
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Hum. Neurosci. , 24 August 2022
Sec. Brain Imaging and Stimulation
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.933718
This article is part of the Research Topic Normalization of Human Social and Emotional Dysfunction Using Noninvasive Brain Modulation Approaches: From Proof-Of-Concept to Clinical Studies View all 8 articles
Dysregulated frontostriatal circuitries are viewed as a common target for the treatment of aberrant behaviors in various psychiatric and neurological disorders. Accordingly, experimental neurofeedback paradigms have been applied to modify the frontostriatal circuitry. The human frontostriatal circuitry is topographically and functionally organized into the “limbic,” the “associative,” and the “motor” subsystems underlying a variety of affective, cognitive, and motor functions. We conducted a systematic review of the literature regarding functional magnetic resonance imaging-based neurofeedback studies that targeted brain activations within the frontostriatal circuitry. Seventy-nine published studies were included in our survey. We assessed the efficacy of these studies in terms of imaging findings of neurofeedback intervention as well as behavioral and clinical outcomes. Furthermore, we evaluated whether the neurofeedback targets of the studies could be assigned to the identifiable frontostriatal subsystems. The majority of studies that targeted frontostriatal circuitry functions focused on the anterior cingulate cortex, the dorsolateral prefrontal cortex, and the supplementary motor area. Only a few studies (n = 14) targeted the connectivity of the frontostriatal regions. However, post-hoc analyses of connectivity changes were reported in more cases (n = 32). Neurofeedback has been frequently used to modify brain activations within the frontostriatal circuitry. Given the regulatory mechanisms within the closed loop of the frontostriatal circuitry, the connectivity-based neurofeedback paradigms should be primarily considered for modifications of this system. The anatomical and functional organization of the frontostriatal system needs to be considered in decisions pertaining to the neurofeedback targets.
Neurofeedback (NF) is a biofeedback method that enables individuals to modify the relevant neural targets for treatment purposes. Magnetic resonance imaging-based NF, which has been continuously advanced over the last two decades (Weiskopf, 2012; Sulzer et al., 2013; Watanabe et al., 2017), can induce an altered activation level in the targeted brain region or modify connectivity between different brain regions. The main goal of this effort is the amelioration of aberrant activation and connectivity patterns in clinical populations. The fundamental prerequisite for NF treatment is the selection of an appropriate target. NF techniques have been probed on various structures as a potential target for behavioral improvement and treatment of neuropsychiatric and movement disorders (Linden and Turner, 2016; Linhartová et al., 2019; Lipp and Cohen Kadosh, 2020; Anil et al., 2021). The search for an optimal NF target ought to involve the existing knowledge about the anatomical and functional organization of the underlying neurocircuitry. Various components of the frontostriatal circuitry (FSC) have been common targets for probing NF-induced modifications.
The FSC is known to be involved in a variety of affective, cognitive, and motor functions, underpinning complex human behavior (Bonelli et al., 2007; Beste et al., 2012). According to clinical studies, alterations within the FSC may underlie the pathophysiology of various psychiatric and neurological disorders, including major depressive disorder (MDD) (Baxter et al., 1989; Greicius et al., 2007), schizophrenia (Li et al., 2020), substance-use disorders (SUD) (Fettes et al., 2017), anxiety disorders including obsessive-compulsive disorder (OCD) (Graybiel and Rauch, 2000; Dunlop et al., 2016), post-traumatic stress disorder (PTSD), and eating disorders (Foerde et al., 2015). Additionally, abnormalities within this circuitry have been proposed to contribute to the pathophysiology of many primary movement disorders, such as Huntington's disease (HD), Parkinson's disease (PD), and tic disorders (Galvan et al., 2015; Blumenstock and Dudanova, 2020). It is therefore not surprising that the components of this circuitry, such as the prefrontal regions, the thalamus and the striatal nuclei, have been modification targets for trials probing new treatment strategies like transcranial magnetic stimulation (TMS) (Alkhasli et al., 2019; Lefaucheur et al., 2020) and deep brain stimulation (DBS) (Aum and Tierney, 2018; Andrade et al., 2020).
However, the question is which targets are effective in improving the symptoms being studied. Have prospective controlled trials already provided an answer to this question? Indeed, there is no universal consensus on the best target for NF in patients suffering from neuropsychiatric symptoms. On the other hand, NF interventions are highly dependent on precise localization of the target regions or networks (Linden et al., 2012). It is timely to make sure that the available knowledge about the organization of the FSC is well integrated into the selection of brain targets for NF modifications.
The FSC forms a closed-loop system with direct projections from the frontal cortex to the striatum and indirect projections from the striatum (via the thalamus) back to the frontal cortex (Figure 1) (Öngür and Price, 2000). Already in 1986, Alexander et al. proposed a three-part organization, with distinct “limbic,” “associative,” and “motor” subsystems, within the circuitry linking the frontal cortical regions and the striatum (Alexander et al., 1986). Since then, subdivisions of the frontostriatal projection system have been extensively investigated based on various approaches through anatomical links, histochemical properties, patterns of gene expression and biochemical variations. The findings converge in a functional organization, subserving affective, cognitive, and motor functions (Basile et al., 2021). It is also likely to be topographically organized along medio-lateral and ventral-dorsal axes (Jarbo and Verstynen, 2015; Haber, 2016). The three major functional subsystems share common features and the main anatomical structures. All three circuits originate in the frontal cortex, connect with the striatum (putamen, ventral striatum, or caudate), project to the globus pallidus and the substantia nigra and from there form connections with the thalamus. Each subsystem forms a loop and connects back to the frontal cortex (Bonelli et al., 2007).
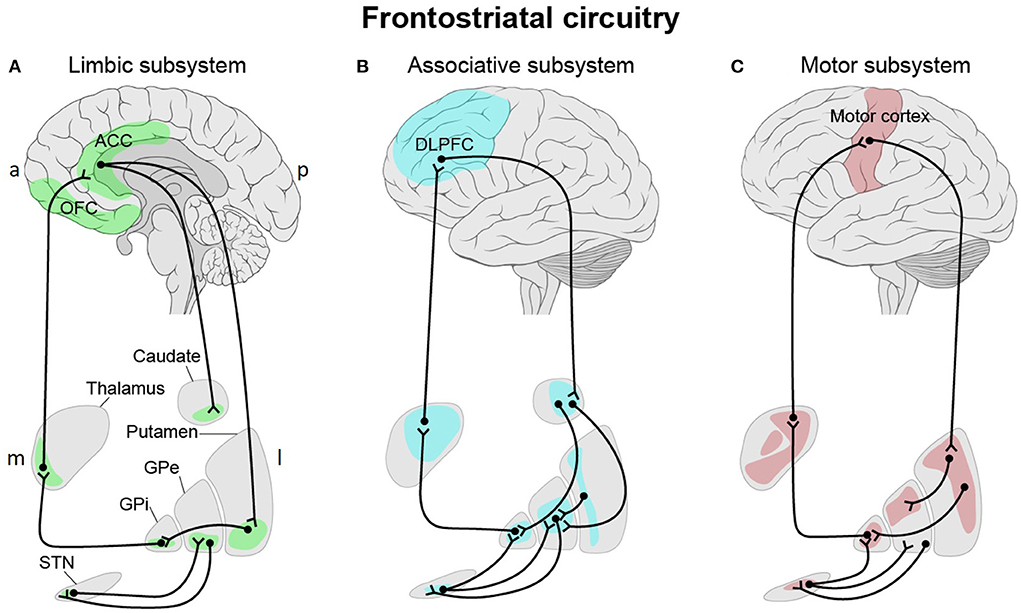
Figure 1. Three-part organization of the frontostriatal circuitry with distinct “limbic” (A), “associative” (B), and “motor” (C) subsystems linking the frontal cortical regions and the striatum. (A) The “limbic” subsystem is divided into two parts. The first part originates in the lateral orbitofrontal cortex (OFC) and projects to the ventromedial sector of the caudate nucleus. This region innervates the dorsomedial globus pallidus interna (GPi) and rostromedial substantia nigra (SNr, not shown). The latter projects to the ventral anterior thalamic nucleus, magnocellular part and the mediodorsal thalamic nucleus, magnocellular part before it forms a closed loop with the lateral OFC. Both the anterior cingulate cortex (ACC) and medial OFC project to the ventral striatum (ventromedial caudate, ventral putamen, nucleus accumbens and olfactory tubercle) which in turn project to the rostrolateral GPi and the rostrodorsal SNr. Via the mediodorsal thalamic nucleus, magnocellular part, the SNr sends fibers back to the ACC and medial OFC. (B) Within the “associative” subsystem, the dorsolateral prefrontal cortex (DLPFC) projections terminate in the dorsolateral head of the caudate nucleus. The caudate nucleus projects to the dorsomedial part of the GPi and globus pallidus externa (GPe) and from there to the rostral portions of the SNr. The GPi closes the loop via the parvocellular portion of the ventral anterior thalamic nucleus to the DLPFC. (C) Motor-relevant cortical areas (motor, premotor, supplementary motor, and somatosensory cortices) innervate the caudal putamen, which sends input to the ventrolateral GPi and GPe and to the caudolateral portions of the SNr. The GPi sends input to the ventrolateral nucleus of the thalamus, which in turns forms a closed loop with the motor cortex. In all three subsystems, the subthalamic nucleus (STN) modulates input to the thalamus via the GPi and/or GPe. a, anterior; p, posterior; m, medial; l, lateral. This figure adapted from Obeso et al. (2008).
The anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC) (“limbic” subsystem) project most densely to the ventral striatum, which includes the ventromedial caudate, the ventral putamen, and the nucleus accumbens (Mega et al., 1997). The lateral OFC sends fibers to the ventromedial caudate nucleus (Tekin and Cummings, 2002; Barbas, 2007), while the medial OFC and the ACC project to the ventral striatum (ventromedial caudate, ventral putamen and nucleus accumbens) (Mega et al., 1997; Haber, 2003; Levy and Dubois, 2006; Bonelli et al., 2007).
The ACC, as part of the limbic and affective system, monitors cognitive regulation of emotions (Delevich et al., 2015). Additionally, this region seems to be involved in action selection and expression of emotion- and fear-related evaluation (Stevens et al., 2011). The OFC is a key brain area in emotional reappraisal and the generation of affective states (Fettes et al., 2017). Additionally, this brain area is involved in the representation of rewarded and non-rewarded values (Rolls, 2019), in reward-based learning (Kringelbach, 2005), and in reward prediction error (Boorman et al., 2013).
Neuroimaging studies have revealed abnormal activations in the ACC and the OFC leading to the dysregulation of their projections in the ventral striatum in various pathological conditions such as MDD (Biver et al., 1994; Frodl et al., 2010), OCD (Graybiel and Rauch, 2000; Menzies et al., 2008; Radua et al., 2010), substance-use disorders (Everitt and Robbins, 2005; Burton et al., 2015), schizophrenia (Wang et al., 2015), Tourette (Neuner et al., 2014; O'Neill et al., 2019), PTSD (Chen et al., 2019), and attention deficit hyperactivity disorder (ADHD) (Bledsoe et al., 2013).
Neurons from the dorsolateral prefrontal cortex (DLPFC) send their input most densely to the dorsolateral head of the caudate nucleus (“associative” subsystem) (Parent et al., 1984; Parent and Hazrati, 1995).
The DLPFC plays a key node in dorsal attention networks, which supports basic cognitive selection of sensory information and responses (Corbetta and Shulman, 2002; Kuo and Nitsche, 2012). It is involved in executive functions including working memory, selective attention, control of cognitive processes (Curtis and D'Esposito, 2003), and decision making (Krawczyk, 2002). The “associative” subsystem is also involved in anticipation, monitoring, and use of feedback in task performance as part of executive function (Alvarez and Emory, 2006). Even though no direct projections to the emotion generating areas exist, the DLPFC influences emotional response (Ochsner et al., 2012).
Dysfunction within this circuitry has been associated with SUD (Abernathy et al., 2010; Hu et al., 2015), MDD (Koenigs and Grafman, 2009; Furman et al., 2011), schizophrenia (Callicott et al., 2000), OCD (Figee et al., 2013), eating disorders (Hayes et al., 2015), and PTSD (Ke et al., 2015).
Neurons from the motor-related cortical areas (motor, premotor, supplementary motor, and somatosensory cortices) innervate the caudal putamen (“motor” subsystem) in a topographic pattern (Lehéricy et al., 2006; DeLong and Wichmann, 2007). This subsystem is mainly associated with planning, preparation, control, and execution of movement (Luppino and Rizzolatti, 2000; Nachev et al., 2008; Svoboda and Li, 2018).
Dysfunctions of this subsystem are associated with both common psychiatric disorders such as schizophrenia (Walther, 2015), MDD (Exner et al., 2009), OCD, and bipolar disorder (Hirjak et al., 2018), and major movement disorders such as PD (Galvan et al., 2015) and HD (Blumenstock and Dudanova, 2020). The abnormalities of this system seem to be an intersection between movement disorders and psychiatric conditions (Cummins et al., 2015). Frequently, affective disorders or psychosis predate the onset of motor symptoms in these patients (Ishihara and Brayne, 2006; Duff et al., 2007; Xu et al., 2016). Tourette's syndrome, a tic disorder with chronic motor and/or vocal tics and psychiatric impairments and comorbidities, also emphasizes this frontostriatal subsystem as an interface for neurological and psychiatric pathologies (Neuner et al., 2014).
The current review article is dedicated to NF interventions that aim at introducing changes in the activations of brain regions within the FSC. Several neuromodulation techniques have been investigated in recent decades, including invasive methods such as DBS (Dougherty, 2018) and non-invasive methods such as (repetitive) TMS (Lefaucheur et al., 2020), transcranial direct current stimulation (tDCS) (Palm et al., 2016), and electroconvulsive therapy (ECT) (Park et al., 2021). Almost all of these techniques have been focused on inducing activation changes in brain regions within the FSC. Various localizations have been tested to find an optimal stimulation target. However, multiple targets have been shown to be effective in improving the related symptoms (Sadleir et al., 2012; Honey et al., 2017; Barbour et al., 2019).
Unlike the direct neurostimulation techniques like DBS and TMS, functional magnetic resonance imaging (fMRI) NF enables targeting the connectivity of two or more brain regions, which may enhance the treatment effect (Watanabe et al., 2017). For this reason, fMRI NF, as a non-invasive neuromodulation technique, should be considered as a potential technique for neuromodulation of the FSC in treatment of psychiatric conditions and movement disorders. This technique is a form of biofeedback in which the participant receives real-time information about their ongoing brain activation, allowing for self-regulation training that can lead to clinical improvement and symptom reduction (Weiskopf, 2012). In a NF paradigm, participants learn to regulate their own neural activation guided by feedback to facilitate a desired neuropsychological pattern. Previous reviews have analyzed various mechanisms underlying NF (Sitaram et al., 2017; Shibata et al., 2019; Muñoz-Moldes and Cleeremans, 2020). Learning processes (Strehl, 2014) like operant conditioning (Birbaumer et al., 2013) and reinforcement learning (Lubianiker et al., 2022) have been broadly accepted as the neuropsychological mechanism for NF-based training. While learning control over specific neural substrates is assumed to underlie changing specific behaviors, the role of awareness (Stirner et al., 2022), metacognition and various forms of implicit and explicit learning in NF-based training have not been uncovered yet. Understanding the learning processes involved in NF in terms of frontostriatal functioning and monoaminergic modulation is essential for developing efficient NF interventions for brain and mental disorders. Recording of fMRI as an indirect measure of brain activity might be influenced by neurotransmitter modulation. Surprisingly, there is only one study that directly investigated the effect of NF on the endogenous release of dopamine (Ros et al., 2021). Imaging of the neurotransmission system in association with NF training using single-photon emission tomography and positron emission tomography might be leading the way to investigate the role of brain's key neuromodulatory systems in NF-based modulations.
