
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 02 June 2015
Sec. Antigen Presenting Cell Biology
Volume 6 - 2015 | https://doi.org/10.3389/fimmu.2015.00254
This article is part of the Research Topic Dendritic Cell and Macrophage nomenclature and classification View all 18 articles
Intestinal mononuclear phagocytes find themselves in a unique environment, most prominently characterized by its constant exposure to commensal microbiota and food antigens. This anatomic setting has resulted in a number of specializations of the intestinal mononuclear phagocyte compartment that collectively contribute the unique steady state immune landscape of the healthy gut, including homeostatic innate lymphoid cells, B, and T cell compartments. As in other organs, macrophages and dendritic cells (DCs) orchestrate in addition the immune defense against pathogens, both in lymph nodes and mucosa-associated lymphoid tissue. Here, we will discuss origins and functions of intestinal DCs and macrophages and their respective subsets, focusing largely on the mouse and cells residing in the lamina propria.
Intestinal mononuclear phagocytes are located in a unique anatomic environment that necessitated the evolution of special functional adaptations of these cells. Exposure to commensal bacteria and harmful pathogens, as well as nutrients and food antigens, in the intestinal lumen force the immune system to continuously weigh tolerogenic and protective immune response. Disruption of this critical and delicate balance can result in devastating inflammatory reactions, e.g., hyper-reactivity to food components (1) or inflammatory bowel diseases (IBD), such as Crohn’s disease or ulcerative colitis (2).
Both dendritic cells (DC) and macrophages are found spread throughout the connective tissue that underlies the epithelial layer of the gut, the lamina propria. Moreover, representatives of the two main mononuclear phagocyte families are also located in mucosa-associated lymphoid tissue (MALT), including Peyers’ Patches and isolated lymphoid follicles (ILFs) (3). DC and macrophages have distinct, yet complementary roles in maintaining gut homeostasis and immune defense. In keeping with their migratory capacity, DC translocate from the lamina propria via the lymphatics to the gut-draining mesenteric lymph nodes (MsnLNs), where they present antigens to naïve T cells, polarize them toward effector fates, and thus establish the adaptive branch of the immune system (4).
Macrophages, on the other hand, are believed to contribute to the local clearance of bacteria from the tissue, translate alert signals to other immune cells, secrete cytokines to establish the local homeostatic immune cell network, and participate in T cell re-stimulation and maintenance within the lamina propria (5).
DC and macrophages can, as discussed in detail below, be divided into several subpopulations with defined origins, overlapping and distinct surface marker profiles, functions and locations. The best characterized DC and macrophage subsets and their key features are summarized in Table 1.
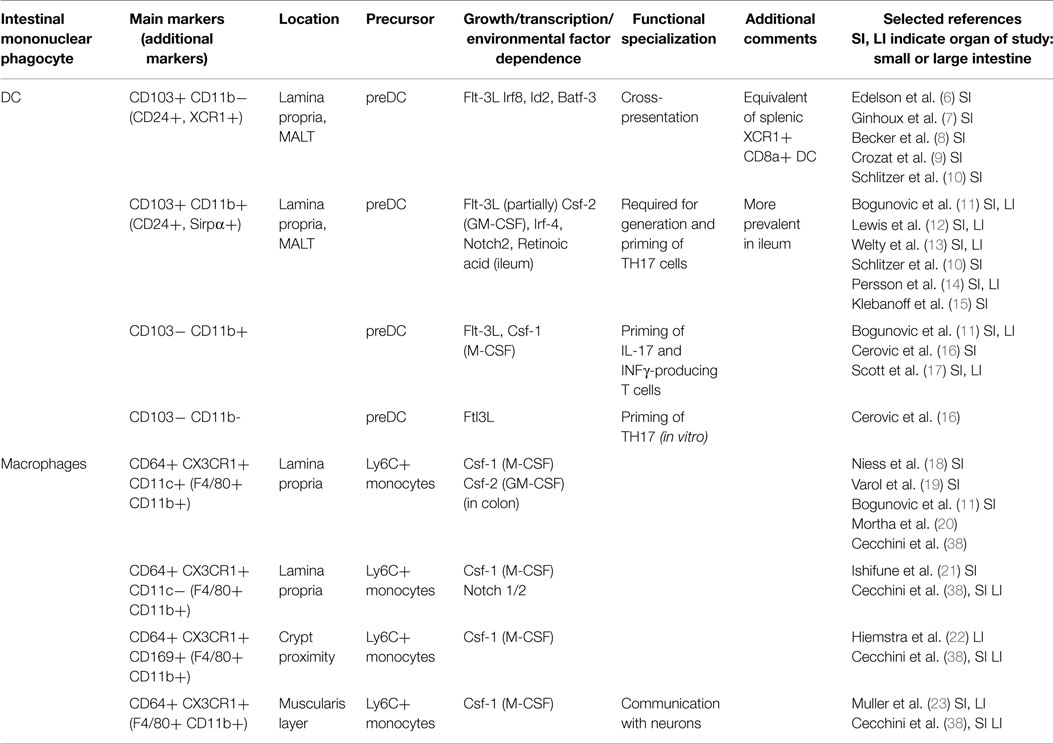
Table 1. Mononuclear phagocytes and their respective subsets in the lamina propria of the mouse intestine.
With this review, we provide an overview on the characteristics and function of intestinal macrophages and DC in the mouse, including specific roles of their subpopulations. We will discuss distinct origins, roles in maintaining gut homeostasis, and the interactions between these cells and other immune cells. Finally, we will review their communication with their non-immune microenvironment and elaborate on emerging roles of macrophages and DC in inflammation.
Macrophages are the most abundant mononuclear phagocytes in the steady-state gut lamina propria (3, 24). Intestinal macrophages are currently best characterized by their expression of CD64, the Fcγ receptor 1 (FcγRI) (25), and the chemokine receptor CX3CR1 (18), as well as the F4/80 antigen (EGF-like module containing mucin-like hormone receptor-like 1-EMR1) and the integrins CD11b and CD11c (26). Due to the high surface expression levels of the chemokine receptor CX3CR1 by gut macrophages, these cells can also be readily detected, isolated, and studied in situ using intra-vital microscopy on mice harboring a GFP reporter gene inserted into the CX3CR1 locus (27).
