
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hum. Neurosci., 21 April 2023
Sec. Brain Imaging and Stimulation
Volume 17 - 2023 | https://doi.org/10.3389/fnhum.2023.1160237
This article is part of the Research TopicDeep Brain Stimulation Think Tank: Updates in Neurotechnology and Neuromodulation, Volume IVView all 17 articles
 Marshall T. Holland1
Marshall T. Holland1 Abraham Alvarado-Gonzalez2,3
Abraham Alvarado-Gonzalez2,3 Joshua K. Wong2,3
Joshua K. Wong2,3 Leonardo Brito de Almeida4
Leonardo Brito de Almeida4 Aparna Wagle Shukla2,3
Aparna Wagle Shukla2,3 Wissam Deeb5
Wissam Deeb5 Addie Patterson2,3
Addie Patterson2,3 Michael S. Okun2,3
Michael S. Okun2,3 Kelly D. Foote2,3*
Kelly D. Foote2,3*Deep brain stimulators (DBS) may fail for a multitude of reasons. We present a 79-year-old Parkinson's disease patient who suffered a DBS failure following impulse generator (IPG) replacement surgery due to the IPG flipping within an expanded capsular pocket. This creation of the pocket was unintentional, and the pocket formed around an undiagnosed postoperative hemorrhage. The syndrome could be considered “Twiddler-like” because it resulted in device flipping. There were, however, many characteristic differences between our case and classical Twiddler's syndrome. DBS neurostimulator failure due to hematoma induced device flipping should be suspected when device interrogation is impossible or there are abnormally high impedances across multiple DBS lead contacts. A plain film X-ray series should be ordered and can be useful in providing radiological evidence of device flipping. In cases like ours the extensive braiding encountered in Twiddler's syndrome may be absent. Anchoring the IPG to a deep fascial layer as well as the use of an antimicrobial pouch are two methods that may be employed to prevent or to treat this complication.
A deep brain stimulator (DBS) device consists of an intracranially placed lead, a subcutaneous extension wire, and implantable pulse generator (IPG). The IPG is most commonly placed in the subclavicular location. A minority of patients will undergo IPG placement in an abdominal location. The clinician must be able to connect and to facilitate communication with the device through a handheld programmer in order to pursue device maintenance and programming. In the case of a rechargeable device, the patient must be able to connect to the device to enable the wireless charging function. If the device flips over, it may not be accessible for programming or for charging. We report an unusual case of neurostimulator device failure due to “flipping” over in an expanded subcutaneous capsular pocket that formed around an undiagnosed postoperative hematoma.
A 79-year-old woman with advanced Parkinson's disease (PD) and BMI of 32.3 presented for troubleshooting of her DBS device. Her first symptom of PD was a right upper extremity tremor. As her disease progressed, she developed worsening tremor in both upper extremities, dystonia, and cramping of the right foot. She also progressed to develop motor fluctuations, severe bradykinesia, and dyskinesias. She did not have any significant psychiatric co-morbidities such as obsessive compulsive disorder. Significant dementia was not identified on neuropsychological screening and she was deemed a good candidate for DBS. The patient did not have any history of bleeding diathesis and was not taking any antiplatelet or anticoagulation medications. She underwent bilateral globus pallidus interna (GPi) DBS implantation without complication and the IPG (non-rechargeable) was placed in her abdomen which was her preference for cosmesis. During elective replacement of the IPG for battery depletion she requested placement of a rechargeable neurostimulator. Prior to replacement, the patient had not experienced any recent weight loss. A new, more superficial, pocket for the device was created to accommodate charging. The device was tested intraoperatively and found to have normal impedances and full wireless pairing with the external charging device was confirmed. The device was anchored to the underlying tissue with 2-0 silk at the two anchoring sites in the standard fashion.
