/day, with these doses subdivided to be taken three times in a day. Combination therapy with synthetic T3 and LT4 thyroid hormones (T3 12.5–35 µg and LT4 50–75 µg) was prescribed to four patients and one patient received biological thyroid hormone extract (LT4 + T3). Satisfactory results were achieved within 3–36 months (average 10.1), but a complete curative response was documented in six patients within a shorter time, between 3 and 6 months (Table 2). The initiation of T3-based therapy relieved all of the symptoms in all of the nine patients. T3-based therapy lessened SI symptoms to a variable degree in all nine patients; they tolerated stress much better especially with the adrenal support (55–58). It is noteworthy that those patients who presented with HV, psychological (Psy), or infection sensitivity (Inf) symptoms during LT4 monotherapy became asymptomatic during T3-based therapy (Figures 1 and 2). Body temperature normalized and patients who presented with allergic or airway symptoms became asymptomatic in the majority of cases. Those patients (3/9) who ignored adherence to a strict naturally gluten-free diet continued to have GI symptoms despite of thyroid hormone correction. Patients with SI may have a comorbidity with latent adrenal insufficiency and they benefited from adrenal support provided as supplementation with physiological dosages of hydrocortisone and/or DHEA (Table 3).
At present, there is no unanimous consensus on diagnostic criteria or efficacious treatment modalities for mold-exposed individuals. It has been estimated that as many as 14.5% of residents in Finland suffer from sick building syndrome (59). The Finnish authorities are reluctant to clarify the reasons for this problem. Instead, they claim that the patients’ symptoms are medically unrelated, unexplained, or possibly psychiatric, with one exception which has accepted the possibility that a moldy environment may evoke airway symptoms (60).
Hypothyroidism and NTIS appear to be common among patients with dampness and mold hypersensitivity syndrome (DMHS) (unpublished observation); this is often disregarded and neglected. It is well known that hypothyroidism is more common in females. In this study, all patients were females. The reasons may be that more women than men work in public institutions, which are often infested with indoor air molds. I have tackled this problem by treating patients with active T3 hormone, as well as supporting adrenal gland function and providing nutritional advice.
In an animal model of primary hypothyroidism, it has been demonstrated that the LT4 monotherapy could not achieve systemic euthyroidism (44). Thyroidectomized rodents treated with LT4 at doses that normalized serum TSH concentrations exhibited relatively lower serum T3 and higher serum T4 levels. Furthermore, markers of hypothyroidism could be identified in their brains, skeletal muscles, and livers. Measurements of thyroid hormone levels in serum do not correlate with the hormone levels in tissues; this is the case not only in animal studies in vivo but also in human studies in vitro (44). In humans, it has been shown that NTIS, the level of rT3, and the T3/rT3 ratio were correlated with postmortem tissue DIO enzyme activity (58). It is of utmost importance to understand the crucial role of DIO2, as well as measuring FT3/rT3 ratio, which are useful laboratory markers.
Successful treatment of DMHS patients required stringent adherence to a gluten-free diet. They were allowed to eat only naturally gluten-free cereals, but not gluten-stripped cereals marketed as “gluten-free.” The rationale for this approach is that wheat, rye, spelt, and barley can contain various toxic compounds, such as trichothecene mycotoxins (61) and ergot alkaloids (62–64), as well as metabolites from Aspergillus and Penicillium and other fungi which exist in cereals (65). The ergot alkaloid producing fungi, Claviceps purpurea, often live in symbiosis with gluten-containing cereal grains and Penicillium and Aspergillus strains have been demonstrated to produce ergot alkaloids in broth culture (66). It has been claimed that simultaneous inhalation and oral digestion of these toxins seem to potentiate the mold-induced symptoms. Furthermore, possibly gastric enzyme activity is impaired by the amylase and trypsin inhibitors (ATI) present in gluten cereals (67). These ATI structures in grains hinder digestion in the gastrointestinal tract. Gluten-containing cereals make up a large part of the everyday diet of most Europeans. It has been postulated that even a low toxin load is harmful; chronic exposure to mycotoxins has been claimed to be a crucial factor in causing a dysfunction of mitochondrial energy production (68). The importance of T3 for stimulating mitochondrial activity was presented by Forini et al. in their animal model, T3 decreased the size of cardiovascular infarction and prevented heart failure (69).
