- 1Neuroscience Center, Brigham Young University, Provo, UT, United States
- 2Department of Psychology, Brigham Young University, Provo, UT, United States
- 3Pacific Northwest University of Health Sciences, Yakima, WA, United States
- 4University of Utah School of Medicine, Salt Lake City, UT, United States
- 5Children’s National Hospital, Washington, DC, United States
- 6Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, NC, United States
- 7Departments of Psychiatry and Neurology, University of Utah, Salt Lake City, UT, United States
Introduction: Concussion in children and adolescents is a public health concern with higher concussion incidence than adults and increased susceptibility to axonal injury. The corpus callosum is a vulnerable location of concussion-related white matter damage that can be associated with short- and long-term effects of concussion. Interhemispheric transfer time (IHTT) of visual information across the corpus callosum can be used as a direct measure of corpus callosum functioning that may be impacted by adolescent concussion with slower IHTT relative to matched controls. Longitudinal studies and studies testing physiological measures of IHTT following concussion in adolescents are lacking.
Methods: We used the N1 and P1 components of the scalp-recorded brain event-related potential (ERP) to measure IHTT in 20 adolescents (ages 12–19 years old) with confirmed concussion and 16 neurologically-healthy control participants within 3 weeks of concussion (subacute stage) and approximately 10 months after injury (longitudinal).
Results: Separate two-group (concussion, control) by two-time (3 weeks, 10 months) repeated measures ANOVAs on difference response times and IHTT latencies of the P1 and N1 components showed no significant differences by group (ps ≥ 0.25) nor by time (ps ≥ 0.64), with no significant interactions (ps ≥ 0.15).
Discussion: Results from the current sample suggest that measures of IHTT may not be strongly influenced at 3 weeks or longitudinally following adolescent concussion using the current IHTT paradigm.
1. Introduction
Over 2 million traumatic brain injuries (TBIs) occur each year (Coronado et al., 2015; Taylor et al., 2017; Centers for Disease Control and Prevention, 2019), with 70–90% of the injuries in the mild range—also referred to as concussions (Kay et al., 1993; Cassidy et al., 2004). Up to 32% of concussions occur in adolescents (ages 10–19; World Health Organization [WHO], 2022), representing the highest incidence rate among age groups (Cassidy et al., 2004; Dewan et al., 2016; Zhang et al., 2016). Concussions are often unreported or down-played due to strong external motivators in adolescence such as resuming a sports program or not missing school (Kimbler et al., 2011; Kroshus et al., 2015; Halstead et al., 2018). In addition, adolescents are more susceptible than adults to axonal injury following concussion (Reeves et al., 2005; Giza and Hovda, 2014; Halstead et al., 2018), potentially exacerbating cognitive symptoms (Xiong et al., 2014; Tate et al., 2017). Increased susceptibility to axonal injury in adolescents may be attributed to incomplete neuronal myelination, as unmyelinated axons display greater vulnerability to damage than myelinated axons following brain injury in animal models (Reeves et al., 2005; Bartzokis et al., 2010; Kochunov et al., 2012; Giza and Hovda, 2014; Halstead et al., 2018).
The corpus callosum is a frequent location of axonal damage following concussion (Viano et al., 2005; Henry et al., 2011; Jang et al., 2019). The corpus callosum is the largest white matter commissure in the brain and facilitates the interhemispheric transfer of motor, somatosensory, and cognitive information (Hinkley et al., 2012; Roland et al., 2017). The rotational and acceleration forces experienced during a concussive event cause extreme strain throughout the brain, which can lead to localized shearing in white matter structures, including the corpus callosum (Romeu-Mejia et al., 2019). These rotational forces are exacerbated in the corpus callosum as the falx cerebri acts as a fulcrum between hemispheres during rotational events and applies increased force to the corpus callosum (Hernandez et al., 2019).
Callosal injury and decreased callosal integrity are inversely associated with post-injury cognitive performance, including difficulties with executive and memory functioning (Kraus et al., 2007; Jorge et al., 2012; Hayes et al., 2016). The vulnerability of the corpus callosum to damage and its implication in potential cognitive sequelae following concussion make it a possible indicator of concussion presence and severity (Jorge et al., 2012; Hayes et al., 2016; Jang et al., 2019). Notably, the splenium of the corpus callosum, which is the posterior portion where interhemispheric transfer of visual information primarily occurs, may be particularly susceptible to concussion-related damage (Aoki et al., 2012) because the falx cerebri extends closer to the posterior corpus callosum than the anterior corpus callosum, increasing the pressure and forces particularly during rotational events and leading to increased axonal shearing (Shiramizu et al., 2008; Hernandez et al., 2019). Thus, the current study aimed to test the differences in corpus callosum functioning in concussed and non-concussed adolescents using physiological [scalp-recorded brain event-related potentials (ERP)] and behavioral (response time) measures of interhemispheric transfer time (IHTT) across the corpus callosum both in the subacute time period following injury (within approximately 3 weeks) and longitudinally (approximately 10 months post-injury).
Visual IHTT is the duration in milliseconds it takes for a visual stimulus presented to a single visual field to pass from one hemisphere to the other via connections primarily located in the splenium of the corpus callosum, though research on lesion and partial callosotomy patients also suggests that other ventral and more anterior callosal fibers may also contribute to visual interhemispheric transfer (e.g., Greenblatt et al., 1980; Risse et al., 1989; Clarke et al., 2000). Tasks designed to measure visual IHTT use a visual stimulus presented to one visual hemifield, which then travels posteriorly from the retina through a direct pathway until it is initially processed in the contralateral visual cortex (Brown and Jeeves, 1993). Visual stimuli are additionally processed in the primary visual cortex ipsilateral to the stimulus after being transferred through the posterior portion of the corpus callosum (Brown and Jeeves, 1993). The transfer of neural information through the direct pathway and then subsequently the corpus callosum is known as the indirect pathway. The difference in time elapsed between the direct and indirect pathways (indirect minus direct) is equivalent to the time taken for visual information to cross from one hemisphere to the other through the corpus callosum.
Decreased callosal connectivity following concussion is associated with slower interhemispheric transfer of information (Mathias et al., 2004; Dennis et al., 2017; Meissner et al., 2017; Womack et al., 2017). Thus, slowed interhemispheric transfer of visual information represents a potential indicator for the presence and severity of callosal damage or, possibly, concussion (Register-Mihalik et al., 2013; Smith et al., 2017; Dobney et al., 2018). Few studies have tested physiological measures of IHTT, such as event-related potentials (ERP) derived from electroencephalogram (EEG) data, following concussion. Diffusion weighted imaging has shown potential as an objective measure for the presence of white matter damage following concussion (Jorge et al., 2012; Asken et al., 2018; Jang et al., 2019; Churchill et al., 2021). However, the measurement of IHTT using ERPs is both more cost-effective and provides a direct measure of neural transfer time relative to the indirect and inferential diffusion-weighted MRI measures and merits further testing (Lystad and Pollard, 2009).
Interhemispheric transfer time can be directly measured with millisecond resolution using P1 and N1 ERP component latencies. P1 is a positive-deflecting ERP component occurring within the first 200 ms following the presentation of a visual stimulus (Brown and Jeeves, 1993; Clark and Hillyard, 1996); N1 occurs within the first 250 ms following the visual stimulus and is a large negative deflection following the P1 (Brown and Jeeves, 1993; Clark and Hillyard, 1996). These components appear maximally at posterior electrode sites contralateral to the visual hemifield containing the stimulus (Luck et al., 1990). P1 is thought to be generated in the extra-striate cortex of the ventrolateral occipital lobe and reflects the initial visual processing of a stimulus (Luck et al., 1990; Clark and Hillyard, 1996). N1 is generated in the occipitoparietal and inferior parietal cortex and represents continued contextual processing of a task-relevant stimulus (Luck et al., 1990; Kimura et al., 2010). IHTT is derived from the difference between the latency of the N1 or P1 component from the direct pathway subtracted from the latency of the N1 or P1 of the indirect pathway.
Previous studies of IHTT in individuals who experienced concussion primarily used response times as a measure of IHTT in adults (Mathias et al., 2004; Womack et al., 2017). For example, Mathias et al. (2004) found adults with concussion to have slower IHTT response times compared to healthy controls. Womack et al. (2017) showed greater difference between direct and indirect pathway response times to be correlated with decreased integrity of the posterior corpus callosum, indexed by lower mean diffusivity, following concussion in adults. Although these results are supportive of the potential clinical utility of IHTT, individual motor variability is a potential confound when using this indirect, behavioral measurement of IHTT (Moes et al., 2007; Meissner et al., 2017).
In adolescents and children, a series of studies have used ERPs to directly measure IHTT following moderate-to-severe traumatic brain injury (Dennis et al., 2015, 2017; Ellis et al., 2016; Olsen et al., 2020). These studies showed a bimodal distribution of IHTT ERP values, in which approximately 50% of children with moderate-to-severe traumatic brain injury displayed significantly slower IHTTs and significantly worse cognitive functioning in domains including processing speed, working memory, verbal learning, and executive functioning compared to controls, while the other 50% did not differ from control children significantly. Additionally, lower fractional anisotropy in the corpus callosum was significantly correlated with worse cognitive functioning (Dennis et al., 2015). A subsequent study on the same sample showed that children with moderate-to-severe traumatic brain injury and slower IHTT had significantly lower white matter organization, indicated by lower fractional anisotropy and higher mean diffusivity and radial diffusivity, than healthy controls (Dennis et al., 2017). This same group of children with TBI experienced a progressive decline in white matter organization from an initial assessment at 2–5 months following the injury to a second assessment 13–19 months after injury (Dennis et al., 2017). These studies provide preliminary evidence that slower IHTT may predict white matter degeneration and associated cognitive deficits following moderate-to-severe traumatic brain injury. Although these results highlight the potential utility of ERP measures of IHTT as a potential indicator of moderate-to-severe traumatic brain injury severity and prognosis, further research is needed to understand the relationship between more mild concussive events and measures of IHTT.
For the current study, we compared ERP and response time measures of IHTT between adolescents with concussion and healthy controls both within 3 weeks of injury and longitudinally (approximately 10 months following injury). We hypothesized that adolescents with concussion would display slowed behavioral IHTT (indicated by slower difference between the direct and indirect response times and greater difference accuracies) and slowed electrophysiological IHTT (indexed by the P1 and N1 direct and indirect difference latencies) in comparison to controls approximately 3 weeks following concussion. We further hypothesized that these effects of response time and latency would still be evident following approximately a 10 months recovery period. As a secondary aim, we tested correlations between IHTT and cognitive performance both within 3 weeks of injury and longitudinally. We hypothesized that slower IHTT would significantly correlate with more severe neurocognitive deficits following concussion.
2. Materials and methods
All data and code are posted on the Open Science Framework and can be found at https://osf.io/aqf9v/?view_only=0624935781604da1adf5e9099baf121c.
2.1. Participants
Study procedures were approved by the Brigham Young University Institutional Review Board. Consistent with the focus on adolescent concussion, all participants were between 12 and 19 years of age (World Health Organization [WHO], 2022). Prior to participation, parent or guardian written consent along with adolescent assent were obtained for all participants ages 17 years and below; adolescents ages 18 and 19 years provided written consent. Individuals with concussion were recruited via flyers and advertisements placed in the community, at local hospitals, concussion clinics, and via athletic trainers who provided study information to parents of adolescents. Control participants were recruited using flyers and advertisements in the local community. We used these varied recruitment strategies to enhance the ecological validity and representativeness of our concussion sample, as many individuals (including adolescents) with concussion do not report to an emergency department, and those who do present to the emergency department tend to experience more severe injuries (Cassidy et al., 2004; Babcock et al., 2013).
