
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 25 January 2017
Sec. Microbial Immunology
Volume 8 - 2017 | https://doi.org/10.3389/fimmu.2017.00022
This article is part of the Research Topic Immunobiotics: Interactions of Beneficial Microbes With the Immune System View all 26 articles
Inflammatory bowel diseases (IBDs), including ulcerative colitis and Crohn’s disease, are chronic inflammatory diseases characterized by dysregulated immune responses of the gastrointestinal tract. In recent years, the incidence of IBDs has increased in developed nations, but their prophylaxis/treatment is not yet established. Site-directed delivery of molecules showing anti-inflammatory properties using genetically modified (gm)-probiotics shows promise as a new strategy for the prevention and treatment of IBD. Advantages of gm-probiotics include (1) the ability to use bacteria as a delivery vehicle, enabling safe and long-term use by humans, (2) decreased risks of side effects, and (3) reduced costs. The intestinal delivery of anti-inflammatory proteins such as cytokines and enzymes using Lactococcus lactis has been shown to regulate host intestinal homeostasis depending on the delivered protein-specific machinery. Additionally, clinical experience using interleukin 10-secreting Lc. lactis has been shown to be safe and to facilitate biological containment in IBD therapy. On the other hand, some preclinical studies have demonstrated that gm-strains of immunobiotics (probiotic strains able to beneficially regulate the mucosal immunity) provide beneficial effects on intestinal inflammation as a result of the synergy between the immunoregulatory effects of the bacterium itself and the anti-inflammatory effects of the delivered recombinant proteins. In this review, we discuss the rapid progression in the development of strategies for the prophylaxis and treatment of IBD using gm-probiotics that exhibit immune regulation effects (gm-immunobiotics). In particular, we discuss the type of strains used as delivery agents.
Inflammatory bowel disease (IBD) is a chronic inflammatory disease that occurs in the gastrointestinal tract (GIT); IBDs are largely classified as ulcerative colitis (UC) and Crohn’s disease (CD). There has been an increase in the number of cases of IBD in recent years, mainly in Western countries (1). IBD causes inflammatory obstruction of the GIT, resulting in symptoms such as stomach cramps, pain, diarrhea, constipation, and vomiting over an extended period of time. These symptoms cause considerable reduction in quality of life. While IBD is not a direct cause of mortality, the disease can increase the risk of colorectal cancer (2). The precise etiology of IBD has yet to be clarified, but causal factors are thought to include the environment, genetics, and microorganisms (3). The chronic inflammation seen in IBD is characterized by dysregulated immune response of the host as a result of marked changes in the intestinal environment (3). Consequently, favorable regulation of the compromised immune homeostasis is effective in the prognosis and treatment of IBD. Corticosteroids, thiopurines, and anti-tumor necrosis factor (TNF) antibody (Ab), which exhibit immune-regulatory effects, can control IBD to a certain extent, and these treatments are widely used in clinical settings as therapeutic drugs (4). However, there are individual-specific differences in the effectiveness of these drugs, and there are also issues such as the possibility of serious side effects and high costs (4, 5).
There is currently a great deal of interest in the use of probiotics that have been genetically modified (gm) to produce proteins with IBD therapeutic potential as novel drug substitutes. Probiotics, defined as “live microorganisms that, when administrated in adequate amounts, confer a health benefit on the host” (6), have been reported to attenuate inflammation in the host GIT through immune system regulation, strengthening of barrier function, and improvement of the changed intestinal microbiota (7). Probiotics comprise primarily lactic acid bacteria (LAB) and bifidobacteria, and also include non-pathogenic Escherichia coli. Probiotics have been used in food for a long time, and many of the bacteria included in probiotics fall under the Generally Recognized As Safe assessment designated by the United States Food and Drug Administration and meet the Qualified Presumption of Safety designation of the European Food Safety Authority. Genetic modification technology has undergone considerable advances in recent years, and Lactococcus (Lc.) lactis in particular has been established as an efficient expression system for recombinant proteins (RPs) (8) (Figure 1A). Thus, probiotics, which have excellent safety and health advantages, are likely to be very useful as producers of IBD therapeutic proteins and as agents for delivering such proteins to the GIT (Figure 1B). gm-Probiotics that produce or secrete various different anti-inflammatory proteins have been constructed in recent years, and their anti-inflammatory effectiveness when administered orally has been verified using in vivo experiments in animal models of IBD (9, 10) (Table 1; Table S1 in Supplementary Material). In this context, it is important to note that the delivery of IBD therapeutic proteins to the GIT using gm-probiotics is expected (1) to allow the therapeutic protein to act locally, with greater effectiveness and decreased risk of medical error or side effects compared to conventional systemic administration of the molecule by injection, and (2) to be considerably cheaper than refined drugs (10, 11). It is of particular interest that many of the molecules selected as anti-inflammatory proteins target the host immune system. Many studies to date have used Lc. lactis as a model strain, but methods using lactobacilli, bifidobacteria, streptococci, and E. coli Nissle 1917 (EcN), bacteria that have more beneficial health effects than Lc. lactis, as delivery agents have been attempted in recent years (Figure 1A). Many of these studies (12–26) employ bacteria that have been termed “immunobiotics,” which have been defined as probiotic strains that are able to beneficially regulate mucosal immunity (27, 28). Immunobiotics are recognized by the pattern recognition receptors of epithelial and antigen-presenting cells such as dendritic cells and macrophages, and these immunobiotics are known to beneficially regulate innate and adoptive immune responses (Figure 1C); there have been tremendous advances in the clarification of strain-specific immune regulation functions at the cellular and molecular levels (28–32).