Like other tissue macrophages (28), also intestinal macrophages are first established before birth from precursors originating in the yolk sac or fetal liver (29). However, unlike macrophages in most other tissues, these embryo-derived cells are replaced in the gut shortly after birth by cells that derive from Ly6C+ blood monocytes (29). The adult monocyte-derived cells display a uniquely short half-life for macrophages (30) indicating their continuous renewal. The monocytic origin of intestinal macrophages was first established in adoptive transfer experiments, involving the transfer of CX3CR1gfp monocyte-precursors and monocytes into CD11c-DTR transgenic mice, whose CD11c-expressing cells, including intestinal macrophages, were depleted by a diphtheria toxin challenge (11, 19, 31). During their differentiation into gut macrophages, monocytes lose Ly6C expression, while other surface markers, such as MHCII, F4/80, CD64, CD11c, and CX3CR1 are up-regulated (25, 32, 33). Moreover, the cells acquire a characteristic anti-inflammatory gene expression profile (32, 34), whose timely establishment and maintenance are critical for gut homeostasis (35). This includes the expression of IL-10, TREM-2, IRAK-M, and tumor necrosis factor (TNF)AIP3 genes, but also of TNFα, which has both pro- and -anti-inflammatory activity (32). Of note, this expression profile is robust, as it seems to withstand acute challenges, such as the ones associated with oral dextran sulfate sodium (DSS) exposure (32). The molecular cues that drive the “education” of the macrophages in various regions of the gut remain to be defined, but the epithelium is likely to play a role in this process. Epithelial cells could control macrophage differentiation by secretion of immune-regulatory factors, such as thymic stromal lymphopoietin (TSLP), transforming growth factor-β (TGF-β), and prostaglandin E-2 (PGE-2) (36). In addition, recent findings suggested that semaphorin 7A, which is secreted by epithelial cells, contributes to the induction of IL-10 expression by CX3CR1+ intestinal macrophages (37). Also, colony-stimulating factor 2 (Csf-1; previously named macrophage colony-stimulating factor, M-CSF) and colony-stimulating factor 2 (Csf-2; previously named granulocyte-macrophage colony-stimulating factor, GM-CSF) play a role in the development of macrophages. Csf-1 is a crucial factor for monocyte development, as Csf-1-deficient osteopetrotic (op/op) mice display reduced levels of F4/80+ cells in the small and large intestine after the first few days of life (28, 38, 39). Csf-2-depleted mice were shown have reduced numbers of CD11c+ colonic macrophages (20).
Of note, Ly6C+ monocytes fail to acquire the characteristic macrophage quiescence during intestinal inflammation, but under this condition respond to local factors that trigger pattern recognition receptors, such as TLRs and NLRs, giving rise to pro-inflammatory macrophages (32). These pro-inflammatory cells, which in acute inflammation outnumber the resident macrophage population, secrete IL-12, IL-23, TNF-α, and inducible nitric oxide synthase (iNOS) (32).
A key suppressor of macrophage-associated inflammation is the IL-10/IL-10 receptor (IL-10R) axis, as mice bearing mutations in IL10-Ra in intestinal CX3CR1+ macrophages developed severe colitis (35) comparable to the pathology reported for IL-10-deficient animals (40). This central critical role of IL-10 in maintaining the non-inflammatory state of macrophages, and thereby, gut homeostasis is also supported by research conducted on samples from humans with loss of function mutations in IL-10R (41). The latter provides an explanation for the severe early onset of colitis observed in pediatric patients harboring nonsense and missense mutations in IL-10R, which reduce IL-10R expression and hamper its signaling cascades (42). Interestingly though, IL-10 production by intestinal macrophages, although also prominent, seems to be redundant for the maintenance of gut homeostasis (35); rather the system seems to rely on alternative IL-10 sources, such as Treg cells (43).
Homeostatic monocyte recruitment to the gut is thought to depend on the chemokine receptor CCR2, as CCR2-deficient mice display less intestinal macrophages and CCR2-deficient intestinal macrophages are underrepresented in mixed bone marrow chimeras (24, 25). The exact factors and mechanisms that ensure homeostatic Ly6C+ monocyte recruitment to the steady state gut are, however, still unknown. While they are likely related to the microbiota exposure of the tissue, analysis of germ-free animals has yielded conflicting results (29, 34, 44, 45). The latter could be due to intestinal embryo-derived macrophages that might persist in the absence of arising competition by an adult monocyte influx.
Interestingly, emerging evidence suggests that intestinal macrophages are more heterogeneous than previously thought. Monocyte-derived CD11b+ CX3CR1+ cells in the gut comprise both CD11c+ and CD11c− cells. While differential functions of these cells remain to be established, studies into this matter might profit from the recent finding that generation of CD11c+, but not CD11c− CX3CR1+ intestinal macrophages requires Notch signaling (21). A subpopulation of CD169-expressing CX3CR1+ macrophages has been reported to be associated with the intestinal crypts (22), although these cells will require further functional characterization. Bogunovic and colleagues recently reported an intriguing CX3CR1+ macrophage subpopulation that resides in the muscularis layer and communicates with enteric neurons to regulate gastrointestinal motility (23). Importantly, we and others have recently shown that macrophages isolated from distinct tissues, such as the liver, lung, brain, and peritoneum, differ considerably with respect to their gene expression profile (46, 47). As expected, this diversity is also prominently reflected in the differential enhancer usage of these cells, as inferred from highly divergent histone modifications (47). Moreover, given that the number of regulatory elements by far exceeds the number of genes (48, 49), this heterogeneity is even more pronounced, including both active and poised enhancer states (47). This applies, albeit to a lesser extent, also to macrophages located in proximal and distal segments of the gut (47). Epigenetic heterogeneity of intestinal macrophages likely reflects monocyte exposure to distinct environmental cues in ileum and colon during their local differentiation (32, 47). In-depth understanding of how these macrophage identities are established, including the hierarchy of induced transcription factors, could yield valuable insights into monocyte differentiation that might be applicable to other tissues and inflammatory settings. PU.1 is a pioneering factor, which induces c-fms transcription and is hence required for macrophage differentiation (50). Intestinal macrophages are furthermore characterized by prominent expression of the Runt-related transcription factor 3 (Runx-3) (47). Interestingly, mice that harbor Runx3 deficiency develop spontaneous colitis (51). Other candidates that might be involved in the establishment of the intestinal macrophage signature are the interferon regulatory factors 4 and 5 (Irf-4, Irf-5), shown to be associated with classical and alternative macrophage activation, respectively (52–54).
