
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 26 August 2014
Sec. T Cell Biology
Volume 5 - 2014 | https://doi.org/10.3389/fimmu.2014.00400
This article is part of the Research Topic Recent Advances in HBV and HCV Immunology View all 10 articles
Discovered 30 years ago, gamma delta (γδ) T-lymphocytes remain an intriguing and enigmatic T-cell subset. Although in humans they comprise a small fraction of the total circulating T-lymphocyte pool, they represent an important T-cell subset in tissues such as the liver, with roles bridging the innate and adaptive immune systems. The associations of γδ T-lymphocytes with chronic liver disease have been explored – however, there remain conflicting data as to whether these T-cells are pathogenic or protective. In patients with some forms of liver disease, their expansion in the periphery and especially in the liver may indeed help pathogen clearance, while in other conditions their presence may, in contrast, contribute to disease progression. γδ T-cells can also express CD161, a C-type lectin, and such cells have been found to be involved in the pathogenesis of inflammatory disease. CD161+ T-cells of diverse subsets are known to be enriched in the livers of patients with chronic hepatitis C. This article serves to provide a review of the γδ T-cell population and its role in hepatitis C and other chronic liver diseases, and also explores a potential role of the CD161+ γδ T-cells in liver diseases.
The liver is the largest organ in the body and also one of the most complex with multiple functional roles. Its dual vascular supply includes the portal vein and the hepatic artery, and thus, it has a prime location draining blood from the gut via the portal vein with a constant exposure to antigens (Ag) and bacterial products from the gut. However, it is not only harmful pathogens that challenge the liver, it must recognize and tolerate harmless foreign (food-derived) and self-Ags. Thus, the liver serves as a checkpoint to promote tolerance to self and non-pathogenic stimuli and protect against pathogens via the gut, and is therefore at risk of damage from viruses and bacteria.
The liver can be affected by many acute insults such as drugs, toxins, and acute viral infections; for example alcoholic hepatitis is one of the most florid manifestations of liver disease (1). When the insult is a continuous or recurrent over a period of time, a more chronic liver disease picture can develop leading initially to fibrosis and eventually, if the stimulus persists, cirrhosis. The most common causes of chronic liver disease globally include: hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH), primary sclerosing cholangitis (PSC), and primary biliary cirrhosis (PBC). Chronic liver disease caused by any of these – with HCV a good example – can lead to a broad spectrum of liver injury from mild fibrosis to severe fibrosis, compensated cirrhosis and finally decompensated cirrhosis with major complications (e.g. esophageal variceal bleeding and spontaneous bacterial peritonitis) and hepatocellular carcinoma (HCC) development (2).
It is increasingly recognized that the liver has its own regional immune system providing a local immune-surveillance network, especially relevant to the gut. Blood from the gut drains via the superior and inferior mesenteric veins into the portal vein and then via the portal tracts and into the liver sinusoids and leaves via the central vein. In the liver sinusoids, there is a complex network of specialized immune-surveillance/antigen presenting cells (including liver sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells), which possess mechanisms allowing induction and recruitment of innate and adaptive responses. Irrespective of the causative agent of liver disease, once activated, the intrahepatic immune system is typically thought to play a dual role both in local host defense, and also in causing and/or propagating liver disease.
The protective role of intrahepatic cellular immune responses can be clearly seen in viral hepatitis. Spontaneous control of HCV infection is linked to the activation of intrahepatic cellular immunity and, conversely, the progression of HCV from an acute to chronic infection (occurring in the majority of cases) relies on evasion of cellular immunity (3). In acute HBV infection, a vigorous response with robust generation of interferon (IFN-γ) and tumor necrosis factor-alpha (TNF-α) can lead to clearance in 95% of cases in adults (4). Clearance of the virus in both these contexts is typically accompanied by a flare in the hepatocyte-derived amino transaminases, suggesting an aggressive immune mediated response within the liver. Indeed, the degree of liver damage correlates with the odds ratio of HCV clearance (5). The relevant intrahepatic lymphocyte (IHL) populations have been broadly characterized in humans, with the dominant intrahepatic population being the αβ T-cell populations. However, there also exist many other subsets and gamma delta (γδ) T-cells comprise 15% of mouse and human IHLs (6).
Gamma delta T-cells represent approximately 2–10% of all human T-cells in the peripheral blood (7) and are also found in diverse tissues, most abundantly in gut epithelial tissue, and also skin and bronchial epithelia. The position of γδ T-cells at epithelial barriers is strategic, i.e. continuously exposed to Ag and bacteria. A specific subset of CD161+ γδ T-cell has been implicated in inflammatory diseases such as multiple sclerosis (8, 9). CD161+ T-cells, however, have been found to be enriched in the livers of patients with chronic HCV (10–15) and thus the specific features of CD161+ γδ T-cells are of interest.
This article focuses on the γδ T-cell lymphocyte population in HCV, drawing also on data from other related chronic liver diseases.
Gamma delta T-cells were discovered after isolation of the TCR γ chain gene (16) and represent the subgroup of T-cells that have γ- and δ-glycoprotein chains linked by disulfide bonds rather than the “conventional” αβ chain. Large numbers of different receptors can be generated where different segments of genes: the variable (V), diversity (D), and joining (J) segments combine at random to produce numerous gene sequences – “VDJ recombination.” This re-arrangement allows the generation of different receptors to a variety of different Ags (17). γδ T-cells express CD3 – a complex forming part of the TCR, but are normally CD4/CD8 negative (double negative), although they may express variably CD4 or CD8 (18, 19). γδ T-cells, in contrast to αβ T-cells, do not require major histocompatibility complex (MHC) presentation of Ag. Two main subsets of γδ T-cells exist in humans determined by their Vδ chain re-arrangement (although there are six subsets in total in humans). Vδ1 cells are predominantly found in the gut and other peripheral tissues (such as lung, kidney, and spleen), whereas Vδ2 cells represent the major fraction of circulating γδ cells in blood (20).
