- 1Department of Pathology, University of Massachusetts Medical School, Worcester, MA, USA
- 2Program in Immunology and Virology, University of Massachusetts Medical School, Worcester, MA, USA
- 3Department of Pathology, University of Chicago, Chicago, IL, USA
The signaling lymphocyte activation molecule (SLAM) family receptor, 2B4/CD244, was first implicated in anti-viral immunity by the discovery that mutations of the SLAM-associated protein, SAP/SH2D1A, impaired 2B4-dependent stimulation of T and natural killer (NK) cell anti-viral functions in X-linked lymphoproliferative syndrome patients with uncontrolled Epstein–Barr virus infections. Engagement of 2B4 has been variably shown to either activate or inhibit lymphocytes which express this receptor. While SAP expression is required for stimulatory functions of 2B4 on lymphocytes, it remains unclear whether inhibitory signals derived from 2B4 can predominate even in the presence of SAP. Regardless, mounting evidence suggests that 2B4 expression by NK and CD8 T cells is altered by virus infection in mice as well as in humans, and 2B4-mediated signaling may be an important determinant of effective immune control of chronic virus infections. In this review, recent findings regarding the expression and function of 2B4 as well as SAP on T and NK cells during virus infection is discussed, with a focus on the role of 2B4–CD48 interactions in crosstalk between innate and adaptive immunity.
Introduction
Immunity to virus infection involves the complex interplay of many different leukocytes, including natural killer (NK) cells (Bukowski et al., 1983; Biron et al., 1989) and CD8 T cells (Zinkernagel and Welsh, 1976). Pro-inflammatory cytokines, including type I interferon (IFN), can stimulate the activation and proliferation of these cytolytic and cytokine-producing effector cells. Moreover, while CD8 T cell responses are induced by virus-derived peptides in the context of class I major histocompatibility complex (MHC) molecules, NK cell activity can be provoked by interactions of stimulatory NK cell receptors (NKR) such as Ly49H with virally encoded proteins (Brown et al., 2001; Daniels et al., 2001; Lee et al., 2001; Arase et al., 2002; Smith et al., 2002).
Natural killer cell anti-viral function is further regulated by a variety of activating and inhibitory receptors that engage stress-induced proteins (Raulet, 2003) or self-specific MHC antigens (Brutkiewicz and Welsh, 1995) on virus-infected cells. Notably, expression of some NKR is also up-regulated on activated T cells and this expression is often sustained at a high level on virus-specific T cells that have lost functionality or become exhausted in the context of chronic virus infection (Vivier and Anfossi, 2004; Crawford and Wherry, 2009). One particular NKR, 2B4 (CD244/slamf4), contributes to the regulation of both NK cell anti-viral activity and virus-specific CD8 T cell functionality (McNerney et al., 2005b), and has recently been implicated in NK cell regulation of virus-specific T cell responses (Waggoner et al., 2010).
SLAM Family Receptors in Virus Infection
2B4 is a member of the signaling lymphocyte activation molecule (SLAM) family of CD2-related receptors, which includes SLAM (Cd150/slamf1), CD48 (slamf2), Ly9 (CD229/slamf3), CD84 (slamf5), NK-T-B-antigen (NTB-A in human, Ly108 in mouse, CD352/slamf6), and CD2-like receptor activating cytotoxic cells (CRACC/CS1/CD319/slamf7; Cannons et al., 2011). Each of these receptors, except CD48, encodes two or more cytoplasmic immunoreceptor tyrosine-based switch motifs (ITSM). Tyrosine phosphorylation of this motif facilitates binding of SLAM-associated protein (SAP) family adaptors SAP (sh2d1a), EWS–Fli1-activated transcript-2 (EAT-2/sh2d1b), and EAT-2-related transducer (ERT/sh2d1c; Veillette et al., 2009). Although most SLAM family receptors engage in homotypic interactions, 2B4 interacts with CD48.
A number of SLAM family receptors are implicated in the pathogenesis of virus infections. The prototypical family member, SLAM, is a cellular receptor for measles virus (Tatsuo et al., 2000). CD48 is highly up-regulated on the surface of Epstein–Barr virus (EBV)-infected lymphoblasts and may contribute to viral trafficking (Thorley-Lawson et al., 1982). The UL7 protein of human cytomegalovirus contains an immunoglobulin-like domain exhibiting remarkable sequence similarity to Ly9/CD229 (Engel et al., 2011). Expression of UL7 in myeloid cells attenuated pro-inflammatory cytokine production.
In contrast to SLAM receptors that are exploited by viruses, NTB-A and 2B4 contribute to effector cell-mediated killing of virus-infected cells. Co-engagement of NTB-A and NKG2D on human NK cells promotes release of lytic granules and lysis of HIV-infected CD4 T cells (Ward et al., 2007). However, HIV-1 has evolved mechanisms to evade this SLAM receptor recognition through Vpu-mediated down-modulation of NTB-A expression on infected CD4 T cells (Shah et al., 2010). Thus, some SLAM receptors are sufficiently important to the host anti-viral response that viruses may encode proteins to negate their functions.
SAP Family Adaptors and XLP
Interactions between the ITSM motif of SLAM family receptors and the adaptor protein SAP enables recruitment of the Src family tyrosine kinase Fyn, which facilitates downstream signaling to modulate effector functionality (Veillette, 2006). EAT-2 and ERT serve a similar but mechanistically distinct role (Cannons et al., 2011).
The SH2D1A gene that encodes SAP is mutated in patients with X-linked lymphoproliferative (XLP) disease such that the SAP polypeptide is absent or dysfunctional (Coffey et al., 1998). These individuals often present with uncontrolled EBV-induced infectious mononucleosis, characterized by lymphoproliferation, organ failure and death. Infection of sh2d1a-deficient mice with lymphocytic choriomeningitis virus (LCMV), Toxoplasma gondii, or murine gammaherpesvirus-68 recapitulates many of the disease phenotypes observed in EBV-infected XLP patients, including hyperproliferation of CD8 T cells (Czar et al., 2001; Wu et al., 2001; Yin et al., 2003; Chen et al., 2005) and fatal immunopathology (Crotty et al., 2006). SAP-deficiency also causes defects in long-term humoral immunity (Czar et al., 2001; Crotty et al., 2003; Malbran et al., 2004) and NKT cell development (Chung et al., 2005; Nichols et al., 2005; Pasquier et al., 2005).
