- 1Graduate School of Jiangxi University of Traditional Chinese Medicine, Nanchang, China
- 2Department of Rehabilitation Medicine, Hongdu Hospital of Traditional Chinese Medicine, Nanchang, China
Stroke is a significant cardiovascular and cerebrovascular condition and is among the primary causes of prolonged neurological impairment globally. Approximately 55%–75% of stroke survivors will experience some form of long-term sensorimotor impairment. Post-stroke, the upper limb typically exhibits restricted mobility, complicating daily chores for 70% of patients and impairing normal limb utilization. Repetitive Transcranial Magnetic Stimulation (rTMS), a prominent non-invasive neuromodulation technique designed to enhance functional recovery post-stroke, has garnered significant attention in clinical studies. Likewise, constraint-induced movement therapy (CIMT) has been extensively employed in therapeutic settings to promote neuroplasticity. However, there remain several issues with it in practical application. Recently, considerable focus has been directed toward a novel treatment known as rTMS in conjunction with obligatory motor therapy. This can circumvent the issues associated with conventional treatments and optimize the advantages of both. This article discusses the present status of clinical research with rTMS and CIMT.
1 Introduction
Stroke, a significant cerebrovascular disorder, is typically induced by the rupture or obstruction of blood arteries, leading to impairment of cerebral function and disruption of regulatory mechanisms. The prevalence of this disease in China is comparatively elevated on a global scale, making it one of the primary contributors to mortality and disability among adults (Wang, 2021). Common functional deficits following a stroke include motor impairments, sensory anomalies, speech difficulties, discomfort, and restricted capacity to perform activities of daily living (ADLs). Patients may experience mobility challenges, which might complicate their everyday activities and significantly diminish their quality of life (Ruan and Ye, 2024). Researchers have mostly examined the efficacy of either CIMT or rTMS in facilitating the restoration of normal bodily function in stroke patients (Abdullahi et al., 2023), few research examine the synergistic effects of constraint-induced mobility therapy (Duan et al., 2024). Prior studies have mostly examined the efficacy of constraint-induced movement therapy and rTMS in aiding individuals with motor impairment following a stroke. Nonetheless, no extensive research has investigated the synergistic effects of these two therapies in a clinical setting. This article examines studies on the application of repeated transcranial magnetic stimulation in conjunction with constraint-induced movement therapy for the treatment of dyskinesia following a stroke. The objective is to provide readers with an innovative perspective on rehabilitation methods that can assist stroke patients experiencing limb difficulties.
2 Repeated transcranial magnetic stimulation
Various forms of magnetic stimulation, namely single-pulse and paired-pulse, have been employed to investigate the reorganization of the brain’s cortex following a stroke. Researchers have discovered that varying stimulation parameters in rTMS can either decrease or increase cortical excitability (Han et al., 2025), which has prompted the development of rTMS therapy. Contemporary mainstream research indicates that the mechanism of action of rTMS in post-stroke dyskinesia may be as follows: Cheng et al. (2023) employed fMRI to investigate the effects of rTMS on brain networks and discovered that it could enhance interhemispheric connectivity by increasing activity in ipsilateral injury regions, augmenting excitatory connectivity from ipsilateral injury to contralateral brain areas, and reducing stroke-induced inhibition across the corpus callosum, thereby facilitating motor performance recovery. Zong et al. (2020) observed that with prolonged rTMS treatment, levels of anti-inflammatory cytokines and mitochondrial MnSOD were elevated in the vicinity of the infarct. This preserved the integrity of the mitochondrial membrane and inhibited the cystatin-9/3 apoptotic pathway occurring spontaneously in the infarcted cortex, hence enhancing the local neuronal microenvironment. The transition of microglia to the M1/M2 phenotype and the shift from A1 to A2 in astrocyte phenotype were associated with accelerated nerve healing facilitated by rTMS. The quantity of neurotrophic factor: Brain-derived neurotrophic factor (BDNF) facilitates neuronal survival, growth, migration, and differentiation, while also enhancing dendritic and axonal growth and synaptic formation following central nervous system injury. Research indicates that the external magnetic field generated by rTMS influences BDNF concentrations in serum and cerebrospinal fluid (Ozkan et al., 2024). Luo et al. (2017) discovered that high-frequency rTMS stimulation elevated blood BDNF levels and its affinity for the TrkB receptor (neuronal receptor tyrosine kinase-2), resulting in increased BDNF and phosphorylated TrkB protein levels. In conclusion, the precise pathways through which rTMS produces its therapeutic effects remain inadequately elucidated, necessitating future research for validation.
Repetitive transcranial magnetic stimulation, a non-invasive and painless neuromodulation technique, is regarded as a treatment that does not harm the internal cranial structures (Klomjai et al., 2015) and is frequently utilized for limb dysfunction, speech loss, visual impairment, swallowing difficulties, motor neurodeficiencies in limbs, and psychiatric disorders following a stroke. Research indicates that over 70% of stroke patients experience varied degrees of motor impairment following the onset of the stroke (Cieza et al., 2020). The effectiveness of rTMS is contingent upon the stimulation frequency, with low-frequency stimulation (≤ 1 Hz) exerting an inhibitory effect and high-frequency stimulation (> 5 Hz) inducing brain excitement (Simonetta-Moreau, 2014). Presently, studies indicate the comparable efficacy of rTMS at 3 Hz and 10 Hz in addressing upper-limb motor dysfunction following a stroke (Xiao et al., 2019). A study conducted by Vabalaite et al. (2021) demonstrated that rTMS at 10 Hz was more effective than low-frequency stimulation on the FMA-UL rating scale. Presently, the majority of individuals believe that low-frequency stimulation is equally effective as high-frequency stimulation for upper extremity rehabilitation (Kim et al., 2014). Two meta-analyses indicate that rTMS enhanced lower limb dyskinesia post-stroke (Ghayour-Najafabadi et al., 2019) and significantly augmented walking speed (Li et al., 2018). Numerous studies have validated the efficacy of rTMS in treating lower limb dyskinesia. The ideal therapeutic target of rTMS remains ambiguous, necessitating further research to assess and quantify the various stroke phases of the pathological substrates, thereby determiningat the exact neural grid localization at the dorsolateral prefrontal cortex to find the optimal therapeutic target. It involves focal magnetic stimulation applied externally to the scalp, typically at the dorsolateral prefrontal cortex, which induces electrical stimulation in underlying cortical tissue (Cash et al., 2021). High-frequency magnetic stimulation, rTMS, alters the excitability of cortical neurons, such as those in M1. This is what enables neuroplasticity in the brain (Chen et al., 2024). Constraint-Induced Movement Therapy (CIMT) seeks to transform the activation of brain pathways into functional motor performance. By compelling the impacted limb to engage in high-intensity task training, we convert neural remodeling into tangible motor improvements (Zhang et al., 2024).