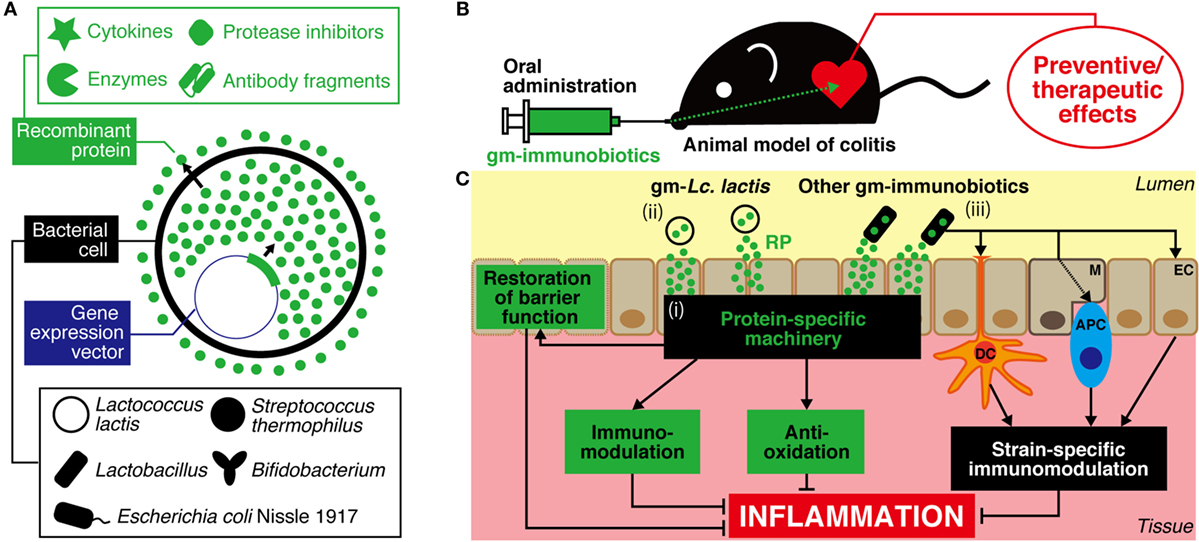
Figure 1. A strategy for prevention and treatment of IBD using genetically modified (gm)-immunobiotics. (A) Different bioactive proteins such as cytokines, enzymes, protease inhibitors, and antibody fragments can be produced/secreted by gm-strains. (B) After oral administration, viable cells of gm-immunobiotics transit through the gastric environment and reach the intestine. Then, gm-immunobiotics provide preventive/therapeutic effects against experimental colitis in animal as a result of the exertion of anti-inflammatory effects in situ. (C) General mechanisms of action of gm-immunobiotics on anti-inflammatory effects in the intestine. Physiologically meaningful amounts of recombinant proteins are yielded by gm-immunobiotics via secretion or cell lysis, and exert host anti-inflammatory effects through a protein-specific machinery including immunomodulation, anti-oxidation, and restoration of epithelial barrier functions (i). Lactococcus (Lc.) lactis has been most widely used as a safe and effective vector in this strategy (ii). Lc. lactis has little or no effect on either the improvement or aggravation of the intestinal inflammation and does not colonize the intestine. Other gm-immunobiotics (including some strains of Lactobacillus, Bifidobacterium, and Streptococcus salivarius subsp. thermophilus, and Escherichia coli Nissle 1917) provide beneficial effects on intestinal inflammation as a result of the synergy between the immunoregulatory effects of the bacterium itself and the anti-inflammatory effects of the delivered recombinant proteins (iii). Immunobiotics interact with pattern recognition receptors of host epithelial cells and antigen-presenting cells such as dendritic cells and macrophages to exert strain-specific immunomodulatory effects. Some strains of immunobiotics may colonize the intestine. IBD, inflammatory bowel disease; RP, recombinant protein; EC, epithelial cell; M, microfold cell; DC, dendritic cell; APC, antigen-presenting cell.
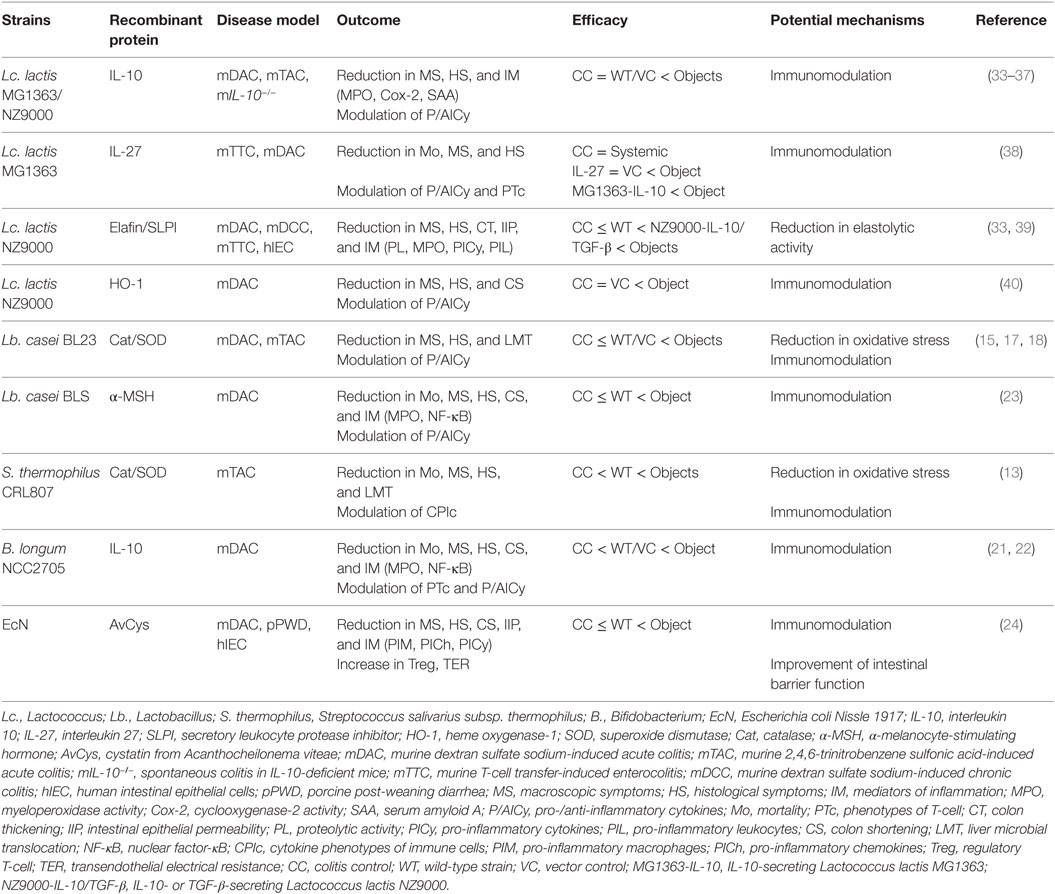
Table 1. Selected preclinical evidence showing beneficial effects of gm-immunobiotics in treatment of gastrointestinal tract inflammation.
In this review, we describe recent developments in preventive and therapeutic strategies for the treatment of IBD using gm-probiotics. In particular, our discussion focuses on gm-probiotics that exhibit immune regulation effects (gm-immunobiotics) and bacterial species that are used as protein delivery agents.
Lactococcus lactis is a species of LAB used universally in cheese and other fermented dairy products. To date, Lc. lactis MG1363 (MG1363) and its derivatives have been widely used to produce RPs and as carriers for delivery to mucous membranes (Figure 1). Lc. lactis was the first LAB species to have its whole genome sequenced, and there exists a wealth of genetic data on this species (8, 41, 42). In addition, Lc. lactis genetic modification is straightforward, and there are a great number of useful gene expression systems for this organism (8). Furthermore, Lc. lactis is able to pass through the GIT alive but does not establish itself in the GIT and is easy to control pharmacokinetically (43, 44). It is important to note that Lc. lactis itself has little or no effect on either the improvement or aggravation of GIT inflammation in animals and humans and is therefore highly safe for use against IBD (14, 33–35, 38–40, 45–52) (http://ClinicalTrials.gov Identifier: NCT00729872). The research to date into gm-Lc. lactis has been compiled into a number of review articles (9–11, 53). In the present review, we will deal with a series of landmark studies that showed the usefulness and practicality of the present strategy, and we will examine the latest findings.