Exclusion criteria were assessed via interview with a parent or guardian and included pregnancy, reported substance or alcohol use within the past year, neurological disease (stroke, epilepsy, Tourette’s syndrome, etc.), current antiepileptic medication use, and involvement in current litigation surrounding the concussion. One control participant reported a diagnosis of Tourette’s syndrome and was excluded. Ten participants (nine concussion, one control) reported current psychiatric treatment. Two participants with concussion had a formal diagnosis of ADHD and/or learning disability. One control participant reported a previous diagnosis of meningitis years before participation. No control participants had a history of previous concussion.
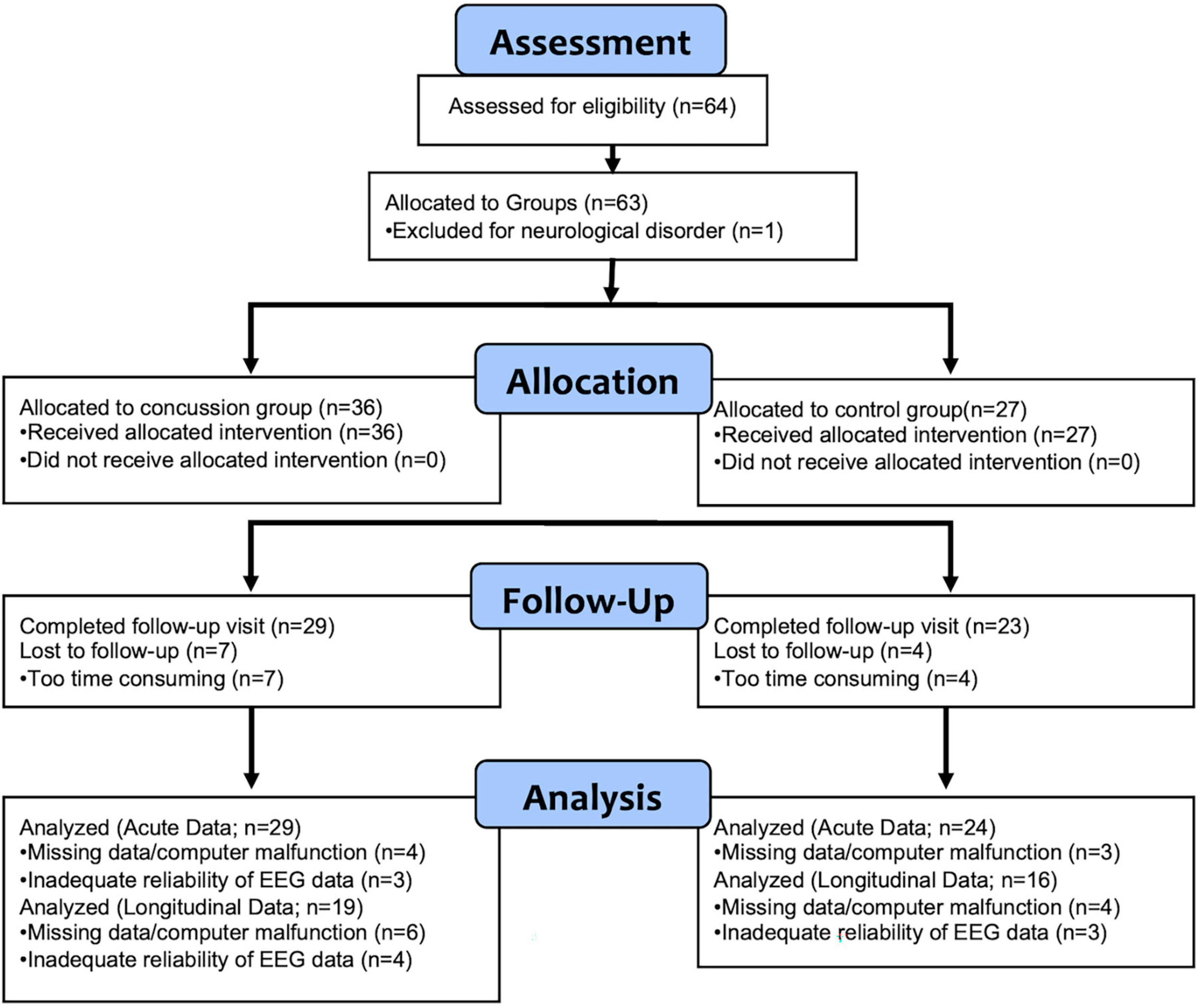
Sixty-four participants were originally enrolled in the study (36 concussion; 28 control). A consort diagram (Figure 1) provides detail about participant attrition over time and reasons for data loss. Participants were excluded for unreliable or missing EEG data, physiologically improbable negative IHTT values (Meissner et al., 2017), and Test of Memory Malingering (TOMM) and Reliable Digit Span (RDS) scores in the invalid range (see below). Following exclusion, 35 participants with concussion and 27 healthy controls were included for subacute (within 3 weeks of injury) analyses of response times. A total of 29 concussion and 24 control participants were included for subacute P1 and N1 latency analyses. For longitudinal analyses (approximately 10 months post-injury), 28 concussion and 23 control participants were included for response times and 19 concussion and 16 control participants were included for longitudinal analyses of P1 and N1 ERP IHTT.
All participants were asked to commit to attend two sessions: the first within 3 weeks following concussion and the second approximately 10 months after the first. We chose this 10 months follow-up session consistent with research that indicates that white matter pathology and concussive symptoms can persist up to 1 year post-injury (Yeates et al., 2009; Ewing-Cobbs et al., 2018; Churchill et al., 2021). For those with concussion, the initial session was completed as close to concussion as possible, the majority within 3 weeks of the injury [M (SD) = 2.6 (1.7) weeks]. Due to scheduling conflicts, not all follow up sessions occurred within 10 months, and we extended this time as needed. The majority of follow up sessions occurred within 1 year from the first session [M (SD)Concussion = 10.4 (1.3) months; RangeConcussion = 9.3, 13.1 months; M (SD)Control = 10.0 (0.8) months; RangeControl = 8.3, 11.6 months].
Participants with concussion were required to have one or more of the following after a biomechanical force to the head or neck: confusion/disorientation, loss of consciousness for 30 min or less, or post-traumatic amnesia less than 24 h (Kay et al., 1993; Cassidy et al., 2004) and consistent with current guidelines (e.g., McCrory et al., 2017). A licensed clinical neuropsychologist (MJL) or clinical neuropsychology graduate student (AC) confirmed the presence of concussion and the estimated duration of loss of consciousness and/or post-traumatic amnesia (if present) in a structured interview with the parent or guardian using the systematic concussion interview guidelines described by Ruff et al. (2009). Glasgow Coma Scale data were not available for most participants due to lack of an emergency room visit.
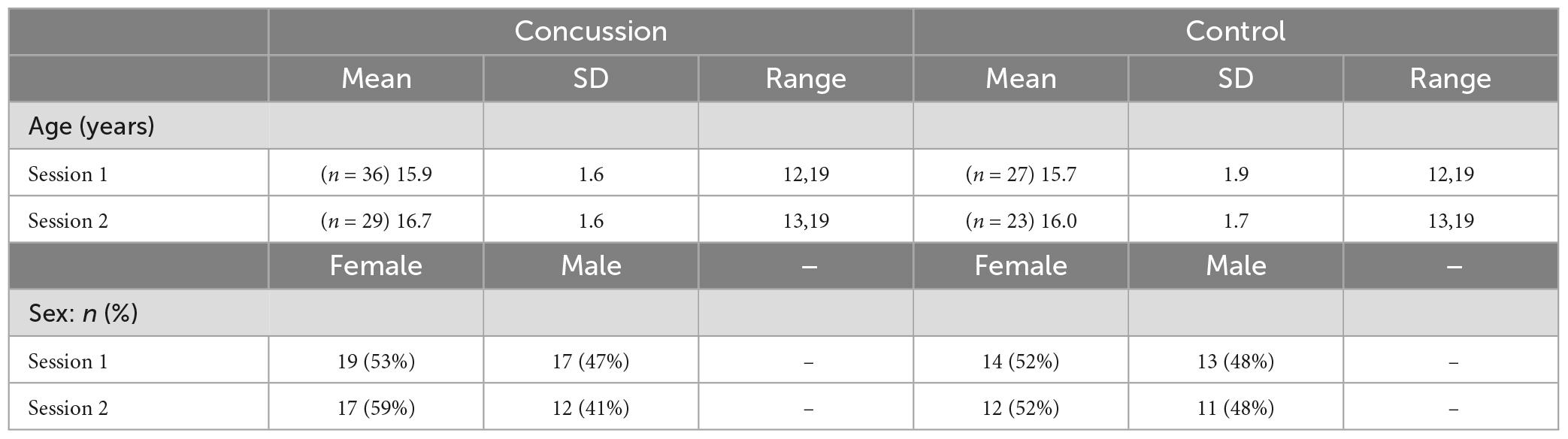
In the initial sample of 63 participants that met inclusion criteria (36 concussion, 27 control), there were no significant differences between groups in age [Session 1: t(61) = –0.6, p = 0.57, d = –0.1; Session 2: t(50) = –1.4, p = 0.16, d = –0.4], male/female ratio between groups [Session 1: χ2(1) = 0.0, p = 1.00; Session 2: χ2(1) = 0.0, p = 0.85], or task accuracy [Session 1: t(61) = 0.1, p = 0.94, d = 0.0; Session 2: t(50) = –0.9, p = 0.40, d = –0.2]. Table 1 displays means, standard deviations, and ranges for participant age and proportions for participant sex and session 1 and session 2 for participants with useable EEG data for at least one session.
In the initial sample of 36 individuals with concussion, the mechanism of injury was primarily recreation/sport-related injury (29 participants; 80.6%), followed by motor vehicle accidents (five participants; 13.9%), and falls (two participants; 5.5%). Nine participants (25.0%) experienced a loss of consciousness at the time of the injury, and self-reported lengths of loss of consciousness were all under 1 min. A total of 16 participants with concussion (44.4%) reported more than one previous concussion [M (SD) = 1.8 (1.1) concussions, range = 1–5 concussions].
2.2. Experimental protocol
Following the interview to confirm concussion, 18 years-old participants and parents or guardians completed a demographics questionnaire and the self-report Behavior Rating Inventory of Executive Function Parent Questionnaire (BRIEF) or the self-report BRIEF Adult Version depending on participant age. All participants then completed the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) to assess concussion symptoms, and measures of performance validity, including the TOMM and RDS after which the EEG net was fitted and the IHTT task completed.
Participants completed a modified version of a letter matching task while wearing the EEG net (Brown and Jeeves, 1993). Stimuli consisted of a random matching or non-matching letter pair of upper or lower case “A”s or “B”s (AB, Ab, etc.). Two of the same letters were considered a match regardless of case. Two letters were presented on each trial in two of four possible letter locations forming the corners of an imaginary rectangle surrounding a visible fixation point (“:”). Stimuli were presented in Courier 13-point white font on a black background on a 17 inch computer monitor set at approximately 20 inches from the participant’s head. At this distance, letter height was 27’ of the visual angle and the central fixation (“:”) was 25’ by 40’. Letter locations subtended 2°19’ to the left or right of the fixation and 1°56’ above or below the fixation. Please see Clawson et al. (2015) for figure. Dimmed direct-current LED lights were held constant for each participant.
Each trial began with a fixation point for 500 ms. The stimulus was then presented for 60 ms, followed by a jittered intertrial interval lasting between 1,500 and 2,000 ms, during which participants could respond. The task consisted of eight blocks of 48 trials (384 total trials) as well as 10 initial practice trials. On a traditional keyboard, participants were instructed to press “m” for matching letter pairs and “n” for non-matching pairs. The hand that participants used to respond at the beginning of the task was randomized between participants and counterbalanced between the two sessions. Participants were asked to focus on the fixation cross and respond as quickly and accurately as possible. Participants also switched the response hand halfway through the task. Since the current study was meant to assess the transfer of visual information across the corpus callosum, only trials with letters in a single visual field (left or right) were included in data analyses (Clawson et al., 2015). Additionally, only correct trials were included in data analyses to ensure accurate stimulus perception and increase the likelihood that the participant was focusing on the visual stimulus (Moes et al., 2007).
