
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 03 April 2025
Sec. Brain Imaging and Stimulation
Volume 19 - 2025 | https://doi.org/10.3389/fnhum.2025.1578853
This article is part of the Research TopicExploring the complexities of the human nervous system through advanced brain imaging and stimulationView all articles
Background: Deep tiny flow voids (DTFVs) have recently been identified as a novel form of collateral circulation linked to chronic steno-occlusive atherosclerotic middle cerebral artery (MCA) lesions, detectable via high-resolution magnetic resonance imaging (HR-MRI). To date, no study has focused on the presence and clinical significance of DTFVs in acute MCA atherosclerotic occlusion.
Materials and methods: This retrospective study included patients with acute MCA atherosclerotic occlusion from two multicenter HR-MRI cohorts. The incidence of DTFVs and its association with baseline National Institute of Health Stroke Scale (NIHSS) scores, infarct volume, and the proportion of patients with a favorable 90-day clinical outcome defined as a modified Rankin Scale (mRS) ≤ 2 were analyzed.
Results: Sixty-six patients (mean age 58.2 ± 9.2 years; 71.2% men) were included. The median time from stroke onset to image was 44.5 (27.3–67.0) hours. DTFVs were identified in 57.6% of patients with MCA atherosclerotic occlusion. After adjusting the potential confounders, DTFVs were significantly associated with lower baseline NIHSS scores (β, −3.68; 95% CI, −6.30, –1.07; p = 0.007), smaller infarct volume (β, −40.88; 95% CI, −70.15, −11.60; p = 0.007), and a higher proportion of patients with favorable 90-day clinical outcome (OR, 6.03; 95% CI, 1.39–26.19; p = 0.017).
Conclusion: The presence of DTFVs was correlated with a favorable outcome in patients with acute MCA atherosclerotic occlusion. Improved recognition and awareness of this imaging marker of collaterals could help understand the varying infarct evolution seen in MCA occlusion and contribute to more individualized management and treatment.
Acute ischemic stroke due to middle cerebral artery (MCA) occlusion is common in stroke practice. Without reperfusion therapy, up to 87% of patients with proximal MCA occlusion and 47% with distal MCA occlusion have poor outcomes (Hernández-Pérez et al., 2014). Although intravenous thrombolysis within 9 h or endovascular treatment within 24 h from stroke onset can improve the prognosis (Liyis et al., 2023; Nogueira et al., 2018; Albers et al., 2018; Ma et al., 2019; Berge et al., 2021; Liu et al., 2023), a large number of patients do not present within these critical time windows (Tong et al., 2012; Yuan et al., 2023). Understanding the determinants of prognosis in such cases is essential for developing individualized treatment strategies and informing future clinical trials.
Collateral circulation is widely recognized as a key pathophysiological factor influencing the natural progression of ischemic brain injury (Lin et al., 2021; Lima et al., 2010). These collaterals primarily involve tertiary pathways, including the Circle of Willis, leptomeningeal vessels, and neovascularization (Leng and Leung, 2023). In the context of MCA occlusion, leptomeningeal collaterals have been strongly associated with favorable clinical outcomes (Lima et al., 2010; Madelung et al., 2018; Singer et al., 2015), whereas the role of the Circle of Willis remains controversial due to significant anatomical variability among individuals (Westphal et al., 2021; Sadeh-Gonik et al., 2024). Research on neovascularization, however, has been limited by the resolution constraints of conventional imaging techniques (Leng and Leung, 2023).
Recently, deep tiny flow voids (DTFVs) have been identified as a novel form of collateral circulation linked to steno-occlusive atherosclerotic MCA lesions, detectable via high-resolution magnetic resonance imaging (HR-MRI) (Xu et al., 2016; Xu et al., 2014). The prevalence of DTFVs increases with the severity of MCA stenosis and is higher in asymptomatic compared to symptomatic MCA occlusion cases (Xu et al., 2016). They were hypothesized to originate from new vessel network formation in response to chronic cerebral ischemia (Xu et al., 2016; Cho et al., 2013). Despite these insights, the incidence of DTFVs in acute MCA atherosclerotic occlusion and their impact on prognosis remain unexplored.
This study aims to investigate the presence and clinical significance of DTFVs in patients with acute ischemic stroke caused by MCA atherosclerotic occlusion. By doing so, we hope to shed light on their potential role in influencing outcomes and guiding therapeutic interventions.
