
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 28 February 2025
Sec. Brain Health and Clinical Neuroscience
Volume 19 - 2025 | https://doi.org/10.3389/fnhum.2025.1557661
This article is part of the Research TopicNeurobiological mechanisms of addiction: bridging Neuroscience and clinical implicationsView all articles
 Roberto U. Cofresí1*
Roberto U. Cofresí1* Spencer Upton1
Spencer Upton1 Devon Terry1
Devon Terry1 Alexander A. Brown1
Alexander A. Brown1 Thomas M. Piasecki2
Thomas M. Piasecki2 Bruce D. Bartholow3
Bruce D. Bartholow3 Brett Froeliger1,4
Brett Froeliger1,4Introduction: Lower sensitivity (LS) to acute alcohol promotes hazardous alcohol use, increasing risk for alcohol use disorder (AUD). Compared to peers with high sensitivity (HS), LS individuals exhibit amplified responses to alcohol cues and difficulty exerting inhibitory control (IC) over those cued responses. However, it is unclear whether LS and HS individuals differ in neural or behavioral responses when exerting IC over affectively neutral prepotent responses (i.e., domain-general IC). This fMRI pilot study examined domain-general IC and its neural correlates in young adult LS and HS individuals.
Methods: Participants (N = 32, Mage = 20.3) were recruited based on their Alcohol Sensitivity Questionnaire responses (HS: n = 16; LS: n = 16; 9 females/group) to complete an event-related fMRI IC task in a sober state. Retrospective assessments of alcohol craving, consumption, and problems were taken outside the lab.
Results: Although IC performance (accuracy) was numerically lower for the LS group (M[SD] = 0.527[0.125]) compared to the HS group (M[SD] = 0.595[0.124]), no significant difference was detected [t(30) = 1.55, p = 0.132]. Across groups, IC-related activity was observed in bilateral fronto-cortico-striatal circuitry, including dorsal striatum (DS) and dorsal/supragenual anterior cingulate cortex (dACC). Within group HS, IC-related dACC activity was greater among individuals reporting less intense (b-95 CI = [−0.201, −0.041], p = 0.004) and less frequent alcohol craving experiences (b-95 CI = [−0.131, 0.005], p = 0.068), whereas in group LS, IC-related dACC activity was greater among individuals reporting more intense (b-95 CI = [0.009, 0.140], p = 0.028) and more frequent alcohol craving experiences (b-95 CI = [0.022, 0.128], p = 0.007).
Discussion: In sum, while LS and HS individuals demonstrated similar domain-general IC performance and recruited similar neural resources to perform IC, findings suggest that compensatory over-activation of frontocortical nodes of the fronto-cortico-striatal IC circuitry may be related to affective-motivational aspects of AUD symptomatology (craving in daily life) among LS individuals. Based on these preliminary findings, future studies with larger samples are warranted to determine the extent to which domain-general IC performance associated with fronto-cortico-striatal IC circuit activation contributes to the alcohol use pathophysiology, and whether therapeutic interventions (e.g., non-invasive brain stimulation) targeting fronto-cortico-striatal IC circuitry may decrease AUD symptomatology.
Alcohol use is highly prevalent and socially acceptable compared to the use of other addictive substances, yet poses a myriad of acute and chronic harms to individuals and society (Connor, 2017; Duke et al., 2017; Griswold et al., 2018). Chronic use of alcohol, like other addictive substances, is associated with deficits in multiple forms of executive functioning (EF) (Brion et al., 2017; Fernández-Serrano et al., 2011). Individual differences in inhibitory control (IC), one facet of EF, are strongly implicated in risk for onset and relapse across various substance use disorders (SUDs), including alcohol use disorders (AUD) (Gunawan et al., 2024; Morein-Zamir and Robbins, 2015; Smith et al., 2014; Zilverstand et al., 2018). IC encompasses the cognitive ability to stop a prepotent response (Friedman and Miyake, 2004, 2017; Miyake et al., 2000). Lower IC task performance is associated with heavier and more hazardous patterns of alcohol use (Hu et al., 2016; López-Caneda et al., 2014; Mullan et al., 2011; Murphy and Garavan, 2011; O’Halloran et al., 2020) and higher likelihood of relapse (return to use) among individuals attempting to abstain from drinking alcohol (Czapla et al., 2016; Rupp et al., 2016). The associations between IC and AUD risk may be driven by individual differences in susceptibility to the acute effects of alcohol, including IC impairment, as well as by individual differences in IC abilities in the sober state.
Implementation of IC involves at least one of two potentially cooperative frontocortical-basal ganglia circuits (Bundt and Huster, 2024; Hannah and Aron, 2021; Jahanshahi et al., 2015; Wessel and Anderson, 2024): the indirect and hyperdirect pathways. The indirect IC pathway involves frontocortical excitatory drive onto cells in dorsal striatum (caudate/putamen) with inhibitory projections onto cells in globus pallidus pars externa (GPe) that in turn have inhibitory projections onto cells in globus pallidus pars interna (GPi) and substantia nigra pars reticulata (SNr) that provide inhibitory tone on thalamic cells with excitatory projections to the primary motor cortex (M1) in the precentral gyrus. The hyperdirect IC pathway involves frontocortical excitatory drive onto cells in the subthalamic nucleus (STN) with excitatory projections onto the inhibitory cells in GPi/SNr, providing a faster mechanism (in terms of fewer synapses) for inhibition of M1. Meta-analyses suggest that key frontocortical nodes for IC implementation include dorsal and ventral lateral frontal cortices, such as anterior insula, middle and inferior frontal gyrus, as well as more medial cortices, such as anterior cingulate and superior frontal gyrus, depending on task-specific demands (Criaud and Boulinguez, 2013; Gavazzi et al., 2021, 2023; Isherwood et al., 2021; Simmonds et al., 2008; Zhang et al., 2017).
Individuals with active SUDs tend to exhibit hypo-activation of frontocortical IC circuit nodes during successful IC compared to healthy control cases (Goldstein and Volkow, 2002; Le et al., 2021; Luijten et al., 2014; Moeller et al., 2016; Zilverstand et al., 2018), suggesting that, functionally, “under-recruitment” of IC circuits lies at the core of SUD-related IC deficits. However, the story is complicated by potential differences between SUDs as well as by differential relationships to different aspects of risk (e.g., craving, consumption, and consequences) across the lifespan and/or substance use trajectory (Heitzeg et al., 2015; Hildebrandt et al., 2021; Luijten et al., 2014; Moeller et al., 2016). Indeed, as noted by Moeller et al. (2016), hyper- rather than hypo-activation of frontocortical IC circuit nodes during successful IC has been associated with craving and relapse (return to use) risk in clinical samples (Froeliger et al., 2017; Grieder et al., 2022; Prisciandaro et al., 2013; Stein et al., 2021), as well as with risky substance use patterns (e.g., binge drinking) in non-clinical samples (Herman et al., 2019; Suárez-Suárez et al., 2020). To reconcile such findings with the over-arching idea that SUD-related IC deficits are due to “under-recruitment” of IC circuits, it has been proposed that to achieve certain levels of IC performance some individuals compensate for hypo-active IC circuits by expending additional effort or neural resources (Heitzeg et al., 2015; Hildebrandt et al., 2021), leading to apparent “over-recruitment” of IC circuits.
Individual differences in susceptibility to alcohol intoxication are known to moderate risk for AUD onset and progression. Specifically, lower sensitivity (LS) to acute alcohol predicts heavier alcohol use and more alcohol use-related problems, including AUD (Schuckit et al., 2007, 2017, 2021). Several mechanisms have been proposed to account for LS-related AUD risk, including paradoxical hyper-sensitivity to appetitive effects of alcohol (Fleming et al., 2016; King et al., 2021, 2022), including cue reactivity (Bartholow et al., 2010; Cofresí et al., 2022b), and hypo-sensitivity to aversive effects (Davis et al., 2021; Fleming et al., 2016; Hone et al., 2017; Piasecki et al., 2012). Despite continued empirical and theoretical progress in understanding LS-related AUD risk (Parker et al., 2020; Ray et al., 2010; Schuckit et al., 2021), the role of potential sober-state differences in EF facets like IC in LS-related AUD risk remains under-explored.
Early indication of potential sober-state differences in EF-related processes as a function of alcohol sensitivity phenotype came from an attentional IC (flanker task) study using event-related brain potentials. This study found that, despite similar task performance, the P300 was smaller among LS individuals relative to HS peers (Bartholow et al., 2003). The P300 is an attention-related brain potential that integrates activity across multiple distributed neural systems (Linden, 2005; Polich, 2007). To our knowledge, there has been only one prior fMRI study of IC performance as a function of alcohol sensitivity phenotype, that is, only one prior study that would be able to identify the specific neural substrates of potential sober-state differences in IC processes. This study found that, at matched IC performance levels, sober LS individuals “over-recruit” anterior (frontal, cingulate, precentral) cortical areas during successful IC compared to their HS peers (Schuckit et al., 2012). Consequently, LS and HS individuals may or may not differ in IC abilities per se, yet may differ in terms of frontocortical circuitry recruitment to successfully implement IC.
Replicating the latter finding and establishing the extent to which IC performance and its neural substrates differ between LS and HS individuals is important for understanding the potential role of domain-general (viz., “core”) self-control abilities in shaping LS and HS individuals’ different alcohol craving and consumption topographies in the natural environment (Kohen et al., 2023; Piasecki et al., 2012; Trela et al., 2016, 2018). Furthermore, potential differences in the neural substrates of IC as a function of alcohol sensitivity phenotype can help inform early-stage indicators for risk of developing AUD, as well as provide guidance on neuroanatomical locations for testing non-invasive brain stimulation (NIBS) to modulate IC in the context of AUD.
A functional magnetic resonance imaging (fMRI) pilot study was conducted to examine potential differences in sober-state domain-general IC performance and its neural underpinnings among young adults who regularly use alcohol and report relatively extreme LS or HS to acute alcohol. Based on (Schuckit et al., 2012), it was hypothesized that IC performance would be similar between groups, but that successful IC performance would be associated with elevated activity, as indexed by the blood oxygen-level dependent (BOLD) response, in the frontocortical nodes of the IC circuits for group LS compared to HS. Given an extensive literature linking LS to alcohol with elevated alcohol craving, consumption, and consequences, alcohol sensitivity phenotype-based effects on IC circuit activation were examined while accounting for potential moderation by between-person differences in alcohol use and problem levels or daily experiences with alcohol craving.
This report presents primary analysis of behavioral task performance and brain activity measures derived from a domain-general IC task completed in the context of a functional neuroimaging pilot study focused on alcohol cue reactivity among young adults with relatively extreme LS or HS to acute alcohol. Detailed description of the sample, including its sociodemographics and alcohol use behavior, as well as in-depth coverage of laboratory visit procedures were published in our recent report on the alcohol cue reactivity results of the study (Cofresí et al., 2024). Below, we provide brief coverage where details are available in our recent report, and in-depth coverage of aspects relevant to the IC task and its analysis.
