1
Department of Psychiatry, Beth Israel Deaconess Medical Center, Massachusetts Mental Health Center, Harvard Medical School, Boston, MA, USA
2
Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
3
Department of Psychiatry, University of Illinois, Chicago, IL, USA
Neurocognitive deficits in schizophrenia (SZ) are thought to be stable trait markers that predate the illness and manifest in relatives of patients. Adolescence is the age of maximum vulnerability to the onset of SZ and may be an opportune “window” to observe neurocognitive impairments close to but prior to the onset of psychosis. We reviewed the extant studies assessing neurocognitive deficits in young relatives at high risk (HR) for SZ and their relation to brain structural alterations. We also provide some additional data pertaining to the relation of these deficits to psychopathology and brain structural alterations from the Pittsburgh Risk Evaluation Program (PREP). Cognitive deficits are noted in the HR population, which are more severe in first-degree relatives compared to second-degree relatives and primarily involve psychomotor speed, memory, attention, reasoning, and social-cognition. Reduced general intelligence is also noted, although its relationship to these specific domains is underexplored. Premorbid cognitive deficits may be related to brain structural and functional abnormalities, underlining the neurobiological basis of this illness. Cognitive impairments might predict later emergence of psychopathology in at-risk subjects and may be targets of early remediation and preventive strategies. Although evidence for neurocognitive deficits in young relatives abounds, further studies on their structural underpinnings and on their candidate status as endophenotypes are needed.
Schizophrenia (SZ) was originally described over a century ago with the earlier name “dementia praecox,” which literally means “cognitive decline with onset in youth.” Cognitive impairment is highly prevalent in patients with SZ as determined by the majority of patients who show cognitive decrement relative to parental education (Keefe et al., 2005
) or to their own estimate of premorbid intelligence measured by single word identification (Kremen et al., 1995
). Meta-analyses show that cognitive impairment distinguishes patients with SZ from healthy comparison subjects to a robust degree (i.e., an effect size of approximately one with approximately one standard deviation); these deficits are apparent at the first episode and roughly are equal to those observed in chronic cases (Mesholam-Gately et al., 2009
). Average effect sizes for cognitive impairments in SZ are about twice as large as those obtained in structured magnetic resonance imaging studies (Heinrichs, 2005
). Cognitive impairment is a stable, trait-related aspect of SZ, being present in the early phase of the illness and persisting during the long-term course (Rund, 1998
). Cognitive impairment is a predictor of social and vocational outcome as evaluated longitudinally (Green et al., 2004
). Recent studies suggest that social cognition may have a particularly strong relation to functional outcome (Green et al., 2004
). Finally, cognitive impairment may differ to some extent between SZ and other psychiatric disorders (MacDonald et al., 2005
). Cognitive deficits in patients with SZ are generally more severe and pervasive compared to patients with psychotic and non-psychotic affective disorders (Seidman et al., 2002a
; Hill et al., 2004a
). All of the above observations firmly point to cognitive deficits being a core feature of SZ and clearly a key path toward understanding the etiopathology of this illness.
Genetic factors are the best established etiological determinants of SZ (Keshavan et al., 2005
) as suggested by a heritability of 0.41–0.87 (Cannon et al., 1998
). The risk of SZ is proportional to genetic dose (number of affected relatives and relatedness with the proband). This suggests that studies of relatives at genetic high-risk are a very valuable approach to elucidate the genetic underpinnings of this illness. Offspring of patients have a 10-to 15-fold increase in risk of developing the illness. Having two parents with SZ increases the risk to about 40% (Keshavan et al., 2004
). While studies of unaffected relatives of SZ patients help us understand the genetic underpinnings of this illness, all such relatives may not necessarily be at high-risk; studies of young relatives who are within, or younger than the age range of risk for SZ are more likely to illuminate neurocognitive indicators of risk. The view that SZ is a neurodevelopmental disorder (Feinberg, 1982
; Murray and Lewis, 1987
; Weinberger, 1987
; Keshavan et al., 2006
) suggests that neurocognitive and neurobiological alterations may be detectable in the premorbid phase before the typical onset of the features of the illness (e.g., psychosis) during childhood, adolescence, or early adulthood. These alterations may also represent endophenotypes (i.e. markers intermediate between phenotypic manifestations of the disease and the genotype) (Gottesman and Gould, 2003
).
In this paper, we review studies that have examined various cognitive domains including attention, learning and memory, general intelligence, social-cognition, speed of processing and executive-function (Henry and Crawford, 2005
) in unaffected young relatives presumed to be at high genetic risk. We did an extensive PubMed search using keywords “schizophrenia”, “relatives” and “cognition.” In particular, several high-risk studies conducted over the last three decades were reviewed (Table 1
). We also summarize findings from our ongoing studies related to neurocognition, as well as provide some additional data on the nature of cognitive deficits and their relation to neurobiological alterations as well as the dose of familial risk (first vs. second-degree relatives) in young relatives at risk for SZ.
Table 1. Findings in cognitive domains in high-risk relatives.