We conducted this study based on two multicenter cohort studies with similar designs: the Stroke Imaging Package Study (SIPS) (Zhang et al., 2023) and the Stroke Imaging Package Study of Intracranial Atherosclerosis (SIPS-ICAS) (Lin et al., 2020). The purpose of these two cohort studies was to evaluate the clinical value of HR-MRI in acute ischemic stroke, and the details of the study design were published elsewhere (Zhang et al., 2023; Lin et al., 2020). These two studies included patients who experienced their first-ever acute ischemic stroke within 7 days of symptom onset (Zhang et al., 2023; Lin et al., 2020). Age, sex, risk factors, onset to HR-MRI time, baseline NIHSS, reperfusion therapy, other treatment, mRS scores at 90 days and other information were recorded in detail. Patients with acute ischemic stroke due to MCA (including M1 or M2 segments) atherosclerotic occlusion, complete clinical data, and good image quality were selected for this study. Both SIPS and SIPS-ICAS were approved by the institutional review board at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (JS872 and JS-1699), and the local participating centers’ ethics board. The patients enrolled in both studies provided written informed consent.
Patients were excluded if they had any of the following characteristics: (1) coexistent with ipsilateral extracranial or intracranial internal carotid artery stenosis (≥ 50%), as assessed by MRA, Doppler ultrasonography, or CTA; (2) evidence of cardioembolism, including atrial fibrillation or flutter, left atrial thrombus, prosthetic valve, severe mitral stenosis, concomitant acute myocardial infarction, congestive heart failure, infective endocarditis, and/or sick-sinus syndrome as assessed by electrocardiogram or echocardiography; (3) evidence of vasculitis or arterial dissection, diagnosed by clinical evaluation, laboratory work, and vascular imaging; (4) Patients with undetermined etiology; (5) poor image quality.
Both SIPS and SIPS-ICAS used the same imaging protocol, including conventional MRI (T1-weighted imaging, T2-weighted imaging, T2-weighted fluid attenuation inversion recovery imaging, diffusion-weighted imaging, three-dimensional time-of-flight magnetic resonance angiography, and susceptibility-weighted imaging or T2*-weighted imaging) and 3D T1-weighted HR-MRI (Zhang et al., 2023; Lin et al., 2020). Arterial spin labeling MRI, two-dimensional T2-weighted HR-MRI of intracranial arteries (bilateral middle cerebral arteries and basilar artery), and contrast-enhanced 3D T1-weighted HR-MRI were optional (Zhang et al., 2023; Lin et al., 2020).
As described previously, DTFVs were defined as three or more flow voids along the affected MCA on at least two consecutive T2-weighted image slices on HR-MRI (Figure 1) (Xu et al., 2016; Xu et al., 2014). Infarct volume was calculated using a self-developed algorithm based on the MATLAB platform. The total infarct volume for each patient was determined by multiplying the sum of the lesion areas in each slice by the slice thickness. For each slice containing infarct lesions, the radiologist selected a seed point within each lesion. The lesion was then segmented using a region-growing algorithm (Zhang et al., 2023; Adams and Bischof, 1994; Yuan et al., 2024).
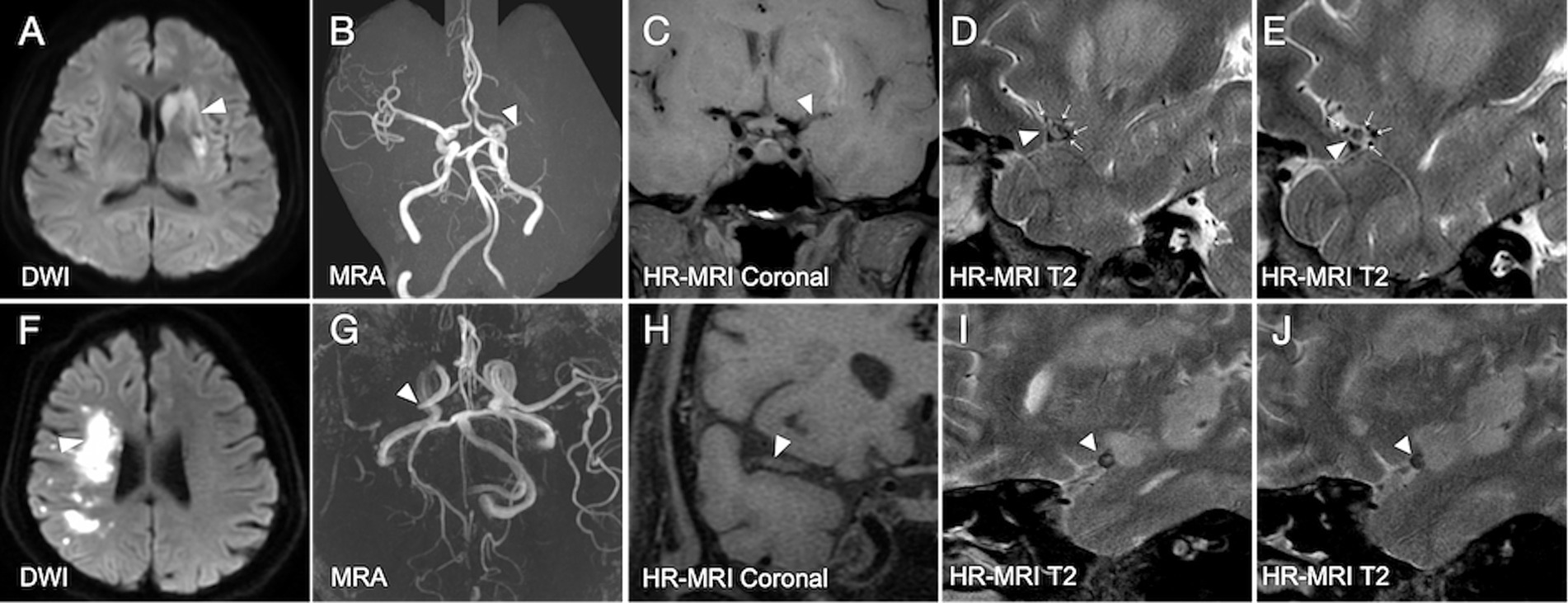
