- 1Center for Oncology and Cell Biology, The Feinstein Institute for Medical Research, Manhasset, NY, United States
- 2Compass Therapeutics LLC, Cambridge, MA, United States
- 3Department of Medicine, The Hofstra Northwell School of Medicine, Manhasset, NY, United States
- 4Department of Molecular Medicine, The Hofstra Northwell School of Medicine, Manhasset, NY, United States
B-1a cells are innate-like B-lymphocytes producing natural antibodies. Activation-induced cytidine deaminase (AID), a product of the Aicda gene, plays a central role in class-switch recombination and somatic hypermutation in B cells. Although a role for Aicda in B-1a cells has been suggested on the basis of experiments with knock out (KO) mice, whether B-1a cells express Aicda, and if so, which B-1a cell subpopulation expresses Aicda, remains unknown. Here, we demonstrate that B-1 cells express Aicda, but at a level below that expressed by germinal center (GC) B cells. We previously reported that B-1a cells can be subdivided based on CD25 expression. We show here that B-1a cell Aicda expression is concentrated in the CD25+ B-1a cell subpopulation. These results suggest the possibility that previous studies of memory B cells identified on the basis of Aicda expression may have inadvertently included an unknown number of CD25+ B-1a cells. Although B-1a cells develop normally in the absence of Aicda, a competitive reconstitution assay reveals enhanced vigor for AID KO B-1a cell bone marrow (BM) progenitors, as compared with wild-type BM B-1 cell progenitors. These results suggest that AID inhibits the development of B-1a cells from BM B-1 cell progenitors in a competitive environment.
Introduction
B-1 cells are innate-like B-lymphocytes that spontaneously and constitutively produce natural antibodies, which provide immediate protection against infection and rapid removal of dying cell debris (1, 2). Mouse B-1 cells are distinguished from B-2 cells both by phenotype and by function (3). Phenotypically, B-1 cells are characterized as IgMhi, IgDlo, B220lo, CD23−, and CD43+ (and CD11b+ in the peritoneal cavity) (3). B-1 cells are either CD5+ (B-1a) or CD5− (B-1b). Recent studies have shown B-1 cells can be further subdivided based on the expression of CD25 (4), CD73 (5), PD-L2 (CD273) (6–9), or ENPP1 (PC1) (10), suggesting different roles for each subpopulation.
Activation-induced cytidine deaminase (AID) plays a central role in class-switch recombination and somatic hypermutation (11). AID is expressed abundantly in germinal center (GC) B cells (12) and at a low level in immature B cells (13–15). It was recently reported that B-1 cells accumulate immunoglobulin somatic hypermutation and increase class switching from 1 week of age up to 6 months of age, and these changes are diminished in the absence of AID (16). Nonetheless, AID expression in B-1 cells has not been documented and is yet to be directly addressed, raising the possibility that B-1 cell changes in AID KO mice may represent indirect effects.
We previously found that expression of CD25 on B-1a cells is activation dependent and these CD25+ B-1a cells express leukemia inhibitory factor receptor as well as increased levels of activated STAT3 as compared to CD25− B-1a cells (4). We explored the possibility that B-1 cells express AID and found that AID is expressed in B-1a cells and that this expression is concentrated in the activated, CD25+ B-1a cell pool.
Materials and Methods
Mice
Male BALB/c-ByJ and C57BL/6 mice were obtained from The Jackson Laboratory at 6–8 weeks of age. CB17-SCID or CB17 mice of 6–8 weeks of age were obtained from Taconic. AID KO mice on a BALB/c background were obtained from Dr. Michel Nussenzweig with Dr. Tasuku Honjo’s permission. All mice were used for experimentation at 8–14 weeks of age. All studies were approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research. Mice were cared for and handled in accordance with the National Institutes of Health and institutional guidelines.