2.3. Measures
2.3.1. Questionnaires
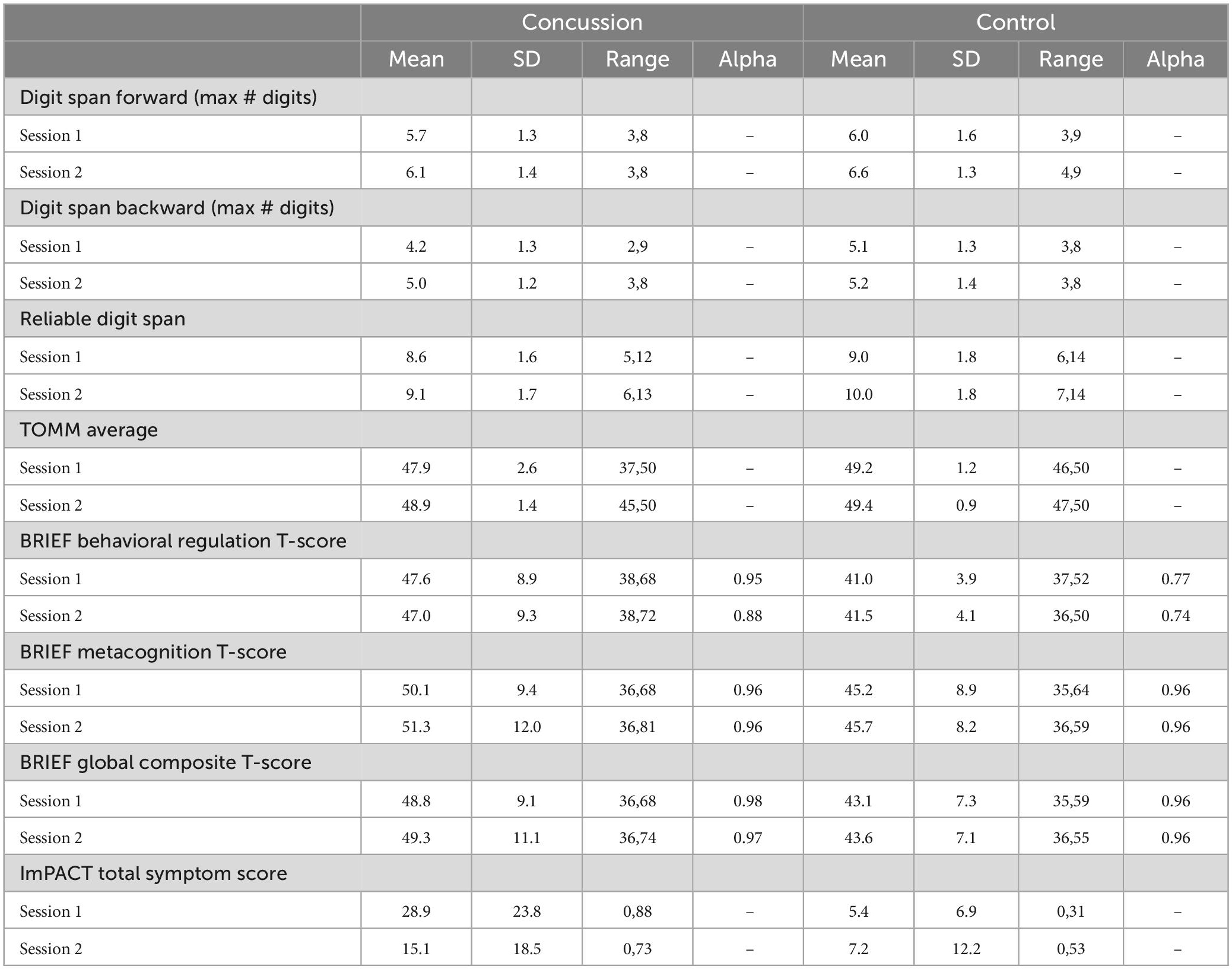
Parents of minors completed the Behavior Rating Inventory of Executive Function (BRIEF) parent questionnaire at both sessions (Gioia et al., 2000). A total of 18 years-old participants completed the comparable BRIEF Adult Questionnaire at both sessions (BRIEF-A; Roth et al., 2013). The BRIEF consists of 86 items, scored on a three-point scale (0 = never, 1 = sometimes, and 2 = often; Gioia et al., 2000). The BRIEF-A includes 75 items, scored on the same scale (Roth et al., 2013). Scores on individual items in both the BRIEF and BRIEF-A are separated and summed into eight subscales measuring different aspects of executive function such as inhibition, organization, and emotional control (Gioia et al., 2000; Roth et al., 2013). Subscale scores are then summed together to provide a behavioral regulation index score and a metacognition index score (Gioia et al., 2000; Roth et al., 2013). The sum of these two index scores provides a global composite score for executive function (Gioia et al., 2000; Roth et al., 2013). The BRIEF and BRIEF-A have excellent internal consistency (Cronbach’s αBRIEF = 0.80–0.98; Cronbach’s αBRIEF–A = 0.93–0.96; Gioia et al., 2000; Roth et al., 2013). Standardized T-scores for the BRIEF and BRIEF-A allowed us to combine results from these two questionnaires for data analyses. Means, standard deviations, ranges, and Cronbach’s alphas for the BRIEF and BRIEF-A in our study are listed in Table 2.
2.3.2. Neuropsychological tests
Along with the completion of the previously mentioned questionnaires, participants completed the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT), TOMM and the RDS. The ImPACT is a computerized neuropsychological test battery used to assess cognitive functioning following concussion (Iverson et al., 2003; Covassin et al., 2009). The test includes six modules that, respectively measure attentional processes, verbal recognition memory, visual working memory, visual processing speed, reaction time, numerical sequencing ability, and learning (Covassin et al., 2009). In adolescents, the ImPACT displays high sensitivity rates in correctly categorizing those with concussion (81.9%) and high specificity rates in distinguishing patients without concussion (89.4%; Schatz et al., 2006). It also displays high test-retest reliability in adolescents with intraclass correlation coefficients ranging from 0.62 to 0.85 across the various composite scores produced by this measure (Elbin et al., 2011). However, not all subscales of this measure display significant convergent and discriminant validity in adolescent populations (Koehl et al., 2019).
To assess performance validity, participants completed the TOMM as well as the RDS. Means, SDs, and ranges for these tests are displayed in Table 3. Inadequate performance validity was determined by a score less than 45 on either of the first two recognition trials of the TOMM (Tombaugh, 1997) together with a score less than or equal to six on the RDS (Axelrod et al., 2006; Kirkwood et al., 2011; Schroeder et al., 2012). For participants that received a score of 45 or higher for the first learning trial of the TOMM, the subsequent trials were not administered as this score correlates strongly with overall performance (Kraemer et al., 2020). Seven participants with concussion received a score less than six on the RDS and two participants with concussion had TOMM scores less than 45 on all three trials in their first session. Of these participants, only one had scores in the invalid range on both the TOMM and the RDS (Highest TOMM = 37, RDS = 5) and was thus excluded from analyses. Two control participants were missing TOMM scores but had acceptable RDS scores. No control participants displayed overall inadequate performance validity. The combination of the TOMM and RDS were used to determine participant exclusion, as this combination adequately distinguishes between a simulated malingering group and individuals with concussion, while the RDS alone may not (Bashem et al., 2014). Groups did not significantly differ in RDS scores for either session [Session 1: tRDS(61) = 0.9, p = 0.37, d = 0.2; Session 2: tRDS(50) = 1.8, p = 0.07, d = 0.5]. We did observe a significant difference between concussion and control groups in TOMM scores for the first session, with the control group displaying higher scores than the concussion group [tTOMM(59) = 2.3, p = 0.03, d = 0.6]. However, we observed no significant differences in TOMM scores for the second session [tTOMM(50) = 1.7, p = 0.10, d = 0.5]. Means, standard deviations, and ranges for TOMM and RDS scores are displayed in Table 2.
2.4. Electroencephalogram recording and reduction
Electroencephalogram (EEG) data were recorded from 128 equidistant Ag/AgCl scalp electrode sites using a hydrocele sensor net from Electrical Geodesics, Inc., (EGI; Eugene, OR, United States) and a NetAmps 300 amplifier system (20 K gain, nominal bandpass = 0.01–100 Hz). The sensor montage of the 128-electrode net is shown in Clayson and Larson (2011). Data were referenced to the Cz electrode during data collection and digitized continuously at 1,000 Hz with a 16-bit analog-to-digital converter. An electrode on the posterior midline 2 cm below the reference electrode served as common ground. Electrode impedances were maintained below 50 kΩ per the manufacturer’s recommendation.
Offline, all EEG data were filtered and segmented in NetStation (version 5.3.0.1). Data were high-pass filtered at 0.1 Hz and low-pass filtered at 15 Hz. Data were then segmented to include correct trials for each visual field, collapsed across match and non-match trials. Trial numbers are displayed in Table 6 for each IHTT dependent variable divided by group and session. Data were segmented from 200 ms before stimulus onset to 1,000 ms after stimulus onset. All subsequent data processing steps used the ERP PCA Toolkit in Matlab (Dien, 2010). Eye movements and blink artifacts were corrected using independent components analysis (ICA) where single trial epochs were rejected if voltages exceeded 100 μV, transitional (sample-to-sample) thresholds were greater than 100 μV, or eye-channel amplitudes were above 70 μV. If any ICA component displayed a correlation of 0.9 or higher with two blink templates (one being derived from the authors and one coming from the ERP PCA Toolkit) that component was removed from the data. Additionally, if the fast average amplitude of any channel was greater than 100 μV or if the differential average amplitude was greater than 50 μV, that channel was labeled as a bad channel and the six nearest neighboring electrodes were then used to interpolate the data for the specified electrode. Finally, trials for participants were averaged together, re-referenced using an average reference, and baseline adjusted from 200 to 0 ms before stimulus onset for both the P1 and the N1. Data reduction and analysis pipeline choices were based on previous multiverse analyses from lab data showing maximal data quality and psychometric reliability (Clayson et al., 2021).
Noise levels and trial numbers for each IHTT dependent variable divided by group and session are available on OSF.1 Noise values represent the root mean square of residual noise after consistent ERP data is removed by inverting every other trial (Schimmel, 1967). One control participant was excluded for noise values greater than 10. Trial numbers ranged from 5 to 77 for participants across groups and sessions.
To measure IHTT response times, we calculated the differences between averaged indirect values (e.g., stimulus in the right visual field and response with the left hand) and averaged direct values (e.g., stimulus in the right visual field and response with the right hand). This produced indirect minus direct difference response time values for each participant (Meissner et al., 2017; Womack et al., 2017).
We extracted P1 and N1 peak latencies from electrodes 65 (left posterior region) and 90 (right posterior region; Clayson and Larson, 2011) using Matlab and R (version 4.0.2). These electrodes were chosen as they are adjacent to electrodes O1 and O2 on a 10–20 EEG system over the occipital areas and occipital electrode sites produce large visual ERPs which yield clear P1 and N1 ERPs for measuring IHTT (Andreassi et al., 1975). For peak latency, the peak for P1 was defined as the most positive peak between 0 and 200 ms and the peak for N1 was defined as the most negative peak between 150 and 250 ms (Brown and Jeeves, 1993; Clark and Hillyard, 1996). To ensure that the time windows correctly captured the peak latency of interest (and not the edge of the epoch), each individual waveform was inspected. If the extracted peak latency was the edge of the epoch and not at the true P1 or N1 peak, or if the peak fell outside the originally designated time window, the window was adjusted using visual inspection to capture the peak deflection. P1 and N1 IHTT for each participant were calculated by averaging together direct pathway latencies (i.e., left visual field to right-occipital electrode 90 and right visual field to left-occipital electrode 65) for each component and subtracting them from averaged indirect pathway latencies (i.e., left visual field to left-occipital electrode 65 and right visual field to right-occipital electrode 90).
