- 1Cell Biology and Immunology Group, Wageningen University, Wageningen, Netherlands
- 2Top Institute Food and Nutrition, Wageningen, Netherlands
- 3Gut Health and Food Safety, Institute of Food Research, Norwich, UK
- 4Host Microbe Interactomics, Wageningen University, Wageningen, Netherlands
- 5Laboratory of Microbiology, Wageningen University, Wageningen, Netherlands
- 6Human Nutrition, Wageningen University, Wageningen, Netherlands
- 7Department of Molecular Genetics, Erasmus University Medical Center, Rotterdam, Netherlands
- 8CECAD Forschungszentrum, Universität zu Köln, Köln, Germany
- 9University of Groningen, Groningen, Netherlands
- 10Department of Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
- 11Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
- 12Department of Pulmonary Medicine, Erasmus University Medical Center, Rotterdam, Netherlands
Although it is clear that probiotics improve intestinal barrier function, little is known about the effects of probiotics on the aging intestine. We investigated effects of 10-week bacterial supplementation of Lactobacillus plantarum WCFS1, Lactobacillus casei BL23, or Bifidobacterium breve DSM20213 on gut barrier and immunity in 16-week-old accelerated aging Ercc1−/Δ7 mice, which have a median lifespan of ~20 weeks, and their wild-type littermates. The colonic barrier in Ercc1−/Δ7 mice was characterized by a thin (< 10 μm) mucus layer. L. plantarum prevented this decline in mucus integrity in Ercc1−/Δ7 mice, whereas B. breve exacerbated it. Bacterial supplementations affected the expression of immune-related genes, including Toll-like receptor 4. Regulatory T cell frequencies were increased in the mesenteric lymph nodes of L. plantarum- and L. casei-treated Ercc1−/Δ7 mice. L. plantarum- and L. casei-treated Ercc1−/Δ7 mice showed increased specific antibody production in a T cell-dependent immune response in vivo. By contrast, the effects of bacterial supplementation on wild-type control mice were negligible. Thus, supplementation with L. plantarum – but not with L. casei and B. breve – prevented the decline in the mucus barrier in Ercc1−/Δ7 mice. Our data indicate that age is an important factor influencing beneficial or detrimental effects of candidate probiotics. These findings also highlight the need for caution in translating beneficial effects of probiotics observed in young animals or humans to the elderly.
Introduction
Aging is accompanied by multiple age-related diseases (1), posing a major burden to public health care (2). With age, a decline in the regenerative potential of tissues due to stem cell exhaustion occurs (3). Turnover in epithelial cells is rapid, and mounting evidence indicates that intestinal stem cells are compromised with aging (4). For example, a crucial component of the intestinal barrier is mucus secreted by goblet cells (5). The Muc2 glycoprotein regulates immunity by inducing tolerogenic signals in mucosal dendritic cells (6) and is important in host–microbe interactions (7). Thus, changes in mucus quantity and integrity influence immunity (6, 8).
Aging is accompanied by the development of a low-grade inflammation (“inflammaging”), which is characterized by elevated IL-6 and TNF serum levels in elderly (9). Involution of the thymus and the bone marrow (BM) leads to decreased T and B cell production (10, 11). By contrast, the production of myeloid cells is enhanced with aging, characterized by a progressive increase of neutrophil frequencies in the circulation (12).
Probiotics are defined as live bacteria that confer health benefits to the host, for example, by competing with pathogens, enhancing intestinal barrier function, and regulating immunity (13, 14). They might, therefore, prevent some of the undesired age-related intestinal barrier and immune effects. Probiotic supplementation of elderly subjects led to changes in fecal microbiota composition (15–17), and affected the distribution and function of NK cells, macrophages, granulocytes, and T cells in the circulation (18, 19). Supplementation of aged mice with Lactobacillus paracasei resulted in increased IgG2a serum titers after antigenic challenge (20). Middle-aged mice that were supplemented with Bifidobacterium animalis showed decreased colon permeability, extended lifespan, and improved quality of life (21). Besides these studies, little is known about how exposure to probiotics impacts on the aging intestinal barrier and immune system. Moreover, it is unknown whether the beneficial effects of probiotics are age dependent.
In this report, we have used an accelerated aging mouse model to evaluate the effects of candidate probiotics in aging. Based on a variety of histological, functional, metabolomic, and proteomic data, it has been concluded that Ercc1−/Δ7 mice resemble normal murine aging (22). Recently, we have shown that the immune system of Ercc1−/Δ7 mice resembles the immune system of aged WT mice. For instance, we showed a similar decrease in B cell precursors and naïve T cells, and a similar increase in memory T cells and regulatory T cells (23). The ERCC1 protein is involved in multiple DNA repair pathways. Ercc1−/Δ7 mice (median lifespan ~20 weeks) are deficient for fully functional ERCC1 protein. The expression of ERCC1-XPF (excision repair cross-complementation group 1-xeroderma pigmentosum group F) DNA repair endonuclease is reduced to ~5% compared with Ercc1+/+ mice. Moreover, the residual ERCC1-XPF protein present is expressed from a truncated allele, and lacks the last seven amino acids. A reduction of ERCC1 protein activity leads to increased accumulation of DNA damage and, hence, results in an accelerated aging phenotype (24, 25).
The aim of this study was to investigate the potential of supplementation with candidate probiotic strains to ameliorate the effects of aging on the intestinal barrier and the immune system. Previously, probiotic activity was documented for Lactobacillus plantarum WCFS1 (26–28), Lactobacillus casei BL23 (29, 30), and relatives of Bifidobacterium breve DSM20213 (31). We selected these strains on the basis of induced IL-10/TNF ratios in young and aged immune cells in vitro (32). The three strains can be classified as potential pro-inflammatory (L. plantarum), regulatory (L. casei), or anti-inflammatory (B. breve), based on low, intermediate, or high IL-10/TNF ratios, respectively.
For this study, we supplemented 6-week-old Ercc1+/+ mice and Ercc1−/Δ7 mice with L. plantarum, L. casei, or B. breve for 10 weeks. Mucus barrier, microbiota composition, and gene regulation in the colon were analyzed, as well as the distribution of immune cells in various mucosal and peripheral lymphoid organs. We determined immune competence by antigenic challenge.
Materials and Methods
Mice
The generation and characterization of Ercc1+/Δ7 and Ercc1−/+ mice has been previously described (25). Ercc1−/Δ7 mice were obtained by crossing Ercc1+/Δ7 with Ercc1−/+ mice of pure C57Bl6/J and FVB backgrounds to yield Ercc1−/Δ7 with an F1 C57Bl6J/FVB hybrid background. Genotyping was performed as described previously (33). Wild-type littermates (C57Bl6J/FVB F1) were used as controls. Four-month-old and 18-month-old C57Bl6/J mice were purchased from Harlan (Horst, The Netherlands; only used in Figure 1).
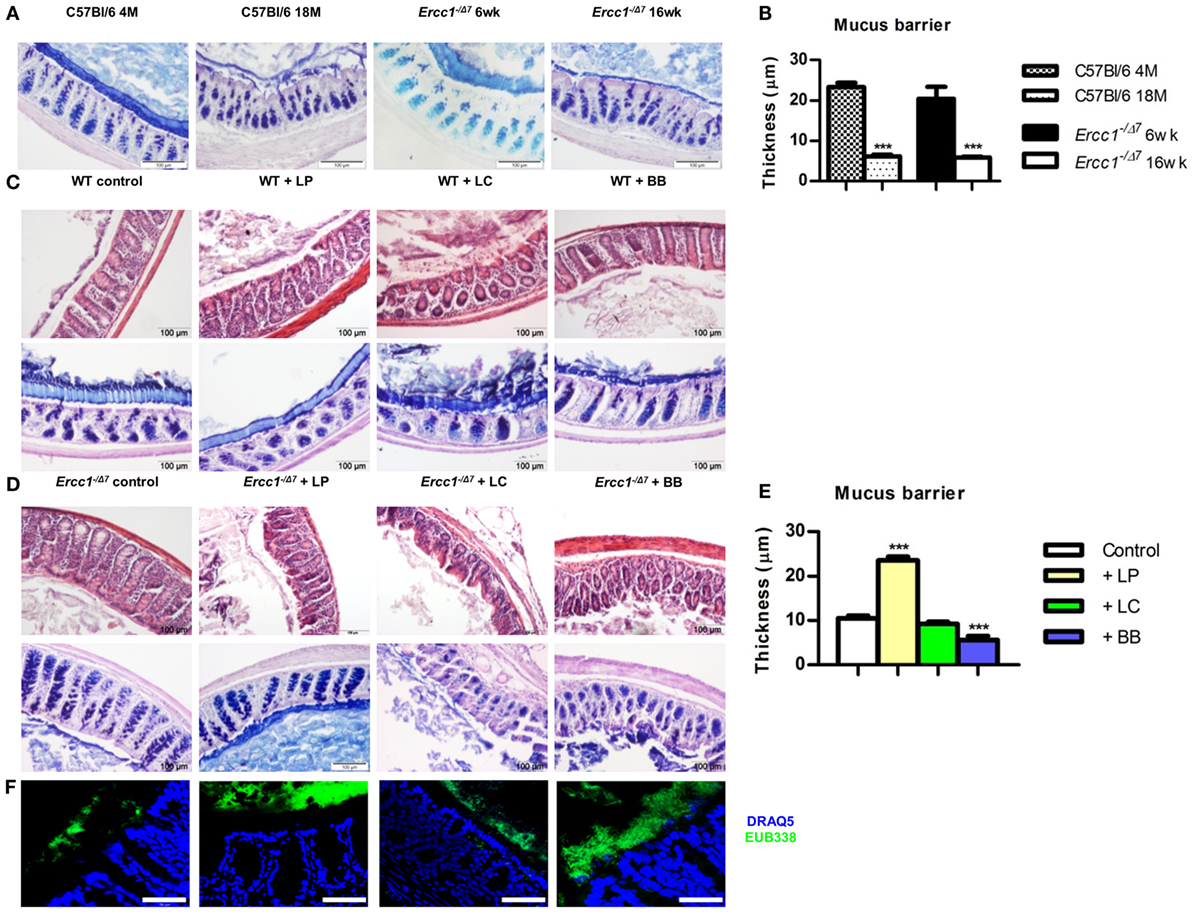
Figure 1. Treatment with L. plantarum prevented the age-related decline in colonic mucus barrier of accelerated aging Ercc1−/Δ7 mice. (A) Representative pictures of proximal colon stained with PAS/Alcian Blue of 4-month-old (4 M) or 18-month-old (18 M) WT mice and 6-week-old (6 weeks) or 16-week-old (16 weeks) Ercc1−/Δ7 mice. (B) Quantitative measurement of mucus thickness in young and old WT and Ercc1−/Δ mice by ImageJ. (C) Representative pictures of proximal colon stained with H&E or PAS/Alcian Blue of Ercc1+/+ mice supplemented with control treatment, L. plantarum WCFS1 (LP), L. casei BL23 (LC), or B. breve DSM20213 (BB). (D) Representative pictures of proximal colon stained with H&E and PAS/Alcian Blue of Ercc1−/Δ7 mice supplemented with control treatment, LP, LC, or BB. (E) Quantitative measurement of mucus thickness in Ercc1−/Δ7 mice by ImageJ. (F) Fluorescence in situ hybridization (FISH) of proximal colon from Ercc1−/Δ7 mice. Data represent the mean + SEM from four to six animals per group. ***p < 0.001. Scale bars histological pictures: 100 μm; scale bars FISH: 50 μm.
