
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 13 October 2015
Sec. Immunological Memory
Volume 6 - 2015 | https://doi.org/10.3389/fimmu.2015.00527
This article is part of the Research TopicInnate immune cell determinants of T cell immunity: from basic mechanisms to clinical implications.View all 14 articles
 Jens Geginat1*
Jens Geginat1* Giulia Nizzoli1
Giulia Nizzoli1 Moira Paroni1
Moira Paroni1 Stefano Maglie1
Stefano Maglie1 Paola Larghi1
Paola Larghi1 Steve Pascolo2
Steve Pascolo2 Sergio Abrignani1,3
Sergio Abrignani1,3
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that have a key role in immune responses because they bridge the innate and adaptive arms of the immune system. They mature upon recognition of pathogens and upregulate MHC molecules and costimulatory receptors to activate antigen-specific CD4+ and CD8+ T cells. It is now well established that DCs are not a homogeneous population but are composed of different subsets with specialized functions in immune responses to specific pathogens. Upon viral infections, plasmacytoid DCs (pDCs) rapidly produce large amounts of IFN-α, which has potent antiviral functions and activates several other immune cells. However, pDCs are not particularly potent APCs and induce the tolerogenic cytokine IL-10 in CD4+ T cells. In contrast, myeloid DCs (mDCs) are very potent APCs and possess the unique capacity to prime naive T cells and consequently to initiate a primary adaptive immune response. Different subsets of mDCs with specialized functions have been identified. In mice, CD8α+ mDCs capture antigenic material from necrotic cells, secrete high levels of IL-12, and prime Th1 and cytotoxic T-cell responses to control intracellular pathogens. Conversely, CD8α− mDCs preferentially prime CD4+ T cells and promote Th2 or Th17 differentiation. BDCA-3+ mDC2 are the human homologue of CD8α+ mDCs, since they share the expression of several key molecules, the capacity to cross-present antigens to CD8+ T-cells and to produce IFN-λ. However, although several features of the DC network are conserved between humans and mice, the expression of several toll-like receptors as well as the production of cytokines that regulate T-cell differentiation are different. Intriguingly, recent data suggest specific roles for human DC subsets in immune responses against individual pathogens. The biology of human DC subsets holds the promise to be exploitable in translational medicine, in particular for the development of vaccines against persistent infections or cancer.
Human beings are constantly exposed to a myriad of pathogens, including bacteria, fungi, and viruses. These foreign invaders or cohabitants contain molecular structures that are sensed by the innate immune system, which mounts a first-line defense and also activates a pathogen-specific, adaptive immune response. The adaptive immune system is composed of B cells that produce specific antibodies, CD8+ T cells that can kill pathogen-infected cells, and CD4+ T cells that produce effector cytokines and coordinate the immune response. T cells express antigen receptors (T-cell antigen receptors, TCR) that recognize specific peptides presented on MHC molecules. CD8+ T cells recognize peptides presented by MHC class-I molecules that are ubiquitously expressed, whereas CD4+ T cells are activated by peptide-MHC class-II complexes, which are largely restricted to antigen-presenting cells (APCs). Dendritic cells (DCs) can express very high levels of MHC and costimulatory molecules, and it is generally accepted that they are the relevant cells to induce the activation (“priming”) of antigen-specific “naive” T cells (1, 2) and induce their differentiation into various types of effector T cells.
The elimination or containment of different types of pathogens requires dedicated classes of adaptive immune responses (3). Thus, pathogens like viruses or intracellular bacteria require CD4+ and CD8+ T cells that produce IFN-γ and kill infected cells (Th1 and CTL, respectively). IL-12 is the critical cytokine that induces this type of response, but IL-12 production by DC is tightly controlled and requires several stimuli derived from pathogens and from CD4+ helper T cells (4–9). Conversely, extracellular bacteria and fungi require a different type of response that can be mediated by Th17 cells (10–12). These effector cells are induced by proinflammatory cytokines produced by DC and macrophages (13) and attract neutrophils that in turn phagocytose extracellular bacteria (14). A third type of effector response is the Th2 response, which is required to expel extracellular parasites such as helminths by activating eosinophils and basophils and by inducing antibodies of the IgE class (15). IL-4 is the critical cytokine that induces this response (16), but IL-4 is normally not produced by DC (17, 18). Finally, these different effector responses have to be controlled by specialized regulatory T cells, in particular by IL-10-producing T cells (“Tr1 cells”), which are generated from effector cells and are important to avoid excessive tissue damage by adaptive immune responses (19–22). Cytokines that promote this type of regulatory T-cell response are IFN-α, IL-27, and IL-10 (23–25), and all these cytokines can be produced by DCs (26, 27).
Professional APCs have to present pathogen-derived peptides on MHC molecules to activate antigen-specific T cells. DCs are phagocytic in the immature state, i.e., under steady-state conditions and upon initial pathogen encounter, and can take up antigenic material by pinocytosis or by surface receptor-mediated internalization (28). Proteins from pathogens are then shuttled to lysosomes where they are chopped to peptides and loaded on MHC class-II molecules (29, 30). These peptide–MHC complexes are then transported to the plasma membrane to activate specific CD4+ T cells. The presentation of peptides derived from exogenous proteins on MHC class-I, a process called cross-presentation (31, 32), is a largely unique feature of DCs and is particularly important to activate CD8+ T cells in viral infections. Virus-infected cells express viral proteins in the cytosol where they are degraded to peptides by the proteasome, translocated to the endoplasmic reticulum by TAP proteins, and loaded on MHC class-I molecules (31). However, since DCs are not necessarily infected by viruses, they must be able to process virus-derived proteins also from external sources, such as virus-infected cells, to activate CD8+ T cells. The mechanism of cross-presentation is still incompletely understood, but two distinct pathways via vacuoles and peptide translocation from phagolysosomes to the cytosol have been described (32). It is believed that cross-presentation is the most important pathway leading to the induction of cytotoxic T-cell responses, and excellent reviews have been published on this relevant topic (31–33).
Naive T cells have a very high activation threshold (34), and only professional APCs that express high levels of MHC and costimulatory molecules such as DCs are able to induce proliferation of naive T cells (35). Several receptor–ligand interactions contribute to naive T-cell activation (36–38), but CD28 costimulation is particularly important to amplify the signal transduced by the TCR (39). Monocytes efficiently present peptides derived from extracellular proteins on MHC class-II to activate antigen-experienced CD4+ T cells (34), and this capacity can be exploited to selectively expand antigen-specific memory T cells (40). However, monocytes have an approximately 1000-fold lower capacity to prime naive CD4+ T cells as compared to DCs (Nizzoli et al., under review) and home to non-lymphoid tissues in the steady state. However, upon inflammation, they can differentiate to inflammatory DCs (41) and home to lymph nodes where they can activate T cells (42, 43). In addition, there is some evidence that CD16+ subsets of human blood monocytes might contain DCs (27, 44, 45). Naive T cells constantly recirculate in the blood and migrate through secondary lymphoid organs (46), but are largely excluded from non-lymphoid tissues. In secondary lymphoid tissues, they migrate to the T-cell zone, where they encounter DCs (47). B cells are also present in secondary lymphoid organs and can potently present antigen to T cells when they internalize and process antigens that have specifically bound to their B-cell receptor (48). However, B cells are physically separated from naive T cells in lymph nodes and only following TCR activation naive T cells migrate to the B-cell zone where they interact with antigen-specific B cells to induce antibody production (49, 50). Thus, antigen presentation by B cells appears to be important for the activation of antigen-experienced T cells rather than for naive T-cell priming.
