
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Imaging, 29 March 2023
Sec. Imaging Applications
Volume 2 - 2023 | https://doi.org/10.3389/fimag.2023.1117798
 Syed Rahmanuddin1*
Syed Rahmanuddin1* Daniel D. Von Hoff2
Daniel D. Von Hoff2 Ammar Chaudhry1
Ammar Chaudhry1 Danielle Guidaben1
Danielle Guidaben1 Marjaan Khan1
Marjaan Khan1 William Boswell1
William Boswell1 Derek Cridebring2
Derek Cridebring2 Jordyn Brase1
Jordyn Brase1 Yuman Fong1
Yuman Fong1 Pejman Motarjem1
Pejman Motarjem1 Erkut Borazanci3
Erkut Borazanci3Purpose: Neoadjuvant therapy and surgical resection can improve the survival rate of patients who receive a diagnosis of pancreatic cancer and shows to be potentially curative. The aim of this study is to define a novel CT perfusion analytical method by observing the treatment response of pancreatic cancer patients in a neoadjuvant-treated population to determine surgical candidacy.
Experimental design: This prospective study involved 22 adult patients with pancreatic ductal adenocarcinoma (PDAC). Participants received neoadjuvant therapy (paricalcitol, paclitaxel protein-bound, cisplatin, and gemcitabine) for up to 6 months. The study examined differences in density between the arterial and venous phases of CT scans using a mathematical analysis called the Marley equation. The data was used to assess treatment responses and determined whether a patient can become a surgical candidate. The consideration for surgical candidacy was defined by Dr. Rahmanuddin, termed the “R” score and graphically depicted as the “R” Clock. The R score determined the number of tumor-linked blood vessels. Any vessel associated with tumor involvement received a score of 1. Patients who received a score of 5 or less were eligible for surgery. 3D Tumor volumetric analyses were performed using GE AW 3D software to assess the treatment response associated with tumor perfusion.
Results: Visual differences in vascular involvement between baseline and final imaging were associated with a higher likelihood of proceeding to surgery. After administration of the neoadjuvant therapy, 81% of patients (18 of 22) received an R score of 5 or less, deeming all of them eligible for surgery. A total of 59% of patients (13 of 22) proceeded with the surgery. Changes in arterial and venous perfusion reflected tumor aggressiveness as defined by the Marley equation.
Conclusion: CT vessel perfusion using the R score and Marley Equation might be helpful in defining the surgical candidacy of PDAC patients when used in conjunction with 3D tumor volumetric quantification. The parameters defined by the R score determined higher perfusion scores as having greater vascular growth, and patients with tumor involvement of more than six vessels were deemed surgically unresectable. The Marley equation demonstrated tumor aggression via changes in arterial and venous perfusion. Additional studies are needed to further validate these methodologies and assess their clinical utility.
Statistically, pancreatic cancer is the third leading cause of cancer death in the United States, with a 5-year survival rate below 10% (Siegel et al., 2016). Worldwide, it represents the 11th most diagnosed cancer (Bray et al., 2018). Pancreatic cancer is most prevalent in developed countries, with risk factors including family history, obesity, diabetes mellitus, high-fat diets, excessive alcohol use, tobacco smoking, and physical inactivity. Due to non-specific symptoms and limited early detection tools, most pancreatic cancer diagnoses are identified in advanced stages and are typically unresectable (Vincent et al., 2011).
Pancreatic ductal adenocarcinoma (PDAC) accounts for over 85% of pancreatic malignancies (Hidalgo et al., 2015). Effective early detection tools for pancreatic cancer are lacking in high-risk populations, including those with >5% lifetime risk (Zhang et al., 2018). Early screening methods for PDAC, including endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI), have not been shown to improve a patient's long-term survival rate. However, both modalities are sensitive and specific for detecting small lesions or early cancer without the risks of radiation exposure. EUS performs better for small solid lesions and provides an opportunity for tissue diagnosis by fine needle aspiration (FNA); however, it is more invasive (Zhang et al., 2018).
For the few patients that have a PDAC diagnosis detected in its early stages, resection of the tumor is the only treatment with curative potential. The prognosis of PDAC remains poor, even in those with resectable disease with an overall 1-year survival rate of 24% (Siegel et al., 2016). Approximately 50–55% of patients at initial presentation have metastatic disease, which is considered unresectable, and only 20% have resectable disease. The remaining 25–30% have what is considered a borderline resectable disease (BR). In BR disease, the tumor remains within the pancreas but also invades nearby vasculature, including the superior mesenteric vein, superior mesenteric artery, celiac vessels, and portal vein, while, in general, resectable disease is considered a tumor limited to the pancreas with no arterial invasion (Versteinjine et al., 2022). However, there is great variability within these classifications (Soweid, 2017).
A recent study reviewed patients with resectable PDAC treated with neoadjuvant chemotherapy with a median overall survival rate of 31.5 months after surgical resection compared to 8.1 months without resection (Christians et al., 2016). Neoadjuvant therapy usually involves either FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) or albumin-bound paclitaxel plus gemcitabine (Seufferlein et al., 2012). In another study involving patients with stage I or II pancreatic cancer, those who received neoadjuvant chemotherapy and resection had a median overall survival rate of 26 months compared to 21 months in patients who had upfront resection without chemotherapy (Mokdad et al., 2017). Studies have repeatedly illustrated the importance of neoadjuvant therapy in reducing tumor size prior to surgery and limiting micrometastatic disease as well as the importance of resection in general for overall survival (Brown et al., 2022). There is growing evidence to suggest these patients with BR disease, in particular, may benefit from neoadjuvant chemotherapy before surgery to increase the odds of R0 or negative margins after resection (Shinchi et al., 2002). However, classifying PDAC into resectable, borderline unresectable, and unresectable is not always straightforward. The decision on resectability is typically multidisciplinary, with variable definitions of what is truly resectable among varying institutions (NCCN, 2022). Most definitions, however, do focus on vascular invasion on imaging with the criteria created by the National Comprehensive Cancer Network (NCCN) probably being the most used.