Figure 1. Illustrated cases of acute ischemic stroke due to middle cerebral artery occlusion with and without deep tiny flow voids. (A–E) In a patient with acute ischemic stroke (white arrowhead, A) due to the occlusive MCA (white arrowheads, B–E), DTFVs are revealed (white arrows, D,E). The 90-day mRS score for this patient is 0. (F–J) In another patient with acute ischemic stroke (white arrowhead, F) due to the occlusive MCA (white arrowheads, G–J), no DTFVs are revealed (I,J). The 90-day mRS score for this patient is 3. DTFVs, deep tiny flow voids; MCA, middle cerebral artery; mRS, modified Rankin Scale.
Image analysis was performed using commercial software (Osirix MD, v.9.02). One experienced reader (Dr. Ding), blinded to the clinical information, interpreted the cross-sectional image slices of the bilateral MCA on HR-MRI. Dr. Ding and another experienced reader (Dr. Yuan) independently measured the DTFVs around occlusive MCA of the initial 20 consecutive patients two months later to assess interobserver and intraobserver variability. The intra-observer (κ = 0.89, p < 0.001) and inter-observer (κ = 0.80, p < 0.001) reproducibility of DTFVs measurements demonstrated substantial agreement.
The disability at baseline and day 90 was evaluated by the National Institute of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS), respectively. A favorable 90-day clinical outcome was defined as a mRS score ≤ 2. The study outcomes included the incidence of DTFVs and the association of DTFVs with the baseline NIHSS, infarct volume, and the proportion of patients with a favorable 90-day clinical outcome.
Continuous variables were described as mean (standard deviation [SD]) or median (interquartile range [IQR]), and categorical variables were presented as the frequency (percentage). We used Cohen’s ĸ coefficient to assess both interobserver and intraobserver agreement for DTFVs. Comparison between groups was performed using parametric (t-test or χ2 squared) or nonparametric (Wilcoxon or Fisher’s exact) tests. To adjust the potential confounders, including age, sex, hypertension, diabetes, hyperlipidemia, smoking, infarct volume, baseline NIHSS, and reperfusion therapy, we used linear or logistic regression analysis to investigate the association of DTFVs with baseline NIHSS, infarct volume, and the proportion of patients with a favorable 90-day clinical outcome. First, a univariate model was applied, including potential confounders, and the results were expressed in the regression coefficient (β) or odds ratio (OR) and 95% confidence interval (CI). All variables with p < 0.1 in the univariate analysis were included in the multivariate analysis. Variables with p < 0.05 were maintained in the analysis. A two-tailed p-value of less than 0.05 was considered significant for all statistical tests. All data analysis was conducted using SPSS Version 26.0 (IBM, Armonk, NY, United States).
Among 1,011 patients with acute ischemic stroke in two cohort studies, MCA (including M1 and M2) occlusion was observed in 127 patients based on the result of MRA. After excluding 20 patients with embolic-related occlusion, 4 patients with undetermined etiology, 33 patients without T2-weighted HR-MRI, 3 patients with poor image quality, and 1 with incomplete clinical data, 66 patients were enrolled for analysis, including 46 from SIPS and 20 from SIPS-ICAS (Figure 2). The mean age was 58.2 years (standard deviation [SD], 9.2 years), and 47 patients (71.2%) were male. The median time from symptom onset to imaging was 44.5 h (IQR, 27.3–67.0 h). The characteristics of this cohort are presented in Table 1.