There are prominent impairments in SZ in several domains of cognition, including psychomotor speed, memory, attention, reasoning, and social cognition (Table 1
). These may be easily remembered by the mnemonic SMART [Speed of processing, Memory, Attention, Reasoning and Tact (or social cognition)]. Studies have suggested some inter-correlation between cognitive performance on these domains, although there is no clear consensus regards the degree of shared variance across domains. Studies have shown both significant (Dodrill, 1997
) and non-significant correlations between these domains (Nuechterlein et al., 2008
) in patients with SZ. A common “general intelligence” factor, correlating with all domains may explain the lack of independence of cognitive performance on these domains. Evidence suggests correlation of IQ (an index of general intelligence) with performance across domains in SZ patients (Bell and Roper, 1998
; Tremont et al., 1998
; Horton, 1999
; Jung et al., 2000
; Kremen et al., 2008
) and may represent this common general intelligence factor. Alternatively, the inter-dependence of specific cognitive domains could be due to similarities across the different neuropsychological tests used to assess different domains (Larrabee, 2000
). Although IQ deficits generally share variance with specific cognitive deficits, deficits in some domains such as speed of processing and verbal memory have been found to be independent of the IQ deficits. It is therefore unclear if domain-specific deficits can be fully accounted for by a super-ordinate factor like general intelligence.
Speed of Processing
This domain measures cognitive efficiency and involves the ability to automatically and fluently perform relatively easy or repetitive cognitive tasks. Shakow (1963)
originally described this deficit in SZ studying reaction time slowing. Speed of processing has been posited as a predictor of global functioning, autonomy, self care and hence of illness outcome and quality of living (Sánchez et al., 2009
). Reaction time, an indicator of speed of processing, is increased in relatives of patients (Birkett et al., 2007
). Young relatives at risk for SZ have reduced processing speed even after controlling for IQ as shown by the Edinburgh High-Risk Study (EHRS) (p = 0.044) (Byrne et al., 2003
; Cunningham Owens and Johnstone, 2006
; O’Connor et al., 2009
), as well as our studies which will be described later. These deficits might be state-independent given that psychotic symptoms do not alter the severity of speed of processing deficits in patients (O’Connor et al., 2009
). Evidence suggests that processing speed may depend on testing conditions. In a study with varying cognitive processing loads, while the fastest reaction times (that happen during low-cognitive load tasks) were not increased in relatives (Birkett et al., 2007
), mean reaction time was slower, suggesting slower reaction times during high-cognitive-load tasks. Slowed performance on the various psychomotor measures has been shown to be independent of medication (Morrens et al., 2007
). Speed of processing has also been found to predict negative symptoms and impaired functional outcomes (Niendam et al., 2006
; Morrens et al., 2007
).
A genetic-load effect is noted with performance of relatives being intermediate between that of healthy controls and patients on this domain (Birkett et al., 2007
; Gur et al., 2007b
; Bertisch et al., 2009
). Reaction time has been proposed as a putative endophenotype of the illness (Wang et al., 2007
). However, a study showed that both patients with and without reaction time deficits on the Continuous Performance Test (CPT) have relatives showing these deficits (Birkett et al., 2007
). Thus, the candidacy of reaction time deficits as an endophenotype, as well as their role as premorbid vulnerability indicators deserve further consideration.
Memory
Working memory (WM) involves holding information online for brief periods of time, and typically involves processes like information manipulation, maintenance and monitoring in verbal, visual and spatial domains (Kellogg et al., 2007
). Maintenance involves retaining information in a sequential manner, manipulation deals with rearrangement of the information sequence while monitoring checks and updates the contents of WM to determine the next step in a sequential task. WM in all subsystems is impaired in first episode patients (Zanello et al., 2009
) and unaffected first degree relatives (Conklin et al., 2005
; Saperstein et al., 2006
; Horan et al., 2008
). Relatives of SZ patients perform poorly on spatial WM (Awh et al., 1998
; Saperstein et al., 2006
) and spatial memory capacity (O’Connor et al., 2009
). Several studies report impairments in verbal, spatial and object WM domains with a graded pattern of impairment; deficits in patients > relatives > controls are observed for verbal WM (Niendam et al., 2003
; Conklin et al., 2005
). Deficits in WM appear to correlate with negative symptoms (Chkonia and Tsverava, 2007
). Impaired WM has been proposed as a putative endophenotype for SZ (Niendam et al., 2003
).
Verbal declarative or long-term memory is significantly reduced in patients (Chkonia and Tsverava, 2007
), is associated with earlier disease onset, is related to social functioning and negative symptoms (Niendam et al., 2006
), and is proposed to be a predictor of later SZ in high-risk individuals (Niemi et al., 2003
; Groom et al., 2007
) (Table 1
). However, a study reported no verbal-memory deficits in high-risk offspring after controlling for education (Chkonia and Tsverava, 2007
). In the EHRS, deficits in Rey’s auditory verbal learning test predicted later SZ but deficits in Rivermead Behavioral Memory Test did not (Byrne et al., 1999
, 2003
; Cosway et al., 2000
; Johnstone et al., 2002
, 2005
; Whyte et al., 2006
; Whalley et al., 2007
). The New York High-Risk Project (NYHRP) reported that verbal memory deficits predict 83% of high-risk subjects who subsequently received a diagnosis of SZ. The Harvard and Hillside adolescent high-risk study (Seidman et al., 2006
) showed that verbal memory impairment may have promise as a premorbid predictive marker in those at genetic risk for the illness, but further investigation is needed into confounding mediator factors such as affective symptomatology, education and environmental factors in these deficits. Another multi-site study (The Consortium on the Genetics of Schizophrenia) has proposed verbal WM deficits to be putative inherited endophenotypes of SZ (Greenwood et al., 2007
; Horan et al., 2008
). In general, verbal memory has been shown to be one of the most robust deficits in studies of relatives.
Visual memory has been studied less frequently than verbal memory in patients, and impairments in the visual domain among family members appear to be somewhat less severe than in the verbal domain (Snitz et al., 2006
). A study reported verbal recall deficits over short and long delays in both patients and relatives of patients but visual recall deficits only in patients (Heinrichs and Zakzanis, 1998
; Whyte et al., 2005
; Delawalla et al., 2006
). Visual recall deficits have been thought to be state dependent while verbal memory deficits may be heritable stable trait markers (Skelley et al., 2008
). Visio-spatial memory deficits in relatives correlate with their proximity to probands (genetic loading) (Robles et al., 2008
). Also, visual recall deficits in delayed recognition tasks have been observed in high-risk relatives (Byrne et al., 1999
).
