
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Hum. Neurosci. , 03 September 2024
Sec. Cognitive Neuroscience
Volume 18 - 2024 | https://doi.org/10.3389/fnhum.2024.1401895
The law assumes that healthy adults are generally responsible for their actions and have the ability to control their behavior based on rational and moral principles. This contrasts with some recent neuroscientific accounts of action control. Nevertheless, both law and neuroscience acknowledge that strong emotions including fear and anger may “trigger” loss of normal voluntary control over action. Thus, “Loss of Control” is a partial defense for murder under English law, paralleling similar defenses in other legal systems. Here we consider the neuroscientific evidence for such legal classifications of responsibility, particularly focussing on how emotional states modulate voluntary motor control and sense of agency. First, we investigate whether neuroscience could contribute an evidence-base for law in this area. Second, we consider the societal impact of some areas where legal thinking regarding responsibility for action diverges from neuroscientific evidence: should we be guided by normative legal traditions, or by modern understanding of brain functions? In addressing these objectives, we propose a translation exercise between neuroscientific and legal terms, which may assist future interdisciplinary research.
All known human societies have some concept of responsibility for action. Healthy adults are held responsible for actions that they voluntarily choose and execute, but they are not generally responsible for actions that are involuntary. In common law1 systems, criminal responsibility requires mens rea: the agent must have intended the criminal action. This concept recalls psychological theories of action in which individuals are capable of appropriate and rational action because they plan their actions on the basis of goals and outcomes (Fishbein and Ajzen, 1975; Ajzen and Fishbein, 1980; Ajzen, 1985). Importantly, the law recognizes that not all human actions conform to this model. Recent psychological and neuroscientific studies likewise agree that the capacity of human volition is both profoundly limited (Libet et al., 1983; Wegner, 2003), and also routinely overestimated. For example, Libet argued that the timing of subjective intention came too late to provide active control of action generation, and was limited, at best, to a form of veto. Similarly, self-serving biases (Bandura, 1984, 2002) and misperceptions about the sources of our own actions may lead people to overestimate their own volitional agency.
In fact, human behaviors rarely have simple causes. Most action choices reflect complex interplays between neurocognitive factors within an individual on the one hand, and social/environmental processes on the other. Nevertheless, the criminal law must judge individual actions in a reasonable and consistent way, including classifying them as voluntary or not. Here we consider how this legal classification relates to neurocognitive concepts of the initiation, control, and awareness of actions. We focus on the partial defenses of loss of control and of diminished responsibility in England and Wales, though the points we raise may also be relevant to many other jurisdictions where similar principles apply. English law generally assumes that healthy adults are responsible for their actions, because they have the ability to control their behavior based on rational and moral principles (Moore, 2020). This view of action differs from more mechanistic views found in recent neuroscientific research. That research often stresses specific brain subsystems that drive behavior. For example, actions can arise from habit (Graybiel, 2008), or from specific calculations for reward maximization (Talmi et al., 2009). Some “neurolaw” approaches emphasize neural correlates of individual traits (Philippi et al., 2015), or the relation between stable measures of individuals’ brain function and their offending behavior (Kiehl et al., 2018), in addition to the standard emphasis on actions as individual events (Morse, 2004, 2023). Such general traits are assumed to contribute to the generation of particular culpable actions. Here, we take a complementary event-based approach, by focussing on actions themselves. The relation between specific actions and stable enduring traits of the agent is outside our scope.
Specifically, we consider the neural mechanisms that may underlie a specific action that constitutes a crime. We then try to relate these mechanisms to the legal treatment of the same action. In particular, we ask how a scientific understanding of the neural mechanisms involved in causing a specific action should influence the legal treatment of the action. We develop this approach in the context of Loss of Control defenses. Figure 1 and Table 1 show the different definitions and terminology that have been used in neuroscience and in law in discussing responsibility for actions.
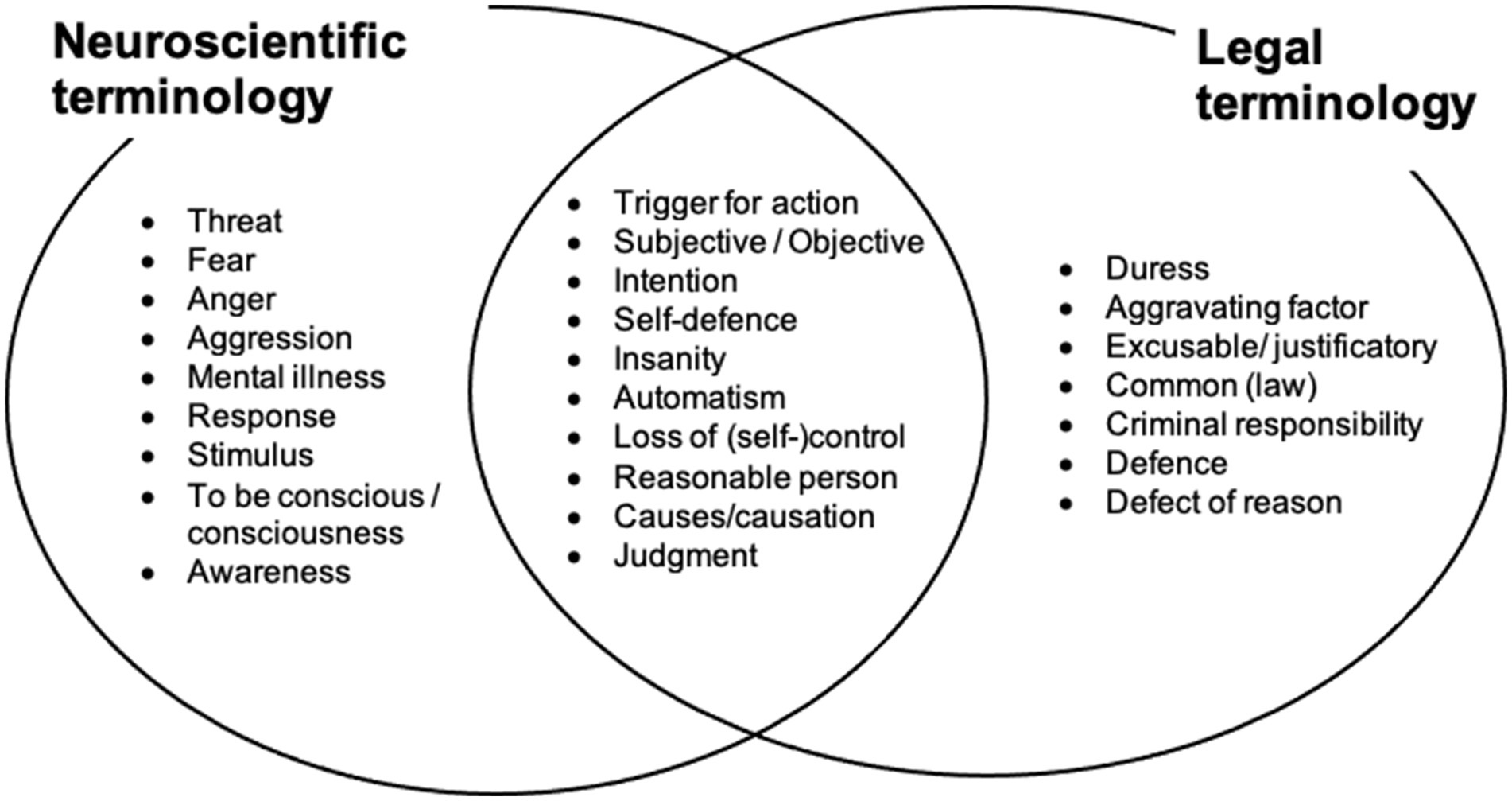
Figure 1. Key terminology for neuroscience and law. Each discipline has specific vocabulary that differs from the definition of these words in everyday language. Some terms are shared between the two disciplines, but each discipline has their own definition of those terms. Friction in interdisciplinary teams can occur when terms are not used in the discipline-specific way, or, when scholars are unaware of the presence of specific definitions of terms within the “other” discipline. The left column lists concepts that are relevant to responsibility and have a technical meaning within neuroscience, but which lack a corresponding technical definition in law, and would therefore fall back to the meaning based on everyday usage, potentially leading to incommensurability. The right column lists concepts that have a technical meaning in law, but which create difficulties in neuroscience because they cannot be readily related to a brain circuit or cognitive process, and so fall back to meaning based on everyday usage. The central, intersection column lists key terms that are important for both the neuroscience of responsibility and for legal responsibility, but which have a meaning restricted to just one discipline, or have distinctly different meanings within each discipline. Table 1 part C clarifies these concepts by directly contrasting neuroscientific and legal definitions.