Another important mechanism in NTIS is DIO2 polymorphism. DIO2 is present in all cells of the human body. Patients who are heterozygotes (CT) or homozygotes (CC) have defective DIO2 activity, and they will benefit from T3-based therapy compared to LT4 monotherapy (70). Presumably an improved DIO2 enzyme activity explains why part of the patients recovered on nutritional supplementation especially with selenium. T3-based therapy is indicated in situations when the FT3/rT3 ratio is abnormal due to overproduction of rT3 and when the patient presents with symptoms compatible with hypothyroidism or NTIS (71). According to U.S. Food and Drug Administration, all thyroid hormone drugs, including T3 monotherapy are safe, and can be utilized as replacement or supplemental therapy in patients of any age, and also during pregnancy (72–79).
This article describes the successful treatment of patients with hypothyroidism and NTIS. The disease developed after prolonged and cumulative or massive exposure to indoor air dampness microbiota. The treatment was based on full laboratory assessment of thyroid and adrenal hormones as well as careful interviewing and clinical monitoring. DMHS with NTIS is a devastating condition caused by OS and toxicosis, which should be recognized and treated appropriately.
Ethical clearance was not needed because this study was a retrospective study using the medical records. Samples were taken during treatment and the patient provided written informed consent and provided permission to use their data for scientific purposes.
TS has interviewed, treated, and evaluated all the patients and wrote the study.
TS is one of the owners of a private clinic in Tampere, Finland.
I acknowledge the support and help from Jani Somppi, Merja Lindström, Marjo Sukeva-Hakanpää, Eeva Lundell, and Hilkka Mononen.
1. Brewer JH, Thrasher JD, Hooper D. Chronic illness associated with mold and mycotoxins: is naso-sinus fungal biofilm the culprit? Toxins (Basel) (2013) 6(1):66–80. doi:10.3390/toxins6010066
2. Jaakkola MS, Quansah R, Hugg TT, Heikkinen SA, Jaakkola JJ. Association of indoor dampness and molds with rhinitis risk: a systematic review and meta-analysis. J Allergy Clin Immunol (2013) 132(5):1099–110.e1018. doi:10.1016/j.jaci.2013.07.028
3. Empting LD. Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health (2009) 25(9–10):577–81. doi:10.1177/0748233709348393
4. Shoemaker RC, House D, Ryan JC. Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water-damaged buildings: a volumetric MRI study using NeuroQuant(R). Neurotoxicol Teratol (2014) 45:18–26. doi:10.1016/j.ntt.2014.06.004
5. Stanzani M, Orciuolo E, Lewis R, Kontoyiannis DP, Martins SL, St John LS, et al. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood (2005) 105(6):2258–65. doi:10.1182/blood-2004-09-3421
6. Ryan JC, Wu Q, Shoemaker RC. Transcriptomic signatures in whole blood of patients who acquire a chronic inflammatory response syndrome (CIRS) following an exposure to the marine toxin ciguatoxin. BMC Med Genomics (2015) 8:15. doi:10.1186/s12920-015-0089-x
7. Wolff H, Mussalo-Rauhamaa H, Raitio H, Elg P, Orpana A, Piilonen A, et al. Patients referred to an indoor air health clinic: exposure to water-damaged buildings causes an increase of lymphocytes in bronchoalveolar lavage and a decrease of CD19 leucocytes in peripheral blood. Scand J Clin Lab Invest (2009) 69(5):537–44. doi:10.1080/00365510902770061