Attention
Attention involves the appropriate allocation of processing resources to relevant stimuli, and includes sub-processes like sustained attention and selective attention. A frequently used test to assess attention-performance is the CPT. Several CPT versions vary with regards to modality (auditory or visual), type of stimulus (letters, numbers, colors, or geometric forms), and nature of the task (Miranda et al., 2008
). Attentional abnormalities have been well documented in SZ; attentional deficits are associated with negative and disorganized symptoms and persist despite treatment. Impaired sustained attention indexed by perceptual sensitivity (d′) in the CPT task strongly discriminates high-risk relatives from healthy controls (Erlenmeyer-Kimling et al., 2000
); attention deficits are consistent, temporally stable, and independent of environmental factors or onset of psychotic symptoms (Freedman et al., 1998
; Cornblatt et al., 1999
; Erlenmeyer-Kimling et al., 2000
). Attention deficits predicted more than half (58%) of the high-risk offspring who developed SZ in their future (Erlenmeyer-Kimling et al., 2000
). Measures of attention deviance predicted social outcomes while poor neurobehavioral functioning predicted future SZ spectrum disorders (Marcus et al., 1987
; Erlenmeyer-Kimling et al., 2000
). Attention deficits have been observed in unaffected relatives in the prodromal as well as premorbid phases and have been considered as “endophenotypes” for later emergence of SZ (Cornblatt and Malhotra, 2001
), using poor attentional performance as a marker of vulnerability to SZ could provide a valuable measure of genetic risk.
Reasoning and Executive Function
Executive functions refer to cognitive processes that bear specific tasks related to problem solving. Abstraction (extracting a common feature from various perceptions), reasoning, set shifting (ability to modify ongoing behavior in response to changing goals or environmental input), and error monitoring are critical aspects of executive function. Perseverative and non-perseverative errors on the Wisconsin Card Sorting Test (WCST) are indicators of deficits in cognitive set shifting and generalized reasoning, respectively (Franke et al., 1992
; Gur et al., 2007c
). Relatives of SZ patients show higher perseverative errors but relatively normal non-perseverative errors than controls, suggesting cognitive set shifting to be a vulnerability marker of the illness. Additionally, patients have deficits in non-perseverative errors indicating these to be state dependent; poorer performance on reasoning and problem solving is associated with reduced global functioning (Niendam et al., 2006
). By factor analysis in first degree relatives, perseverative errors, set-shifting difficulties, and idiosyncratic sorting were identified as orthogonal (uncorrelated) dimensions assessed by the WCST (Koren et al., 1998
). In young high-risk relatives of SZ patients, EHRS found deficits in executive function (Johnstone et al., 2002
; Byrne et al., 2003
), while another study displayed poor performance on WCST in relatives of patients having a family history of SZ compared to relatives of patients without a family history of SZ (Birkett et al., 2008
). Some studies have been equivocal (Stratta et al., 1997
), suggesting further investigation.
Social Cognition
Social cognition involves faculties allowing tactful and socially appropriate behavior that involve affect perception, emotion regulation, and the ability to infer other people’s mental states (Theory of Mind). These functions are reported to be compromised in individuals with SZ (Kindermann et al., 1997
; Brune, 2005
). Impairments in social cognition are only partly correlated with and largely independent of neurocognitive dysfunction (Corrigan et al., 1994
; Sergi et al., 2007
), and may underlie symptoms of SZ and disability (Bentall et al., 2001
; Sergi et al., 2006
). Studies have shown that many of these domains of cognitive impairment are stable over time and are present after the cessation of schizophrenic symptoms (Rund, 1998
; Hill et al., 2004b
).
Social cognitive deficits may have predictive value for later SZ (Niendam et al., 2006
, 2007b
; Simon et al., 2006
; Calkins et al., 2007
; Matsumoto et al., 2007
; Suzuki et al., 2007
; Yui et al., 2007
; Chung et al., 2008
; Fornito et al., 2008
; Meisenzahl et al., 2008
; Muñoz Maniega et al., 2008
; Shim et al., 2008
; O’Brien et al., 2009
; Sun et al., 2009
). A large body of evidence shows social cognition aberrations to be the predominant cognitive deficit in the prodrome, a phase that often progresses to psychotic disorder (Moller and Husby, 2000
; Cohen et al., 2006
; Niendam et al., 2006
, 2007a
; Simon et al., 2006
; Cannon et al., 2008
; Chung et al., 2008
). Social dysfunction is a predictor of future positive symptoms (Moller and Husby, 2000
; Cohen et al., 2006
; Niendam et al., 2006
; Cannon et al., 2008
) and influences prodromal morbidity and functioning more than other neurocognitive deficits (Niendam et al., 2006
, 2007b
; Simon et al., 2006
; Calkins et al., 2007
; Shim et al., 2008
; O’Brien et al., 2009
). Structural alterations in regions mediating social cognition (McDonald et al., 2004
; Braff and Light, 2005
; Bender et al., 2007
; Braff et al., 2007
; Gur et al., 2007b
,c
; Keshavan et al., 2007
; Prasad and Keshavan, 2008
; Kallimani et al., 2009
) might therefore be promising predictors of SZ.