Cell Purification and Flow Cytometry
Peritoneal washout cells and splenocytes were obtained from 8- to 14-week-old wild-type (WT) or AID knock out (KO) mice and were stained with fluorescence-labeled antibodies to B220, CD5, CD25, CD23, and GL-7 and with peanut agglutinin (PNA). B-cell populations (peritoneal B-1a cells: B220lo/CD5+, peritoneal CD25+ B-1a cells: B220lo/CD5+CD25+, peritoneal CD25− B-1a cells: B220lo/CD5+CD25−, splenic B2 cells: B220hiCD5−CD23+, or GC B cells: B220+/GL-7+/PNAhigh) were isolated using the Influx cell sorter (BD Biosciences). Post-sort, reanalysis of the B-cell populations showed them to be ≥98% pure. Cells were blocked with rat anti-mouse CD16/CD32 antibody (clone 2.4G2), stained with immunofluorescent antibodies, and then analyzed on a FACSCalibur flow cytometer (BD Biosciences) with appropriate gating. Images were constructed with FlowJo 6.0 software (Tree Star). PE-conjugated rat anti-mouse CD25 (clone PC61) was obtained from BD Pharmingen. The following antibodies were obtained from Biolegend: perCP-Cy5.5-conjugated rat anti-mouse CD45R/B220 (clone RA3-6B2); Alexa 647-conjugated rat anti-mouse CD5 (clone 53-7.3); and Alexa 647-conjugated rat anti-mouse GL-7. FITC-PNA was obtained from Sigma. CD23-PE-Cy7 (clone 2G8) was obtained from Abcam.
Gene Expression
Gene expression was assayed by real-time PCR as previously described (17). Briefly, RNA was prepared from B cells using the RNeasy mini kit (Qiagen), according to the manufacturer’s instructions. cDNA was prepared using avian myeloblastosis virus reverse transcriptase (Bio-Rad). Gene expression was then measured by real-time PCR using iTaq SYBR Green (Bio-Rad) and normalized with β2-microglobulin. The following primer sets were used: β2-microglobulin (F-CCCGCCTCACATTGAAATCC/R-GCGTATGTATCAGTCTCAGTGG); AID (AGAAAGTCACGCTGGAGACC/CTCCTCTTCACCACGTAGCA). Gene expression was also measured by real-time PCR using TaqMan chemistry. Primer and probe sets were obtained from Applied Biosystems for Aicda (Mm01184115_m1) and β-actin, which was used for normalization.
Adoptive Transfer
Bone marrow (BM) was obtained from 2-month-old BALB/c-ByJ (IgMa) mice and 2-month-old CB17 mice (IgMb). BM B-1a cell progenitors (lineage negative, CD19+B220lo/−AA4.1+) were sort-purified using the Influx cell sorter (BD Biosciences), washed twice in 1× PBS, resuspended in 1× PBS, and then injected (i.v.) into recipient CB17-SCID mice at 0.6 × 106 cells per mouse in 0.2 ml. Recipient mice were not irradiated prior to transfer. Serum samples, spleens, and peritoneal washout cells were collected from euthanized CB17-SCID recipients 6 weeks post transfer.
Statistics
Comparisons were conducted between WT and AID KO mice using Graphpad Prism 6.0 with two-tailed tests as indicated in the figure legends.
Results
B-1a Cells Express Aicda and Gene Expression Is Restricted to the CD25+ B-1a Cell Subset
The expression level of Aicda was evaluated in sort-purified peritoneal B-1a cells, peritoneal CD25+ B-1a cells (4), peritoneal CD25− B-1a cells, splenic B2 cells, and GC B cells from unmanipulated mice. The sorting strategy for isolating these populations is shown in Figure 1A. GC B cells displayed a high level of Aicda expression, which is consistent with previous reports (12), in contrast to splenic B-2 cells that expressed very little Aicda. We found that peritoneal B-1a cells expressed more Aicda than that by splenic B-2 cells, but less than that by GC B cells (Figure 1B). We then examined CD25+ B-1a cells in comparison to CD25− B-1a cells and found that CD25+ B-1a cells demonstrated a higher level of Aicda expression than did CD25− B-1a cells, total B-1a cells, and splenic B-2 cells, although this was still less than the level expressed by GC B cells. These results were confirmed using Taqman primers and probe (Figure S1 in Supplementary Material). Peritoneal CD25+ B-1a cells from C57BL/6 mice were also found to express Aicda in greater amounts than that by CD25− B-1a cells (Figure S2 in Supplementary Material). The mean level of Aicda expression in BALB/c CD25+ B-1a cells was 18-fold more than that of splenic B-2 cells but 40-fold less than that of GC B cells. Thus, B-1a cells, especially CD25+ B-1a cells, express Aicda.
