
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 16 May 2012
Sec. Inflammation
Volume 3 - 2012 | https://doi.org/10.3389/fimmu.2012.00112
This article is part of the Research Topic Mast Cells in Immunity View all 14 articles
Mast cells play a key role in allergic reaction and disorders through the high affinity receptor for IgE (FcεRI) which is primarily activated by IgE and antigen complex. In humans, mast cells express two types of FcεRI on the cell surface, tetrameric αβγ2 and trimeric αγ2, whereas in mice, the tetrameric αβγ2 type is exclusively expressed. In human allergic inflammation lesions, mast cells increase in number and preferentially express the αβγ2 type FcεRI. By contrast, in the lesion of non-allergic inflammation, mast cells mainly express the αγ2type. Since the β chain amplifies the expression and signaling of FcεRI, mast cell effector functions and allergic reaction in vivo are enhanced in the presence of the β chain. In contrast, a truncated β chain-isoform (βT) inhibits FcεRI surface expression. The human FcεRIβ gene contains seven exons and a repressor element located in the forth intron, through which FcεRIβ transcription is repressed in the presence of GM-CSF. Regarding the additional signal regulatory function of the β chain, the β chain ITAM has dual (positive and negative) functions in the regulation of the mast cell activation. Namely, the FcεRIβ chain ITAM enhances the mast cell activation signal triggered by a low-intensity (weak) stimulation whereas it suppresses the signal triggered by high-intensity (strong) stimulation. In an oxazolone-induced mouse CHS model, IgE-mediated mast cell activation is required and the β chain ITAM is crucially involved. Adenosine receptor, one of the GPCRs, triggers a synergistic degranulation response with FcεRI in mast cells, for which the β chain ITAM critically plays positive role, possibly reflecting the in vivo allergic response. These regulatory functions of the FcεRIβ ITAM finely tune FcεRI-induced mast cell activation depending on the stimulation strength, enabling the FcεRIβ chain to become a potential molecular target for the development of new strategies for therapeutic interventions for allergies.
Mast cells reside in virtually all organs, among which they are distributed in a great number particularly in tissues at the interface between inside and outside environments, such as the skin and mucosal membrane of the airway and intestine, where they are in close contact with the outside environment and play a key role in allergic reaction and disorders. In IgE-mediated allergic reaction mast cells are activated by the aggregation of the IgE-bound high affinity receptor for IgE (FcεRI) with multivalent antigen.
The FcεRI consists of three subunits, an α chain, a β chain, and a disulfide-linked dimeric γ chain. The α chain binds IgE with a high affinity, while the β and γ chains transduce extracellular signals into the cell through an immunoreceptor tyrosine-based activation motif (ITAM). The γ chain is essential for FcεRI cell surface expression while the β chain is dispensable in humans. J. Hopkin, W. Cockson, and T. Shirakawa originally demonstrated the β chain gene as an “atopy responsible gene” based on the results of a genetic association study (Sandford et al., 1993; Shirakawa et al., 1994). However, functional evidence of the β chain gene as an “atopy gene” has not yet been demonstrated. The human FcεRIβ gene contains seven exons and two spliced products were recently found to produce two truncated proteins, βT and MS4A2truc (Donnadieu et al., 2003; Fiebiger et al., 2005; Cruse et al., 2010). A repressor element was found in the fourth intron and a molecular mechanism for the regulation of FcεRIβ gene expression has been discussed (Takahashi et al., 2006).
The expression of the β chain in specimens from patients with atopic disease was recently investigated and the ratio of αβγ2 FcεRI to αγ2 FcεRI was compared to that in specimens from patients with non-atopic disease (Matsuda et al., 2009).
The consensus sequence of ITAM contains two canonical tyrosine residues (YxxL-x7-YxxL), but the β chain has another non-canonical tyrosine residue (Y225) between the two canonical residues (Y219EELHVY225SPIY229SEL). Using β chain knockout (KO) mice (Dombrowicz et al., 1998; Hiraoka et al., 1999), the function of the non-canonical tyrosine residue (Y225) of the β chain ITAM was investigated (Furumoto et al., 2004). The β chain ITAM was recently shown to work in tandem with the stimulation-intensity (strength), such as the antigen concentration (Nunomura et al., 2005; Xiao et al., 2005). Adenosine with IgE antigen stimulation at a low concentration has also been suggested to enhance mast cell activation remarkably, and this synergistic activation may also be dependent on the β chain ITAM (Nunomura et al., 2010).
We think that this type of β chain-induced enhancement may play a crucial role in allergic reactions occurring in vivo, such as in bronchial asthma. The IgE-FcεRI-mast cell system was shown to be critically involved in an oxazolone-induced contact hypersensitivity (CHS) mouse model (Kobayashi et al., 2010). The role of the β ITAM has also been examined using this mouse model.
In this review we have mainly focused on recent findings regarding the roles of the FcεRIβ chain, especially the dual (positive and negative) regulatory roles of the FcεRIβ chain ITAM, both in vitro and in vivo. Detailed reviews on FcεRI signaling, including this topic, are available (Kraft et al., 2004; Rivera and Gilfillan, 2006; Kraft and Kinet, 2007; Rivera et al., 2008). Findings regarding the novel roles of the FcεRIβ chain in the fine-tuning of mast cell activation will contribute to investigation in new areas for the development of therapeutic interventions for allergic diseases.
