- 1Department of Obstetrics and Gynecology, Università Cattolica Del Sacro Cuore, Policlinico A. Gemelli, Rome, Italy
- 2Emergency Department, Università Cattolica Del Sacro Cuore, Policlinico A. Gemelli, Rome, Italy
- 3Department of Surgical Sciences, Università degli Studi di Torino, Ospedale S. Anna, Turin, Italy
Preeclampsia (PE) is defined as a hypertensive and coagulative disorder affecting about 2–8% of all pregnancies and is one of the main causes of maternal and fetal morbidity and mortality. Despite the great amount of studies run in this field, little is known about the precise pathogenic mechanisms behind PE. While endothelial and trophoblast dysfunctions, exaggerated inflammatory response, and hypercoagulative state have been shown to play a key role in the occurrence of PE, the primary trigger is still unknown. One of the hypotheses is that some infectious agents may represent a trigger for PE onset. Consistently, higher seroprevalence of Helicobacter pylori (HP) infection, a Gram-negative bacterium with a specific tropism for human gastric mucosa, has been shown in women with PE. Even tighter association has been found between PE and infection with cytotoxin-associated gene-A (CagA)-positive strains of HP. Recent in vitro studies have shown that anti-CagA antibodies cross-react with human trophoblast cells and determine a functional impairment in terms of cell invasiveness, thus, providing the first pathogenic model of HP infection-mediated placental damage. Since in the early process of implantation and placental development, trophoblast invasion of maternal decidua is a crucial step, the proposed autoimmune mechanism induced by HP infection, negatively interfering with the fetal side of the early developing placenta, may represent a mechanism explaining the higher seropositivity for HP infection among PE women. However, the contribution of HP infection to the pathogenesis of PE or to the worsening of its clinical presentation need to be further investigated as well as the possible impact of pre-pregnancy screening and eradication of HP infection on the incidence of the syndrome.
Introduction
Preeclampsia (PE) is generally defined as new hypertension and substantial proteinuria at or after 20 weeks’ gestation (1). Complicating 2–8% of pregnancies, PE is a major cause of severe maternal morbidity and mortality and adverse perinatal outcomes worldwide (2, 3).
In the last 20 years, the incidence of PE has risen in the Western Countries, probably due to an increased prevalence of predisposing factors, such as advanced maternal age, chronic hypertension, diabetes, obesity, and the growing use of assisted reproductive techniques (4, 5).
Despite the great socio-economic impact of PE and the amount of studies carried out in this field, the pathogenic mechanisms leading to PE onset still remains unclear as well as an effective preventive intervention is still lacking (6).
The placental origins of PE have long been recognized and then formalized in the two stage model of the syndrome (7). The first stage is represented by inadequate development of the early placenta and its maternal blood supply, called poor placentation, which is established before 20 weeks and before clinical signs appear. During physiological placental development, extensive remodeling of maternal spiral arteries takes place in order to supply increased need of maternal blood in the second two trimesters of pregnancy. That process depends on extravillous cytotrophoblasts that invade the lining of the pregnant uterus from weeks 6 to 18 of gestation, expanding the vascular capacity of the utero-placental circulation (8). In many cases of PE, trophoblast invasion has been shown to be inadequate with poorly remodeled arteries and reduced capacity of the utero-placental circulation (9).
In the second stage, a dysfunctional and hypoxic placenta is considered to release factors into the maternal circulation that cause the clinical features of this condition, including hypertension and proteinuria, as well as clotting and liver dysfunction. These appear to arise from a generalized systemic inflammatory response, of which endothelial dysfunction is a prominent component (7).
Thus, nowadays, one of the biggest challenges in the research field of PE is to identify possible primary triggers of poor placentation, then leading to clinical PE, in order to develop effective preventative interventions. In that scenario, a possible role for infections has been widely suggested.
Epidemiologic Association between Helicobacter pylori Infection and Preeclampsia
In the last few years, an epidemiological link between Helicobacter pylori (HP) infection and PE has been observed (10–13).
