
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Hum. Neurosci. , 03 August 2022
Sec. Brain Imaging and Stimulation
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.907716
This article is part of the Research Topic All Roads Lead to Rome: Harnessing Thalamic Neuromodulation for Difficult-to-treat Neurological Disorders View all 7 articles
Idiopathic generalized epilepsy (IGE) is a common type of epilepsy and despite an increase in the number of available anti-seizure medications, approximately 20–30% of people with IGE continue to experience seizures despite adequate medication trials. Unlike focal epilepsy, resective surgery is not a viable treatment option for IGE; however, neuromodulation may be an effective surgical treatment for people with IGE. Thalamic stimulation through deep brain stimulation (DBS) and responsive neurostimulation (RNS) have been explored for the treatment of generalized and focal epilepsies. Although the data regarding DBS and RNS in IGE is limited to case reports and case series, the results of the published studies have been promising. The current manuscript will review the published literature of DBS and RNS within the centromedian nucleus of the thalamus for the treatment of IGE, as well as highlight an illustrative case.
Epilepsy is defined as a disease of the brain characterized by an enduring predisposition to generate seizures. Epilepsy is defined by the presence of two or more unprovoked seizures separated by 24 h, one unprovoked seizure with a high probability for seizure recurrence, and/or the presence of a discrete electroclinical syndrome (Fisher et al., 2014). Epilepsy is the fourth most common neurologic condition with a prevalence in the United States of 1.2%, affecting around 3.4 million individuals (Zack and Kobau, 2017).
In a broad classification, generalized epilepsy is the second most common type of epilepsy after focal epilepsy. The term idiopathic generalized epilepsy (IGE) is used to describe a group of epilepsies with presumed genetic basis, manifesting as generalized-onset seizures, most commonly absence, myoclonic and/or generalized tonic-clonic seizures (GTCS). IGE syndromes share several clinical features including normal cognitive development, absence of gross neurological pathology, age dependency, and responsivity to certain anti-seizure medications (ASMs) (Andermann, 2006). Electrographically, they are characterized by the occurrence of generalized spike-and-wave discharges (GSWDs) or polyspike-and-wave discharges on an otherwise normal EEG background. Based on seizure types and age of onset, IGE can be further classified into syndromes, the most prevalent of which are childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), juvenile myoclonic epilepsy (JME) and IGE with GTCS alone (IGE-GTC).
Lennox-Gastaut syndrome (LGS) is an epileptic encephalopathy characterized by multiple seizure types including atypical absence, tonic-clonic, myoclonic, tonic and atonic seizures. Typically, EEG shows GSWDs and on a slow background. Due to its generalized clinical and EEG features, LGS was previously referred to as a “symptomatic generalized epilepsy,” a term that is no longer endorsed by the International League Against Epilepsy. LGS is often due to brain injury that almost always involves, but is not necessarily limited to, the cerebral cortex (such as hypoxic-ischemic encephalopathy, central nervous system infections, tuberous sclerosis complex, and metabolic disorders).
Despite an increase in the number of ASMs available to treat epilepsy seizures over the last 20–30 years, up to 20–30% of people with IGE are unable to control their seizures with medications alone (Kwan and Brodie, 2000; Kokkinos et al., 2020). Patients who continue to have seizures despite 2 adequately chosen and dosed ASMs are diagnosed with drug-resistant epilepsy (DRE) (Kwan and Brodie, 2000; Kwan et al., 2010; Chen et al., 2018). In IGE, there are several risk factors that may contribute to DRE, such as multiple seizure types (absence seizures, myoclonic seizures, GTCS), comorbid psychiatric disorders, generalized paroxysmal fast activity (GPFA) on EEG, catamenial seizures, high frequency of GTCS, and photosensitivity (Gelisse et al., 2001; Seneviratne et al., 2012; Cerulli Irelli et al., 2020; Pietrafusa et al., 2021; Kamitaki et al., 2022). Whereas surgical resection is often the optimal treatment for refractory focal epilepsy, patients with IGE are not candidates for resective surgery.
The first neuromodulation modality approved for the treatment of epilepsy was vagus nerve stimulation (VNS). VNS received FDA approval in 1997 for the treatment of focal-onset seizures (DeGiorgio et al., 2000), which was then extended to children age 4 and older in 2017 (Jain and Arya, 2021). In the United States, responsive neurostimulation (RNS) was approved by the FDA in 2013 for the treatment of focal epilepsy, while deep brain stimulation (DBS) of the anterior thalamic nucleus (ANT) was approved for the treatment of focal epilepsy in 2018 (2010 in Europe) (Ryvlin and Jehi, 2022).
Neuromodulation modalities have been utilized in the treatment of drug-resistant IGE. More recently, DBS and RNS targeting the centromedian nucleus (CM) of the thalamus have been explored as surgical therapies for people with drug-resistant IGE. Although VNS has been used in the treatment of drug-resistant IGE with some success (Kostov et al., 2007), this review will focus on the treatment of drug-resistant IGE through intracranial neuromodulation of the CM with DBS and RNS. An illustrative case is also provided to highlight the efficacy of CM-RNS in the treatment of IGE.