The cDNA for the FcεRIβ chain was identified from a cDNA library derived from a rat mucosal mast cell tumor in 1988 (Kinet et al., 1988). Subsequent studies identified mouse and human FcεRIβ chain counterparts (Blank et al., 1989; Ra et al., 1989; Küster et al., 1992). The human and mouse FcεRIβ chain genes contain seven exons. The start and stop codons are located in exon 1 and exon 7, respectively (Figure 1A). The homology among the amino acid sequences of the rat, mouse, and human β chain proteins is approximately 69% (Küster et al., 1992).
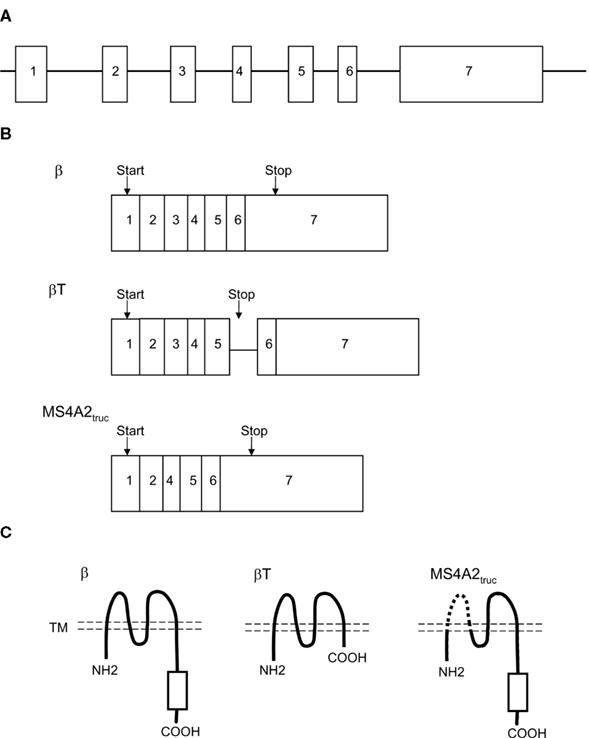
Figure 1. FcεRIβ gene encoding the full-length form of the protein (β) and two truncated alternative splicing products (βT and MS4A2truc). (A) FcεRIβ gene structure. (B) Transcript structure. (C) FcεRIβ protein structure. βT and MS4A2truc lack the C-terminal cytoplasmic region and transmembrane (TM) region (dashed line), respectively.
Recent studies have demonstrated that the human FcεRIβ chain gene encodes two additional spliced products (Donnadieu et al., 2003; Fiebiger et al., 2005; Cruse et al., 2010). These splicing variants produce two truncated proteins, which are designated βT and MS4A2truc (Figures 1B,C). βT retains the fifth intron, which contains a stop codon. Unlike βT, MS4A2truc, a novel β isoform, does not retain this intron sequence and lacks exon 3. Whether the murine FcεRIβ chain gene also encodes βT and/or MS4A2truc is currently unclear.
In humans and mice, the FcεRIβ chain is a component of the tetrameric FcεRI complex, which is expressed in mast cells and basophils. The tetrameric form (αβγ2) of FcεRI is composed of an α chain, a β chain, and a homodimer of γ chains. The full-length FcεRIβ chain protein spans the plasma membrane four times in a manner such that both its N- and C-terminal regions protrude toward the cytoplasm. The C-terminal cytoplasmic region of the FcεRIβ chain possesses an ITAM, which is immediately phosphorylated upon FcεRI crosslinking.
A sequence located in the fourth intron has been shown to serve as a repressor element by screening for cis-acting elements over the entire region of the human FcεRIβ gene (Takahashi et al., 2003). This element binds the transcription factor MZF-1. The MZF-1 antisense inhibits the suppressive effect of the element on the FcεRIβ promoter and increases the quantity of FcεRIβ mRNA, indicating that MZF-1 represses human FcεRIβ gene expression via the element in the fourth intron. This transcriptional repression by MZF-1 requires FHL3 as a cofactor (Takahashi et al., 2005). Furthermore, MZF-1 and FHL3 form a complex with a high molecular mass by binding additional proteins in the nucleus. We identified NFY, which reportedly binds HDACs, as a constituent of the repressor complex in the fourth intron (Takahashi et al., 2006).
GM-CSF, which reportedly decreases FcεRI expression, induces the accumulation of FHL3 in the nucleus, in accordance with the repressive role of FHL3 in FcεRIβ expression. In the presence of GM-CSF, the C-subunit of NFY forms a ternary complex with MZF-1/FHL3 and recruits HDAC1 and HDAC2 on the fourth intron of FcεRIβ gene in human mast cells. As a result, HDACs repress FcεRIβ transcription by deacetylating histones (Figure 2). These mechanisms are involved not only in the cell type-specific repression of FcεRIβ expression in differentiating hematopoietic cells but also in the repression of FcεRIβ expression in peripheral cells, such as mast cells, under specific circumstances.
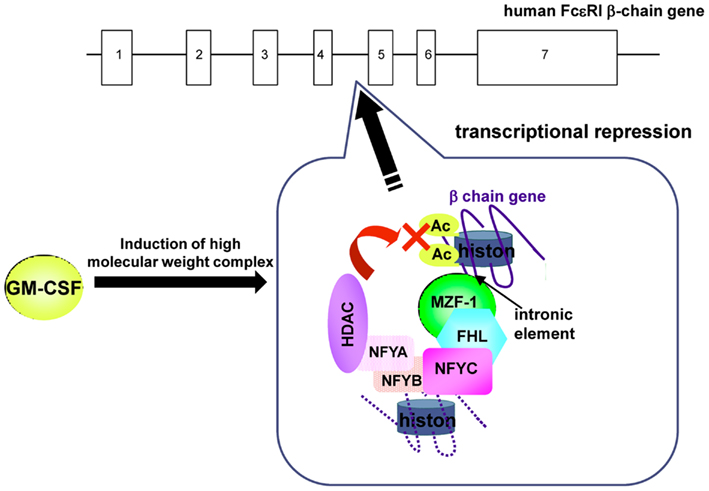
Figure 2. GM-CSF-induced repression of FcεRIβ gene expression in human mast cells. HDACs, which were recruited to the human FcεRIβ gene through the element in the fourth intron by MZF-1/FHL3/NFY, repressed FcεRIβ transcription through the deacetylation of histones in the presence of GM-CSF.
