
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Hum. Neurosci., 24 June 2022
Sec. Motor Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.914340
This article is part of the Research TopicAdvances in Rehabilitation for Motor Symptoms in Neurodegenerative DiseaseView all 8 articles
 Chiara Rambelli1,2
Chiara Rambelli1,2 Davide Mazzoli1*
Davide Mazzoli1* Martina Galletti1
Martina Galletti1 Giacomo Basini1
Giacomo Basini1 Paolo Zerbinati1,3
Paolo Zerbinati1,3 Paolo Prati1
Paolo Prati1 Francesca Mascioli1
Francesca Mascioli1 Stefano Masiero2
Stefano Masiero2 Andrea Merlo1
Andrea Merlo1Introduction: Charcot-Marie-Tooth disease (CMT) is a slow and progressive peripheral motor sensory neuropathy frequently associated with the cavo-varus foot deformity. We conducted a scoping review on the clinical scales used to assess foot deviations in CMT patients and analyzed their metric properties.
Evidence Acquisition: A first search was conducted to retrieve all scales used to assess foot characteristics in CMT patients from the Medline, Web of Science, Google Scholar, Cochrane, and PEDro databases. A second search was conducted to include all studies that evaluated the metric properties of such identified scales from the same databases. We followed the methodologic guidelines specific for scoping reviews and used the PICO framework to set the eligibility criteria. Two independent investigators screened all papers.
Evidence Synthesis: The first search found 724 papers. Of these, 41 were included, using six different scales: “Foot Posture Index” (FPI), “Foot Function Index”, “Maryland Foot Score”, “American Orthopedic Foot & Ankle Society's Hindfoot Evaluation Scale”, “Foot Health Status Questionnaire”, Wicart-Seringe grade. The second search produced 259 papers. Of these, 49 regarding the metric properties of these scales were included. We presented and analyzed the properties of all identified scales in terms of developmental history, clinical characteristics (domains, items, scores), metric characteristics (uni-dimensionality, inter- and intra-rater reliability, concurrent validity, responsiveness), and operational characteristics (normative values, manual availability, learning time and assessors' characteristics).
Conclusions: Our results suggested the adoption of the six-item version of the FPI scale (FPI-6) for foot assessment in the CMT population, with scoring provided by Rasch Analysis. This scale has demonstrated high applicability in different cohorts after a short training period for clinicians, along with good psychometric properties. FPI-6 can help health professionals to assess foot deformity in CMT patients over the years.
Charcot-Marie-Tooth (CMT) disease is a peripheral progressive motor sensory neuropathy. It represents the most frequent hereditary neuromuscular disorder with an estimated prevalence of up to 40 cases per 100,000 individuals (Martini et al., 1998; Pareyson and Marchesi, 2009). This pathological condition is characterized by the progressive deterioration of peripheral nervous system fibers, causing loss of both motor and sensory functions. Lower limb afflictions are often the earliest ones to arise, including distal muscle atrophy and weakness, which could result in foot drop, sensory loss, absent tendon reflexes, muscle cramps, and cavo-varus foot deformity. The average age of onset is between 10 and 20 years of age (Pareyson and Marchesi, 2009). The cavo-varus foot deformity usually represents the first clinical symptom of the disease. Consequently, the presence of bilateral cavus foot deformity in a healthy subject should be investigated for CMT when other etiologies have already been excluded (Stino et al., 2019). CMT patients exhibiting a foot deformity account for 71% of the total: in children aged 0–5 the planovalgus foot is the prevailing one, whereas in older patients cavo-varus associated with claw-toes and ankle instability is more widespread. Differences in timing and severity of muscle involvement cause an imbalance between agonistic and antagonistic muscles, resulting in a vicious circle of ensuing denervation and biomechanical alterations (Stino et al., 2019). Foot deformity, in association with muscle weakness, leads to the development of gait alterations typical of CMT, besides negatively affecting the patient's quality of life (Crosbie et al., 2008). Gait deviation also relates to limitations in everyday activities (Fulk et al., 2017; Mazzoli et al., 2019).
To properly assess foot characteristics in the CMT population, a scale should be designed for this kind of neurological condition. The design and validation of a clinical scale is a challenging and time-consuming process (Boateng et al., 2018). Identifying a set of items that reasonably describe the desired outcome is the first of a list of consecutive steps that must be followed and that deal with face and construct validity, internal consistency, item reduction and scaling, reliability, and validity (Boateng et al., 2018). These are commonly referred to as the metric properties of a scale.
In 2002 Razeghi and Batt critically reviewed the different methods used to classify foot types and observed a poor correlation between radiographic and observational indicators of foot morphology and its functional characteristics during walking (Razeghi and Batt, 2002). In the last decades, many different tools have been developed to assess foot characteristics in a wide spectrum of pathologies. Some of these were developed for purely surgical purposes, in order to aid with a radiographic evaluation of the ankle/foot complex injuries (Leigheb et al., 2016). Other tools were developed by clinicians or podiatrists to assess and monitor the patient's perceived disability in systemic chronic pathologies such as rheumatoid arthritis (Saag et al., 1996), milder afflictions of the foot like cutaneous and nail disorders (Bennett et al., 1998) and neurological conditions including CMT.
Although several clinical assessment tools were developed, to the best of our knowledge, no systematic or scoping review has been developed to identify the existing tools and their characteristics and metric properties. Therefore, the aim of this scoping review is collect all clinical scales used up till now for the clinical evaluation of the foot in CMT patients and to analyse their metric properties and characteristics to identify the most appropriate tool to be used with these patients.
This scoping review was conducted and progressively updated until July 2021, in accordance with the JBI methodology guidelines for scoping reviews, which are an extension of PRISMA guidelines specific for scoping reviews (Tricco et al., 2018; Peters et al., 2020).
We followed a two-step procedure consistent with our two leading questions: (I) What were the clinical scales previously adopted in literature to assess foot characteristics in CMT patients? (II) What are their psychometric properties?
An initial limited search of Medline was undertaken to identify the appropriate articles. The text words contained in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a first full search strategy for the Medline, Cochrane, PEDro, and Web of Science databases. Google Scholar was also considered. The search strategy, including all identified keywords and index terms, was adapted for each database. The reference list of all included findings was screened for additional studies to be included by manual search.
The first search strategy for the first step was: [(Charcot Marie Tooth) OR (Charcot Marie Tooth disease [MeSH Terms])] AND [(foot joints[MeSH Terms]) OR (ankle joints[MeSH Terms]) OR (ankle[MeSH Terms]) OR foot OR ankle] AND [assessment OR evaluation OR measure OR (outcome measure) OR scale OR (outcome assessment health care[MeSH Terms]) OR (disability evaluation[MeSH Terms])].
We used the PICO framework to set the eligibility criteria. Since two steps with two different searches were conducted, two different PICOs were set up.
We included studies on CMT patients, both adults and children. No restrictions on study design were set. We included all types of studies such as reviews, experimental studies, and observational studies. Studies published both in English and Italian were included and no time limitations were set. Exclusion criteria were studies on animals, genetic studies, studies concerning other pathologies and withdrawn papers. Abstracts from conferences, where full text was not available or did not exist, were also excluded.
Studies could involve any type of intervention.
No limitations were set for this parameter.
Papers had to use at least one clinical scale specific for the assessment of foot characteristics in the CMT population. Scales including foot assessment as part of an overall assessment of disease severity were not included.
In the second stage, we analyzed the metric properties of the scales identified in Step 1. This was carried out through an advanced search on Medline, Cochrane, PEDro, Web of Science and Google Scholar by looking for the name of each scale found in the previous step and associated with the terms “validity”, “validation”, “consistency”, “reliability” and “responsiveness” in either the title or in the abstract, and excluding translated studies and cultural adaptations of the scale (“NOT translation”, “NOT adaptation”). Again, the bibliography of the included papers was screened to further identify papers to be included.
The second full search string was: (((scale name[Title/Abstract]) OR (scale acronym[Title/Abstract])) AND (foot[Title/Abstract])) AND (validity[Title/Abstract] OR validation[Title/Abstract] OR consistency[Title/Abstract] OR reliability[Title/Abstract] OR responsiveness[Title/Abstract]) NOT (fascia[Title/Abstract] OR fasciopathy[Title/Abstract] OR fasciitis) NOT (translation[Title/Abstract] OR adaptation[Title/Abstract] OR elderly[Title/Abstract] OR treatment[Title/Abstract] OR trial[Title/Abstract]).
We considered the populations on which metric properties of the included clinical scales had been analyzed. To focus on studies necessary to our aims – i.e. foot assessment CMT patients – we excluded those treating pathologies of the plantar fascia, hallux valgus and studies on the geriatric population.
