- 1Department of Psychology, Université de Montréal, Montreal, QC, Canada
- 2Centre de recherche de l'institut universitaire de gériatrie de Montréal, CIUSSS du Centre-Sud-de-l'Île-de-Montréal, Montreal, QC, Canada
- 3Notre-Dame Hospital, Centre intégré universitaire de santé et de services sociaux du Centre-Sud-de-l'Île-de-Montréal (CCSMTL), Montreal, QC, Canada
- 4Department of Life Sciences, Brunel University London, Uxbridge, United Kingdom
- 5Department of Medicine and Surgery, University of Parma, Parma, Italy
- 6Department of Cell Biology and Anatomy, Louisiana State University Health Sciences Center, New Orleans, LA, United States
- 7Department of Psychology, University of South Africa, Pretoria, South Africa
- 8Department of Psychology, Scientific College of Greece, Athens, Greece
- 9Department of Psychiatry and Addiction, Université de Montréal, Montreal, QC, Canada
Introduction
For decades, scholars across disciplines, including researchers, scientists, and clinicians, have exhibited a keen interest in exploring the neuroscientific underpinnings of sexual and gender diversity. The purpose of this paper is thus to provide an overview and opinion on the evolution of related research. To achieve this, the authors conducted a bibliographic search of the literature in MEDLINE and PsycInfo from inception to September 2023 with specific keywords, including “neuroscience,” and variations of “lesbian, gay, bisexual, transgender, queer, intersex, asexual, and people with other sexual orientations and forms of gender expression (LGBTQIA+).”
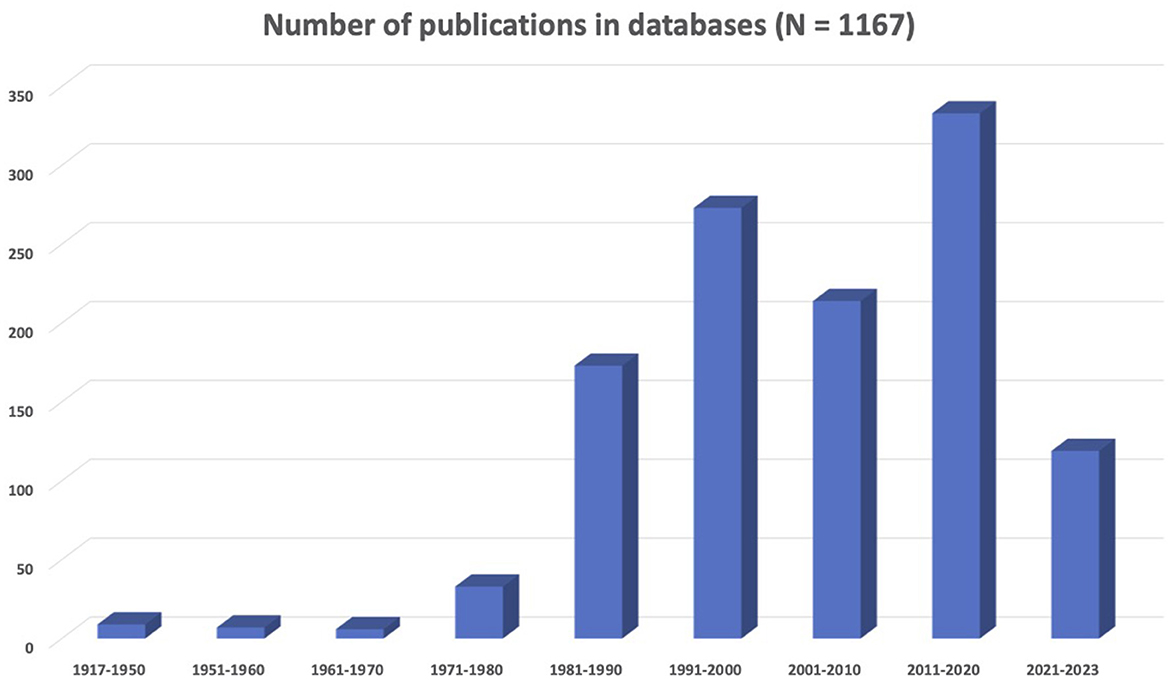
The results presented in Figure 1 show that there is an increase in neuroscience and LGBTQIA+ concerns represented in the scientific literature. In particular, the decade from 1991 to 2000 saw a significant growth in related publications, with the field of neuroscience establishing itself as distinct from psychiatry. However, 2011–2020 was the most prolific decade in the history of sexual and gender diversity in neuroscience. In summary, there were 1,167 records (i.e., scientific articles, book chapters, opinion letters, among others) corresponding to a collective sample of 444,249 individuals on the LGBTQIA+ spectrum. Most research centered on gay men (32%) or transgender people (24.3%), and only a few addressed lesbian (1.1%), bisexual (1.3%), or other identities (1.1%). When mixed samples were included, the majority corresponded to gay and lesbian people (15.3%). The most common topics were brain development (34.6%), general biological aspects (31.8%), and human immunodeficiency virus (HIV)-related research (27.5%). In addition, articles focused on social (19%), psychological (15.9%), and neuropsychological (13.3%) aspects of sexual and gender identity. Animal studies accounted for 7.8% of research.
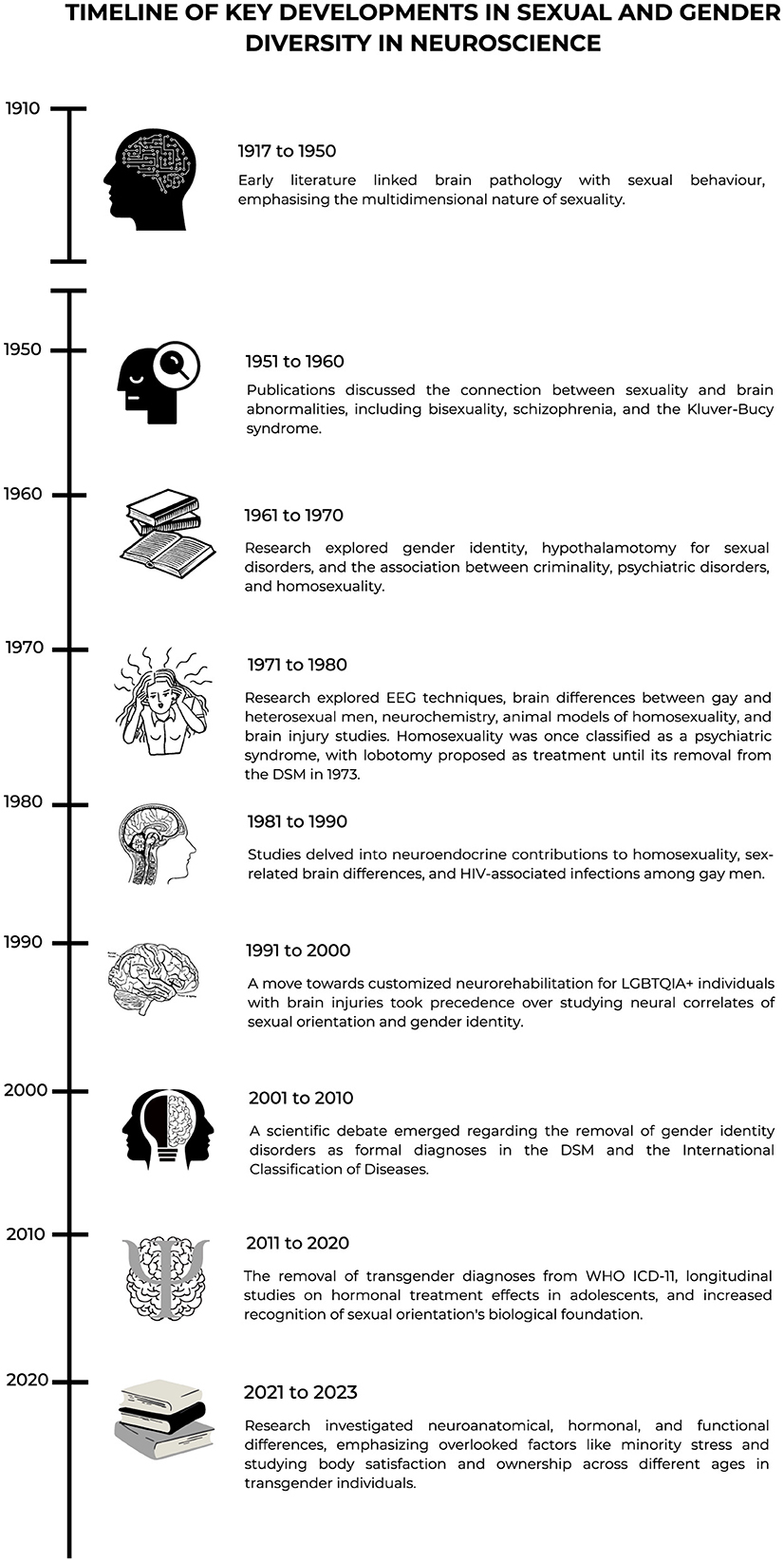
Before considering the future of neuroscience of sexual and gender diversity, we first provide a chronological overview of select related key developments in the field.
