
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 22 May 2024
Sec. Motor Neuroscience
Volume 18 - 2024 | https://doi.org/10.3389/fnhum.2024.1316117
This article is part of the Research TopicCurrent Perspectives on Developmental Coordination Disorder (DCD), volume IIView all 11 articles
Introduction: Cognitive Orientation to daily Occupational Performance (CO-OP) is a cognitive-based, task-specific intervention recommended for children with developmental coordination disorder (DCD). We recently showed structural and functional brain changes after CO-OP, including increased cerebellar grey matter. This study aimed to determine whether CO-OP intervention induced changes in cortical grey matter volume in children with DCD, and if these changes were associated with improvements in motor performance and movement quality.
Methods: This study is part of a randomized waitlist-control trial (ClinicalTrials.gov ID: NCT02597751). Children with DCD (N = 78) were randomized to either a treatment or waitlist group and underwent three MRIs over 6 months. The treatment group received intervention (once weekly for 10 weeks) between the first and second scan; the waitlist group received intervention between the second and third scan. Cortical grey matter volume was measured using voxel-based morphometry (VBM). Behavioral outcome measures included the Performance Quality Rating Scale (PQRS) and Bruininks-Oseretsky Test of Motor Proficiency-2 (BOT-2). Of the 78 children, 58 were excluded (mostly due to insufficient data quality), leaving a final N = 20 for analyses. Due to the small sample size, we combined both groups to examine treatment effects. Cortical grey matter volume differences were assessed using a repeated measures ANOVA, controlling for total intracranial volume. Regression analyses examined the relationship of grey matter volume changes to BOT-2 (motor performance) and PQRS (movement quality).
Results: After CO-OP, children had significantly decreased grey matter in the right superior frontal gyrus and middle/posterior cingulate gyri. We found no significant associations of grey matter volume changes with PQRS or BOT-2 scores.
Conclusion: Decreased cortical grey matter volume generally reflects greater brain maturity. Decreases in grey matter volume after CO-OP intervention were in regions associated with self-regulation and motor control, consistent with our other studies. Decreased grey matter volume may be due to focal increases in synaptic pruning, perhaps as a result of strengthening networks in the brain via the repeated learning and actions in therapy. Findings from this study add to the growing body of literature demonstrating positive neuroplastic changes in the brain after CO-OP intervention.
Developmental coordination disorder (DCD) is classified as a neurodevelopmental disorder in the Diagnostic and Statistical Manual 5th edition (DSM-5). This motor disorder affects approximately 450,000 Canadian school-aged children (American Psychiatric Association, 2013; Statistics Canada, 2021). The related gross and fine motor difficulties affect important childhood activities such as tying shoelaces, printing, or riding a bicycle (Kirby and Sugden, 2007; Zwicker et al., 2012; Blank et al., 2019). Early intervention is important, as children with DCD typically continue to experience motor difficulties well into adolescence and adulthood if adequate intervention is not provided throughout childhood (Kirby et al., 2013).
Traditionally, interventions have been process-oriented and focused on addressing the sensorimotor dysfunction that was thought to contribute to their motor impairments (Polatajko et al., 2001; Mandich et al., 2002; Polatajko and Mandich, 2004). Newer approaches leverage current theories of cognitive and motor learning and advocate for problem-solving focused intervention (Sugden, 2007). One such intervention, the Cognitive Orientation approach to daily Occupational Performance (CO-OP), was developed by occupational therapists in Canada (Polatajko et al., 2001). This task-specific intervention is a cognitive-based, problem-solving approach that uses verbal mediation and identifies strategies to support motor skill acquisition (Polatajko et al., 2001). Several systematic reviews have been conducted that further demonstrate the effectiveness of CO-OP intervention for children with DCD (Smits-Engelsman et al., 2012, 2018; Scammell et al., 2016; Yu et al., 2018), making CO-OP one of the recommended treatments in the international clinical practice guidelines for DCD (Blank et al., 2019).
While CO-OP has been deemed effective, the underlying mechanisms or neural bases for clinical improvements were unknown. Our research group recently used magnetic resonance imaging (MRI) to investigate brain changes associated with CO-OP intervention. In a study that focused on the cerebellum, we showed increases in grey matter volume in the brainstem and in cognitive (right crus II) and motor regions (right and left lobule VIIIb and lobule IX) of the cerebellum following the intervention (Gill et al., 2022). Improvements in actual movement performance predicted the increases in cerebellar grey matter volume. In addition, increased functional connectivity in the default mode network and right anterior cingulate cortex were observed after CO-OP intervention (Izadi-Najafabadi et al., 2022b), as well as improved white matter microstructure in several regions, including the bilateral anterior thalamic radiations, bilateral sensorimotor tracts, bilateral cingulum, and corpus callosum (Izadi-Najafabadi and Zwicker, 2021). These brain regions are associated with attention, self-regulation, motor planning, and inter-hemispheric communication (Izadi-Najafabadi and Zwicker, 2021).
Cortical brain structures undergo developmental changes during childhood (Giedd et al., 1999; Wierenga et al., 2014; Gilmore et al., 2018), and some therapies (e.g., behavioral, medications) have been associated with changes in cortical volume in children with neurodevelopmental disorders (Spencer et al., 2013; Sterling et al., 2013). To date, it is unknown whether CO-OP induces neuroplastic change in cortical brain structure. The aims of this study were to determine: (1) whether CO-OP intervention induces changes in cortical grey matter volume of children with DCD; and (2) if any grey matter volume changes are associated with improvements in motor performance and movement quality. We hypothesized that following CO-OP intervention, we would find: (1) increased cortical grey matter volume in regions of the brain associated in coordinating motor and executive functioning skills (i.e., parietal and frontal lobe); and (2) positive associations between changes in grey matter volume, motor performance, and movement quality improvements.
This study was a part of a randomized waitlist-control trial using multiple neuroimaging modalities to assess brain changes with CO-OP intervention (ClinicalTrials.gov ID: NCT02597751). For the purpose of this study, structural MRI data were collected before and after CO-OP intervention to investigate changes in grey matter volume in children with DCD and in children with DCD and co-occurring ADHD (DCD + ADHD). Participants received treatment either after the first MRI (treatment group) or after a 3-month waiting period (waitlist group). A statistician randomized participants using computer-generated sequential blocks of 4 to 6. The randomization codes, either treatment or waitlist, were sealed in opaque envelopes until study enrollment. After screening and recruitment, all parents or legal guardians provided written consent and children assented to participate in the study. The study design (randomized waitlist-control trial) for the purpose of this paper is shown in Figure 1. All aspects of the study were approved by the Children’s and Women’s Health Centre/University of British Columbia Research Ethics Board (#H14-00397).
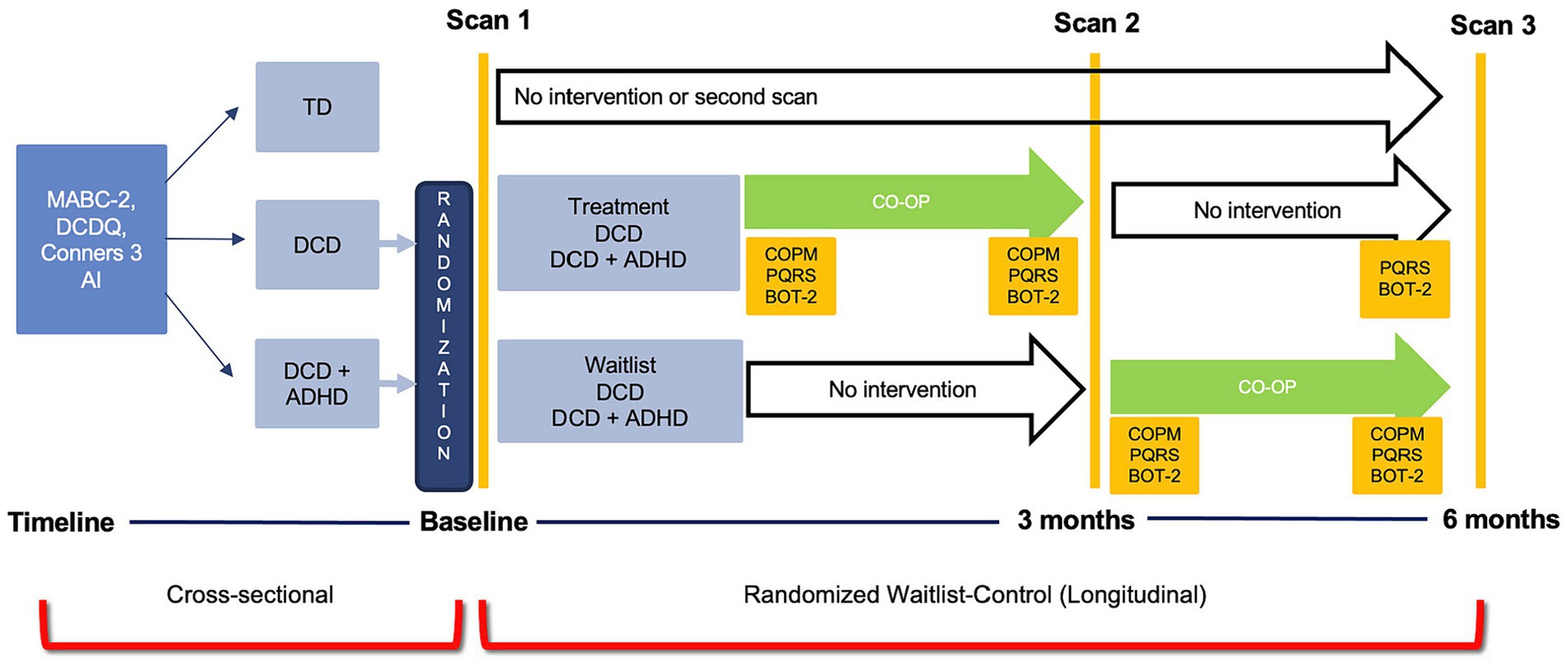
Figure 1. Study design for the cross-sectional and randomized waitlist-control trial for the Zwicker Lab DCD imaging study.