One month following the neurostimulator replacement surgery, the patient alerted the clinic that she had (for a week) been unable to communicate with or charge her device. During evaluation in clinic the device could not be interrogated, and programming was not possible. There were no abnormal physical exam findings. Plain film X-rays revealed the IPG had flipped 180° along its long axis (see Figure 1). Upon palpation of pulse generator site, we were unable to manually induce a flip of the device. The patient returned to the surgical suite that week where upon exploration of the IPG pocket, a large brown liquefied hematoma was observed. Following drainage, a large capsule was encountered. The IPG was restored to the correct orientation and replaced in a revised pocket, which was made smaller, and the IPG was anchored to the anterior abdominal wall with 2-0 silk at both anchoring sites on the IPG. Intraoperative interrogation of the device demonstrated normal functioning. Subsequent discussion with the patient revealed that there was swelling at the surgical site several days following the IPG replacement surgery, but denied any significant bruising. However, she did not seek care for the swelling until there was a failure to charge the device. Additionally, the patient could not recall any trauma to the abdomen or surgical site following the index operation prior to revision surgery. Six months after the intervention, the patient continued to be able to interrogate and charge her device and was receiving good therapeutic benefit from the system.
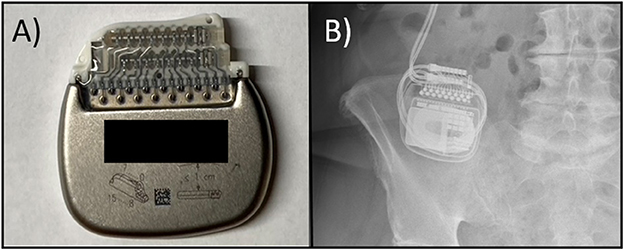
Figure 1. (A) Photo of a rechargeable IPG with the expected normal positioning of the DBS leads which exit to the right of the device; the device label is noted to have an upward orientation with the manufacturing label intentionally obscured, (B) A plain film X-ray series from our case demonstrating the abnormal insertion of the leads on the left side suggestive of flipping.
We observed a rare pocket hematoma resulting in neurostimulator (IPG) flipping with device failure. This complication manifested with an inability of the patient to recharge her device. We hypothesize that hemorrhage created an acute gelatinous hematoma that locked the IPG in a partially flipped position, facilitating formation of a fibrous capsule around the IPG and hematoma. The evolution into a chronic liquid hematoma likely allowed the IPG to mobilize within the enlarged capsule, rotate, and then become lodged in a suboptimal position for device communication, charging, and programming. Figure 2 provides a summary of the events likely leading to device failure. One could also speculate that the device became mobile following surgery, flipped, and initiated a slow bleed leading to a hematoma and enlarged pulse generator capsule. This would result in a similar situation of hypermobility, flipping of the device, and ultimately device failure due to an inability to charge or program the device once settled in the inappropriate position. Both the abdominal placement of the IPG and the patient's obese BMI of 32.3 put her at risk for this to occur (Burdick et al., 2010).