Helicobacter pylori is a Gram-negative bacterium with a specific tropism for the gastric mucosa (14); it is the main cause of chronic gastritis and peptic ulcer, as well as a risk factor for MALT-lymphoma and gastric cancer (15). Only some strains of HP possess determinants of pathogenicity, able to modulate the local and systemic inflammatory response (16), like the cytotoxin-associated gene-A (CagA), which encodes for a hydrophilic, surface-exposed protein (17). CagA-positive strains of HP have been shown to induce an inflammatory response in the gastric mucosa greater than that induced by CagA-negative ones (18). Owing to its capability to stimulate the immune system, HP has also been proposed to play a role in some extra-gastric diseases; in particular, the epidemiological association between HP infection and vascular diseases has been shown, including ischemic heart diseases, primary Raynaud’s phenomenon and migraine, all conditions characterized by endothelial dysfunction (19, 20).
Interestingly, anti-CagA antibodies seem to be able to cross-react with antigens localized on the surface of human endothelial cells in either normal or atherosclerotic arteries, thus providing a possible mechanism explaining this association (21, 22).
Daví and co-authors have shown an association between HP infection and high levels of in vivo markers of lipid peroxidation and platelet activation, urinary 8-iso-PGF2 and 11-dehydro-TXB2, respectively. Interestingly, successful eradication of HP infection led to a significant reduction in both markers, suggesting a novel mechanism by which an infectious agent could contribute to atherothrombosis (23).
A few years ago, Ponzetto et al. showed, for the first time, higher seropositivity for HP infection in 47 mothers with PE (51.1%) compared with 47 women with uneventful pregnancy (31.9%). The difference was even greater when considering positivity for CagA-positive strains of HP (80.9 and 14.9%, respectively) (10).
This epidemiologic association has subsequently been confirmed by several studies (11, 13) (Table 1), and a correlation between persistent and virulent infections (VacA/CagA seropositive patients) for HP and PE complicated by fetal intrauterine growth restriction (IUGR) has also been shown (13).
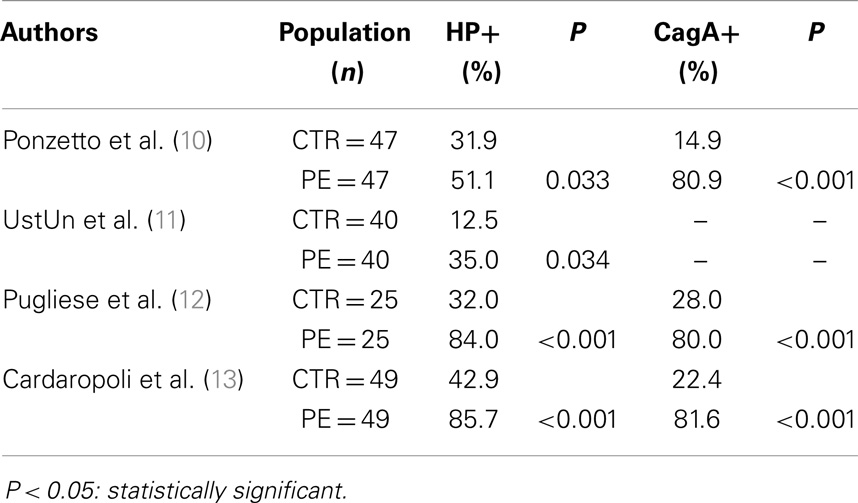
Table 1. Studies investigating the prevalence of HP infection in general, and CagA+ strains HP infection, in particular, in healthy pregnant women (CTR) in comparison with preeclamptic women (PE).
Thus, since the association between HP infection and PE occurrence has been widely confirmed, we hypothesized that this bacterial infection might have a role as possible trigger in the etiopathogenesis of PE.
