
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 29 September 2022
Sec. Brain Health and Clinical Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.972538
This article is part of the Research Topic Dissociations Between Neural Activity and Conscious State: A Key to Understanding Consciousness View all 7 articles
Objective: When regaining consciousness, patients who emerge from a minimally conscious state (EMCS) present with different levels of functional disability, which pose great challenges for treatment. This study investigated the frontoparietal activity in EMCS patients and its effects on functional disability.
Materials and methods: In this preliminary study, 12 EMCS patients and 12 healthy controls were recruited. We recorded a resting-state scalp electroencephalogram (EEG) for at least 5 min for each participant. Each patient was assessed using the disability rating scale (DRS) to determine the level of functional disability. We analyzed the EEG power spectral density and sensor-level functional connectivity in relation to the patient’s functional disability.
Results: In the frontoparietal region, EMCS patients demonstrated lower relative beta power (P < 0.01) and higher weighted phase lag index (wPLI) values in the theta (P < 0.01) and gamma (P < 0.01) bands than healthy controls. The frontoparietal theta wPLI values of EMCS patients were positively correlated with the DRS scores (rs = 0.629, P = 0.029). At the whole-brain level, EMCS patients only had higher wPLI values in the theta band (P < 0.01) than healthy controls. The whole-brain theta wPLI values of EMCS patients were also positively correlated with the DRS scores (rs = 0.650, P = 0.022). No significant difference in the power and connectivity between the frontoparietal region and the whole brain in EMCS patients was observed.
Conclusion: EMCS patients still experience neural dysfunction, especially in the frontoparietal region. However, the theta connectivity in the frontoparietal region did not increase specifically. At the level of the whole brain, the same shift could also be seen. Theta functional connectivity in the whole brain may underlie different levels of functional disability.
Severe brain injury may lead to prolonged impairment of consciousness, that is, disorders of consciousness (DoC). Frequently, the recovery trajectory of DoC is a vegetative state or “unresponsive wakefulness syndrome” (VS/UWS) (Laureys et al., 2010), minimally conscious state (MCS), and emergence from MCS (EMCS, also called confusional state) (Giacino et al., 2002).
When regaining consciousness (able to use objects or communicate functionally) (Giacino et al., 2002), 98% of EMCS patients have cognitive impairment and are largely dependent on caregivers for basic activities of daily living (Nakase-Richardson et al., 2009; Murphy, 2018; Bodien et al., 2020). These sequelae of brain injury create great challenges for evaluation, therapy, and prognosis. However, Bodien et al. observed that EMCS patients had a large spread in the total scores for the disability rating scale (DRS), which is used to assess disability outcomes for severely brain-injured patients (Rappaport et al., 1982; Rappaport, 2005), and categorized several levels of dysfunction ranging from VS to moderate disability (Bodien et al., 2020). This implies that some EMCS patients could return to society, while others remained unable to take care of themselves. However, the cause of EMCS patients having different levels of functional disability after regaining consciousness remains unclear. Objective measurements are needed to assess cortical function, which may help to explain this phenomenon.
Several differential features between EMCS and MCS or VS/UWS have been reported (Rodriguez Moreno et al., 2010; Lesenfants et al., 2016; Aubinet et al., 2018). The degree of impairment of the frontoparietal network observed using positron emission tomography, magnetic resonance imaging, and electroencephalography is often used to determine the level of consciousness in patients (Fernández-Espejo et al., 2012; Stender et al., 2015; Wu et al., 2020). EMCS patients with a higher level of consciousness demonstrate more preserved metabolism in the frontoparietal network than VS/UWS and MCS patients, while they have lower regional cerebral glucose metabolism rates than healthy controls (HCs; the maximum difference is in the frontoparietal cortex) (Thibaut et al., 2012; Stender et al., 2015; Bodart et al., 2017). The frontoparietal network comprises two subnetworks as follows: the default mode network (DMN), which is involved in internal awareness and self-reflection, and the executive control network (ECN), which processes external stimuli and mediates attention (Vogt and Laureys, 2005; Vanhaudenhuyse et al., 2011; Buckner and DiNicola, 2019). The connectivity between the two subnetworks is correlated with metabolic activity and has been demonstrated to clearly characterize EMCS patients and indicate a neural correlate of consciousness (Di Perri et al., 2016). Ongoing impairments within these subnetworks may result in various levels of dysfunction. Clinically, EMCS patients experience confusion and perceptual disturbances (Nakase-Richardson et al., 2009; Bodien et al., 2020). Thus, we hypothesized that there may be impairment in the frontoparietal function of patients and that this may be associated with functional disability.
In this study, we aimed to verify the hypothesis that electroencephalogram (EEG) measurements of the frontoparietal area may be helpful in characterizing EMCS patients and to establish an objective method to quantify the degree of functional disability. We used EEG measurements to preliminarily explore differences in frontoparietal cortical function between EMCS patients and HCs, and examined the relationship between patients’ EEG activities and their levels of disability.
In total, 12 EMCS patients (mean age 47.2 ± 14.4 years, five men) and 12 HCs (mean age 46.0 ± 12.8 years, seven men) were recruited for this preliminary study. The EMCS patient assessments were confirmed using the Coma Recovery Scale-Revised (CRS-R), and patients had one or both of the following behaviors: functional interactive communication or functional use of two different objects (Giacino et al., 2002, 2004). The patients enrolled in the study did not receive any treatment with sedatives or psychostimulants, such as clonazepam, midazolam, zolpidem, or modafinil for at least 2 days before data collection. Patients with a history of epilepsy or other neuropsychological diseases were excluded from this study. Written informed consent to participate in this study was obtained from the HCs and legally authorized representatives of patients. The ethics committee of Zhujiang Hospital approved all aspects of the study.
Before the recording session, each patient was assessed three times by two experienced raters with the CRS-R within a week and on the last day of EEG recording (Bodart et al., 2017). The best result was maintained as a behavioral diagnosis. The DRS is designed to assess functional disability in patients with brain injury. The scores range from no disability (0) to death (30), and have ten disability categories. Higher scores reflect poorer functioning (Rappaport et al., 1982; Rappaport, 2005). In this study, the DRS score was observed within 3 days of EMCS diagnosis (Bodien et al., 2020).