Gamma delta-T-cells in the gut epithelium (predominantly the Vδ1 subset) comprise up to 50% of the intra-epithelial lymphocyte population (21), a population that is expanded in certain disease states such as celiac disease (22). The Vδ1 T-cell population is also expanded in intracellular bacterial infections (Mycobacterium tuberculosis and Listeria monocytogenes), extracellular infections (Borrelia burgdorferi) and have been shown to proliferate in response to stimulation with Ags from cytomegalovirus (CMV) (23, 24). Vδ1-expressing γδ T-cells have also found to be expanded in the periphery in patients with acquired immunodeficiency syndrome (25). The Vδ1 subset recognize stress-inducible MHC class 1 chain-related Ags (MIC) A/B (26) or self-lipid presented Ags by CD1c (27). These γδ T-cells can also be modulated by toll-like receptor (TLR) ligands. TLR 1, 2, 3, 5, and 6 ligands can act in combination with TCR stimulation to enhance chemokine and cytokine production (28). The Vδ1 subset is not restricted to the gut, but found at other sites including the skin (29). It has recently been described that a subset of such cells can respond to CD1d-lipid complexes in a manner analogous to iNKT cells (30).
The Vδ2 subset (also known as Vδ2Vγ9) are unique to humans and primates making up to 50–95% of the total γδ population in the blood and further expanding in acute infections such as M. tuberculosis, Salmonella, Listeria, Brucella, Malaria, and Toxoplasmosis even before other lymphocyte populations expand (31, 32). The Vδ2 subset can, however, also play an important role in local disease processes in barriers such as the skin (33) acting as a pro-inflammatory subset likely involved in the pathogenesis of psoriatic lesions in the skin, for example, reduced circulating levels of these cells are found in the periphery compared to healthy controls or patients with atopic dermatitis.
The Vδ2 subset recognizes small non-peptide pyrophosphomonoesters Ag, collectively known as “Phos-Ag” [the main one being the microbial compound (e)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate (HMB-PP) – an essential metabolite in pathogenic bacteria but not in the commensal human microbiota]. Bacteria synthesizing isopentenyl pyrophosphate (IPP) via the classical mevalonate pathway such as Streptococcus and Staphylococcus do not produce HMB-PP and thus do not recruit γδ T-cells, whereas other bacteria such as L. monocytogenes and the parasite Toxoplasma gondii, which synthesize IPP via the alternative non-mevalonate pathway do indeed produce HMB-PP and thus recruit γδ T-cells. The aminobisphosphonates (used in clinical practice for bone strengthening in osteoporosis by the inhibition of bone digestion by osteoclasts) are structurally related to IPP, and thus, act as Vδ2 agonists (34). There have been only a few single non-peptide ligands that have been shown to be both necessary and sufficient to stimulate γδ T-cells, and indeed T-cell activation by Phos-Ag often additionally requires cell-to-cell contact (7). Recent data have indicated that butyrophilin 3A1 is a surface molecule required for the presentation of Phos-Ag to γδ T-cells, although the exact nature of the presenting pathway still needs to be fully defined (35).
In addition to conventional type 1 T-cell functions, recently γδ T-cells have been found to be a source of IL-17 (36) promoting granulopoiesis and neutrophil recruitment to aid pathogen clearance. The production of IL-17 by γδ cells in the gut epithelium can lead to tight junction formation and mucin secretion in the face of insults to the gut epithelium. They have also been found to produce IL-17 during TB infection in the lung (37). Along with IL-17, γδ T-cells may also produce IL-22, granzyme B, perforin, TNF-α, and IFN-γ (38–40). IL-17 producing γδ-T-cells may contribute to the efficacy of anticancer chemotherapy (41); chemotherapy leads to a rapid invasion of γδ-T-cells into the tumor bed, preceding accumulation of Tc-1 cytotoxic T-lymphocytes. The anticancer effect of the γδ-T-cells can be lost when there is a lack/deficiency of IL-17A or IL-1R1.
Cytotoxicity of γδ T-cells has been widely studied in the context of tumors. Ex vivo expanded γδ T-cells from healthy volunteers have been shown to be cytotoxic to high-grade glioblastomas in vitro and in vivo (42). γδ T-cells have also been shown to mediate killing of other tumor cells and represent an important effector of the immune system with an anti-tumor peripheral surveillance role (43). The Vδ2 T-cells are triggered by Phos-Ags (which are indeed increased in malignancy) and produce cytokines typical of Th-1, Th-2, or Th-17 cells (44–46), cross-talk with DCs (47) and also have a direct cytotoxic effect via: perforin/granzyme, Fas/FasL, TNF/TNF-R, and TRAIL-TRAIL-R pathways (29). The killing capacity of the Vδ2 T-cells was improved by pre-treatment of tumor target cells with aminobisphosphonates. The role of γδ T-cells in the future of anticancer (including HCC) therapy may be either via adoptive transfer (48) or in vivo stimulation and recruitment through the aminobisphosphonates (49).
Gamma delta T-cells localize preferentially in the liver compared to blood (14) – thus, their contribution to liver disease remains of great interest (see Table 1). Kenna and colleagues (13) showed marked enrichment of γδ T-cells in normal liver specimens from healthy donors compared to blood. In their study, they found a clear enrichment of the Vδ3 subset (mean in liver 21%) compared to blood, where it is very rarely found (0.5%). In healthy donors, the dominant Vδ population was still found to be Vδ2, as in blood, but relatively enriched compared to Vδ1 cells.