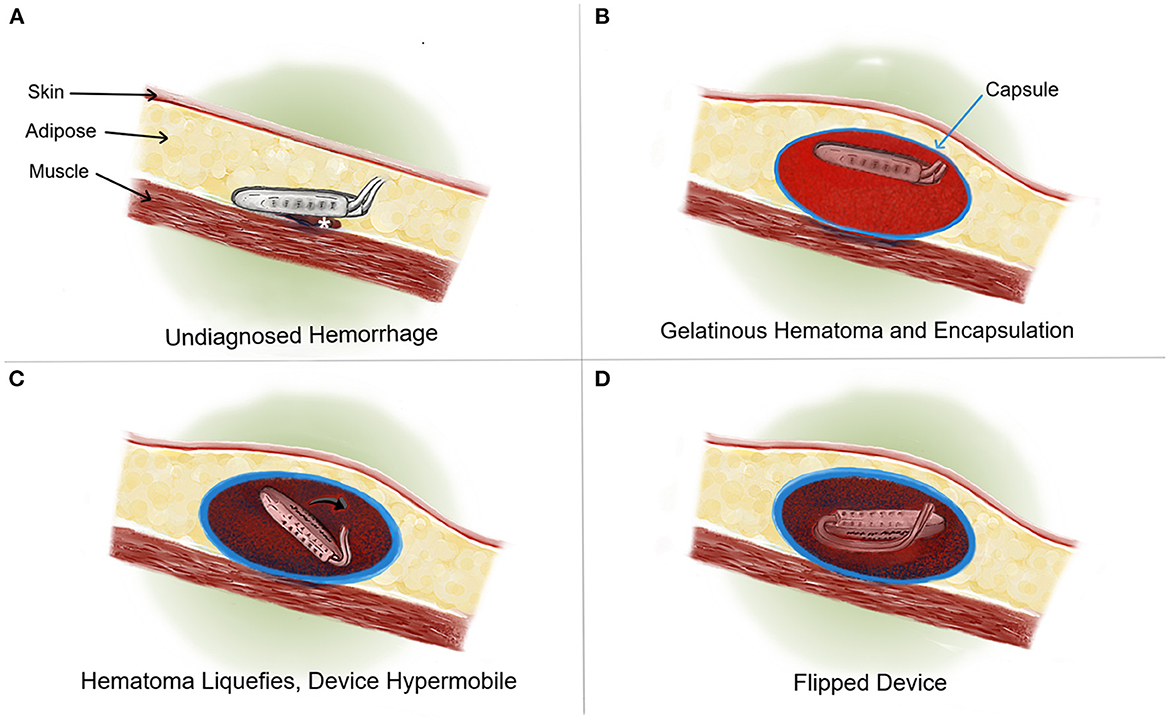
Figure 2. Cross sectional rostral to caudal view medical illustration. (A) An example of an IPG implanted with a resulting bleed (*). (B) Formation of a solid, gelatinous clot which expands until cessation of bleeding. A fibrous capsule is formed around the IPG and clot over the course of several weeks. At this time the IPG remains in appropriate orientation that facilitates interrogation and charging. (C) Over time, the blood clot evolves, breaking down into liquid blood. This scenario results in the hypermobility of the IPG (→) within a now enlarged and well-formed capsule. (D) The hypermobile IPG is “flipped” and cannot be charged by the patient or programmed by the clinician.
This phenomenon resembles Twiddler's syndrome (TS), however based on its characteristics it would not qualify for this diagnosis. First reported in 1968, shortly after the introduction of implantable pacemakers (Bayliss et al., 1968), TS is a rare complication caused by repeated flipping of the neurostimulator within the implanted pocket. Traditionally, TS flipping due to the unintentional manipulating or picking of the device (i.e., “twiddling”). The estimated TS prevalence in cardiac pacemakers is 1% of all implantation malfunctions (Hill, 1987). In neurostimulation, TS has been estimated to account for 1.3% of all DBS malfunctions (Burdick et al., 2010). TS has been demonstrated in the spinal cord stimulation (Son et al., 2018) and the vagal nerve stimulation literature (Trout et al., 2013). Risk factors for TS include surgical technique, unconscious flipping of the device by the patient (Menghetti et al., 2014), obese body habitus (Femenia et al., 2010), early return to exercise (Bracke et al., 2005), and the shape of the device (Gul et al., 2017). Obsessive compulsive symptoms may also contribute and the TS patient may not be conscious that they are flipping the device (Femenia et al., 2010; Moliz et al., 2015). It has been postulated that the abdominal pulse generator location, as was the location in our patient, may be more prone to flipping then the chest site (Boyle et al., 1998; Burdick et al., 2010; Gelabert-Gonzalez et al., 2010). Given the many potential reasons that could lead to device hypermobility, flipping, and failure, we believe the field should evaluate the use of the term TS to describe this phenomenon.
The repetitive flipping of the devices usually presents as a loss of benefit. It may lead to hardware failure, often associated with out-of-range elevated impedances discovered to be present across all electrode contacts (open-circuit), and potentially associated with an inability to communicate with or to charge the device. TS can result in lead fracture or migration. Although the flipping occurs in the pocket, the lead damage is often rostral to the IPG and may be in the nuchal region. Though our case may not be due to twiddling, as our patient denied any manipulation of the device after surgery, the result of the single flip in orientation due to a hematoma resulted in Twiddler-like manifestations.