Animals were housed in individual ventilated cages under SPF conditions. Experiments were performed in accordance with the Principles of Laboratory Animal Care and with Dutch legislation. This study was carried out in accordance with the recommendations of the Dutch Ethical Committee of Wageningen that approved the work. Blood was taken from mice being sacrificed, and serum was frozen in −80°C for later use. After mice (n = 4–6) were sacrificed, feces from colon was collected and snap-frozen. Distal ileum and proximal colon sections were isolated and fixed in Carnoy or snap-frozen in liquid nitrogen. BM, thymus, spleen, mesenteric lymph nodes (MLN), and Peyer’s patches (PPs) were isolated.
Bacterial Cultures and Supplementation
L. plantarum WCFS1, L. casei BL23, and B. breve DSM20213 were grown on MRS medium (Merck, Darmstadt, Germany) until stationary phase, frozen in glycerol, and stored in −80°C until use. Upon use, bacteria were thawed and 10× diluted in NaHCO3/PBS buffer. Around 2 × 108 CFU in 200 μL were administered to mice by gavage, three times per week. Treatment of mice started at 6 weeks of age until 1 day before sacrifice at 16 weeks or until death.
Histology and Fluorescence In Situ Hybridization
Carnoy-fixed proximal colon sections were embedded in paraffin. Paraffin sections (5 μm) were attached to poly-l-lysine-coated glass slides (Thermo Scientific, Germany). After overnight incubation at 37°C, slides were de-waxed and rehydrated. Sections were stained with hematoxylin and eosin (H&E) and PAS/Alcian blue. Mucus layer thickness was measured using ImageJ software (NIH, MD, USA), as previously published (34). For detection of bacteria, tissue sections were used for fluorescence in situ hybridization (FISH), as previously published (8).
MIT-Chips/16S Sequencing
Microbiota composition in colonic content was analyzed by Mouse Intestinal Tract Chip (MITChip), as described previously (35). The data were normalized and analyzed using a set of R-based scripts in combination with a custom-designed relational database, which operates under the MySQL database management system. For the microbial profiling, the Robust Probabilistic Averaging signal intensities of 2667 specific probes for the 94 genus-level bacterial groups detected on the MITChip were used (36). Diversity calculations were performed using a microbiome R-script package (https://github.com/microbiome). Multivariate statistics, redundancy analysis (RDA), and principal response curves were performed in Canoco 5.0 and visualized in triplots or a principal response curves plot (37).
RNA Isolation and Transcriptome Analysis
Total RNA was isolated from proximal colon (n = 3–6 per group) using the RNeasy kit (Qiagen) with a DNase digestion step according to the manufacturer’s protocol. Transcriptome analysis on individual samples was performed as previously described (8). The gene expression datasets were deposited in NCBI’s Gene Expression Omnibus (GEO) and are accesible through GEO Series accession number: GSE87368.
General Flow Cytometry Procedures
Single-cell suspensions of BM were obtained by crushing femurs, tibias, iliac crests, and sternum with mortar and pestle. BM cells were then filtered on a 40-μm cell strainer. A proportion of the BM cells was frozen for later use in in vitro cultures. Spleen, MLN, PP, thymus, and peritoneal cavity single-cell suspensions were obtained by gently pushing cells through a 40-μm cell strainer with a syringe. All cells were stained for extracellular markers and dead cells were identified with fixable live/dead stain (Ebioscience, San Diego, CA, USA), after which intracellular staining was enabled by fixing and permeabilizing cells with Fix/Perm buffer (Ebioscience) according to manufacturer’s instructions. Antibodies used for flow cytometric measurements are listed in Supplementary Table 1 in Data Sheet 1. All flow cytometric measurements were performed on a Canto II flow cytometer (BD Biosciences, Erembodegem, Belgium). FlowJo vX.07 software (Tree Star) was used for data analysis. Gating of all presented immune cell populations was based on single live cells.
Spleen Cell Cultures
Splenic cells were cultured at 106 cells/mL for 4 days in the absence or presence of 5 μg/mL concanavalin A (ConA). Proliferation was measured by Ki-67 (Ebioscience). Supernatants were stored at −20°C. After thawing, levels of IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, and TNF were measured with the Cytometric Bead Array Th1/Th2/Th17 Kit (BD Biosciences), according to manufacturer’s instructions. Samples were acquired on a Canto II flow cytometer. Data were analyzed using FCAP Array version 3.0 (BD Biosciences) software.
Antibody Titers in Serum
Levels of IgM, IgG1, IgG2a, IgG2b, IgG3, IgE, and IgA were analyzed in serum using ProcartaPlex Mouse Antibody Isotyping Panel kit on the Luminex platform (Affymetrix, Santa Clara, CA, USA) according to the manufacturer’s instructions. Data were acquired on a BioPlex 200 (Bio-Rad, Hercules, CA, USA) and analyzed with BioPlex software (version 5.0, Bio-Rad).
In Vivo Immunization and Antibody Detection
Primary and secondary T cell-dependent (TD) immune responses against TNP-KLH were measured 7 days after primary i.p. immunization and 7 days after i.p. booster immunization. The primary immunization was performed at 8 weeks of age (TNP-KLH in alum), booster doses were injected at 12 weeks of age (TNP-KLH in PBS). Total and TNP-specific Ig subclasses were determined by sandwich ELISA as previously described (38).
Statistical Analysis
Values are expressed as mean + SEM. Normal distribution of the data was confirmed using the Kolmogorov–Smirnov test. Statistical comparisons were performed using the two-sided Student’s t-test. Where non-Gaussian distribution was demonstrated, we applied the non-parametric Mann–Whitney U test. Where no equal variances were observed, we applied the two-sided Student’s t-test with Welch’s correction. Statistical comparisons for lifespan data were performed using the log-rank (Mantel–Cox) test. Statistical comparisons for serum immunoglobulins were performed using two-way ANOVA, with subsequent Bonferroni posttests. Values of p < 0.05 were considered to be statistically significant. Values between p > 0.05 and p < 0.10 were considered as a trend.
Results
The Mucus Layer in the Colon Declines with Age
To assess the mucus barrier in normal and accelerated aging, we compared the proximal colon of 4-month-old (young) with 18-month-old (aged) C57Bl/6 mice, and of 6-week-old (young) with 16-week-old (aged) Ercc1−/Δ7 mice. We observed that in aged C57Bl/6 and Ercc1−/Δ7 mice, a thinner mucus layer was present, compared with young C57Bl/6 and Ercc1−/Δ7 mice (Figure 1A). With ImageJ, we measured the thickness of the mucus layer. In young C57Bl/6 and Ercc1−/Δ7 mice, a mucus layer of ~20 μm was present, whereas in normal and accelerated aged mice, a significantly thinner mucus layer of less than 10 μm was observed (p < 0.001; Figure 1B).
Bacterial Supplementations Do Not Change the Mucus Layer in Colon of Young WT Mice
To determine the effects of the three selected bacterial strains in the young intestine, we analyzed proximal colon tissues of WT mice that were treated with L. plantarum WCFS1, L. casei BL23, or B. breve DSM20213 for 10 weeks. No change in tissue integrity (H&E) or mucus layer (PAS/Alcian Blue) was observed in the colon after supplementation with bacterial strains (Figure 1C).