Overview of select key developments in sexual and gender diversity in neuroscience
Early work
The first record we identified appeared in 1917 in the context of mental disorders suggesting that brain pathology could be related with sexual behavior (Meyer, 1917). Until 1950, only nine publications captured by this search focused on human sexuality, and as complex and multi-determined by biological, psychological, and sociological aspects (Morselli, 1931). This literature indicated that structural damages have an impact on sexual behavior. For instance, “bad thyroid gland may cause queer behavior in the human” (Moss, 1932). During the same period, homosexuality was, in fact, classified as part of psychiatric syndromes (Rosanoff, 1938) and this continued to be the case until 1973. Lobotomy was offered as an option for treating the so-called “sex psychopaths” (Banay and Davidoff, 1942).
From 1951 to 1960, seven publications addressed topics linking sexuality to brain abnormalities. For instance, records theorized upon associations linking disturbed cerebral functioning with bisexuality and schizophrenia (Potzl, 1951) and the Klüver-Bucy syndrome with “homosexual advances” following the removal of the temporal lobes (Terzian and Dalle Ore, 1955). Later, scientific research on the same syndrome indicates that it is characterized by increased nonselective sexual behavior, and not homosexuality itself. However, it was interpreted as homosexual behavior at the time the study was conducted. Between 1961 and 1970, six records were found on topics such as gender identity in gay males and psychosexual functioning of the brain (Money, 1965), the use of hypothalamotomy for sexual disorders (Anonymous, 1969), and literature suggesting a link between criminality and psychiatric disorders, with homosexuality being considered one of them (Guze et al., 1969).
Redefining research and classification systems (1970s and 1990s)
Informed by changing social and scientific attitudes and theories about sexual orientation, in 1973, homosexuality as a mental disorder was officially removed from the Diagnostic and Statistical Manual of Mental Disorders (DSM). From 1971 to 1980, 33 records included topics studying the use of electroencephalographic techniques to document pleasure in men (Heath, 1972) and transgender people (Nussselt and Kockott, 1976), as well as brain functioning differences between gay men and heterosexual men (Wilson et al., 1973).
During this time, neurochemistry studies flourished. This included the role of brain monoamines in male sexual behavior (Gessa and Tagliamonte, 1974), the role of hormones as an explanation for homosexuality in men (Dorner et al., 1975), and animal models of homosexuality in rats and rabbits that experimented on serotonin levels (Fratta et al., 1977). Brain injury studies included the link between homosexuality and early brain damage (Holzer, 1976) and there were studies linking “latent homosexuality” with schizophrenia in males (Sigal, 1978). In addition, we find the first reference to “patients with the problem of intersexuality” when endocrine problems can “masculinize” females and “demasculinize” males (Ehrhardt and Meyer-Bahlburg, 1979).
From the period between 1981 and 1990, the search led to 173 records. And yet, there was only one study addressing neuroendocrine contributions to male and female homosexuality (MacCulloch and Waddington, 1981). A few studies also provided a more nuanced approach and considered sex-related brain differences as a mosaic of both male and female “characteristics” influenced by hormones (Neumann and Elger, 1981). The 1980s also marked the beginning of investigations on samples of gay men to document opportunistic infections of the central nervous system associated with HIV (Anderson et al., 1983; Handler et al., 1983).
Despite changes in psychiatric nomenclature, psychopathological views persisted. For instance, a study suggested a relationship between decreased serotonergic activity and “delusional ideas of homosexual content” in individuals with paranoid schizophrenia (Rinieris et al., 1985). Yet, during this decade, researchers also started to question studies focusing on the biological basis of homosexuality as tainted by personal beliefs and cultural prejudices (Ricketts, 1984). Furthermore, the traditional nature/nurture debate around the genesis of sexual behaviors (homosexual, bisexual, and heterosexual) was enriched by new concepts, such as “critical-period/nurture” in brain development and social influences on behavior (Money, 1986). Another study suggested a change in sexual preference following brain injuries involving limbic system structures (Miller et al., 1986).
More integrative theories started to appear classifying sexual orientation as a complex phenomenon determined by genetic-hormonal, pharmacological, maternal stress, immunological, and social experiential variables (Ellis and Ames, 1987). An integrative book consolidating multiple perspectives to explain sexual orientation was published during this decade (Money, 1988). Interestingly, this period was also the beginning of theories linking a cluster of cells located in the preoptic area of the hypothalamus with sexual orientation and gender identity (Swaab and Hofman, 1988).
The decade from 1991 to 2000 showed a significant explosion in scientific productivity as compared to the previous decades with 273 records. A new clinical interest started to emerge leading to neurorehabilitation approaches specifically tailored for the needs of gay and lesbian people with brain injuries (Mapou, 1990). Investigating the neural correlates of sexual orientation and gender identity were not as important anymore as finding alternatives to meet LGBTQIA+ health and wellness needs following a brain injury.
With the proliferation of neuroscience emerging as a field separate from psychiatry, the results of neuroanatomical and neuropsychological studies began to be used in perspectives that were less pathologizing. In this manner, there were studies suggesting a neurobiological component related to hemispheric functional lateralization with an over-representation of left-handedness in gay men and lesbian women (McCormick et al., 1990). The results of a neuroanatomical study also showed that the midsagittal plane of the anterior commissure in gay men was 18% larger than in heterosexual women and 34% larger than in heterosexual men (Allen and Gorski, 1992). In addition, the number of cells in the suprachiasmatic nucleus of the hypothalamus in gay men was indicated as twice as large in comparison to heterosexual individuals (Swaab et al., 1995), while a female-sized volume of the central subdivision of the bed nucleus of the stria terminalis was found in male-to-female transsexuals1 (Zhou et al., 1997; Kruijver et al., 2000).
Critiques of genetic (Hamer and Copeland, 1994) and hormonal studies (Garnets and Kimmel, 1993) concerning human sexual orientation started to appear during this time. This perspective essentially argued against “nature” as a single biological determinant of sexual orientation (Byne and Parsons, 1993; Banks and Gartrell, 1995; Gooren, 1995) and instead considered a multitude of biopsychosocial factors (Friedman and Downey, 1993; Bancroft, 1994; Doell, 1995; Looy, 1995; Byne, 1997). These changes were also reflected in animal models of homosexuality with an alternative bio-social program of research on the development of sexual behavior in animals (Fausto-Sterling, 1995). Notwithstanding, genetic studies with Drosophila models of sexual orientation continued to flourish (Ferveur et al., 1995; Ito et al., 1996; Yamamoto et al., 1996). There were also animal models of homosexuality in rams and sheep (Perkins and Fitzgerald, 1997), and rat models of bisexuality (Aron, 1999).
Critics also called attention to the fact that this research excluded females/women and ethnic minorities and denied the political, cultural, and historical dimensions of sexuality (Hegarty, 1997). Reflecting a refocus on care, neuropsychological literature in humans insisted on the exploration with the client and their support system and how their sexual orientation affected assessment and rehabilitation (Morales, 2000). Also, when a neuropsychological assessment was conducted, the sexual orientation of the individual influenced outcomes and treatment when external psychosocial stressors were considered in rehabilitation. Those stressors became commonly known as “minority stress” caused by stigma, prejudice, and discrimination being responsible for a sustained unwelcoming stressful social environment having a deleterious impact on the health of sexually and gender-diverse people.
Increasing criticism to binarism in neuroscientific research
Between the decade of 2001 and 2010, the search identified a total of 214 records. Toward the end of this decade, there was a scientific debate about the removal of gender identity disorders as formal diagnoses in both the DSM and the World Health Organization (WHO) International Classification of Diseases (ICD) (Bockting, 2009). The following examples show that the interest during this time was in demonstrating the anatomical and hormonal differences in sexually and gender-diverse individuals. It is also important to note concerns regarding issues of replication. Indeed, studies regarding differences in the anterior commissure of the brain could not be replicated (Lasco, 2001). A study concluded that compared with heterosexual women, lesbians display less gray matter bilaterally in the temporo-basal cortex, ventral cerebellum, and left ventral premotor cortex (Ponseti et al., 2007).
A few reports were also published on the common occurrence of phantom genitalia following gender confirmation surgery, with a post-surgical incidence of 30% of phantom penises following gender confirmation surgery in female-to-male transgender participants, as compared to 60% in men following penectomy for cancer treatment (Ramachandran and McGeoch, 2007, 2008). A study found the sex reversal of one of the interstitial nuclei of the anterior hypothalamus in transgender people (Garcia-Falgueras and Swaab, 2008). Studies investigating the activation of the hypothalamus when participants were watching erotic videos found a lack of hypothalamic activation and intense autonomic response following exposure to videos of the “opposite” sexual orientation (Paul et al., 2008). A positron emission tomography (PET)-magnetic resonance imaging (MRI) study indicated sex-atypical cerebral asymmetry and functional connections in homosexual subjects showing sex-atypical amygdala connections (Savic and Lindstrom, 2008).