The workup for device flipping or TS includes interrogation of the device, specifically searching for an open or short circuit, as well as obtaining plain film X-rays. The imaging usually reveals an inappropriate position of the neurostimulator that “flipped” along the long axis (see Figure 1). The electrical leads may be dislodged or displaced and there may possibly be twisting or braiding of the extension wire although any braiding or twisting is usually minimal as the device usually flips only once and at the rotation is 180 degrees or less. Prevention and treatment are similar with the goal of firmly securing the neurostimulator within the device pocket. Anchoring the IPG to a strong fascial layer with a non-absorbable suture has been recommended (Sobstyl et al., 2017). An antimicrobial pouch can also be used to decrease IPG mobility (Osoro et al., 2018). The pouch is thought to manifest its effectiveness by occupying more space in the pocket, and thus inducing a more robust inflammatory response. It may also work through increasing friction between the IPG-surface and the surrounding tissue (Shandling et al., 1991; Osoro et al., 2018).
This report demonstrates a previously undescribed and unusual presentation of device failure due to a postoperative implantation hematoma and expanded capsule. Prior literature demonstrates that all neurostimulator systems retain the risk for device flipping or patient twiddling leading to device failure. A strength of this report is our presentation of mitigation techniques to prevent this complication. While we are limited to our timepoints of evaluation in this case, as we did not have the opportunity to evaluate the patient when she noted the swelling, we were able to observe the consequences of this with the expanded device capsule.
In conclusion, we report a novel cause for IPG flipping. It is unknown whether this complication will be more common in the abdominal or subclavicular IPG location and future reports may help to clarify this point. Utilizing a technique to reduce mobility of the neurostimulator may reduce this potential complication.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MH, AA-G, MO, and KF conceptualized the study. MH and AA-G gathered the data. MH wrote the first draft of the study. All authors provided crucial input about the study, critically evaluated, edited, and approved the final version of the manuscript.
MO serves as a consultant for the Parkinson's Foundation and has received research grants from NIH, Parkinson's Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. MO's DBS research was supported by: NIH R01 NR014852 and R01NS096008. MO is PI of the NIH R25NS108939 Training Grant. MO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford, and Cambridge (movement disorders books). MO is an associate editor for New England Journal of Medicine Journal Watch Neurology. MO has participated in CME and educational activities on movement disorders sponsored by the Academy for Healthcare Learning, PeerView, Prime, QuantiaMD, WebMD/Medscape, Medicus, MedNet, Einstein, MedNet, Henry Stewart, American Academy of Neurology, Movement Disorders Society, and by Vanderbilt University. The institution and not MO receives grants from Medtronic, Abbvie, Boston Scientific, Abbott, and Allergan and the PI has no financial interest in these grants. MO has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations. LA has worked as an educational consultant and has participated in advisory board, therefore receiving honoraria for his activities, for Medtronic and Boston Scientific. WD has received training grant funding from the Tourette Association of America, American Brain Foundation, and Dystonia Medical Research Foundation. He received royalties for publication from Robert Rose, Inc. He received honoraria for a consultation for Medtronic, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2023.1160237/full#supplementary-material
Bayliss, C. E., Beanlands, D. S., and Baird, R. J. (1968). The pacemaker-twiddler's syndrome: A new complication of implantable transvenous pacemakers. Can. Med. Assoc. J. 99, 371–373.
Boyle, N. G., Anselme, F., Monahan, K. M., Beswick, P., Schuger, C. D., Zebede, J., et al. (1998). Twiddler's syndrome variants in ICD patients. Pacing Clin. Electrophysiol. 21, 2685–2687. doi: 10.1111/j.1540-8159.1998.tb00049.x
Bracke, F., van Gelder, B., Dijkman, B., and Meijer, A. (2005). Lead system causing twiddler's syndrome in patients with an implantable cardioverter-defibrillator. J. Thorac. Cardiovasc. Surg. 129, 231–232. doi: 10.1016/j.jtcvs.2004.10.008
Burdick, A. P., Okun, M. S., Haq, I. U., Ward, H. E., Bova, F., Jacobson, C. E., et al. (2010). Prevalence of Twiddler's syndrome as a cause of deep brain stimulation hardware failure. Stereotact. Funct. Neurosurg. 88, 353–359. doi: 10.1159/000319039
Femenia, F., Florentino, C., Arrieta, M., and Arce, M. (2010). Iatrogenic Twiddler's syndrome: Case report and proposed experimental model. Indian Pacing Electrophysiol. J. 10, 517–521.