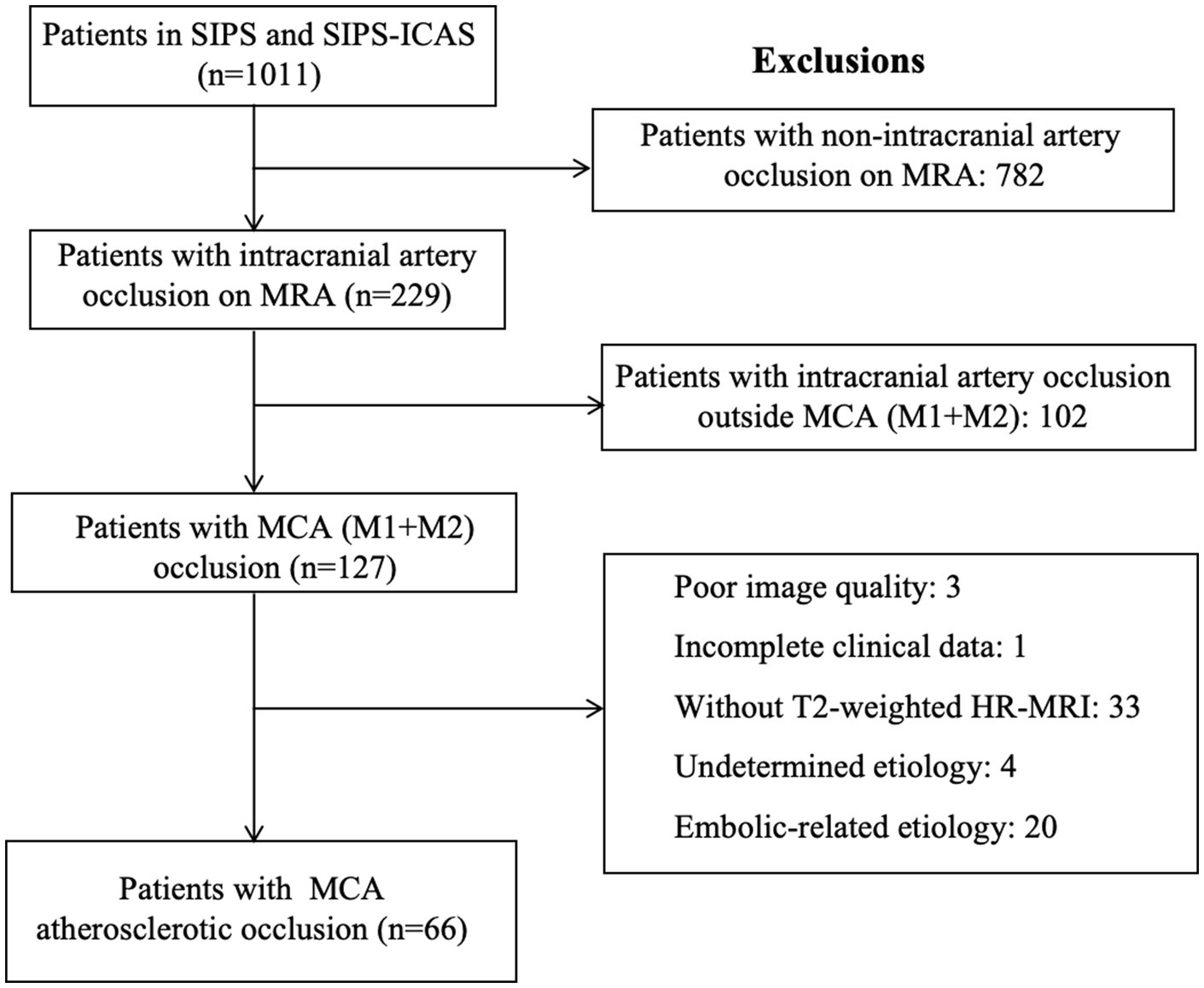
Figure 2. Flowchart of included/excluded patients. This flowchart presents the process of screening patients for enrollment from SIPS and SIPS-ICAS. MCA, middle cerebral artery; MRA, magnetic resonance angiography; SIPS, Stroke Imaging Package Study; SIPS-ICAS, Stroke Imaging Package Study of Intracranial Atherosclerosis.
Among these 66 patients, 38 (57.6%) had DTFVs (Table 1). Table 1 shows the characteristics of patients with and without DTFVs. There are no significant differences between demographic characteristics, risk factors, onset to HR-MRI time, and all treatments between the two groups. Compared with patients without DTFVs, the patients with DTFVs had much lower NIHSS scores (4.0 [1.0–8.0] vs. 11.5 [7.3–14.8], p < 0.001), smaller infarct volumes (4.9 [2.6–10.4] cm3 vs. 20.9 [8.2–75.2] cm3, p < 0.001), and higher percentage patients having favorable clinical outcomes at day 90 (94.3% vs. 35.7%, p < 0.001) (Table 1; Figure 3).