For each participant, resting-state EEG was recorded for at least 5 min (completed between 30 and 85 trials, 10 s per trial). During recording, the patients sat in a wheelchair with their eyes open, and the standard CRS-R arousal facilitation protocol was used to maintain an arousal state. The controls were asked to relax, be awake with their eyes open, and not engage in any specific activity. The brain activity of the participants was recorded using a 66 channel system (SynAmps2TM 8500; Neuroscan, USA) with a 2,500-Hz sampling frequency, followed by the International 10–20 System. The machine used Ag/AgCl pin electrodes with band-pass filtering at a direct current of 1,000 Hz. During recording, the electrode impedance was maintained at <5 kΩ. To objectively assess the open state of the eyes, we measured vertical eye blink-related activity by electrooculography (EOG) similar to that used in a previous study (Chennu et al., 2014; Supplementary Figure 1).
Electroencephalogram preprocessing was conducted using the EEGLAB toolbox (13_0_0b) in MATLAB (version 2013b; MathWorks Inc., Natick, MA, USA). The EEG data were downsampled to 500 Hz and filtered between 0.5 and 45 Hz. The EEG signals were divided into epochs of 10 s. Artifacts derived from nearby muscle activity and eye movements were eliminated using independent component analysis. Epochs containing evident artifacts were manually removed by visually inspecting the data of each participant. Epochs with activity exceeding ± 100 μV were rejected using a semi-automated procedure, and the artifact-free signals were averaged. After preprocessing, the data of the participants were retained between 22 and 56 trials. Therefore, we selected 22 trials for each participant separately to match the trial numbers across the groups for further analysis.
The Welch method was used to compute the power spectral density (PSD, with a 50% overlap between 1-s Hamming windowed segments) for each signal epoch. The absolute powers of the delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–45 Hz) bands were estimated. To normalize and compute the relative power, the absolute PSD values at each frequency band relative to the total power across the entire frequency spectrum were calculated. To verify our hypothesis, the relative power for each band was averaged across channels in both the frontoparietal region and whole brain: frontoparietal region (frontal: Fp1, Fp2, FPz, AF3, AF4, Fz, F1, F2, F3, F4, F5, F6, F7, F8, parietal: CPz, CP1, CP2, CP3, CP4, CP5, CP6, Pz, P1, P2, P3, P4, P5, P6).
A functional connectivity analysis was performed using the weighted phase lag index (wPLI). The wPLI measure is more robust and partially invariant to volume conduction in phase relationships (Peraza et al., 2012). wPLI is defined as the phase difference between two signals from the frontal and parietal regions, weighted by the magnitude of the imaginary component of the cross-spectrum within the band of interest. Calculations were performed according to a previous study (Vinck et al., 2011). The values of wPLI range from 0 to 1, with 1 indicating strong functional connectivity and 0 indicating no connectivity. The mean connectivity of the frontoparietal region and whole brain was measured for all frequency bands in each group.
Due to the non-normality of the data, we used a non-parametric permutation test to identify the differences in EEG relative power and the wPLIs within the frontoparietal region and the whole brain between EMCS patients and HCs in the frequency ranges (number of permutations > 1,000; P < 0.05). Bonferroni correction was used for multiple comparison of the correction for each frequency band (n = 5). Pairwise comparisons of connectivity between every electrode were performed using two-sample t-tests, and the network-based statistic was performed after multiple comparisons using the graph theory network analysis toolbox (Wang et al., 2015). An edge p-value of 0.01 was set for the t matrix. Next, statistical significance was estimated with 5,000 permutation tests and a family-wise error corrected level of P < 0.05. Therefore, we performed Spearman’s correlations to separately estimate the relationship between DRS scores and significant EEG parameters in defined regions of interest in EMCS patients.
No significant differences in age and sex were observed between EMCS patients and HCs (P > 0.05). The details of EMCS patients, including etiology, post-injury (months), lesions, cranioplasty, EMCS behaviors, CRS-R sub-scale, CRS-R total scores, and DRS total scores are presented in Table 1. The DRS total scores were categorized as follows: moderately severe disability (scores 7–11), severe disability (scores 12–16), and extremely severe disability (scores 17–21). None of the enrolled patients demonstrated functional object use or communication in the CRS-R. Regarding scores, one patient had 11, 13, and 18, two had 21, three had 20, and four had 19. Furthermore, we observed no significant difference between the average EOG activity in the first and second halves of the recording in EMCS patients (P > 0.05; Supplementary Figure 1). The EEG data between patients who received cranioplasty and those who did not revealed no significant differences in the five frequency bands (all P-values > 0.05; Supplementary Figure 2).
In terms of the frontoparietal region, the EMCS patients demonstrated lower relative power in the beta band than the HCs (P = 0.007; Figures 1A,B). Furthermore, the wPLI increased in the theta (P = 0.008) and gamma (P = 0.004) bands in this region in EMCS patients. Figures 2A,B present the average functional connectivity of channels in the frontoparietal region. Fisher-Z transformation was used to normalize the distribution of the values. Figure 2C depicts the mean ± SEM of the wPLI values in the frontoparietal region. After multiple comparisons of each connectivity value, the significantly altered connectivity was consistent with the average connectivity findings (Figure 2D). Compared with the HC group, the increased connectivity of the theta band occured between the frontal and parietal regions and within these regions in the EMCS patients. Increased connectivity of the gamma band was mostly observed between the frontal and parietal regions in the EMCS group. A significant correlation was identified between theta functional connectivity and the total DRS score in the frontoparietal area. The wPLI in the theta band was positively correlated with DRS scores (rs = 0.629, P = 0.029). However, gamma wPLI and beta relative power did not correlate with the DRS scores (rs = –0.047, P = 0.885; rs = –0.445, P = 0.147).