Age-Related Decline in the Mucus Barrier is Prevented by Supplementation of Ercc1−/Δ7 Mice with L. plantarum
Because the mucus layer declines with age, we questioned whether bacterial supplementation of Ercc1−/Δ7 mice prevents the decline in mucus barrier. Colon tissue of 10-week treated Ercc1−/Δ7 mice was checked for tissue integrity and mucus layer thickness. In contrast to our findings in WT mice, bacterial supplementation had significant effects on tissue integrity and the mucus layer. In Ercc1−/Δ7 mice supplemented with L. plantarum, the colon showed a thicker mucus layer than their controls (Figure 1D). L. plantarum supplementation completely prevented age-related decline in the mucus layer compared with controls (p < 0.001; Figure 1E), resulting in a mucus thickness comparable to young WT mice. Spatial compartmentalization of bacteria in the colon was improved after L. plantarum supplementation (Figure 1F), as demonstrated by FISH analyses. On the contrary, Ercc1−/Δ7 mice supplemented with L. casei or B. breve showed loss of tissue integrity (Figure 1D). No difference in mucus thickness was observed after supplementation with L. casei (Figure 1E). B. breve supplementation resulted in a deteriorated mucus layer and a loss in mucus thickness (p < 0.001; Figure 1E). B. breve supplementation also resulted in less spatial compartmentalization of bacteria in the colon of Ercc1−/Δ7 mice (Figure 1F).
Collectively, these data show that L. plantarum supplementation improves the mucus layer in the aged (but not young) colon. In addition, supplementation with L. casei or B. breve exacerbates the age-related decline of mucus barrier in the colon.
Bacterial Supplementation Associated with Minor Alterations in Colonic Microbiota Composition
As we introduced bacteria by bacterial supplementations into the intestinal microbial community, we investigate whether changes in the microbiota composition were underlying the observed changes in the mucus barrier of Ercc1−/Δ7 mice. Microbiota composition was determined by performing 16S rRNA gene microbiota profiles of colonic content. The bacterial supplementations did not significantly alter microbial diversity nor richness (data not shown).
Redundancy analysis showed that 10.1% of the total variability of the gut microbiota can be related to the bacterial supplementations (Figure 2). No statistical significance was established. The first ordination axis explained 4.9% of the variability and separated Ercc1−/Δ7 mice supplemented with either of the three bacterial strains from the control Ercc1−/Δ7 mice. The second ordination axis explained 3.6% of the variability but did not result in a separation between groups. The third ordination axis explained an additional 1.6% of the variability (data not shown).
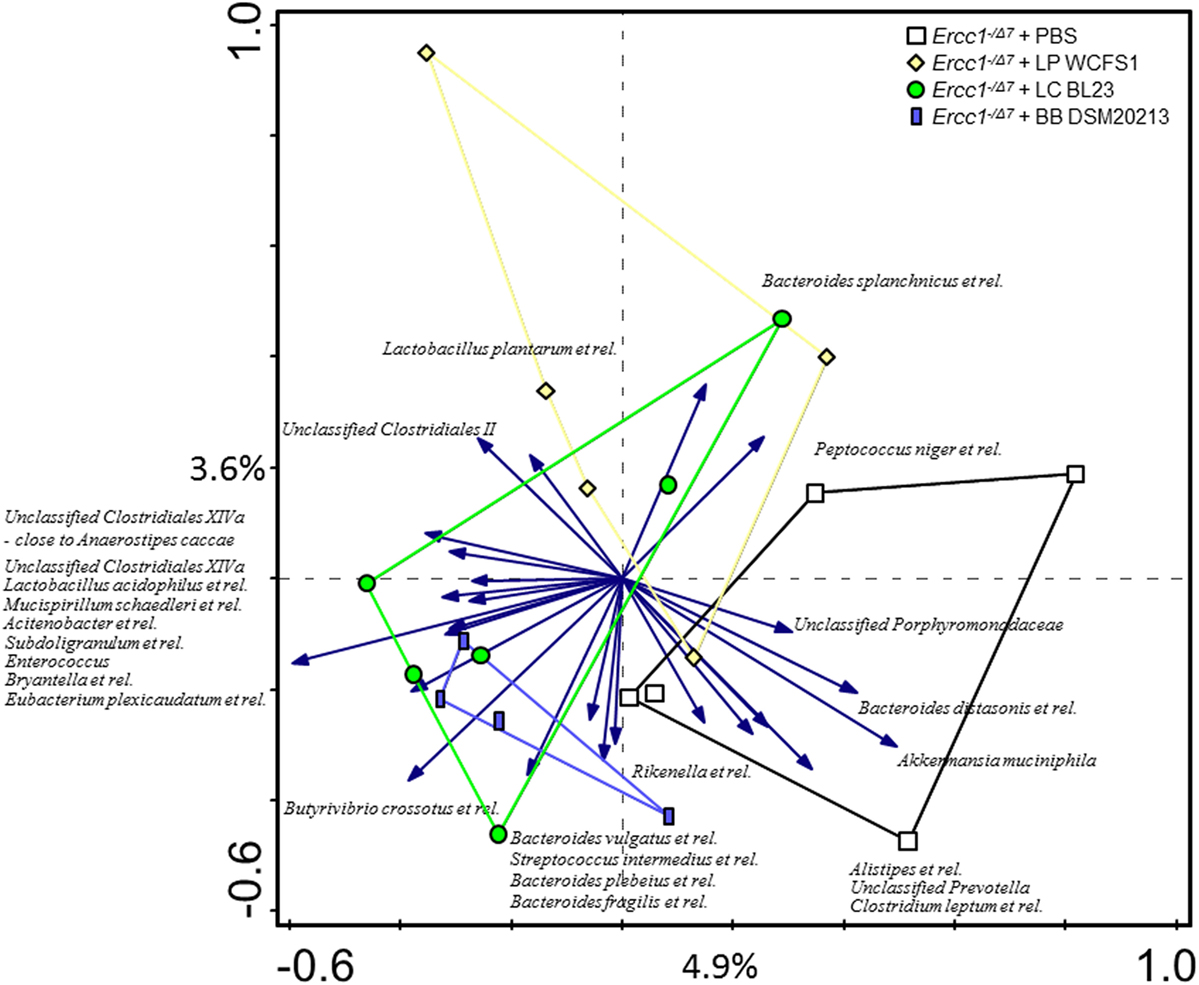
Figure 2. The effect of bacterial supplementations on colonic microbiota composition in Ercc1−/Δ7 mice. Redundancy analysis of the microbial composition after bacterial supplementations, on genus-like level of the MITChip analysis. Mice belonging to control-, LP-, LC-, and BB-treated groups are indicated by white squares, yellow diamonds, green circles, and blue rectangles, respectively. First and second ordination axes are plotted, showing 4.9 and 3.6% of the variability in the dataset, respectively. No significant changes were observed. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
To assess whether significant changes in the microbial genus-like bacterial groups existed after different bacterial supplementations in Ercc1−/Δ7 mice, we performed the Wilcoxon test. Subdoligranulum was higher in mice supplemented with L. casei (p < 0.05), whereas it tended to be higher in mice supplemented with B. breve (p = 0.05), as compared with control mice (Figure 3). Akkermansia muciniphila tended to be less present (p = 0.06) in mice supplemented with L. plantarum compared with control mice. Eubacterium plexicaudatum and a close relative to Anaerostipes caccae tended to be higher (p = 0.06) in Ercc1−/Δ7 mice supplemented with L. casei.
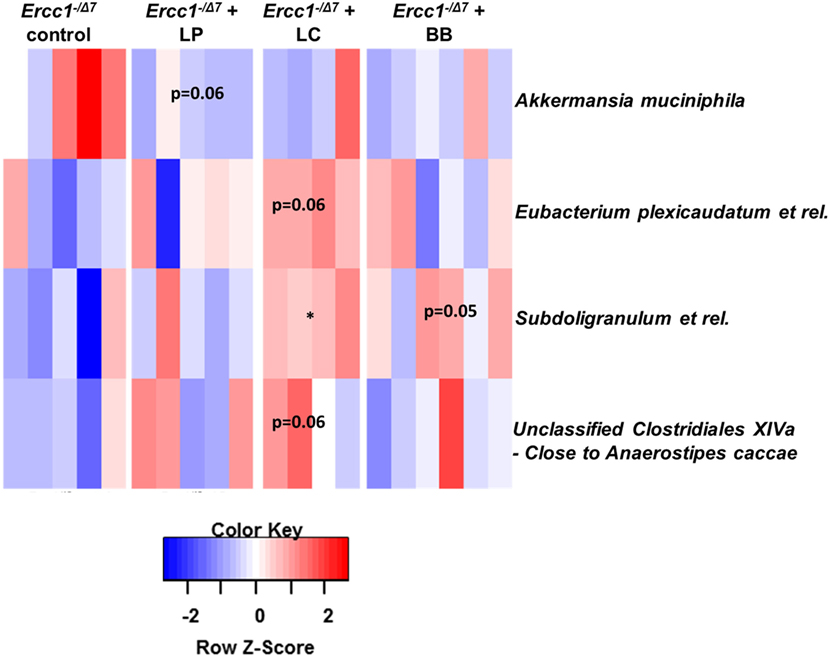
Figure 3. Bacterial supplementation induced changes in bacterial taxa in the colon of Ercc1−/Δ7 mice. Wilcoxon tests comparing mice treated with bacterial strains with control group. Data represent n = 4–6 mice per group. *p < 0.05.
These data demonstrate some differences in microbial species between control-treated Ercc1−/Δ7 mice and Ercc1−/Δ7 mice treated with bacterial supplementations.
Distinct Gene Expression Profiles in Colon after Each Bacterial Supplementation
To understand the mechanisms by which bacterial supplementation changes the mucus barrier, we performed transcriptome analysis on the proximal colon of Ercc1−/Δ7 mice. Gene expression microarrays on total proximal colon samples from Ercc1−/Δ7 mice treated with bacterial supplementations or control treatment revealed relatively low numbers of differentially expressed genes: 84 by L. plantarum, 238 by L. casei, and 384 by B. breve. Only a few genes were overlapping between two or three different bacterial supplementations, whereas most of the differentially expressed genes were distinctly regulated by one of the treatments (Figure 4).
