- 1Department of Psychosocial Rehabilitation, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
- 2Department of Rehabilitation, School of Allied Health Sciences, Kitasato University, Sagamihara, Kanagawa, Japan
Backgrounds: Cancer survivors suffer from specific symptoms known as chemotherapy-induced cognitive impairments (CICIs). CICIs are difficult to capture with existing assessments such as the brief screening test for dementia. Although recommended neuropsychological tests (NPTs) exist, international consensus and shared cognitive domains of assessment tools are unknown. The aim of this scoping review was as follows: (1) to identify studies that assess CICIs in cancer survivors; (2) to identify shared cognitive assessment tools and domains by mapping the domains reported in studies using the International Classification of Functioning, Disability and Health (ICF) framework.
Methods: The study followed the recommendations made by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews. We searched the following three databases through October 2021: PubMed, CINAHL, and Web of Science. Prospective longitudinal or cross-sectional studies were selected to determine CICI-specific assessment tools for adult cancer survivors.
Results: Sixty-four prospective studies (36 longitudinal studies and 28 cross-sectional studies) were included after checking for eligibility. The NPTs were divided into seven main cognitive domains. The specific mental functions were often used in the order of memory, attention, higher-level cognitive functions, and psychomotor functions. Perceptual functions were used less frequently. In some ICF domains, shared NPTs were not clearly identified. In some different domains, the same NPTs were used, such as the trail making test and the verbal fluency test. When the association between the publishing year and the amount of NPT use was examined, it was found that the amount of tool use tended to decline over the publication years. The Functional Assessment of Cancer Therapy-Cognitive function (FACT-Cog) was a shared consensus tool among the patient-reported outcomes (PROs).
Conclusion: Chemotherapy-induced cognitive impairments are currently gaining interest. Shared ICF domains such as memory and attention were identified for NPTs. There was a gap between the publicly recommended tools and the tools actually used in the studies. For PROs, a clearly shared tool, FACT-Cog, was identified. Mapping the domains reported in studies using the ICF can help in the process of reviewing consensus on which NPTs may be used to target cognitive domains.
Systematic review registration: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000053710, identifier UMIN000047104.
1. Introduction
There has been a continuous increase in cancer patients worldwide, with 19.3 million new cancer patients, and almost 10 million cancer deaths, as estimated in 2020 (Sung et al., 2021). With the early detection of cancer and state-of-the-art treatments, the number of cancer survivors has tended to increase. Common impairments in cancer survivors are general physical impairments (such as somatic pain, fatigue, weakness, and deconditioning), specific physical impairments (such as lymphedema, shoulder pain, and sensory deficits), and psychosocial functional impairments (Silver et al., 2013). With many cancer survivors expected to return to work, or engage with society, the presence of cognitive impairments can largely inhibit their ability to do so.
“Cancer-related cognitive impairment (CRCI)” has been used as a general term for impairments in patients with cancer. This includes the many factors influencing cognitive function including cancer, cancer treatments (surgery, radiotherapy, chemotherapy, targeted therapy, hormonal therapy, and immunotherapy), psychological factors (depression, anxiety, and fatigue), genetic polymorphisms, and psychosocial factors (education level, cognitive reserve, and age) (Lange et al., 2019). Understanding the phenomenon of CRCI is getting more difficult due to the confounding factors. CICIs are the most frequently encountered and unresolved treatment-related consequence. Several review studies have investigated not only the effects of chemotherapy, but also the cognitive dysfunction associated with concomitant radiation and hormone therapy (Lindner et al., 2014; Bernstein et al., 2017; Bray et al., 2018). Focusing on CICIs may lead to a stereotypical understanding of the phenomenon of CRCI.
Previous studies have reported that cancer survivors experience cognitive decline in the range of 16–75%, before and after chemotherapy (Wieneke and Dienst, 1995; Wefel et al., 2004; Fan et al., 2005; Janelsins et al., 2017). This discrepancy in the range of estimates of cognitive impairments may be due to lack of widely shared cognitive assessment tools (Whitford et al., 2020). Neuropsychological tests (NPTs) and patient-reported outcomes (PROs) are frequently used to assess the CICIs. NPTs provide an objective evaluation of generalized or specific cognitive function in brain-damaged patients. Three NPTs have been recommended for detecting the CRCI by the International Cognition and Cancer Task Force (ICCTF): trail making test (TMT), Hopkins Verbal Learning Test-revised (HVLT-R), and the controlled oral word test (COWA) (Wefel et al., 2011). In contrast, subjective evaluations, such as cognitive complaints, are acknowledged as crucial evaluations. This is because there have been reports of difficulties in real-life situations even though the traditional NPTs have not detected clear cognitive impairments. PROs are a subjective assessment tool that can most sensitively capture cognitive changes and complaints. On the downside, PROs are sensitive to psychosocial effects such as anxiety and depression (Janelsins et al., 2017). Due to cultural and regional diversity, the NPTs and PROs in use are expected to have a wide range of variations. In this respect, it is necessary to investigate what assessment tools are being used as international consensus tools. Additionally, the ICCTF recommends that CRCI studies be investigated as follows: (1) longitudinal studies, (2) compared to healthy controls, and (3) correlated with neuroimaging (Deprez et al., 2018). In particular, neuroimaging assessment can reveal structural changes in diffusion tensor imaging associated with chemotherapy (Deprez et al., 2013). Therefore, we included the studies that employed neuroimaging evaluations in this review.
Although various classification methods have already been proposed for cognitive domains, the use of the International Classification of Functioning, Disability and Health (ICF) framework is a valuable attempt. The ICF framework is the World Health Organization (WHO) classification scheme used internationally to describe the functional status about health conditions (Cieza et al., 2002). The framework consists of the following components: Body functions (b) and Structures (s), Activities and Participation (d), Environmental factors (e), and personal factors. These components are coded using letters and numbers and are widely applied in various research fields including health, medical, welfare, and education (Cieza et al., 2002) (e.g., attention functions, b140). We would be able to share our insights with healthcare professionals from various fields if we could categorize cognitive assessment tools using the cognitive domains of the ICF framework.
The aims of this review were (1) to identify studies that evaluate CICIs; (2) to identify cognitive-related assessment tools used in these studies and the domains they target; and (3) to identify common tools by mapping the cognitive domains reported in the studies using a common ICF framework.
2. Materials and methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) recommendations (the completed checklists were provided in Supplementary material A; Tricco et al., 2018). Our protocol was registered with the University Medical Information Network (UMIN) Center (UMIN000047104).
2.1. Eligibility criteria/information sources
We searched three online databases: PubMed, CINAHL, and Web of Science. Search limits included peer-reviewed studies, whether they were published in English, and were published by October 1, 2021. The inclusion criterion for the present review was that the studies must have principally investigated the effects of chemotherapy on cognition in adults with cancer. However, studies that investigated other cancer treatments separately from chemotherapy were included if they had a distinct chemotherapy treatment group. The exclusion criteria for our study were as follows: (1) no abstract, (2) languages other than English, (3) review article, interventional trial, and case study, (4) experiments with animals, (5) primary brain tumor or brain metastasis, (6) unclear distinction between hormonal therapy and radiotherapy, (7) having any other reason that the reviewer finds to be an obvious content discrepancy. An example of this content inconsistency is when there is a claim to an exclusive examination of the effect of chemotherapy treatment on cognitive impairment, but reviewers have considered the impairment to be strongly influenced by depression, anxiety, fatigue, or anemia.
2.2. Search strategy/data charting
We used the Medical Subject Heading (MeSH) and keyword terms as the targeted search strategy for literature. Subject headings and synonyms were used to expand the search and were combined using boolean operators (i.e., AND, OR) and truncations (i.e., cognit*). The MeSH terms used to search the literature using PubMed and CINAHL online databases were as follows: “cognition disorders”[MeSH Terms] OR (“cognition”[All Fields] AND “disorders”[All Fields]) OR “cognition disorders”[All Fields], AND “neoplasm’s”[All Fields] OR “neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “neoplasm”[All Fields], AND “drug therapy”[Subheading] OR (“drug”[All Fields] AND “therapy”[All Fields]) OR “drug therapy”[All Fields] OR “drug therapy”[MeSH Terms] OR (“drug”[All Fields] AND “therapy”[All Fields]). The keywords hierarchized by MeSH terms are shown in Supplementary material B. Other search terms in the online databases were as follows: “cancer” AND “cognit*” AND “adjuvant chemotherapy” AND “related” OR “induced.”