The law recognizes that certain environmental situations can provide ‘qualifying triggers’. These situations may lead directly, and presumably unavoidably, to action, and lie outside of an agent’s normal voluntary control. Neuroscientifically speaking, this would imply a basic neuromotor mechanism intruding into the sphere of rational, responsible action. The ‘loss of control’ defense is a legal defense arguing for reduced responsibility, on the grounds that one of these qualifying triggers is present. Actions performed out of fear or out of anger provide the most ready examples of the ‘loss of control’ defense. In particular, when an action is performed in situations of fear or anger, criminal liability for a resulting death may be reduced from murder to manslaughter. The crucial difference between murder and voluntary manslaughter is the intent to kill. The partial defenses provide for a reduction in culpability where D (the defendant) intends to kill, but there is an explanation for the behavior that may reduce legal culpability. In the “partial defenses” (a legal technical term that refers to this particular type of defense), the criminal law is concerned primarily with the nature and the severity of those causes of the action that derive from the situation (i.e., the factors that lead to the agent being afraid or angry). Therefore, the law seems to assume that agents can be less intentional when they are strongly afraid, or strongly angry. In principle, neuroscientific knowledge could either support this assumption or call it into question.
In section 1, we outline a neurocognitive classification of human behavior, focussing on behaviors that are legally relevant. We explore whether the complementary legal and neuroscientific approaches to human behavior should be seen as compatible but alternative conceptual schemes that merely require some interdisciplinary translation, or whether they rather involve fundamentally incommensurable ways of thinking about the same events. In section 2, we consider the societal impact of the areas where neuroscience and law disagree, and we use these as a blueprint for future interdisciplinary research.
A legal plea of ‘Not guilty’ is a personal statement about agency: ‘I did not do it’. There are at least 4 factors that could motivate this claim.
1. The agent in question truly did not do it.
2. The claim reflects secondary gain considerations (e.g., the agent did it, and knows that they did it, but they are lying).
3. The agent did it, but they do not experience that they did it, because some neurocognitive mechanism reduced their ability to control their action, and/or their experience of agency with respect to the action (Haggard et al., 2002; Yoshie and Haggard, 2013; Christensen et al., 2016). In this case, the agent’s claim ‘I did not do it’ may be coherent from their first-person perspective.
4. The agent did it, but either does not or did not realize it was legally wrong.
These four situations attract very different attention from the law, although they may not separate very clearly in any particular case. Broadly speaking, however, if scenario 1 can be established, the agent is clearly not criminally liable. If scenario 2 can be established, the agent is criminally liable. Scenario 4 is also recognized as a “mistake of law,” although there is a general assumption that agents should have some knowledge, simply through being members of society, about what actions are proscribed and what are allowed (Matthews, 1983). The most interesting situation for our current neurolaw discussion is situation 3, since it speaks directly to the loss of control defense. The law is rightly skeptical regarding situation 3, because claiming situation 3 is very attractive when situation 2 actually applies. Consider an agent who knew at the time what they were doing, did it nevertheless, and still knows now that they have done it. This agent has a strong motivation to claim that their action was, in fact, beyond their voluntary control. Clearly, any claim of situation 3 needs to present convincing evidence that the agent did not have a conscious experience of willing and controlling the action. Here we consider whether neuroscientific evidence can potentially clarify the circumstances under which situation 3 is likely, and also when it is unlikely. We do so in the context of one particular legal argument, namely loss of control (see Table 2). The partial defense to murder of loss of control is laid down in sections 54 and 55 of the Coroners and Justice Act 2009.2 We will not consider situation 4.
The loss of control defense repeals the previous common law partial defense of provocation and applies in cases where a person claims to have lost self-control [section 54(1)(a)] if this conduct was not motivated by a desire for revenge [section 54(4)]. Furthermore, it must have been caused by a so-called qualifying trigger, i.e., by a fear of serious violence [section 55(3), ‘fear’ trigger] and/or by a thing or things done or said (or both) which constituted circumstances of an extremely grave character, which caused the person to have a justifiable sense of being seriously wronged [section 55(4), ‘anger’ trigger: we take anger as the neurocognitive emotional state that results from being ‘wronged’]. However, the defendant cannot rely on one or both of the triggers if they themselves incited the trigger situation as an excuse to use violence [section 55(6)(a) and (b)], e.g., if A taunts B to make B angry, A could not then claim that fear of B’s serious violence is a qualifying trigger. In addition, section 55(6)(c) provides another important exclusion from the ‘anger’ trigger: it excludes things said or done constituting ‘sexual infidelity’. Sexual infidelity is, thus, not relevant to whether someone has been ‘seriously wronged’. The final requirement within the loss of control defense is a so-called ‘objective test’. The defendant’s reaction must have been objectively understandable in a way that a person of the defendant’s sex and age, with a normal degree of tolerance and self-restraint and in the circumstances of the defendant, might have reacted in the same or in a similar way [section 54(1)(c)]. Interestingly, this so-called ‘objective’ test in fact introduces a norm-related element to the defense: would it be ‘normal’ to behave in this way, given the situation? The mention of a person’s sex as part of this norm is particularly striking. On the one hand, it could be seen as biological determinism, or as an unwarranted, gendered bias. On the other hand, one legal text speculates that it reflects an odd legacy from case law, given that there is no evidence for different levels of normal tolerance or self-restraint between the sexes (Child and Ormerod, 2015). If all these requirements are met, the defense will reduce the defendant’s liability from murder to manslaughter. Importantly, this defense therefore avoids the mandatory life sentence for murder.
From the neuroscientific perspective, there is something unusual in the way the law deals with criminal acts. Both law and neuroscience explicitly acknowledge two states that may lead to a loss of normal voluntary control over action (out of fear, vs. out of anger). However, the two disciplines use very different means to establish whether these states were present or not.
The first scientific assessments of fear and anger states were made by Cannon (1927, 1931) who postulated that visceral reactions in emotional states are unspecific (i.e., they do not show a specific pattern depending on the particular emotional state). Ax (1953) combined several psychophysiological measures of autonomous nervous system activity (e.g., heartrate, ECG; respiratory rate; galvanic skin response, GSR; electromyography, EMG, etc.) to assess the difference in psychophysiological arousal between fear and anger states. Ax made volunteer participants, in turn, fearful and angry. Anger caused higher diastolic blood pressure, higher EMG levels, and more frequent galvanic skin responses than fear. Conversely, when participants were in a fearful state, they showed an increased tonic baseline level of GSR, an increased number of phasic EMG peaks and a higher respiration rate. These differential effects were broadly replicated later by Schachter (1957).
However, the law has no a posteriori evidence about such neurophysiological states at the time of the act. Therefore, these differential neurobiological qualities are useful chiefly because they correlate with the different subjective experiences of fear and anger, respectively. In fact, fear and anger frequently co-occur in loss of control situations, but they have different effects on capacity for voluntary action, and are indeed treated differently in section 55 of the Coroners and Justice Act (2009), see Table 2.