In the early 2000s, electroencephalography (EEG) was the first and only method for providing real-time information about brain activation in the NF setting. However, the use of EEG does not permit accurate localization (Cohen et al., 2011). In particular, studying affective disorders using EEG may be inadequate given that abnormal brain activity is also found in subcortical areas such as the thalamus, the striatum, and the amygdala. Luckily, it has been shown that fMRI NF can be employed to detect changes in blood oxygen level-dependent (BOLD) activity in these brain regions of interest in real time (Sulzer et al., 2013). Due to its whole-brain coverage and high resolution, fMRI NF has gained considerable popularity over the past decade (Weiskopf, 2012), with 99 articles, according to a recent review (Thibault et al., 2018), having been published based on real-time fMRI (rt-fMRI) studies.
One of the main issues in designing NF intervention studies is the choice of target regions. The structural and functional correlates of most major psychiatric disorders are becoming better characterized, owing to expanding databases of neuroimaging studies and developing quantitative meta-analytic algorithms. Brain areas and networks within the FSC, which are affected across various psychiatric disorders (Casey et al., 1997; Cubillo et al., 2012; Pulcu and Elliott, 2015), may represent promising targets for NF intervention studies. Providing a systematic review of the studies that have investigated NF interventions based on target regions within the FSC, we aimed to address the following questions: (1) Which target regions within the FSC have been selected for rt-fMRI NF? (2) Which behavioral/clinical parameters have been addressed by the rt-fMRI NF targeting the FSC? Furthermore, issues pertaining to the design of rt-fMRI NF studies and suggestions for future studies are discussed.
In this systematic review we followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009).
We searched the following electronic databases for peer-reviewed studies published until February 22, 2022: Pubmed/MEDLINE, Web of Science/Web of Knowledge, EMBASE, Scopus, Cochrane Library, and PsycINFO. Further internet-based searches were carried out on the “Real-Time Functional Imaging and Neurofeedback database” and “ClinicalTrials.gov.” The Cochrane Library and ClinicalTrials.gov was used to identify currently ongoing or planned studies.
The following search terms and syntax in title, abstract and keywords were used: (Neurofeedback OR Biofeedback) AND (fMRI OR “functional MRI” OR “functional magnetic resonance imaging”) AND (thalamus OR striat* OR putamen OR accumbens OR cauda* OR subthalamic OR pallidu* OR “Olfactory tubercle” OR “substantia nigra” OR cingulate OR ACC OR OFC OR IFG OR *PFC OR *FRONTAL OR “*motor cortex” OR SMA). The search was conducted by the author LO.
All study designs applying fMRI-based NF techniques to modulate regions belonging to the FSC were considered. All pilot and feasibility studies, randomized controlled trials, clinical studies, and cohort and case control studies in original research format were selected (reviews, book chapters, and conference abstracts were excluded). We only included studies with humans with no limitations in sex, age, ethnicity, and nationality. Only studies published in the English language were included.
After removing duplicates, study selection was conducted by two reviewers (LO and JM). First, titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened to identify studies that would potentially meet the inclusion criteria outlined above. Then full texts of these potentially eligible studies were retrieved and assessed for eligibility. Studies originating from the same author group and/or research group were carefully screened to avoid duplication of data. A total of 79 studies met the above-mentioned criteria and were considered for data analysis.
Data from the selected studies was extracted by the authors LO and JM and stored in an excel sheet based on the following template:
1. Information on study population
• sample size
• average age
• gender distribution
• healthy volunteers and/or clinical population
• (clinical) population specification
2. Information on the NF Intervention
• region(s) of interest (ROI)
• regulation direction (increase or decrease)
• number and duration of sessions and runs
• feedback timescale (intermittent or continuous), feedback type (numerical, social, scale, or curve)
3. Statistical differences
• between group comparison randomized
• between group comparison not randomized
• within group comparison randomized
• within group comparison not randomized
4. Control condition
• placebo control (yoked/computer-generated sham feedback, alternative ROI feedback)
• feedback from contralateral ROI
• feedback based on opposite regulation direction
• control without feedback intervention (with or without fMRI)
• no control condition
• other
5. Other study information
• blinding (single-blinded, double-blinded, or non-blinded; if a study did not specify blinding, it was also classified as not blinded)
• pre-registration
6. Imaging findings regarding the real-time NF [changes in brain activity and connectivity of the target region(s)]
7. Post-hoc imaging findings pre and post NF intervention: Whole-brain changes in brain activity and connectivity
8. Behavioral outcome of the NF intervention
9. Clinical outcome of the NF intervention (if any)
10. Follow-up effects of NF intervention (if any)
All extracted data were mutually checked by LO and JM. Conflicting results were discussed among authors to achieve a consensus.
Tables 1–4 show the aforementioned extracted data. Additional information on the studies is provided in Supplementary Table 1.
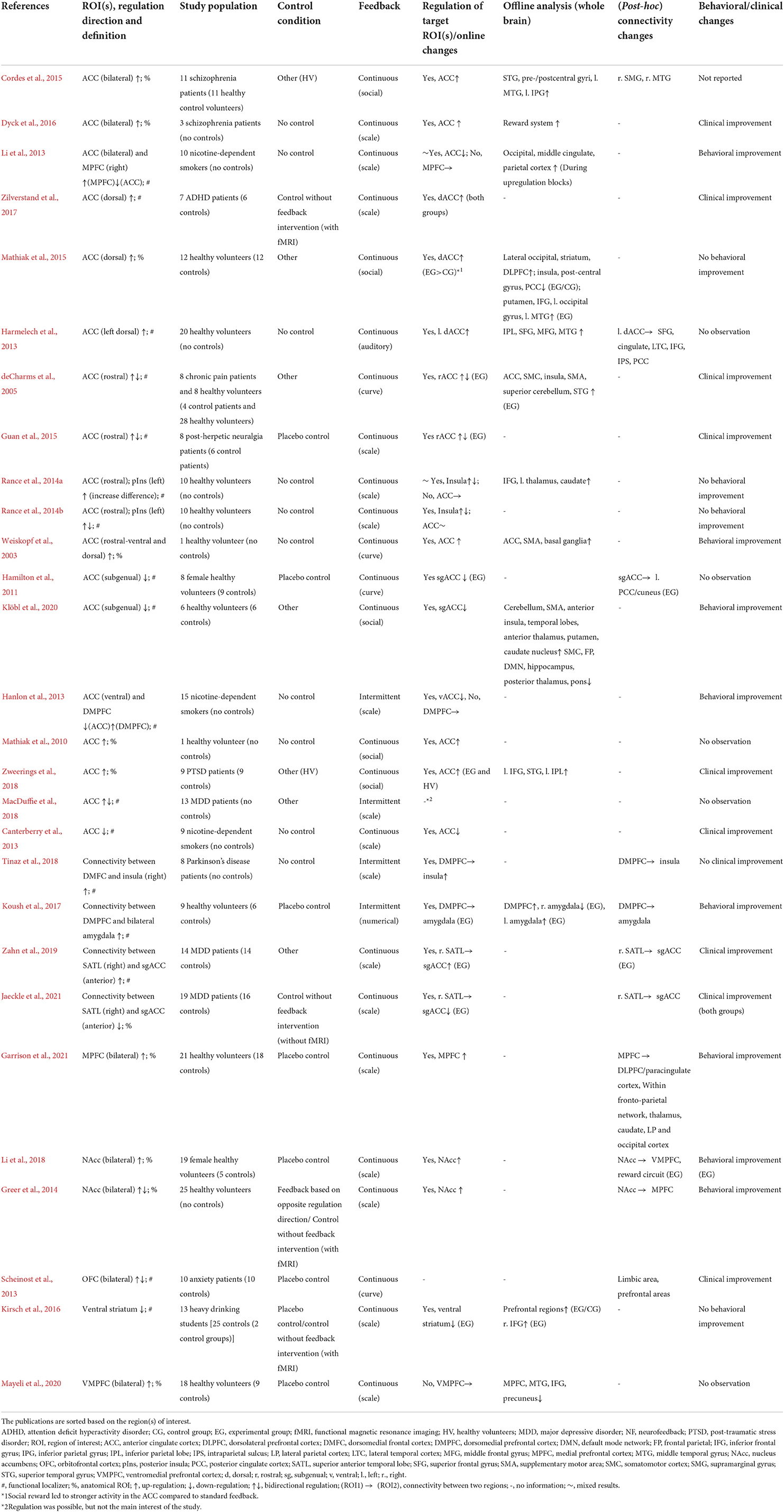
Table 1. Details of real-time fMRI neurofeedback studies with regulation target in the “limbic subsystem.”
For the sake of completeness, we also surveyed pre-registered studies, which have yet to publish results. The corresponding information is presented in Supplementary Table 2.
Our initial search retrieved 1,537 citations. After eliminating duplicates, 673 articles were assessed based on the search criteria in their title and/or abstract. Thirty-five additional records could be identified through other sources such as reference lists. A total of 463 articles were excluded because they did not meet the inclusion criteria. The final number of studies included in the review was 79. Figure 2 shows a flow diagram of the article selection process.
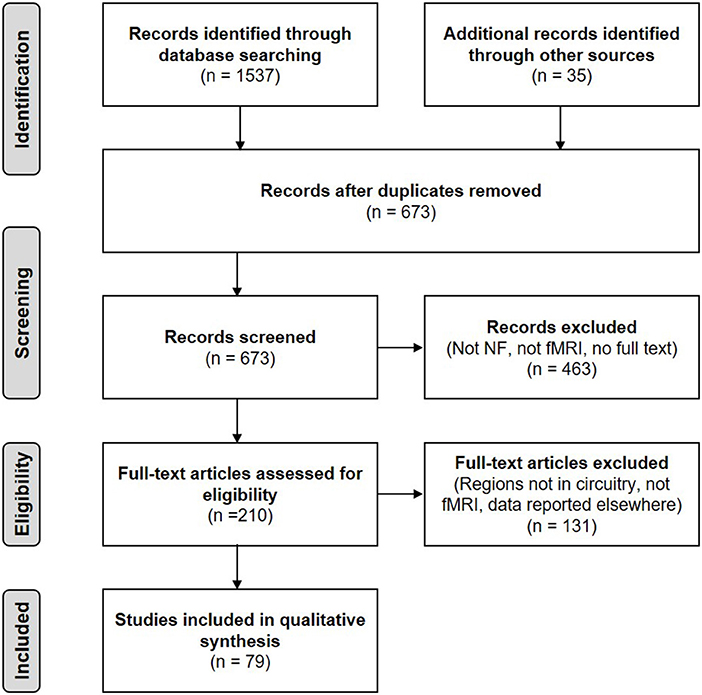
Figure 2. PRISMA flow diagram of the literature search (adapted from Moher et al., 2009).
The majority of studies originate from the last 10 years (2012–2021) (n = 68, 86%), with the first publication investigating fMRI-based NF in the SMA in 2003 (Weiskopf et al., 2003). Based on our search criteria, the survey confirmed a growing tendency, which has been growing steadily over the past decade, toward research in rt-fMRI NF (Tursic et al., 2020).
The data on study populations were extracted from the selected publications into major categories. In most publications, the potential of rt-fMRI NF is investigated in healthy volunteers (n = 41, 52%). The clinical populations include SUD (n = 9, 11%), depression (n = 6, 8%), anxiety disorders (n = 4, 5%), schizophrenia (n = 3, 4%), PD (n = 3, 4%), paralysis after stroke (n = 3, 4%), PTSD (n = 2, 3%), eating disorders (n = 2, 3%), HD (n = 2, 3%), chronic pain disorders (n = 2, 3%), ADHD (n = 1, 1%), and Tourette's syndrome (n = 1, 1%) (Figure 3). The total number of participants ranges from 1 to 76 with the sample sizes of the published studies growing over the last 10 years.
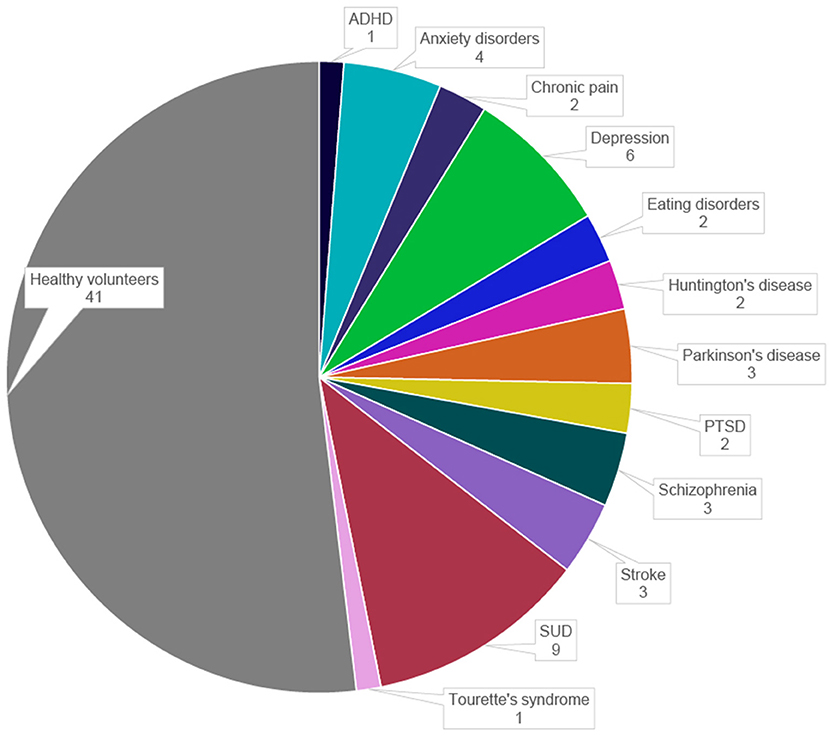
Figure 3. Distribution of study populations in the selected publications. ADHD, attention deficit hyperactivity disorder; PTSD, post-traumatic stress disorder; SUD, substance-use disorders.
In the clinical groups the age of the participants was various with the mean age of 38 years for depression, about 20 years for anxiety, 30 years for eating disorders, 37 years for schizophrenia, 37 years for ADHD, and 43 years for PTSD. Participants in NF studies with neurological diseases were older and aged above 39 years for PD, above 49 years for stroke and around 50 years for HD. In the NF studies that examined patients with chronic pain, the average age was around 50 years. In the study investigating NF in patients with Tourette's syndrome the average age was 16. In one of the studies with anxiety patients, age was not mentioned (Scheinost et al., 2013).
Target regions were selected from all three subsystems of the FSC (Figure 4). The most studied target region in the publications is the ACC (n = 22, 28%) belonging to the limbic subsystem (Table 1), followed by the supplementary motor area (SMA) (n = 8, 10%) belonging to the motor subsystem (Table 3). The associative subsystem (Table 2) has been investigated in 16 publications (27%), targeting different parts of the prefrontal cortex (PFC), the DLPFC and the ventrolateral PFC, and the inferior frontal gyrus (IFG). Most of the studies have focused on the frontal side of the FSC with only four publications (5%) investigating NF modulation in subcortical components of the FSC, namely the striatum (Greer et al., 2014; Kirsch et al., 2016; Li et al., 2018) and the thalamus (Zotev et al., 2018). Notably, 18% of the publications have investigated the effect of NF on interconnections of the FSC regions (n = 14).