3 Constraint-induced movement therapy
Constraint-induced movement therapy is a well-established rehabilitation program that is crucial for addressing limb motor impairment in stroke patients. Taub (1976) indicated research indicates that primate trials reveal that severing somatosensory afferent nerves leads to a phenomenon of learnt disuse, subsequently constraint-induced movement therapy. Researchers have conducted comprehensive studies from various perspectives and identified several potential mechanisms of action: The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) serves as the principal ionotropic glutamate receptor (GluR) inside the central nervous system of animals. AMPARs are located in cortical neurons and hippocampus pyramidal neurons, primarily consisting of GluR1/2 and GluR3/4 heterodimers. As noted by Hu et al. (2020), CIMT therapy resulted in an augmentation of GluR2-containing synapses and an elevation of GluR2 expression in the ipsilateral sensorimotor cortex synapses. rTMS particularly high-frequency stimulation, might enhance the aggregation of NMDAR receptors at the postsynaptic membrane by causing long-term potentiation (Kweon et al., 2024), with both mechanisms potentially complementing each other in the context of synaptic plasticity. CIMT enhances synaptic connection in behavioral training, whereas rTMS directly alters the synaptic protein production pathway via electromagnetic stimulation. The CST (corticospinal tract), a significant white matter bundle comprising the pyramidal tract, internal capsule, and cerebral tegmentum, functions as a primary neuronal conduit for voluntary movement. Hu et al. (2019) examined the anisotropy scores and mean diffusion coefficients of the CST in wind mice before and after CIMT using diffusion tensor imaging, concluding that CIMT enhances functional recovery post-ischemic stroke by aiding the rebuilding of the ipsilateral CST. rTMS also elevates cerebral blood flow in the targeted region and ameliorates hypoxia within the CST milieu (Heejae, 2024), these effects may synergistically promote axon guidance and myelin regeneration in the CST. A study utilizing bioinformatics prediction and luciferase assays discovered that CIMT may enhance neuronal and myelin remodeling in the motor cortex by partially inhibiting the c-Jun/miR-182-5p/Nogo-A pathway. This enhances the capacity for comprehension and equilibrium (Tang et al., 2023). Researchers have identified that rTMS directly suppresses the promoter activity of Nogo-A and stimulates the cAMP-PKA-CREB pathway (Bao et al., 2021). Two distinct inhibitory actions may occur simultaneously: CIMT reduces Nogo-A post-transcriptionally, while rTMS inhibits its synthesis during transcription. While the role of CIMT in stroke patients has been evidenced in multiple investigations, the specific mechanism by which it has a predominant effect remains unestablished, necessitating future research for clarification.
It aims to enhance movement abnormalities by promoting the utilization of limbs with compromised motor function following a stroke. Constraint-induced movement therapy has demonstrated significant efficacy and is frequently employed as a standard protocol in stroke rehabilitation (Shen et al., 2019). Frequently employed as a directive protocol in stroke therapy exercises, it has shown considerable effectiveness. To investigate enhancements in lower limb rehabilitation, Aloraini (2022) conducted a randomized controlled, single-blind clinical investigation involving 38 individuals experiencing dyskinesia post-stroke. The patients were randomly allocated to the CIMT group or the control group. In comparison to the control group, patients in the CIMT group exhibited enhanced recovery of lower limb mobility, postural stability, and gait velocity, with these advancements maintained 3 months following the conclusion of the treatment regimen. 3 months post-treatment, these enhancements persisted; numerous researchers have investigated CIMT therapy in upper limb rehabilitation. Thirty cases of persistent hemiplegia patients who had CIMT therapy have been included. The treatment group exhibited a significant enhancement in upper limb function relative to the control group (Rocha et al., 2021). It can be seen that both CIMT therapies can effectively promote the rehabilitation of upper and lower limb motor functions.
4 Repetitive transcranial magnetic stimulation combined with constraint-induced movement therapy
4.1 Potential mechanisms
Conventional stroke therapy methods primarily emphasize peripheral rehabilitation tactics to facilitate the relearning and recovery of motor functions. Nonetheless, these techniques exhibit issues including prolonged rehabilitation duration, restricted clinical efficacy, and elevated therapy stagnation rates, with certain patients demonstrating minimal progress in motor dysfunction or remaining at baseline for extended periods (French et al., 2016; Legg et al., 2017). The research commenced with closed-loop rehabilitation theory and then transitioned to the central-peripheral synergistic intervention paradigm. This methodology directly addresses brain injury via “top-down” neuromodulation and integrates it with peripheral functional training to establish a bidirectional closed-loop intervention (Jia, 2016). Non-invasive neuromodulation techniques, including transcranial direct current stimulation (tDCS), rTMS, and mirror therapy (MT), are employed in central interventions to stimulate the neuroplasticity of the motor cortex and reconstruct neural network connections. In contrast, peripheral interventions incorporate CIMT and additional strengthening training tools to facilitate functional remodeling of the limbs through task-oriented training. The synergistic effect may arise from the optimization of limb movement through central intervention and the enhancement of behavioral output via peripheral intervention, thereby transcending the constraints of singular therapy and offering a more focused intervention strategy for stroke rehabilitation (Yan, 2021).