Pioneering studies by Rescignio and colleagues revealed that certain intestinal mononuclear phagocytes can penetrate the intestinal epithelium by virtue of expression of tight junction proteins and formations of dendritic projections (55). These structures, later termed trans-epithelial dendrites (TEDs) (56), were subsequently ascribed to macrophages expressing CX3CR1 (18) and allegedly allow these non-migratory cells to sense, and potentially sample, the luminal content (18, 56). TED formation by macrophages in the terminal region of the ileum was found to be dependent on expression of both CX3CR1 macrophages and its membrane-tethered ligand CX3CL1/Fractalkine by selected epithelial cells (57). CX3CR1-deficient and CX3CL1-deficient mice were reported to be relatively protected from acute, DSS-induced colitis (58) – a phenotype that might be related to TED formation (57). Likewise, CX3CR1-deficient mice were shown to display impaired oral tolerance, which was related to impaired IL-10 production by intestinal macrophages, though not their TED formation (59). Finally, there is evidence for a potential role of CX3CR1+ macrophages in the capture of luminal bacteria (60) and even the transport of the latter to lymph nodes, at least under conditions of dysbiosis (61). However, the exact definition of macrophage contributions in their native tissue context remains challenging, because it requires their accurate discrimination from closely related and phenotypically similar monocyte-derived DC.
Apart from their role in maintaining intestinal immune homeostasis, gut macrophages also contribute critically to epithelial wound healing. Macrophages associated with the crypts of Lieberkuehn in the colon were reported to assist, following tissue damage, the proliferation and survival of epithelial progenitor cells in a Myd88-dependent manner (62–64). Moreover, in a murine model of acute epithelial regeneration in the colon, activated macrophages supported tissue repair by up-regulating expression of IL-3 and IL-4, while inhibiting secretion of TNF and interferon-γ (IFN-γ) in the lamina propria (3, 65). Macrophages also appear to be able to influence the permeability of the epithelium barrier via the secretion of IL-6 and NO, thereby potentially increasing the invasion of pathogens (66).
Macrophages are inferior to DC in their ability to prime naïve T cells (67). This might be due to their rapid degradation of ingested proteins, which impairs their ability to retain antigens for presentation (68). Moreover, at least in steady state, intestinal CX3CR1+ macrophages lack expression of CCR7, i.e., the chemokine receptor required for migration to the MsnLNs (25, 69). Rather, the cells that reside in the lamina propria have been proposed to maintain the functionality of FoxP-3+ T regulatory cells that migrated back from the MsnLNs into the tissue (59). Thus, while Treg cell generation of CX3CR1-deficient mice is unimpaired, these animals harbor reduced Treg cell numbers in the lamina propria, a phenotype that is associated with impaired oral tolerance (59). In light of other data (70), the authors of this study linked the reduced FoxP-3+ Treg cell numbers to impaired production of IL-10 by CX3CR1+ macrophages (59). However, the latter might have to be revised, since CX3CR1Cre:IL10fl/fl mice were shown to harbor unimpaired FoxP-3+ Treg cell numbers (35). Also, interactions between CX3CR1+ macrophages and Th17 cells, which are rarely found in intestinal lymphoid tissues and, though primed in the MsnLN, might terminally differentiate in the lamina propria, remain incompletely defined. On one hand, it was shown that intestinal CD70hi CX3CR1+ macrophages are activated by commensal-derived ATP and drive the in vitro differentiation of Th17 cells (71, 72). On the other hand, intestinal macrophages were reported to counteract Th17 generation that is promoted by CD103+CD11b+ DC (73, 74). Of note, CD103+CD11b+ DCs and Th17 cells co-localize in the intestinal tract, as the number of both cells drop from the duodenum to the ileum, and they are scarce in the colon. By contrast, CX3CR1+ macrophages and FoxP3+ Treg cells are most abundant in the colon (74).
Recent findings revealed an intriguing cross-talk between intestinal macrophages and innate lymphoid cells (ILC). Thus, in response to luminal stimuli and using a signaling pathway involving the TLR adaptor Myd88, macrophages were shown to secrete IL-1β and in turn induce production of csf-2 by RORγt+ type 3 ILC (20). Mice lacking Csf-2 display reduced numbers of colonic macrophages and DC, associated with a hampered Treg cell compartment (20). Moreover, in a Citrobacter infection model CX3CR1+ macrophages were shown to promote ILC production of IL-22 via secretion of IL-23 (75), in line with another report (76). Interestingly, CX3CR1+ macrophage-derived IL-23 not only induces IL-22 but also seems to concomitantly suppress IL-12 production by CD103+ CD11b− DC and thereby prevents otherwise detrimental immunopathology (77). Notably, the latter finding provides first evidence for the existence of a direct cross-talk among intestinal mononuclear phagocytes in tissue context, a topic that clearly deserves further study.
Dendritic cells are specialized in communicating with T cells, curbing autoreactivity and activating T cell immunity in response to threats. Specifically, DC provide T cells with antigenic peptides that are presented in MHC context, co-stimulation and instructing cytokines that govern T cell polarization into effector cells (67). In order to maintain homeostasis and avoid inflammatory responses toward innocuous antigens, gut DC employ tolerogenic mechanisms that allow them to dampen adaptive immunity. MsnLN- and lamina propria-resident CD103+ DC secrete, for example, retinoic acid (RA) and transforming growth factor-β (TGF-β), which promote the generation of Foxp3+ Treg cells and contribute to the differentiation of plasma cells, which secrete IgA (78, 79).
Intestinal DC in mice are characterized by the surface expression of the integrins CD11c (αX) and CD103 (αEβ7) (11, 19, 69). More recently, CD24 and Sirpα have been introduced for the better discrimination of DC from macrophages (8, 10). CD103+ DC in the gut arise from dedicated DC precursors, or preDC, and accordingly, mice deficient for fms-related tyrosine kinase-3 receptor (Flt-3) or its ligand Flt-3L have significantly decreased levels of intestinal DC (7, 19). Other, currently though less well-characterized DC progenitors are α4β7+ so-called “pre-μDC,” which are generated in the bone marrow and were shown to give rise to classical CD103+ DC and CCR9+ plasmacytoid DC (80).