Although many thalamic nuclei may be involved in the generation of generalized epileptiform discharges and seizures, the CM and ANT appear to be activated during the initial spike-wave pattern in generalized epilepsy (Tyvaert et al., 2009).
GSWDs and generalized polyspike-and-wave discharges are the typical interictal signature of all IGE syndromes. Since the first description (Gibbs et al., 1935) of GSWDs in absence seizures, investigators have been intrigued by their distinctiveness from focal ictal patterns, particularly their abrupt onset and offset and synchronous bilateral distribution. This led to the speculation that GSWDs are generated by a midline subcortical pacemaker (Jasper and Kershman, 1941), possibly involving the diencephalon and mesencephalon (Penfield and Erickson, 1941). Studies in the 1940s demonstrated that patterns similar to GSWDs can be recorded over the cortex after stimulation of the thalamus in animals (Lewy and Gammon, 1940; Jasper and Drooglever-Fortuyn, 1947) and can be accompanied by clinical manifestations similar to human absence seizures (Hunter and Jasper, 1949). GSWDs were later recorded from thalami of patients with generalized epilepsy (Spiegel and Wycis, 1950; Williams, 1953).
Penfield and Jasper theorized that generalized-onset seizures originate in a centrencephalic system that includes the thalamus with the diencephalon and the brainstem (Penfield and Jasper, 1954), a concept dubbed as the centrencephalic theory. In the following decade, there were animal studies suggesting that GSWDs rather arise in the cortex (Marcus and Watson, 1966; Fischer-Williams et al., 1968) and can be triggered by electrical stimulation of the human frontal cortex (Bancaud et al., 1974), which sparked a long-standing debate between proponents of the centrocephalic and the cortical focus theories (Blumenfeld, 2005). Gloor subsequently introduced the theory of generalized corticoreticular epilepsy in which seizures are attributed to abnormal oscillations within corticoreticular networks (Gloor, 1968), and it eventually became widely accepted that thalamocortical network interactions are pivotal in generating typical generalized seizures (Blumenfeld, 2002; Avoli, 2012). Indeed, evidence from animal models suggested that neither cortex nor thalamus alone can generate GSWDs (Pellegrini et al., 1979; Avoli and Gloor, 1981; Danober et al., 1998; Blumenfeld, 2005). On the cellular level, it was suggested that reciprocal excitatory and inhibitory interaction between thalamocortical neurons and thalamic nucleus reticularis neurons give rise to the alternating excitatory (spike) and GABAA-mediated inhibitory (wave) activity during GSWDs (Blumenfeld, 2005).
More recently, evidence implicating thalamocortical networks emerged from magnetoencephalography studies (Amor et al., 2009; Stefan et al., 2009; Tenney et al., 2013) and from functional magnetic resonance imaging (fMRI) studies showing thalamic activation during GSWDs in patients with various IGE syndromes (Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Moeller et al., 2008; Tyvaert et al., 2009). Importantly, one fMRI study examining thalamic nuclei activity during GSWDs in patients with CAE, JAE, and JME found that activation of the CM and parafasciular nuclei preceded that of the anterior nucleus, suggesting a predominant role of the CM-parafasciular complex in seizure generation or early propagation (Tyvaert et al., 2009). Studies of magnetic resonance spectroscopy suggested the presence of thalamic dysfunction in patients with JME and IGE with tonic-clonic seizures only, as evidenced by a reduction in thalamic N-acetyl aspartate/creatine ratio compared to controls in patients of mixed IGE syndromes (Bernasconi et al., 2003), a homogenous groups of patients with JME (Mory et al., 2003) and JAE (Kabay et al., 2010). Evidence of thalamocortical network dysfunction also came from a study using voxel-based morphometry and seed-based functional connectivity analysis in a mixed group of IGE patients (almost all with JME or IGE-GTC) (Kim et al., 2014).
GSWDs are also the hallmark of LGS, yet they typically occur at a lower frequency and are hence termed slow spike-and-wave discharges (SSWDs). Another electrographic signature of LGS is fast rhythmic waves or paroxysmal fast activity, often associated with tonic seizures (Blume, 2001; Verrotti et al., 2018). Although both patterns are primarily generated in the neocortex, the thalamus seems to be significantly involved (Blume, 2001). The neuronal substrates contributing to GSWDs in IGE and SSWDs in LGS are believed to be similar (Steriade, 2006). It has been shown that increased firing in the thalamocortical pathway may lead to a more sustained firing pattern of thalamic nucleus reticularis cells effecting conversion from GABAA- to GABAB-mediated inhibitory potentials of longer duration (Blumenfeld and McCormick, 2000). Accordingly, the greater cortical excitability in LGS compared to IGE may underlie the “slow” rate of SSWDs in LGS and its medical intractability (Blume, 2001).
The CM is considered a “diffuse-projecting” nucleus in the thalamus, with the lateral third of the CM exhibiting reciprocal connections with the premotor, motor, and primary somatosensory cortices (Ilyas et al., 2019). Tractography studies have also suggested the CM may be associated with the anterior insula and frontal operculum, with further projections to other thalamic nuclei, including the reticular nucleus (Ilyas et al., 2019). These diffuse projections may make it an ideal target for neuromodulation.