Dendritic cells are generated from committed precursors in the bone marrow that are released into the circulation to seed peripheral organs (51–55). Both monocytes and DCs can be derived from common myeloid progenitors (CMPs), but committed precursors that selectively give rise to monocytes or DCs (51) or even selected DC subsets (53, 54) have been identified in humans and mice. DCs are poorly stimulatory in the immature state and can induce a partial T-cell activation, leading to deletion of autoreactive CD8+ T cells (56–59). In addition, they promote self-tolerance by inducing Foxp3+ regulatory CD4+ T cells that suppress autoreactive T cells (60). Pathogens induce the maturation of DCs that consequently acquire the capacity to produce polarizing cytokines and to prime pathogen-specific effector T-cell responses. Pathogen-derived molecular patterns [PAMPs (61, 62)] are recognized by DCs and lead to the efficient presentation of antigens to T cells (63). There are different classes of pathogen-sensing receptors, including Toll-like receptors (62, 64), nucleotide-binding oligomerization domain (NOD)-like receptors (65), retinoic acid-inducible gene 1 (RIG-I)-like receptors (66), and C-type lectins (67). TLRs recognize different PAMPs, including nucleic acids or cell wall components such as proteins and lipoproteins (68, 69). In the case of viruses, nucleic acids are sensed not only by different TLRs in endosomes but also by cytosolic receptors like RIG-I (66, 70) and induce a potent activation of DCs. Importantly, subsets of DCs express different patterns of pathogen-sensing receptors and might thus preferentially respond to individual pathogens (71, 72). DNA viruses such as cytomegalovirus (CMV) and herpes simplex virus (HSV) and also bacteria can activate DCs via unmethylated CpG-containing DNA (69), which is sensed by TLR9. Double- and single-stranded RNAs, which are generated by both DNA and RNA viruses, are sensed by DCs via TLR3 (73) and TLR7/8 (74, 75), respectively. Of note, TLR3 is restricted to mDCs (71) and induces cross-presentation capacities (76). Viruses such as respiratory syncytial virus (RSV) and hepatitis C virus (HCV) can also activate DCs via TLR2 or TLR4, which are expressed on the plasma membrane and recognize viral proteins (77). TLR2 is also involved in immune responses to fungi (78) and Gram-positive bacteria (79, 80) while TLR4 recognizes lipopolysaccharide (LPS) (81), a cell membrane compound of Gram-negative bacteria. Many pathogens like viruses activate DCs via multiple TLRs (77). Moreover, other immune cells, including T cells themselves, feed-back on DCs to regulate the ongoing response. In particular, CD40 stimulation by CD4+ helper T cells is crucial for CD8+ T-cell stimulation and IL-12 production (4, 5). Moreover, IFN-γ (6) and paradoxically also IL-4 (7, 8) that can be provided by T cells further enhance IL-12 production (9).
Surface TLRs such as TLR2 and TLR4 signal via the adaptor protein Myd88 (82) to induce the activation of Map kinases and the nuclear translocation of the transcription factor NF-κB, which in turn induces the transcription of proinflammatory cytokines (62). Endosomal TLRs 7, 8, and 9 also signal via Myd88 but activate IRF7, which in turn induces type-1 interferon production (83, 84). TLR3 is an exception since it does not signal via Myd88 but utilizes TRIF (85) to activate IRF3 (86, 87) or IRF7 (88). How all these complex signaling pathways are integrated by DCs to induce the appropriate T-cell response is still incompletely understood (88–90).
Dendritic cells in mice can be subdivided into distinct subsets with specific functions. Some DCs are stably resident in lymph nodes while others are positioned in non-lymphoid tissues to sense tissue-invading pathogens, but are migratory and are recruited via the lymph following pathogen encounter in a CCR7-dependent manner (91, 92). In secondary lymphoid tissues, two major DC subsets are myeloid DCs (mDCs) and plasmacytoid DCs (pDCs; Table 1) (72, 93, 94). Both pDCs and mDCs upregulate MHC and costimulatory molecules like CD80 and CD86 upon maturation (72) that bind to CD28 and are required to induce full T-cell stimulation (39). However, pDCs are poorly phagocytic and have a different regulation of MHC class-II turnover upon maturation as compared to mDCs (95). Thus, mDCs stop phagocytosis and peptide loading on MHC upon pathogen recognition and stably present peptides from antigenic material they had acquired upon pathogen encounter (30, 96, 97). This maturation-induced stabilization of peptide–MHC complexes enhances the priming of pathogen-specific T cells by mDCs. In contrast, pDCs continue to present new peptides on MHC complexes even in the mature stage (95). On the one hand, this limits their capacity to stimulate pathogen-specific T cells; on the other hand, this enables them to present also late-expressed viral antigens when they are actively infected. This diverse regulation of MHC–peptide stability in mDCs and pDCs suggests that they present different antigens to T cells.
Plasmacytoid DCs are present in lymph nodes and are largely absent from non-lymphoid organs, but they can be recruited upon inflammation (98). The role of pDC in T-cell priming is still debated (99). There is consensus that they are poorly stimulatory in their resting state (100, 101), but while some groups proposed that they become potent APCs following TLR stimulation and prime CD4+ and cross-prime CD8+ T-cell responses (102–105), others concluded that also mature pDCs have only low priming and cross-priming capacities and might rather be tolerogenic (101). The rapid and abundant production of type-1 interferon by pDC suggests a pivotal role in viral infections, even if their capacity to prime virus-specific T cells directly appears to be limited. IFN-α can also be produced by other immune cells and by virus-infected cells, but the early and systemic IFN-α response is believed to depend on pDCs (101). Consistently, in the case of HSV infections, it was shown that pDCs were important for systemic but not local protection (106). However, in several other viral infections in mice, including vesicular stomatitis virus (VSV), lymphocytic choriomeningitis virus (LCMV), RSV, and mouse cytomegalovirus (MCMV), pDCs do not seem to play a major role (100). In marked contrast, in mouse hepatitis virus (MHV) infection, the antiviral response against this coronavirus was largely pDC dependent (107) (Figure 1). Finally, pDCs have been found by several groups to induce the production of the anti-inflammatory cytokine IL-10 by CD4+ T cells, suggesting that they might be important to inhibit excessive T-cell responses. Several proteins expressed by pDCs were found to promote IL-10 induction in T cells, including the Notch ligand Delta-like 4 (108), ICOSL (109, 110), as well as IFN-α (23, Nizzoli et al., under review).