Such differential diagnosis is borne out by neuroimaging studies. In a meta-analysis, Vytal and Hamann (2010) identified 13 clusters of brain activity, mainly located in left inferior frontal gyrus (IFG), as specific to anger states. Fear > Anger contrasts (comparisons) revealed 11 significant clusters, mainly located in left amygdala. Threat processing that leads to fear is confined to specific circuits, including the amygdala (Davis, 1994 LeDoux, 2003, 2012; Adolphs et al., 1994), while anger states recruit higher-level brain circuitries such as PFC. While it is true that no ‘distinct systems’ exist in the brain, and all systems are in some way connected with one another (Pessoa, 2017, 2023a,b; Pessoa and McMenamin, 2017), put simply, anger is a cortical response, and therefore participates in the wider cognitive systems for rational control of appropriate action. In contrast, fear is a subcortical response, linked to evolutionarily ancient systems for control of basic survival-oriented behaviors.
Fear has been described as a ‘physiological state that intervenes between stimuli and responses (…). Conscious fear can occur when conditions are favorable, but such conscious states come about through different processes that involve different [neural] circuits. The function of the neural circuit [of fear] …is to coordinate brain and body resources to increase the chance of surviving the encounter (…)’ (Ledoux, 2014, p. 2873). Neuroscientific accounts of brain mechanisms in humans are often based on comparative research with other animals. In the case of fear, the crucial role of the amygdala circuit has been established in studies with rodents (LeDoux, 2003). Responses to threats are stereotyped and species-specific. Briefly, the central nucleus of the amygdala is connected via the periacqueductal gray with the brain’s motor systems, to activate specific defense responses (“fight, flight or freeze” responses). When danger is detected by a sensory system such as vision or hearing, the information is processed by the thalamus and relayed directly to the amygdala. This evaluation activates the autonomous nervous system (e.g., increasing heart rate and blood pressure), and the neuroendocrine system (e.g., triggering stress hormone release by action on the pituitary and adrenal glands). These changes prepare the organism for the optimal behavioral response to threat. The amygdala also directly impacts the motor systems to produce fight and flight behaviors by acting on the hypothalamic–pituitary–adrenal axis, releasing neurotransmitters implicated in motor control and thus provoking motor responses such as trembling. See Figure 2 for an illustration.
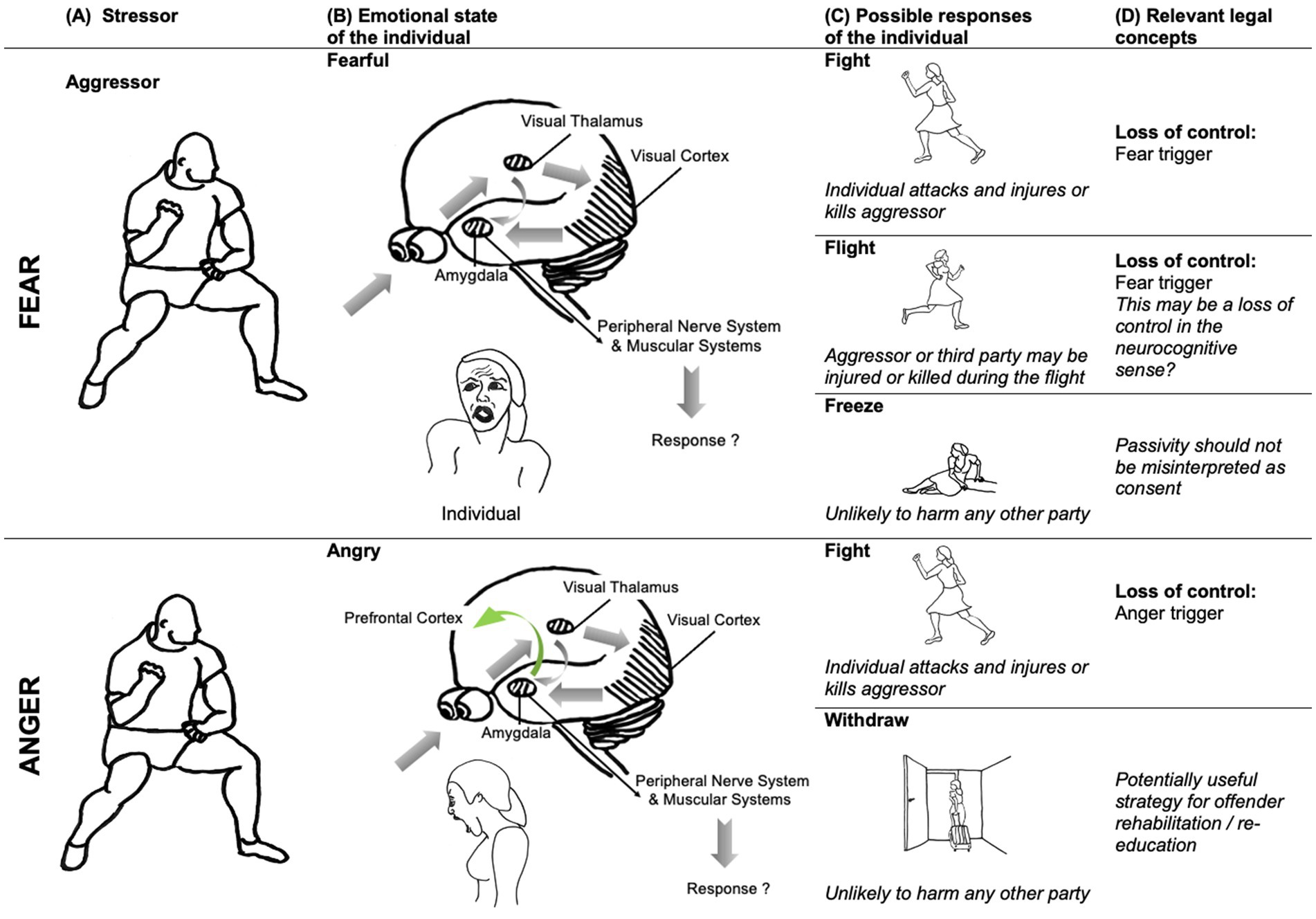
Figure 2. The neural fear/threat circuit and its legal implications. Colomn A: An aggressor is considered a stressor stimulus that is detected by an individual’s sensory systems. Column B: Visual information relating to the stressor is relayed via the visual thalamus to the visual cortex and directly to the central nucleus of the amygdala. The amygdala has direct projections with the peripheral nerve and muscular systems of the body, which prepare the body for appropriate response to the stressor, by activating specific and stereotyped defense behaviors. Depending on the sensory input, the individual’s appraisal will lead to different emotions: fear or anger. Column C: In fear contexts, three distinct response types are prominent: “fight, flight or freeze.” In anger contexts, reactive aggression responses predominate, but can be downregulated by the prefrontal cortex (PFC) in most circumstances, as when the individual withdraws. Column D: The individual’s response is legally significant: for example, the individual’s fight response may lead to injury, or even death, of the aggressor. The flight response might unintentionally harm a third party. Classically, legal considerations of loss of control apply to fight responses, and depend on identifying a fear or anger trigger. Neuroscientific evidence that multiple responses can arise from the same circuitry raises the question of how to treat these responses consistently in law. Illustration, text and neural models elaborated on basis of illustration from Ledoux (2003). Drawings: © Main Author anonymized.
Human experimental studies are largely based on showing fearful or angry faces to healthy volunteers while recording their brain activity. Such threatening stimuli activate similar neural circuits in humans to those in animals (LeDoux, 2003; Denson et al., 2009b). The subcortical circuits underlying threat-processing and fear are thought to be evolutionarily-conserved. However, humans have a strong projection from the prefrontal cortex to the amygdala. This projection is thought to play an important role in learned control of fear, and is a focus for cognitive therapies in anxiety disorders.
The legal concept of loss of control seems to suggest that brain circuits for fear should influence brain circuits for voluntary action. Indeed, some scientific experiments have investigated the effects of fear on control of voluntary action in humans. Transcranial magnetic stimulation (TMS) applied over the motor cortex during the observation of fearful body positions results in lower motor excitability in hand muscles responsible for grasping (first dorsal interosseous), than during observation of happy and neutral body positions (Borgomaneri et al., 2015). Conversely, excitability increases when participants see angry bodily expressions (Hortensius et al., 2016). This suggests a visuo-motor route within the brain that could modulate the tendency to approach a stimulus, with the modulation operating in different directions in the case of fear and anger.