Defining the CM as a stereotactic target is primarily accomplished through indirect targeting based on the anterior commissure-posterior commissure line, as the thalamic nuclei are not visible on conventional imaging and intraoperative electrophysiology is not useful to guide placement. Accordingly, studies have attempted to define both optimal targeting techniques as well as success based on active contact location (Velasco M. et al., 2000; Velasco et al., 2007; Ilyas et al., 2022). Newer imaging modalities and improved MRI sequences may provide better delineation of the CM and allow for direct targeting in future studies (Warren et al., 2020; Middlebrooks et al., 2021).
Whereas the treatment of focal seizures with ANT-DBS has received considerable attention and FDA approval (Fisher et al., 2010), the treatment of generalized seizures has yet to have an approved neuromodulation modality. There is a long history of CM stimulation for the treatment of intractable epilepsy, starting with reports by Velasco et al. describing the CM as a feasible and efficacious target (Velasco et al., 1987, 1995).
Three controlled trials assessing the efficacy of CM-DBS have been performed (Fisher et al., 1992; Velasco F. et al., 2000; Dalic et al., 2022). The first was a small study of 7 patients with drug-resistant focal epilepsy. There was a trend toward reduction of seizure frequency as a percentage of baseline, but this did not reach statistical significance (Fisher et al., 1992). In the study by Velasco et al., which included 15 patients with LGS, stimulation significantly decreased both the total number of seizures as well as the seizure subtypes, although no significant differences were identified when stimulation was turned off during the 3-month blinded follow-up (Velasco F. et al., 2000). A recent randomized controlled trial included 19 patients with LGS (Dalic et al., 2022). Half of the patient in the treatment group had ≥50% reduction in diary-recorded seizures after 3 months of stimulation, compared to 22% in the placebo group, but this difference was not statistically significant. A significant reduction in electrographic seizures on 24-h ambulatory EEGs was observed, a more objective measure. None of the aforementioned controlled trials included patients with IGE.
There have also been several small uncontrolled studies on the use of CM-DBS, most which focused on patients with LGS. Velasco et al. reported a significant difference in seizure reduction, and specifically >87% reduction in patients with appropriate electrode placement (Velasco et al., 2006). Son et al. reported a mean seizure reduction of 63.8% in a subset of four patients with LGS, compared to 69.2% in multilobar epilepsy, which was not significant (Son et al., 2016). Seizure reduction in a study reported by Kim et al. (2017) was 72% in a cohort of LGS and multifocal epilepsy. In a prospective, open label study on 20 patients with LGS or LGS-like syndrome, 90% were classified responders (Cukiert et al., 2020). Alcala et al. retrospectively reported a 60% in reduction in seizure frequency with simultaneous CM- and ANT-DBS compared with a 56% reduction in CM-DBS alone for the management of DRE in LGS or focal epilepsy (Alcala-Zermeno et al., 2021).
A recent systematic review of DBS targets in epilepsy reported a mean seizure reduction of 60.8, 73.4, and 67.8%, respectively with stimulation of the ANT, CMT, and hippocampus (Vetkas et al., 2022). The analysis included 8 studies of DBS-CMN with 90 patients in total. These authors suggested that the potentially most efficient DBS targets are the ANT for treatment of focal seizures, CM for generalized seizures, and hippocampus for temporal lobe seizures (Vetkas et al., 2022).
Reports on the use of CM-DBS for IGE are scarce; with the majority of reported cases being in patients with focal epilepsy, LGS or seizure types suggestive of LGS. There are only 6 reported cases of using CM-DBS in LGS. These are summarized in Table 1 (Cukiert et al., 2009; Valentin et al., 2013).
Cukiert et al. (2009) reported on a series of 4 patients, 2 with IGE and 2 with LGS, who have previously undergone callosal section surgery prior to CM-DBS placement. The two patients with IGE reported a 70 and 85% reduction in seizure at 1 year post DBS-CMN. Additionally, the authors noted a clinically relevant increase in attention levels in all patients in an extended SNAP-IV questionnaire. In these patients, continuous stimulation was progressively increased by 0.2 V increments every 2 weeks until final parameters of 2 V, 130 Hz, and 300 μs were reached. Seizure frequency reduction was noted when simulation intensity reached 1.2 V, which progressively increased up to 2 V.
Valentin et al. (2013) reported the utility of CM-DBS in patients with IGE, LGS, and frontal lobe epilepsy. All 4 patients with IGE experienced a >50% reduction in seizure frequency during the blinded portion of the study. Interestingly, one patient remained seizure-free with the stimulator off at follow-up of 60 months following unblinding. Another patient was seizure-free for 1 year following implantation with the device turned off, then had 5 seizures in 1 month; following the initiation of stimulation, this patient remained seizure-free for 45 months. The blinded phase consisted of continuous stimulation of 60 Hz, 90 μs, and up to 5 V. Frequency of 130 Hz was initially used, although was adjusted to 60 Hz following unclear clinical efficacy, and to be consistent with previous CM-DBS studies (Fisher et al., 1992).