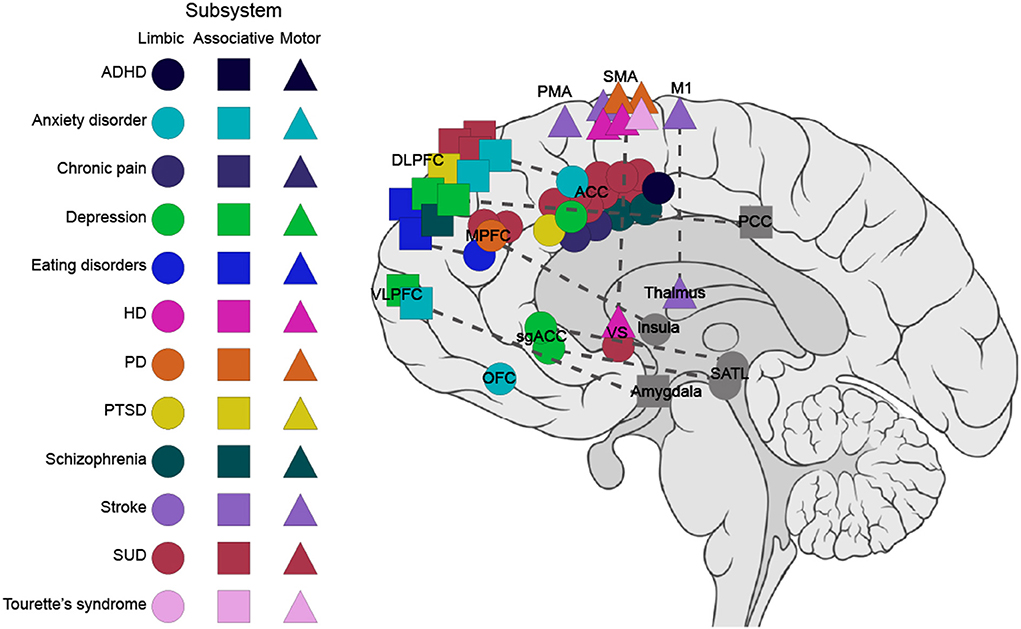
Figure 4. Targets within the frontostriatal circuitry (FSC) for fMRI-based NF in clinical populations. This graph depicts the targeted regions within the FSC for the three subsystems. The circle, square, and triangle refer to the regions in limbic, associative, and motor subsystems, respectively. The dashed line represents connectivity feedback. Regions outside the FSC with connectivity feedback to FSC regions are marked in gray. ACC, anterior cingulate cortex; ADHD, attention deficits hyperactivity disorder; DLPFC, dorsolateral prefrontal cortex; HD, Huntington's disease; IFG, inferior frontal gyrus; M1, primary motor cortex; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; PD, Parkinson's disease; PMA, premotor area; PTSD, post-traumatic stress disorder; SATL, superior anterior temporal lobe; sgACC, subgenual cingulate; SMA, supplementary motor area; SUD, substance-use disorders; VS, ventral striatum; VLPFC, ventrolateral prefrontal cortex.
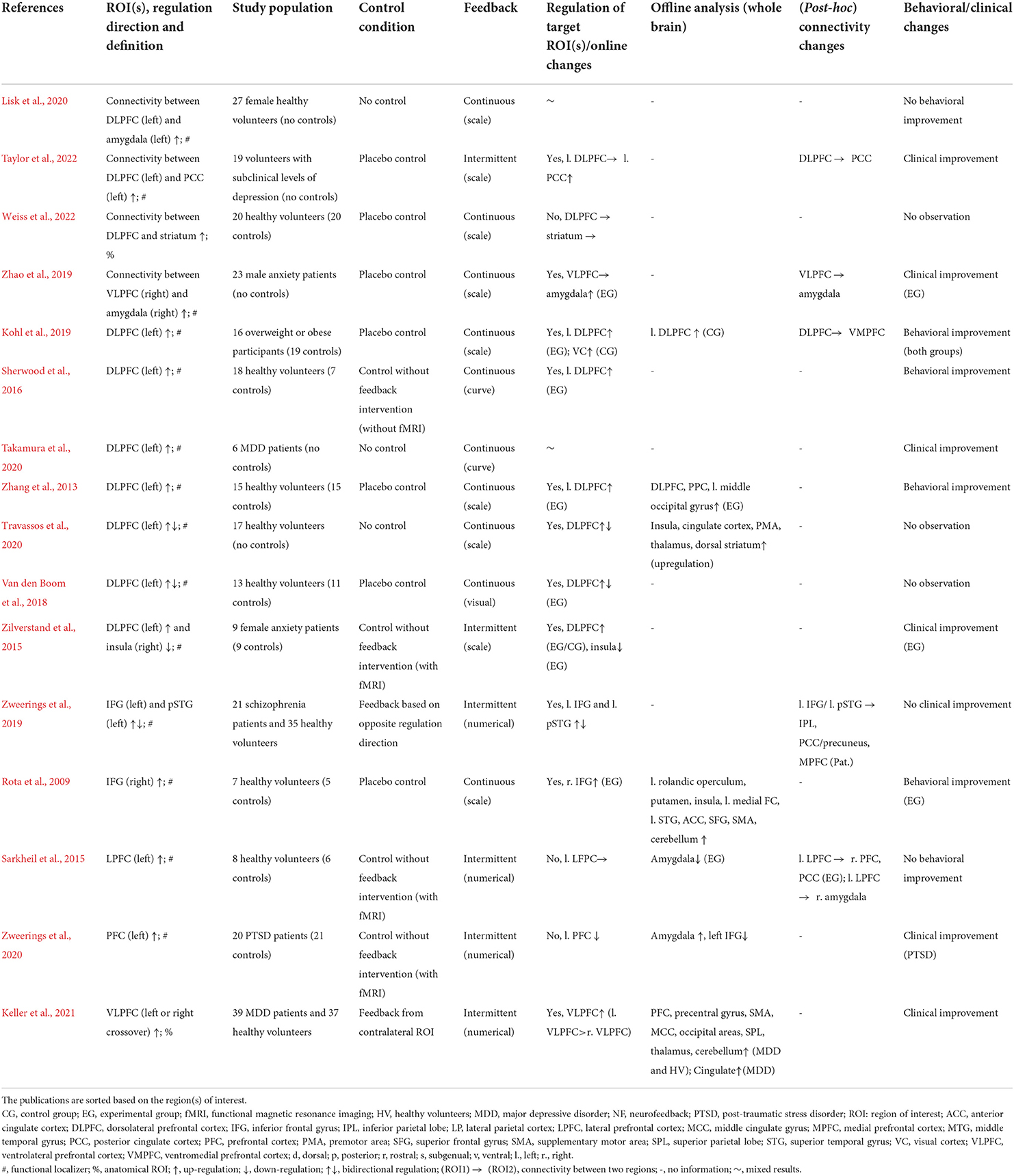
Table 2. Details of real-time fMRI neurofeedback studies with regulation target in the “associative subsystem.”
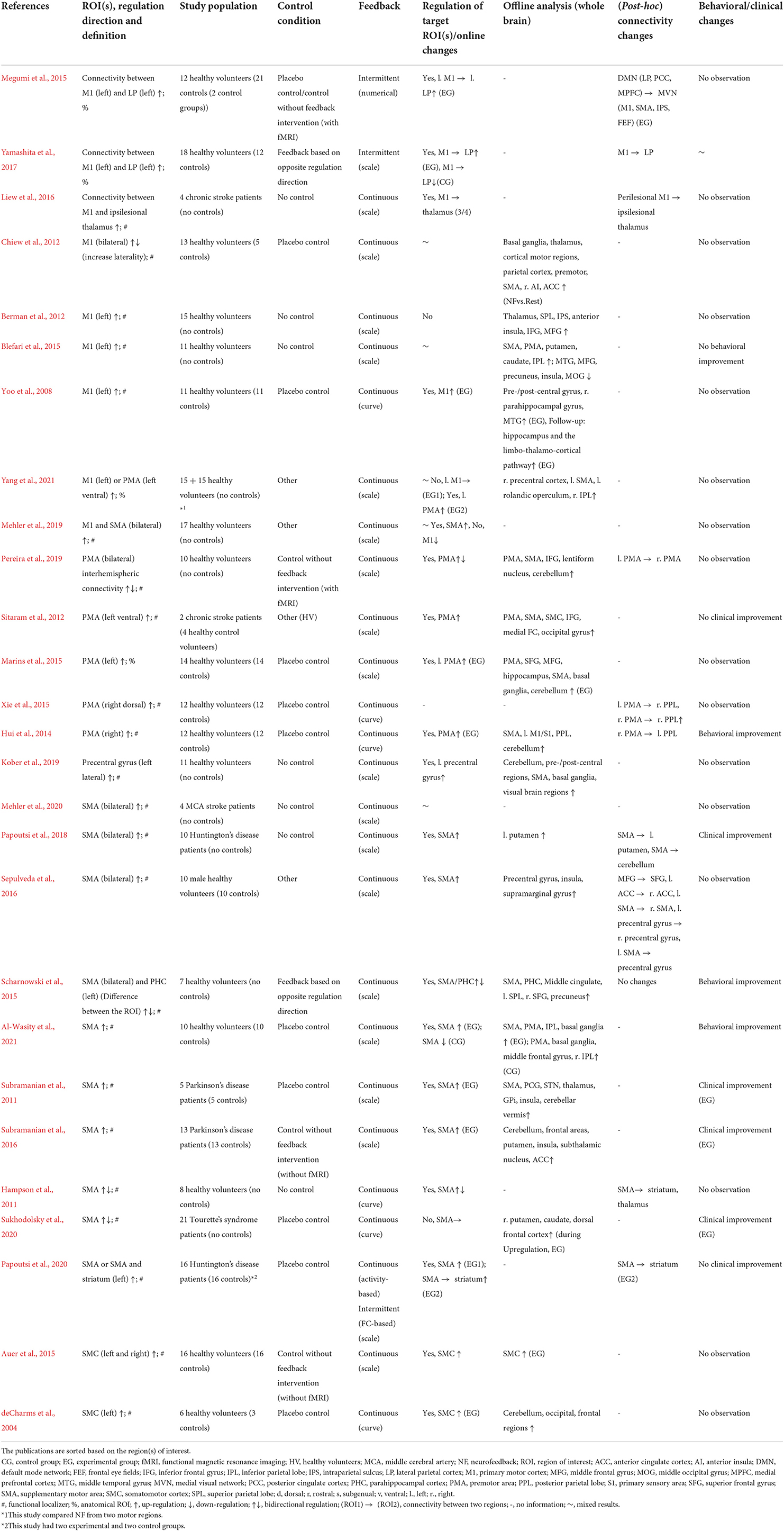
Table 3. Details of real-time fMRI neurofeedback studies with regulation target in the “motor subsystem.”
Most studies have used bilateral ROIs (n = 23, 29%), mainly targeting medial regions such as the ACC, the SMA, and the medial PFC (MPFC). Regions in the left hemisphere (n = 23, 29%) have been used significantly more often than in the right hemisphere (n = 6, 8%). Regions targeted in the left hemisphere mainly include the DLPFC and the motor regions such as the primary motor cortex (M1) and the premotor area (PMA). Eight studies (10%) have targeted multiple regions located in different hemispheres or have bilateral and lateral ROI. Only 36 of the 61 studies mentioned have clearly indicated the choice of laterality. In 18 studies (23%), participants received NF from an individually localized region. Laterality is either not mentioned or is different among participants in these studies.
Control conditions are considered to be essential for demonstrating specific NF effects in clinical trials (Sorger et al., 2019). The majority (n = 58, 73%) of the selected studies apply a method for controlling the unspecific effects of NF interventions (Tables 1–4). No control group or control condition is chosen in 27% of the studies (n = 21) with only 18% of the publications being pre-registered (n = 14). For more details and additional information on the study population, see Supplementary Table 1.
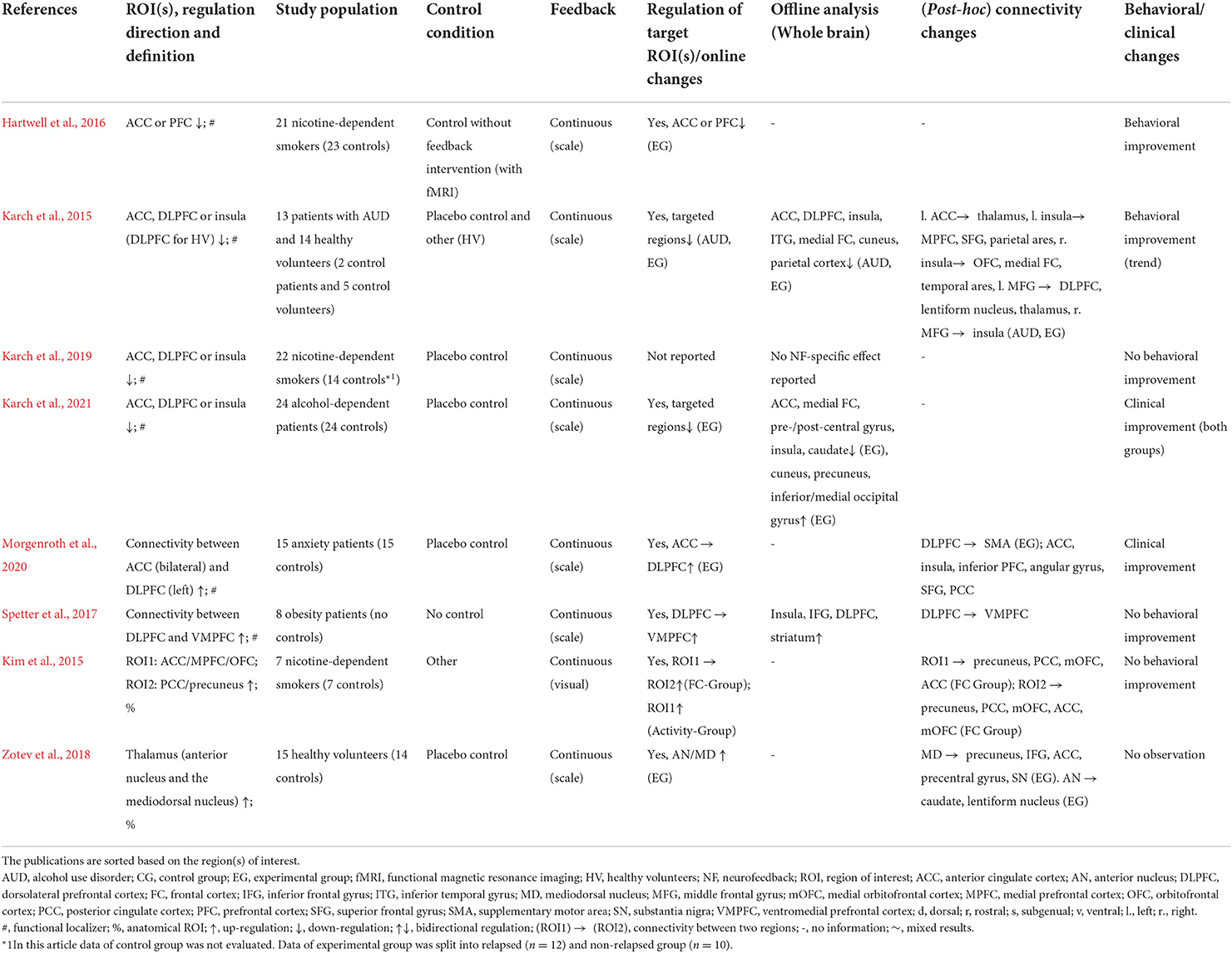
Table 4. Details of real-time fMRI neurofeedback studies with regulation target in mixed subsystems.
Among the controlled studies (n = 58), blinding as concealment of group allocations and a method to overcome performance biases (Pildal et al., 2007) has been implemented in 31 studies. Twenty-three studies report being single-blinded with 8 incorporating double-blinding. Twenty-one studies do not include a control condition, precluding any possible indication with respect to blinding. Thirty-two of the 58 controlled studies were randomized.
Barring one study (Harmelech et al., 2013) with auditory feedback, all studies provided a visual feedback to shape the regional activation in the desired direction. While most studies implemented continuous feedback (n = 67, 85%), intermittent feedback (every 12–153 s) was also used frequently (Tables 1–4). Commonly, the feedback was some form of scale like a thermometer (n = 53, 67 %), although other feedback forms such as social feedback (emotional faces), numeric values, and graphs demonstrating the feedback course (as a curve) were also employed.
As a form of neuromodulation, fMRI NF has been investigated as a new therapy method in a number of proof-of-principle studies and clinical trials involving individuals with disorders such as schizophrenia, depression, anxiety disorders, and SUDs. This is a welcome development in treatment of mental health disorders given the limited effectiveness of current treatment recommendations, i.e., psychotherapy and pharmacotherapy (Leichsenring et al., 2022), and the high number of difficult-to-treat cases. That being said, the target brain regions for NF applications are still unclear. Fortunately, there is decades of functional neuroimaging research that can underpin NF target selection. The frontostriatal circuit that underlies a variety of affective, cognitive, and motor functions (Haber, 2016) has been identified as a NF training target for improving psychiatric deficits. Based on our systematic review, we provide an overview of the choice of behavioral/clinical targets, ROI and study design, discuss the findings and shortcomings, and make suggestions for future research.