Genetic deficiency of any single SLAM family receptor can recapitulate only a subset of the pathologies of SAP-deficiency and XLP disease (Cannons et al., 2011). The defects in humoral immunity are associated with changes in T cell production of cytokines (Ma et al., 2005; Yusuf et al., 2010), including IL-10, and destabilization of interactions between CD4 T cells and cognate B cells (Qi et al., 2008). CD84 and Ly108 play important roles in the conjugation of follicular CD4 T cells and germinal center B cells (Cannons et al., 2010). The defect in the NKT cell lineage in the absence of SAP can be elicited by combined deletion of SLAM and Ly108, suggesting that these receptors are important in the development of NKT cells (Griewank et al., 2007). Furthermore, inhibitory signals derived from Ly108 engagement in the absence of SAP substantially impaired NKT cell development (Kageyama et al., 2012).
A remarkable facet of XLP is the specific sensitivity of these patients to infections with EBV. NK and CD8 T cells from these individuals have an impaired capacity to lyse EBV-infected B cells (Parolini et al., 2000; Bottino et al., 2001; Sharifi et al., 2004; Dupre et al., 2005; Hislop et al., 2010). Among the different SLAM receptors expressed by NK and CD8 T cells, NTB-A and 2B4 substantially contribute to the cytolytic defects of these effectors in XLP (Nakajima et al., 2000; Parolini et al., 2000; Tangye et al., 2000b; Bottino et al., 2001). Analysis of female XLP carriers with heterozygous expression of SAP revealed that while memory CD8 T cells specific for cytomegalovirus or influenza can be SAP-expressing or SAP-deficient, EBV-specific T cells are exclusively SAP+ due to NTB-A- and 2B4-mediated inhibitory signaling in the absence of SAP when ligands of these SLAM receptors are engaged on antigen-bearing B cells (Palendira et al., 2011). Thus, in the context of SAP-deficiency, 2B4 and NTB-A mediate potent inhibition of effector cell cytotoxic function.
The Dual-Function Receptor, 2B4/CD244
2B4 is expressed by all NK cells, γδ T cells, basophils, and monocytes as well as a subset of memory-phenotype CD8+ αβ T cells (McNerney et al., 2005b). It is currently well accepted that engagement of 2B4 by CD48 can mediate activating as well as inhibitory signals, although the nature of functional dichotomy is a contentious issue. In humans, ligation of 2B4 with specific antibodies or CD48-expressing target cells provides an activating signal for NK cells (Tangye et al., 2000a). In XLP patients who lack functional SAP protein, 2B4 ligation can inhibit NK and CD8 T cell function (Nakajima et al., 2000; Parolini et al., 2000; Tangye et al., 2000b).
The understanding of the dual nature of 2B4 signaling has been complicated by studies in mice. In contrast to full-length 2B4 which bears four ITSM motifs, alternative splicing in mice generates a short form of 2B4 that contains only one ITSM (Stepp et al., 1999). Whereas overexpression of full-length 2B4 impaired NK cell cytotoxicity, the short form of 2B4 facilitated redirected lysis of tumor cells (Schatzle et al., 1999). Two isoforms of human 2B4 that differ in a small portion of the extracellular domain were also described, where one facilitated lysis of CD48-expressing target cells and the other did not (Mathew et al., 2009). In vitro studies have provided conflicting results regarding the effect of 2B4 ligation on murine NK and CD8 T cells, with both activating and inhibitory roles described (Kubin et al., 1999; Kambayashi et al., 2001; Lee et al., 2003; Assarsson et al., 2004; Mooney et al., 2004). Mutual expression of both 2B4 and CD48 by the effector and target cells in those assays could facilitate bi-directional signaling as well as interactions between 2B4 and CD48 expressed on neighboring effector cells (Kambayashi et al., 2001; Lee et al., 2003). Kumar and colleagues (Chlewicki et al., 2008) have proposed that the dual-function of 2B4 is dynamically regulated by the ligand density of CD48, 2B4 receptor expression levels, and availability of intracellular SAP protein in NK cells (Figure 1). Importantly, inhibition of NK cell lysis of self within the hematopoietic compartment is mediated by both 2B4 and MHC class I receptors in a non-redundant fashion (McNerney et al., 2005a). Loss of either 2B4- or MHC-mediated inhibition permitted a partial increase in NK cell lysis, whereas loss of ligands for both systems resulted in greatly elevated NK cell lysis of target cells.