Other research during this time suggested that the isthmal area corresponding to the posterior region of the callosal body connecting parieto-temporal cortical regions was larger in gay men and even predicted 96% of sexual orientation in men based on neuroanatomical and cognitive variables (Witelson et al., 2008). Also, a study showed that male-to-female (MTF) transsexuals showed a significantly larger volume of regional gray matter in the right putamen compared to cisgender men (Luders et al., 2009).
There was increasing debate on the way science had contributed to the discussion on sex differences. Indeed, distorted or exaggerated evidence sometimes reflected social and political opinions (Rogers, 2001), interests, and values (Saravi, 2007). During this time, neuroscientists discussed the social, forensic, and therapeutic implications of their findings (Wolpe, 2004). Neuropharmacological approaches included a study on the differential cerebral response of antidepressants in gay and heterosexual men (Kinnunen, 2002), and the effect of prenatal exposure to therapeutic drugs on brain feminization/demasculinization (Ellis and Hellberg, 2005).
A neuroimmunological theory emerged as the “maternal immune hypothesis.” Similar to the fraternal birth order effect, this model suggested that homosexuality in human males was predicted by higher numbers of older brothers reflecting the progressive immunization of some mothers to male-specific antigens and their effects on the sexual differentiation of the brain (Blanchard, 2004; Blanchard and Bogaert, 2004). In the meantime, intersex individuals were studied under the perspective of hormonal abnormalities (Hines, 2004a,b). Notably, female-to-male transsexuals (FTM) tested before and after 6 months of androgen treatment significantly improved their performance on a visual memory task (Gomez-Gil et al., 2009), with an fMRI study showing that differences in activation patterns remained stable over the course of hormonal treatment (Schoning et al., 2010).
A new era of reformulation in research and classification systems
The decade from 2011 to 2020 included 333 records, which is the most prolific decade in the history of sexual and gender diversity in neuroscience to date. Transgender-related diagnoses were removed from the ICD (11th edition) chapter on mental and behavioral disorders in 2018, taking effect clinically in 2022. Longitudinal case reports of positive effects of hormonal treatment in adolescents (Cohen-Kettenis et al., 2011) and the conviction that a person's sexual orientation arises in large part from biological processes that are already underway before birth led scientists to increasingly see sexual and gender diversity as something to be valued, celebrated, and welcomed into society (LeVay, 2011).
An increasing recognition emerged that monocausal explanations were unable to effectively address the complexity of transgender identity development and the integration of neurobiological findings into other disciplines were deemed to be the best avenue forward (Nieder et al., 2011). As such, it was recommended that genetic, neuroendocrinological, neurostructural, and neurofunctional findings must be integrated within a multidisciplinary framework to reach a more comprehensive vision of transgenderism. Still, diffusion tensor imaging showed that the white matter microstructure pattern in untreated MTF transgender participants fell halfway between the pattern of male and female cisgender individuals (Rametti et al., 2011). A fMRI study in FTM transsexuals showed that making a brain “more male” by the application of androgens not only reduced the activity of a core neural hub (frontal, temporal, and striatal regions), but also altered the organization of the brain network with increased connectivity among limbic regions supporting emotional and social cognitive processes related to empathy and mentalizing (Ye et al., 2011). MTF transsexuals showed significantly larger gray matter volume in the right putamen compared to cisgender men (Luders et al., 2012). Another study ascertained that FTM participants showed evidence of subcortical gray matter “masculinization,” while MTF individuals showed evidence of cortical thickness “feminization” (Zubiaurre-Elorza et al., 2013), but these findings were not replicated in non-Western samples (Sorouri Khorashad et al., 2020). Compared to heterosexual men, gay men and heterosexual women had similar thickness values in visual cortices and thalamic volumes (Abe et al., 2014). A study showed decreased hemispheric connectivity ratios of subcortical/limbic areas for both MTF and FTM transgender groups (Hahn et al., 2015).
The activation of regions within the temporo-parietal junction linked to empathy was also observed in individuals sexually attracted to men (heterosexual women and gay men) showing greater empathy levels than participants attracted to women (heterosexual men and lesbians) (Perry et al., 2013). Other identities such as bigender appeared in the context of medical literature referring to brain plasticity (Case and Ramachandran, 2012). A call for a feminist/queer critical neuroscience framework based on interdisciplinarity emerged to address controversies in the literature (Kraus, 2012). The first reports of a link between individuals living on the autism spectrum with gender diversity (Jones et al., 2012; Pasterski et al., 2014) as well as the neurodiversity movement (Jaarsma and Welin, 2012; Bertilsdotter Rosqvist et al., 2020) appeared in this decade. Good summaries addressing both ultimate (e.g., evolutionary) and proximate causes influenced the spectrum of sexual orientations (Hill et al., 2013). In terms of cognitive abilities and brain activation patterns, a study showed higher verbal fluency scores in FTM adolescents as compared to MTF adolescents and cisgender boys and girls, with no significant brain activation differences (Soleman et al., 2013). Genetic research suggested the influence of the sex hormone-related genes, estrogen receptor beta (ERbeta) in the “defeminization” of the female brain in FTM individuals (Fernández et al., 2014).
A focus shift on social determinants of health brought research documenting the deleterious effect of structural stigma causing a chronic activation of the hypothalamic-pituitary-adrenocortical axis in LGB young adults who were raised in highly stigmatizing environments as adolescents evidencing a blunted cortisol response (Hatzenbuehler and McLaughlin, 2014). In addition, more sophisticated techniques continued to address functional and neuroanatomical differences by gender diversity. For instance, a study showed that a rightward asymmetry of the serotonin transporter distribution observed via PET imaging in the midcingulate cortex of cisgender males was absent in females and MTF transsexuals (Kranz et al., 2014). Electroencephalogram (EEG) and event-related potentials to study implicit levels of discomfort toward homosexuality showed differences in the processing of visual images that occur as early as 200 milliseconds and may be moderated by familiarity in heterosexual participants (Dickter et al., 2015). A study on white matter microstructure suggested that the neuroanatomical signature of transgenderism was related to brain areas processing self-perception and body ownership, whereas homosexuality seemed to be associated with less pronounced cerebral sexual differentiation of white matter tracts (Burke et al., 2017; Manzouri et al., 2017). A study showed different functional connectivity patterns in the brains of transgender compared with cisgender girls, boys, and adolescents (Nota et al., 2017). New developments included a model to explain functional neuroimaging correlates of transgender identity development and gender dysphoria (Altinay and Anand, 2020). Animal models of homosexuality became available with the use of aromatase inhibitors affecting the estrogen synthesis during the critical periods of brain sexual differentiation (Olvera-Hernandez and Fernandez-Guasti, 2015).
During this decade, critics focused on disparities involving LGBT communities who remained understudied in medicine, including neurology (Rosendale and Josephson, 2015). The rationale behind this argument seemed based on a binary paradigm of sexual and gender diversity disregarding the effects of minority stress and creating limitations to culturally competent care in medicine. Even when minority stress was taking a more important place in the neuroscience of sexual and gender diversity, there were reports suggesting a direct link with biological aspects as a case report of a transgender person that became cisgender following a status epilepticus (Parkinson, 2015).
In another study, heterosexual women and gay men showed more left-brain lateralization for processing female faces as compared to heterosexual men with more right-brain lateralization (Rahman and Yusuf, 2015). Neuropsychological studies indicated that compared to heterosexual men, heterosexual women and gay men showed higher scores in processing speed that became similar to those of heterosexual men with aging (Faris, 2016). A meta-analysis revealed that gay men performed like heterosexual women in both male-favoring (e.g., spatial cognition) and female-favoring (e.g., verbal fluency) cognitive tests, while lesbians performed like heterosexual men only in male-favoring tests, with larger magnitudes for spatial abilities (Xu et al., 2017). Neuropsychological and psychological testing controversies highlighted problems when choosing the appropriate gender norms in transgender individuals as many tests had gender-based norms (Keo-Meier and Fitzgerald, 2017; Trittschuh et al., 2018). In addition, a call for a change in the culture of neurodisability and ableism emerged as recommendations were made to improve health care in LGBTQIA+ individuals (Moreno et al., 2017). Another line of research demonstrates that cross-sex hormone treatment affects cerebral tissue in transgender people using longitudinal MRI measurements of cortical thickness (Kilpatrick et al., 2019). Their study demonstrated that compared to controls, both transgender men and women showed significant decreases in the mesial prefrontal and parietal cortices.
There is still a small number of brain imaging studies in vivo in transgender people that are difficult to integrate because they differ in terms of techniques, research design, and samples (Kreukels and Guillamon, 2016). Also, there are different developmental trajectories confirmed by the fact that not all children with gender incongruence become transgender adolescents or adults. Conversely, not all transgender adults have been children with gender incongruence.