The presence of γδ T-cells in chronic hepatitis biopsies has been explored by Kasper and colleagues (51). In biopsies from 18 HBV and 25 HCV patients, they found the predominant portal tract infiltrate to be αβ T-cells; however, the lobular infiltration frequencies between αβ and γδ T-cells were approximately equal. Tseng and colleagues (52) studied T-cell lines generated from HCV+ or HBV+ patient liver biopsies in vitro and found significant numbers of γδ T-cells compared to expanded cells from the non-virally infected liver. These γδ T-cells had high levels of non-MHC-restricted cytotoxicity activity against primary hepatocytes and also produced high levels of IL-8, IFN-γ, and TNF-α when activated by anti-CD3. Similar findings were described by Kanayama and colleagues (50), who found increased γδ T-cells in immunohistochemical staining of liver tissue from patients with chronic liver disease. Thus, although not the dominant T-cell infiltrate in the liver, the γδ T-cell population has been found to be enriched in the livers of patients with liver disease.
The intrahepatic γδ T-cell population was further described by Agrati and colleagues (53), who studied 35 matched liver/blood samples from patients with chronic HCV. There was a specific compartmentalization of Vδ1 cells in preference to Vδ2 within the liver with the cells expressing a memory/effector phenotype (CD62L− CD45RO+ CD95+). On mitogenic stimulation of these cells they produced IFN-γ and IL-4. A higher frequency of IFN-γ producing Vδ1 cells was associated with higher degree of necro-inflammation, suggesting that these cells may indeed contribute to intrahepatic pathogenesis and disease progression in HCV patients. Similar observations were made in HCV/HIV co-infected patients, correlating Vδ1 infiltration with hepatic inflammation even in the setting of HAART (54).
The same group (53) further analyzed the antiviral functions of the Vδ2 T-cells on Huh7 hepatoma cells carrying the subgenomic HCV replicon. Activation of the Vδ2 cells was associated with a marked reduction of HCV RNA levels. The neutralization of IFN-γ by antibodies revealed the importance of this cytokine in inhibiting HCV replication. The inhibition of HCV was increased by the use of aminobisphosphonates that activate γδ T-cells – thus, pointing toward a potential future immunotherapeutic strategy. These studies suggested that depending on the type of Vδ chain expressed by the γδ T-cells, the cells might either have a beneficial or deleterious effect in patients with chronic HCV infection.
Par and colleagues (55) studied γδ T-cell responses in blood in patients who had HCV disease compared to healthy controls, and those patients who had cleared the virus with treatment. Patients with chronic HCV showed a decreased percentage of Vδ2 cells, as well as reduced perforin-positive T-lymphocytes compared to controls or to those who had cleared HCV infection. These data again suggested that impaired function and number of the Vδ2 γδ T-cells (although measured here in the periphery) may be involved in viral persistence. The γδ T-cell populations have also been studied in patients with chronic HBV (56). In patients with chronic HBV, the percentage of Vδ2 T-cells negatively correlated with serum alanine aminotransferase, aspartate transferase, and bilirubin suggesting that reduction in the Vδ2 population in the periphery had a role in the chronicity of HBV infection. Also, IFN-γ and TCR-γδ T-cell cytotoxicity was lower in patients with chronic HBV compared to healthy controls.
Relevant data also come from the liver transplant setting; increased numbers of γδ T-cells have been identified in the circulation of patients with stable liver graft function (57, 58, 60). Similarly, other authors (59) identified an increase in the total circulating peripheral γδ T-cell population in patients post-transplant when compared to healthy controls. The main, stable, circulating fraction of γδ T-cells was the Vδ1 T-cell population irrespective of type of transplant (single liver or combined liver/kidney) or type of immunosuppression. Interestingly, the physiological reversal of the normal peripheral blood distribution (Vδ2 > Vδ1) was particularly seen in patients with HCV and CMV positivity (rather than Epstein–Barr virus or herpes simplex virus positive patients). Thus, it was proposed that patients may have increased Vδ1 populations in the transplant setting as a consequence of certain chronic viral triggers. This Vδ1 cell population expressed CD4, CD8, NKG2D, perforin, and IL-17A.
In another study, biological traits were compared between patients who had immunosuppressive tolerance (i.e., who did not develop rejection on immunosuppression withdrawal), those who required ongoing immunosuppression and also healthy controls (60). Genes encoding for γδ T-cells were found in those with immune tolerance and these individuals were found to have significantly greater numbers of circulating (Vδ1) γδ T-cells than either non-tolerant patients or healthy individuals. Thus, the γδ T-cells may indeed play an important role in the post-liver transplant setting, especially in patients who may manage to withdraw their immunosuppression. The Vδ1 subset may expand in the setting of post-transplantation due to chronic viral triggers in the face of immunosuppression, or potentially the relative loss of Vδ2 cells may reflect recruitment to the liver.
Gamma delta T-cells have also been studied in the context of autoimmune liver disease (61). The γδ T-cell population frequency was found to be comparable between the PBMCs of patients with AIH (irrespective of disease state, i.e., active vs. remission) and healthy control groups. However, interestingly they also observed a reversal in the physiological Vδ2:Vδ1 ratio with more Vδ1 cells found in the periphery (p = 0.001), as in post-liver transplantation population previously described (59). In patients with AIH, the γδ T-cell population expressed significantly more granzyme B and the number of Vδ1 cells (but not Vδ2) producing IFN-γ was significantly higher in patients with AIH compared to healthy controls.
Since their function has been established in responses to certain tumors, γδ T-cells have also been studied in targeting HCC – a feature of advanced liver disease associated with HCV (64). In a recent study (63), γδ T-cells expanded ex vivo with an aminobisphosphonate were able to recognize and lyse HCC cell lines in co-culture assays – thus, providing the rationale for targeted immunotherapy in patients with HCC in the future.
Overall, these data suggest a clear disturbance of the normal distribution of γδ T-cells during chronic hepatitis C, with an apparently consistent redistribution of γδ T-cells to the liver, especially the lobular infiltrates. The impact of chronic hepatitis C appears to be differential according to the Vδ chain usage, although this needs to be confirmed by further studies. Such impacts are not restricted to HCV infection, and although a clear potential as a pro-inflammatory subset in chronic inflammation exists, in the absence of a relevant animal model where they can be depleted, this is not currently proven. The summary of the role and function of γδ T-cells in health and liver disease can be seen in Figure 1, with the published studies in the chronic liver disease setting summarised in Table 1.