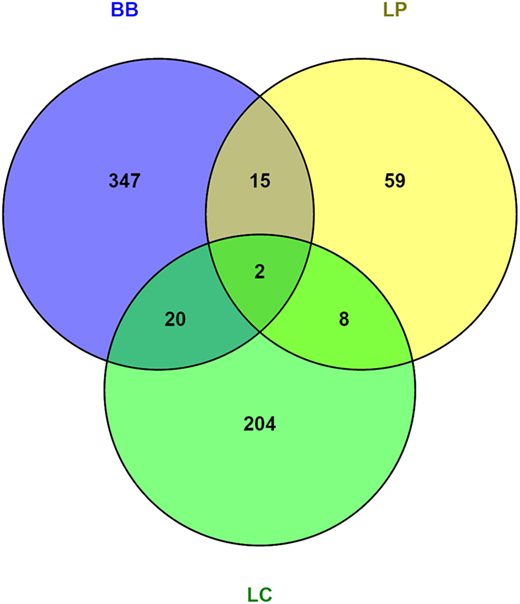
Figure 4. Venn diagrams of differentially regulated genes in colon of Ercc1−/Δ7 mice after bacterial supplementations. Total number of genes altered in the proximal colon of Ercc1−/Δ7 mice treated with L. plantarum WCFS1 (LP), L. casei BL23 (LC), or B. breve DSM20213 (BB) compared with control-treated Ercc1−/Δ7 mice. Venn diagram of the total number of genes upregulated and downregulated in the proximal colon of Ercc1−/Δ7 mice treated with LP, BB, or LC (p < 0.05 and >1.2-fold difference).
Several growth- and immune-related genes were differentially expressed after bacterial supplementation. Apolipoprotein (APO) A-1, APOA-4, suppressor of cytokine signaling (SOCS) 3, and toll-like receptor (TLR) 4 were upregulated more than 1.2-fold after L. plantarum supplementation (Data Sheet 2 in the Supplementary Material). Several immunoglobulin variable genes and TLR13 were upregulated after administration of L. casei, whereas defensin 40β was 1.3-fold downregulated. Defensin 24α, amphiregulin, and keratinocyte growth factor 7 (FGF7) were upregulated more than 1.4-fold after administration of B. breve, while TLR6, TLR7, and CCL3 (MIP-1α) were more than 1.2-fold downregulated (Data Sheet 2 in the Supplementary Material). Remarkably, we found no significant up- or downregulation in any mucin.
Bacterial Supplementation Alters Growth- and Immune-Related Pathways in Colon
Because we found relatively low numbers of differentially expressed genes, we applied a gene set enrichment analysis (GSEA) (39) to gain insight into the regulated pathways by bacterial supplementations. Upstream regulators that can explain the observed changes in gene expression were identified using Ingenuity Upstream Regulator Analysis.
Gene set enrichment analysis revealed that L. plantarum supplementation significantly enhanced several processes involved in growth and cell cycle, and immunity (Supplementary Table 2 in Data Sheet 1), such as “Type II Interferon Signaling,” “VIP pathway,” and “IL8/CXCR1 pathway.” Interestingly, in the top-10 of upregulated pathways, three pathways were linked to DNA repair: the “Fanconi Pathway,” “ATRBRCA pathway,” and the “Fanconi anemia pathway.” Several growth factors were activated after L. plantarum supplementation: leptin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF) BB, early growth response protein (EGR) 1, and insulin-like growth factor (IGF) 1 (Table 1). Inflammatory cytokines (IFN-γ, IL-1β, IL-4, TNF) and CD40L (CD154) were activated in colon of mice supplemented with L. plantarum, compared with colon of mice supplemented with control treatment.
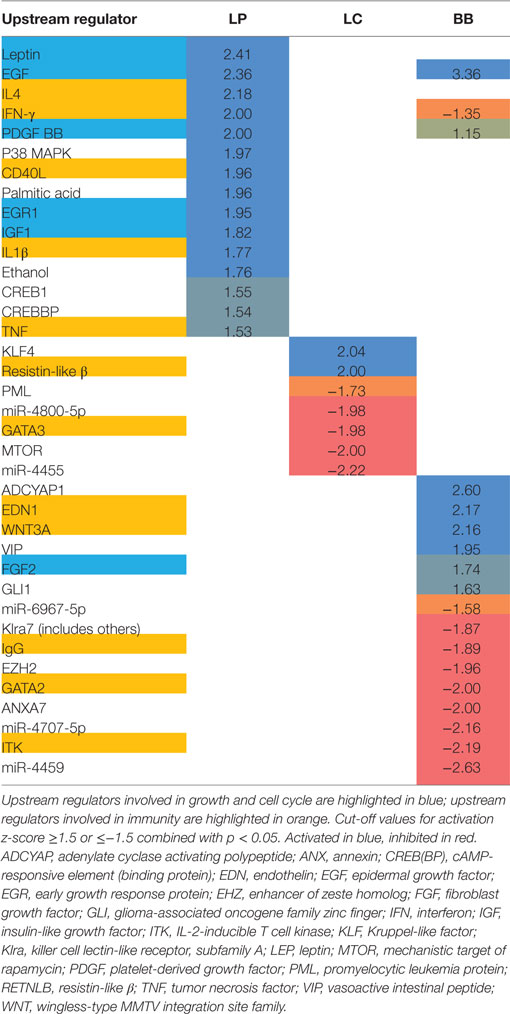
Table 1. Activation z-scores of upstream regulators in proximal colon of Ercc1−/Δ7 mice after bacterial supplementations L. plantarum WCFS1 (LP), L. casei BL23 (LC), or B. breve DSM20213 (BB) as determined by Ingenuity.
Lactobacillus casei supplementation enhanced several processes involved in growth and cell cycle, like “Mitotic G1-G1 S Phases,” “DNA replication,” “Synthesis of DNA,” and “G1 S transition” (Supplementary Table 2 in Data Sheet 1). In addition, the “NOD-like receptor signaling pathway” was enhanced after L. casei supplementation, as well as and the “Unfolded protein response” (UPR), indicated endoplasmatic reticulum (ER) stress. Upstream regulators resistin-like β (RTNLB; activated) and GATA3 (inhibited) were regulated in the colon of mice supplemented with L. casei (Table 1).
Several metabolic pathways were enhanced in colon of B. breve-supplemented mice (Supplementary Table 2 in Data Sheet 1). Of note, “Protein folding” was upregulated. In contrast to L. plantarum and L. casei supplementation, B. breve supplementation significantly inhibited several processes involved in immunity, such as “IL2 STAT5 pathway,” “Immunoregulatory interactions between lymphoid/non-lymphoid cells,” “Type II Interferon signaling,” “IL4 2 pathway,” and “IL6 7 pathway” (Supplementary Table 3 in Data Sheet 1). B. breve supplementation activated EGF and inhibited fibroblast growth factor (FGF) 2. IgG, GATA2, and IL-2-inducible T cell kinase (ITK) were inhibited in colon of mice supplemented with B. breve. In line with GSEA, IFN-γ was inhibited as well after B. breve supplementation.
These data indicate that immune pathways in the colon are enhanced by L. plantarum and L. casei, but are inhibited by B. breve supplementation.
L. plantarum and L. casei Supplementation Induce Regulatory T Cells in MLN
Based on the regulation of immune genes by bacterial supplementations, we tested whether the distribution of immune cells was altered in mucosal immune organs of Ercc1−/Δ7 mice.
First, we evaluated changes in distribution of immune cells in PPs and MLN. B cell frequencies were reduced in PP and MLN (p < 0.05) after L. casei supplementation in Ercc1−/Δ7 mice (Figure 5A). By contrast, frequencies of T cells were increased in PP (p < 0.01) and MLN (p < 0.05; Figure 5B). The frequencies of regulatory T (Treg) cells in MLN were increased after L. plantarum and L. casei supplementation (p < 0.05; Figures 5C,D). No changes in distribution of B and T cells were observed upon bacterial supplementation in WT mice, except for a tendency to decreased Treg cells after L. casei supplementation (p = 0.09; Supplementary Figure 1 in Data Sheet 1).
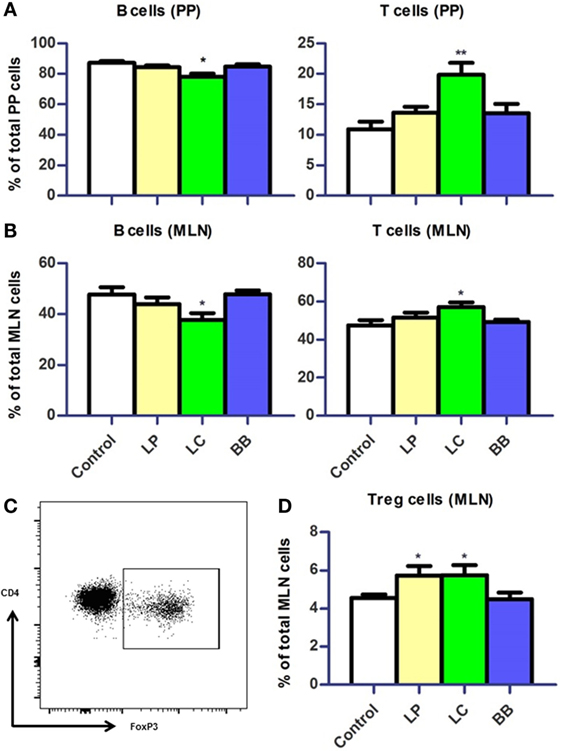
Figure 5. Distribution of B cells and T cells in Peyer’s patches (PP) and mesenteric lymph nodes (MLN) upon bacterial supplementation in Ercc1−/Δ7 mice. (A,B) Mean frequencies of B and T cells in PP and MLN were determined by flow cytometry. B cells were defined as CD19+, T cells were defined as CD3+. (C) Flow cytometric analysis of CD3+CD4+CD8− regulatory T (Treg) cells in MLN. (D) Mean frequencies of Treg cells in MLN. Data represent the mean + SEM from four to six animals per group. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213. *p < 0.05; **p < 0.01.