Participants for the fMRI pilot study were recruited from a 95 pool of individuals who were actively involved in or had recently (past year) completed a NIH-funded longitudinal study (AA025451) characterizing alcohol sensitivity across early emerging adulthood. Eligibility criteria for the longitudinal (“parent”) study have been previously reported (Cofresí et al., 2022a; Cofresí et al., 2022b; Kohen et al., 2023, 2024). Briefly, inclusion criteria at time of enrollment in the parent study included: (1) being age 18–20; (2) English language proficiency; (3) normal or corrected-to-normal vision; and (4) regular alcohol use (at least monthly use across past year, and at least 1 binge drinking episode in past 6 months). Exclusion criteria at time of enrollment in the parent study included: (1) a history of unsuccessful attempts to moderate or quit alcohol use; (2) any current or past psychosis; (4) any history of major chronic illness or neurological disease (e.g., epilepsy); (5) any history of head injury that resulted in loss of consciousness; or any (6) EEG contraindications (e.g., highly sensitive skin, hairstyle preventing scalp access for electrode placements). Individuals were excluded from potential participation in the fMRI pilot study if they reported any of the following at subsequent screening: (1) no longer living in or near Columbia, MO or inability or unwillingness to travel to the lab again for a new study; (2) MRI contraindications (e.g., claustrophobia, non-removable medical electronics or ferrous metal in the body, sensitivity to loud noises); (3) new history of unsuccessful attempts to moderate or quit alcohol use; (4) irregular (less than monthly) or no alcohol use in past year; (5) any current or new history past psychosis; (6) new history of major chronic illness or neurological disease; (5) new history of head injury that resulted in loss of consciousness. From the remaining pool of 76 otherwise eligible individuals, 15 (7 females, 8 males) were excluded from potential participation due to moderate alcohol sensitivity phenotype (see “2.3.1. Alcohol Sensitivity Questionnaire (ASQ)” for details). This left 61 prospective participants, only 36 of whom could possibly be enrolled due to the limited funds available for the fMRI pilot study. Prospective participants were contacted and enrolled strategically to ensure similarly sized HS and LS groups, and similar numbers of females and males within these groups. Of 34 individuals scheduled for participation prior to the end of the enrollment (and funding) period, only 33 visited the lab (1 person failed to present themselves and did not reply to rescheduling attempts). Additional exclusion criteria at the time of the MRI scan were: (1) alcohol intoxication; and for female participants: (2) pregnancy, trying to become pregnant, and/or breastfeeding. One individual who visited the lab to participate in the fMRI pilot study was able to tolerate procedures in the training/mock scanner, but unable to do so in the actual MRI scanner due to unexpected claustrophobia—this person’s data were excluded from all analyses. Ultimately, 32 participants (age 18–23 years at time of MRI scans; 56% female, 94% Non-Hispanic White, 91% Right-Handed), 16 HS and 16 LS, were included in the final analytic sample for this study. For more sociodemographic details, see our recent report also based on this sample (Cofresí et al., 2024).
All procedures were approved by the University of Missouri Institutional Review Board. Laboratory visits lasted ∼2 h and took place at the University of Missouri Cognitive Neuroscience Systems Core facility. Sobriety was verified with a breath alcohol test upon arrival and then informed consent was obtained. To rule out claustrophobia in the MRI environment, participants underwent training in a mock MRI scanner. Urine samples were collected and tested for cotinine (Healgen One Step COT, Healgen Scientific LLC, Houston, TX, USA), and for female participants, pregnancy (Sure-Vue hCG-STAT, Fisher Healthcare, Pittsburgh, PA, USA). All participants then underwent the MRI phase of the study, which included a ∼7-min IC task (see “2.4.3. IC fMRI task” for details) and a ∼30-min cue reactivity task (previously reported: Cofresí et al., 2024). Finally, outside of the scanner, participants completed an 8-day TimeLine Follow-Back calendar (Sobell and Sobell, 1992), were debriefed, and compensated ($50 USD).
Alcohol sensitivity and AUD risk levels were assessed before the lab visit using electronically administered surveys (Harris et al., 2009), whereas recent alcohol craving experiences were assessed at the lab visit between the mock MRI scanner training and the MRI phase of the study. Assessments were conducted using validated, standardized questionnaire instruments (described next). For more details about alcohol and other substance use in this sample, see our recent report (Cofresí et al., 2024).
Each participant’s general sensitivity to the acute effects of alcohol was assessed using the ASQ (Fleming et al., 2016). The ASQ’s 15 items each query whether the respondent has experienced a specific effect from drinking alcohol (e.g., feeling buzzed; passing out), and for all endorsed effects respondents indicate the number of standard drinks they typically require to experience it. For current purposes, responses (i.e., numbers of drinks) across all 15 items were averaged to produce the ASQ total score. These scores were used to classify participants as either LS or HS based on upper and lower terciles of the sex-stratified ASQ score distribution. More details about the sex-stratified thresholds are available in Cofresí et al. (2024). Internal consistency reliability (ICR) for ASQ scores was excellent (α = 0.91–0.95). Group LS comprised females with ASQ total scores > 4.50, and males with ASQ total scores > 5.50. Group HS comprised females with ASQ total scores < 3.00, and males with ASQ total scores < 4.50.
Past year alcohol use and alcohol-related problem levels were assessed using the AUDIT (Bohn et al., 1995; Saunders et al., 1993) subscales for Consumption and Problems (Peng et al., 2012). Use levels were indexed by summing responses to AUDIT items 1–3 into AUDIT Consumption subscale scores. Problem levels were indexed by summing responses to AUDIT items 4–10 into AUDIT Problem subscale scores. ICR for these AUDIT scores was fair-to-good (α = 0.78–0.88). Supplementary Table 1 shows AUDIT subscale scores were elevated in group LS compared to HS, as previously reported (Cofresí et al., 2024).
The frequency and strength of alcohol craving in the past week were assessed using subscales of the Frequency and Strength forms of the ACEQ (May et al., 2014; Statham et al., 2011). The Frequency form focused on the frequency of craving experiences (weak or strong) during the past week. The Strength form focused on the strength of the most intense craving experience within the past week by instructing participants to think about the time in the past week when they “most wanted” to use alcohol and to refer to that experience when responding. Craving frequency levels were indexed by summing responses to 3 items (stem: “how often did you…”; items: “want it?”, “need it?,” “have a strong urge for it?”) on the Frequency form into ACEQ-Frequency subscale scores. Peak craving intensity or strength levels were indexed by summing responses to 3 items (stem: “At that time…”; items: “how much did you want it?,” “how much did you need it?,” “how strong was the urge to have it?”) on the Strength form into ACEQ-Strength subscale scores. ICR for these ACEQ subscale scores was good (α = 0.82–0.83). Supplementary Table 1 shows ACEQ subscale scores were elevated in group LS compared to HS, as previously reported (Cofresí et al., 2024).
MRI scans were acquired using a 3T Siemens Prisma scanner using a 32-channel head coil with padding to restrict head movements. A high-resolution, T1-weighted magnetization prepared-rapid gradient echo (MPRAGE) sequence (TR = 2,300 ms, TE = 2.26 ms, flip angle = 9°, 192 slices, 1-mm isotropic voxels, FOV = 256 mm) was used to acquire anatomical images. Following the acquisition of a B0 field map, functional T2*-weighted images were acquired to measure BOLD responses using a simultaneous multi-slice (SMS) echo-planar imaging (EPI) sequence (acceleration factor = 3, TR = 2,000 ms, TE = 36 ms, flip angle = 70°, 69 slices, 2.2-mm isotropic voxels, FOV = 207 mm).
Functional and structural images underwent standard preprocessing using statistical parametric mapping (SPM) package version 12 (Penny et al., 2007) in Matlab version 2021b (The Mathworks Inc., Natick, MA, USA). Preprocessing included: B0 correction; realignment; slice timing correction; co-registration to structural images; segmentation of structural images; normalization to MNI space using forward deformations with resampling to 1.5-mm3 voxels; and smoothing with a 6-mm3 full-width at half maximum (FWHM) Gaussian filter.
IC was assessed with the “Go/Go/NoGo” task (Chikazoe et al., 2009), which has been validated in SUD populations (Bell and Froeliger, 2021; Brown A. A. et al., 2023; Froeliger et al., 2017; Newman-Norlund et al., 2020; Upton et al., 2023a; Upton et al., 2023b). Using handheld response pads, participants were instructed to press a button in response to common (gray circles: 75.8% of trials) and rare (yellow circles: 12.1% of trials) Go stimuli and to inhibit responding to rare NoGo stimuli (blue circles: 12.1% of trials). The task provided errors of omission and reaction times during Go trials, errors of commission on NoGo trials (blue circles) and controlled for novelty detection via Rare Go trials (yellow circles). Total task time was 7 min (538 trials, 400 ms stimulus, 400 ms blank); completed in 1 run.
IC performance was indexed by adjusted NoGo trial accuracy. As in Bell and Froeliger (2021), Froeliger et al. (2017), Newman-Norlund et al. (2020),and Upton et al. (2023a),b, NoGo trial accuracy was adjusted to control for transient attentional lapses unrelated to IC by scoring NoGo trials with null response as incorrect when the participant did not respond to the Go trial immediately preceding it. IC performance and other task-derived behavioral performance measures [e.g., Go or Rare Go correct response time (RT)] were examined for group differences using two-tailed independent samples Student’s t-tests and Wilcoxon rank sum tests, as appropriate.
Preprocessed functional images were entered into a 1st-level analysis using the general linear model (GLM) to examine the BOLD response during each of 5 event types: NoGocorrect (successful IC), NoGoincorrect (error of commission), RareGocorrect (novel-target detection), RareGoincorrect (novel-target error of omission), and Goincorrect (error of omission). Each event was modeled as an impulse at event onset (event duration = 0 s) and convolved with a canonical hemodynamic response function. If a person’s data were missing for a given event type (e.g., NoGoincorrect because the person always correctly omitted responses to the NoGo stimulus), the specific event type was not included in the 1st-level model of that person’s data. Intra-run motion was removed through rigid body rotation and translation, and 6 motion parameters (x, y, z, roll, pitch, yaw) were included as nuisance covariates. A high-pass filter (128 s; 0.008 Hz) was applied to remove slow signal drift. A whole brain mask was applied. To isolate brain activity during successful IC while controlling for novelty detection, a NoGocorrect–RareGocorrect contrast image (IC contrast) was generated and fed-forward to 2nd level analyses.
A whole-brain 2nd level model was fit to the 1st level model-derived IC contrast images to identify voxel clusters showing significant IC-related activity across the full sample (ignoring alcohol sensitivity phenotype groups). The voxel intensity-based statistical threshold was set to family-wise error (FWE)-corrected p < 0.05 using the random field theory (RFT) method in SPM, which accounts for image smoothing and the statistical dependency of signal from neighboring voxels. Furthermore, the spatial extent-based statistical (cluster-forming) threshold was set to kE ≥ 3 voxels to avoid detecting intensely activated but spatially isolated voxels since these are more likely to be false positives. Person-level IC contrast beta coefficients were then extracted using MarsBaR version 0.45 (Brett et al., 2002). The average IC contrast beta coefficient in a 5 mm radius sphere centered on the peak voxels in each cluster was extracted. Peak voxel locations are reported using the Montreal Neurological Institute (MNI) coordinate system.