2.5. Statistical analyses
All statistical analyses of the data were decided on a priori and were conducted in R (version 4.0.2). We defined outliers as participants with primary dependent variable values (indirect minus direct difference response times, P1 IHTT difference latencies, and N1 IHTT difference latencies) greater than 1.5 times the interquartile range from the median at either session. Three participants (one at session 1 and two at session 2) with outlier difference response time values were excluded from response time analyses, and one participant at session 1 with outlier N1 IHTT values was excluded from N1 analyses. No participants had outlier P1 IHTT values. We performed all analyses both including and excluding outlier participants to test if there were different patterns or results. Only one session 2 response time manipulation check was affected by the removal of outliers, which is discussed further in our results and discussion. Removing outliers did not affect the statistical significance of the outcomes of any additional analyses.
2.5.1. BRIEF and ImPACT analyses
To test session and group differences in the BRIEF subscale scores (metacognition, behavioral regulation, and global composite) and ImPACT total symptom scores, we performed separate two-session (session 1, session 2) by two-group (concussion, control) ANOVAs for each questionnaire. Generalized eta squared is reported as a measure of effect size for all ANOVAs (η2).
2.5.2. Interhemispheric transfer time analyses
To ensure that IHTT was observed in the current data we performed paired-samples t-tests between direct and indirect P1 and N1 latencies and direct and indirect response times collapsed across groups. Cohen’s dz was used as a measure of effect size for all within-subjects t-tests (dz; Cohen, 2013). These analyses served as a manipulation check to ensure that IHTT occurred, evidenced by significantly greater indirect latencies than direct latencies and significantly greater indirect response times in comparison to direct response times. Following manipulation checks we tested the initial differences in response times and ERPs between groups using independent-samples t-tests with Cohen’s d presented as a measure of effect size. To test for longitudinal changes in IHTT we performed two-session (session 1, session 2) by two-group (concussion, control) ANOVAs for indirect minus direct response times and P1 and N1 ERP component indirect minus direct difference IHTT latencies.
2.5.3. Correlational analyses
We conducted Pearson’s correlations between self-reported concussion symptomology as measured by ImPACT total symptom scores and BRIEF metacognition, behavioral regulation, and global composite subscales with indirect minus direct difference response times and difference P1 and N1 IHTT at both sessions. We also conducted Pearson’s correlations between indirect minus direct difference response times and difference P1 and N1 IHTT at both sessions. We set alpha at 0.01 for correlational analyses to provide some alpha correction for the large number of correlational analyses. We chose a less conservative p of 0.01 given the relative lack of studies in this area relative to a more conservative Bonferroni or similar correction.
2.5.4. Sensitivity analysis
We performed a sensitivity analysis in G*Power (v3.1.9.3) to determine what size of an effect we were powered to detect given the study sample size. For the independent samples t-tests, with 29 participants with concussion and 24 control participants who completed the first session, and an alpha level of 0.05, we were powered to detect large effects at 80% power (Cohen’s d = 0.80). For the two-session by two-group ANOVAs, with a total sample size of 35, a correlation of 0.3 between repeated measures, and an alpha level of 0.05, we were powered to detect medium to large effects at 80% power (f = 0.28). Thus, the current study is limited in its ability to detect small effects if present. This limitation is discussed in detail in the discussion.
3. Results
3.1. BRIEF and ImPACT results
Means, standard deviations, ranges, and Cronbach’s alphas for all questionnaires are displayed in Table 2.
For the BRIEF questionnaire, participants with concussion had significantly higher (i.e., worse functioning) scores than healthy controls for the behavioral regulation [F(1,46) = 12.5, p < 0.001, η2 = 0.1], metacognition [F(1,46) = 4.6, p = 0.04, η2 = 0.1], and global composite [F(1,46) = 6.8, p = 0.01, η2 = 0.1] subscales, although we note these scores are within the average range. There were no significant main effects of session (ps ≥ 0.61) nor significant interaction effects (ps ≥ 0.66) for the three BRIEF subscales.
Analyses of ImPACT total symptom score showed significant main effects of group [F(1,51) = 14.5, p < 0.001, η2 = 0.2] and session [F(1,51) = 7.5, p = 0.01, η2 = 0.1]. There was also a significant group by session interaction [F(1,51) = 12.2, p < 0.01, η2 = 0.1]. Follow up t-tests showed that total symptom scores significantly decreased for individuals with concussion between sessions 1 and 2 [t(29) = 4.0, p < 0.001, dz = 0.7], but scores remained the same between sessions for controls [t(22) = –0.7, p = 0.48, dz = –0.1], showing the expected symptom improvement in concussion participants over time. Participants with concussion had significantly higher total symptom scores than controls at session 1 [t(35.2) = –5.3, p < 0.001, d = –1.3; degrees of freedom adjusted for lack of homogeneity of variance], but no significant differences between concussion participants and controls at session 2 [t(51) = –1.7, p = 0.10, d = –0.5].
3.2. Manipulation checks
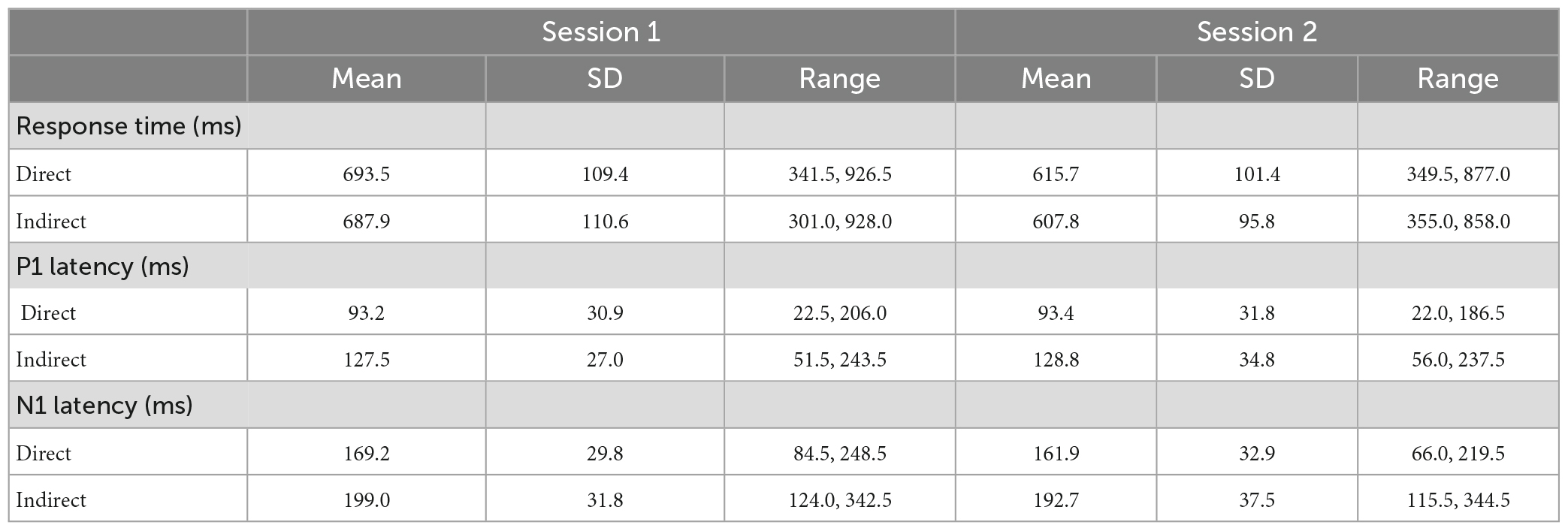
Means, standard deviations, and ranges for P1 and N1 latencies and response times are displayed in Table 3.
There were no significant differences in response times for either session between the direct and indirect pathways [tSession1(61) = 1.1, p = 0.27, dz = 0.1; tSession2(51) = 1.9, p = 0.07, dz = 0.3]. However, following the removal of outliers, session 2 response time was significantly longer for the direct pathway than the indirect pathway [tRT(49) = 2.9, p = 0.01, dz = 0.4]. The removal of outliers did not affect the significance of outcomes of any other analyses. P1 ERP latency was significantly faster for the direct pathway compared to the indirect pathway at both sessions, as expected [tSession1(52) = –14.3, p < 0.001, dz = –2.0; tSession2(43) = –12.1, p < 0.001, dz = –1.8]. N1 latency was also significantly faster for the direct pathway compared to the indirect pathway for both sessions [tSession1(52) = –12.0, p < 0.001, dz = –1.6; tSession2(41) = –9.5, p < 0.001, dz = –1.5].
3.3. Subacute results
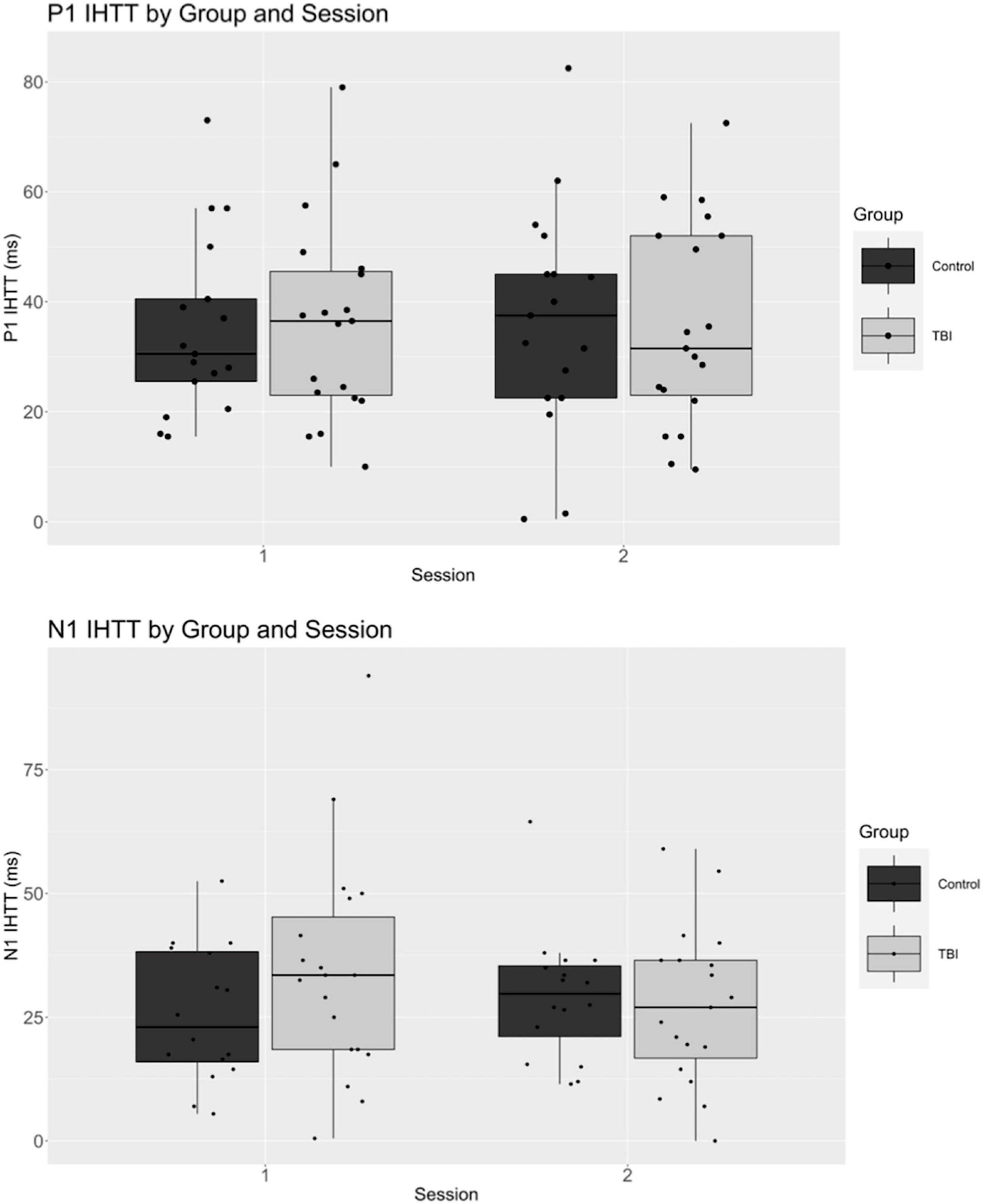
Means, standard deviations, and ranges for all dependent variables are displayed in Table 4. ERP waveforms separated by group and session are displayed in Figure 2 and boxplots of P1 and N1 latency measures are displayed in Figure 3.