Studies had to involve the analysis of the metric properties of at least one clinical scale from those retrieved in the first step.
No limitations were set for this parameter.
Metric properties of the scales, such as inter/intra-rater reliability, concurrent validity, or internal consistency.
All identified citations were collected, and duplicates were removed. Titles and abstracts were screened, and potentially relevant sources were retrieved. Two independent reviewers (authors CR and AM) assessed the full text and decided whether to include selected papers. Reasons for exclusion of sources of evidence were recorded and reported in the scoping review. Any disagreements that arose between the reviewers at each given stage of the selection process was discussed. Relevant data from each paper were collected in tables and presented in a narrative synthesis.
The first step of the search found 724 papers. Of these, 41 were included in the review because they met the aim of this research. Of the 683 excluded studies, only three required a discussion between both investigators. The selection procedure is presented in Figure 1. The selected studies used six different scales: “Foot Posture Index” (FPI), “Foot Function Index” (FFI), “Maryland Foot Score” (MFS), “American Orthopedic Foot & Ankle Society's Hindfoot Evaluation Scale” (AOFAS-AHES), “Foot Health Status Questionnaire” (FHSQ), and “Wicart-Seringe grade” (WSG).
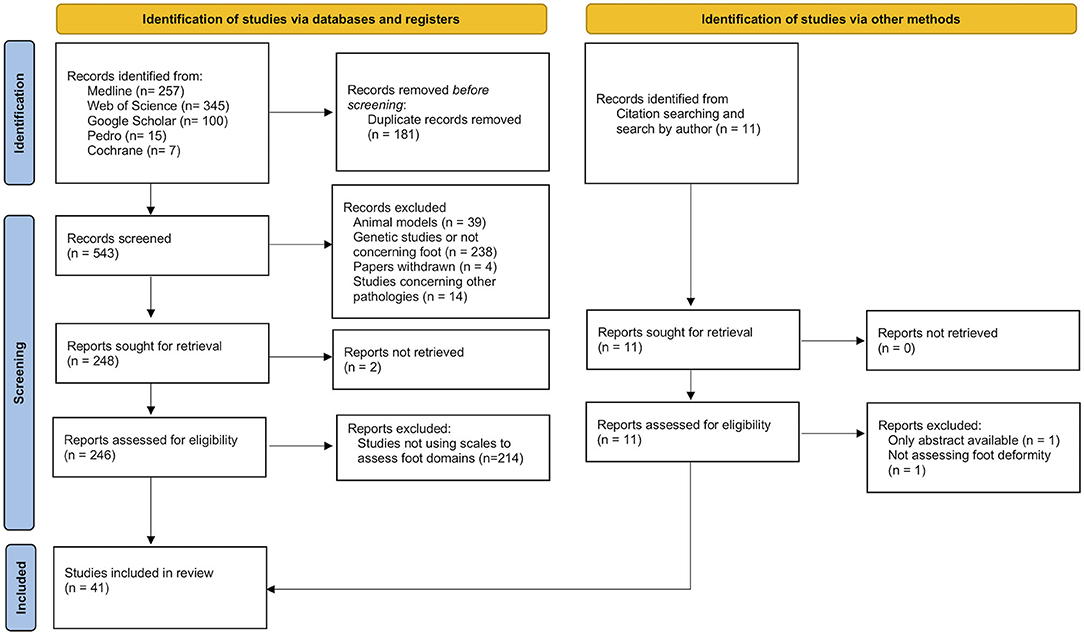
Figure 1. Flow diagram of the selection process of papers using clinical scales to assess foot deformity in CMT patients.
All included papers are listed in Table 1 and grouped by the foot assessment scale used. The main characteristics of each scale are also summarized in Table 1. Papers in which more than one tool was used were included more than once.
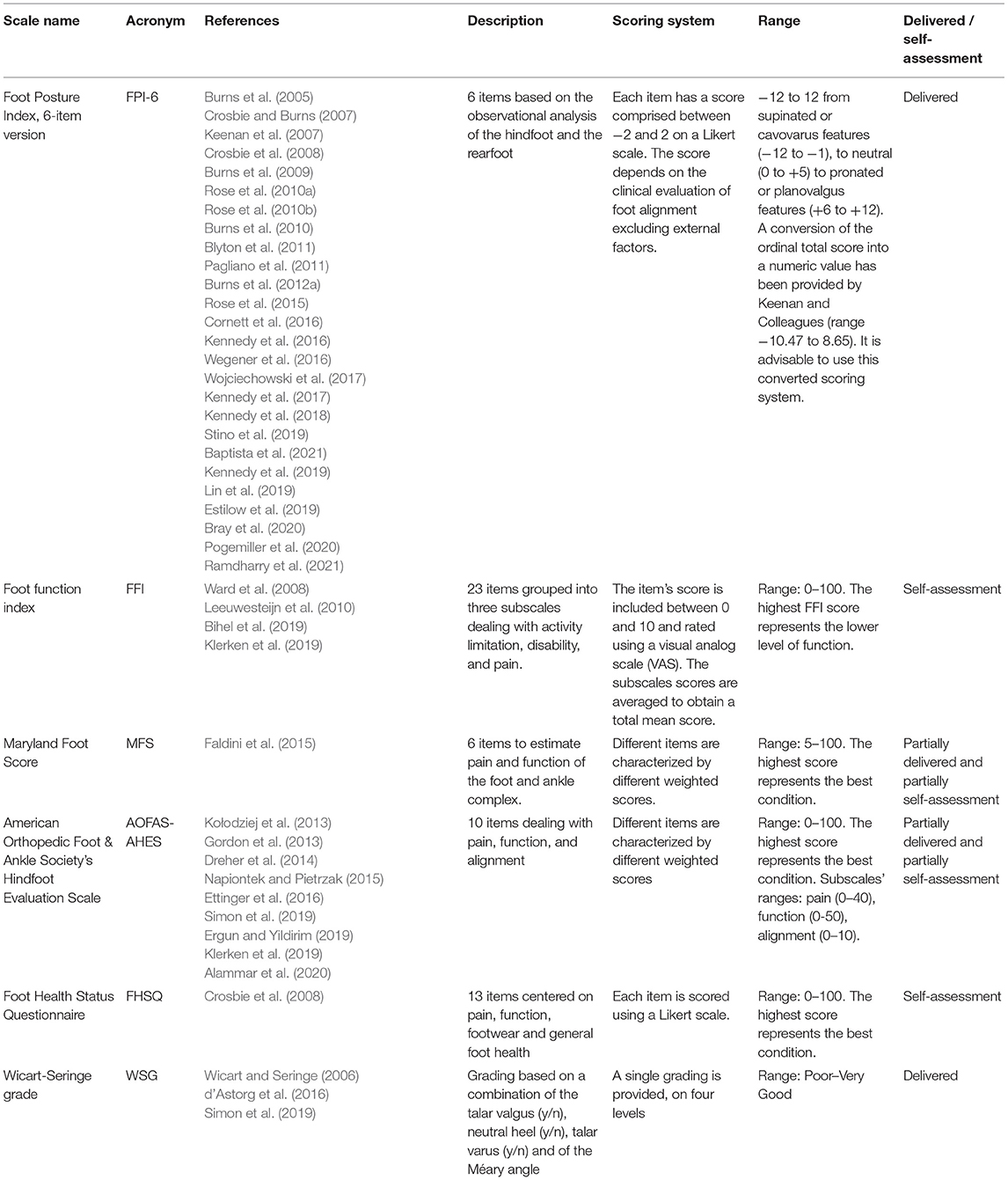
Table 1. Scales used in literature for foot assessment in CMT patients, related articles, and main characteristics.
FPI was developed to evaluate the overall foot position considering its three-dimensional nature (Redmond et al., 2006). A comprehensive review of the literature surrounding the clinical evaluation of foot was performed, leading to a list of 36 items. According to the best practices for item selection when developing and validating clinical scales (Boateng et al., 2018), this list was narrowed down to eight items (FPI-8) (Evans et al., 2003; Scharfbillig et al., 2004; Redmond et al., 2006; Keenan et al., 2007). Next, two items not belonging to the foot function domain were also removed, leading to the final FPI-6 version (Redmond et al., 2006). Twenty-six papers used FPI to assess CMT patients in different settings in both the adult and pediatric populations (Burns et al., 2005, 2009, 2010, 2012a; Crosbie and Burns, 2007; Keenan et al., 2007; Crosbie et al., 2008; Rose et al., 2010a,b, 2015; Blyton et al., 2011; Pagliano et al., 2011; Cornett et al., 2016; Kennedy et al., 2016, 2017, 2018, 2019; Wegener et al., 2016; Wojciechowski et al., 2017; Estilow et al., 2019; Lin et al., 2019; Stino et al., 2019; Bray et al., 2020; Pogemiller et al., 2020; Baptista et al., 2021; Ramdharry et al., 2021).