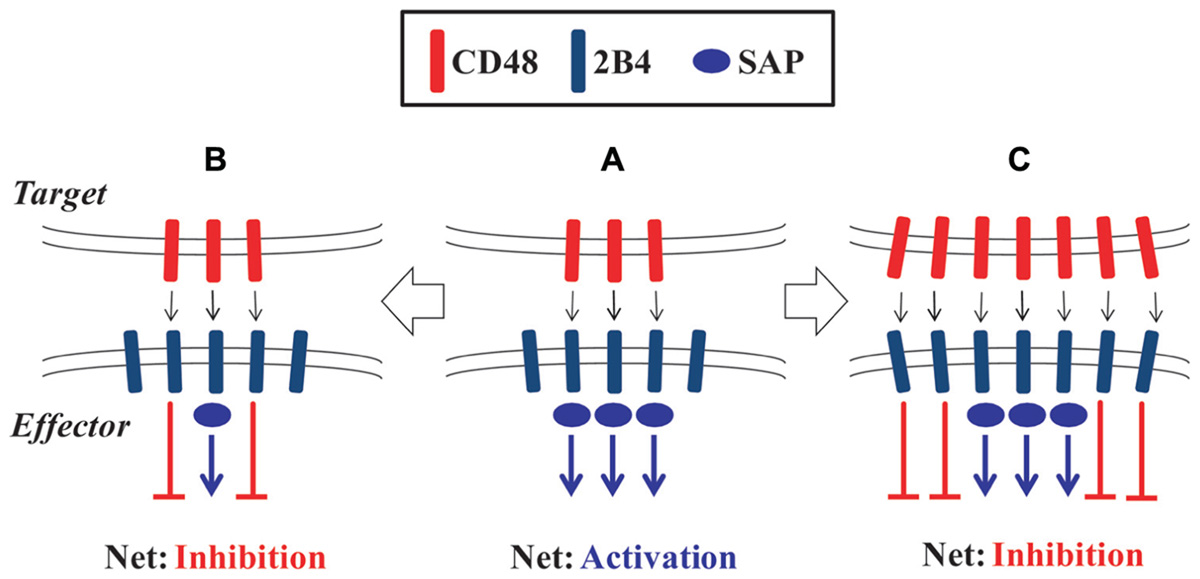
FIGURE 1. Molecular model of the dual-function of 2B4. (A) Engagement of 2B4 by CD48 in the presence of sufficient SAP protein leads to activation of NK or CD8 T cell effector cells. (B) Insufficient levels of SAP protein leads to predominantly inhibitory signals downstream of 2B4 on effector cells. (C) Increased ligand density or receptor expression can also make SAP expression limiting and result in inhibition of effector cell function.
Control of NK Cell Function by 2B4
Given that 2B4 could mediate activation of NK and CD8 T cells, it was somewhat surprising that the generation of 2B4-deficient mice underscored an important inhibitory role for murine 2B4 (Lee et al., 2004; Vaidya et al., 2005). 2B4-deficient NK cells demonstrated an enhanced capacity to kill CD48-expressing target cells in vitro and in vivo (Lee et al., 2004; Vaidya et al., 2005). In the absence of 2B4, NK cells not only lyse hematopoietic tumor cells more efficiently but also begin to target other NK cells (Taniguchi et al., 2007) as well as activated CD8 T cells (Waggoner et al., 2010), suggesting that 2B4 is involved in the maintenance of self-tolerance (McNerney et al., 2005a). While 2B4-deficient mice have strengthened the assertion that 2B4 is an important inhibitor of NK cell cytotoxic function (Figure 2A), no 2B4 deficiencies have been identified in humans, and it is unclear whether ablation of 2B4 expression on human NK cells would produce similar effects to those observed in mice.
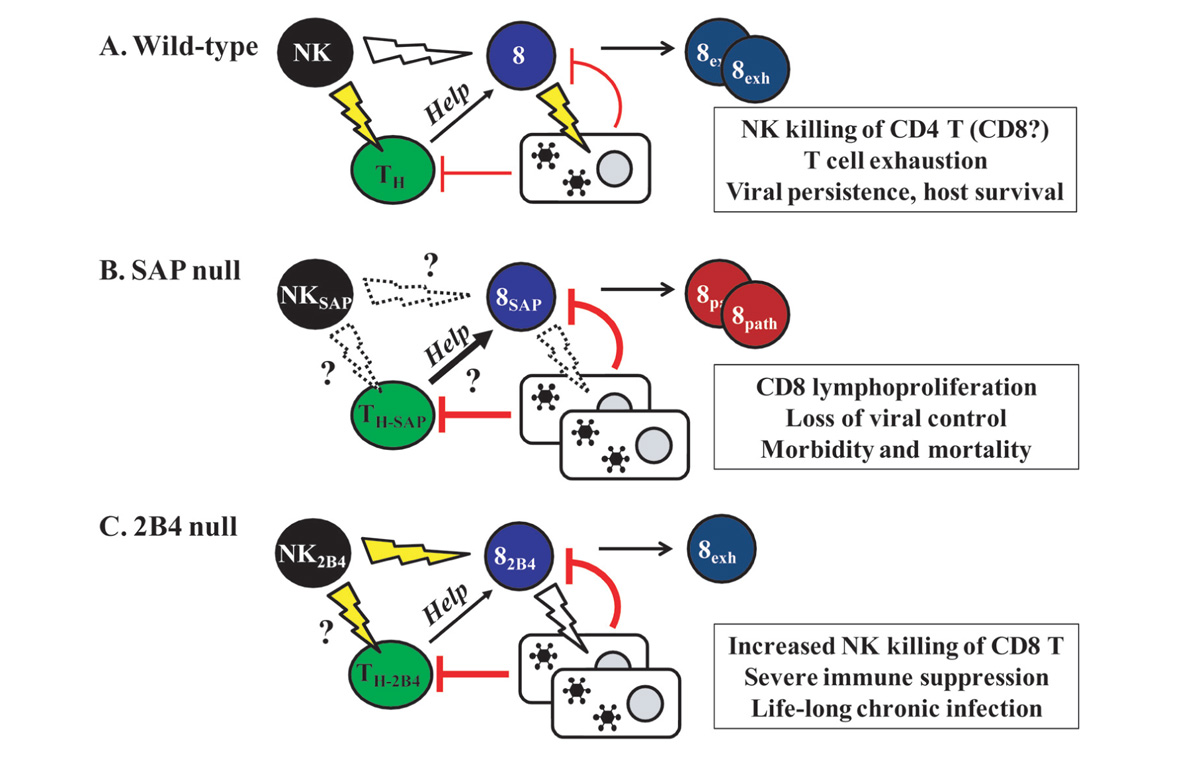
FIGURE 2. Modeling the consequences of 2B4 and SAP expression in immunity to persistent virus infection in mice. (A) In a wild-type scenario, virus infection may trigger NK cell lysis of activated CD4 T helper cells and to a lesser extent activated CD8 T cells. This results in less help from CD4 T cells, thereby favoring a loss of CD8 T cell anti-viral function in the context of high levels of replicating virus. Exhaustion of CD8 T cells permits viral persistence with limited immunopathology. (B) In the absence of SAP, NK and CD8 T cells exhibit an impaired ability to lyse virus-infected cells, resulting in a loss of viral control. Moreover, we postulate that the immunoregulatory lysis of activated T cells by NK cells may be suppressed in the absence of SAP, thereby contributing to the exaggerated expansion of CD8 T cells and associated fatal immune pathology observed in XLP. (C) NK cell lysis of CD8 T cells is enhanced in the absence of 2B4. This causes a severe immune suppression in the context of high viral load, which in turn results in a life-long chronic infection and diminished T cell-mediated tissue damage.