Preparing for the future
The last period from 2021 to September 2023 included 119 records with different topics addressing neuroanatomical, hormonal, and functional differences. A distinguishing feature in the interpretation of findings is characterized by the inclusion of previously overlooked variables, such as minority stress. Studies also focused on body satisfaction and body ownership in transgender individuals of different ages. A large MRI study showed that transgender participants seemingly presented with their own unique brain phenotype and not only a male-female shift (Mueller et al., 2021). Neuroanatomical differences were also documented in a study with a neuroimaging-genetics dataset suggesting that genetic factors related to same-sex behavior may contribute to structural variation in certain brain structures (Abe et al., 2021). A study showed that, compared with cisgender individuals, transgender people showed lower cortical gyrification index limited to the occipito-parietal cortex and the sensory motor cortex regions, encoding own body image and body ownership (Wang et al., 2021). Another study in transgender youth showed that hormonal treatment was associated with significantly lower body image dissatisfaction and greater functional connectivity between the amygdala and ventromedial prefrontal cortex during a task designed to engage the amygdala (Grannis et al., 2023).
Additional research focused on the role of minority stress having an effect in emotion processing among transgender individuals in a study using fMRI and magnetic resonance spectroscopy (Kiyar et al., 2022). A meta-analysis of neuroimaging studies showed that minority stress associated with alterations within intrinsic connectivity networks was examined in only one study. Moreover, other studies were limited to investigating the neurobiological basis of sexual orientation (Nicholson et al., 2022). Some contributions focused on intersexuality including one study measuring the impact of HIV on brain health for racial/ethnic older adult LGBTQ people of color (Ramos, 2021). Further developments included a model of sexual differentiation where genes, hormones, and the environment act together in multiple parallel pathways leading to male or female phenotypes (Rouse and Hamilton, 2021).
From the point of view of neurocognitive disorders, a study showed that the prevalence of Alzheimer's disease and related dementias was higher in transgender adults as compared to cisgender adults (Guo et al., 2022). Recently, machine learning algorithms are being used to detect sexual orientation based on gray matter volumes with 62% accuracy and 92% with resting-state functional connectivity (Clemens et al., 2023), but this approach has been criticized in the prediction of gender identity with high risk of misleading conclusions (Wiersch et al., 2023). Researchers are now taking position in neuroimaging studies stating that their goal is not to uncover a mechanism that can be “fixed” to prevent gender diversity, but to make progress toward destigmatization, greater acceptance, and improved quality of life for individuals with diverse gender identities (Xerxa et al., 2023).
Having provided a historical overview and timeline, divided into decades, of key developments in sexual and gender diversity in neuroscience, also reflected in Figure 2, toward concluding the opinion paper, we now look to the future.
Concluding remarks: the future of neuroscience of sexual and gender diversity
Any historic account is incomplete without asking whose histories are being told and by whom (Hegarty and Ruterford, 2019; Horne et al., 2019; Moreno et al., 2019). The selected research presented chronologically above may indeed suggest a White cisgender male, and a Northern American and British skew in who conducted the research and where it was published. Yet, this account demonstrates how competing theories and concepts were interpreted based on ideologies, values, and political opinions that have generally evolved throughout the 20th and the first decades of the 21st century. Unsurprisingly, some published research was subsequently retracted by editors reconsidering long discredited beliefs and unethical practice, such as conversion therapy; notably, some of these studies are kept in archives only for their historical value (i.e., see Glover, 1951).
Large parts of the scientific world have significantly shifted from an obsession with pathology and causation to one in which sexual and gender diversity is increasingly affirmed and even celebrated. However, it will serve us well to consider how some countries are on record for excluding candidates for psychiatric residences or doctoral programs based on their “militant homosexuality” (Moreno Robles, 1975; Kooden, 2021), and so-doing limited sexual and gender diversity perspectives informing scientific research in this field. Still today, some journals and scientific fields may unintentionally, or otherwise, operate from a hetero-cis-normative epistemological position (Pillay et al., 2022). Also in neuroscience, biased science, research neglect, and exclusion have had negative consequences leading to a narrow comprehension of the full spectrum of sexual and gender diversity. A clear example is the persistent neglect of lesbian, bisexual, and intersex concerns evidenced by the reduced number of studies targeting these specific communities, as compared to the large number of studies with gay and transgender individuals.
Throughout its brief history, neuroscience has been used to understand the biological foundations of sexual and gender expressions. While an invaluable contribution, unfortunately, this quest does not automatically lead to positive social changes. For example, a parallel can be established for skin color, the biological foundations of which have clearly been established (Naik and Farrukh, 2022). Yet, while race relations have changed dramatically in recent years, this has not prevented racism in our societies. Dated and unjustified beliefs, homo-, bi-, and transphobia, and even scientifically discredited practices, such as conversion therapies are not consigned to history and are difficult to eradicate and can perpetuate healthcare disparities (White and Chanoff, 2011; Academy of Science of South Africa, 2015).
And regardless of cumulative research demonstrating the multidimensional nature of human sexuality, very real health disparities exist among LGBTQIA+ individuals across their lifespans. The same applies to hetero-cis-normative assumptions as dangerous political and social determinants of health, and rigid conceptual binaries of, among others, heterosexual and homosexual, male and female, masculine and feminine, transgender and cisgender (Pillay et al., 2022). It is important to move away from a focus on etiology to a recognition of the effects of minority stress on the brain and on mental health (Edmiston and Juster, 2022). Avoiding a rigidly binary conceptualization of biological sex, either explicit or implicit (Rouse and Hamilton, 2021), and shifting away from research that focuses solely on the “etiology” or origins of LGBT identities (Edmiston and Juster, 2022) seem to be two promising approaches to push forward the neuroscience of sexual and gender diversity.
The historical overview presented in this opinion paper may assist scientists to reflect on how their research can maintain and/or reinforce stereotypes and harmful ideas regarding sexual and gender diversity. Explicit or implicit biases can contaminate research, amplify disparities, and translate into significant negative health consequences for LGBTQIA+ people. Given the social responsibility of science, researchers must clearly state the theoretical models behind the rationale of their studies and anticipate the consequences of their findings to reduce negative stereotypes, disparities, and stigma. As such, researcher reflexivity and justification ought to be encouraged by funding agencies supporting research on sexual and gender diversity. Ultimately, related research should serve to create an environment where LGBTQIA+ people feel comfortable disclosing their sexual orientation and gender identity to destigmatize care (Colin, 2015).
In including previously overlooked variables, such as minority stress, in the interpretation of neuroscientific findings, lessons are to be learnt from recent shifts to multisystemic, interpersonal, contextual, and affirmative understandings in LGBTQIA+ Psychology, also in relation to resilience science (Wilks et al., 2022). Notably, psychological science has been at the forefront of advancing affirmative practice guidelines development in many parts of the world to contribute to service provider cultural competence and ethical practice in working with LGBTQIA+ client populations (Horne et al., 2019; Moreno et al., 2019; Pillay et al., 2022; Wilks et al., 2022). Similarly, it will serve the neuroscientific field well to incorporate an intersectional lens more deliberately in future research, that is, discourses of capacity (and ableism), race (and racism), gender (and sexism and cisgenderism), class (and classism), and sexuality (and heterosexism) (Hegarty and Ruterford, 2019). There is also an urgent need to expand understandings of sexual and gender diversity from the non-WEIRD (Western, educated, industrialized, rich, and democratic) world (Henrich et al., 2010). Moving away from the binary model, the significant definitional shifts in understanding sexual orientation (Park, 2022), sexual orientation development and the science of sexual and gender fluidity (Diamond, 2020, 2021), ought to be considered. Also, to remain current, terminologies now employed in the healthcare of trans and gender-diverse people (Tomson et al., 2021) should be adopted.
Hegarty (2018, p. 99) posed the rhetorical question: “What discovery made us feel that neuroscience might be a politically progressive narrative in the decade of the brain?” As we look to the future, several questions preoccupy the authors. Given a historical focus on pathology, is there any value in applying neuroscientific research to “explain” the origins of sexual and gender diversity? Given the issue of replicability, are findings just isolated results? Beyond scientific knowledge, is this quest deprived of value and concrete positive repercussions for LGBTQIA+ people? From a translational perspective, what is the value of animal research, animal models and genetics in explaining sexual orientation or gender identity in humans? Is this still relevant to sexual and gender diversity and how can sociocultural factors be studied in animal models to mimic the human condition? In thinking about emerging technologies, how is artificial intelligence going to be used to increase predictions of sexual and gender identity in neuroscience? If we abandoned lobotomies to control sexual and gender diversity, could science be used to control the sexualities of individuals using more sophisticated ways, such as artificial intelligence, considering that such data will be feeding algorithms? How can researchers, scientists, and clinicians demonstrate responsibility and persist in the prevention of abuse and harm?