Anti-CagA Antibodies Class IgG-Mediated Trophoblast Invasion Inhibition: An In vitro Model of HP-Induced Poor Placentation
To try to answer that question, we investigated whether HP infection might induce an immune humoral response able to trigger an autoantibody-mediated placental cellular damage. In particular, since anti-CagA antibodies are able to cross-react with antigens of endothelial cells (21) and cytotrophoblast cells show an endothelial origin, we tested murine anti-CagA antibodies class IgG – the only class of immunoglobulins able to cross placental barrier on human primary trophoblast cultures in order to find a possible cross-reaction. Interestingly, we observed that anti-CagA antibodies are able to bind, on the surface of trophoblast cells, to β-actin protein, one of the main components of cell cytoskeleton (24). Consistently, immunofluorescence performed on trophoblast cells using either anti-CagA or anti-β-actin antibodies showed an identical pattern of reaction, thus confirming β-actin to be the real cross-reacting protein. Interestingly, actin, in either endothelial or trophoblast cells, is not only important for maintaining the cell structure but it is also crucial for intercellular adhesion (25, 26) and it is now well established that actin-associated adhesions contribute to placental anchorage (26). We observed that anti-CagA antibodies show a dose-dependent binding activity as well and, as biological effect, a dose-dependent impairment of cytotrophoblast invasiveness in vitro, a crucial point for PE development. Furthermore, to better understand the molecular mechanisms involved in the antibody-mediated functional impairment of trophoblast cells, we examined the effect of anti-CagA on ERK activation and NF-kB nuclear translocation, two important factors activated during trophoblast proliferation, and we observed that anti-CagA antibodies are able to inhibit the activation of both elements (24).
As a whole, these observations provided a possible autoimmune pathogenic mechanism induced by HP infection, negatively interfering with the fetal side of placental development. This pathogenic model of autoimmune-mediated placental impairment is the first one linking HP infection, poor placentation, and PE (Figure 1).
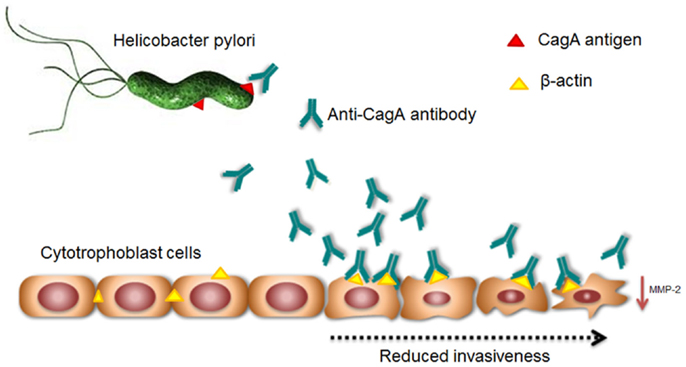
Figure 1. Supposed mechanism of Helicobacter pylori infection-induced molecular mimicry leading to cross-reaction of anti-CagA antibodies to trophoblast cells and poor placentation.
Discussion
Although the cause of PE remains largely unknown, the leading hypotheses strongly rely on disturbed placental function early in pregnancy (27). Impaired remodeling of the spiral arteries has especially been considered as an early defect causing PE (28).
Inadequate placentation may lead to impaired intervillous perfusion and to the establishment of placental hypoxic status, causing oxidative stress of trophoblast cells and the release in maternal circulation of anti-angiogenic factors and trophoblast debris, believed to mediate maternal systemic inflammatory response, endothelial dysfunction, and hypercoagulability in PE syndrome (28, 29).
Several studies suggested a strong association between PE and HP infections (10–13). Our in vitro studies showed an anti-CagA antibody-mediated mechanism of placental impairment at fetal side of early placental development. Thus, it is likely that antibodies directed against bacterial CagA protein might cross-react with antigens expressed on trophoblast cell, and in particular with β-actin, through an immunologic mechanism called molecular mimicry, leading to autoimmune response. This binding could inhibit significantly trophoblast invasiveness, probably negatively interfering with intracellular signaling ad intercellular connections, potentially leading to inadequate placental development. That could represent an intriguing model of infection-induced autoimmune triggering for poor placentation and PE onset, giving an explanation to the higher prevalence of HP infection among women developing PE.
Conclusion
More studies are needed to further investigate the impact of HP infection in triggering PE onset or, eventually, in worsening its clinical presentation.