The strategy of reducing intestinal inflammation by using gm-probiotics for delivery of RPs to the GIT was first proposed in 2000 by Steidler et al. (35), who created a MG1363 strain that secreted interleukin (IL)-10 (LL-mIL10). IL-10 is a cytokine that plays a central role in the suppression of inflammation (54), and mutation of the endogenous gene has been shown to be involved in the onset of murine enterocolitis (55, 56) and infantile-onset IBD (57, 58). Steidler et al. showed that daily oral administration of LL-mIL10 resulted in a dramatic reduction of colitis onset and progression in a murine IBD model (35). Notably, the effective amount of IL-10 was 1/10,000th of the amount used in conventional systemic administration. This enhancement may be regarded as the greatest advantage of the present strategy. The reduction in the amount administered has also been demonstrated in the delivery systems of other RPs (38, 49, 50). Next, Steidler et al. constructed LL-Thy12, in which the thymidylate synthase gene (thyA) of the Lc. lactis genome was replaced by the human IL-10-encoding gene (59). The results of a phase 1 clinical study in CD patients confirmed the safety, biological containment, and significant therapeutic effect of LL-Thy12 (52). However, no statistically significant therapeutic effect was found in the subsequent phase 2a clinical study (http://ClinicalTrials.gov Identifier: NCT00729872). The authors suggested that the lack of therapeutic effect was due to low concentration of IL-10 in the intestine. Nonetheless, bearing in mind that this first clinical study using gm-LAB suggested the safety and usefulness of this delivery system, the results were remarkable.
IL-27 is an anti-inflammatory cytokine belonging to the IL-12 family, a group of molecules that has been shown to attenuate murine experimental colitis by suppressing the development of T helper 17 (Th17) cells (60). In addition, the involvement of low-expressing variants of the IL-27-encoding gene in early-onset IBD has been demonstrated (61). In 2014, Hanson et al. showed that daily oral administration of MG1363 that secretes IL-27 (LL-IL-27) almost completely cured murine T-cell transfer-induced enterocolitis and reduced the associated mortality rate (38). LL-IL-27 treatment caused a reduction in the level of inflammatory cytokines that had increased in the GIT as a result of enterocolitis and a reduction in the number of colitis pathogenic IL-17-producing T-cells. In addition, the results indicated that increased local production of IL-10 by LL-IL-27 in the GIT was effective in providing a therapeutic effect. It is important to note that oral administration of LL-IL-27 demonstrated a notably greater therapeutic effect than systemic administration of IL-27 or oral administration of IL-10-secreting MG1363.
In 2015, a study comparing Lc. lactis NZ9000 (NZ9000) that secreted serine protease inhibitors (elafin or secretory leukocyte protease inhibitor) to NZ9000 that secreted the anti-inflammatory cytokines IL-10 or transforming growth factor-β showed that the former significantly attenuated the symptoms of dextran sodium sulfate (DSS)-induced colitis (33). Prior to that study, Motta et al. showed that the expression of elafin was lower in IBD patients than in healthy people, and that this decreased expression correlated with the increased elastolytic activity of the colonic mucosa in IBD patients (39). Also, delivery of elafin to the GIT using a gm-NZ9000 resulted in marked improvement of acute and chronic colitis in murine models (39). Elafin-secreting NZ9000 restored the colonic elastolytic homeostasis that had broken down as a result of colitis, reduced the number of immune cells infiltrating the colon, and repaired the barrier function of the intestinal epithelium (39).
In 2015, we successfully constructed a gm-NZ9000 strain (designated NZ-HO) that secretes biologically active heme oxygenase-1 (HO-1). HO-1 is an enzyme that catalyzes heme catabolism in vivo. HO-1 is induced endogenously by stimuli such as inflammation or oxidative stress, and the enzyme exhibits anti-inflammatory and cytoprotective effects mediated by the generation of heme breakdown products (62, 63). We showed that daily oral administration of NZ-HO markedly attenuated the symptoms of DSS colitis (40). Interestingly, NZ-HO increased the production of IL-10, decreased inflammatory cell infiltration, and decreased expression of IL-6 and IL-1α in the colonic tissue of murine colitis models (40). In 2014, Zhang et al. showed that intraperitoneal injection of an HO-1 inducer-induced IL-10-producing regulatory T cells (Treg) (rather than IBD pathogenic Th17) by inhibiting IL-6/IL-6 receptor signaling, thus ameliorating DSS colitis (64). This result suggested that NZ-HO regulates the immune responses of the inflamed colon in a beneficial fashion to ameliorate DSS colitis.
In 2015, Aubry et al. found that preventive oral administration of MG1363 that secreted thymic stromal lymphopoietin caused a transient increase in the number of CD4+ CD25+ FoxP3+ Treg cells in the mesenteric lymph node and attenuated DSS colitis in mice (45). Quevrain et al. found that MG1363 that secreted an anti-inflammatory protein (MAM) isolated from a strain of Faecalibacterium prausnitzii, a species that is deficient in CD patients and alleviated dinitrobenzene sulfonic acid-induced colitis in mice (47). MAM-secreting MG1363 markedly reduced the production of pro-inflammatory cytokines (IL-17A and interferon-γ) in the colonic tissue of colitis mice (47).
IL-6 is an important pathogenic factor in various different inflammatory diseases, including IBD. By regulating the function and proliferation of T cells, IL-6 exacerbates GIT inflammation in IBD (65). In addition, studies using murine models of colitis and CD patients showed that inhibition of IL-6 signaling using antibodies improved the symptoms (66, 67). However, the cost of Ab drugs is very high. We therefore created a NZ9000 derivative that secretes a single-chain variable fragment Ab against IL-6 (IL6scFv) (68). Importantly, we showed that the recombinant IL6scFv produced by gm-NZ9000 is immunoreactive, as demonstrated by binding to IL-6 (68). Thus, IL6scFv-secreting NZ9000 is an attractive gm-LAB for research and development of a low-cost IBD therapeutic drug that can yield site-directed delivery of anti-IL-6 antibodies.
Bacteria of the genus Lactobacillus, which are classified as LAB, are the best-known type of probiotics. Several strains belonging to this genus are commensal bacteria that reside within the human GIT. To date, many preclinical studies have indicated that strains belonging to genus Lactobacillus regulate GIT inflammation in a favorable fashion through strain-specific, health-beneficial mechanisms (9). In addition, clinical research to date has shown that a probiotic mixture containing four species of Lactobacillus (VSL#3) and Lactobacillus reuteri ATCC 55730 exhibits benefits in the treatment of active UC (69–72). Bacteria belonging to genus Lactobacillus are used predominantly in probiotic formulations that are useful for the prevention and drug therapy of GIT-related diseases selected by the World Gastroenterology Organization (73).