Expression of 2B4 is dynamically regulated on human NK cells during virus infection. One study found that 2B4 expression on NK cells was transiently reduced after HIV infection (Ostrowski et al., 2005). Likewise, 2B4 expression was reduced on NK cells in hepatitis C virus (HCV)-infected individuals who demonstrated enhanced control of viral titers after type I IFN therapy (Ahlenstiel et al., 2011). Thus, reduced 2B4 expression on NK cells may be indicative of the direct anti-viral activity of NK cells. Nonetheless, human NK cells up-regulated 2B4 expression and displayed enhanced functional activity following in vitro exposure to or intramuscular vaccination with influenza A virus (Jost et al., 2011). In addition, heightened transforming growth factor-beta (TGF-β) expression during the immune tolerant phase of persistent hepatitis B virus (HBV) infection was associated with reduced expression of both 2B4 and SAP by NK cells (Sun et al., 2012). These low levels of 2B4 and SAP were further correlated with impaired cytotoxic and IFN-γ-producing activities of NK cells. Of note, TGF-β has recently been shown to contribute to the immaturity of NK cells and susceptibility to virus infection during murine infancy (Marcoe et al., 2012). Together, these studies support the idea that 2B4 functions as an activating receptor on human NK cells during virus infection.
Expression of 2B4 by Virus-Specific T Cells
A subpopulation of CD8 T cells with an activated/memory-phenotype express 2B4, which is postulated to play a co-stimulatory role in T cell activation (Assarsson et al., 2004; Altvater et al., 2009). In the absence of functional SAP (Figure 2B), 2B4 can also profoundly impair CD8 T cell cytotoxicity against EBV-infected B cells (Hislop et al., 2010; Palendira et al., 2011). In a similar fashion, human T-lymphotropic virus I (HTLV-I)-specific CD8 T cells express higher levels of both 2B4 and SAP in patients with HTLV-I-associated neurological disease than in asymptomatic carriers of HTLV-I (Enose-Akahata et al., 2009). Antibody-mediated blockade of 2B4 or knockdown of SAP expression impaired degranulation and IFN-γ production by the HTLV-I-specific CD8 T cells which mediate disease. While this seems to indicate a role for 2B4 in stimulating virus-specific CD8 T cell function, sustained overexpression of 2B4 may also contribute to dysfunction of exhausted virus-specific CD8 T cells during chronic infection.
Chronic viral antigen presentation can drive functional exhaustion and even deletion of virus-specific T cells in both humans and mice (Shin and Wherry, 2007). High level expression of 2B4 and other inhibitory receptors has also been observed on exhausted CD8 T cells specific for LCMV (Wherry et al., 2007), HIV (Aldy et al., 2011), HCV (Bengsch et al., 2010), and HBV (Raziorrouh et al., 2010). Blockade of 2B4–CD48 interactions restored murine LCMV- (Blackburn et al., 2009) and human HBV-specific (Raziorrouh et al., 2010) CD8 T cell effector functions during in vitro culture. Moreover, 2B4-deficient LCMV-specific CD8 memory transgenic T cells were maintained at greater levels than wild-type controls during clone 13 infection of mice (West et al., 2011). Although these results point to a prominent contribution of 2B4 to functional impairment or deletion of virus-specific CD8 T cells (Figure 2A), another study found that cross-linking of 2B4 on HCV-specific CD8 T cells could either inhibit or enhance effector functionality depending on the expression levels of both 2B4 and SAP within T cells (Schlaphoff et al., 2011). In addition, antibody-mediated engagement of 2B4 counteracted PD-1 blockade-induced enhancement of HCV-specific CD8 T cell proliferation during in vitro culture of human PBMCs. The complex nature of 2B4/PD-1 mediated control of human T cell exhaustion could potentially be explained by our recent findings regarding the role of 2B4 on NK cells in preventing NK-mediated lysis of activated CD8 T cells (Waggoner et al., 2010).
Role of 2B4 in NK–T Cell Crosstalk and Viral Pathogenesis
In mice, NK cells play an important role in restricting virus replication during murine cytomegalovirus (MCMV) infection (Bukowski et al., 1983). NK cells may also contribute to immunity by regulating the magnitude and function of MCMV-specific T cell responses (Bukowski et al., 1984; Su et al., 2001; Robbins et al., 2007; Lee et al., 2009; Andrews et al., 2010; Stadnisky et al., 2011; Mitrovic et al., 2012; Narni-Mancinelli et al., 2012). NK cells could impair T cell responses by restricting antigen presentation (Andrews et al., 2010; Mitrovic et al., 2012) or through production of anti-inflammatory cytokines like IL-10 (Lee et al., 2009). Conversely, other studies reported that NK cells sustained conventional dendritic cell populations to enhance anti-viral T cell responses (Robbins et al., 2007; Stadnisky et al., 2011). However, these immunoregulatory contributions of NK cells are difficult to distinguish from the important role of NK cells in direct control of MCMV replication.
In contrast to MCMV, NK cells are largely dispensable in early control of LCMV infection (Bukowski et al., 1983; Welsh et al., 1991). We and others recently described an important role for NK cells in determination of viral clearance and disease associated with LCMV infection that involves direct lysis of virus-specific T cells (Waggoner et al., 2010, 2012; Lang et al., 2012). During infection with the clone 13 strain of LCMV, NK cells aided in the development of persistent infection rather than fatal immunopathology through perforin-mediated lysis of activated CD4 T cells, a population which acts to sustain function of the virus-specific CD8 T cells that mediate virus clearance and tissue damage (Waggoner et al., 2012). NK cells also regulate CD4 responses during LCMV Armstrong infection of CD8 T cell-deficient mice (Su et al., 2001).