The predicted group difference (LS > HS) in IC-related neural activity was tested at all extracted functional ROIs using a multiple linear regression (MLR) approach. This approach enables testing and controlling for moderating effects of sex and previously reported group differences (LS > HS) in alcohol use, problems, and cravings (see Supplementary Table 1). Between-person differences in alcohol use (AUDIT Consumption), problems (AUDIT Problem), and cravings (frequency: ACEQ-F; strength: ACEQ-S) were thus tested as moderators of the predicted group difference (LS > HS) in IC-related neural activity. Testing these moderators in separate models was necessary to mitigate collinearity issues arising from large intercorrelations (see Supplementary Table 2). They were entered into the MLR models as grand mean-centered continuous variables. Sex also was tested as a potential moderator in all models using an effect-coded binary variable. Handedness was included in all MLR models as a nuisance covariate using an effect-coded binary variable. A model selection process was used to find the best-fitting MLR model at each functional ROI, defined as the most parsimonious model that explains a significant amount of between-person variance (per model F-test). This iterative process began with a 3-way interaction model (e.g., Group × AUDIT Problem × Sex) and involved dropping non-significant interaction effects followed by non-significant main effects. The threshold for significance was p < 0.05. If the process arrived at a best-fitting MLR model containing a significant main or interaction effect of Group or one of its potential moderators, then the robustness of that MLR model to exclusion of statistical outliers or highly influential datapoints was examined. Only MLR models for which the model F-test and the relevant beta coefficient t-test remained significant after exclusion of such datapoints were considered sufficiently robust to report. Decomposition of significant effects in reported MLR models involved pairwise comparisons of simple slopes or model-estimated means. Bonferroni correction for multiple comparisons was applied to control the Type 1 inferential error rate.
As shown in Figure 1, IC performance adjusted for attentional lapses was numerically lower for group LS compared to HS. However, as shown in Table 1, no significant group differences were detected on IC performance or other aspects of task behavior (e.g., Go or Rare Go correct RT, premature response counts).
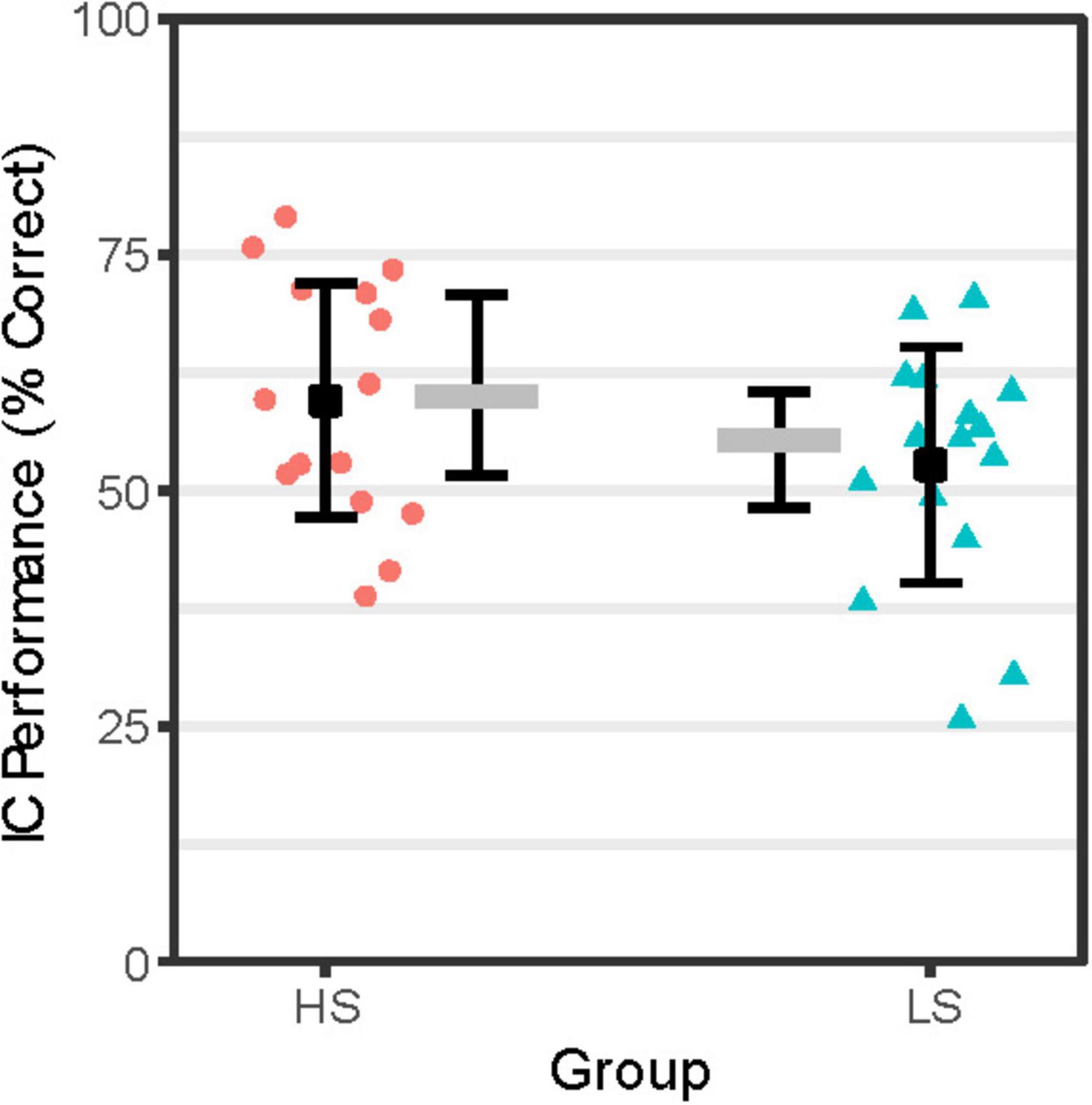
Figure 1. IC Performance by Group. IC performance (% correct = proportion correct × 100) reflects accuracy on NoGo trials adjusted for attentional lapses by scoring NoGo trial null responses coded as incorrect if response to immediately prior FreqGo trial was omitted. Person-level IC performance scores for the High Alcohol Sensitivity (n = 16; HS) and Low Alcohol Sensitivity (n = 16; LS) groups are shown as red-filled circles and teal-filled triangles, respectively. Group-level mean IC performance scores are shown as black-filled squares flanked by error bars representing ± 1 SE. Group-level median IC performance scores are shown as gray-filled rectangles flanked by error bars indicating the interquartile range.
As shown in Table 2, the whole-brain 2nd level model of successful IC-related activity detected 8 clusters spanning cortex, striatum, and cerebellum. Cortical clusters were located primarily in anterior cingulate. Subcortical clusters were located primarily in rostral dorsal striatum (caudate and putamen). Among these functional ROIs, only two located in the dorsal/supragenual anterior cingulate cortex (dACC) exhibited robust effects of alcohol sensitivity phenotype (Group). These Group effects were moderated by biological sex and between-person differences in alcohol craving frequency/strength outside the lab. We present and decompose these Group effects below.
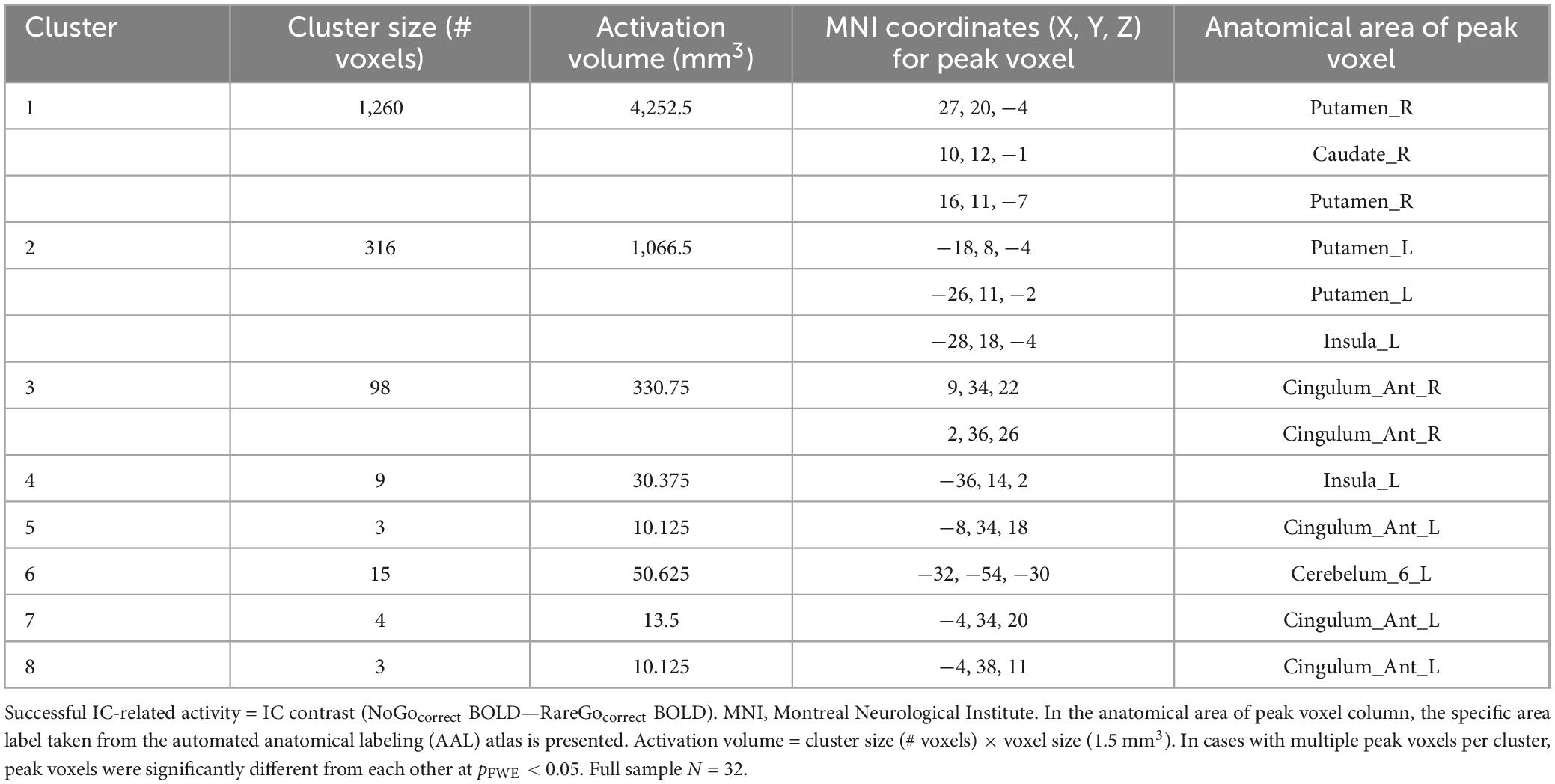
Table 2. Regions of successful IC-related activity identified using whole-brain 2nd level model across full sample.