Unaffected relatives of patients show deficits in emotion recognition (Kee et al., 2004
; Bediou et al., 2007
; Eack and Mermon, 2009
), and theory of mind tasks (Anselmetti et al., 2009
). A study on siblings of SZ patients (Leppanen et al., 2008
) demonstrated significant performance deficits in the recognition of facial anger. Recently, one study (Addington et al., 2008
) found that individuals clinically at high risk (HR) for developing SZ (i.e., those with prodromal symptoms) performed as poorly as first episode patients on an emotion identification task. Theory of mind deficits also have been shown to be compromised in relatives and together with emotion perception may predict functioning in the community (Irani et al., 2006
; Marjoram et al., 2006
; Pijnenborg et al., 2009
). As reviewed in Phillips and Seidman (2008)
, emotion perception deficits in relatives are consistently present, as well as social anhedonia and negative affect. Some studies have found high-risk offspring to have poor social competence (Goodman, 1987
; Marcus et al., 1987
; Dworkin et al., 1993
).
Verbal Fluency
Language related cognitive deficits, verbal memory (Goldberg et al., 1998
; Riley et al., 2000
), verbal fluency (Goldberg et al., 1998
; Riley et al., 2000
), semantic memory (Lorente-Rovira et al., 2007
), comprehension (Condray et al., 2002
), and receptive language (Condray et al., 2002
) are found to be deficient in patients with SZ and are also present in at-risk children (Keefe et al., 1994
; Chen et al., 2000
; Weiser et al., 2007
). Category verbal fluency indexes semantic memory, lexical access, and executive function while letter fluency may index psychomotor speed (Benton and Hamscher, 1978
). Although verbal fluency is shown to be altered in relatives of SZ patients (Bhojraj et al., 2009
), few studies have assessed young relatives (Broome et al., 2009
). A recent meta-analysis revealed a large effect size (0.68) in category fluency (Snitz et al., 2006
). Verbal fluency may be significantly correlated with intelligence (Gilvarry et al., 2001
); another study reported deficits in verbal fluency and executive function among relatives of SZ patients (Keefe et al., 1994
). The possibility of verbal fluency deficits in young relatives was assessed by the Pittsburgh High-Risk Study (see below) which found significant deficits at the baseline assessment.
General Intelligence
Intelligence deficits in relatives at risk for SZ are equivocal with studies both showing (Mednick and Schulsinger, 1968
; Rieder et al., 1977
; Dworkin et al., 1993
; Byrne et al., 1999
; Goldstein et al., 2000
) and not showing significant IQ deficits (Sameroff et al., 1987
). IQ deficits tend to progress with time as evidenced by some studies (Worland et al., 1982
) while others did not find such a pattern (Goodman, 1987
; Dworkin et al., 1993
). Some studies with HR offspring bearing IQ deficits predicted adult SZ (Cosway et al., 2000
) while others could not (Dworkin et al., 1993
). A study reported low social status and severity of maternal illness to be strong predictors of low IQ in offspring of patients (Sameroff et al., 1993
). Worland et al. (1982)
reported a time by parental diagnosis interaction on verbal IQ among HR offspring, children of mothers with SZ showed more deficits than children of fathers with SZ during a 16-year follow-up, and also children of SZ parents had the lowest stability on IQ scores. The question of whether the liability to SZ is mainly related to a generalized intellectual defect or whether there exists unique cognitive domains with selectively more prominent impairments remains unclear (Woodberry et al., 2008
).
SZ patients show enduring structural gray matter volumetric deficits of the subcortical regions (Ellison-Wright et al., 2008
), medial-temporal, cingulate, prefrontal temporal, and parietal cortices (Shenton et al., 2001
). These alterations may be heritable and have been posited as stable trait markers or endophenotypes of SZ (Keshavan et al., 2007
; Prasad and Keshavan, 2008
). As structural alterations may reflect genetic liability to SZ, brain regions altered in patients may also be altered in their relatives (Keshavan et al., 2007
; Prasad and Keshavan, 2008
). High-risk relatives show alterations of amygdalae, hippocampus (Keshavan et al., 1997
, 2002
; Seidman et al., 1997
, 1999
, 2002b
; O’Driscoll et al., 2001
; Lawrie et al., 2002
; Boos et al., 2007
; Lawrie et al., 2008
), thalami (Seidman et al., 1999
), basal ganglia (Staal et al., 1998
), anterior cingulate gyros (Diwadkar et al., 2006
; Fornito et al., 2008
), and ventricular enlargement (Boos et al., 2007
; Lawrie et al., 2008
). HR subjects have been reported to show structural alterations in white matter: reduced levels of FA (Fractional Anisotropy – an indicator of white matter integrity) (Hoptman et al., 2008
) in anterior limb of internal capsule (Muñoz Maniega et al., 2008
) and in bilateral cingulate and angular gyri (Hoptman et al., 2008
) but relatively increased orbitofrontal white matter volumes (Fan et al., 2008
). Deficits in left posterior cingulate, right inferior parietal, orbitofrontal cortex, and right middle frontal agree with results from the EHRS (Job et al., 2005
) which found an exaggerated longitudinal volume decline in these regions in relatives using voxel based approaches. A left > right decrement of the hippocampal amygdalar complex (Keshavan et al., 2002
; Tanskanen et al., 2005
) in relatives of patients is also reported (Seidman et al., 2002b
). The left parahippocampal gyrus is noted to be altered in those at genetic risk (Seidman et al., 2003
). As reviewed earlier, studies in young relatives of SZ patients have found deficits (Sitskoorn et al., 2004
; Heydebrand, 2006
; Snitz et al., 2006
; Gur et al., 2007c
) in executive-function (Diwadkar et al., 2001
), working-memory, attention (Vanderzeypen et al., 2003
; Klemm et al., 2006
; Lencz et al., 2006
; Seidman et al., 2006
; Schubert and McNeil, 2007
), language (Byrne et al., 1999
; Cosway et al., 2000
; Erlenmeyer-Kimling, 2001
; Schubert and McNeil, 2007
; Thermenos et al., 2007
), speed of processing (Konrad et al., 2008
) and social cognition ( Irani et al., 2006
; Gur et al., 2007c
; Baas et al., 2008
; Mazza et al., 2008
). It is proposed that speed of processing (reaction time) depends on nerve conduction velocity which is in turn based on the myelination of white matter fibers (Begré et al., 2008
). Subjects at risk for SZ have altered white matter volumes, and may lead to slower reaction time (Konrad et al., 2008
). Preliminary studies show that presence of genetic polymorphisms affecting the integrity of white-matter tracts may correlate with reaction-time deficits (McIntosh et al., 2007
).