Figure 2. Event-related potential (ERP) waveforms for the direct and indirect P1 and N1 components by concussion and control group for session 1 [(top); within 3 weeks of concussion] and session 2 [(bottom); approximately 10 months after injury].
At session 1, no significant differences were observed between participants with concussion and controls in indirect minus direct response times [t(60)RT = –0.6, p = 0.53, d = –0.2]. Additionally, there were no significant differences between participants with concussion and controls for P1 nor N1 IHTT latency at session 1 [t(51)P1 = –1.1, p = 0.26, d = –0.3; t(51)N1 = –1.1, p = 0.27, d = –0.3].
3.4. Longitudinal results
When examining our data longitudinally w, there ere no significant differences in difference response time between groups [F(1,49) = 1.4, p = 0.25, η2 = 0.0] or sessions [F(1,49) = 0.0, p = 0.88, η2 < 0.001], and there was no significant group by session interaction [F(1,49) = 0.1, p = 0.73, η2 = 0.0]. P1 IHTT did not significantly differ between groups [F(1,34) = 0.0, p = 0.96, η2 < 0.001] or sessions [F(1,34) = 0.0, p = 0.90, η2 < 0.001], and we observed no group by session interaction [F(1,34) = 0.0, p = 0.83, η2 < 0.001]. Likewise, N1 IHTT did not significantly differ between groups [F(1,33) = 0.6, p = 0.43, η2 = 0.0] or sessions [F(1,33) = 0.2, p = 0.64, η2 = 0.0], with no significant group by session interaction [F(1,33) = 2.1, p = 0.15, η2 = 0.0].
3.5. Correlations between IHTT and questionnaire scores
Correlation coefficients and p-values are presented in Tables 5–7. We did not observe any significant correlations between BRIEF behavioral regulation, metacognition, or global composite subscale scores and indirect minus direct difference response times or P1 or N1 IHTT in either the first or second session (ps ≥ 0.08). Likewise, we did not observe any significant correlations between ImPACT total symptom scores and indirect minus direct difference response times or P1 or N1 IHTT in either session (ps ≥ 0.10). Lastly, we did not observe any significant correlations between indirect minus direct difference response time and P1 or N1 IHTT.
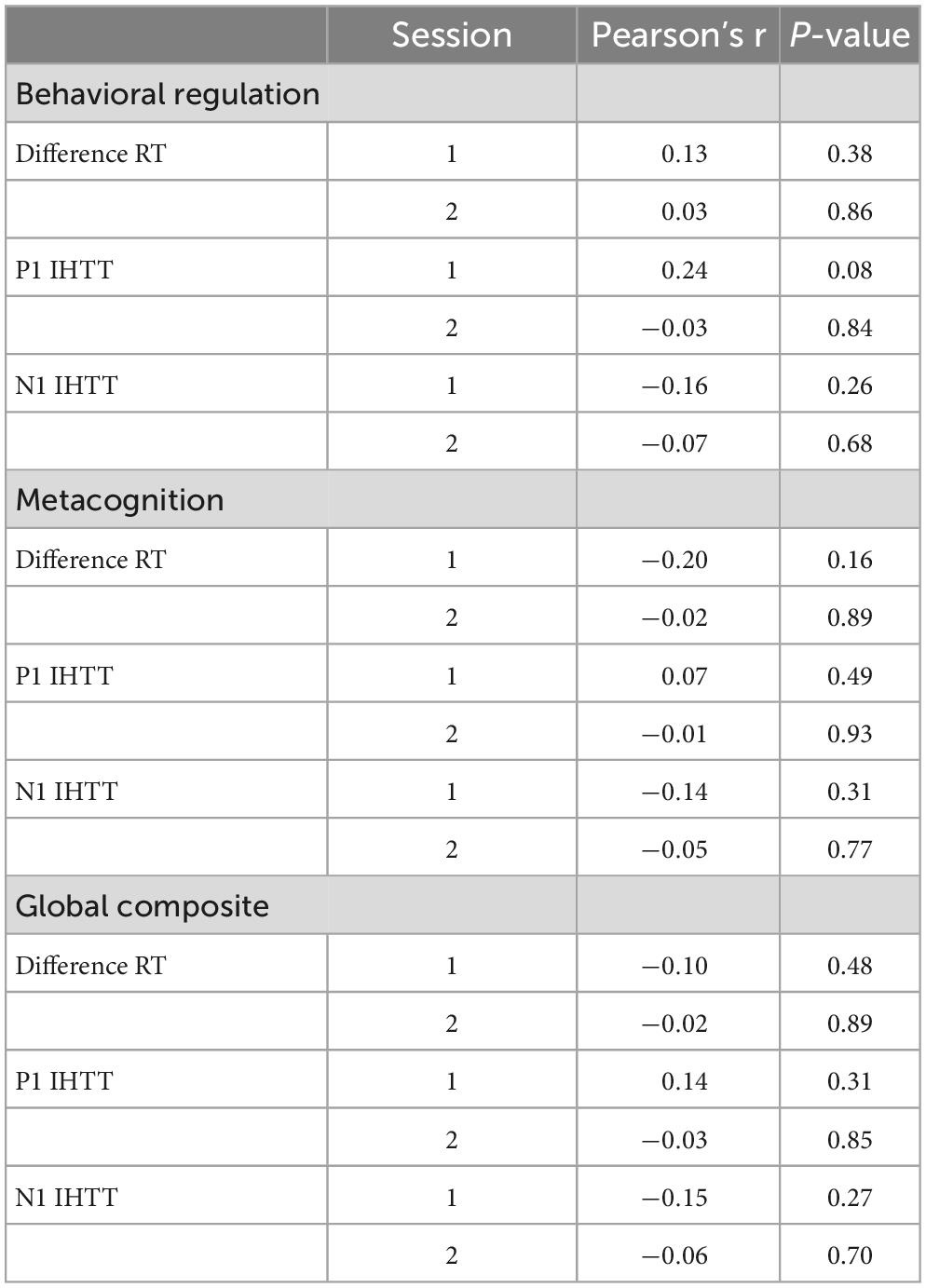
Table 5. Behavior Rating Inventory of Executive Function Parent Questionnaire (BRIEF) subscales and P1 and N1 interhemispheric transfer time (IHTT) correlations.
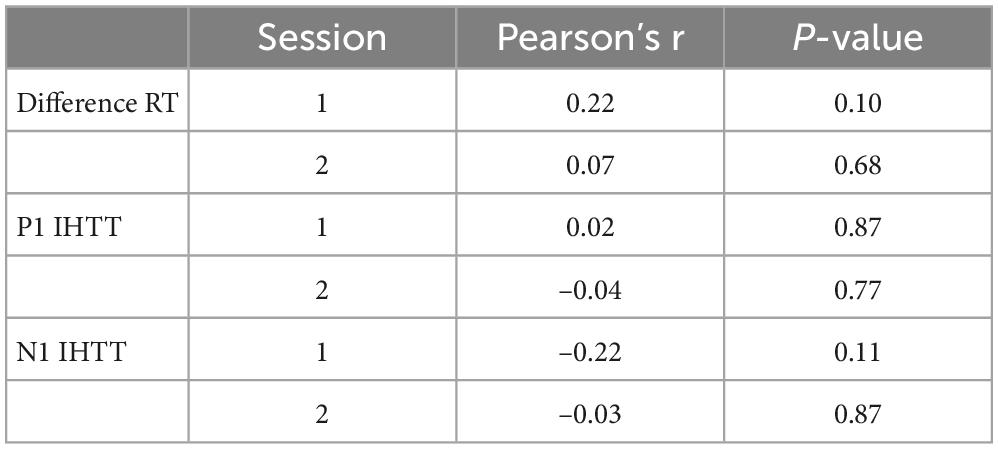
Table 6. Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) total symptom score and P1 and N1 interhemispheric transfer time (IHTT) correlations.
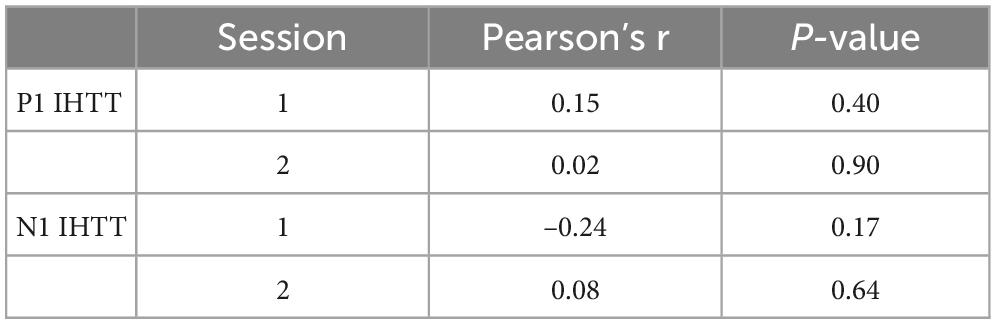
Table 7. Correlations between indirect minus direct differences responses times and P1 and N1 interhemispheric transfer time (IHTT).
4. Discussion
We tested possible differences in IHTT between adolescents with concussion and healthy controls both within 3 weeks of injury and longitudinally within 10 months of injury. Concussion symptoms as measured by the ImPACT were significantly higher (i.e., more severe) in the concussion group than the control group in the subacute period following concussion. ImPACT scores decreased significantly between sessions for the concussion group showing the expected symptom improvement over time. Additionally, the concussion group displayed significantly worse, though not clinically significant, reported executive functioning indicated by higher scores on the BRIEF than the control group averaging across time points. Thus, adolescents with concussion displayed neurocognitive difficulties in the period after injury that decreased in severity, and neurocognitive vulnerabilities that persisted, approximately 10 months following injury.
Participants with concussion and controls did not differ on response time and electrophysiological measures of interhemispheric transfer. Specifically, we observed no significant group differences between concussion and control participants in indirect minus direct difference response times or P1 or N1 IHTT either within 3 weeks or at approximately 10 months. Thus, our first hypothesis that participants in the subacute stage following concussion would show slowed interhemispheric transfer was not supported, as we observed no significant differences between groups for difference response times or ERP measures of IHTT at session 1. Notably, we also did not observe significantly shorter direct response times in comparison to indirect response times at either session. The lack of significant differences in this manipulation check call into question the utility of this surrogate measure of IHTT. Recent studies using indirect minus direct difference response times to infer IHTT generally have not reported response time manipulation checks to validate IHTT measurement (Mathias et al., 2004; Meissner et al., 2017; Womack et al., 2017). Westerhausen et al. (2006) examined behavioral IHTT in healthy, adult males. They found a significant visual field and response hand interaction in which direct response times (RVF to RH) were significantly faster than indirect response times (LVF to RH) for the right hand (Westerhausen et al., 2006). However, they did not observe significant differences between direct and indirect response times for the left hand (Westerhausen et al., 2006). Thus, the current literature contains variable findings about the utility of indirect minus direct difference response times in measuring IHTT.