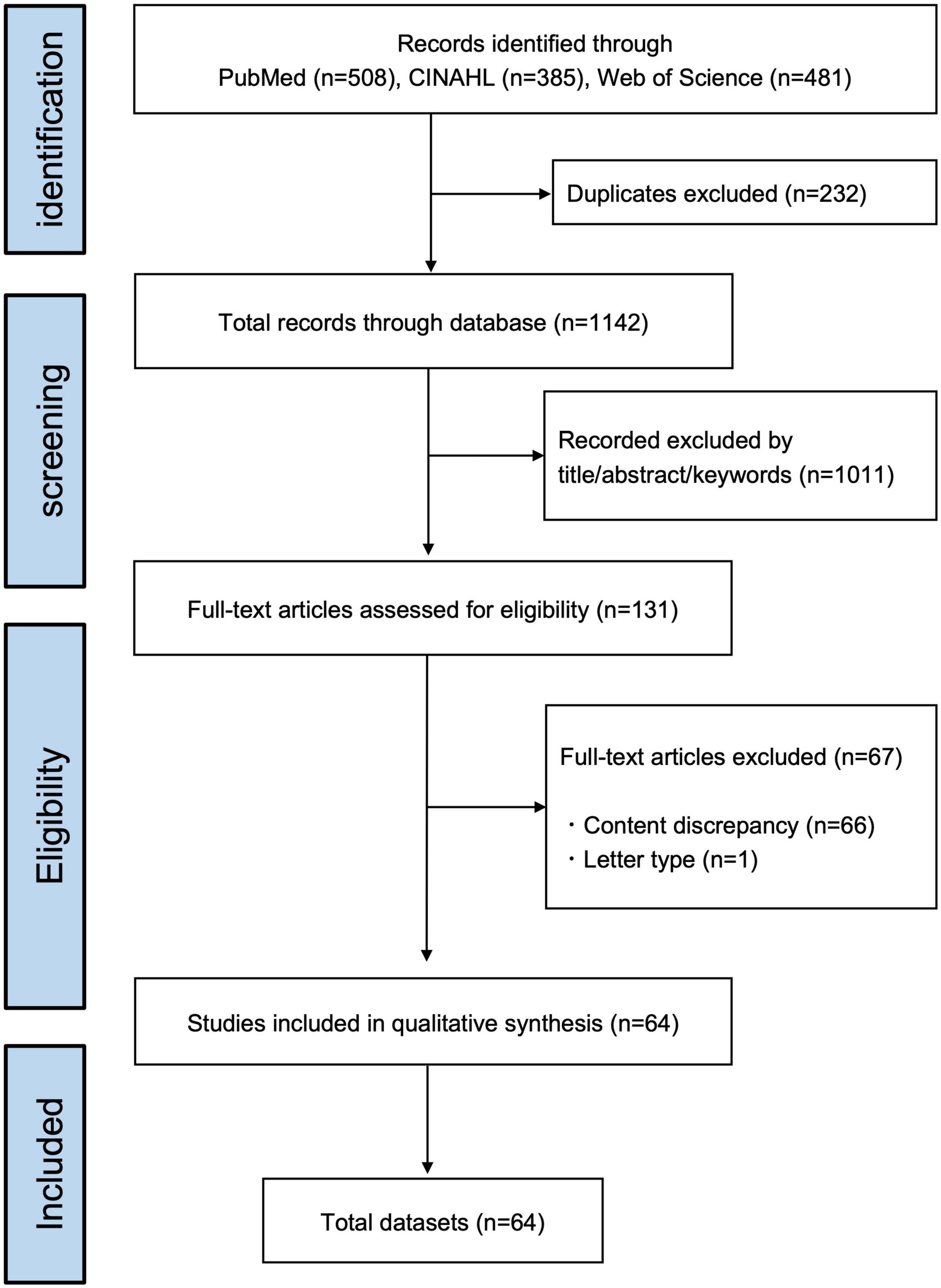
Duplicate articles were excluded from all papers obtained by this search method. The articles were then independently reviewed by two reviewers (KS and SA) to check for the inclusion/exclusion criteria by title, abstract, and keywords. After downloading or ordering the full text of the report, the data were again thoroughly examined and extracted by the two reviewers. In cases where the necessary information for charting was not available, we contacted the authors concerned to identify additional sources. Disagreements between the two reviewers at each step were either resolved by consensus after reviewing the full text or by a procedure in which a third senior researcher (HO) made the final decision.
2.3. Data items/synthesis of results
All data were extracted in equal parts from the reports of two of the review authors (KS and SA). The following information was extracted from each article: first author, year of publication, country of the first author, type of study, sample size at baseline, type of cancer, and full description of the cognitive assessment performed (cognitive assessment tools and instruments used, cognitive domains considered by the authors). The sample size was shown after excluding healthy participants who did not receive chemotherapy. The cognitive assessment tools were organized by the components of standard NPTs as objective assessments, and PROs as subjective assessments. The type of neuroimaging devices used was counted. Psychological assessment tools for depression and anxiety, activities of daily living, and quality of life were not included in the extracted items for this review.
The ICF category codes contained in “mental functions (b110–b199)” were used to classify the domains of NPTs (Cieza et al., 2002). Cognitive assessments were identified as either full assessments, or subtests used as stand-alone assessments. The NPTs were categorized to the following purposes: (1) diagnostic, (2) screening. If the cognitive domains intended by the authors were different, the same assessment tools were organized by the cognitive areas intended by each study. That is, the same evaluation tool may be described in different cognitive domains.
The number of cases in each cognitive domain and the assessment tools in each domain was calculated as percentages. For all data pre-processing, agreement calculations, and figure creations, we used the statistical software JMP® Pro (version 16.1.0) and statistical package R (version 4.1.2)1 (R Core Team, 2018).
3. Results
3.1. Literature search results
The present study yielded a total of 1,374 records published through October 2021. After excluding duplicates (n = 232), 1,142 records remained. Independent screening by title, abstract, and keywords resulted in an initial 131 articles eligible for full-text papers. The information necessary for data charting was available without having to contact the authors. Reasons for exclusion criteria were publications of the content discrepancy (66 studies) and the publication type of letter (one study). After studying the eligibility, 64 papers were included by the two reviewers. The information data sets, which included all extracted data from each article, were summarized in Figure 1 and Supplementary Table 1 (table of characteristics of included studies with reference information).
3.2. Publication characteristics
3.2.1. Years and regions of the publication
The number of studies in the year of issue (divided into 5-year intervals) is shown in Figure 2. In order of most frequently reported, were North America (n = 22, 34.4%), Europe (n = 18; 28.1%), Asia (n = 18; 28.1%), Africa (n = 3, 4.7%), multi-region (n = 2, 3.1%), and South America (n = 1; 1.6%). The categories of regions were described in Supplementary material C.