Despite both rTMS and CIMT being grounded in neuroplasticity, they exhibit substantial distinctions in their mechanisms and intervention targets. rTMS employs electromagnetic pulses to directly activate the cerebral cortex. It regulates neuronal firing and alters the functionality of neural networks at the central level (Kwakkel et al., 2015; Lefaucheur et al., 2017). CIMT alters brain function by engaging peripheral behavioral interventions and motor feedback pathways. This reverses the phenomenon of “learned disuse”. Due to the modifiable parameters, rTMS is applicable at all phases of brain injury, ranging from acute to chronic (Zhou et al., 2023). CIMT requires patients to possess fundamental motor abilities and is primarily utilized during the chronic or functional plateau phase. The disparity in mechanisms may stem from rTMS directly influencing central electrical activity to disrupt neural inhibition in pathological conditions, whereas CIMT enhances motor learning via peripheral training, targeting the deficiencies within the “central-peripheral” bidirectional control system. In the initial trial, rTMS was administered exclusively while the individuals were at rest. The impacts of various activities occurring during, before to, and after to the stimulation were disregarded. The relationship between physical training and rTMS remains inadequately investigated in the existing literature (Beaulieu and Milot, 2018). Clarifying the link between the two is crucial for investigating the CNS adaptation mechanism. CIMT promotes motor function compensation and neuronal reconfiguration via behavioral interventions by restricting training on the unaffected side of the limb and enhancing it on the affected side. This leads to both functional compensation and brain remodeling. Research has revealed that rTMS applied during prolonged voluntary tonic activity enhances the synchronization of motor neurons. The integration of behavioral stimulation enhances neuronal plasticity in the motor cortex beyond that achieved by rTMS alone and assists rTMS treatments in determining the optimal mix of intensity, duration, frequency, and location (Mills and Schubert, 1995). Furthermore, in terms of neurotrophic factors, according to Livingston-Thomas, the mechanism of action of CIMT in the restoration of motor function in the upper limb may be closely related to its modulation of microglial cell function: although post-stroke rehabilitation did not alter the total proportion of BDNF-expressing cells in the infarcted area, it was found, by immunofluorescence assay, that CIMT induced a significant increase in the proportion of non-neuronal/non-astrocyte-derived BDNF was significantly increased, and a functional transformation of the subpopulation of BDNF-expressing microglia was also observed (Livingston-Thomas et al., 2014), the cellular phenotype may have shifted from a pro-inflammatory M1-type to a reparative M2-type, and this phenotypic polarization may have occurred either through inhibition of TAK1 -NF-κB-p38 MAPK signaling pathway, thereby attenuating neuroinflammation and enhancing tissue repair (Vinnakota et al., 2024). This technique exhibits synergistic potential with high-frequency rTMS, which facilitates neurological recovery by stimulating the BDNF/TrkB signaling pathway, so establishing a closed-loop “central-peripheral-central” regulation (Luo et al., 2017). Consequently, the synergistic mechanism of rTMS may involve the enhancement of central neuroplasticity by modulating motor cortex excitability and the BDNF signaling pathway, while simultaneously inhibiting the inflammatory signaling pathway to facilitate the transformation of microglia to the M2 phenotype. In contrast, CIMT activates sensory-motor feedback at the peripheral level through intensive exercise training, thereby further reinforcing central remodeling and sustaining the reparative phenotype of microglia. This multimodal intervention produces a compounded effect across the three aspects of neuroinflammation management, neurotrophic factor release, and synaptic plasticity repair, hence overcoming the limitations of single therapy efficacy.
Synergistic potential of rTMS paired with CIMT in stroke recovery, numerous problems persist within this program: Attention must be given to potential safety concerns and parameter optimization, such as the risk of electromagnetic interference between TMS and implanted devices (Rossi et al., 2021). Additionally, excessive use of CIMT may lead to muscle strain in the training limb or psychological resistance in patients, thereby increasing risk. The therapeutic synergies, specifically the temporal alignment between the neuromodulation after-effect window (approximately 60 min) of rTMS and the intensity of CIMT training, remain unclear. Furthermore, individual adaptability is a concern, as patients with severe motor disorders (Fugl-M) may experience central fatigue due to over-stimulation. The relationship between the therapeutic synergy of rTMS’s neuromodulation after-effect window (about 30–60 min) and the intensity of CIMT training remains ambiguous, and excessive stimulation may lead to central weariness (Zhu et al., 2023). Individuals with significant movement problems (Fugl-Meyer score < 30) may lack the capacity for effective CIMT, hence diminishing the treatment’s efficacy. The combination treatment needs two and a half to three hours daily, incurs significant costs, necessitates extensive equipment, and consequently poses challenges for clinical promotion. Stringent requirements provide obstacles to clinical dissemination (Sharma et al., 2023). Addressing these issues necessitates enhanced patient classification, adaptive parameter adjustment mechanisms, and validation through multicenter, large-scale research.