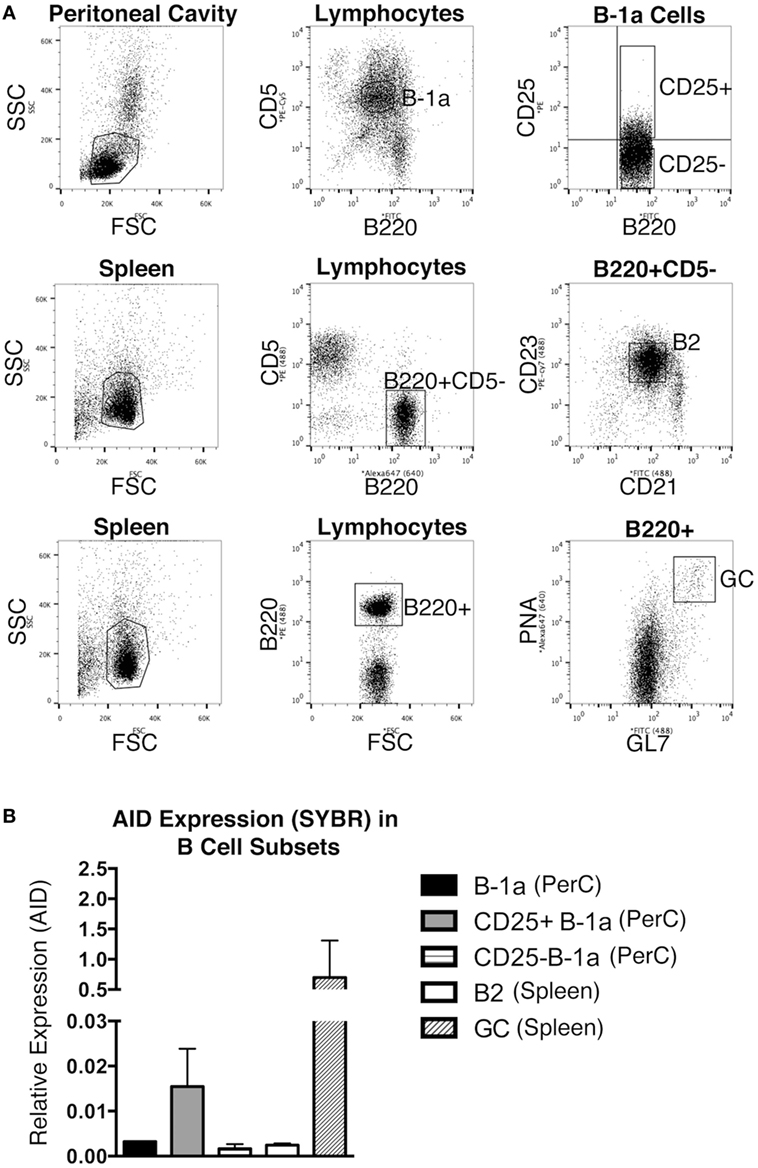
Figure 1. Aicda gene expression in B cells. Peritoneal washout cells and spleen cells were obtained from 3-month-old BALB/c-ByJ mice, immunofluorescently stained, and sorted for peritoneal B-1a (B220loCD5+), CD25+ B-1a (B220loCD5+CD25+), CD25− B-1a (B220loCD5+CD25−), splenic B2 (B220+CD5−CD23+), and germinal center (GC, B220+/GL-7+/PNAhigh) cells. The sorting strategy for these populations is shown in (A). RNA was prepared from each sort-purified B cell subset and reverse transcribed. (A) The level of Acida relative to β2-microglobulin was determined by real-time PCR (SYBR Green) with the primers described in Section “Materials and Methods.” The means of three independent experiments are shown in (B), along with lines indicating SEMs.