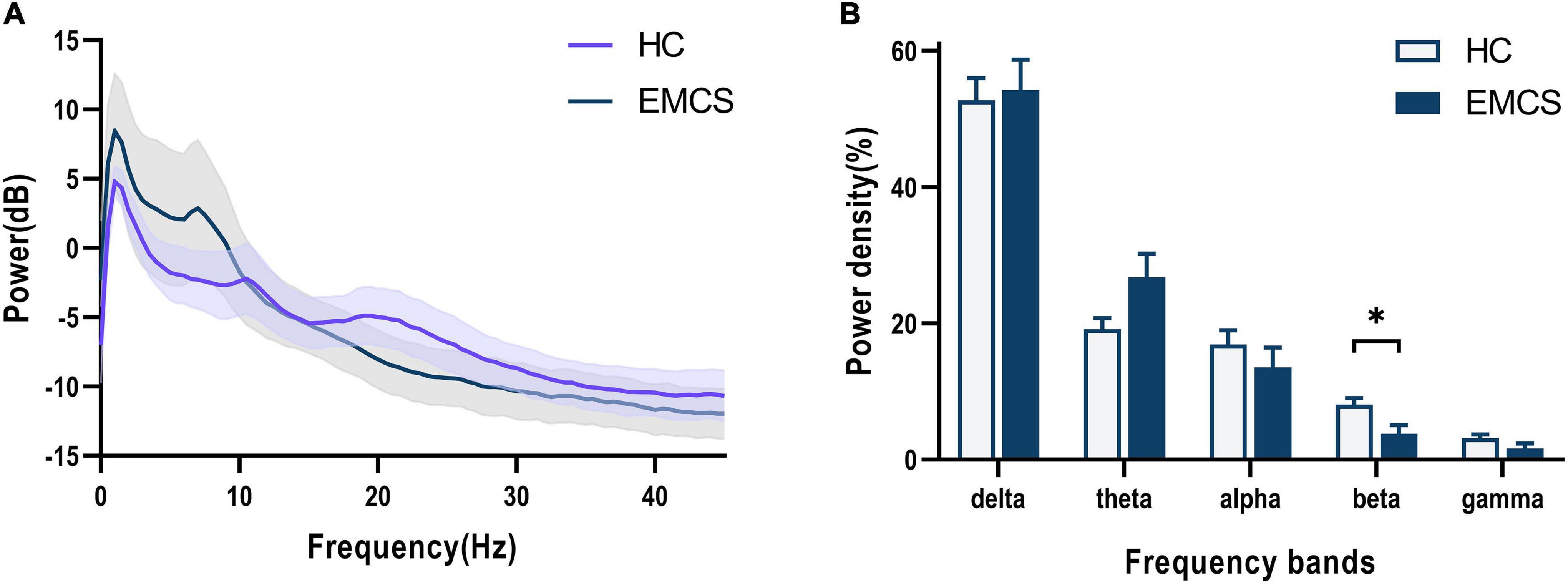
Figure 1. Frontoparietal changes in the EEG power spectra in the emergence from minimally conscious state (EMCS) group compared with the healthy control (HC) group. (A) The average absolute EEG power in HCs (purple line) and EMCS patients (blue line). The Y-axis represented the log-transformed power. (B) The relative power in five frequency bands. A marked decrease in the relative power in beta frequencies was observed in the EMCS group compared with the power in the HC group (*P < 0.01, after Bonferroni correction for frequency bands). The data were expressed as the means ± SEM.
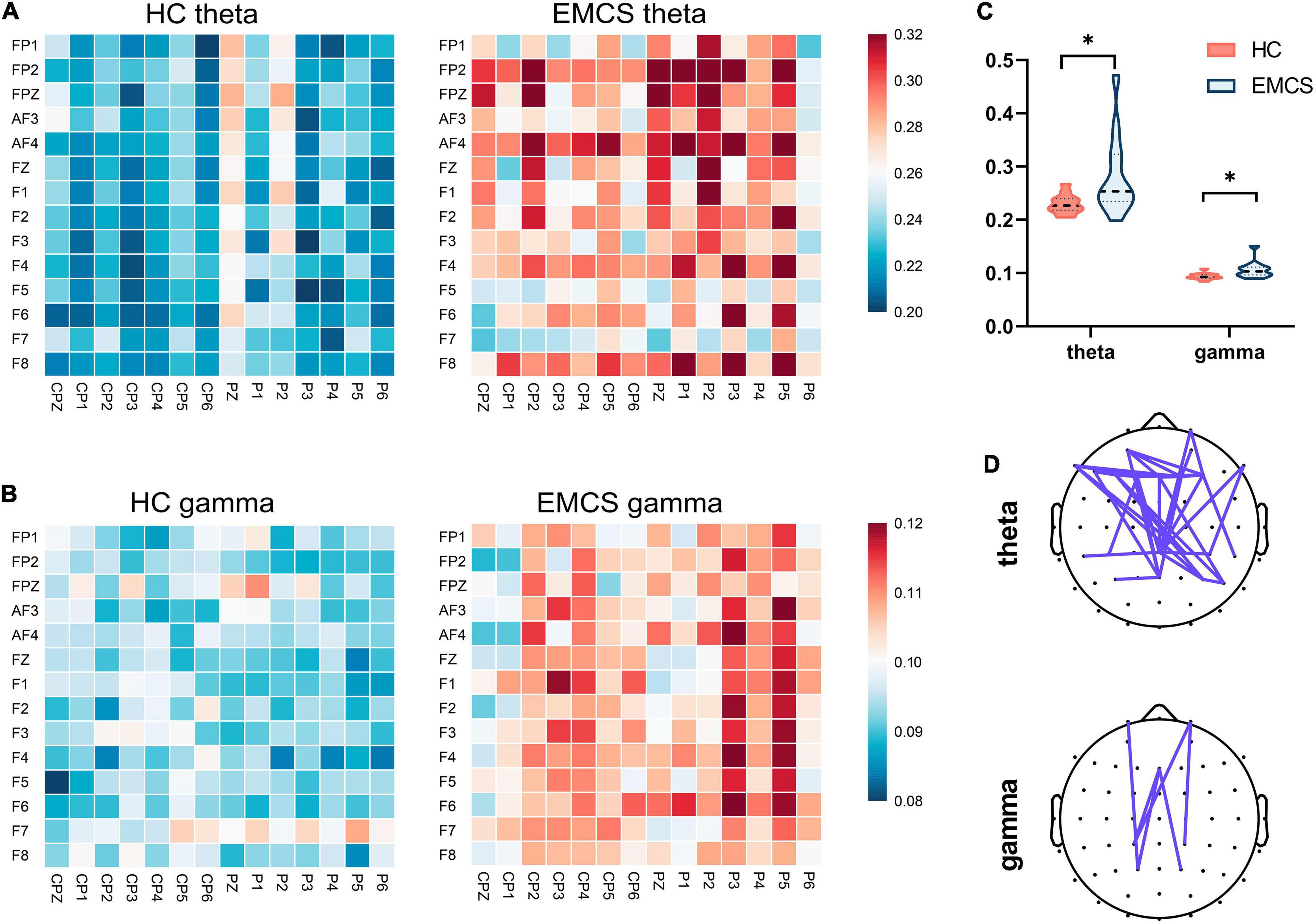
Figure 2. Frontoparietal weight phase lag index (wPLI) among healthy control (HC) group and emergence from minimally conscious state (EMCS) group. (A) Heatmaps of averaged wPLI values at rest for theta band between the groups. (B) Heatmaps of averaged wPLI values at rest for gamma band. The wPLI values were implemented Fisher-Z transformed. (C) Violin plot of wPLI values between frontal and parietal areas in theta and gamma bands (*P < 0.01, after Bonferroni correction). (D) The top panel shows significantly altered connectivities between the two groups. The purple line means significantly increased connectivity in EMCS patients.