These are important questions that we do not have answers to currently. As we move forward respectfully and inclusively, we must ensure that the power of neuroscience is used for good and not harm. We, the authors, hold the conviction that studies on how stigma, stress, and strain shape the brain are of greater value to LGBTQIA+ communities than fixating and focusing on sexually dimorphic nuclei to explain why people are different. Rather, by showing how socially constructed pressures can impact neural functions, we provide the strongest possible evidence that the environment impacts the brain and that more progressive spaces can be promoted so that people can live authentic lives without prejudice. During this time of war, climate crisis, and pandemic recovery, we fear that conservative voices are becoming louder in their hate toward LGBTQIA+ people. With this in mind, it is important to build upon neuroscientific research on sexual and gender diversity in a manner that includes the unique lived experiences of the communities we study and serve.
Several efforts have been made in neuroscience resulting from a significant interaction between clinical observations, technical advancement, and social attitudes. Science is self-corrective, and neuroscientists are challenging erroneous views and beliefs limiting the credibility of their findings. Scientific transparency and the willingness to change attitudes toward sexual and gender minorities along with increased knowledge will enable more trustworthy results. Indeed, funding agencies are also playing their role introducing clear guidelines supporting equity, diversity, and inclusion in research.
Author contributions
JAM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. RM: Writing – review & editing. LA-S: Writing – review & editing, Funding acquisition. JN: Writing – review & editing. IS: Data curation, Resources, Writing – review & editing. ZV: Resources, Writing – review & editing. R-PJ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. JAM's laboratory (Innovation, Technology, and Cognition - INTECOG) receives financial assistance provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) (DH-2024-00194) and Gouvernement du Québec (Ministère de l'Économie, de l'Innovation et de l'Énergie) in Quebec, Canada (2023-2028-PSOv2a-UDEM-IS-68102). JAM also receives financial support from the Office des personnes handicapées du Québec. JAM is supported by an AGE-WELL-EPIC-AT Fellowship (EPIC-AT-2024-F24) and the Réseau Québécois de Recherche sur le Vieillissement (RQRV), a Research Network financed by Fonds de recherche du Québec (EPIC-AT-2024-F24). RM is supported by an Alzheimer's Association Grant (AARF-22-919481).
Acknowledgments
The authors would like to thank Marc-Olivier Croteau, M. Sc., M.S.I. (Librarian at Université de Montréal) who helped to refine and perform the search strategy for the literature review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^While the authors acknowledge significant shifts in trans-related terminologies, those utilized at the time of the cited research have been retained.
References
Abe, C., Johansson, E., Allzen, E., and Savic, I. (2014). Sexual orientation related differences in cortical thickness in male individuals. PLoS ONE 9:e114721. doi: 10.1371/journal.pone.0114721
Abe, C., Lebedev, A., Zhang, R., Jonsson, L., Bergen, S. E., Ingvar, M., et al. (2021). Cross-sex shifts in two brain imaging phenotypes and their relation to polygenic scores for same-sex sexual behavior: a study of 18,645 individuals from the UK Biobank. Hum. Brain Mapp. 42, 2292–2304. doi: 10.1002/hbm.25370
Academy of Science of South Africa (2015). Diversity in Human Sexuality: Implications for Policy in Africa. Pretoria, South Africa: Academy of Science of South Africa.
Allen, L. S., and Gorski, R. A. (1992). Sexual orientation and the size of the anterior commissure in the human brain. Proc. Natl. Acad. Sci. USA. 89, 7199–7202. doi: 10.1073/pnas.89.15.7199
Altinay, M., and Anand, A. (2020). Neuroimaging gender dysphoria: a novel psychobiological model. Brain Imaging Behav. 14, 1281–1297. doi: 10.1007/s11682-019-00121-8
Anderson, K. P., Atlas, E., Ahern, M. J., and Weisbrot, I. M. (1983). Central nervous system toxoplasmosis in homosexual men. Am. J. Medicine 75, 877–881. doi: 10.1016/0002-9343(83)90420-5
Aron, C. (1999). “An animal model for a physiological interpretation of human bisexuality,” in Animal models of human emotion and cognition, eds. H. Marc, W. Richard (Washington, DC: American Psychological Association), 157–174. doi: 10.1037/10335-010
Banay, R., and Davidoff, L. (1942). Apparent recovery of a sex psychopath after lobotomy. J. Crim. Psychopathol. 4, 59–66.
Bancroft, J. (1994). Homosexual orientation: the search for a biological basis. Br. J. Psychiat. 164, 437–440. doi: 10.1192/bjp.164.4.437
Banks, A., and Gartrell, N. K. (1995). Hormones and sexual orientation: a questionable link. J. Homosex. 28, 247–268. doi: 10.1300/J082v28n03_04
Bertilsdotter Rosqvist, H., Chown, N., and Stenning, A. (2020). “Neurodiversity studies: a new critical paradigm. ST - Routledge advances in sociology,” in Neurodiversity studies: A new critical paradigm xiv (New York, NY, US: Routledge/Taylor and Francis Group), 241. doi: 10.4324/9780429322297
Blanchard, R. (2004). Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. J. Theor. Biol. 230, 173–187. doi: 10.1016/j.jtbi.2004.04.021
Blanchard, R., and Bogaert, A. F. (2004). Proportion of homosexual men who owe their sexual orientation to fraternal birth order: an estimate based on two national probability samples. Am. J. Hum. Biol. 16, 151–157. doi: 10.1002/ajhb.20006
Bockting, W. (2009). Are gender identity disorders mental disorders? Recommendations for revision of the World Professional Association for Transgender Health's Standards of Care. Int. J. Transgender. 11, 53–62. doi: 10.1080/15532730902799987
Burke, S. M., Manzouri, A. H., and Savic, I. (2017). Structural connections in the brain in relation to gender identity and sexual orientation. Sci. Rep. 7:17954. doi: 10.1038/s41598-017-17352-8
Byne, W. (1997). Why we cannot conclude that sexual orientation is primarily a biological phenomenon. J. Homosex. 34, 73–80. doi: 10.1300/J082v34n01_07
Byne, W., and Parsons, B. (1993). Human sexual orientation: the biologic theories reappraised. Arch. Gen. Psychiatry 50, 228–239. doi: 10.1001/archpsyc.1993.01820150078009
Case, L. K., and Ramachandran, V. S. (2012). Alternating gender incongruity: a new neuropsychiatric syndrome providing insight into the dynamic plasticity of brain-sex. Med. Hypotheses 78, 626–631. doi: 10.1016/j.mehy.2012.01.041
Clemens, B., Lefort-Besnard, J., Ritter, C., Smith, E., Votinov, M., Derntl, B., et al. (2023). Accurate machine learning prediction of sexual orientation based on brain morphology and intrinsic functional connectivity. Cerebral Cortex 33, 4013–4025. doi: 10.1093/cercor/bhac323
Cohen-Kettenis, P. T., Schagen, S. E., Steensma, T. D., de Vries, A. L., and Delemarre-van de Waal, H. A. (2011). Puberty suppression in a gender-dysphoric adolescent: a 22-year follow-up. Arch. Sex. Behav. 40, 843–847. doi: 10.1007/s10508-011-9758-9
Colin, O. (2015). Acceptance of lesbian, gay, bisexual, and transgender people. JAMA Neurol. 72:1209. doi: 10.1001/jamaneurol.2015.2128
Diamond, L. M. (2020). Gender fluidity and nonbinary gender identities among children and adolescents. Child Dev. Perspect. 14, 110–115. doi: 10.1111/cdep.12366
Diamond, L. M. (2021). The new genetic evidence on same-gender sexuality: Implications for sexual fluidity and multiple forms of sexual diversity. J. Sex Res. 58, 818–837. doi: 10.1080/00224499.2021.1879721
Dickter, C. L., Forestell, C. A., and Mulder, B. E. (2015). Neural attention and evaluative responses to gay and lesbian couples. Soc. Neurosci. 10, 308–319. doi: 10.1080/17470919.2014.999161
Dorner, G., Rohde, W., Stahl, F., Krell, L., and Masius, W. G. (1975). A neuroendocrine predisposition for homosexuality in men. Arch. Sex. Behav. 4, 1–8. doi: 10.1007/BF01541882
Edmiston, E. K., and Juster, R. P. (2022). Refining research and representation of sexual and gender diversity in neuroscience. Biol. Psychiat. 7, 1251–1257. doi: 10.1016/j.bpsc.2022.07.007
Ehrhardt, A. A., and Meyer-Bahlburg, H. F. (1979). Prenatal sex hormones and the developing brain: effects on psychosexual differentiation and cognitive function. Annu. Rev. Med. 30, 417–430. doi: 10.1146/annurev.me.30.020179.002221
Ellis, L., and Ames, M. (1987). Neurohormonal functioning and sexual orientation: a theory of homosexuality-heterosexuality. Psychol. Bull. 101, 233–258. doi: 10.1037/0033-2909.101.2.233
Ellis, L., and Hellberg, J. (2005). Fetal exposure to prescription drugs and adult sexual orientation. Pers. Individ. Dif. 38, 225–236. doi: 10.1016/j.paid.2004.04.004
Faris, A. N. (2016). The aging brain: Differences in WAIS-IV coding subtest performance of older gay and straight men and women. Doctoral dissertation, John F. Kennedy University.