The Number of CD25+ B-1a Cells Is Unchanged in AID KO
Mice lacking the AID gene on the BALB/c background were assessed for numbers of total peritoneal cells, total peritoneal lymphocytes, B-1a cells, CD25+ B-1a cells, and CD25− B-1a cells. There was no significant difference in the total number of peritoneal lymphocytes in AID KO mice (4.3 × 106 ± 0.71) compared to that in WT mice (3.0 × 106 ± 0.17) (Figure 2A), although the total number of cells in the peritoneal cavities of AID KO mice was greater than the number in WT mice, presumably due to differences in a non-lymphoid population, such as myeloid cells. Next, the total numbers of B-1a, CD25+ B-1a, and CD25− B-1a cells were assessed in WT and AID KO mice. The results demonstrated that there is no significant difference in the total numbers of peritoneal B-1a, CD25+ B-1a, or CD25− B-1a cells from AID KO mice compared to those in WT controls (Figure 2B). Thus, AID does not appear to be required for the development of early appearing CD25+ or CD25− B-1a cells.
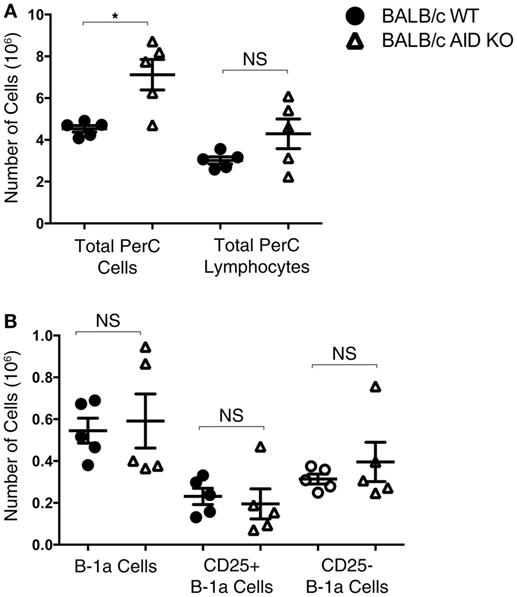
Figure 2. Number of B-1a cells in activation-induced cytidine deaminase (AID) knock out (KO) mice. Peritoneal washout cells were obtained from 3-month-old wild-type (WT) and AID KO mice on the BALB/c-ByJ background. (A) The total number of peritoneal cells and the total number of lymphocytes (based on the lymphocyte gate) are shown. (B) Peritoneal washout cells were stained with anti-B220-pCP-Cy5.5, anti-CD5-Alexa 647, anti-CD25-PE, and CD23-PE-Cy7. Based on staining for B-1a (B220loCD5+), CD25+ B-1a (B220loCD5+CD25+), and CD25− B-1a (B220loCD5+CD25−), the absolute number of each subset is shown. Results are shown as means of five independent mice, along with lines indicating the SEMs.
AID Impairs BM B-1a Cell Development
It has been previously shown that Aicda deficiency impairs B-cell development (13); however, it is unknown whether this effect extends to B-1a cell development. To directly test the extent to which Aicda affects B-1a cell development, we set up a mixed chimera system. Figure 3A illustrates the experimental design, which involved adoptive transfer of B-1 cell-specific progenitors (Lin−B220lo/−CD19+AA4.1+) obtained from the BM of AID KO mice and WT mice. Three groups of chimera mice were set up: (1) SCID mice were injected with 600,000 B-1-specific progenitors from the BM of BALB/c AID KO mice plus 600,000 B-1-specific progenitors from the BM of CB17 WT mice; (2) SCID mice were injected with 600,000 B-1-specific progenitors from the BM of BALB/c AID KO mice; and (3) SCID mice were injected with 600,000 B-1-specific progenitors from the BM of CB17 WT mice. Allotypic differences between BALB/c-ByJ (IgMa) and CB17 (IgMb) mice were used to assess the individual contributions of WT (IgMb)- and AID KO (IgMa)-derived B-1a cells to the B-1a cell pool.