Multiphase multi-detector computed tomography (MDCT) is the first-line imaging modality for the diagnosis of pancreatic cancer. Pancreas-specific protocols involve images in the pre-contrast, early arterial (CT angiography), portal venous, and pancreatic phases. The early arterial phase is useful in delineating the superior mesenteric artery and aorta, while the portal venous phase can assess venous involvement and liver metastases. There is conflicting data on the optimal phase for the enhancement of pancreatic lesions. Due to its hypovascularity, some studies suggest better visualization of PDAC as low-attenuation lesions during the arterial phase, whereas others demonstrated better pancreatic enhancement and attenuation differences between normal pancreas and tumor in the portal venous phase (Choi et al., 1997; Graf et al., 1997). Pancreatic tumors are often ill-defined, with irregular texture and abnormal morphology. Current imaging techniques fall short when it comes to predicting resection due to radiographically occult malignancies and are much better at predicting unresectable disease (Jimenez et al., 2000).
In this study, we obtained multiphase CT images from patients undergoing treatment for PDAC and used GE AW 3D software to characterize vascular invasion and radiodensity of tumors at different stages. Because current imaging techniques can miss the opportunity for resection, the goal of this study was to develop a novel and clinically applicable radiologic scoring system that accurately reflected treatment response and tumor resectability in the hope of increasing survival.
There is a study looking at a similar radiologic scoring system for cholangiocarcinoma based on current guidelines but with the addition of vascular invasion. This demonstrated a possible increased predictive accuracy for surgical resection (Bird et al., 2019). Another study looked at arterial involvement and resectability before and after neoadjuvant chemotherapy in patients with PDAC. Scores on a 5-point scale were assigned to determine arterial involvement, and scores on a 4-point scale were given based on resectability. Total scores were then used to help differentiate between R0 and R1 disease after resection rather than predicting resectability itself (Noda et al., 2022). There was also a study that evaluated the diagnostic performance of CT-determined resectability after neoadjuvant chemotherapy based on the NCCN guidelines for resectability. However, this study revealed that the NCCN criteria were relatively non-specific and insensitive for predicting R0 resection in patients with PDAC after neoadjuvant chemotherapy, indicating that there is a need for additional radiologic scoring of PDAC resectability (Jang et al., 2020).
A total of 22 adult PDAC patients were used in this prospective study. Major inclusion criteria involved patients with histologically or cytologically confirmed resectable, borderline resectable, or locally advanced (unresectable) PDAC. All patients were enrolled in a clinical trial at HonorHealth Research Institute (Scottsdale, AZ) between 2015 and 2018. Table 1 represents the main exclusion conditions for patients set forth by the study. Patients with prior history of PDAC radiation or chemotherapy were omitted from the trials. Patients with organ failure or coagulopathy were also omitted. However, patients on anticoagulation were included at the discretion of the study investigator.
Study participants were divided into three groups based on resectability: resectable, borderline resectable, and locally advanced/unresectable. Groups were treated with neoadjuvant chemotherapy for up to 6 months. The neoadjuvant regimen consisted of 21-day cycles of paricalcitol, albumin-bound paclitaxel, cisplatin, and gemcitabine. Albumin-bound paclitaxel was given as an IV infusion at a dose of 125 mg/m2 over 30 min on days 1 and 8 of each cycle. Cisplatin was given at a dose of 25 mg/m2 diluted in 500 ml of 0.9% sodium chloride over 60 min on days 1 and 8 of each cycle. Gemcitabine was given at a dose of 1,000 mg/m2 in 250 ml 0.9% of sodium chloride for 30 min on days 1 and 8 of each cycle.
Patients were restaged after three cycles and removed from the study if there was evidence of disease progression. Patients whose CA 19-9 levels had normalized were referred for surgical evaluation, and those with resectable tumors were removed from the study to undergo standard care.
Multiphase CT was performed for most patients on a GE scanner using a standard pancreatic protocol, including a pre-injection phase, arterial phase, and porto-venous phase. The iodinated contrast agent Isovue 370 was dosed at 150 cc + 40 cc saline and was then injected at the rate of 5 cc/s into the antecubital vein.
The areas of interest were defined as the region above the diaphragm to the inferior liver margin for arterial phase images and from above the diaphragm to the superior aspect of the iliac crest for pre-contrast and venous phase images. Scanning parameters were 120 kV with 0.5 s rotation time. Pre-contrast phase images were reconstructed into 3 mm slices, and images from arterial and venous phases were reconstructed into 2 mm slices. The images collected were reviewed by experienced radiologists. Initial images were reconstructed into 0.625 × 1.25 mm slices for 3D post-processing and were uploaded from the picture archiving and communication system (PACS) to advanced imaging software (GE Advantage Workstation 3.2) for 3D volumetric analysis.
GE AW software is a 3D advanced imaging system of GE healthcare. It performs advanced visualization of CT and MRI advanced modality. GE AW 3D was used to perform volumetric analysis and quantify vascular invasion on the CT images of 22 study participants. This software has the ability to highlight the specifically identified vessels attached to a tumor before treatment (known as the “Baseline Vessel”) and after treatment. Such vessels were then categorized as having a “Major” or “Minor” role in tumor development. Changes in the number of vessels involved and tumor volume demonstrated disease progression or regression. Regions of interest were identified between the arterial and venous phases, which were automatically defined and manually edited for volumetric tumor quantification. Figure 1 depicts the images that were used to assess tumor growth and vessel involvement. 3D tumor volumetric assessments were performed to assess treatment response linked directly with tumor perfusion. In a few cases, we could not perform the 3D volumetric due to the stent, which did not allow us to differentiate the margin due to density issues. We decided not to perform the volumetric quantification in those cases due to the concern for accuracy because every fraction of volume is significant in this study for tumor response.