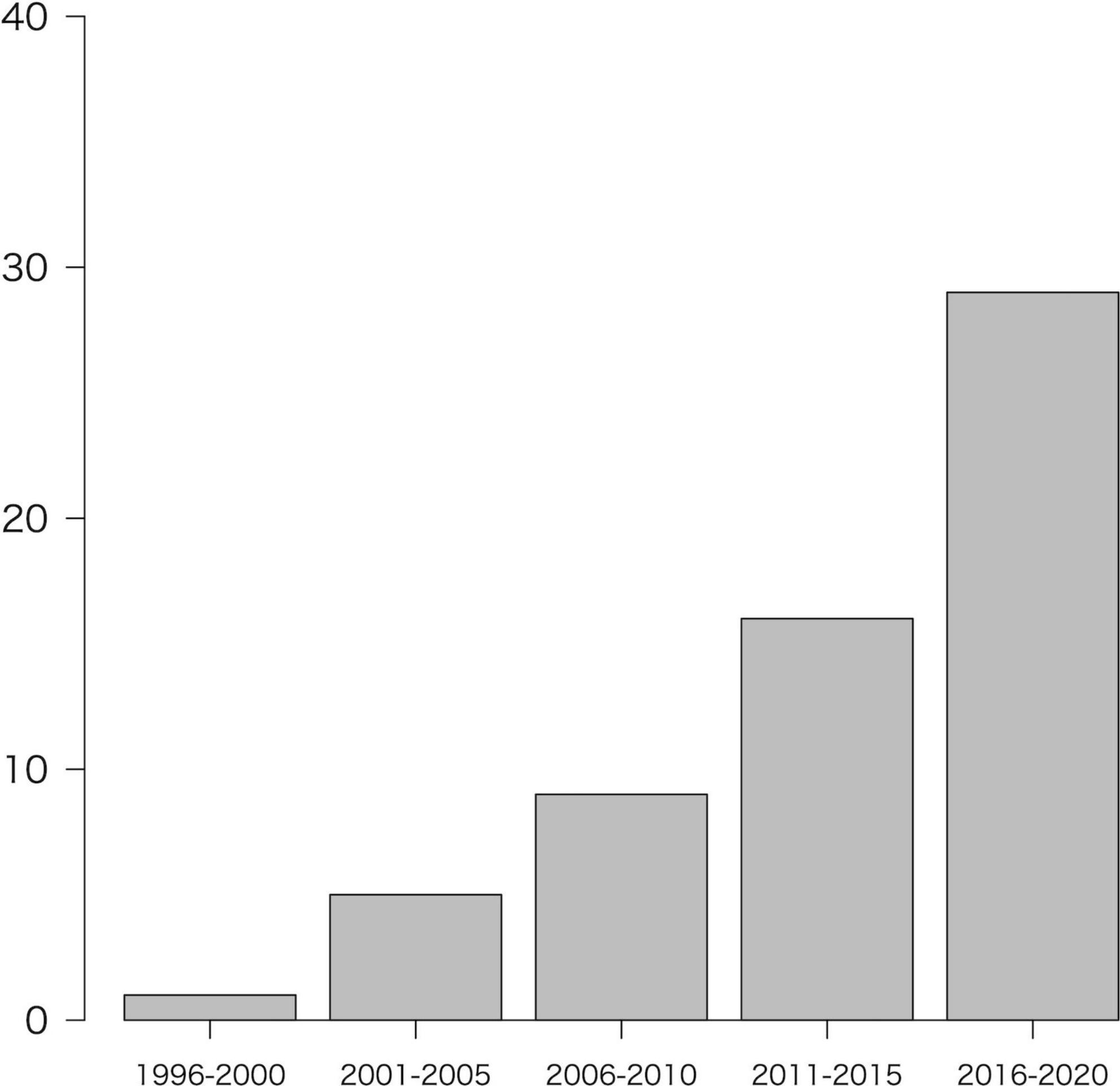
Figure 2. Number of included studies by year of publication. Note that the year 2021 (n = 4) was not included in the figure because it is less than 1 year.
3.2.2. Study designs of publication
With respect to the publication type, 36 articles were longitudinal studies, and 28 articles were cross-sectional studies. Among them, 44 and 20 articles were single-center and multicenter studies, respectively.
3.2.3. Sample size of publication
The total sample size at the baseline evaluation was distributed as follows: less than 50 (n = 32; 50.0%), 50–99 (n = 14; 21.9%), 100–699 (n = 17; 26.6%), and more than 700 (n = 1; 1.6%).
3.2.4. Cancer types studied in publications
The most common types of cancer were breast cancer (n = 44; 68.8%), followed by colorectal cancer (n = 5; 7.8%), lymphoma (n = 2; 3.1%), testicular cancer (n = 2; 3.1%), ovarian cancer (n = 1; 1.6%), and lung cancer (n = 1; 1.6%). The percentage of studies that included different cancer types was 14% (n = 9).
3.3. Neuropsychological assessment tools (NPTs) and cognitive domains
Neuropsychological tests were classified according to the function intended by authors into the following domains: intellectual functions (b117), attention functions (b140), memory function (b144), psychomotor functions (b147), perceptual functions (b156), higher-level cognitive functions (b164), and mental function of language (b167). Those that did not match any of these categories were classified as mental functions, unspecified (b199). The classification procedure was based on the domains that were reported by the authors, and then the semantic categorized terms to the ICF domains were mapped using constant rules. For example, the semantic categories “verbal memory,” “visual memory” and “learning” were mapped to the memory functions domain. The semantic categories “executive function” and “cognitive flexibility” were mapped onto the higher-level cognitive functions domain (Please see Supplementary material D for other classification methods).
Figure 3 showed the distribution of cognitive domains in the NPTs, with their results in the following order: memory functions were the most common (23.8%), followed by attention functions (20.0%), higher-level cognitive functions (14.8%), intellectual functions (14.5%), psychomotor functions (13.2%), mental functions of language (7.1%), and perceptual functions (3.4%).

Figure 3. International Classification of Functioning, Disability and Health (ICF) domain sorting of neuropsychological tools. The ICF codes corresponding to the selected domains are as follows: b114 (memory functions), b140 (attention functions), b164 (higher-level cognitive functions), b117 (intellectual functions), b147 (psychomotor functions), b167 (mental functions of language), and b156 (perceptual functions).
Figure 4 shows the map of the cognitive domains using an ICF framework. The top five tools in each domain were targeted, and those that were used only once or twice were excluded. Among the seven domains, the TMT was frequently used in three domains (attention; n = 7, psychomotor; n = 19, higher-level cognitive function; n = 19). The TMT-part A tool (the task of connecting numbers in sequence) was mainly used in the attention and psychomotor function domains, whereas the TMT-part B tool (the task that connects numbers and letters in alternating sequence) was mainly used in the higher-level cognitive function domain. The verbal fluency test (VFT) and the COWA were highly used in two domains: mental functions of language (VFT; n = 7, COWA; n = 7) and higher-level cognitive function (VFT; n = 10, COWA; n = 3) domains. Both tools assess word fluency, but in terms of the number of VFT and COWA used, COWA was less used in the higher-level cognitive function domain. These tools were mainly used to assess phonemic fluency (i.e., letter words) in the mental functions of language domain and as semantic fluency (i.e., animal words) in the higher-level cognitive function domains. Similarly, the Rey-Osterrieth Complex Figure test (ROCF) was used in both memory function (n = 12) and perceptual function (n = 6) domains. In the perceptual function domain, all used the ROCF-copy subtest, while in the memory functions domain, the ROCF-delayed recall subtest was the tool that was most used. Of the 64 studies included for the current study, 33 used the screening tools (eight used the screening tools only); 54 used the diagnosis tools (29 used the diagnosis tools only). In terms of screening tools, the Mini-Mental State Examination (MMSE) was used most frequently (n = 23) followed by the National Adult Reading Test (NART, n = 7).
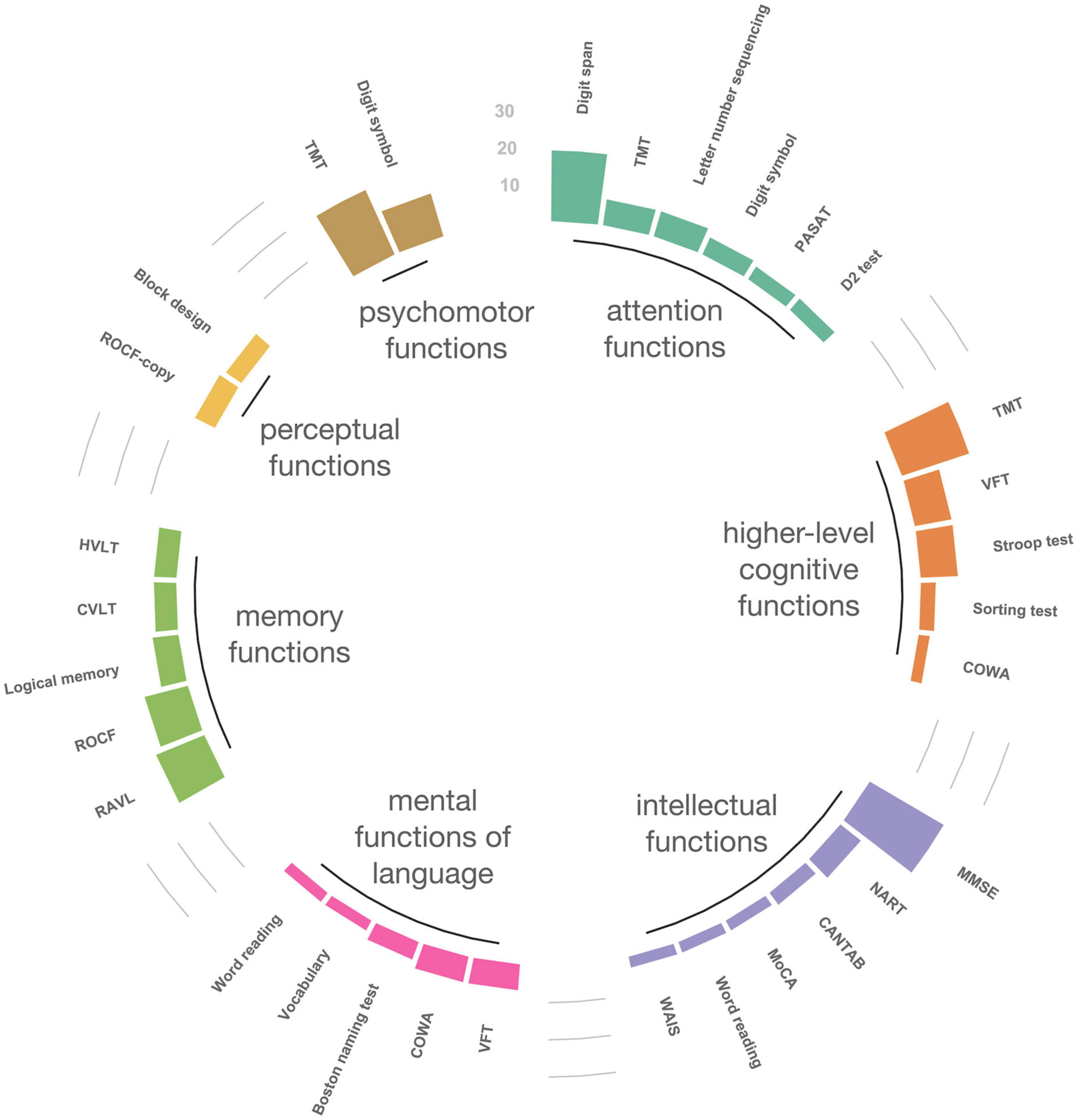
Figure 4. International Classification of Functioning, Disability and Health domain sorting map of neuropsychological tools. The top five tools in each domain were targeted, and those that were used only once or twice were excluded. TMT, trail making test; PASAT, paced auditory serial addition test; VFT, verbal fluency test; COWA, controlled oral word association test; MMSE, Mini-Mental State Examination; NART, National Adult Reading Test; CANTAB, Cambridge Neuropsychological Test Automated Battery; MoCA, Montreal Cognitive Assessment; WAIS, Wechsler Adult Intelligence Scale; RAVL, Rey Auditory Verbal Learning test; ROCF, Rey-Osterrieth Complex Figure test; CVLT, California Verbal Learning Test; HVLT, Hopkins Verbal Learning Test; Digit symbol, digit symbol substitution (coding) test. The following tools are subtests of the WAIS: letter number sequencing, vocabulary, block design. The word reading is a subtest of the Wide Range Achievement Test. Logical memory is a subtest of the Wechsler Memory Scale.