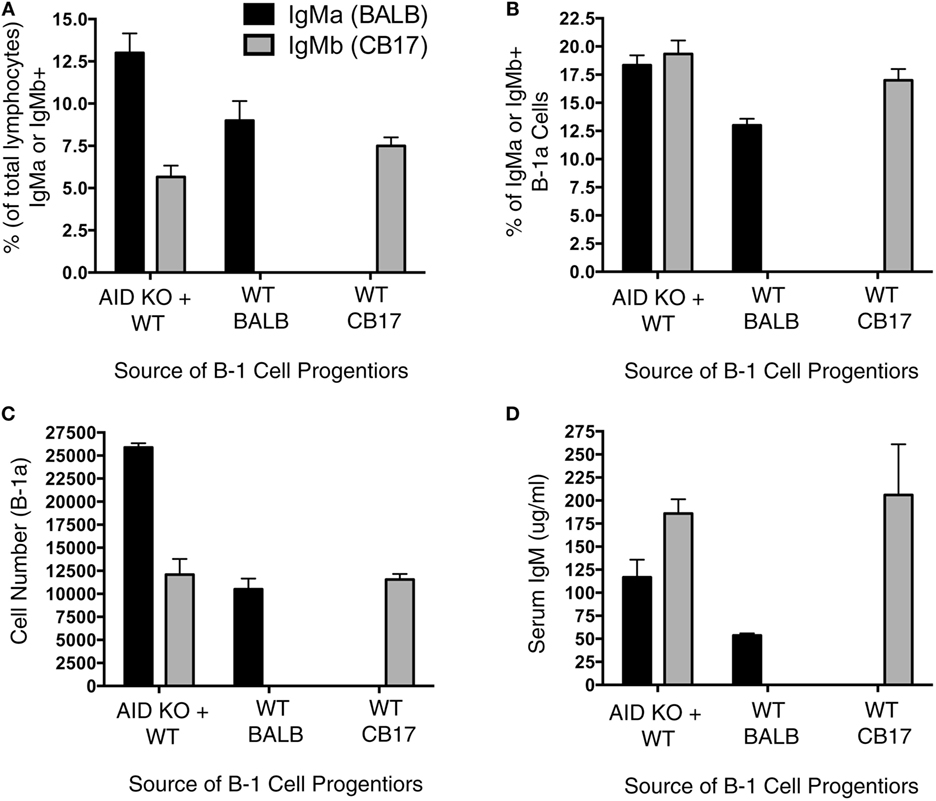
Figure 3. Aicda impairs B-1a cell development. Allotype mixed chimeras were set up by injecting (i.v.): (1) 600,000 B-1 cell-specific progenitors (Lin−B220lo/−CD19+AA4.1+) obtained from activation-induced cytidine deaminase (AID) knock out (KO)-BALB/c bone marrow (BM) (IgMa) along with 600,000 B-1 cell-specific progenitors from wild-type (WT)-CB17 BM (IgMb); (2) 600,000 B-1 cell-specific progenitors obtained from WT-BALB/c BM (IgMa); or (3) 600,000 B-1 cell-specific progenitors from WT-CB17 BM (IgMb) into CB17-SCID recipients. Six weeks after the transfer, peritoneal cells were collected for the flow analysis. (A) The percent of live lymphocytes positive for IgMa (black bars) or IgMb (gray bars) in the collected washout cells was assessed. (B) The percent of live lymphocytes that phenotyped as IgMa (black bars) or IgMb (gray bars) B-1a cells in the collected washout cells was assessed. (C) The total number of peritoneal B-1a cells derived from BALB/c (IgMa, black bars) or CB17 (IgMb, gray bars) mice was assessed.