The amygdalae, hippocampi, and orbito and medial prefrontal regions mediate social-cognition (Bechara et al., 2003
; Britton et al., 2006
; Tsukiura and Cabeza, 2008
). The inferior parietal lobule (Hunter et al., 2003
), and the inferior frontal cortex (Papathanassiou et al., 2000
; Maess et al., 2006
; Kawasaki et al., 2008
) perform language processing while the thalamus, caudate-nucleus (Salgado-Pineda et al., 2003
; Gur et al., 2007a
), middle frontal gyrus, and superior parietal cortex (Wager and Smith, 2003
) have been shown to mediate attention, working-memory, and executive function (Seidman et al., 1994
; Menon et al., 2001
; Shad et al., 2004
; Owen et al., 2005
). Frontal release signs, indices of prefrontal pathology are correlated with executive function and attention (Hyde et al., 2007
).
Premorbid cognitive deficits may map onto observed structural deficits in brain regions mediating corresponding cognitions. Relations between cognitive deficits and brain structural alterations in high-risk relatives have not been systematically examined. If such relations are established, cognitive and brain structural deficits, both considered to be endophenotypes of SZ, might be more parsimoniously explained by the “extended endophenotype” concept (Kippenhan et al., 2005
).
In an ongoing longitudinal study, the Pittsburgh Risk Evaluation Program (PREP), we assess young (10–25 years) first- and second-degree relatives of SZ probands and healthy controls. The participants were identified at the Western Psychiatric Institute and Clinic (WPIC), Pittsburgh or related clinical sites. Young HR relatives were recruited by first approaching patients with SZ with eligible relatives in our outpatient clinical services and via advertisements in community locations. Participants were included if they had a first or second degree relative with SZ or schizoaffective disorder, had an IQ ≥ 80, did not have any lifetime evidence of psychotic disorders, antipsychotic medication exposure, history of substance use, and neurological or medical condition. Age and gender matched healthy controls were recruited from the same community neighborhoods as HR subjects. The study design, demographic, and clinical characteristics of these subjects have been described elsewhere (Keshavan et al., 2008
). We report herein summary observations in key neurocognitive domains and their neuroimaging correlates.
Previously published findings from the PREP study involve deficits in memory, attention, verbal fluency, executive function, social cognition, and general intelligence. High-risk offspring performed poorer compared to controls on spatial WM, sustained attention, category verbal fluency (Eack et al., 2008
), executive function (Keshavan et al., 2004
, 2005
; Eack et al., 2008
), and general intelligence (Eack et al., 2008
). Social cognition deficits in facial emotion recognition were also noted (Eack and Mermon, 2009
). Relatives were found to over-attribute negative valence to neutral faces and took longer to identify neutral faces. These deficits were independent of other neurocognitive dysfunction and correlated with positive symptoms and general psychopathology scores (Keshavan et al., 2004
). Compared to healthy controls, relatives of SZ patients were more prone to develop attention deficit hyperkinetic disorder (Keshavan et al., 2003
) and schizotypal personality traits. Using a multivariate psychobiological prediction model comprised of neuroimaging, neurocognitive, and psychosis proneness measures, these variables together predicted 71% chance to develop psychopathology, in contrast to individuals not identified to develop psychopathology by the model who only had a 17% chance of developing psychopathology (Eack et al., 2008
). In this review, we provide additional data on (a) neurocognitive findings and their familial dose effects and (b) brain structural correlates of neurocognitive deficits in young relatives at risk for SZ.
Neurocognitive Deficits and Genetic Dose Effects
Neurocognitive scores (measured in parentheses) were collected from a neuropsychological battery including IQ (Wechsler Abbreviated Scale of Intelligence; Wechsler, 1999
); WM (Cogtest Spatial Working Memory Test; distance median after a 12-s delay; Cogtest, 2009
); executive functioning (Wisconsin Card Sorting Test perseverative error scores; Heaton et al., 1993
); attention (Continuous Performance Test, IP version visual d prime; Cornblatt et al., 1988
); and verbal fluency (Benton and Hamscher total correct from the category/letter fluency task; Benton and Hamscher, 1978
).