However, it should be considered that, nowadays, in obstetrical practice, diseases with lower incidence than PE, like Rh alloimmunization or Down’s syndrome, are commonly screened. Thus, if HP will be confirmed as a contributing factor to PE, it will have important positive implications for the public health system, since the infection is treatable, and the future challenge would be to assess whether pre-pregnancy screening and preventive HP eradication would reduce the incidence of PE or moderate the severity of its clinical presentation.
Author Contributions
Giovanni Scambia, Tullia Todros, Francesco Franceschi, Simona Cardaropoli, and Nicoletta Di Simone were responsible for the manuscript concept, design, and supervision. Chiara Tersigni performed the literature searches and extraction of data. Chiara Tersigni, Tullia Todros, and Nicoletta Di Simone drafted the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Prof. Antonio Gasbarrini, Department of Internal Medicine, Università Cattolica del Sacro Cuore, Rome, Italy, for his precious scientific support to our research.
References
1. ACOG Committee on Practice Bulletins – Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol (2002) 99:159–67. doi: 10.1016/S0029-7844(01)01747-1
2. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet (2006) 367:1066–74. doi:10.1016/S0140-6736(06)68397-9
3. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol (2009) 33:130–7. doi:10.1053/j.semperi.2009.02.010
4. Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstet Gynecol (2009) 113:1075–81. doi:10.1097/AOG.0b013e3181a09fc0
5. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens (2008) 21:521–6. doi:10.1038/ajh.2008.20
6. Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol (2014) 10:531–40. doi:10.1038/nrneph.2014.103
7. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science (2005) 308:1592–4. doi:10.1126/science.1111726
8. Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest (2004) 114:744–54. doi:10.1172/JCI200422991
9. Redman CW. Current topic: pre-eclampsia and the placenta. Placenta (1991) 12:301–8. doi:10.1016/0143-4004(91)90339-H
10. Ponzetto A, Cardaropoli S, Piccoli E, Rolfo A, Gennero L, Kanduc D, et al. Pre-eclampsia is associated with Helicobacter pylori seropositivity in Italy. J Hypertens (2006) 24:2445–9. doi:10.1097/HJH.0b013e3280109e8c
11. UstUn Y, Engin-UstUn Y, Ozkaplan E, Otlu B, SaitTekerekoGlu M. Association of Helicobacter pylori infection with systemic inflammation in preeclampsia. J Matern Fetal Neonatal Med (2010) 23:311–4. doi:10.3109/14767050903121456
12. Pugliese A, Beltramo T, Todros T, Cardaropoli S, Ponzetto A. Interleukin-18 andgestosis: correlation with Helicobacter pylori seropositivity. Cell Biochem Funct (2008) 26:817–9. doi:10.1002/cbf.1503
13. Cardaropoli S, Rolfo A, Piazzese A, Ponzetto A, Todros T. Helicobacter pylori’s virulence and infection persistence define pre-eclampsia complicated by fetal growth retardation. World J Gastroenterol (2011) 17:5156–65. doi:10.3748/wjg.v17.i47.5156
14. Marshall BJ, Barrett LJ, Prakash C, McCallum RW, Guerrant RL. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology (1990) 99:697–702.
15. Gasbarrini G, Malfertheiner P, Deltenre M, Mégraud F, O’Morain C, Pajares-García J, et al. New concepts concerning management of Helicobacter pylori infection: 2 years after the Maastricht consensus report. Ital J Gastroenterol Hepatol (1998) 30:S244–7.