4.2 Application and progress
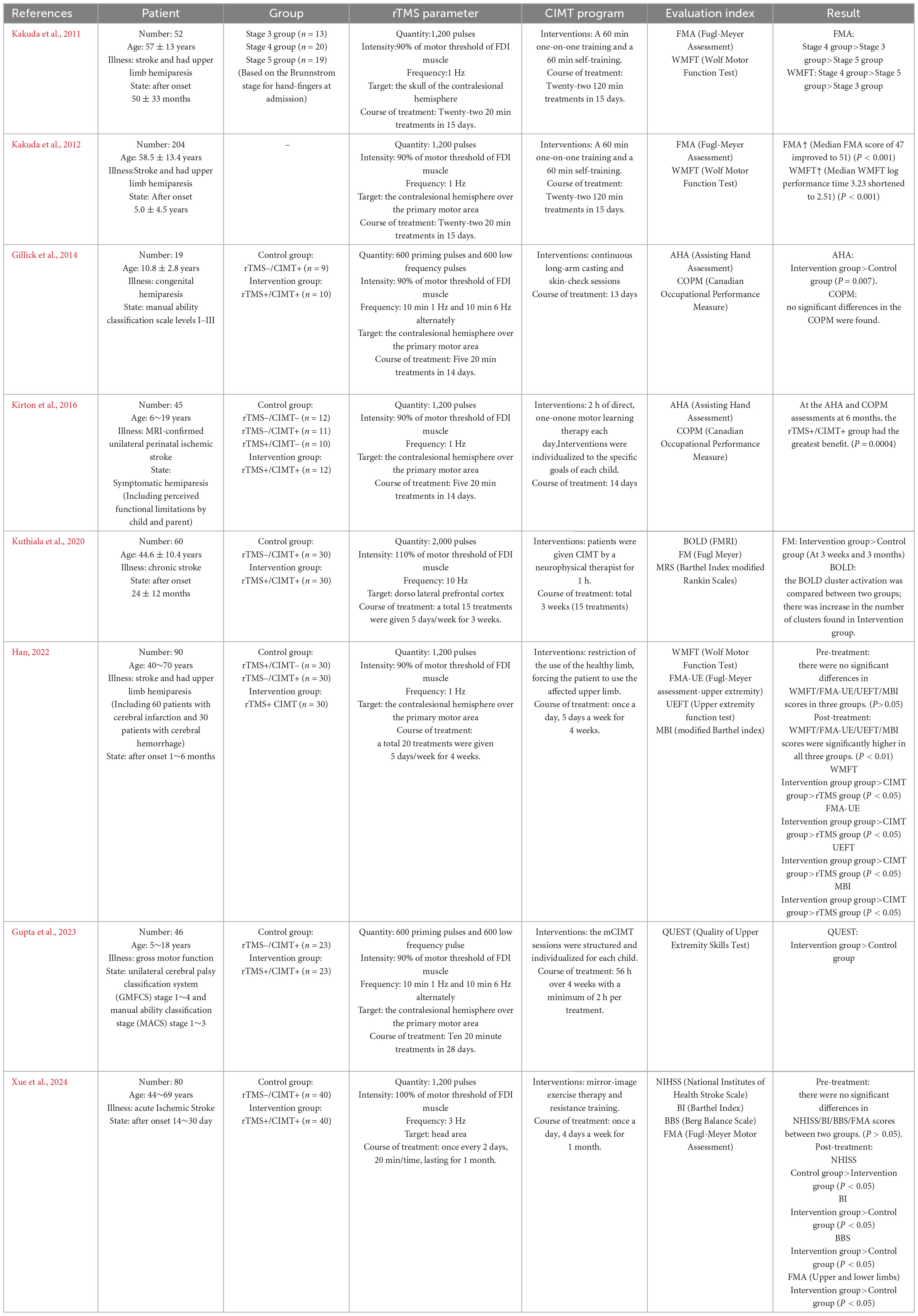
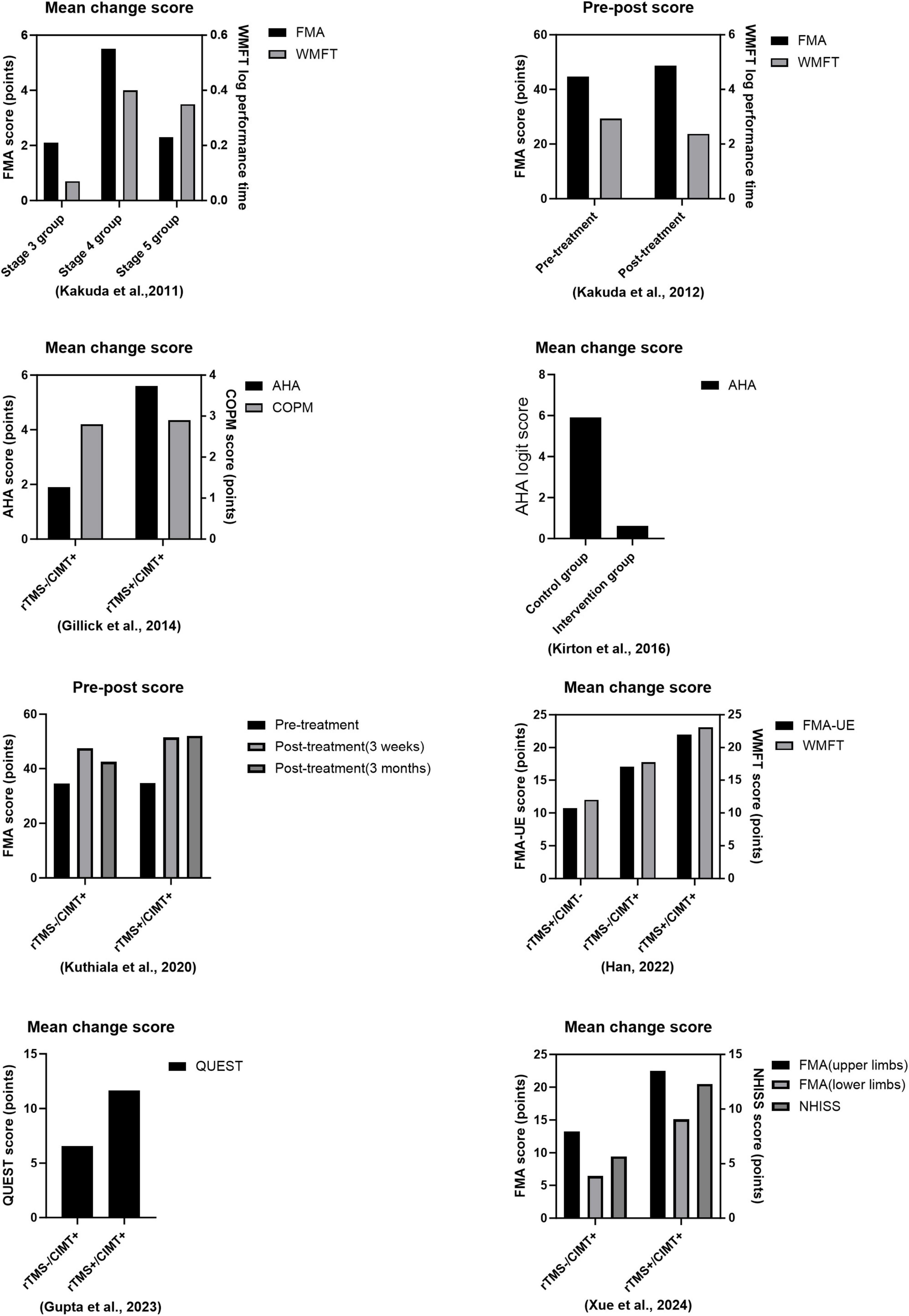
Table 1 and Figure 1 give more details of relevant combination therapies studies, so only more critical points are discussed here. A study including 52 stroke patients with upper limb hemiparesis shown that a 15 days treatment utilizing a combined protocol of CIMT and 1 Hz rTMS to stimulate the contralesional hemisphere was effective. The results indicated a substantial correlation between the extent of improvement in the Fugl-Meyer and Wolf Motor Function Test (WMFT) and the initial Brunnstrom stage, implying that the intervention’s efficacy is contingent upon the initial severity of hemiparesis, thereby affirming the safety of the combination therapy and offering an evidence-based foundation for the formulation of personalized rehabilitation strategies (Kakuda et al., 2011). Kakuda et al. (2012) conducted a follow-up study examining the efficacy of 1 Hz rTMS in conjunction with CIMT in individuals with upper extremity hemiparesis post-stroke. They aimed to direct rTMS stimulation to the contralesional hemisphere above the major motor region for a duration of 15 days. The findings indicated that the patients’ motor function score for the upper extremity (median FMA score increased from 47 to 51) and motor efficiency (median WMFT log performance time decreased from 3.23 to 2.51) exhibited significant improvement at discharge compared to baseline levels at admission, thereby affirming the clinical efficacy of the combination protocol in enhancing limb motor control and functional recovery. Hemiplegic cerebral palsy is a prevalent subtype typically caused by a prenatal stroke. Perinatal strokes exemplify how human development can evolve over time, as they occur at specific intervals in healthy brains (Peter et al., 2007). To elucidate the feasibility and efficacy of rTMS in conjunction with CIMT for the rehabilitation of paralyzed hands in children with cerebral palsy (CP), Gillick et al. (2014) stratified 19 children aged 8–17 years into two cohorts, each undergoing five consecutive sessions of either real or sham rTMS and CIMT alternately, targeting the contralesional hemisphere over the primary motor area for a duration of 14 days. They employed Fisher’s exact test to analyze alterations in individuals’ raw AHA scores with a four-point smallest discernible difference (SDD). The primary measure utilized was the Assisted Hand Assessment (AHA), while the Canadian Occupational Performance Measure (COPM) and Stereognostic indicators served as additional measures. The findings revealed that eight of 10 participants in the rTMS/CIMT group exhibited improvements exceeding the SDD, whereas only two of nine in the sham rTMS/CIMT group demonstrated similar progress. This suggests that low-frequency rTMS combined with CIMT may be safe, feasible, and effective for treating pediatric hemiparesis. It is noteworthy that the rTMS protocol employed by the team was particularly innovative, utilizing a combination of 1 Hz low-frequency and 6 Hz high-frequency alternating stimulation patterns, each with distinct stimuli. The team’s rTMS program is highly novel since it transcends the constraints of single-frequency stimulation modes, such as a solitary 1 Hz, by alternating between 10 min of 1 Hz low-frequency and 6 Hz high-frequency stimulation modes. This dual-frequency timing combination may synergistically enhance motor function remodeling by dynamically regulating the inhibitory balance between the two hemispheres and offer fresh evidence for optimizing therapeutic parameters of rTMS in conjunction with CIMT. In an analytical randomized controlled study, 45 hemiplegic youngsters were assigned to one of five groups: CIMT, 1 Hz daily rTMS, neither group, or both groups. Researchers conducted rTMS for 14 days, focusing on the contralesional hemisphere at the primary motor region as the stimulation site. The rTMS was administered for 14 days, and the COPM scale was utilized to assess the quality of life in the rTMS combined with the CIMT group at the 6 months mark. This demonstrated that the two interventions were more effective in conjunction, enhancing the children’s engagement in functional activities and improving their quality of life, surpassing the outcomes of the individual therapies administered separately (Kirton et al., 2016).
