
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Hum. Neurosci., 29 August 2023
Sec. Brain Imaging and Stimulation
Volume 17 - 2023 | https://doi.org/10.3389/fnhum.2023.1227194
This article is part of the Research Topic15 Years of Frontiers in Human Neuroscience: NeuromodulationView all 6 articles
 Rachael M. Harrington1,2,3*
Rachael M. Harrington1,2,3* Lisa C. Krishnamurthy4,5,6,7
Lisa C. Krishnamurthy4,5,6,7 Alexandra Ossowski3,8
Alexandra Ossowski3,8 Mykayla Jeter3,4
Mykayla Jeter3,4 Adriane Davis3
Adriane Davis3 Ewelina Bledniak3,8
Ewelina Bledniak3,8 Ashley L. Ware3,8,9
Ashley L. Ware3,8,9 Robin Morris1,3,5,8
Robin Morris1,3,5,8 C. Nikki Arrington3,5,8
C. Nikki Arrington3,5,8Theta-burst stimulation (TBS) is a repetitive transcranial magnetic stimulation technique that can be used to upregulate or downregulate different brain regions. However, the timing of its effects and the differing effects of continuous TBS (cTBS) versus intermittent TBS (iTBS) in the reading system have not been explored. This study assessed how stimulation type and post-stimulation timing affected change in performance during a phonological discrimination and sight word recognition task after stimulation of supramarginal gyrus (SMG). Fourteen right-handed young adults (age 18–27 years; 44% male) were block-randomized to receive either iTBS or cTBS to the supramarginal gyrus. Participants then performed a pseudoword discrimination task and an orthographic awareness task (behavioral control) at four different time points and change in reaction time compared to baseline was measured from each time point. There was no effect of stimulation type on change in reaction time [t(16) = −0.2, p = 0.9], suggesting that both types of TBS caused similar effects. Percent change in reaction time decreased over time in the pseudoword task [t(50) = −5.9, p < 0.001], indicating faster pseudoword processing speed with better performance 60–70 min after stimulation. In contrast, no change was demonstrated over time for the behavioral control task [t(43) = −0.6, p = 0.6], suggesting that the change over time seen in the test condition was not a learning effect. These findings provide insight into the effects of TBS on the reading system and can guide future study designs.
Neural plasticity is induced and amplified by increased neuronal activity (Kleim and Jones, 2008; Magee and Grienberger, 2020). Early studies of plasticity in the hippocampus showed that synaptic connectivity between neurons increased following trains of high-frequency electrical stimulation and that the connectivity decreased following trains of low-frequency stimulation (Hebb, 2005). Transcranial magnetic stimulation (TMS) has been used to non-invasively replicate these principles in humans (Thickbroom, 2007). TMS causes a brief magnetic pulse at the scalp that induces an electrical current in the targeted, underlying cortical brain region (Harris-Love, 2012). When done repetitively (rTMS), at high or low frequencies, stimulation results in increases or decreases in cortical excitability which affect behavior, mimicking the effects of long-term potentiation or depression (Rossini et al., 2015).
One form of rTMS, theta-burst stimulation (TBS) performs this repetitive stimulation in 50 Hz “bursts,” decreasing the needed time for stimulation and making it a more tolerable methodology for human studies (Huang et al., 2005). TBS uses a rapid train of single TMS pulses to change the state of the underlying cortical excitability (Bestmann and Krakauer, 2015; Suppa et al., 2016). In the motor system, continuous TBS (cTBS) depresses the amplitude of motor evoked potentials (MEPs), while intermittent TBS (iTBS) increases the amplitude of MEPs (Suppa et al., 2016). This broadly suggests that iTBS is excitatory and that cTBS is inhibitory (Suppa et al., 2016). The literature also suggests differing effects of timing between each type of TBS. On average, cTBS has decreased MEP amplitude for up to 50 min and iTBS has increased MEP amplitude for an average of 60 min (Wischnewski and Schutter, 2015). However, the greatest change in MEP amplitude occurs 18–33 min post-stimulation with iTBS or 5 min after cTBS (Hinder et al., 2014; Vernet et al., 2014). These principles of the time course of TBS have not been examined in more complex cognitive networks, such as the reading network.
The reading network is inherently more complex than the network required to produce an MEP. Despite this, the effects of TBS on the reading network have thus far been assumed to be on time scales that are similar to the motor system (Arrington et al., 2022). Stimulation of the motor system results in an approximate 1:1 relationship between stimulation of cortical neurons and production of an MEP. Two motor neurons (corticospinal tract and alpha motor neuron) are all that is needed to evoke the desired behavioral response (Brasil-Neto et al., 2009). Stimulation of a more complex cognitive system, like the reading network, is theoretically less straightforward. The reading network integrates visuospatial pattern-recognition, language, attention, memory, executive function, and articulatory motor networks. Therefore, the more complex neural architecture and number of networks recruited during reading may reflect a less linear relationship between stimulation of cortical neurons and reading output than the system needed to produce an isolated MEP (Krishnamurthy et al., 2019). The reading network is not one system, but the coordination of three distinct systems supporting underlying cognitive outcomes (Pugh et al., 1996). The coordination of these networks is more effortful and the spread of stimulation likely requires a longer time course, leading the effects of TBS to potentially peak later and last longer in the reading network than the time course reported in the motor literature. In fact, a review found that effects of TBS are much more variable when affecting complex cognitive systems than motor and somatosensory function (Demeter, 2016). Understanding the time course effects of TBS on complex cognitive systems is critical for evaluating the effects of stimulation on cognitive functions such as reading. It also has implications for the use of TBS in treatment paradigms.
According to conventional models of reading, the reading system is comprised of three primary subnetworks: dorsal, ventral, and anterior (Pugh et al., 1996). The dorsal stream is classically thought to be involved in phonological decoding, or mapping sounds to visual representation, with a primary node in the supramarginal gyrus (SMG) (Pugh et al., 2001). The ventral stream facilitates reading fluency and sight word recognition, with a primary node in middle temporal gyrus (Pugh et al., 2001). The anterior stream is involved in articulatory recoding with a primary node in pars triangularis (Pugh et al., 2001). Tasks that involve phonological discrimination have been shown to engage the dorsal stream while tasks that involve sight word recognition have been shown to engage the ventral stream (Pugh et al., 2001). The SMG sits within the dorsal stream and is expected to influence phonological decoding but not sight word recognition allowing these two distinct components of reading to be both functionally and structurally differentiated. Here, we use this model to examine the facilitation and timing principles of TBS that have yet to be fully understood in the complex reading system. Specifically, this study assessed change in phonological discrimination and sight word recognition reaction time post-stimulation to SMG. We aimed to address the following research questions within the context of well-studied reading networks:
1. Do cTBS and iTBS to SMG lead to differential effects on change in post-stimulation reaction time during a phonological decoding task? We hypothesize that cTBS will inhibit and iTBS will facilitate reading behavior as demonstrated by a change in reaction time during a phonological discrimination task.