IC-related activity at a site in the L-dACC (Figure 2A) was best explained by a MLR model of Group × ACEQ-F interaction, model F(4, 27) = 3.23, p = 0.027, adj. R2 = 0.22, improvements in model fit relative to a main effects only model: Δ model F = 10.76, Δ residual df = 1, p = 0.003, Δ adj. R2 = 0.27. As shown in Figure 2B, follow-up tests revealed that this L-dACC activity was associated with the frequency of daily life alcohol craving experiences, albeit in different directions depending on alcohol sensitivity. Specifically, for group LS, L-dACC activity during IC increased as the frequency of alcohol craving experiences increased; this tendency was statistically robust (simple slope b ± SE = 0.075 ± 0.026, 95% CI: [0.022, 0.128], p = 0.007, Bonferroni-corrected p = 0.014). In contrast, for group HS, L-dACC activity during IC decreased as the frequency of alcohol craving experiences increased, but this tendency was not statistically robust (simple slope b ± SE = −0.063 ± 0.033, 95% CI: [−0.131, 0.005], p = 0.068, Bonferroni-corrected p = 0.136).
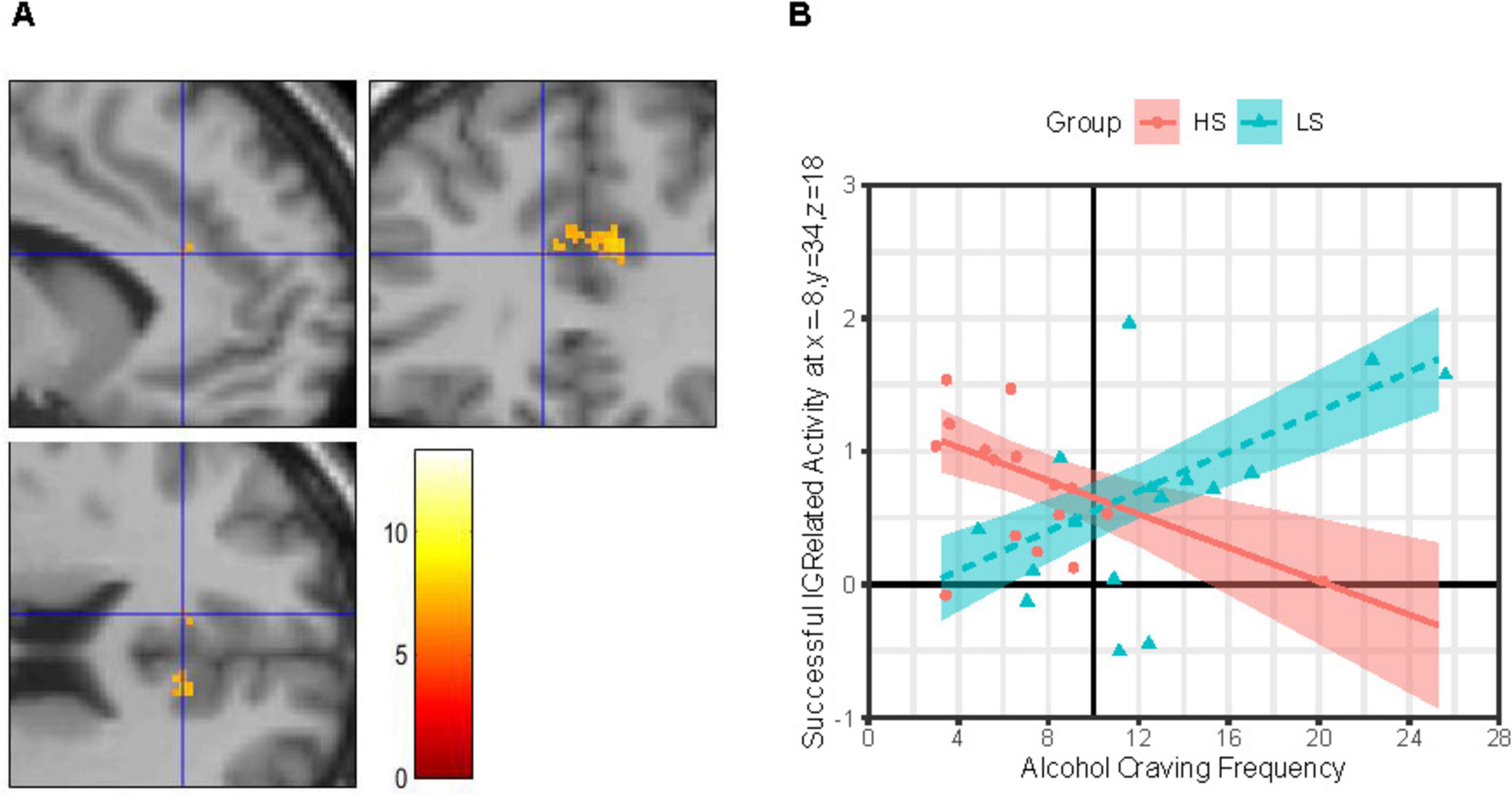
Figure 2. Group × alcohol craving frequency effects on IC-related activity in left dorsal anterior cingulate. (Panel A) Images (scale: 80 mm × 80 mm; clockwise: sagittal, coronal, axial views) showing clusters of significant IC contrast (NoGocorrect BOLD—RareGocorrect BOLD) in the dorsal anterior cingulate cortex (dACC) across the full sample (N = 32). Blue crosshair in each image shows the approximate location of an IC-related activity peak at voxel x = –8, y = 34, z = 18 in the left dACC. Color bar shows T-scores. (Panel B) Successful IC-related activity = IC contrast. Alcohol Craving Frequency = ACEQ-F subscale scores. Person-level average IC contrast beta coefficients across a 5-mm radius sphere centered on voxel x = –8, y = 34, z = 18 are shown for the High Alcohol Sensitivity (n = 16; HS) and Low Alcohol Sensitivity (n = 16; LS) groups are shown as red-filled circles and teal-filled triangles, respectively. Multiple linear regression (MLR) model predicted IC activity values (means) across ACEQ-F scores are shown for group HS and LS as a red solid line and dashed teal line, respectively, with color-matched areas around them showing ± 1 SE. Results were robust to removal of statistical outliers. ACEQ-F score was entered into the MLR model as a grand-mean centered predictor. The grand-mean ACEQ-F score is shown as a solid black vertical line intersecting the x-axis.
IC-related activity at a site in the R-dACC (Figure 3A) was best explained by a MLR model including both a Group × ACEQ-S and a Group × Sex interaction, model F(6, 25) = 3.71, p = 0.009, adj. R2 = 0.34, improvements in model fit relative to a main effects only model: Δ model F = 8.51, Δ residual df = 2, p = 0.001, Δ adj. R2 = 0.36. Both the Group × Sex interaction, b ± SE = 0.332 ± 0.143, t(25) = 2.32, p = 0.029, and Group × ACEQ-S interaction, b ± SE = 0.098 ± 0.025, t(25) = 3.91, p = 0.001, were significant. Follow-up on each is presented next.
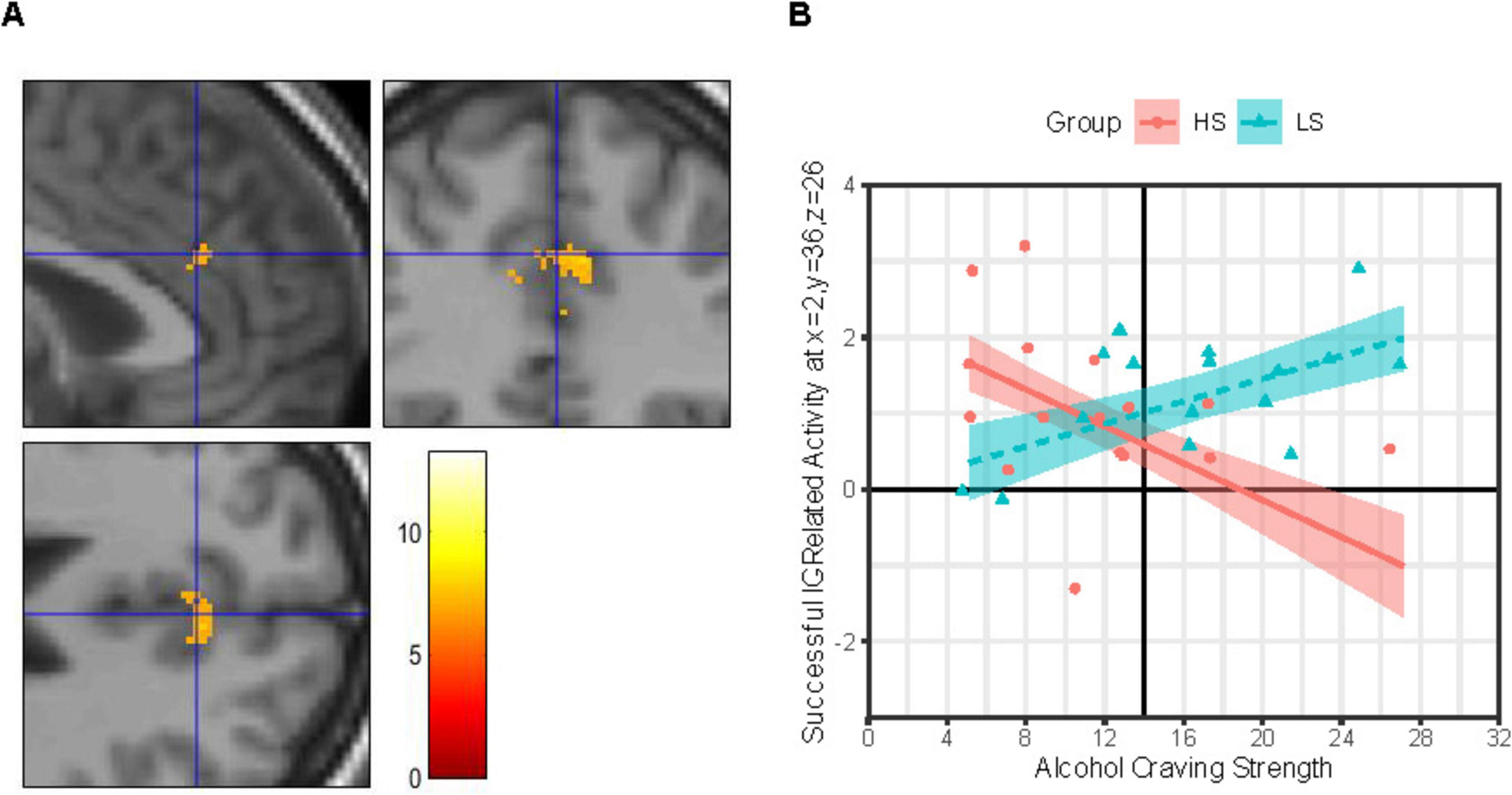
Figure 3. Group × alcohol craving strength effects on IC-related activity in right dorsal anterior cingulate. (Panel A) Images (scale: 80 mm × 80 mm; clockwise: sagittal, coronal, axial views) showing clusters of significant IC contrast (NoGocorrect BOLD—RareGocorrect BOLD) in the dorsal anterior cingulate cortex (dACC) across the full sample (N = 32). Blue crosshair in each image shows the approximate location of an IC-related activity peak at voxel x = 2, y = 36, z = 26 in right dACC. Color bar shows T-scores. (Panel B) Successful IC-related activity = IC contrast. Alcohol Craving Strength = ACEQ-S subscale scores. Person-level average IC contrast beta coefficients across a 5-mm radius sphere centered on voxel x = 2, y = 36, z = 26 are shown for the High Alcohol Sensitivity (n = 16; HS) and Low Alcohol Sensitivity (n = 16; LS) groups are shown as red-filled circles and teal-filled triangles, respectively. Multiple linear regression (MLR) model predicted IC activity values (means) across ACEQ-S scores are shown for group HS and LS as a red solid line and dashed teal line, respectively, with color-matched areas around them showing ± 1 SE. Results were robust to removal of statistical outliers. ACEQ-S score was entered into the MLR model as a grand-mean centered predictor. The grand-mean ACEQ-S score is shown as a solid black vertical line intersecting the x-axis.