Fausto-Sterling, A. (1995). Animals models for the development of human sexuality: a critical evaluation. J. Homosex. 28, 217–236. doi: 10.1300/J082v28n03_02
Fernández, R., Esteva, I., Gómez-Gil, E., Rumbo, T., Almaraz, M. C., Roda, E., et al. (2014). The (CA)n polymorphism of ERβ gene is associated with FTM transsexualism. J. Sex. Med. 11, 720–728. doi: 10.1111/jsm.12398
Ferveur, J. F., Stortkuhl, K. F., Stocker, R. F., and Greenspan, R. J. (1995). Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 267, 902–905. doi: 10.1126/science.7846534
Fratta, W., Biggio, G., and Gessa, G. L. (1977). Homosexual mounting behavior induced in male rats and rabbits by a tryptophan-free diet. Life Sci. 21, 379–384. doi: 10.1016/0024-3205(77)90518-5
Friedman, R. C., and Downey, J. (1993). Neurobiology and sexual orientation: current relationships. J. Neuropsychiat. Clin. Neurosci. 5, 131–153. doi: 10.1176/jnp.5.2.131
Garcia-Falgueras, A., and Swaab, D. F. (2008). A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain 131, 3132–3146. doi: 10.1093/brain/awn276
Garnets, L. D., and Kimmel, D. C. (1993). Psychological Perspectives on Lesbian and Gay Male Experiences. New York: Columbia University Press.
Gessa, G. L., and Tagliamonte, A. (1974). Role of brain monoamines in male sexual behavior. Life Sci. 14, 425–436. doi: 10.1016/0024-3205(74)90357-9
Glover, B. H. (1951). Observations on homosexuality among university students. J. Nerv. Mental Dis. 113, 377–387. doi: 10.1097/00005053-195111350-00001
Gomez-Gil, E., Canizares, S., Torres, A., de la Torre, F., Halperin, I., and Salamero, M. (2009). Androgen treatment effects on memory in female-to-male transsexuals. Psychoneuroendocrinology 34, 110–117. doi: 10.1016/j.psyneuen.2008.08.017
Gooren, L. J. (1995). Biomedical concepts of homosexuality: folk belief in a white coat. J. Homosex. 28, 237–246. doi: 10.1300/J082v28n03_03
Grannis, C., Mattson, W. I., Leibowitz, S. F., Nahata, L., Chen, D., Strang, J. F., et al. (2023). Expanding upon the relationship between gender-affirming hormone therapy, neural connectivity, mental health, and body image dissatisfaction. Psychoneuroendocrinology 156:106319. doi: 10.1016/j.psyneuen.2023.106319
Guo, Y., Li, Q., Yang, X., Jaffee, M. S., Wu, Y., Wang, F., et al. (2022). Prevalence of Alzheimer's and related dementia diseases and risk factors among transgender adults, Florida, 2012-2020. Am. J. Public Health 112, 754–757. doi: 10.2105/AJPH.2022.306720
Guze, S. B., Goodwin, D. W., and Crane, J. (1969). Criminality and psychiatric disorders. Arch. Gen. Psychiatry 20, 583–591. doi: 10.1001/archpsyc.1969.01740170087013
Hahn, A., Kranz, G. S., Kublbock, M., Kaufmann, U., Ganger, S., Hummer, A., et al. (2015). Structural connectivity networks of transgender people. Cerebral Cortex 25, 3527–3534. doi: 10.1093/cercor/bhu194
Hamer, D. H., and Copeland, P. (1994). The Science of Desire: The Search for the Gay Gene and the Biology of Behavior. New York, NY, US: Simon and Schuster.
Handler, M., Ho, V., Whelan, M., and Budzilovich, G. (1983). Intracerebral toxoplasmosis in patients with acquired immune deficiency syndrome. J. Neurosurg. 59, 994–1001. doi: 10.3171/jns.1983.59.6.0994
Hatzenbuehler, M. L., and McLaughlin, K. A. (2014). Structural stigma and hypothalamic-pituitary-adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Ann. Behav. Med. 47, 39–47. doi: 10.1007/s12160-013-9556-9
Heath, R. G. (1972). Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. J. Nerv. Mental Dis. 154, 3–18. doi: 10.1097/00005053-197201000-00002
Hegarty, P. (1997). Materializing the hypothalamus: a performative account of the “gay brain.” Femin. Psychol. 7, 355–372. doi: 10.1177/0959353597073009
Hegarty, P. (2018). A Recent History of Lesbian and Gay Psychology: From Homophobia to LGBT. New York, NY: Routledge/Taylor and Francis. doi: 10.4324/9781315563442
Hegarty, P., and Ruterford, A. (2019). Histories of psychology after Stonewall: introduction to the special issue. Am. Psychol. 74, 857–867. doi: 10.1037/amp0000571
Henrich, J., Heine, S. J., and Norenzayan, A. (2010). The weirdest people in the world? Behav. Brain Sci. 33, 61–83. doi: 10.1017/S0140525X0999152X
Hill, A. K., Dawood, K., and Puts, D. A. (2013). “Biological foundations of sexual orientation,” in Handbook of Psychology and Sexual Orientation, eds. C. Patterson and A. R., D'Augelli (New York, NY: Oxford University Press), 55–68. doi: 10.1093/acprof:oso/9780199765218.003.0005
Hines, M. (2004b). “Psychological gender development in individuals born with ambiguous genitalia,” in Paediatric and adolescent gynaecology: A multidisciplinary approach, ed. A. H. Balen (New York, NY: Cambridge University Press), 492–508. doi: 10.1017/CBO9780511527036.038
Holzer, D. (1976). Minimal early brain damage as cause of a pathologic development of sexuality. Prax. Kinderpsychol. Kinderpsychiatr. 25, 173–175.
Horne, S. G., Maroney, M. R., Nel, J. A., Chaparro, R. A., and Manalastas, E. J. (2019). Emergence of a transnational LGBTI psychology: commonalities and challenges in advocacy and activism. Am. Psychol. 74, 967–986. doi: 10.1037/amp0000561
Ito, H., Fujitani, K., Usui, K., Shimizu-Nishikawa, K., Tanaka, S., and Yamamoto, D. (1996). Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA. 93, 9687–9692. doi: 10.1073/pnas.93.18.9687
Jaarsma, P., and Welin, S. (2012). Autism as a natural human variation: reflections on the claims of the neurodiversity movement. Health Care Analy. 20, 20–30. doi: 10.1007/s10728-011-0169-9
Jones, R. M., Wheelwright, S., Farrell, K., Martin, E., Green, R., Di Ceglie, D., et al. (2012). Brief report: female-to-male transsexual people and autistic traits. J. Autism Dev. Disord. 42, 301–306. doi: 10.1007/s10803-011-1227-8
Keo-Meier, C. L., and Fitzgerald, K. M. (2017). Affirmative psychological testing and neurocognitive assessment with transgender adults. Psychiatr. Clin. North Am. 40, 51–64. doi: 10.1016/j.psc.2016.10.011
Kilpatrick, L. A., Holmberg, M., Manzouri, A., and Savic, I. (2019). Cross sex hormone treatment is linked with a reversal of cerebral patterns associated with gender dysphoria to the baseline of cisgender controls. Eur. J. Neurosci. 50, 3269–3281. doi: 10.1111/ejn.14420
Kinnunen, L. H. (2002). Evidence of a neurochemical difference between the brains of exclusively homosexual and exclusively heterosexual men: Differential effects of fluoxetine on cerebral glucose metabolism. Dissertation Abstracts International: Section B: The Sciences and Engineering, 4831.
Kiyar, M., Kubre, M. A., Collet, S., Van Den Eynde, T., T'Sjoen, G., Guillamon, A., et al. (2022). Gender-affirming hormonal treatment changes neural processing of emotions in trans men: an fMRI study. Psychoneuroendocrinology 146:105928. doi: 10.1016/j.psyneuen.2022.105928
Kooden, H. (2021). “The scarecrow's search for a brain: A gay man's odyssey for validation as a psychotherapist,” in Different Paths Towards Becoming a Psychoanalyst and Psychotherapist (New York, NY: Routledge/Taylor and Francis Group), 212–260. doi: 10.4324/9781003057383-13
Kranz, G. S., Hahn, A., Baldinger, P., Haeusler, D., Philippe, C., Kaufmann, U., et al. (2014). Cerebral serotonin transporter asymmetry in females, males and male-to-female transsexuals measured by PET in vivo. Brain Struct. Funct. 219, 171–183. doi: 10.1007/s00429-012-0492-4
Kraus, C. (2012). Critical studies of the sexed brain: a critique of what and for whom? Neuroethics 5, 247–259. doi: 10.1007/s12152-011-9107-7
Kreukels, B. P., and Guillamon, A. (2016). Neuroimaging studies in people with gender incongruence. Int. Rev. Psychiat. 28, 120–128. doi: 10.3109/09540261.2015.1113163
Kruijver, F. P., Zhou, J. N., Pool, C. W., Hofman, M. A., Gooren, L. J., and Swaab, D. F. (2000). Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J. Clin. Endocrinol. Metabol. 85, 2034–2041. doi: 10.1210/jcem.85.5.6564
Lasco, M. S. (2001). Differences of Sex and Sexual Orientation in the Human Anterior Commissure. New York: New York University.