Unlike DBS, RNS is a technology that operates in a closed-loop circuit, via detection of epileptic discharges that are defined based on the patient’s unique electrographic seizure characteristics. When these preset discharges are recognized and detected by the device, responsive therapy is delivered via electrical current. Therefore, RNS provides individualized therapy that can be modified and adjusted based on the patient’s electrocorticography (ECoG) data and seizures. The publication of the RNS pivotal trial in 2011 (Morrell, 2011) resulted in FDA approved for its use in patients ≥18 years with ≤2 seizure foci. There has since been substantial interest in targeting the CM with RNS for the treatment of drug-resistant IGE; however, the data regarding the use of RNS in IGE are limited. There are only 2 case reports and a single case series illustrating the potential efficacy of this therapy (Table 1; Kokkinos et al., 2020; Welch et al., 2021; Sisterson et al., 2022).
In the first reported case, a 19-year-old female with eyelid myoclonia with absences underwent bilateral CM-RNS implantation (Kokkinos et al., 2020). Following implantation, her seizures decreased from a mean of 60 seizures per day to less than 10 seizures per day at 18-month follow-up. Interestingly, after RNS implantation within the bilateral CM, she retained awareness during her absence seizures. This patient was reported on in a subsequent publication (Sisterson et al., 2022), with greater than 90% seizure frequency reduction maintained at 33-month follow-up. Stimulation of 1–2 mA, 125 Hz, 160 μs for 5,000 ms was employed. Three additional patients in this series with IGE underwent bilateral CM-RNS implantation, with similar significant reduction in seizure frequency that was sustained between 24–27 months of follow-up (Sisterson et al., 2022). Stimulation amplitude varied between 0.2 and 2.0 mA between patients, with amplitude increasing over successive epochs; however, patients received differing amounts of overall daily stimulation and total charge duration. All stimulation paradigms were delivered in bursts lasting 5,000 ms.
In another case, a 16-year-old male childhood absence epilepsy underwent bilateral CM-RNS (Welch et al., 2021). After implantation and turning on stimulation, seizure frequency was improved, although with continued multiple weekly absence seizures. At 6-month follow-up, there was resolution of his daily absence seizures. In addition, the frequency of his GTCS decreased from 3–4 per month to 1 per month. The stimulation parameters at 6 month were 1.5 mA, 125 Hz, 160 μS for 5,000 ms.
In addition to focal epilepsy and IGE, other studies have evaluated different indications for the use of CM-RNS as well. Burdette et al. (2020, 2021) have demonstrated the utility of thalamic stimulation in regional neocortical epilepsy with RNS. In one study of neocortical epilepsies, one depth lead was implanted into the CMT and the other lead into the most active neocortical region, with 67% mean reduction in all seizures and 80% reduction in disabling seizures over 17 months (Burdette et al., 2020). In another unique case series of posterior quadrant epilepsies, one lead was placed in the pulvinar nucleus with a second lead over the ipsilateral posterior quadrant, with all patients having ≥50% seizure reduction and 2 of 3 patients having ≥90% seizure reduction at last follow-up (Burdette et al., 2021). Bilateral CM-RNS has also been demonstrated to significantly improve seizure frequency in pediatric patients with LGS and autism spectrum disorder (Kwon et al., 2020).
The patient is a 41-year-old right-handed female with a history of drug resistant IGE manifesting as typical absence seizures, myoclonic seizures, GTCS. She had a family history of epilepsy (father with focal epilepsy). There were no other epilepsy risk factors. Her seizures began at the age of 4 years old. Over time, she continued to experience frequent GTCS that were often followed by non-convulsive status epilepticus (NCSE, absence status epilepticus). At the time of her initial evaluation in our comprehensive epilepsy center, she would have at least 1 GTCS per year but often would have 1 GTCS every 2–3 months. From 2018 to 2020, she had 11 GTCS that were consistently followed by 1–2 days of NCSE, requiring hospital admissions. Throughout her epilepsy course, she failed medication trials of levetiracetam, topiramate, perampanel, and ethosuximide.
In light of her DRE, she was evaluated for epilepsy surgery. A brain MRI was unremarkable for discrete epileptic lesions. Inpatient video-EEG monitoring revealed generalized, frontally predominant, 3–4 Hz, spike- and polyspike-and-wave discharges as well as bursts of GPFA (Figure 1). Her case was reviewed at our patient management conference. Considering her history, semiology, and diagnostic studies, resective surgery was not a reasonable treatment option; however, neuromodulation was thought to be a reasonable treatment consideration. Our experience with VNS in IGE was underwhelming, while our experience with RNS and DBS (albeit in focal epilepsy) was quite positive. Overall, our group favors the ability of RNS to record ECoG and adjust detection and treatment parameters based on these data. In light of new case reports illustrating the potential safety and efficacy of RNS in people with IGE, neuromodulation was offered and she elected to pursue RNS implantation. She then underwent RNS implantation in February 2021 with depth electrodes placed into the bilateral CM. At the time of RNS implantation, her medication regimen consisted of lamotrigine, brivaracetam, cannabidiol, and clonazepam polytherapy.