Significant advancements have recently been achieved in the rehabilitation of individuals with upper limb hemiplegia following a stroke. Neuromodulation techniques and behavioral training synergistically yield significant advantages. Several astute researchers integrated rTMS with CIMT. The frequency employed was 10 Hz, representing 110% of the motor threshold of the first dorsal interosseous muscle. The dorsolateral prefrontal cortex served as the target for rTMS stimulation. The group exhibited a more pronounced enhancement in Fugl-Meyer motor function scale (FM) scores 3 weeks post-intervention in comparison to the CIMT group alone. This enhancement was also observed at the 3 weeks and 3 months follow-ups post-treatment. Functional magnetic resonance imaging (fMRI) revealed significantly greater activation clusters of blood oxygen level-dependent (BOLD) signaling in the brains of patients in the experimental group. This indicates that high-frequency rTMS may enhance the functional connection of the DLPFC with the motor cortex. This underscores the significance of integrating central modulation with peripheral motor training in a comprehensive rehabilitation approach for chronic phase stroke patients, demonstrating clinical applicability (Kuthiala et al., 2020). A study involving 90 stroke patients (60 with cerebral infarction and 30 with cerebral hemorrhage) demonstrated that a 4 weeks intervention protocol utilizing 1 Hz rTMS targeting the contralesional hemisphere over the primary motor area, in conjunction with CIMT, resulted in enhanced scores on the Wolf Motor Function Test (WMFT), Fugl-Meyer Assessment-Upper Extremity (FMA-UE), upper extremity function test (UEFT), and modified Barthel index (MBI). This resulted from a substantial synergistic effect. The study results demonstrated that the mean scores of the combined treatment group across the four scales were significantly elevated compared to the control group receiving only forced exercise or single rTMS (p < 0.05), thereby substantiating the clinical benefits of the central-peripheral synergistic intervention model in the rehabilitation of patients with varying degrees of stroke (Han, 2022). A study on pediatric cerebral palsy (CP) by Gupta et al. (2023) sought to apply rTMS in conjunction with modified CIMT (mCIMT) at 6 Hz, focusing on the contralesional hemisphere above the major motor region. The Quality of Upper Extremity Skills Test (QUEST) verified that the mean change in the QUEST total score for the intervention group was considerably greater than that of the control group at 4 weeks, demonstrating that 6 Hz rTMS paired with CIMT is both safe and practical. The group’s mean alteration in the QUEST score significantly exceeded that of the control group. This indicates that the application of rTMS at 6 Hz in conjunction with CIMT is both safe and feasible. This provides a framework for identifying the optimal treatment plan by modulating the intensity of the CIMT intervention and the parameters of task-oriented training. Broadening the research scope is a significant aspect of the present advancements. Xue et al. (2024) has achieved a significant advancement in stroke rehabilitation. The study used 3 Hz rTMS, with a stimulation intensity set at 100% of the movement threshold for the first dorsal interosseous muscle, targeting the cranial region. It employs an innovative CIMT program in conjunction with a multidimensional assessment system [National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI), Berg Balance Scale (BBS), and Fugl-Meyer Motor Function Assessment Scale (FMA)] to systematically evaluate the clinical efficacy of the combined intervention. One month later, the study’s results indicated that, with the exception of the NIHSS, the combined therapy group had considerably superior BI, BBS, and FMA scores compared to the control group, which got only behavioral training. The FMA subgroup analysis indicated that the enhancement of motor function in both upper and lower limbs occurred simultaneously, which constituted an additional advantage. This was the inaugural instance in which the combined therapy was systematically demonstrated to enhance the overall motor function of stroke patients. The combination therapy may exert a comprehensive regulatory influence via the trans-hemispheric neuronal network. The findings indicate that the combination therapy may exert a worldwide regulatory influence via the trans-hemispheric neural network. This provides us with data that can be utilized in the future to determine the optimal therapeutic parameters for combination therapy. These insights have enabled the development of neuroplasticity-based models for integrated central and peripheral therapies. In the future, researchers will concentrate on identifying the precise location of optimal stimulation parameters.
5 Limitations
Historically, rTMS has been shown to be safe and effective as a rehabilitation therapy, and its safety requires special attention to the following factors: metal implants in the patient’s body need to be kept at a safe distance to avoid magnetic field interference, people with a history of epilepsy or those who are taking medications to lower the epileptic threshold need to be carefully assessed for risk, and parameter settings need to be in accordance with international safety guidelines in order to reduce the probability of epileptic seizure triggers (Luo, 2020); and CIMT has also proved to be safe for treating limb dysfunction after a stroke. CIMT has also been shown to be safe in the treatment of post-stroke limb dysfunction, with safety considerations including avoidance of overexertion or strain of the affected limb, attention to the risk of skin compression injuries, and its non-invasive nature is more easily accepted by patients and can effectively facilitate their participation in daily activities (Zhang et al., 2023). Nonetheless, low-frequency rTMS combo therapy is more tolerable for patients, alleviating pain, muscle spasms, and discomfort compared to high-frequency rTMS (Kaur et al., 2019). A recent randomized controlled experiment with blinded assessors demonstrated that children with unilateral cerebral palsy can benefit from low-frequency rTMS in conjunction with CIMT. This therapy is also secure for neurorehabilitation (Wu et al., 2022). A study including 45 stroke patients has shown that an intervention utilizing 10 Hz rTMS in conjunction with exercise treatment is safe (Wang et al., 2020). Lomarev et al. (2007) evaluated the safety of 20 and 25 Hz rTMS applied to the motor cortex of the afflicted hemisphere by assessing the electrical activity of the hand and arm muscles. They observed transient, significant alterations in the EMG, indicating that high-frequency rTMS is safe for healthy volunteers but may increase the risk of seizures in individuals with a history of stroke.