3.4. Subjective cognitive tools/patient-reported outcomes (PROs)
Among PROs, the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) was used in 14 studies, accounting for 56% of all PROs. Other PROs reported in two studies were the Patient Assessment of Own Functioning Inventory (PAOFI), the Cognitive Failures Questionnaire, the Everyday Cognition questionnaire, the Retrospective Memory and Prospective Memory questionnaires, and the Cognitive Problems in Daily Life.
3.5. Neuroimaging devices and measuring methods
Among neuroimaging devices, magnetic resonance imaging (MRI) was the most used (n = 19), followed by positron emission tomography (n = 2) and electroencephalography (n = 1). In the segment of MRI devices, structural and functional imaging evaluations were reported in ten and nine reports, respectively. Regarding measurement methods, seven studies consisted of the voxel-based morphometry (VBM) and three consisted of the diffusion tensor imaging (DTI) for structural MRI studies. Three studies were based on functional MRI (fMRI), and six studies on resting-state fMRI.
3.6. Association between the publishing year and the amount of tool use (including study design and sample size)
Figure 5 showed the association between the publishing year and the amount of tool use, including study design and sample size. In the early 2000s, both single-center and multicenter studies used more than 13 tools in a study. Around 2020, multicenter, longitudinal studies with large sample sizes were being conducted, and one to at most eight tools were used in the studies (Dhillon et al., 2018; Ng et al., 2018; Hormozi et al., 2019; Magnuson et al., 2019; Atallah et al., 2020; Van Dyk et al., 2021).
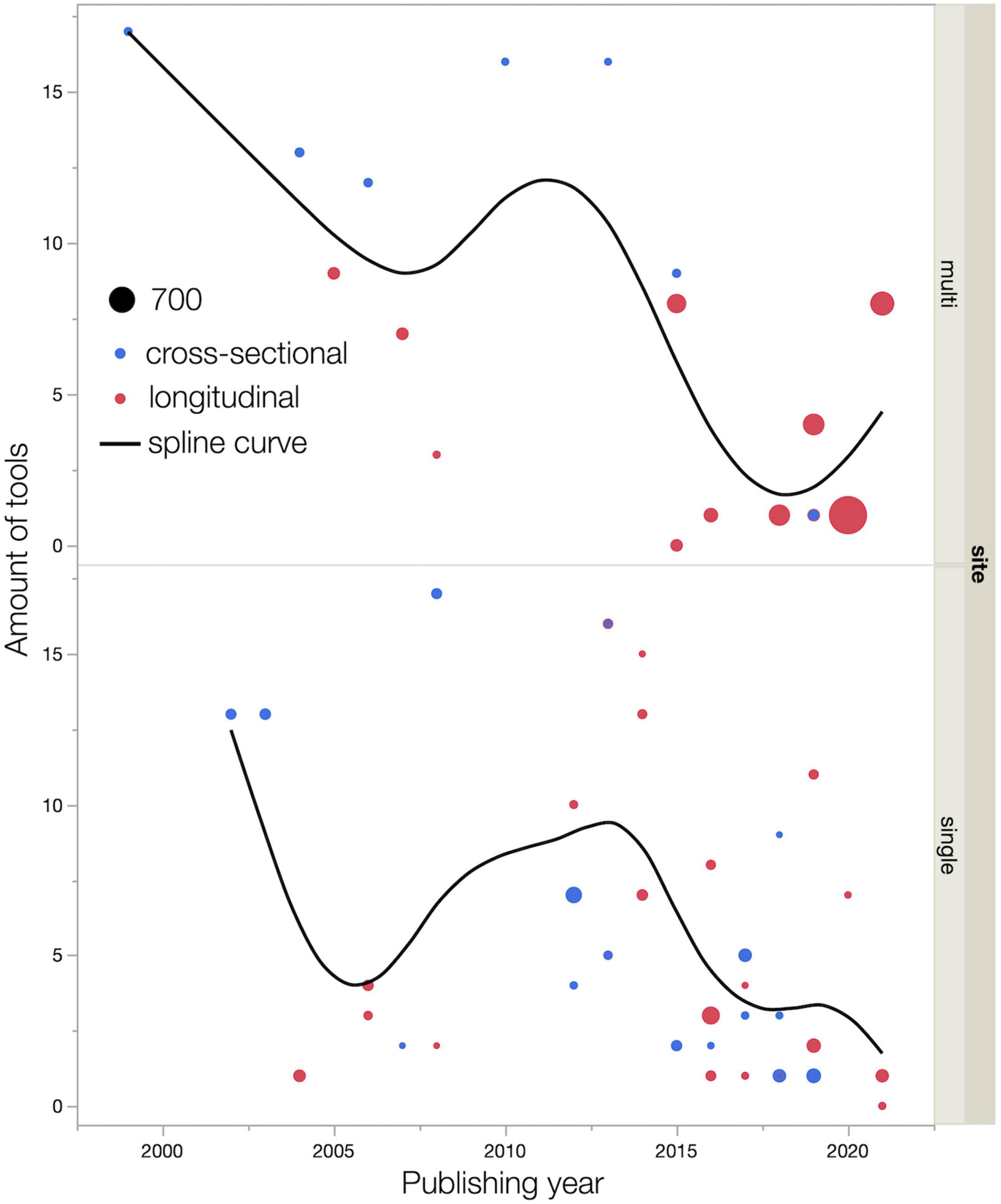
Figure 5. Relationships among publishing year, amount of tools use, and sample size. Dot size indicates sample size. Smoothing spline curves are used for estimating functional relationships between publishing year and amount of tools use. The smoothing parameter λ (lambda) is set at 0.05.
4. Discussion
In the present study, 36 longitudinal studies and 28 cross-sectional studies were systematically identified to investigate the assessment tools and cognitive domains used to assess CICIs, in accordance with PRISMA-ScR recommendations. Mapping the domains reported in studies using the ICF framework in NPTs revealed the extent of agreement between studies on the cognitive domains when each tool was used (Figure 4). The amount of tool use tended to diminish over the publication years. FACT-Cog was commonly used for PROs. Structural and functional studies using MRI together with brain imaging dominated the studies.