FFI was developed in 1991 to assess foot function in patients with rheumatoid arthritis, without fixed foot deformities or prior foot surgery (Budiman-Mak et al., 1991). FFI metric properties have been assessed mainly in patients with orthopedic foot pathologies (Budiman-Mak et al., 1991; Saag et al., 1996; Kuyvenhoven et al., 2002; Agel et al., 2005; Madeley et al., 2012; Pinsker et al., 2015; Bihel et al., 2019). Four papers used FFI in the evaluation of CMT patients (Ward et al., 2008; Leeuwesteijn et al., 2010; Bihel et al., 2019; Klerken et al., 2019). Of these, one focused on the metric properties of FFI when used with patients with type 1A CMT disease.
AOFAS-AHES and MFS were developed for trauma and orthopedic patients, and their scores merged aspects of alignment, pain, and loss of activity (Heffernan et al., 2000; SooHoo et al., 2003; Ibrahim et al., 2007; Pena et al., 2007; Schepers et al., 2008; Madeley et al., 2012; Cöster et al., 2014; Pinsker et al., 2015; Conceição et al., 2016; Ponkilainen et al., 2020). In these tools the walking impairment is presumed to be a direct consequence of an acute foot pathology. FHSQ is a self-administered questionnaire developed and validated to measure the quality of life related to foot health in a population suffering from minor foot conditions, such as skin and nail disorders (Bennett et al., 1998). AOFAS-AHES was used in nine orthopedic studies to evaluate a cohort of CMT patients (Gordon et al., 2013; Kołodziej et al., 2013; Dreher et al., 2014; Napiontek and Pietrzak, 2015; Ettinger et al., 2016; Ergun and Yildirim, 2019; Klerken et al., 2019; Simon et al., 2019; Alammar et al., 2020) while MFS and FHSQ were both employed only once (Crosbie et al., 2008; Faldini et al., 2015).
WGS was used for the first time in a study by Simon and colleagues in 2019 (Simon et al., 2019) to assess the effects of osteotomy surgery in children with cavo-varus deformity. WGS was then used in another two studies conducted by the same research team (Wicart and Seringe, 2006; d'Astorg et al., 2016).
As detailed in the method section, a further analysis of their psychometric properties was conducted for each of the scales found in the previous search and selection. This second search initially produced 259 papers. After the removal of duplicates, 148 further papers were excluded because they did not add any extra information about the metric properties of the tools and/or because of their application in pathologies very different from CMT, resulting in 45 primary studies. Four more primary studies were added based on the bibliographic references of two other reviews (Evans and Rome, 2011; Budiman-Mak et al., 2013), leading to a total of 49 primary studies. The selection procedure is presented in Figure 2.
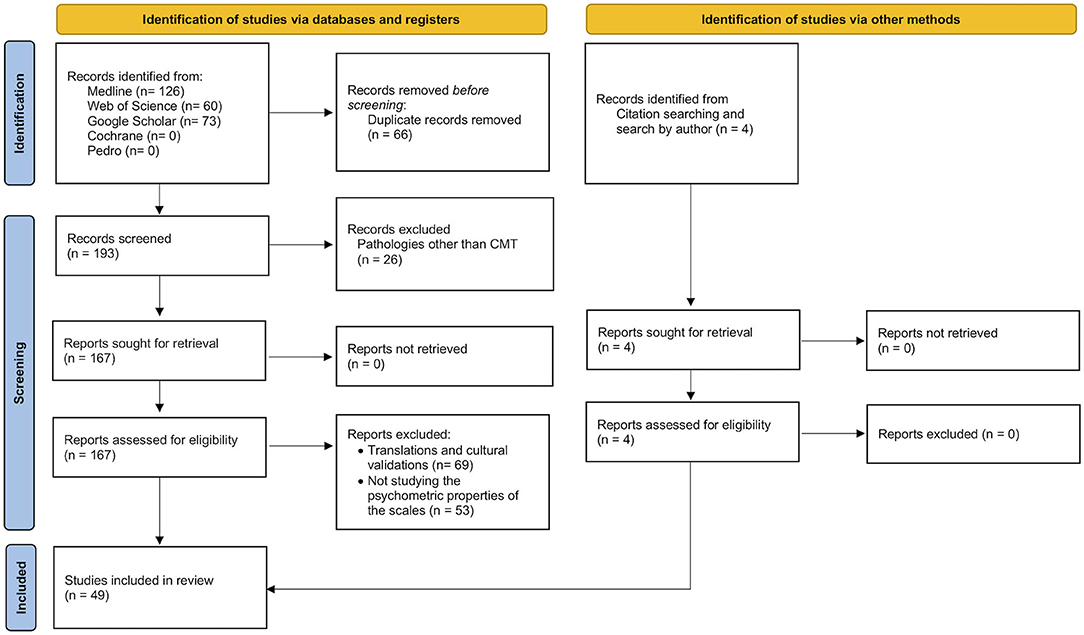
Figure 2. Flow diagram of the selection process for studies assessing the psychometric properties of the scales identified previously.
The metric properties of the scales have been collected in terms of number of raters and their expertise, indicators of internal consistency (e.g., Cronbach's alpha), intra-rater and inter-rater reliability (e.g. ICC), and of concurrent validity (e.g. r, R2). These are listed in Table 2.
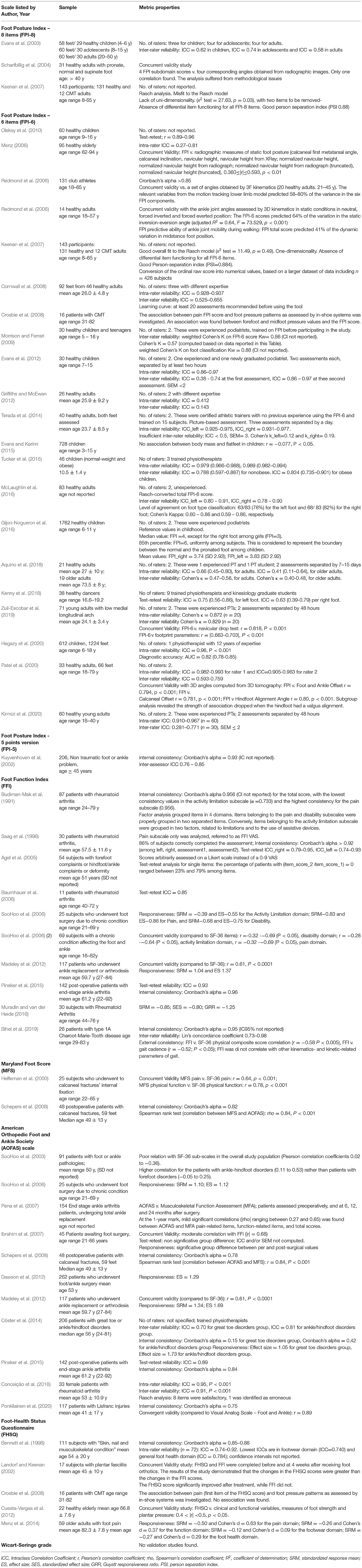
Table 2. Sample characteristics and metric properties of the scales as reported in all studies included.
Several versions of FPI were developed and analyzed before defining which was best, following an iterative process typical in the construction of assessment scales (Kuyvenhoven et al., 2002; Evans et al., 2003; Scharfbillig et al., 2004; Keenan et al., 2007). FPI-8 has been used in the early 2000s and was tested by three authors in samples of both healthy subjects and CMT patients aged from 4 to 65. It showed medium inter-rater reliability (ICC: 0.58–0.74) and a lack of uni-dimensionality. After the removal of two items not linked to the foot deviation domain, the metric properties of the new FPI-6 were analyzed in 21 studies from 2006 to 2020. Study samples ranged from 14 to 1762 healthy adults and children. Only one study considered 12 CMT adults from an assessed sample of 143 participants (Keenan et al., 2007). The scale showed an appropriate internal consistency (Cronbach's alpha > 0.85) (Redmond et al., 2008), and the Rasch analysis by Keenan et al. confirmed its uni-dimensionality. Moreover, the scale was found to be well suited to be used at the single-patient level, because of a Person Separation Index > 0.85 (Keenan et al., 2007). Finally, the Rasch procedure converts the ordinal FPI-6 score to a numerical value, which is proportional to the amount of foot deviation (Keenan et al., 2007). Inter-rater reliability was 0.59 – 0.97 when the scale was administered by expert assessors. Raters in the included studies were podiatrists, athletic trainers, physiotherapists, and kinesiologists. Concurrent validity was demonstrated by comparing the FPI-6 score to Xray and tomography related measures, 3D kinematics, and other clinical measurements such as the navicular drop test. No study addressed the responsiveness of the scale, yet.