Gelabert-Gonzalez, M., Relova-Quinteiro, J. L., and Castro-Garcia, A. (2010). “Twiddler syndrome” in two patients with deep brain stimulation. Acta Neurochir. 152, 489–491. doi: 10.1007/s00701-009-0366-6
Gul, E. E., Boles, U., Haseeb, S., Glover, B., Simpson, C., Baranchuk, A., et al. (2017). “Spontaneous Twiddler's” syndrome: The importance of the device shape. Pacing Clin. Electrophysiol. 40, 326–329. doi: 10.1111/pace.12974
Hill, P. E. (1987). Complications of permanent transvenous cardiac pacing: A 14-year review of all transvenous pacemakers inserted at one community hospital. Pacing Clin. Electrophysiol. 10, 564–570. doi: 10.1111/j.1540-8159.1987.tb04521.x
Menghetti, C., Zekaj, E., Saleh, C., Porta, M., and Servello, D. (2014). How to avoid Twiddler's syndrome in deep brain stimulation for dystonia? Neuromodulation 17, 198–199. doi: 10.1111/ner.12067
Moliz, N., Katati, M. J., Iañez, B., García, A., Yagui, E., and Horcajadas, Á. (2015). Twiddler's syndrome in a patient with obsessive-compulsive disorder treated with deep brain stimulation. Neurocirugia 26, 196–199. doi: 10.1016/j.neucir.2014.11.001
Osoro, M., Lorson, W., Hirsh, J. B., and Mahlow, W. J. (2018). Use of an antimicrobial pouch/envelope in the treatment of Twiddler's syndrome. Pacing Clin. Electrophysiol. 41, 136–142. doi: 10.1111/pace.13259
Shandling, A. H., Ellestad, M. H., Castellanet, M. J., and Messenger, J. C. (1991). Dacron-woven pacemaker pouch. Influence on long-term pacemaker mobility. Chest 99, 660–662. doi: 10.1378/chest.99.3.660
Sobstyl, M. R., Zabek, M., Brzuszkiewicz-Kuzmicka, G., and Pasterski, T. (2017). Dual anchor internal pulse generator technique may lower risk of Twiddler's syndrome: A case series and literature review. Neuromodulation 20, 606–612. doi: 10.1111/ner.12581
Son, B. C., Choi, J. G., and Ha, S. W. (2018). Twiddler's syndrome: A rare hardware complication in spinal cord stimulation. Asian J. Neurosurg. 13, 403–406. doi: 10.4103/ajns.AJNS_147_16
Keywords: deep brain stimulation, Twiddler's syndrome, neurostimulator failure, pocket hematoma, impulse generator
Citation: Holland MT, Alvarado-Gonzalez A, Wong JK, Almeida LBd, Wagle Shukla A, Deeb W, Patterson A, Okun MS and Foote KD (2023) Hematoma-induced Twiddler-like phenomenon as a presentation of DBS hardware failure: Case report. Front. Hum. Neurosci. 17:1160237. doi: 10.3389/fnhum.2023.1160237
Received: 06 February 2023; Accepted: 09 March 2023;
Published: 21 April 2023.
Edited by:
Umer Akbar, Brown University, United StatesReviewed by:
Oliver Phillips, Cleveland Clinic, United StatesCopyright © 2023 Holland, Alvarado-Gonzalez, Wong, Almeida, Wagle Shukla, Deeb, Patterson, Okun and Foote. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly D. Foote, Zm9vdGVAbmV1cm9zdXJnZXJ5LnVmbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.