Classical CD103+ DC are divided into two major subpopulations according to their expression of CD11b (αM) (81). CD103+ CD11b+ DC and CD103+ CD11b− DC display distinct abundance in small and large intestine, present different additional surface markers, and require different growth factors for their development (82, 83).
CD103+ CD11b+ DC are developmentally related to CD11b+ CD8α− splenic DCs (15) and found in the lamina propria of the small and large intestine. They can migrate in CCR7-dependent manner (84) to the MsnLNs, where they present luminal antigens to T cells. CD103+ CD11b+ DCs likely represent a heterogeneous population, as a fraction of them is Csf-2-dependent (3). Development of CD103+ CD11b+ DC, but not of CD103+ CD11b− DC, is hampered in Csf-2R-deficient mice (85) and when expression of Notch-2 (12, 76) or IRF-4 (14) is impaired. Moreover, CD103+ CD11b+ DC numbers are also reduced in absence of RA and under conditions of vitamin A deprivation (15).
CD103+ CD11b− DC are more prevalent in lymphoid organs – the Peyer’s Patches, MsnLNs, and ILFs (7, 69). However, they can be found also in animals lacking these structures, and are hence not limited to lymphoid tissues (3). Similar to classical CD8α+ DC in the spleen, CD103+ CD11b− DC depend on the expression of the transcription factors BatF-3 and Irf-8 (6, 15). Like the former, they also express the chemokine receptor XCR1 that has emerged as a universal marker for this DC subset in mouse and human (8, 9). The connection between CD103+ CD11b− DC and CD8α+ DC is also supported by the fact that the number of CD103+ CD11b− DC was shown to increase, alongside with splenic CD8α+ DC, in mice that display constitutive β-catenin activation (86). Moreover, like splenic CD8α+ DC (87), also CD103+ CD11b− DC are specialized in cross-presentation (88).
The exact definition of intestinal DC is complicated, since monocyte-derived cells can acquire phenotypic and functional DC hallmarks. Studies have described a population of CD103−CX3CR1+CD11b+ DC, which resides in the lamina propria (11, 16). These cells are CSFR-1 dependent and appear to be derived from Ly6Chigh monocytes (11). Recent studies also reported that under inflammatory conditions, these CD103−CX3CR1+CD11b+ DC expressed CCR7 and migrated in the intestinal lymph, similar to classical intestinal DC, and induced the differentiation of IL-17 and IFN-γ producing T cells (16, 17).
CD103+ DC, present in the lamina propria and associated with the intestinal epithelium lining the villi, provide surveillance of the luminal environment (30). They detect foreign and inflammatory signals, acquire and present antigens and interact with T cells by migrating to secondary lymphoid organs (3). Located deep in the core of the villous lamina propria, CD103+CD11b+ DC would seemingly have limited access to luminal signals, unless antigens or bacteria cross the epithelium or are imported into the lamina propria by other cells, e.g., macrophages, epithelial M cells, or small intestine goblet cells (36, 89, 90). However, lamina propria-resident CD103+ DC were shown to migrate into the epithelial cell layer and capture bacterial antigens (90).
Mucosal T cell priming, arguably one of the primary roles of gut DC, is believed to be restricted to lymphoid tissues (3). Intestinal DC are hence bound to migrate from the lamina propria to the MsnLNs, or within Peyer’s Patches into T cell zones. Indeed, CD103+ DC were detected in the intestinal lymph under homeostatic conditions (69, 84). In addition, after systemic BrdU administration, labeled CD103+ DC were found in the lamina propria before they could be discerned in the MsnLNs (30). LN-resident CD103+ DC are thus derived from the tissue and constantly immigrate (30, 91). Interestingly, steady state migration of intestinal CD103+ DC does not appear to be induced by the microbiota or by TLR signaling (92), but may rather depend on a low, tonic release of inflammatory cytokines, or result from spontaneous DC maturation. Nevertheless, entry of CD103+ DC into the MsnLNs is of course considerably enhanced by pro-inflammatory cytokines or TLR ligands (93, 94). Migration of intestinal DC depends on CCR7, both in steady state and under inflammatory conditions. Accordingly, CCR7 expression is up-regulated in DC before their migration from the tissue into the MsnLN (84) and CCR7 deficient DC fail to migrate (69, 84, 95). Moreover, it was recently shown that DC can also migrate from the lamina propria into the epithelial layer (90) and can thus gain direct access to antigen and luminal bacteria. Hence, following challenge with Salmonella, accumulation of the bacteria was first observed in DC of the epithelial fraction and only subsequently in DC in the lamina propria (90).
DC intimately interact with the epithelial layer of the intestine by a variety of mechanisms. Small intestinal goblet cells were shown to transfer small soluble antigens from the intestinal lumen to CD103+ DC (89). Chemokines secreted by enterocytes in response to TLR ligand exposure can induce the above-mentioned relocation of lamina propria DC to the epithelium (90). In addition, it is becoming more and more evident that epithelial cells play a critical role in maintaining DC in a tolerogenic state, compatible with gut homeostasis. Epithelial and stromal cells secrete factors, which are thought to induce DC tolerance, such as RA, TGF-β, PGE-2, and TSLP (3, 82, 96–99). In parallel to ILC (20), intestinal epithelial cells regulate retinal dehydrogenase (RALDH) expression by CD103+ DC that the cells need to metabolize retinoids. Specifically, epithelial cells express a critical cytosolic retinoid chaperone, the cellular retinol binding protein II, which is required for in vivo imprinting of gut DC by lumenal retinoids (99, 100). Supporting this notion, the in vitro co-culture of bone marrow- or spleen-derived DC with epithelial cells results in the up-regulation of CD103 and RALDH, together with TGF-β imprinted homing potential on T cells (101–103). These data establish the potential of intestinal epithelial cells to educate intestinal DC, although further in vivo studies and higher resolution, with respect to cell subsets, are required to better elucidate the underlying mechanisms.