L. casei Elevates Systemic Inflammatory Markers
Next, we assessed distribution of immune cells in the spleen. We noted that the relative spleen weight increased after L. casei supplementation in Ercc1−/Δ7 mice (Supplementary Figure 2A in Data Sheet 1). Absolute numbers of spleen cells were not affected by bacterial supplementations (data not shown). Splenic B cell frequencies tended to be decreased after L. casei supplementation (p = 0.06; Figure 6A), but no changes in T cell frequencies were observed (Figure 6B). Treg cell frequencies in the spleen were increased after L. casei supplementation in Ercc1−/Δ7 mice (p < 0.05; Figure 6C).
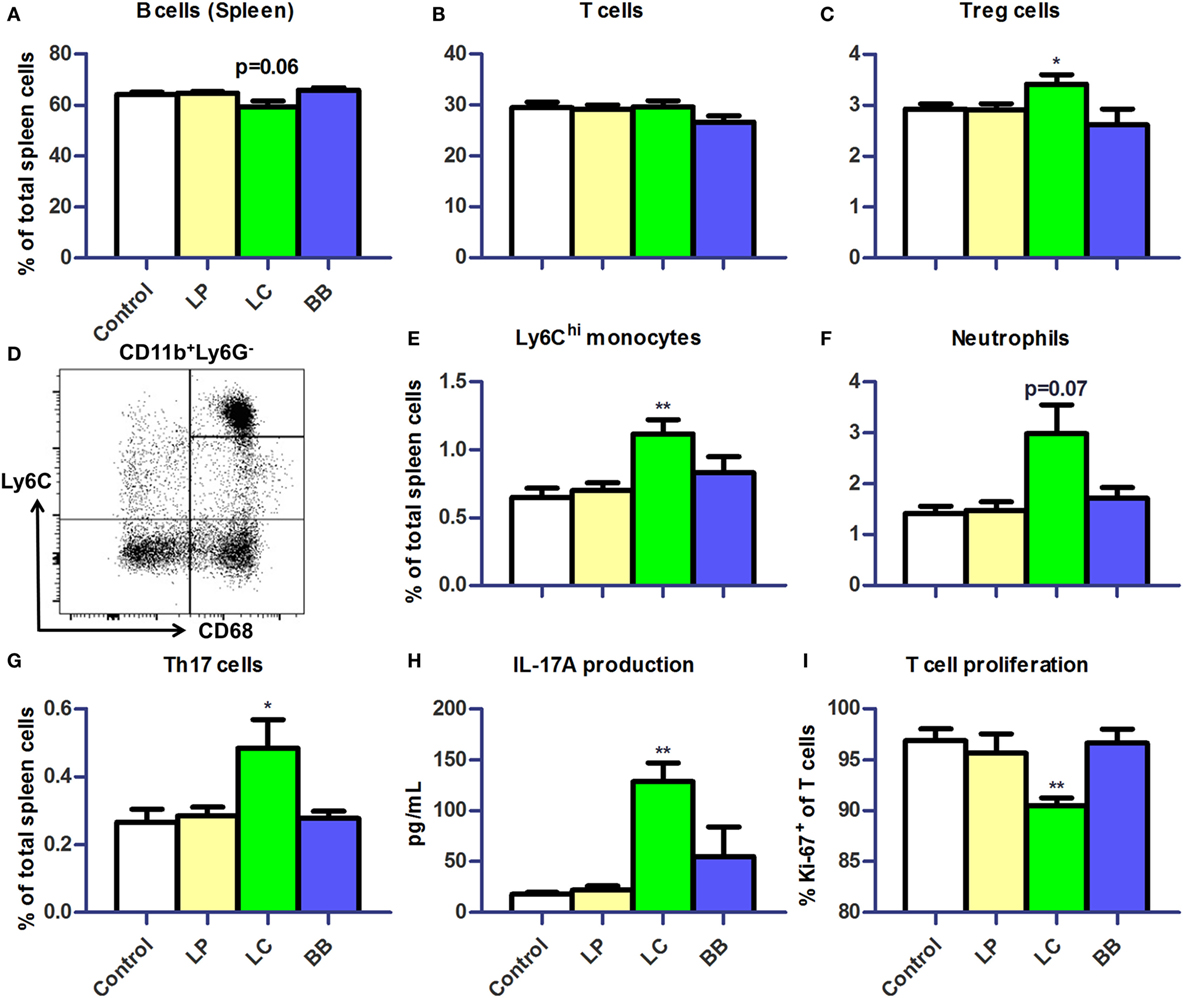
Figure 6. L. casei supplementation of Ercc1−/Δ7 mice raised inflammatory markers in spleen. (A,B) Mean frequencies of B and T cells in spleen were determined by flow cytometry. B cells were defined as CD19+, T cells were defined as CD3+. (C) Mean frequencies of Treg cells in spleen. (D) Flow cytometric analysis of splenic monocytes. CD11b+Ly6G−CD68+ cells were divided in Ly6Chi, Ly6Cint, and Ly6Clo monocytes. (E–G) Mean frequencies of Ly6Chi monocytes, neutrophils, and CD3+CD4+CD8−Rorγt+ Th17 cells were determined by flow cytometry. (H) Mean concentration of IL-17A production by splenocytes stimulated with ConA for 4 days, as determined by Cytometric Bead Array. (I) Mean proliferating T cells (Ki-67+) in splenocyte culture stimulated with ConA for 4 days, as determined by flow cytometry. Data represent the mean + SEM from four to six animals per group. *p < 0.05; **p < 0.01. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
Increased frequencies of CD11b+Ly6G−CD68+Ly6Chi monocytes (p < 0.01; Figures 6D,E) and a tendency to increased frequencies of CD11b+CD68intLy6CintLy6G+ neutrophils were observed after L. casei supplementation (p = 0.07; Figure 6F). In addition, the proportions of CD3+CD4+RORγt+ Th17 cells (Supplementary Figure 2B in Data Sheet 1) were increased after L. casei supplementation (p < 0.05; Figure 6G). A 4-day culture of splenocytes stimulated with concanavalin A (ConA), also showed increased IL-17A production (p < 0.01; Figure 6H) and a decreased T cell proliferation in splenocytes derived from L. casei-treated mice (p < 0.01; Figure 6I). None of these changes were observed in Ercc1−/Δ7 mice treated with L. plantarum or B. breve, and in WT mice treated with each of the bacterial supplementations (Supplementary Figure 3 in Data Sheet 1). These data suggest that L. casei, in contrast to L. plantarum and B. breve, raises several inflammatory markers in Ercc1−/Δ7 mice.
Lymphocyte and Myeloid Development Affected after L. plantarum or L. casei Supplementation
We subsequently investigated the development of B cells and myeloid cells in BM and of T cells in thymus of Ercc1−/Δ7 mice, as the observed changes in cell distribution in PP, MLN, and spleen might be explained by an altered migration or production. Absolute numbers in the BM were unchanged after bacterial supplementation (data not shown). In the BM, we observed significantly higher Lin−CD117hiCD11c−CD135−CD16/32+ granulocyte–monocyte precursor (GMP), CD11b+Ly6G+ neutrophil, and Ly6ChiCD31− monocyte frequencies after L. casei supplementation (Figures 7A–C). Frequencies of total CD19+CD45R+ B-lineage cells were decreased after L. plantarum (p < 0.05) and L. casei supplementation (p < 0.001), but not after B. breve supplementation (Figure 7D). We observed a reduction in all B-lineage subsets, except pro-B cells, after L. casei and L. plantarum supplementation (data not shown). In thymus, only L. casei supplementation caused changes in cell distribution, with significantly reduced CD3−CD4+CD8+ double-positive (DP) cell numbers (Figures 7E–H).