In 2007, Rochat et al. showed that daily oral administration of Lactobacillus casei BL23 (BL23) attenuated murine DSS colitis (17). The same year, Foligne et al. demonstrated that BL23 induced an immune reaction with dominance of anti-inflammatory IL-10 over pro-inflammatory IL-12 in human peripheral blood mononuclear cells and reduced the symptoms of murine 2,4,6-trinitrobenzenesulfonic acid (TNBS) colitis (74). In 2010, Watterlot et al. orally administered superoxide dismutase (SOD)-producing and SOD-non-producing BL23 to mice and found that the former resulted in marked amelioration of DSS-induced histological damage to the colon, while the latter gave only slight amelioration (18). An excess of reactive oxygen species causes considerable tissue damage, which suggests a link to IBD development, and the use of antioxidative enzymes to eliminate reactive oxidative species is expected to have potential as an IBD treatment strategy (75). Oral delivery of SOD using gm-LAB has actually been shown to reduce colitis in rodents (12, 14). In 2011, LeBlanc et al. orally administered BL23 that produced an antioxidative enzyme (SOD or catalase) to mice, and their results showed that the mortality rate, weight loss, histological colon damage, and liver microbial translocation induced by TNBS administration were markedly reduced (15). However, in the studies performed by Watterlot et al. (18) and LeBlanc et al. (15), wild-type (WT) BL23 had only mild anti-inflammatory properties and did not induce marked IL-10 production in colon tissue, indicating that the amelioration effects on murine colon inflammation are limited. In 2014, Hou et al. showed that oral administration of SOD-producing Lactobacillus fermentum I5007 (I5007) improved lipid peroxidation and immune parameters in the colon, thus ameliorating murine TNBS colitis (26). A partial, but significant, improvement effect was also observed with WT-I5007. I5007 was isolated from healthy porcine intestinal mucosa and has been used as a growth stimulator for livestock. The above series of studies proposed a novel IBD preventive strategy combining the two different intestinal inflammation amelioration mechanisms: the immunobiotic effects of lactobacilli and the antioxidative effects of delivered proteins (Figure 1C).
In 2008, α-melanocyte-stimulating hormone (α-MSH)-secreting Lb. casei BLS (BLS) was created (23). α-MSH is a neuropeptide with immunosuppressant effects that has been reported to exhibit anti-inflammatory effects in animal models of various diseases, including IBD (76). Orally administered gm-BLS shows curative effects for the symptoms of murine DSS colitis (23). This improvement involves decreased secretion of inflammatory cytokines (TNF-α, IL-1β, and IL-6) and increased secretion of immune-regulatory cytokines (IL-4 and IL-10) in ex vivo cultures of colonic tissue (23). It is interesting to note that gm-BLS brought about considerable improvement in a number of parameters when compared to the WT strain (23).
Streptococcus thermophilus is a LAB that has traditionally been used as a yogurt starter. Preclinical studies to date have clarified the roles of specific S. thermophilus strains as immunobiotics (77–82). For example, Ogita et al. showed that S. thermophilus ST28 (ST28) derived from milk regulated IL-17 production in murine splenocytes in Th17-skewed conditions by induction of counteracting interferon-γ (82). Moreover, oral administration of ST28 to mice markedly decreased DSS-induced intestinal lesions, and this treatment markedly decreased IL-17 secretion and the frequency of accumulation of Th17, the numbers of which had increased in the lamina propria as a result of DSS (81). S. thermophilus is a component of a probiotic mixture agent (VSL#3) that has been found to be effective for induction and maintenance of remission in UC and prevention and maintenance of remission in pouchitis (73). It is interesting to note that several S. thermophilus strains are known to be autolytic, a useful trait for strains used as gm-immunobiotics (83).
In 2014, an immunobiotic strain, S. thermophilus CRL807 (CRL807), which exhibits immunosuppressant action in vitro and in vivo, was selected from a mixed yogurt starter; CRL807’s usefulness as a delivery agent for SOD and catalase then was investigated (13). CRL807 significantly increased the ratios of IL-10:inflammatory cytokine (IL-12, IL-17, or interferon-γ) in human peripheral blood mononuclear cells and the digestive tract of healthy mice. Oral administration of antioxidative enzyme-producing gm-CRL807 and WT-CRL807 to mice markedly potentiated the ratio of IL-10-positive:IL-17-positive cells, a ratio that had been reduced by TNBS administration, and provided amelioration of colitis. Notably, administration of either or both SOD-producing and catalase-producing CRL807 improved antioxidative enzyme activity in the colon, demonstrating greater anti-inflammatory action than WT-CRL807 administration. Experimental long-term (30-day) oral administration of gm-CRL807 and WT-CRL807 in healthy mice showed the safety of CRL807 (84).
The genus Bifidobacterium comprises indigenous bacteria that make up the intestinal flora and in particular are present in significant numbers in healthy infants. In IBD patients, on the other hand, it is known that there is a decreased number of Bifidobacterium and an increase in pro-inflammatory E. coli and Bacteroides in the intestinal mucosa (85–89). Preclinical studies to date have shown that various strains of genus Bifidobacterium bring about beneficial effects in the prevention and treatment of colitis, mediated by different effects [immunoregulation effects (90, 91), improvement of the barrier function of intestinal epithelium (92, 93), and improvement of the intestinal flora (94, 95)]. It is interesting that Bifidobacterium longum subsp. infantis 35624 has been shown to selectively drive specialization of FoxP3+ Treg cells and/or induce IL-10 production in animal disease models and in humans (96–99). In addition, clinical studies of patients with UC and other inflammatory diseases showed that, compared to placebo, oral administration of this immunobiotic strain resulted in a marked decrease in the level of plasma C-type protein, an inflammatory biomarker that increases with the disease (100). It has also been shown that the symptoms of UC patients are ameliorated by a single Bifidobacteria strain (101), probiotic mixtures that include Bifidobacteria (69, 71, 72, 102, 103), and symbiotics (probiotic/prebiotic mixtures) in which Bifidobacteria is the main constituent (104–106).
In 2011, an immunobiotic strain, B. longum NCC2705 (NCC2705), was engineered to secrete biologically active IL-10, and the strain’s curative effects in DSS colitis were investigated (21). Improvement of the symptoms of DSS colitis (aggravation of gross symptoms, colon shortening, histopathological changes accompanying tissue damage, and myeloperoxidase activation) was observed with oral administration of WT-NCC2705 alone. Considerable improvement was found with IL-10-secreting gmNCC2705 when compared to WT-NCC2705 treatment (21). In addition, this study found that WT-NCC2705 and gm-NCC2705 reduced the expression of nuclear factor-κB and pro-inflammatory cytokines in the colon and the peripheral blood, and restored the proportion of CD4+ CD25+ FoxP3+ Treg cells (21). These effects were markedly stronger with gm-NCC2705. In 2015, Zhang et al. showed that the Treg/Th17 balance that had broken down as a result of DSS colitis was fully restored by gm-NCC2705 through the inhibition of two intracellular signaling pathways for Th17 induction (22). In 2016, the intestinal inflammation amelioration action of different strains of B. longum that produced human α-MSH was reported (19, 20). In the first of these reports, preventive daily oral administration of α-MSH-secreting B. longum HB15 (HB15) markedly reduced histopathological damage, increased myeloperoxidase activity, corrected an inflammatory/anti-inflammatory cytokine imbalance, and induced production of the pro-inflammatory factor nitrogen monoxide, overcoming effects caused by DSS colitis in rats. Administration of WT-HB15 improved all the parameters with the exception of nitrogen monoxide production, but to a considerably lower degree than that seen with the recombinant strain (19). In the second report, α-MSH-secreting B. longum HB25 (HB25) was created. Therapeutic daily oral administration of this recombinant strain markedly improved murine DSS colitis. Interestingly, no curative effects were observed from oral administration of the vector control strain (20). The two serial studies above indicated that immunobiotic Bifidobacteria that secrete proteins exhibiting immunomodulatory effects beneficial to IBD amelioration (IL-10 or α-MSH) are capable of stronger prevention/cure of UC-like colitis in mice than are WT strains, with effects presumably mediated through synergistic effects on various functions (Figure 1C).