2. What are the effects of time post-stimulation on change in reading behavior after stimulation of SMG? We expected a prolonged timing effect post-stimulation on reading behavior.
Adults age 18–30 (22.2 (4) years) were recruited via fliers posted at universities around the Greater Atlanta area as part of a neuroimaging study of reading networks in typical readers. The joint Georgia State University/Georgia Institute of Technology Center for Advanced Brain Imaging Institutional Review Board approved the protocol and procedures. Written consent was obtained from each participant.
Participants met the following inclusion criteria: between the ages of 18–60 years, a native speaker of English, right-handed, have a Full Scale IQ score of low average or higher (FSIQ-2=>80) based on the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence [WASI-2 (Wechsler, 2011)], and have reading performance within age expected norms (=>90) based on the Woodcock Johnson-3 [WJ-III (Schrank et al., 2014)] Broad and Basic Reading Scores and the Test of Word Reading Efficiency [TOWRE-2 (Torgesen et al., 2012)] Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE) measures (Table 1). These tests are normed for the age range of participants and are well-established measures of reading ability (Wendling et al., 2007). Participants were excluded if they did not meet the criteria for TMS or MRI safety (e.g., history of seizure, metal in the body), or had history of a severe neurological or psychological disorder (Rossi et al., 2009). MRI contraindications were necessary as participants underwent two MRI scans (one for neuronavigation and one for post-stimulation data collection) as part of a larger parent study.
Relevant demographic and reading history were collected at a pre-screening appointment. Inclusion criterion tests were completed at that time (see above).
Eligible participants were then block-randomized into two potential stimulation conditions (cTBS or iTBS). There were four participants who completed both conditions in accordance with their randomization in the parent study. A minimum wash-out period (range = 12 to 63 days) was maintained to minimize carryover effects between stimulation sessions. Participants also completed a T1-weighted MRI scan (TR: 2,250 ms, voxel size: 1.0 mm × 1.0 mm × 1.0 mm) on a 3 Tesla Siemens Tim Trio scanner at the GSU/GA Tech Center for Advanced Brain Imaging, which was used for neuronavigation during stimulation.
Participants completed two baseline computerized reading tasks during the initial MRI scan.
Pseudoword discrimination (PD) task: The visual pseudoword discrimination task is a computerized forced-choice, reaction time assessment of phonological processing. It measures the speed and accuracy of participants’ ability to phonetically decode a string of letters. This task is simple with high accuracy and has proven highly sensitive to individual differences in phonological decoding skills (Olson et al., 1994). Participants are presented with a letter string on the screen and are asked to judge via button press whether the letter string can be pronounced like a “real word” (e.g., “roze”) or not (e.g., “heaf”). Pronounceable and unpronounceable pseudowords are intermixed pseudo-randomly and response times and accuracy rates are recorded on a total of 50 trials. The median response time and accuracy rate across correct trials is calculated for each participant. This task is expected to activate the dorsal stream and engage SMG. This task served as our primary outcome measure.
Orthographic awareness (OA) task: This computerized forced-choice, reaction time assessment measures how quickly and automatically participants can identify valid orthographic patterns. This well-validated and well-replicated experimental task is used to measure the speed at which participants can identify whether or not a string of letters represents a correctly spelled real word (Olson et al., 1994). Participants see a letter string on the screen that either represents a correctly spelled real word (e.g., “flute”) or an incorrectly spelled word (e.g., “roze”). They are asked to judge via button press whether the letter string is a “real word” or “not a real word.” Correctly and incorrectly spelled words are intermixed pseudo-randomly and response times and accuracy rates are recorded on a total of 32 trials. The median response time across correct trials is calculated for each participant. This task is expected to engage the ventral stream. This task is not expected to engage the dorsal stream and therefore should not be impacted by stimulation of SMG, serving as our behavioral control measure.
During TMS stimulation, participants were seated in a comfortable chair with legs uncrossed. A TMS cap was placed on the participant and tied securely under the chin. The starting point for establishing the motor thresholding hotspot was identified as the halfway point between the nasion and inion and 1/3 of the length between the center point (vertex) and the tragus of the left ear. Primary motor cortex (motor hotspot) was identified in this area using visual confirmation of muscle activation in the relaxed first dorseus interosseus (FDI) muscle following single-pulse TMS from a figure-of-eight coil (C-B60) connected to a MagVenture MagPro X1100 Magnetic Stimulator. Once the hotspot was identified, participants were asked to raise their right arm at a 90-degree angle in front of their torso and contract their right hand into an “OK” symbol. Active motor threshold (AMT), or the minimum stimulator output required to cause muscle contracture in the actively contracted hand, was determined using the adaptive parameter estimation by sequential testing (PEST) paradigm from the Medical University of South Carolina and visual confirmation of muscle contraction in the FDI (Awiszus and Borckardt, 2011).
Once AMT was identified, fiducial sensors were placed on the participant’s head and registered to their skull. Using Localite Neuronavigation software, SMG (apex of ascending ramus of superior parietal gyrus) was anatomically defined on the participant’s T1-weighted MRI for neuronavigation (Jung et al., 2016). Theta-burst conditions were delivered at 80% of the individual-determined AMT with a static cooled figure-of-eight magnetic coil (MCF-B65). Excitatory iTBS consisted of a series of 20 sets of 3-pulse bursts given at 50 Hz repeated every 200 ms for a total of 600 pulses (Huang et al., 2005). Inhibitory cTBS consisted of 40 s of uninterrupted 3-pulse bursts. One session of inhibitory stimulation delivers a total of 600 pulses (Huang et al., 2005). Participants were asked to read silently TOWRE-2 SWE Form-A during stimulation to activate the reading network.
Immediately following stimulation, a timer was started and participants completed a second MRI scan that included repeated testing on the PD and OA tasks. These tasks were performed at four time points post-stimulation: (i) ∼15 min post (timepoint A), (ii) ∼30 min post (timepoint B), (iii) ∼60 min post (timepoint C), and (iv) ∼75 min (timepoint D) (see Table 2).