Response time measures of IHTT may also not differentiate individuals with concussion and healthy controls. Mathias et al. (2004) observed that healthy controls had significantly faster response times than individuals with concussion on tasks that required interhemispheric processing. However, this study did not use indirect minus direct difference response times as an outcome measure, and thus did not specifically measure IHTT (Mathias et al., 2004). Although Womack et al. (2017) found a significant negative correlation between difference response times and corpus callosum integrity as indexed by diffusion MRI mean diffusivity, they did not observe significant differences between concussion and control participants for behavioral IHTT. They did note significantly greater indirect minus direct difference response times in a subset of participants with concussion compared to the control group (Womack et al., 2017). Furthermore, indirect minus direct difference response times did not significantly correlate with electrophysiological IHTT in our data as well as in data from other studies, indicating that behavioral and electrophysiological IHTT may not reflect identical neural phenomena (Braun, 1992; Westerhausen et al., 2006; Friedrich et al., 2017; Meissner et al., 2017). A study by Hammond-Tooke et al. (2010) indicated that response time deficits in individuals with concussion may be attributed to damage to intrahemispheric motor networks and not damage to the corpus callosum. Thus, indirect minus direct difference response times may not be useful in inferring corpus callosum damage following concussion.
Regarding electrophysiological results within 3 weeks of injury, our data showed interhemispheric transfer as indicated by significantly faster P1 and N1 direct latencies in comparison to indirect latencies. However, there were no significant differences in IHTT between participants with concussion and control participants as indexed by P1 and N1 indirect minus direct difference latencies. Although studies comparing IHTT in individuals with moderate-to-severe TBI and controls have found significant between group differences (Dennis et al., 2015, 2017; Ellis et al., 2016), ours is the first study in adolescents with concussion and suggests that the corpus callosum is not as consistently and significantly functionally impaired following concussion.
Different TBI severities may possess differing likelihoods of contributing to damage of the corpus callosum (Romeu-Mejia et al., 2019), possibly leading to differences in IHTT. A common form of injury following TBI that commonly affects the integrity of the corpus callosum is diffuse axonal injury (DAI; Johnson et al., 2013; Jang et al., 2019). This white matter damage is characterized by disruption in axonal transport together with swelling and progressive degeneration of myelinated axons (Johnson et al., 2013). Although DAI can be present in all levels of TBI severity, it tends to be more pronounced in more severe TBI and is often not seen at all in individuals with concussion (Adams et al., 1989; Choe and Barlow, 2018; Romeu-Mejia et al., 2019). Our results may suggest that concussion may not cause reliable or sufficient damage to the corpus callosum to lead to delayed interhemispheric transfer. Additionally, history of concussion may lead to chronic changes in corpus callosum microstructure, as indicated by increased mean diffusivity, even if these changes do not reach the severity of DAI (Churchill et al., 2021). Alternatively, our null results may reflect insufficient sensitivity of IHTT to detect changes in corpus callosum microstructure. Lastly, while our study focused on adolescent concussion (ages 12–19), the studies on moderate-to-severe TBI focused on a broader pediatric population (ages 8–18; Dennis et al., 2015; Ellis et al., 2016; Dennis et al., 2017). As IHTT may decrease with age, our older sample may have also contributed to our different results (Hagelthorn et al., 2000; Meissner et al., 2017).
Our second hypothesis that individuals with concussion would have significantly slower IHTT than healthy controls at a 10 months follow-up session was also not supported. To our knowledge, this study is the first to examine behavioral and electrophysiological IHTT longitudinally in individuals with concussion. Other studies that have examined IHTT over time in healthy individuals show that behavioral measures of IHTT display low reliability (Friedrich et al., 2017; Meissner et al., 2017). Specifically, in a study by Friedrich et al. (2017), indirect minus direct difference response times displayed low test-retest reliability across time points and internal consistency at both time points in comparison to electrophysiological IHTT. Likewise, Meissner et al. (2017) showed that behavioral IHTT lacked significant test-retest reliability. Therefore, our study’s lack of significant effects of indirect minus direct difference response time may be partially attributed to the unreliable nature of these surrogate measures of IHTT.
In relation to our longitudinal electrophysiological results, Dennis et al. (2017) showed that individuals with moderate-to-severe TBI and slowed IHTT displayed poorer white matter organization, indicated by lower fractional anisotropy and higher mean and radial diffusivity, which worsened over time. Studies examining white matter structure using diffusion tensor imaging following concussion also indicate corpus callosum damage (Henry et al., 2011; Jang et al., 2019). Specifically, Henry et al. (2011) observed increased fractional anisotropy in concussed participants both 1–6 days following injury and 6 months later. Jang et al. (2019) observed significantly lower fractional anisotropy and fiber numbers and significantly higher apparent diffusion coefficient in concussed participants compared to healthy controls within 1 month post-injury. More longitudinally however, individuals with a history of concussion show no significant differences in corpus callosum microstructure from individuals without a history of concussion approximately two and a half years post-injury (Sohn et al., 2020). Thus, IHTT may not be sufficiently sensitive to detect the structural changes following concussion within our study’s approximately 10 months time period.
Although previous research supports the presence of structural white matter changes following concussion, most post-concussion symptoms remit within weeks-to-months, potentially due to less white matter damage (Cassidy et al., 2004; Hall et al., 2005; Babcock et al., 2015; Barlow et al., 2015). However, as many as 20–30% of children and adolescents with concussion symptoms persist for up to 1 year following injury (Yeates et al., 2009; Ewing-Cobbs et al., 2018). Adolescents with persistent symptoms may be at increased risk for a variety of health-related quality of life issues compared to other age groups, including decreased scholastic performance and mental health concerns (Ewing-Cobbs et al., 2018). Thus, the modifying factors of symptom persistence following concussion, including white matter integrity, are an important subject of ongoing investigation. Regardless, long-lasting functional consequences following concussion appear rare (Yeates et al., 2009; Ewing-Cobbs et al., 2018).
Finally, we hypothesized that slower IHTT would significantly correlate with more severe neurocognitive deficits following concussion, as indicated by BRIEF and ImPACT scores, but our data did not support this hypothesis. Although individuals with concussion displayed significantly poorer functioning than healthy controls on all BRIEF subscales and subacute ImPACT total symptom scores, we did not observe any correlations between BRIEF subscales or ImPACT total symptom scores and P1 or N1 latency measures of IHTT at either session.
One possible alternative explanation for the current findings is that the choice of P1 and N1 ERPs may have been confounded by using a task that required focused attention and cognitive processing to determine the match or non-match of the stimuli. Manipulating visual attention differentially invokes the magnocellular and parvocellular pathways (e.g., Brown, 2009). Studies using visual evoked potentials and other methodologies suggest persistent and specific difficulties following concussion in magnocellular pathway stimulation (e.g., Schrupp et al., 2009; Fimreite et al., 2015; Poltavski et al., 2019), particularly in people with poor visual attention (Yadav and Ciuffreda, 2015). Future studies using tasks that can compare or manipulate transient magnocellular pathways relative to persistent parvocellular pathways (e.g., by manipulating variables such as luminance, contrast, and reversal rate) may lead to alternate findings more specific to concussion-related changes in visual and inter-hemispheric processing.
Some limitations in our study may further explain our results. There was attrition in our sample reducing the statistical power. For subacute analyses, a sensitivity analysis based on our final sample size showed that we were powered to detect large effects at 80% power. For longitudinal analyses, a power analysis showed that we were powered to detect medium to large effects at 80% power. Due to our sample size, potentially present small-to-medium effects may not have been seen. Additionally, our task differed from more traditional IHTT tasks in that it required participants to think about whether the two letters matched before making a response (Braun, 1992). This additional cognitive processing required by our task may have masked the effect of visual pathway on response times (Braun, 1992). Heterogeneity of the number of concussions and mechanism of concussion may have limited our results. Participants ranged from having experienced 1–5 concussions and participants experienced a variety of different types of concussion categorized as deceleration, pure deceleration with blunt head trauma, and acceleration and deceleration with and without blunt head trauma. The variety in number and types of concussion is a potential confound in our experiment, as different numbers and types of concussion could have varied effects on corpus callosum integrity and IHTT. Regarding the IHTT task, we did not have gaze-tracking or video monitoring capabilities to ensure that participants maintained their fixation during each trial. As a result, the exact gaze position is unknown in the current paradigm representing a weakness of the study.
Although our study may have been impacted by its limitations, there are also strengths. The current study is the first of which we are aware to study the effect of concussion in adolescents on IHTT, both in the subacute stage and longitudinally. Our study also ensured the reliability of our measures of IHTT through relevant manipulation checks, as well as ecological validity through our varied recruiting methods, and performance validity as determined by the TOMM and RDS. Our results provide a foundation for future research seeking to better understand the impacts of concussion on the corpus callosum in adolescents. Furthermore, our study adds to the important body of research on concussion in the high-incidence age group of adolescents. Finally, not only does our study provide information about subacute results of concussion, but it also provides important longitudinal evidence about the impacts of concussion on the corpus callosum. As brain damage following concussion frequently changes over time, the longitudinal aspect of our study may be a particularly valuable addition to the current literature.
In summary, participants with concussion in our sample showed increased concussion symptoms and decreased neuropsychological performance on the IMPACT. However, our data showed no significant differences on measures of IHTT between individuals with concussion and healthy control participants. The lack of significant between-groups differences suggests that concussion may not contribute to functional impairment of the corpus callosum in adolescents. Thus, based on the current study, IHTT may not possess clinical utility as an indicator for the presence and severity of concussion in an adolescent population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Open Science Framework at https://osf.io/aqf9v/?view_only=0624935781604da1adf5e9099baf121c.
Ethics statement
The studies involving human participants were reviewed and approved by Brigham Young University Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
AC, TF, EB, and ML: conceptualization, methodology, project administration, and supervision. BAC, BC, EC, TJ, NA, AC, TF, and ML: data curation. BAC, BC, AM, and ML: formal analysis. EB and ML: funding acquisition, resources, and software. EC, TJ, NA, AC, TF, and ML: investigation. BAC, BC, AM, WA, EC, TJ, and NA: validation. BAC, BC, AM, WA, and ML: visualization. BAC, BC, AM, WA, EC, and ML: writing–original draft. All authors contributed to the writing–review and editing and approved the submitted version.
Funding
This study was funded by the Brigham Young University College of Family, Home, and Social Sciences and an anonymous donor who provided funds for research assistants and had no influence on study design, procedures, or outcomes.