A convenience sampling strategy was used to recruit participants between September 2014 to July 2019. The following sources were used to recruit participants for the intervention: (1) Dr. Zwicker’s research-integrated DCD clinic at Sunny Hill Health Centre for Children; (2) BC Children’s Hospital ADHD Clinic; (3) from caseloads of occupational and/or physical therapists from Sunny Hill and the Vancouver Regional Pediatric Team who service schools in the Vancouver and surrounding districts; and (4) the community. Community recruitment was done by using bulletin boards at BC Children’s Hospital, UBC, and Vancouver schools. TD children were recruited through advertisements in Vancouver schools and community centres, and by word-of-mouth.
Inclusion criteria were based on the DSM-5 diagnostic criteria (American Psychiatric Association, 2013) and international clinical practice recommendations for DCD diagnosis (Blank et al., 2019) as follows: (1) scores ≤16th percentile on the MABC-2 (Henderson et al., 2007); (2) score in the suspected or indicative range on the Developmental Coordination Disorder Questionnaire (DCDQ) (Wilson and Crawford, 2007); (3) parent-reported motor difficulties from a young age; and (4) no other medical condition that could explain motor difficulties as per parent-reports, clinical reports and/or medical examination. Participants were excluded if they were born preterm (gestational age week <37 weeks) or diagnosed with other neurodevelopmental disorders, such as autism spectrum disorder.
The control group (TD children) included children 8–12-years old with no history of motor difficulties and a MABC-2 score ≥25th percentile. Exclusion criteria included being born preterm (gestation week <37 weeks) or diagnosed with any other neurodevelopmental disorder, such as autism spectrum disorder. Children assigned to the TD group were excluded if they were diagnosed with ADHD.
Prior to enrollment, all participants were administered the Movement Assessment Battery for Children (MABC-2) to quantify the level of motor impairment (Henderson et al., 2007) and whether the participants met the inclusion criteria for the study. In addition, the DCDQ parent-completed questionnaire was used to identify motor impairments of the participants in comparison to their peers (Wilson and Crawford, 2007). Lastly, the Conners 3 ADHD Index parent form was used to assess for attentional performance (Conners, 2009). Scores 70 and above are considered to be clinically significant (poorer attentional performance indicates greater attentional difficulties).
Both scanning and intervention took place at BC Children Hospital Research Institute. All children participated in a magnetic resonance imaging (MRI) safety screening and were informed about the MRI procedure. An MRI simulator session was done to familiarize the children with the scanning environment (noise, confined space, and head coil). They were also provided with strategies from the research team to help reduce potential for anxiety. The simulator session helped to answer the child’s and/or parent’s questions and inform the research team about the child’s ability to remain still in the MRI scan, as the scans are sensitive to motion (Zaitsev et al., 2015).
After the first MRI session, children were randomly assigned to either the treatment or waitlist group, so that the research team was blinded to group allocation until after the first MRI. Children in both groups had three MRI sessions: (1) scan 1 occurred at enrollment; (2) scan 2 was conducted 3 months after the first scan (to measure treatment effect in the treatment group and maturation in the waitlist group); and (3) scan 3 occurred 6 months after the first scan (to assess follow-up 3 months after intervention in the treatment group and to measure the treatment effect of the waitlist group). Following the first MRI session, children in the treatment group received CO-OP intervention (led by an occupational therapist) once weekly for 10 weeks; they then had a post-intervention scan, and another follow-up scan 3 months later. Children in the waitlist group waited for 3 months for their second MRI and then began CO-OP intervention for 10 weeks; they had a third MRI after intervention (Figure 1). Caregivers were encouraged to attend treatment sessions so that therapists could instruct them how to facilitate strategy use between treatment sessions. Prior to intervention, children selected three functional motor goals (e.g., printing, tying shoes, performing sport-related movements) that they wanted to achieve over the 10 weeks of therapy; each session lasted an hour. Outcomes measures included the Canadian Occupational Performance Measure (COPM) (Law et al., 2014), Performance Quality Rating Scale (PQRS) (Martini et al., 2014), and Bruininks-Oseretsky Test of Motor Proficiency-2 (BOT-2) (Bruininks and Bruininks, 2005). The COPM and BOT-2 were administered by an occupational therapist not involved in the intervention. The PQRS was video recorded by the treating therapist before and after intervention but was scored by the assessing therapist who was blinded to pre-test/post-test status.
The COPM (Law et al., 2014) is a client-centered questionnaire that was administered by an occupational therapist before and after the completion of the 10-week CO-OP intervention. It allows the individual to rate their performance and satisfaction for each of their self-chosen goals on a scale of 1 to 10, where a higher score indicates increased levels of performance and satisfaction with their self-chosen goals (Law et al., 2014). A two-point change is considered clinically meaningful (Carswell et al., 2004; Law et al., 2014). The COPM is considered a valid, reliable, and responsive outcome measure (Carswell et al., 2004; Dedding et al., 2004; Eyssen et al., 2011).
The PQRS is a 10-point performance rating scale to evaluate changes in observed movement quality during task performance; higher scores indicate better movement quality (Miller et al., 2001; Polatajko and Mandich, 2004). The PQRS has moderate to substantial inter-rater reliability, excellent test-retest reliability, and good internal responsiveness (Miller et al., 2001; Martini et al., 2014). Before and after CO-OP intervention, children were video-recorded performing their chosen goals. An occupational therapist who was not engaged in the delivery of the intervention and was blinded to the pre/post assessment sessions scored the videos. The child’s actual performance quality was rated on a scale of 1 to 10 (1 being “cannot do the skill at all” and 10 being “does the skill very well”) (Martini et al., 2014). An increase of three points is considered clinically significant (Martini et al., 2014). The PQRS complements the COPM by measuring the actual, rather than perceived, performance of the child’s self-chosen goals.
The short form of BOT-2 (Bruininks and Bruininks, 2005) was completed for this study. This short form consists of one or two items from each of the eight areas: bilateral coordination, balance, running speed and agility, strength, fine-motor precision, fine-motor integration, manual dexterity, and upper extremity coordination (Bruininks and Bruininks, 2005). The BOT-2 is a standardized, norm-referenced assessment that measures motor performance (Deitz et al., 2007), where a higher percentile scoring indicates better motor performance. This assessment is reported to have moderate to strong inter-rater/test–retest reliability (Deitz et al., 2007), excellent concurrent validity with other motor measures, and adequate construct and content validity (Slater et al., 2010). The total percentile scores of the BOT-2 short-form were used for analysis.
All brain images were acquired at the MRI Research Facility at BC Children’s Hospital Research Institute in Vancouver, Canada. MRI scans were obtained on a 3-Tesla General-Electric Discovery MR 750 scanner. T1-weighted 3D structural scans were acquired with the following parameters: three-dimensional spoiled gradient recalled acquisition in steady state (3D SPGR), echo time = 30 ms, repetition time = 3,000 ms, FOV = 256, matrix size = 256 × 256, flip angel = 12°, number of slices = 256, slice thickness = 1 mm, interleaved with no gaps (voxel size 0.9375 × 0.9375 × 1 mm). Using T1 weighted scans allows for reliable segmentation of tissues (grey matter, white matter, and cerebrospinal fluid) and permits reliable identification of underlying regions (Lerch et al., 2017).
All scans were visually inspected for truncation, motion, aliasing-related and other artifacts (Krupa and Bekiesińska-Figatowska, 2015; Reuter et al., 2015). Specifically, image quality was assessed for head coverage, wrapping artifact, radiofrequency noise, signal inhomogeneity, susceptibility artifact, and ringing artifact (Reuter et al., 2015). An ordinal score was given to each image based on motion artifacts and image quality (pass, questionable, or fail) using standardized methodology (Harvard Center for Brain Science, 2014). Two trainees assessed the scans independently; the level of agreement for the categorization of each scan assessed by each trainee was 96%. Only scans that passed the final quality check from both trainees were included in the analysis. Additionally, quantitative measures of motion were calculated using the software package MRIQC (10.1371/journal.pone.0184661). In particular, we measured coefficient of joint variation (CJV), where higher values are related to the presence of heavy head motion and large intensity non-uniformity (10.3389/fninf.2016.00010). Forty-five participants with DCD with significant motion artifact or poor grey to white matter differentiation were excluded from the larger sample to produce the final dataset of 20 participants with two good quality scans before and after intervention.
Twenty-two TD participants with similar data quality artifacts were excluded, resulting in nine participants with good quality scans acquired 3 months apart.
Data were converted from DICOM (Digital Imaging and Communications in Medicine) to NIfTI (Neuroimaging Informatics Technology Initiative) format using the dcm2nii tool from MRIcron.1 T1 images were processed using voxel-based morphometry (VBM), a computational technique that measures differences in grey matter volume through a voxel-wise comparison (Ashburner and Friston, 2000; Whitwell, 2009). VBM uses T1-weighted MRI scans and performs a voxel-by-voxel statistical analysis across each image to identify volume differences between patients and controls (Ashburner and Friston, 2000). All pre-processing and longitudinal VBM data analysis were carried out using the Computational Anatomy Tool Box (CAT12, v1742, The Structural Brain Mapping Group, Jena, Germany, http://dbm.neuro.uni-jena.de/cat12/), through Statistical Parametric Mapping 12 software (SPM12, v7771, The Wellcome Centre for Human Neuroimaging, London, United Kingdom, https://www.fil.ion.ucl.ac.uk/spm/) in MATLAB R2020b (Mathworks, Natick, Massachusetts, United States).