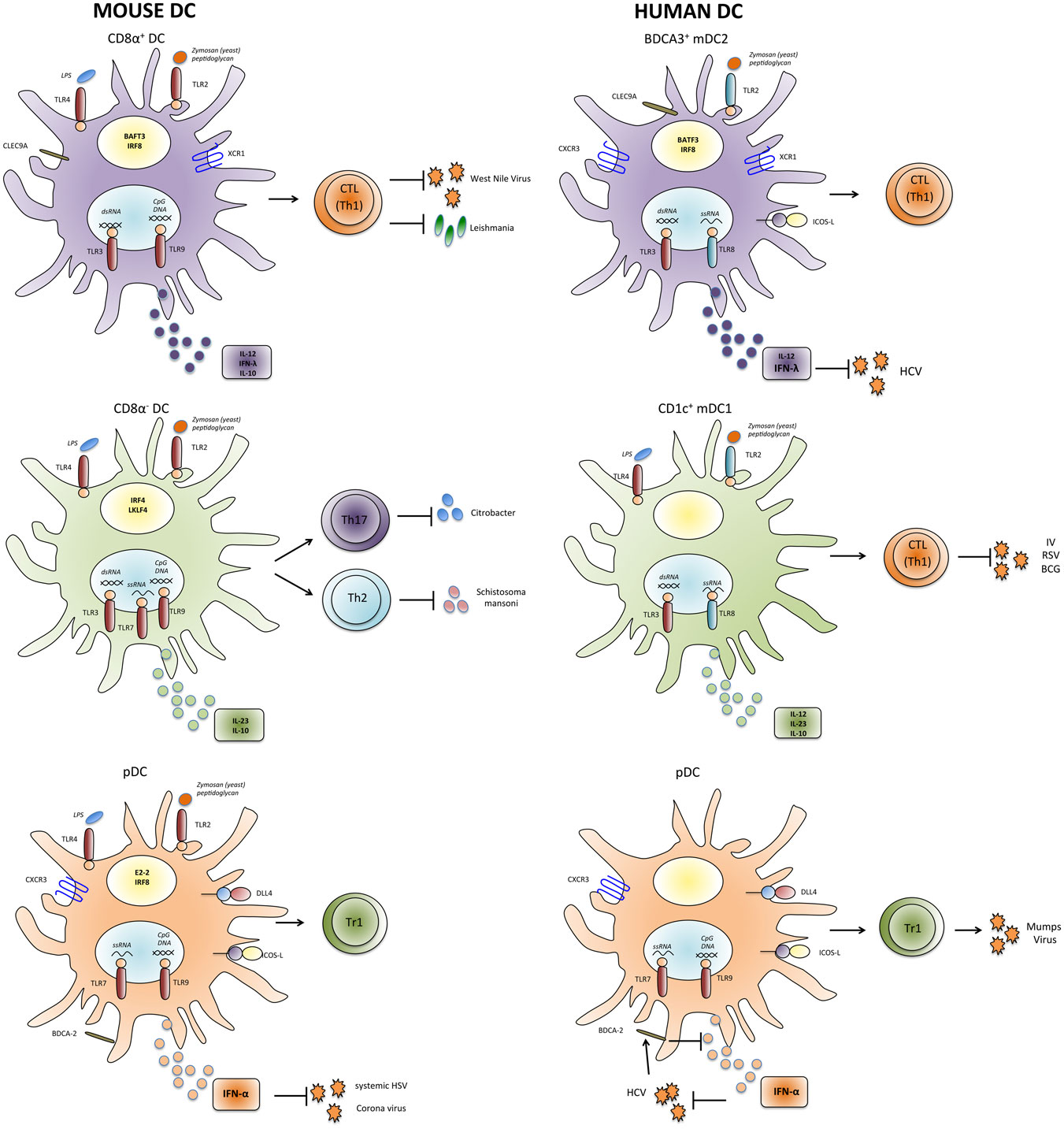
Figure 1. Properties and functions of human and mouse DC subsets. Human and mouse mDC and pDC subsets express partially different patterns of pathogen-sensing receptors and cytokines and might thus have unique functions in inducing appropriate types of T-cell responses against individual pathogens. IV, influenza virus; HCV, hepatitis C virus; RSV, respiratory syncytial virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; BCG, Bacillus Calmette–Guérin.
Myeloid DCs are a heterogeneous population, and different mDC subsets can be identified that preferentially initiate different types of adaptive immune responses (Figure 1). In the spleen of mice, mDCs can be subdivided into CD8α+ and CD8α− subsets (Table 1). CD8α+ mDCs produce high levels of bioactive IL-12p70 and efficiently cross-prime CD8+ T-cell responses (111). They express CLEC9A, a C-type lectin, that enables them to take up antigenic material from dying cells, and their generation was shown to rely on the transcription factors BATF3 and IRF8 (112, 113). Moreover, they express the chemokine receptor XCR1 that favors their colocalization with CD8+ T cells (114). Altogether the present evidence indicates that CD8α+ DCs are specialized to induce Th1 and CTL responses in response to intracellular pathogens (115, 116). Notably, DCs in the gut that express CD103 have similar characteristics and are closely related to CD8α+ DC (117, 118). CD8α− DCs express CD11b and can be further subdivided into CD4+ and CD4−CD8− subsets. They preferentially prime CD4+ T-cell responses (119) and promote Th17 responses, but they can also induce Th2 cells (113, 120). Interestingly, CD11b+ DCs produce IL-23 in the gut and are required for protection against Citrobacter rodentium (121). Their generation depends on the transcription factor IRF4, while KLF4 expression is required for Th2, but not for Th17 induction (122). Notably, however, CD8α− DCs and also pDCs can cross-prime CD8+ T-cell responses under certain conditions (102–104, 123). Moreover, it was shown that upon appropriate microbial stimulation all mDC subsets have the potential to promote either Th1 or Th2 responses (124). Thus, although the proposed functional specialization of DC subsets is an intriguing and helpful concept, it might also be an oversimplification, since DC subsets have considerable plasticity and the induction of a specific type of immune response critically depends on the stimuli they receive from pathogens as well as from other immune cells (125).
High numbers of human DCs can be generated in vitro by culturing monocytes with cytokines (41), and the large majority of studies on human DCs have been done with these monocyte-derived DCs. They are primary cells and show many behaviors of in vivo occurring DCs, including cytokine production as well as stable and potent antigen presentation upon maturation with TLR ligands (125). However, monocyte-derived DCs are not the appropriate model to study the role of specialized DC subsets in human immune responses.
Dendritic cells circulating at low frequency in human peripheral blood share several features with murine splenic DC subsets (126) (Table 1). Human pDCs have been identified more than 15 years ago as the natural IFN-α-producing cells (127, 128). They express TLR7 and TLR9 and produce large amounts of IFN-α in response to CpG DNA or influenza virus. Similar to their murine counterparts, they are poorly stimulatory (94), express the C-type lectin BDCA-2 (93), and induce IL-10 production in CD4+ T cells (129). In addition, subsets of mDCs can also be found in human blood and in tissues (130–133). As their murine homologues, they express CD11c and potently prime CD4+ and CD8+ T-cell responses. The expression of CD1c/BDCA-1 and CD141/BDCA-3 identifies two subsets among human mDCs in peripheral blood (93) and also in secondary lymphoid organs (105, 132, 134, 135). BDCA-3+ “mDC2” (Table 1) are rare, but it could recently be demonstrated that they represent the human counterpart of murine CD8α+ DCs (136–140). Thus, as CD8α+ DCs, they selectively express CLEC9A and XCR1 and are dependent on the transcription factor BATF3 (112, 136, 138, 140, 141). Importantly, they can cross-present exogenous antigens on MHC class-I to CD8+ T cells and produce IL-12 (134–136). CD1c+ “mDC1” (Table 1) are more frequent and share some features with CD8α− DC, including CD11b expression and IL-23 production (121, 142, Nizzoli et al., under review). Also TLR3 expression in DC subsets appears to be similar in humans and mice, since it is expressed at high levels on CD8α+ DCs and mDC2, at lower levels on CD8α− DCs and mDC1, and absent on pDC. Surprisingly, TLR3 in mice is not required for immune responses against several viruses, including LCMV, VSV, MCMV, and Reovirus, suggesting that TLR3 has not a pivotal role in antiviral immune defense (143). Consistently, TLR3 deficiency in humans selectively leads to uncontrolled HSV1 infections in the central nervous system (CNS) (144).
Different subsets of DC have also been identified in human non-lymphoid tissues where they are strategically positioned to recognize invading pathogens, in particular at barrier surfaces. These migratory DC subsets play a crucial role to transport antigenic material of pathogens that invade specific tissues to draining lymph nodes and thus to initiate a tissue-specific T-cell response (130, 145, 146). Human Langerhans cells were first described more than a century ago and reside in the epidermis and are thus the first DCs that encounter skin-invading pathogens. Upon activation, they mature and migrate to draining lymph nodes to activate CD4+ and CD8+ T cells. In the dermis, different subsets of interstitial DCs are present and can be classified according to CD14, CD1a, and CD141 expression. Dermal CD14+ cells might represent monocyte-derived macrophages rather then DCs (147), but CD1a+ and CD141+ DCs, respectively, resemble the CD1c+ and CD141+ DC subsets in peripheral blood (148). Also in the lung and the liver, DC subsets that are related to CD1c+ and CD141+ DCs could be identified (133). Finally, in the human intestine, DC subsets that express CD11b and CD103 are similar to CD1c+ and CD141+ DCs, respectively, and these intestinal DC subsets are also largely conserved between humans and mice (149).