Acknowledgments
We gratefully acknowledge the assistance of Mike Diede, Ph.D., ATC, the BYU Athletic Training students and staff, and Brandon Carter, DC, for assistance in participant recruitment. We are also grateful to Denise Lafont-Tanner for compiling data for some measures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Adams, J. H., Doyle, D., Ford, I., Gennarelli, T. A., Graham, D. I., and McLellan, D. R. (1989). Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology 15, 49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x
Andreassi, J. L., Okamura, H., and Stern, M. (1975). Hemispheric asymmetries in the visual cortical evoked potential as a function of stimulus location. Psychophysiology 12, 541–546. doi: 10.1111/j.1469-8986.1975.tb00043.x
Aoki, Y., Inokuchi, R., Gunshin, M., Yahagi, N., and Suwa, H. (2012). Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 83, 870–876. doi: 10.1136/jnnp-2012-302742
Asken, B. M., DeKosky, S. T., Clugston, J. R., Jaffee, M. S., and Bauer, R. M. (2018). Diffusion tensor imaging (DTI) findings in adult civilian, military, and sport-related mild traumatic brain injury (mTBI): A systematic critical review. Brain Imaging Behav. 12, 585–612. doi: 10.1007/s11682-017-9708-9
Axelrod, B. N., Fichtenberg, N. L., Millis, S. R., and Wertheimer, J. C. (2006). Detecting incomplete effort with digit span from the Wechsler adult intelligence scale–third edition. Clin. Neuropsychol. 20, 513–523. doi: 10.1080/13854040590967117
Babcock, L., Byczkowski, T., Wade, S. L., Ho, M., Mookerjee, S., and Bazarian, J. J. (2013). Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 167, 156–161. doi: 10.1001/jamapediatrics.2013.434
Babcock, L., Yuan, W., Leach, J., Nash, T., and Wade, S. (2015). White matter alterations in youth with acute mild traumatic brain injury. J. Pediatr. Rehabil. Med. 8, 285–296. doi: 10.3233/PRM-150347
Barlow, K. M., Crawford, S., Brooks, B. L., Turley, B., and Mikrogianakis, A. (2015). The incidence of postconcussion syndrome remains stable following mild traumatic brain injury in children. Pediatr. Neurol. 53, 491–497. doi: 10.1016/j.pediatrneurol.2015.04.011
Bartzokis, G., Lu, P. H., Tingus, K., Mendez, M. F., Richard, A., Peters, D. G., et al. (2010). Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol. Aging 31, 1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015
Bashem, J. R., Rapport, L. J., Miller, J. B., Hanks, R. A., Axelrod, B. N., and Millis, S. R. (2014). Comparisons of five performance validity indices in bona fide and simulated traumatic brain injury. Clin. Neuropsychol. 28, 851–875. doi: 10.1080/13854046.2014.927927
Braun, C. M. J. (1992). Estimation of interhemispheric dynamics from simple unimanual reaction time to extrafoveal stimuli. Neuropsychol. Rev. 3, 321–365. doi: 10.1007/BF01108415
Brown, J. M. (2009). Visual streams and shifting attention. Prog. Brain Res. 176, 47–63. doi: 10.1016/S0079-6123(09)17604-5
Brown, W. S., and Jeeves, M. A. (1993). Bilateral visual field processing and evoked potential interhemispheric transmission time. Neuropsychologia 31, 1267–1281. doi: 10.1016/0028-3932(93)90097-J
Cassidy, D. J., Carroll, L. J., Peloso, P. M., Borg, J., von Holst, H., Holm, L., et al. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 36, 28–60. doi: 10.1080/16501960410023732
Centers for Disease Control and Prevention (2019). Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths–United States, 2014. Washington, DC: U.S. Department of Health and Human Services.
Choe, M., and Barlow, K. M. (2018). Pediatric traumatic brain injury and concussion. Continuum 24, 300–311. doi: 10.1212/CON.0000000000000569
Churchill, N. W., Hutchison, M. G., Graham, S. J., and Schweizer, T. A. (2021). Acute and chronic effects of multiple concussions on midline brain structures. Neurology 97, e1170–e1181. doi: 10.1212/WNL.0000000000012580
Clark, V. P., and Hillyard, S. A. (1996). Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J. Cogn. Neurosci. 8, 387–402. doi: 10.1162/jocn.1996.8.5.387
Clarke, S., Maeder, P., Meuli, R., Staub, F., Bellmann, A., Regli, L., et al. (2000). Interhemispheric transfer of visual motion information after a posterior callosal lesion: A neuropsychological and fMRI study. Exp. Brain Res. 132, 127–133. doi: 10.1007/s002219900327
Clawson, A., Clayson, P. E., South, M., Bigler, E. D., and Larson, M. J. (2015). An electrophysiological investigation of interhemispheric transfer time in children and adolescents with high-functioning autism spectrum disorders. J. Autism Dev. Disord. 45, 363–375. doi: 10.1007/s10803-013-1895-7
Clayson, P. E., and Larson, M. J. (2011). Effects of repetition priming on electrophysiological and behavioral indices of conflict adaptation and cognitive control. Psychophysiology 48, 1621–1630. doi: 10.1111/j.1469-8986.2011.01265.x
Clayson, P. E., Baldwin, S. A., Rocha, H. A., and Larson, M. J. (2021). The data-processing multiverse of event-related potentials (ERPs): A roadmap for the optimization and standardization of ERP processing and reduction pipelines. Neuroimage 245:118712. doi: 10.1016/j.neuroimage.2021.118712C
Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Cambridge, MA: Academic Press.
Coronado, V. G., Haileyesus, T., Cheng, T. A., Bell, J. M., Haarbauer-Krupa, J., Lionbarger, M. R., et al. (2015). Trends in sports- and recreation- related traumatic brain injuries treated in US emergency departments: The national electronic injury surveillance system-all injury program (NEISS-AIP) 2001-2012. J. Head Trauma Rehabil. 30, 185–197. doi: 10.1097/HTR.0000000000000156
Covassin, T., Elbin, R. J., Stiller-Ostrowski, J. L., and Kontos, A. P. (2009). Immediate post-concussion assessment and cognitive testing (ImPACT) practices of sports medicine professionals. J. Athl. Train. 44, 639–644. doi: 10.4085/1062-6050-44.6.639
Dennis, E. L., Ellis, M. U., Marion, S. D., Jin, Y., Moran, L., Olsen, A., et al. (2015). Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J. Neurosci. 35, 10202–10211. doi: 10.1523/JNEUROSCI.1595-15.2015
Dennis, E. L., Rashid, F., Ellis, M. U., Babikian, T., Vlasova, R. M., Villalon-Reina, J. E., et al. (2017). Diverging white matter trajectories in children after traumatic brain injury: The RAPBI study. Neurology 88, 1392–1399. doi: 10.1212/WNL.0000000000003808
Dewan, M. C., Mummareddy, N., Wellons, J. C., and Bonfield, C. M. (2016). Epidemiology of global pediatric traumatic brain injury: Qualitative review. World Neurosurg. 91, 497–509.e1. doi: 10.1016/j.wneu.2016.03.045
Dien, J. (2010). The ERP PCA toolkit: An open source program for advanced statistical analysis of event-related potential data. J. Neurosci. Methods 187, 138–145. doi: 10.1016/j.jneumeth.2009.12.009
Dobney, D. M., Thomas, S. G., Taha, T., and Keightley, M. (2018). Physiological and performance measures for baseline concussion assessment. J. Sport Rehabil. 27, 312–318. doi: 10.1123/jsr.2017-0038
Elbin, R. J., Schatz, P., and Covassin, T. (2011). One-year test-retest reliability of the online version of ImPACT in high school athletes. Am. J. Sports Med. 39, 2319–2324. doi: 10.1177/0363546511417173
Ellis, M. U., DeBoard Marion, S., McArthur, D. L., Babikian, T., Giza, C., Kernan, C. L., et al. (2016). The UCLA study of children with moderate-to-severe traumatic brain injury: Event-related potential measure of interhemispheric transfer time. J. Neurotrauma 33, 990–996. doi: 10.1089/neu.2015.4023
Ewing-Cobbs, L., Cox, C. S., Clark, A. E., Holubkov, R., and Keenan, H. T. (2018). Persistent postconcussion symptoms after injury. Pediatrics 142:e20180939. doi: 10.1542/peds.2018-0939
Fimreite, V., Ciuffreda, K. J., and Yadav, N. K. (2015). Effect of luminance on the visually-evoked potential in visuallynormal individuals and in mTBI/concussion. Brain Inj. 29, 1199–1210. doi: 10.3109/02699052.2015.1035329
Friedrich, P., Ocklenburg, S., Mochalski, L., Schlüter, C., Güntürkün, O., and Genc, E. (2017). Long-term reliability of the visual EEG Poffenberger paradigm. Behav. Brain Res. 330, 85–91. doi: 10.1016/j.bbr.2017.05.019
Gioia, G. A., Isquith, P. K., Guy, S. C., and Kenworthy, L. (2000). Test review behavior rating inventory of executive function. Child Neuropsychol. 6, 235–238. doi: 10.1076/chin.6.3.235.3152
Giza, C. C., and Hovda, D. A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, S24–S33. doi: 10.1227/NEU.0000000000000505
Greenblatt, S. H., Saunders, R. L., Culver, C. M., and Bogdanowicz, W. (1980). Normal interhemispheric visual transfer with incomplete section of the splenium. Arch. Neurol. 37, 567–571. doi: 10.1001/archneur.1980.00500580063010
Hagelthorn, K. M., Brown, W. S., Amano, S., and Asarnow, R. (2000). Normal development of bilateral field advantage and evoked potential interhemispheric transmission time. Dev. Neuropsychol. 18, 11–31. doi: 10.1207/S15326942DN1801_2
Hall, R. C. W., Hall, R. C. W., and Chapman, M. J. (2005). Definition, diagnosis, and forensic implications of postconcussional syndrome. Psychosomatics 46, 195–202. doi: 10.1176/appi.psy.46.3.195
Halstead, M. E., Walter, K. D., Moffatt, K., and Council on Sports Medicine and Fitness (2018). Sport-related concussion in children and adolescents. Pediatrics 142:e20183074. doi: 10.1542/peds.2018-3074
Hammond-Tooke, G. D., Goei, J., du Plessis, L. J., and Franz, E. A. (2010). Concussion causes transient dysfunction in cortical inhibitory networks but not the corpus callosum. J. Clin. Neurosci. 17, 315–319. doi: 10.1016/j.jocn.2009.06.026
Hayes, J. P., Bigler, E. D., and Verfaellie, M. (2016). Traumatic brain injury as a disorder of brain connectivity. J. Int. Neuropsychol. Soc. 22, 120–137. doi: 10.1017/S1355617715000740
Henry, L. C., Tremblay, J., Tremblay, S., Lee, A., Brun, C., Lepore, N., et al. (2011). Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 28, 2049–2059. doi: 10.1089/neu.2011.1836
Hernandez, F., Giordano, C., Goubran, M., Parivash, S., Grant, G., Zeineh, M., et al. (2019). Lateral impacts correlate with falx cerebri displacement and corpus callosum trauma in sports-related concussions. Biomech. Model. Mechanobiol. 18, 631–649. doi: 10.1007/s10237-018-01106-0
Hinkley, L. B. N., Marco, E. J., Findlay, A. M., Honma, S., Jeremy, R. J., Strominger, Z., et al. (2012). The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One 7:e39804. doi: 10.1371/journal.pone.0039804
Iverson, G. L., Lovell, M. R., and Collins, M. W. (2003). Interpreting change on ImPACT following sport concussion. Clin. Neuropsychol. 17, 460–467. doi: 10.1076/clin.17.4.460.27934
Jang, S. H., Kim, O. L., Kim, S. H., and Lee, H. D. (2019). Differences in corpus callosum injury between cerebral concussion and diffuse axonal injury. Medicine 98:e17467. doi: 10.1097/MD.0000000000017467
Johnson, V. E., Stewart, W., and Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43. doi: 10.1016/j.expneurol.2012.01.013
Jorge, R. E., Acion, L., White, T., Tordesillas-Gutierrez, D., Pierson, R., Crespo-Facorro, B., et al. (2012). White matter abnormalities in veterans with mild traumatic brain injury. Am. J. Psychiatry 169, 1284–1291. doi: 10.1176/appi.ajp.2012.12050600
Kay, T., Harrington, D. E., Adams, R., Anderson, T., Berrol, S., Cicerone, K., et al. (1993). Definition of mild traumatic brain injury. J. Head Trauma. Rehabil. 8, 86–87.