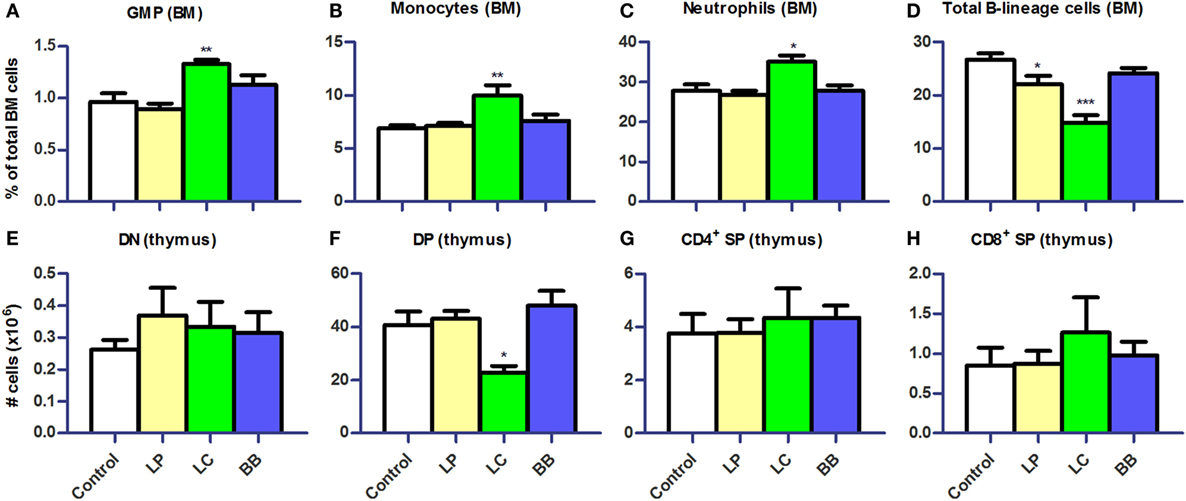
Figure 7. L. casei or L. plantarum supplementation altered myeloid and lymphoid development in bone marrow and thymus of Ercc1−/Δ7 mice. (A–D) Mean frequencies in bone marrow (BM) were determined by flow cytometry. Granulocyte–monocyte precursors (GMP) were defined as Lin−CD117hiCD11c−CD135−CD16/32+, neutrophils as CD11b+Ly6G+, monocytes as Ly6ChiCD31−, and B-lineage cells as CD19+CD45R+. (E–H) Mean absolute numbers were determined by cell counts and flow cytometry. Double-negative (DN) cells were defined as CD3−CD4−CD8−, double-positive (DP) cells as CD3−CD4+CD8+, CD4+ single-positive (SP) as CD3+CD4+CD8−, and CD8+ SP as CD3+CD4−CD8+. Data represent the mean + SEM from four to six animals per group. *p < 0.05; **p < 0.01; ***p < 0.001. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
In WT mice, we found no effect of bacterial supplementations on distribution of immune cells in BM or thymus, except for B-lineage cells after L. plantarum and L. casei supplementation (Supplementary Figure 4 in Data Sheet 1).
Bacterial Supplementations Do Not Alter Lifespan of Ercc1−/Δ7 Mice
The accelerated aging of Ercc1−/Δ7 mice enabled us to assess the potential life-extending properties of the bacterial strains. No significant change in lifespan of Ercc1−/Δ7 mice was observed after lifelong supplementation with L. plantarum or L. casei (Supplementary Figure 5 in Data Sheet 1).
L. casei Supplementation Increases IgG Serum Titers
Because L. casei supplementation led to decreased B cell proportions in several immune organs of Ercc1−/Δ7 mice, we tested whether serum antibody titers in Ercc1−/Δ7 mice were altered. Total IgG1 and IgG2b (but not IgG2a, IgG3, IgE, and IgA) titers were significantly increased after L. casei supplementation (Figure 8). L. plantarum and B. breve supplementation did not significantly change titers of any Ig subclass.
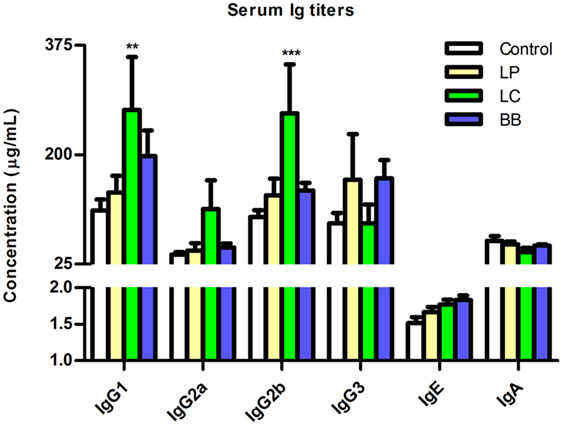
Figure 8. L. casei supplementation increased IgG1 and IgG2b titers in Ercc1−/Δ7 mice. Mean titers of IgG1, IgG2a, IgG2b, IgG3, IgE, and IgA in serum, as determined by Luminex. Data represent the mean + SEM from three to six animals per group. **p < 0.01; ***p < 0.001. LP = L. plantarum WCFS1; LC = L. casei BL23; BB = B. breve DSM20213.
Immune Competence Improved by L. casei and L. plantarum Supplementation
To test whether changes in immune cell distribution also impact immune competence, we analyzed the B cell response of Ercc1−/Δ7 mice to the T cell-dependent antigen TNP-KLH. Specific anti-TNP-KLH Ig titers of the three tested isotype classes (IgM, IgG1, IgG2a) after primary and booster immunization were consistently higher after L. plantarum and L. casei supplementation (Figure 9). In particular, IgG1 titers after booster immunization increased in both L. plantarum- and L. casei-supplemented mice compared with control-treated mice (p < 0.001).
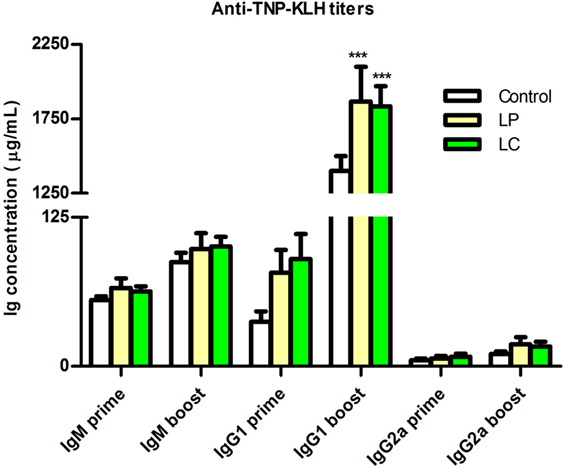
Figure 9. Supplementation of L. plantarum and L. casei increased specific anti-TNP-KLH antibody responses of Ercc1−/Δ7 mice. Mean TNP-specific IgM, IgG1, and IgG2a concentrations in serum were determined by ELISA, 7 days after primary immunization (prime, age 9 weeks), or 7 days after booster immunization (boost, age 13 weeks). Data represent the mean + SEM of 6–12 animals per group. ***p < 0.001. LP, L. plantarum WCFS1; LC, L. casei BL23.
From these findings, we conclude that L. plantarum and L. casei enhance T cell-dependent B cell responses in Ercc1−/Δ7 mice.
Discussion
The effects of bacterial supplementations on the intestinal barrier and cellular parameters of immunity were studied in fast aging Ercc1−/Δ7 mice. We observed that the mucus layer in the colon declines with age and that bacterial supplementation may prevent or exacerbate the age-related decline in the mucus layer, dependent on the specific bacterial strain. Additionally, we demonstrated a marked difference in the response to bacterial supplementations between Ercc1−/Δ7 mice and WT mice. Finally, supplementation with L. casei BL23 profoundly changed the distribution of immune cells and supplementation with L. plantarum WCFS1 or L. casei BL23 improved immune competence in Ercc1−/Δ7 mice.
Recently, we showed the age-related decline in mucus barrier of C57Bl/6 mice as well (Sovran et al., unpublished data). Importantly, we report that the mucus barrier declines with age, in aged C57Bl/6 and Ercc1−/Δ7 mice (Figure 1). This finding adds another age-related phenotype to the wide spectrum of age-related phenotypes observed in Ercc1−/Δ7 mice (40). Moreover, we report that the age-related decline in mucus barrier can be modulated by bacterial supplementation. L. plantarum prevented the decline in mucus barrier. L. plantarum is able to bind to mucus with a mannose-specific adhesin, which is described as a potential probiotic feature (41). In total, L. plantarum harbors four mucus-binding proteins (42). Based on the improved spatial compartmentalization of bacteria after L. plantarum supplementation, we postulate that L. plantarum adheres to the mucus. In addition, we found that L. plantarum supplementation tended to decrease the abundance of Akkermansia muciniphila (Figure 3), which is known as a mucus degrader (43). Thus, it would be conceivable that mucus degradation is decreased after L. plantarum supplementation. By contrast, B. breve is known as a mucus degrader (44), and could, therefore, be directly responsible for the decrease in mucus thickness in the colon of B. breve-treated mice. Interestingly, several pathways involved in protein folding and the UPR were upregulated after L. casei and B. breve supplementation (Data Sheet 2 in the Supplementary Material). A high demand for synthesis of secretory proteins (such as mucins) induces ER stress, which in turn induces the UPR (45). The close proximity of bacteria to the epithelium in L. casei- and B. breve-treated mice might induce a high demand for mucin production and secretion, leading to induction of ER stress and UPR. There is indeed evidence that defects in MUC2 mucin and a subsequent defective mucus layer lead to ER stress and UPR (46).
Microbiota profiling showed that only few microbial species are slightly altered by bacterial supplementation (Figures 2 and 3). Therefore, most of the observed effects in the mucus barrier and immune system may be directly linked to the supplementation of each of the bacterial strains.
We found that the different bacterial strains elicited characteristically different responses in gene regulation in the colon (Figure 4). L. plantarum is known for its moderately pro-inflammatory profile, and relatively high IL-10 induction, when tested in human PBMC cultures (28, 47). In line with these studies, several upstream regulators predicted to be activated after L. plantarum supplementation included the inflammatory cytokines IFN-γ, IL-1β, IL-4, and TNF. The association between increased activation of inflammatory cytokines and the improved integrity of the colon after L. plantarum supplementation raises the possibility that it might be beneficial to locally increase inflammatory cytokine levels. This suggestion is corroborated by the absence of activation of these inflammatory cytokines after L. casei or B. breve supplementation, which did not improve or exacerbate the age-related decline in mucus integrity. A “tonic” level of constitutive TLR activation by commensal bacteria was previously shown to be crucial in the recovery from DSS-induced epithelial damage due to the role of NF-κB in epithelial repair processes (48). This notion that “physiological pro-inflammatory signals” is required for intestinal homeostasis is also supported by studies using epithelium-specific iκB kinase-γ (or NEMO) ablation in mice. These mice develop spontaneous colitis due to the failure of NF-κB to induce epithelial repair and steady-state production of innate effector mechanisms in the intestine (49). TLR2 signaling has been implicated in tight junction regulation in vivo and in vitro (13). Thus, it is possible that aged mice have sub-optimal level of TLR stimulation in the intestine to promote innate barrier defenses and that this is enhanced by L. plantarum, but not by L. casei and B. breve.