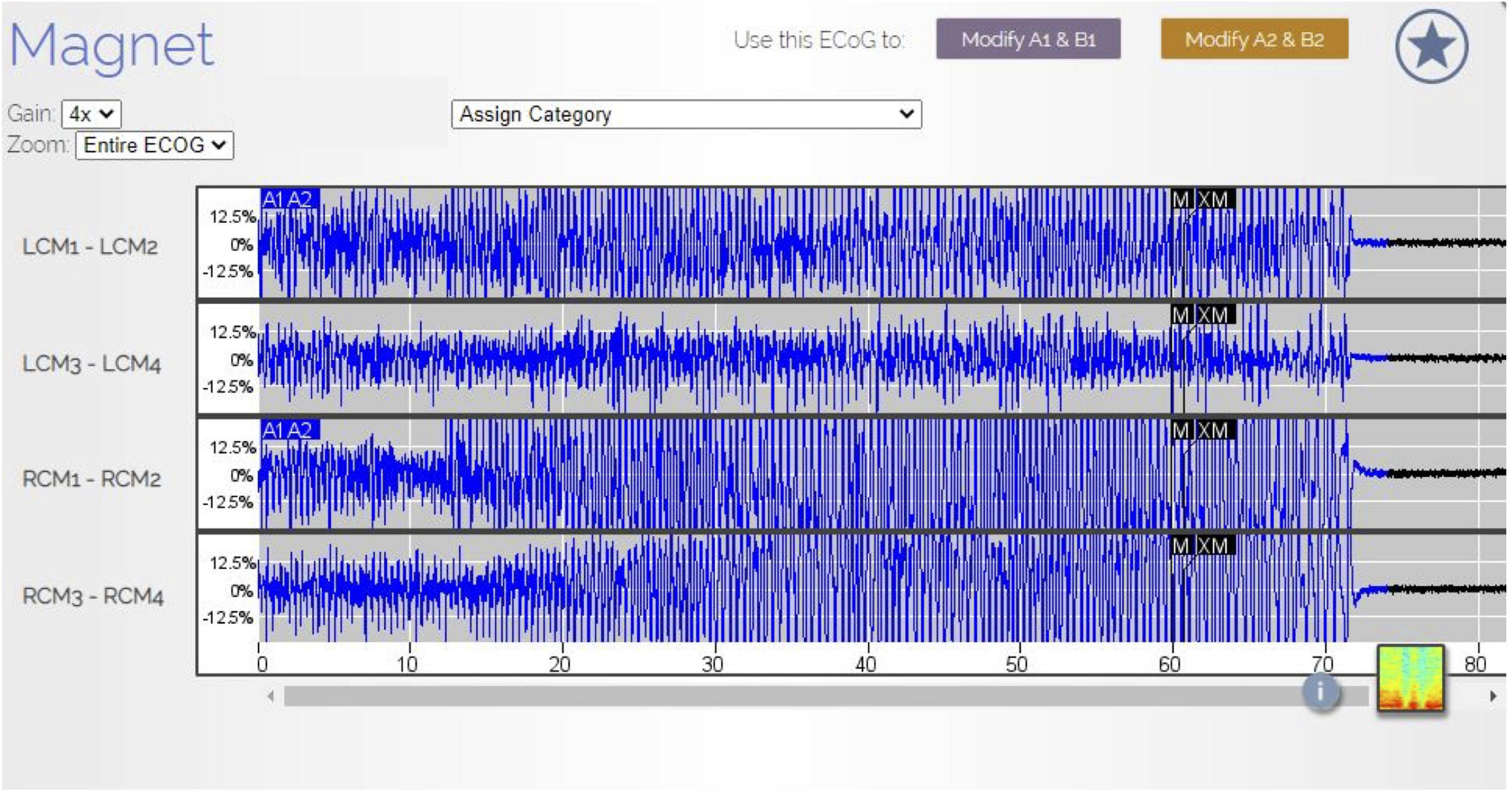
Figure 1. RNS electrothalamogram (ETG) of GTCS. In August 2021, the patient experienced a GTCS. Her family used the RNS magnet (black labels M and XM in the ETS) to store this ETS data in the patient data management system (PDMS). The ETG represents 1 long episode (LE) lasting 90 s. The A1A2 blue labels are the RNS detection of this seizure. The seizure is characterized by rhythmic, beta range, spike-wave discharges (Gain 4×). Note, the patient was not undergoing scalp EEG at the time of this seizure and no direct comparisons between scalp EEG findings and RNS ETS were available.
Initially, following RNS implantation, she continued to experience frequent GTCS with approximately 1 GTCS every 1–3 months. Six months after RNS implantation, she experienced a cluster of GTCS that was followed by absence status epilepticus (Figures 1–3). During the subsequent hospital admission, she was treated with intravenous lacosamide (400 mg loading dose, followed by 200 mg twice daily). An adjunctive trial of valproate was initiated (2,000 mg loading dose and 1,000 mg twice daily). Her RNS therapy settings were also changed (Table 2). No changes were made to her detection parameters. Following treatment with lacosamide and valproate and changes to her RNS therapy settings, her seizures resolved; however, with time, treatment with lacosamide and valproate was poorly tolerated. These medications were tapered and discontinued. No changes were made to her RNS therapy settings during these medication changes. She has remained seizure-free for 10 months with her current treatment regimen. In addition, on review of her RNS data, she has experienced a marked reduction in the number of long episodes (LEs) per month and therapies per day. Prior to the RNS changes, she would have 98 LEs per month with 80 therapies per day (total of 154 days of treatment). After the RNS changes, her LEs decreased to 0.2 per month and her therapies per day decreased to 15 per day (total of 287 days of treatment).