The metric properties of FFI have been assessed in ten studies, dated 1991–2019. Samples usually included patients with rheumatoid arthritis, foot deformities or who underwent arthrodesis, with sample sizes ranging from 11 to 142 individuals. Authors found high internal consistency and inter-rater reliability (ICC: 0.74 – 0.95). Bihel and colleagues investigated the metric properties of FFI in 26 CMT patients, and found excellent internal validity (Cronbach's α = 0.95) and satisfactory reproducibility (Lin's concordance coefficient = 0.82) (Bihel et al., 2019). Test-retest ICC was in the order of 0.8-0.9 (Saag et al., 1996; Baumhauer et al., 2006). However, FFI activity subscale demonstrated low external validity when compared with gait patterns. Adequate responsiveness of the scale to the variations determined by surgery was found in the three studies addressing this topic (SooHoo et al., 2006; Madeley et al., 2012; Muradin and van der Heide, 2016).
Two studies tested MFS in a population of 25–48 patients who suffered from calcaneal fracture. Schepers and colleagues found high internal consistency (Cronbach's alpha = 0.82) (Schepers et al., 2008), but moderate concurrent validity when the scale was compared to SF-36 (Heffernan et al., 2000). Information on the raters characteristics were not available.
Eleven authors investigated the metric properties of the AOFAS from 2003 to 2019. Samples ranged from 25 to 262 patients suffering from diverse orthopedic or rheumatic pathologies. Among authors, only Cöster specified that trained physiotherapists administered the test (Cöster et al., 2014). Internal consistency was different among studies, ranging from 0.15 to 0.78. Authors found low to moderate concurrent validity with other clinical measures, such as the Visual Analogical Scale, the SF-36, or the Musculoskeletal Functional Assessment, and moderate inter-rater reliability (ICC = 0.70–0.91). Responsiveness was satisfactory (see Table 2), as found in three studies on foot surgery involving large samples (SooHoo et al., 2006; Dawson et al., 2012; Madeley et al., 2012), also thanks to the large effect of surgery on foot alignment and pain.
FHSQ was investigated in five studies from 1998 to 2014. Bennet and colleagues found high internal consistency (Cronbach's alpha = 0.85 – 0.88). The overall foot condition in patients with plantar fasciitis, or secondary skin and nail issues was assessed, and FHSQ was found to have moderate to good reliability (Bennett et al., 1998) and moderate concurrent validity with a set of clinical and functional variables and with measures of foot strength and plantar pressure (Landorf and Keenan, 2002; Cuesta-Vargas et al., 2012). Crosbie adopted FHSQ in a cohort of CMT patients with cavus foot deformity and found no relationship between the FHSQ score and the amount of cavus deformity assessed with sensors for plantar pressure and foot-ground contact duration (Crosbie et al., 2008). Inadequate responsiveness of the whole tool was also reported by Menz and colleagues in a study on the effect of specific footwear on foot status in older adults with persistent foot pain (Menz et al., 2014). While the subscales assessing pain and function detected improvements, the remaining subscales on footwear and general foot health did not, leading to a low global responsiveness.
Our search did not find any studies investigating the metric properties of WSG.
The aim of this study was to provide an overall view of the clinical scales used to assess the foot in CMT patients, and help clinicians choose the best scale to employ in their daily practice. For this reason, we conducted a scoping review collecting all clinical scales used so far in literature, describing the scales' development and metric properties. Scoping reviews are better suited, as they do not aim at answering a specific question—as systematic reviews do—but aim at mapping existing evidence and analyzing any gap in knowledge (Tricco et al., 2018; Peters et al., 2020).
We found 42 studies using six different scales for foot assessment in the CMT population (Table 1) and 49 studies assessing their metric properties (Table 2). Their history, internal consistency, inter-rate and intra-rate reliability, and assessing modalities are summarized in Table 2.
The literature search revealed two different types of scales: those built specifically for neurologic foot assessment, including CMT patients, and those borrowed from the orthopedic or rheumatologic fields and then used to assess the neuropathic foot.
FPI-6 is the only scale specifically developed for CMT patients being the most widely used scale assessing foot deformity and was employed in 27 studies included in the current review. Its broad use is mainly due to its uni-dimensionality (i.e., the power to address a single construct) and satisfactory psychometric properties (e.g., inter-rater reliability). This version is a product of fine-tuning previous versions (Martin and Irrgang, 2007) and following the criteria for the creation of a new assessment scale.
Other scales such as FFI, MFS, AOFAS-AHES, FHSQ, and WSG have poor psychometric properties when used with neurologic patients (see Table 2). This might be traced back some missteps during their set up. In fact, unlike FPI, these scales were developed and evaluated only for orthopedic or rheumatologic patient cohorts. For this reason, the assessment of foot deviation provided by these scales probably does not include all the aspects that should be considered when dealing with more neurologically complex patients. Moreover, these scales were mainly developed during clinical practice and did not undergo all the steps necessary to build a new measurement scale (Boateng et al., 2018).
FFI was developed for rheumatic patients and not all domains have proven to possess good external validity, such as the activity subscale in patients with rheumatoid arthritis. Responsiveness of this scale has been demonstrated and this supports the use of FFI to monitor outcomes in patients with orthopedic conditions. However, this subscale is reasonably linked to several factors other than foot deviation consequent to peripheral neuropathy. Another issue of this tool relies on the exclusion of subjects with fixed foot deformities during its validation, while 71% of CMT patients present this kind of foot deformity (Saag et al., 1996; Stino et al., 2019). Hence, its use with CMT patients remains questionable.
AOFAS-AHES and MFS were mainly developed for orthopedic patients with specific issues caused by an acute ankle-foot injury. Responsiveness was satisfactory, also thanks to the large effect of surgery on foot alignment and pain. However, when dealing with CMT patients, many factors must be considered when assessing walking impairment, such as muscle atrophy and weakness, sensory deficiency, and foot deformity. Therefore, scales designed for orthopedic patients should not be used with neurologic patients.
Finally, FHSQ was developed to test patients with plantar fasciitis or non-serious skin pathologies. When used with CMT, no correlation was found between its score and the percentage of cavus deformity (Crosbie et al., 2008; Cuesta-Vargas et al., 2012). Although the subscales assessing pain and function were found to have good responsiveness, the remaining subscales did not. This is a common drawback in tools assessing multiple domains. The use of this tool in a cohort of patients should be adopted with caution, as mentioned by Landorf and colleagues (Landorf and Keenan, 2002), who suggested limiting the use of FHSQ for pathologies where walking ability is not compromised.
Most of the scales considered in this scoping review (FFI, MFS, AOFAS and FHQS) focus on a general assessment of the whole lower limb function, investigating pain, perception of stability and limping, difficulty while performing ADLs (walking indoors or outdoors, climbing stairs, getting up from a chair, stepping over an obstacle), and use of appropriate walking aids and shoes (see Table 1). The AOFAS includes a subscale specific for foot alignment, while FPI and WGS focus on the single domain of foot posture. When focusing on foot deformity and on the effect of any corrective interventions, unidimensional scales assessing foot posture should be used. At the same time, from an ICF classification perspective, the impact of foot posture and pain on functional activities should also be assessed. In line with the aims of this scoping review and for the above-mentioned reasons, we suggest the use of FPI-6 when assessing foot deformities in CMT patients.
Currently, FPI-6 is the most appropriate tool to be used for foot assessment in CMT patients. The FPI-6 is scale involving six items related to rearfoot and forefoot components, used to quantify the degrees of foot pronation or supination while standing. It investigates the position of the talar head, the calcanear inversion/eversion, the lateral malleolus, the talo-navicular congruence, the medial arch height, and the forefoot abduction/adduction. Each item in scored on between−2 to +2, and the item scores are summed up to obtain a global score. A positive final score > 5 points reveals a pronated foot, a negative final score suggests a supinated foot, while a score of 0–5 indicates a neutral foot position (Redmond et al., 2006, 2008). This was specifically designed to assess foot deformities in neurological patients and proved to be sensitive to disease-related postural changes (Scharfbillig et al., 2004; Redmond et al., 2006).
Gijon-Nogueron et al. (2016) investigated the FPI distribution score and its variations linked to age in more than 1,500 healthy children, thus setting the reference values for children. The CMT cohort investigated by Redmond et al. (2008) showed a correlation between FPI-6 values and age, with significantly higher FPI-6 scores in the young and the elderly compared to the adult population with a 'U' shaped distribution curve. The availability of normative values of FPI-6 is a further element favoring the adoption of this scale (Redmond et al., 2008).