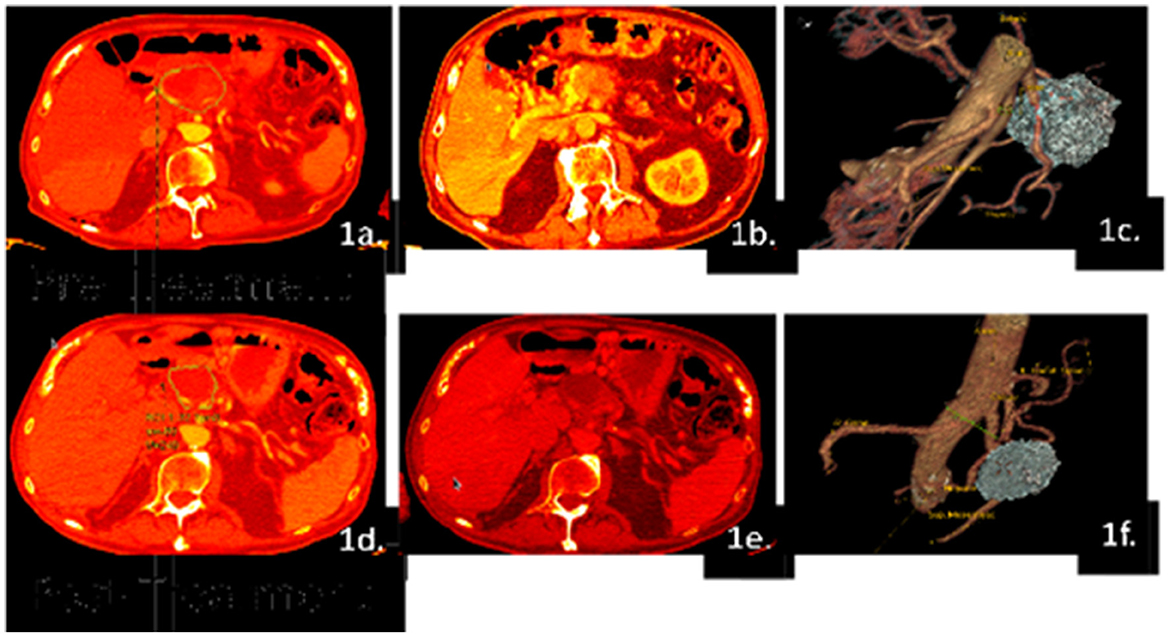
Figure 1. Example of volumetric analysis using Patient #01 images. CT scans were taken prior to and after the treatment regimen, which were analyzed in the arterial (a, d) and venous phases (b, e). A 3D-generated image of the patient's baseline tumor and final tumor size were created to visualize perfusion differences and determine tumor volume (c, f). The patient had a pre-treated tumor volume of 86 cc and a post-treated tumor volume of 31 cc, which indicates the regression of the tumor after therapy, but surgical candidacy is still questionable.
The Marley equation defined by Rahmanuddin et al. (2021) describes the difference between arterial and venous perfusion to predict tumor aggressiveness (Figure 2A). The difference between the arterial phase (first 44 s after contrast injection) and the venous phase (up to 96 s after contrast injection) delineates pancreatic cancer, which is typically hyperintense in venous phase imaging. The difference in duration of these two phases might reflect the aggressiveness of a tumor (Kim et al., 2014). This equation, in conjunction with the Rahmanuddin scoring method, illustrates the tumor's aggressive nature and assisted in the determination of surgical candidacy. The Marley equation is the primary initial step to detect perfusion characteristics that are not necessarily required to get the R score. It defines the imaging-based graphical criteria and predicts the progression or regression of the disease by highlighting the differences in the multi-phase imaging. The secondary step is to count the number of vessels and determine the R score. Multi-phase CT imaging is the key to diagnosing pancreatic cancer. It requires pre-contrast, arterial phase, venous, and delay phase exams. In pancreatic cancer, our focus is more to measure the venous phase timings, which can be detected using the Marley equation formula. The Marley equation is a methodology applicable to use technique detecting perfusion changes and aggressivity of tumors on 3D volumetrics. The higher the perfusion in the venous phase, the more aggressive the disease will be, and the higher volume will be detected as well as the higher the R score. The numbers can be detected on a perfusion-based dynamic imaging graph, which shows the higher and lower contrast time on multi-phase imaging.
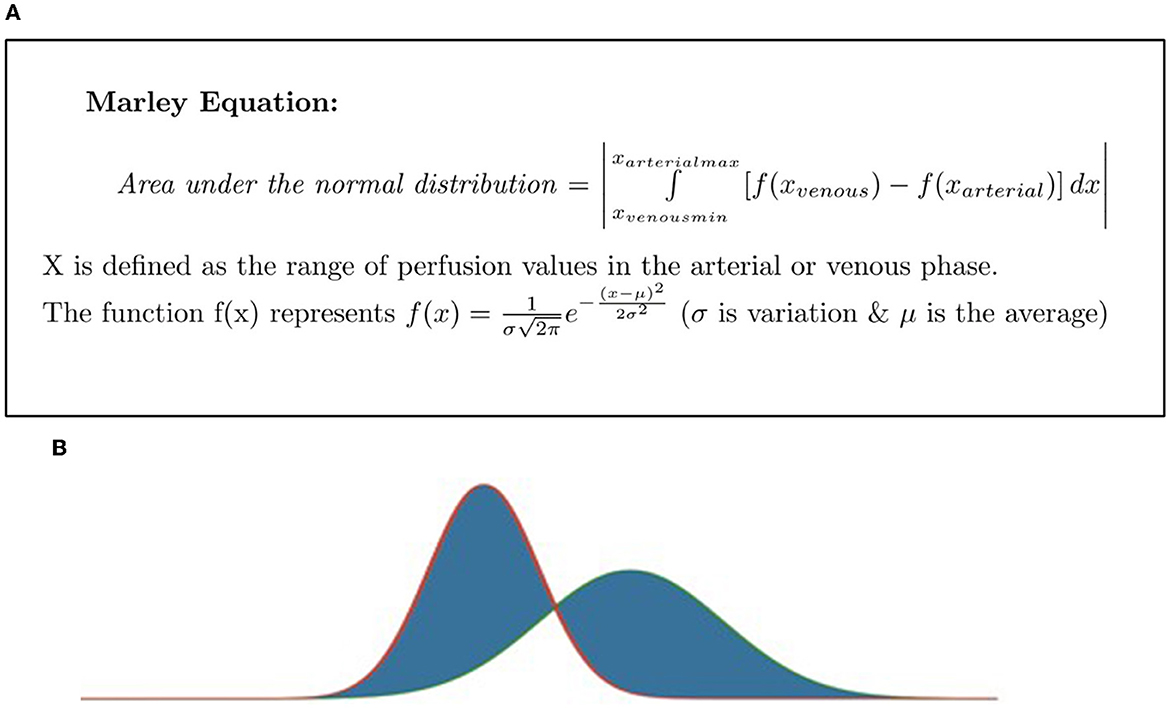
Figure 2. (A) The Marley equation, as defined by Rahmanuddin et al. (2021). ∫ defines the x as arterial max and x venous min. (B) Rahmanuddin scoring graph.