Remarkably, none of the significantly regulated genes were directly linked to mucus production. However, while performing Upstream Regulator Analysis, growth factors, such as EGF, IGF1, and EGR1, were predicted to be activated after L. plantarum supplementation. Together, these findings may indicate that mucus production by goblet cells is not directly enhanced, but is part of general epithelial integrity, supported by a number of growth factors.
Because many regulated genes involved immune-related genes, we additionally analyzed the makeup of the immune system after bacterial supplementation. Whereas supplementation with B. breve exacerbated the age-related decline in mucus barrier in colon, it did not cause any changes in mucosal or systemic immunity (Figures 5–8). Oppositely, L. casei supplementation caused various signs of inflammation, such as Ly6Chi monocyte and neutrophil influx and production in spleen and BM, respectively. These inflammatory signs were coincided with the general decrease in B cell frequencies (also in the BM) and double-positive thymocytes. There is evidence that neutrophils in the BM are primed by microbial ligands (50). The effects of microbiota-derived signals on priming B and T cells in the BM have not been previously described. Our study suggests an, up to now, unknown link between microbiota, intestinal barrier, and B and T cell precursors. Specific precursor stages (i.e., small resting pre-B cells) were significantly decreased after L. casei supplementation, and to a lesser extent after L. plantarum supplementation. In the case of L. plantarum supplementation, we suggest that improved intestinal barrier function might alter circulating microbiota-derived products, such as peptidoglycan (PGN) and lipopolysaccharide (LPS). For instance, hematopoietic stem cells are damaged after chronic exposure to LPS (51). Interestingly, the decrease in small resting pre-B cells after L. casei supplementation (and to lesser extent by L. plantarum) was the only finding that could be reproduced in WT mice supplemented with these bacterial strains (Supplementary Figure 4 in Data Sheet 1). This may indicate that the effect of L. casei and L. plantarum supplementation on B cell development is independent of age.
A previous study showed lifespan extension after B. animalis supplementation (21). Therefore, we performed a lifespan study for L. plantarum and L. casei, which indicated that neither of them is shortening or extending lifespan (Supplementary Figure 5 in Data Sheet 1).
Surprisingly, anti-TNP-KLH IgG1 titers in serum increased not only after L. plantarum but also after L. casei supplementation (Figure 9). This increase suggests that a demise in B cell development and B cell distribution does not necessarily translate into impaired B cell function. Previously, it has been shown that antigen-specific antibody titers can be enhanced by probiotic supplementation in aged mice (20), but data on B cell development are lacking.
The effects of the candidate probiotic strains were pronounced on the mucus barrier in the colon of Ercc1−/Δ7 mice compared with WT mice. It has been shown in previous studies that strains, such as L. casei and B. breve, have beneficial effects on immunological parameters and intestinal barrier function in young mice (29–31). In our hands, L. casei and B. breve had no effect on mucus barrier or systemic immunity in young WT mice (except for the above-discussed finding on B cell development). A severe deteriorating effect, however, was observed on the mucus barrier or systemic immunity in Ercc1−/Δ7 mice. These findings highlight the need for caution in translating beneficial effects of probiotics observed in young animals or humans to the elderly.
Our study has a number of limitations. We observed remarkable changes in the mucus layer, but could not pinpoint a single gene that is directly linked to the mucus layer. Furthermore, we did not include commercially available probiotic bacterial strains, such as Lactobacillus rhamnosus GG, or a non-probiotic bacterial strain. Nevertheless, our study reveals a previously unknown effect of age on the mucus barrier. We also show that it is possible to modulate this age-related decline in the mucus barrier by supplementation of bacterial strains, with coinciding effects on systemic immunity. More research is warranted to elucidate the interplay between bacteria, the aged gut epithelium, and the immune system.
Our data provide evidence that a comprehensive analysis of the intestinal barrier and immunity are needed in order to evaluate how bacterial supplementation contributes to the restoration of the age-related decline in intestinal barrier. A positive finding was that probiotic strains, such as L. plantarum, might contribute to maintenance of intestinal integrity by preventing age-related deterioration of the colonic mucus layer.
Author Contributions
AB, BS, JHH, WV, PV, JW, PL, CN, RH, and HS conceived the study. AB, BS, FH, BM, CB, JAH, VM, CP, and MB performed the experiments. AB wrote the manuscript. BS, FH, BM, CB, JAH, JHH, WV, PV, JW, PL, CN, RH, and HS contributed to the revisions of the draft manuscripts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SS and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
Authors thank Steven Aalvink, Marjolein de Jong-de Bruijn, and Jenny Jansen for technical help. We acknowledge the excellent help of all animal caretakers.
Funding
This work was funded by TI Food and Nutrition, a public-private partnership on precompetitive research in food and nutrition. The public partners are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The private partners have contributed to the project through regular discussion.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2016.00408
Data Sheet 1.
Supplementary Table 1. Used antibodies in flow cytometry.
Supplementary Table 2. Top-10 biological processes upregulated (as determined with GSEA) by bacterial supplementations in proximal colon of Ercc1−/Δ7 mice treated with L. plantarum WCFS1 (LP), L. casei BL23 (LC), or B. breve DSM20213 (BB).
Supplementary Table 3. Top-10 biological processes downregulated (as determined with GSEA) by bacterial supplementations in proximal colon of Ercc1−/Δ7 mice treated with L. plantarum WCFS1 (LP), L. casei BL23 (LC), or B. breve DSM20213 (BB).
Supplementary Figure 1. Distribution of B cells and T cells in Peyer’s patches and mesenteric lymph nodes not changed upon bacterial supplementation in Ercc1+/+ mice. (A/B) Mean frequencies were determined by flow cytometry. B cells were defined as CD19+, T cells were defined as CD3+. (C) Mean frequencies of CD3+CD4+CD8−FoxP3+ regulatory T (Treg) cells in MLN. Data represent the mean + SEM from four to six animals per group. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
Supplementary Figure 2. Increased relative spleen weight after L. casei supplementation of Ercc1−/Δ7 mice. (A) Spleen weights relative to body weight. Data represent mean spleen weights + SEM of four to six animals per group. (B) Flow cytometric analysis of splenic Th17 cells. CD3+CD4+CD8− cells were gated for RORγt and FSC (forward scatter).
Supplementary Figure 3. Bacterial supplementation of Ercc1+/+ mice did not change splenic parameters. (A) Mean frequencies of Treg cells in spleen. (B–D) Mean frequencies of Ly6Chi monocytes, neutrophils, and CD3+CD4+CD8−Rorγt+ Th17 cells were determined by flow cytometry. (E) Mean concentration of IL-17A production by splenocytes stimulated with ConA for 4 days, as determined by Cytometric Bead Array. (F) Mean proliferating T cells (Ki-67+) in splenocyte culture stimulated with ConA for 4 days, as determined by flow cytometry. Data represent the mean + SEM from four to six animals per group. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
Supplementary Figure 4. L. casei supplementation altered B cell development in bone marrow of Ercc1+/+ mice. (A–E) Mean frequencies in bone marrow (BM) were determined by flow cytometry. Granulocyte–monocyte precursors (GMP) were defined as Lin−CD117hiCD11c−CD135−CD16/32+, neutrophils as CD11b+Ly6G+, monocytes as Ly6ChiCD31−, B-lineage cells as CD19+CD45R+, and small resting pre-B cells as sIgκ/λ−cIgM+CD2+. (F) Mean absolute numbers were determined by cell counts and flow cytometry. Double-positive (DP) cells were defined as CD3−CD4+CD8+. Data represent the mean + SEM from four to six animals per group. *p<0.05. LP, L. plantarum WCFS1; LC, L. casei BL23; BB, B. breve DSM20213.
Supplementary Figure 5. Bacterial supplementations did not change lifespan of Ercc1−/Δ7 mice. Data represent 11–12 animals per group (with an additional 6 animals per group censored at 16 weeks). LP, L. plantarum WCFS1; LC, L. casei BL23.
Data Sheet 2. Total file with differentially expressed genes after bacterial supplementations.