Although the existence of both FcεRIαβγ2 and αγ2 receptor subtypes was theoretically anticipated, the distribution of the FcεRIαβγ2 and αγ2 isoforms in human mast cells in vivo has not been determined. The precise pathophysiological roles of FcεRIβ in human atopic diseases remain unknown.
Atopic keratoconjunctivitis (AKC; Foster and Calonge, 1990; Tuft et al., 1991) and vernal keratoconjunctivitis (VKC) (Bonini et al., 2000) are the most severe form of chronic allergic conjunctivitis, showing the massive infiltration of mast cells and significantly high serum and tear IgE levels compared with those in normal controls (Tuft et al., 1991). VKC and AKC tend to form giant papillae at the upper tasal conjunctiva (Abu el-Asrar et al., 1989; Tuft et al., 1991; Bonini et al., 2000). Histopathological analyses using an anti-FcεRIβ specific antibody (Matsuda et al., 2008) and performed by our group revealed that the densities of FcεRIβ+ cells, FcεRIα+ cells, tryptase+ cells, and the ratio of FcεRIβ+/tryptase+ cells were significantly increased in giant papillae compared with conjunctiva from non-allergic conjunctivitis patients with conjunctivochalasis and superior limbic keratoconjunctivitis (Matsuda et al., 2009; Figure 3). The ratio of the FcεRIβ+ mast cell number/FcεRIα+ mast cell number in the giant papillae was also significantly higher than that in the non-allergic conjunctivitis patients. FcεRIβ+ cells were preferentially localized within and around the epithelial tissue, suggesting that the FcεRIβ+ mast cells around the epithelium in the mucosa of allergic patients are easily able to access allergens.
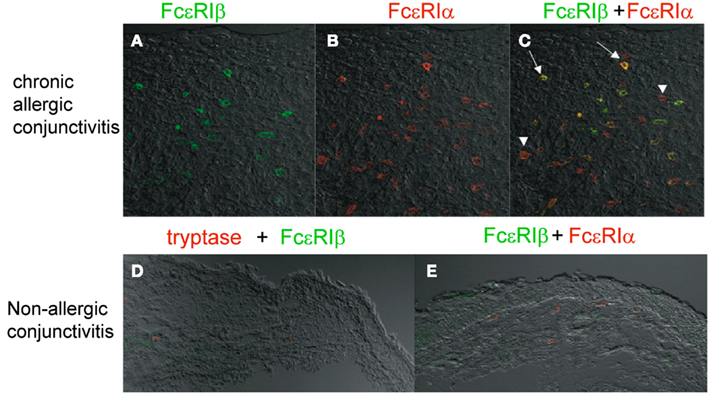
Figure 3. Preferential FcεRIβ expression in the mast cells of giant papillae from patients with atopic keratoconjunctivitis. Anti-FcεRIβ antibody immunostaining of giant papillae. Immunohistochemical staining was carried out with giant papillae specimens obtained from patients with AKC or VKC (A–C) and from patients with superior limbic keratoconjunctivitis (D,E) using the anti-FcεRIβ antibody (green) and the anti-FcεRIα antibody (red) or anti-tryptase antibody (red). FcεRIβ was merged with FcεRIα (C,E). The arrows indicate FcεRIα/β double-positive cells (yellow) and the arrowheads indicate FcεRIα single-positive (red) cells. The figures were reproduced from “Invest Ophth Vis Sci 50(6):2871–2877, 2009” with written permission from ARVO.
Because the shRNA-mediated diminution of the FcεRIβ chain in human mast cells significantly downregulated cell surface FcεRI expression, and IgE-dependent mediator release/production (unpublished data), FcεRIαβγ2 mast cells are thought to contribute to the pathophysiology of AKC/VKC.
The requirement of the FcεRIβ chain for FcεRI cell surface expression differs between rodents and humans. While the FcεRIβ chain is required for surface expression of the receptor in rodents, human FcεRI can be expressed on the cell surface in the absence of the FcεRIβ chain. Therefore, human trimeric FcεRI (αγ2) can be expressed in β chain-deficient cell types, such as monocytes, Langerhans cells, and dendritic cells.
However, the FcεRIβ chain can enhance FcεRI cell surface expression in humans by promoting the maturation (glycosylation) of the FcεRIα chain protein (Donnadieu et al., 2000b). Donnadieu et al. showed that immature FcεRIα chain protein accumulates in the ER in the absence of the FcεRIβ chain protein. Moreover, the FcεRIβ chain increases the stability of surface FcεRI complexes (Donnadieu et al., 2000b). Trimeric FcεRI complexes are unstable when exposed to a strong detergent (Triton-X100), whereas tetrameric FcεRI complexes remain stable when exposed to the same detergent.