We first examined the percent of total lymphocytes in the peritoneal washouts that were either IgMa+ or IgMb+. Interestingly, in the chimera mice receiving B-1 cell progenitors from both AID KO and WT BM, there were more AID KO-derived (IgMa+) B cells than WT-derived (IgMb+) B cells (p = 0.01) (Figure 3A). On the other hand, there was no difference in the percent of IgMa+ or IgMb+ lymphocytes that were peritoneal B-1a cells (Figure 3B) in mice receiving B-1 cell progenitors from both AID KO and WT BM. Thus, the total number of peritoneal B-1a cells derived from WT BM B-1 cell progenitors was significantly lower than those derived from AID KO BM B-1 cell progenitors (p = 0.01) (Figure 3C). As a control, BM B-1 cell progenitors from WT mice or AID KO mice were transferred alone into SCID recipients to detect any differences in reconstitution when the progenitors from these two sources (BALB/c or CD17 mice) were transferred alone. The results of the single transfers demonstrated no significant differences in the overall reconstitution of WT or AID KO mouse B-1 cell progenitors when transferred individually (Figures 3A–C). Together, these results demonstrate that Aicda inhibits the development of B-1a cells from BM B-1 cell progenitors in a competitive environment.
Discussion
We found that B-1a cells, particularly CD25+ B-1a cells, express Aicda. This Aicda expression occurs in the absence of intentional stimulation and without participation in GCs. Our results support the findings of Herzenberg and colleagues regarding the loss of somatic mutation and isotype switching from B-1 cell immunoglobulin in the absence of AID (16). Thus, we have extended previously reported functional results by directly demonstrating that WT B-1a cells express Aicda, and we have shown that Aicda expression is concentrated within the CD25+ B-1a cell subpopulation.
Recent studies have utilized AID reporter constructs to identify memory B cells that developed in GCs (18–20). Our results inject a note of caution regarding the interpretation of these kinds of experiments by showing that some mature B cells, specifically CD25+ B-1a cells, express Aicda and thus could register as reporter-positive despite not having resided in a GC. Further complicating this issue is the recent evidence that some B-1 cells may themselves be memory B cells (21). It is clear from the work reported here and by others that further study will be needed to tease out the extent to which Aicda expression marks naïve B-1a as well as memory B-2 cells and/or marks memory B cells regardless of whether they are B-1a or B-2.
We previously found that about 20% of peritoneal B-1a cells express CD25, a component of the high-affinity IL-2 receptor, and CD25+ B-1a cells express increased levels of activated signaling intermediates (4). However, these B-1a cells lack expression of CD122 and are not responsive to IL-2 (4). In some systems, AID expression appears to impact viability. Immature murine B cells express AID and AID-deficient immature B cells are more resistant to apoptosis than immature B cells that express AID (15). Moreover, AID-deficient GC B cells are more resistant to apoptosis (22). These data suggest a role for AID in regulating cell viability. Along these lines, we found more B-1 cells in the peritoneal cavities of SCID mice after reconstitution with AID KO B-1 progenitor cells, and this was accentuated in BM chimeras wherein competition exists between WT and KO B-1 cell progenitors. Thus, AID is likely involved in the development and/or viability of B-1a cells.
The phenotype of mouse-like human B-1 cells has recently been redefined (23) from its previous focus on CD5 (24), an unreliable marker for B-1 cells in Homo sapiens and other species (25–27). It has been reported that AID-deficient hyper IgM syndrome patients are prone to develop autoimmune or inflammatory diseases such as diabetes mellitus, polyarthritis, autoimmune hepatitis, hemolytic anemia, and immune thrombocytopenia (28, 29). Our results suggest that B-1 cells should be considered as the pathogenesis of AID-deficient autoimmunity is probed.
Author Contributions
HK, NH, and JT designed and performed the research, analyzed and interpreted data, and wrote the manuscript; TR interpreted data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by Public Health Service grant AI029690 awarded to TR by the National Institutes of Health.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00672/full#supplementary-material.