The two vertical bars around our mathematical expression and in this equation show the absolute value. For example, how far it ranges from 0. The Marley equation takes the distribution for the venous phase and arterial phase and calculates the area of the non-overlapping sections, as shown in Figure 2B.
The Rahmanuddin or “R” radiologic scoring system is based on the degree of tumors' vascular invasion, where a score digit represents the number of major and minor vessels involved in either or both arterial or venous phases. For instance, the major vessels include, celiac, hepatic, splenic, superior mesenteric, etc., whereas minor vessels include all branches of pancreatoduodenal, dorsal pancreatic, and transverse pancreatic, and others. Each vessel that has tumor invasion earns 1 point. Patients who score 6 or higher were considered ineligible for surgery because of high vascular invasion, which deemed them unresectable. There are no such perfusion imaging-based criteria available at this time, but the R score could be helpful and reliable if the validation can be done with a higher number of cases. The R score criteria are based on vascular perfusion, which can be detected using the Marley equation. Each vessel scored 1 point in R scoring. For example, 2 vessel involvement means an R score of 2. We have major and minor vessel involvement with a total of ~10 vessels in R score generation. If the score is higher than 5, then surgery is not possible, but a score of 5 or below is suitable for surgical candidacy. The depiction of the R score can be seen in Figures 3A, B termed, as the “R Clock” of resectability.

Figure 3. (A) “R” clock of resectability. As per the scoring criteria, the patients who secure a score of 5 or less are considered suitable candidates for the surgery, as they involve minor vessels. However, patients securing scores of 6 or higher are not suitable candidates for the surgery as they involve major vessels. In (A), the long yellow arrow represents the patient's baseline score before treatment and the short yellow arrow represents the patient's final score after the treatment. Moreover, increased post-treatment scores indicate progressed disease, whereas decreased scores indicate regressed disease. The higher the score above 5, the lower the chances for surgery. As in a clock, as a patient's scores increase or move in a clockwise fashion, the less chance for surgical resection. However, after neoadjuvant chemotherapy, the hope is for the patient's scores to move anticlockwise or drop below a score of 5, thereby increasing the chances of surgical resection. (B) “R” score criterion: the above graph indicates precision criteria based on perfusion and volumetrics.
This R scoring criterion is truly based on 3D volumetrics and depicts that digit 5 is the game changer. Thus, a score of 5 and below predicts that the surgical intervention can be easily conducted as it can be determined that no major vessels will be involved, and the tumor will be highly perfused. This is because the minor vessels have a less significant effect on the tumor and show fewer morphological aggressive changes compared to high perfusion areas. On the other hand, a score of 6 and above clearly indicates the involvement of the major vessels such as celiac or hepatic thereby turning down the surgical candidacy. Moreover, the criteria of score 6 and above are also based on the involvement of major and minor tumor volume changes. Hence, if there is a major tumor volume change, the surgical procedure could have a better outcome when completed. If there is a minor tumor volume change, progression or regression status is critical; therefore, surgery cannot be completed. Progression with a minor increase is a good indication for surgical candidacy but surgical candidacy in the case of regression with a minor change is based on the decision of the surgeon/oncologist. They either need to perform surgery or continue chemotherapy.
A study of the administration of the neoadjuvant NABPLAGEM-NEO 2017 to 22 PDAC patients, was used to gauge the effectiveness of the Marley Equation and R scoring method on tumor resectability. A total of 27% of study participants (six of 22) had a positive response to the neoadjuvant therapy, as demonstrated by decreasing tumor volume in Table 2 and Figure 4. Notably, Patient 1 demonstrated complete remission.
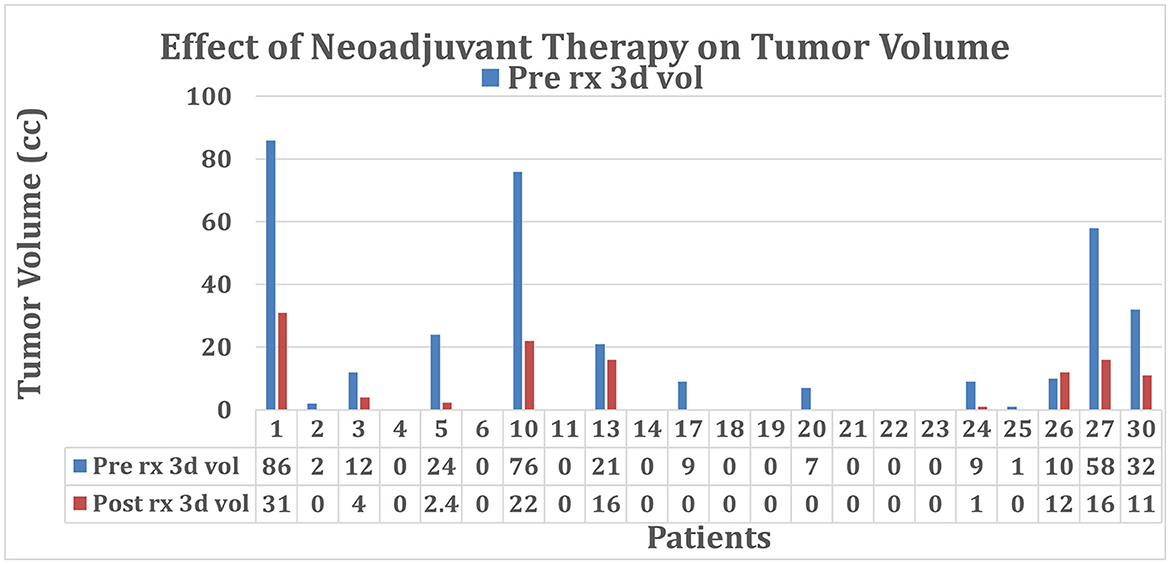
Figure 4. Tumor volume before and after treatment with neoadjuvant NABPLAGEM-NEO 2017. Patients who received a pre-treatment volume of 0 cc had stent involvement causing issues with the estimation of the tumor size. Cases with 0 cc post-treatment either had stent involvement or received surgery during the clinical trial. Refer to Table 2.