6 Perspectives and future direction
Numerous clinical trials have demonstrated that rTMS in conjunction with CIMT therapy is both safe and effective. Significant opportunities remain for research and public understanding regarding the rehabilitation of mobility problems following a stroke. This is an innovative method for post-stroke rehabilitation that will provide fresh insights for future recovery strategies. However, this study presents some issues: (1) The mechanism of action of the combination therapy is not elucidated; (2) additional studies employing standardized combination protocols are necessary to evaluate the efficacy of the treatment; (3) the sample size for this combination therapy is limited; and (4) the optimal stimulation parameters, such as intensity, frequency, therapeutic target, duration, and interval, remain ambiguous. Future research may investigate the standardization of combination therapy, particularly regarding stimulation parameters. Diverse assessment criteria employed for combination therapy complicate the comparison of findings across various research. It is hoped that assessment standards might be standardized in the future. As rehabilitation technology undergoes continuous iterative updates and the research team matures, further validation and enhancement of synergistic rehabilitation therapy techniques will more effectively restore the functional abilities of patients with post-stroke movement disorders.
Author contributions
ZL: Writing – original draft. QY: Writing – original draft. FZ: Writing – review and editing. MY: Writing – review and editing. HS: Writing – review and editing. MZ: Funding acquisition, Project administration, Supervision, Writing – review and editing. TP: Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jiangxi Province young and middle-aged backbone talents training project of traditional Chinese medicine [No: Gan TCM Ke Jiao Zi (2020) No. 5] and National advantageous specialty construction project of traditional Chinese medicine (Department of rehabilitation) [zyh (2024) No. 90].
Acknowledgments
We gratefully acknowledge the financial supports by the Jiangxi Province young and middle-aged backbone talents training project of traditional Chinese medicine [No: Gan TCM Ke Jiao Zi (2020) No. 5] and well as the National advantageous specialty construction project of traditional Chinese medicine (Department of rehabilitation) [zyh (2024) No. 90].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullahi, A., Wong, T. W., Van Criekinge, T., and Ng, S. S. (2023). Combination of noninvasive brain stimulation and constraint-induced movement therapy in patients with stroke: A systematic review and meta-analysis. Expert. Rev. Neurother. 23, 187–203. doi: 10.1080/14737175.2023.2177154
Aloraini, S. M. (2022). Effects of constraint-induced movement therapy for the lower extremity among individuals post-stroke: A randomized controlled clinical trial. Neurorehabilitation 51, 421–431. doi: 10.3233/NRE-220139
Bao, Z., Bao, L., Han, N., Hou, Y., and Feng, F. (2021). rTMS alleviates AD-induced cognitive impairment by inhibitng apoptosis in SAMP8 mouse. Aging (albany Ny) 13, 26034. doi: 10.18632/aging.203796
Beaulieu, L.-D., and Milot, M.-H. (2018). Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: A systematic review of randomized controlled trials. Ann. Phys. Rehabil. Med. 61, 224–234. doi: 10.1016/j.rehab.2017.04.003
Cash, R. F. H., Weigand, A., Zalesky, A., Siddiqi, S. H., Downar, J., Fitzgerald, P. B., et al. (2021). Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol. Psychiatry 90, 689–700. doi: 10.1016/j.biopsych.2020.05.033
Chen, S.-Y., Tsou, M.-H., Chen, K.-Y., Liu, Y.-C., and Lin, M.-T. (2024). Impact of repetitive transcranial magnetic stimulation on cortical activity: A systematic review and meta-analysis utilizing functional near-infrared spectroscopy evaluation. J. NeuroEng. Rehabil. 21:108. doi: 10.1186/s12984-024-01407-9
Cheng, S., Xin, R., Zhao, Y., Wang, P., Feng, W., and Liu, P. (2023). Evaluation of fMRI activation in post-stroke patients with movement disorders after repetitive transcranial magnetic stimulation: A scoping review. Front. Neurol. 14:1192545. doi: 10.3389/fneur.2023.1192545
Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., and Vos, T. (2020). Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet 396, 2006–2017. doi: 10.1016/S0140-6736(20)32340-0
Duan, X., Huang, D., Zhong, H., Wu, J., Xiao, Z., Yang, P., et al. (2024). Efficacy of rTMS in treating functional impairment in post-stroke patients: A systematic review and meta-analysis. Neurol. Sci. 45, 3887–3899. doi: 10.1007/s10072-024-07455-2
French, B., Thomas, L. H., Coupe, J., McMahon, N. E., Connell, L., Harrison, J., et al. (2016). Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. CD006073. doi: 10.1002/14651858.CD006073.pub3
Ghayour-Najafabadi, M., Memari, A.-H., Hosseini, L., Shariat, A., and Cleland, J. A. (2019). Repetitive transcranial magnetic stimulation for the treatment of lower limb dysfunction in patients poststroke: A systematic review with meta-analysis. J. Stroke Cerebrovasc. Dis. 28:104412. doi: 10.1016/j.jstrokecerebrovasdis.2019.104412
Gillick, B. T., Krach, L. E., Feyma, T., Rich, T. L., Moberg, K., Thomas, W., et al. (2014). Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: A randomized controlled trial. Dev. Med. Child Neurol. 56, 44–52. doi: 10.1111/dmcn.12243
Gupta, J., Gulati, S., Singh, U. P., Kumar, A., Jauhari, P., Chakrabarty, B., et al. (2023). Brain stimulation and constraint induced movement therapy in children with unilateral cerebral palsy: A randomized controlled trial. Neurorehabil. Neural Repair. 37, 266–276. doi: 10.1177/15459683231174222
Han, X. (2022). Repetitive Transcranial Magnetic Stimulation Combined with Compulsory Exercise Therapy for the Treatment of Upper Extremity Motor Dysfunction in Stroke. Jinan: Shandong University of Traditional Chinese Medicine, doi: 10.27282/d.cnki.gsdzu.2021.000619
Han, Y., Wei, Z.-Y., Zhao, N., Zhuang, Q., Zhang, H., Fang, H.-L., et al. (2025). Transcranial magnetic stimulation in attention-deficit/hyperactivity disorder: A systematic review and meta-analysis of cortical excitability and therapeutic efficacy. Front. Psychiatry 16:1544816. doi: 10.3389/fpsyt.2025.1544816
Heejae, K. (2024). Effect of low-frequency repetitive magnetic transcranial stimulation in hemichorea-hemiballismus with ipsilateral basal ganglia hemorrhage: A case report. Turk. J. Phys. Med. Rehabil. 70, 397–401. doi: 10.5606/tftrd.2024.13022
Hu, J., Li, C., Hua, Y., Liu, P., Gao, B., Wang, Y., et al. (2020). Constraint-induced movement therapy improves functional recovery after ischemic stroke and its impacts on synaptic plasticity in sensorimotor cortex and hippocampus. Brain Res. Bull. 160, 8–23. doi: 10.1016/j.brainresbull.2020.04.006
Hu, J., Li, C., Hua, Y., Zhang, B., Gao, B.-Y., Liu, P.-L., et al. (2019). Constrained-induced movement therapy promotes motor function recovery by enhancing the remodeling of ipsilesional corticospinal tract in rats after stroke. Brain Res. 1708, 27–35. doi: 10.1016/j.brainres.2018.11.011
Jia, J. (2016). “Central-peripheral-central” closed-loop rehabilitation: A new concept of hand function rehabilitation after stroke. Chin. J. Rehabil. Med. 31, 1180–1182.