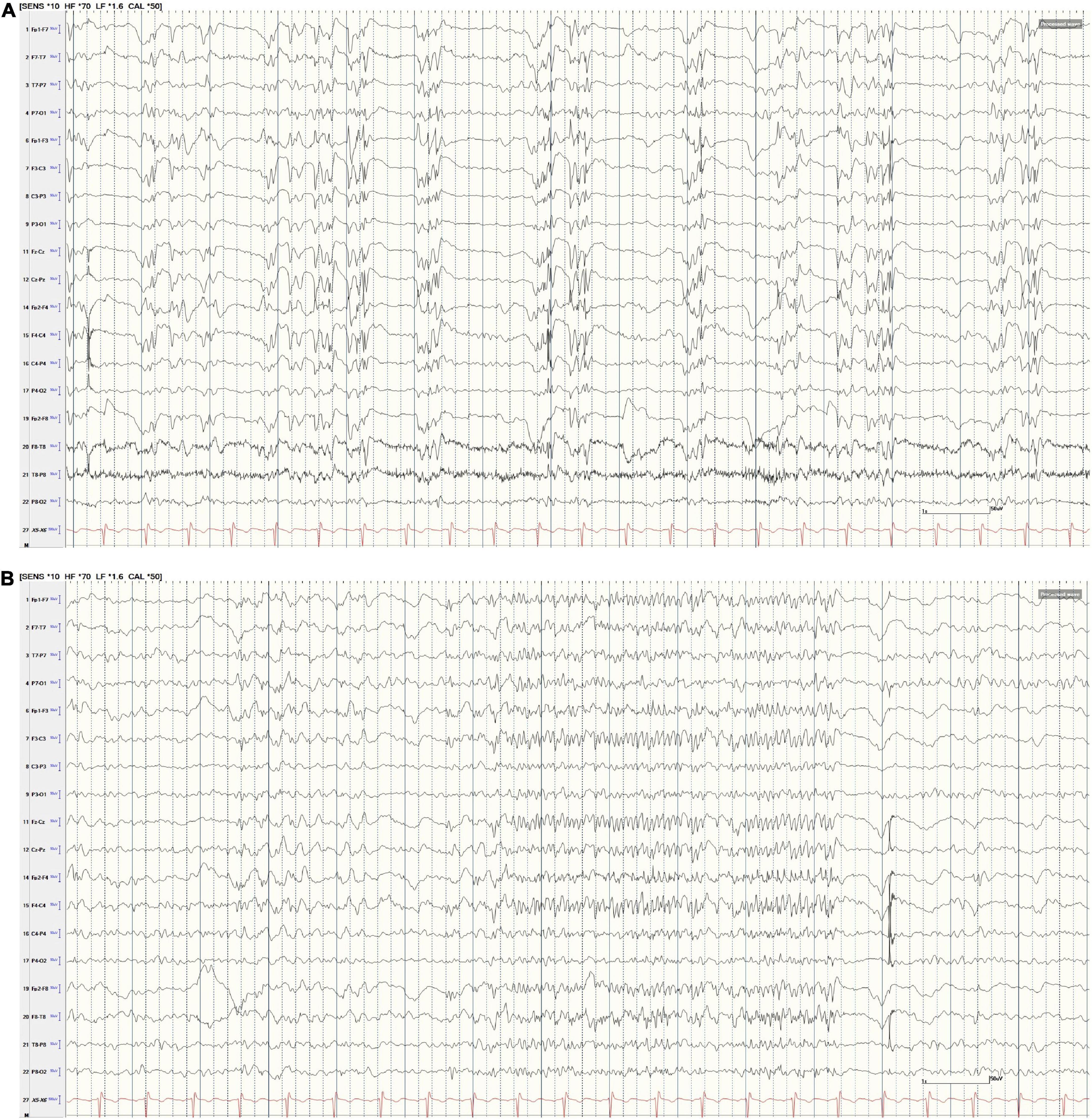
Figure 2. (A,B) Scalp EEG following GTCS. (A) AP Bipolar montage (15 s; HFF 70 Hz, LFF 1.5 Hz, Sen 10 uV). The tracing reveals nearly continuous generalized polyspike-and-wave discharges. At times, these discharges were intermixed with generalized paroxysmal fast activity (GPFA, B). Note, the ETG captured by RNS and illustrated in Figure 3 do not correspond to these specific scalp EEG examples in (A,B).