At the whole-brain level, relative power did not differ between the two groups. Compared with the HCs, the EMCS patients had increased wPLI values in the theta band (P = 0.004). Figure 3A presents the average functional connectivity of channels across the brain. After multiple comparisons of each connectivity value, the significantly altered connectivity was consistent with the average connectivity findings (Figure 3B). The theta wPLI value in the whole brain revealed a positive correlation with DRS scores (rs = 0.650, P = 0.022, Figure 4).
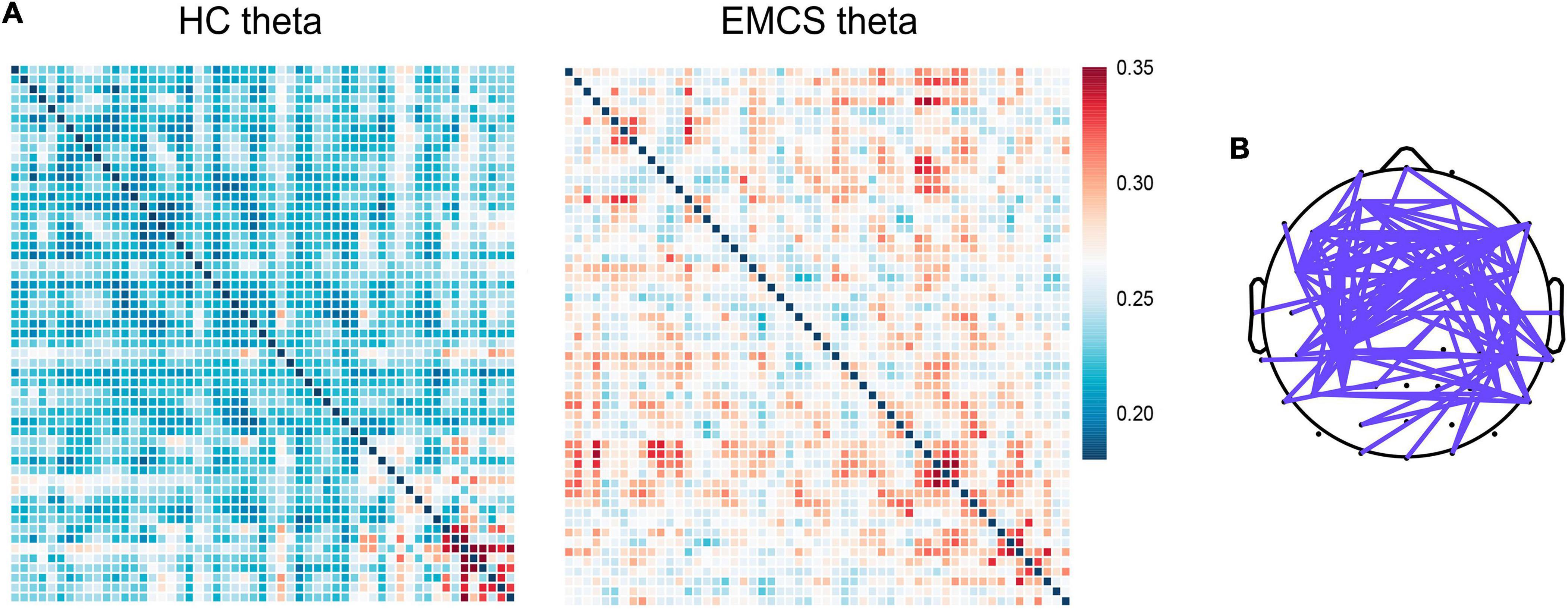
Figure 3. Global weight phase lag index (wPLI) among healthy control (HC) group and emergence from minimally conscious state (EMCS) group. (A) Heatmaps of averaged wPLI values at rest for theta band between the groups. The wPLI values were implemented Fisher-Z transformed. (B) The top panel shows significantly altered connectivities between the two groups. The purple line means significantly increased connectivity in EMCS patients.