We screened for factors associated with smaller infarct volume, lower baseline NIHSS score, and favorable outcome on day 90 by univariate regression analysis, respectively, in patients with acute MCA atherosclerotic occlusion. Multivariate regression analyses were performed for variables with p < 0.1 in univariate regression analyses. After adjusting for age and smoking, DTFVs (β, −40.88; 95% CI, −70.15, −11.60; p = 0.007) were significantly associated with smaller infarct volume. After adjusting for hyperlipidemia and infarct volume, DTFVs (β, −3.68; 95% CI, −6.30, −1.07; p = 0.007) were significantly associated with lower baseline NIHSS score. After adjusting for smoking, infarct volume, and NIHSS score, DTFVs (OR, 6.03; 95% CI, 1.39, 26.19; p = 0.017) were significantly associated with a favorable outcome on day 90 (Table 2). Overall, DTFVs were significantly associated with smaller infarct volume, lower baseline NIHSS score, and a favorable outcome on day 90.
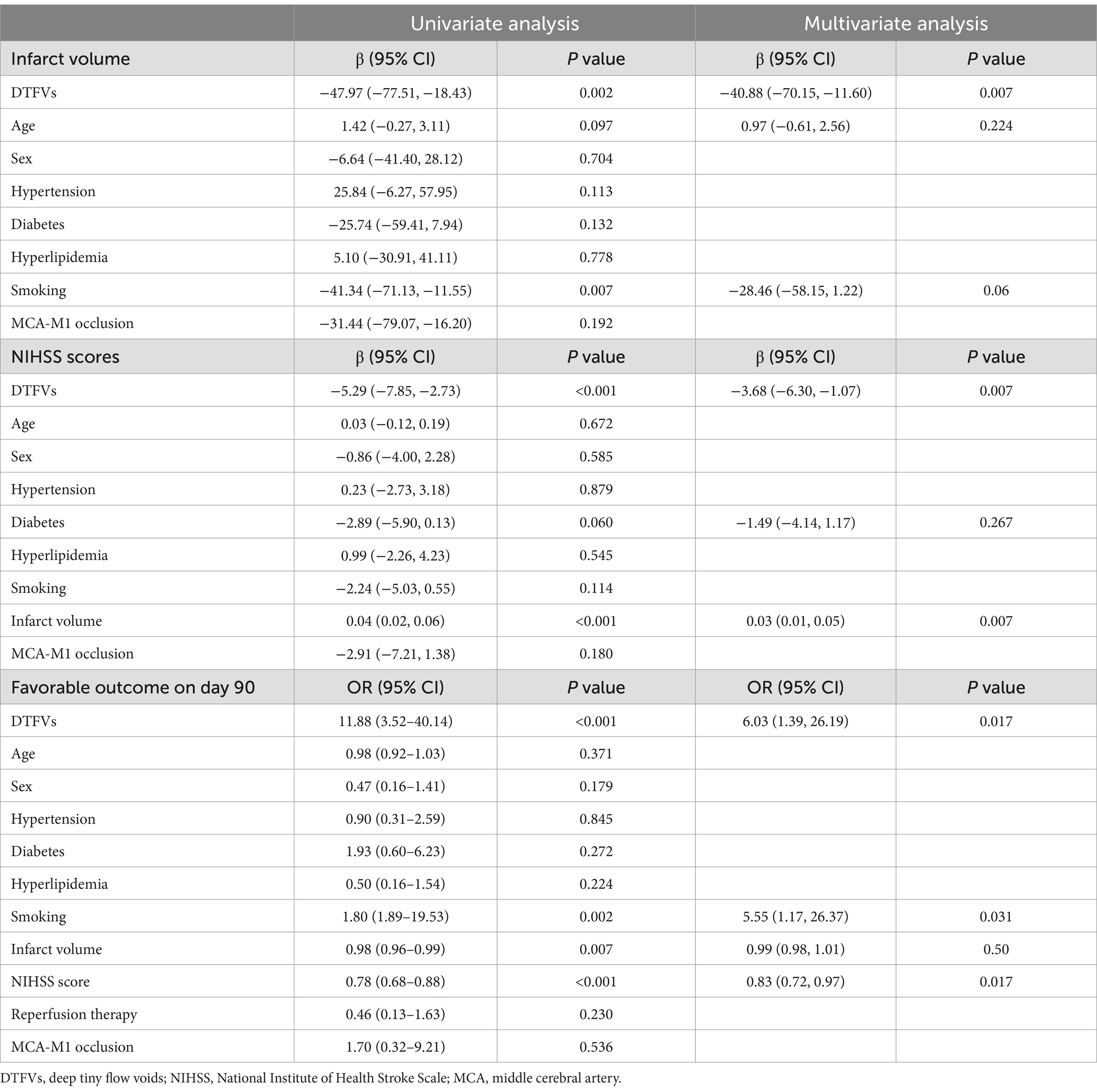
Table 2. Univariate and multivariate analyses of the association between DTFVs and clinical outcomes.