The presence of a full-length FcεRIβ chain is thus widely believed to result in a fourfold to sixfold enhancement of FcεRI surface expression. The truncated form of βT lacks the C-terminal cytoplasmic region, including the ITAM. Interestingly, the βT protein is unable to support the maturation of the nascent FcεRIα chain. Therefore, FcεRI surface expression was found to be unaltered following introduction of βT cDNA into CHO cells expressing trimeric FcεRI (αγ2) (Fiebiger et al., 2005). However, the participation of the FcεRIβ chain ITAM domain in the maturation of the FcεRIα chain remains unclear. Further investigation is required to elucidate the role of the FcεRIβ chain ITAM in this maturation process.
Upon the engagement of FcεRI with IgE and a multivalent antigen, the rapid tyrosyl phosphorylation of the FcεRIβ and γ chain ITAMs is initiated; this, in turn, leads to effector functions, such as degranulation, the de novo synthesis of lipid mediators, and cytokine production. The tyrosine phosphorylation of the FcεRIβ chain ITAM occurs through trans-phosphorylation by the src family tyrosine kinase (PTK) Lyn.
Earlier studies found that the FcεRIβ chain acts as an amplifier of FcεRIγ-mediated signaling. The mutation of two canonical tyrosines in the FcεRIβ chain ITAM has been shown to abolish the phosphorylation of both the FcεRIβ and γ chain ITAMs in a rodent mast cell line (Jouvin et al., 1994). Similarly, Lyn KO mast cells showed a reduction in the phosphorylation of the FcεRIβ and γ chains (Nishizumi and Yamamoto, 1997). Furthermore, in fibroblasts expressing human FcεRI, it was revealed that upon receptor engagement, cells expressing the trimeric form of FcεRI (αγ2) exhibit less Lyn-dependent tyrosine phosphorylation of the FcεRIγ chain ITAM and less subsequent tyrosine phosphorylation of Syk kinase (Lin et al., 1996) compared to cells expressing the tetrameric form of FcεRI (αβγ2).
These studies show that the FcεRIβ chain is associated with a fivefold- to sevenfold increase in FcεRI signaling through the FcεRIγ chain ITAM. Researchers have long recognized the classical function of the FcεRIβ chain ITAM as a signal amplifier. However, studies by our group recently revealed novel functions of the FcεRIβ chain ITAM and Lyn in the negative regulation of cell activation and effector functions.
The generation of FcεRIβ chain KO mice (Hiraoka et al., 1999) and the development of retroviral gene transfer have contributed greatly to the establishment of FcεRI reconstitution systems in murine mast cells. This system allows us to investigate the biological functions of the FcεRIβ chain in mast cells. Polymorphisms (I181L, V183L, and E237G) in the coding region of the FcεRIβ chain have been found to be associated with allergic disorders. However, reconstitution studies did not find any effects of these variants on mast cell effector functions (Donnadieu et al., 2000a; Furumoto et al., 2000).
Interestingly, the ITAM sequence of the FcεRIβ chain is unique, differing from the consensus ITAM sequence. While the FcεRIγ chain ITAM (YTGLNTRSQETYETL) contains the consensus sequence (YxxL-x7-YxxL), the FcεRIβ chain ITAM (Y219EELHVY225SPIY229SEL) contains a third non-canonical tyrosine (Y225) between two canonical tyrosine residues (Y219 and Y229; Figure 4). A mutational analysis of these tyrosine residues in the ITAM (tyrosine replaced with phenylalanine, Y→F) performed by our group revealed novel functions of the FcεRIβ chain (Furumoto et al., 2004).
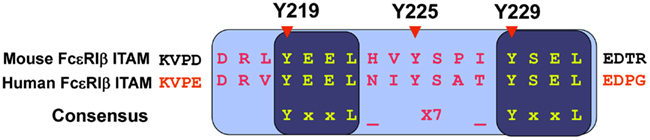
Figure 4. FcεRIβ chain ITAM sequence. The FcεRIβ chain contains a non-canonical tyrosine residue at position 225, which is involved in the negative regulation for mast cell activation.
The N-terminal canonical tyrosine (Y219) in the FcεRIβ chain ITAM is essential for the modulation of the effects of the FcεRIβ chain because of its ability to associate with Lyn upon FcεRI engagement, whereas the other canonical tyrosine (Y229) is dispensable for the interaction of the FcεRIβ chain with Lyn. Mast cells (αβFYFγ2) harboring mutations in Y219 and Y229 showed a reduction in degranulation and cytokine production when stimulated with antigen at sufficient concentration, triggering an optimal degranulation response. Under the same stimulation conditions, the additional introduction of mutation of the middle non-canonical tyrosine (Y225) in αβFYFγ2 mast cells (αβFFFγ2) unexpectedly resulted in a marked increase in cytokine production (IL-6 and IL-13) with no affect on degranulation. This finding was associated with a reduction in the tyrosine phosphorylation of SHIP-1, a negative regulator of signaling. Y225 plays a crucial role in the interaction between the FcεRIβ chain and SHIP-1 following FcεRI stimulation. Thus, the FcεRIβ chain ITAM may mediate negative signals affecting mast cell responses.
Further studies have demonstrated that the strength of FcεRI engagement determines whether the FcεRIβ chain functions as a positive or negative regulator (Figure 5). Upon exposure to low-intensity stimuli, the Lyn-dependent amplifying signals through FcεRIβ can mediate weak positive signals but are insufficient to induce negative signals including SHIP-1; meanwhile, upon exposure to high-intensity stimuli, the FcεRIβ chain increases Lyn-dependent signals considerably, robustly activating both the positive and negative signals simultaneously. Consequently, the FcεRIβ chain increases degranulation and cytokine production in the presence of low-intensity stimuli, whereas it decreases degranulation and cytokine production in the presence of high-intensity stimuli (Nunomura et al., 2005; Xiao et al., 2005).