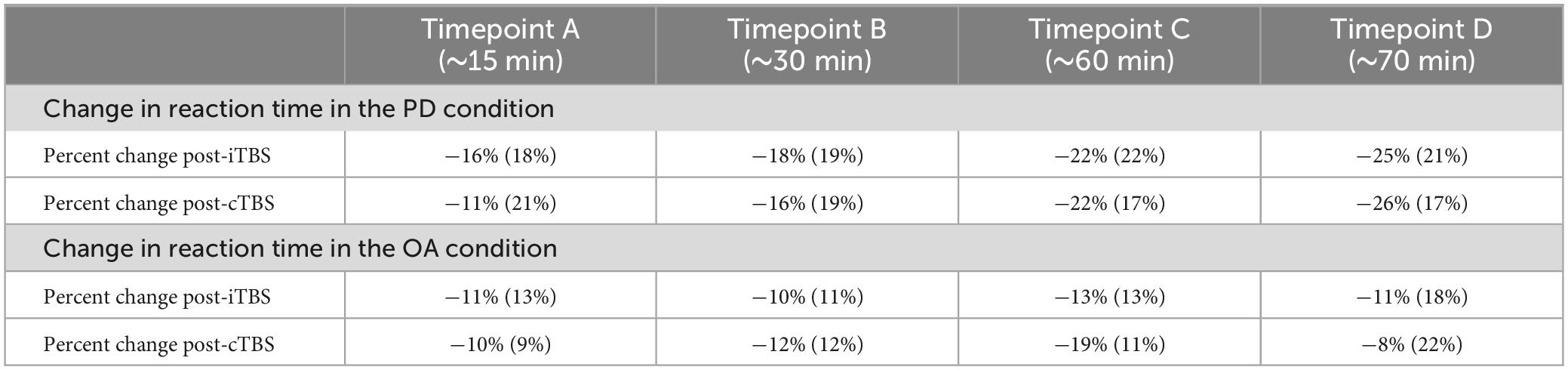
Table 2. Descriptive statistics for percent change in reaction time in the phonological decoding (PD) and orthographic awareness (OA) conditions after intermittent TBS (iTBS) and continuous TBS (cTBS).
Participant responses during the pre- and post-stimulation PD and OA tasks were binarized by accuracy (i.e., correct versus incorrect). The median reaction time for each task iteration was calculated based on the reaction time of correct responses only (Willems et al., 2011). Post-stimulation reaction time at each time point was normalized against pre-stimulation reaction time by calculating the percent change as follows:
Where %△ is the percent change in reaction time at time point I (i.e., A, B, C, or D), x1 is the median reaction time from the baseline (pre-stimulation) session, and x2 is the post-stimulation median reaction time from time point i.
Descriptive statistics (mean and standard deviation) were calculated for demographic variables, timing data, and performance for participants in each stimulus condition (cTBS or iTBS). Comparisons were conducted using chi-square and t-test techniques in R v4.1.3.
Multiple linear mixed-effects models were computed using the lmer function from the lme4 package in R (Bates et al., 2015). Comparison values were calculated using lmerTest (Kuznetsova et al., 2017). %Δ in either the OA or PD tasks was included as the dependent variable with stimulation type (cTBS or iTBS) and/or time (minutes) post-stimulation entered as fixed effects and participant as a random effect (Pinheiro et al., 2022). Including participant as a random effect accounted for repeated measures in some subjects. To determine the significance of type and time post-stimulation, likelihood ratio tests (LRT) were used to compare main effects models of stimulation type:
Or time post-stimulation:
Against a model with both fixed effects:
And an intercept only model:
We conducted an analysis to estimate Cohen’s d as a measure of effect size using the t_to_d package from the effectsize package (Ben-Shachar et al., 2020).
Pairwise comparisons of means were conducted using Tukey contrasts to examine differences in %ΔPD and %ΔOA across the four timepoint categories within the model. The estimates, standard errors (SE), z-values, and p-values are reported. The significance level was set at α = 0.05. Adjusted p-values were reported using the false discovery rate (FDR) method.
Fourteen participants were included in the analysis. Of those, 4 participated in both stimulation conditions, separated by a washout period, for a total of 18 datasets between the two conditions. For each participant, repeated measures were obtained with four time points for both the PD and OA tasks. Eight participants were included in the cTBS condition and 10 in the iTBS condition.
Participants who completed cTBS did not differ from those who completed iTBS in terms of age, gender, race, or years of education (see Table 1).
The results of the LRT show that the addition of type of stimulation did not improve the fit of the model above the intercept in either the PD [χ2(1) = 0.03, p = 0.9] or OA conditions [χ2(1) = 0.06, p = 0.8] indicating that type of stimulation did not affect Δ%PD or Δ%OA (see Tables 2–5).
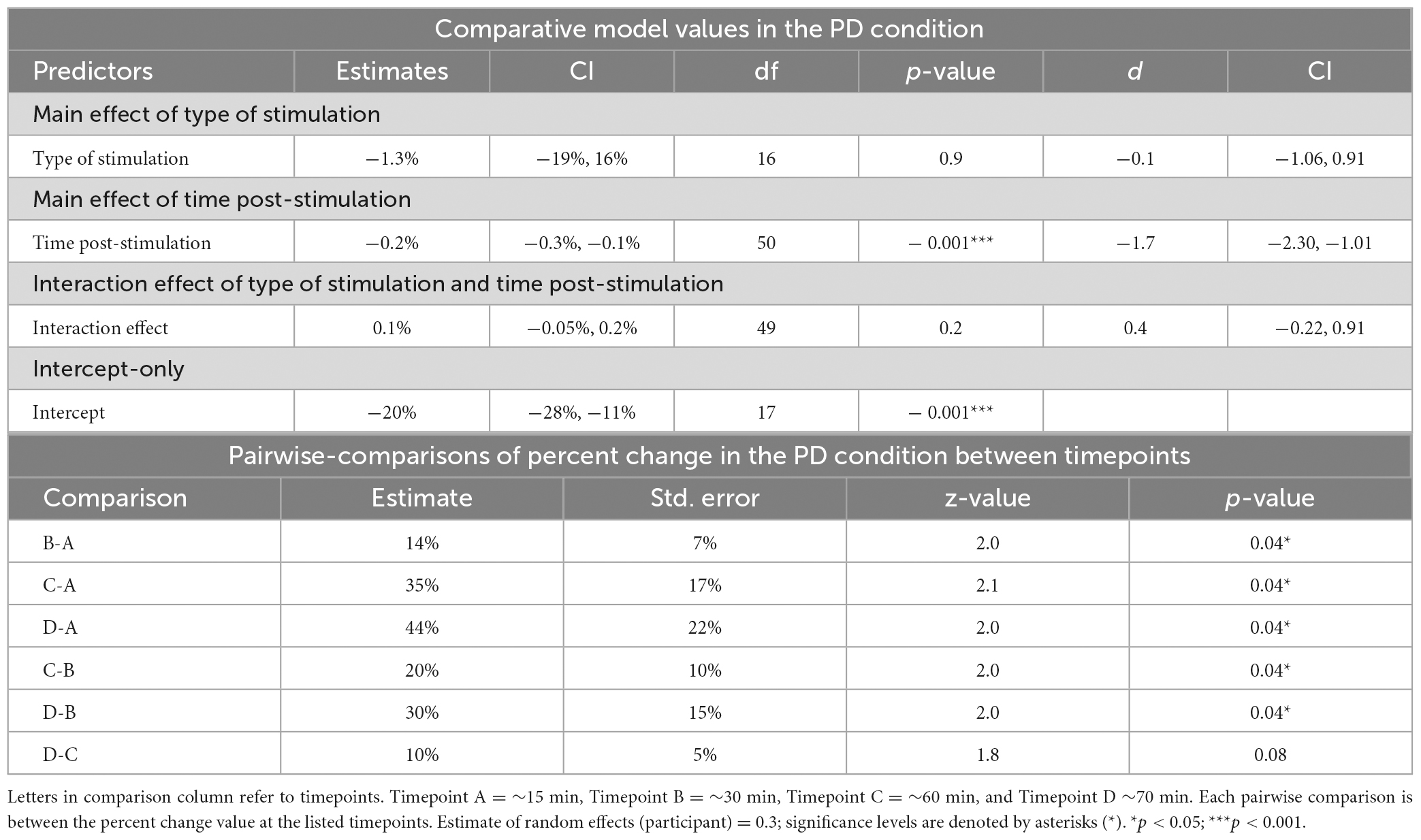
Table 3. Mixed-linear effects modeling and post-hoc analysis for change in reaction time in the phonological decoding (PD) condition.