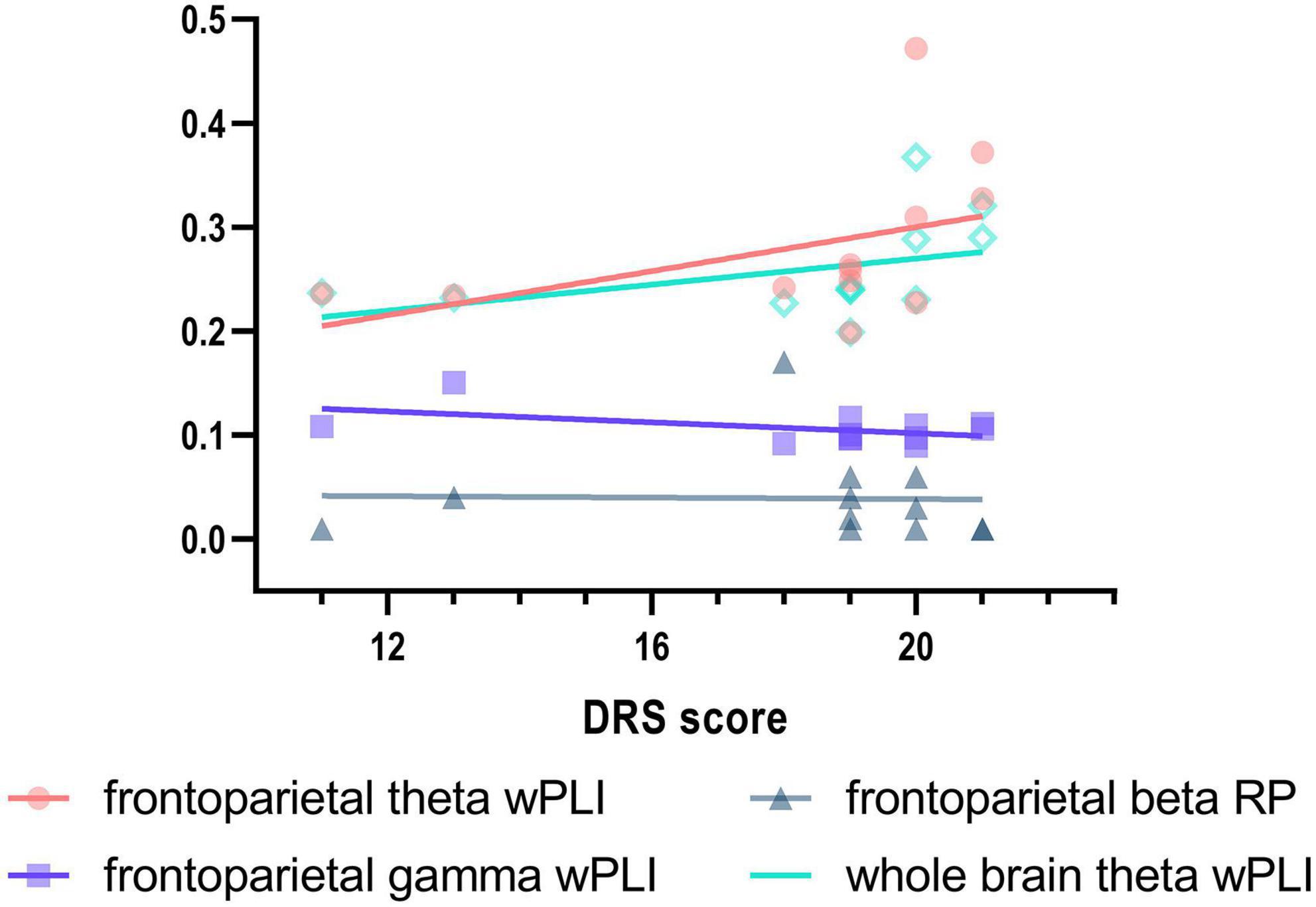
Figure 4. Correlation between Disability Rating Scale (DRS) scores and EEG activity in EMCS patients. Spearman’s correlations between DRS and frontoparietal theta wPLI (rs = 0.629, P = 0.029), frontoparietal gamma wPLI (rs = –0.047, P = 0.885), frontoparietal beta relative power (rs = –0.445, P = 0.147), and whole brain theta wPLI (rs = 0.650, P = 0.022) in EMCS patients. Linear regression was used to generate fitted lines for each data set.
To clarify whether such EEG changes were specifically altered in the frontoparietal region, we also compared measures in the whole brain and frontoparietal region in the EMCS group. No significant differences in power and functional connectivity were observed between the two groups at any frequency (Supplementary Figure 3).
Emergence from minimally conscious state is a late stage in the typical recovery of DoC patients with severe brain injury etiologies. Patients at this stage have different levels of dysfunction. Once patients regain consciousness, clinicians often focus on treating cognitive impairment, lack of speech, and motor dysregulation (Lancioni et al., 2014). Therefore, a reliable and objective assessment is necessary to characterize this population. Our study evaluated patients who experienced EMCS using EEG activity and analyzed the effects of EMCS on functional disability. This study had three main preliminary findings as follows: EMCS patients had varying degrees of severe disability; EMCS patients still had abnormal EEG activity, especially in the frontoparietal region; and theta wPLI correlated with functional disability in EMCS at the whole-brain level.
In terms of functional disability, EMCS patients were associated with severe disability on the DRS, ranging from moderate to extremely severe disability. They demonstrated a restricted range of cognition and behavior. The DRS scores were expected and in line with those of earlier investigations (Murphy, 2018; Bodien et al., 2020).
Additionally, the relative beta power was lower in the frontoparietal region in EMCS patients than in HCs, which is inconsistent with the “ABCD” model suggested by Schiff that EMCS patients have dominant peaks in higher frequency ranges (i.e., 15–40 Hz) (Schiff, 2016). There could be three possible reasons for this inconsistency. First, lower beta band may appear in EMCS patients in contrast to that in HCs. EMCS patients have two EEG types in the ABCD model, the first characterized by oscillations at 5–9 Hz and 15–40 Hz (C-type) and the second characterized by oscillations at 8–12 Hz and 15–40 Hz (D-type or healthy). C-Type is produced by burst central thalamic activity, while D-type is driven by tonic firing thalamic activity. Insufficient firing and moderate deafferentation in thalamocortical connectivity may reduce the beta power (Llinás et al., 2005). However, the beta power may be relatively preserved when compared to other patients with DoC or unrecovered patients with severe brain injury, who have quiescent central thalamic activity and more severe deafferentation. Second, EMCS patients could have local electrophysiological abnormalities even in the D-type spectrum, such as an increased delta to alpha power ratio (Shah et al., 2017b; Edlow et al., 2021). A previous study has reported that regional beta power is reduced in mild traumatic brain injury patients who have functional impairment (Zhang et al., 2020). Similarly, patients who recover full consciousness have deficits in attention owing to the suppression of frontal beta power (Shah et al., 2017a). Third, the observed pattern may not fit the ABCD model. According to a recent study, some EEG activity (10.6%) in patients with acute traumatic brain injury with DoC did not correspond to any ABCD type. Though not all data contained peak combinations from the ABCD model, the dataset could still significantly predict the recovery of consciousness (Frohlich et al., 2022). Beta oscillations are associated with motor function and various cognitive functions, including predictive coding, working memory, perception, and emotion (Roa Romero et al., 2015; von Lautz et al., 2017; Chang et al., 2018; Betti et al., 2021; Schubring and Schupp, 2021). The decrease in the beta power in EMCS patients may indicate reduced cortical function.