Intestinal CD103+ DC, found in lamina propria, Peyer’s Patches, and the MsnLNs program T cells to express the gut-homing factors CCR9 and α4β7 integrin (101, 104, 105). Concomitantly, DC can also induce the development of FoxP-3+ and IL-10 producing Treg cells (106) and prime Th17 cells (17, 107, 108). The majority of these DC-governed priming events require TGF-β signaling and RA, which are generated in the DC by enzymatic conversion of all-trans-retinal, a derivative of vitamin A, using RALDH2 (101, 109, 110). Indeed, RA has emerged as the critical conditioning factor for intestinal DC, as vitamin A is crucial for the activity of the enzyme RALDH in DC. Without RALDH, the ability of DC to imprint T cells is hampered, and restored only after vitamin A administration (111). The balance between RA and TGF-β levels seems to determine the fate of Treg cells primed by DC, as presence of both RA and TGF-β favor the development of FoxP-3+ cells, while RA induces the generation of IL-10 producing T cells (106).
Other enzymes that influence the outcome of T cell priming are indoleamine 2,3 dioxygenase (IDO) and TSLP. IDO is expressed also by DC in other tissues and was shown to inhibit the development of effector T cells and promote Treg cell generation (112, 113). TSLP is, as mentioned above, secreted by epithelial cells, but also by the intestinal DC, themselves. In the presence of TSLP, Th17 responses are restricted due to a reduced ability to produce IL-17, and Treg cell differentiation is up-regulated (107). The ability of the intestinal DC compartment to generate Th17 cells seems to be associated with CD103+ CD11b+ DC, as the frequency of Th17 cells is reduced in mice lacking these DC due to either IRF-4 or Notch-deficiency (10, 12, 14), or as a result of conditional ablation of this DC subset (13). Interestingly though, a recent study showed that also another subpopulation of DC, i.e. CCR2+ CD103− CD11b+ DC can induce IL-17a production in CD4+ T cells and effectively prime Th17 cells, probably via IL-12/IL-23p40 secretion (17).
In steady state, intestinal DC are probably mainly tolerogenic. Under inflammatory conditions, however, they can become highly effective T cell activators (114). Induction of experimental colitis results in the accumulation of CD103+ DC with an inflammatory profile in the MsnLNs (114). These DC express less RALDH and TGF-β and instead of promoting Treg cell formation, now induce Th1 inflammatory responses (114). While Th17 polarization might be carried out by CD103+ CD11b+ DC (12), differentiation of CD8+ effector T cells under inflammatory conditions seems to be dependent on CD103+ CD11b− CD8α+ DC that migrated into the lymph (88).
Flagellin stimulation causes TLR-5+ CD103+ DC in the small intestine to promote differentiation of Th17 cells and secrete IL-23, which in turn induces IL-22 production by ILC3 and subsequent epithelial up-regulation of antibacterial peptides (115).
In summary, DC are major players in maintaining homeostasis in the intestine. While tolerogenic at steady state, under inflammatory conditions they tip the scales and activate the immune system. They can migrate between different compartments of the intestine – from the lamina propria to the epithelium and into the MsnLNs – and execute different immune responses in each tissue. Further research regarding the location of DC, their functions and characteristics should shed new light on the role of these cells in the intestine.
In summary, macrophages and DC critically contribute to intestinal homeostasis and immune defense. Both cellular compartments have been subdivided into discrete subpopulations, which though currently mainly phenotypically defined, in some cases have been assigned distinct activities. The challenge ahead is to better define precise roles of these subsets both in health and under inflammatory conditions, first in the mouse but then also in the human. This task is complicated by the fact that many of the used markers used to distinguish between subpopulations of DC and macrophages are shared by the two types of mononuclear phagocytes. Moreover, under inflammatory conditions monocyte-derived cells further blur the picture. Collectively, this highlights the need to define cells by multiple parameters, including both surface and intracellular markers. Single cell transcriptome analysis is likely to help with this task (116, 117). However, classic flow cytometry analysis using fluorescent dye-coupled antibodies allows only a very limited simultaneous panel of markers due to the few dyes available and the spectral overlap of their emission. This problem might, in the near future, be solved by spectral cytometry systems that use ultrafast optical spectroscopy combined with flow cytometry to differentiate between the emission curves of different fluorophores, thus enabling the use of dozens of antibodies in one sample (118). Moreover, a new cell analyzer has been introduced, which uses mass cytometry instead of flow cytometry and is termed cytometry by Time-Of-Flight, or CyTOF (119). Instead of conjugations to fluorophores, this machine uses conjugations to heavy metal isotopes. Such metals do not exist naturally in the cells, so background is insignificant. The stained cells are injected into the CyTOF and are evaporated in a plasma chamber. The metals are ionized, hit the TOF detector, and their mass is measured, allowing the machine to determine the expression levels of the markers on each cell. This multiple-parameter approach enables to explore entire immune cell populations and subpopulations from the same tissue. As exemplified in Figure 1, such global analysis methods might well hold the key for the better definition and understanding of the cellular make-up of the intestine. No doubt, that with the recent development in the fields of cell cytometry and RNA sequencing, more pieces of this complex puzzle of the characteristics and roles of mononuclear phagocytes in the gut will be detected and put in place.