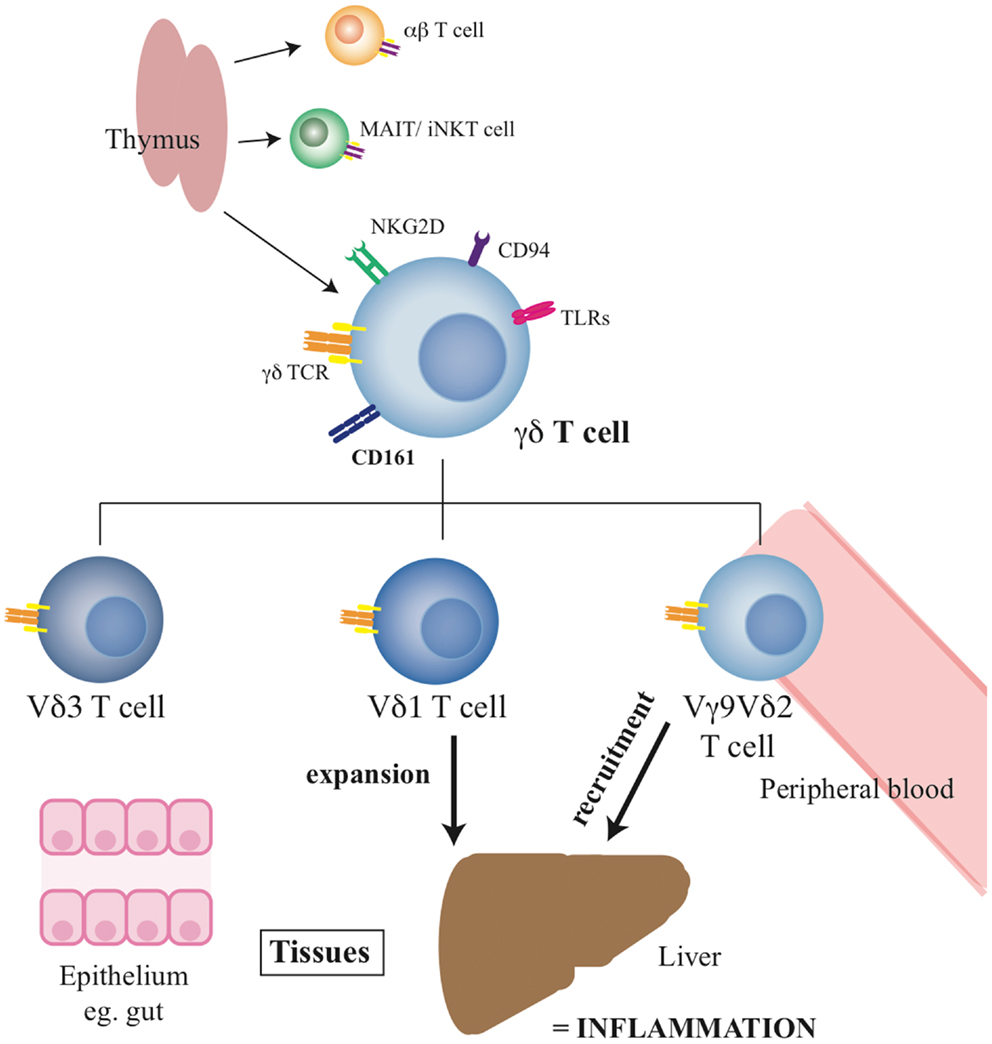
Figure 1. Summary of γδ T-cell functions and the roles of specific subsets. The diagram indicates the origin of the γδ T-cell pool, their phenotype, and the distribution of the key subsets in tissues.
CD161 [also known as killer lectin receptor subfamily B member 1 (KLRB1)] is a C-type lectin membrane glycoprotein that is expressed on the majority of NK cells, and approximately 24% of peripheral T-cells (65). CD161 was originally identified as the human homolog of the NKRP1A glycoproteins expressed on rodent NK cells, demonstrating 46–47% homology with its rodent counterparts. It is composed of a disulfide-linked homodimer of 40 kDa subunits, and has been shown to be expressed on both αβ and γδ T-cells (66).
CD161 is present on CD4 and CD8 αβ T-cells with higher surface density on CD8 T-cells (65). T-cells expressing CD161 can be divided into further populations based on expression levels with high and intermediate levels (also known as CD161 “bright” and CD161 “mid” populations) (10, 67). CD161 T-cell expression has consistently been associated with a memory phenotype in adult circulations not only in αβ T-cell populations (67–70) but also in the γδ T-cell population (66). CD161 is expressed early in development: a subset of T-cells in fetal liver express CD161 as does a population of CD3+ thymocytes (65, 71). CD161+ CD4 T-cells and CD8+ T-cells have been found in umbilical cord blood (69, 71, 72).
CD161 expression is highly associated with liver-homing T cell populations (11, 14). CD161 positivity has been found particularly associated with mucosal-associated invariant T-cells (MAIT) (73) that are abundant in humans (comprising up to 45% of liver lymphocytes). Staining with anti-Vα7.2 mAb and either CD161 or IL-18R unequivocally identifies MAIT cells in the γδ−CD4−CD3+ compartment. CD161 is also expressed on a significant subset of HCV and HBV-specific T-cells (15), especially those in the IHL compartment. CD161-expressing CD4+ T-cells are also enriched in the liver (12). This cell population, like its CD8 counterpart, is associated with RORγt expression and the potential for IL-17 secretion (10, 69). Indeed, expression of CD161 marks all human T-cells, whether CD4+ or CD8+, αβ, or γδ, capable of producing IL-17 (66).