For image preprocessing, all T1 images were manually registered to the anterior commissure at the origin of the Montreal Neurological Institute (MNI) coordinate system (Jahn, 2019). Initially in longitudinal VBM analysis, intra-participant co-registration was performed on the pre- and post-intervention images. The co-registered images were then realigned across participants and bias-corrected with reference to the mean images computed from each participant’s pre- and post-intervention images. The images were then segmented into grey matter, white matter, and cerebrospinal fluid (CSF) with the customized pediatric tissue probability maps from Template-O-Matic Toolbox (TOM8 https://neuro-jena.github.io/software.html#tom) as an initial estimate. All images were included if their weight average Image Quality Rating (IQR) was greater than 80%, corresponding to a “good” image quality. Mean correlations between all volumes were visualized through CAT12. Volumes with a correlation below two standard deviations from the sample mean were again visually inspected for artifacts. Next, good quality pre- and post-average affine-registered white and grey matter tissue segments were extracted to construct a customized Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) study-specific template registered to the MNI-International Consortium for brain Mapping (ICBM) space. This alternative to the adult-based template provided by CAT12 was used to achieve a more accurate inter-participant registration to improve the realignment of small inner structures for an overall better segmentation (Good et al., 2001; Yassa and Stark, 2009). Likewise, this additional step is similar to VBM studies done in other pediatric neurodevelopmental disorder studies that created a study-specific average template for their sample (Reynolds et al., 2017; Wang et al., 2017; Sáenz et al., 2020). The images were then normalized using an affine spatial normalization and a further modulation was applied to convert the voxel values of tissue concentration to measures of volume. Finally, the normalized grey matter maps were smoothed with an isotropic Gaussian kernel (full width at half maximum = 6 mm). Total intracranial volume (TIV) was calculated for the pre and post grey matter, white matter, and CSF images for each participant using CAT12 module “Total intracranial volume.”
The Structural Brain Mapping Group at the University of Jena (Jena, Germany) designed the automatic and easy-to-use toolbox CAT12 as an extension to the SPM software. CAT12 follows a standard VBM analysis pipeline similar to VBM8. We used segmentation through SPM’s extension CAT12 rather than FreeSurfer or FSL as SPM produces a more robust segmentation for those with limited image quality (Fellhauer et al., 2015). This decision was further supported through a comparison to previous toolboxes, with CAT12 providing a more accurate and robust volumetric analysis (Farokhian et al., 2017) and advanced segmentation tool (Tavares et al., 2020). It has also been used in neurodevelopmental disorders that commonly co-occur with DCD (Wang et al., 2017; Mei et al., 2020; Sáenz et al., 2020) where the workflow was adapted to accommodate a pediatric population as recommended for VBM analysis.
Jeffreys’s Amazing Statistics Program (JASP https://jasp-stats.org/) was used to summarize participant characteristics [age, sex, TIV, MABC-2 (motor ability), DCDQ (motor function), and Conners 3 ADHD Index (ADHD symptomatology)] and pre-post intervention outcome measures. The behavioral data for the entire cohort have been reported by Izadi-Najafabadi et al. (2022a). Here, we report motor outcome data for the sub-sample in this paper. The Wilcoxon Signed-Rank Test (non-parametric) was used to compare the before and after effect of CO-OP intervention on average COPM performance and satisfaction scores, average PQRS total actual performance scores, and BOT-2 motor percentile ranks. The alpha level was set to 0.05 with Bonferroni correction used to correct for multiple comparisons when comparing pre- post values to avoid type 1 errors.
All statistical models were to be set up using general linear modeling through SPM. Initially, our goal for the analysis was to conduct a treatment vs. waitlist comparison; however due to the smaller than anticipated sample size for each group (n = 7 treatment, n = 13 waitlist), we combined scans 1 and 2 of the treatment group and scans 2 and 3 of the waitlist group to examine grey matter volume differences before and after intervention. Paired participant smoothed grey matter volumes were entered into a second level analysis using the “Flexible factorial” module in CAT12. To estimate differences in pre-post grey matter in children with DCD, a repeated measure analysis of variance (ANOVA) design with 5,000 permutations with an alpha level of 0.05 was used, with whole and within exchangeability blocks. Threshold-Free Cluster Enhancement (TFCE) thresholding was conducted using the TFCE Toolbox Version r2142 with 5,000 permutations (Draper–Stoneman method) with equal variance (patients) with an E = 0.5 and H = 0.2. TIV was mean-centered and used as a covariate/nuisance variable as recommended in VBM analysis to account for intra-individual differences. In order to conserve degrees of freedom, age, attentional performance, and sex were not included as covariates in this analysis. Initially, a regression analysis was proposed to examine the relationship between grey matter volume and COPM, PQRS, and BOT-2. However, PQRS and COPM were significantly positively correlated (r = 0.39, p = 0.046) for this sample (Hinkle et al., 2003). Instead, we used a regression analysis with only grey matter volume, BOT-2 (motor performance), and PQRS (actual performance quality), controlling for the effect of intracranial volume. PQRS was used instead of COPM as it is a more objective measure, despite COPM being our primary outcome. Structural images were analyzed using TFCE due to its increased sensitivity compared to voxel- or cluster-based statistics (Smith and Nichols, 2009; Salimi-Khorshidi et al., 2011; Radua et al., 2014). We assessed statistical significance with the permutation test included in SPM.
All results are reported with TFCE thresholding uncorrected for multiple comparisons (no pFDR-corrected or pFWE-corrected) but corrected for the number of planned comparisons (pre > post, pre < post). Results are presented at p < 0.001 with cluster size threshold at 50 voxels. Cluster size threshold was based on current literature regarding cluster thresholding. Given our N < 50, we opted for a more stringent cluster threshold of 50 compared to lower thresholds of 10 (Lieberman and Cunningham, 2009; Woo et al., 2014). This is also comparable to previous publications of cerebellar VBM with samples of children with neurodevelopmental disabilities (D’Mello et al., 2015). Uncorrected results (p < 0.001) minimize false negative results but do increase the false-positive rate (Durnez et al., 2014).
This study recruited 80 children with DCD+/−ADHD from which 60 were excluded because they either had co-occurring ASD or preterm birth (n = 9), declined to participate in the intervention (n = 2), discontinued intervention (n = 4), or had insufficient data quality for both scans (before and after intervention) to conduct VBM analysis (n = 45) (Figure 2). In order to preserve power, the DCD and DCD + ADHD group were combined. Our final sample for voxel-based morphometry analysis after quality checks comprised of 20 children with DCD [mean (SD) age = 9.9 (1.6) years], of which 70% were males.
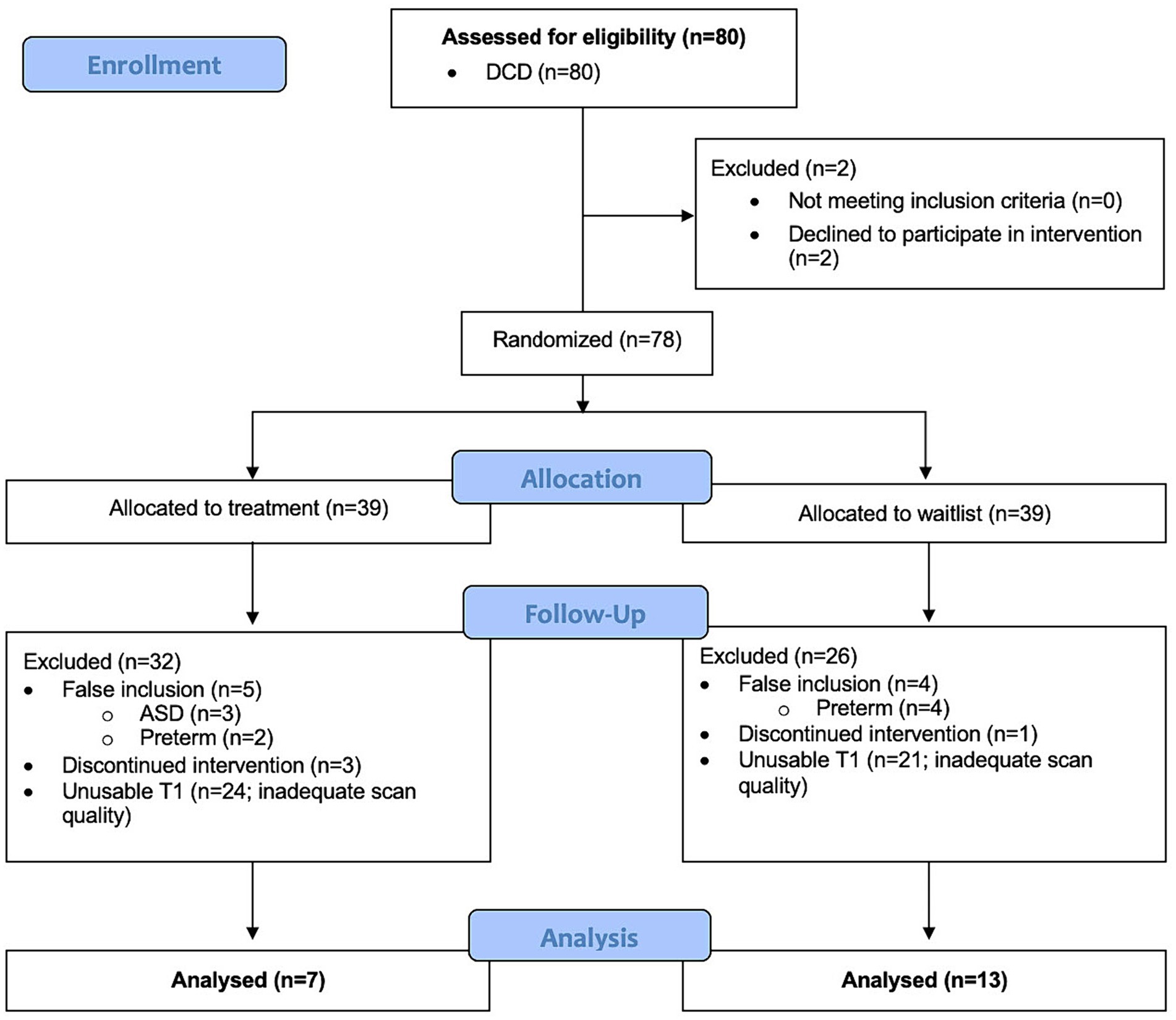
Figure 2. Flow diagram of participant inclusion and exclusion for longitudinal voxel-based morphometry analysis.