The treatment of different symptoms may require different NF targets with different underlying brain circuits (Figure 4). Identifying the appropriate targets is a central issue with respect to treatment efficacy. Previous NF studies have targeted various FSC structures distributed in all three subsystems. The ACC, the SMA and the DLPFC are the most commonly chosen regions as NF targets in the FSC (Tables 1–4). The selection of these structures has been supported mainly by neuroimaging findings of their anatomical and functional positions.
The ACC is the most studied region in the publications reviewed here. Extensive connections to the medial frontal cortex, the posterior cingulate cortex (PCC), the anterior medial temporal lobe, the dorsal medial thalamus, the nucleus accumbens and the brainstem nuclei (Rolls, 2019) indicate the ACC as a central node in the large-scale neural networks that may be dysfunctional in patients, either directly or through a downstream structure (Monosov et al., 2020).
In almost all the reviewed studies, which targeted the ACC for NF interventions, regulations of the targeted ROIs were achieved. The efficacy of NF training in the ACC to ameliorate behavioral and clinical symptoms, i.e., craving (Canterberry et al., 2013; Hanlon et al., 2013; Li et al., 2013; Karch et al., 2015; Hartwell et al., 2016), hallucination severity and affective state (Dyck et al., 2016), and pain perception (deCharms et al., 2005; Guan et al., 2015) was also demonstrated (Tables 2, 4).
NF interventions in the ACC also induced changes in the activity pattern of other regions, which were revealed in the post-hoc whole-brain analyses. Activity changes in relation to ACC modulations were observed in the thalamus (Rance et al., 2014a; Klöbl et al., 2020), the striatum (Rance et al., 2014a; Mathiak et al., 2015; Dyck et al., 2016), the PCC (Mathiak et al., 2015), and the insula (deCharms et al., 2005; Klöbl et al., 2020), suggesting a broad interaction with large-scale brain networks such as the default mode network (DMN) and the salience network (SN) (Uddin, 2015).
On the whole, the ACC within the limbic subsystem of FSC plays a significant role in mediating cognitive influences on emotion. As noted above, over- or under-activation of regions within the ACC and its connection appears to be associated with particular psychopathologies. Furthermore, NF modulations of the ACC were possible and were effective in reducing some of the aberrant behaviors. This evidence converges on the conclusion that the ACC should be considered as an effective NF target, enhancing the cognitive control improvements particularly in dysregulated emotional states.
The SMA is a cortical region in the dorsomedial frontal cortex and just anterior to the primary motor cortex that contributes to movement (Kaas and Stepniewska, 2002). With connections to the limbic system, the ACC, the basal ganglia, the cerebellum, the thalamus, and the superior parietal lobe (Nguyen et al., 2014; Bozkurt et al., 2017), the SMA complex is thought to play a role in the initiation and coordination of movements (Nachev et al., 2008).
An altered SMA activity has been measured in movement disorders like HD (Klöppel et al., 2009), PD (Nachev et al., 2008), and Tourette's syndrome (Neuner et al., 2014), a psychiatric condition with tic disorders. Movement disturbance has also been regarded as an essential feature of depression (Sobin and Sackeim, 1997) and has been described in other psychiatric disorders like schizophrenia (Walther and Strik, 2012). Aberrant SMA activity profiles have been shown in depression (Sarkheil et al., 2020).
Understandably, the efficacy of SMA modulations through NF was investigated in several of the reviewed studies (Subramanian et al., 2011, 2016; Papoutsi et al., 2018, 2020; Mehler et al., 2019, 2020). Following NF training, SMA activity was found to be successfully regulated by the study populations afflicted with PD (Subramanian et al., 2011, 2016) and HD (Papoutsi et al., 2018, 2020) with improvements in motor performance. The clinical symptoms of Tourette's syndrome also showed improvements after NF training of SMA activity (Sukhodolsky et al., 2020).
Some post-hoc whole-brain analyses tapped into the SMA-striatal connections within the FSC. Several studies that targeted the SMA with NF paradigms showed activity changes in the striatal regions (Hampson et al., 2011; Scharnowski et al., 2015; Subramanian et al., 2016; Papoutsi et al., 2018; Sukhodolsky et al., 2020; Al-Wasity et al., 2021). An attenuated functional connectivity of the SMA-striatal neurocircuitry has already been reported in depression (Sarkheil et al., 2020). Altogether, these findings highlight a potential for NF modulations of the motor subsystem within the FSC to improve the (psycho)motor symptoms of psychiatric disorders.
The importance of the DLPFC within the FSC has been outlined in the reviewed studies. Besides its role in attention, cognitive control, and executive function, this region is involved in emotional response and has extensive connections to the thalamus, the dorsal caudate nucleus, the hippocampus, the OFC, and the posterior temporal, parietal, and occipital areas (Kobayashi, 2009).
The controlling role of this area has motivated its selection as a target region for NF modulations in the context of SUD (Karch et al., 2015), MDD (Takamura et al., 2020), anxiety disorders (Zilverstand et al., 2015), eating behavior in obesity (Spetter et al., 2017; Kohl et al., 2019), and in improving working memory in healthy participants (Sherwood et al., 2016).
The corresponding studies have demonstrated successful regulations of the DLPFC. Further post-hoc analyses have revealed changes on the whole brain level, e.g., the dorsal striatum, the thalamus, the parietal cortex, the occipital cortex, and the cuneus (Zhang et al., 2013; Kohl et al., 2019; Travassos et al., 2020; Karch et al., 2021).
The improvement of clinical and behavioral parameters was reported in almost all studies (Zhang et al., 2013; Karch et al., 2015; Sherwood et al., 2016; Kohl et al., 2019; Morgenroth et al., 2020; Takamura et al., 2020; Taylor et al., 2022). As for the long-term effects, considerable responsiveness and clinical improvement were observed 4 weeks (Kohl et al., 2019) and 3 months (Zilverstand et al., 2015; Karch et al., 2021) after NF training, which suggest a promising potential for NF in treatment of clinical conditions.
The subcortical components of the FSC and their connections have been extensively described based on the available functional and anatomical knowledge (Smith et al., 2014). NF studies have already contributed to the identification of the frontostriatal connections by showing alterations in the thalamostriatal regions linked to the NF modulations of the frontal cortical areas (Hampson et al., 2011; Rance et al., 2014a; Klöbl et al., 2020; Travassos et al., 2020; Garrison et al., 2021).
In comparison to NF training of the frontal cortical regions, few researchers have investigated whether humans can voluntarily control the striatal activity. This is probably due to the technical challenges like small size, dynamic activity changes, and deep subcortical location. The ventral striatum, as a key part of the reward system, is particularly interesting for feedback-based paradigms as it is related to learning by means of reward feedback (O'Doherty, 2004) and predicting rewards (Knutson and Cooper, 2005).
Given the importance of the ventral striatum in psychiatric disorders such as schizophrenia (Sorg et al., 2013), depression (Pan et al., 2017), and ADHD (Plichta and Scheres, 2014), three studies investigated the NF training of the ventral striatum. Two studies investigated the feasibility of NF training in healthy participants (Greer et al., 2014; Li et al., 2018), demonstrating successful voluntary regulation of the ventral striatum and resultant improvement in motivation and positive arousal. Post-hoc connectivity analyses revealed connectivity changes within the reward circuit (ventromedial frontal cortex and MPFC) (Knutson and Greer, 2008). Kirsch et al. used the ventral striatum as a regulation target to reduce craving in non-addicted heavy drinking students (Kirsch et al., 2016) and showed that participants were able to successfully regulate activation of the ventral striatum, with a transfer effect in measurement runs without feedback.
The thalamus, as part of the FSC, was probed as a target for NF training during retrieval of happy autobiographical memories (Zotev et al., 2018). The anterior and mediodorsal nuclei of the thalamus were chosen based on their involvement in episodic memory function and activation during recall of autobiographical memories. This group also investigated the potential of thalamus NF training and its connection to posterior alpha EEG power. Participants were able to significantly increase the BOLD activity of the thalamus nuclei, which were correlated with increased EEG alpha power indicating their involvement in the DMN (Sestieri et al., 2011). Overcoming the technical challenges, the aforementioned studies demonstrate the potential for NF modulations in subcortical regions within the FSC. Given the importance of subcortical regions within the FSC in many psychiatric disorders, more NF research in these areas should follow.
Connectivity feedback has been used in the context of anxiety, depression, PD, HD, and paralysis after stroke. Only 18% of studies have applied this method. Studying connectivity is closely linked to functional imaging methods like fMRI. The intersection between brain connectivity and fMRI-based NF is growing and has been recognized in the NF literature (Ruiz et al., 2014). With functional imaging, it is technically possible to consider the remote impact on brain regions connected to the NF target. For example, the connectivity within the frontostriatal system, including the MPFC-ventral striatum circuitry, has been shown to be facilitated by ventral striatum NF training (Greer et al., 2014; Li et al., 2018). The SMA-striatal connectivity has been shown to be influenced by SMA NF (Hampson et al., 2011).
Connectivity imaging allows the targeting of two or more regions, instead of focusing in the local activity of isolated brain regions, to achieve improvement. Two of the reviewed studies investigated the modulation of connectivity between the subgenual ACC and the superior anterior temporal lobe in MDD patients (Zahn et al., 2019; Jaeckle et al., 2021), demonstrating successful connectivity modulation and clinical improvements. In their recent study, Morgenroth et al. (2020) used a connectomic approach for ACC-based NF to investigate the potential of modulating the connectivity between the ACC and the DLPFC in patients suffering from high levels of trait anxiety. The patients were able to increase this connectivity and thereby improve clinical symptoms.
Connectivity NF, as the concept of using neuromodulation to target distributed brain networks is in line with the previous findings regarding different treatments strategies in normalizing pathological functional connectivity, including pharmacotherapy (Goveas et al., 2011; Abbott et al., 2013; Gudayol-Ferré et al., 2015), repetitive TMS (Beynel et al., 2020), ECT (Perrin et al., 2012), and DBS (Figee et al., 2013).
Cerebral lateralization refers to the functional specialization of the two cerebral hemispheres (Geschwind and Galaburda, 1985). The left cerebral cortex is dominant for motor control and verbal processing, whereas the right cerebral cortex is dominant for spatial cognition, visualization, and depth perception (Mutha et al., 2012). The left and right hemispheres also have different functions in relation to emotions (Silberman and Weingartner, 1986). While the left hemisphere is responsible for handling positive emotions, the right hemisphere is more responsible for controlling emotional expressions, recognizing emotions, and negative emotions (Sackeim et al., 1982). A laterality of the thalamostriatal brain regions can also be assumed given the critical role of information integration and processing in the cortical motor and cognitive functions. There is considerable neurobiological (Glick et al., 1982; Cheesman et al., 2005), structural (Kooistra and Heilman, 1988) and electrophysiological (Eitan et al., 2013) evidence to suggest a laterality of the thalamostriatal brain regions.
Accordingly, it is not only the choice of the ROI but also the laterality that is critical for the NF effects. Our review indicates that researchers have investigated mainly left-sided ROIs and the lateralized effects of NF have not been adequately studied. Some studies have used a functional localizer to determine the target region without reference to a rationale of the choice of laterality.
More than half of the reviewed studies engaged healthy volunteers to probe the feasibility of NF training in FSC regions. These studies sought to modify behavioral aspects such as emotion regulation (Sarkheil et al., 2015), motor performance (Hui et al., 2014; Blefari et al., 2015; Scharnowski et al., 2015; Al-Wasity et al., 2021), motivation (Li et al., 2018), working memory (Zhang et al., 2013; Sherwood et al., 2016), speech processing (Rota et al., 2009) and social avoidance (Lisk et al., 2020) with their results underscoring the relevance of the functional organization of the FSC. The studies that aimed to improve motor performance focused on regions in the motor subsystem, such as the M1, the SMA, and the PMA. Improvement of working memory was investigated by alteration in the DLPFC. The ACC as a part of the pain processing network (Qu et al., 2011) was targeted to improve pain perception, while the nucleus accumbens, an area underlying motivation and reward (Berridge, 2007), was the target region for motivation improvement.
NF studies involving healthy populations are crucially important given that the notion of mental health is not just the absence of mental illness. According to the World Health Organization (WHO), mental health is “a state of wellbeing in which the individual realizes his or her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to his or her community” (World Health Organization, 2004). The main domains of mental health, including regulation of negative and positive valence systems, cognitive functioning, and social processes and interaction (Insel et al., 2010; Cuthbert and Insel, 2013), rely on the FSC (Dalley et al., 2008; Morris et al., 2016; Vaghi et al., 2017) and can be addressed by NF training.
As shown in Figure 3, various clinical populations have been addressed in the reviewed NF studies. According to our review, patients with depression were successful in modulating self-esteem, brooding rumination, and depressive symptoms in general (Zahn et al., 2019; Takamura et al., 2020; Jaeckle et al., 2021; Keller et al., 2021; Taylor et al., 2022). Additionally, studies of patients suffering from anxiety reported clinical improvements in self-report of the anxiety level or control over contamination anxiety (Scheinost et al., 2013; Zilverstand et al., 2015; Zhao et al., 2019; Morgenroth et al., 2020). While SUD patients were found to successfully change their brain activity in the regulation targets, in some studies they were unable to significantly reduce craving (Kim et al., 2015; Kirsch et al., 2016; Karch et al., 2019). On the other hand, patients with Tourette's syndrome showed reduced symptoms after rt-fMRI NF, but no changes in the ROI were reported (Sukhodolsky et al., 2020). Altogether, the majority of the reviewed studies presented not only successful regulations of brain activity within the FSC, but also improvements in clinical symptoms. Collectively, these results suggest that NF based on the FSC may be a promising target for NF interventions in neuropsychiatric disorders. Nonetheless, the potential of NF in clinical populations needs to be fully exploited by means of further adjustments. As will be discussed later, there is room for improvement with respect to several aspects of study design of some of these studies.
NF-based interventions of FSC have been probed for a potential effect on treatment of psychiatric disorders, which highlight the importance of replicability and reliability for this line of research. Statistical power should be considered as an important marker for reliability of the results (Nord et al., 2017). Along with the systematically underpowered studies in neuroscience (Button et al., 2013; Stanley et al., 2018), almost half of the studies included in this review had only small sample sizes. A priori sample size estimation was performed in only four studies (Papoutsi et al., 2020; Garrison et al., 2021; Jaeckle et al., 2021; Weiss et al., 2022) and two studies performed a post-hoc power analysis (Mayeli et al., 2020; Morgenroth et al., 2020). Additionally, the studies lacked clarity with respect to effect size estimations. A recent meta-analysis (Fernández-Alvarez et al., 2022) has calculated an effect size of (Hedges' g) 0.303 for the efficacy of biofeedback for depressive symptoms. In the absence of the sample size estimations both under- and overpowered studies may occur, which causes a heterogeneity in the statistical power. Despite the well-known importance of a priori power analyses, and the low statistical power of psychological studies (Cohen, 1962), the reviewed studies failed to systematically calculate the statistical power a priori.
Pre-registrations in international online databases such as clinicaltrials.gov can motivate a priori power analyses. As a practice, pre-registration should be more widely used as it can also guarantee transparency among collaborators and prevent accusations of p-hacking. The oldest pre-registered study of the current review is from 2016 (Subramanian et al., 2016). While the number of pre-registered studies is on the rise, only 14 of the 43 studies between 2016 and now have mentioned pre-registrations. Another important aspect of probing an intervention method is the choice of an appropriate control condition. It is common knowledge that, in a study without a control condition, non-specific effects, such as placebo effects, motivation, and exercise effects (Sorger et al., 2019) cannot be excluded. For example, when monitoring symptom changes over time, the results of a non-controlled study should be closely examined with respect to natural recovery. Currently, there is no consensus regarding a control condition, which largely depends on particular aspects of the NF training design that need to be controlled. The current review has revealed the application of a variety of control conditions.