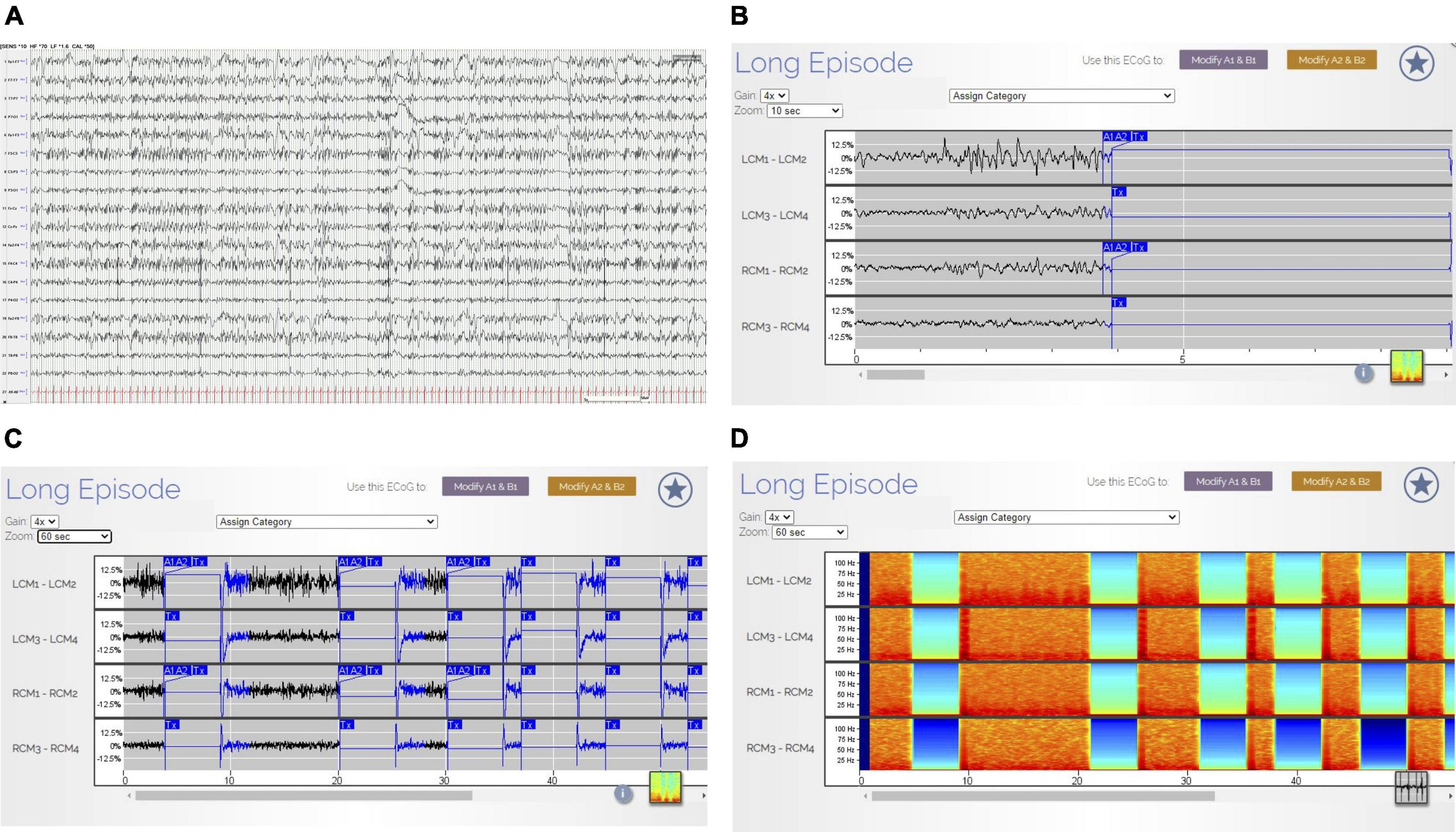
Figure 3. (A–D) Non-convulsive status epilepticus (NCSE) on scalp EEG and RNS Findings. The tracing in (A) (AP Bipolar montage, 60 s; HFF 70 Hz, LFF 1.6 Hz, Sen 10 uV) illustrates continuous generalized polyspike-and-wave discharges. Clinically, the patient exhibited impaired cognition and responsiveness. There were no motor features during these EEG changes. The ETG in RNS (B–D) corresponds directly to the EEG 60 s epoch in (A). B reveals the first 10 s of ETG data (gain 4×) corresponding to the first 10 s of scalp EEG data displayed in (A). There are continuous, spike-wave discharges within the left and right CMN contacts. (C) (Gain 4×) reveals the 60 s of ETG data corresponding to the 60 s of scalp EEG data. The blue A1/A2 boxes denote RNS detection followed by a delivered therapy (Tx). During therapy, there is interruption in the ETS background. In (D) (gain 4×), the density spectral array demonstrates bursts of high-power activity that corresponds to the ictal activity (rhythmic, beta range spike-waves) in the ETG tracing (C). The low power (blue/green) in the density spectral array corresponds to delivered therapies (identified in (C) by the Tx blue box).
To date, the published literature regarding neuromodulation in IGE is primarily limited to case reports and case series. As such, there are many questions related to patient selection, safety, neuromodulation settings, and outcomes. Although the failure of two appropriately chosen medications at appropriate doses denotes drug resistant epilepsy, should patients with IGE have a trial of valproic acid before proceeding to surgery? Are certain electroclinical syndromes more responsive to neuromodulation, e.g., epilepsy with generalized tonic-clonic seizures alone? What are the optimal DBS and RNS settings for IGE? For RNS, the baseline simulation settings in previously published reports have largely adhered to those established for ANT-DBS (Fisher et al., 2010); however, the heterogeneity in individual settings and treatment outcomes warrants further exploration.