Our study investigated the presence and clinical significance of DTFVs in acute MCA atherosclerotic occlusion. We found that nearly half of the patients exhibited DTFVs. Compared to patients without DTFVs, those with DTFVs were more likely to present with lower NIHSS scores, smaller infarct volumes, and better 90-day prognoses. These findings highlight the potential role of DTFVs as a marker of favorable outcomes in acute MCA atherosclerotic occlusion.
We observed that patients with DTFVs exhibited lower baseline NIHSS scores and smaller infarct volumes. These findings align with our previous study, which reported a higher prevalence of DTFVs in patients with asymptomatic MCA occlusion compared to symptomatic cases (68% vs. 41.7%) (Adams and Bischof, 1994). This consistency suggests that DTFVs may serve as a protective factor in ischemic stroke. Similar to other collateral pathways, such as leptomeningeal vessels, DTFVs appear to mitigate the risk of stroke in large artery occlusive diseases and help reduce ischemic injury when a stroke occurs (Leng and Leung, 2023). Notably, our study demonstrated that DTFVs were significantly associated with favorable clinical outcomes, underscoring their potential value in guiding clinical decision-making. For instance, the presence of DTFVs in patients with acute MCA occlusion may indicate that aggressive or invasive therapies are less necessary. Conversely, patients without DTFVs may represent a subgroup that could benefit from more intensive interventions and should be prioritized in future clinical trials.
The underlying pathophysiology for DTFVs in acute MCA atherosclerotic occlusion remains incompletely understood. In our previous study, we found that the incidence of DTFVs increased with the degree of MCA stenosis, particularly in cases with 70% or greater stenosis or complete occlusion. This is consistent with the understanding that neovascularization—through mechanisms such as vasculogenesis, angiogenesis, and/or arteriogenesis—develops in response to chronic tissue hypoxia and injury (Ergul et al., 2014). This is also consistent with the phenomenon observed in coronary artery disease, that is, coronary lesion severity is the only independent pathogenetic variable related to collateral flow, which promotes enough collateral flow to prevent myocardial ischemia during coronary occlusion (Pohl et al., 2001). We hypothesize that patients without DTFVs may have had less severe MCA stenosis before acute occlusion compared to those with DTFVs. This hypothesis is supported by a similar phenomenon observed in coronary artery disease. In cases of mild to moderate coronary atherosclerosis, acute occlusion often leads to severe and potentially fatal myocardial infarction with large infarct areas due to the lack of well-developed collateral circulation (Hackett et al., 1988; Stone et al., 2023). In contrast, patients with severe coronary atherosclerosis may be asymptomatic or present with stable angina rather than acute infarction, as the chronic hypoxic stimulus promotes the formation of extensive collateral vessels, which can mitigate the effects of sudden occlusion (Pohl et al., 2001; Hackett et al., 1988; Stone et al., 2023). This parallel suggests that the absence of DTFVs in acute MCA occlusion may reflect insufficient pre-existing stenosis to trigger robust neovascularization, leaving the brain more vulnerable to ischemic injury when acute occlusion occurs. Conversely, patients with DTFVs may have had more severe and prolonged stenosis, leading to the development of protective collateral vessels that limit infarct size and improve outcomes. These insights highlight the potential role of DTFVs as a marker of pre-existing collateral development and their importance in understanding the variability of outcomes in acute MCA occlusion.
Our study has several limitations that should be acknowledged. First, as a retrospective study, the results may be influenced by selection bias, particularly given the characteristics of the included population. The patients were relatively young (mean age 58.2 years) and had small infarct volumes (median 7.9 cm3). The pre-designed extended MRI acquisition time may have further skewed the cohort toward those with mild to moderate symptoms, as patients with severe strokes may not have been stable enough to undergo prolonged imaging. To address this, future studies should aim to include a broader range of patients, encompassing a wider spectrum of ages, infarct sizes, and stroke severities. Second, HR-MRI is not routinely used in clinical practice, which may limit the immediate applicability of our findings. However, this limitation does not diminish the significance of our conclusions regarding the pathophysiology and clinical relevance of DTFVs. With advancements in accelerated MRI techniques and the integration of artificial intelligence in medical imaging (Pohl et al., 2001; Hackett et al., 1988), HR-MRI is poised to become more accessible and widely adopted in clinical settings. These technological developments hold promise for expanding the use of HR-MRI in routine stroke evaluation, enabling more precise identification of DTFVs and other collateral pathways. Third, the small sample size of our study may limit statistical power and increase bias risk. Nevertheless, our findings provide preliminary evidence suggesting that the presence of DTFVs is associated with favorable clinical outcomes in patients with acute ischemic stroke due to middle cerebral artery occlusion. Future multi-center studies with larger sample sizes are warranted to validate these observations and explore potential effect modifiers.