Kimbler, D. E., Murphy, M., and Dhandapani, K. M. (2011). Concussion and the adolescent athlete. J. Neurosci. Nurs. 43, 286–290.
Kimura, M., Ohira, H., and Schröger, E. (2010). Localizing sensory and cognitive systems for pre-attentive visual deviance detection: An sLORETA analysis of the data of Kimura et al. (2009). Neurosci. Lett. 485, 198–203. doi: 10.1016/j.neulet.2010.09.011
Kirkwood, M. W., Hargrave, D. D., and Kirk, J. W. (2011). The value of the WISC-IV digit span subtest in detecting noncredible performance during pediatric neuropsychological examinations. Arch. Clin. Neuropsychol. 26, 377–384.
Kochunov, P., Williamson, D. E., Lancaster, J., Fox, P., Cornell, J., Blangero, J., et al. (2012). Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol. Aging 33, 9–20. doi: 10.1016/j.neurobiolaging.2010.01.014
Koehl, L. M., Walls, B. D., Brothers, S. L., Morris, S. N., Glueck, A. C., Schmitt, F. A., et al. (2019). Convergent and discriminant validity of the Immediate postconcussion assessment and cognitive testing battery (ImPACT) in young athletes. Appl. Neuropsychol. Child 8, 253–263. doi: 10.1080/21622965.2018.1436440
Kraemer, L. D. R., Soble, J. R., Phillips, J. I., Webber, T. A., Fullen, C. T., Highsmith, J. M., et al. (2020). Minimizing evaluation time while maintaining accuracy: Cross-validation of the test of memory malingering (TOMM) trial 1 and first 10-item errors as briefer performance validity tests. Psychol. Assess. 32, 442–450. doi: 10.1037/pas0000802
Kraus, M. F., Susmaras, T., Caughlin, B. P., Walker, C. J., Sweeney, J. A., and Little, D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130, 2508–2519. doi: 10.1093/brain/awm216
Kroshus, E., Garnett, B., Hawrilenko, M., Baugh, C. M., and Calzo, J. P. (2015). Concussion under-reporting and pressure from coaches, teammates, fans, and parents. Soc. Sci. Med. 134, 66–75. doi: 10.1016/j.socscimed.2015.04.011
Luck, S. J., Heinze, H. J., Mangun, G. R., and Hillyard, S. A. (1990). Visual event-related potentials index focused attention within bilateral stimulus arrays. II. functional dissociation of P1 and N1 components. Electroencephalogr. Clin. Neurophysiol. 75, 528–542. doi: 10.1016/0013-4694(90)90139-B
Lystad, R. P., and Pollard, H. (2009). Functional neuroimaging: A brief overview and feasibility for use in chiropractic research. J. Can. Chiropr. Assoc. 53, 59–72.
Mathias, J. L., Beall, J. A., and Bigler, E. D. (2004). Neuropsychological and information processing deficits following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 10, 286–297. doi: 10.1017/S1355617704102117
McCrory, P., Meeuwisse, W., Dvořák, J., Aubry, M., Bailes, J., Broglio, S., et al. (2017). Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 51, 838–847. doi: 10.1136/bjsports-2017-097699
Meissner, T. W., Friedrich, P., Ocklenburg, S., Genç, E., and Weigelt, S. (2017). Tracking the functional development of the corpus callosum in children using behavioral and evoked potential interhemispheric transfer times. Dev. Neuropsychol. 42, 172–186. doi: 10.1080/87565641.2017.1315582
Moes, P. E., Brown, W. S., and Minnema, M. T. (2007). Individual differences in interhemispheric transfer time (IHTT) as measured by event related potentials. Neuropsychologia 45, 2626–2630. doi: 10.1016/j.neuropsychologia.2007.03.017
Olsen, A., Babikian, T., Dennis, E. L., Ellis-Blied, M. U., Giza, C., Marion, S. D., et al. (2020). Functional brain hyperactivations are linked to an electrophysiological measure of slow interhemispheric transfer time after pediatric moderate/severe traumatic brain injury. J. Neurotrauma 37, 397–409. doi: 10.1089/neu.2019.6532
Poltavski, D., Bernhardt, K., Mark, C., and Biberdorf, D. (2019). Frontal theta-gamma ratio is a sensitive index of concussion history in athletes on tasks of visuo-motor control. Sci. Rep. 9:17565. doi: 10.1038/s41598-019-54054-9
Reeves, T. M., Phillips, L. L., and Povlishock, J. T. (2005). Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 196, 126–137. doi: 10.1016/j.expneurol.2005.07.014
Register-Mihalik, J. K., Guskiewicz, K. M., Mihalik, J. P., Schmidt, J. D., Kerr, Z. Y., and McCrea, M. A. (2013). Reliable change, sensitivity, and specificity of a multidimensional concussion assessment battery: Implications for caution in clinical practice. J. Head Trauma Rehabil. 28, 274–283. doi: 10.1097/HTR.0b013e3182585d37
Risse, G. L., Gates, J., Lund, G., Maxwell, R., and Rubens, A. (1989). Interhemispheric transfer in patients with incomplete section of the corpus callosum. Anatomic verification with magnetic resonance imaging. Arch. Neurol. 46, 437–443. doi: 10.1001/archneur.1989.00520400097026
Roland, J. L., Snyder, A. Z., Hacker, C. D., Mitra, A., Shimony, J. S., Limbrick, D. D., et al. (2017). On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc. Natl. Acad. Sci. U.S.A. 114, 13278–13283. doi: 10.1073/pnas.1707050114
Romeu-Mejia, R., Giza, C. C., and Goldman, J. T. (2019). Concussion pathophysiology and injury biomechanics. Curr. Rev. Musculoskelet. Med. 12, 105–116. doi: 10.1007/s12178-019-09536-8
Roth, R. M., Lance, C. E., Isquith, P. K., Fischer, A. S., and Giancola, P. R. (2013). Confirmatory factor analysis of the behavior rating inventory of executive function-adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Arch. Clin. Neuropsychol. 28, 425–434. doi: 10.1093/arclin/act031
Ruff, R. M., Iverson, G. L., Barth, J. T., Bush, S. S., Broshek, D. K., and Nan Policy and Planning Committee (2009). Recommendations for diagnosing a mild traumatic brain injury: A national academy of neuropsychology education paper. Arch. Clin. Neuropsychol. 24, 3–10. doi: 10.1093/arclin/acp006
Schatz, P., Pardini, J. E., Lovell, M. R., Collins, M. W., and Podell, K. (2006). Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch. Clin. Neuropsychol. 21, 91–99. doi: 10.1016/j.acn.2005.08.001
Schimmel, H. (1967). The (±) reference: Accuracy of estimated mean components in average response studies. Science 157, 92–94. doi: 10.1126/science.157.3784.92
Schroeder, R. W., Twumasi-Ankrah, P., Baade, L. E., and Marshall, P. S. (2012). Reliable digit span: A systematic review and cross-validation study. Assessment 19, 21–30. doi: 10.1177/1073191111428764
Schrupp, L. E., Ciuffreda, K. J., and Kapoor, N. (2009). Foveal versus eccentric retinal critical flicker frequency in mild traumatic brain injury. Optometry 80, 642–650. doi: 10.1016/j.optm.2009.04.097
Shiramizu, H., Masuko, A., Ishizaka, H., Shibata, M., Atsumi, H., Imai, M., et al. (2008). Mechanism of injury to the corpus callosum, with particular reference to the anatomical relationship between site of injury and adjacent brain structures. Neurol. Med. Chir. 48, 1–7. doi: 10.2176/nmc.48.1
Smith, A. M., Stuart, M. J., Roberts, W. O., Dodick, D. W., Finnoff, J. T., Jorgensen, J. K., et al. (2017). Concussion in ice hockey: Current gaps and future directions in an objective diagnosis. Clin. J. Sport Med. 27, 503–509. doi: 10.1097/JSM.0000000000000412
Sohn, M. N., Virani, S., Carlson, H. L., MacPhail, S., Low, T. A., Plourde, V., et al. (2020). Microstructure of the corpus callosum long after pediatric concussion. J. Int. Neuropsychol. Soc. 26, 763–775. doi: 10.1017/S1355617720000223
Tate, D. F., Gusman, M., Kini, J., Reid, M., Velez, C. S., Drennon, A. M., et al. (2017). Susceptibility weighted imaging and white matter abnormality findings in service members with persistent cognitive symptoms following mild traumatic brain injury. Mil. Med. 182, e1651–e1658. doi: 10.7205/MILMED-D-16-00132
Taylor, C. A., Bell, J. M., Breiding, M. J., and Xu, L. (2017). Traumatic brain injury–related emergency department visits, hospitalizations, and deaths–United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16. doi: 10.15585/mmwr.ss6609a1
Tombaugh, T. N. (1997). The test of memory malingering (TOMM): Normative data from cognitively intact and cognitively impaired individuals. Psychol. Assess. 9, 260–268. doi: 10.1037/1040-3590.9.3.260
Viano, D. C., Casson, I. R., Pellman, E. J., Zhang, L., King, A. I., and Yang, K. H. (2005). Concussion in professional football: Brain responses by finite element analysis: Part 9. Neurosurgery 57, 891–916. doi: 10.1227/01.NEU.0000186950.54075.3B
Westerhausen, R., Kreuder, F., Woerner, W., Huster, R. J., Smit, C. M., Schweiger, E., et al. (2006). Interhemispheric transfer time and structural properties of the corpus callosum. Neurosci. Lett. 409, 140–145. doi: 10.1016/j.neulet.2006.09.028
Womack, K. B., Paliotta, C., Strain, J. F., Ho, J. S., Skolnick, Y., Lytton, W. W., et al. (2017). Measurement of peripheral vision reaction time identifies white matter disruption in patients with mild traumatic brain injury. J. Neurotrauma 34, 1539–1545. doi: 10.1089/neu.2016.4670
Xiong, K., Zhu, Y., Zhang, Y., Yin, Z., Zhang, J., Qiu, M., et al. (2014). White matter integrity and cognition in mild traumatic brain injury following motor vehicle accident. Brain Res. 1591, 86–92. doi: 10.1016/j.brainres.2014.10.030
Yadav, N. K., and Ciuffreda, K. J. (2015). Objective assessment of visual attention in mild traumatic brain injury (mTBI) using visual-evoked potentials (VEP). Brain Inj. 29, 352–365. doi: 10.3109/02699052.2014.979229
Yeates, K. O., Taylor, H. G., Rusin, J., Bangert, B., Dietrich, A., Nuss, K., et al. (2009). Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123, 735–743. doi: 10.1542/peds.2008-1056
Keywords: concussion, corpus callosum, interhemispheric transfer time, adolescent, event-related potential, neurocognitive functioning
Citation: Christensen BA, Clark B, Muir AM, Allen WD, Corbin EM, Jaggi T, Alder N, Clawson A, Farrer TJ, Bigler ED and Larson MJ (2023) Interhemispheric transfer time and concussion in adolescents: A longitudinal study using response time and event-related potential measures. Front. Hum. Neurosci. 17:1161156. doi: 10.3389/fnhum.2023.1161156
Received: 08 February 2023; Accepted: 07 March 2023;
Published: 28 March 2023.
Edited by:
Dahlia Zaidel, University of California, Los Angeles, United StatesReviewed by:
Leonardo Jose Trejo, Pacific Development and Technology, LLC, United StatesDmitri Poltavski, University of North Dakota, United States
Copyright © 2023 Christensen, Clark, Muir, Allen, Corbin, Jaggi, Alder, Clawson, Farrer, Bigler and Larson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Larson, bWljaGFlbF9sYXJzb25AYnl1LmVkdQ==
 Benjamin A. Christensen1,2
Benjamin A. Christensen1,2 Erin D. Bigler
Erin D. Bigler Michael J. Larson
Michael J. Larson