Most of the studies considered in this review stated the role and the level of expertise of the assessors using FPI-6 (see Table 2). A variety of healthcare professionals were present including physicians, physiotherapists, podiatrists, and physiotherapy and osteopathy students. Significant score differences arose based on the difference in expertise levels. Both intra-rater and inter-rater reliability increased after a short training period. This proves the need for a short training period, requiring about 20-30 supervised evaluations (Cornwall et al., 2008; Evans et al., 2012), further proving the validity of FPI-6. The most difficult items to be properly assessed were those related to the differences between the neutral and pronated foot types (McLaughlin et al., 2016). Following a brief learning period, FPI-6 proved to be a satisfactory tool in all the studies considered: intra-rater reliability results were very good among studies, with intra-rater ICC > 0.90 (Evans et al., 2012; Terada et al., 2014; Kirmizi et al., 2020; Patel et al., 2020) or Cohen's k > 0.85 (Zuil-Escobar et al., 2019) or Pearson's r ≥ 0.89 (Oleksy et al., 2010). The inter-rater ICC varied among the studies, ranging from fair to very good when untrained or trained raters were respectively included (see Table 2) (Menz, 2006; Cornwall et al., 2008; Evans et al., 2012; Griffiths and McEwan, 2012; Terada et al., 2014; Evans and Karimi, 2015; Tucker et al., 2015; McLaughlin et al., 2016; Aquino et al., 2018; Kenny et al., 2018; Hegazy et al., 2020; Kirmizi et al., 2020; Patel et al., 2020). To support operator training, Kirmizi and colleagues suggested implementing the FPI-6 operative manual by including drawings that fully described each possible foot deviation and its associated score (Kirmizi et al., 2020).
A current limitation with FPI-6 is the lack of studies addressing its responsiveness to change after a treatment. Internal responsiveness is the ability to detect a change between the pre- and post-intervention condition, and external responsiveness is the ability to detect a change that truly affects the patient's health status (Menz et al., 2014). The sensitivity of the scale to changes was addressed by Redmond et al. (2006) by applying wedges under specific parts of the foot and verifying the modification in the score. However, no studies specifically designed to assess FPI-6 responsiveness are available. The lack of this information is a current limitation of the scale and should be addressed by future studies.
The natural evolution of CMT is characterized by atrophy of the intrinsic foot muscles and their imbalance with the antagonist extrinsic foot muscles. This leads to foot deformity and a progressive decrease in ankle range of motion (Burns, 2006). Foot deformity can be tracked and quantified by using the FPI-6. Since FPI-6 supplies a measurement for foot alignment alone, this should be combined with other scales addressing further domains related to the patient's functionality.
In light of these observations, we suggest using FPI-6 to assess foot deformities and measuring the patient's functional and impairment levels by using specific tools developed for neuropathies, such as the Charcot-Marie-Tooth Disease Pediatric Scale in Children (Burns et al., 2012b), the CMT Neuropathy Score (Burns et al., 2012b; Zuccarino et al., 2020) along with measures of strength, pain, balance and walking ability. In clinical practice, it can be used to follow the evolution of the cavovarus foot deformity in CMT patients and to assess the effect of foot surgery in restoring the physiological tibiotarsal and foot joint posture.
In research studies, when algebraic operations are required such as the computation of longitudinal differences or the computation of the ensemble average, the use of the linear, numerical version of the FPI-6 score obtained by the Rasch Analysis procedure (Redmond et al., 2006) is advisable.
This is the first scoping review about scales used for the clinical evaluation of foot deviations in CMT patients to be found in literature. The main strengths of this review are the comprehensive analysis of the development processes and the psychometric properties of the scales, including a discussion of both their usability and learning curve when available.
The main limitation is that CMT is a rare disease, so studies on this topic compared to other pathologies are few. Moreover, differently from systematic reviews, protocols for scoping reviews cannot be uploaded on dedicated repositories, such as the PROSPERO database (Page et al., 2018). Consequently, a preliminary peer-review of the procedures we used in this scoping review is missing. Even if the string search was built following an iterative process aimed at improving the sensibility of the search, as suggested by scoping reviews guidelines, we could have missed some papers during the database search.
In this study, we did not control for eventual methodological errors in the included studies, according to the procedure for scoping reviews. For managing this limitation, readers are invited to always pay attention to sample numerosity reported in Table 2 and to the use of the proper statistical indicator when assessing the scale metric properties.
The results of our scoping review suggest the adoption of FPI-6 for foot assessment in the CMT population. The scale demonstrated a high applicability in different cohorts, good psychometric properties, uni-dimensionality, and the ability to differentiate between single patients. FPI-6 requires a short training period for the assessors. We suggest its use in clinical practice as it can be a helpful tool for clinicians in assessing foot deformities in the CMT population, along with functional scales, specifically designed for patients with CMT or similar neuropathies, and suited to assess the patient functional and impairment levels. Future studies should address the responsiveness of FPI-6 when used with different treatments delivered to specific cohorts of patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
CR, AM, DM, and SM contributed to conception and design of the study. AM, PP, and FM organized the database. CR and AM wrote the first draft of the manuscript. DM, MG, GB, and PZ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was entirely funded by our Institution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AOFAS-AHES, American Orthopedic Foot & Ankle Society's Hindfoot Evaluation Scale; CMT, Charcot Marie Tooth; FFI, Foot Function Index; FHSQ, Foot Health Status Questionnaire; FPI, Foot Posture Index; FPI-6, Foot Posture Index-6 items; ICC, Intraclass Correlation Coefficient; MFS, Maryland Foot Score; SF-36, Medical Outcomes Study Questionnaire Short Form 36 Health Survey; WSG, Wicart-Seringe grade.
Agel, J., Beskin, J. L., Brage, M., Guyton, G. P., Kadel, N. J., Saltzman, C. L., et al. (2005). Reliability of the foot function index: a report of the AOFAS outcomes committee. Foot Ankle Int. 26, 962–967. doi: 10.1177/107110070502601112.
Alammar, Y., Sudnitsyn, A., Neretin, A., Leonchuk, S., and Kliushin, N. M. (2020). Closed arthrodesis in infected neuropathic ankles using Ilizarov ring fixation. Bone Jt. J. 102B. doi: 10.1302/0301-620X.102B4.BJJ-2019-1158.R1