The average age of the participants varied across the studies. Most studies examined adults, which reflects the manifestation age of the symptoms. Two studies looked at adolescents, one with patients with Tourette's syndrome and one with healthy volunteers. Critically, there is a strong association between dysfunction of frontostriatal regions and brain disorders in in adolescents, like SUD (Alegria et al., 2016; Bjork, 2020; Tervo-Clemmens et al., 2020), eating disorders (Marsh et al., 2009; Berner and Marsh, 2014) and anxiety disorders (Newman et al., 2016; Merz et al., 2018). In general, the age factor should be considered more carefully in selection of the study cohorts. Based on the developmental aspects of the FSC, there is a clear need for NF studies that focus on children or adolescents.
Most of the reviewed studies used continuous feedback as opposed to intermittent feedback. However, the feedback time scale is a matter of discussion (Emmert et al., 2017). Comprehensiveness and timing account for the efficiency of the feedback-based training. Both rt-fMRI measurement techniques and implementations of psychological strategies impose limitations that are relevant for feedback timing. A timely and frequent feedback based on every MRI scan (continuous feedback) may facilitate learning. On the other hand, an intermittent feedback based on averaged MRI signals over an interval may provide more reliable and comprehensive information with respect to training. Empirical tests may help us find the best balance between comprehensiveness and frequency of feedback for each paradigm. We recommend integrating the identified moderators of the feedback-performance relationship (Kluger and DeNisi, 1996) in designing the feedback type in NF paradigms. For example, previous research has indicated an increased efficacy of positive feedback (Arbel et al., 2014) or feedback after successful trials (Chiviacowsky and Wulf, 2007), and normative feedback (Hartwell and Campion, 2016), which refers to information on one's performance compared to others. Interestingly, feedback has proved to be more effective when provided by a computer (Kluger and DeNisi, 1996).
There is no clear definition about NF training dose. However, repeating sessions have been suggested for different NF modalities (Fede et al., 2020; Domingos et al., 2021). Indeed, NF studies indicate that there is a correlation between the number of NF training sessions and the overall clinical effects in the treatment group (Trambaiolli et al., 2021).
In addition to the optimal number of sessions, the length of follow-up periods is important. Longer follow-up periods are desirable. According to clinical studies, effects following NF interventions last up to several months after the last NF session. Some studies have found effects to even improve over time (Mehler et al., 2018; Rance et al., 2018; Goldway et al., 2019).
Personalized interventions for people suffering from mental health conditions are currently the focus of research in this field. To this end, genomic-based and imaging-based subtyping are seen as the main avenues. NF can contribute to this effort by using individualized targeting in patients. This could lead to brain-based treatment by clarifying the neural basis of disordered behavior through real-time observations. Large NF studies using imaging-based subtyping may be a possibility for the future. Here, studies in healthy controls can be considered relevant for validation of controllability of specific targets. fMRI NF can also be used to clarify brain-behavior relationships that are critical to understanding and treating brain disorders. As a clinical neuroimaging tool, fMRI NF can potentially to be used for clinical diagnosis and to track the natural history of disease and the treatment progress. Finally, fMRI NF has the potential to make predictions not only about clinical outcomes but also about response to NF training. As this field is still lacking detailed investigation of neuronal mechanism of NF, augmentations by further imaging modalities such as positron emission tomography or single-photon spectroscopy might be a perspective for future. Importantly, fMRI NF can be integrated with other approaches aimed at modulating brain behavior pathways such as DBS and tDCS, offering another means of augmenting a desirable connectivity outcome.
The three-part organization of the FSC provides important reference for delineating brain circuits that can be differentially targeted for optimal intervention in various neuropsychiatric symptoms.
Another important question pertaining to future research has do with how the observed transient effects of NF may be translated to sustainable recovery in the clinical setting. The continuation of first-line treatments, such as psychotherapy, may be a useful strategy, as cognitive behavioral therapy is associated with changes in the frontostriatal connectivity (Yoshimura et al., 2017; Han et al., 2018). NF complements other existing neurotherapeutic technologies, including DBS and transcranial stimulation, by providing a non-invasive alternative for brain disorders. In addition, it may add value over psychotherapy alone by providing information about how and where cognitive changes in brain function are produced. Continuation of the previous treatment regime along with NF could be compared to single NF. Longitudinal follow-up studies with large samples are needed to probe the effect of psychotherapy and other strategies.
Because of its importance in various psychiatric and neurological disorders, the FSC has been targeted by various neuromodulation techniques. NF, which describes the biofeedback of brain activity, can help individuals learn how to self-regulate their brain activity, thereby potentially inducing behavioral changes or improvements in clinical symptoms. The current review has shown that NF modulation of FSC structures has a great potential for interventions in neuropsychiatric disorders. The FSC can be divided into three spatially segregated loops, each being involved in different aspects of human behavior. We suggest that the topographical organization of the FSC should be considered in target selection for NF interventions. The network aspect of the FSC encourages investigation and selection of the functional connectivity as NF target. Further measures such as standardization of feedback, adjusting the training duration and interval and targeting the connectivity are expected to be helpful in optimizing the results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LO: conceptualization, study search, data selection/extraction, data interpretation, visualization, writing manuscript, and correction and revision. JM: data selection/extraction, data interpretation, and correction and revision of the manuscript. RG and IN: data interpretation and correction and revision of the manuscript. PS: conceptualization, data interpretation, correction and revision of the manuscript, and supervision. All authors contributed to the article and approved the submitted version.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)–269953372/GRK2150 and 448334688.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.933718/full#supplementary-material
Abbott, C. C., Jaramillo, A., Wilcox, C. E., and Hamilton, D. A. (2013). Antipsychotic drug effects in schizophrenia: A review of longitudinal FMRI investigations and neural interpretations. Curr. Med. Chem. 20, 428–437. doi: 10.2174/0929867311320030014
Abernathy, K., Chandler, L. J., and Woodward, J. J. (2010). Alcohol and the prefrontal cortex. Int. Rev. Neurobiol. 91, 289–320. doi: 10.1016/S0074-7742(10)91009-X
Alegria, A. A., Radua, J., and Rubia, K. (2016). Meta-analysis of fMRI studies of disruptive behavior disorders. Am. J. Psychiatry 173, 1119–1130. doi: 10.1176/appi.ajp.2016.15081089
Alexander, G. E., Delong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Alkhasli, I., Sakreida, K., Mottaghy, F. M., and Binkofski, F. (2019). Modulation of fronto-striatal functional connectivity using transcranial magnetic stimulation. Front. Hum. Neurosci. 13, 190. doi: 10.3389/fnhum.2019.00190
Alvarez, J. A., and Emory, E. (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42. doi: 10.1007/s11065-006-9002-x
Al-Wasity, S., Vogt, S., Vuckovic, A., and Pollick, F. E. (2021). Upregulation of supplementary motor area activation with fmri neurofeedback during motor imagery. eNeuro 8, 1–14. doi: 10.1523/ENEURO.0377-18.2020
Andrade, P., Heiden, P., Hoevels, M., Schlamann, M., Baldermann, J. C., Huys, D., et al. (2020). Modulation of fibers to motor cortex during thalamic DBS in tourette patients correlates with tic reduction. Brain Sci. 10, 302. doi: 10.3390/brainsci10050302
Anil, K., Hall, S. D., Demain, S., Freeman, J. A., Ganis, G., and Marsden, J. (2021). A systematic review of neurofeedback for the management of motor symptoms in Parkinson's disease. Brain Sci. 11, 292. doi: 10.3390/brainsci11101292
Arbel, Y., Murphy, A., and Donchin, E. (2014). On the utility of positive and negative feedback in a paired-associate learning task. J. Cogn. Neurosci. 26, 1445–1453. doi: 10.1162/jocn_a_00617
Auer, T., Schweizer, R., and Frahm, J. (2015). Training efficiency and transfer success in an extended real-time functional MRI neurofeedback training of the somatomotor cortex of healthy subjects. Front. Hum. Neurosci. 9, 547. doi: 10.3389/fnhum.2015.00547
Aum, D. J., and Tierney, T. S. (2018). Deep brain stimulation foundations and future trends. Front. Biosci. 23, 4586. doi: 10.2741/4586
Barbas, H. (2007). Specialized elements of orbitofrontal cortex in primates. Ann. N. Y. Acad. Sci. 1121, 10–32. doi: 10.1196/annals.1401.015
Barbour, T., Lee, E., Ellard, K., and Camprodon, J. (2019). Individualized TMS target selection for MDD: clinical outcomes, mechanisms of action and predictors of response. Brain Stimul. Basic, Transl. Clin. Res. Neuromodulation 12, 516. doi: 10.1016/j.brs.2018.12.695
Basile, G. A., Bertino, S., Bramanti, A., Ciurleo, R., Anastasi, G. P., Milardi, D., et al. (2021). Striatal topographical organization: bridging the gap between molecules, connectivity and behavior. Eur. J. Histochem. 65, 3284. doi: 10.4081/ejh.2021.3284
Baxter, L. R., Schwartz, J. M., Phelps, M. E., Mazziotta, J. C., Guze, B. H., Selin, C. E., et al. (1989). Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry 46, 243–250. doi: 10.1001/archpsyc.1989.01810030049007
Berman, B. D., Horovitz, S. G., Venkataraman, G., and Hallett, M. (2012). Self-modulation of primary motor cortex activity with motor and motor imagery tasks using real-time fMRI-based neurofeedback. Neuroimage 59, 917–925. doi: 10.1016/j.neuroimage.2011.07.035
Berner, L. A., and Marsh, R. (2014). Frontostriatal circuits and the development of bulimia nervosa. Front. Behav. Neurosci. 8, 395. doi: 10.3389/fnbeh.2014.00395
Berridge, K. C. (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 191, 391–431. doi: 10.1007/s00213-006-0578-x
Beste, C., Ness, V., Lukas, C., Hoffmann, R., Stüwe, S., Falkenstein, M., et al. (2012). Mechanisms mediating parallel action monitoring in fronto-striatal circuits. Neuroimage 62, 137–146. doi: 10.1016/j.neuroimage.2012.05.019
Beynel, L., Powers, J. P., and Appelbaum, L. G. (2020). Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: a systematic review. Neuroimage 211, 116596. doi: 10.1016/j.neuroimage.2020.116596
Birbaumer, N., Ruiz, S., and Sitaram, R. (2013). Learned regulation of brain metabolism. Trends Cogn. Sci. 17, 295–302. doi: 10.1016/j.tics.2013.04.009
Biver, F., Goldman, S., Delvenne, V., Luxen, A., De Maertelaer, V., Hubain, P., et al. (1994). Frontal and parietal metabolic disturbances in unipolar depression. Biol. Psychiatry 36, 381–388. doi: 10.1016/0006-3223(94)91213-0
Bjork, J. M. (2020). The ups and downs of relating nondrug reward activation to substance use risk in adolescents. Curr. Addict. reports 7, 421–429. doi: 10.1007/s40429-020-00327-7
Bledsoe, J. C., Semrud-Clikeman, M., and Pliszka, S. R. (2013). Anterior cingulate cortex and symptom severity in attention-deficit/hyperactivity disorder. J. Abnorm. Psychol. 122, 558–565. doi: 10.1037/a0032390
Blefari, M. L., Sulzer, J., Hepp-Reymond, M. C., Kollias, S., and Gassert, R. (2015). Improvement in precision grip force control with self-modulation of primary motor cortex during motor imagery. Front. Behav. Neurosci. 9, 18. doi: 10.3389/fnbeh.2015.00018
Blumenstock, S., and Dudanova, I. (2020). Cortical and striatal circuits in Huntington's disease. Front. Neurosci. 14, 82. doi: 10.3389/fnins.2020.00082
Bonelli, R. M., Cummings, J. L., Bonelli, M., Cummings, J. L., Bonelli, R. M., and Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues Clin. Neurosci. 9, 141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli
Boorman, E. D., O'Doherty, J. P., Adolphs, R., and Rangel, A. (2013). The behavioral and neural mechanisms underlying the tracking of expertise. Neuron 80, 1558–1571. doi: 10.1016/j.neuron.2013.10.024
Bozkurt, B., Yagmurlu, K., Middlebrooks, E. H., Cayci, Z., Cevik, O. M., Karadag, A., et al. (2017). Fiber connections of the supplementary motor area revisited: methodology of fiber dissection, DTI, and three dimensional documentation. J. Vis. Exp. 123, 55681. doi: 10.3791/55681
Burton, A. C., Nakamura, K., and Roesch, M. R. (2015). From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol. Learn. Mem. 117, 51–59. doi: 10.1016/j.nlm.2014.05.003
Button, K. S., Ioannidis, J. P. A., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S. J., et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Callicott, J. H., Bertolino, A., Mattay, V. S., Langheim, F. J. P., Duyn, J., Coppola, R., et al. (2000). Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex 10, 1078–1092. doi: 10.1093/cercor/10.11.1078
Canterberry, M., Hanlon, C. A., Hartwell, K. J., Li, X., Owens, M., LeMatty, T., et al. (2013). Sustained reduction of nicotine craving with real-time neurofeedback: exploring the role of severity of dependence. Nicotine Tob. Res. 15, 2120–2124. doi: 10.1093/ntr/ntt122
Casey, B. J., Castellanos, F. X., Giedd, J. N., Marsh, W. L., Hamburger, S. D., Schubert, A. B., et al. (1997). Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 36, 374–383. doi: 10.1097/00004583-199703000-00016
Cheesman, A. L., Barker, R. A., Lewis, S. J. G., Robbins, T. W., Owen, A. M., and Brooks, D. J. (2005). Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 76, 1204. doi: 10.1136/jnnp.2004.055079
Chen, H. J., Zhang, L., Ke, J., Qi, R., Xu, Q., Zhong, Y., et al. (2019). Altered resting-state dorsal anterior cingulate cortex functional connectivity in patients with post-traumatic stress disorder. Aust. New Zeal. J. Psychiatry 53, 68–79. doi: 10.1177/0004867418812674
Chiew, M., LaConte, S. M., and Graham, S. J. (2012). Investigation of fMRI neurofeedback of differential primary motor cortex activity using kinesthetic motor imagery. Neuroimage 61, 21–31. doi: 10.1016/j.neuroimage.2012.02.053
Chiviacowsky, S., and Wulf, G. (2007). Feedback after good trials enhances learning. Res. Q. Exerc. Sport 78, 40–47. doi: 10.1080/02701367.2007.10599402
Cohen, J. (1962). The statistical power of abnormal-social psychological research: a review. J. Abnorm. Soc. Psychol. 65, 145. doi: 10.1037/h0045186