Natural killer cells can also mediate direct lysis of activated CD8 T cells (Rabinovich et al., 2003; Soderquest et al., 2011), but this lysis is much more effective when NK cells are not restrained by 2B4 expression (Waggoner et al., 2010). Through the use of different strains of LCMV and NK cell-deficient mice, Ohashi and colleagues (Lang et al., 2012) also demonstrated that NK cells control viral persistence and immunopathology during LCMV infection, but attributed this regulatory effect to perforin-dependent restriction of CD8 T cell expansion without addressing a role for CD4 T cells. Using a modified in vivo cytotoxicity assay, we showed that virus infections and pro-inflammatory stimuli (e.g., polyI:C) triggered a rapid, perforin-dependent lysis of activated, but not naive CD4 T cells (Waggoner et al., 2012). NK cell-dependent elimination of CD8 T cells was limited in these assays, suggesting that activated CD4 and CD8 T differ in their susceptibility to NK cell lysis. Notably, activated CD8 T cells expressed more CD48 than their activated CD4 T cell counterparts during LCMV clone 13 infection (Waggoner et al., 2012), and in the absence of 2B4, NK cells displayed an enhanced ability to lyse activated CD8 T cells (Waggoner et al., 2010). As a consequence of dysregulated NK cell lysis of activated CD8 T cells, 2B4-deficient mice suffered a life-long chronic infection with LCMV clone 13 (Figure 2C). Thus, 2B4–CD48 interactions can prevent NK cell lysis of CD8 T cells and the resulting loss of viral control. In combination with the effects of 2B4 expression by either NK cells or CD8 T cells on the individual anti-viral activities of these effector cells described in the preceding sections, the role of 2B4 in NK cell/T cell crosstalk may be important to consider when evaluating the use of antibody-mediated blockade of 2B4–CD48 interactions in the rescue of the exhausted virus-specific T cell response (Blackburn et al., 2009; Schlaphoff et al., 2011).
Of note, spontaneous clearance of HCV infection (Khakoo et al., 2004), delayed progression to AIDS during HIV infection (Martin et al., 2007), and resistance to HIV infection in chronically exposed sex workers (Jennes et al., 2006) have all been associated with expression of inhibitory killer immunoglobulin-like receptor (KIR) and their cognate HLA ligands. One interpretation of this observation is that stronger inhibition of NK cell immunoregulatory activity by an inhibitory KIR, or perhaps an inhibitory SLAM receptor such as 2B4, could enhance the virus-specific T cell response against the virus. In fact, the potency of NK cell responsiveness was inversely correlated with the magnitude of HIV-specific T cell responses in a cohort of elite controllers (Tomescu et al., 2012).
Concluding Remarks
Immunity to virus infection often involves NK cells and virus-specific T cells, whose responses must be regulated in order to prevent excessive lymphoproliferation that could lead to immunopathology, autoimmunity, and cancer. Growing evidence suggests NK cells may be one mechanism which restrains potentially pathogenic effector T cell responses and that such immunoregulation may itself be regulated by the SLAM receptor 2B4. Heightened expression of 2B4 on NK and CD8 T cells during persistent virus infection may contribute to viral pathogenesis by regulating the anti-viral cytokine-producing and cytolytic functions of these anti-viral effectors. Interactions between 2B4 and CD48 may also contribute to anti-viral immunity in an apparent indirect fashion by controlling the ability of NK cells to regulate virus-specific T cell responses. Evidence from a number of different virus infections demonstrates that 2B4-mediated signals can contribute in both beneficial and detrimental manners to viral clearance and immune-mediated disease. An improved understanding of how 2B4 regulates both effector cell function and NK cell/T cell crosstalk would facilitate development of a number of translational avenues. For example, blockade of 2B4/CD48 interactions may enhance effector functions of NK or CD8 T cells during chronic infections. However, one must temper this prospect with concerns over whether 2B4 blockade would ultimately impair adaptive immunity by unleashing NK cell responses against T cells. The latter possibility itself has translational potential. An improved understanding of how and to what extent 2B4 regulates NK cell lysis of activated T cells could be used to generate NK cell-based therapeutic strategies for autoimmune diseases where T cells are active mediators of disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH training grant AI07349 and the Ellison Medical Foundation to Stephen N. Waggoner.
Abbreviations
EBV, Epstein–Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HTLV-I, human T-lymphotropic virus I; IFN, interferon; ITSM, immunoreceptor tyrosine-based switch motif; KIR, killer immunoglobulin-like receptor; LCMV, lymphocytic choriomeningitis virus; MCMV, murine cytomegalovirus; MHC, major histocompatibility complex; NK, natural killer; NKR, NK cell receptor; NTB-A, NK-T-B-antigen; PD-1, programed death-1; SAP, SLAM-associated protein; SLAM, signaling lymphocyte activation molecule; TGF-β, transforming growth factor-beta; XLP, X-linked lymphoproliferative.
References
Ahlenstiel, G., Edlich, B., Hogdal, L. J., Rotman, Y., Noureddin, M., Feld, J. J., et al. (2011). Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 141, 1231–1239.
Aldy, K. N., Horton, N. C., Mathew, P. A., and Mathew, S. O. (2011). 2B4+ CD8+ T cells play an inhibitory role against constrained HIV epitopes. Biochem. Biophys. Res. Commun. 405, 503–507.
Altvater, B., Landmeier, S., Pscherer, S., Temme, J., Juergens, H., Pule, M. et al. (2009). 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells. Cancer Immunol. Immunother. 58, 1991–2001.
Andrews, D. M., Estcourt, M. J., Andoniou, C. E., Wikstrom, M. E., Khong, A., Voigt, V., et al. (2010). Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J. Exp. Med. 207, 1333–1343.
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B., and Lanier, L. L. (2002). Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326.
Assarsson, E., Kambayashi, T., Schatzle, J. D., Cramer, S. O., von Bonin, A., Jensen, P. E., et al. (2004). NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J. Immunol. 173, 174–180.