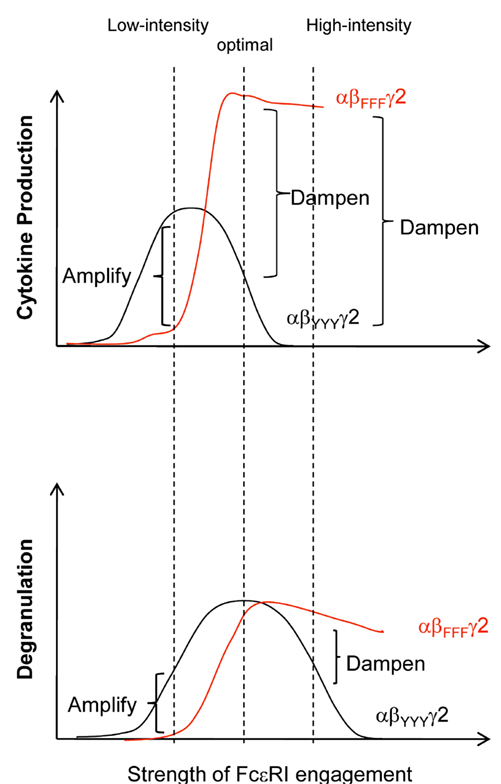
Figure 5. Positive and negative regulation of FcεRI-dependent mast cell activation by the FcεRIβ ITAM. Y→F β (αβFFFγ2) mast cells exhibit reduced degranulation and cytokine production following exposure to a “low-intensity” stimulus (left dashed line), whereas the cells exhibit increased degranulation and cytokine production following exposure to a “high-intensity” stimulus (right dashed line). The FcεRI stimulation that induces the optimal degranulation response of wild-type (αβYYYγ2) mast cells (middle dashed line) triggered a normal degranulation response but increased cytokine production in αβFFFγ2 mast cells.
Hck and phospholipase C β3 (PLCβ3) are reportedly involved in regulation of mast cell functions by the FcεRIβ chain and Lyn (Hong et al., 2007; Xiao et al., 2011). Hck is a PTK expressed at levels of 30-fold to 50-fold less than Lyn in mast cells. However, Hck KO mast cells exhibit a sustained increase in Lyn activity following “high-intensity” FcεRI stimulation. In this context, Hck counteracts the negative roles of Lyn and thereby acts as a positive regulator of mast cell activation. Consequently, degranulation and cytokine production in Hck KO mast cells are decreased.
Similar to Hck KO mast cells, PLCβ3 KO mast cells also show increased Lyn activity. However, although PLCβ3 KO mast cells also exhibit reduced cytokine production, degranulation is normal in these cells. A constitutive interaction among PLCβ3 and the FcεRIβ chain, Lyn, and the SHP-1 protein phosphatase has been observed. In this context, PLCβ3 and SHP-1 regulate mast cell cytokine production by suppressing Lyn and SHIP-1 activity. Importantly, the FcεRIβ chain can provide a docking site for the formation of a negative signalosome that includes Lyn, SHP-1, and SHIP-1.
With regard to the roles of Lyn in FcεRI-dependent mast cell degranulation response, however, contradicting conclusions have been reported. Nishizumi et al. and J. Rivera’s group independently demonstrated that Lyn KO mast cells (129/sv or 129/sv × C57BL/6 [less than N8]) exhibit enhanced degranulation response following high-intensity FcεRI engagement (Nishizumi and Yamamoto, 1997; Odom et al., 2004). However, more recent studies have reported that Lyn KO mast cells (C57BL/6 or 129/sv × C57BL/6 [N8]) are poorly degranulated upon high-intensity FcεRI engagement. Together, these findings raise the possibility that the genetic background of these mice may affect the positive and negative roles of Lyn on the degranulation response. In contrast, the enhanced cytokine responses of Lyn KO mast cells are independent of the genetic background of the mice.
The simultaneous stimulation of FcεRI and adenosine receptors in mast cells triggers a synergistic degranulation response, even when the FcεRI stimulation is of “lower intensity” than the threshold strength (Laffargue et al., 2002). Additionally, an early-phase allergic reaction in asthmatic subjects but not in non-asthmatic subjects is induced by the inhalation of a low-dose mite allergen (Bryant and Burns, 1976; Dohi et al., 1990; M’Raihi et al., 1990). These findings suggest that the augmentation of “low-intensity” FcεRI stimulus-mediated degranulation by an exacerbating factor, such as adenosine, may be responsible for the high susceptibility of asthmatic patients to low-dose allergens.
We recently reported a positive role for the FcεRIβ chain ITAM in the regulation of the synergistic degranulation response following “low-intensity” FcεRI stimulation and adenosine receptor stimulation, possibly reflecting in vivo allergic reactions (Nunomura et al., 2010). In this report, we demonstrated that adenosine fails to increase the degranulation response in αβFFFγ2 mast cells. Conversely, the degranulation response of αβYYYγ2, αβYFYγ2, and αβFYFγ2 mast cells was enhanced, suggesting that the two canonical tyrosine residues Y219 and Y229) in the FcεRIβ ITAM are sufficient for the amplification of the degranulation response by adenosine. This phenomenon was found to be associated with increased phosphorylation of Thr308 in Akt, reflecting PI3K activity.
However, the question of how the FcεRIβ chain ITAM regulates the amplification of the degranulation response and PI3K signaling remains. Of particular note, the tyrosine phosphorylation of the FcεRIβ chain was synergistically increased upon costimulation with FcεRI and adenosine receptors, representing one mechanism that mediates the synergy between the two signaling cascades. However, how adenosine receptor signaling enhances the FcεRI-mediated tyrosine phosphorylation of the FcεRIβ chain remains unclear. Further studies are needed to assess the potential role of adenosine receptors in this process.