8. Dennis D, Robertson D, Curtis L, Black J. Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. Toxicol Ind Health (2009) 25(9–10):669–80. doi:10.1177/0748233709348266
9. Matheson MC, Reece JC, Kandane-Rathnayake RK, Tang ML, Simpson JA, Feather IH, et al. Mould-sensitized adults have lower Th2 cytokines and a higher prevalence of asthma than those sensitized to other aeroallergens. Allergy (2016) 71(12):1701–11. doi:10.1111/all.12964
10. Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One (2012) 7(11):e47526. doi:10.1371/journal.pone.0047526
11. Shiue I. Indoor mildew odour in old housing was associated with adult allergic symptoms, asthma, chronic bronchitis, vision, sleep and self-rated health: USA NHANES, 2005–2006. Environ Sci Pollut Res Int (2015) 22(18):14234–40. doi:10.1007/s11356-015-4671-8
12. Fisk WJ, Eliseeva EA, Mendell MJ. Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health (2010) 9:72. doi:10.1186/1476-069X-9-72
13. Lieberman A, Curtis L, Campbell A. Development of new onset chronic inflammatory demyelinating polyneuropathy (CIDP) following exposure to a water damaged home with high airborne mold levels: a report of two cases and a review of the literature. J Neurol Res (2017) 7(3):59–62. doi:10.14740/jnr413e
14. Morris G, Berk M, Walder K, Maes M. The putative role of viruses, bacteria, and chronic fungal biotoxin exposure in the genesis of intractable fatigue accompanied by cognitive and physical disability. Mol Neurobiol (2016) 53(4):2550–71. doi:10.1007/s12035-015-9262-7
15. Gordon WA, Cantor JB, Johanning E, Charatz HJ, Ashman TA, Breeze JL, et al. Cognitive impairment associated with toxigenic fungal exposure: a replication and extension of previous findings. Appl Neuropsychol (2004) 11(2):65–74. doi:10.1207/s15324826an1102_1
16. Campbell AW, Thrasher JD, Madison RA, Vojdani A, Gray MR, Johnson A. Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch Environ Health (2003) 58(8):464–74. doi:10.3200/AEOH.58.8.464-474
17. Shenassa ED, Daskalakis C, Liebhaber A, Braubach M, Brown M. Dampness and mold in the home and depression: an examination of mold-related illness and perceived control of one’s home as possible depression pathways. Am J Public Health (2007) 97(10):1893–9. doi:10.2105/AJPH.2006.093773
18. Brewer JH, Thrasher JD, Straus DC, Madison RA, Hooper D. Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins (Basel) (2013) 5(4):605–17. doi:10.3390/toxins5040605
19. Hoyt AE, Borish L, Gurrola J, Payne S. Allergic fungal rhinosinusitis. J Allergy Clin Immunol Pract (2016) 4(4):599–604. doi:10.1016/j.jaip.2016.03.010
20. Dennis DP. Chronic sinusitis: defective T-cells responding to superantigens, treated by reduction of fungi in the nose and air. Arch Environ Health (2003) 58(7):433–41. doi:10.1080/00039896.2003.11879144
21. Smoragiewicz W, Cossette B, Boutard A, Krzystyniak K. Trichothecene mycotoxins in the dust of ventilation systems in office buildings. Int Arch Occup Environ Health (1993) 65(2):113–7. doi:10.1007/BF00405729
22. Gray MR, Thrasher JD, Crago R, Madison RA, Arnold L, Campbell AW, et al. Mixed mold mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health (2003) 58(7):410–20. doi:10.1080/00039896.2003.11879142
23. Park JH, Cox-Ganser JM. Mold exposure and respiratory health in damp indoor environments. Front Biosci (Elite Ed) (2011) 3:757–71. doi:10.2741/e284