As shown in Figure 3B, IC-related activity at this R-dACC site was associated with the strength of daily life alcohol craving experiences, albeit in different directions depending on alcohol sensitivity. Specifically, for group LS, this R-dACC activity increased as the strength of alcohol craving experiences increased, but this tendency was not statistically robust (simple slope b ± SE = 0.074 ± 0.032, 95% CI: [0.009, 0.140], p = 0.028, Bonferroni-corrected p = 0.056). In contrast, for group HS, R-dACC activity decreased as the strength of alcohol craving experiences increased, and this tendency was statistically robust (simple slope b ± SE = −0.121 ± 0.039, 95% CI: [−0.201, −0.041], p = 0.004, Bonferroni-corrected p = 0.009).
There was significantly less IC-related activity at this R-dACC site for group HS males compared to group HS females (MD ± SED = 1.40 ± 0.45, t[25] = 3.12, p = 0.004, Bonferroni-corrected p = 0.018). Additionally, this R-dACC activity was numerically lower for males in group HS than LS, but the difference was not statistically robust (MD ± SED = 1.08 ± 0.46, t[25] = 2.33, p = 0.028, Bonferroni-corrected p = 0.112). In contrast, no sex difference in R-dACC activity level was detected in group LS (MD ± SED = 0.68 ± 0.39, t[25] = 0.17, p = 0.863, Bonferroni-corrected p = 1), and R-dACC activity levels were similar for females in group HS compared to group LS (MD ± SED = 0.24 ± 0.37, t[25] = 0.655, p = 0.519, Bonferroni-corrected p = 1).
The present study found that groups LS and HS were similarly successful at IC task performance, and that at most “hotspots” of IC-related activity detected across the whole brain in the full sample, which implicated the indirect rather than hyperdirect IC pathway, groups LS and HS exhibited similar levels of IC-related activity. There were only two exceptions to the latter finding, both dependent upon accounting for lived experiences of alcohol craving, which can contribute to AUD symptomatology. First, the frequency of alcohol cravings experienced outside the lab was associated with IC-related activity at a site in the left anterior cingulate; this association was positive for group LS but negative for group HS. Second, and similarly, as the strength of alcohol cravings experienced outside the lab increased, IC-related activity at a site in the right anterior cingulate tended to increase for group LS but to decrease for group HS. The similarity of these association patterns in the dACC across both hemispheres, and across frequency vs. strength dimensions of alcohol craving, suggests a more general link between alcohol craving and anterior cingulate activity, and that this more general link may differ by alcohol sensitivity phenotype. There also appeared to be a sex difference (females > males) in the level of IC-related activity in the right dACC for group HS but not LS. Together, the present findings indicate that, despite a similar capacity to exert control over prepotent responses in the sober state, there may be covert neurofunctional differences in the implementation of IC as a function of alcohol sensitivity phenotype and potential nuances as a function of biological sex.
The present study replicates and extends the one prior fMRI study (to our knowledge) that reported on domain-general IC performance and its neural substrates among LS and HS young adults (Schuckit et al., 2012). Findings from that study indicated similar IC task performance between LS and HS groups but higher activity in left superior frontal gyrus and anterior cingulate during successful IC in group LS compared to group HS. Despite different methods across studies (e.g., recruitment and screening, alcohol sensitivity assessment, IC task, MRI scanner and head coil, and fMRI acquisition parameters), the present study’s findings converge with those reported by Schuckit et al. (2012) in suggesting differential recruitment of, or activation thresholds for, the frontocortical neural substrates of IC in the sober state as a function of alcohol sensitivity phenotype. Although the extent of overlap is unclear, both studies detected this difference at a site in the left anterior cingulate. Furthermore, the present study detected a potential group difference at a site in the right anterior cingulate. This convergence of potential differences being localized bilaterally to anterior cingulate is consistent with the anterior cingulate’s proposed role in IC, which is based on its ability to detect conflict and broadcast cognitive control demand (Botvinick et al., 2004; Shenhav et al., 2016).
More broadly, our convergent fMRI findings with respect to the IC facet of EF reinforce Schuckit et al.’ proposal (2012) that, to perform EF tasks while sober at the same level as HS peers, LS individuals may “over-recruit” the frontocortical nodes of the relevant neural circuits. Additional support for this proposal comes from Schuckit and colleagues’ prior fMRI studies of a different facet of EF: working memory. These studies uncovered greater working memory load-related activation of frontocortical nodes such as anterior cingulate, dorsolateral prefrontal cortex, and inferior frontal gyrus (ventrolateral prefrontal cortex) in the sober state among LS youth compared to HS peers despite similar task performance (Paulus et al., 2006; Tapert et al., 2004; Trim et al., 2010). These sober-state differences between LS and HS individuals in task-related frontocortical activation may even extend from the cognitive to affective task domain (Paulus et al., 2012).
The present study’s findings have implications for prevention and treatment of AUD. Specifically, the findings suggest that emerging adults reporting LS to alcohol may expend more neural effort or resources to exert control over prepotent responses (in general) than do their peers reporting HS. Prior studies suggest that, compared to their HS peers, LS individuals exhibit amplified approach responses to alcohol cues (Cofresí et al., 2022a; Fleming and Bartholow, 2014) and increased internal conflict or difficulty with IC in alcohol cue-saturated contexts (Bailey and Bartholow, 2016; Cofresí et al., 2022a; Fleming and Bartholow, 2014). Thus, LS persons may be at a disadvantage in the sustainability of their intentions to abstain or moderate alcohol use via deliberate acts of self-control in-the-moment. Supporting abstinence or moderation goals in clients or patients reporting LS to alcohol could involve fostering skills or strategies that minimize the need for deliberate acts of control, or training countervailing prepotent responses to personally relevant alcohol cues. Alternatively, it could involve repeated non-invasive stimulation of the neural circuits supporting self-control, which has been shown to have short- and long-term benefits in AUD and other SUDs (Mehta et al., 2024; Song et al., 2019; Upton et al., 2023a; Upton et al., 2023b). With respect to the latter, the present study suggests that LS individuals may need higher doses or longer courses of treatment than HS peers. Additionally, the present study suggests that LS individuals may derive more benefit from stimulation of the anterior cingulate than from stimulation of other frontocortical sites implicated in self-control that are more common treatment targets, such as the dorsolateral prefrontal cortex (Vanderhasselt et al., 2020; Wietschorke et al., 2016). Alcohol sensitivity phenotype differences also could help explain some of the inconsistency in treatment response thought to underlie inconsistent clinical trial results for non-invasive stimulation of the more common treatment targets (den Uyl et al., 2017, 2018). Non-invasive stimulation targeting the anterior cingulate may bolster LS individuals’ ability to inhibit attentional, approach, or craving responses to alcohol cues, as it has been shown to do generally in certain clinical trials (De Ridder et al., 2011; Kearney-Ramos et al., 2018), potentially by decreasing functional connectivity between anterior cingulate and dorsal striatum (Harel et al., 2022), both of which were found to be activated during successful IC in the present study.
The present study’s findings need to be considered in light of the study’s limitations. First and foremost, this study was designed as a preliminary exploration, and therefore its sample size was small. However, individuals with relatively extreme LS and HS phenotypes were recruited to capitalize on phenotypic differences in neurocognitive processes of interest, and yet, the resulting groups also differed on aspects of alcohol craving, consumption, and consequences. The analytic approach provided an efficient means by which to test alcohol sensitivity phenotype-based differences across sites in the brain exhibiting significant IC-related activation while statistically accounting for phenotype-linked covariates indexing AUD risk, such as craving, consumption, and consequences. Nonetheless, future studies seeking to isolate alcohol sensitivity phenotype effects from chronic alcohol use effects (Karoly et al., 2024; Pérez-García et al., 2022) would benefit from stratifying the study sample for alcohol sensitivity and alcohol use levels. Larger studies are necessary to obtain uniform representation across the alcohol use and sensitivity phenotype spectra. As a proposed AUD risk-conferring endophenotype, it is also important to determine which neurocognitive or neurofunctional vulnerabilities associated with LS to alcohol are causes or consequences of alcohol use. Cross-sectional observations like the present study cannot speak to the cause/consequence conundrum. Longitudinal observations are necessary to parse cause from consequence. Furthermore, future studies should consider the extent to which LS to alcohol is associated with specific neuroanatomical variations (e.g., gray matter density in frontal cortices, integrity of white matter tracts linking cortical regions or subcortical nuclei) and their overlap with neuroanatomical variations associated with problematic alcohol use (for review, see Honarvar et al., 2023).
Future studies also should assess other proposed and often related AUD risk-conferring phenotypes (e.g., positive family history, trait disinhibition/impulsivity) and disentangle their contributions from those of alcohol sensitivity phenotype. This is especially important with respect to positive family history (FHP) of AUD because: (i) LS to alcohol was first proposed as a sub-mechanism of FHP-based risk for AUD (Eng et al., 2005; Schuckit, 1980), and (ii) LS to alcohol is a highly heritable source of risk for AUD (Heath et al., 1999; Heath and Martin, 1991; Viken et al., 2003). Prospective studies of AUD onset in large samples indicate that FHP and LS operate as distinct contributors of risk for AUD (Schuckit et al., 2006; Schuckit and Smith, 2000, 2001), which suggests distinct genetic bases. Nonetheless, to the extent that FHP-based risk for AUD and LS-based risk for AUD share common genetic bases (Schuckit, 2018), the present study’s findings also converge with prior fMRI studies of IC as a function of family history of AUD. These prior studies found elevated IC-related activity in anterior cingulate as well as middle and inferior frontal gyri among FHP individuals compared to peers with no family history of AUD [(Heitzeg et al., 2010; Jamadar et al., 2012; Kareken et al., 2013), but also see (Schweinsburg et al., 2004)]. Given that these and other important constructs are being assessed alongside structural and functional neuroimaging repeatedly across development for a nationally representative and extremely large sample [e.g., the NIH Adolescent Brain Cognitive Development (ABCD) Study (Brown S. A. et al., 2023)], a more comprehensive picture of the inter-relatedness or uniqueness of endophenotypic risk factors for AUD onset may soon emerge. These large-scale neuroimaging studies also stand to illuminate the inter-relatedness or uniqueness of AUD risk conferred by neurofunctional (Morales et al., 2024) vs. neuroanatomical characteristics across development (Honarvar et al., 2023; Jones et al., 2023; Miller et al., 2024).