To date, there are no published studies of CD161+ γδ T-cells in liver disease. However, some intriguing data are emerging from other studies of tissue inflammation. CD161+γδ T-cells were first studied by Battistini et al. (74), with CD94 the most common NK receptor found on γδ T-cells. However, some γδ T-cells were found also to express CD161 and hence, it was proposed that the CD161 phenotype could be acquired in vitro; this was confirmed in culture experiments. On cross-linking CD161 on γδ T-cells with antibody, the γδ T-cells expressed large amounts of IFN-γ indicating the molecule could deliver a stimulatory signal.
These data reveal that γδ T-cells are capable of regulation via both the TCR and NK receptors, such as CD161, and hence act in both adaptive and innate manners, respectively. In this way, γδ T-cells have been described as part of the family of innate-like lymphocytes, which includes NKT and MAIT cells. Recently, MAIT cells have been described to respond in an innate-like manner to cytokine stimulation, independently of the TCR. The combination of IL-12 and IL-18 induced IFNγ from the CD161++ CD8+ T cell subset, including both MAIT and non-MAIT cells (75). Innate-like γδ T-cells, which similarly respond selectively to IL-12 and IL-18, have also been identified by Wencker et al. (76). This TCR-independent response may suggest a mechanism by which γδ T-cells can be activated in diverse infection settings, even in the absence of specific ligands. Indeed, response of γδ T-cells to various cytokines, and cytokine combinations, has previously been reported (77, 78).
In addition to a costimulatory role, prior experiments have also shown an involvement of CD161 in trans-endothelial migration (79), with CD161+ CD4 T-cells migrating further across endothelial cell monolayers compared to CD161−ve CD4 T-cells, an effect blocked by an anti-CD161 monoclonal antibody. CD161 expression was also found to be up-regulated on Vδ2 cells when cultured with IL-12 [known to up-regulate CD161 (80)]. Transmigration was then studied by culturing γδ T-cells with IL-2 or IL-12, and on day 6 assessing transmigration through HUVEC monolayers using a double transwell system. Vδ2 cell transmigration was higher and faster and enhanced by culture with IL-12. When blocking the cells with an anti-NKRP1A (191b8) monoclonal Ab, there was a significant reduction in trans-endothelial migration. It was postulated that the CD161+ γδ T-cells did indeed localize to sites of inflammation, and on exposure to IL-12 up-regulate expression of CD161. In contrast, further experiments revealed that the Vδ1 subset used platelet endothelial cell adhesion molecule (PCAM1 or CD31: an adhesion molecule involved in lymphocyte extravasion), rather than CD161, for trans-endothelial migration (81).
These data indicate a specific functional role for CD161 on γδ T-cells, notably the Vδ2 cell subset, and a specific set of functions on CD161+ γδ T-cells in tissue inflammation. Since CD161 appears to be a general marker for liver-homing populations, the specific role of CD161+ γδ T-cells in liver disease is therefore of some interest for future studies. A summary of the role of CD161+ γδ T-cells can be seen in Figure 2.
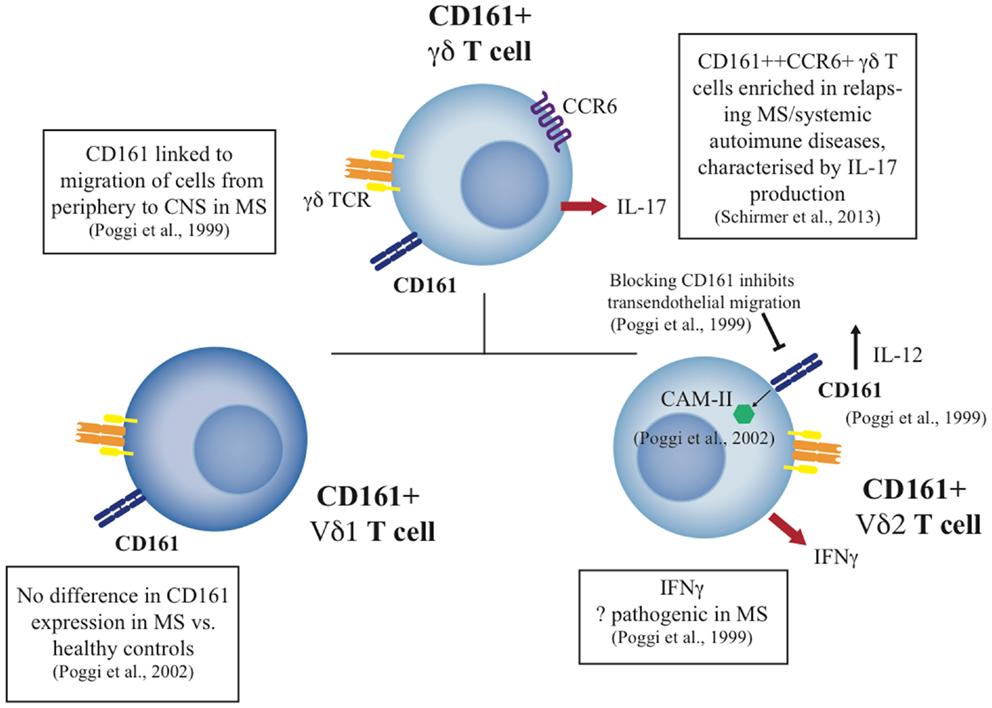
Figure 2. Summary of CD161+ γδ T cell studies to date in health and disease. The diagram indicates the phenotype and function of CD161+ γδ T cell subsets, including the Vδ1 and Vδ2 subsets, and their potential role in disease where published data exist.