Kakuda, W., Abo, M., Kobayashi, K., Takagishi, T., Momosaki, R., Yokoi, A., et al. (2011). Baseline severity of upper limb hemiparesis influences the outcome of low-frequency rTMS combined with intensive occupational therapy in patients who have had a stroke. Pmr 3, 516–522. doi: 10.1016/j.pmrj.2011.02.015
Kakuda, W., Abo, M., Shimizu, M., Sasanuma, J., Okamoto, T., Yokoi, A., et al. (2012). A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J. NeuroEng. Rehabil. 9:4. doi: 10.1186/1743-0003-9-4
Kaur, M., Michael, J. A., Fitzgibbon, B. M., Hoy, K. E., and Fitzgerald, P. B. (2019). Low-frequency rTMS is better tolerated than high-frequency rTMS in healthy people: Empirical evidence from a single session study. J. Psychiatr. Res. 113, 79–82. doi: 10.1016/j.jpsychires.2019.03.015
Kim, C., Choi, H. E., Jung, H., Lee, B.-J., Lee, K. H., and Lim, Y.-J. (2014). Comparison of the effects of 1 hz and 20 hz rTMS on motor recovery in subacute stroke patients. Ann. Rehabil. Med. 38, 585–591. doi: 10.5535/arm.2014.38.5.585
Kirton, A., Andersen, J., Herrero, M., Nettel-Aguirre, A., Carsolio, L., Damji, O., et al. (2016). Brain stimulation and constraint for perinatal stroke hemiparesis: The PLASTIC CHAMPS trial. Neurology 86, 1659–1667. doi: 10.1212/WNL.0000000000002646
Klomjai, W., Katz, R., and Lackmy-Vallée, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann. Phys. Rehabil. Med. 58, 208–213. doi: 10.1016/j.rehab.2015.05.005
Kuthiala, N., Ashu, B., Rahul, S., Padma, S., Senthil, K., and Sakshi, S. (2020). rTMS and CIMT for neurofunctional recovery in chronic stroke. Int. J Neurorehabil. 7, 1–7. doi: 10.37421/2376-0281.2020.7.e002
Kwakkel, G., Veerbeek, J. M., Van Wegen, E. E. H., and Wolf, S. L. (2015). Constraint-induced movement therapy after stroke. Lancet Neurol. 14, 224–234. doi: 10.1016/S1474-4422(14)70160-7
Kweon, J., Vigne, M., Fukuda, A. M., Ren, B., Carpenter, L. L., and Brown, J. C. (2024). NMDA and GABA receptor-mediated plasticity induced by 10-hz repetitive transcranial magnetic stimulation. Res. Sq. doi: 10.21203/rs.3.rs-4630964/v1
Lefaucheur, J.-P., Andrea, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92. doi: 10.1016/j.clinph.2016.10.087
Legg, L. A., Lewis, S. R., Schofield-Robinson, O. J., Drummond, A., and Langhorne, P. (2017). Occupational therapy for adults with problems in activities of daily living after stroke. Cochrane Database Syst. Rev. 7:CD003585. doi: 10.1002/14651858.CD003585.pub3
Li, Y., Fan, J., Yang, J., He, C., and Li, S. (2018). Effects of repetitive transcranial magnetic stimulation on walking and balance function after stroke: A systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 97:773. doi: 10.1097/PHM.0000000000000948
Livingston-Thomas, J. M., McGuire, E. P., Doucette, T. A., and Tasker, R. A. (2014). Voluntary forced use of the impaired limb following stroke facilitates functional recovery in the rat. Behav. Brain Res. 261, 210–219. doi: 10.1016/j.bbr.2013.12.032
Lomarev, M. P., Kim, D. Y., Richardson, S. P., Voller, B., and Hallett, M. (2007). Safety study of high-frequency transcranial magnetic stimulation in patients with chronic stroke. Clin. Neurophysiol. 118, 2072–2075. doi: 10.1016/j.clinph.2007.06.016
Luo, J., Zheng, H., Zhang, L., Zhang, Q., Li, L., Pei, Z., et al. (2017). High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int. J. Mol. Sci. 18:455. doi: 10.3390/ijms18020455
Luo, X. (2020). Study on the effect and safety of compulsory exercise therapy fo!rehabilitation treatment of stroke hemiplegia. Smart Healthcare 6, 72–73. doi: 10.19335/j.cnki.2096-1219.2020.34.028
Mills, K. R., and Schubert, M. (1995). Short term synchronization of human motor units and their responses to transcranial magnetic stimulation. J. Physiol. 483, 511–523. doi: 10.1113/jphysiol.1995.sp020602
Ozkan, B. N., Bozali, K., Boylu, M. E., Velioglu, H. A., Aktas, S., Kirpinar, I., et al. (2024). Altered blood parameters in “major depression” patients receiving repetitive transcranial magnetic stimulation (rTMS) therapy: A randomized case-control study. Transl. Psychiatry 14:264. doi: 10.1038/s41398-024-02942-8
Peter, R., Nigel, P., Alan, L., Murray, G., and Martin, B. (2007). A report: The definition and classification of cerebral palsy april 2006. Dev. Med. Child Neurol. 49, 8–14. doi: 10.1111/j.1469-8749.2007.tb12610.x
Rocha, L. S. O., Gama, G. C. B., Rocha, R. S. B., Rocha, L. D. B., Dias, C. P., Santos, L. L. S., et al. (2021). Constraint induced movement therapy increases functionality and quality of life after stroke. J. Stroke Cerebrovasc. 30:105774. doi: 10.1016/j.jstrokecerebrovasdis.2021.105774
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmöller, J., et al. (2021). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert guidelines. Clin. Neurophysiol. 132, 269–306. doi: 10.1016/j.clinph.2020.10.003
Ruan, P., and Ye, X. (2024). Current status of rehabilitation for motor dysfunction in stroke. Zhejiang Clin. Med. J. 26, 1415–1417.