To our knowledge, however, no studies with human participants have investigated whether and how intense fear-states in the individual themselves might lead to a loss of voluntary action control: such experiments would quite probably be unethical. Experimental studies confirm that fear and anger indeed influence the subjective experience of voluntary action control, and particularly the ‘sense of agency’ (i.e., the subjective experience of controlling one’s own voluntary actions, and, through them, their consequences in the external world). Using an implicit measure of sense of agency, based on time perception (the “intentional binding” paradigm; Haggard et al., 2002; Haggard, 2017), a laboratory analog of “fear” was induced (by means of the threat-of-shock paradigm; Davis et al., 2010; Robinson et al., 2013). Then, participants were asked to estimate the interval between their action and the subsequent outcome. Their time estimation (intentional binding) in the condition of fear was compared to their performance in a neutral condition, with no emotion induction. Longer time estimates in the fear condition were taken to indicate a reduced sense of agency. Thus, fear apparently undermined participants’ subjective sense of control over their ations (Christensen et al., 2019, exp. 1&2). Conversely, other research has shown that positive emotion and arousal induction lead to an increase in the subjective sense of control (Wen et al., 2015; Minohara et al., 2016). Thus, the concept that fear can lead to loss of control comes from experimental human work on relatively weak fear-inducing stimuli. Conversely, strong evidence from animal research with non-human mammals suggests a cornered, frightened animal will nevertheless attack when possibilities of escape have been exhausted (Seligman, 1971; Pichon et al., 2012).
This argument has interesting wider implications. If neuroscience demonstrates a mechanism that directly and powerfully shapes human behavior, it seems logical that engagement of this mechanism must be relevant if it occurs in legal contexts. An interesting future challenge for neurolaw will involve considering whether a single, identified neural mechanism, in this case the fear/threat response circuit, demands consistent legal treatments across the different behaviors, and thus legal cases, in which it figures.
The case of anger is less clear. Anger has been defined as a ‘syndrome of relatively specific feelings, cognitions and physiological reactions linked associatively with an urge to injure some target’ (Berkowitz and Harmon-Jones, 2004). However, no single, dedicated low-level circuit for anger, analogous to that for fear (LeDoux, 2012), has been identified in animals. Research with animals shows that aggression out of anger can be viewed as a set of several distinct neuromotor elements, rather than a unitary nervous state. These elements include a defensive aggression circuitry (self-protection against harm), reproductive aggression circuitry (against competing individuals) and a predative aggression circuitry (against a possible prey) (Chi and Flynn, 1971; LeDoux, 2012). Interestingly, these responses are highly species-specific, and are often triggered by the need to defend territory, mates, kin or resources from conspecifics (Brosnan and de Waal, 2003; Roma et al., 2006).
Studies with humans have struggled to produce convincing methods for eliciting genuine anger in experimental situations, again perhaps because ethical reasons discourage making volunteer participants truly angry (Anderson and Bushman, 1997). Instead, milder experimental paradigms have been used, based on inducing anger through frustration. In human social experiments, anger has been induced, for example, by social humiliation (Ritter and Eslea, 2005; Leary et al., 2006), by economic inequity (Beyer et al., 2015; Klimecki et al., 2018), or by posing an “impossible task” to participants (Buss, 1961; Taylor, 1967; Pawliczek et al., 2013). These studies show widespread neural activity throughout the brain associated with frustration/anger, including both cognitive and ‘limbic’ (emotional) neural systems (e.g., Denson et al., 2009b; Blair, 2012). In the aforementioned study on the effects of fear on participants’ sense of agency, also a lab analog of “anger” was induced in a different group of participants (using an “impossible task”-frustration paradigm; Buss, 1961; Taylor, 1967). Similar to the fear condition explained above, as compared to a neutral condition, participants’ subjective sense of control over their own actions was again reduced when angry (Christensen et al., 2019; exp. 3). All three of these experiments showed a significant mismatch between the objective facts of an action, and participants’ subjective sense of control over it, both when participants were fearful, or angry.
In criminal law proceedings, one cannot retrospectively establish exactly what happened at the moment of a crime and whether ‘loss of control’ applies, so the factors relevant to a particular legal scenario can never be definitively found in the experimental literature. But law and neuroscience have rather different ways to deal with these knowledge gaps. The law is concerned with the mind-set of the person (mens rea) at the moment of the act (actus reus). The law assumes that people generally act with intent, while recognizing that intended actions may have unintended outcomes. In this context intent or intention has a very different meaning for law and neuroscience. Intention in the law typically refers to intended outcomes. The question is whether or not the person had the intention to kill at the time of the killing. In contrast, neuroscientists use the term intention or intentional to refer to the neural pathways that drive motor action, rather than the representation of the final outcome of the action. For example, intentional actions necessarily involve the cerebral cortex, and the final common path from the brain to the body through the primary motor cortex. This contrasts with non-intentional forms of action, such as reflexes, which may bypass the brain entirely. We elaborate on this disinction in Table 3.
In both neuroscience and law, the classification of different subtypes of action appeals to both ‘objective’ and ‘subjective’ evidence. However, the meaning of these terms also differs between the disciplines. For neuroscience, ‘subjective’ typically refers to reports of experiences of action and mental states. Neuroscientific interest in subjective experience is generally based on linking specific subjective experiences with specific physiological mechanisms. Reports of subjective experience also reflect general cognitive biases and distortions, such as self-serving bias and confirmation bias. Neuroscientific investigations often seek to control for these wider aspects of subjective data ‘Objective’ evidence in neuroscience, instead, refers to brain events and measurements of brain function. These include neurophysiological measures (e.g., fMRI, EEG), and some implicit behavioral measures. Asking people to describe their subjective experience directly, for example, in direct reports, or through a questionnaire, would not constitute an objective measure, though their experience might also show important correlations with objective measures of brain function.
In contrast, for law, a ‘subjective’ element describes a behavior or situation as it was perceived by the person. An ‘objective’ element considers whether a reasonable neutral observer would have acted or perceived a situation in the same or in a similar way. There is a further ‘subjective’ requirement in the cases considered here: the homicidal action must have resulted from a loss of self-control. That is, the defendant must claim and prove that they ‘lost it’. Unfortunately, it remains unclear what amounts to a loss of self-control – there is no definition in the Coroners and Justice Act (2009). In R v Jewell, however, loss of control was defined as the ‘loss of the ability to act in accordance with considered judgment or a loss of normal powers of reasoning’ (R v Jewell [2014] EWCA Crim 414 [10]). One approach might involve defining loss of control by exclusion. For example, the processes of planning and deliberation that are considered necessary for murder might at first sight seem to be incompatible with the concept of loss of control. For example, one might describe pre-action conditions such as reasoning and deliberation that accompany normal human voluntary actions. Absence of these pre-action conditions woud then be necessary (but not sufficient) for demonstrating loss of control. However, this approach based on exclusion is not the path taken by the Coroners and Justice Act (2009).
Another ‘subjective’ requirement can be found in section 55(3). The defendant must act out of fear of serious violence. As with the ‘anger’ trigger in section 55(4) there are both ‘subjective’ and ‘objective’ requirements. The subjective requirement, for anger is that the defendant must have felt seriously wronged. The objective requirement for anger is that the feeling of being seriously wronged is justifiable. Here, ‘objective’ simply means that a person of normal tolerance and self-restraint might have reacted in the same or in a similar way to the defendant.