Regarding the validity of the extracted articles, recent review articles reported that between 17 and 101 cases of chemotherapy-induced cognitive dysfunction were extracted (Bray et al., 2018; Cerulla Torrente et al., 2020; Dijkshoorn et al., 2021). Because of differences in target disease and assessment tools, it would be difficult to make general comparisons. These studies extracted generally the same articles at the screening phase, and we believe that our search methodology is reasonable. In this study, we adopted studies that used prospective assessment tools to capture chemotherapy-induced cognitive dysfunction. Additionally, the exclusion of studies that did not distinguish between radiation or hormonal therapy was unique; the study by Li et al. (2018) used particularly ideal criteria. Hormonal therapy is particularly difficult to distinguish in the treatment process in breast cancer and prostate cancer. The present study did not include studies on hormone therapy, which may have led to the different eligibility selection of papers from other studies. However, considering the pathogenesis, a clear distinction should be recognized between hormonal therapy and chemotherapy (Mounier et al., 2020). On the other hand, it is noteworthy that breast cancer studies were the most frequently selected, even if hormonal therapy was omitted as much as possible. As a result, this review article may be as specific as possible to the assessment of chemotherapy-induced cognitive dysfunction. The scoping review by Vizer et al. (2022) should be referenced since it focused on studies on the Adolescent and Young Adult (AYA) generation, which was not the focus of the present review.
The most commonly used cognitive domain was memory functions, followed by attention functions, higher-level cognitive functions, and psychomotor functions. Perceptual function was relatively minimal. The results of the present study agreed with those of previous reviews of other domain classifications that have investigated the CRCI (Vardy et al., 2008; Wefel et al., 2011; Cerulla Torrente et al., 2020). Interestingly, the present study found that shared NPTs were not clearly identified in some ICF domains. The same NPTs were used among domains, as shown by the results in Figure 4. This indicates that it can be difficult to categorize a single NPT into a single cognitive domain, and it is worth noting that a single NPT can affect multiple cognitive domains. In the cognitive domains based on the ICF classification we used, the TMT covered three domains (attention, psychomotor, and higher-level cognitive function), while the VFT and COWA covered two cognitive domains, language and higher-level cognitive function. This indicates that most of the cognitive domains to investigate CRCI can be covered by using the three tools (TMT, COWA, HVLT-R) recommended by the ICCTF. In this respect, these NPTs recommended by the ICCTF are reasonable assessments, as they allow for a multi-dimensional view of chemotherapy-induced cognitive functions. Meanwhile, the present review indicates that fewer HVLT-R and COWA were used compared to TMTs. The HVLT-R has similar concepts of the Rey Auditory Verbal Learning test (RAVLT), and the COWA evaluates the same concept as the VFT. Thus, although the names of the NPTs are different, there are cases where the concept to be evaluated is the same. This may be more likely to occur because of language-mediated procedures. Compared to NPTs that do not rely as much on language, NPTs that use language are more difficult to translate and adapt across languages and cultures, resulting in different NPTs with the same concept.
As Figures 2, 5 shows, the number of reports increase over the years, but sample sizes for both cross-sectional and longitudinal studies are generally small. This indicates that, as with CRCI, the research trend on CICIs has attracted interest, but the studies revealed a trend of poor quality of study design. Figure 5 shows that the number of tools used tends to gradually decrease over the years for both single and multicenter studies. Our study indicates that in the early 2000s, more than 13 tools were used to identify CICIs in exploratory research. We also believe that in the 2020s, the number of multicenter longitudinal studies have increased, and the number of tools has decreased. This indicates a trend toward a clear understanding of the NPTs and cognitive domains that need more attention for the CICIs, which was explored by studies in the 2000s. Recent reports have used tools that can assess multiple domains with a single tool, such as the Cambridge Neuropsychological Test Automated Battery (CANTAB) (Vardy et al., 2015; Magnuson et al., 2016; Dhillon et al., 2018; Williams et al., 2018). The results indicate that these personal computer-based assessment batteries are a standardized, multidisciplinary, well-developed single tool that could be widely used in future studies of the CICIs.
Different chemotherapeutics and cancer types may influence different cognitive domains. Some review articles suggest that different chemotherapeutics and cancer types may influence different cognitive domains (Subramaniam et al., 2020; Orszaghova et al., 2021). A previous study of breast cancer survivors reported that taxanes, compared to other cognitive domains, affected attention, psychomotor speed, and memory function (Cerulla et al., 2017). One study of breast cancer survivors with anthracyclines reported effects on verbal memory function using HVLT-R (Kesler and Blayney, 2016). Consecutive colorectal cancer patients receiving 5-fluorouracil and oxaliplatin-based adjuvant chemotherapy showed impaired higher-level cognitive function (executive function), including the VFT, compared to other cognitive domains (Sales et al., 2019). On the level of the biological control, the mechanisms of CICIs are mediated by a variety of factors including: direct neurotoxicity, inhibition of hippocampal neurogenesis, white and gray matter reduction, oxidative stress response, reduced cerebral blood flow, blood-brain barrier damage, neuroinflammation, hormonal changes, decreased hypothalamic-pituitary-adrenal axis activity, and microbiota-gut-brain axis dysfunction (Mounier et al., 2020; Subramaniam et al., 2020; Orszaghova et al., 2021). In recent years, chronic inflammation and neuroinflammatory pathways have been considered the main factors of CICIs. Inflammation-related problems have been shown to be closely related to attentional function in the cognitive domains (Oppegaard et al., 2021). The frequent use of attention domain tools in our current review may show that many researchers in the field support the idea of inflammation-induced attention deficit.
Neuroimaging assists in a more accurate understanding of the NPT results. In a PET study examining different brain activity areas during short-term memory tasks, the most significant difference between the chemotherapy and control groups was in the inferior frontal gyrus (Silverman et al., 2007). MRI-VBM analysis showed decreased gray matter density in the inferior and middle frontal gyrus and cerebellum in breast cancer survivors after chemotherapy. Furthermore, the decrease in gray matter density in the right middle frontal gyrus was related to the dose of chemotherapy for VFT (Li et al., 2018). In other words, decreased activity in the prefrontal cortex may be important for capturing the symptoms of the CICIs. These neuroimaging studies have the potential to identify specific cognitive domains that should be targeted by the NPTs. However, while PET and MRI-based studies are useful in that they can reveal structural brain changes and functional brain changes, they are likely to be costly and unsuitable for general use in clinical care. To address this issue, we anticipate the possibility of adopting portable EEG and near-infrared spectroscopy (NIRS) instruments that are less expensive and constrained in the future to aid in diagnosis as a way to overcome these problems (Jean-Pierre, 2014; Suhaimi et al., 2020).
4.1. Strengths and limitations
This scoping review reported cognitive assessment tools used to assess CICIs in the CRCI. To this end, a cognitive assessment tool for cancer in the domains of the ICF framework was summarized by peer review using the chart method. The ICF framework was used for the international consensus. In recent years, it has also been applied to scoping reviews for the assessment of cognitive function after a stroke (Saa et al., 2019). Furthermore, this study has the unique advantage of examining the trends in reporting not only NPTs, but also PROs and neuroimaging devices for CICIs. However, this scoping review has several limitations.
The main limitation of the present study is that we summarized several reports on different diseases and chemotherapeutic agents rather than focusing on one agent. Second, we did not investigate the influences of cancer-induced fatigue, anxiety, and depression on cognitive function. Other factors such as age, educational history, hormone levels, sleep disturbances, and anemia are also likely to influence cognitive function (Lange et al., 2019; Orszaghova et al., 2021). Therefore, in the future, more rigorous studies controlling for the effects of confounding factors are warranted. Regarding the charting method, this review does not include gray literature. Although gray literature is an important source of information, this study targeted peer-reviewed articles with standard quality assurance of content. Furthermore, we did not assess the quality of the methodology for the selected studies. The review articles are biased toward the female gender as most studies were those of breast cancer patients or were focused on breast cancer. It would be desirable to see more reports on target populations other than breast cancer patients in the future.
5. Conclusion
Interest in the CICIs has increased over time, and the cognitive assessment tools used were mapped in this study. The memory and attention functions were found to be common ICF domains in the NPT. There was a gap between commonly recommended NPTs and tools employed in the study. For PROs, the FACT-Cog was clearly the shared tool used. We believe that integrating assessment tools for CRCIs from the perspective of the ICF domains can address interdisciplinary issues. This research is crucial for multidisciplinary care and rehabilitation treatments for cancer survivors with CICIs.
Author contributions
KS and SA performed the material preparation, data collection, and analysis. KS wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript, contributed to the study conception and design, and read and approved the final manuscript.