Finally, the present study involved healthy, predominantly White, highly educated participants (university students, presumably from higher socioeconomic status backgrounds) in either late adolescence or early emerging adulthood, which limits generalizability of its findings regarding IC performance and its neural substrates. This limitation also applies to prior fMRI studies of alcohol sensitivity phenotype-based group differences in mental functions (Paulus et al., 2006, 2012; Schuckit et al., 2012; Tapert et al., 2004; Trim et al., 2010). Data from an extremely large and nationally representative sample like the ABCD Study can be leveraged to examine measurement invariance across ethnic, racial, and socioeconomic status groups for alcohol sensitivity and AUD risk as well as for IC performance and its neural substrates.
Preliminary findings from the present fMRI pilot study suggest that the ability to implement IC over prepotent responses to cues (broadly) among individuals with LS to alcohol may depend on compensatory over-activation of the anterior cingulate cortex, and that this compensatory activation tracks dimensions of their alcohol craving experiences in daily life. Prior fMRI studies of IC as a function of LS to alcohol or positive family history of AUD using different samples, assessments, and tasks also point to compensatory over-activation of anterior cingulate cortex or functionally related frontocortical regions. Longitudinal fMRI studies with larger and more demographically diverse samples are needed to examine the extent to which covert functional differences in the neural substrates of IC can account for differential AUD onset or progression risk as a function of alcohol sensitivity phenotype.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the University of Missouri Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RC: Writing – original draft, Writing – review and editing. SU: Writing – review and editing. DT: Writing – review and editing. AB: Writing – review and editing. TP: Writing – review and editing. BB: Writing – review and editing. BF: Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. Funding for the project was provided by an internal seed grant from the University of Missouri Cognitive Neuroscience Systems Core Facility Board of Directors. BB and TP’s contributions were supported by the NIH grant that funded the parent study (AA025451). SU’s contributions were supported by an NIH T32 traineeship (AA013526). RC’s contributions were supported by NIH Diversity Supplement (AA025451-[04/05]S1) and by NIH K99/R00 (AA029169).
Results from preliminary analyses of these data were presented in abstracts for the 2022 scientific meeting of the Research Society on Alcohol and the 2023 scientific meeting of the Society for Neuroscience. Ms. Mikayla Rodgers is thanked for her assistance with project coordination.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1557661/full#supplementary-material
Bailey, K., and Bartholow, B. D. (2016). Alcohol words elicit reactive cognitive control in low-sensitivity drinkers. Psychophysiology 53, 1751–1759. doi: 10.1111/psyp.12741
Bartholow, B. D., Lust, S. A., and Tragesser, S. L. (2010). Specificity of P3 Event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psychol. Addict. Behav. 24, 220–228. doi: 10.1037/a0017705
Bartholow, B. D., Pearson, M. A., Sher, K. J., Wieman, L. C., Fabiani, M., and Gratton, G. (2003). Effects of alcohol consumption and alcohol susceptibility on cognition: A psychophysiological examination. Biol. Psychol. 64, 167–190. doi: 10.1016/S0301-0511(03)00108-X
Bell, S., and Froeliger, B. (2021). Associations between smoking abstinence, inhibitory control, and smoking behavior: An fMRI study. Front. Psychiatry 12:592443. doi: 10.3389/fpsyt.2021.592443
Bohn, M. J., Babor, T. F., and Kranzler, H. R. (1995). The alcohol use disorders identification test (AUDIT): Validation of a screening instrument for use in medical settings. J. Stud. Alcohol. 56, 423–432. doi: 10.3922/j.psns.2009.1.12
Botvinick, M. M., Cohen, J. D., and Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 8, 539–546. doi: 10.1016/j.tics.2004.10.003
Brett, M., Anton, J.-L., Valabregue, R., and Poline, J.-B. (2002). “Region of interest analysis using an SPM toolbox,” in 8th International Conference on Functional Mapping of the Human Brain. Academic Press.
Brion, M., D’Hondt, F., Pitel, A. L., Lecomte, B., Ferauge, M., de Timary, P., et al. (2017). Executive functions in alcohol-dependence: A theoretically grounded and integrative exploration. Drug Alcohol Dependence 177, 39–47. doi: 10.1016/j.drugalcdep.2017.03.018
Brown, A. A., Upton, S., Craig, S., and Froeliger, B. (2023). Associations between right inferior frontal gyrus morphometry and inhibitory control in individuals with nicotine dependence. Drug Alcohol Dependence 244:109766. doi: 10.1016/j.drugalcdep.2023.109766
Brown, S. A., Jernigan, T. L., and Dowling, G. J. (2023). The adolescent brain cognitive development study. Health Psychol. 42, 840–841. doi: 10.1037/hea0001353
Bundt, C., and Huster, R. J. (2024). Corticospinal excitability reductions during action preparation and action stopping in humans: Different sides of the same inhibitory coin? Neuropsychologia 195:108799. doi: 10.1016/j.neuropsychologia.2024.108799
Chikazoe, J., Jimura, K., Asari, T., Yamashita, K. I., Morimoto, H., Hirose, S., et al. (2009). Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex 19, 146–152. doi: 10.1093/cercor/bhn065
Cofresí, R. U., Kohen, C. B., Motschman, C. A., Wiers, R. W., Piasecki, T. M., and Bartholow, B. D. (2022a). Behavioral response bias and event-related brain potentials implicate elevated incentive salience attribution to alcohol cues in emerging adults with lower sensitivity to alcohol. Addiction 117, 892–904. doi: 10.1111/add.15728
Cofresí, R. U., Piasecki, T. M., and Bartholow, B. D. (2022b). Acute sensitization of the P3 event-related potential response to beverage images and the risk for alcohol use disorder. Addict. Neurosci. 4:100041. doi: 10.1016/j.addicn.2022.100041
Cofresí, R. U., Upton, S., Brown, A. A., Piasecki, T. M., Bartholow, B. D., and Froeliger, B. (2024). Mesocorticolimbic system reactivity to alcohol use-related visual cues as a function of alcohol sensitivity phenotype: A pilot fMRI study. Addict. Neurosci. 11:100156. doi: 10.1016/j.addicn.2024.100156
Connor, J. (2017). Alcohol consumption as a cause of cancer. Addiction 112, 222–228. doi: 10.1111/add.13477
Criaud, M., and Boulinguez, P. (2013). Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 37, 11–23. doi: 10.1016/j.neubiorev.2012.11.003
Czapla, M., Simon, J. J., Richter, B., Kluge, M., Friederich, H.-C. C., Herpertz, S. S. C., et al. (2016). The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: Implications for psychotherapeutic treatment. Addict. Biol. 21, 873–884. doi: 10.1111/adb.12229
Davis, C. N., Piasecki, T. M., Bartholow, B. D., and Slutske, W. S. (2021). Effects of alcohol sensitivity on alcohol- - Induced blackouts and passing out: An examination of the alcohol sensitivity questionnaire among underage drinkers. Alcohol. Clin. Exp. Res. 45, 1149–1160. doi: 10.1111/acer.14607
De Ridder, D., Vanneste, S., Kovacs, S., Sunaert, S., and Dom, G. (2011). Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: An fMRI and LORETA EEG study. Neurosci. Lett. 496, 5–10. doi: 10.1016/j.neulet.2011.03.074
den Uyl, T. E., Gladwin, T. E., Lindenmeyer, J., and Wiers, R. W. (2018). A clinical trial with combined transcranial direct current stimulation and attentional bias modification in alcohol-dependent patients. Alcohol. Clin. Exp. Res. 42, 1961–1969. doi: 10.1111/acer.13841
den Uyl, T. E., Gladwin, T. E., Rinck, M., Lindenmeyer, J., and Wiers, R. W. (2017). A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict. Biol. 22, 1632–1640. doi: 10.1111/adb.12463
Duke, A. A., Smith, K. M. Z., Oberleitner, L. M. S., Westphal, A., and McKee, S. A. (2017). Alcohol, drugs, and violence: A meta-meta-analysis. Psychol. Violence 8, 238–249. doi: 10.1037/vio0000106
Eng, M. Y., Schuckit, M. A., and Smith, T. L. (2005). The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Dependence 79, 83–93. doi: 10.1016/j.drugalcdep.2005.01.002
Fernández-Serrano, M. J., Pérez-García, M., and Verdejo-García, A. (2011). What are the specific vs. Generalized effects of drugs of abuse on neuropsychological performance? Neurosci. Biobehav. Rev. 35, 377–406. doi: 10.1016/j.neubiorev.2010.04.008
Fleming, K. A., and Bartholow, B. D. (2014). Alcohol cues, approach bias, and inhibitory control: Applying a dual process model of addiction to alcohol sensitivity. Psychol. Addict. Behav. 28, 85–96. doi: 10.1037/a0031565
Fleming, K. A., Bartholow, B. D., Hilgard, J., McCarthy, D. M., O’Neill, S. E., Steinley, D., et al. (2016). The alcohol sensitivity questionnaire: Evidence for construct validity. Alcohol. Clin. Exp. Res. 40, 880–888. doi: 10.1111/acer.13015
Friedman, N. P., and Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. General 133, 101–135. doi: 10.1037/0096-3445.133.1.101
Friedman, N. P., and Miyake, A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex 86, 186–204. doi: 10.1016/j.cortex.2016.04.023
Froeliger, B., McConnell, P. A., Bell, S., Sweitzer, M., Kozink, R. V., Eichberg, C., et al. (2017). Association between baseline corticothalamic-mediated inhibitory control and smoking relapse vulnerability. JAMA Psychiatry 74, 379–386. doi: 10.1001/jamapsychiatry.2017.0017
Gavazzi, G., Giovannelli, F., Currò, T., Mascalchi, M., and Viggiano, M. P. (2021). Contiguity of proactive and reactive inhibitory brain areas: A cognitive model based on ALE meta-analyses. Brain Imaging Behav. 15, 2199–2214. doi: 10.1007/s11682-020-00369-5
Gavazzi, G., Giovannelli, F., Noferini, C., Cincotta, M., Cavaliere, C., Salvatore, M., et al. (2023). Subregional prefrontal cortex recruitment as a function of inhibitory demand: An fMRI metanalysis. Neurosci. Biobehav. Rev. 152:105285. doi: 10.1016/j.neubiorev.2023.105285
Goldstein, R. Z., and Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. doi: 10.1176/appi.ajp.159.10.1642
Grieder, M., Soravia, L. M., Tschuemperlin, R. M., Batschelet, H. M., Federspiel, A., Schwab, S., et al. (2022). Right inferior frontal activation during alcohol-specific inhibition increases with craving and predicts drinking outcome in alcohol use disorder. Front. Psychiatry 13:909992. doi: 10.3389/fpsyt.2022.909992
Griswold, M. G., Fullman, N., Hawley, C., Arian, N., Zimsen, S. R. M., Tymeson, H. D., et al. (2018). Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 392, 1015–1035. doi: 10.1016/S0140-6736(18)31310-2
Gunawan, T., Luk, J. W., Schwandt, M. L., Kwako, L. E., Vinson, T., Horneffer, Y., et al. (2024). Factors underlying the neurofunctional domains of the Addictions Neuroclinical Assessment assessed by a standardized neurocognitive battery. Transl. Psychiatry 14:271. doi: 10.1038/s41398-024-02987-9
Hannah, R., and Aron, A. R. (2021). Towards real-world generalizability of a circuit for action-stopping. Nat. Rev. Neurosci. 22, 538–552. doi: 10.1038/s41583-021-00485-1
Harel, M., Perini, I., Kämpe, R., Alyagon, U., Shalev, H., Besser, I., et al. (2022). Repetitive transcranial magnetic stimulation in alcohol dependence: A randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol. Psychiatry 91, 1061–1069. doi: 10.1016/j.biopsych.2021.11.020
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G. (2009). Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Informatics 42, 377–381. doi: 10.1016/j.jbi.2008.08.010
Heath, A. C., and Martin, N. G. (1991). The inheritance of alcohol sensitivity and of patterns of alcohol use. Alcohol Alcoholism 1, 141–145.