Bengsch, B., Seigel, B., Ruhl, M., Timm, J., Kuntz, M., Blum, H. E., et al. (2010). Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6:e1000947. doi: 10.1371/journal.ppat.1000947
Biron, C. A., Byron, K. S., and Sullivan, J. L. (1989). Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735.
Blackburn, S. D., Shin, H., Haining, W. N., Zou, T., Workman, C. J., Polley, A., et al. (2009). Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37.
Bottino, C., Falco, M., Parolini, S., Marcenaro, E., Augugliaro, R., Sivori, S., et al. (2001). NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein–Barr virus-infected B cells in X-linked lymphoproliferative disease. J. Exp. Med. 194, 235–246.
Brown, M. G., Dokun, A. O., Heusel, J. W., Smith, H. R., Beckman, D. L., Blattenberger, E. A., et al. (2001). Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292, 934–937.
Brutkiewicz, R. R., and Welsh, R. M. (1995). Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J. Virol. 69, 3967–3971.
Bukowski, J. F., Woda, B. A., Habu, S., Okumura, K., and Welsh, R. M. (1983). Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J. Immunol. 131, 1531–1538.
Bukowski, J. F., Woda, B. A., and Welsh, R. M. (1984). Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 52, 119–128.
Cannons, J. L., Qi, H., Lu, K. T., Dutta, M., Gomez-Rodriguez, J., Cheng, J., et al. (2010). Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32, 253–265.
Cannons, J. L., Tangye, S. G., and Schwartzberg, P. L. (2011). SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29, 665–705.
Chen, G., Tai, A. K., Lin, M., Chang, F., Terhorst, C., and Huber, B. T. (2005). Signaling lymphocyte activation molecule-associated protein is a negative regulator of the CD8 T cell response in mice. J. Immunol. 175, 2212–2218.
Chlewicki, L. K., Velikovsky, C. A., Balakrishnan, V., Mariuzza, R. A., and Kumar, V. (2008). Molecular basis of the dual functions of 2B4 (CD244). J. Immunol. 180, 8159–8167.
Chung, B., Aoukaty, A., Dutz, J., Terhorst, C., and Tan, R. (2005). Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J. Immunol. 174, 3153–3157.
Coffey, A. J., Brooksbank, R. A., Brandau, O., Oohashi, T., Howell, G. R., Bye, J. M., et al. (1998). Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20, 129–135.
Crawford, A., and Wherry, E. J. (2009). The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr. Opin. Immunol. 21, 179–186.
Crotty, S., Kersh, E. N., Cannons, J., Schwartzberg, P. L., and Ahmed, R. (2003). SAP is required for generating long-term humoral immunity. Nature 421, 282–287.
Crotty, S., McCausland, M. M., Aubert, R. D., Wherry, E. J., and Ahmed, R. (2006). Hypogammaglobulinemia and exacerbated CD8 T-cell-media-ted immunopathology in SAP-defici-ent mice with chronic LCMV infection mimics human XLP disease. Blood 108, 3085–3093.
Czar, M. J., Kersh, E. N., Mijares, L. A., Lanier, G., Lewis, J., Yap, G., et al. (2001). Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lym-phoproliferative disease gene SH2D-1A/DSHP/SAP. Proc. Natl. Acad. Sci. U.S.A. 98, 7449–7454.
Daniels, K. A., Devora, G., Lai, W. C., O’Donnell, C. L., Bennett, M., and Welsh, R. M. (2001). Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194, 29–44.
Dupre, L., Andolfi, G., Tangye, S. G., Clementi, R., Locatelli, F., Arico, M., et al. (2005). SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood 105, 4383–4389.
Engel, P., Perez-Carmona, N., Alba, M. M., Robertson, K., Ghazal, P., and Angulo, A. (2011). Human cytomegalovirus UL7, a homologue of the SLAM-family receptor CD229, impairs cytokine production. Immunol. Cell Biol. 89, 753–766.
Enose-Akahata, Y., Matsuura, E., Oh, U., and Jacobson, S. (2009). High expression of CD244 and SAP regulated CD8 T cell responses of patients with HTLV-I associated neurologic disease. PLoS Pathog. 5:e1000682. doi: 10.1371/journal.ppat.1000682
Griewank, K., Borowski, C., Rietdijk, S., Wang, N., Julien, A., Wei, D. G., et al. (2007). Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity 27, 751–762.
Hislop, A. D., Palendira, U., Leese, A. M., Arkwright, P. D., Rohrlich, P. S., Tangye, S. G., et al. (2010). Impaired Epstein–Barr virus-specific CD8+ T-cell function in X-linked lymphoproliferative disease is restricted to SLAM family-positive B-cell targets. Blood 116, 3249–3257.
Jennes, W., Verheyden, S., Demanet, C., Adje-Toure, C. A., Vuylsteke, B., Nkengasong, J. N., et al. (2006). Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J. Immunol. 177, 6588–6592.
Jost, S., Reardon, J., Peterson, E., Poole, D., Bosch, R., Alter, G., et al. (2011). Expansion of 2B4+ natural killer (NK) cells and decrease in NKp46+ NK cells in response to influenza. Immunology 132, 516–526.
Kageyama, R., Cannons, J. L., Zhao, F., Yusuf, I., Lao, C., Locci, M., et al. (2012). The receptor Ly108 functions as a SAP adaptor-dependent on–off switch for T cell help to B cells and NKT cell development. Immunity 36, 986–1002.
Kambayashi, T., Assarsson, E., Chambers, B. J., and Ljunggren, H. G. (2001). Cutting edge: regulation of CD8+ T cell proliferation by 2B4/CD48 interactions. J. Immunol. 167, 6706–6710.
Khakoo, S. I., Thio, C. L., Martin, M. P., Brooks, C. R., Gao, X., Astemborski, J., et al. (2004). HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874.
Kubin, M. Z., Parshley, D. L., Din, W., Waugh, J. Y., Davis-Smith, T., Smith, C. A., et al. (1999). Molecular cloning and biological characterization of NK cell activation-inducing ligand, a counterstructure for CD48. Eur. J. Immunol. 29, 3466–3477.