Cohen, M. X., Cavanagh, J. F., and Slagter, H. A. (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity: commentary. Hum. Brain Mapp. 32, 2270–2271. doi: 10.1002/hbm.21358
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. doi: 10.1038/nrn755
Cordes, J. S., Mathiak, K. A., Dyck, M., Alawi, E. M., Gaber, T. J., Zepf, F. D., et al. (2015). Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Front. Behav. Neurosci. 9, 169. doi: 10.3389/fnbeh.2015.00169
Cubillo, A., Halari, R., Smith, A., Taylor, E., and Rubia, K. (2012). A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex 48, 194–215. doi: 10.1016/j.cortex.2011.04.007
Cummins, G., Zandi, M., and Barker, R. A. (2015). Movement disorders and psychiatry: Five new things. Neurol. Clin. Pract. 5, 143–149. doi: 10.1212/CPJ.0000000000000113
Curtis, C. E., and D'Esposito, M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cong. Sci. 7, 415–423. doi: 10.1016/S1364-6613(03)00197-9
Cuthbert, B. N., and Insel, T. R. (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126. doi: 10.1186/1741-7015-11-126
Dalley, J. W., Mar, A. C., Economidou, D., and Robbins, T. W. (2008). Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 90, 250–260. doi: 10.1016/j.pbb.2007.12.021
deCharms, R. C., Christoff, K., Glover, G. H., Pauly, J. M., Whitfield, S., and Gabrieli, J. D. E. (2004). Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage 21, 436–443. doi: 10.1016/j.neuroimage.2003.08.041
deCharms, R. C., Maeda, F., Glover, G. H., Ludlow, D., Pauly, J. M., Soneji, D., et al. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl. Acad. Sci. 102, 18626–18631. doi: 10.1073/pnas.0505210102
Delevich, K., Tucciarone, J., Huang, Z. J., and Li, B. (2015). The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J. Neurosci. 35, 5743 LP−5753. doi: 10.1523/JNEUROSCI.4565-14.2015
DeLong, M. R., and Wichmann, T. (2007). Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 64, 20–24. doi: 10.1001/archneur.64.1.20
Domingos, C., Peralta, M., Prazeres, P., Nan, W., Rosa, A., and Pereira, J. G. (2021). Session frequency matters in neurofeedback training of athletes. Appl. Psychophysiol. Biofeedback 46, 195–204. doi: 10.1007/s10484-021-09505-3
Dougherty, D. D. (2018). Deep brain stimulation: clinical applications. Psychiatr. Clin. North Am. 41, 385–394. doi: 10.1016/j.psc.2018.04.004
Duff, K., Paulsen, J. S., Beglinger, L. J., Langbehn, D. R., and Stout, J. C. (2007). Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol. Psychiatry 62, 1341–1346. doi: 10.1016/j.biopsych.2006.11.034
Dunlop, K., Woodside, B., Olmsted, M., Colton, P., Giacobbe, P., and Downar, J. (2016). Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology 41, 1395–1403. doi: 10.1038/npp.2015.292
Dyck, M. S., Mathiak, K. A., Bergert, S., Sarkheil, P., Koush, Y., Alawi, E. M., et al. (2016). Targeting treatment-resistant auditory verbal hallucinations in schizophrenia with fMRI-based neurofeedback – exploring different cases of schizophrenia. Front. Psychiatry 7, 37. doi: 10.3389/fpsyt.2016.00037
Eitan, R., Shamir, R. R., Linetsky, E., Rosenbluh, O., Moshel, S., Ben-Hur, T., et al. (2013). Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front. Syst. Neurosci. 7, 69. doi: 10.3389/fnsys.2013.00069
Emmert, K., Kopel, R., Koush, Y., Maire, R., Senn, P., Van De Ville, D., et al. (2017). Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real-time fMRI - A pilot study. NeuroImage Clin. 14, 97–104. doi: 10.1016/j.nicl.2016.12.023
Everitt, B. J., and Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489. doi: 10.1038/nn1579
Exner, C., Lange, C., and Irle, E. (2009). Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J. Affect. Disord. 119, 156–162. doi: 10.1016/j.jad.2009.03.015
Fede, S. J., Dean, S. F., Manuweera, T., and Momenan, R. (2020). A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: a systematic review. Front. Hum. Neurosci. 14, 60. doi: 10.3389/fnhum.2020.00060
Fernández-Alvarez, J., Grassi, M., Colombo, D., Botella, C., Cipresso, P., Perna, G., et al. (2022). Efficacy of bio- and neurofeedback for depression: a meta-analysis. Psychol. Med. 52, 201–216. doi: 10.1017/S0033291721004396
Fettes, P., Schulze, L., and Downar, J. (2017). Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front. Syst. Neurosci. 11, 25. doi: 10.3389/fnsys.2017.00025
Figee, M., Luigjes, J., Smolders, R., Valencia-Alfonso, C. E., Van Wingen, G., De Kwaasteniet, B., et al. (2013). Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat. Neurosci. 16, 386–387. doi: 10.1038/nn.3344
Foerde, K., Steinglass, J. E., Shohamy, D., and Walsh, B. T. (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci. 18, 1571–1573. doi: 10.1038/nn.4136
Frodl, T., Bokde, A. L. W., Scheuerecker, J., Lisiecka, D., Schoepf, V., Hampel, H., et al. (2010). Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol. Psychiatry 67, 161–167. doi: 10.1016/j.biopsych.2009.08.022
Furman, D. J., Hamilton, J. P., and Gotlib, I. H. (2011). Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 1, 11. doi: 10.1186/2045-5380-1-11
Galvan, A., Devergnas, A., and Wichmann, T. (2015). Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 9, 5. doi: 10.3389/fnana.2015.00005
Garrison, J. R., Saviola, F., Morgenroth, E., Barker, H., Lührs, M., Simons, J. S., et al. (2021). Modulating medial prefrontal cortex activity using real-time fMRI neurofeedback: Effects on reality monitoring performance and associated functional connectivity. Neuroimage 245, 118640. doi: 10.1016/j.neuroimage.2021.118640
Geschwind, N., and Galaburda, A. M. (1985). Cerebral lateralization: biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch. Neurol. 42, 428–459. doi: 10.1001/archneur.1985.04060050026008
Glick, S. D., Ross, D. A., and Hough, L. B. (1982). Lateral asymmetry of neurotransmitters in human brain. Brain Res. 234, 53–63. doi: 10.1016/0006-8993(82)90472-3
Goldway, N., Ablin, J., Lubin, O., Zamir, Y., Keynan, J. N., Or-Borichev, A., et al. (2019). Volitional limbic neuromodulation exerts a beneficial clinical effect on Fibromyalgia. Neuroimage 186, 758–770. doi: 10.1016/j.neuroimage.2018.11.001
Goveas, J. S., Xie, C., Ward, B. D., Wu, Z., Li, W., Franczak, M., et al. (2011). Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer's disease patients treated with donepezil assessed by resting-state fMRI. J. Magn. Reson. Imaging 34, 764–773. doi: 10.1002/jmri.22662
Graybiel, A. M., and Rauch, S. L. (2000). Toward a neurobiology of obsessive-compulsive disorder. Neuron 28, 343–347. doi: 10.1016/S0896-6273(00)00113-6
Greer, S. M., Trujillo, A. J., Glover, G. H., and Knutson, B. (2014). Control of nucleus accumbens activity with neurofeedback. Neuroimage 96, 237–244. doi: 10.1016/j.neuroimage.2014.03.073
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437. doi: 10.1016/j.biopsych.2006.09.020
Guan, M., Ma, L., Li, L., Yan, B., Zhao, L., Tong, L., et al. (2015). Self-regulation of brain activity in patients with postherpetic neuralgia: a double-blind randomized study using real-time fMRI neurofeedback. PLoS ONE 10, e0123675. doi: 10.1371/journal.pone.0123675
Gudayol-Ferré, E., Peró-Cebollero, M., González-Garrido, A. A., and Guàrdia-Olmos, J. (2015). Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front. Hum. Neurosci. 9, 582. doi: 10.3389/fnhum.2015.00582
Haber, S. N. (2003). The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 26, 317–330. doi: 10.1016/j.jchemneu.2003.10.003
Haber, S. N. (2016). Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7–21. doi: 10.31887/DCNS.2016.18.1/shaber
Hamilton, J. P., Glover, G. H., Hsu, J. J., Johnson, R. F., and Gotlib, I. H. (2011). Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum. Brain Mapp. 32, 22–37. doi: 10.1002/hbm.20997
Hampson, M., Scheinost, D., Qiu, M., Bhawnani, J., Lacadie, C. M., Leckman, J. F., et al. (2011). Biofeedback of real-time functional magnetic resonance imaging data from the supplementary motor area reduces functional connectivity to subcortical regions. Brain Connect. 1, 91–98. doi: 10.1089/brain.2011.0002
Han, X., Wang, Y., Jiang, W., Bao, X., Sun, Y., Ding, W., et al. (2018). Resting-State Activity of Prefrontal-Striatal Circuits in Internet Gaming Disorder: Changes With Cognitive Behavior Therapy and Predictors of Treatment Response. Front. psychiatry 9, 341. doi: 10.3389/fpsyt.2018.00341
Hanlon, C. A., Hartwell, K. J., Canterberry, M., Li, X., Owens, M., LeMatty, T., et al. (2013). Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res. Neuroimaging 213, 79–81. doi: 10.1016/j.pscychresns.2013.03.003
Harmelech, T., Preminger, S., Wertman, E., and Malach, R. (2013). The day-after effect: long term, hebbian-like restructuring of resting-state fMRI patterns induced by a single epoch of cortical activation. J. Neurosci. 33, 9488–9497. doi: 10.1523/JNEUROSCI.5911-12.2013
Hartwell, C. J., and Campion, M. A. (2016). Getting on the same page: the effect of normative feedback interventions on structured interview ratings. J. Appl. Psychol. 101, 757–778. doi: 10.1037/apl0000099
Hartwell, K. J., Hanlon, C. A., Li, X., Borckardt, J. J., Canterberry, M., Prisciandaro, J. J., et al. (2016). Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J. Psychiatry Neurosci. 41, 48–55. doi: 10.1503/jpn.140200
Hayes, D. J., Lipsman, N., Chen, D. Q., Woodside, D. B., Davis, K. D., Lozano, A. M., et al. (2015). Subcallosal cingulate connectivity in anorexia nervosa patients differs from healthy controls: a multi-tensor tractography study. Brain Stimul. 8, 758–768. doi: 10.1016/j.brs.2015.03.005
Hirjak, D., Meyer-Lindenberg, A., Fritze, S., Sambataro, F., Kubera, K. M., and Wolf, R. C. (2018). Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neurosci. Biobehav. Rev. 95, 315–335. doi: 10.1016/j.neubiorev.2018.09.009
Honey, C. R., Hamani, C., Kalia, S. K., Sankar, T., Picillo, M., Munhoz, R. P., et al. (2017). Deep brain stimulation target selection for Parkinson's disease. Can. J. Neurol. Sci. 44, 3–8. doi: 10.1017/cjn.2016.22
Hu, Y., Betty, J. S., Gu, H., Stein, E. A., and Yang, Y. (2015). Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction supplemental content at jamapsychiatry.com. JAMA Psychiatry 72, 584–592. doi: 10.1001/jamapsychiatry.2015.1
Hui, M., Zhang, H., Ge, R., Yao, L., and Long, Z. (2014). Modulation of functional network with real-time fMRI feedback training of right premotor cortex activity. Neuropsychologia 62, 111–123. doi: 10.1016/j.neuropsychologia.2014.07.012
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379
Ishihara, L., and Brayne, C. (2006). A systematic review of depression and mental illness preceding Parkinson's disease. Acta Neurol. Scand. 113, 211–220. doi: 10.1111/j.1600-0404.2006.00579.x
Jaeckle, T., Williams, S. C. R., Barker, G. J., Basilio, R., Carr, E., Goldsmith, K., et al. (2021). Self-blame in major depression: a randomised pilot trial comparing fMRI neurofeedback with self-guided psychological strategies. Psychol. Med. 1–11. doi: 10.1017/S0033291721004797. [Epub ahead of print].
Jarbo, K., and Verstynen, T. D. (2015). Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J. Neurosci. 35, 3865 LP−3878. doi: 10.1523/JNEUROSCI.2636-14.2015
Kaas, J. H., and Stepniewska, I. (2002). “Motor cortex,” in Encyclopedia of the Human Brain, ed. V. S. B. T.-E. of the H. B. Ramachandran (New York, NY: Elsevier), 159–169.
Karch, S., Keeser, D., Hümmer, S., Paolini, M., Kirsch, V., Karali, T., et al. (2015). Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS ONE 10, e0133034. doi: 10.1371/journal.pone.0133034
Karch, S., Krause, D., Lehnert, K., Konrad, J., Haller, D., Rauchmann, B. S., et al. (2021). Functional and clinical outcomes of FMRI-based neurofeedback training in patients with alcohol dependence: A pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 272, 557–569. doi: 10.1007/s00406-021-01336-x
Karch, S., Paolini, M., Gschwendtner, S., Jeanty, H., Reckenfelderbäumer, A., Yaseen, O., et al. (2019). Real-time fMRI neurofeedback in patients with tobacco use disorder during smoking cessation: functional differences and implications of the first training session in regard to future abstinence or relapse. Front. Hum. Neurosci. 13, 65. doi: 10.3389/fnhum.2019.00065
Ke, J., Zhang, L., Qi, R., Xu, Q., Li, W., Hou, C., et al. (2015). Altered blood oxygen level-dependent signal variability in chronic post-traumatic stress disorder during symptom provocation. Neuropsychiatr. Dis. Treat. 11, 1805–15. doi: 10.2147/NDT.S87332
Keller, M., Zweerings, J., Klasen, M., Zvyagintsev, M., Iglesias, J., Mendoza Quiñones, R., et al. (2021). fMRI neurofeedback-enhanced cognitive reappraisal training in depression: a double-blind comparison of left and right vlPFC regulation. Front. Psychiatry 12, 715898. doi: 10.3389/fpsyt.2021.715898
Kim, D.-Y., Yoo, S.-S., Tegethoff, M., Meinlschmidt, G., and Lee, J.-H. (2015). The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. J. Cogn. Neurosci. 27, 1552–1572. doi: 10.1162/jocn_a_00802
Kirsch, M., Gruber, I., Ruf, M., Kiefer, F., and Kirsch, P. (2016). Real-time functional magnetic resonance imaging neurofeedback can reduce striatal cue-reactivity to alcohol stimuli. Addict. Biol. 21, 982–992. doi: 10.1111/adb.12278
Klöbl, M., Michenthaler, P., Godbersen, G. M., Robinson, S., Hahn, A., and Lanzenberger, R. (2020). Reinforcement and punishment shape the learning dynamics in fMRI neurofeedback. Front. Hum. Neurosci. 14, 304. doi: 10.3389/fnhum.2020.00304
Klöppel, S., Henley, S. M., Hobbs, N. Z., Wolf, R. C., Kassubek, J., Tabrizi, S. J., et al. (2009). Magnetic resonance imaging of Huntington's disease: preparing for clinical trials. Neuroscience 164, 205–219. doi: 10.1016/j.neuroscience.2009.01.045
Kluger, A. N., and DeNisi, A. (1996). The effects of feedback interventions on performance: a historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychol. Bull. 119, 254–284. doi: 10.1037/0033-2909.119.2.254
Knutson, B., and Cooper, J. C. (2005). Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 18, 411–417. doi: 10.1097/01.wco.0000173463.24758.f6
Knutson, B., and Greer, S. M. (2008). Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 3771–3786. doi: 10.1098/rstb.2008.0155
Kobayashi, S. (2009). “Reward neurophysiology and primate cerebral cortex,” in Encyclopedia of Neuroscience (Elsevier), 325–333. doi: 10.1016/B978-008045046-9.01559-X
Kober, S. E., Grössinger, D., and Wood, G. (2019). Effects of motor imagery and visual neurofeedback on activation in the swallowing network: a real-time fMRI study. Dysphagia 34, 879–895. doi: 10.1007/s00455-019-09985-w
Koenigs, M., and Grafman, J. (2009). The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 201, 239–243. doi: 10.1016/j.bbr.2009.03.004
Kohl, S. H., Veit, R., Spetter, M. S., Günther, A., Rina, A., Lührs, M., et al. (2019). Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: a randomized controlled trial in overweight and obese subjects. Neuroimage 191, 596–609. doi: 10.1016/j.neuroimage.2019.02.033
Kooistra, C. A., and Heilman, K. M. (1988). Motor dominance and lateral asymmetry of the globus pallidus. Neurology 38, 388–390. doi: 10.1212/WNL.38.3.388
Koush, Y., Meskaldji, D. E., Pichon, S., Rey, G., Rieger, S. W., Linden, D. E. J., et al. (2017). Learning control over emotion networks through connectivity-based neurofeedback. Cereb. Cortex 27, 1193–1202.