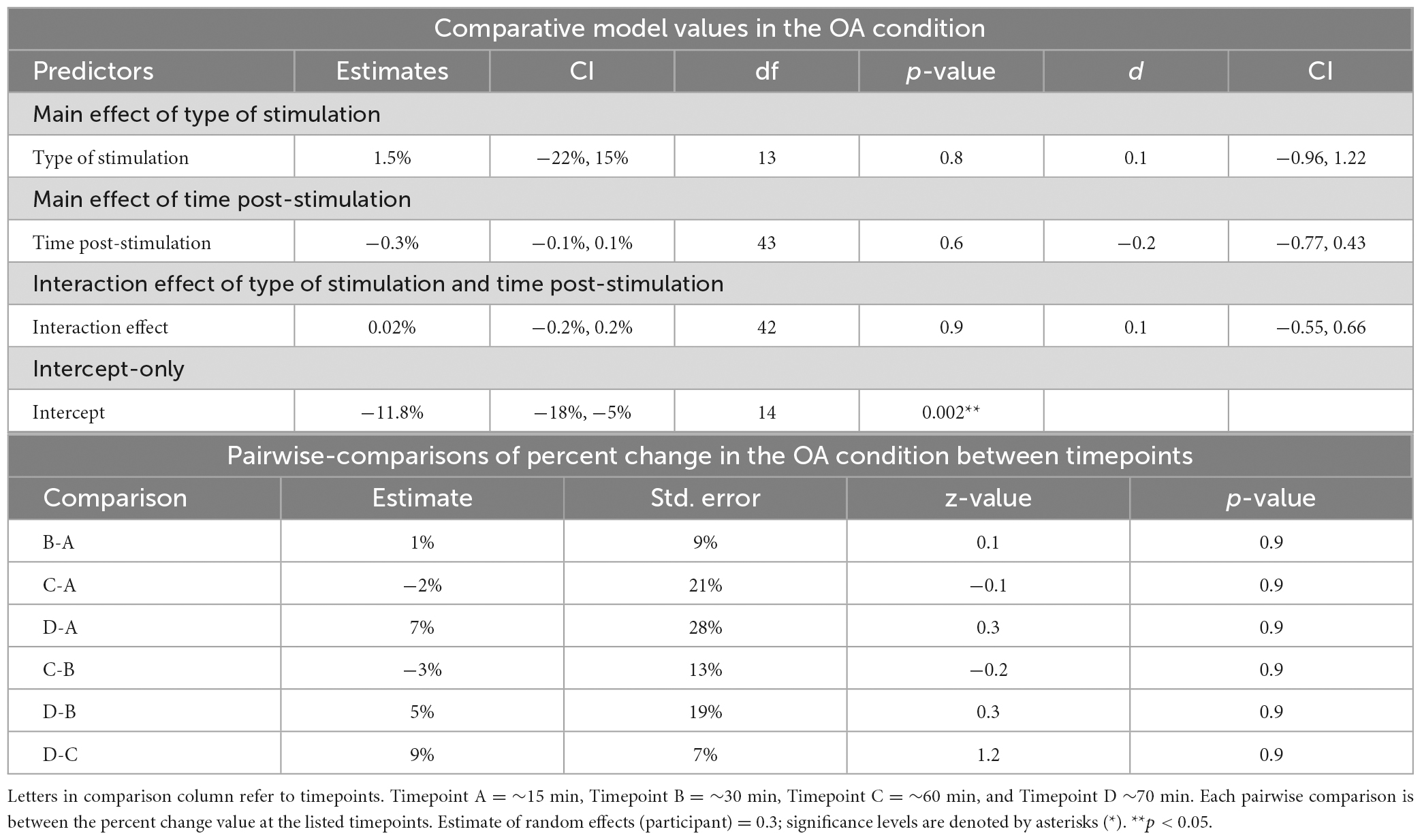
Table 4. Mixed-linear effects modeling and post-hoc analysis for change in reaction time in the OA condition.
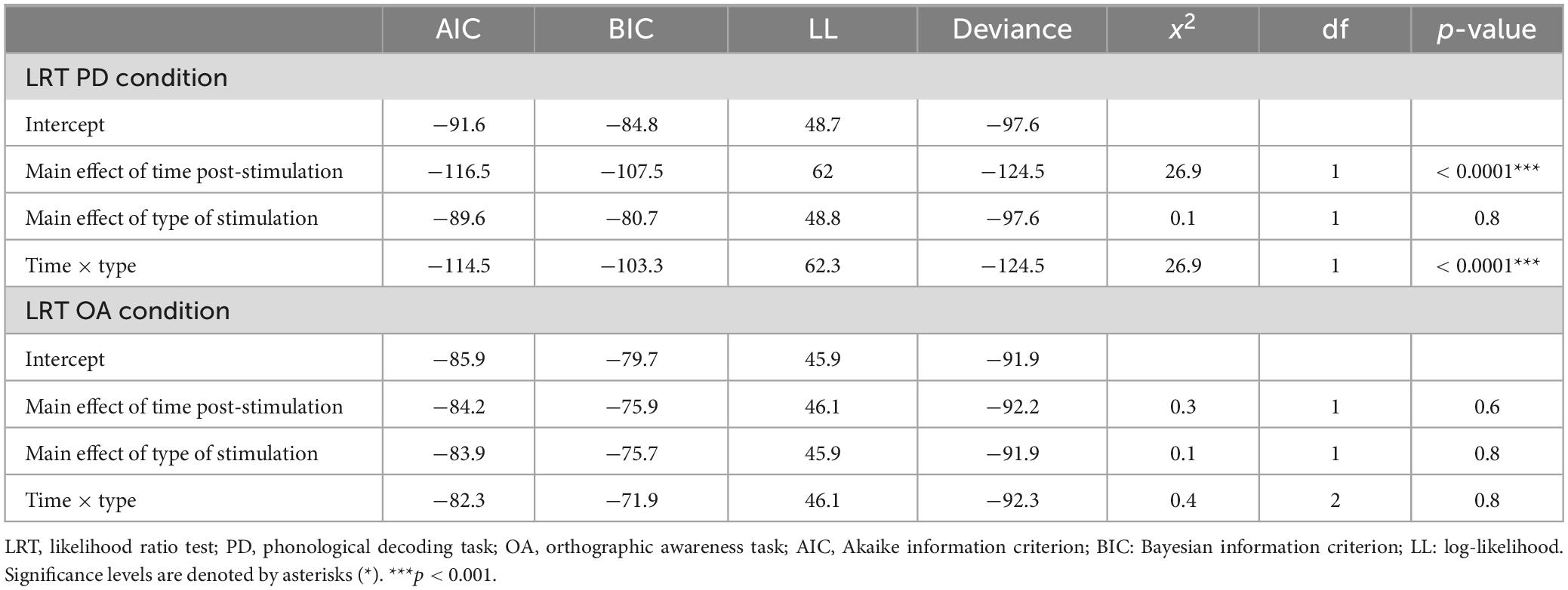
Table 5. Results of likelihood ratio test (LRT) for the phonological decoding (PD) and orthographic awareness (OA) conditions.