Figure S1. Aicda gene expression in B cells by Taqman assay. Peritoneal washout cells and spleen cells were obtained from 3-month-old BALB/c-ByJ mice, immunofluorescently stained, and sorted for peritoneal B-1a (B220loCD5+), CD25+ B-1a (B220loCD5+CD25+), CD25− B-1a (B220loCD5+CD25−), splenic B2 (B220+CD5−CD23+), and GC (B220+/GL-7+/PNAhigh) cells, as shown in Figure 1. RNA was prepared from each sort-purified B cell subset and reverse transcribed. The level of Aicda relative to actin was determined by real-time PCR (Taqman) with the primers described in Section “Materials and Methods.” The means of three independent experiments are shown, along with lines indicating SEMs.
Figure S2. Aicda gene expression in C57BL/6 B cells. Peritoneal washout cells and spleen cells were obtained from 3-month-old C57BL/6J mice, immunofluorescently stained, and sorted for peritoneal B-1a (B220loCD5+), CD25+ B-1a (B220loCD5+CD25+), CD25− B-1a (B220loCD5+CD25−), splenic B2 (B220+CD5−CD23+), and GC (B220+/GL-7+/PNAhigh) cells, as shown in Figure 1. RNA was prepared from each sort-purified B cell subset and reverse transcribed. The level of Acida relative to β2-microglobulin was determined by real-time PCR (SYBR Green) with the primers described in Section “Materials and Methods.” The means of three independent experiments are shown in (A), along with lines indicating SEMs. The level of Aicda relative to actin was determined by real-time PCR (Taqman) with the primers described in Section “Materials and Methods.” The means of three independent experiments are shown in (B), along with lines indicating SEMs.
References
1. Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Ann N Y Acad Sci (2013) 1285:97–114. doi: 10.1111/nyas.12137
2. Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A (2000) 97(3):1184–9. doi:10.1073/pnas.97.3.1184
3. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol (2011) 11(1):34–46. doi:10.1038/nri2901
4. Tumang JR, Holodick NE, Vizconde TC, Kaku H, Frances R, Rothstein TL. A CD25(−) positive population of activated B1 cells expresses LIFR and responds to LIF. Front Immunol (2011) 2:6. doi:10.3389/fimmu.2011.00006
5. Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol (2014) 193(12):5904–13. doi:10.4049/jimmunol.1400336
6. Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol (2007) 37(9):2405–10. doi:10.1002/eji.200737461
7. Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum (2009) 60(12):3734–43. doi:10.1002/art.25015
8. Kaku H, Rothstein TL. Octamer binding protein 2 (Oct2) regulates PD-L2 gene expression in B-1 cells through lineage-specific activity of a unique, intronic promoter. Genes Immun (2010) 11(1):55–66. doi:10.1038/gene.2009.68
9. Zhong X, Rothstein TL. L2pB1: a new player in autoimmunity. Mol Immunol (2011) 48(11):1292–300. doi:10.1016/j.molimm.2010.12.006
10. Wang H, Shin DM, Abbasi S, Jain S, Kovalchuk AL, Beaty N, et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proc Natl Acad Sci U S A (2012) 109(49):20077–82. doi:10.1073/pnas.1212428109
11. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell (2000) 102(5):553–63. doi:10.1016/S0092-8674(00)00078-7
12. Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem (1999) 274(26):18470–6. doi:10.1074/jbc.274.26.18470
13. Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, et al. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A (2011) 108(28):11560–5. doi:10.1073/pnas.1102571108
14. Kuraoka M, McWilliams L, Kelsoe G. AID expression during B-cell development: searching for answers. Immunol Res (2011) 49(1–3):3–13. doi:10.1007/s12026-010-8185-7