Funding
This study was partly supported by the JSPS KAKENHI (Grant number: JP21K20286).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2023.1063674/full#supplementary-material
Footnotes
References
Abebe, E., Tollesa, T., Assefa, M., Tilahun, Z., Dinku, Y., Abebaw, S., et al. (2021). Cognitive functioning and its associated factors among breast cancer patients on chemotherapy at Tikur Anbessa specialized hospital, Addis Ababa Ethiopia: An institution-based comparative cross-sectional study. BMC Cancer 21:1052. doi: 10.1186/s12885-021-08799-0
Ahles, T. A., Li, Y., Mcdonald, B. C., Schwartz, G. N., Kaufman, P. A., Tsongalis, G. J., et al. (2014). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: The impact of APOE and smoking. Psychooncology 23, 1382–1390. doi: 10.1002/pon.3545
Ahles, T. A., Saykin, A. J., Furstenberg, C. T., Cole, B., Mott, L. A., Skalla, K., et al. (2002). Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. 20, 485–493. doi: 10.1200/JCO.2002.20.2.485
Ahles, T. A., Saykin, A. J., Noll, W. W., Furstenberg, C. T., Guerin, S., Cole, B., et al. (2003). The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12, 612–619. doi: 10.1002/pon.742
Andreis, F., Ferri, M., Mazzocchi, M., Meriggi, F., Rizzi, A., Rota, L., et al. (2013). Lack of a chemobrain effect for adjuvant FOLFOX chemotherapy in colon cancer patients. A pilot study. Support. Care Cancer 21, 583–590. doi: 10.1007/s00520-012-1560-2
Andryszak, P., Wiłkość, M., Żurawski, B., and Izdebski, P. (2018). Verbal memory in breast cancer patients treated with chemotherapy with doxorubicin and cyclophosphamide. Eur. J. Cancer Care 27:e12749. doi: 10.1111/ecc.12749
Atallah, M., Cooper, B., Muñoz, R. F., Paul, S. M., Anguera, J., Levine, J. D., et al. (2020). Psychological symptoms and stress are associated with decrements in attentional function in cancer patients undergoing chemotherapy. Cancer Nurs. 43, 402–410. doi: 10.1097/NCC.0000000000000713
Bai, X., Zheng, J., Zhang, B., and Luo, Y. (2021). Cognitive dysfunction and neurophysiologic mechanism of breast cancer patients undergoing chemotherapy based on resting state functional magnetic resonance imaging. World Neurosurg. 149, 406–412. doi: 10.1016/j.wneu.2020.10.066
Baudino, B., D’agata, F., Caroppo, P., Castellano, G., Cauda, S., Manfredi, M., et al. (2012). The chemotherapy long-term effect on cognitive functions and brain metabolism in lymphoma patients. Q. J. Nucl. Med. Mol. Imaging 56, 559–568.
Bender, C. M., Sereika, S. M., Berga, S. L., Vogel, V. G., Brufsky, A. M., Paraska, K. K., et al. (2006). Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology 15, 422–430. doi: 10.1002/pon.964
Bernstein, L. J., Mccreath, G. A., Komeylian, Z., and Rich, J. B. (2017). Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci. Biobehav. Rev. 83, 417–428. doi: 10.1016/j.neubiorev.2017.10.028
Biglia, N., Bounous, V. E., Malabaila, A., Palmisano, D., Torta, D. M., D’alonzo, M., et al. (2012). Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. Eur. J. Cancer Care 21, 485–492. doi: 10.1111/j.1365-2354.2011.01320.x
Bray, V. J., Dhillon, H. M., and Vardy, J. L. (2018). Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J. Cancer Surviv. 12, 537–559. doi: 10.1007/s11764-018-0692-x
Castellon, S. A., Ganz, P. A., Bower, J. E., Petersen, L., Abraham, L., and Greendale, G. A. (2004). Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J. Clin. Exp. Neuropsychol. 26, 955–969. doi: 10.1080/13803390490510905
Cerulla Torrente, N., Navarro Pastor, J. B., and de la Osa Chaparro, N. (2020). Systematic review of cognitive sequelae of non-central nervous system cancer and cancer therapy. J. Cancer Surviv. 14, 464–482. doi: 10.1007/s11764-020-00870-2
Cerulla, N., Arcusa, A., Navarro, J. B., Garolera, M., Enero, C., Chico, G., et al. (2017). Role of taxanes in chemotherapy-related cognitive impairment: A prospective longitudinal study. Breast Cancer Res. Treat. 164, 179–187. doi: 10.1007/s10549-017-4240-6
Chen, B. H. T., Sethi, S. K., Jin, T. H., Patel, S. K., Ye, N. R., Sun, C. L., et al. (2018a). Assessing brain volume changes in older women with breast cancer receiving adjuvant chemotherapy: A brain magnetic resonance imaging pilot study. Breast Cancer Res. 20:38. doi: 10.1186/s13058-018-0965-3
Chen, B. T., Ghassaban, K., Jin, T., Patel, S. K., Ye, N., Sun, C. L., et al. (2018b). Subcortical brain iron deposition and cognitive performance in older women with breast cancer receiving adjuvant chemotherapy: A pilot MRI study. Magn. Reson. Imaging 54, 218–224. doi: 10.1016/j.mri.2018.07.016
Cheng, H. D., Li, W., Gan, C., Zhang, B., Jia, Q. Q., and Wang, K. (2016). The COMT (rs165599) gene polymorphism contributes to chemotherapy-induced cognitive impairment in breast cancer patients. Am. J. Transl. Res. 8, 5087–5097.
Cheng, H. D., Li, W., Gong, L., Xuan, H., Huang, Z. L., Zhao, H., et al. (2017). Altered resting-state hippocampal functional networks associated with chemotherapy-induced prospective memory impairment in breast cancer survivors. Sci. Rep. 7:45135. doi: 10.1038/srep45135
Cheng, H., Yang, Z., Dong, B., Chen, C., Zhang, M., Huang, Z., et al. (2013). Chemotherapy-induced prospective memory impairment in patients with breast cancer. Psychooncology 22, 2391–2395. doi: 10.1002/pon.3291
Cheung, Y. T., Ng, T., Shwe, M., Ho, H. K., Foo, K. M., Cham, M. T., et al. (2015). Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 26, 1446–1451. doi: 10.1093/annonc/mdv206
Cieza, A., Brockow, T., Ewert, T., Amman, E., Kollerits, B., Chatterji, S., et al. (2002). Linking health-status measurements to the international classification of functioning, disability and health. J. Rehabil. Med. 34, 205–210. doi: 10.1080/165019702760279189
Collins, B., Mackenzie, J., Tasca, G. A., Scherling, C., and Smith, A. (2013). Cognitive effects of chemotherapy in breast cancer patients: A dose-response study. Psychooncology. 22, 1517–1527. doi: 10.1002/pon.3163
Conroy, S. K., Mcdonald, B. C., Smith, D. J., Moser, L. R., West, J. D., Kamendulis, L. M., et al. (2013). Alterations in brain structure and function in breast cancer survivors: Effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res. Treat. 137, 493–502. doi: 10.1007/s10549-012-2385-x
Correa, D. D., Root, J. C., Kryza-Lacombe, M., Mehta, M., Karimi, S., Hensley, M. L., et al. (2017). Brain structure and function in patients with ovarian cancer treated with first-line chemotherapy: A pilot study. Brain Imaging Behav. 11, 1652–1663. doi: 10.1007/s11682-016-9608-4
Cruzado, J. A., López-Santiago, S., Martínez-Marín, V., José-Moreno, G., Custodio, A. B., and Feliu, J. (2014). Longitudinal study of cognitive dysfunctions induced by adjuvant chemotherapy in colon cancer patients. Support. Care Cancer 22, 1815–1823. doi: 10.1007/s00520-014-2147-x
Deprez, S., Billiet, T., Sunaert, S., and Leemans, A. (2013). Diffusion tensor MRI of chemotherapy-induced cognitive impairment in non-CNS cancer patients: A review. Brain Imaging Behav. 7, 409–435. doi: 10.1007/s11682-012-9220-1
Deprez, S., Kesler, S. R., Saykin, A. J., Silverman, D. H. S., de Ruiter, M. B., and Mcdonald, B. C. (2018). International cognition and cancer task force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. J. Natl. Cancer Inst. 110, 223–231. doi: 10.1093/jnci/djx285
Dhillon, H. M., Tannock, I. F., Pond, G. R., Renton, C., Rourke, S. B., and Vardy, J. L. (2018). Perceived cognitive impairment in people with colorectal cancer who do and do not receive chemotherapy. J. Cancer Surviv. 12, 178–185. doi: 10.1007/s11764-017-0656-6