The LRT results indicated that the main effect model that included time post-stimulation was the best fitting model for Δ%PD [χ2(1) = 26.9, p < 0.0001] (see Tables 2, 3, 5). These results show that time post-stimulation has a significant effect on Δ%PD. A large effect size (d = −1.7, CI = −2.30, −1.01) was calculated for this predictor, indicating a large and significant effect on the response variable.
Time post-stimulation did not improve the fit of the model above the intercept in the OA condition [χ2(1) = 0.3, p = 0.6]. These results show that the effect of time post-stimulation seen in the PD condition was not a learning effect (see Tables 2, 4, 5).
The interaction of type of stimulation and time post-stimulation did not improve the fit of the model above the intercept in the OA condition [χ2(1) = 0.4, p = 0.8]. Including both effects did improve the fit of the model above the intercept in the PD condition [χ2(1) = 26.9, p < 0.0001], however, this appears to be primarily driven by the effect of time, not by the interaction effect (see Tables 3–5).
Pairwise comparisons were conducted to examine percent change in the PD condition between the four different timepoints (∼15, 30, 60 and 70 min after stimulation). The comparisons revealed significant differences in all pairs except for the comparison between timepoints C and D, which did not reach statistical significance (Table 3).
No significant differences between timepoints were found in the OA condition (Table 4).
The goal of this study was to provide preliminary evidence about the facilitation type and timing effects of TBS in the reading network. In 14 young adults, we found that there were no differences in performance (i.e., percent change in reaction time) between cTBS and iTBS for our active condition or our behavioral control tasks. Thus, the facilitation and inhibition effects of TBS are not seen in behavioral reading measures as has been previously reported in the motor system. Instead, within our sample, both types of stimulation were associated with near equal facilitation or speeding of phonological processing. In contrast, the effect of TBS was significantly influenced by time post-stimulation, with the greatest effect demonstrated 60–70 min, and exceeding 80 min, post-stimulation. We did not see this effect of time in our behavioral control condition. This indicates that, in this study, a timing effect exists outside of a repeated exposure learning effect or task habituation. When considering the neural bases of these findings, the SMG is situated in the dorsal stream and therefore stimulation of this area should have more of an effect on performance during PD than in OA, as is seen in our results.
Interestingly, there was no significant difference between facilitation of change in reaction time on the PD task following cTBS and iTBS. In the motor system, cTBS is thought to mimic long-term depression, leading to an inhibitory effect, while iTBS is thought to mimic long-term potentiation that causes an excitatory effect (Chung et al., 2016). There is evidence, however, that this effect is inconsistent. Previous studies have found that MEP amplitude was consistently affected by on-line, single-pulse stimulation. However, when TBS was performed prior to single-pulse stimulation, the MEP response was variable, and for individual participants, the elicited MEP responses could not be reliably reproduced across sessions (Wischnewski and Schutter, 2015). This previous study added to a growing body of evidence that cTBS can elicit an excitatory response within the motor system (Demeter, 2016; Ozdemir et al., 2021). In our sample, this excitatory response after cTBS was found in the reading system with both forms of TBS resulting in general facilitation of change in phonological processing speed in all participants. Inhibitory effects were not seen in any participant.
Several studies have suggested that the effects of TBS observed in the motor cortex might not necessarily apply to other cortical regions (Tupak et al., 2013; Tsang et al., 2014; Painter et al., 2015). There are limited studies available to contextualize the timing results found in this study within the cognitive system. One previous study found that trains of TBS applied during the retention phase of a working memory task increased response time more than trains of TBS applied during the retrieval phase, indicating that, at least for short bursts of stimulation, some time is required for TBS to have an effect (Luber et al., 2007). This difference, however, was over the course of seconds—not on the order of minutes as seen in our results. No other studies have reported the effects of time for TBS and cognition (Demeter, 2016). Several studies, however, have called for future work examining the facilitation and timing effects of TBS in cognitive tasks (Luber et al., 2007; Demeter, 2016).
There are several potential explanations for the discrepancy between response to iTBS and cTBS seen in this study and those seen in previous literature. The first is that the difference is dependent on the relative size and complexity of the networks (Pugh et al., 1996). The reading network is comprised of three streams working in parallel across all lobes of the brain while the network that is required for production of an MEP is relatively simple, in comparison (Pugh et al., 1996). In addition, cognitive tasks, such as reading, require the coordination of multiple brain regions which increases the computational likelihood of variability of neuronal response (Malins et al., 2018). Finally, cognitive tasks have more competition from neural noise which may also increase individual variability and play a role in the discrepancies seen in this study (Hancock et al., 2017). The competition of neural noise within the reading network and the individual variability has been demonstrated in imaging studies of reading impairment (Hancock et al., 2017; Malins et al., 2018; Arrington et al., 2019).
Many studies simply infer that a behavioral outcome is due to excitatory or inhibitory stimulation without neurophysiological measures to confirm that assertion (Ozdemir et al., 2021). Some studies, however, have gathered neurophysiological data along with cTBS outcomes. These studies have used cTBS to suppress neural activation or reduce functional connectivity and have had varying degrees of success in this effort (Ott et al., 2011; Valchev et al., 2015; Steel et al., 2016; Shang et al., 2020). This effect has been demonstrated in the semantic system, which is intertwined with the reading system (Jung and Lambon Ralph, 2016, 2021). It is worth noting that many of the studies that have more consistent results demonstrating inhibition after cTBS stimulation are performed in the motor system with stimulation of the primary motor cortex (Chung et al., 2016). Functional data analyses of the current sample is ongoing to provide neuroimaging data to add to these results.