Table 2
denotes deficits seen in first- and second-degree relatives (HR) compared to HC controlling for age at baseline assessment of the PREP study. Significant deficits were noted in HR in IQ (p < 0.000). Higher order cognitive domains like executive function and spatial-WM (Nuechterlein et al., 2008
) were not as prominently affected in HR as were simpler domains such as psychomotor speed, sustained attention, and verbal fluency. Deficits in both attention and spatial WM were attenuated and those in verbal fluency lost significance after controlling for psychomotor speed, suggesting that higher order cognitive deficits may be mediated by deficits in hierarchically more basic cognitive processes such as speed of processing (Nuechterlein et al., 2004
). We assessed familial-loading effects by comparing groups of first-degree relatives (n = 122), second-degree relatives (n = 23) and healthy controls (n = 109) using ANCOVA models. Familial-loading effects were seen at p < 0.05 for psychomotor speed (F = 5.89, p = 0.043), executive-function (F = 4.56, p = 0.05) and verbal-fluency (F = 3.91 p = 0.078) with first-degree relatives performing poorer than second-degree relatives on all domains except WM. Figure 1
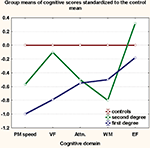
shows that first-degree relatives have the more prominent deficits, while second-degree relatives have impairment intermediate to that of first-degree relatives and healthy controls in all domains except WM. No moderating effects of gender on the main effect of study group (HR vs. HC) were noted.
Table 2. Neurocognitive fi ndings in young relatives of schizophrenia patients in the Pittsburgh High-Risk Study.
Figure 1. Proximity of relatives to patients predicts poorer cognition. Cognitive scores for each group were z-transformed to the control mean. Group-means of the z-scores are plotted on the y-axis. PM, psychomotor; VF, verbal fluency; EF, executive function; Attn, attention; WM, working memory.
As the exact relation of IQ deficits with domain specific deficits is unclear, we conducted parallel analyses controlling, as well as not controlling for IQ. Deficits in sustained attention (F = 5.1, p = 0.025), speed of processing (F = 5.2, p = 0.023), and verbal fluency (6.2, p = 0.011) in relatives survived controlling for IQ. All neurocognitive scores were significantly correlated with IQ (r ranging from 0.30 to 0.43). Studies in patients have shown most neurocognitive deficits, except for psychomotor speed and verbal memory, to be mediated by a latent “cognitive ability factor” (Weickert et al., 2000
; Dickinson et al., 2008
). This agrees with findings of attention and verbal fluency deficits but not psychomotor speed deficits losing significance after controlling for IQ in the PREP study. A latent cognitive ability factor as underpinning all neurocognitive deficits is debatable as the latent factor was revealed using a correlation method in a cross-sectional design (Dodrill, 1997
, 1999
; Bell and Roper, 1998
; Tremont et al., 1998
; Horton, 1999
; Jung and Haier, 2007
; Dickinson et al., 2008
). Longitudinal studies have shown cognitive deficits to precede generalized cognitive deficits like IQ (Weickert et al., 2000
). Controlling for IQ when assessing cognitive deficits may be unnecessarily conservative, especially given the equivocal evidence about the role of IQ in cognitive deficits (Dickinson et al., 2008
). IQ deficit may be an inherent, natural property of subjects at genetic risk instead of a confound and hence controlling it may have the effect of throwing the baby out with the bathwater (Miller and Chapman, 2001
). Also, correlations between a dependent variable and a putative confound argue against controlling for that confound as it may obscure real group differences of the dependent variables (Miller and Chapman, 2001
).
The spatial-working-memory deficits noted at the 12-s delay were absent for a 2-s delay. This supports previous evidence suggesting a task difficulty by group interaction when comparing SZ patients with healthy controls where memory deficits in patients are evident only at high difficulty levels. Disproportionately high BOLD response in the DLPFC during low difficulty level WM tasks may interfere with the capacity of patients to increase DLPFC activity compared to baseline when presented with high difficulty tasks (Callicott et al., 1998
, 2000
; Tan et al., 2006
). Longitudinal neurocognitive assessments are needed to explore further temporal decline in attention, verbal fluency, and psychomotor to detect a possible emergence of executive function and spatial WM deficits.
Brain Structural Correlates of Neurocognitive Deficits
The Pittsburgh High-Risk Study also involved a structural brain-imaging component. Relatives were categorized into low cognitive scoring and high cognitive scoring groups based on verbal fluency, attention, psychomotor speed, and executive-function scores using K-means cluster analysis. This method is an iterative procedure, which clusters cases into two groups. The iterations seek to minimize within cluster variance and maximize variability between clusters in an ANOVA-like fashion. Brain regions involved in these cognitions and implicated in SZ were compared across low and high scoring clusters of relatives.
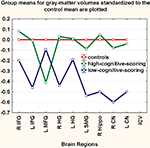
As seen in Figure 2
, the low scoring subset of relatives (n = 59) had lower volumes in critical brain regions compared to the high scoring subset (n = 35), with the exception of the middle frontal gyrus. Relatives of patients show cognitive deficits that co-occur with alterations of regions mediating these compromised cognitions. This association may tentatively suggest structural alterations to underpin cognitive deficits seen in relatives. Brain regional abnormalities with their “downstream” attendant cognitive deficits may together be considered as “extended endophenotypes”, a parsimonious conceptualization of SZ (Prasad and Keshavan, 2008
).
Figure 2. The low scoring subset of relatives had volumetric deficits in critical brain regions compared to the high scoring subset. Regional gray-matter-volumes (right and left combined) for each group were z-transformed to the control mean. Group-means of the z-scores are plotted on the y-axis in the low-scoring and the high-scoring groups (see text for description of approach to this classification). HG, Heschl’s gyrus; SMG, supramarginal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; Hippo, hippocampus; CN, caudate nucleus.