Similarly, 35 TD children were initially recruited, from which 26 were excluded because they either declined to participate (n = 1), had ADHD (n = 1), were born preterm (n = 1), had a MABC-2 ≤16th percentile (n = 1), or had insufficient data quality for both scans (3 months separation) to conduct VBM analysis (n = 22).
No difference was found between children with DCD with good or bad quality T1 images based on BOT2 or PQRS scores. Difference in Connors T-score was close to significant [p = 0.086, CI95% = (−8.67, 0.57)]. The loss of children due to poor image quality was similar between the TD and DCD cohorts—71 and 70%, respectively—suggesting that getting quality T1 images from children this age is difficult in general.
Children with DCD whose data was excluded due to motion had an average CJV of 0.68 (±0.13 SD), while those that were kept had an average CJV of 0.61 (±0.09 SD). These values were significantly different [p = 0.002; CI95% = (0.03, 0.11)]. Of the participants that were included for analysis, there was no difference in CJV between the TD and DCD cohorts. Furthermore, for the children with DCD included for analysis, no correlation was found between CJV and MACB-2 scores [CI 95% = (−0.36, 0.06)].
Table 1 presents demographic and behavioral characteristics of the sample. Our final sample included 20 participants with DCD (Figure 2), of which 19 (95%) had attentional difficulties as indicated by a score of 70 or greater on the Conners ADHD Index. This is further supported by current literature which suggests a greater than 50% overlap between the DCD and ADHD (Kadesjö and Gillberg, 1998; Goulardins et al., 2015; Lange, 2017). Lastly, our sample of DCD included 14 males (70%), which aligns with previous literature indicating high prevalence of DCD in males (American Psychiatric Association, 2013). We also included 9 TD participants to investigate if any grey matter changes were due to maturation over a 3 months period.
The Wilcoxon Signed-Rank Test (non-parametric) was used to compare the before and after effect of CO-OP intervention. Participants showed statistically significant improvements (p < 0.001) in their motor goals (COPM), movement quality (PQRS), and motor skills (BOT-2) after CO-OP intervention (Table 2).
When comparing pre-post scans, children with DCD had significantly decreased grey matter [cluster size (k) >50, puncorrected < 0.001] in the right hemisphere in the following regions: middle cingulate gyrus, posterior cingulate gyrus, and the superior frontal gyrus following CO-OP intervention (Table 3 and Figure 3). There were no regions where there was increased grey matter when comparing pre- intervention to post-intervention—the inverse contrast [cluster size (k) <50]. A follow-up analysis with age as a covariate can be found in Supplementary Figure S1.
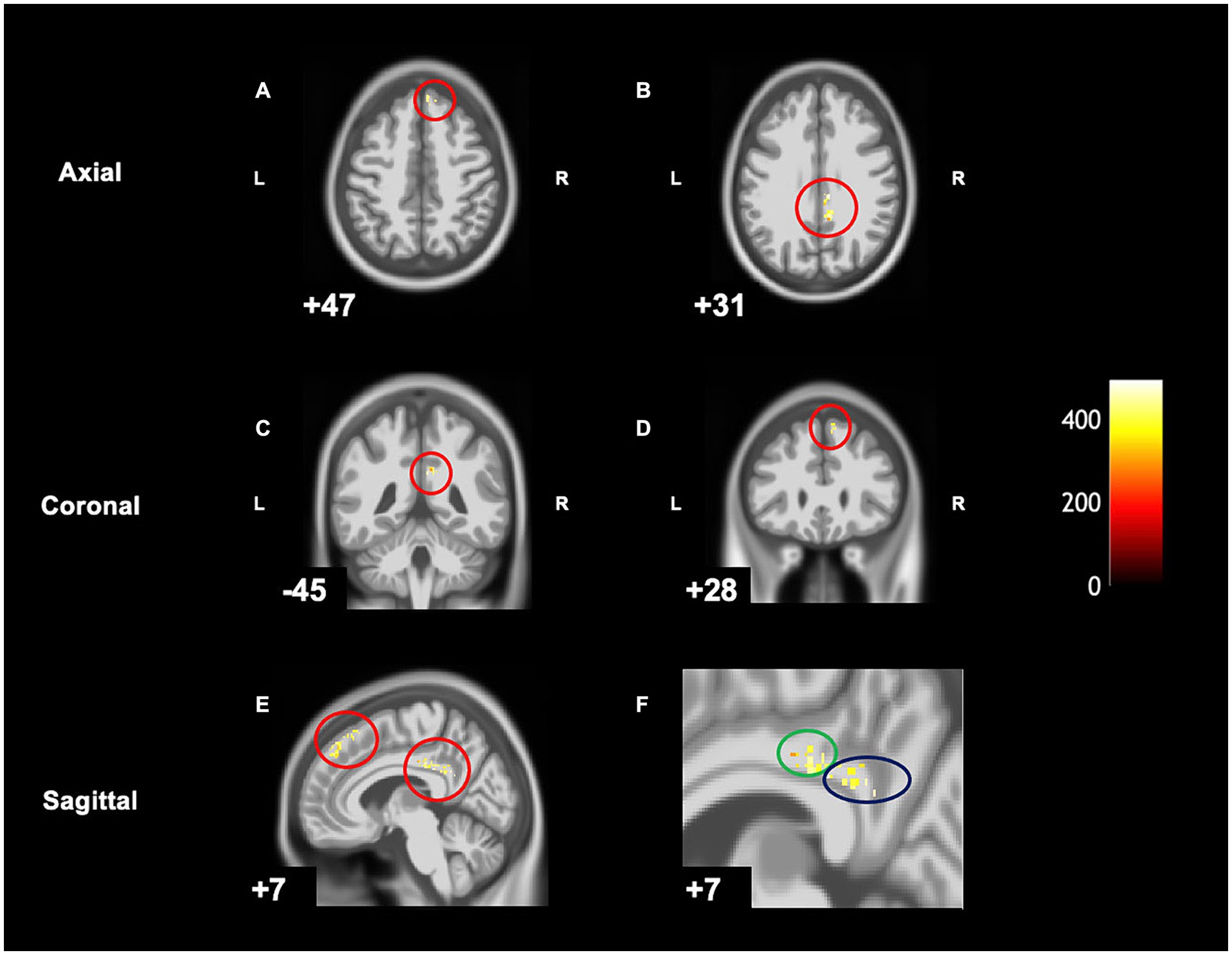
Figure 3. Within-group differences show significantly decreased grey matter in children with DCD after intervention on CAT T1 IXI555 GS. (A) Right superior frontal gyrus. (B) Middle and posterior cingulate gyrus. (C) Right middle and posterior cingulate gyrus. (D) Right superior frontal gyrus. (E) Right superior frontal gyrus, middle and posterior cingulate gyrus. (F) Zoomed image of E: middle (green) and posterior cingulate gyrus (blue).
When looking at TD children over the same period of time, no changes were found in the grey matter [cluster size (k) >50, puncorrected < 0.001].
There were no significant positive or negative (inverse contrast) association between overall motor performance on the BOT-2 percentile scores and grey matter volume changes [cluster size (k) <50]. In addition, there was no significant positive or negative (inverse contrast) association between actual performance quality on motor goals after 10 weeks of intervention (as measured by PQRS scores) and grey matter volume changes [cluster size (k) <50].
In this randomized waitlist-control study, we found that, following CO-OP intervention, children with DCD had decreased grey matter volume in the right superior frontal gyrus, right middle cingulate gyrus, and right posterior cingulate gyrus. We reiterate that due to sample size, we did not correct for multiple comparisons, and further work and replication of these findings is needed. The regions that showed volumetric changes are different from those identified in our previous work that investigated brain volume differences in DCD versus typically developing children using the pre-treatment scans (Malik et al., 2023). There, we showed that children with DCD had greater grey matter volume in left STG at baseline. The post-treatment findings in the right hemisphere reported here may reflect the lateralization of the brain in the early stages of learning to problem solve, or it might relate to emotional regulation (Shobe, 2014; Blais et al., 2017), both of which could be modulated via the CO-OP intervention. In other words, the intervention changes did not “normalize” the previously observed group-based or diagnostic differences in volume, though they may relate to important changes and learning functions implicated in the therapy. To contextualize and interpret the results reported in this study, we will discuss motor learning theories and background literature on the frontal and parietal regions that were found to have decreased grey matter volume.