In the connectivity analysis, the EMCS patients demonstrated higher theta wPLI values both in the frontoparietal region and the whole brain, and higher wPLI values in gamma bands within the frontoparietal region than the HCs. Cavaliere et al. (2018) also observed that patients have predominant posterior theta activity. Theta activity is generally considered to reflect fundamental cognitive functions (Biel et al., 2022; Cowley et al., 2022). An association between increased theta connectivity and cognitive dysfunction has been reported in some diseases such as Alzheimer’s disease, Parkinson’s disease, and schizophrenia (Andreou et al., 2015; Iyer et al., 2020; Yan et al., 2021). Increased gamma connectivity can facilitate synchronization during cognitive processing (Başar, 2013). The EMCS patients who demonstrate higher theta and gamma connectivity may have difficulty maintaining basic cognition in a resting state. Thus, they need to apply more neural resources to integrate information. Gamma oscillations originating from inhibitory interneurons by spreading gamma-aminobutyric acid (GABA) are considered an efficient mechanism for functional connectivity (Fingelkurts et al., 2004; Jafari et al., 2020). Theta activity also requires GABAergic and cholinergic input (Başar, 2013). A high level of energy expenditure in the theta and gamma bands at rest may implicate ongoing neural dysfunction in neurotransmitter systems such as GABA.
Our results revealed that increased theta band wPLI values in the frontoparietal region were positively correlated with total DRS scores. However, this correlation was also observed at the whole-brain level. Interestingly, EMCS patients had similar power and connectivity changes between the frontoparietal region and the whole brain, suggesting that theta connectivity increases are not specific to the frontoparietal regions. However, the current results are insufficient to support the hypothesis that abnormalities in the frontoparietal region affect dysfunction. The reasons for similar changes in patients need further evaluation. Additionally, the different effects of beta power and gamma connectivity within the frontoparietal region might be driven by the differences between the frontoparietal region and the whole brain in HCs.
Nevertheless, theta wPLI correlated with functional disability at the whole-brain level in the EMCS. This suggests that increased theta functional connectivity may indicate severe dysfunction in EMCS patients. The DRS includes the assessment of physical and cognitive items, reflecting functional disability in patients with severe brain injury (Rappaport et al., 1982). Alterations in theta oscillations are not only linked with cognition but also with impulse control disorders (Zhu et al., 2019). Therefore, the correlation we observed has a plausible biological interpretation. Due to the small sample size, we did not directly link the EEG results to specific DRS items. In fact, most EMCS patients have cognitive impairments (Bodien et al., 2020). Physical disorders are a frequent complication of severe brain injury (Thibaut et al., 2015). Further studies should directly assess cognition in EMCS patients to verify the relationship between functional details and EEG activity.
Several limitations of this study should be noted. First, the broad range of etiologies and multiple structural lesions were not considered. Patients with traumatic brain injury often experience greater rehabilitation efforts (Colantonio et al., 2011). Second, this population was heterogeneous (Marino and Whyte, 2022). Complex stratification in our study could have led to inaccurate results because of the lower number of patients in the experimental group. If available, it may be more appropriate to select patients with brain injury having the same etiology and history of no DoC as controls, which may reduce the impact of brain damage on the EEG results. Third, although we preliminarily observed frontoparietal activity in this study as a previous study has reported one of the maximum differences between EMCS patients and healthy individuals in this region (Cavaliere et al., 2018), the spatial resolution of EEG is limited. Multimodal neuroimaging methods can be used to analyze the correlation between brain function (e.g., ECN and DMN source connectivity) and functional disability, which has a higher spatial and temporal resolution and may provide more meaningful information.
Emergence from minimally conscious state patients with a higher consciousness level could experience ongoing neural dysfunction, especially in the frontoparietal region. Theta functional connectivity at the whole-brain level may be a mechanism for the different levels of functional disability in this population. Exploration of brain features may contribute to a more detailed characterization of EMCS patients, and sustained treatment of such patients should be considered. In the future, patients could undergo individual cognitive evaluation and rehabilitation programs.
The datasets presented in this article are not readily available because the data also forms part of an ongoing study. Requests to access the datasets should be directed to the corresponding author QXe.
The studies involving human participants were reviewed and approved by Zhujiang Hospital, Southern Medical University. The patients/participants provided their written informed consent to participate in this study.
WW contributed to the conception and design of the study, performed the statistical analysis, and wrote the first draft of the manuscript. WW, CX, QXa, XZ, XH, HZ, and QL organized the database. CX, XH, QXa, and XZ wrote sections of the manuscript. QXe contributed to the conceptualization, funding acqusition, resources, supervision, and writing–review and editing. All authors contributed to the manuscript revision, read, and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81801119, 81974154, and 82171174) and Key Scientific and Brain-like research (Grant No. 202007030005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.972538/full#supplementary-material
CRS-R, coma recovery scale-revised; DMN, default mode network; DRS, disability rating scale; ECN, executive control network; EEG, electroencephalogram; EMCS, emergence from minimally conscious state; EOG, electrooculography; GABA, gamma-aminobutyric acid; HC, healthy control; MCS, minimally conscious state; PSD, power spectral density; VS/UWS, vegetative state/unresponsive wakefulness syndrome; wPLI, weighted phase lag index.