Heath, A. C., Madden, P. A. F., Bucholz, K. K., Dinwiddie, S. H., Slutske, W. S., Bierut, L. J., et al. (1999). Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol. Med. 29, 1069–1081. doi: 10.1017/S0033291799008909
Heitzeg, M. M., Cope, L. M., Martz, M. E., and Hardee, J. E. (2015). Neuroimaging risk markers for substance abuse: Recent findings on inhibitory control and reward system functioning. Curr. Addict. Rep. 2, 91–103. doi: 10.1007/s40429-015-0048-9
Heitzeg, M. M., Nigg, J. T., Yau, W.-Y. W., Zucker, R. A., and Zubieta, J.-K. (2010). Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry 68, 287–295. doi: 10.1016/j.biopsych.2010.02.020
Herman, A. M., Critchley, H. D., and Duka, T. (2019). Binge drinking is associated with attenuated frontal and parietal activation during successful response inhibition in fearful context. Eur. J. Neurosci. 50, 2297–2310. doi: 10.1111/ejn.14108
Hildebrandt, M. K., Dieterich, R., and Endrass, T. (2021). Neural correlates of inhibitory control in relation to the degree of substance use and substance-related problems – A systematic review and perspective. Neurosci. Biobehav. Rev. 128, 1–11. doi: 10.1016/j.neubiorev.2021.06.011
Honarvar, F., Arfaie, S., Edalati, H., Ghasroddashti, A., Solgi, A., Mashayekhi, M. S., et al. (2023). Neuroanatomical predictors of problematic alcohol consumption in adolescents: A systematic review of longitudinal studies. Alcohol Alcohol. 58, 455–471. doi: 10.1093/alcalc/agad049
Hone, L. S. E., Bartholow, B. D., Piasecki, T. M., and Sher, K. J. (2017). Women’s alcohol sensitivity predicts alcohol-related regretted sex. Alcohol. Clin. Exp. Res. 41, 1630–1636. doi: 10.1111/acer.13447
Hu, S., Zhang, S., Chao, H. H., Krystal, J. H., and Li, C. S. R. (2016). Association of drinking problems and duration of alcohol use to inhibitory control in nondependent young adult social drinkers. Alcohol. Clin. Exp. Res. 40, 319–328. doi: 10.1111/acer.12964
Isherwood, S. J. S., Keuken, M. C., Bazin, P. L., and Forstmann, B. U. (2021). Cortical and subcortical contributions to interference resolution and inhibition – An fMRI ALE meta-analysis. Neurosci. Biobehav. Rev. 129, 245–260. doi: 10.1016/j.neubiorev.2021.07.021
Jahanshahi, M., Obeso, I., Rothwell, J. C., and Obeso, J. A. (2015). A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 16, 719–732. doi: 10.1038/nrn4038
Jamadar, S., DeVito, E. E., Jiantonio, R. E., Meda, S. A., Stevens, M. C., Potenza, M. N., et al. (2012). Memantine, an NMDA receptor antagonist, differentially influences Go/No-Go performance and fMRI activity in individuals with and without a family history of alcoholism. Psychopharmacology 222, 129–140. doi: 10.1007/s00213-011-2628-2
Jones, S. A., Morales, A. M., Harman, G., Dominguez-Savage, K. A., Gilbert, S., Baker, F. C., et al. (2023). Associations between alcohol use and sex-specific maturation of subcortical gray matter morphometry from adolescence to adulthood: Replication across two longitudinal samples. Dev. Cogn. Neurosci. 63:101294. doi: 10.1016/j.dcn.2023.101294
Kareken, D. A., Dzemidzic, M., Wetherill, L., Eiler, W., Oberlin, B. G., Harezlak, J., et al. (2013). Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology 228, 335–345. doi: 10.1007/s00213-013-3038-4
Karoly, H. C., Kirk-Provencher, K. T., Schacht, J. P., and Gowin, J. L. (2024). Alcohol and brain structure across the lifespan: A systematic review of large-scale neuroimaging studies. Addict. Biol. 29:e13439. doi: 10.1111/adb.13439
Kearney-Ramos, T. E., Dowdle, L. T., Lench, D. H., Mithoefer, O. J., Devries, W. H., George, M. S., et al. (2018). Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 599–609. doi: 10.1016/j.bpsc.2018.03.016
King, A. C., Vena, A., Hasin, D. S., DeWit, H., O’Connor, S. J., and Cao, D. (2021). Subjective responses to alcohol in the development and maintenance of alcohol use disorder. Am. J. Psychiatry 178, 560–571. doi: 10.1176/appi.ajp.2020.20030247
King, A. C., Vena, A., Howe, M. M., Feather, A., and Cao, D. (2022). Haven’t lost the positive feeling: A dose-response, oral alcohol challenge study in drinkers with alcohol use disorder. Neuropsychopharmacology 47, 1892–1900. doi: 10.1038/s41386-022-01340-2
Kohen, C. B., Cofresí, R. U., Bartholow, B. D., and Piasecki, T. M. (2023). Alcohol craving in the natural environment: Moderating roles of cue exposure, drinking, and alcohol sensitivity. Exp. Clin. Psychopharmacol. 31, 57–71. doi: 10.1037/pha0000540
Kohen, C. B., Cofresí, R. U., Piasecki, T. M., and Bartholow, B. D. (2024). Predictive utility of the P3 event-related potential (ERP) response to alcohol cues for ecologically assessed alcohol craving and use. Addict. Biol. 29:e13368. doi: 10.1111/adb.13368
Le, T. M., Potvin, S., Zhornitsky, S., and Li, C. S. R. (2021). Distinct patterns of prefrontal cortical disengagement during inhibitory control in addiction: A meta-analysis based on population characteristics. Neurosci. Biobehav. Rev. 127, 255–269. doi: 10.1016/j.neubiorev.2021.04.028
Linden, D. E. J. (2005). The P300: Where in the brain is it produced and what does it tell us? Neuroscientist 11, 563–576. doi: 10.1177/1073858405280524
López-Caneda, E., Rodríguez Holguín, S., Cadaveira, F., Corral, M., and Doallo, S. (2014). Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: A review. Alcohol Alcohol. 49, 173–181. doi: 10.1093/alcalc/agt168
Luijten, M., Machielsen, M. W. J., Veltman, D. J., Hester, R., de Haan, L., and Franken, I. H. A. (2014). Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J. Psychiatry Neurosci. 39, 149–169. doi: 10.1503/jpn.130052
May, J., Andrade, J., Kavanagh, D. J., Feeney, G. F. X., Gullo, M. J., Statham, D. J., et al. (2014). The craving experience questionnaire: A brief, theory-based measure of consummatory desire and craving. Addiction 109, 728–735. doi: 10.1111/add.12472
Mehta, D. D., Praecht, A., Ward, H. B., Sanches, M., Sorkhou, M., Tang, V. M., et al. (2024). A systematic review and meta-analysis of neuromodulation therapies for substance use disorders. Neuropsychopharmacology 49, 649–680. doi: 10.1038/s41386-023-01776-0
Miller, A. P., Baranger, D. A. A., Paul, S. E., Garavan, H., Mackey, S., Tapert, S. F., et al. (2024). Neuroanatomical variability and substance use initiation in late childhood and early adolescence. JAMA Netw. Open 7:e2452027. doi: 10.1001/jamanetworkopen.2024.52027
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 41, 49–100. doi: 10.1006/cogp.1999.0734
Moeller, S. J., Bederson, L., Alia-klein, N., and Goldstein, R. Z. (2016). Neuroscience of inhibition for addiction medicine: From prediction of initiation to prediction of relapse. Progr. Brain Res. 223, 165–188. doi: 10.1016/bs.pbr.2015.07.007.Neuroscience
Morales, A. M., Jones, S. A., Carlson, B., Kliamovich, D., Dehoney, J., Simpson, B. L., et al. (2024). Associations between mesolimbic connectivity, and alcohol use from adolescence to adulthood. Dev. Cogn. Neurosci. 70:101478. doi: 10.1016/j.dcn.2024.101478
Morein-Zamir, S., and Robbins, T. W. (2015). Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 1628, 117–129. doi: 10.1016/j.brainres.2014.09.012
Mullan, B., Wong, C., Allom, V., and Pack, S. L. (2011). The role of executive function in bridging the intention-behaviour gap for binge-drinking in university students. Addict. Behav. 36, 1023–1026. doi: 10.1016/j.addbeh.2011.05.012
Murphy, P., and Garavan, H. (2011). Cognitive predictors of problem drinking and AUDIT scores among college students. Drug Alcohol Dependence 115, 94–100. doi: 10.1016/j.drugalcdep.2010.10.011
Newman-Norlund, R. D., Gibson, M., McConnell, P. A., and Froeliger, B. (2020). Dissociable effects of theta-burst repeated transcranial magnetic stimulation to the inferior frontal gyrus on inhibitory control in nicotine addiction. Front. Psychiatry 11:260. doi: 10.3389/fpsyt.2020.00260
O’Halloran, L., Rueda-Delgado, L. M., Jollans, L., Cao, Z., Boyle, R., Vaughan, C., et al. (2020). Inhibitory-control event-related potentials correlate with individual differences in alcohol use. Addict. Biol. 25, 1–11. doi: 10.1111/adb.12729
Parker, C. C., Lusk, R., and Saba, L. M. (2020). Alcohol sensitivity as an endophenotype of alcohol use disorder: Exploring its translational utility between rodents and humans. Brain Sci. 10, 1–28. doi: 10.3390/brainsci10100725
Paulus, M. P., Schuckit, M. A., Tapert, S. F., Tolentino, N. J., Matthews, S. C., Smith, T. L., et al. (2012). High versus low level of response to alcohol: Evidence of differential reactivity to emotional stimuli. Biol. Psychiatry 72, 848–855. doi: 10.1016/j.biopsych.2012.04.016
Paulus, M. P., Tapert, S. F., Pulido, C., and Schuckit, M. A. (2006). Alcohol attenuates load-related activation during a working memory task: Relation to level of response to alcohol. Alcohol. Clin. Exp. Res. 30, 1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x
Peng, C.-Z., Wilsnack, R. W., Kristjanson, A. F., Benson, P., and Wilsnack, S. C. (2012). Gender differences in the factor structure of the Alcohol Use Disorders Identification Test in multinational general population surveys. Drug Alcohol Dependence 124, 50–56. doi: 10.1016/j.drugalcdep.2011.12.002
Penny, W., Friston, K., Ashburner, J., Kiebel, S., and Nichols, T. (2007). Statistical Parametric Mapping: The Analysis of Functional Brain Images, eds W. D. Penny, K. J. Friston, and J. T. Ashburner (Amsterdam: Elsevier), doi: 10.1016/B978-0-12-372560-8.X5000-1