15. Kuraoka M, Kelsoe G. A novel role for activation-induced cytidine deaminase: central B-cell tolerance. Cell Cycle (2011) 10(20):3423–4. doi:10.4161/cc.10.20.17693
16. Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, et al. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife (2015) 4:e09083. doi:10.7554/eLife.09083
17. Tumang JR, Frances R, Yeo SG, Rothstein TL. Cutting edge: spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol (2005) 174(6):3173–7. doi:10.4049/jimmunol.174.6.3173
18. Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, et al. Regulation of AID expression in the immune response. J Exp Med (2007) 204(5):1145–56. doi:10.1084/jem.20061952
19. Mullins CD, Su MY, Hucthagowder V, Chu L, Lu L, Kulkarni S, et al. Germinal center B-cells resist transformation by Kras independently of tumor suppressor Arf. PLoS One (2013) 8(6):e67941. doi:10.1371/journal.pone.0067941
20. Healy JA, Nugent A, Rempel RE, Moffitt AB, Davis NS, Jiang X, et al. GNA13 loss in germinal center B cells leads to impaired apoptosis and promotes lymphoma in vivo. Blood (2016) 127(22):2723–31. doi:10.1182/blood-2015-07-659938
21. Yang Y, Ghosn EE, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A (2012) 109(14):5388–93. doi:10.1073/pnas.1121627109
22. Boulianne B, Rojas OL, Haddad D, Zaheen A, Kapelnikov A, Nguyen T, et al. AID and caspase 8 shape the germinal center response through apoptosis. J Immunol (2013) 191(12):5840–7. doi:10.4049/jimmunol.1301776
23. Quach TD, Rodriguez-Zhurbenko N, Hopkins TJ, Guo X, Hernandez AM, Li W, et al. Distinctions among circulating antibody-secreting cell populations, including B-1 cells, in human adult peripheral blood. J Immunol (2016) 196(3):1060–9. doi:10.4049/jimmunol.1501843
24. Casali P, Notkins AL. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol (1989) 7:513–35. doi:10.1146/annurev.iy.07.040189.002501
25. Freedman AS, Freeman G, Whitman J, Segil J, Daley J, Levine H, et al. Expression and regulation of CD5 on in vitro activated human B cells. Eur J Immunol (1989) 19(5):849–55. doi:10.1002/eji.1830190511
26. Raman C, Knight KL. CD5+ B cells predominate in peripheral tissues of rabbit. J Immunol (1992) 149(12):3858–64.
27. Guelpa-Fonlupt V, Tonnelle C, Blaise D, Fougereau M, Fumoux F. Discrete early pro-B and pre-B stages in normal human bone marrow as defined by surface pseudo-light chain expression. Eur J Immunol (1994) 24(1):257–64. doi:10.1002/eji.1830240140
28. Jesus AA, Duarte AJ, Oliveira JB. Autoimmunity in hyper-IgM syndrome. J Clin Immunol (2008) 28(Suppl 1):S62–6. doi:10.1007/s10875-008-9171-x
Keywords: AID, B-1a cells, CD25, B-1 cell subset, peritoneal cavity
Citation: Kaku H, Holodick NE, Tumang JR and Rothstein TL (2017) CD25+ B-1a Cells Express Aicda. Front. Immunol. 8:672. doi: 10.3389/fimmu.2017.00672
Received: 16 September 2016; Accepted: 23 May 2017;
Published: 20 June 2017
Edited by:
Harry W. Schroeder, University of Alabama at Birmingham, United StatesReviewed by:
Paulo Vieira, Institut Pasteur de Paris, FrancePaolo Casali, The University of Texas Health Science Center San Antonio, United States
Copyright: © 2017 Kaku, Holodick, Tumang and Rothstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroaki Kaku, aGlyb2FraS5rYWt1QG1lZC53bWljaC5lZHU=
†Present address: Hiroaki Kaku, Nichol E. Holodick and Thomas L. Rothstein, Center for Immunobiology, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, MI, United States;
Joseph R. Tumang, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, United States
‡These authors have contributed equally to this work.
 Hiroaki Kaku
Hiroaki Kaku Nichol E. Holodick
Nichol E. Holodick Joseph R. Tumang
Joseph R. Tumang Thomas L. Rothstein
Thomas L. Rothstein