Mouse mutants for c-Kit that genetically lack mast cell populations can undergo engraftment with wild-type or genetically altered mast cells (Tsai et al., 2005; Metz et al., 2007). WBB6F1-W/Wv and KitW-sh/W-sh mice are two representative examples of mast cell-deficient mouse strains. Using the adoptive transfer of mast cells into these mast cell-deficient mice, several groups have investigated the role of mast cells in hapten-induced CHS. For instance, mast cells are required for the optimal elicitation of the cutaneous inflammation response associated with mouse models of oxazolone-induced CHS (Bryce et al., 2004; Nakae et al., 2005, 2006; Kakurai et al., 2006). Although the requirement of mast cells for the elicitation of CHS differs with the type and concentration of haptens, the CHS model employing oxazolone is suitable for investigating the in vivo effector functions of mast cells. A recent study by our group revealed that the abrogation of IgE-mediated mast cell activation in the effector phase prevents oxazolone-mediated CHS without affecting the immune response in the sensitization phase (Kobayashi et al., 2010).
Furthermore, using the adoptive transfer of αβYYYγ2 and αβFFFγ2 mast cells into WBB6F1-W/Wv mice, we investigated whether the FcεRIβ chain ITAM regulates the CHS response to oxazolone in mice. In the study, an amplifying role was demonstrated for the FcεRIβ chain ITAM in IgE-mediated in vivo mast cell effector functions, suggesting that the in vivo activation of mast cells may occur through “low-intensity FcεRI stimulation.”
The major focus of this review was the novel roles of the FcεRIβ chain both in vitro and in vivo, especially the dual function of the β chain ITAM. In the β chain ITAM, an additional non-canonical tyrosine residue (Y225) is present between the two canonical residues (Y219, Y229). The β chain positively and negatively regulates FcεRI signaling in response to low-intensity and high-intensity (weak and strong) stimuli, respectively. Lyn kinase associates with the β chain ITAM (Y219) and has a dual-function in the regulation of FcεRI signaling (Furumoto et al., 2004). Hck and PLCβ3 suppress the negative roles of Lyn in mast cell activation (Hong et al., 2007; Xiao et al., 2011). Interactions among PLCβ3 and the β chain, Lyn, and SHP-1 have been reported; in this context, PLCβ3 and SHP-1 regulate mast cell cytokine production by suppressing Lyn and SHIP-1 activity. In this compartment, the β chain may provide a docking platform for the formation of a negative signalosome that includes Lyn, SHP-1 and SHIP-1. Importantly the non-canonical tyrosine residue in the β chain ITAM (Y225) plays a crucial role in interaction between the β chain and SHIP-1 following FcεRI stimulation and in the negative regulation of FcεRI signaling (Furumoto et al., 2004). Regarding the molecular mechanisms for the bidirectional (positive and negative) regulation of the β chain in FcεRI-induced mast cell activation, unknown players and compartments requiring further investigation may exist. The elucidation of the underlying mechanisms responsible for bidirectional response to the strength of the stimuli, such as the type and concentration of antigen, is particularly important. Deeper insights into the activation mechanisms for mast cells are needed for the development of mast cells biology and the pathophysiology of allergy.
In allergic inflammation lesions, such as giant papillae in AKC and atopic dermatitis, mast cells preferentially express the tetrameric αβγ2 type FcεRI (Matsuda et al., 2009), indicating that these mast cells are much more sensitive to antigen stimulation. In contrast, mast cells mainly express the trimeric αγ2 type FcεRI in specimens from non-allergic patients. A repressor element was found in the fourth intron of the FcεRIβ gene and a molecular mechanism to repress the FcεRIβ gene through this element has been elucidated (Takahashi et al., 2006). Further investigation of the regulation of FcεRIβ expression at both the translational and post translational levels, is required, especially for elucidating the mechanisms by which the β chain associates with cell surface-expressed FcεRI. When shRNA for the β chain or phosphorylated ITAM peptide of the β chain was introduced into human mast cells, Ag·IgE-induced histamine, PGD2, and cytokine release were almost completely abolished (unpublished data).
Recent findings regarding the roles of the FcεRIβ chain in fine-tuning of FcεRI signaling indicate that the β chain may be a novel molecular target for the development of new strategies for therapeutic interventions for allergies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work is supported in part by the Grant-in-Aid for Scientific research from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government [Project No. (C) 20591195 and (C) 23591470, awarded to Yoshimichi Okayama and (B) 22390202 awarded to Chisei Ra), from the Grants-in-Aid for private universities from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government, and Nihon University Joint Research Grant for 2008, 2009 (awarded to Chisei Ra), Multidisciplinary Research Grant for 2004 (awarded to Chisei Ra), 2010-2011 (awarded to Yoshimichi Okayama).
Abu el-Asrar, A. M., Van den Oord, J. J., Geboes, K., Missotten, L., Emarah, M. H., and Desmet, V. (1989). Immunopathological study of vernal keratoconjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 227, 374–379.
Blank, U., Ra, C., Miller, L., White, K., Metzger, H., and Kinet, J. (1989). Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 337, 187–189.
Bonini, S., Bonini, S., Lambiase, A., Marchi, S., Pasqualetti, P., Zuccaro, O., Rama, P., Magrini, L., Juhas, T., and Bucci, M. G. (2000). Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology 107, 1157–1163.
Bryant, D., and Burns, M. (1976). Bronchial histamine reactivity: its relationship to the reactivity of the bronchi to allergens. Clin. Allergy 6, 523–532.