According to general principles of motor learning and neuroplasticity, interventions that involve people in active, repetitive training can not only increase motor function (Salbach et al., 2004; Michaelsen et al., 2006; Arya et al., 2011) but can also lead to neuroplastic changes (Takeuchi and Izumi, 2013; Maier et al., 2019). Compared to TD children, children with DCD use different strategies and brain regions to improve motor performance and motor learning (Zwicker et al., 2010, 2011; Biotteau et al., 2016). Children with DCD also tend not to improve their motor skills with practice alone (Schoemaker and Smits-Engelsman, 2015), but rather benefit from using cognitive strategies and problem-solving skills to facilitate motor skill acquisition (Mandich et al., 2003; Jokić et al., 2013). CO-OP is a task-oriented intervention that combines both motor learning theories with cognitive approaches (i.e., problem-solving and self-evaluation) (Polatajko et al., 2001; Jackman et al., 2018). Additional studies underscore the self-regulation processes. For example, self-regulation is thought to be a mediator to improve motor skills (Jokić et al., 2013; Green and Payne, 2018). Brain imaging data to date seem to support this hypothesis, as brain regions associated with self-regulation showed greater structural (most findings were in default mode network) (Izadi-Najafabadi and Zwicker, 2021) and functional connectivity (default mode network and the right pregenual anterior cingulate cortex) (Izadi-Najafabadi et al., 2022b) after CO-OP intervention. Two of the brain regions that showed decreased grey matter volume after intervention, the right posterior cingulate gyrus and the right superior frontal gyrus, further support the hypothesis that changes in self-regulation may mediate improved motor function.
The posterior cingulate gyrus is one of the most densely connected regions in the brain, and has been discussed as one of the “least well-understood regions of the cortex” (Leech and Smallwood, 2019, p. 73). It functions as a hub for the default mode network (DMN), arguably one of the most complex networks in the brain (Alves et al., 2019; Buckner and DiNicola, 2019). The DMN has been implicated in a wide array of higher-order functions, most of which rely on internally constructed information (Buckner et al., 2008; Leech and Sharp, 2014; Pan et al., 2018). Perhaps the most relevant DMN functions in the current context would be network suppression during active tasks and learning combined with self-referential processing and self-regulation (Brewer et al., 2013). During CO-OP intervention, children with DCD are guided to use self-regulatory skills such as goal setting, planning, and self-monitoring to address their motor performance difficulties (Hyland and Polatajko, 2012; Jokić et al., 2013). As self-regulation is thought to mediate motor skill improvements observed with CO-OP intervention (Jokić et al., 2013; Green and Payne, 2018), it makes clinical sense that neuroplastic changes would be observed in brain regions associated with self-regulation. We propose that CO-OP intervention may reinforce synaptic connections while pruning less efficient pathways, resulting in a decrease in grey matter volume. While speculative, our interpretation is consistent with theories and findings relating to experience-dependent neuroplasticity (Giedd et al., 1999; Lenroot et al., 2007; Kleim and Jones, 2008; Wierenga et al., 2014).
The right superior frontal gyrus also showed decreased grey matter volume after CO-OP intervention. Activation of the right superior frontal gyrus is thought to modulate inhibitory control (Hu et al., 2016). Inhibitory control is defined as the suppression of behavior in response to internal or external influences (Morasch and Bell, 2011). It is a cognitive function that plays an important role in tasks such as riding a bike, where it is often necessary to prevent an action from being performed inappropriately (Coxon et al., 2007). As children with DCD have difficulty with inhibitory control of attention and executive function (Wilson et al., 1997; Wilson and Maruff, 1999; Mandich et al., 2003; Tsai, 2009; Wilson et al., 2020), impairment in inhibitory control might play a role in underlying motor coordination problems (Tsai, 2009). Our finding of decreased grey matter in the frontal region is consistent with neurodevelopmental and lesion neuroimaging studies that have identified dorsolateral and medial prefrontal cortices (responsible for unwanted response) (Rubia et al., 2005) and the right inferior frontal gyrus and basal ganglia (for cancellation of prepared or ongoing movements) (Aron et al., 2003; Chambers et al., 2006; Chikazoe et al., 2008) are involved in inhibitory control. Inhibitory control tends to improve with active intervention (Tsai, 2009), which may be associated with decreased grey matter volume in the superior frontal gyrus. As above, we speculate that this may be due to synaptic pruning of pathways that were reinforced during intervention.
The middle cingulate gyrus is known by two names: (1) dorsal anterior cingulate cortex (Palomero-Gallagher et al., 2008, 2009; Apps et al., 2013); or (2) middle cingulate cortex, which is further split into an anterior and posterior middle cingulate cortex (Apps et al., 2013; Brewer et al., 2013; Vogt, 2016). The different terminology may lead many studies to inaccurately discuss the functional properties of the middle cingulate cortex (Apps et al., 2013). Here, we adopt the more recent nomenclature of the middle cingulate gyrus, which itself is divided into dorsal, middle and posterior subsections. It has extensive connections with cognitive (e.g., lateral prefrontal) and motor-related (e.g., premotor and primary motor) areas of the cortex (Vogt and Morecraft, 2009; Stevens et al., 2011). Based on the seminal study conducted in nonhuman primates by Paus (2001), the most dorsal portion of the middle cingulate gyrus is important for the execution of voluntary motor control through its cognitive and motor connections and processing of abstract thinking and intention in motor execution. In humans, the dorsal middle cingulate gyrus is activated with motor-related tasks (Beckmann et al., 2009) and during the internal generation of movements (pre-frontal, pre-motor, parietal, basal ganglia) (Deiber et al., 1999; Cunnington et al., 2002; Debaere et al., 2003; Lau et al., 2004; van Eimeren et al., 2006; Jankowski et al., 2009). The posterior middle cingulate cortex is part of the caudal cingulate premotor area which is involved in multisensory orientation of the individual in space and in sensing the force and direction of movements in space (Vogt, 2016). Reflecting on the methodology of the intervention, to achieve the self-chosen motor goals, CO-OP uses cognitive-based strategies during task-specific intervention to facilitate motor skill acquisition. The problem-solving aspect of the intervention promotes the thought process of “what” and “how” to do a particular action. This thinking, in addition to the cognitive connections of middle cingulate gyrus may have promoted a decrease in grey matter (through increased synaptic pruning) in the middle cingulate gyrus and cognitive (posterior cingulate gyrus) and motor regions (superior frontal gyrus) found in this study.
Previously, we reported that children with DCD had greater grey matter volume in the left superior frontal gyrus compared to typically developing children (Malik et al., 2023). This is posited to be a result of aberrant brain maturation, specifically a delay or dysfunction in cortical thinning through the mechanisms (synaptic pruning) described earlier. It is thought that similar to other neurodevelopmental disorders, dysfunction of synaptic pruning may result in increased grey matter as the neural connections are not maturing as expected. This leads to difficulties in working memory and higher order cognitive functions. After having undergone 10 weeks of CO-OP intervention, the decrease in grey matter volume in the right middle and posterior cingulate gyrus and right superior frontal gyrus may be a result of increased synaptic pruning.
The short form of BOT-2 percentile (measure of motor performance) significantly increased after intervention with this sub-set of participants; however, this was not the case in the larger sample (Izadi-Najafabadi et al., 2022a). We did not see any association between grey matter changes and this measure of overall motor performance in this small sample. One possible explanation for this has to do with the nature of the intervention and neuroplasticity. CO-OP is a task-oriented intervention that focuses on using a problem-solving based framework to acquire specific motor skills; it does not address the underlying motor impairment (Polatajko et al., 2001; Blank et al., 2012; Smits-Engelsman et al., 2012). Given the principles of neuroplasticity (Kleim and Jones, 2008), we would only expect to see brain changes associated with the specific target actions, not overall motor performance; thus, it is not surprising that we did not observe a relationship between grey matter volume changes and BOT-2 scores. However, we also investigated the association between PQRS and grey matter volume changes in the brain. The PQRS is a measure of actual performance quality for the child-chosen goals that were addressed in therapy over 10 weeks. We also did not find a relationship between movement quality and grey matter volume. Given our small sample size, we are likely under-powered to detect this expected relationship.
Several limitations are present in this study. First, our sample size was limited. Of the 80 participants scanned, several scans were lost due to insufficient quality for VBM analysis at either or both time points. After stringent quality checks and exclusion criteria, the final sample was small (N = 20). As mentioned by Sáenz et al. (2020), this may lead to a biased sample as the significant head motion can be associated with clinical traits and the scans of the most severely impaired participants therefore might have been excluded; the exclusion of poor-quality scans also limits the generalizability of results (Sáenz et al., 2020). Relatedly, we did not correct for multiple comparisons in the analyses, and thus the results are potentially more vulnerable to false positives. The fact that the results are sensible (i.e., the identified brain regions relate to key aspects of the intervention) suggests the results may be valid, but replication is needed. Second, there are no standardized quantification guides to measure degree of motion artifact in T1 scans. We used visual inspection by two trained, independent raters based on established guidelines (Harvard Center for Brain Science, 2014), ensuring that only high-quality scans that were deemed acceptable by both raters were included. Third, while we intended to analyze children with DCD and DCD + ADHD separately, our sample for VBM analysis was smaller than expected and included children with DCD and co-occurring ADHD (n = 6) could have confounded our findings. However, the sample had similar Connors ADHD Index scores, suggesting the children with diagnosed ADHD were more similar than different to the DCD group. We were also unable to compare the treatment group with the waitlist group, which would have controlled for maturation, nor were we able to examine follow-up effects in the treatment group. Fourth, we did not control for age, sex, or medications, in order to conserve power. Finally, there are some limitations regarding volume-based measures. Since grey matter includes surface area and thickness, each of which have their own developmental trajectories, the interpretation of grey matter volume becomes difficult without examining surface area or thickness individually (Frye et al., 2010).