LeVay, S. (2011). Gay, Straight, and the Reason Why: The Science of Sexual Orientation. Oxford: Oxford University Press.
Looy, H. (1995). Born gay? A critical review of biological research on homosexuality. J. Psychol. Christ. 14, 197–214.
Luders, E., Sanchez, F. J., Gaser, C., Toga, A. W., Narr, K. L., Hamilton, L. S., et al. (2009). Regional gray matter variation in male-to-female transsexualism. Neuroimage 46, 904–907. doi: 10.1016/j.neuroimage.2009.03.048
Luders, E., Sanchez, F. J., Tosun, D., Shattuck, D. W., Gaser, C., Vilain, E., et al. (2012). Increased cortical thickness in male-to-female transsexualism. J. Behav. Brain Sci. 2, 357–362. doi: 10.4236/jbbs.2012.23040
MacCulloch, M. J., and Waddington, J. L. (1981). Neuroendocrine mechanisms and the aetiology of male and female homosexuality. Br. J. Psychiat. 139, 341–345. doi: 10.1192/bjp.139.4.341
Manzouri, A., Kosidou, K., and Savic, I. (2017). Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cerebral Cortex 27, 998–1010. doi: 10.1093/cercor/bhv278
Mapou, R. L. (1990). Traumatic brain injury rehabilitation with gay and lesbian individuals. J. Head Trauma Rehabil. 5, 67–72. doi: 10.1097/00001199-199005020-00011
McCormick, C. M., Witelson, S. F., and Kingstone, E. (1990). Left-handedness in homosexual men and women: neuroendocrine implications. Psychoneuroendocrinology 15, 69–76. doi: 10.1016/0306-4530(90)90048-E
Meyer, A. (1917). “Modern conceptions of mental disease,” in Suggestions of Modern Science Concerning Education, eds. H. S. Jennings, J. B. Watson, A. Meyer, and W. I. Thomas (New York, NY: MacMillan Co.), 201–211. doi: 10.1037/10964-005
Miller, B. L., Cummings, J. L., McIntyre, H., Ebers, G., and Grode, M. (1986). Hypersexuality or altered sexual preference following brain injury. J. Neurol. Neurosurg. Psychiat. 49, 867–873. doi: 10.1136/jnnp.49.8.867
Money, J. (1986). Homosexual genesis, outcome studies, and a nature/nurture paradigm shift. Am. J. Soc. Psychiat. 6, 95–98.
Money, J. (1988). Gay, Straight, and In-Between: The Sexology of Erotic Orientation. New York: Oxford University Press.
Morales, P. C. (2000). “Neuropsychological assessment of gays and lesbians,” in Handbook of Cross-Cultural Neuropsychology, eds. E. Fletcher-Janzen, T. L. Strickland, and C. R. Reynolds (Dordrecht, Netherlands: Kluwer Academic Publishers), 55–71. doi: 10.1007/978-1-4615-4219-3_5
Moreno Robles, A. (1975). Importance of preselection of incoming candidates in psychotherapeutic training centers. Rev. Psicol. Univ. Monter. 4, 4–8.
Moreno, A., Ardila, R., Zervoulis, K., Nel, J. A., Light, E., and Chamberland, L. (2019). Cross-cultural perspectives of LGBTQ Psychology from five different countries: current state and recommendations. Preaching to the choir special issue. Psychol. Sexual. 11, 5–31. doi: 10.1080/19419899.2019.1658125
Moreno, A., Laoch, A., and Zasler, N. D. (2017). Changing the culture of neurodisability through language and sensitivity of providers: Creating a safe place for LGBTQIA+ people. NeuroRehabilitation 41, 375–393. doi: 10.3233/NRE-172187
Moss, F. A. (1932). “Mending broken personalities,” in Psychology today: Lectures and study manual, ed. W. V. Bingham (Chicago, IL: The University of Chicago Press), 126–134. doi: 10.1037/13342-014
Mueller, S. C., Guillamon, A., Zubiaurre-Elorza, L., Junque, C., Gomez-Gil, E., Uribe, C., et al. (2021). The neuroanatomy of transgender identity: mega-analytic findings from the ENIGMA Transgender Persons Working Group. J. Sexual Med. 18, 1122–1129. doi: 10.1016/j.jsxm.2021.03.079
Naik, P. P., and Farrukh, S. N. (2022). Influence of ethnicities and skin color variations in different populations: a review. Skin Pharmacol. Physiol. 35, 65–76. doi: 10.1159/000518826
Neumann, F., and Elger, W. (1981). Critical evaluation of the current concept of brain differentiation - relevance for the primate brain. Exper. Brain Res. 3, 246–261. doi: 10.1007/978-3-642-45525-4_20
Nicholson, A. A., Siegel, M., Wolf, J., Narikuzhy, S., Roth, S. L., Hatchard, T., et al. (2022). A systematic review of the neural correlates of sexual minority stress: towards an intersectional minority mosaic framework with implications for a future research agenda. Eur. J. Psychotraumatol. 13:2002572. doi: 10.1080/20008198.2021.2002572
Nieder, T. O., Jordan, K., and Richter-Appelt, H. (2011). On the neurobiology of transsexual developments-A discussion of findings on sexual differentiation, gender atypical behaviour and gender identity. Zeitschrift Sexualforschung 24, 199–227. doi: 10.1055/s-0031-1283716
Nota, N. M., Kreukels, B. P., den Heijer, M., Veltman, D. J., Cohen-Kettenis, P. T., Burke, S. M., et al. (2017). Brain functional connectivity patterns in children and adolescents with gender dysphoria: sex-atypical or not? Psychoneuroendocrinology 86, 187–195. doi: 10.1016/j.psyneuen.2017.09.014
Nussselt, L., and Kockott, G. (1976). Electroencephalographic changes in transsexualism (author's transl). EEG-EMG Zeitschrift fur Elektroenzephalographie Elektromyographie und Verwandte Gebiete 7, 42–48.
Olvera-Hernandez, S., and Fernandez-Guasti, A. (2015). Perinatal administration of aromatase inhibitors in rodents as animal models of human male homosexuality: similarities and differences. Advances in Neurobiology, 10, 381–406. doi: 10.1007/978-1-4939-1372-5_18
Park, A. (2022). Defining sexual orientation: a proposal for a new definition. Mich. J. Gender L. 29:1. doi: 10.36641/mjgl.29.1.defining
Parkinson, J. (2015). Gender dysphoria “cured” by status epilepticus. Austral. Psychiat. 23, 166–168. doi: 10.1177/1039856214568223
Pasterski, V., Gilligan, L., and Curtis, R. (2014). Traits of autism spectrum disorders in adults with gender dysphoria. Arch. Sex. Behav. 43, 387–393. doi: 10.1007/s10508-013-0154-5
Paul, T., Schiffer, B., Zwarg, T., Kruger, T. H., Karama, S., Schedlowski, M., et al. (2008). Brain response to visual sexual stimuli in heterosexual and homosexual males. Hum. Brain Mapp. 29, 726–735. doi: 10.1002/hbm.20435
Perkins, A., and Fitzgerald, J. A. (1997). “Sexual orientation in domestic rams: some biological and social correlates,” in Sexual orientation: Toward biological understanding, L. Ellis, L. Ebertz (Westport, CT: Praeger Publishers/Greenwood Publishing Group), 107–127.
Perry, D., Walder, K., Hendler, T., and Shamay-Tsoory, S. G. (2013). The gender you are and the gender you like: Sexual preference and empathic neural responses. Brain Res. 1534, 66–75. doi: 10.1016/j.brainres.2013.08.040
Pillay, S. R., Ntetmen, J. M., and Nel, J. A. (2022). Queering global health: an urgent call for LGBT+ affirmative practices. Lancet Global Health 10, E574–E578. doi: 10.1016/S2214-109X(22)00001-8
Ponseti, J., Siebner, H. R., Kloppel, S., Wolff, S., Granert, O., Jansen, O., et al. (2007). Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS ONE 2:e762. doi: 10.1371/journal.pone.0000762
Potzl, O. (1951). Contributions to the problem of sexual-metabolic delusions in schizophrenia. Z. Psychother. Med. Psychol. 1, 221–227.