Dijkshoorn, A. B. C., van Stralen, H. E., Sloots, M., Schagen, S. B., Visser-Meily, J. M. A., and Schepers, V. P. M. (2021). Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psychooncology 30, 635–648. doi: 10.1002/pon.5623
Eberhardt, B., Dilger, S., Musial, F., Wedding, U., Weiss, T., and Miltner, W. (2006). Medium-term effects of chemotherapy in older cancer patients. Support. Care Cancer 14, 216–222. doi: 10.1007/s00520-005-0894-4
Fan, H. G., Houédé-Tchen, N., Yi, Q. L., Chemerynsky, I., Downie, F. P., Sabate, K., et al. (2005). Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J. Clin. Oncol. 23, 8025–8032. doi: 10.1200/JCO.2005.01.6550
Hermelink, K., Untch, M., Lux, M. P., Kreienberg, R., Beck, T., Bauerfeind, I., et al. (2007). Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer 109, 1905–1913. doi: 10.1002/cncr.22610
Hormozi, M., Hashemi, S. M., and Shahraki, S. (2019). Investigating relationship between pre- and post- chemotherapy cognitive performance with levels of depression and anxiety in breast cancer patients: A cross-sectional study. Asian Pac. J. Cancer Prev. 20, 3831–3837. doi: 10.31557/APJCP.2019.20.12.3831
Iconomou, G., Mega, V., Koutras, A., Iconomou, A. V., and Kalofonos, H. P. (2004). Prospective assessment of emotional distress, cognitive function, and quality of life in patients with cancer treated with chemotherapy. Cancer 101, 404–411. doi: 10.1002/cncr.20385
Janelsins, M. C., Heckler, C. E., Peppone, L. J., Kamen, C., Mustian, K., Mohile, S. G., et al. (2017). Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide. Multicenter, prospective longitudinal study. J. Clin. Oncol. 35, 506–514. doi: 10.1200/JCO.2016.68.5826
Jansen, C. E., Dodd, M. J., Miaskowski, C. A., Dowling, G. A., and Kramer, J. (2008). Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology 17, 1189–1195. doi: 10.1002/pon.1342
Jean-Pierre, P. (2014). Integrating functional near-infrared spectroscopy in the characterization, assessment, and monitoring of cancer and treatment-related neurocognitive dysfunction. Neuroimage 85, 408–414. doi: 10.1016/j.neuroimage.2013.06.075
Keetile, N. M., Osuch, E., and Lentoor, A. G. (2021). Chemotherapy-related subjective cognitive impairment in breast cancer patients in semi-rural South Africa. Health SA 26:1605. doi: 10.4102/hsag.v26i0.1605
Kesler, S. R., and Blayney, D. W. (2016). Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2, 185–192. doi: 10.1001/jamaoncol.2015.4333
Khan, M. A., Garg, K., Bhurani, D., and Agarwal, N. B. (2016). Early manifestation of mild cognitive impairment in B-cell non-Hodgkin’s lymphoma patients receiving CHOP and rituximab-CHOP chemotherapy. Naunyn Schmiedebergs Arch. Pharmacol. 389, 1253–1265. doi: 10.1007/s00210-016-1290-y
Khan, O. F., Cusano, E., Raissouni, S., Pabia, M., Haeseker, J., Bosma, N., et al. (2019). Immediate-term cognitive impairment following intravenous (IV) chemotherapy: A prospective pre-post design study. BMC Cancer 19:150. doi: 10.1186/s12885-019-5349-2
Koppelmans, V., Breteler, M. M. B., Boogerd, W., Seynaeve, C., Gundy, C., and Schagen, S. B. (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 30, 1080–1086. doi: 10.1200/JCO.2011.37.0189
Kotb, M. G., Soliman, A. E., Ibrahim, R. I., Said, R. M. M., and El Din, M. M. W. (2019). Chemotherapy-induced cognitive impairment in hematological malignancies. Egypt. J. Neurol. Psychiatr. Neurosurg. 55:56. doi: 10.1186/s41983-019-0104-9
Lange, M., Heutte, N., Rigal, O., Noal, S., Kurtz, J. E., Lévy, C., et al. (2016). Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist 21, 1337–1348. doi: 10.1634/theoncologist.2016-0014
Lange, M., Joly, F., Vardy, J., Ahles, T., Dubois, M., Tron, L., et al. (2019). Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 30, 1925–1940. doi: 10.1093/annonc/mdz410
Lepage, C., Smith, A. M., Moreau, J., Barlow-Krelina, E., Wallis, N., Collins, B., et al. (2014). A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus 3:10. doi: 10.1186/2193-1801-3-444
Li, W., Gan, C., Lv, Y., Wang, S. H., and Cheng, H. D. (2017). Chemotherapy-induced prospective memory impairment in breast cancer patients with different hormone receptor expression. Medicine 96:e6514. doi: 10.1097/MD.0000000000006514
Li, X., Chen, H. J., Lv, Y., Chao, H. H., Gong, L., Li, C. S. R., et al. (2018). Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep. 8:13801. doi: 10.1038/s41598-018-32257-w
Lindner, O. C., Phillips, B., Mccabe, M. G., Mayes, A., Wearden, A., Varese, F., et al. (2014). A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 28, 726–740. doi: 10.1037/neu0000064
Liu, Z., Han, X., Tian, C., Chen, J., Lei, L., Liang, Q., et al. (2018). A study on the relationship between chemotherapy-induced cognitive impairment and age in patients with breast cancer. J. BUON 23, 1601–1605.
Magnuson, A., Lei, L., Gilmore, N., Kleckner, A. S., Lin, F. V., Ferguson, R., et al. (2019). Longitudinal relationship between frailty and cognition in patients 50 years and older with breast cancer. J. Am. Geriatr. Soc. 67, 928–936. doi: 10.1111/jgs.15934
Magnuson, A., Mohile, S., and Janelsins, M. (2016). Cognition and cognitive impairment in older adults with cancer. Curr. Geriatr. Rep. 5, 213–219. doi: 10.1007/s13670-016-0182-9
Miao, H., Li, J., Hu, S., He, X., Partridge, S. C., Ren, J., et al. (2016). Long-term cognitive impairment of breast cancer patients after chemotherapy: A functional MRI study. Eur. J. Radiol. 85, 1053–1057. doi: 10.1016/j.ejrad.2016.03.011
Minisini, A. M., De Faccio, S., Ermacora, P., Andreetta, C., Fantinel, R., Balestrieri, M., et al. (2008). Cognitive functions and elderly anticancer treatment: A cancer patients receiving prospective study. Crit. Rev. Oncol. Hematol. 67, 71–79. doi: 10.1016/j.critrevonc.2008.02.004
Mo, C. Q., Lin, H. L., Fu, F. M., Lin, L., Zhang, J., Huang, M., et al. (2017). Chemotherapy-induced changes of cerebral activity in resting-state functional magnetic resonance imaging and cerebral white matter in diffusion tensor imaging. Oncotarget 8, 81273–81284. doi: 10.18632/oncotarget.18111
Mounier, N. M., Abdel-Maged, A. E. S., Wahdan, S. A., Gad, A. M., and Azab, S. S. (2020). Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sci. 258:118071. doi: 10.1016/j.lfs.2020.118071
Natori, A., Ogata, T., Sumitani, M., Kogure, T., Yamauchi, T., and Yamauchi, H. (2015). Potential role of pNF-H, a biomarker of axonal damage in the central nervous system, as a predictive marker of chemotherapy-induced cognitive impairment. Clin. Cancer Res. 21, 1348–1352. doi: 10.1158/1078-0432.CCR-14-2775
Ng, T., Phey, X. Y., Yeo, H. L., Shwe, M., Gan, Y. X., Ng, R., et al. (2018). Impact of adjuvant anthracycline-based and taxane-based chemotherapy on plasma VEGF levels and cognitive function in breast cancer patients: A longitudinal study. Clin. Breast Cancer 18, e927–e937. doi: 10.1016/j.clbc.2018.03.016
Ng, T., Teo, S. M., Yeo, H. L., Shwe, M., Gan, Y. X., Cheung, Y. T., et al. (2016). Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro. Oncol. 18, 244–251. doi: 10.1093/neuonc/nov162
Nguyen, C. M., Yamada, T. H., Beglinger, L. J., Cavanaugh, J. E., Denburg, N. L., and Schultz, S. K. (2013). Cognitive features 10 or more years after successful breast cancer survival: Comparisons across types of cancer interventions. Psychooncology 22, 862–868. doi: 10.1002/pon.3086
Oh, P. J., and Moon, S. M. (2019). Changes of cognitive function and fatigue following chemotherapy in patients with gastrointestinal cancer: A prospective controlled study. Asian Oncol. Nurs. 19, 126–134. doi: 10.5388/aon.2019.19.3.126