16. Crabtree JE, Kersulyte D, Li SD, Lindley IJ, Berg DE. Modulation of Helicobacter pylori induced interleukin-8 synthesis in gastric epithelial cells mediated by cag PAI encoded VirD4 homologue. J Clin Pathol (1999) 52:653–7. doi:10.1136/jcp.52.9.653
17. Nguyen LT, Uchida T, Tsukamoto Y, Trinh TD, Ta L, Mai HB, et al. Clinical relevance of cag PAI intactness in Helicobacter pylori isolates from Vietnam. Eur J Clin Microbiol Infect Dis (2010) 29:651–60. doi:10.1007/s10096-010-0909-z
18. Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and-18 mRNA expression in Helicobacter pylori induced Mongolian gerbils. Cancer Sci (2009) 100:2152–9. doi:10.1111/j.1349-7006.2009.01291.x
19. Pellicano R, Franceschi F, Saracco G, Fagoonee S, Roccarina D, Gasbarrini A. Helicobacters and extragastric diseases. Helicobacter (2009) 14:58–68. doi:10.1111/j.1523-5378.2009.00699.x
20. Franceschi F, Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol (2007) 2:325–34. doi:10.1016/j.bpg.2006.10.003
21. Franceschi F, Sepulveda AR, Gasbarrini A, Pola P, Silveri NG, Gasbarrini G, et al. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation (2002) 106:430–4. doi:10.1161/01.CIR.0000024100.90140.19
22. Franceschi F, Niccoli G, Ferrante G, Gasbarrini A, Baldi A, Candelli M, et al. CagA antigen of Helicobacter pylori and coronary instability: insight from a clinico-pathological study and a meta-analysis of 4241 cases. Atherosclerosis (2009) 202:535–42. doi:10.1016/j.atherosclerosis.2008.04.051
23. Daví G, Neri M, Falco A, Festi D, Taraborelli T, Ciabattoni G, et al. Helicobacter pylori infection causes persistent platelet activation in vivo through enhanced lipid peroxidation. Arterioscler Thromb Vasc Biol (2005) 25:246–51. doi:10.1161/01.ATV.0000147128.10278.99
24. Franceschi F, Di Simone N, D’Ippolito S, Castellani R, Di Nicuolo F, Gasbarrini G, et al. Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia? Helicobacter (2012) 17:426–34. doi:10.1111/j.1523-5378.2012.00966.x
25. Pardridge WM, Nowlin DM, Choi TB, Yang J, Calaycay J, Shively JE. Brain capillary 46,000 dalton protein is cytoplasmic actin and is localized to endothelial plasma membrane. J Cereb Blood Flow Metab (1989) 9:675–80. doi:10.1038/jcbfm.1989.95
26. Aplin JD, Jones CJP, Harris LK. Adhesion molecules in human trophoblast – a review. I. Villous trophoblast. Placenta (2009) 30:293–8. doi:10.1016/j.placenta.2008.12.001
27. Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet (2010) 376:631–44. doi:10.1016/S0140-6736(10)60279-6
28. Brosens I, Robertson WB, Dixon HG. The role of spiral arteries in the pathogenesis of preeclampsia. In: Wynn RM, editor. Obstetrics and Gynecology Annual. New York: Appleton-Century-Crofts (1972). p. 177–91.
Keywords: preeclampsia, Helicobacter pylori, infection, placenta, anti-CagA antibody
Citation: Tersigni C, Franceschi F, Todros T, Cardaropoli S, Scambia G and Di Simone N (2014) Insights into the role of Helicobacter pylori infection in preeclampsia: from the bench to the bedside. Front. Immunol. 5:484. doi: 10.3389/fimmu.2014.00484
Received: 25 August 2014; Paper pending published: 09 September 2014;
Accepted: 22 September 2014; Published online: 09 October 2014.
Edited by:
Sinuhe Hahn, University Hospital Basel, SwitzerlandReviewed by:
Stefan Gebhardt, Stellenbosch University, South AfricaStavros Giaglis, University of Basel, Switzerland
Copyright: © 2014 Tersigni, Franceschi, Todros, Cardaropoli, Scambia and Di Simone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicoletta Di Simone, Department of Obstetrics and Gynecology, Policlinico A. Gemelli, L.go A. Gemelli 8, Rome 00168, Italy e-mail:bmljb2xldHRhZGlzaW1vbmVAcm0udW5pY2F0dC5pdA==
 Chiara Tersigni
Chiara Tersigni Francesco Franceschi2
Francesco Franceschi2 Simona Cardaropoli
Simona Cardaropoli Nicoletta Di Simone
Nicoletta Di Simone