24. Park JH, Kreiss K, Cox-Ganser JM. Rhinosinusitis and mold as risk factors for asthma symptoms in occupants of a water-damaged building. Indoor Air (2012) 22(5):396–404. doi:10.1111/j.1600-0668.2012.00775.x
25. Rea WJ, Didriksen N, Simon TR, Pan Y, Fenyves EJ, Griffiths B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch Environ Health (2003) 58(7):399–405. doi:10.1080/00039896.2003.11879140
26. Campbell AW, Thrasher JD, Gray MR, Vojdani A. Mold and mycotoxins: effects on the neurological and immune systems in humans. Adv Appl Microbiol (2004) 55:375–406. doi:10.1016/S0065-2164(04)55015-3
27. Baldo JV, Ahmad L, Ruff R. Neuropsychological performance of patients following mold exposure. Appl Neuropsychol (2002) 9(4):193–202. doi:10.1207/S15324826AN0904_1
28. Crago BR, Gray MR, Nelson LA, Davis M, Arnold L, Thrasher JD. Psychological, neuropsychological, and electrocortical effects of mixed mold exposure. Arch Environ Health (2003) 58(8):452–63. doi:10.3200/AEOH.58.8.452-463
29. Kilburn KH. Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. Toxicol Ind Health (2009) 25(9–10):681–92. doi:10.1177/0748233709348390
30. Chester AC, Levine PH. Concurrent sick building syndrome and chronic fatigue syndrome: epidemic neuromyasthenia revisited. Clin Infect Dis (1994) 18(Suppl 1):S43–8. doi:10.1093/clinids/18.Supplement_1.S43
31. Täubel M, Sulyok M, Vishwanath V, Bloom E, Turunen M, Järvi K, et al. Co-occurrence of toxic bacterial and fungal secondary metabolites in moisture-damaged indoor environments. Indoor Air (2011) 21(5):368–75. doi:10.1111/j.1600-0668.2011.00721.x
32. Gottschalk C, Bauer J, Meyer K. Detection of satratoxin G and H in indoor air from a water-damaged building. Mycopathologia (2008) 166(2):103–7. doi:10.1007/s11046-008-9126-z
33. Brasel TL, Martin JM, Carriker CG, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl Environ Microbiol (2005) 71(11):7376–88. doi:10.1128/AEM.71.11.7376-7388.2005
34. Thrasher JD, Crawley S. The biocontaminants and complexity of damp indoor spaces: more than what meets the eyes. Toxicol Ind Health (2009) 25(9–10):583–615. doi:10.1177/0748233709348386
35. Straus DC. Molds, mycotoxins, and sick building syndrome. Toxicol Ind Health (2009) 25(9–10):617–35. doi:10.1177/0748233709348287
36. Kilburn KH. Mold and mycotoxin symposium: towards healthy homes. Toxicol Ind Health (2009) 25(9–10):569. doi:10.1177/0748233709348259
37. Lu C, Deng Q, Li Y, Sundell J, Norbäck D. Outdoor air pollution, meteorological conditions and indoor factors in dwellings in relation to sick building syndrome (SBS) among adults in China. Sci Total Environ (2016) 560–561:186–96. doi:10.1016/j.scitotenv.2016.04.033
38. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm (2016) 2016:6757154. doi:10.1155/2016/6757154
39. Polizzi V, Delmulle B, Adams A, Moretti A, Susca A, Picco AM, et al. JEM spotlight: fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged buildings. J Environ Monit (2009) 11(10):1849–58. doi:10.1039/b906856b
40. Arrojo E, Drigo R, Bianco AC. Type 2 deiodinase at the crossroads of thyroid hormone action. Int J Biochem Cell Biol (2011) 43(10):1432–41. doi:10.1016/j.biocel.2011.05.016
41. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest (2006) 116(10):2571–9. doi:10.1172/JCI29812
42. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta (2013) 1830(7):3937–45. doi:10.1016/j.bbagen.2012.05.007
43. Moura Neto A, Parisi MC, Tambascia MA, Alegre SM, Pavin EJ, Zantut-Wittmann DE. The influence of body mass index and low-grade systemic inflammation on thyroid hormone abnormalities in patients with type 2 diabetes. Endocr J (2013) 60(7):877–84. doi:10.1507/endocrj.EJ13-0030
44. Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest (1995) 96(6):2828–38. doi:10.1172/JCI118353
45. Mancini A, Raimondo S, Di Segni C, Persano M, Gadotti G, Silvestrini A, et al. Thyroid hormones and antioxidant systems: focus on oxidative stress in cardiovascular and pulmonary diseases. Int J Mol Sci (2013) 14(12):23893–909. doi:10.3390/ijms141223893
46. Chopra IJ, Solomon DH, Hepner GW, Morgenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med (1979) 90(6):905–12. doi:10.7326/0003-4819-90-6-905
47. Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol (2015) 6:472–85. doi:10.1016/j.redox.2015.09.005
48. Markkanen Penttinen P, Pelkonen J, Tapanainen M, Mäki-Paakkanen J, Jalava PI, Hirvonen MR. Co-cultivated damp building related microbes Streptomyces californicus and Stachybotrys chartarum induce immunotoxic and genotoxic responses via oxidative stress. Inhal Toxicol (2009) 21(10):857–67. doi:10.1080/08958370802526873
49. Ramli NSF, Mat Junit S, Leong NK, Razali N, Jayapalan JJ, Abdul Aziz A. Analyses of antioxidant status and nucleotide alterations in genes encoding antioxidant enzymes in patients with benign and malignant thyroid disorders. PeerJ (2017) 5:e3365. doi:10.7717/peerj.3365
50. Chopra IJ. An assessment of daily production and significance of thyroidal secretion of 3,3′,5′-triiodothyronine (reverse T3) in man. J Clin Invest (1976) 58:32–40. doi:10.1172/JCI108456
51. Moura Neto A, Zantut-Wittmann DE. Abnormalities of thyroid hormone metabolism during systemic illness: the low T3 syndrome in different clinical settings. Int J Endocrinol (2016) 2016:2157583. doi:10.1155/2016/2157583
52. Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Front Physiol (2015) 6:36. doi:10.3389/fphys.2015.00036
53. Araujo RL, Carvalho DP. Bioenergetic impact of tissue-specific regulation of iodothyronine deiodinases during nutritional imbalance. J Bioenerg Biomembr (2011) 43(1):59–65. doi:10.1007/s10863-011-9327-x
54. Verdier T, Coutand M, Bertron A, Roques C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build Environ (2014) 80:136–49. doi:10.1016/j.buildenv.2014.05.030
55. Yamamoto T. Comorbid latent adrenal insufficiency with autoimmune thyroid disease. Eur Thyroid J (2015) 4(3):201–6. doi:10.1159/000433532
56. Yamamoto T. History of stress-related health changes: a cue to pursue a diagnosis of latent primary adrenal insufficiency. Intern Med (2014) 53(3):183–8. doi:10.2169/internalmedicine.53.1156
57. McKenzie R, O’Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, et al. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. JAMA (1998) 280(12):1061–6. doi:10.1001/jama.280.12.1061
58. Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,3’,5’-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab (2005) 90(8):4559–65. doi:10.1210/jc.2005-0535
59. Reijula K, Ahonen G, Alenius H, Holopainen R, Lappalainen S, Palomäki E, et al. Rakennusten kosteus- ja homeongelmat. Eduskunnan Tarkastusvaliokunnan Julkaisu (2012) 1:207.