Rahman, Q., and Yusuf, S. (2015). Lateralization for processing facial emotions in gay men, heterosexual men, and heterosexual women. Arch. Sex. Behav. 44, 1405–1413. doi: 10.1007/s10508-014-0466-0
Ramachandran, V. S., and McGeoch, P. D. (2007). Occurrence of phantom genitalia after gender reassignment surgery. Med. Hypotheses 69, 1001–1003. doi: 10.1016/j.mehy.2007.02.024
Ramachandran, V. S., and McGeoch, P. D. (2008). Phantom penises in transsexuals. J. Conscious. Stud. 15, 5–16. doi: 10.1080/13554790903081767
Rametti, G., Carrillo, B., Gomez-Gil, E., Junque, C., Segovia, S., Gomez, A., et al. (2011). White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J. Psychiat. Res. 45, 199–204. doi: 10.1016/j.jpsychires.2010.05.006
Ramos, S. (2021). “HIV and brain health in LGBTQ PoC,” in Heart, brain and mental health disparities for LGBTQ People of Color, ed. J. J. Garcia (New York, NY: Palgrave Macmillan/Springer Nature), 93–106. doi: 10.1007/978-3-030-70060-7_8
Ricketts, W. (1984). Biological research on homosexuality: Ansell's cow or Occam's razor? J. Homosex. 9, 65–93. doi: 10.1300/J082v09n04_06
Rinieris, P., Markianos, M., Hatzimanolis, J., and Stefanis, C. (1985). A psychoendocrine study in male paranoid schizophrenics with delusional ideas of homosexual content. Acta Psychiatr. Scand. 72, 309–314. doi: 10.1111/j.1600-0447.1985.tb02612.x
Rosanoff, A. J. (1938). “Neuropsychiatric Syndromes,” in Manual of psychiatry and mental hygiene, ed. A. J. Rosanoff (New York: John Wiley & Sons, Inc.), 71–168. doi: 10.1037/11375-003
Rosendale, N., and Josephson, A. (2015). Acceptance of lesbian, gay, bisexual, and transgender people-Reply. JAMA Neurol. 72, 1209–1210. doi: 10.1001/jamaneurol.2015.2131
Rouse, M. Jr., and Hamilton, E. (2021). Rethinking sex and the brain: How to create an inclusive discourse in neuroscience. Mind, Brain, Educ. 15, 163–167. doi: 10.1111/mbe.12285
Saravi, F. (2007). “The elusive search for a 'gay gene'DP - 2007,” in Tall tales about the mind and brain: Separating fact from fiction, ed. S. S. Della (New York, NY: Oxford University Press), 461–477. doi: 10.1093/acprof:oso/9780198568773.003.0029
Savic, I., and Lindstrom, P. (2008). PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. PNAS 105, 9403–9408. doi: 10.1073/pnas.0801566105
Schoning, S., Engelien, A., Bauer, C., Kugel, H., Kersting, A., Roestel, C., et al. (2010). Neuroimaging differences in spatial cognition between men and male-to-female transsexuals before and during hormone therapy. J. Sexual Med. 7, 1858–1867. doi: 10.1111/j.1743-6109.2009.01484.x
Sigal, M. (1978). Relationship between presumed etiological factors and clinical picture in 100 schizophrenic males. Psychiatr. Clin. 11, 139–146. doi: 10.1159/000283744
Soleman, R. S., Schagen, S. E., Veltman, D. J., Kreukels, B. P., Cohen-Kettenis, P. T., Lambalk, C. B., et al. (2013). Sex differences in verbal fluency during adolescence: a functional magnetic resonance imaging study in gender dysphoric and control boys and girls. J. Sexual Med. 10, 1969–1977. doi: 10.1111/jsm.12083
Sorouri Khorashad, B., Khazai, B., Talaei, A., Acar, F., Hudson, A. R., Borji, N., et al. (2020). Neuroanatomy of transgender persons in a Non-Western population and improving reliability in clinical neuroimaging. J. Neurosci. Res. 98, 2166–2177. doi: 10.1002/jnr.24702
Swaab, D. F., Gooren, L. J., and Hofman, M. A. (1995). Brain research, gender and sexual orientation. J. Homosex. 28, 283–301. doi: 10.1300/J082v28n03_07
Swaab, D. F., and Hofman, M. A. (1988). Sexual differentiation of the human hypothalamus: ontogeny of the sexually dimorphic nucleus of the preoptic area. Brain Res. Dev. Brain Res. 44, 314–318. doi: 10.1016/0165-3806(88)90231-3
Terzian, H., and Dalle Ore, G. (1955). Syndrome of Kluver and Bucy, reproduced in man by bilateral removal of the temporal lobes. Neurology 5, 374–380. doi: 10.1212/WNL.5.6.373
Tomson, A., McLachlan, C., Wattrus, C., et al. (2021). Southern African HIV Clinicians Society gender-affirming healthcare guideline for South Africa. South. Afr. J. HIV Med. 22:a1299. doi: 10.4102/sajhivmed.v22i1.1299
Trittschuh, E. H., Parmenter, B. A., Clausell, E. R., Mariano, M. J., and Reger, M. A. (2018). Conducting neuropsychological assessment with transgender individuals. Clin. Neuropsychol. 32, 1393–1410. doi: 10.1080/13854046.2018.1440632
Wang, Y., Khorashad, B. S., Feusner, J. D., and Savic, I. (2021). Cortical gyrification in transgender individuals. Cerebral Cortex 31, 3184–3193. doi: 10.1093/cercor/bhaa412
White A. A. III. and Chanoff D. (2011). Seeing Patients: Unconscious Bias in Health Care. Cambridge: Harvard University Press. doi: 10.2307/j.ctvjk2xx5
Wiersch, L., Hamdan, S., Hoffstaedter, F., Votinov, M., Habel, U., Clemens, B., et al. (2023). Accurate sex prediction of cisgender and transgender individuals without brain size bias. Sci. Rep. 13:13868. doi: 10.1038/s41598-023-37508-z
Wilks, M., Papakyriakou, B., and Nel, J. A. (2022). Positioning resilience science more centrally in affirming LGBTIQA+ persons and communities. South African J. Psychol. 52, 351–363. doi: 10.1177/00812463211073872
Wilson, W. P., Zung, W. W., and Lee, J. C. (1973). Arousal from sleep of male homosexuals. Biol. Psychiatry 6, 81–84.
Witelson, S. F., Kigar, D. L., Scamvougeras, A., Kideckel, D. M., Buck, B., Stanchev, P. L., et al. (2008). Corpus callosum anatomy in right-handed homosexual and heterosexual men. Arch. Sex. Behav. 37, 857–863. doi: 10.1007/s10508-007-9276-y
Wolpe, P. R. (2004). Ethics and social policy in research on the neuroscience of human sexuality. Nat. Neurosci. 7, 1031–1033. doi: 10.1038/nn1324
Xerxa, Y., White, T., Busa, S., Trasande, L., Hillegers, M. H. J., Jaddoe, V. W., et al. (2023). Gender diversity and brain morphology among adolescents. JAMA Netw. Open 6:e2313139. doi: 10.1001/jamanetworkopen.2023.13139
Xu, Y., Norton, S., and Rahman, Q. (2017). Sexual orientation and neurocognitive ability: a meta-analysis in men and women. Neurosci. Biobehav. Rev. 83, 691–696. doi: 10.1016/j.neubiorev.2017.06.014
Yamamoto, D., Ito, H., and Fujitani, K. (1996). Genetic dissection of sexual orientation: behavioral, cellular, and molecular approaches in Drosophila melanogaster. Neurosci. Res. 26, 95–107. doi: 10.1016/S0168-0102(96)01087-5
Ye, Z., Kopyciok, R., Mohammadi, B., Kramer, U. M., Brunnlieb, C., Heldmann, M., et al. (2011). Androgens modulate brain networks of empathy in female-to-male transsexuals: an fMRI study. Zeitschrift Neuropsychol. 22, 263–277. doi: 10.1024/1016-264X/a000056
Zhou, J., Hofman, M., Gooren, L., and Swaab, D. (1997). A sex difference in the human brain and its relation to transsexuality. Nature 378, 68–70. doi: 10.1038/378068a0
Keywords: key developments, LGBTQIA+, neuroscience, literature review, research trends, sexual and gender diversity, SOGI, LGBTQIA+ history
Citation: Moreno JA, Manca R, Albrechet-Souza L, Nel JA, Spantidakis I, Venter Z and Juster R-P (2024) A brief historic overview of sexual and gender diversity in neuroscience: past, present, and future. Front. Hum. Neurosci. 18:1414396. doi: 10.3389/fnhum.2024.1414396
Received: 10 May 2024; Accepted: 14 August 2024;
Published: 13 September 2024.
Edited by:
Rene Luis Vidal, Universidad Mayor, ChileReviewed by:
Ivanka Savic, Karolinska Institutet (KI), SwedenCopyright © 2024 Moreno, Manca, Albrechet-Souza, Nel, Spantidakis, Venter and Juster. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jhon Alexander Moreno, amhvbi5hbGV4YW5kZXIubW9yZW5vLjFAdW1vbnRyZWFsLmNh
 Jhon Alexander Moreno
Jhon Alexander Moreno Riccardo Manca
Riccardo Manca Lucas Albrechet-Souza
Lucas Albrechet-Souza Juan A. Nel
Juan A. Nel Ioannis Spantidakis
Ioannis Spantidakis Zindi Venter
Zindi Venter Robert-Paul Juster
Robert-Paul Juster