Pérez-García, J. M., Suárez-Suárez, S., Doallo, S., and Cadaveira, F. (2022). Effects of binge drinking during adolescence and emerging adulthood on the brain: A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 137:104637. doi: 10.1016/j.neubiorev.2022.104637
Piasecki, T. M., Alley, K. J., Slutske, W. S., Wood, P. K., Sher, K. J., Shiffman, S., et al. (2012). Low sensitivity to alcohol: Relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. J. Stud. Alcohol. Drugs 73, 925–932. doi: 10.15288/jsad.2012.73.925
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 118, 2128–2148. doi: 10.1016/j.clinph.2007.04.019
Prisciandaro, J. J., Myrick, H., Henderson, S., McRae-Clark, A. L., and Brady, K. T. (2013). Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Dependence 131, 44–49. doi: 10.1016/j.drugalcdep.2013.04.008
Ray, L. A., Mackillop, J., and Monti, P. M. (2010). Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use Misuse 45, 1742–1765. doi: 10.3109/10826084.2010.482427
Rupp, C. I., Beck, J. K., Heinz, A., Kemmler, G., Manz, S., Tempel, K., et al. (2016). Impulsivity and alcohol dependence treatment completion: Is there a neurocognitive risk factor at treatment entry? Alcohol. Clin. Exp. Res. 40, 152–160. doi: 10.1111/acer.12924
Saunders, J. B., Aasland, O. G., Babor, T. F., De La Fuente, J. R., and Grant, M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88, 791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
Schuckit, M. A. (1980). Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J. Stud. Alcohol. 41, 242–249. doi: 10.15288/jsa.1980.41.242
Schuckit, M. A. (2018). A critical review of methods and results in the search for genetic contributors to alcohol sensitivity. Alcohol. Clin. Exp. Res. 42, 822–835. doi: 10.1111/acer.13628
Schuckit, M. A., and Smith, T. L. (2000). The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J. Stud. Alcohol. 61, 827–835. doi: 10.15288/jsa.2000.61.827
Schuckit, M. A., and Smith, T. L. (2001). The clinical course of alcohol dependence associated with a low level of response to alcohol. Addiction 96, 903–910. doi: 10.1046/j.1360-0443.2001.96690311.x
Schuckit, M. A., Smith, T. L., and Clarke, D. F. (2021). Cross-sectional and prospective associations of drinking characteristics with scores from the Self-report of the effects of alcohol questionnaire and findings from alcohol challenges. Alcohol. Clin. Exp. Res. 45, 1–12. doi: 10.1111/acer.14710
Schuckit, M. A., Smith, T. L., Danko, G. P., Anthenelli, R. M., Schoen, L., Kawamura, M., et al. (2017). A prospective comparison of how the level of response to alcohol and impulsivity relate to future DSM-IV alcohol problems in the COGA youth panel. Alcohol. Clin. Exp. Res. 41, 1329–1339. doi: 10.1111/acer.13407
Schuckit, M. A., Smith, T. L., Danko, G. P., Pierson, J., Hesselbrock, V. M., Bucholz, K. K., et al. (2007). The ability of the self-rating of the effects of alcohol (SRE) scale to predict alcohol-related outcomes five years later. J. Stud. Alcohol Drugs 68, 371–378. doi: 10.15288/jsad.2007.68.371
Schuckit, M. A., Smith, T. L., Pierson, J., Danko, G. P., and Beltran, I. A. (2006). Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol. Clin. Exp. Res. 30, 1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x
Schuckit, M. A., Tapert, S., Matthews, S. C., Paulus, M. P., Tolentino, N. J., Smith, T. L., et al. (2012). fMRI differences between subjects with low and high responses to alcohol during a stop signal task. Alcohol. Clin. Exp. Res. 36, 130–140. doi: 10.1111/j.1530-0277.2011.01590.x
Schweinsburg, A. D., Paulus, M. P., Barlett, V. C., Killeen, L. A., Caldwell, L. C., Pulido, C., et al. (2004). An fMRI study of response inhibition in youths with a family history of alcoholism. Ann. N. Y. Acad. Sci. 1021, 391–394. doi: 10.1196/annals.1308.050
Shenhav, A., Cohen, J. D., and Botvinick, M. M. (2016). Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci. 19, 1286–1291. doi: 10.1038/nn.4384
Simmonds, D. J., Pekar, J. J., and Mostofsky, S. H. (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46, 224–232. doi: 10.1016/j.neuropsychologia.2007.07.015
Smith, J. L., Mattick, R. P., Jamadar, S. D., and Iredale, J. M. (2014). Deficits in behavioural inhibition in substance abuse and addiction: A meta-analysis. Drug Alcohol Dependence 145, 1–33. doi: 10.1016/j.drugalcdep.2014.08.009
Sobell, L. C., and Sobell, M. B. (1992). Timeline follow-back, in Measuring alcohol consumption, eds R. Z. Litten and J. P. Allen (Totowa, NJ: Humana Press). doi: 10.1007/978-1-4612-0357-5_3
Song, S., Zilverstand, A., Gui, W., Li, H., and Zhou, X. (2019). Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimul. 12, 606–618. doi: 10.1016/j.brs.2018.12.975
Statham, D. J., Connor, J. P., Kavanagh, D. J., Feeney, G. F. X., Young, R. M., May, J., et al. (2011). Measuring alcohol craving: Development of the alcohol craving experience questionnaire. Addiction 106, 1230–1238. doi: 10.1111/j.1360-0443.2011.03442.x
Stein, M., Steiner, L., Fey, W., Conring, F., Rieger, K., Federspiel, A., et al. (2021). Alcohol-related context modulates neural correlates of inhibitory control in alcohol dependent patients: Preliminary data from an fMRI study using an alcohol-related Go/NoGo-task. Behav. Brain Res. 398, 112973. doi: 10.1016/j.bbr.2020.112973
Suárez-Suárez, S., Doallo, S., Pérez-García, J. M., Corral, M., Rodríguez Holguín, S., and Cadaveira, F. (2020). Response inhibition and binge drinking during transition to university: An fMRI study. Front. Psychiatry 11:535. doi: 10.3389/fpsyt.2020.00535
Tapert, S. F., Pulido, C., Paulus, M. P., Schuckit, M. A., and Burke, C. (2004). Level of response to alcohol and brain response during visual working memory. J. Stud. Alcohol. 65, 692–700. doi: 10.15288/jsa.2004.65.692
Trela, C. J., Hayes, A. W., Bartholow, B. D., Sher, K. J., Heath, A. C., and Piasecki, T. M. (2018). Moderation of alcohol craving reactivity to drinking-related contexts by individual differences in alcohol sensitivity: An ecological investigation. Exp. Clin. Psychopharmacol. 26, 354–365. doi: 10.1037/pha0000206
Trela, C. J., Piasecki, T. M., Bartholow, B. D., Heath, A. C., and Sher, K. J. (2016). The natural expression of individual differences in self-reported level of response to alcohol during ecologically assessed drinking episodes. Psychopharmacology 233, 2185–2195. doi: 10.1007/s00213-016-4270-5
Trim, R. S., Simmons, A. N., Tolentino, N. J., Hall, S. A., Matthews, S. C., Robinson, S. K., et al. (2010). Acute Ethanol Effects on Brain Activation in Low- and High-Level Responders to Alcohol. Alcohol. Clin. Exp. Res. 34, 1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x
Upton, S., Brown, A. A., Garland, E. L., and Froeliger, B. (2023a). Right Inferior frontal gyrus theta-burst stimulation reduces smoking behaviors and strengthens fronto-striatal-limbic resting-state functional connectivity: A randomized crossover trial. Front. Psychiatry 14:1166912. doi: 10.3389/fpsyt.2023.1166912/abstract
Upton, S., Brown, A. A., Ithman, M., Newman-Norlund, R., Sahlem, G., Prisciandaro, J. J., et al. (2023b). Effects of hyperdirect pathway theta burst transcranial magnetic stimulation on inhibitory control, craving, and smoking in adults with nicotine dependence: A double-blind, randomized crossover trial. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8, 1156–1165. doi: 10.1016/j.bpsc.2023.07.014
Vanderhasselt, M. A., Allaert, J., De Raedt, R., Baeken, C., Krebs, R. M., and Herremans, S. (2020). Bifrontal tDCS applied to the dorsolateral prefrontal cortex in heavy drinkers: Influence on reward-triggered approach bias and alcohol consumption. Brain Cogn. 138:105512. doi: 10.1016/j.bandc.2019.105512
Viken, R. J., Rose, R. J., Morzorati, S. L., Christian, J. C., and Li, T.-K. (2003). Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol. Clin. Exp. Res. 27, 795–803. doi: 10.1097/01.ALC.0000067974.41160.95
Wessel, J. R., and Anderson, M. C. (2024). Neural mechanisms of domain-general inhibitory control. Trends Cogn. Sci. 28, 124–143. doi: 10.1016/j.tics.2023.09.008
Wietschorke, K., Lippold, J., Jacob, C., Polak, T., and Herrmann, M. J. (2016). Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J. Neural Transmiss. 123, 1173–1178. doi: 10.1007/s00702-016-1541-6
Zhang, R., Geng, X., and Lee, T. M. C. (2017). Large-scale functional neural network correlates of response inhibition: An fMRI meta-analysis. Brain Struct. Funct. 222, 3973–3990. doi: 10.1007/s00429-017-1443-x
Keywords: craving, executive function, cingulate, cognitive control, response inhibition
Citation: Cofresí RU, Upton S, Terry D, Brown AA, Piasecki TM, Bartholow BD and Froeliger B (2025) Inhibitory control in the sober state as a function of alcohol sensitivity: a pilot functional magnetic resonance imaging (fMRI) study. Front. Hum. Neurosci. 19:1557661. doi: 10.3389/fnhum.2025.1557661
Received: 08 January 2025; Accepted: 13 February 2025;
Published: 28 February 2025.
Edited by:
Chella Kamarajan, Downstate Health Sciences University, United StatesReviewed by:
Mohammad Mofatteh, Queen’s University Belfast, United KingdomCopyright © 2025 Cofresí, Upton, Terry, Brown, Piasecki, Bartholow and Froeliger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto U. Cofresí, Y29mcmVzaXJAbWlzc291cmkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.