Aquino, M. R. C., Avelar, B. S., Silva, P. L., Ocarino, J. M., and Resende, R. A. (2018). Reliability of foot posture index individual and total scores for adults and older adults. Musculoskelet. Sci. Pract. 36, 92–95. doi: 10.1016/j.msksp.2018.02.002
Baptista, C. R. De., Nascimento-Elias, A. H., Garcia, B., Testa, A., Domingues, P. C., Martinez, E. Z., et al. (2021). Physical function and performance measures of children and adolescents with Charcot-Marie-Tooth disease. Physiother. Theory Pract. 37, 73–80. doi: 10.1080/09593985.2019.1603257
Baumhauer, J. F., Nawoczenski, D. A., DiGiovanni, B. F., and Wilding, G. E. (2006). Reliability and validity of the american orthopaedic foot and ankle society clinical rating scale: a pilot study for the hallux and lesser toes. Foot Ankle Int. 27, 1014–1019. doi: 10.1177/107110070602701202
Bennett, P. J., Patterson, C., Wearing, S., and Baglioni, T. (1998). Development and validation of a questionnaire designed to measure foot-health status. J. Am. Podiatr. Med. Assoc. 88, 419–428. doi: 10.7547/87507315-88-9-419
Bihel, L., Reynaud, V., Givron, P., Clavelou, P., Cornut-Chauvinc, C., Pereira, B., et al. (2019). Foot function index: a promising questionnaire for individuals with charcot-marie-tooth disease type 1A. Arch. Phys. Med. Rehabil., 4–7. doi: 10.1016/j.apmr.2019.06.003
Blyton, F., Ryan, M. M., Ouvrier, R. A., and Burns, J. (2011). Muscle cramp in pediatric charcot-marie-tooth disease type 1A: prevalence and predictors. Neurology 77. doi: 10.1212/WNL.0b013e31823d76aa
Boateng, G. O., Neilands, T. B., Frongillo, E. A., Melgar-Quiñonez, H. R., and Young, S. L. (2018). Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front. Public Heal. 6, 1–18. doi: 10.3389/fpubh.2018.00149
Bray, P., Cornett, K. M. D., Estilow, T., Pareyson, D., Zuccarino, R., Skorupinska, M., et al. (2020). Reliability of the charcot-marie-tooth functional outcome measure. J. Peripher. Nerv. Syst. 25, 288–291. doi: 10.1111/jns.12406
Budiman-Mak, E., Conrad, K. J., Mazza, J., and Stuck, R. M. (2013). A review of the foot function index and the foot function index - revised. J. Foot Ankle Res. 6, 5. doi: 10.1186/1757-1146-6-5
Budiman-Mak, E., Conrad, K. J., and Roach, K. E. (1991). The foot function index: a measure of foot pain and disability. J. Clin. Epidemiol. 44. doi: 10.1016/0895-4356(91)90220-4
Burns, J. (2006). Pes cavus pathogenesis in Charcot-Marie-Tooth disease type 1A. Brain 129, E50–E50. doi: 10.1093/brain/awl116
Burns, J., Ouvrier, R., Estilow, T., Shy, R., Laur,á, M., Eichinger, K., et al. (2012a). Symmetry of foot alignment and ankle flexibility in paediatric Charcot–Marie–Tooth disease. Clin. Biomech. 27, 744–747. doi: 10.1016/j.clinbiomech.2012.02.006
Burns, J., Ouvrier, R., Estilow, T., Shy, R., Laura, M., Pallant, J., et al. (2012b). Validation of the CMT Pediatric Scale as an outcome measure of disability. Ann Neurol. 71, 642–652. doi: 10.1002/ana.23572
Burns, J., Redmond, A., Ouvrier, R., and Crosbie, J. (2005). Quantification of muscle strength and imbalance in neurogenic pes cavus, compared to health controls, using hand-held dynamometry. Foot Ankle Int. 26. doi: 10.1177/107110070502600708
Burns, J., Ryan, M. M., and Ouvrier, R. A. (2009). Evolution of foot and ankle manifestations in children with CMT1A. Muscle Nerve. 39. doi: 10.1002/mus.21140
Burns, J., Scheinberg, A., Ryan, M. M., Rose, K. J., and Ouvrier, R. A. (2010). Randomized trial of botulinum toxin to prevent pes cavus progression in pediatric charcot-marie-tooth disease type 1A. Muscle Nerve. 42, 262–267. doi: 10.1002/mus.21685
Conceição, C. S., da Neto, M. G., Neto, A. C., Mendes, S. M. D., Baptista, A. F., and Sá, K. N. (2016). Analysis of the psychometric properties of the American Orthopaedic Foot and Ankle Society Score (AOFAS) in rheumatoid arthritis patients: application of the Rasch model. Rev. Bras. Reumatol. (English Ed. 56, 8–13. doi: 10.1016/j.rbr.2014.12.003
Cornett, K. M. D., Menezes, M. P., Bray, P., Halaki, M., Shy, R. R., Yum, S. W., et al. (2016). Phenotypic variability of childhood Charcot-Marie-Tooth disease. JAMA Neurol. 73. doi: 10.1001/jamaneurol.2016.0171
Cornwall, M. W., McPoil, T. G., Lebec, M., Vicenzino, B., and Wilson, J. (2008). Reliability of the modified foot posture index. J. Am. Podiatr. Med. Assoc. 98, 7–13. doi: 10.7547/0980007
Cöster, M. C., Rosengren, B. E., Bremander, A., Brudin, L., and Karlsson, M. K. (2014). Comparison of the self-reported foot and ankle score (SEFAS) and the American orthopedic foot and ankle society score (AOFAS). Foot Ankle Int. 35, 1031–1036. doi: 10.1177/1071100714543647
Crosbie, J., and Burns, J. (2007). Predicting outcomes in the orthotic management of painful, idiopathic Pes Cavus. Clin. J. Sport Med. 17, 337–342. doi: 10.1097/JSM.0b013e31814c3e9e
Crosbie, J., Burns, J., and Ouvrier, R. A. (2008). Pressure characteristics in painful pes cavus feet resulting from Charcot–Marie–Tooth disease. Gait Posture. 28, 545–551. doi: 10.1016/j.gaitpost.2008.03.011
Cuesta-Vargas, A. I., Galan-Mercant, A., Martín-Borras, M. C., and González-Sánchez, M. (2012). Criterion-related validity of the foot health status questionnaire regarding strength and plantar pressure measurements in elderly people. Foot Ankle Spec. 5, 366–373. doi: 10.1177/1938640012463056
d'Astorg, H., Rampal, V., Seringe, R., Glorion, C., and Wicart, P. (2016). Is non-operative management of childhood neurologic cavovarus foot effective? Orthop. Traumatol. Surg. Res. 102, 1087–1091. doi: 10.1016/j.otsr.2016.09.006
Dawson, J., Boller, I., Doll, H., Lavis, G., Sharp, R., Cooke, P., et al. (2012). Responsiveness of the Manchester–Oxford foot questionnaire (MOXFQ) compared with AOFAS, SF-36 and EQ-5D assessments following foot or ankle surgery. J. Bone Joint Surg. Br. 94-B, 215–221. doi: 10.1302/0301-620X.94B2.27634
Dreher, T., Wolf, S. I., Heitzmann, D., Fremd, C., Klotz, M. C., and Wenz, W. (2014). Tibialis posterior tendon transfer corrects the foot drop component of cavovarus foot deformity in charcot-marie-tooth disease. J. Bone Jt. Surg. 96, 456–462. doi: 10.2106/JBJS.L.01749
Ergun, S., and Yildirim, Y. (2019). The cole midfoot osteotomy clinical and radiographic retrospective review of five patients (six feet) with different etiologies. J. Am. Podiatr. Med. Assoc. 109, 180–186. doi: 10.7547/17-056
Estilow, T., Glanzman, A. M., Burns, J., Harrington, A., Cornett, K., Menezes, M. P., et al. (2019). Balance impairment in pediatric charcot–marie–tooth disease. Muscle Nerve. 60, 242–249. doi: 10.1002/mus.26500
Ettinger, S., Stukenborg-Colsman, C., Plaass, C., Yao, D., Claassen, L., Berger, S., et al. (2016). Tibiocalcaneal arthrodesis as a limb salvage procedure for complex hindfoot deformities. Arch. Orthop. Trauma Surg. 136, 457–462. doi: 10.1007/s00402-016-2420-1
Evans, A. M., Copper, A. W., Scharfbillig, R. W., Scutter, S. D., and Williams, M. T. (2003). Reliability of the foot posture index and traditional measures of foot position. J. Am. Podiatr. Med. Assoc. 93, 203–213. doi: 10.7547/87507315-93-3-203
Evans, A. M., and Karimi, L. (2015). The relationship between paediatric foot posture and body mass index: do heavier children really have flatter feet? J. Foot Ankle Res. 8, 46. doi: 10.1186/s13047-015-0101-x
Evans, A. M., and Rome, K. (2011). A Cochrane review of the evidence for non-surgical interventions for flexible pediatric flat feet. Eur. J. Phys. Rehabil. Med. 47, 69–89.