It is clear that although a small non-dominant T-cell subset in the human T-cell lineage, γδ T-cells play an important role in defense against foreign pathogens. Their role as innate sentinels, guarding against microbial invasion places them as a key T-cell subset in the battle against pathogens not only in the gut at an epithelial level but also, or potentially moreso, in the liver. Evidence is emerging that depending on the type of liver disease, γδ T-cells may play a role in disease pathogenesis. They have been shown to be effective killers of cancer cells and thus patients with HCV-driven HCC may be amenable to γδ T-cell-based immunotherapy.
Intriguingly, depending on their Vδ chain expression, the cells may provide protection or induce tissue damage in the liver; in particular, Vδ1 T-cells correlate with a higher necroinflammatory score in patients with chronic HCV (53). Further delineation of the relevant subsets may be of relevance in future. We postulate this particularly applies to CD161 expression as this has been linked to distinct functional attributes of the T cell, including pro-inflammatory IL-17 production, and is also closely associated with liver-homing populations. Finally, further studies of these and related intrahepatic T cell populations are relevant not only to chronic HCV but also to other chronic liver disease settings, especially those where therapeutic interventions are more limited.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. Christian Willberg for his help with the CD161 project. Dr. Neil Rajoriya was funded by the Wellcome Trust for his D.Phil degree (January 2009–2012, WT 084451/Z/07/Z) and is currently funded by Oxford University Hospital Trust. Joannah Ruth Fergusson is supported by the Wellcome Trust Immunology and Translational Medicine Programme. Dr. Joanna A. Leithead is funded by University of Birmingham and University Hospital Birmingham Trust. Professor Paul Klenerman is funded by the Wellcome Trust (WT 091663MA), the NIHR Biomedical Research Centre, Oxford, UK, the Oxford Martin School, and the NIH (2 U19 AI 082630–06).
1. Forrest EH, Morris AJ, Stewart S, Phillips M, Oo YH, Fisher NC, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut (2007) 56(12):1743–6. doi:10.1136/gut.2006.099226
2. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med (2001) 345(1):41–52. doi:10.1056/NEJM200107053450107
3. Klenerman P, Thimme R. T-cell responses in hepatitis C: the good, the bad and the unconventional. Gut (2012) 61(8):1226–34. doi:10.1136/gutjnl-2011-300620
4. Mackay IR. Contemporary liver immunology: Obstacles and Opportunities. Liver Immunology Principles and Practice. Totowa, NJ: Humania Press (2007).
5. Thomson EC, Fleming VM, Main J, Klenerman P, Weber J, Eliahoo J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1 infected men. Gut (2011) 60(6):837–45. doi:10.1136/gut.2010.217166
6. Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology (2001) 120:250–60. doi:10.1053/gast.2001.20947
7. Chien YH, Bonneville M. Gamma delta T cell receptors. Cell Mol Life Sci (2006) 63(18):2089–94. doi:10.1007/s00018-006-6020-z
8. Stinissen P, Vandevyver C, Medaer R, Vandegaer L, Nies J, Tuyls L, et al. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J Immunol (1995) 154(9):4883–94.
9. Poggi A, Zocchi MR, Costa P, Ferrero E, Borsellino G, Placido R, et al. IL-12-mediated NKRP1A up-regulation and consequent enhancement of endothelial transmigration of V delta 2+ TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol (1999) 162(7):4349–54.
10. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A (2010) 107(7):3006–11. doi:10.1073/pnas.0914839107
11. Iiai T, Watanabe H, Suda T, Okamoto H, Abo T, Hatakeyama K. CD161+ T (NT) cells exist predominantly in human intestinal epithelium as well as in liver. Clin Exp Immunol (2002) 129(1):92–8. doi:10.1046/j.1365-2249.2002.01886.x
12. Kang YH, Seigel B, Bengsch B, Fleming VM, Billerbeck E, Simmons R, et al. CD161(+)CD4(+) T-cells are enriched in the liver during chronic hepatitis and associated with co-secretion of IL-22 and IFN-γ. Front Immunol (2012) 20(3):346. doi:10.3389/fimmu.2012.00346
13. Kenna T, Golden-Mason L, Norris S, Hegarty JE, O’Farrelly C, Docherty DG. Distinct subpopulations of gamma delta T cells are present in normal and tumour bearing human liver. Clin Immunol (2004) 113(1):56–63. doi:10.1016/j.clim.2004.05.003
14. Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol (1999) 60(1):20–31. doi:10.1016/S0198-8859(98)00098-6
15. Northfield JW, Kasprowicz V, Lucas M, Kersting N, Bengsch B, Kim A, et al. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology (2008) 47(2):396–406. doi:10.1002/hep.22040
16. Saito H, Kranz DM, Tagaki Y, Hayday AC, Eisen HN, Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature (1984) 309(5971):757–62. doi:10.1038/309757a0
17. Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human gammadelta T lymphocytes. Int Arch Allergy Immunol (2000) 122(1):1–77. doi:10.1159/000024353
18. Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, et al. Identification of a putative second T-cell receptor. Nature (1986) 322(6075):145–9. doi:10.1038/322145a0
19. Kabelitz D, Bender A, Schondelmaier S, da Silva Lobo ML, Janssen O. Human cytotoxic lymphocytes. V. Frequency and specificity of gamma delta+ cytotoxic lymphocyte precursors activated by allogeneic or autologous stimulator cells. J Immunol (1990) 145(9):2827–32.
20. Triebel F, Hercend T. Subpopulations of human peripheral T gamma delta lymphocytes. Immunol Today (1989) 10(6):186–8. doi:10.1016/0167-5699(89)90321-6
21. Viney J, MacDonald TT, Spencer J. Gamma/delta T cells in the gut epithelium. Gut (1990) 31(8):841–4. doi:10.1136/gut.31.8.841
22. Halstensen TS, Scott H, Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol (1989) 30(6):665–72. doi:10.1111/j.1365-3083.1989.tb02474.x
23. Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, et al. Implication of gamma delta T cells in the human immune response to cytomegalovirus. J Clin Invest (1999) 103(10):1437–49. doi:10.1172/JCI5409
24. Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood (2010) 116(12):2164–72. doi:10.1182/blood-2010-01-255166
25. Boullier S, Dadaglio G, Lafeuillade A, Debord T, Gougeon ML. V delta 1 T cells expanded in the blood throughout HIV infection display a cytotoxic activity and are primed for TNF-alpha and IFN-gamma production but are not selected in lymph nodes. J Immunol (1997) 159(7):3629–37.
26. Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science (1998) 279(5357):1737–40.
27. Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, et al. Self recognition of CD1 by gamma/delta T-cell: implications for innate immunity. J Exp Med (2000) 191:937–48. doi:10.1084/jem.191.6.937
28. Wesch D, Peters C, Oberg HH, Pietschmann K, Kabelitz D. Modulation of γδ T-cell responses by TLR ligands. Cell Mol Life Sci (2011) 68:2357–70. doi:10.1007/s00018-011-0699-1
29. Bonneville M, O’Brien RL, Born WK. Gammadelta T-cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol (2010) 10(7):467–78. doi:10.1038/nri2781
30. Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, et al. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol (2013) 14(11):1137–45. doi:10.1038/ni.2713
31. De Paoli P, Basaglia G, Gennari D, Crovatto M, Modolo ML, Santini G. Phenotypic profile and functional characteristics of human gamma and delta T cells during acute toxoplasmosis. J Clin Microbiol (1992) 30(3):729–31.
32. Romero P, Eberl G, Casanova JL, Cordey AS, Widmann C, Luescher IF, et al. Immunization with synthetic peptides containing a defined malaria epitope induces a highly diverse cytotoxic T lymphocyte response. Evidence that two peptide residues are buried in MHC molecule. J Immunol (1992) 148(6):1871–8.
33. Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol (2011) 187(5):2783–93. doi:10.4049/jimmunol.1100804
34. Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N, et al. Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother (2009) 58(4):493–502. doi:10.1007/s00262-008-0571-9
35. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol (2013) 14(9):908–16. doi:10.1038/ni.2665
36. Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol (2010) 184(12):7268–80. doi:10.4049/jimmunol.1000600
37. Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol (2008) 5(3):203–8. doi:10.1038/cmi.2008.25
38. García VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y, et al. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol (1997) 159(3):1328–35.
39. Bade B, Boettcher HE, Lohrmann J, Hink-Schauer C, Bratke K, Jenne DE, et al. Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int Immunol (2005) 17(11):1419–28. doi:10.1093/intimm/dxh320
40. Wang L, Das H, Kamath A, Bukowski JF. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol (2001) 167(11):6195–201. doi:10.4049/jimmunol.167.11.6195
41. Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta-T cells to the efficacy of anticancer chemotherapy. J Exp Med (2011) 208:491–501. doi:10.1084/jem.20100269
42. Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S. Characterization and immunotherapeutic potential of gamma delta T-cells in patients with glioblastoma. Neuro Oncol (2009) 11(4):357–67. doi:10.1215/15228517-2008-111
43. Hayday AC. γδ T-cells and the lymphoid stress-surveillance response. Immunity (2009) 31(2):184–96. doi:10.1016/j.immuni.2009.08.006
44. Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, et al. Distinct cytokine-driven responses of activated blood gammadelta T-cells: insights into unconventional T-cell pleitropy”. J Immunol (2007) 178:4304–14. doi:10.4049/jimmunol.178.7.4304
45. Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, et al. Differentiation, phenotype and function of interleukin-17 producing human V γ9Vδ2 T-cells. Blood (2011) 118:129–38. doi:10.1182/blood-2011-01-331298
46. Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood γδ T-cells towards Th1- or Th2-phenotype. Cell Immunol (2001) 212:110–7. doi:10.1006/cimm.2001.1850
47. Meraviglia S, El Daker S, Dieli F, Martini F, Martino A. γδ T-cells cross-link innate and adaptive immunity in Mycobacterium tuberculosis infection. Clin Dev Immunol (2011) 2011:587315. doi:10.1155/2011/587315
48. Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, et al. A phase 1 study of adoptive immunotherapy for recurrent non-small cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg (2010) 37:1191–7. doi:10.1016/j.ejcts.2009.11.051
49. Lucas C, Ingoure S, Soule E, Faria B, Audibert F, Blery M, et al. IPH1101, the first specific gamma-delta T-cell agonist, shows potent immuno-biological efficacy in low grade follicular lymphoma patients when combined with rituximab: results from a phase II study. ASH Annu Meet (2009) 114:583.
50. Kanayama K, Morise K, Nagura H. Immunohistochemical study of T cell receptor gamma delta cells in chronic liver disease. Am J Gastroenterol (1992) 87:1018–22.
51. Kasper HU, Ligum D, Cucus J, Stippel DL, Dienes HP, Drebber U. Liver distribution of gammadelta-T-cells in patients with chronic hepatitis of different etiology. APMIS (2009) 117(11):779–85. doi:10.1111/j.1600-0463.2009.02540.x
52. Tseng CT, Miskovsky E, Houghton M, Klimpel GR. Characterization of liver T-cell receptor gammadelta T cells obtained from individuals chronically infected with hepatitis C virus (HCV): evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology (2001) 33(5):1312–20. doi:10.1053/jhep.2001.24269
53. Agrati C, D’Offizi G, Narciso P, Abrignani S, Ippolito G, Colizzi V, et al. Vδ1 T lymphocytes expressing a Th1 phenotype are the major gammadelta T cell subset infiltrating the liver of HCV-infected persons. Mol Med (2001) 7(1):11–9.