Escherichia coli Nissle 1917 has no pathogenic factors (adhesion molecules, invasiveness, enterotoxin, cytotoxins, etc.). This strain’s genetics, physiology, and biological activities as a probiotics were largely characterized some time ago; as an alternative medicine (Mutaflor) for IBD and other GIT-related diseases, EcN currently serves as one of the most useful bacterial strains (104). In randomized controlled trials of UC remission maintenance, oral administration of EcN was as effective as treatment with mesalazine in preventing relapse of the disease (105–107). In studies using IBD model animals, EcN was proven to ameliorate colitis symptoms by regulation of the immune system and intestinal barrier function (108–111). In addition, the utility of this immunobiotic strain as a production platform for vaccines and pharmaceutics and as an intestinal delivery system continues to grow (112). Studies of gm-EcN that produces pathogenic bacteria/virus antigens (113–115) and immunomodulatory molecules such as cytokines and proteins derived from parasites (24, 25) have been reported, and disease preventive/curative effects have been verified in animals.
In 2012, Gardlik et al. developed IL-10-secreting EcN and verified this strain’s anti-inflammatory effects using DSS colitis (25). Oral administration of IL10-secreting EcN was shown to improve inflammation parameters (reduced stool consistency, colon shortening, decreased oxidative and carbonyl stress), but these effects were of the same degree as obtained with WT-EcN or IL-10-secreting MG1363. In 2014, EcN that secretes a protease inhibitor protein derived from nematodes (AvCys) was created (24). AvCys’ immune-regulatory action is mediated mainly by targeting macrophages, and this inhibitory protein exhibits anti-inflammatory action in murine models of IBD and allergies (116–119). Oral administration of AvCys-secreting EcN (EcN-AvCys) on alternate days attenuated DSS colitis by beneficial regulation of the immune system in the inflamed colon (regulation of the proportion and function of pro-inflammatory macrophages, increase in the proportion of FoxP3+ Treg cells, and decrease in inflammatory cytokines and chemokines). In addition, in experiments using pigs (whose GITs closely resemble those of humans), oral administration of EcN-AvCys on alternate days to post-weaning piglets reduced spontaneous colon inflammation. Interestingly, the results of that study suggested that EcN-AvCys ameliorates inflammation in this piglet model by improving intestinal barrier function rather than by regulating the intestinal immune system. WT-EcN shows some benefits in ameliorating murine intestinal inflammation, inducing Treg cells, and increasing transepithelial resistance in a culture of a human colonic epithelial cell strain, but the efficacies were significantly milder than those obtained with EcN-AvCys.
Site-directed delivery of proteins that exhibit anti-inflammatory effects using gm-immunobiotics is extremely attractive as an effective preventive/curative strategy for IBD (Figure 1). A series of studies using IL-10-secreting Lc. lactis, ranging from basic to clinical, established a milestone by indicating the effectiveness and the feasibility of clinical application of this concept. Subsequently, gm-Lc. lactis strains that efficiently produce cytokines, enzymes, and protease inhibitors with a range of anti-inflammatory properties have been developed, and anti-inflammatory properties of these strains have been verified using rodent models of IBD (Table 1; Table S1 in Supplementary Material). Recent research into intestinal delivery of serine protease inhibitors and IL-27 has shown that these strains provide markedly more beneficial amelioration of murine intestinal inflammation than do strains that deliver IL-10. In addition, the research strongly implies that MG1363 and its derivatives do not have any negative impact on GIT inflammation or health maintenance, regardless of whether the strains are WT or recombinant. It may therefore be concluded that Lc. lactis is the bacterium that holds the most promise as a delivery agent for proteins with IBD therapeutic potential. In addition, work has also advanced to verify the potential for application of immunobiotics in this strategy. Interestingly, these studies show marked amelioration of GIT inflammation in animals as a result of the synergy between the immunoregulatory effects of the immunobiotic bacterium itself and the anti-inflammatory effects of the delivered RPs (Figure 1C; Table 1; Table S1 in Supplementary Material). This observation implies that the strategy of using immunobiotics is an effective means toward the development of IBD therapeutics with greater efficacy. For future work, it would be desirable to carry out comparative investigations of the therapeutic effects on GIT inflammation of different gm-strains that produce the same RP.
Clinical trials that include verification of safety and efficacy will be essential for developing gm-immunobiotics as therapeutic drugs for IBD. To date, there have been no findings that demonstrate any danger in the use of gm-probiotics including gm-immunobiotics. At the same time, there is little evidence to prove the safety of these agents in clinical use, and it remains possible that gm-probiotic organisms may be spread into the environment. Thus, there is some skepticism regarding the use of these agents. However, two clinical studies using IL-10-secreting Lc. lactis have demonstrated tremendous breakthroughs (59, 120, 121). In addition, in a recent phase 1b trial, oral administration of AG013 (an oral rinse containing trefoil factor 1-secreting MG1363 as the main component) was shown to be safe and well tolerated in cancer patients while also exhibiting efficacy against oral mucositis (122). Guidelines toward clinical use of gm-Lc. lactis have been proposed (123), and the feasibility of the clinical application of gm-Lc. lactis is strongly implied. With other probiotics, aspects such as the time for passage through the GIT, establishment in the GIT, health benefits, or the danger of side effects will differ from those of Lc. lactis, so safety evaluations will be needed and biological containment strategies will have to be developed. The establishment of effective gm-immunobiotics for prevention and treatment of IBD is near at hand, and it is to be hoped that this strategy will be facilitated by advances in the scientific understanding of gene recombination techniques in the future.
SS and TS conceived, designed, and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SA and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
The study was funded by a Grant-in-Aid for the Japan Society for the Promotion of Science Fellows (No. 14J06317) to SS and by a grant from Kato Memorial Bioscience Foundation to TS.
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00022/full#supplementary-material.
1. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology (2012) 142(1):46.e42–54.e42. doi:10.1053/j.gastro.2011.10.001
2. Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res (2009) 29(7):2727–37.
3. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol (2016) 13(1):13–27. doi:10.1038/nrgastro.2015.186
4. Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol (2014) 20(12):3146–52. doi:10.3748/wjg.v20.i12.3146
5. Rutgeerts P, Van Assche G, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease – seven years on. Aliment Pharmacol Ther (2006) 23(4):451–63. doi:10.1111/j.1365-2036.2006.02786.x
6. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol (2014) 11(8):506–14. doi:10.1038/nrgastro.2014.66
7. Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol (2010) 7(9):503–14. doi:10.1038/nrgastro.2010.117
8. Wyszynska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK. Lactic acid bacteria – 20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol (2015) 99(7):2967–77. doi:10.1007/s00253-015-6498-0
9. de Moreno de LeBlanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P, et al. Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract (2015) 2015:146972. doi:10.1155/2015/146972
10. Wells J. Mucosal vaccination and therapy with genetically modified lactic acid bacteria. Annu Rev Food Sci Technol (2011) 2:423–45. doi:10.1146/annurev-food-022510-133640
11. Cano-Garrido O, Seras-Franzoso J, Garcia-Fruitos E. Lactic acid bacteria: reviewing the potential of a promising delivery live vector for biomedical purposes. Microb Cell Fact (2015) 14:137. doi:10.1186/s12934-015-0313-6
12. Carroll IM, Andrus JM, Bruno-Barcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol (2007) 293(4):G729–38. doi:10.1152/ajpgi.00132.2007
13. Del Carmen S, de Moreno de LeBlanc A, Martin R, Chain F, Langella P, Bermudez-Humaran LG, et al. Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl Environ Microbiol (2014) 80(3):869–77. doi:10.1128/aem.03296-13
14. Han W, Mercenier A, Ait-Belgnaoui A, Pavan S, Lamine F, van S II, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing superoxide dismutase. Inflamm Bowel Dis (2006) 12(11):1044–52. doi:10.1097/01.mib.0000235101.09231.9e
15. LeBlanc JG, del Carmen S, Miyoshi A, Azevedo V, Sesma F, Langella P, et al. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J Biotechnol (2011) 151(3):287–93. doi:10.1016/j.jbiotec.2010.11.008
16. Qiu ZB, Chen J, Chen JJ, Rong L, Ding WQ, Yang HJ, et al. Effect of recombinant Lactobacillus casei expressing interleukin-10 in dextran sulfate sodium-induced colitis mice. J Dig Dis (2013) 14(2):76–83. doi:10.1111/1751-2980.12006
17. Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, et al. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact (2007) 6:22. doi:10.1186/1475-2859-6-22
18. Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefevre F, et al. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol (2010) 144(1):35–41. doi:10.1016/j.ijfoodmicro.2010.03.037
19. Wei P, Yang Y, Ding Q, Li X, Sun H, Liu Z, et al. Oral delivery of Bifidobacterium longum expressing α-melanocyte-stimulating hormone to combat ulcerative colitis. J Med Microbiol (2016) 65(2):160–8. doi:10.1099/jmm.0.000197
20. Wei P, Yang Y, Liu Z, Huang J, Gong Y, Sun H. Oral Bifidobacterium longum expressing α-melanocyte-stimulating hormone to fight experimental colitis. Drug Deliv (2016) 23(6):2058–64. doi:10.3109/10717544.2015.1122672
21. Yao J, Wang JY, Lai MG, Li YX, Zhu HM, Shi RY, et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol Pharm (2011) 8(2):488–97. doi:10.1021/mp100331r
22. Zhang D, Wei C, Yao J, Cai X, Wang L. Interleukin-10 gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17 cell pathway. Exp Biol Med (Maywood) (2015) 240(12):1622–9. doi:10.1177/1535370215584901
23. Yoon SW, Lee CH, Kim JY, Kim JY, Sung MH, Poo H. Lactobacillus casei secreting α-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c Mice. J Microbiol Biotechnol (2008) 18(12):1975–83. doi:10.4014/jmb.0800.445
24. Whelan RA, Rausch S, Ebner F, Gunzel D, Richter JF, Hering NA, et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol Ther (2014) 22(10):1730–40. doi:10.1038/mt.2014.125
25. Gardlik R, Palffy R, Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol (Praha) (2012) 58(6):238–45.
26. Hou CL, Zhang J, Liu XT, Liu H, Zeng XF, Qiao SY. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol (2014) 116(6):1621–31. doi:10.1111/jam.12461
27. Clancy R. Immunobiotics and the probiotic evolution. FEMS Immunol Med Microbiol (2003) 38(1):9–12. doi:10.1016/S0928-8244(03)00147-0
28. Kitazawa H, Villena J, Alvarez S. Probiotics: Immunobiotics and Immunogenics. Boca Raton, FL: CRC Press (2014).
29. Kitazawa H, Villena J. Modulation of respiratory TLR3-anti-viral response by probiotic microorganisms: lessons learned from Lactobacillus rhamnosus CRL1505. Front Immunol (2014) 5:201. doi:10.3389/fimmu.2014.00201
30. Villena J, Kitazawa H. Modulation of intestinal TLR4-inflammatory signaling pathways by probiotic microorganisms: lessons learned from Lactobacillus jensenii TL2937. Front Immunol (2014) 4:512. doi:10.3389/fimmu.2013.00512
31. Villena J, Aso H, Kitazawa H. Regulation of toll-like receptors-mediated inflammation by immunobiotics in bovine intestinal epitheliocytes: role of signaling pathways and negative regulators. Front Immunol (2014) 5:421. doi:10.3389/fimmu.2014.00421
32. Laino J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms (2016) 4(3):27. doi:10.3390/microorganisms4030027
33. Bermudez-Humaran LG, Motta JP, Aubry C, Kharrat P, Rous-Martin L, Sallenave JM, et al. Serine protease inhibitors protect better than IL-10 and TGF-beta anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb Cell Fact (2015) 14:26. doi:10.1186/s12934-015-0198-4
34. Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, et al. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology (2007) 133(3):862–74. doi:10.1053/j.gastro.2007.06.018
35. Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science (2000) 289(5483):1352–5. doi:10.1126/science.289.5483.1352
36. del Carmen S, Martin Rosique R, Saraiva T, Zurita-Turk M, Miyoshi A, Azevedo V, et al. Protective effects of lactococci strains delivering either IL-10 protein or cDNA in a TNBS-induced chronic colitis model. J Clin Gastroenterol (2014) 48(Suppl 1):S12–7. doi:10.1097/mcg.0000000000000235
37. Waeytens A, Ferdinande L, Neirynck S, Rottiers P, De Vos M, Steidler L, et al. Paracellular entry of interleukin-10 producing Lactococcus lactis in inflamed intestinal mucosa in mice. Inflamm Bowel Dis (2008) 14(4):471–9. doi:10.1002/ibd.20346
38. Hanson ML, Hixon JA, Li W, Felber BK, Anver MR, Stewart CA, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology (2014) 146(1):210.e13–21.e13. doi:10.1053/j.gastro.2013.09.060
39. Motta JP, Bermudez-Humaran LG, Deraison C, Martin L, Rolland C, Rousset P, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med (2012) 4(158):158ra44. doi:10.1126/scitranslmed.3004212
40. Shigemori S, Watanabe T, Kudoh K, Ihara M, Nigar S, Yamamoto Y, et al. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb Cell Fact (2015) 14:189. doi:10.1186/s12934-015-0378-2
41. Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res (2001) 11(5):731–53. doi:10.1101/gr.169701
42. Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, et al. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol (2007) 189(8):3256–70. doi:10.1128/jb.01768-06
43. Drouault S, Corthier G, Ehrlich SD, Renault P. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl Environ Microbiol (1999) 65(11):4881–6.