The “fear trigger” is considered through the lens of the person who experienced the fear response but has objective requirements. The fear reaction is assessed from the standpoint of someone of the same age and sex with a normal degree of tolerance and self-restraint3. This creates a problem for juries applying the objective test. An illustration of this is in a case where the defendant made a frenzied attack on a friend after consuming alcohol. The defendant claimed that he experienced fear of serious violence when his friend attacked him with a hammer. The appeal court decided that a jury might find when assessing the objective test that whilst the initial response might arise from fear they might conclude that the length of the attack, which was estimated at over five minutes meant that it carried on when the accused was no longer in fear R v Goodwin [2018] EWCA 2287.
Based on the neuroscientific literature, it is assumed that both fear and anger may lead to aggressive behaviors (Geen, 1990). However, these behaviors are likely to differ in important ways. The involvement of conscious awareness, and, thus, the potential for executive control, is more prominent in anger than in fear. Aggressions that happen out of fear are relatively automatic, brief, typically involve stereotyped fight or flight responses, and cease when the underlying threat ends. Typically, a fear reaction will only result in aggression if no other escape option is available: in animals flight often dominates over fight (Porges, 1995, 1997). Other studies suggest that behavioral arrest (freezing), mediated by the periaqueductal gray, occurs as a last resort only when escape (flight) and aggression (fight) possibilities are exhausted (Dhawan and Haggard, 2023). One theory (Porges, 1995, 1997) proposes that the extreme freezing response reflects a specific and ancient neural response by a dedicated brain circuit.
In contrast, anger states strongly predispose the individual to aggress. However, anger states also recruit a broader cortical network than fear (Denson et al., 2009a). They engage the amygdala projections to several cortical regions, notably the inferior frontal gyrus (IFG) (Vytal et al., 2014). The IFG is importantly involved in behavioral control, inhibition and risk aversion (Aron et al., 2003; Christopoulos et al., 2009). This link could play a key role in the capacity to check, manage or suppress anger, or at least to suppress the aggressive behaviors that it tends to cause (Berkowitz, 1993). The stop-signal reaction time task is a well-established laboratory task in which participants must stop an action they are about to make. Doing so appears to involve a specific control signal transmitted from the inferior frontal gyrus to the subcortical structures that release rapid responses and impulsive action. Lesions in the IFG area lead to difficulty in stopping a prepotent action (Aron et al., 2003; Aron and Poldrack, 2006). It seems plausible that loss of control in situations of anger could represent failures of the inferior frontal gyrus circuit to prevent subcortically-driven prepotent responses. The loss of control defense could be seen as a normative recognition that this circuit can be insufficient.
In addition, aggression out of anger is often prolonged in time, outlasting the event that triggers it, and outlasting other reactions such as fear. Angry rumination typically maintains and even auguments anger and the likelihood of aggression (Fabiansson et al., 2012). Besides, some research indicates that retaliatory aggression after a provocation is mediated by a feeling of reward, a strong motivation toward aggression, traceable also at neural level. In one fMRI study, retaliatory aggression was associated with activity in the nucleus accumbens. Further, this association was related to participants’ level of real-world violence (Chester and DeWall, 2016). Finally, in contrast to fear, aggression out of revenge-seeking appears linked to the personality traits of impulsivity and sadism (Chester and DeWall, 2018).
Proactive aggression is goal-directed and endogenous, rather than provoked by any external cue. In animals, predation is the paradigmatic form of proactive aggression. Animal research points to a second form of aggression, which can occur even without any clear threat in the environment. In mice, such spontaneous aggressive behavior is associated with activity of neurons in the ventrolateral part of the ventromedial hypothalamus (VMHvl): inactivating this region reduces spontaneous aggressive behavior (Lee et al., 2014; Falkner et al., 2016). To our knowledge, little is known about such spontaneous aggression in humans. Yet, neuroscientific evidence with humans suggest that the dorsolateral prefrontal cortex plays a crucial role in anger control and management (Denson et al., 2009b; Achterberg et al., 2016). Furthermore, lesions to these regions of the frontal cortex produce a difficulty in controlling anger (Blair, 2012).
In contrast, reactive aggression involves responding to some external cue, and comes closer to the everyday concept of evoked anger. The legal concept of ‘qualifying trigger’ clearly suggests a reactive, rather than proactive, behavior, so we focus here only on reactive aggression. In particular, we ask how neuroscientific evidence could shape our understanding of aggressive reactions to threat, and of the capacity to control such reactions.
The neural mechanisms mediating reactive aggression out of anger are not as specific as they are for aggression out of fear, because the neural mechanisms and circuits of reactive aggression are also implicated in the regulation of other social behaviors (Newman, 1999; Nelson and Trainor, 2007). Research with rodents and non-human primates suggests that the key network underlying reactive aggression includes medial preoptic area, lateral septum, anterior hypothalamus, ventromedial hypothalamus, periaqueductal gray, medial amygdala orbitofrontal cortex and the stria terminalis. See Figure 3.
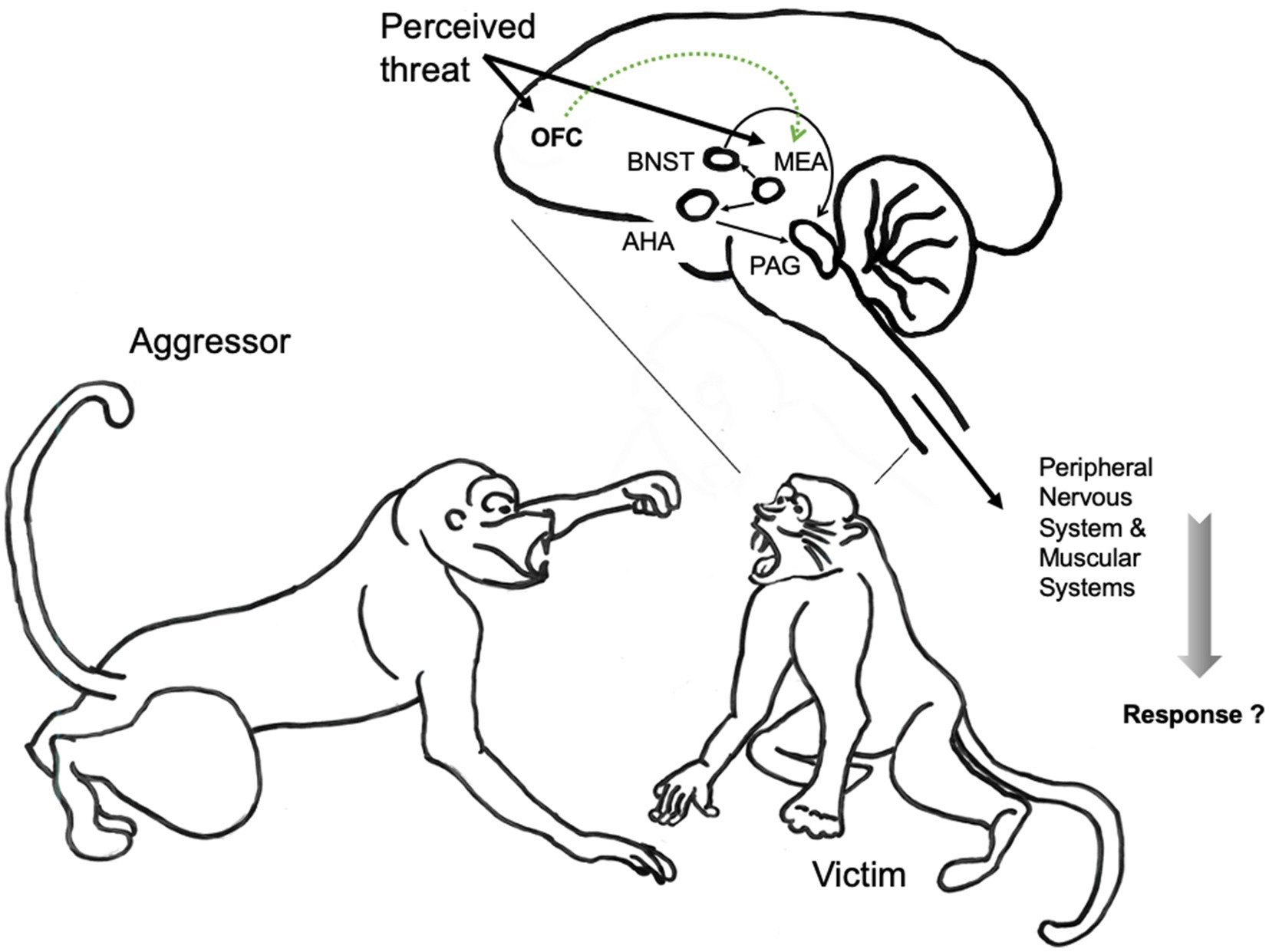
Figure 3. The neuroanatomical pathways of reactive aggression illustrated in the non-human primate brain (Wilson et al., 2014). Reactive aggression is often evoked by a vocal or visual signal, in this case from an aggressive conspecific. Activation of the medial amygdala (MEA) is thought to result in the activation of the bed nucleus of the stria terminalis (BNST) and the anterior hypothalamic area (AHA), which in turn activate the periaqueductal gray (PAG). In general, the orbital frontal cortex (OFC) appears to be important for the interpretation of social cues, and inhibitory inputs from the OFC might inhibit aggression by reducing responsiveness in the amygdala. Thick arrows represent inputs and outputs to and from the brain; thin arrows represent connections within the brain; dotted lines represent inhibitory connections. Illustration, text and neural models of non-human primate aggression elaborated on basis of illustration from Nelson and Trainor (2007). Drawings: © Main Author anonymized.