54. Agrati C, D’Offizi G, Narciso P, Selva C, Pucillo LP, Ippolito G, et al. Gammadelta T cell activation by chronic HIV infection may contribute to intrahepatic Vdelta1 compartmentalization and hepatitis C virus disease progression independent of highly active antiretroviral therapy. AIDS Res Hum Retroviruses (2001) 17(14):1357–63. doi:10.1089/08892220152596614
55. Pár G, Rukavina D, Podack ER, Horányi M, Szekeres-Barthó J, Hegedüs G, et al. Decrease in CD3-negative-CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol (2002) 37(4):514–22. doi:10.1016/S0168-8278(02)00218-0
56. Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, et al. Characteristics of circulating T cell receptor gamma-delta T cells from individuals chronically infected with hepatitis B virus (HBV): an association between V(delta)2 subtype and chronic HBV infection. J Infect Dis (2008) 198(11):1643–50. doi:10.1086/593065
57. Malan-Borel I, Racca A, Garcia MI, Bailat A, Quiroga F, Soutullo A, et al. Gammadelta T cells and interleukin-6 levels could provide information regarding the progression of human renal allograft. Scand J Immunol (2003) 58(1):99–105. doi:10.1046/j.1365-3083.2003.01275.x
58. Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol (2007) 17(2):94–7. doi:10.1016/j.trim.2006.10.004
59. Puig-Pey I, Bohne F, Benítez C, López M, Martínez-Llordella M, Oppenheimer F, et al. Characterization of γδ T cell subsets in organ transplantation. Transpl Int (2010) 23(10):1045–55. doi:10.1111/j.1432-2277.2010.01095
60. Martinez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant (2007) 7(2):309–19. doi:10.1111/j.1600-6143.2006.01621.x
61. Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, et al. A multifaceted imbalance of T-cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology (2010) 52(3):999–1007. doi:10.1002/hep.23792
62. Martins EB, Graham AK, Chapman RW, Fleming KA. Elevation of γδ T lymphocytes in peripheral blood of patients with Primary Sclerosing cholangitis and other autoimmune liver diseases. Hepatology (1996) 23:988–93. doi:10.1002/hep.510230508
63. Hoh A, Dewerth A, Vogt F, Wenz J, Baeuerle PA, Warmann SW, et al. The activity of γδ T cells against paediatric liver tumour cells and spheroids in cell culture. Liver Int (2013) 33(1):127–36. doi:10.1111/liv.12011
64. Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintière C, Daniel P, et al. Vgamma9Vdelta2 T-cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother (2008) 57(4):531–9. doi:10.1007/s00262-007-0391-3
65. Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol (1994) 153(6):2417–28.
66. Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol (2010) 40(8):2174–81. doi:10.1002/eji.200940257
67. Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol (2006) 176(1):211–6. doi:10.4049/jimmunol.176.1.211
68. Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med (2007) 204(8):1849–61. doi:10.1084/jem.20070663
69. Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med (2008) 205(8):1903–16. doi:10.1084/jem.20080397
70. Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med (2009) 206(3):525–34. doi:10.1084/jem.20081712
71. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol (2009) 7(3):e1000054. doi:10.1371/journal.pbio.1000054
72. Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, et al. Human MAIT and CD8αα cells develop from a pool of type-17 pre-committed CD8+ T cells. Blood (2011) 119(2):422–33. doi:10.1182/blood-2011-05-353789
73. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood (2011) 117(4):1250–9. doi:10.1182/blood-2010-08-303339
74. Battistini L, Borsellino G, Sawicki G, Poccia F, Salvetti M, Ristori G, et al. Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. J Immunol (1997) 159(8):3723–30.
75. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol (2014) 44(1):195–203. doi:10.1002/eji.201343509
76. Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol (2014) 15(1):80–7. doi:10.1038/ni.2773
77. Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol (1995) 154(11):5832–41.
78. Das H, Sugita M, Brenner MB. Mechanisms of Vdelta1 gammadelta T cell activation by microbial components. J Immunol (2004) 172(11):6578–86. doi:10.4049/jimmunol.172.11.6578
79. Poggi A, Costa P, Zocchi MR, Moretta L. Phenotypic and functional analysis of CD4+ NKRP1A+ human T lymphocytes. Direct evidence that the NKRP1A molecule is involved in transendothelial migration. Eur J Immunol (1997) 27(9):2345–50. doi:10.1002/eji.1830270932
80. Poggi A, Costa P, Tomasello E, Moretta L. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur J Immunol (1998) 28(5):1611–6. doi:10.1002/(SICI)1521-4141(199805)28:05<1611::AID-IMMU1611>3.0.CO;2-6
81. Poggi A, Zocchi MR, Carosio R, Ferrero E, Angelini DF, Galgani S, et al. Transendothelial migratory pathways of V delta 1+TCR gamma delta+ and V delta 2+TCR gamma delta+ T lymphocytes from healthy donors and multiple sclerosis patients: involvement of phosphatidylinositol 3 kinase and calcium calmodulin-dependent kinase II. J Immunol (2002) 168(12):6071–7. doi:10.4049/jimmunol.168.12.6071
Keywords: gamma delta T-lymphocytes, γδ T-cells, CD161
Citation: Rajoriya N, Fergusson JR, Leithead JA and Klenerman P (2014) Gamma delta T-lymphocytes in hepatitis C and chronic liver disease. Front. Immunol. 5:400. doi: 10.3389/fimmu.2014.00400
Received: 30 May 2014; Accepted: 05 August 2014;
Published online: 26 August 2014.
Edited by:
Lynn B. Dustin, University of Oxford, UKReviewed by:
Antonio Bertoletti, DUKE-NUS Graduate Medical School, SingaporeCopyright: © 2014 Rajoriya, Fergusson, Leithead and Klenerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neil Rajoriya and Paul Klenerman, Peter Medawar Building for Pathogen Research, University of Oxford, South Parks Road, Oxford, OX1 3SY, UK e-mail:bmVpbC5yYWpvcml5YUBnbWFpbC5jb20=;cGF1bC5rbGVuZXJtYW5AbmRtLm94LmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.