References
1. CDC. The State of Aging and Health in America 2013. Atlanta, GA: (2013). Available from: http://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf
2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2013) 380(9859):2095–128. doi:10.1016/S0140-6736(12)61728-0
3. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell (2013) 153(6):1194–217. doi:10.1016/j.cell.2013.05.039
4. Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol (2014) 289(1):112–8. doi:10.1016/j.cellimm.2014.04.001
5. Johansson ME, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci (2011) 68(22):3635–41. doi:10.1007/s00018-011-0822-3
6. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science (2013) 342(6157):447–53. doi:10.1126/science.1237910
7. Johansson ME, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc Natl Acad Sci U S A (2011) 108(Suppl 1):4659–65. doi:10.1073/pnas.1006451107
8. Sovran B, Loonen LM, Lu P, Hugenholtz F, Belzer C, Stolte EH, et al. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm Bowel Dis (2015) 21(3):531–42. doi:10.1097/MIB.0000000000000319
9. Franceschi C, Bonafè M, Valensin S, Olivieri F, de Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci (2000) 908(1):244–54. doi:10.1111/j.1749-6632.2000.tb06651.x
10. Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol (2006) 176(2):1007–12. doi:10.4049/jimmunol.176.2.1007
11. George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today (1996) 17(6):267–72. doi:10.1016/0167-5699(96)80543-3
12. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol (2016) 83(4):255–66. doi:10.1111/sji.12413
13. Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer R-JM, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol (2010) 298(6):G851–9. doi:10.1152/ajpgi.00327.2009
14. Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract (2009) 24(1):10–4. doi:10.1177/0884533608329231
15. Akatsu H, Iwabuchi N, Xiao J-Z, Matsuyama Z, Kurihara R, Okuda K, et al. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J Parenter Enteral Nutr (2012) 37(5):631–40. doi:10.1177/0148607112467819
16. Spaiser SJ, Culpepper T, Nieves C Jr, Ukhanova M, Mai V, Percival SS, et al. Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J Am Coll Nutr (2015) 34(6):459–69. doi:10.1080/07315724.2014.983249
17. Rampelli S, Candela M, Severgnini M, Biagi E, Turroni S, Roselli M, et al. A probiotics-containing biscuit modulates the intestinal microbiota in the elderly. J Nutr Health Aging (2013) 17(2):166–72. doi:10.1007/s12603-012-0372-x
18. Gill H, Rutherfurd K, Cross M. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol (2001) 21(4):264–71. doi:10.1023/A:1010979225018
19. Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr (2001) 74(6):833–9.
20. Vidal K, Benyacoub J, Moser M, Sanchez-Garcia J, Serrant P, Segura-Roggero I, et al. Effect of Lactobacillus paracasei NCC2461 on antigen-specific T-cell mediated immune responses in aged mice. Rejuvenation Res (2008) 11(5):957–64. doi:10.1089/rej.2008.0780
21. Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One (2011) 6(8):e23652. doi:10.1371/journal.pone.0023652
22. Gurkar AU, Niedernhofer LJ. Comparison of mice with accelerated aging caused by distinct mechanisms. Exp Gerontol (2015) 68:43–50. doi:10.1016/j.exger.2015.01.045
23. van Beek AA, Hugenholtz F, Meijer B, Sovran B, Perdijk O, Vermeij WP, et al. Tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1−/Δ7 mice. J Leukoc Biol (2016). doi:10.1189/jlb.1HI0216-062RR
24. Dollé ME, Kuiper RV, Roodbergen M, Robinson J, de Vlugt S, Wijnhoven SW, et al. Broad segmental progeroid changes in short-lived Ercc1−/Δ7 mice. Pathobiol Aging Age Relat Dis (2011) 1:7219. doi:10.3402/pba.v1i0.7219
25. Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers C, et al. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol (1997) 7(6):427–39. doi:10.1016/S0960-9822(06)00190-4
26. Ivanovic N, Minic R, Dimitrijevic L, Skodric SR, Zivkovic I, Djordjevic B. Lactobacillus rhamnosus LA68 and Lactobacillus plantarum WCFS1 differently influence metabolic and immunological parameters in high fat diet-induced hypercholesterolemia and hepatic steatosis. Food Funct (2015) 6:558–65. doi:10.1039/c4fo00843j
27. Meijerink M, Wells JM, Taverne N, Zeeuw Brouwer ML, Hilhorst B, Venema K, et al. Immunomodulatory effects of potential probiotics in a mouse peanut sensitization model. FEMS Immunol Med Microbiol (2012) 65(3):488–96. doi:10.1111/j.1574-695X.2012.00981.x
28. Snel J, Vissers Y, Smit B, Jongen J, Van der Meulen E, Zwijsen R, et al. Strain-specific immunomodulatory effects of Lactobacillus plantarum strains on birch-pollen-allergic subjects out of season. Clin Exp Allergy (2011) 41(2):232–42. doi:10.1111/j.1365-2222.2010.03650.x
29. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol (2007) 13(2):236–43. doi:10.3748/wjg.v13.i2.236
30. Bäuerl C, Pérez-Martínez G, Yan F, Polk DB, Monedero V. Functional analysis of the p40 and p75 proteins from Lactobacillus casei BL23. J Mol Microbiol Biotechnol (2010) 19(4):231–41. doi:10.1159/000322233
31. Hougee S, Vriesema A, Wijering S, Knippels L, Folkerts G, Nijkamp F, et al. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: a bacterial strain comparative study. Int Arch Allergy Immunol (2009) 151(2):107–17. doi:10.1159/000236000
32. Van Beek AA, Hoogerland JA, Belzer C, De Vos P, De Vos W, Savelkoul HF, et al. Interaction of mouse splenocytes and macrophages with bacterial strains in vivo: the effect of age in the immune response. Benef Microbes (2016) 7(2):275–87. doi:10.3920/BM2015.0094
33. Cho JS, Kook SH, Robinson AR, Niedernhofer LJ, Lee BC. Cell autonomous and nonautonomous mechanisms drive hematopoietic stem/progenitor cell loss in the absence of DNA repair. Stem Cells (2013) 31(3):511–25. doi:10.1002/stem.1261
34. Sovran B, Lu P, Loonen LM, Hugenholtz F, Belzer C, Stolte EH, et al. Identification of commensal species positively correlated with early stress responses to a compromised mucus barrier. Inflamm Bowel Dis (2016) 22(4):826–40. doi:10.1097/MIB.0000000000000688
35. Rajilić-Stojanović M, Heilig HG, Molenaar D, Kajander K, Surakka A, Smidt H, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol (2009) 11(7):1736–51. doi:10.1111/j.1462-2920.2009.01900.x
36. Lahti L, Elo LL, Aittokallio T, Kaski S. Probabilistic analysis of probe reliability in differential gene expression studies with short oligonucleotide arrays. IEEE/ACM Trans Comput Biol Bioinform (2011) 8(1):217–25. doi:10.1109/TCBB.2009.38
37. Braak CJF, Šmilauer P. CANOCO Reference Manual and User’s Guide: Software for Ordination (Version 5.0). Ithaca: Biometris (2012).
38. van Loo PF, Dingjan GM, Maas A, Hendriks RW. Surrogate-light-chain silencing is not critical for the limitation of pre-B cell expansion but is for the termination of constitutive signaling. Immunity (2007) 27(3):468–80. doi:10.1016/j.immuni.2007.07.018
39. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A (2005) 102(43):15545–50. doi:10.1073/pnas.0506580102
40. Vermeij WP, Hoeijmakers J, Pothof J. Genome integrity in aging: human syndromes, mouse models, and therapeutic options. Annu Rev Pharmacol Toxicol (2016) 56(11):427–45. doi:10.1146/annurev-pharmtox-010814-124316
41. Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, et al. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J Bacteriol (2005) 187(17):6128–36. doi:10.1128/JB.187.17.6128-6136.2005
42. Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology (2006) 152(1):273–80. doi:10.1099/mic.0.28415-0
43. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol (2004) 54(5):1469–76. doi:10.1099/ijs.0.02873-0
44. Rokhsefat S, Lin A, Comelli EM. Mucin–microbiota interaction during postnatal maturation of the intestinal ecosystem: clinical implications. Dig Dis Sci (2016) 61(6):1473–86. doi:10.1007/s10620-016-4032-6
45. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol (2012) 13(2):89–102. doi:10.1038/nrm3270
46. Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med (2008) 5(3):e54. doi:10.1371/journal.pmed.0050054
47. van Hemert S, Meijerink M, Molenaar D, Bron PA, de Vos P, Kleerebezem M, et al. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol (2010) 10(1):293. doi:10.1186/1471-2180-10-293
48. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell (2004) 118(2):229–41. doi:10.1016/j.cell.2004.07.002
49. Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature (2007) 446(7135):557–61. doi:10.1038/nature05698
50. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med (2010) 16(2):228–31. doi:10.1038/nm.2087
Keywords: aging, probiotics, immunity, mucus, intestinal barrier, microbiota
Citation: van Beek AA, Sovran B, Hugenholtz F, Meijer B, Hoogerland JA, Mihailova V, van der Ploeg C, Belzer C, Boekschoten MV, Hoeijmakers JHJ, Vermeij WP, de Vos P, Wells JM, Leenen PJM, Nicoletti C, Hendriks RW and Savelkoul HFJ (2016) Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1−/Δ7 Mice. Front. Immunol. 7:408. doi: 10.3389/fimmu.2016.00408
Received: 04 August 2016; Accepted: 22 September 2016;
Published: 07 October 2016
Edited by:
Julio Villena, CERELA-CONICET, ArgentinaReviewed by:
Jennelle Maree Kyd, Swinburne University of Technology, AustraliaSusana Salva, CERELA-CONICET, Argentina
Copyright: © 2016 van Beek, Sovran, Hugenholtz, Meijer, Hoogerland, Mihailova, van der Ploeg, Belzer, Boekschoten, Hoeijmakers, Vermeij, de Vos, Wells, Leenen, Nicoletti, Hendriks and Savelkoul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huub F. J. Savelkoul, aHV1Yi5zYXZlbGtvdWxAd3VyLm5s
†Present address: Adriaan A. van Beek, Department of Gastroenterology and Hepatology, Erasmus University Medical Center, Rotterdam, Netherlands
 Adriaan A. van Beek
Adriaan A. van Beek Bruno Sovran
Bruno Sovran Floor Hugenholtz2,5
Floor Hugenholtz2,5 Joanne A. Hoogerland
Joanne A. Hoogerland Corine van der Ploeg
Corine van der Ploeg Clara Belzer
Clara Belzer Paul de Vos
Paul de Vos Jerry M. Wells
Jerry M. Wells Pieter J. M. Leenen
Pieter J. M. Leenen Claudio Nicoletti
Claudio Nicoletti Rudi W. Hendriks
Rudi W. Hendriks