Future studies should continue to explore intervention-induced changes in grey matter volume in children with DCD but in a larger sample of children and measured overtime to see if these changes are maintained. Exploring cortical thickness and volume in one study longitudinally would provide more insight into the brain’s structural morphology in this disorder. Likewise, results could be stratified by age, sex and/or medication use to provide further insights (Caviness et al., 1996; De Bellis et al., 2001; Spencer et al., 2013). Lastly, to further explore synaptic pruning, molecular, and animal model studies should be conducted to examine the long-term intervention-induced changes in synaptic pruning in this population.
Overall, CO-OP is one of the recommended task-oriented rehabilitation interventions in the international clinical practice guidelines for DCD (Blank et al., 2019). This intervention is effective in improving children’s perceptions of their motor performance of the specific skills they wanted to learn, as well as improved motor quality while performing these skills as rated by a therapist. In conclusion, our data indicate that CO-OP induces neuroplasticity of the grey matter in the right superior frontal gyrus (inhibitory control), right posterior cingulate gyrus (self-regulation), and right middle cingulate gyrus (voluntary thinking and cognitive and motor connections) in children with DCD. We speculate that these neuroplastic changes result from upregulated synaptic pruning that occurs within the repeated actions and learning of the intervention, leading to the maturation of synapses in DCD-related circuits. This study further supports our team’s findings on how CO-OP induces changes in structure and function of brain regions associated with self-regulation, providing initial evidence for the brain-based impact of this intervention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Children’s and Women’s Health Centre/University of British Columbia. The studies were conducted in accordance with the local legislation and institutional requirements. Parents/legal guardians provided informed written consent and children assented to participate in the study.
MM: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. DL: Formal analysis, Writing – review & editing. TV: Formal analysis, Writing – review & editing. JZ: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. AW: Formal analysis, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Canadian Institutes of Health Research (PI Zwicker; FDN-143258). Malik was funded by a UBC Faculty of Medicine Graduate Award (#6442) and the Syd Vernon Graduate Student Award. Zwicker was funded by the Canadian Institutes of Health Research New Investigator Award (201512MSH-360883-141638), Michael and Smith Foundation for Health Research Scholar Award (#5762), Canadian Child Health Clinician Scientist Program Career Development/Enhancement Award. Weber, Vanderwal, and Zwicker are funded by BC Children’s Hospital Research Institute and Foundation.
We are thankful for the children and families who took the time to participate in our study. We thank our research team—Gisela Gosse, Janet Rigney, Sara Leckie, Meisan Brown-Lum, and Alyssa Barrie—for their assistance with data collection and management, and the many occupational therapists who provided the intervention.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2024.1316117/full#supplementary-material
Alves, P. N., Foulon, C., Karolis, V., Bzdok, D., Margulies, D. S., Volle, E., et al. (2019). An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol. 2:370. doi: 10.1038/s42003-019-0611-3
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5. 5th Edn. Washington, DC: American Psychiatric Association.
Apps, M. A. J., Lockwood, P. L., and Balsters, J. H. (2013). The role of the midcingulate cortex in monitoring others’ decisions. Front. Neurosci. 7:251. doi: 10.3389/fnins.2013.00251
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J., and Robbins, T. W. (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 6, 115–116. doi: 10.1038/nn1003
Arya, K. N., Pandian, S., Verma, R., and Garg, R. K. (2011). Movement therapy induced neural reorganization and motor recovery in stroke: a review. J. Bodyw. Mov. Ther. 15, 528–537. doi: 10.1016/j.jbmt.2011.01.023
Ashburner, J., and Friston, K. J. (2000). Voxel-based morphometry—the methods. NeuroImage 11, 805–821. doi: 10.1006/nimg.2000.0582
Beckmann, M., Johansen-Berg, H., and Rushworth, M. F. S. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190. doi: 10.1523/jneurosci.3328-08.2009
Biotteau, M., Chaix, Y., Blais, M., Tallet, J., Péran, P., and Albaret, J.-M. (2016). Neural signature of DCD: a critical review of MRI neuroimaging studies. Front. Neurol. 7:227. doi: 10.3389/fneur.2016.00227
Blais, M., Amarantini, D., Albaret, J.-M., Chaix, Y., and Tallet, J. (2017). Atypical inter-hemispheric communication correlates with altered motor inhibition during learning of a new bimanual coordination pattern in developmental coordination disorder. Dev. Sci. 21:e12563. doi: 10.1111/desc.12563
Blank, R., Barnett, A. L., Cairney, J., Green, D., Kirby, A., Polatajko, H., et al. (2019). International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 61, 242–285. doi: 10.1111/dmcn.14132
Blank, R., Smits-Engelsman, B., Polatajko, H., and Wilson, P.European Academy for Childhood Disability (2012). European Academy for Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version). Dev. Med. Child Neurol. 54, 54–93. doi: 10.1111/j.1469-8749.2011.04171.x
Brewer, J. A., Garrison, K. A., and Whitfield-Gabrieli, S. (2013). What about the “self” is processed in the posterior cingulate cortex. Front. Hum. Neurosci. 7:647. doi: 10.3389/fnhum.2013.00647
Bruininks, R., and Bruininks, B. (2005). Bruininks-Oseretsky test of motor proficiency. Minneapolis, MN: NCS Pearson.
Buckner, R. L., Andrews-Hanna, J. R., and Schacter, D. L. (2008). The brain’s default network. Ann. N. Y. Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Buckner, R. L., and DiNicola, L. M. (2019). The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608. doi: 10.1038/s41583-019-0212-7
Carswell, A., McColl, M. A., Baptiste, S., Law, M., Polatajko, H., and Pollock, N. (2004). The Canadian Occupational Performance Measure: a research and clinical literature review. Can. J. Occup. Ther. 71, 210–222. doi: 10.1177/000841740407100406
Caviness, V. S., Kennedy, D. N., Richelme, C., Rademacher, J., and Filipek, P. A. (1996). The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb. Cortex 6, 726–736. doi: 10.1093/cercor/6.5.726
Chambers, C. D., Bellgrove, M. A., Stokes, M. G., Henderson, T. R., Garavan, H., Robertson, I. H., et al. (2006). Executive ‘brake failure’ following deactivation of human frontal lobe. J. Cogn. Neurosci. 18, 444–455. doi: 10.1162/089892906775990606
Chikazoe, J., Jimura, K., Asari, T., Yamashita, K., Morimoto, H., Hirose, S., et al. (2008). Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex 19, 146–152. doi: 10.1093/cercor/bhn065
Coxon, J. P., Stinear, C. M., and Byblow, W. D. (2007). Selective inhibition of movement. J. Neurophysiol. 97, 2480–2489. doi: 10.1152/jn.01284.2006
Cunnington, R., Windischberger, C., Deecke, L., and Moser, E. (2002). The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage 15, 373–385. doi: 10.1006/nimg.2001.0976
D’Mello, A. M., Crocetti, D., Mostofsky, S. H., and Stoodley, C. J. (2015). Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage Clin. 7, 631–639. doi: 10.1016/j.nicl.2015.02.007
De Bellis, M. D., Keshavan, M. S., Beers, S. R., Hall, J., Frustaci, K., Masalehdan, A., et al. (2001). Sex differences in brain maturation during childhood and adolescence. Cereb. Cortex 11, 552–557. doi: 10.1093/cercor/11.6.552
Debaere, F., Wenderoth, N., Sunaert, S., Van Hecke, P., and Swinnen, S. P. (2003). Internal vs. external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage 19, 764–776. doi: 10.1016/s1053-8119(03)00148-4
Dedding, C., Cardol, M., Eyssen, I. C., and Beelen, A. (2004). Validity of the Canadian Occupational Performance Measure: a client-centred outcome measurement. Clin. Rehabil. 18, 660–667. doi: 10.1191/0269215504cr746oa
Deiber, M.-P., Honda, M., Ibañez, V., Sadato, N., and Hallett, M. (1999). Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J. Neurophysiol. 81, 3065–3077. doi: 10.1152/jn.1999.81.6.3065
Deitz, J. C., Kartin, D., and Kopp, K. (2007). Review of the Bruininks-Oseretsky Test of Motor Proficiency, second edition (BOT-2). Phys. Occup. Ther. Pediatr. 27, 87–102. doi: 10.1080/j006v27n04_06
Durnez, J., Moerkerke, B., and Nichols, T. E. (2014). Post-hoc power estimation for topological inference in fMRI. NeuroImage 84, 45–64. doi: 10.1016/j.neuroimage.2013.07.072
Eyssen, I. C. J. M., Steultjens, M. P. M., Oud, T. A. M., Bolt, E. M., Maasdam, A., and Dekker, J. (2011). Responsiveness of the Canadian Occupational Performance Measure. J. Rehabil. Res. Dev. 48, 517–528. doi: 10.1682/jrrd.2010.06.0110
Farokhian, F., Beheshti, I., Sato, N., and Matsuda, H. (2017). Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Front. Neurol. 8:428. doi: 10.3389/fneur.2017.00428
Fellhauer, I., Zöllner, F. G., Schröder, J., Degen, C., Kong, L., Essig, M., et al. (2015). Comparison of automated brain segmentation using a brain phantom and patients with early Alzheimer’s dementia or mild cognitive impairment. Psychiatry Res. 233, 299–305. doi: 10.1016/j.pscychresns.2015.07.011
Frye, R. E., Liederman, J., Malmberg, B., McLean, J., Strickland, D., and Beauchamp, M. S. (2010). Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb. Cortex 20, 2625–2635. doi: 10.1093/cercor/bhq010
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863. doi: 10.1038/13158
Gill, K. K., Lang, D., and Zwicker, J. G. (2022). Cerebellar differences after rehabilitation in children with developmental coordination disorder. Brain Sci. 12:856. doi: 10.3390/brainsci12070856
Gilmore, J. H., Knickmeyer, R. C., and Gao, W. (2018). Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 19, 123–137. doi: 10.1038/nrn.2018.1
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N. A., Friston, K. J., and Frackowiak, R. S. J. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14, 21–36. doi: 10.1006/nimg.2001.0786
Goulardins, J. B., Rigoli, D., Licari, M., Piek, J. P., Hasue, R. H., Oosterlaan, J., et al. (2015). Attention deficit hyperactivity disorder and developmental coordination disorder: two separate disorders or do they share a common etiology. Behav. Brain Res. 292, 484–492. doi: 10.1016/j.bbr.2015.07.009
Green, D., and Payne, S. (2018). Understanding organisational ability and self-regulation in children with developmental coordination disorder. Curr. Dev. Disord. Rep. 5, 34–42. doi: 10.1007/s40474-018-0129-2
Harvard Center for Brain Science (2014). Quality control. Available at: http://cbs.fas.harvard.edu/science/core-facilities/neuroimaging/information-investigators/qc
Henderson, S. E., Sugden, D. A., and Barnett, A. L. (2007). Movement assessment battery for children. 2nd Edn. London: Psychological Corporation.