Although the similarities between human and mouse DC subsets are often emphasized, there are also some important differences in pathogen sensing by DCs in humans and mice (150). Importantly, the expression of several relevant TLRs is not conserved (Figure 1), presumably because humans and mice have evolved under the selective pressure of different pathogens. Thus, in mice, TLR7 and TLR9 are expressed by both pDC and mDC subsets (71), whereas in humans, they are restricted to pDCs (72). Also TLR4 expression is more restricted in human DCs, since it is expressed by mDC1 but not by mDC2 (136). Moreover, TLR8 is not expressed by human pDCs (72), and some agonists of human TLR8 such as the resiquimod R848 do not activate murine TLR8 (75, 151). Another relevant difference seems to be the role of the adaptor protein Myd88, which transduces signals from all TLRs with the notable exception of TLR3. Thus, mice deficient for Myd88 are highly susceptible to several infections by bacteria, viruses, parasites, and fungi. Conversely, Myd88-deficient patients are selectively affected by infections with pyogenic bacteria in childhood (152). Finally, human CD1c+ DCs and also Langerhans cells seem to have superior capacities to cross-present antigens and to induce CTL responses as compared to their murine homologues (105, 134, 153, 154). Overall, these differences in pathogen sensing and T-cell activation between human and murine DCs are likely to have an important impact on their role in immune responses against specific pathogens.
Dendritic cell subsets in humans and mice express not only different patterns of toll-like receptors, but they have also partially distinct cytokine profiles (Figure 1). In particular, human mDC1 have a complex and quite unique regulation of cytokine production. Thus, while LPS triggers only low levels of cytokine production by mDC1, dual TLR stimulation with LPS or Poly-I:C (TLR3 ligand) in combination with R848 induces very high levels of a broad panel of cytokines, including TNF, IL-6, IL-10, IL-12, and IL-23 (Nizzoli et al., under review). The very potent cytokine-producing capacity of mDC1 has been missed in several studies where mDC1 were activated with single TLR ligands (45, 155, 156). Of note, single TLR stimulation is sufficient to induce antiviral cytokines by mDC2 and pDCs (see below) and proinflammatory cytokines by monocytes. Although mDC1 can secrete several proinflammatory cytokines that promote Th17 cell generation including IL-23 (142), it is unclear if they are the physiological inducers of Th17 cells or if monocyte-derived, inflammatory DCs do the job (12, 157). Also the identity of the DC subset that induces human Th2 responses is still enigmatic. It was originally proposed that mDCs induce Th1 polarization and pDCs Th2, but later it was shown that also pDCs can drive Th1 responses (158, 159). More recently, mDC2 but not mDC1 were found to induce Th2 cells in an aberrant response to influenza virus (160).
In apparent contrast to CD8α− DCs, mDC1 can produce high levels of IL-12 (134, 135), suggesting a relevant role in immune responses against intracellular pathogens. Moreover, the production of the anti-inflammatory cytokine IL-10, which can be produced by all mDCs in mice, is largely restricted to mDC1 in humans (Nizzoli et al., under review). Stimulation of mDC1 with the intestinal bacterium Escherichia coli or with LPS alone induces IL-10 and was proposed to induce a tolerogenic state in mDC1 (155). Although IL-10 is indeed a tolerogenic cytokine and a well-established negative regulator of DC maturation and cytokine production (161), it can paradoxically also have positive effects, in particular on CD8+ T-cell responses (162, 163). Consistently, we found that IL-10 produced by mDC1 completely blocked the cross-priming of low-affinity CTL and enhanced the responsiveness of CD8+ memory T cells to the homeostatic cytokine IL-15. Thus, mDC1-derived IL-10 appears to play an important positive role in CTL responses, since it selects high affinity cells upon priming and inhibits CTL memory attrition at the same time (Nizzoli et al., under review).
While mDC1 can secrete a broad panel of pro- and anti-inflammatory cytokines, mDC2 and pDC are largely dedicated to secrete high levels of antiviral cytokines. The subset-specific production of IFN-α by pDC (128) and of IFN-λ by CD8α+ and mDC2 (134, 137) appears to be largely conserved between humans and mice. The very potent IFN-λ-producing capacities of BDCA-3+ DC (134, 137) suggest that analogous to pDCs they might be the relevant source of early and systemic IFN-λ in viral infections. Notably, IFN-λ has antiproliferative and antiviral activities similar to type-I interferon, but the expression of the IFN-λ receptor is much more restricted and found mainly on epithelial cells at barrier surfaces and in the liver (164). MDC2 can also secrete selected isoforms of IFN-α (165) and some IL-12 (134–136, 138), consistent with the view that they play an important role in antiviral immune responses. As previously mentioned for murine pDCs, IFN-α is not only a powerful antiviral cytokine that activates several different types of immune cells, but it also induces IL-10 production in CD4+ T cells, suggesting that pDCs induce Tr1-like regulatory T cells also in humans (21, 23, 108, Nizzoli et al., under review).
The more restricted expression of TLRs and the specific cytokine-producing capacities of human DC subset suggest that they play unique roles in immune responses against individual pathogens. The roles of human DC subsets in pathogen-specific immune response are however difficult to address directly because patients that selectively lack a DC subset of interest have not been identified so far. Nevertheless, some interesting findings were reported. In particular, mDC2 appear to be highly relevant in HCV infection. Single-nucleotide polymorphisms in the IFN-λ3 gene locus are strongly associated with spontaneous clearance and response to therapy in HCV patients (166). All DC subsets can secrete some IFN-λ1 (134, 167), but mDC2 produce much higher amounts. Moreover, IFN-λ2/3 are largely restricted to mDC2, and importantly HCV induces IFN-λ3 production by mDC2 (168, Nizzoli et al., under review). Thus, mDC2 appear to be a highly relevant source for protective IFN-λ3 in HCV infection (169). Interestingly, an important role for mDC1 rather than for mDC2 was recently proposed in tuberculosis (170, 171). Thus, mDC1 were more efficiently infected with the Bacillus Calmette–Guérin (BCG) vaccine than other DCs and induced the activation of pDCs and CD8+ T cells. Notably, mDC1 could not be replaced by mDC2 in this system, suggesting that mDC1 could play a non-redundant role in the defense against selected intracellular pathogens. MDC1 and mDC2 have also been suggested to play different roles in RSV infection (172, 173). Thus, mDC subsets produced different cytokines in response to RSV, consistent with their different cytokine profiles upon stimulation with purified TLR ligands (134, Nizzoli et al., under review). Moreover, they induced different classes of T-cell responses, with mDC1 inducing preferentially Th1 cells and mDC2 inducing predominantly Th2 and T-regulatory cells. Similarly, mDC2, but not mDC1, were found to induce Th2 response to influenza virus (160). Also the capacity of pDCs to induce IL-10-producing regulatory T cells has been documented with a relevant pathogen, since pDCs were shown to induce IFN-γ and IL-10 production in antigen-experienced CD4+ T cells specific for mumps virus (129). Conversely, CD11c+ mDCs, which contain both mDC1 and mDC2, induced IFN-γ and, surprisingly, IL-5.
It is largely accepted that pDC-derived IFN-α is important to contain human viral infections. Thus, stabilized pegylated IFN-α is a widely used therapy for HCV patients. IFN-λ appears to be similar effective, but is less toxic presumably because of the more restricted expression of its receptor (164). Interestingly, the HCV glycoprotein E2 is a ligand for BDCA-2, which is specifically expressed on pDCs (Table 1) and inhibits IFN-α production (174, 175). In this way, HCV might inhibit IFN-α production to establish chronic infection. Finally, pDCs are also targeted by human immunodeficiency virus (HIV), but whether they play a protective or detrimental role is still unclear (176).