Oppegaard, K., Harris, C. S., Shin, J., Paul, S. M., Cooper, B. A., Chan, A., et al. (2021). Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 148:155653. doi: 10.1016/j.cyto.2021.155653
Orszaghova, Z., Mego, M., and Chovanec, M. (2021). Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci. 8:770413. doi: 10.3389/fmolb.2021.770413
Perrier, J., Viard, A., Levy, C., Morel, N., Allouache, D., Noal, S., et al. (2020). Longitudinal investigation of cognitive deficits in breast cancer patients and their gray matter correlates: Impact of education level. Brain Imaging Behav. 14, 226–241. doi: 10.1007/s11682-018-9991-0
R Core Team (2018). A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Saa, J. P., Tse, T., Baum, C., Cumming, T., Josman, N., Rose, M., et al. (2019). Longitudinal evaluation of cognition after stroke - A systematic scoping review. PLoS One 14:e0221735. doi: 10.1371/journal.pone.0221735
Sales, M. V. C., Suemoto, C. K., Apolinario, D., Serrao, V., Andrade, C. S., Conceição, D. M., et al. (2019). Effects of adjuvant chemotherapy on cognitive function of patients with early-stage colorectal cancer. Clin. Colorectal Cancer 18, 19–27. doi: 10.1016/j.clcc.2018.09.002
Schagen, S. B., Boogerd, W., Muller, M. J., Huinink, W. T., Moonen, L., Meinhardt, W., et al. (2008). Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol. 47, 63–70. doi: 10.1080/02841860701518058
Schagen, S. B., Van Dam, F. S., Muller, M. J., Boogerd, W., Lindeboom, J., and Bruning, P. F. (1999). Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85, 640–650. doi: 10.1002/(SICI)1097-0142(19990201)85:3<640::AID-CNCR14>3.0.CO;2-G
Scherwath, A., Mehnert, A., Schleimer, B., Schirmer, L., Fehlauer, F., Kreienberg, R., et al. (2006). Neuropsychological function in high-risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: Evaluation of long-term treatment effects. Ann. Oncol. 17, 415–423. doi: 10.1093/annonc/mdj108
Shen, C. Y., Chen, V. C., Yeh, D. C., Huang, S. L., Zhang, X. R., Chai, J. W., et al. (2019). Association of functional dorsal attention network alterations with breast cancer and chemotherapy. Sci. Rep. 9:104. doi: 10.1038/s41598-018-36380-6
Shilling, V., Jenkins, V., Morris, R., Deutsch, G., and Bloomfield, D. (2005). The effects of adjuvant chemotherapy on cognition in women with breast cancer–preliminary results of an observational longitudinal study. Breast 14, 142–150. doi: 10.1016/j.breast.2004.10.004
Silver, J. K., Baima, J., and Mayer, R. S. (2013). Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J. Clin. 63, 295–317. doi: 10.3322/caac.21186
Silverman, D. H. S., Dy, C. J., Castellon, S. A., Lai, J., Pio, B. S., Abraham, L., et al. (2007). Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res. Treat. 103, 303–311. doi: 10.1007/s10549-006-9380-z
Simo, M., Gurtubay-Antolin, A., Vaquero, L., Bruna, J., and Rodriguez-Fornells, A. (2018). Performance monitoring in lung cancer patients pre- and post-chemotherapy using fine-grained electrophysiological measures. Neuroimage Clin. 18, 86–96. doi: 10.1016/j.nicl.2017.12.032
Stouten-Kemperman, M. M., De Ruiter, M. B., Boogerd, W., Veltman, D. J., Reneman, L., and Schagen, S. B. (2015). Very late treatment-related alterations in brain function of breast cancer survivors. J. Int. Neuropsychol. Soc. 21, 50–61. doi: 10.1017/S1355617714001015
Subramaniam, C. B., Bowen, J. M., Gladman, M. A., Lustberg, M. B., Mayo, S. J., and Wardill, H. R. (2020). The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci. Biobehav. Rev. 116, 470–479. doi: 10.1016/j.neubiorev.2020.07.002
Suhaimi, N. S., Mountstephens, J., and Teo, J. (2020). EEG-based emotion recognition: A state-of-the-art review of current trends and opportunities. Comput. Intell. Neurosci. 2020:8875426. doi: 10.1155/2020/8875426
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Tao, L., Lin, H., Yan, Y., Xu, X., Wang, L., Zhang, J., et al. (2017). Impairment of the executive function in breast cancer patients receiving chemotherapy treatment: A functional MRI study. Eur. J. Cancer Care 26:e12553. doi: 10.1111/ecc.12553
Tricco, A. C., Lillie, E., Zarin, W., O’brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Van Dyk, K., Zhou, X. T., Small, B. J., Ahn, J., Zhai, W. T., Ahles, T., et al. (2021). Protective effects of APOE epsilon 2 genotype on cognition in older breast cancer survivors: The thinking and living with cancer study. JNCI Cancer Spectr. 5:12. doi: 10.1093/jncics/pkab013
Vardy, J. L., Dhillon, H. M., Pond, G. R., Rourke, S. B., Bekele, T., Renton, C., et al. (2015). Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J. Clin. Oncol. 33, 4085–4092. doi: 10.1200/JCO.2015.63.0905
Vardy, J., Wefel, J. S., Ahles, T., Tannock, I. F., and Schagen, S. B. (2008). Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice cognitive workshop. Ann. Oncol. 19, 623–629. doi: 10.1093/annonc/mdm500
Vizer, L. M., Mikles, S. P., and Piepmeier, A. T. (2022). Cancer-related cognitive impairment in survivors of adolescent and young adult non-central nervous system cancer: A scoping review. Psychooncology 31, 1275–1285. doi: 10.1002/pon.5980
Wefel, J. S., Lenzi, R., Theriault, R. L., Davis, R. N., and Meyers, C. A. (2004). The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100, 2292–2299. doi: 10.1002/cncr.20272
Wefel, J. S., Vardy, J., Ahles, T., and Schagen, S. B. (2011). International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12, 703–708. doi: 10.1016/S1470-2045(10)70294-1
Wefel, J. S., Vidrine, D. J., Marani, S. K., Swartz, R. J., Veramonti, T. L., Meyers, C. A., et al. (2014). A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psychooncology 23, 626–633. doi: 10.1002/pon.3453
Whitford, H. S., Kalinowski, P., Schembri, A., Grimison, P., Stockler, M., Martin, A., et al. (2020). The impact of chemotherapy on cognitive function: A multicentre prospective cohort study in testicular cancer. Support. Care Cancer 28, 3081–3091. doi: 10.1007/s00520-019-05095-3
Wieneke, M. H., and Dienst, E. R. (1995). Neuropsychological assessment of cognitive-functioning following chemotherapy for breast-cancer. Psychooncology 4, 61–66. doi: 10.1002/pon.2960040108
Williams, A. M., Shah, R., Shayne, M., Huston, A. J., Krebs, M., Murray, N., et al. (2018). Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 314, 17–23. doi: 10.1016/j.jneuroim.2017.10.005
Yamada, T. H., Denburg, N. L., Beglinger, L. J., and Schultz, S. K. (2010). Neuropsychological outcomes of older breast cancer survivors: Cognitive features ten or more years after chemotherapy. J. Neuropsychiatry Clin. Neurosci. 22, 48–54. doi: 10.1176/jnp.2010.22.1.48
Keywords: cognitive disorder, cognitive function, cancer-related cognitive impairment (CRCI), ICF, neoplasm, chemo brain, CICIs
Citation: Saita K, Amano S, Kaneko F and Okamura H (2023) A scoping review of cognitive assessment tools and domains for chemotherapy-induced cognitive impairments in cancer survivors. Front. Hum. Neurosci. 17:1063674. doi: 10.3389/fnhum.2023.1063674
Received: 07 October 2022; Accepted: 30 January 2023;
Published: 20 February 2023.
Edited by:
Vijaya Majumdar, Swami Vivekananda Yoga Anusandhana Samsthana, IndiaReviewed by:
Martin Rakuša, Maribor University Medical Centre, SloveniaNilton Custodio, Peruvian Institute of Neurosciences (IPN), Peru
Copyright © 2023 Saita, Amano, Kaneko and Okamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoru Amano,  cy1hbWFub0BraXRhc2F0by11LmFjLmpw
cy1hbWFub0BraXRhc2F0by11LmFjLmpw
 Kazuya Saita
Kazuya Saita Satoru Amano
Satoru Amano Fumiko Kaneko1
Fumiko Kaneko1 Hitoshi Okamura
Hitoshi Okamura