Sharma, P., Gupta, M., and Kalra, R. (2023). Recent advancements in interventions for cerebral palsy – A review. J. Neurorestoratol. 11:100071. doi: 10.1016/j.jnrt.2023.100071
Shen, H., Wang, G., and Wang, X. (2019). A meta-analysis of the effects of modified mandatory exercise therapy on upper extremity motor function in stroke patients with hemiplegia. Chinese J. Rehabil. Med. 34, 1216–1223.
Simonetta-Moreau, M. (2014). Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann. Phys. Rehabil. Med. 57, 530–542. doi: 10.1016/j.rehab.2014.08.003
Tang, H., Pan, J., Xu, Y., Liu, L., Yang, X., Huang, S., et al. (2023). Constraint therapy promotes motor cortex remodeling and functional improvement by regulating c-jun/miR-182–5p/nogo – A signals in hemiplegic cerebral palsy mice. Ann. Anat. 250:152136. doi: 10.1016/j.aanat.2023.152136
Taub, E. (1976). Movement In nonhuman primates deprived of somatosensory feedback. Exercise Sport Sci. Rev. 4, 335–374.
Vabalaite, B., Petruseviciene, L., Savickas, R., Kubilius, R., Ignatavicius, P., and Lendraitiene, E. (2021). Effects of high-frequency (HF) repetitive transcranial magnetic stimulation (rTMS) on upper extremity motor function in stroke patients: A systematic review. Medicina (Mex.) 57:1215. doi: 10.3390/medicina57111215
Vinnakota, J. M., Biavasco, F., Schwabenland, M., Chhatbar, C., Adams, R. C., Erny, D., et al. (2024). Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome. Nat Cancer 5, 1227–1249. doi: 10.1038/s43018-024-00764-7
Wang, L. (2021). Effects of repetitive transcranial magnetic stimulation with different frequencies on limb function in hemiplegic patients after stroke. Henan Med. Res. 30, 66–68.
Wang, Q., Zhang, D., Zhao, Y.-Y., Hai, H., and Ma, Y.-W. (2020). Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul. 13, 979–986. doi: 10.1016/j.brs.2020.03.020
Wu, Q., Peng, T., Liu, L., Zeng, P., Xu, Y., Yang, X., et al. (2022). The effect of constraint-induced movement therapy combined with repetitive transcranial magnetic stimulation on hand function in preschool children with unilateral cerebral palsy: A randomized controlled preliminary study. Front. Behav. Neurosci. 16:876567. doi: 10.3389/fnbeh.2022.876567
Xiao, C., Pan, C., Chen, Y., Huang, S., Li, Q., Fu, Z., et al. (2019). Effects of high-frequency repetitive transcranial magnetic stimulation inDifferent frequencies on upper limb function after lschemic stroke. Chin. J. Rehabil. Theory Pract. 25, 557–563. doi: 10.3969/j.issn.1006-9771.2019.05.011
Xue, J., Liu, Y., and Pan, J. (2024). Application effect of repetitive transcranial magnetic stimulationcombined with limb movement therapy in the patients with acuteischemic stroke. Reflexol. Rehabil. Med. 47, 41–43.
Yan, T. (2021). Actively carrying out research on the clinical application of “brain-limb synergistic treatment technology”. Chinese J. Rehabil. Med. 36, 1195–1197.
Zhang, A., Xing, Y., Zheng, J., Li, C., Hua, Y., Hu, J., et al. (2024). Constraint-induced movement therapy modulates neuron recruitment and neurotransmission homeostasis of the contralesional cortex to enhance function recovery after ischemic stroke. ACS Omega 9, 21612–21625. doi: 10.1021/acsomega.4c02537
Zhang, J., Xiao, X., Jin, Q., Li, J., Zhong, D., Li, Y., et al. (2023). The effect and safety of constraint-induced movement therapy for post-stroke motor dysfunction: A meta-analysis and trial sequential analysis. Front. Neurol. 14:1137320. doi: 10.3389/fneur.2023.1137320
Zhou, G., Liu, D., Huang, X., Wu, Q., Feng, W., Zeng, Y., et al. (2023). High-frequency repetitive transcranial magnetic stimulation protects against cerebral ischemia/reperfusion injury in rats: Involving the mitigation of ferroptosis and inflammation. Brain Behav. 13:e2988. doi: 10.1002/brb3.2988
Zhu, G., Chen, S., Huo, C., Li, X., Chen, Y., He, X., et al. (2023). Expert consensus on peripheral combined with central dual target magnetic stimulation promoting rehabilitation of motor dysfunction after stroke. Chin. J. Rehabil. Med. 38, 880–884.
Keywords: post-stroke dyskinesia, repetitive transcranial magnetic stimulation, constraint-induced movement therapy, combination therapy, review
Citation: Liu Z, Yu Q, Zhou F, Yu M, Shu H, Zhu M and Peng T (2025) Repetitive transcranial magnetic stimulation and constraint-induced movement therapy combined in the treatment of post-stroke movement disorders: a narrative review. Front. Hum. Neurosci. 19:1578258. doi: 10.3389/fnhum.2025.1578258
Received: 17 February 2025; Accepted: 21 March 2025;
Published: 07 April 2025.
Edited by:
Naama Schwartz, Reichman University, IsraelReviewed by:
Joel Oster, Tufts Medical Center, United StatesFangling Sun, Capital Medical University, China
Jie Lyu, Shanghai University of Medicine and Health Sciences, China
Rita Huan-Ting Peng, University of Illinois at Urbana-Champaign, United States
Copyright © 2025 Liu, Yu, Zhou, Yu, Shu, Zhu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianzhong Peng, cGVuZ3RpYW56aG9uZ0AxMjYuY29t; Manhua Zhu, MzYzNjI5MDY2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zhennan Liu
Zhennan Liu Qingying Yu2†
Qingying Yu2†