The preliminary timing effects seen in this analysis have potential implications for future study design. As discussed previously, the available guidance on timing effects suggest windows of effectiveness up to 50 (cTBS) and 60 (iTBS) minutes post-stimulation with a peak effect after 18–33 min (iTBS) or 5 min (cTBS) post-stimulation (Hinder et al., 2014; Vernet et al., 2014; Wischnewski and Schutter, 2015). Our results suggest that in the more complex reading network, this window is much longer with a potentially much later peak. SMG is situated in the dorsal stream and therefore stimulation of this area should have more of an effect on performance during PD than in OA, as is seen in our results.
Understanding the timing of TBS effects is needed to design studies that target behavioral change or measure outcomes during the peak effect of stimulation. Inaccurate understanding of timing effects could decrease the effectiveness of TBS as an adjuvant to behavioral change techniques or lead to null or inaccurate results with outcome measures collected outside of an optimal range. This effect is particularly robust when you consider that these data were collected in a sample of typically developed readers. It is unusual to get a large effect from this population as there is often a ceiling effect for change in an otherwise well-established and automatized behavior. These results should be replicated in a larger sample size before firm conclusions about future study design are drawn. It would also be useful, in larger samples, to look at some of the known influences on response to TBS (genetic variability, developmental factors, age, cortical network activity, and neurotransmitter and receptor variation) to better understand what is driving the observed effects. We were unable to test for these potential causes of variability within this sample.
Despite promising implications, this study has several limitations that impact generalizability. Most importantly, the parent study was not initially designed to answer the question of timing and facilitation effects. As such, there is a range of time post-stimulation for each time point, and some overlap is observed in timepoints A to B and timepoints C to D. We have addressed this limitation by analyzing the data as a continuous-time variable via mixed-effects linear modeling instead of binned time points. Additionally, our control condition for learning effect was limited. We employed a behavioral control rather than sham TMS in this pilot sample. Though sham would have been preferred, our lab lacked sham capabilities during the period in which this data were collected. The effects of the behavioral control, however, are robust and show that the increased processing speed seen in the PD task after stimulation of the SMG is not solely a learning or habituation effect. In fact, it appears that the learning effect hovers between 10 and 15% while behavioral change occurs between 15 and 25% change in reaction time. Participants also performed the baseline and primary outcome measures while lying in the MRI scanner. Because this setting was consistent across baseline and outcome measure settings, we do not believe that this influenced the results. The sample size is relatively small, but repeated measures across participants help to address this limitation and Cohen’s d shows a very large effect of time in the PD condition. TBS is known to have wide inter-individual variability (Hinder et al., 2014). We controlled for this by including each participant in the mixed linear effects model as a random effect. We do not believe, however, that this variability was a contributing factor to our results, as demonstrated by individual spaghetti plots as shown in Figure 1.
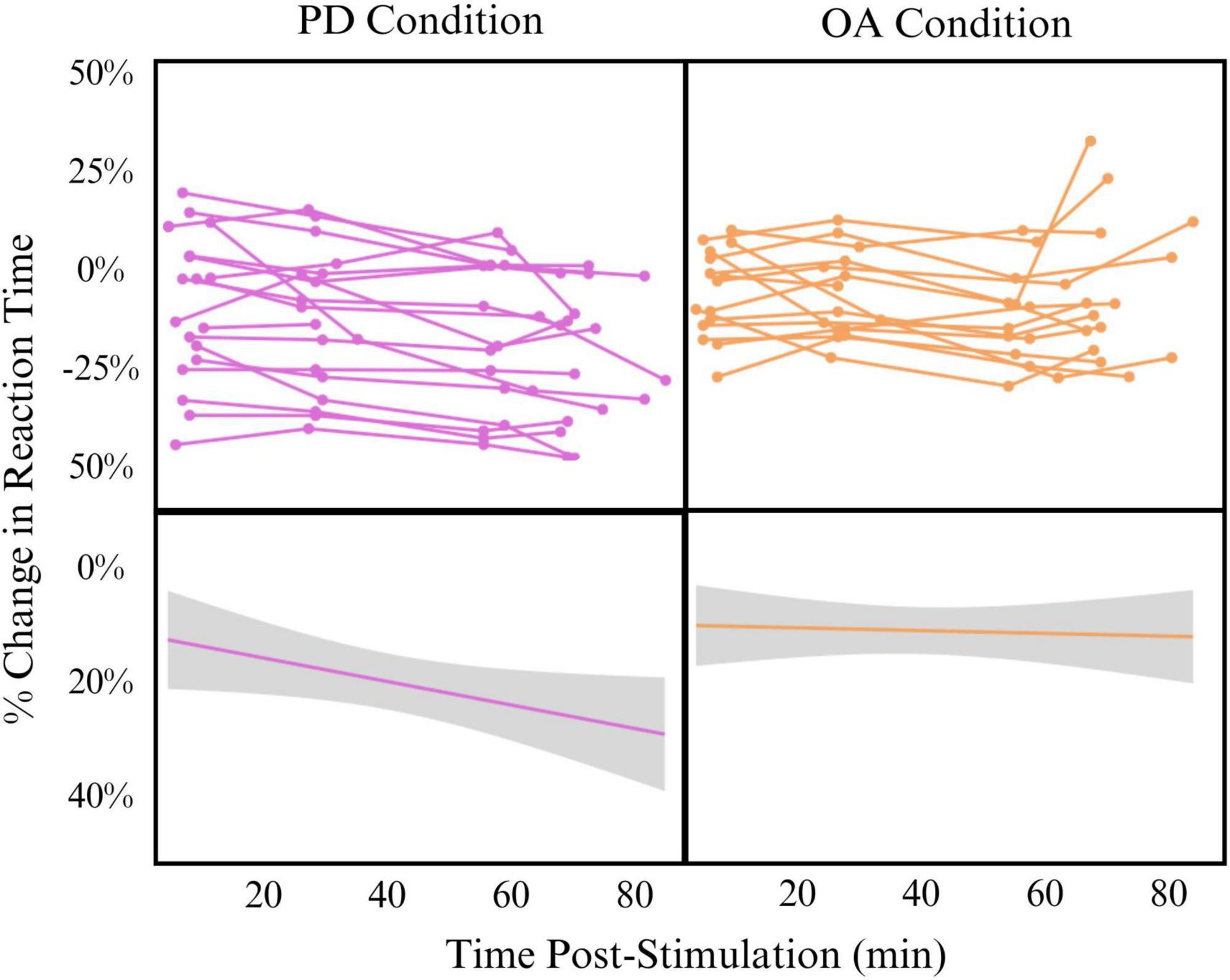
Figure 1. Effect of time post-stimulation on percent change in reaction time in phonological decoding (PD) (left) and orthographic awareness (OA) (right) conditions Top: individual spaghetti plots showing participant change in reaction time across time post-stimulation. Bottom: linear model of all participants change in reaction time across time post-stimulation (Change∼Time). Model is smoothed with standard error.