Bryce, P., Miller, M., Miyajima, I., Tsai, M., Galli, S., and Oettgen, H. (2004). Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity 20, 381–392.
Cruse, G., Kaur, D., Leyland, M., and Bradding, P. (2010). A novel FcεRIβ-chain truncation regulates human mast cell proliferation and survival. FASEB J. 24, 4047–4057.
Dohi, M., Okudaira, H., Sugiyama, H., Tsurumachi, K., Suko, M., Nakagawa, T., Morita, Y., Ito, K., Nakayama, H., and Miyamoto, T. (1990). Bronchial responsiveness to mite allergen in atopic dermatitis without asthma. Int. Arch. Allergy Appl. Immunol. 92, 138–142.
Dombrowicz, D., Lin, S., Flamand, V., Brini, A. T., Koller, B. H., and Kinet, J. P. (1998). Allergy-associated FcRβ is a molecular amplifier of IgE- and IgG-mediated in vivo responses. Immunity 8, 517–529.
Donnadieu, E., Cookson, W. O., Jouvin, M. H., and Kinet, J. P. (2000a). Allergy-associated polymorphisms of the FcεRI β subunit do not impact its two amplification functions. J. Immunol. 165, 3917–3922.
Donnadieu, E., Jouvin, M. H., and Kinet, J. P. (2000b). A second amplifier function for the allergy-associated FcεRI-β subunit. Immunity 12, 515–523.
Donnadieu, E., Jouvin, M. H., Rana, S., Moffatt, M. F., Mockford, E. H., Cookson, W. O., and Kinet, J. P. (2003). Competing functions encoded in the allergy-associated FcεRIβ gene. Immunity 18, 665–674.
Fiebiger, E., Tortorella, D., Jouvin, M. H., Kinet, J. P., and Ploegh, H. L. (2005). Cotranslational endoplasmic reticulum assembly of FcεRI controls the formation of functional IgE-binding receptors. J. Exp. Med. 201, 267–277.
Furumoto, Y., Hiraoka, S., Kawamoto, K., Masaki, S., Kitamura, T., Okumura, K., and Ra, C. (2000). Polymorphisms in FcεRI β chain do not affect IgE-mediated mast cell activation. Biochem. Biophys. Res. Commun. 273, 765–771.
Furumoto, Y., Nunomura, S., Terada, T., Rivera, J., and Ra, C. (2004). The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IkappaB kinase phosphorylation and mast cell cytokine production. J. Biol. Chem. 279, 49177–49187.
Hiraoka, S., Furumoto, Y., Koseki, H., Takagaki, Y., Taniguchi, M., Okumura, K., and Ra, C. (1999). Fc receptor β subunit is required for full activation of mast cells through Fc receptor engagement. Int. Immunol. 11, 199–207.
Hong, H., Kitaura, J., Xiao, W., Horejsi, V., Ra, C., Lowell, C. A., Kawakami, Y., and Kawakami, T. (2007). The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood 110, 2511–2519.
Jouvin, M. H., Adamczewski, M., Numerof, R., Letourneur, O., Vallé, A., and Kinet, J. P. (1994). Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J. Biol. Chem. 269, 5918–5925.
Kakurai, M., Monteforte, R., Suto, H., Tsai, M., Nakae, S., and Galli, S. (2006). Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am. J. Pathol. 169, 1713–1721.
Kinet, J. P., Blank, U., Ra, C., White, K., Metzger, H., and Kochan, J. (1988). Isolation and characterization of cDNAs coding for the beta subunit of the high-affinity receptor for immunoglobulin E. Proc. Natl. Acad. Sci. U.S.A. 85, 6483–6487.
Kobayashi, M., Nunomura, S., Gon, Y., Endo, D., Kishiro, S., Fukunaga, M., Kitahata, Y., Terui, T., and Ra, C. (2010). Abrogation of high-affinity IgE receptor-mediated mast cell activation at the effector phase prevents contact hypersensitivity to oxazolone. J. Invest. Dermatol. 130, 725–731.
Kraft, S., and Kinet, J. P. (2007). New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 7, 365–378.
Kraft, S., Rana, S., Jouvin, M. H., and Kinet, J. P. (2004). The role of the FcepsilonRI beta-chain in allergic diseases. Int. Arch. Allergy Immunol. 135, 62–72.
Küster, H., Zhang, L., Brini, A. T., MacGlashan, D. W., and Kinet, J. P. (1992). The gene and cDNA for the human high affinity immunoglobulin E receptor beta chain and expression of the complete human receptor. J. Biol. Chem. 267, 12782–12787.
Laffargue, M., Calvez, R., Finan, P., Trifilieff, A., Barbier, M., Altruda, F., Hirsch, E., and Wymann, M. (2002). Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity 16, 441–451.
Lin, S., Cicala, C., Scharenberg, A. M., and Kinet, J. P. (1996). The Fc(epsilon)RIbeta subunit functions as an amplifier of Fc(epsilon)RIgamma-mediated cell activation signals. Cell 85, 985–995.
Matsuda, A., Okayama, Y., Ebihara, N., Yokoi, N., Gao, P., Hamuro, J., Hopkin, J. M., and Kinoshita, S. (2008). High-affinity IgE receptor-beta chain expression in human mast cells. J. Immunol. Methods 336, 229–234.
Matsuda, A., Okayama, Y., Ebihara, N., Yokoi, N., Hamuro, J., Walls, A. F., Ra, C., Hopkin, J. M., and Kinoshita, S. (2009). Hyperexpression of the high-affinity IgE receptor-β chain in chronic allergic keratoconjunctivitis. Invest. Ophthalmol. Vis. Sci. 50, 2871–2877.