Out of the 22 patients, 18 received a baseline R score of 5 or less, deeming them potential surgical candidates. There were nine unknown baseline tumor volumes due to the presence of stents. After treatment, 18 patients were considered resectable, and 13 patients proceeded with surgery. Figures 4, 5 provided the graphical representation of the neoadjuvant therapy outcomes on tumor volume and R score, respectively.
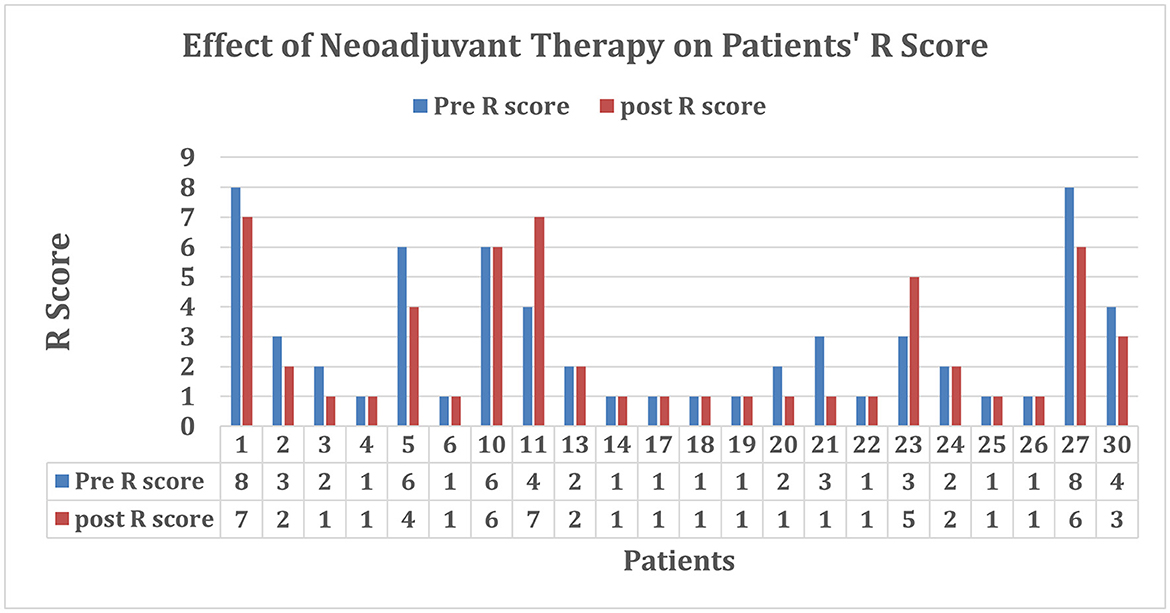
Figure 5. R scores before and after treatment. Patients who received a baseline R score of 0 had visible tumors with no vessel involvement. Those who received a final R score of 0 either had no vessel involvement, no visible tumor, or missing images or proceeded to surgery before the end of the study.
Negative responses to treatment were recorded when there was no shrinkage in tumor volume and an elevated perfusion rate. Mild progression was seen in patient #26 (Figure 6), where the tumor volume pre-treatment was 10 cc and post-treatment was 12cc. This slight increase in size suggested that the therapy was not doing well, and the patient was moved to surgery before the cancer could progress further since this patient had a stent and took longer to edit and quantify the tumor volume.
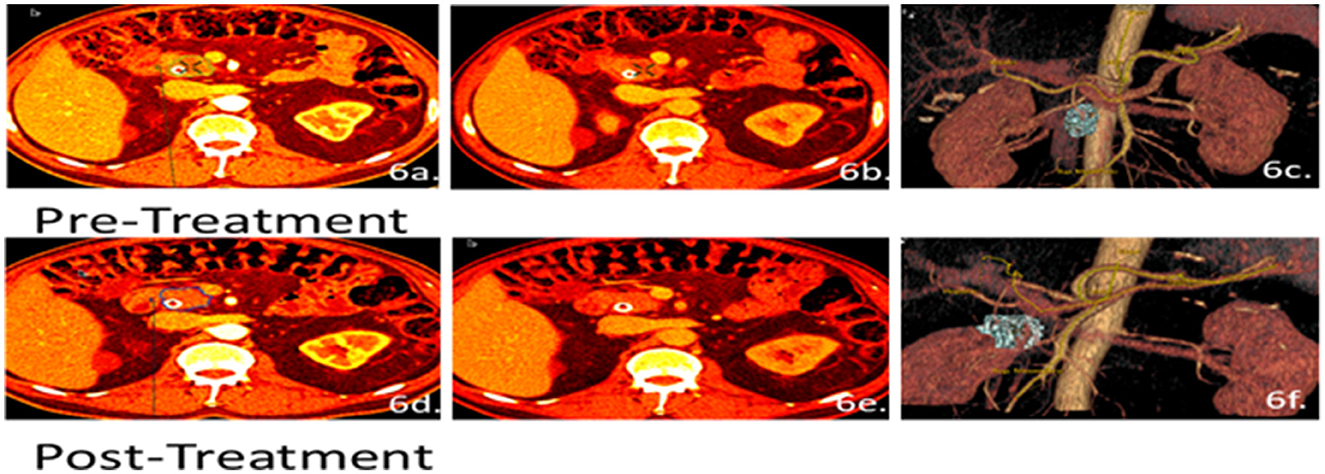
Figure 6. Patient 26 CT images for volumetric and perfusion analysis. Arterial (a, d) and venous phases (b, e) were used to develop the 3D analysis (c, f) of the patient's tumor. The patient's pre-treated tumor volume was estimated at 10 cc and the post-treated tumor volume was 12 cc. However, no change in the R score was observed from a baseline score of 1 to a final score of 1. Even though the tumor size was increasing, the patient still qualified for surgery due to low vascular invasion.