For future analyses, we strive to further understand the neurobiology that supports TBS-induced changes by pairing the behavioral information reported in this study with gathered functional imaging data post-stimulation. We plan to extend this study design to a population of participants with aphasia, alexia, and dyslexia. These novel and exciting results from TBS in the reading system show that mechanisms derived from the motor system should not be assumed to be applicable to the more complex cognitive networks. If our results are replicated, it will be necessary to reevaluate how we have interpreted past results using cTBS as an inhibitory technique in the language and reading system, particularly as an intervention and rehabilitation technique, and reevaluate how we plan future intervention and rehabilitation studies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://osf.io/bewxv/.
The studies involving humans were approved by the Georgia State University/Georgia Tech Joint Center for Advanced Brain Imaging Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RH designed analysis, gathered data, performed analysis, and wrote manuscript. CA designed protocol, gathered data, designed analysis, performed analysis, and edited manuscript. LK designed analysis and edited manuscript. AO and AD gathered data, performed analysis, designed analysis, and edited manuscript. MJ gathered data, performed analysis, and edited manuscript. EB performed analysis and edited manuscript. AW contributed to analysis and edited manuscript. RM oversaw project, designed protocol, designed analysis, and edited manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the American Heart Association 20POST35050048 (PI: RH), the Oak Foundation OCAY-16-526 (PI: RM), the National Center for Neuromodulation for Rehabilitation NC NM4R P2CHD086844 MUSC18-114-8B465 (PI: CA), NIH P01HD070837 (PI: RM), NSF2112455 (Co-PI: RM), and NIH R01HD096261 (Investigators: RM and CA).
We would like to acknowledge our funding sources: American Heart Association, Oak Foundation, National Institutes of Health, National Science Foundation, and National Center for Neuromodulation for Rehabilitation. We would like to acknowledge Ryan Slaby, Eileen Persechetti, and Humza Baig for help with data collection and Dr. Adianes Herrera-Diaz for help with editing. We would like to acknowledge Dr. Kaitlyn Stanhope and Dr. Lee Branum-Martin for help with statistical analysis. We would like to acknowledge David Loewy and Brett Maddox for technical assistance. We would also like to acknowledge Dr. Colleen Hanlon for help with study design.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arrington, C. N., Malins, J. G., Winter, R., Mencl, W. E., Pugh, K. R., and Morris, R. (2019). Examining individual differences in reading and attentional control networks utilizing an oddball fMRI task. Dev. Cogn. Neurosci. 38:100674. doi: 10.1016/j.dcn.2019.100674
Arrington, C. N., Ossowski, A. E., Baig, H., Persichetti, E., and Morris, R. (2022). The impact of transcranial magnetic stimulation on reading processes: A systematic review. Neuropsychol. Rev. 33, 255–277. doi: 10.1007/s11065-022-09534-7
Awiszus, F., and Borckardt, J. (2011). TMS motor threshold assessment tool (MTAT 2.0). Brain Stimulation Laboratory. Charleston, SC: Medical University of South Carolina.
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Ben-Shachar, M. S., Lüdecke, D., and Makowski, D. (2020). Effectsize: Estimation of effect size indices and standardized parameters. J. Open Source Softw. 5:2815.
Bestmann, S., and Krakauer, J. W. (2015). The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 233, 679–689. doi: 10.1007/s00221-014-4183-7
Brasil-Neto, J. P., Cammarota, A., Valls-Solé, J., Pascual-Leone, A., Hallett, M., and Cohen, L. G. (2009). Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurol. Scand. 92, 383–386. doi: 10.1111/j.1600-0404.1995.tb00151.x
Chung, S. W., Hill, A. T., Rogasch, N. C., Hoy, K. E., and Fitzgerald, P. B. (2016). Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 63, 43–64. doi: 10.1016/j.neubiorev.2016.01.008
Demeter, E. (2016). Enhancing cognition with theta burst stimulation. Curr. Behav. Neurosci. Rep. 3, 87–94. doi: 10.1007/s40473-016-0072-7
Hancock, R., Pugh, K. R., and Hoeft, F. (2017). Neural noise hypothesis of developmental dyslexia. Trends Cogn. Sci. 21, 434–448. doi: 10.1016/j.tics.2017.03.008
Harris-Love, M. (2012). Transcranial magnetic stimulation for the prediction and enhancement of rehabilitation treatment effects. J. Neurol. Phys. Therapy 36, 87–93. doi: 10.1097/NPT.0b013e3182564d26
Hebb, D. O. (2005). The organization of behavior: A neuropsychological theory. London: Psychology Press.
Hinder, M. R., Goss, E. L., Fujiyama, H., Canty, A. J., Garry, M. I., Rodger, J., et al. (2014). Inter- and intra-individual variability following intermittent theta burst stimulation: Implications for rehabilitation and recovery. Brain Stimul. 7, 365–371. doi: 10.1016/j.brs.2014.01.004
Huang, Y.-Z., Edwards, M. J., Rounis, E., Bhatia, K. P., and Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. doi: 10.1016/j.neuron.2004.12.033
Jung, J., and Lambon Ralph, M. A. (2016). Mapping the dynamic network interactions underpinning cognition: A CTBS-fmri study of the flexible adaptive neural system for semantics. Cereb. Cortex 26, 3580–3590. doi: 10.1093/cercor/bhw149
Jung, J., and Lambon Ralph, M. A. (2021). The immediate impact of transcranial magnetic stimulation on brain structure: Short-term neuroplasticity following one session of cTBS. NeuroImage 240:118375. doi: 10.1016/j.neuroimage.2021.118375
Jung, J., Bungert, A., Bowtell, R., and Jackson, S. R. (2016). Vertex stimulation as a control site for transcranial magnetic stimulation: A concurrent TMS/fMRI study. Brain Stimul. 9, 58–64. doi: 10.1016/j.brs.2015.09.008
Kleim, J. A., and Jones, T. A. (2008). Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, S225–S239. doi: 10.1044/1092-4388(2008/018)
Krishnamurthy, L. C., Krishnamurthy, V., Crosson, B., Rothman, D. L., Schwam, D. M., Greenberg, D., et al. (2019). Strength of resting state functional connectivity and local GABA concentrations predict oral reading of real and pseudo-words. Sci. Rep. 9, 1–12.