In summary, findings from the PREP study are consistent with previous reports of cognitive deficits in relatives of SZ patients and suggest that these deficits may be related to neuroanatomical deficits of corresponding brain regions. The existence of distinct subgroups of low and high cognitive scoring subjects within the sample of relatives is a critical finding from the PREP study. The clustering of structural alterations within the low-scoring subgroup tentatively suggests a neuroanatomically and cognitively compromised “hypervulnerable” subset within relatives with a familial diathesis for SZ. The risk of SZ and SZ spectrum disorders in genetically liable relatives of patients is 11–15% and about 40% (Diwadkar et al., 2006
) respectively. This further suggests a heterogeneous risk-profile of the genetically vulnerable population for future psychotic illness and the occurrence of “hypervulnerable” subgroups (Diwadkar et al., 2006
). The latent genetic heterogeneity in SZ explains the existence of these subgroups rather than a uniform vulnerability for SZ within genetically predisposed populations (Diwadkar et al., 2006
; Eack et al., 2008
).
In summary, cognitive deficits are a core feature of the premorbid vulnerability to SZ. Impairments are seen in several cognitive domains in unaffected relatives of patients including attention, WM, verbal memory, visual memory, executive function, speed of information processing, social cognition, and general intelligence. In general, the abnormalities appear more severe in first-degree relatives, and are associated with more prominent brain structural alterations. These observations are of clinical as well as pathophysiological significance.
An important question of clinical relevance is whether premorbid cognitive deficits can predict the emergence of later SZ in non-symptomatic at-risk subjects. As reviewed, the NYHRP and EHRS studies suggest that deficits in memory, attention, and social cognition in young relatives of SZ patients may predict later psychosis. Attention deficits in young relatives of SZ patients frequently have features of attention deficit disorder (Keshavan et al., 2003
, 2008
). This often leads to the clinical practice of treating such individuals with stimulant medications, which may have the undesirable effect of triggering psychosis in these vulnerable individuals. It is important to distinguish attentional impairments that are the precursors of a serious illness such as SZ and treat them with the disease appropriate interventions. Thus, children and adolescents newly presenting with attentional impairments should not, as often happens, be automatically diagnosed as having attention deficit disorders, but should be assessed to rule out early features of SZ (such as prodromal symptoms and schizotypy) or bipolar disorder (mood dysregulation). Inquiring for family histories of major psychiatric disorders is also important. Investigating premorbid neurocognitive deficits is also of importance for early intervention.
Further research is needed to evaluate the efficacy of cognitive remediation approaches, shown to benefit early phases of SZ (Eack et al., 2007
), in at-risk individuals with cognitive deficits. Pharmacological interventions, including low dose atypical antipsychotics, have also been piloted in cognitively impaired relatives at risk for SZ (Tsuang et al., 1999
).
Cognitive deficits, being core impairments in the premorbid phase of SZ, offer the best way to define the neurobiology of the vulnerability to this illness. As reviewed in this paper, cognitive deficits are robust, highly prevalent, stable, easily quantifiable, correlate with defined biological alterations in the illness, and are present in both those with the illness and those at risk. These features qualify cognitive impairments as endo- (or intermediate) phenotypes, which are beginning to pave the way to identification of the susceptibility gene(s) (Gur et al., 2007c
).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by NIMH grants MH 64023, MH 01180 and a NARSAD Established Investigator award (MSK). We thank Drs. Konasale Prasad and R. P. Rajarethinam who helped with research design issues; Diana Mermon who did the assessments; and Jean Miewald who helped with the data management and analyses.
Bertisch, H., Mesen-Fainardi, A., Martin, M. V., Pérez-Vargas, V., Vargas-Rodríguez, T., Delgado, G., Delgado, C., Llach, M., LaPrade, B., and Byerley, W. (2009). Neuropsychological performance as endophenotypes in extended schizophrenia families from the Central Valley of Costa Rica. Psychiatr. Genet. 19, 45–52.
Bhojraj, T. S., Francis, A. N., Rajarethinam, R., Eack, S., Kulkarni, S., Prasad, K. M., Montrose, D. M., Dworakowski, D., Diwadkar, V., and Keshavan, M. S. (2009). Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophr. Res. (in press).
Broome, M. R., Matthiasson, P., Fusar-Poli, P., Woolley, J. B., Johns, L. C., Tabraham, P., Bramon, E., Valmaggia, L., Williams, S. C., Brammer, M. J., Chitnis, X., and McGuire, P. K. (2009). Neural correlates of executive function and working memory in the ‘at-risk mental state’. Br. J. Psychiatry 194, 25–33.
Calkins, M. E., Dobie, D. J., Cadenhead, K. S., Olincy, A., Freedman, R., Green, M. F., Greenwood, T. A., Gur, R. E., Gur, R. C., Light, G. A., Mintz, J., Nuechterlein, K. H., Radant, A. D., Schork, N. J., Seidman, L. J., Siever, L. J., Silverman, J. M., Stone, W. S., Swerdlow, N. R., Tsuang, D. W., Tsuang, M. T., Turetsky, B. I., and Braff, D. L. (2007). The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr. Bull. 33, 33–48.
Callicott, J. H., Ramsey, N. F., Tallent, K., Bertolino, A., Knable, M. B., Coppola, R., Goldberg, T., van Gelderen, P., Mattay, V. S., Frank, J. A., Moonen, C. T., and Weinberger, D. R. (1998). Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 18, 186–196.
Cannon, T. D., Cadenhead, K., Cornblatt, B., Woods, S. W., Addington, J., Walker, E., Seidman, L. J., Perkins, D., Tsuang, M., McGlashan, T., and Heinssen, R. (2008). Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry 65, 28–37.
Cogtest, I. (2009). Cogtest: Computerised Cognitive Battery for Clinical Trials (2002). Accessed 24 March. http://www.cogtest.com
Cosway, R., Byrne, M., Clafferty, R., Hodges, A., Grant, E., Abukmeil, S. S., Lawrie, S. M., Miller, P., and Johnstone, E. C. (2000). Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol. Med. 30, 1111–1121.