Regression or shrinking was observed in 31% of patients (seven of 22). Notably, patient 05 (Figure 7) initially received a baseline R score of 6 and a pre-treated tumor volume at 24 cc. After treatment, the patient received an R score of 4 and a post-treated tumor volume of 2.4 cc. Due to the significant regression of the cancer, the patient was eligible to receive surgery. This case is unique because both the tumor volume and the vessel involvement score indicated surgery as a potential option. However, the surgery was not done as it was believed the patient might continue responding to the chemotherapy.
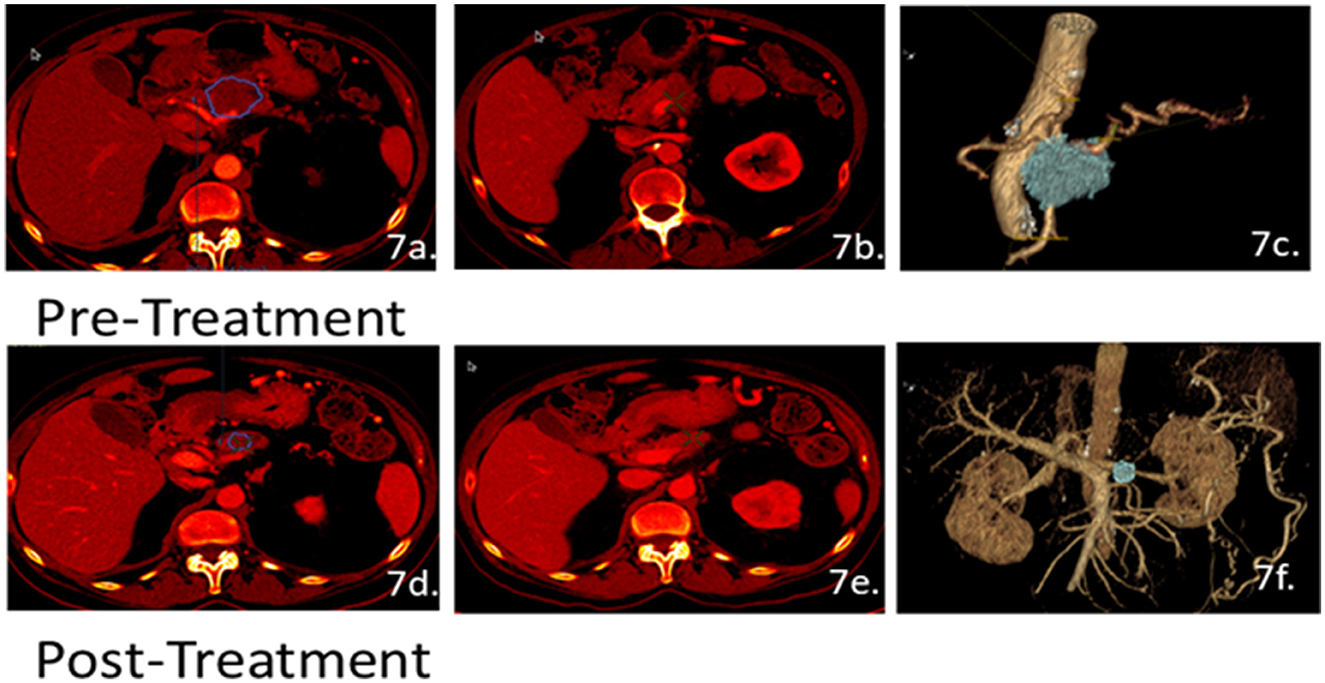
Figure 7. Patient 05 CT scans for volumetric and perfusion analysis. Scans of the arterial (a, d) and venous phases (b, e) were used to develop the 3D images (c, f) for 3D analysis. The patient's pre-treated tumor volume was estimated at 24 cc and the post-treated tumor volume size was 2.4 cc. Initially, patient 05 had a baseline R score of 6 and, through the clinical trials, received a final R score of 4. Due to the significant reduction in both tumor size and receiving an R score of 4, the patient was an eligible candidate for surgery.
The Rahmanuddin scoring method (Figure 3) proved to be a good indicator of whether a patient might proceed to surgery. Patient 01 (seen in Table 1 and Figures 1, 4, 5) received a baseline R score of 8 and a pre-treated tumor volume of 86 cc. Post-therapy, the patient had improved to an R score of 7 with a tumor volume of 31 cc. While this was a dramatic drop in volume, the R score did not drop to the necessary score of 5, indicating that surgery was not a viable option. Other treatment possibilities were considered for the patient.
The main objective of this research was to define a novel CT imaging perfusion analytical method by observing the treatment response of pancreatic cancer patients in a neoadjuvant-treated population to determine whether patients can become appropriate candidates for surgery.
Observing reduction in vascular involvement between pre-treatment and post-treatment imaging was associated with a higher likelihood of proceeding with surgery. If the patient responds to treatment with chemotherapy, the surgical intervention is decided by the medical oncologist and surgeon depending on the R score. For example, in patient number 05, the score went down from 6 to 4, which shows the patient responded well to chemotherapeutic intervention. Therefore, in this case, the clinicians decided not to move forward with surgical intervention. The same rules were applied to all the cases.