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Luber, B., Kinnunen, L. H., Rakitin, B. C., Ellsasser, R., Stern, Y., and Lisanby, S. H. (2007). Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: Frequency- and time-dependent effects. Brain Res. 1128, 120–129. doi: 10.1016/j.brainres.2006.10.011
Magee, J. C., and Grienberger, C. (2020). Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 43, 95–117. doi: 10.1146/annurev-neuro-090919-022842
Malins, J. G., Pugh, K. R., Buis, B., Frost, S. J., Hoeft, F., Landi, N., et al. (2018). Individual differences in reading skill are related to trial-by-trial neural activation variability in the reading network. J. Neurosci. 38, 2981–2989.
Olson, R., Forsberg, H., Wise, B., and Rack, J. (1994). “Measurement of word recognition, orthographic, and phonological skills,” in Frames of reference for the assessment of learning disabilities: New views on measurement issues, ed. G. R. Lyon (Baltimore, MA: Paul H. Brookes Publishing Co), 243–277.
Ott, D. V. M., Ullsperger, M., Jocham, G., Neumann, J., and Klein, T. A. (2011). Continuous theta-burst stimulation (cTBS) over the lateral prefrontal cortex alters reinforcement learning bias. NeuroImage 57, 617–623. doi: 10.1016/j.neuroimage.2011.04.038
Ozdemir, R. A., Boucher, P., Fried, P. J., Momi, D., Jannati, A., Pascual-Leone, A., et al. (2021). Reproducibility of cortical response modulation induced by intermittent and continuous theta-burst stimulation of the human motor cortex. Brain Stimulation 14, 949–964. doi: 10.1016/j.brs.2021.05.013
Painter, D. R., Dux, P. E., and Mattingley, J. B. (2015). Causal involvement of visual area MT in global feature-based enhancement but not contingent attentional capture. NeuroImage 118, 90–102. doi: 10.1016/j.neuroimage.2015.06.019
Pinheiro J., Bates D., and R Core Team (2022). nlme: Linear and nonlinear mixed effects models. R package version 3.1-157. Vienna: R Core Team.
Pugh, K. R., Mencl, W. E., Jenner, A. R., Katz, L., Frost, S. J., Lee, J. R., et al. (2001). Neurobiological studies of reading and reading disability. J. Commun. Disord. 34, 479–492. doi: 10.1016/S0021-9924(01)00060-0
Pugh, K. R., Shaywitz, B. A., Shaywitz, S. E., Constable, R. T., Skudlarski, P., Fulbright, R. K., et al. (1996). Cerebral organization of component processes in reading. Brain 119, 1221–1238.
Rossi, S., Hallett, M., Rossini, P. M., and Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039. doi: 10.1016/j.clinph.2009.08.016
Rossini, P. M., Burke, D., Chen, R., Cohen, L. G., Daskalakis, Z., Di Iorio, R., et al. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107. doi: 10.1016/j.clinph.2015.02.001
Schrank, F., McGrew, K., Mather, N., Wendling, B., and LaForte, E. (2014). Woodcock-Johnson IV tests of achievement. Rolling Meadows, IL: Riverside Publishing Company.
Shang, Y., Chang, D., Zhang, J., Peng, W., Song, D., Gao, X., et al. (2020). Theta-burst transcranial magnetic stimulation induced functional connectivity changes between dorsolateral prefrontal cortex and default-mode-network. Brain Imag. Behav. 14, 1955–1963. doi: 10.1007/s11682-019-00139-y
Steel, A., Song, S., Bageac, D., Knutson, K. M., Keisler, A., Saad, Z. S., et al. (2016). Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex 74, 134–148. doi: 10.1016/j.cortex.2015.10.004
Suppa, A., Huang, Y.-Z., Funke, K., Ridding, M. C., Cheeran, B., Di Lazzaro, V., et al. (2016). Ten years of theta burst stimulation in humans: Established knowledge. Unknowns and prospects. Brain Stimul. 9, 323–335. doi: 10.1016/j.brs.2016.01.006
Thickbroom, G. W. (2007). Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp. Brain Res. 180, 583–593.
Torgesen, J. K., Wagner, R. K., and Rashotte, C. A. (2012). TOWRE-2 examiner’s manual. Austin, TX: Pro-Ed.
Tsang, P., Jacobs, M. F., Lee, K. G. H., Asmussen, M. J., Zapallow, C. M., and Nelson, A. J. (2014). Continuous theta-burst stimulation over primary somatosensory cortex modulates short-latency afferent inhibition. Clin. Neurophysiol. 125, 2253–2259. doi: 10.1016/j.clinph.2014.02.026
Tupak, S. V., Dresler, T., Badewien, M., Hahn, T., Ernst, L. H., Herrmann, M. J., et al. (2013). Inhibitory transcranial magnetic theta burst stimulation attenuates prefrontal cortex oxygenation. Hum. Brain Mapp. 34, 150–157. doi: 10.1002/hbm.21421
Valchev, N., Ćurčić-Blake, B., Renken, R. J., Avenanti, A., Keysers, C., Gazzola, V., et al. (2015). CTBS delivered to the left somatosensory cortex changes its functional connectivity during rest. NeuroImage 114, 386–397. doi: 10.1016/j.neuroimage.2015.04.017
Vernet, M., Bashir, S., Yoo, W.-K., Oberman, L., Mizrahi, I., Ifert-Miller, F., et al. (2014). Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin. Neurophysiol. 125, 320–326. doi: 10.1016/j.clinph.2013.07.004
Wechsler, D. (2011). Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). Bloomington, MN: NCS Pearson.
Wendling, B. J., Schrank, F. A., and Schmitt, A. J. (2007). Educational interventions related to the Woodcock-Johnson III tests of achievement (Assessment Service Bulletin No. 8). Rolling Meadows, IL: Riverside Publishing.
Willems, R. M., Labruna, L., D’Esposito, M., Ivry, R., and Casasanto, D. (2011). A functional role for the motor system in language understanding: Evidence from theta-burst transcranial magnetic stimulation. Psychol. Sci. 22, 849–854. doi: 10.1177/0956797611412387
Keywords: reading, transcranial magnetic stimulation, theta-burst stimulation, Neuromodulation, repetitive transcranial magnetic stimulation, reading facilitation
Citation: Harrington RM, Krishnamurthy LC, Ossowski A, Jeter M, Davis A, Bledniak E, Ware AL, Morris R and Arrington CN (2023) Preliminary evidence of prolonged timing effects of theta-burst stimulation in the reading system. Front. Hum. Neurosci. 17:1227194. doi: 10.3389/fnhum.2023.1227194
Received: 22 May 2023; Accepted: 31 July 2023;
Published: 29 August 2023.
Edited by:
Chella Kamarajan, Downstate Health Sciences University, United StatesReviewed by:
Ying-hui Chou, University of Arizona, United StatesCopyright © 2023 Harrington, Krishnamurthy, Ossowski, Jeter, Davis, Bledniak, Ware, Morris and Arrington. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachael M. Harrington, cmhhcnJpbmd0b24yQGdzdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.