Lang, P. A., Lang, K. S., Xu, H. C., Grusdat, M., Parish, I. A., Recher, M., et al. (2012). Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc. Natl. Acad. Sci. U.S.A. 109, 1210–1215.
Lee, K. M., Bhawan, S., Majima, T., Wei, H., Nishimura, M. I., Yagita, H., et al. (2003). Cutting edge: the NK cell receptor 2B4 augments antigen-specific T cell cytotoxicity through CD48 ligation on neighboring T cells. J. Immunol. 170, 4881–4885.
Lee, K. M., McNerney, M. E., Stepp, S. E., Mathew, P. A., Schatzle, J. D., Bennett, M., et al. (2004). 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 199, 1245–1254.
Lee, S. H., Girard, S., Macina, D., Busa, M., Zafer, A., Belouchi, A., et al. (2001). Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat. Genet. 28, 42–45.
Lee, S. H., Kim, K. S., Fodil-Cornu, N., Vidal, S. M., and Biron, C. A. (2009). Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J. Exp. Med. 206, 2235–2251.
Ma, C. S., Hare, N. J., Nichols, K. E., Dupre, L., Andolfi, G., Roncarolo, M. G., et al. (2005). Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J. Clin. Invest. 115, 1049–1059.
Malbran, A., Belmonte, L., Ruibal-Ares, B., Bare, P., Massud, I., Parodi, C., et al. (2004). Loss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood 103, 1625–1631.
Marcoe, J. P., Lim, J. R., Schaubert, K. L., Fodil-Cornu, N., Matka, M., McCubbrey, A. L., et al. (2012). TGF-beta is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat. Immunol. 13, 843–850.
Martin, M. P., Qi, Y., Gao, X., Yamada, E., Martin, J. N., Pereyra, F., et al. (2007). Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39, 733–740.
Mathew, S. O., Rao, K. K., Kim, J. R., Bambard, N. D., and Mathew, P. A. (2009). Functional role of human NK cell receptor 2B4 (CD244) isoforms. Eur. J. Immunol. 39, 1632–1641.
McNerney, M. E., Guzior, D., and Kumar, V. (2005a). 2B4 (CD244)–CD48 interactions provide a novel MHC class I-independent system for NK-cell self-tolerance in mice. Blood 106, 1337–1340.
McNerney, M. E., Lee, K. M., and Kumar, V. (2005b). 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol. Immunol. 42, 489–494.
Mitrovic, M., Arapovic, J., Jordan, S., Fodil-Cornu, N., Ebert, S., Vidal, S. M., et al. (2012). The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8+ T-cell response. J. Virol. 86, 2165–2175.
Mooney, J. M., Klem, J., Wulfing, C., Mijares, L. A., Schwartzberg, P. L., Bennett, M., et al. (2004). The murine NK receptor 2B4 (CD244) exhibits inhibitory function independent of signaling lymphocytic activation molecule-associated protein expression. J. Immunol. 173, 3953–3961.
Nakajima, H., Cella, M., Bouchon, A., Grierson, H. L., Lewis, J., Duckett, C. S., et al. (2000). Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur. J. Immunol. 30, 3309–3318.
Narni-Mancinelli, E., Jaeger, B. N., Bernat, C., Fenis, A., Kung, S., De Gassart, A., et al. (2012). Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 335, 344–348.
Nichols, K. E., Hom, J., Gong, S. Y., Ganguly, A., Ma, C. S., Cannons, J. L., et al. (2005). Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 11, 340–345.
Ostrowski, S. R., Ullum, H., Pedersen, B. K., Gerstoft, J., and Katzenstein, T. L. (2005). 2B4 expression on natural killer cells increases in HIV-1 infected patients followed prospectively during highly active antiretroviral therapy. Clin. Exp. Immunol. 141, 526–533.
Palendira, U., Low, C., Chan, A., Hislop, A. D., Ho, E., Phan, T. G., et al. (2011). Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 9:e1001187. doi: 10.1371/journal.pbio.1001187
Parolini, S., Bottino, C., Falco, M., Augugliaro, R., Giliani, S., Franceschini, R., et al. (2000). X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein–Barr virus-infected cells. J. Exp. Med. 192, 337–346.
Pasquier, B., Yin, L., Fondaneche, M. C., Relouzat, F., Bloch-Queyrat, C., Lambert, N., et al. (2005). Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201, 695–701.
Qi, H., Cannons, J. L., Klauschen, F., Schwartzberg, P. L., and Germain, R. N. (2008). SAP-controlled T–B cell interactions underlie germinal centre formation. Nature 455, 764–769.
Rabinovich, B. A., Li, J., Shannon, J., Hurren, R., Chalupny, J., Cosman, D., et al. (2003). Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J. Immunol. 170, 3572–3576.
Raulet, D. H. (2003). Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3, 781–790.
Raziorrouh, B., Schraut, W., Gerlach, T., Nowack, D., Gruner, N. H., Ulsenheimer, A., et al. (2010). The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 52, 1934–1947.
Robbins, S. H., Bessou, G., Cornillon, A., Zucchini, N., Rupp, B., Ruzsics, Z., et al. (2007). Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 3:e123. doi: 10.1371/journal.ppat.0030123
Schatzle, J. D., Sheu, S., Stepp, S. E., Mathew, P. A., Bennett, M., and Kumar, V. (1999). Characterization of inhibitory and stimulatory forms of the murine natural killer cell receptor 2B4. Proc. Natl. Acad. Sci. U.S.A. 96, 3870–3875.
Schlaphoff, V., Lunemann, S., Suneetha, P. V., Jaroszewicz, J., Grabowski, J., Dietz, J., et al. (2011). Dual function of the NK cell receptor 2B4 (CD244) in the regulation of HCV-specific CD8+ T cells. PLoS Pathog. 7:e1002045. doi: 10.1371/journal.ppat.1002045q
Shah, A. H., Sowrirajan, B., Davis, Z. B., Ward, J. P., Campbell, E. M., Planelles, V., et al. (2010). Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8, 397–409.