In conclusion, our study demonstrates that the presence of DTFVs is associated with favorable outcomes in patients with acute ischemic stroke caused by MCA atherosclerotic occlusion. These findings highlight the potential role of DTFVs as an imaging marker of collateral circulation, which may help explain the variability in infarct evolution observed in MCA occlusion. Improved recognition and understanding of DTFVs could enhance our ability to predict patient outcomes and guide more individualized management and treatment strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the institutional review board at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (JS872 and JS-1699), and the local participating centers’ ethics board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
M-QD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. W-ZY: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing. Z-JW: Writing – review & editing. Y-LZ: Methodology, Writing – review & editing. M-LL: Software, Supervision, Writing – review & editing. YX: Conceptualization, Methodology, Supervision, Writing – review & editing. W-HX: Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-C-001; 2022-PUMCH-D-007), the National Science Fund for Distinguished Young Scholars (82025013), and the CAMS Innovation Fund for Medical Sciences (2022-I2M-1-002), and the Beijing Natural Science Foundation (7222131).
The authors appreciate the data collection and judgment provided by Yu-Yuan Xu, MD, Xue Man, MD, Yu-Wen Chen, MD, Yao Meng, MD, Yi-Tong Liu, MD, Zong-Mu-Yu Zhang, MD. Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College; Han-Jing Kong, Ph.D., Academy for Advanced Interdisciplinary Studies & College of Engineering, Peking University; Bo Wu, MD, Department of Neurology, West China Hospital, Sichuan University, Cheng-Lin Tian, MD, Department of Neurology, Chinese PLA General Hospital, and the statistical consultation provided by Yue-Lun Zhang, Ph.D., Institute of Clinical Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1578853/full#supplementary-material
Adams, R., and Bischof, L. (1994). Seeded region growing. IEEE Trans. Pattern Anal. Mach. Intell. 16, 641–647. doi: 10.1109/34.295913
Albers, G. W., Marks, M. P., Kemp, S., Christensen, S., Tsai, J. P., Ortega-Gutierrez, S., et al. (2018). Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 378, 708–718. doi: 10.1056/NEJMoa1713973
Berge, E., Whiteley, W., Audebert, H., de Marchis, G. M., Fonseca, A. C., Padiglioni, C., et al. (2021). European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 6, II–LXII. doi: 10.1177/2396987321989865
Cho, Z. H., Lee, Y. B., Kang, C. K., Yang, J. W., Jung, I. H., Park, C. A., et al. (2013). Microvascular imaging of asymptomatic MCA steno-occlusive patients using ultra-high-field 7T MRI. J. Neurol. 260, 144–150. doi: 10.1007/s00415-012-6604-5
Ergul, A., Abdelsaid, M., Fouda, A. Y., and Fagan, S. C. (2014). Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J. Cereb. Blood Flow Metab. 34, 553–563. doi: 10.1038/jcbfm.2014.18
Hackett, D., Davies, G., and Maseri, A. (1988). Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur. Heart J. 9, 1317–1323. doi: 10.1093/oxfordjournals.eurheartj.a062449
Hernández-Pérez, M., Pérez de la Ossa, N., Aleu, A., Millán, M., Gomis, M., Dorado, L., et al. (2014). Natural history of acute stroke due to occlusion of the middle cerebral artery and intracranial internal carotid artery. J. Neuroimaging 24, 354–358. doi: 10.1111/jon.12062
Leng, X., and Leung, T. W. (2023). Collateral flow in intracranial atherosclerotic disease. Transl. Stroke Res. 14, 38–52. doi: 10.1007/s12975-022-01042-3
Lima, F. O., Furie, K. L., Silva, G. S., Lev, M. H., Camargo, É. C. S., Singhal, A. B., et al. (2010). The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 41, 2316–2322. doi: 10.1161/STROKEAHA.110.592303
Lin, Q., Liu, X., Chen, B., Tian, D., Liu, C., Du, A., et al. (2020). Design of stroke imaging package study of intracranial atherosclerosis: a multicenter, prospective, cohort study. Ann. Transl. Med. 8:13. doi: 10.21037/atm.2019.11.111
Lin, L., Yang, J., Chen, C., Tian, H., Bivard, A., Spratt, N. J., et al. (2021). Association of Collateral Status and Ischemic Core Growth in patients with acute ischemic stroke. Neurology 96, e161–e170. doi: 10.1212/WNL.0000000000011258