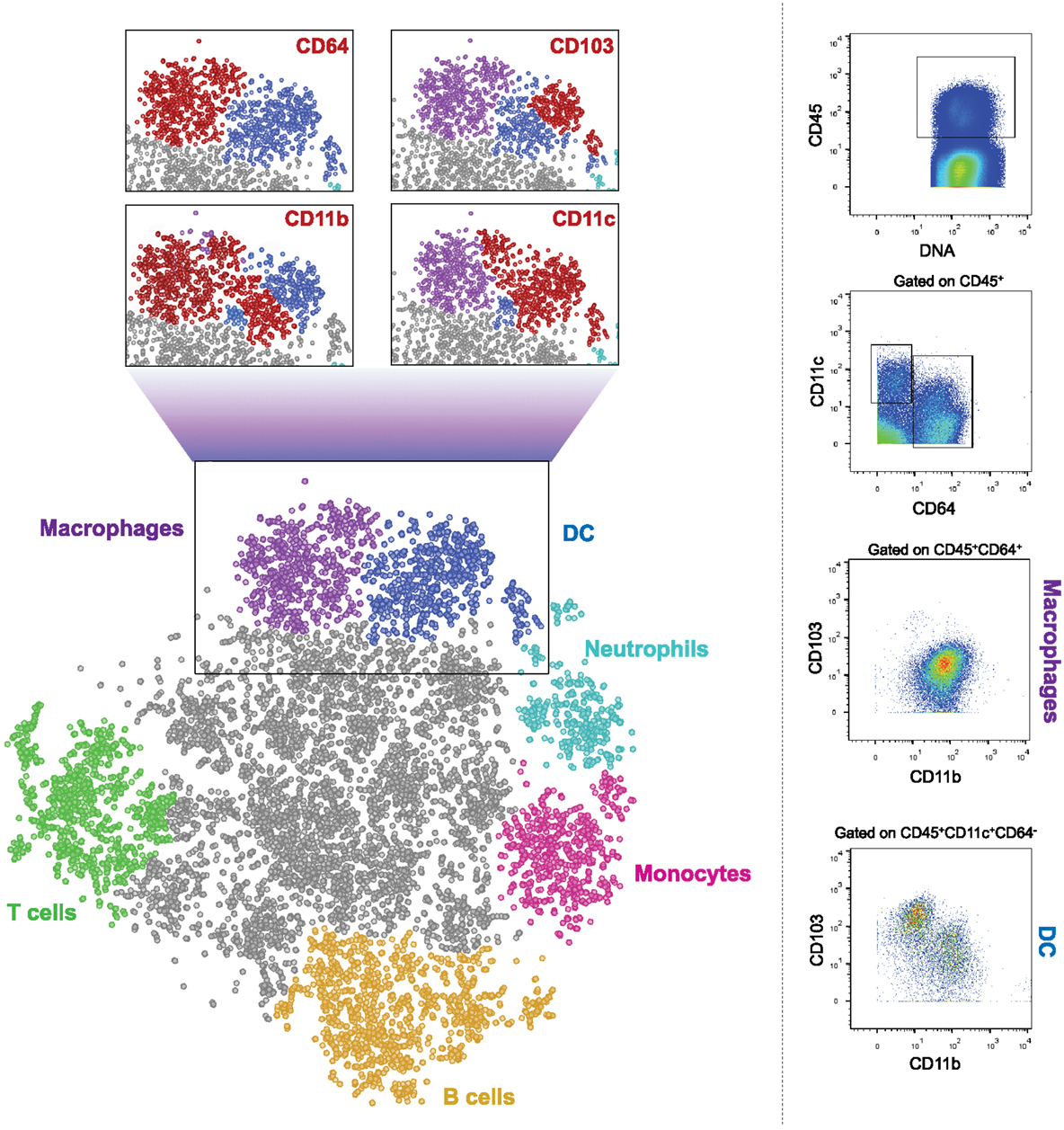
Figure 1. CyTOF analysis of CD45+ cells from murine colon. Cells were isolated from colon of 6–9 weeks old WT female C57Bl/6 mice and stained with a panel of 26 cell surface markers. The results were gated for live, single, CD45+ cells. Bh-SNE analysis and clustering were performed by Accense (http://www.cellaccense.com/) and the results were processed by GIMP. Colors indicate high levels of the following markers: green – TCRβ, CD3e (T cells), Orange – B220 (B cells), light blue – Ly6G (granulocytes), pink –Ly6C (monocytes), purple – CD64, F4/80 (macrophages), blue – clustered by Accence, different DC populations, gray – non-identified or non-specific cells. Red populations in zoom-in black squares indicate high levels of the marker written. Representative of at least four separate, independent experiments.
The Guest Associate Editor Martin Guilliams declares that, despite having collaborated on a paper with Steffen Jung in May 2013, the review process was handled objectively. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the European Research Council (340345).
1. Meresse B, Malamut G, Cerf-Bensussan N. Celiac disease: an immunological jigsaw. Immunity (2012) 36:907–19. doi: 10.1016/j.immuni.2012.06.006
2. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature (2007) 448:427–34. doi:10.1038/nature06005
3. Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol (2013) 91:232–9. doi:10.1038/icb.2012.79
4. Bekiaris V, Persson EK, Agace WW. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol Rev (2014) 260:86–101. doi:10.1111/imr.12194
5. Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol (2013) 34:162–8. doi:10.1016/j.it.2013.02.001
6. Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med (2010) 207:823–36. doi:10.1084/jem.20091627
7. Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med (2009) 206:3115–30. doi:10.1084/jem.20091756
8. Becker M, Guttler S, Bachem A, Hartung E, Mora A, Jakel A, et al. Ontogenic, phenotypic, and functional characterization of XCR1(+) dendritic cells leads to a consistent classification of intestinal dendritic cells based on the expression of XCR1 and SIRPalpha. Front Immunol (2014) 5:326. doi:10.3389/fimmu.2014.00326
9. Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207:1283–92. doi:10.1084/jem.20100223
10. Schlitzer A, Mcgovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity (2013) 38:970–83. doi:10.1016/j.immuni.2013.04.011
11. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity (2009) 31:513–25. doi:10.1016/j.immuni.2009.08.010
12. Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity (2011) 35:780–91. doi:10.1016/j.immuni.2011.08.013
13. Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyarto BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J Exp Med (2013) 210:2011–24. doi:10.1084/jem.20130728
14. Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity (2013) 38:958–69. doi:10.1016/j.immuni.2013.03.009
15. Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med (2013) 210:1961–76. doi:10.1084/jem.20122508
16. Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, et al. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol (2013) 6:104–13. doi:10.1038/mi.2012.53
17. Scott CL, Bain CC, Wright PB, Sichien D, Kotarsky K, Persson EK, et al. CCR2CD103 intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol (2014) 8(2):327–39. doi:10.1038/mi.2014.70
18. Niess JH, Brand S, Gu X, Landsman L, Jung S, Mccormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science (2005) 307:254–8. doi:10.1126/science.1102901
19. Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity (2009) 31:502–12. doi:10.1016/j.immuni.2009.06.025
20. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science (2014) 343:1249288. doi:10.1126/science.1249288
21. Ishifune C, Maruyama S, Sasaki Y, Yagita H, Hozumi K, Tomita T, et al. Differentiation of CD11c+ CX3CR1+ cells in the small intestine requires notch signaling. Proc Natl Acad Sci U S A (2014) 111:5986–91. doi:10.1073/pnas.1401671111
22. Hiemstra IH, Beijer MR, Veninga H, Vrijland K, Borg EG, Olivier BJ, et al. The identification and developmental requirements of colonic CD169(+) macrophages. Immunology (2014) 142:269–78. doi:10.1111/imm.12251
23. Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell (2014) 158:300–13. doi:10.1016/j.cell.2014.04.050
24. Bain CC, Mowat AM. Intestinal macrophages – specialised adaptation to a unique environment. Eur J Immunol (2011) 41:2494–8. doi:10.1002/eji.201141714
25. Tamoutounour S, Henri S, Lelouard H, De Bovis B, De Haar C, Van Der Woude CJ, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol (2012) 42:3150–66. doi:10.1002/eji.201242847
26. Pabst O, Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol (2010) 40:2107–11. doi:10.1002/eji.201040557
27. Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol (2000) 20:4106–14. doi:10.1128/MCB.20.11.4106-4114.2000
28. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol (2014) 14:392–404. doi:10.1038/nri3671
29. Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol (2014) 15:929–37. doi:10.1038/ni.2967
30. Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med (2008) 205:2139–49. doi:10.1084/jem.20080414
31. Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med (2007) 204:171–80. doi:10.1084/jem.20061011
32. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity (2012) 37:1076–90. doi:10.1016/j.immuni.2012.08.026
33. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol (2013) 6:498–510. doi:10.1038/mi.2012.89
34. Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med (2012) 209:139–55. doi:10.1084/jem.20101387
35. Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity (2014) 40:720–33. doi:10.1016/j.immuni.2014.03.012
36. Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol (2008) 8:411–20. doi:10.1038/nri2316
37. Kang S, Okuno T, Takegahara N, Takamatsu H, Nojima S, Kimura T, et al. Intestinal epithelial cell-derived semaphorin 7A negatively regulates development of colitis via alphavbeta1 integrin. J Immunol (2012) 188:1108–16. doi:10.4049/jimmunol.1102084
38. Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development (1994) 120:1357–72.
39. Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood (2002) 99:111–20. doi:10.1182/blood.V99.1.111
40. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell (1993) 75:263–74. doi:10.1016/0092-8674(93)80068-P
41. Shouval DS, Biswas A, Goettel JA, Mccann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity (2014) 40:706–19. doi:10.1016/j.immuni.2014.03.011
42. Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med (2009) 361:2033–45. doi:10.1056/NEJMoa0907206
43. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity (2008) 28:546–58. doi:10.1016/j.immuni.2008.02.017
44. Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol (2010) 184:2026–37. doi:10.4049/jimmunol.0901936
45. Ueda Y, Kayama H, Jeon SG, Kusu T, Isaka Y, Rakugi H, et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol (2010) 22:953–62. doi:10.1093/intimm/dxq449
46. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol (2012) 13:1118–28. doi:10.1038/ni.2419
47. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell (2014) 159:1312–26. doi:10.1016/j.cell.2014.11.018
48. Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity (2010) 32:317–28. doi:10.1016/j.immuni.2010.02.008
49. Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell (2010) 38:576–89. doi:10.1016/j.molcel.2010.05.004
50. Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood (2003) 101:1155–63. doi:10.1182/blood-2002-02-0569
51. Brenner O, Levanon D, Negreanu V, Golubkov O, Fainaru O, Woolf E, et al. Loss of Runx3 function in leukocytes is associated with spontaneously developed colitis and gastric mucosal hyperplasia. Proc Natl Acad Sci U S A (2004) 101:16016–21. doi:10.1073/pnas.0407180101
52. Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol (2010) 11:936–44. doi:10.1038/ni.1920
53. Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol (2011) 12:231–8. doi:10.1038/ni.1990
54. Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell (2014) 157:832–44. doi:10.1016/j.cell.2014.04.016
55. Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol (2001) 2:361–7. doi:10.1038/86373
56. Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol (2006) 176:2465–9. doi:10.4049/jimmunol.176.4.2465
57. Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood (2011) 118:e156–67. doi:10.1182/blood-2011-04-348946
58. Kostadinova FI, Baba T, Ishida Y, Kondo T, Popivanova BK, Mukaida N. Crucial involvement of the CX3CR1-CX3CL1 axis in dextran sulfate sodium-mediated acute colitis in mice. J Leukoc Biol (2010) 88:133–43. doi:10.1189/jlb.1109768
59. Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity (2011) 34:237–46. doi:10.1016/j.immuni.2011.01.016
60. Hapfelmeier S, Muller AJ, Stecher B, Kaiser P, Barthel M, Endt K, et al. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med (2008) 205:437–50. doi:10.1084/jem.20070633
61. Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature (2013) 494:116–20. doi:10.1038/nature11809
62. Slavin J, Nash JR, Kingsnorth AN. Effect of transforming growth factor beta and basic fibroblast growth factor on steroid-impaired healing intestinal wounds. Br J Surg (1992) 79:69–72. doi:10.1002/bjs.1800790124
63. Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol (2005) 288:G1055–65. doi:10.1152/ajpgi.00328.2004
64. Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A (2005) 102:99–104. doi:10.1073/pnas.0405979102
65. Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A (2009) 106:256–61. doi:10.1073/pnas.0803343106
66. Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol (2013) 58:1125–32. doi:10.1016/j.jhep.2013.01.038
67. Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity (2014) 40:642–56. doi:10.1016/j.immuni.2014.04.016
68. Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science (2005) 307:1630–4. doi:10.1126/science.1108003
69. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med (2009) 206:3101–14. doi:10.1084/jem.20091925
70. Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol (2009) 10:1178–84. doi:10.1038/ni.1791
71. Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature (2008) 455:808–12. doi:10.1038/nature07240
72. Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest (2011) 121:4787–95. doi:10.1172/JCI59150
73. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol (2007) 8:1086–94. doi:10.1038/ni1511
74. Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol (2011) 187:733–47. doi:10.4049/jimmunol.1002701
75. Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med (2014) 211:1571–83. doi:10.1084/jem.20140678
76. Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol (2013) 14:937–48. doi:10.1038/ni.2679
77. Aychek T, Mildner A, Yona S, Kim KW, Lampl N, Reich-Zeliger S, et al. IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology. Nat Commun (2015) 6:6525. doi:10.1038/ncomms7525
78. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med (2007) 204:1775–85. doi:10.1084/jem.20070602
79. Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol (2008) 8:435–46. doi:10.1038/nri2335
80. Zeng R, Oderup C, Yuan R, Lee M, Habtezion A, Hadeiba H, et al. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol (2012) 6:847–56. doi:10.1038/mi.2012.123
81. Milling S, Yrlid U, Cerovic V, Macpherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev (2010) 234:259–67. doi:10.1111/j.0105-2896.2009.00866.x
82. Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol (2008) 20:61–7. doi:10.1016/j.coi.2007.10.009
83. Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol (2014) 14:667–85. doi:10.1038/nri3738
84. Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol (2006) 176:803–10. doi:10.4049/jimmunol.176.2.803
85. Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol (2014) 35:270–7. doi:10.1016/j.it.2014.04.003
86. Cohen SB, Smith NL, Mcdougal C, Pepper M, Shah S, Yap GS, et al. Beta-catenin signaling drives differentiation and proinflammatory function of IRF8-dependent dendritic cells. J Immunol (2014) 194(1):210–22. doi:10.4049/jimmunol.1402453
87. den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med (2000) 192:1685–96. doi:10.1084/jem.192.12.1685
88. Cerovic V, Houston SA, Westlund J, Utriainen L, Davison ES, Scott CL, et al. Lymph-borne CD8alpha dendritic cells are uniquely able to cross-prime CD8 T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol (2014) 8(1):38–48. doi:10.1038/mi.2014.40
89. McDole JR, Wheeler LW, Mcdonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature (2012) 483:345–9. doi:10.1038/nature10863
90. Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity (2013) 38:581–95. doi:10.1016/j.immuni.2013.01.009
91. Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkotter HJ. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur J Immunol (2005) 35:1418–27. doi:10.1002/eji.200425726
92. Wilson NS, Young LJ, Kupresanin F, Naik SH, Vremec D, Heath WR, et al. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or toll-like receptor signaling. Immunol Cell Biol (2008) 86:200–5. doi:10.1038/sj.icb.7100125
93. Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A (2001) 98:13722–7. doi:10.1073/pnas.241308598
94. Yrlid U, Milling SW, Miller JL, Cartland S, Jenkins CD, Macpherson GG. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-alpha and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol (2006) 176:5205–12. doi:10.4049/jimmunol.176.9.5205
95. Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol (2008) 8:362–71. doi:10.1038/nri2297
96. Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol (2005) 6:507–14. doi:10.1038/ni1192
97. Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology (2008) 123:197–208. doi:10.1111/j.1365-2567.2007.02687.x
98. Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut (2009) 58:1481–9. doi:10.1136/gut.2008.175166
99. Stock A, Booth S, Cerundolo V. Prostaglandin E2 suppresses the differentiation of retinoic acid-producing dendritic cells in mice and humans. J Exp Med (2011) 208:761–73. doi:10.1084/jem.20101967
100. McDonald KG, Leach MR, Brooke KW, Wang C, Wheeler LW, Hanly EK, et al. Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids. Am J Pathol (2012) 180:984–97. doi:10.1016/j.ajpath.2011.11.009
101. Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med (2005) 202:1063–73. doi:10.1084/jem.20051100
102. Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol (2008) 181:3745–9. doi:10.4049/jimmunol.181.6.3745
103. Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol (2009) 2:340–50. doi:10.1038/mi.2009.13
104. Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature (2003) 424:88–93. doi:10.1038/nature01726
105. Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med (2005) 202:1051–61. doi:10.1084/jem.20040662
106. Bakdash G, Vogelpoel LT, Van Capel TM, Kapsenberg ML, De Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol (2014) 8(2):265–78. doi:10.1038/mi.2014.64
107. Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol (2012) 5:184–93. doi:10.1038/mi.2011.64
108. Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity (2014) 40:594–607. doi:10.1016/j.immuni.2014.03.005
109. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med (2007) 204:1757–64. doi:10.1084/jem.20070590
110. Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med (2009) 15:401–9. doi:10.1038/nm.1925
111. Molenaar R, Knippenberg M, Goverse G, Olivier BJ, De Vos AF, O’Toole T, et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol (2011) 186:1934–42. doi:10.4049/jimmunol.1001672
112. Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis (2009) 15:1391–6. doi:10.1002/ibd.20910
113. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut (2010) 59:595–604. doi:10.1136/gut.2009.185108
114. Laffont S, Siddiqui KR, Powrie F. (2010). Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol 40: 1877–1883. doi:10.1002/eji.200939957
115. Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity (2012) 36:276–87. doi:10.1016/j.immuni.2011.12.011
116. Jaitin DA, Kenigsberg E, Keren-Shaul H, Elefant N, Paul F, Zaretsky I, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science (2014) 343:776–9. doi:10.1126/science.1247651
117. Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science (2015) 347:1138–42. doi:10.1126/science.aaa1934
118. Gregori G, Rajwa B, Patsekin V, Jones J, Furuki M, Yamamoto M, et al. Hyperspectral cytometry. Curr Top Microbiol Immunol (2014) 377:191–210. doi:10.1007/82_2013_359
Keywords: gut, dendritic cells, macrophages, homeostasis, inflammation, IBD
Citation: Gross M, Salame T-M and Jung S (2015) Guardians of the gut – murine intestinal macrophages and dendritic cells. Front. Immunol. 6:254. doi: 10.3389/fimmu.2015.00254
Received: 23 March 2015; Accepted: 07 May 2015;
Published: 02 June 2015
Edited by:
Martin Guilliams, Ghent University – VIB, BelgiumReviewed by:
Masaaki Murakami, Hokkaido University, JapanCopyright: © 2015 Gross, Salame and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffen Jung, Weizmann Institute of Science, Department of Immunology, Rehovot 76100, Israel,cy5qdW5nQHdlaXptYW5uLmFjLmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.