Sharifi, R., Sinclair, J. C., Gilmour, K. C., Arkwright, P. D., Kinnon, C., Thrasher, A. J., et al. (2004). SAP mediates specific cytotoxic T-cell functions in X-linked lymphoproliferative disease. Blood 103, 3821–3827.
Shin, H., and Wherry, E. J. (2007). CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19, 408–415.
Smith, H. R., Heusel, J. W., Mehta, I. K., Kim, S., Dorner, B. G., Naidenko, O. V., et al. (2002). Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U.S.A. 99, 8826–8831.
Soderquest, K., Walzer, T., Zafirova, B., Klavinskis, L. S., Polic, B., Vivier, E., et al. (2011). Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J. Immunol. 186, 3304–3308.
Stadnisky, M. D., Xie, X., Coats, E. R., Bullock, T. N., and Brown, M. G. (2011). Self MHC class I-licensed NK cells enhance adaptive CD8 T-cell viral immunity. Blood 117, 5133–5141.
Stepp, S. E., Schatzle, J. D., Bennett, M., Kumar, V., and Mathew, P. A. (1999). Gene structure of the murine NK cell receptor 2B4: presence of two alternatively spliced isoforms with distinct cytoplasmic domains. Eur. J. Immunol. 29, 2392–2399.
Su, H. C., Nguyen, K. B., Salazar-Mather, T. P., Ruzek, M. C., Dalod, M. Y., and Biron, C. A. (2001). NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 31, 3048–3055.
Sun, C., Fu, B., Gao, Y., Liao, X., Sun, R., Tian, Z., et al. (2012). TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 8:e1002594. doi: 10.1371/journal.ppat.1002594
Tangye, S. G., Cherwinski, H., Lanier, L. L., and Phillips, J. H. (2000a). 2B4-mediated activation of human natural killer cells. Mol. Immunol. 37, 493–501.
Tangye, S. G., Phillips, J. H., Lanier, L. L., and Nichols, K. E. (2000b). Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J. Immunol. 165, 2932–2936.
Taniguchi, R. T., Guzior, D., and Kumar, V. (2007). 2B4 inhibits NK-cell fratricide. Blood 110, 2020–2023.
Tatsuo, H., Ono, N., Tanaka, K., and Yanagi, Y. (2000). SLAM (CDw150) is a cellular receptor for measles virus. Nature 406, 893–897.
Thorley-Lawson, D. A., Schooley, R. T., Bhan, A. K., and Nadler, L. M. (1982). Epstein–Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell 30, 415–425.
Tomescu, C., Duh, F. M., Hoh, R., Viviani, A., Harvill, K., Martin, M. P., et al. (2012). Impact of protective KIR/HLA genotypes on NK cell and T cell function in HIV-1 infected controllers. AIDS 26, 1869–1878.
Vaidya, S. V., Stepp, S. E., McNerney, M. E., Lee, J. K., Bennett, M., Lee, K. M., et al. (2005). Targeted disruption of the 2B4 gene in mice reveals an in vivo role of 2B4 (CD244) in the rejection of B16 melanoma cells. J. Immunol. 174, 800–807.
Veillette, A. (2006). NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol. Rev. 214, 22–34.
Veillette, A., Dong, Z., Perez-Quintero, L. A., Zhong, M. C., and Cruz-Munoz, M. E. (2009). Importance and mechanism of ‘switch’ function of SAP family adapters. Immunol. Rev. 232, 229–239.
Vivier, E., and Anfossi, N. (2004). Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat. Rev. Immunol. 4, 190–198.
Waggoner, S. N., Cornberg, M., Selin, L. K., and Welsh, R. M. (2012). Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398.
Waggoner, S. N., Taniguchi, R. T., Mathew, P. A., Kumar, V., and Welsh, R. M. (2010). Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J. Clin. Invest. 120, 1925–1938.
Ward, J., Bonaparte, M., Sacks, J., Guterman, J., Fogli, M., Mavilio, D., et al. (2007). HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110, 1207–1214.
Welsh, R. M., Brubaker, J. O., Vargas-Cortes, M., and O’Donnell, C. L. (1991). Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J. Exp. Med. 173, 1053–1063.
West, E. E., Youngblood, B., Tan, W. G., Jin, H. T., Araki, K., Alexe, G., et al. (2011). Tight regulation of memory CD8+ T cells limits their effectiveness during sustained high viral load. Immunity 35, 285–298.
Wherry, E. J., Ha, S. J., Kaech, S. M., Haining, W. N., Sarkar, S., Kalia, V., et al. (2007). Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684.
Wu, C., Nguyen, K. B., Pien, G. C., Wang, N., Gullo, C., Howie, D., et al. (2001). SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2, 410–414.
Yin, L., Al-Alem, U., Liang, J., Tong, W. M., Li, C., Badiali, M., et al. (2003). Mice deficient in the X-linked lymphoproliferative disease gene sap exhibit increased susceptibility to murine gammaherpesvirus-68 and hypo-gammaglobulinemia. J. Med. Virol. 71, 446–455.
Yusuf, I., Kageyama, R., Monticelli, L., Johnston, R. J., Ditoro, D., Hansen, K., et al. (2010). Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol. 185, 190–202.
Zinkernagel, R. M., and Welsh, R. M. (1976). H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J. Immunol. 117, 1495–1502.
Keywords: NK cells, T cells, virus infection, XLP, SLAM receptors, 2B4
Citation: Waggoner SN and Kumar V (2012) Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front. Immun. 3:377. doi: 10.3389/fimmu.2012.00377
Received: 28 September 2012; Paper pending published: 19 October 2012;
Accepted: 26 November 2012; Published online: 11 December 2012.
Edited by:
Eric Vivier, Centre d’Immunologie de Marseille-Luminy, FranceReviewed by:
Georges Leclercq, Ghent University, BelgiumAlfonso Martin-Fontecha, Boehringer Ingelheim, Germany
Copyright: © 2012 Waggoner and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Stephen N. Waggoner, Department of Pathology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655, USA. e-mail:c3RlcGhlbi53YWdnb25lckB1bWFzc21lZC5lZHU=