Lesions to the OFC cause impairment in the executive emotional systems, and increase reactive aggressive behavior (Damasio, 1996; Blair, 2001). Conversely, the frontal cortex inhibits reactive aggression: individuals with low basal frontal activity are more prone to aggress (Anderson et al., 1999).
From a neurocognitive point of view, aggressive behavior can be associated with either anger or fear. It therefore becomes important to understand the extent to which people can control fear or anger. There is an extensive literature on therapeutic control of anxiety (Banks et al., 2007), using either reappraisal (Buhle et al., 2013) or acceptance (Hayes-Skelton et al., 2013) techniques. This literature tends to focus on therapeutic situations and on chronic anxiety, rather than on the capacity to control fear while in the situation of immediate threat. Another literature deals with fear suppression during actual threatening situations, often focussing on occupational groups such as firefighters (Smith et al., 2013). This literature suggests that preparation and training can allow individuals to continue to produce functional behaviors (e.g., fighting the fire) even under conditions of extreme threat, but does not really remove the emotion of fear or the physiological responses associated with it. Taken as a whole, this literature suggests that the physiological and behavioral responses associated with intense situational fear and threat may be difficult to control.
In contrast, aggression out of anger relies on neural mechanisms that are broadly cortical and involve association areas that are broadly identified with controlled processing. This might suggest a higher degree of voluntary control over actions during anger states than during fear states. The law specifically mentions fear and as potential qualifying triggers for a reduced responsibility for action, at least in the context of loss of control partial defense for murder (Coroners and Justice Act, 2009).
Importantly, neuroplasticity, culture and social action all allow that laws could be ‘progressive’. The law recognizes that, just as some features of our neurobiological nature make us aggressive, other features of our neurobiology also make us rational, and capable of self-control. The law expects a degree of control over behavior based on our neurobiological capacity to be rational, not just on our neurobiological capacity to be aggressive. Our investigation has one concrete implication: the law should sharply distinguish between loss of control from anger, and loss of control from fear. Anger involves a widespread neural circuit that is substantially cortical. This may explain why anger can be controlled. Fear involves specific subcortical pathways – the extent to which cortical circuits associated with volition, autonomy and rationality have control over these subcortical circuits remains a matter of research debate (Berkowitz et al., 2007). The law might reasonably require agents to suppress any tendency to aggressive acts performed in anger, while showing greater awareness of loss of control for aggressive acts performed out of fear.
JC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. CR: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing. LC: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. PH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by AHRC Science in Culture grant to PH (Award number: 162746). PH was additionally supported by an ESRC Professorial Fellowship, and by ERC Advanced Grant (HUMVOL grant agreement number 323943). JC was additionally supported by the Max Planck Society, Germany.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Words written in bold are terms which have important different meanings in neuroscience and law; see Figure 1 and Table 1.
2. ^In addition, there are two further partial defenses to murder (diminished responsibility and suicide pact). There are also general complete defenses such as self-defense, automatism, and insanity that may lead to a full acquittal. These all lie beyond the scope of the present article.
Achterberg, M., van Duijvenvoorde, A. C., Bakermans-Kranenburg, M. J., and Crone, E. A. (2016). Control your anger! The neural basis of aggression regulation in response to negative social feedback. Soc. Cogn. Affect. Neurosci. 11, 712–720. doi: 10.1093/scan/nsv154
Adolphs, R., Tranel, D., Damasio, H., and Damasio, A. R. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672. doi: 10.1038/372669a0
Ajzen, I. (1985). “From intentions to actions: a theory of planned behavior” in Action control: From cognition to behavior. eds. J. Kuhl and J. Beckmann (Berlin: Springer-Verlag), 11–39.
Ajzen, I., and Fishbein, M. (1980). Understanding attitudes and predicting social behavior. Hoboken, NJ: Prentice-Hall.
Anderson, S. W., Bechara, A., Damasio, H., Tranel, D., and Damasio, A. R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat. Neurosci. 2, 1032–1037. doi: 10.1038/14833
Anderson, C. A., and Bushman, B. J. (1997). External validity of “trivial” experiments: the case of laboratory aggression. Rev. Gen. Psychol. 1, 19–41. doi: 10.1037/1089-2680.1.1.19
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J., and Robbins, T. W. (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 115–116. doi: 10.1038/nn1003
Aron, A. R., and Poldrack, R. A. (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433. doi: 10.1523/jneurosci.4682-05.2006
Ax, A. F. (1953). The physiological differentiation between fear and anger in humans. Psychosom. Med. 15, 433–442. doi: 10.1097/00006842-195309000-00007
Bandura, A. (1984). Recycling misconceptions of perceived self-efficacy. Cogn. Ther. Res. 8, 231–255. doi: 10.1007/BF01172995
Bandura, A. (2002). Social cognitive theory: an agentic perspective. Annu. Rev. Psychol. 52, 1–26. doi: 10.1146/annurev.psych.52.1.1
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., and Phan, K. L. (2007). Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2, 303–312. doi: 10.1093/scan/nsm029
Bechara, A., Damasio, H., and Damasio, A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10, 295–307. doi: 10.1093/cercor/10.3.295
Berkowitz, R. L., Coplan, J. D., Reddy, D. P., and Gorman, J. M. (2007). The human dimension: how the prefrontal cortex modulates the subcortical fear response. Rev. Neurosci. 18, 191–207. doi: 10.1515/revneuro.2007.18.3-4.191
Berkowitz, L., and Harmon-Jones, E. (2004). Toward an understanding of the determinants of anger. Emotion 4, 107–130. doi: 10.1037/1528-3542.4.2.107
Beyer, F., Münte, T. F., Göttlich, M., and Krämer, U. M. (2015). Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb. Cortex 25, 3057–3063. doi: 10.1093/cercor/bhu101
Blair, R. (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry 71, 727–731. doi: 10.1136/jnnp.71.6.727
Blair, R. J. R. (2012). Considering anger from a cognitive neuroscience perspective. Wiley Interdiscip. Rev. Cogn. Sci. 3, 65–74. doi: 10.1002/wcs.154
Borgomaneri, S., Vitale, F., and Avenanti, A. (2015). Early changes in corticospinal excitability when seeing fearful body expressions. Sci. Rep. 5:14122. doi: 10.1038/srep14122
Brosnan, S. F., and de Waal, F. B. M. (2003). Monkeys reject unequal pay [10.1038/nature01963]. Nature 425, 297–299. doi: 10.1038/nature01963
Buhle, J. T., Silvers, J. A., Wager, T. D., Lopez, R., Onyemekwu, C., Kober, H., et al. (2013). Cognitive reappraisal of emotion: a Meta-analysis of human neuroimaging studies. Cereb. Cortex 24, 2981–2990. doi: 10.1093/cercor/bht154
Buss, A. (1961). Investigating aggression in the laboratory. The psychology of aggression. New York: John Wiley and Sons Inc.