Hinkle, D. E., Wiersma, W., and Jurs, S. G. (2003). Applied statistics for the behavioral sciences. 5th Edn. Boston: Houghton Mifflin.
Hu, S., Ide, J. S., Zhang, S., and Li, C. R. (2016). The right superior frontal gyrus and individual variation in proactive control of impulsive response. J. Neurosci. 36, 12688–12696. doi: 10.1523/jneurosci.1175-16.2016
Hyland, M., and Polatajko, H. J. (2012). Enabling children with developmental coordination disorder to self-regulate through the use of dynamic performance analysis: evidence from the CO-OP approach. Hum. Mov. Sci. 31, 987–998. doi: 10.1016/j.humov.2011.09.003
Izadi-Najafabadi, S., Gunton, C., Dureno, Z., and Zwicker, J. G. (2022a). Effectiveness of Cognitive Orientation to Occupational Performance intervention in improving motor skills of children with developmental coordination disorder: a randomized waitlist-control trial. Clin. Rehabil. 36, 776–788. doi: 10.1177/02692155221086188
Izadi-Najafabadi, S., Rinat, S., and Zwicker, J. G. (2022b). Brain functional connectivity in children with developmental coordination disorder following rehabilitation intervention. Pediatr. Res. 91, 1459–1468. doi: 10.1038/s41390-021-01517-3
Izadi-Najafabadi, S., and Zwicker, J. G. (2021). White matter changes with rehabilitation in children with developmental coordination disorder: a randomized controlled trial. Front. Hum. Neurosci. 15:673003. doi: 10.3389/fnhum.2021.673003
Jackman, M., Novak, I., Lannin, N., Froude, E., Miller, L., and Galea, C. (2018). Effectiveness of Cognitive Orientation to daily Occupational Performance over and above functional hand splints for children with cerebral palsy or brain injury: a randomized controlled trial. BMC Pediatr. 18:248. doi: 10.1186/s12887-018-1213-9
Jahn, A. (2019). “SPM tutorial #7: setting the origin” in Andy’s brain book. Available at: https://andysbrainbook.readthedocs.io/en/latest/SPM/SPM_Short_Course/SPM_07_SettingTheOrigin.html
Jankowski, J., Scheef, L., Hüppe, C., and Boecker, H. (2009). Distinct striatal regions for planning and executing novel and automated movement sequences. NeuroImage 44, 1369–1379. doi: 10.1016/j.neuroimage.2008.10.059
Jokić, C. S., Polatajko, H., and Whitebread, D. (2013). Self-regulation as a mediator in motor learning: the effect of the Cognitive Orientation to Occupational Performance approach on children with DCD. Adapt. Phys. Act. Q. 30, 103–126. doi: 10.1123/apaq.30.2.103
Kadesjö, B., and Gillberg, C. (1998). Attention deficits and clumsiness in Swedish 7-year-old children. Dev. Med. Child Neurol. 40, 796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x
Kirby, A., and Sugden, D. A. (2007). Children with developmental coordination disorders. J. R. Soc. Med. 100, 182–186. doi: 10.1258/jrsm.100.4.182
Kirby, A., Sugden, D., and Purcell, C. (2013). Diagnosing developmental coordination disorders. Arch. Dis. Child. 99, 292–296. doi: 10.1136/archdischild-2012-303569
Kleim, J. A., and Jones, T. A. (2008). Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, S225–S239. doi: 10.1044/1092-4388(2008/018)
Krupa, K., and Bekiesińska-Figatowska, M. (2015). Artifacts in magnetic resonance imaging. Pol. J. Radiol. 80, 93–106. doi: 10.12659/PJR.892628
Lange, S. M. (2017). ADHD and comorbid developmental coordination disorder: implications and recommendations for school psychologists. Contemp. Sch. Psychol. 22, 30–39. doi: 10.1007/s40688-017-0122-5
Lau, H. C., Rogers, R. D., Ramnani, N., and Passingham, R. E. (2004). Willed action and attention to the selection of action. NeuroImage 21, 1407–1415. doi: 10.1016/j.neuroimage.2003.10.034
Law, M., Baptiste, S., Carswell, A., McColl, M. A., Polatajko, H. J., and Pollock, N. (2014). Canadian occupational performance measure (COPM). 5th Edn. Ottawa, ON: CAOT Publications ACE.
Leech, R., and Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. doi: 10.1093/brain/awt162
Leech, R., and Smallwood, J. (2019). The posterior cingulate cortex: Insights from structure and function. Handb. Clin. Neurol. 166, 73–85. doi: 10.1016/B978-0-444-64196-0.00005-4
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36, 1065–1073. doi: 10.1016/j.neuroimage.2007.03.053
Lerch, J. P., van der Kouwe, A. J. W., Raznahan, A., Paus, T., Johansen-Berg, H., Miller, K. L., et al. (2017). Studying neuroanatomy using MRI. Nat. Neurosci. 20, 314–326. doi: 10.1038/nn.4501
Lieberman, M. D., and Cunningham, W. A. (2009). Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 4, 423–428. doi: 10.1093/scan/nsp052
Maier, M., Ballester, B. R., and Verschure, P. F. M. J. (2019). Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front. Syst. Neurosci. 13:74. doi: 10.3389/fnsys.2019.00074
Malik, M. A., Lang, D., Vanderwal, T., and Zwicker, J. G. (2023). Cortical grey matter volume differences in children with developmental coordination disorder compared to typically developing children. Front. Hum. Neurosci. 16:921505. doi: 10.3389/fnhum.2022.921505
Mandich, A., Buckolz, E., and Polatajko, H. (2002). On the ability of children with developmental coordination disorder (DCD) to inhibit response initiation: the Simon effect. Brain Cogn. 50, 150–162. doi: 10.1016/s0278-2626(02)00020-9
Mandich, A. D., Polatajko, H. J., and Rodger, S. (2003). Rites of passage: understanding participation of children with developmental coordination disorder. Hum. Mov. Sci. 22, 583–595. doi: 10.1016/j.humov.2003.09.011
Martini, R., Rios, J., Polatajko, H., Wolf, T., and McEwen, S. (2014). The Performance Quality Rating Scale (PQRS): reliability, convergent validity, and internal responsiveness for two scoring systems. Disabil. Rehabil. 37, 231–238. doi: 10.3109/09638288.2014.913702
Mei, T., Llera, A., Floris, D. L., Forde, N. J., Tillmann, J., Durston, S., et al. (2020). Gray matter covariations and core symptoms of autism: the EU-AIMS Longitudinal European Autism Project. Mol. Autism. 11:86. doi: 10.1186/s13229-020-00389-4
Michaelsen, S. M., Dannenbaum, R., and Levin, M. F. (2006). Task-specific training with trunk restraint on arm recovery in stroke. Stroke 37, 186–192. doi: 10.1161/01.str.0000196940.20446.c9
Miller, L. T., Polatajko, H. J., Missiuna, C., Mandich, A. D., and Macnab, J. J. (2001). A pilot trial of a cognitive treatment for children with developmental coordination disorder. Hum. Mov. Sci. 20, 183–210. doi: 10.1016/s0167-9457(01)00034-3
Morasch, K. C., and Bell, M. A. (2011). The role of inhibitory control in behavioral and physiological expressions of toddler executive function. J. Exp. Child Psychol. 108, 593–606. doi: 10.1016/j.jecp.2010.07.003
Palomero-Gallagher, N., Mohlberg, H., Zilles, K., and Vogt, B. (2008). Cytology and receptor architecture of human anterior cingulate cortex. J. Comp. Neurol. 508, 906–926. doi: 10.1002/cne.21684
Palomero-Gallagher, N., Vogt, B. A., Schleicher, A., Mayberg, H. S., and Zilles, K. (2009). Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 30, 2336–2355. doi: 10.1002/hbm.20667
Pan, J., Zhan, L., Hu, C., Yang, J., Wang, C., Gu, L., et al. (2018). Emotion regulation and complex brain networks: association between expressive suppression and efficiency in the fronto-parietal network and default-mode network. Front. Hum. Neurosci. 12:70. doi: 10.3389/fnhum.2018.00070
Paus, T. (2001). Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2, 417–424. doi: 10.1038/35077500
Polatajko, H. J., and Mandich, A. (2004). Enabling occupation in children: the Cognitive Orientation to daily Occupational Performance (CO-OP) approach. Ottawa, ON: CAOT Publications ACE.