Vaccines have been a major breakthrough for human health. Attenuated or killed pathogens are highly efficient to induce protective cellular and humoral immune responses, and the induced protective memory can last for a lifetime (177, 178). However, since these pathogen-based vaccines also have considerable side effects, proteins in combination with adjuvants that activate APCs are more often used. Protein vaccines induce CD4+ T-cell responses and neutralizing antibodies, but they are poorly efficient in inducing cytotoxic T-cell responses and are also rather inefficient in inducing Th1 cells (179, 180). Frequently used adjuvants are alum, oil-in-water emulsions like MF59, and more recently also monophosphoryl lipid A (MPL), a detoxified form of LPS. In mice, different adjuvants were shown to induce different proinflammatory cytokines. Thus, alum acts via uric acid on inflammatory DCs (181), which leads to NOD-like receptor protein-3 (NALP3)-dependent IL-1β production (182). Conversely, MPL does not induce IL-1β (183) but induces specific antibodies through an IL-6-dependent mechanism (184), while MF-59 and alum act independently of IL-6 (185). However, the different TLR expression and cytokine production by human APC subsets should be considered when translating this knowledge from animal models to patients. A recent interesting report analyzed the response of APC subsets to 13 different vaccines and concluded that different vaccines activate indeed different APC populations (186). More direct information on the effect of DCs was obtained by vaccinations with peptide-pulsed monocyte-derived DCs in cancer patients, which can induce tumor-specific CD8+ T cells (187), but the clinical responses were so far largely insufficient. MDCs might be more potent and are currently tested in clinical trials.
Nucleic acid-sensing TLRs are particularly potent to induce CD8+ T-cell responses in mice (188) and have recently been employed as adjuvants in vaccines. Examples are CpG-DNA that stimulates TLR9 (189), and the TLR7 ligand imiquimod, which is used as a cream to stimulate DC locally in the skin, and was shown to induce CD8+ T-cell responses in situ (190). Vaccines consisting of plasmid DNA coding for relevant protein antigens are a novel approach that efficiently induces humoral and cellular immune responses in animals. However, in humans, these DNA vaccines are often poorly immunogenic (191), presumably because they have only low adjuvant activity and stimulate mainly cytosolic DNA sensors rather than TLR9 (192), which in addition is restricted to pDCs and B cells in humans. An alternative promising approach is the vaccination with mRNA (193, 194), which delivers not only the antigenic protein directly to the cytosol, thereby bypassing the requirements for cross-presentation (195), but also induces mDC and pDC maturation and cytokine production via TLR7/8 at the same time (196). Indeed, intradermal injection of naked mRNA results in local uptake and translation of the nucleic acid (197) followed by the development of an adaptive immunity in mice (198) and in humans (199, 200). Since also lymph node-resident DCs are expected to be appropriate APCs to process antigens encoded by mRNA, direct injection of nucleic acid into lymph nodes has also been evaluated. In animal models, intra-lymph node injections of mRNA result in expression of the protein encoded by the mRNA in DCs. Furthermore, the injected mRNA activated lymph node-resident APCs and induced potent CD4+ and CD8+ T-cell responses as well as prophylactic and therapeutic antitumor immunity (201). The approach is currently being evaluated through two clinical studies exploring the efficacy of intra-lymph node mRNA vaccination in advanced melanoma patients. As a further development, systemic administration of a liposomal formulation of mRNA that delivers the nucleic acids to APCs present in secondary lymphoid organs is also being evaluated. Using the functional diversity of DCs in vivo, and their specific capabilities in generating appropriate adaptive immune responses, those systemic synthetic vaccines might recapitulate the natural mechanisms of immunity developed during pathogen infection and guarantee the development of therapeutically efficacious immune responses.
Dendritic cells continue to attract much interest of immunologists because they are the most potent APCs in the immune system and are the principal inducers of naive T-cell differentiation. Intensive research in the last years has established that different subsets of DC exist in mice that have specialized functions and preferentially induce different types of immune responses. In humans, much has been learned from in vitro differentiated monocyte-derived DCs, and more recently, also different subsets of DC populating human tissues have been analyzed at the molecular and functional levels. It is fundamental to further define the biology of these in vivo occurring human DC subsets to understand and cure pathogenic immune-mediated processes in so different settings as autoimmunity, infections, and cancer. In particular, appropriate targeting of DC subsets by vaccines holds the promise to induce cytotoxic T-cell responses to eradicate persistent intracellular pathogens or tumors.
Steve Pascolo is the founder and CEO of Miescher Pharma GmbH, a company exploiting the immunostimulating potential of RNA. The remaining co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
JG, GN, and SA are supported by the CARIPLO foundation, SA is supported by the European Research Council, and the INGM is supported by the “Romeo ed Enrica Invernizzi” foundation.
1. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol (1991) 9:271–96. doi: 10.1146/annurev.iy.09.040191.001415
2. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol (2000) 18:767–811. doi:10.1146/annurev.immunol.18.1.767
3. Geginat J, Paroni M, Facciotti F, Gruarin P, Kastirr I, Caprioli F, et al. The CD4-centered universe of human T cell subsets. Semin Immunol (2013) 25(4):252–62. doi:10.1016/j.smim.2013.10.012
4. Cella M, Scheidegger D, Palmer Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med (1996) 184(2):747–52. doi:10.1084/jem.184.2.747
5. Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med (1996) 184:741–6. doi:10.1084/jem.184.2.741
6. Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol (2001) 166:2961–9. doi:10.4049/jimmunol.166.5.2961
7. Kalinski P, Smits HH, Schuitemaker JH, Vieira PL, van Eijk M, de Jong EC, et al. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol (2000) 165:1877–81. doi:10.4049/jimmunol.165.4.1877
8. Hochrein H, O’Keeffe M, Luft T, Vandenabeele S, Grumont RJ, Maraskovsky E, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med (2000) 192:823–34. doi:10.1084/jem.192.6.823
9. Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J Immunol (2001) 166(9):5448–55.
10. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med (2006) 203(10):2271–9. doi:10.1084/jem.20061308
11. Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med (2008) 205(7):1551–7. doi:10.1084/jem.20080218
12. Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol (2007) 8(6):639–46. doi:10.1038/ni1467
13. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol (2007) 8(9):942–9. doi:10.1038/ni1496
14. Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol (2008) 38(10):2636–49. doi:10.1002/eji.200838535
15. Anderson GP, Coyle AJ. TH2 and ‘TH2-like’ cells in allergy and asthma: pharmacological perspectives. Trends Pharmacol Sci (1994) 15(9):324–32. doi:10.1016/0165-6147(94)90027-2
16. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (1993) 362:245–8. doi:10.1038/362245a0
17. Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity (2014) 40(3):425–35. doi:10.1016/j.immuni.2014.01.011
18. Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol (2013) 14(6):536–42. doi:10.1038/ni.2617
19. Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med (2007) 204(2):273–83. doi:10.1084/jem.20062175
20. Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature (1997) 389(6652):737–42. doi:10.1038/39614
21. Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med (2009) 206(5):1009–17. doi:10.1084/jem.20082238
22. Huber S, Gagliani N, Esplugues E, O’Connor W Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity (2011) 34(4):554–65. doi:10.1016/j.immuni.2011.01.020
23. Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol (2001) 166(9):5530–9. doi:10.4049/jimmunol.166.9.5530
24. Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol (2009) 183(4):2435–43. doi:10.4049/jimmunol.0900568
25. Le Buanec H, Gougeon ML, Mathian A, Lebon P, Dupont JM, Peltre G, et al. IFN-alpha and CD46 stimulation are associated with active lupus and skew natural T regulatory cell differentiation to type 1 regulatory T (Tr1) cells. Proc Natl Acad Sci USA (2011) 108(47):18995–9000. doi:10.1073/pnas.1113301108
26. Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett (2009) 125(1):7–14. doi:10.1016/j.imlet.2009.05.002
27. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood (2010) 116(6):935–44. doi:10.1182/blood-2009-07-234872
28. Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med (1995) 182:389–400. doi:10.1084/jem.182.2.389
29. Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med (2000) 191:927–36. doi:10.1084/jem.191.6.927
30. Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science (2000) 288:522–7. doi:10.1126/science.288.5465.522
31. Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol (2001) 1(2):126–34. doi:10.1038/35100512
32. Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol (2012) 12(8):557–69. doi:10.1038/nri3254
33. Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol (1993) 11:403–50. doi:10.1146/annurev.iy.11.040193.002155
34. Sanders ME, Makgoba MW, June CH, Young HA, Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol (1989) 19(5):803–8. doi:10.1002/eji.1830190504
35. Mehta Damani A, Markowicz S, Engleman EG. Generation of antigen-specific CD4+ T cell lines from naive precursors. Eur J Immunol (1995) 25(5):1206–11. doi:10.1002/eji.1830250511
36. Geginat J, Clissi B, Moro M, Dellabona P, Bender JR, Pardi R. CD28 and LFA-1 contribute to cyclosporin A-resistant T cell growth by stabilizing the IL-2 mRNA through distinct signaling pathways. Eur J Immunol (2000) 30(4):1136–44. doi:10.1002/(SICI)1521-4141(200004)30:4<1136::AID-IMMU1136>3.0.CO;2-3
37. Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood (1998) 92(9):3338–45.
38. DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol (1999) 163(9):4833–41.
39. Johnson JG, Jenkins MK. Accessory cell-derived signals required for T cell activation. Immunol Res (1993) 12(1):48–64. doi:10.1007/BF02918368
40. Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med (2004) 200(6):725–35. doi:10.1084/jem.20040774
41. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med (1994) 179:1109–18. doi:10.1084/jem.179.4.1109
42. Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell (2010) 143(3):416–29. doi:10.1016/j.cell.2010.09.039
43. Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol (2009) 10(4):394–402. doi:10.1038/ni.1707
44. Schakel K, von Kietzell M, Hansel A, Ebling A, Schulze L, Haase M, et al. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity (2006) 24(6):767–77. doi:10.1016/j.immuni.2006.03.020
45. Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, et al. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood (2007) 109(12):5371–9. doi:10.1182/blood-2006-08-038422
46. Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med (1990) 171:801–17. doi:10.1084/jem.171.3.801
47. Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev (1997) 156:25–37. doi:10.1111/j.1600-065X.1997.tb00956.x
48. Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature (1985) 314(6011):537–9. doi:10.1038/314537a0
49. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med (2000) 192(11):1545–52. doi:10.1084/jem.192.11.1545
50. Muller G, Lipp M. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Microcirculation (2003) 10(3–4):325–34. doi:10.1080/mic.10.3-4.325.334
51. Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity (2014) 40(5):642–56. doi:10.1016/j.immuni.2014.04.016
52. Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science (2009) 324(5925):392–7. doi:10.1126/science.1170540
53. Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med (2015) 212(3):401–13. doi:10.1084/jem.20141441
54. Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, et al. Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med (2015) 212(3):385–99. doi:10.1084/jem.20141442
55. Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol (2007) 8(11):1207–16. doi:10.1038/ni1518
56. Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A (2002) 99(1):351–8. doi:10.1073/pnas.231606698
57. Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med (2001) 194(6):769–79. doi:10.1084/jem.194.6.769
58. Hernandez J, Aung S, Redmond WL, Sherman LA. Phenotypic and functional analysis of CD8(+) T cells undergoing peripheral deletion in response to cross-presentation of self-antigen. J Exp Med (2001) 194(6):707–17. doi:10.1084/jem.194.6.707
59. Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol (2003) 4(4):355–60. doi:10.1038/ni908
60. Bacchetta R, Gregori S, Roncarolo MG. CD4+ regulatory T cells: mechanisms of induction and effector function. Autoimmun Rev (2005) 4(8):491–6. doi:10.1016/j.autrev.2005.04.005
61. Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today (1992) 13:11–6. doi:10.1016/0167-5699(92)90198-G
62. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature (1997) 388(6640):394–7. doi:10.1038/41131
63. Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol (1997) 9:10–6. doi:10.1016/S0952-7915(97)80153-7
64. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila toll. Proc Natl Acad Sci U S A (1998) 95(2):588–93. doi:10.1073/pnas.95.2.588
65. Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev (2015) 95(1):149–78. doi:10.1152/physrev.00009.2014
66. Bowie AG, Fitzgerald KA. RIG-I: tri-ing to discriminate between self and non-self RNA. Trends Immunol (2007) 28(4):147–50. doi:10.1016/j.it.2007.02.002
67. Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity (2011) 34(5):651–64. doi:10.1016/j.immuni.2011.05.001
68. Muzio M, Polentarutti N, Bosisio D, Prahladan MK, Mantovani A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J Leukoc Biol (2000) 67(4):450–6.
69. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A toll-like receptor recognizes bacterial DNA. Nature (2000) 408:740–5. doi:10.1038/35047123
70. Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J (1995) 14(24):6095–106.
71. Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol (2003) 33(4):827–33. doi:10.1002/eji.200323797
72. Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol (2001) 31(11):3388–93. doi:10.1002/1521-4141(200111)31:11<3388::AID-IMMU3388>3.0.CO;2-Q
73. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature (2001) 413(6857):732–8. doi:10.1038/35099560
74. Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science (2004) 303(5663):1529–31. doi:10.1126/science.1093616
75. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science (2004) 303(5663):1526–9. doi:10.1126/science.1093620
76. Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature (2005) 433(7028):887–92. doi:10.1038/nature03326
77. Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol J (2008) 2:49–59. doi:10.2174/1874285800802010049
78. Goodridge HS, Underhill DM. Fungal recognition by TLR2 and dectin-1. Handb Exp Pharmacol (2008) 183:87–109. doi:10.1007/978-3-540-72167-3_5
79. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity (1999) 11:443–51. doi:10.1016/S1074-7613(00)80119-3
80. Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem (2004) 88(3):746–58. doi:10.1046/j.1471-4159.2003.02202.x
81. Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol (1999) 162(7):3749–52.
82. Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell (1998) 2(2):253–8. doi:10.1016/S1097-2765(00)80136-7
83. Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature (2005) 434(7034):772–7. doi:10.1038/nature03464
84. Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol (2004) 5(10):1061–8. doi:10.1038/ni1118
85. Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the toll-like receptor signaling. J Immunol (2002) 169(12):6668–72. doi:10.4049/jimmunol.169.12.6668
86. Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity (2002) 17(3):251–63. doi:10.1016/S1074-7613(02)00390-4
87. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science (2003) 301(5633):640–3. doi:10.1126/science.1087262
88. O’Neill LA. How toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol (2006) 18(1):3–9. doi:10.1016/j.coi.2005.11.012
89. Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol (2007) 28(5):227–33. doi:10.1016/j.it.2007.03.008
90. Liu Q, Zhu Y, Yong WK, Sze NS, Tan NS, Ding JL. Cutting edge: synchronization of IRF1, JunB, and C/EBPbeta activities during TLR3-TLR7 cross-talk orchestrates timely cytokine synergy in the proinflammatory response. J Immunol (2015) 195(3):801–5. doi:10.4049/jimmunol.1402358
91. Sozzani S, Allavena P, D’Amico G, Luini W, Bianchi G, Kataura M, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol (1998) 161(3):1083–6.
92. Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol (1998) 28:2760–9. doi:10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N
93. Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol (2000) 165(11):6037–46. doi:10.4049/jimmunol.165.11.6037
94. MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood (2002) 100(13):4512–20. doi:10.1182/blood-2001-11-0097
95. Sadaka C, Marloie-Provost MA, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood (2009) 113(10):2127–35. doi:10.1182/blood-2008-10-178152
96. Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature (1997) 388:787–92. doi:10.1038/42039
97. Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol (1997) 15:821–50. doi:10.1146/annurev.immunol.15.1.821
98. Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol (2004) 16(7):915–28. doi:10.1093/intimm/dxh093
99. Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity (2008) 29(3):352–61. doi:10.1016/j.immuni.2008.09.002
100. Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol (2011) 29:163–83. doi:10.1146/annurev-immunol-031210-101345
101. Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol (2011) 11(8):558–65. doi:10.1038/nri3027
102. Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood (2008) 112(9):3713–22. doi:10.1182/blood-2008-03-146290
103. Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, Lebon P, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity (2007) 27(3):481–92. doi:10.1016/j.immuni.2007.07.021
104. Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Lambeck AJ, et al. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T-cells, despite lower Ag uptake than myeloid dendritic cell subsets. Blood (2013) 121(3):459–67. doi:10.1182/blood-2012-06-435644
105. Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med (2013) 210(5):1035–47. doi:10.1084/jem.20121103
106. Swiecki M, Wang Y, Gilfillan S, Colonna M. Plasmacytoid dendritic cells contribute to systemic but not local antiviral responses to HSV infections. PLoS Pathog (2013) 9(10):e1003728. doi:10.1371/journal.ppat.1003728
107. Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood (2007) 109(3):1131–7. doi:10.1182/blood-2006-05-023770
108. Kassner N, Krueger M, Yagita H, Dzionek A, Hutloff A, Kroczek R, et al. Cutting edge: plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol (2010) 184(2):550–4. doi:10.4049/jimmunol.0903152
109. Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature (1999) 397:263–6. doi:10.1038/16717
110. Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med (2007) 204(1):105–15. doi:10.1084/jem.20061660
111. Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev (2010) 234(1):18–31. doi:10.1111/j.0105-2896.2009.00870.x
112. Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood (2012) 119(25):6052–62. doi:10.1182/blood-2012-01-406967
113. Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol (2013) 120:239–67. doi:10.1016/B978-0-12-417028-5.00009-0
114. Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Guttler S, et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity (2009) 31(5):823–33. doi:10.1016/j.immuni.2009.08.027
115. Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(-) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol (2001) 167(8):4345–50. doi:10.4049/jimmunol.167.8.4345
116. Ashok D, Schuster S, Ronet C, Rosa M, Mack V, Lavanchy C, et al. Cross-presenting dendritic cells are required for control of Leishmania major infection. Eur J Immunol (2014) 44(5):1422–32. doi:10.1002/eji.201344242
117. Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med (2010) 207(4):823–36. doi:10.1084/jem.20091627
118. Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest (2009) 119(9):2441–50. doi:10.1172/JCI39134
119. Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science (2007) 315(5808):107–11. doi:10.1126/science.1136080
120. Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, et al. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med (1999) 189:587–92. doi:10.1084/jem.189.3.587
121. Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol (2013) 14(9):937–48. doi:10.1038/ni.2679
122. Tussiwand R, Everts B, Grajales-Reyes GE, Kretzer NM, Iwata A, Bagaitkar J, et al. Klf4 expression in conventional dendritic cells is required for T helper 2 cell responses. Immunity (2015) 42(5):916–28. doi:10.1016/j.immuni.2015.04.017
123. den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med (2002) 196(6):817–27. doi:10.1084/jem.20020295
124. Manickasingham SP, Edwards AD, Schulz O, Reis e Sousa C. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur J Immunol (2003) 33(1):101–7. doi:10.1002/immu.200390001
125. Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res (2002) 4(Suppl 3):S127–32. doi:10.1186/ar567
126. O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology (1994) 82(3):487–93.
127. Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science (1999) 284:1835–7. doi:10.1126/science.284.5421.1835
128. Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med (1999) 5:919–23. doi:10.1038/11360
129. Farkas L, Kvale EO, Lund-Johansen F, Jahnsen FL. Plasmacytoid dendritic cells induce a distinct cytokine pattern in virus-specific CD4+ memory T cells that is modulated by CpG oligodeoxynucleotides. Scand J Immunol (2006) 64(4):404–11. doi:10.1111/j.1365-3083.2006.01792.x
130. Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med (2012) 209(4):653–60. doi:10.1084/jem.20111457
131. Narbutt J, Lesiak A, Sysa-Jedrzejowska A, Smolewski P, Robak T, Zalewska A. The number and distribution of blood dendritic cells in the epidermis and dermis of healthy human subjects. Folia Histochem Cytobiol (2006) 44(1):61–3.
132. Tabarkiewicz J, Rybojad P, Jablonka A, Rolinski J. CD1c+ and CD303+ dendritic cells in peripheral blood, lymph nodes and tumor tissue of patients with non-small cell lung cancer. Oncol Rep (2008) 19(1):237–43.
133. Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity (2012) 37(1):60–73. doi:10.1016/j.immuni.2012.04.012
134. Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T cell responses. Blood (2013) 122(6):932–42. doi:10.1182/blood-2013-04-495424
135. Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, et al. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol (2011) 186(11):6207–17. doi:10.4049/jimmunol.1002632
136. Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med (2010) 207(6):1247–60. doi:10.1084/jem.20092140
137. Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med (2010) 207(12):2703–17. doi:10.1084/jem.20092720
138. Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207(6):1261–71. doi:10.1084/jem.20092618
139. Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med (2010) 207(6):1131–4. doi:10.1084/jem.20100985
140. Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207(6):1283–92. doi:10.1084/jem.20100223
141. Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med (2010) 207(6):1273–81. doi:10.1084/jem.20100348
142. Dillon SM, Rogers LM, Howe R, Hostetler LA, Buhrman J, McCarter MD, et al. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol (2010) 184(12):6612–21. doi:10.4049/jimmunol.1000041
143. Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does toll-like receptor 3 play a biological role in virus infections? Virology (2004) 322(2):231–8. doi:10.1016/j.virol.2004.01.033
144. Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science (2007) 317(5844):1522–7. doi:10.1126/science.1139522
145. Klechevsky E. Human dendritic cells – stars in the skin. Eur J Immunol (2013) 43(12):3147–55. doi:10.1002/eji.201343790
146. Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med (2010) 207(1):189–206. doi:10.1084/jem.20091964
147. McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A, Poyner E, et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity (2014) 41(3):465–77. doi:10.1016/j.immuni.2014.08.006
148. Said A, Weindl G. Regulation of dendritic cell function in inflammation. J Immunol Res (2015) 2015:743169. doi:10.1155/2015/743169
149. Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol (2014) 15(1):98–108. doi:10.1038/ni.2768
150. von Bernuth H, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol (2012) 42(12):3126–35. doi:10.1002/eji.201242683
151. Bauer S, Pigisch S, Hangel D, Kaufmann A, Hamm S. Recognition of nucleic acid and nucleic acid analogs by toll-like receptors 7, 8 and 9. Immunobiology (2008) 213(3–4):315–28. doi:10.1016/j.imbio.2007.10.010
152. von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science (2008) 321(5889):691–6. doi:10.1126/science.1158298
153. Artyomov MN, Munk A, Gorvel L, Korenfeld D, Cella M, Tung T, et al. Modular expression analysis reveals functional conservation between human Langerhans cells and mouse cross-priming dendritic cells. J Exp Med (2015) 212(5):743–57. doi:10.1084/jem.20131675
154. Cohn L, Chatterjee B, Esselborn F, Smed-Sorensen A, Nakamura N, Chalouni C, et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med (2013) 210(5):1049–63. doi:10.1084/jem.20121251
155. Kassianos AJ, Hardy MY, Ju X, Vijayan D, Ding Y, Vulink AJ, et al. Human CD1c (BDCA-1)+ myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur J Immunol (2012) 42(6):1512–22. doi:10.1002/eji.201142098
156. Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol (2005) 6(8):769–76. doi:10.1038/ni1223
157. Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity (2013) 38(2):336–48. doi:10.1016/j.immuni.2012.10.018
158. Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science (1999) 283:1183–6. doi:10.1126/science.283.5405.1183
159. Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat Immunol (2000) 1:305–10. doi:10.1038/79747
160. Yu CI, Becker C, Metang P, Marches F, Wang Y, Toshiyuki H, et al. Human CD141+ dendritic cells induce CD4+ T cells to produce type 2 cytokines. J Immunol (2014) 193(9):4335–43. doi:10.4049/jimmunol.1401159
161. Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev (2010) 21(5):331–44. doi:10.1016/j.cytogfr.2010.09.002
162. Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol (1998) 160(7):3188–93.
163. Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity (2011) 35(5):792–805. doi:10.1016/j.immuni.2011.09.017
164. Donnelly RP, Dickensheets H, O’Brien TR. Interferon-lambda and therapy for chronic hepatitis C virus infection. Trends Immunol (2011) 32(9):443–50. doi:10.1016/j.it.2011.07.002
165. Meixlsperger S, Leung CS, Ramer PC, Pack M, Vanoaica LD, Breton G, et al. CD141+ dendritic cells produce prominent amounts of IFN-alpha after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood (2013) 121(25):5034–44. doi:10.1182/blood-2012-12-473413
166. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature (2009) 461(7262):399–401. doi:10.1038/nature08309
167. Megjugorac NJ, Gallagher GE, Gallagher G. IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra. Blood (2010) 115(21):4185–90. doi:10.1182/blood-2009-09-246157
168. Zhang S, Kodys K, Li K, Szabo G. Human type 2 myeloid dendritic cells produce interferon-lambda and amplify interferon-alpha in response to hepatitis C virus infection. Gastroenterology (2013) 144(2):414.e–25.e. doi:10.1053/j.gastro.2012.10.034
169. Yoshio S, Kanto T, Kuroda S, Matsubara T, Higashitani K, Kakita N, et al. Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-lambda in response to hepatitis C virus. Hepatology (2013) 57(5):1705–15. doi:10.1002/hep.26182
170. Lozza L, Farinacci M, Bechtle M, Staber M, Zedler U, Baiocchini A, et al. Communication between human dendritic cell subsets in tuberculosis: requirements for naive CD4(+) T cell stimulation. Front Immunol (2014) 5:324. doi:10.3389/fimmu.2014.00324
171. Lozza L, Farinacci M, Fae K, Bechtle M, Staber M, Dorhoi A, et al. Crosstalk between human DC subsets promotes antibacterial activity and CD8+ T-cell stimulation in response to bacille Calmette-Guerin. Eur J Immunol (2014) 44(1):80–92. doi:10.1002/eji.201343797
172. Gupta MR, Kolli D, Garofalo RP. Differential response of BDCA-1+ and BDCA-3+ myeloid dendritic cells to respiratory syncytial virus infection. Respir Res (2013) 14:71. doi:10.1186/1465-9921-14-71
173. Gupta MR, Kolli D, Molteni C, Casola A, Garofalo RP. Paramyxovirus infection regulates T cell responses by BDCA-1+ and BDCA-3+ myeloid dendritic cells. PLoS One (2014) 9(6):e99227. doi:10.1371/journal.pone.0099227
174. Florentin J, Aouar B, Dental C, Thumann C, Firaguay G, Gondois-Rey F, et al. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood (2012) 120(23):4544–51. doi:10.1182/blood-2012-02-413286
175. Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med (2001) 194(12):1823–34. doi:10.1084/jem.194.12.1823
176. O’Brien M, Manches O, Bhardwaj N. Plasmacytoid dendritic cells in HIV infection. Adv Exp Med Biol (2013) 762:71–107. doi:10.1007/978-1-4614-4433-6_3
177. Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med (2003) 9(9):1131–7. doi:10.1038/nm917
178. Monath TP. Yellow fever vaccine. Expert Rev Vaccines (2005) 4(4):553–74. doi:10.1586/14760584.4.4.553
179. Divekar AA, Zaiss DM, Lee FE, Liu D, Topham DJ, Sijts AJ, et al. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J Immunol (2006) 176(3):1465–73. doi:10.4049/jimmunol.176.3.1465
180. Parvanova I, Rettig L, Knuth A, Pascolo S. The form of NY-ESO-1 antigen has an impact on the clinical efficacy of anti-tumor vaccination. Vaccine (2011) 29(22):3832–6. doi:10.1016/j.vaccine.2011.03.073
181. Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med (2008) 205(4):869–82. doi:10.1084/jem.20071087
182. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature (2006) 440(7081):237–41. doi:10.1038/nature04516
183. Okemoto K, Kawasaki K, Hanada K, Miura M, Nishijima M. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1beta or activation of caspase-1. J Immunol (2006) 176(2):1203–8. doi:10.4049/jimmunol.176.2.1203
184. Hui G, Hashimoto C. Interleukin-6 has differential influence on the ability of adjuvant formulations to potentiate antibody responses to a Plasmodium falciparum blood-stage vaccine. Vaccine (2007) 25(36):6598–603. doi:10.1016/j.vaccine.2007.10.010
185. Brewer JM, Conacher M, Gaffney M, Douglas M, Bluethmann H, Alexander J. Neither interleukin-6 nor signalling via tumour necrosis factor receptor-1 contribute to the adjuvant activity of Alum and Freund’s adjuvant. Immunology (1998) 93(1):41–8. doi:10.1046/j.1365-2567.1998.00399.x
186. Banchereau R, Baldwin N, Cepika AM, Athale S, Xue Y, Yu CI, et al. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun (2014) 5:5283. doi:10.1038/ncomms6283
187. Palucka K, Ueno H, Fay J, Banchereau J. Harnessing dendritic cells to generate cancer vaccines. Ann N Y Acad Sci (2009) 1174:88–98. doi:10.1111/j.1749-6632.2009.05000.x
188. Mandraju R, Murray S, Forman J, Pasare C. Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo. J Immunol (2014) 192(9):4303–15. doi:10.4049/jimmunol.1302244
189. Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine (2014) 32(48):6377–89. doi:10.1016/j.vaccine.2014.06.065
190. Fehres CM, Bruijns SC, van Beelen AJ, Kalay H, Ambrosini M, Hooijberg E, et al. Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8+ T-cell cross-priming. Eur J Immunol (2014) 44(8):2415–24. doi:10.1002/eji.201344094
191. Vandepapeliere P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine (2008) 26(10):1375–86. doi:10.1016/j.vaccine.2007.12.038
192. Coban C, Kobiyama K, Jounai N, Tozuka M, Ishii KJ. DNA vaccines: a simple DNA sensing matter? Hum Vaccin Immunother (2013) 9(10):2216–21. doi:10.4161/hv.25893
193. Pascolo S. Messenger RNA-based vaccines. Expert Opin Biol Ther (2004) 4(8):1285–94. doi:10.1517/14712598.4.8.1285
194. Pascolo S. The messenger’s great message for vaccination. Expert Rev Vaccines (2015) 14(2):153–6. doi:10.1586/14760584.2015.1000871
195. Teufel R, Carralot JP, Scheel B, Probst J, Walter S, Jung G, et al. Human peripheral blood mononuclear cells transfected with messenger RNA stimulate antigen-specific cytotoxic T-lymphocytes in vitro. Cell Mol Life Sci (2005) 62(15):1755–62. doi:10.1007/s00018-005-5067-6
196. Scheel B, Teufel R, Probst J, Carralot JP, Geginat J, Radsak M, et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol (2005) 35(5):1557–66. doi:10.1002/eji.200425656
197. Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther (2007) 14(15):1175–80. doi:10.1038/sj.gt.3302964
198. Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol (2000) 30(1):1–7. doi:10.1002/1521-4141(200001)30:1<1::AID-IMMU1>3.0.CO;2-#
199. Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother (2008) 31(2):180–8. doi:10.1097/CJI.0b013e31815ce501
200. Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther (2011) 19(5):990–9. doi:10.1038/mt.2010.289
Keywords: dendritic cells, cytokines, toll-like receptors, T-cell differentiation, cytotoxic T cells
Citation: Geginat J, Nizzoli G, Paroni M, Maglie S, Larghi P, Pascolo S and Abrignani S (2015) Immunity to pathogens taught by specialized human dendritic cell subsets. Front. Immunol. 6:527. doi: 10.3389/fimmu.2015.00527
Received: 29 July 2015; Accepted: 28 September 2015;
Published: 13 October 2015
Edited by:
Elisabetta Padovan, University of Basel, SwitzerlandReviewed by:
Marina Cella, Washington University School of Medicine, USACopyright: © 2015 Geginat, Nizzoli, Paroni, Maglie, Larghi, Pascolo and Abrignani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens Geginat, Z2VnaW5hdEBpbmdtLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.