Evans, A. M., Rome, K., and Peet, L. (2012). The foot posture index, ankle lunge test, Beighton scale and the lower limb assessment score in healthy children: a reliability study. J. Foot Ankle Res. 5, 1. doi: 10.1186/1757-1146-5-1
Faldini, C., Traina, F., Nanni, M., Mazzotti, A., Calamelli, C., Fabbri, D., et al. (2015). Surgical treatment of cavus foot in Charcot-Marie-Tooth disease: a review of twenty-four cases: J. Bone Jt. Surg. 97, e30. doi: 10.2106/JBJS.N.00794
Fulk, G. D., He, Y., Boyne, P., and Dunning, K. (2017). Predicting home and community walking activity poststroke. Stroke. 48, 406–411. doi: 10.1161/STROKEAHA.116.015309
Gijon-Nogueron, G., Montes-Alguacil, J., Alfageme-Garcia, P., Cervera-Marin, J. A., Morales-Asencio, J. M., and Martinez-Nova, A. (2016). Establishing normative foot posture index values for the paediatric population: a cross-sectional study. J. Foot Ankle Res. 9, 1. doi: 10.1186/s13047-016-0156-3
Gordon, D., Zicker, R., Cullen, N., and Singh, D. (2013). Open ankle arthrodeses via an anterior approach. Foot Ankle Int. 34. doi: 10.1177/1071100713477385
Griffiths, I. B., and McEwan, I. M. (2012). Reliability of a new supination resistance measurement device and validation of the manual supination resistance test. J. Am. Podiatr. Med. Assoc. 102, 278–289. doi: 10.7547/1020278
Heffernan, G., Khan, F., Awan, N., Riordain, C. O., and Corrigan, J. (2000). A comparison of outcome scores in os calcis fractures. Ir. J. Med. Sci. 169, 127–128. doi: 10.1007/BF03166916
Hegazy, F. A., Aboelnasr, E. A., Salem, Y., and Zaghloul, A. A. (2020). Validity and diagnostic accuracy of foot posture Index-6 using radiographic findings as the gold standard to determine paediatric flexible flatfoot between ages of 6–18 years: a cross-sectional study. Musculoskelet. Sci. Pract. 46, 102107. doi: 10.1016/j.msksp.2020.102107
Ibrahim, T., Beiri, A., Azzabi, M., Best, A. J., Taylor, G. J., and Menon, D. K. (2007). Reliability and validity of the subjective component of the american orthopaedic foot and ankle society clinical rating scales. J. Foot Ankle Surg. 46, 65–74. doi: 10.1053/j.jfas.2006.12.002
Keenan, M. A., Redmond, A. C., Horton, M., Conaghan, P. G., and Tennant, A. (2007). The foot posture index: rasch analysis of a novel, foot-specific outcome measure. Arch. Phys. Med. Rehabil. 88, 88–93. doi: 10.1016/j.apmr.2006.10.005
Kennedy, R., Carroll, K., Paterson, K. L., Ryan, M. M., and McGinley, J. L. (2017). Deterioration in gait and functional ambulation in children and adolescents with Charcot–Marie–Tooth disease over 12 months. Neuromuscul. Disord. 27. doi: 10.1016/j.nmd.2017.04.005
Kennedy, R. A., Carroll, K., and McGinley, J. L. (2016). Gait in children and adolescents with Charcot-Marie-Tooth disease: a systematic review. J. Peripher. Nerv. Syst. 21. doi: 10.1111/jns.12183
Kennedy, R. A., Carroll, K., Paterson, K. L., Ryan, M. M., Burns, J., Rose, K., et al. (2019). Physical activity of children and adolescents with Charcot-Marie-Tooth neuropathies: A cross-sectional case-controlled study. PLoS ONE. 14, e0209628. doi: 10.1371/journal.pone.0209628
Kennedy, R. A., McGinley, J. L., Paterson, K. L., Ryan, M. M., and Carroll, K. (2018). Gait and footwear in children and adolescents with Charcot-Marie-Tooth disease: A cross-sectional, case-controlled study. Gait Posture. 62. doi: 10.1016/j.gaitpost.2018.03.029
Kenny, S. J., Palacios-Derflingher, L., Owoeye, O. B. A., Whittaker, J. L., and Emery, C. A. (2018). Between-day reliability of pre-participation screening components in pre-professional ballet and contemporary dancers. J. Danc. Med. Sci. 22, 54–62. doi: 10.12678/1089-313X.22.1.54
Kirmizi, M., Cakiroglu, M. A., Elvan, A., Simsek, I. E., and Angin, S. (2020). Reliability of different clinical techniques for assessing foot posture. J. Manipulative Physiol. Ther. 43, 901–908. doi: 10.1016/j.jmpt.2020.02.002
Klerken, T., Kosse, N. M., Aarts, C. A. M., and Louwerens, J. W. K. (2019). Long-term results after triple arthrodesis: Influence of alignment on ankle osteoarthritis and clinical outcome. Foot Ankle Surg. 25, 247–250. doi: 10.1016/j.fas.2017.11.003
Kołodziej, Ł., Dobiecki, K., and Sadlik, B. (2013). Surgical treatment of advanced, stiff neurologic cavovarus foot in adults. Ortop. Traumatol. Rehabil. 15. doi: 10.5604/15093492.1073831
Kuyvenhoven, M. M., Gorter, K. J., Zuithoff, P., Budiman-Mak, E., Conrad, K. J., and Post, M. W. M. (2002). The foot function index with verbal rating scales (FFI-5pt): A clinimetric evaluation and comparison with the original FFI. J. Rheumatol. 29, 1023–1028.
Landorf, K. B., and Keenan, A.-M. (2002). An evaluation of two foot-specific, health-related quality-of-life measuring instruments. Foot Ankle Int. 23, 538–546. doi: 10.1177/107110070202300611
Leeuwesteijn, A. E. E. P. M., de Visser, E., and Louwerens, J. W. K. (2010). Flexible cavovarus feet in Charcot-Marie-Tooth disease treated with first ray proximal dorsiflexion osteotomy combined with soft tissue surgery: a short-term to mid-term outcome study. Foot Ankle Surg. 16, 142–147. doi: 10.1016/j.fas.2009.10.002
Leigheb, M., Janicka, P., Andorno, S., Marcuzzi, A., Magnani, C., and Grassi, F. (2016). Italian translation, cultural adaptation and validation of the “American Orthopaedic Foot and Ankle Society's (AOFAS) ankle-hindfoot scale.” Acta Biomed. 87, 38–45.
Lin, T., Gibbons, P., Mudge, A. J., Cornett, K. M. D., Menezes, M. P., and Burns, J. (2019). Surgical outcomes of cavovarus foot deformity in children with Charcot-Marie-Tooth disease. Neuromuscul. Disord. 29, 427–436. doi: 10.1016/j.nmd.2019.04.004
Madeley, N. J., Wing, K. J., Topliss, C., Penner, M. J., Glazebrook, M. A., and Younger, A. S. E. (2012). Responsiveness and validity of the SF-36, ankle osteoarthritis scale, AOFAS ankle hindfoot score, and foot function index in end stage ankle arthritis. Foot Ankle Int. 33, 57–63. doi: 10.3113/FAI.2012.0057
Martin, R. L., and Irrgang, J. J. (2007). A survey of self-reported outcome instruments for the foot and ankle. J. Orthop. Sports Phys. Ther. 37, 72–84. doi: 10.2519/jospt.2007.2403
Martini, R., Zielasek, J., and Toyka, K. V. (1998). Inherited demyelinating neuropathies: From gene to disease. Curr. Opin. Neurol. 11, 545–556. doi: 10.1097/00019052-199810000-00018
Mazzoli, D., Giannotti, E., Rambelli, C., Zerbinati, P., Galletti, M., and Mascioli, F. (2019). Long-term effects on body functions, activity and participation of hemiplegic patients in equino varus foot deformity surgical correction followed by immediate rehabilitation. A prospective observational study. Top. Stroke Rehabil. 26, 518–522. doi: 10.1080/10749357.2019.1642651
McLaughlin, P., Vaughan, B., Shanahan, J., Martin, J., and Linger, G. (2016). Inexperienced examiners and the foot posture index: a reliability study. Man. Ther. 26, 238–240. doi: 10.1016/j.math.2016.06.009
Menz, H. (2006). Letter to the editor-in-chief: validity of 3 clinical techniques for the measurement of static foot posture in older people. J. Orthop. Sports Phys. Ther. 36, 179–179. doi: 10.2519/jospt.2006.0201
Menz, H. B., Auhl, M., Ristevski, S., Frescos, N., and Munteanu, S. E. (2014). Comparison of the responsiveness of the foot health status questionnaire and the manchester foot pain and disability index in older people. Health Qual. Life Outcomes. 12, 158. doi: 10.1186/s12955-014-0158-4
Morrison, S. C., and Ferrari, J. (2009). Inter-rater reliability of the Foot Posture Index (FPI-6) in the assessment of the paediatric foot. J. Foot Ankle Res. 2, 26. doi: 10.1186/1757-1146-2-26
Muradin, I., and van der Heide, H. J. L. (2016). The foot function index is more sensitive to change than the Leeds Foot Impact Scale for evaluating rheumatoid arthritis patients after forefoot or hindfoot reconstruction. Int. Orthop. 40, 745–749. doi: 10.1007/s00264-016-3113-7
Napiontek, M., and Pietrzak, K. (2015). Joint preserving surgery versus arthrodesis in operative treatment of patients with neuromuscular polyneuropathy: questionnaire assessment. Eur. J. Orthop. Surg. Traumatol. 25, 391–397. doi: 10.1007/s00590-014-1498-9
Oleksy, Ł., Mika, A., Łukomska-Górny, A., Marchewka, A., and Machines, Z. (2010). Intrarater reliability of the Foot Posture Index (FPI-6) applied as a tool in foot assessment in children and adolescents. Med. Rehabil. 14, 10–20.