Pancreatic ductal adenocarcinoma (PDAC) accounts for over 85% of pancreatic malignancies and is a leading cause of cancer death with a 5-year survival rate of <5% (Siegel et al., 2016). Most cases of PDAC develop from precursor pancreatic intraepithelial neoplasia lesions that progressively acquire genetic alterations to develop into overt cancer. A minority of these tumors can also develop from cystic neoplasms, including intraductal papillary mucinous neoplasms (Scarpa et al., 2018).
Pancreatic cancer generally presents with non-specific symptoms that can be difficult to distinguish from other diseases. Clinical features that are commonly reported at the time of diagnosis include abnormal liver enzymes (50%), abdominal pain (40–60%), new-onset diabetes (13–20%), dyspepsia (20%), nausea or vomiting (16%), and weight loss (10%; Schmidt-Hansen et al., 2016). This non-specific presentation and difficulty accessing the pancreas are challenges to the early detection of pancreatic cancer. Imaging techniques such as CT, ultrasonography, and MRI are important for diagnosis and preoperative assessment (Hookman and Barkin, 2009; De La Cruz et al., 2014).
Stents are commonly used to maintain the patency of ducts and restore flow through vessels (Serruys et al., 2006). Implanted stents can produce interference during imaging and make it more difficult to accurately delineate tumor margins. For example, post-treatment imaging for one patient seen in this study (patient 17, Figure 5) showed increased tumor perfusion, but tumor volume could not be measured due to stent interference. Of the eight patients in this study who required stent placement, seven patients were referred to surgery after neoadjuvant therapy.
Occult tumors are difficult to detect on imaging. One patient was removed from the study due to the absence of a visible tumor on CT despite having signs and symptoms of pancreatic cancer. A potential future direction for 3D imaging development is the ability to visualize inside the pancreatic duct.
Currently, surgery is the only curative treatment for pancreatic cancer. Increasing evidence has demonstrated the utility of neoadjuvant chemotherapy before surgery (Oettle et al., 2007; Neoptolemos et al., 2010, 2017; Conroy et al., 2018). The Rahmanuddin score describes tumor volume and vessel involvement to accurately predict resectability and has greater implications in prognostication and treatment planning. Similarly, another study was conducted to assess the implication of the radiologic scoring system on acute pancreatitis and found that the scoring system predicts the severity of the disease (Delrue et al., 2010). Another study concluded that the radiological scoring system has increased accuracy in predicting persistent organ failure and mortality (Sharma et al., 2015). Larger studies are needed to validate this scoring tool and demonstrate its clinical application for pancreatic cancer.
CT vessel perfusion using the R score and the Marley Equation might be helpful in defining the surgical candidacy of PDAC patients when used in conjunction with 3D tumor volumetric quantification. The parameters defined by the R score determined higher perfusion scores as having greater vascular growth, and patients with tumor involvement of more than six vessels were deemed surgically unresectable. The Marley equation demonstrated tumor aggression via changes in arterial and venous perfusion. Additional studies are needed to further validate these methodologies and assess their clinical utility. The R score criteria could be very beneficial in assessing the progression or regression of the disease. Our future target is to perform this study on a larger scale using 200 patients' data and validate the study at the next level for clinical practice. It will provide a unique approach for surgeons and medical oncologists to assess the disease status and surgical candidacy.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by HonorHealth. The patients/participants provided their written informed consent to participate in this study.
SR, EB, WB, DC, and YF: study design, data collection and analysis, manuscript writing and editing, and final review. DV: study design, manuscript writing and editing, and final review. AC, DG, JB, PM, and MK: data collection and analysis, manuscript writing and editing, and final review.
This study was made possible by clinical data provided by the HonorHealth Research Institute and funding from the Marley Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bird, N., McKenna, A., Dunne, D., Francis, B., Fenwick, S., Poston, G., et al. (2019). Role of a pre-operative radiological scoring system in determining resectability for potentially resectable hilar cholangiocarcinoma. Eur. J. Surg. Oncol. 45, 192–197. doi: 10.1016/j.ejso.2018.08.018
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A., et al. (2018). Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68, 394–424. doi: 10.3322/caac.21492
Brown, Z. J., Heh, V., Labiner, H. E., Brock, G. N., Ejaz, A., Dillhoff, M., et al. (2022). Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: Meta-analysis. Br. J. Surg. 110, 34–42. doi: 10.1093/bjs/znac354
Choi, B., Chung, M., Han, J., Han, M., and Yoon, Y. (1997). Detection of pancreatic adenocarcinoma: Relative value of arterial and late phases of spiral CT. Abdominal Imag. 22, 199–203. doi: 10.1007/s002619900172
Christians, K. K., Heimler, J. W., George, B., Ritch, P. S., Erickson, B. A., Johnston, F., et al. (2016). Survival of patients with resectable pancreatic cancer who received neoadjuvant therapy. Surgery 159, 893–900. doi: 10.1016/j.surg.2015.09.018
Conroy, T., Hammel, P., Hebbar, M., Ben Abdelghani, M., Wei, A. C., Raoul, J.-L., et al. (2018). FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406. doi: 10.1056/NEJMoa1809775
De La Cruz, M. S. D., Young, A. P., and Ruffin, M. T. (2014). Diagnosis and management of pancreatic cancer. Am. Fam. Phys. 89, 626–632.