44. Kimoto H, Nomura M, Kobayashi M, Mizumachi K, Okamoto T. Survival of lactococci during passage through mouse digestive tract. Can J Microbiol (2003) 49(11):707–11. doi:10.1139/w03-092
45. Aubry C, Michon C, Chain F, Chvatchenko Y, Goffin L, Zimmerli SC, et al. Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS-induced colitis mouse model. Microb Cell Fact (2015) 14:176. doi:10.1186/s12934-015-0367-5
46. Liu S, Li Y, Deng B, Xu Z. Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice. BMC Biotechnol (2016) 16:25. doi:10.1186/s12896-016-0255-z
47. Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut (2016) 65(3):415–25. doi:10.1136/gutjnl-2014-307649
48. Saraiva TD, Morais K, Pereira VB, de Azevedo M, Rocha CS, Prosperi CC, et al. Milk fermented with a 15-lipoxygenase-1-producing Lactococcus lactis alleviates symptoms of colitis in a murine model. Curr Pharm Biotechnol (2015) 16(5):424–9. doi:10.2174/1389201015666141113123502
49. Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, et al. Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol (2010) 3(1):49–56. doi:10.1038/mi.2009.116
50. Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology (2004) 127(2):502–13. doi:10.1053/j.gastro.2004.05.020
51. Wong CC, Zhang L, Li ZJ, Wu WK, Ren SX, Chen YC, et al. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol (2012) 27(7):1205–12. doi:10.1111/j.1440-1746.2012.07158.x
52. Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol (2006) 4(6):754–9. doi:10.1016/j.cgh.2006.03.028
53. Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol (2008) 6(5):349–62. doi:10.1038/nrmicro1840
54. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol (2001) 19:683–765. doi:10.1146/annurev.immunol.19.1.683
55. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell (1993) 75(2):263–74. doi:10.1016/0092-8674(93)80068-P
56. Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med (1998) 187(4):571–8. doi:10.1084/jem.187.4.571
57. Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, et al. Infant colitis – it’s in the genes. Lancet (2010) 376(9748):1272. doi:10.1016/s0140-6736(10)61008-2
58. Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med (2009) 361(21):2033–45. doi:10.1056/NEJMoa0907206
59. Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, et al. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol (2003) 21(7):785–9. doi:10.1038/nbt840
60. Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol (2011) 300(4):G568–76. doi:10.1152/ajpgi.00329.2010
61. Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet (2009) 41(12):1335–40. doi:10.1038/ng.489
62. Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev (2008) 60(1):79–127. doi:10.1124/pr.107.07104
63. Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun (2005) 338(1):558–67. doi:10.1016/j.bbrc.2005.08.020
64. Zhang L, Zhang Y, Zhong W, Di C, Lin X, Xia Z. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem (2014) 289(39):26847–58. doi:10.1074/jbc.M114.590554
65. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol (2015) 16(5):448–57. doi:10.1038/ni.3153
66. Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med (2000) 6(5):583–8. doi:10.1038/75068
67. Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology (2004) 126(4):989–96. doi:10.1053/j.gastro.2004.01.012
68. Shigemori S, Ihara M, Sato T, Yamamoto Y, Nigar S, Ogita T, et al. Secretion of an immunoreactive single-chain variable fragment antibody against mouse interleukin 6 by Lactococcus lactis. Appl Microbiol Biotechnol (2017) 101(1):341–9. doi:10.1007/s00253-016-7907-8
69. Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol (2009) 104(2):437–43. doi:10.1038/ajg.2008.118
70. Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, et al. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther (2012) 35(3):327–34. doi:10.1111/j.1365-2036.2011.04939.x
71. Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol (2009) 7(11):1202–9, 1209.e1. doi:10.1016/j.cgh.2009.07.016
72. Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol (2010) 105(10):2218–27. doi:10.1038/ajg.2010.218
73. Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, et al. World gastroenterology organisation global guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol (2012) 46(6):468–81. doi:10.1097/MCG.0b013e3182549092
74. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol (2007) 13(2):236–43. doi:10.3748/wjg.v13.i2.236
75. Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol (2014) 387(7):605–20. doi:10.1007/s00210-014-0985-1
76. Singh M, Mukhopadhyay K. α-Melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide. Biomed Res Int (2014) 2014:874610. doi:10.1155/2014/874610
77. Donkor ON, Ravikumar M, Proudfoot O, Day SL, Apostolopoulos V, Paukovics G, et al. Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin Exp Immunol (2012) 167(2):282–95. doi:10.1111/j.1365-2249.2011.04496.x
78. Latvala S, Miettinen M, Kekkonen RA, Korpela R, Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin Exp Immunol (2011) 165(1):94–103. doi:10.1111/j.1365-2249.2011.04408.x
79. Shimosato T, Fujimoto M, Tohno M, Sato T, Tateo M, Otani H, et al. CpG oligodeoxynucleotides induce strong up-regulation of interleukin 33 via toll-like receptor 9. Biochem Biophys Res Commun (2010) 394(1):81–6. doi:10.1016/j.bbrc.2010.02.110
80. Shimosato T, Tohno M, Sato T, Nishimura J, Kawai Y, Saito T, et al. Identification of a potent immunostimulatory oligodeoxynucleotide from Streptococcus thermophilus lacZ. Anim Sci J (2009) 80(5):597–604. doi:10.1111/j.1740-0929.2009.00680.x
81. Ogita T, Nakashima M, Morita H, Saito Y, Suzuki T, Tanabe S. Streptococcus thermophilus ST28 ameliorates colitis in mice partially by suppression of inflammatory Th17 cells. J Biomed Biotechnol (2011) 2011:378417. doi:10.1155/2011/378417
82. Ogita T, Tanii Y, Morita H, Suzuki T, Tanabe S. Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. Int J Mol Med (2011) 28(5):817–22. doi:10.3892/ijmm.2011.755
83. Petrarca C, Clemente E, Toto V, Iezzi M, Rossi C, Zanotta S, et al. rBet v 1 immunotherapy of sensitized mice with Streptococcus thermophilus as vehicle and adjuvant. Hum Vaccin Immunother (2014) 10(5):1228–37. doi:10.4161/hv.28155
84. de Moreno de LeBlanc A, Del Carmen S, Chatel JM, Azevedo V, Bermudez-Humaran L, Langella P, et al. Evaluation of the biosafety of recombinant lactic acid bacteria designed to prevent and to treat colitis. J Med Microbiol (2016) 65(9):1038–46. doi:10.1099/jmm.0.000323
85. Burke DA, Axon AT. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ (1988) 297(6641):102–4. doi:10.1136/bmj.297.6641.102
86. Cummings JH, Macfarlane GT, Macfarlane S. Intestinal bacteria and ulcerative colitis. Curr Issues Intest Microbiol (2003) 4(1):9–20.
87. Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe (2014) 15(3):382–92. doi:10.1016/j.chom.2014.02.005
88. Macfarlane S, Furrie E, Cummings JH, Macfarlane GT. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin Infect Dis (2004) 38(12):1690–9. doi:10.1086/420823
89. Mylonaki M, Rayment NB, Rampton DS, Hudspith BN, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis (2005) 11(5):481–7. doi:10.1097/01.MIB.0000159663.62651.4f
90. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog (2012) 8(5):e1002714. doi:10.1371/journal.ppat.1002714
91. McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut (2003) 52(7):975–80. doi:10.1136/gut.52.7.975
92. Srutkova D, Schwarzer M, Hudcovic T, Zakostelska Z, Drab V, Spanova A, et al. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute DSS-induced colitis in strictly strain-specific manner. PLoS One (2015) 10(7):e0134050. doi:10.1371/journal.pone.0134050
93. Takeda Y, Nakase H, Namba K, Inoue S, Ueno S, Uza N, et al. Upregulation of T-bet and tight junction molecules by Bifidobacterium longum improves colonic inflammation of ulcerative colitis. Inflamm Bowel Dis (2009) 15(11):1617–8. doi:10.1002/ibd.20861
94. Setoyama H, Imaoka A, Ishikawa H, Umesaki Y. Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect (2003) 5(2):115–22. doi:10.1016/S1286-4579(02)00080-1
95. Veiga P, Gallini CA, Beal C, Michaud M, Delaney ML, DuBois A, et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci U S A (2010) 107(42):18132–7. doi:10.1073/pnas.1011737107
96. Konieczna P, Akdis CA, Quigley EM, Shanahan F, O’Mahony L. Portrait of an immunoregulatory Bifidobacterium. Gut Microbes (2012) 3(3):261–6. doi:10.4161/gmic.20358
97. Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut (2012) 61(3):354–66. doi:10.1136/gutjnl-2011-300936
98. O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, et al. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog (2008) 4(8):e1000112. doi:10.1371/journal.ppat.1000112
99. O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology (2005) 128(3):541–51. doi:10.1053/j.gastro.2004.11.050
100. Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes (2013) 4(4):325–39. doi:10.4161/gmic.25487
101. Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc (2016) 28(1):67–74. doi:10.1111/den.12553
102. Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol (2005) 100(7):1539–46. doi:10.1111/j.1572-0241.2005.41794.x
103. Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther (2004) 20(10):1133–41. doi:10.1111/j.1365-2036.2004.02268.x
104. Sonnenborn U, Schulze J. The non-pathogenic Escherichia coli strain Nissle 1917 – features of a versatile probiotic. Microb Ecol Health Dis (2009) 21:122–58. doi:10.3109/08910600903444267
105. Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut (2004) 53(11):1617–23. doi:10.1136/gut.2003.037747
106. Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther (1997) 11(5):853–8. doi:10.1046/j.1365-2036.1997.00225.x
107. Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet (1999) 354(9179):635–9. doi:10.1016/S0140-6736(98)06343-0
108. Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One (2007) 2(12):e1308. doi:10.1371/journal.pone.0001308
109. Souza EL, Elian SD, Paula LM, Garcia CC, Vieira AT, Teixeira MM, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis by modulating intestinal permeability, the inflammatory response and clinical signs in a faecal transplantation model. J Med Microbiol (2016) 65(3):201–10. doi:10.1099/jmm.0.000222
110. Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Gottl C, et al. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol (2004) 11(2):372–8. doi:10.1128/cdli.11.2.372-378.2004
111. Kamada N, Inoue N, Hisamatsu T, Okamoto S, Matsuoka K, Sato T, et al. Nonpathogenic Escherichia coli strain Nissle 1917 prevents murine acute and chronic colitis. Inflamm Bowel Dis (2005) 11(5):455–63. doi:10.1097/01.MIB.0000158158.55955.de
112. Ou B, Yang Y, Tham WL, Chen L, Guo J, Zhu G. Genetic engineering of probiotic Escherichia coli Nissle 1917 for clinical application. Appl Microbiol Biotechnol (2016) 100(20):8693–9. doi:10.1007/s00253-016-7829-5
113. Buddenborg C, Daudel D, Liebrecht S, Greune L, Humberg V, Schmidt MA. Development of a tripartite vector system for live oral immunization using a Gram-negative probiotic carrier. Int J Med Microbiol (2008) 298(1–2):105–14. doi:10.1016/j.ijmm.2007.08.008
114. Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, et al. Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci U S A (2005) 102(34):11993–8. doi:10.1073/pnas.0504881102
115. Remer KA, Bartrow M, Roeger B, Moll H, Sonnenborn U, Oelschlaeger TA. Split immune response after oral vaccination of mice with recombinant Escherichia coli Nissle 1917 expressing fimbrial adhesin K88. Int J Med Microbiol (2009) 299(7):467–78. doi:10.1016/j.ijmm.2009.03.003
116. Danilowicz-Luebert E, Steinfelder S, Kuhl AA, Drozdenko G, Lucius R, Worm M, et al. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol (2013) 43(3–4):201–10. doi:10.1016/j.ijpara.2012.10.014
117. Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog (2011) 7(1):e1001248. doi:10.1371/journal.ppat.1001248
118. Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Parasite-specific immunomodulatory functions of filarial cystatin. Infect Immun (2003) 71(5):2422–9. doi:10.1128/IAI.71.5.2422-2429.2003
119. Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol (2008) 180(6):4265–72. doi:10.4049/jimmunol.180.6.4265
120. Huyghebaert N, Vermeire A, Neirynck S, Steidler L, Remaut E, Remon JP. Evaluation of extrusion/spheronisation, layering and compaction for the preparation of an oral, multi-particulate formulation of viable, hIL-10 producing Lactococcus lactis. Eur J Pharm Biopharm (2005) 59(1):9–15. doi:10.1016/j.ejpb.2004.09.003
121. Huyghebaert N, Vermeire A, Neirynck S, Steidler L, Remaut E, Remon JP. Development of an enteric-coated formulation containing freeze-dried, viable recombinant Lactococcus lactis for the ileal mucosal delivery of human interleukin-10. Eur J Pharm Biopharm (2005) 60(3):349–59. doi:10.1016/j.ejpb.2005.02.012
122. Limaye SA, Haddad RI, Cilli F, Sonis ST, Colevas AD, Brennan MT, et al. Phase 1b, multicenter, single blinded, placebo-controlled, sequential dose escalation study to assess the safety and tolerability of topically applied AG013 in subjects with locally advanced head and neck cancer receiving induction chemotherapy. Cancer (2013) 119(24):4268–76. doi:10.1002/cncr.28365
Keywords: probiotics, immunobiotics, IBD, gmLAB, gm-immunobiotics
Citation: Shigemori S and Shimosato T (2017) Applications of Genetically Modified Immunobiotics with High Immunoregulatory Capacity for Treatment of Inflammatory Bowel Diseases. Front. Immunol. 8:22. doi: 10.3389/fimmu.2017.00022
Received: 17 November 2016; Accepted: 05 January 2017;
Published: 25 January 2017
Edited by:
Julio Villena, Reference Centre for Lactobacilli (CERELA-CONICET), ArgentinaReviewed by:
Graciela Liliana Garrote, CIDCA (CONICET-UNLP), ArgentinaCopyright: © 2017 Shigemori and Shimosato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeshi Shimosato, c2hpbW90QHNoaW5zaHUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.