Krawczyk, D. C. (2002). Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci. Biobehav. Rev. 26, 631–664. doi: 10.1016/S0149-7634(02)00021-0
Kringelbach, M. L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702. doi: 10.1038/nrn1747
Kuo, M. F., and Nitsche, M. A. (2012). Effects of transcranial electrical stimulation on cognition. Clin. EEG Neurosci. 43, 192–199. doi: 10.1177/1550059412444975
Lefaucheur, J. P., Aleman, A., Baeken, C., Benninger, D. H., Brunelin, J., Di Lazzaro, V., et al. (2020). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin. Neurophysiol. 131, 474–528. doi: 10.1016/j.clinph.2019.11.002
Lehéricy, S., Bardinet, E., Tremblay, L., Van De Moortele, P. F., Pochon, J. B., Dormont, D., et al. (2006). Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb. Cortex 16, 149–161. doi: 10.1093/cercor/bhi089
Leichsenring, F., Steinert, C., Rabung, S., and Ioannidis, J. P. A. (2022). The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 21, 133–145. doi: 10.1002/wps.20941
Levy, R., and Dubois, B. (2006). Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cereb. Cortex 16, 916–928. doi: 10.1093/cercor/bhj043
Li, A., Zalesky, A., Yue, W., Howes, O., Yan, H., Liu, Y., et al. (2020). A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 26, 558–565. doi: 10.1038/s41591-020-0793-8
Li, X., Hartwell, K. J., Borckardt, J., Prisciandaro, J. J., Saladin, M. E., Morgan, P. S., et al. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict. Biol. 18, 739–748. doi: 10.1111/j.1369-1600.2012.00449.x
Li, Z., Zhang, C., Huang, J., Wang, Y., Yan, C., Li, K., et al. (2018). Improving motivation through real-time fMRI-based self-regulation of the nucleus accumbens. Neuropsychology 32, 764–776. doi: 10.1037/neu0000425
Liew, S.-L., Rana, M., Cornelsen, S., Fortunato de Barros Filho, M., Birbaumer, N., Sitaram, R., et al. (2016). Improving motor corticothalamic communication after stroke using real-time fMRI connectivity-based neurofeedback. Neurorehabil. Neural Repair 30, 671–675. doi: 10.1177/1545968315619699
Linden, D. E. J., Habes, I., Johnston, S. J., Linden, S., Tatineni, R., Subramanian, L., et al. (2012). Real-time self-regulation of emotion networks in patients with depression. PLoS ONE 7, e38115. doi: 10.1371/journal.pone.0038115
Linden, D. E. J., and Turner, D. L. (2016). Real-time functional magnetic resonance imaging neurofeedback in motor neurorehabilitation. Curr. Opin. Neurol. 29, 412–418. doi: 10.1097/WCO.0000000000000340
Linhartová, P., Látalová, A., Kóša, B., Kašpárek, T., Schmahl, C., and Paret, C. (2019). fMRI neurofeedback in emotion regulation: a literature review. Neuroimage 193, 75–92. doi: 10.1016/j.neuroimage.2019.03.011
Lipp, A., and Cohen Kadosh, K. (2020). Training the anxious brain: using fMRI-based neurofeedback to change brain activity in adolescence. Dev. Med. Child Neurol. 62, 1239–1244. doi: 10.1111/dmcn.14611
Lisk, S., Kadosh, K. C., Zich, C., Haller, S. P., and Lau, J. Y. (2020). Training negative connectivity patterns between the dorsolateral prefrontal cortex and amygdala through fMRI-based neurofeedback to target adolescent socially-avoidant behaviour. Behav. Res. Ther. 135, 103760. doi: 10.1016/j.brat.2020.103760
Lubianiker, N., Paret, C., Dayan, P., and Hendler, T. (2022). Neurofeedback through the lens of reinforcement learning. Trends Neurosci. 45, 579–593. doi: 10.1016/j.tins.2022.03.008
Luppino, G., and Rizzolatti, G. (2000). The organization of the frontal motor cortex. Physiology 15, 219–224. doi: 10.1152/physiologyonline.2000.15.5.219
MacDuffie, K. E., MacInnes, J., Dickerson, K. C., Eddington, K. M., Strauman, T. J., and Adcock, R. A. (2018). Single session real-time fMRI neurofeedback has a lasting impact on cognitive behavioral therapy strategies. NeuroImage Clin. 19, 868–875. doi: 10.1016/j.nicl.2018.06.009
Marins, T. F., Rodrigues, E. C., Engel, A., Hoefle, S., Basílio, R., Lent, R., et al. (2015). Enhancing motor network activity using real-time functional MRI neurofeedback of left premotor cortex. Front. Behav. Neurosci. 9, 341. doi: 10.3389/fnbeh.2015.00341
Marsh, R., Maia, T. V., and Peterson, B. S. (2009). Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am. J. Psychiatry 166, 664–674. doi: 10.1176/appi.ajp.2009.08091354
Mathiak, K. A., Alawi, E. M., Koush, Y., Dyck, M., Cordes, J. S., Gaber, T. J., et al. (2015). Social reward improves the voluntary control over localized brain activity in fMRI-based neurofeedback training. Front. Behav. Neurosci. 9, 136. doi: 10.3389/fnbeh.2015.00136
Mathiak, K. A., Koush, Y., Dyck, M., Gaber, T. J., Alawi, E., Zepf, F. D., et al. (2010). Social reinforcement can regulate localized brain activity. Eur. Arch. Psychiatry Clin. Neurosci. 260, 132–136. doi: 10.1007/s00406-010-0135-9
Mayeli, A., Misaki, M., Zotev, V., Tsuchiyagaito, A., Al Zoubi, O., Phillips, R., et al. (2020). Self-regulation of ventromedial prefrontal cortex activation using real-time fMRI neurofeedback—Influence of default mode network. Hum. Brain Mapp. 41, 342–352. doi: 10.1002/hbm.24805
Mega, M. S., Cummings, J. L., Salloway, S., and Malloy, P. (1997). The limbic system: an anatomic, phylogenetic, and clinical perspective. J. Neuropsychiatry Clin. Neurosci. 9, 315–330. doi: 10.1176/jnp.9.3.315
Megumi, F., Yamashita, A., Kawato, M., and Imamizu, H. (2015). Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front. Hum. Neurosci. 9, 160. doi: 10.3389/fnhum.2015.00160
Mehler, D. M. A., Williams, A. N., Krause, F., Lührs, M., Wise, R. G., Turner, D. L., et al. (2019). The BOLD response in primary motor cortex and supplementary motor area during kinesthetic motor imagery based graded fMRI neurofeedback. Neuroimage 184, 36–44. doi: 10.1016/j.neuroimage.2018.09.007
Mehler, D. M. A., Williams, A. N., Whittaker, J. R., Krause, F., Lührs, M., Kunas, S., et al. (2020). Graded fMRI neurofeedback training of motor imagery in middle cerebral artery stroke patients: a preregistered proof-of-concept study. Front. Hum. Neurosci. 14, 226. doi: 10.3389/fnhum.2020.00226
Mehler, D. M. A. A., Sokunbi, M. O., Habes, I., Barawi, K., Subramanian, L., Range, M., et al. (2018). Targeting the affective brain—a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology 43, 2578–2585. doi: 10.1038/s41386-018-0126-5
Menzies, L., Chamberlain, S. R., Laird, A. R., Thelen, S. M., Sahakian, B. J., and Bullmore, E. T. (2008). Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 32, 525–549. doi: 10.1016/j.neubiorev.2007.09.005
Merz, E. C., He, X., and Noble, K. G. (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clin. 20, 243–251. doi: 10.1016/j.nicl.2018.07.020
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi: 10.1136/bmj.b2535
Monosov, I. E., Haber, S. N., Leuthardt, E. C., and Jezzini, A. (2020). Anterior cingulate cortex and the control of dynamic behavior in primates. Curr. Biol. 30, R1442. doi: 10.1016/j.cub.2020.10.009
Morgenroth, E., Saviola, F., Gilleen, J., Allen, B., Lührs, M., Eysenck, W., et al. (2020). Using connectivity-based real-time fMRI neurofeedback to modulate attentional and resting state networks in people with high trait anxiety. NeuroImage Clin. 25, 102191. doi: 10.1016/j.nicl.2020.102191
Morris, L. S., Kundu, P., Dowell, N., Mechelmans, D. J., Favre, P., Irvine, M. A., et al. (2016). Fronto-striatal organization: defining functional and microstructural substrates of behavioural flexibility. Cortex. 74, 118–133. doi: 10.1016/j.cortex.2015.11.004
Muñoz-Moldes, S., and Cleeremans, A. (2020). Delineating implicit and explicit processes in neurofeedback learning. Neurosci. Biobehav. Rev. 118, 681–688. doi: 10.1016/j.neubiorev.2020.09.003
Mutha, P. K., Haaland, K. Y., and Sainburg, R. L. (2012). The effects of brain lateralization on motor control and adaptation. J. Mot. Behav. 44, 455–469. doi: 10.1080/00222895.2012.747482
Nachev, P., Kennard, C., and Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 9, 856–869. doi: 10.1038/nrn2478
Neuner, I., Werner, C. J., Arrubla, J., Stöcker, T., Ehlen, C., Wegener, H. P., et al. (2014). Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 8, 362. doi: 10.3389/fnhum.2014.00362
Newman, E., Thompson, W. K., Bartsch, H., Hagler, D. J., Chen, C.-H., Brown, T. T., et al. (2016). Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct. Funct. 221, 3013–3025. doi: 10.1007/s00429-015-1085-9
Nguyen, V. T., Breakspear, M., and Cunnington, R. (2014). Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J. Neurosci. 34, 16397. doi: 10.1523/JNEUROSCI.2571-14.2014
Nord, C. L., Valton, V., Wood, J., and Roiser, J. P. (2017). Power-up: a reanalysis of “power failure” in neuroscience using mixture modeling. J. Neurosci. 37, 8051–8061. doi: 10.1523/JNEUROSCI.3592-16.2017
Obeso, J. A., Rodríguez-Oroz, M. C., Benitez-Temino, B., Blesa, F. J., Guridi, J., Marin, C., et al. (2008). Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov. Disord. 23, 548–559. doi: 10.1002/mds.22062
Ochsner, K. N., Silvers, J. A., Buhle, J. T., and Ochsner, K. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N.Y. Acad. Sci 1251, E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x
O'Doherty, J. P. (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 14, 769–776. doi: 10.1016/j.conb.2004.10.016
O'Neill, J., Piacentini, J. C., and Peterson, B. S. (2019). Cingulate role in Tourette syndrome. Handb. Clin. Neurol. 166, 165–221. doi: 10.1016/B978-0-444-64196-0.00011-X
Öngür, D., and Price, J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10, 206–219. doi: 10.1093/cercor/10.3.206
Palm, U., Hasan, A., Strube, W., and Padberg, F. (2016). tDCS for the treatment of depression: a comprehensive review. Eur. Arch. Psychiatry Clin. Neurosci. 266, 681–694. doi: 10.1007/s00406-016-0674-9
Pan, P. M., Sato, J. R., Salum, G. A., Rohde, L. A., Gadelha, A., Zugman, A., et al. (2017). Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am. J. Psychiatry 174, 1112–1119. doi: 10.1176/appi.ajp.2017.17040430
Papoutsi, M., Magerkurth, J., Josephs, O., Pépés, S. E., Ibitoye, T., Reilmann, R., et al. (2020). Activity or connectivity? A randomized controlled feasibility study evaluating neurofeedback training in Huntington's disease. Brain Commun. 2, 1–14. doi: 10.1093/braincomms/fcaa049
Papoutsi, M., Weiskopf, N., Langbehn, D., Reilmann, R., Rees, G., and Tabrizi, S. J. (2018). Stimulating neural plasticity with real-time fMRI neurofeedback in Huntington's disease: a proof of concept study. Hum. Brain Mapp. 39, 1339–1353. doi: 10.1002/hbm.23921
Parent, A., Bouchard, C., and Smith, Y. (1984). The striatopallidal and striatonigral projections: two distinct fiber systems in primate. Brain Res. 303, 385–390. doi: 10.1016/0006-8993(84)91224-1
Parent, A., and Hazrati, L.-N. (1995). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 20, 91–127. doi: 10.1016/0165-0173(94)00007-C
Park, M. J., Kim, H., Kim, E. J., Yook, V., Chung, I. W., Lee, S. M., et al. (2021). Recent updates on electro-convulsive therapy in patients with depression. Psychiatry Investig. 18, 1–10. doi: 10.30773/pi.2020.0350
Pereira, J., Direito, B., Sayal, A., Ferreira, C., and Castelo-Branco, M. (2019). Self-modulation of premotor cortex interhemispheric connectivity in a real-time functional magnetic resonance imaging neurofeedback study using an adaptive approach. Brain Connect. 9, 662–672. doi: 10.1089/brain.2019.0697
Perrin, J. S., Merz, S., Bennett, D. M., Currie, J., Steele, D. J., Reid, I. C., et al. (2012). Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc. Natl. Acad. Sci. U. S. A. 109, 5464–5468. doi: 10.1073/pnas.1117206109
Pildal, J., Hrobjartsson, A., Jorgensen, K., Hilden, J., Altman, D. G., Gotzsche, P., et al. (2007). Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int. J. Epidemiol. 36, 847–857. doi: 10.1093/ije/dym087
Plichta, M. M., and Scheres, A. (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 38, 125–134. doi: 10.1016/j.neubiorev.2013.07.012
Pulcu, E., and Elliott, R. (2015). Neural origins of psychosocial functioning impairments in major depression. Lancet Psychiatry 2, 835–843. doi: 10.1016/S2215-0366(15)00237-0
Qu, C., King, T., Okun, A., Lai, J., Fields, H. L., and Porreca, F. (2011). Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 152, 1641–1648. doi: 10.1016/j.pain.2011.03.002
Radua, J., van den Heuvel, O. A., Surguladze, S., and Mataix-Cols, D. (2010). Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs. other anxiety disorders. Arch. Gen. Psychiatry 67, 701–711. doi: 10.1001/archgenpsychiatry.2010.70
Rance, M., Ruttorf, M., Nees, F., Schad, L. R., and Flor, H. (2014a). Neurofeedback of the difference in activation of the anterior cingulate cortex and posterior insular cortex: Two functionally connected areas in the processing of pain. Front. Behav. Neurosci. 8, 357. doi: 10.3389/fnbeh.2014.00357
Rance, M., Ruttorf, M., Nees, F., Schad, L. R., and Flor, H. (2014b). Real time fMRI feedback of the anterior cingulate and posterior insular cortex in the processing of pain. Hum. Brain Mapp. 35, 5784–5798. doi: 10.1002/hbm.22585
Rance, M., Walsh, C., Sukhodolsky, D. G., Pittman, B., Qiu, M., Kichuk, S. A., et al. (2018). Time course of clinical change following neurofeedback. Neuroimage 181, 807–813. doi: 10.1016/j.neuroimage.2018.05.001
Rolls, E. T. (2019). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 224, 3001. doi: 10.1007/s00429-019-01945-2
Ros, T., Kwiek, J., Andriot, T., Michela, A., Vuilleumier, P., Garibotto, V., et al. (2021). PET imaging of dopamine neurotransmission during EEG neurofeedback. Front. Physiol. 11, 590503. doi: 10.3389/fphys.2020.590503
Rota, G., Sitaram, R., Veit, R., Erb, M., Weiskopf, N., Dogil, G., et al. (2009). Self-regulation of regional cortical activity using real-time fmri: the right inferior frontal gyrus and linguistic processing. Hum. Brain Mapp.30, 1605–1614. doi: 10.1002/hbm.20621
Ruiz, S., Buyukturkoglu, K., Rana, M., Birbaumer, N., and Sitaram, R. (2014). Real-time fMRI brain computer interfaces: self-regulation of single brain regions to networks. Biol. Psychol. 95, 4–20. doi: 10.1016/j.biopsycho.2013.04.010
Sackeim, H. A., Greenberg, M. S., Weiman, A. L., Gur, R. C., Hungerbuhler, J. P., and Geschwind, N. (1982). Hemispheric asymmetry in the expression of positive and negative emotions: neurologic evidence. Arch. Neurol. 39, 210–218. doi: 10.1001/archneur.1982.00510160016003