60. Vuokko A, Selinheimo S, Sainio M, Suojalehto H, Järnefelt H, Virtanen M, et al. Decreased work ability associated to indoor air problems: an intervention (RCT) to promote health behavior. Neurotoxicology (2015) 49:59–67. doi:10.1016/j.neuro.2015.04.010
61. McCormick SP, Stanley AM, Stover NA, Alexander NJ. Trichothecenes: from simple to complex mycotoxins. Toxins (Basel) (2011) 3(7):802–14. doi:10.3390/toxins3070802
62. Armenian P, Kearney TE. Pediatric ergot alkaloid exposures reported to the California Poison Control System: 1997–2008. Clin Toxicol (2014) 52(3):214–9. doi:10.3109/15563650.2014.885037
63. European Food Safety Authority. Scientific opinion on ergot alkaloids in food and feed. EFSA J (2012) 10(7):2798. doi:10.2903/j.efsa.2012.2798
64. Tudzynski P, Scheffer J. Claviceps purpurea: molecular aspects of a unique pathogenic lifestyle. Mol Plant Pathol (2004) 5(5):377–88. doi:10.1111/j.1364-3703.2004.00237.x
65. Cole RJ, Kirksey JW, Dorner JW, Wilson DM, Johnson JC Jr, Johnson AN, et al. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. J Agric Food Chem (1977) 25(4):826–30. doi:10.1021/jf60212a015
66. Marcet-Houben M, Gabaldon T. Horizontal acquisition of toxic alkaloid synthesis in a clade of plant associated fungi. Fungal Genet Biol (2016) 86:71–80. doi:10.1016/j.fgb.2015.12.006
67. Schuppan D, Pickert G, Ashfaq-Khan M, Zevallos V. Non-celiac wheat sensitivity: differential diagnosis, triggers and implications. Best Pract Res Clin Gastroenterol (2015) 29(3):469–76. doi:10.1016/j.bpg.2015.04.002
68. Cioffi F, Senese R, Lanni A, Goglia F. Thyroid hormones and mitochondria: with a brief look at derivatives and analogues. Mol Cell Endocrinol (2013) 379(1–2):51–61. doi:10.1016/j.mce.2013.06.006
69. Forini F, Lionetti V, Ardehali H, Pucci A, Cecchetti F, Ghanefar M, et al. Early long-term L-T3 replacement rescues mitochondria and prevents ischemic cardiac remodelling in rats. J Cell Mol Med (2011) 15(3):514–24. doi:10.1111/j.1582-4934.2010.01014.x
70. Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab (2009) 94(5):1623–9. doi:10.1210/jc.2008-1301
71. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol (2015) 11(11):642–52. doi:10.1038/nrendo.2015.155
74. McEvoy G. Thyroid Agents General Statement. Bethesda, MD: American Society of Health-System Pharmacists (2008).
75. Buckshee K, Kriplani A, Kapil A, Bhargava VL, Takkar D. Hypothyroidism complicating pregnancy. Aust N Z J Obstet Gynaecol (1992) 32(3):240–2. doi:10.1111/j.1479-828X.1992.tb01956.x
76. Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. Standards of Care Committee, American Thyroid Association. JAMA (1995) 273(10):808–12. doi:10.1001/jama.273.10.808
77. Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract (2002) 8(6):457–69. doi:10.4158/1934-2403-8.6.457
78. Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics (2006) 117(6):2290–303. doi:10.1542/peds.2006-0915
Keywords: water-damaged building, mycotoxins, hypothyroid symptoms, triiodothyronine, stress intolerance, latent adrenal insufficiency, FT3/rT3 ratio, DIO2
Citation: Somppi TL (2017) Non-Thyroidal Illness Syndrome in Patients Exposed to Indoor Air Dampness Microbiota Treated Successfully with Triiodothyronine. Front. Immunol. 8:919. doi: 10.3389/fimmu.2017.00919
Received: 10 February 2017; Accepted: 20 July 2017;
Published: 07 August 2017
Edited by:
Tamara Tuuminen, University of Helsinki, FinlandReviewed by:
Mario M. D’Elios, University of Florence, ItalyCopyright: © 2017 Somppi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taija Liisa Somppi, dGFpamEuc29tcHBpQGFtcGxpYS5maQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.