Page, M. J., Shamseer, L., and Tricco, A. C. (2018). Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 7, 32. doi: 10.1186/s13643-018-0699-4
Pagliano, E., Moroni, I., Baranello, G., Magro, A., Marchi, A., Bulgheroni, S., et al. (2011). Outcome measures for Charcot-Marie-Tooth disease: Clinical and neurofunctional assessment in children. J. Peripher. Nerv. Syst. 16. doi: 10.1111/j.1529-8027.2011.00357.x
Pareyson, D., and Marchesi, C. (2009). Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 8, 654–667. doi: 10.1016/S1474-4422(09)70110-3
Patel, S., Bernasconi, A., Thornton, J., Buraimoh, O., Cullen, N. P., Welck, M. J., et al. (2020). Relationship between foot posture index and weight bearing computed tomography 3D biometrics to define foot alignment. Gait Posture. 80, 143–147. doi: 10.1016/j.gaitpost.2020.05.038
Pena, F., Agel, J., and Coetzee, J. C. (2007). Comparison of the MFA to the AOFAS outcome tool in a population undergoing total ankle replacement. Foot Ankle Int. 28, 788–793. doi: 10.3113/FAI.2006.0788
Peters, M. D., Godfrey, C., McInerney, P., Munn, Z., and Tricco, A. C. K. H. (2020). JBI reviewer's manual. chapter 11: scoping reviews. Joanna Briggs Inst. Available online at: https://wiki.joannabriggs.org/display/MANUAL/Chapter+11%3A+Scoping+reviews. doi: 10.46658/JBIMES-20-12
Pinsker, E., Inrig, T., Daniels, T. R., Warmington, K., and Beaton, D. E. (2015). Reliability and validity of 6 measures of pain, function, and disability for ankle arthroplasty and arthrodesis. Foot Ankle Int. 36, 617–625. doi: 10.1177/1071100714566624
Pogemiller, K., Garibay, E., Pierz, K., Acsadi, G., and Õunpuu, S. (2020). Comparison of gait patterns and functional measures between Charcot-Marie-Tooth disease type I and II in children to young adults. Gait Posture. 77, 236–242. doi: 10.1016/j.gaitpost.2020.01.027
Ponkilainen, V. T., Uimonen, M., Repo, J. P., Mattila, V. M., and Haapasalo, H. H. (2020). Validity and internal consistency of the American Orthopaedic Foot and Ankle Society Midfoot Scale in patients with Lisfranc injury. Foot Ankle Surg. 26, 523–529. doi: 10.1016/j.fas.2019.06.005
Ramdharry, G., Singh, D., Gray, J., Kozyra, D., Skorupinska, M., Reilly, M. M., et al. (2021). A prospective study on surgical management of foot deformities in Charcot Marie tooth disease. J. Peripher. Nerv. Syst. 26. doi: 10.1111/jns.12437
Razeghi, M., and Batt, M. E. (2002). Foot type classification: a critical review of current methods. Gait Posture 15, 282–291. doi: 10.1016/S0966-6362(01)00151-5
Redmond, A. C., Crane, Y. Z., and Menz, H. B. (2008). Normative values for the Foot Posture Index. J. Foot Ankle Res. 1, 1–9. doi: 10.1186/1757-1146-1-6
Redmond, A. C., Crosbie, J., and Ouvrier, R. (2006). Development and validation of a novel rating system for scoring standing foot posture: The Foot Posture Index. Clin. Biomech. 21, 89–98. doi: 10.1016/j.clinbiomech.2005.08.002
Rose, K. J., Burns, J., and North, K. N. (2010a). Factors associated with foot and ankle strength in healthy preschool-age children and age-matched cases of charcot-marie-tooth disease type 1A. J. Child Neurol. 25. doi: 10.1177/0883073809340698
Rose, K. J., Hiller, C. E., Mandarakas, M., Raymond, J., Refshauge, K., and Burns, J. (2015). Correlates of functional ankle instability in children and adolescents with Charcot-Marie-Tooth disease. J. Foot Ankle Res. 8. doi: 10.1186/s13047-015-0118-1
Rose, K. J., Raymond, J., Refshauge, K., North, K. N., and Burns, J. (2010b). Serial night casting increases ankle dorsiflexion range in children and young adults with Charcot-Marie-Tooth disease: a randomised trial. J. Physiother. 56. doi: 10.1016/S1836-9553(10)70041-2
Saag, K. G., Saltzman, C. L., Brown, C. K., and Budiman-Mak, E. (1996). The foot function index for measuring rheumatoid arthritis pain: evaluating side-to-side reliability. Foot Ankle Int. 17, 506–510. doi: 10.1177/107110079601700814
Scharfbillig, R., Evans, A. M., Copper, A. W., Williams, M., Scutter, S., Iasiello, H., et al. (2004). Criterion validation of four criteria of the foot posture index. J. Am. Podiatr. Med. Assoc. 94, 31–38. doi: 10.7547/87507315-94-1-31
Schepers, T., Heetveld, M. J., Mulder, P. G. H., and Patka, P. (2008). Clinical outcome scoring of intra-articular calcaneal fractures. J. Foot Ankle Surg. 47, 213–218. doi: 10.1053/j.jfas.2008.02.014
Simon, A.-L., Seringe, R., Badina, A., Khouri, N., Glorion, C., and Wicart, P. (2019). Long term results of the revisited Meary closing wedge tarsectomy for the treatment of the fixed cavo-varus foot in adolescent with Charcot-Marie-Tooth disease. Foot Ankle Surg. 25, 834–841. doi: 10.1016/j.fas.2018.11.005
SooHoo, N. F., Shuler, M., and Fleming, L. L. (2003). Evaluation of the validity of the AOFAS clinical rating systems by correlation to the SF-36. Foot Ankle Int. 24, 50–55. doi: 10.1177/107110070302400108
SooHoo, N. F., Vyas, R., and Samini, D. (2006). Responsiveness of the foot function index, AOFAS clinical rating systems, and SF-36 after foot and ankle surgery. Foot Ankle Int. 27, 930–934. doi: 10.1177/107110070602701111
Stino, A. M., Atway, S., Anthony, M., Kline, D., and Kissel, J. T. (2019). Foot measures in patients with pes cavus with and without charcot–marie–tooth disease: a pilot study. Muscle and Nerve 59, 122–125. doi: 10.1002/mus.26309
Terada, M., Wittwer, A. M., and Gribble, P. A. (2014). Intra-rater and inter-rater reliability of the five image-based criteria of the foot posture index-6. Int. J. Sports Phys. Ther. 9, 187–194.
Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169, 467–473. doi: 10.7326/M18-0850
Tucker, J., Moore, M., Rooy, J., Wright, A., Rothschild, C., and Werk, L. N. (2015). Reliability of common lower extremity biomechanical measures of children with and without obesity. Pediatr. Phys. Ther. 27, 250–256. doi: 10.1097/PEP.0000000000000152
Ward, C. M., Dolan, L. A., Bennett, D. L., Morcuende, J. A., and Cooper, R. R. (2008). Long-term results of reconstruction for treatment of a flexible cavovarus foot in Charcot-Marie-Tooth disease. J. Bone Jt. Surg. - Ser. A 90, 2631–2642. doi: 10.2106/JBJS.G.01356
Wegener, C., Wegener, K., Smith, R., Schott, K. H., and Burns, J. (2016). Biomechanical effects of sensorimotor orthoses in adults with charcot-marie-tooth disease. Prosthet. Orthot. Int. doi: 10.1177/0309364615579318
Wicart, P., and Seringe, R. (2006). Plantar opening-wedge osteotomy of cuneiform bones combined with selective plantar release and dwyer osteotomy for pes cavovarus in children. J. Pediatr. Orthop. 26, 100–108. doi: 10.1097/01.bpo.0000189005.78045.17
Wojciechowski, E., Sman, A., Cornett, K., Raymond, J., Refshauge, K., Menezes, M. P., et al. (2017). Gait patterns of children and adolescents with Charcot-Marie-Tooth disease. Gait Posture 56. doi: 10.1016/j.gaitpost.2017.05.005
Zuccarino, R., Prada, V., Moroni, I., Pagliano, E., Foscan, M., Robbiano, G., et al. (2020). Validation of the Italian version of the Charcot-Marie-Tooth disease Pediatric Scale. J. Peripher. Nerv. Syst. 25, 138–142. doi: 10.1111/jns.12383
Keywords: Charcot-Marie-Tooth disease, foot assessment, clinical scales, metric properties, Foot Posture Index
Citation: Rambelli C, Mazzoli D, Galletti M, Basini G, Zerbinati P, Prati P, Mascioli F, Masiero S and Merlo A (2022) Foot Assessment Clinical Scales in Charcot-Marie-Tooth Patients: A Scoping Review. Front. Hum. Neurosci. 16:914340. doi: 10.3389/fnhum.2022.914340
Received: 06 April 2022; Accepted: 30 May 2022;
Published: 24 June 2022.
Edited by:
Akiyoshi Matsugi, Shijonawate Gakuen University, JapanReviewed by:
Ruxu Zhang, Central South University, ChinaCopyright © 2022 Rambelli, Mazzoli, Galletti, Basini, Zerbinati, Prati, Mascioli, Masiero and Merlo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davide Mazzoli, ZC5tYXp6b2xpQHNvbGV0c2FsdXMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.