Sadleir, R. J., Vannorsdall, T. D., Schretlen, D. J., and Gordon, B. (2012). Target optimization in transcranial direct current stimulation. Front. Psychiatry 3, 90. doi: 10.3389/fpsyt.2012.00090
Sarkheil, P., Odysseos, P., Bee, I., Zvyagintsev, M., Neuner, I., and Mathiak, K. (2020). Functional connectivity of supplementary motor area during finger-tapping in major depression. Compr. Psychiatry 99, 152166. doi: 10.1016/j.comppsych.2020.152166
Sarkheil, P., Zilverstand, A., Kilian-Hütten, N., Schneider, F., Goebel, R., and Mathiak, K. (2015). fMRI feedback enhances emotion regulation as evidenced by a reduced amygdala response. Behav. Brain Res. 281, 326–332. doi: 10.1016/j.bbr.2014.11.027
Scharnowski, F., Veit, R., Zopf, R., Studer, P., Bock, S., Diedrichsen, J., et al. (2015). Manipulating motor performance and memory through real-time fMRI neurofeedback. Biol. Psychol. 108, 85–97. doi: 10.1016/j.biopsycho.2015.03.009
Scheinost, D., Stoica, T., Saksa, J., Papademetris, X., Constable, R. T., Pittenger, C., et al. (2013). Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl. Psychiatry 3, e250. doi: 10.1038/tp.2013.24
Sepulveda, P., Sitaram, R., Rana, M., Montalba, C., Tejos, C., and Ruiz, S. (2016). How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum. Brain Mapp. 37, 3153–3171. doi: 10.1002/hbm.23228
Sestieri, C., Corbetta, M., Romani, G. L., and Shulman, G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 31, 4407 LP−4420. doi: 10.1523/JNEUROSCI.3335-10.2011
Sherwood, M. S., Kane, J. H., Weisend, M. P., and Parker, J. G. (2016). Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage 124, 214–223. doi: 10.1016/j.neuroimage.2015.08.074
Shibata, K., Lisi, G., Cortese, A., Watanabe, T., Sasaki, Y., and Kawato, M. (2019). Toward a comprehensive understanding of the neural mechanisms of decoded neurofeedback. Neuroimage 188, 539–556. doi: 10.1016/j.neuroimage.2018.12.022
Silberman, E. K., and Weingartner, H. (1986). Hemispheric lateralization of functions related to emotion. Brain Cogn. 5, 322–353. doi: 10.1016/0278-2626(86)90035-7
Sitaram, R., Ros, T., Stoeckel, L., Haller, S., Scharnowski, F., Lewis-Peacock, J., et al. (2017). Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 18, 86–100. doi: 10.1038/nrn.2016.164
Sitaram, R., Veit, R., Stevens, B., Caria, A., Gerloff, C., and Birbaumer, N. (2012). Acquired control of ventral premotor cortex activity by feedback training an exploratory real-time fMRI and TMS study. Neurorehabil. Neural. Repair 26, 345. doi: 10.1177/1545968311418345
Smith, Y., Galvan, A., Ellender, T., Doig, N., Villalba, R., Ocampo, I., et al. (2014). The thalamostriatal system in normal and diseased states. Front. Syst. Neurosci. 8, 5. doi: 10.3389/fnsys.2014.00005
Sobin, C., and Sackeim, H. A. (1997). Psychomotor symptoms of depression. Am. J. Psychiatry 154, 4–17. doi: 10.1176/ajp.154.1.4
Sorg, C., Manoliu, A., Neufang, S., Myers, N., Peters, H., Schwerthöffer, D., et al. (2013). Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr. Bull. 39, 387. doi: 10.1093/schbul/sbr184
Sorger, B., Scharnowski, F., Linden, D. E. J., Hampson, M., and Young, K. D. (2019). Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Neuroimage 186, 256–265. doi: 10.1016/j.neuroimage.2018.11.004
Spetter, M. S., Malekshahi, R., Birbaumer, N., Lührs, M., van der Veer, A. H., Scheffler, K., et al. (2017). Volitional regulation of brain responses to food stimuli in overweight and obese subjects: a real-time fMRI feedback study. Appetite 112, 188–195. doi: 10.1016/j.appet.2017.01.032
Stanley, T. D., Carter, E. C., and Doucouliagos, H. (2018). What meta-analyses reveal about the replicability of psychological research. Psychol. Bull. 144, 1325–1346. doi: 10.1037/bul0000169
Stevens, F. L., Hurley, R. A., Taber, K. H., Hurley, R. A., Hayman, L. A., and Taber, K. H. (2011). Anterior cingulate cortex: unique role in cognition and emotion. J. Neuropsychiatry Clin. Neurosci. 23, 121–125. doi: 10.1176/jnp.23.2.jnp121
Stirner, M., Gurevitch, G., Lubianiker, N., Hendler, T., Schmahl, C., and Paret, C. (2022). An investigation of awareness and metacognition in neurofeedback with the amygdala electrical fingerprint. Conscious. Cogn. 98, 103264. doi: 10.1016/j.concog.2021.103264
Strehl, U. (2014). What learning theories can teach us in designing neurofeedback treatments. Front. Hum. Neurosci. 8, 894. doi: 10.3389/fnhum.2014.00894
Subramanian, L., Hindle, J. V., Johnston, S., Roberts, M. V., Husain, M., Goebel, R., et al. (2011). Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinso's disease. J. Neurosci. 31, 16309–16317. doi: 10.1523/JNEUROSCI.3498-11.2011
Subramanian, L., Morris, M. B., Brosnan, M., Turner, D. L., Morris, H. R., and Linden, D. E. J. (2016). Functional magnetic resonance imaging neurofeedback-guided motor imagery training and motor training for Parkinson's disease: randomized trial. Front. Behav. Neurosci. 10, 111. doi: 10.3389/fnbeh.2016.00111
Sukhodolsky, D. G., Walsh, C., Koller, W. N., Eilbott, J., Rance, M., Fulbright, R. K., et al. (2020). Randomized, sham-controlled trial of real-time functional magnetic resonance imaging neurofeedback for tics in adolescents with tourette syndrome. Biol. Psychiatry 87, 1063–1070. doi: 10.1016/j.biopsych.2019.07.035
Sulzer, J. S., Haller, S., Scharnowski, F., Weiskopf, N., Birbaumer, N., Blefari, M. L., et al. (2013). Real-time fMRI neurofeedback: progress and challenges. Neuroimage. 76, 386–399. doi: 10.1016/j.neuroimage.2013.03.033
Svoboda, K., and Li, N. (2018). Neural mechanisms of movement planning: motor cortex and beyond. Curr. Opin. Neurobiol. 49, 33–41. doi: 10.1016/j.conb.2017.10.023
Takamura, M., Okamoto, Y., Shibasaki, C., Yoshino, A., Okada, G., Ichikawa, N., et al. (2020). Antidepressive effect of left dorsolateral prefrontal cortex neurofeedback in patients with major depressive disorder: a preliminary report. J. Affect. Disord. 271, 224–227. doi: 10.1016/j.jad.2020.03.080
Taylor, J. E., Yamada, T., Kawashima, T., Kobayashi, Y., Yoshihara, Y., Miyata, J., et al. (2022). Depressive symptoms reduce when dorsolateral prefrontal cortex-precuneus connectivity normalizes after functional connectivity neurofeedback. Sci. Rep. 12, 2581. doi: 10.1038/s41598-022-05860-1
Tekin, S., and Cummings, J. L. (2002). Frontal–subcortical neuronal circuits and clinical neuropsychiatry. J. Psychosom. Res. 53, 647–654. doi: 10.1016/S0022-3999(02)00428-2
Tervo-Clemmens, B., Quach, A., Calabro, F. J., Foran, W., and Luna, B. (2020). Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use. Neuroimage 209, 116476. doi: 10.1016/j.neuroimage.2019.116476
Thibault, R. T., MacPherson, A., Lifshitz, M., Roth, R. R., and Raz, A. (2018). Neurofeedback with fMRI: a critical systematic review. Neuroimage 172, 786–807. doi: 10.1016/j.neuroimage.2017.12.071
Tinaz, S., Para, K., Vives-Rodriguez, A., Martinez-Kaigi, V., Nalamada, K., Sezgin, M., et al. (2018). Insula as the interface between body awareness and movement: a neurofeedback-guided kinesthetic motor imagery study in Parkinson's disease. Front. Hum. Neurosci. 12, 496. doi: 10.3389/fnhum.2018.00496
Trambaiolli, L. R., Tiwari, A., and Falk, T. H. (2021). Affective neurofeedback under naturalistic conditions: a mini-review of current achievements and open challenges. Front. Neuroergonomics 2, 981. doi: 10.3389/fnrgo.2021.678981
Travassos, C., Sayal, A., Direito, B., Castelhano, J., and Castelo-Branco, M. (2020). Volitional modulation of the left DLPFC neural activity based on a pain empathy paradigm—a potential novel therapeutic target for pain. Front. Neurol. 11, 714. doi: 10.3389/fneur.2020.00714
Tursic, A., Eck, J., Lührs, M., Linden, D. E. J., and Goebel, R. (2020). A systematic review of fMRI neurofeedback reporting and effects in clinical populations. NeuroImage. Clin. 28, 102496. doi: 10.1016/j.nicl.2020.102496
Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61. doi: 10.1038/nrn3857
Vaghi, M. M., Vértes, P. E., Kitzbichler, M. G., Apergis-Schoute, A. M., van der Flier, F. E., Fineberg, N. A., et al. (2017). Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol. Psychiatry 81, 708–717. doi: 10.1016/j.biopsych.2016.08.009
Van den Boom, M. A., Jansma, J. M., and Ramsey, N. F. (2018). Rapid acquisition of dynamic control over DLPFC using real-time fMRI feedback. Eur. Neuropsychopharmacol. 28, 1194–1205. doi: 10.1016/j.euroneuro.2018.08.508
Walther, S. (2015). Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. Neuroimaging 233, 293–298. doi: 10.1016/j.pscychresns.2015.06.010
Walther, S., and Strik, W. (2012). Motor symptoms and schizophrenia. Neuropsychobiology 66, 77–92. doi: 10.1159/000339456
Wang, D., Zhou, Y., Zhuo, C., Qin, W., Zhu, J., Liu, H., et al. (2015). Altered functional connectivity of the cingulate subregions in schizophrenia. Transl. Psychiatry 5, 575. doi: 10.1038/tp.2015.69
Watanabe, T., Sasaki, Y., Shibata, K., and Kawato, M. (2017). Advances in fMRI real-time neurofeedback. Trends Cogn. Sci. 21, 997–1010. doi: 10.1016/j.tics.2017.09.010
Weiskopf, N. (2012). Real-time fMRI and its application to neurofeedback. Neuroimage 62, 682–692. doi: 10.1016/j.neuroimage.2011.10.009
Weiskopf, N., Veit, R., Erb, M., Mathiak, K., Grodd, W., Goebel, R., et al. (2003). Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage 19, 577–586. doi: 10.1016/S1053-8119(03)00145-9
Weiss, F., Zhang, J., Aslan, A., Kirsch, P., and Gerchen, M. F. (2022). Feasibility of training the dorsolateral prefrontal-striatal network by real-time fMRI neurofeedback. Sci. Rep. 12, 1669. doi: 10.1038/s41598-022-05675-0
World Health Organization. (2004). Promoting Mental Health: Concepts, Emerging Evidence, Practice: Summary Report / A Report from the World Health Organization, Department of Mental Health and Substance Abuse in collaboration with the Victorian Health Promotion Foundation and the University of Melbourne. World Health Organization. Available online at: https://apps.who.int/iris/handle/10665/42940
Xie, F., Xu, L., Long, Z., Yao, L., and Wu, X. (2015). Functional connectivity alteration after real-time fMRI motor imagery training through self-regulation of activities of the right premotor cortex. BMC Neurosci. 16, 29. doi: 10.1186/s12868-015-0167-1
Xu, C., Yogaratnam, J., Tan, N., and Sim, K. (2016). Psychosis, treatment emergent extrapyramidal events, and subsequent onset of Huntington's disease: a case report and review of the literature. Clin. Psychopharmacol. Neurosci. 14, 302–304. doi: 10.9758/cpn.2016.14.3.302
Yamashita, A., Hayasaka, S., Kawato, M., and Imamizu, H. (2017). Connectivity neurofeedback training can differentially change functional connectivity and cognitive performance. Cereb. Cortex 27, 4960–4970. doi: 10.1093/cercor/bhx177
Yang, H., Hu, Z., Imai, F., Yang, Y., and Ogawa, K. (2021). Effects of neurofeedback on the activities of motor-related areas by using motor execution and imagery. Neurosci. Lett. 746, 135653. doi: 10.1016/j.neulet.2021.135653
Yoo, S.-S., Lee, J.-H., O'Leary, H., Panych, L. P., and Jolesz, F. A. (2008). Neurofeedback fMRI-mediated learning and consolidation of regional brain activation during motor imagery. Int. J. Imaging Syst. Technol. 18, 20139. doi: 10.1002/ima.20139
Yoshimura, S., Okamoto, Y., Matsunaga, M., Onoda, K., Okada, G., Kunisato, Y., et al. (2017). Cognitive behavioral therapy changes functional connectivity between medial prefrontal and anterior cingulate cortices. J. Affect. Disord. 208, 610–614. doi: 10.1016/j.jad.2016.10.017
Zahn, R., Weingartner, J. H., Basilio, R., Bado, P., Mattos, P., Sato, J. R., et al. (2019). Blame-rebalance fMRI neurofeedback in major depressive disorder: a randomised proof-of-concept trial. NeuroImage Clin. 24, 101992. doi: 10.1016/j.nicl.2019.101992
Zhang, G., Yao, L., Zhang, H., Long, Z., and Zhao, X. (2013). Improved working memory performance through self-regulation of dorsal lateral prefrontal cortex activation using real-time fMRI. PLoS ONE 8, 73735. doi: 10.1371/journal.pone.0073735
Zhao, Z., Yao, S., Li, K., Sindermann, C., Zhou, F., Zhao, W., et al. (2019). Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychother. Psychosom. 88, 5–15. doi: 10.1159/000496057
Zilverstand, A., Sorger, B., Sarkheil, P., and Goebel, R. (2015). fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front. Behav. Neurosci. 9, 148. doi: 10.3389/fnbeh.2015.00148
Zilverstand, A., Sorger, B., Slaats-Willemse, D., Kan, C. C., Goebel, R., and Buitelaar, J. K. (2017). fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single-blinded study. PLoS ONE 12, e0170795. doi: 10.1371/journal.pone.0170795
Zotev, V., Misaki, M., Phillips, R., Wong, C. K., and Bodurka, J. (2018). Real-time fMRI neurofeedback of the mediodorsal and anterior thalamus enhances correlation between thalamic BOLD activity and alpha EEG rhythm. Hum. Brain Mapp. 39, 1024–1042. doi: 10.1002/hbm.23902
Zweerings, J., Hummel, B., Keller, M., Zvyagintsev, M., Schneider, F., Klasen, M., et al. (2019). Neurofeedback of core language network nodes modulates connectivity with the default-mode network: a double-blind fMRI neurofeedback study on auditory verbal hallucinations. Neuroimage 189, 533–542. doi: 10.1016/j.neuroimage.2019.01.058
Zweerings, J., Pflieger, E. M., Mathiak, K. A., Zvyagintsev, M., Kacela, A., Flatten, G., et al. (2018). Impaired voluntary control in PTSD: Probing self-regulation of the ACC with real-time fMRI. Front. Psychiatry 9, 219. doi: 10.3389/fpsyt.2018.00219
Keywords: neurofeedback, real-time fMRI, connectivity neurofeedback, neuromodulation, frontostriatal circuitry
Citation: Orth L, Meeh J, Gur RC, Neuner I and Sarkheil P (2022) Frontostriatal circuitry as a target for fMRI-based neurofeedback interventions: A systematic review. Front. Hum. Neurosci. 16:933718. doi: 10.3389/fnhum.2022.933718
Received: 01 May 2022; Accepted: 08 August 2022;
Published: 24 August 2022.
Edited by:
Shuxia Yao, University of Electronic Science and Technology of China, ChinaReviewed by:
Timothy J. Strauman, Duke University, United StatesCopyright © 2022 Orth, Meeh, Gur, Neuner and Sarkheil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda Orth, bG9ydGhAdWthYWNoZW4uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.