Chester, D. S., and DeWall, C. N. (2016). The pleasure of revenge: retaliatory aggression arises from a neural imbalance toward reward. Soc. Cogn. Affect. Neurosci. 11, 1173–1182. doi: 10.1093/scan/nsv082
Chester, D. S., and DeWall, C. N. (2018). Personality correlates of revenge-seeking: multidimensional links to physical aggression, impulsivity, and aggressive pleasure. Aggress. Behav. 44, 235–245. doi: 10.1002/ab.21746
Chi, C. C., and Flynn, J. P. (1971). Neural pathways with hypothalamically elicited attack behaviour in rats. Science 171, 703–706. doi: 10.1126/science.171.3972.703
Child, J., and Ormerod, D. (2015). Smith and Hogan’s essentials of criminal law. Oxford, UK: Oxford University Press.
Christensen, J. F., Di Costa, S., Beck, B., and Haggard, P. (2019). I just lost it! Fear and anger reduce the sense of agency: a study using intentional binding. Exp. Brain Res. 237, 1205–1212. doi: 10.1007/s00221-018-5461-6
Christensen, J. F., Yoshie, M., Di Costa, S., and Haggard, P. (2016). Emotional valence, sense of agency and responsibility: a study using intentional binding. Conscious. Cogn. 43, 1–10. doi: 10.1016/j.concog.2016.02.016
Christopoulos, G. I., Tobler, P. N., Bossaerts, P., Dolan, R. J., and Schultz, W. (2009). Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J. Neurosci. 29, 12574–12583. doi: 10.1523/jneurosci.2614-09.2009
Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. London B Biol. Sci. 351, 1413–1420. doi: 10.1098/rstb.1996.0125
Davis, M. (1994). The role of the amygdala in emotional learning. Int Rev Neurobiol 36, 225–266. doi: 10.1016/s0074-7742(08)60305-0
Davis, M., Walker, D. L., Miles, L., and Grillon, C. (2010). Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. doi: 10.1038/npp.2009.109
Denson, T. F., Pedersen, W. C., Ronquillo, J., and Nandy, A. S. (2009). The angry brain: neural correlates of anger, angry rumination, and aggressive personality. J. Cogn. Neurosci. 21, 734–744. doi: 10.1162/jocn.2009.21051
Dhawan, E., and Haggard, P. (2023). Neuroscience evidence counters a rape myth. Nat. Hum. Behav. 7, 835–838. doi: 10.1038/s41562-023-01598-6
Dougherty, D. D., Shin, L. M., Alpert, N. M., Pitman, R. K., Orr, S. P., Lasko, M., et al. (1999). Anger in healthy men: a PET study using script-driven imagery. Biol. Psychiatry. 46, 466–472. doi: 10.1016/s0006-3223(99)00063-3
Ekman, P., Levenson, R. W., and Friesen, W. V. (1983). Autonomic nervous system activity distinguishes among emotions. Science 221, 1208–1210. doi: 10.1126/science.6612338
Fabiansson, E. C., Denson, T. F., Moulds, M. L., Grisham, J. R., and Schira, M. M. (2012). Don't look back in anger: neural correlates of reappraisal, analytical rumination, and angry rumination during recall of an anger-inducing autobiographical memory. NeuroImage 59, 2974–2981. doi: 10.1016/j.neuroimage.2011.09.078
Falkner, A. L., Grosenick, L., Davidson, T. J., Deisseroth, K., and Lin, D. (2016). Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci. 19, 596–604. doi: 10.1038/nn.4264
Fishbein, M., and Ajzen, I. (1975). Belief, attitude, intention, and behavior: An introduction to theory and research. Boston: Addison-Wesley.
Frankenhaeuser, M. (1971). Behavior and circulating catecholamines. Brain Research 31, 241–262. doi: 10.1016/0006-8993(71)90180-6
Graybiel, A. M. (2008). Habits, rituals, and the evaluative brain. Ann. Rev. Neurosci. 31, 359–387. doi: 10.1146/annurev.neuro.29.051605.112851
Haggard, P. (2017). Sense of agency in the human brain. Nat. Rev. Neurosci. 18, 196–207. doi: 10.1038/nrn.2017.14
Haggard, P., Clark, S., and Kalogeras, J. (2002). Voluntary action and conscious awareness. Nat. Neurosci. 5, 382–385. doi: 10.1038/nn827
Hayes-Skelton, S. A., Orsillo, S. M., and Roemer, L. (2013). An acceptance-based behavioral therapy for individuals with generalized anxiety disorder. Cogn. Behav. Pract. 20, 264–281. doi: 10.1016/j.cbpra.2011.02.005
Hortensius, R., de Gelder, B., and Schutter, D. J. L. G. (2016). When anger dominates the mind: increased motor corticospinal excitability in the face of threat. Psychophysiology 53, 1307–1316. doi: 10.1111/psyp.12685
Kiehl, K. A., Anderson, N. E., Aharoni, E., Maurer, J. M., Harenski, K. A., Rao, V., et al. (2018). Age of gray matters: Neuroprediction of recidivism. NeuroImage 19, 813–823. doi: 10.1016/j.nicl.2018.05.036
Kimbrell, T. A., George, M. S., Parekh, P. I., Ketter, T. A., Podell, D. M., Danielson, A. L., et al. (1999). Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol. Psychiatry. 46, 454–465. doi: 10.1016/s0006-3223(99)00103-1
Klimecki, O. M., Sander, D., and Vuilleumier, P. (2018). Distinct brain areas involved in anger versus punishment during social interactions. Sci. Rep. 8, –10556. doi: 10.1038/s41598-018-28863-3
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421. doi: 10.1016/j.biopsycho.2010.03.010
Leary, M. R., Twenge, J. M., and Quinlivan, E. (2006). Interpersonal rejection as a determinant of anger and aggression. Pers. Soc. Psycholol. Rev. 10, 111–132. doi: 10.1207/s15327957pspr1002_2
LeDoux, J. (2003). The emotional brain, fear and the amygdala. Cell. Mol. Neurobiol. 23, 727–738. doi: 10.1023/A:1025048802629
LeDoux, J. (2012). Rethinking the emotional brain. Neuron 73, 653–676. doi: 10.1016/j.neuron.2012.02.004
Ledoux, J. E. (2014). Coming to terms with fear. Proc. Natl. Acad. Sci. U. S. A. 111, 2871–2878. doi: 10.1073/pnas.1400335111
Lee, H., Kim, D. W., Remedios, R., Anthony, T. E., Chang, A., Madisen, L., et al. (2014). Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. doi: 10.1038/nature13169
Libet, B., Gleason, C. A., Wright, E. W., and Pearl, D. K. (1983). Time of conscious intention to act in relation to onset of cerebral activity (readiness potential): the unconscious initiation of a freely volunatry act. Brain 106, 623–642. doi: 10.1093/brain/106.3.623
Mackay, R., Mitchell, B.J., and Howe, L. (2006). Yet more facts about the insanity defence. Criminal Law Review, May, pp.399–411.
Matthews, P. (1983). Ignorance of the law is no excuse? Leg. Stud. 3, 174–192. doi: 10.1111/j.1748-121X.1983.tb00314.x
Minohara, R., Wen, W., Hamasaki, S., Maeda, T., Kato, M., Yamakawa, H., et al. (2016). Strength of intentional effort enhances the sense of agency [original research]. Front. Psychol. 7:1165. doi: 10.3389/fpsyg.2016.01165
Moore, M. S. (2020). Mechanical choices: The responsibility of the human machine. Oxford, UK: Oxford University Press.