Andreou, C., Leicht, G., Nolte, G., Polomac, N., Moritz, S., Karow, A., et al. (2015). Resting-state theta-band connectivity and verbal memory in schizophrenia and in the high-risk state. Schizophr. Res. 161, 299–307. doi: 10.1016/j.schres.2014.12.018
Aubinet, C., Murphy, L., Bahri, M. A., Larroque, S. K., Cassol, H., Annen, J., et al. (2018). Brain, behavior, and cognitive interplay in disorders of consciousness: A multiple case study. Front. Neurol. 9:665. doi: 10.3389/fneur.2018.00665
Başar, E. (2013). A review of gamma oscillations in healthy subjects and in cognitive impairment. Int. J. Psychophysiol. 90, 99–117. doi: 10.1016/j.ijpsycho.2013.07.005
Betti, V., Della Penna, S., de Pasquale, F., and Corbetta, M. (2021). Spontaneous beta band rhythms in the predictive coding of natural stimuli. Neuroscientist 27, 184–201. doi: 10.1177/1073858420928988
Biel, A. L., Sterner, E., Röll, L., and Sauseng, P. (2022). Modulating verbal working memory with fronto-parietal transcranial electric stimulation at theta frequency: Does it work? Eur. J. Neurosci. 55, 405–425. doi: 10.1111/ejn.15563
Bodart, O., Gosseries, O., Wannez, S., Thibaut, A., Annen, J., Boly, M., et al. (2017). Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin. 14, 354–362. doi: 10.1016/j.nicl.2017.02.002
Bodien, Y. G., Martens, G., Ostrow, J., Sheau, K., and Giacino, J. T. (2020). Cognitive impairment, clinical symptoms and functional disability in patients emerging from the minimally conscious state. NeuroRehabilitation 46, 65–74. doi: 10.3233/NRE-192860
Buckner, R. L., and DiNicola, L. M. (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608. doi: 10.1038/s41583-019-0212-7
Cavaliere, C., Kandeepan, S., Aiello, M., Ribeiro de Paula, D., Marchitelli, R., Fiorenza, S., et al. (2018). Multimodal neuroimaging approach to variability of functional connectivity in disorders of consciousness: A PET/MRI pilot study. Front. Neurol. 9:861. doi: 10.3389/fneur.2018.00861
Chang, A., Bosnyak, D. J., and Trainor, L. J. (2018). Beta oscillatory power modulation reflects the predictability of pitch change. Cortex 106, 248–260. doi: 10.1016/j.cortex.2018.06.008
Chennu, S., Finoia, P., Kamau, E., Allanson, J., Williams, G. B., Monti, M. M., et al. (2014). Spectral signatures of reorganised brain networks in disorders of consciousness. PLoS Comput. Biol. 10:e1003887. doi: 10.1371/journal.pcbi.1003887
Colantonio, A., Gerber, G., Bayley, M., Deber, R., Yin, J., and Kim, H. (2011). Differential profiles for patients with traumatic and non-traumatic brain injury. J. Rehabil. Med. 43, 311–315. doi: 10.2340/16501977-0783
Cowley, B. U., Juurmaa, K., and Palomäki, J. (2022). Reduced power in fronto-parietal theta eeg linked to impaired attention-sampling in adult ADHD. eNeuro 9:ENEURO.0028-21.2021. doi: 10.1523/ENEURO.0028-21.2021
Di Perri, C., Bahri, M. A., Amico, E., Thibaut, A., Heine, L., Antonopoulos, G., et al. (2016). Neural correlates of consciousness in patients who have emerged from a minimally conscious state: A cross-sectional multimodal imaging study. Lancet Neurol. 15, 830–842. doi: 10.1016/S1474-4422(16)00111-3
Edlow, B. L., Claassen, J., Schiff, N. D., and Greer, D. M. (2021). Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 17, 135–156. doi: 10.1038/s41582-020-00428-x
Fernández-Espejo, D., Soddu, A., Cruse, D., Palacios, E. M., Junque, C., Vanhaudenhuyse, A., et al. (2012). A role for the default mode network in the bases of disorders of consciousness. Ann. Neurol. 72, 335–343. doi: 10.1002/ana.23635
Fingelkurts, A. A., Fingelkurts, A. A., Kivisaari, R., Pekkonen, E., Ilmoniemi, R. J., and Kähkönen, S. (2004). Enhancement of GABA-related signalling is associated with increase of functional connectivity in human cortex. Hum. Brain Mapp. 22, 27–39. doi: 10.1002/hbm.20014
Frohlich, J., Crone, J. S., Johnson, M. A., Lutkenhoff, E. S., Spivak, N. M., Dell’Italia, J., et al. (2022). Neural oscillations track recovery of consciousness in acute traumatic brain injury patients. Hum. Brain Mapp. 43, 1804–1820. doi: 10.1002/hbm.25725
Giacino, J. T., Ashwal, S., Childs, N., Cranford, R., Jennett, B., Katz, D. I., et al. (2002). The minimally conscious state: Definition and diagnostic criteria. Neurology 58, 349–353. doi: 10.1212/wnl.58.3.349
Giacino, J. T., Kalmar, K., and Whyte, J. (2004). The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility11no commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated. Arch. Phys. Med. Rehabil. 85, 2020–2029. doi: 10.1016/j.apmr.2004.02.033
Iyer, K. K., Au, T. R., Angwin, A. J., Copland, D. A., and Dissanayaka, N. N. (2020). Theta and gamma connectivity is linked with affective and cognitive symptoms in Parkinson’s disease. J. Affect. Disord. 277, 875–884. doi: 10.1016/j.jad.2020.08.086
Jafari, Z., Kolb, B. E., and Mohajerani, M. H. (2020). Neural oscillations and brain stimulation in Alzheimer’s disease. Prog. Neurobiol. 194:101878. doi: 10.1016/j.pneurobio.2020.101878
Lancioni, G. E., Singh, N. N., O’Reilly, M. F., Sigafoos, J., Olivetti Belardinelli, M., Buonocunto, F., et al. (2014). Technology-aided programs for post-coma patients emerged from or in a minimally conscious state. Front. Hum. Neurosci. 8:931. doi: 10.3389/fnhum.2014.00931
Laureys, S., Celesia, G. G., Cohadon, F., Lavrijsen, J., León-Carrión, J., Sannita, W. G., et al. (2010). Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 8:68. doi: 10.1186/1741-7015-8-68
Lesenfants, D., Habbal, D., Chatelle, C., Schnakers, C., Laureys, S., and Noirhomme, Q. (2016). Electromyographic decoding of response to command in disorders of consciousness. Neurology 87, 2099–2107. doi: 10.1212/WNL.0000000000003333
Llinás, R., Urbano, F. J., Leznik, E., Ramírez, R. R., and van Marle, H. J. F. (2005). Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 28, 325–333. doi: 10.1016/j.tins.2005.04.006
Marino, M. H., and Whyte, J. (2022). Treatment trials in disorders of consciousness: Challenges and future directions. Brain Sci. 12:569. doi: 10.3390/brainsci12050569
Murphy, L. (2018). The cognitive assessment by visual election (CAVE): A pilot study to develop a cognitive assessment tool for people emerging from disorders of consciousness. Neuropsychol. Rehabil. 28, 1275–1284. doi: 10.1080/09602011.2018.1454327
Nakase-Richardson, R., Yablon, S. A., Sherer, M., Nick, T. G., and Evans, C. C. (2009). Emergence from minimally conscious state: Insights from evaluation of posttraumatic confusion. Neurology 73, 1120–1126. doi: 10.1212/WNL.0b013e3181bacf34
Peraza, L. R., Asghar, A. U. R., Green, G., and Halliday, D. M. (2012). Volume conduction effects in brain network inference from electroencephalographic recordings using phase lag index. J. Neurosci. Methods 207, 189–199. doi: 10.1016/j.jneumeth.2012.04.007
Rappaport, M. (2005). The disability rating and coma/near-coma scales in evaluating severe head injury. Neuropsychol. Rehabil. 15, 442–453. doi: 10.1080/09602010443000335
Rappaport, M., Hall, K. M., Hopkins, K., Belleza, T., and Cope, D. N. (1982). Disability rating scale for severe head trauma: Coma to community. Arch. Phys. Med. Rehabil. 63, 118–123.