Delrue, L. J., De Waele, J. J., and Duyck, P. O. (2010). Acute pancreatitis: Radiologic scores in predicting severity and outcome. Abdominal Imag. 35, 349–361. doi: 10.1007/s00261-009-9522-y
Graf, O., Boland, G. W., Warshaw, A. L., Fernandez-del-Castillo, C., Hahn, P. F., and Mueller, P. R. (1997). Arterial vs. portal venous helical CT for revealing pancreatic adenocarcinoma: Conspicuity of tumor and critical vascular anatomy. Am. J. Roentgenol. 169, 119–123. doi: 10.2214/ajr.169.1.9207510
Hidalgo, M., Cascinu, S., Kleeff, J., Labianca, R., Löhr, J. M., Neoptolemos, J., et al. (2015). Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 15, 8–18. doi: 10.1016/j.pan.2014.10.001
Hookman, P., and Barkin, J. S. (2009). Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol. 15, 1554. doi: 10.3748/wjg.15.1554
Jang, J. K., Byun, J. H., Kang, J. H., Son, J. H., Kim, J. H., Lee, S. S., et al. (2020). CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur. Radiol. 31, 813–823. doi: 10.1007/s00330-020-07188-8
Jimenez, R. E., Warshaw, A. L., Rattner, D. W., et al. (2000). Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch. Surg. 135, 409. doi: 10.1001/archsurg.135.4.409
Kim, S. H., Kamaya, A., and Willmann, J. K. (2014). CT perfusion of the liver: Principles and applications in oncology. Radiology 272, 322–344. doi: 10.1148/radiol.14130091
Mokdad, A. A., Minter, R. M., Zhu, H., Augustine, M. M., Porembka, M. R., Wang, S. C., et al. (2017). Neoadjuvant therapy followed by resection vs. upfront resection for resectable pancreatic cancer: A propensity score matched analysis. J. Clin. Oncol. 35, 515–522. doi: 10.1200/JCO.2016.68.5081
NCCN (2022). NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 2, 2022. Available online at: pancreatic.pdf (nccn.org) (accessed January 24, 2023).
Neoptolemos, J. P., Palmer, D. H., Ghaneh, P., Psarelli, E. E., Valle, J. W., Halloran, C. M., et al. (2017). Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024. doi: 10.1016/S0140-6736(16)32409-6
Neoptolemos, J. P., Stocken, D. D., Bassi, C., Ghaneh, P., Cunningham, D., Goldstein, D., et al. (2010). Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. J. Am. Med. Assoc. 304, 1073–1081. doi: 10.1001/jama.2010.1275
Noda, Y., Pisuchpen, N., Mercaldo, N. D., et al. (2022). Arterial involvement and resectability scoring system to predict R0 resection in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation therapy. Eur. Radiol. 32, 2470–2480. doi: 10.1007/s00330-021-08304-y
Oettle, H., Post, S., Neuhaus, P., Gellert, K., Langrehr, J., Ridwelski, K., et al. (2007). Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. J. Am. Med. Assoc. 297, 267–277. doi: 10.1001/jama.297.3.267
Rahmanuddin, S., Korn, R., Cridebring, D., Borazanci, E., Brase, J., Boswell, W., et al. (2021). Role of 3D volumetric and perfusion imaging for detecting early changes in pancreatic adenocarcinoma. Front. Oncol. 11, 678617. doi: 10.3389/fonc.2021.678617
Scarpa, A., Real, F. X., and Luchini, C. (2018). Genetic unrelatedness of co-occurring pancreatic adenocarcinomas and IPMNs challenges current views of clinical management. Gut 67, 1561–1563. doi: 10.1136/gutjnl-2018-316151
Schmidt-Hansen, M., Berendse, S., and Hamilton, W. (2016). Symptoms of pancreatic cancer in primary care: A systematic review. Pancreas 45, 814–818. doi: 10.1097/MPA.0000000000000527
Serruys, P. W., Kutryk, M. J., and Ong, A. T. (2006). Coronary-artery stents. N. Engl. J. Med. 354, 483–495. doi: 10.1056/NEJMra051091
Seufferlein, T., Bachet, J. B., Van Cutsem, E., and Rougier, P. (2012). Pancreatic adenocarcinoma: ESMO–ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23, vii33–vii40. doi: 10.1093/annonc/mds224
Sharma, V., Rana, S. S., Sharma, R. K., Kang, M., Gupta, R., Bhasin, D. K., et al. (2015). A study of radiological scoring system evaluating extrapancreatic inflammation with conventional radiological and clinical scores in predicting outcomes in acute pancreatitis. Ann. Gastroenterol. 28, 399.
Shinchi, H., Takao, S., Noma, H., Matsuo, Y., Mataki, Y., Mori, S., et al. (2002). Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 53, 146–50. doi: 10.1016/s0360-3016(01)02806-1
Siegel, R. L., Miller, K. D., and Jemal, A. (2016). Cancer statistics, 2016. Cancer J. Clin. 66, 7–30. doi: 10.3322/caac.21332
Soweid, A. M. (2017). The borderline resectable and locally advanced pancreatic ductal adenocarcinoma: Definition. Endosc. Ultrasound 6, S76–S78. doi: 10.4103/eus.eus_66_17
Versteinjine, E., van Dam, J. L., Suker, M., Janssen, Q. P., Groothuis, K., Akkermans-Vogelaar, J. M., et al. (2022). Neoadjuvant chemoradiotherapy vs. upfront surgery for resectable and borderline resectable pancreatic cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 40, 1220. doi: 10.1200/JCO.21.02233
Vincent, A., Herman, J., Schulick, R., Hruban, R. H., and Goggins, M. (2011). Pancreatic cancer. Lancet 378, 607–620. doi: 10.1016/S0140-6736(10)62307-0
Keywords: 3D surgical scoring, perfusion, CT, imaging 3D, pancreatic cancer
Citation: Rahmanuddin S, Von Hoff DD, Chaudhry A, Guidaben D, Khan M, Boswell W, Cridebring D, Brase J, Fong Y, Motarjem P and Borazanci E (2023) Utilization of a novel 3D radiologic scoring method to define therapeutic response and surgical candidacy. Front. Imaging. 2:1117798. doi: 10.3389/fimag.2023.1117798
Received: 06 December 2022; Accepted: 02 March 2023;
Published: 29 March 2023.
Edited by:
Marcin Wozniak, Silesian University of Technology, PolandCopyright © 2023 Rahmanuddin, Von Hoff, Chaudhry, Guidaben, Khan, Boswell, Cridebring, Brase, Fong, Motarjem and Borazanci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syed Rahmanuddin, srahmanuddin@coh.org; syedrahmanuddinmd@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.