Metz, M., Grimbaldeston, M. A., Nakae, S., Piliponsky, A. M., Tsai, M., and Galli, S. J. (2007). Mast cells in the promotion and limitation of chronic inflammation. Immunol. Rev. 217, 304–328.
M’Raihi, L., Charpin, D., Thibaudon, M., and Vervloet, D. (1990). Bronchial challenge to house dust can induce immediate bronchoconstriction in allergic asthmatic patients. Ann. Allergy 65, 485–488.
Nakae, S., Suto, H., Iikura, M., Kakurai, M., Sedgwick, J., Tsai, M., and Galli, S. (2006). Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 176, 2238–2248.
Nakae, S., Suto, H., Kakurai, M., Sedgwick, J., Tsai, M., and Galli, S. (2005). Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. U.S.A. 102, 6467–6472.
Nishizumi, H., and Yamamoto, T. (1997). Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 158, 2350–2355.
Nunomura, S., Gon, Y., Yoshimaru, T., Kashiwakura, J., Kawakami, T., and Ra, C. (2010). FcεRI β-chain ITAM amplifies PI3K-signaling to ensure synergistic degranulation response via FcepsilonRI and adenosine receptors. Eur. J. Immunol. 40, 1205–1217.
Nunomura, S., Gon, Y., Yoshimaru, T., Suzuki, Y., Nishimoto, H., Kawakami, T., and Ra, C. (2005). Role of the FcεRI β-chain ITAM as a signal regulator for mast cell activation with monomeric IgE. Int. Immunol. 17, 685–694.
Odom, S., Gomez, G., Kovarova, M., Furumoto, Y., Ryan, J. J., Wright, H. V., Gonzalez-Espinosa, C., Hibbs, M. L., Harder, K. W., and Rivera, J. (2004). Negative regulation of immunoglobulinE-dependent allergic responses by Lyn kinase. J. Exp. Med. 199, 1491–1502.
Ra, C., Jouvin, M. H., and Kinet, J. P. (1989). Complete structure of the mouse mast cell receptor for IgE (FcεRI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 264, 15323–15327.
Rivera, J., Fierro, N. A., Olivera, A., and Suzuki, R. (2008). New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 98, 85–120.
Rivera, J., and Gilfillan, A. M. (2006). Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 17, 1214–1225.
Sandford, A. J., Shirakawa, T., Moffatt, M. F., Daniels, S. E., Ra, C., Faux, J. A., Young, R. P., Nakamura, Y., Lathrop, G. M., and Cookson, W. O. (1993). Localisation of atopy and beta subunit of high-affinity IgE receptor (FcεRI) on chromosome 11q. Lancet 341, 332–334.
Shirakawa, T., Li, A., Dubowitz, M., Dekker, J. W., Shaw, A. E., Faux, J. A., Ra, C., Cookson, W. O., and Hopkin, J. M. (1994). Association between atopy and variants of the β subunit of the high-affinity immunoglobulin E receptor. Nat. Genet. 7, 125–129.
Takahashi, K., Hayashi, N., Kaminogawa, S., and Ra, C. (2006). Molecular mechanisms for transcriptional regulation of human high-affinity IgE receptor β-chain gene induced by GM-CSF. J. Immunol. 177, 4605–4611.
Takahashi, K., Matsumoto, C., and Ra, C. (2005). FHL3 negatively regulates human high-affinity IgE receptor beta-chain gene expression by acting as a transcriptional co-repressor of MZF-1. Biochem. J. 386, 191–200.
Takahashi, K., Nishiyama, C., Hasegawa, M., Akizawa, Y., and Ra, C. (2003). Regulation of the human high affinity IgE receptor beta-chain gene expression via an intronic element. J. Immunol. 171, 2478–2484.
Tsai, M., Grimbaldeston, M. A., Yu, M., Tam, S. Y., and Galli, S. J. (2005). Using mast cell knock-in mice to analyze the roles of mast cells in allergic responses in vivo. Chem. Immunol. Allergy 87, 179–197.
Tuft, S. J., Kemeny, D. M., Dart, J. K., and Buckley, R. J. (1991). Clinical features of atopic keratoconjunctivitis. Ophthalmology 98, 150–158.
Xiao, W., Kashiwakura, J., Hong, H., Yasudo, H., Ando, T., Maeda-Yamamoto, M., Wu, D., Kawakami, Y., and Kawakami, T. (2011). Phospholipase C-β3 regulates FcεRI-mediated mast cell activation by recruiting the protein phosphatase SHP-1. Immunity 34, 893–904.
Keywords: mast cell, FcεRI, FcεRIβ chain, ITAM, signal transduction, allergy
Citation: Ra C, Nunomura S and Okayama Y (2012) Fine-tuning of mast cell activation by FcεRIβ chain. Front. Immun. 3:112. doi: 10.3389/fimmu.2012.00112
Received: 13 January 2012; Accepted: 20 April 2012;
Published online: 16 May 2012.
Edited by:
Toshiaki Kawakamia, La Jolla Institute for Allergy and Immunology, USAReviewed by:
Bridget S. Wilson, University of New Mexico, USACopyright: © 2012 Ra, Nunomura and Okayama. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Chisei Ra, Nihon University Graduate School of Medical Science, Advanced Medical Research Center, Division of Molecular Cell Immunology and Allergology, 30-1 Oyaguchikami-cho, Itabashi-ku, Tokyo 173-8610, Japan. e-mail:cmEuY2hpc2VpQG5paG9uLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.