Roa Romero, Y., Senkowski, D., and Keil, J. (2015). Early and late beta-band power reflect audiovisual perception in the McGurk illusion. J. Neurophysiol. 113, 2342–2350. doi: 10.1152/jn.00783.2014
Rodriguez Moreno, D., Schiff, N. D., Giacino, J., Kalmar, K., and Hirsch, J. (2010). A network approach to assessing cognition in disorders of consciousness. Neurology 75, 1871–1878. doi: 10.1212/WNL.0b013e3181feb259
Schiff, N. D. (2016). “Mesocircuit mechanisms underlying recovery of consciousness following severe brain injuries: model and predictions,” in Brain Function and Responsiveness in Disorders of Consciousness, eds M. M. Monti and W. G. Sannita (Cham: Springer International Publishing), 195–204. doi: 10.1007/978-3-319-21425-2
Schubring, D., and Schupp, H. T. (2021). Emotion and brain oscillations: High arousal is associated with decreases in alpha- and lower beta-band power. Cereb. Cortex 31, 1597–1608. doi: 10.1093/cercor/bhaa312
Shah, S. A., Mohamadpour, M., Askin, G., Nakase-Richardson, R., Stokic, D. S., Sherer, M., et al. (2017b). Focal electroencephalographic changes index post-traumatic confusion and outcome. J. Neurotrauma 34, 2691–2699. doi: 10.1089/neu.2016.4911
Shah, S. A., Goldin, Y., Conte, M. M., Goldfine, A. M., Mohamadpour, M., Fidali, B. C., et al. (2017a). Executive attention deficits after traumatic brain injury reflect impaired recruitment of resources. Neuroimage Clin. 14, 233–241. doi: 10.1016/j.nicl.2017.01.010
Stender, J., Kupers, R., Rodell, A., Thibaut, A., Chatelle, C., Bruno, M.-A., et al. (2015). Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J. Cereb. Blood Flow Metab. 35, 58–65. doi: 10.1038/jcbfm.2014.169
Thibaut, A., Bruno, M.-A., Chatelle, C., Gosseries, O., Vanhaudenhuyse, A., Demertzi, A., et al. (2012). Metabolic activity in external and internal awareness networks in severely brain-damaged patients. J. Rehabil. Med. 44, 487–494. doi: 10.2340/16501977-0940
Thibaut, F. A., Chatelle, C., Wannez, S., Deltombe, T., Stender, J., Schnakers, C., et al. (2015). Spasticity in disorders of consciousness: A behavioral study. Eur. J. Phys. Rehabil. Med. 51, 389–397.
Vanhaudenhuyse, A., Demertzi, A., Schabus, M., Noirhomme, Q., Bredart, S., Boly, M., et al. (2011). Two distinct neuronal networks mediate the awareness of environment and of self. J. Cogn. Neurosci. 23, 570–578. doi: 10.1162/jocn.2010.21488
Vinck, M., Oostenveld, R., van Wingerden, M., Battaglia, F., and Pennartz, C. M. A. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 55, 1548–1565. doi: 10.1016/j.neuroimage.2011.01.055
Vogt, B. A., and Laureys, S. (2005). Posterior cingulate, precuneal and retrosplenial cortices: Cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 150, 205–217. doi: 10.1016/S0079-6123(05)50015-3
von Lautz, A. H., Herding, J., Ludwig, S., Nierhaus, T., Maess, B., Villringer, A., et al. (2017). Gamma and beta oscillations in human meg encode the contents of vibrotactile working memory. Front. Hum. Neurosci. 11:576. doi: 10.3389/fnhum.2017.00576
Wang, J., Wang, X., Xia, M., Liao, X., Evans, A., and He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 9:386. doi: 10.3389/fnhum.2015.00386
Wu, M., Li, F., Wu, Y., Zhang, T., Gao, J., Xu, P., et al. (2020). Impaired frontoparietal connectivity in traumatic individuals with disorders of consciousness: A dynamic brain network analysis. Aging Dis. 11, 301–314. doi: 10.14336/AD.2019.0606
Yan, Y., Zhao, A., Ying, W., Qiu, Y., Ding, Y., Wang, Y., et al. (2021). Functional connectivity alterations based on the weighted phase lag index: An exploratory electroencephalography study on Alzheimer’s Disease. Curr. Alzheimer Res. 18, 513–522. doi: 10.2174/1567205018666211001110824
Zhang, J., Safar, K., Emami, Z., Ibrahim, G. M., Scratch, S. E., da Costa, L., et al. (2020). Local and large-scale beta oscillatory dysfunction in males with mild traumatic brain injury. J. Neurophysiol. 124, 1948–1958. doi: 10.1152/jn.00333.2020
Keywords: emergence from minimally conscious state, disability rating scale, EEG, frontoparietal region, functional connectivity
Citation: Wu W, Xu C, Huang X, Xiao Q, Zheng X, Zhong H, Liang Q and Xie Q (2022) Is frontoparietal electroencephalogram activity related to the level of functional disability in patients emerging from a minimally conscious state? A preliminary study. Front. Hum. Neurosci. 16:972538. doi: 10.3389/fnhum.2022.972538
Received: 18 June 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Joel Frohlich, University of Tübingen, GermanyReviewed by:
Jitka Annen, University of Liège, BelgiumCopyright © 2022 Wu, Xu, Huang, Xiao, Zheng, Zhong, Liang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuyou Xie, eHF5NzE4MEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.