Polatajko, H. J., Mandich, A. D., Missiuna, C., Miller, L. T., Macnab, J. J., Malloy-Miller, T., et al. (2001). Cognitive Orientation to daily Occupational Performance (CO-OP). Phys. Occup. Ther. Pediatr. 20, 107–123. doi: 10.1300/J006v20n02_07
Radua, J., Canales-Rodríguez, E. J., Pomarol-Clotet, E., and Salvador, R. (2014). Validity of modulation and optimal settings for advanced voxel-based morphometry. NeuroImage 86, 81–90. doi: 10.1016/j.neuroimage.2013.07.084
Reuter, M., Tisdall, M. D., Qureshi, A., Buckner, R. L., van der Kouwe, A. J. W., and Fischl, B. (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage 107, 107–115. doi: 10.1016/j.neuroimage.2014.12.006
Reynolds, J. E., Licari, M. K., Reid, S. L., Elliott, C., Winsor, A. M., Bynevelt, M., et al. (2017). Reduced relative volume in motor and attention regions in developmental coordination disorder: a voxel-based morphometry study. Int. J. Dev. Neurosci. 58, 59–64. doi: 10.1016/j.ijdevneu.2017.01.008
Rubia, K., Smith, A. B., Brammer, M. J., Toone, B., and Taylor, E. (2005). Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry 162, 1067–1075. doi: 10.1176/appi.ajp.162.6.1067
Sáenz, A. A., Van Schuerbeek, P., Baijot, S., Septier, M., Deconinck, N., Defresne, P., et al. (2020). Disorder-specific brain volumetric abnormalities in attention-deficit/hyperactivity disorder relative to autism spectrum disorder. PLoS One 15:e0241856. doi: 10.1371/journal.pone.0241856
Salbach, N. M., Mayo, N. E., Wood-Dauphinee, S., Hanley, J. A., Richards, C. L., and Côté, R. (2004). A task-orientated intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clin. Rehabil. 18, 509–519. doi: 10.1191/0269215504cr763oa
Salimi-Khorshidi, G., Smith, S. M., and Nichols, T. E. (2011). Adjusting the effect of nonstationarity in cluster-based and TFCE inference. NeuroImage 54, 2006–2019. doi: 10.1016/j.neuroimage.2010.09.088
Scammell, E. M., Bates, S. V., Houldin, A., and Polatajko, H. J. (2016). The Cognitive Orientation to daily Occupational Performance (CO-OP): a scoping review. Can. J. Occup. Ther. 83, 216–225. doi: 10.1177/0008417416651277
Schoemaker, M. M., and Smits-Engelsman, B. C. M. (2015). Is treating motor problems in DCD just a matter of practice and more practice? Curr. Dev. Disord. Rep. 2, 150–156. doi: 10.1007/s40474-015-0045-7
Shobe, E. R. (2014). Independent and collaborative contributions of the cerebral hemispheres to emotional processing. Front. Hum. Neurosci. 8:230. doi: 10.3389/fnhum.2014.00230
Slater, L. M., Hillier, S. L., and Civetta, L. R. (2010). The clinimetric properties of performance-based gross motor tests used for children with developmental coordination disorder: a systematic review. Pediatr. Phys. Ther. 22, 170–179. doi: 10.1097/pep.0b013e3181dbeff0
Smith, SM., and Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061
Smits-Engelsman, B. C. M., Blank, R., van der Kaay, A.-C., Mosterd-van der Meijs, R., Vlugt-van den Brand, E., Polatajko, H. J., et al. (2012). Efficacy of interventions to improve motor performance in children with developmental coordination disorder: a combined systematic review and meta-analysis. Dev. Med. Child Neurol. 55, 229–237. doi: 10.1111/dmcn.12008
Smits-Engelsman, B., Vinçon, S., Blank, R., Quadrado, V. H., Polatajko, H., and Wilson, P. H. (2018). Evaluating the evidence for motor-based interventions in developmental coordination disorder: a systematic review and meta-analysis. Res. Dev. Disabil. 74, 72–102. doi: 10.1016/j.ridd.2018.01.002
Spencer, T. J., Brown, A., Seidman, L. J., Valera, E. M., Makris, N., Lomedico, A., et al. (2013). Effect of psychostimulants on brain structure and function in ADHD. J. Clin. Psychiatry 74, 902–917. doi: 10.4088/jcp.12r08287
Statistics Canada (2021). Population estimates on July 1st, by age and sex. Statistics Canada. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501
Sterling, C., Taub, E., Davis, D., Rickards, T., Gauthier, L. V., Griffin, A., et al. (2013). Sturctural neuroplatics change after constraint-induced movement therapy in children with cerebral palsy. Pediatrics 131, e1664–e1669. doi: 10.1542/peds.2012-2051
Stevens, F. L., Hurley, R. A., and Taber, K. H. (2011). Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatr. Clin. Neurosci. 23, 121–125. doi: 10.1176/jnp.23.2.jnp121
Sugden, D. (2007). Current approaches to intervention in children with developmental coordination disorder. Dev. Med. Child Neurol. 49, 467–471. doi: 10.1111/j.1469-8749.2007.00467.x
Takeuchi, N., and Izumi, S.-I. (2013). Rehabilitation with poststroke motor recovery: a review with a focus on neural plasticity. Stroke Res. Treat. 2013, 1–13. doi: 10.1155/2013/128641
Tavares, V., Prata, D., and Ferreira, H. (2020). Comparing SPM12 and CAT12 segmentation pipelines: a brain tissue volume-based age and Alzheimer’s disease study. J. Neurosci. Methods 334:108565. doi: 10.1016/j.jneumeth.2019.108565
Tsai, C.-L. (2009). The effectiveness of exercise intervention on inhibitory control in children with developmental coordination disorder: using a visuospatial attention paradigm as a model. Res. Dev. Disabil. 30, 1268–1280. doi: 10.1016/j.ridd.2009.05.001
van Eimeren, T., Wolbers, T., Münchau, A., Büchel, C., Weiller, C., and Roman Siebner, H. (2006). Implementation of visuospatial cues in response selection. NeuroImage 29, 286–294. doi: 10.1016/j.neuroimage.2005.07.014
Vogt, B. A. (2016). Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 74, 28–46. doi: 10.1016/j.jchemneu.2016.01.010
Vogt, B. A., and Morecraft, R. J. (2009). “Cingulate gyrus” in Encyclopedia of neuroscience. eds. M. D. Binder, N. Hirokawa, and U. Windhorst (Berlin: Springer).
Wang, J., Fu, K., Chen, L., Duan, X., Guo, X., Chen, H., et al. (2017). Increased gray matter volume and resting-state functional connectivity in somatosensory cortex and their relationship with autistic symptoms in young boys with autism spectrum disorder. Front. Physiol. 8:588. doi: 10.3389/fphys.2017.00588
Whitwell, J. L. (2009). Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 29, 9661–9664. doi: 10.1523/jneurosci.2160-09.2009
Wierenga, L. M., Langen, M., Oranje, B., and Durston, S. (2014). Unique developmental trajectories of cortical thickness and surface area. NeuroImage 87, 120–126. doi: 10.1016/j.neuroimage.2013.11.010
Wilson, B. N., and Crawford, S. G. (2007). Developmental Coordination Disorder Questionnaire 2007 (DCDQ’07). Available at: http://www.dcdq.ca
Wilson, P. H., and Maruff, P. (1999). Deficits in the endogenous control of covert visuospatial attention in children with developmental coordination disorder. Hum. Mov. Sci. 18, 421–442. doi: 10.1016/s0167-9457(99)00017-2
Wilson, P., Ruddock, S., Rahimi-Golkhandan, S., Piek, J., Sugden, D., Green, D., et al. (2020). Cognitive and motor function in developmental coordination disorder. Dev. Med. Child Neurol. 62, 1317–1323. doi: 10.1111/dmcn.14646
Woo, C.-W., Krishnan, A., and Wager, T. D. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage 91, 412–419. doi: 10.1016/j.neuroimage.2013.12.058
Yassa, M., and Stark, C. (2009). A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage 44, 319–327. doi: 10.1016/j.neuroimage.2008.09.016
Yu, J. J., Burnett, A. F., and Sit, C. H. (2018). Motor skill interventions in children with developmental coordination disorder: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 99, 2076–2099. doi: 10.1016/j.apmr.2017.12.009
Zaitsev, M., Maclaren, J., and Herbst, M. (2015). Motion artifacts in MRI: a complex problem with many partial solutions. J. Magn. Reson. Imaging 42, 887–901. doi: 10.1002/jmri.24850
Zwicker, J. G., Missiuna, C., Harris, S. R., and Boyd, L. A. (2010). Brain activation of children with developmental coordination disorder is different than peers. Pediatrics 126, e678–e686. doi: 10.1542/peds.2010-0059
Zwicker, J. G., Missiuna, C., Harris, S. R., and Boyd, L. A. (2011). Brain activation associated with motor skill practice in children with developmental coordination disorder: an fMRI study. Int. J. Dev. Neurosci. 29, 145–152. doi: 10.1016/j.ijdevneu.2010.12.002
Keywords: developmental coordinator disorder, motor skills disorder, children, CO-OP, rehabilitation, MRI, brain structure, voxel-based morphometry
Citation: Malik MA, Weber AM, Lang D, Vanderwal T and Zwicker JG (2024) Changes in cortical grey matter volume with Cognitive Orientation to daily Occupational Performance intervention in children with developmental coordination disorder. Front. Hum. Neurosci. 18:1316117. doi: 10.3389/fnhum.2024.1316117
Received: 10 October 2023; Accepted: 03 May 2024;
Published: 22 May 2024.
Edited by:
Emily J. Meachon, University of Basel, SwitzerlandReviewed by:
Christian Hyde, Deakin University, AustraliaCopyright © 2024 Malik, Weber, Lang, Vanderwal and Zwicker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jill G. Zwicker, amlsbC56d2lja2VyQHViYy5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.