Morse, S. J. (2004). New neuroscience, old problems: legal implications of brain science. Cerebrum 6, 81–90
Morse, S. J. (2023). Neurolaw: challenges and limits. Handb. Clin. Neurol. 197, 235–250. doi: 10.1016/b978-0-12-821375-9.00003-7
Murphy, F. C., Nimmo-Smith, I., and Lawrence, A. D. (2003). Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience 3, 207–233. doi: 10.3758/cabn.3.3.207
Nelson, R. J., and Trainor, B. C. (2007). Neural mechanisms of aggression. Nat. Rev. Neurosci. 8, 536–546. doi: 10.1038/nrn2174
Newman, S. W. (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 877, 242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x
Patkai, P., and Frankenhaeuser, M. (1965). Constancy of catecholamine excretion. Perceptual Motor Skills 19, 789–790. doi: 10.2466/pms.1964.19.3.789
Pawliczek, C. M., Derntl, B., Kellermann, T., Gur, R. C., Schneider, F., and Habel, U. (2013). Anger under control: neural correlates of frustration as a function of trait aggression. PLoS One 8, –e78503. doi: 10.1371/journal.pone.0078503
Pessoa, L. (2017). A network model of the emotional brain. Trends Cogn. Sci. 21, 357–371. doi: 10.1016/j.tics.2017.03.002
Pessoa, L. (2023b). How many brain regions are needed to elucidate the neural bases of fear and anxiety? Neurosci. Biobehav. Rev. 146:105039. doi: 10.1016/j.neubiorev.2023.105039
Pessoa, L., and McMenamin, B. (2017). Dynamic networks in the emotional brain. Neuroscientist 23, 383–396. doi: 10.1177/1073858416671936
Phan, K. L., Wager, T., Taylor, S. F., and Liberzon, I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348. doi: 10.1006/nimg.2002.1087
Philippi, C. L., Pujara, M. S., Motzkin, J. C., Newman, J., Kiehl, K. A., and Koenigs, M. (2015). Altered resting-state functional connectivity in cortical networks in psychopathy. J. Neurosci. 35, 6068–6078. doi: 10.1523/jneurosci.5010-14.2015
Pichon, S., De Gelder, B., and Grezes, J. (2012). Threat prompts defensive brain responses independently of attentional control. Cereb. Cortex 22, 274–285. doi: 10.1093/cercor/bhr060
Porges, S. W. (1995). Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 32, 301–318
Porges, S. W. (1997). Emotion: an evolutionary by-product of the neural regulation of the autonomic nervous system. Ann. N. Y. Acad. Sci. 807, 62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x
Rainville, P., Bechara, A., Naqvi, N., and Damasio, A. R. (2006). Basic emotions are associated with distinct patterns of cardiorespiratory activity. International Journal of Psychophysiology 61, 5–18. doi: 10.1016/j.ijpsycho.2005.10.024
Rimm-Kaufman, S. E., and Kagan, J. (1996). The psychological significance of changes in skin temperature. Motiv. Emot. 20, 63–78. doi: 10.1007/BF02251007
Ritter, D., and Eslea, M. (2005). Hot sauce, toy guns, and graffiti: a critical account of current laboratory aggression paradigms. Aggress. Behav. 31, 407–419. doi: 10.1002/ab.20066
Robin, O., Alaoui-Ismaïli, O., Dittmar, A., and Vernet-Maury, E. (1998). Emotional responses evoked by dental odors: an evaluation from autonomic parameters. J. Dent. Res. 77, 1638–1646. doi: 10.1177/00220345980770081201
Robinson, O. J., Vytal, K., Cornwell, B. R., and Grillon, C. (2013). The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front. Hum. Neurosci. 7:203. doi: 10.3389/fnhum.2013.00203
Rochman, D., and Diamond, G. M. (2008). From unresolved anger to sadness: identifying physiological correlates. J. Couns. Psychol. 55, 96–105. doi: 10.1037/0022-0167.55.1.96
Roma, P. G., Silberberg, A., Ruggiero, A. M., and Suomi, S. J. (2006). Capuchin monkeys, inequity aversion, and the frustration effect. J. Comp. Psychol. 120, 67–73. doi: 10.1037/0735-7036.120.1.67
Schachter, J. (1957). Pain, fear, and anger in hypertensives and normotensives: a psychophysiological study. Psychosom. Med. 19, 17–29. doi: 10.1097/00006842-195701000-00003
Seligman, M. R. E. P. (1971). Phobias and preparedness. Behav. Ther. 2, 307–320. doi: 10.1016/S0005-7894(71)80064-3
Sinha, R. (1996). Multivariate response patterning of fear and anger. Cognit. Emot. 10, 173–198. doi: 10.1080/026999396380321
Smith, D. L., Barr, D. A., and Kales, S. N. (2013). Extreme sacrifice: sudden cardiac death in the US fire service. Extrem. Physiol. Med. 2:6. doi: 10.1186/2046-7648-2-6
Stemmler, G., Aue, T., and Wacker, J. (2007). Anger and fear: Separable effects of emotion and motivational direction on somatovisceral responses. Int. J. Psychophysiol. 66, 141–153. doi: 10.1016/j.ijpsycho.2007.03.019
Stemmler, G., Heldmann, M., Pauls, C. A., and Scherer, T. (2001). Constraints for emotion specificity in fear and anger: the context counts. Psychophysiol. 38, 275–291. doi: 10.1111/1469-8986.3820275
Strange, B. A., and Dolan, R. J. (2006). Anterior medial temporal lobe in human cognition: memory for fear and the unexpected. Cogn. Neuropsychiatry. 11, 198–218. doi: 10.1080/13546800500305096
Talmi, D., Dayan, P., Kiebel, S. J., Frith, C. D., and Dolan, R. J. (2009). How humans integrate the prospects of pain and reward during choice. J. Neurosci. 29, 14617–14626. doi: 10.1523/jneurosci.2026-09.2009
Taylor, S. P. (1967). Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J. Pers. 35, 297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x
Uchiyama, I. (1992). Differentiation of fear, anger, and joy. Perceptual Motor Skills 74, 663–667. doi: 10.2466/pms.1992.74.2.663
Vytal, K., and Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22, 2864–2885. doi: 10.1162/jocn.2009.21366
Vytal, K. E., Overstreet, C., Charney, D. R., Robinson, O. J., and Grillon, C. (2014). Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. J. Psychiatry Neurosci. 39, 321–329. doi: 10.1503/jpn.130145
Wacker, J., Heldmann, M., and Stemmler, G. (2003). Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion 3, 167–193. doi: 10.1037/1528-3542.3.2.167
Wen, W., Yamashita, A., and Asama, H. (2015). The influence of action-outcome delay and arousal on sense of agency and the intentional binding effect. Conscious. Cogn. 36, 87–95. doi: 10.1016/j.concog.2015.06.004
Whalen, P. J., Shin, L. M., McInerney, S. C., Fischer, H., Wright, C. I., and Rauch, S. L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion 1, 70–83. doi: 10.1037/1528-3542.1.1.70
Wilson, M. L., Boesch, C., Fruth, B., Furuichi, T., Gilby, I. C., Hashimoto, C., et al. (2014). Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417. doi: 10.1038/nature13727
Keywords: volition, action control, human, law, action, loss of control, fear, anger
Citation: Christensen JF, Rödiger C, Claydon L and Haggard P (2024) Volition and control in law and in brain science: neurolegal translation of a foundational concept. Front. Hum. Neurosci. 18:1401895. doi: 10.3389/fnhum.2024.1401895
Received: 16 March 2024; Accepted: 29 July 2024;
Published: 03 September 2024.
Edited by:
Lutz Jäncke, University of Zurich, SwitzerlandReviewed by:
Alfred Mele, Florida State University, United StatesCopyright © 2024 Christensen, Rödiger, Claydon and Haggard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia F. Christensen, SnVsaWEuY2hyaXN0ZW5zZW5AYWUubXBnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.