Eack, S. M., Prasad, K. M. R., Montrose, D. M., Goradia, D. D., Dworakowski, D., Miewald, J., and Keshavan, M. S. (2008). An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1873–1878.
Fornito, A., Yung, A. R., Wood, S. J., Phillips, L. J., Nelson, B., Cotton, S., Velakoulis, D., McGorry, P. D., Pantelis, C., and Yucel, M. (2008). Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol. Psychiatry 64, 758–765.
Goldstein, J. M., Seidman, L. J., Buka, S. L., Horton, N. J., Donatelli, J. L., Rieder, R. O., and Tsuang, M. T. (2000). Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychoses. Schizophr. Bull. 26, 323.
Greenwood, T. A., Braff, D. L., Light, G. A., Cadenhead, K. S., Calkins, M. E., Dobie, D. J., Freedman, R., Green, M. F., Gur, R. E., Gur, R. C. Mintz, J., Nuechterlein, K. H., Olincy, A., Radant, A. D., Seidman, L. J., Siever, L. J., Silverman, J. M., Stone, W. S., Swerdlow, N. R., Tsuang, D. W., Tsuang, M. T., Turetsky, B. I., and Schork, N. J. (2007). Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch. Gen. Psychiatry. 64, 1242–1250.
Horan, W. P., Braff, D. L., Nuechterlein, K. H., Sugar, C. A., Cadenhead, K. S., Calkins, M. E., Dobie, D. J., Freedman, R., Greenwood, T. A., and Gur, R. E. (2008). Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophr. Res. 103, 218–228.
MacDonald, A. W. III, Carter, C. S., Kerns, J. G., Ursu, S., Barch, D. M., Holmes, A. J., Stenger, V. A., and Cohen, J. D. (2005). Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am. J. Psychiatry 162, 475–484.
Meisenzahl, E. M., Koutsouleris, N., Gaser, C., Bottlender, R., Schmitt, G. J., McGuire, P., Decker, P., Burgermeister, B., Born, C., Reiser, M., and Moller, H. J. (2008). Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr. Res. 102, 150–162.
Muñoz Maniega, S., Lymer, G. K. S., Bastin, M. E., Marjoram, D., Job, D. E., Moorhead, T. W. J., Owens, D. G., Johnstone, E. C., McIntosh, A. M., and Lawrie, S. M. (2008). A diffusion tensor MRI study of white matter integrity in subjects at high genetic risk of schizophrenia. Schizophr. Res. 106, 132–139.
O’Brien, M. P., Zinberg, J. L., Ho, L., Rudd, A., Kopelowicz, A., Daley, M., Bearden, C. E., and Cannon, T. D. (2009). Family problem solving interactions and 6-month symptomatic and functional outcomes in youth at ultra-high risk for psychosis and with recent onset psychotic symptoms: a longitudinal study. Schizophr. Res. 107, 198–205.
Pijnenborg, G. H., Withaar, F. K., Evans, J. J., van den Bosch, R. J., Timmerman, M. E., and Brouwer, W. H. (2009). The predictive value of measures of social cognition for community functioning in schizophrenia: implications for neuropsychological assessment. J. Int. Neuropsychol. Soc. 15, 239–247.
Seidman, L. J., Faraone, S. V., Goldstein, J. M., Goodman, J. M., Kremen, W. S., Matsuda, G., Hoge, E. A., Kennedy, D., Makris, N., Caviness, V. S., and Tsuang, M. T. (1997). Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: a pilot magnetic resonance imaging study. Am. J. Med. Genet. 74, 507–514.
Seidman, L. J., Faraone, S. V., Goldstein, J. M., Goodman, J. M., Kremen, W. S., Toomey, R., Tourville, J., Kennedy, D., Makris, N., Caviness, V. S., and Tsuang, M. T. (1999). Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol. Psychiatry 46, 941–954.
Seidman, L. J., Faraone, S. V., Goldstein, J. M., Kremen, W. S., Horton, N. J., Makris, N., Toomey, R., Kennedy, D., Caviness, V. S., and Tsuang, M. T. (2002b). Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch. Gen. Psychiatry 59, 839–849.
Seidman, L. J., Pantelis, C., Keshavan, M. S., Faraone, S. V., Goldstein, J. M., Horton, N. J., Makris, N., Falkai, P., Caviness, V. S., and Tsuang, M. T. (2003). A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophr. Bull. 29, 803–830.
Seidman, L. J., Giuliano, A. J., Smith, C. W., Stone, W. S., Glatt, S. J., Meyer, E., Faraone, S. V., Tsuang, M. T., and Cornblatt, B. (2006). Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr. Bull. 32, 507–524.
Thermenos, H. W., Seidman, L. J., Poldrack, R. A., Peace, N. K., Koch, J. K., Faraone, S. V., and Tsuang, M. T. (2007). Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol. Psychiatry 61, 564–574.
Wang, Q., Chan, R., Sun, J., Yao, J., Deng, W., Sun, X., Liu, X., Sham, P. C., Ma, X., and Meng, H. (2007). Reaction time of the Continuous Performance Test is an endophenotypic marker for schizophrenia: A study of first-episode neuroleptic-naive schizophrenia, their non-psychotic first-degree relatives and healthy population controls. Schizophr. Res. 89, 293–298.
Weiser, M., Reichenberg, A., Rabinowitz, J., Nahon, D., Kravitz, E., Lubin, G., Knobler, H. Y., Davidson, M., and Noy, S. (2007). Impaired reading comprehension and mathematical abilities in male adolescents with average or above general intellectual abilities are associated with comorbid and future psychopathology. J. Nerv. Ment. Dis. 195, 883–890.