Liu, L., Li, Z., Zhou, H., Duan, W., Huo, X., Xu, W., et al. (2023). Chinese Stroke Association guidelines for clinical management of ischaemic cerebrovascular diseases: executive summary and 2023 update. Stroke Vasc Neurol 8:e3. doi: 10.1136/svn-2023-002998
Liyis, B. G., Surya, S. C., Tedyanto, E. H., Pramana, N. A. K., and Widyadharma, I. P. E. (2023). Mechanical thrombectomy in M1 and M2 segments of middle cerebral arteries: a meta-analysis of prospective cohort studies. Clin. Neurol. Neurosurg. 231:107823. doi: 10.1016/j.clineuro.2023.107823
Ma, H., Campbell, B. C. V., Parsons, M. W., Churilov, L., Levi, C. R., Hsu, C., et al. (2019). Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N. Engl. J. Med. 380, 1795–1803. doi: 10.1056/NEJMoa1813046
Madelung, C. F., Ovesen, C., Trampedach, C., Christensen, A., Havsteen, I., Hansen, C. K., et al. (2018). Leptomeningeal collateral status predicts outcome after middle cerebral artery occlusion. Acta Neurol. Scand. 137, 125–132. doi: 10.1111/ane.12834
Nogueira, R. G., Jadhav, A. P., Haussen, D. C., Bonafe, A., Budzik, R. F., Bhuva, P., et al. (2018). Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 378, 11–21. doi: 10.1056/NEJMoa1706442
Pohl, T., Seiler, C., Billinger, M., Herren, E., Wustmann, K., Mehta, H., et al. (2001). Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J. Am. Coll. Cardiol. 38, 1872–1878. doi: 10.1016/S0735-1097(01)01675-8
Sadeh-Gonik, U., Budylev, A., Krivitzky, D., Molad, J., Halevi, H., Jonas-Kimchi, T., et al. (2024). Circle of Willis integrity in acute middle cerebral artery occlusion: does the posterior communicating artery matter? J Neurointerv Surg 16, 801–808. doi: 10.1136/jnis-2023-020326
Singer, O. C., Berkefeld, J., Nolte, C. H., Bohner, G., Reich, A., Wiesmann, M., et al. (2015). Collateral vessels in proximal middle cerebral artery occlusion: the ENDOSTROKE study. Radiology 274, 851–858. doi: 10.1148/radiol.14140951
Stone, P. H., Libby, P., and Boden, W. E. (2023). Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management-the plaque hypothesis: a narrative review. JAMA Cardiol. 8, 192–201. doi: 10.1001/jamacardio.2022.3926
Tong, D., Reeves, M. J., Hernandez, A. F., Zhao, X., Olson, D. W. M., Fonarow, G. C., et al. (2012). Times from symptom onset to hospital arrival in the get with the guidelines--stroke program 2002 to 2009: temporal trends and implications. Stroke 43, 1912–1917. doi: 10.1161/STROKEAHA.111.644963
Westphal, L. P., Lohaus, N., Winklhofer, S., Manzolini, C., Held, U., Steigmiller, K., et al. (2021). Circle of Willis variants and their association with outcome in patients with middle cerebral artery-M1-occlusion stroke. Eur. J. Neurol. 28, 3682–3691. doi: 10.1111/ene.15013
Xu, Y. Y., Li, M. L., Gao, S., Hou, B., Sun, Z. Y., Zhou, H. L., et al. (2016). Non-moyamoya vessel network formation along steno-occlusive middle cerebral artery. Neurology 86, 1957–1963. doi: 10.1212/WNL.0000000000002698
Xu, W. H., Li, M. L., Niu, J. W., Feng, F., Jin, Z. Y., and Gao, S. (2014). Deep tiny flow voids along middle cerebral artery atherosclerotic occlusions: a high-resolution MR imaging study. J. Neurol. Sci. 339, 130–133. doi: 10.1016/j.jns.2014.01.042
Yuan, W., Chen, H. S., Yang, Y., Zhang, M., Fang, L., Wu, S. W., et al. (2024). Quantitative assessment of acute intracranial clot and collaterals on high-resolution magnetic resonance imaging. Cerebrovasc. Dis., 1–9. doi: 10.1159/000540217
Yuan, J., Lu, Z. K., Xiong, X., Li, M., Liu, Y., Wang, L. D., et al. (2023). Age and geographic disparities in acute ischaemic stroke prehospital delays in China: a cross-sectional study using national stroke registry data. Lancet Reg. Health West Pac. 33:100693. doi: 10.1016/j.lanwpc.2023.100693
Keywords: deep tiny flow voids, middle cerebral artery, atherosclerosis, occlusion, prognosis
Citation: Ding M-Q, Yuan W-Z, Wang Z-J, Zhang Y-L, Li M-L, Xu Y and Xu W-H (2025) Association of deep tiny flow voids with prognosis of acute middle cerebral artery atherosclerotic occlusion. Front. Hum. Neurosci. 19:1578853. doi: 10.3389/fnhum.2025.1578853
Received: 18 February 2025; Accepted: 13 March 2025;
Published: 03 April 2025.
Edited by:
Song Qiao, Zhejiang Hospital, ChinaReviewed by:
Shaokuan Fang, The First Hospital of Jilin University, ChinaCopyright © 2025 Ding, Yuan, Wang, Zhang, Li, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Xu, eHV5YW5wdW1jaEBob3RtYWlsLmNvbQ==; Wei-Hai Xu, eHV3aEBwdW1jaC5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.