Drug resistance is common and may be present in up to 20–30% of IGE, and unfortunately there is no FDA-approved treatment for IGE. Although historically surgical options for IGE have been limited, there is increasing evidence that neuromodulation may be an effective treatment option for these patients. Case series utilizing DBS for IGE have been reported and more recently, case reports illustrating the utility of RNS in treating IGE have been published. Bilateral CM-RNS offers an exciting new treatment paradigm in which detection of epileptiform activity in the brain is possible and therapy may be tailored specifically for each patient. The initial promising results from bilateral CM-RNS for the treatment of IGE has led to the initiation of a prospective single blind, multi-center, randomized study (NAUTILUS).1 As case reports and series of CM-RNS in IGE continue to grow and the results of randomized controlled clinical trials become available, RNS may emerge as a viable treatment option for people with drug-resistant IGE.
AZ and MS contributed to conception and design of the review. AZ wrote the case illustration. MS provided supervision and project administration. All authors performed the literature review, contributed to drafting of the manuscript, and read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghakhani, Y., Bagshaw, A., Benar, C., Hawco, C., Andermann, F., Dubeau, F., et al. (2004). fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain 127, 1127–1144. doi: 10.1093/brain/awh136
Alcala-Zermeno, J. L., Gregg, N. M., Wirrell, E. C., Stead, M., Worrell, G. A., Van Gompel, J. J., et al. (2021). Centromedian thalamic nucleus with or without anterior thalamic nucleus deep brain stimulation for epilepsy in children and adults: a retrospective case series. Seizure 84, 101–107. doi: 10.1016/j.seizure.2020.11.012
Amor, F., Baillet, S., Navarro, V., Adam, C., Martinerie, J., and Le Van, et al. (2009). Cortical local and long-range synchronization interplay in human absence seizure initiation. Neuroimage 45, 950–962. doi: 10.1016/j.neuroimage.2008.12.011
Andermann, F. (2006). What is a generalized epilepsy. Generalized Seizures: From Clinical Phenomenology to Underlying Systems and Networks. Montrouge: John Libbey Eurotext. France: John Libbey Eurotext Ltd, 23–32.
Avoli, M. (2012). A brief history on the oscillating roles of thalamus and cortex in absence seizures. Epilepsia 53, 779–789. doi: 10.1111/j.1528-1167.2012.03421.x
Avoli, M., and Gloor, P. (1981). The effects of transient functional depression of the thalamus on spindles and on bilateral synchronous epileptic discharges of feline generalized penicillin epilepsy. Epilepsia 22, 443–452. doi: 10.1111/j.1528-1157.1981.tb06155.x
Bancaud, J., Talairach, J., Morel, P., Bresson, M., Bonis, A., Geier, S., et al. (1974). “Generalized” epileptic seizures elicited by electrical stimulation of the frontal lobe in man. Electroencephal. Clin. Neurophys. 37, 275–282. doi: 10.1016/0013-4694(74)90031-5
Bernasconi, A., Bernasconi, N., Natsume, J., Antel, S. B., Andermann, F., and Arnold, D. L. (2003). Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain 126(Pt 11), 2447–2454. doi: 10.1093/brain/awg249
Blume, W. T. (2001). Pathogenesis of lennox-gastaut syndrome: considerations and hypotheses. Epileptic Dis. 3, 183–196.
Blumenfeld, H. (2002). The thalamus and seizures. Archiv. Neurol. 59, 135–137. doi: 10.1001/archneur.59.1.135
Blumenfeld, H. (2005). Cellular and network mechanisms of spike-wave seizures. Epilepsia 46, 21–33. doi: 10.1111/j.1528-1167.2005.00311.x
Blumenfeld, H., and McCormick, D. A. (2000). Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J. Neurosci. 20, 5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000
Burdette, D., Mirro, E. A., Lawrence, M., and Patra, S. E. (2021). Brain-responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: a case series. Epilepsia Open 6, 611–617. doi: 10.1002/epi4.12524
Burdette, D. E., Haykal, M. A., Jarosiewicz, B., Fabris, R. R., Heredia, G., Elisevich, K., et al. (2020). Brain-responsive corticothalamic stimulation in the centromedian nucleus for the treatment of regional neocortical epilepsy. Epilepsy Behav. 112:107354. doi: 10.1016/j.yebeh.2020.107354
Cerulli Irelli, E., Morano, A., Barone, F. A., Fisco, G., Fanella, M., Orlando, B., et al. (2020). Persistent treatment resistance in genetic generalized epilepsy: a long-term outcome study in a tertiary epilepsy center. Epilepsia 61, 2452–2460. doi: 10.1111/epi.16708
Chen, Z., Brodie, M. J., Liew, D., and Kwan, P. (2018). Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol 75, 279–286. doi: 10.1001/jamaneurol.2017.3949
Cukiert, A., Burattini, J. A., Cukiert, C. M., Argentoni-Baldochi, M., Baise-Zung, C., Forster, C. R., et al. (2009). Centro-median stimulation yields additional seizure frequency and attention improvement in patients previously submitted to callosotomy. Seizure 18, 588–592. doi: 10.1016/j.seizure.2009.06.002
Cukiert, A., Cukiert, C. M., Burattini, J. A., and Mariani, P. P. (2020). Seizure outcome during bilateral, continuous, thalamic centromedian nuclei deep brain stimulation in patients with generalized epilepsy: a prospective, open-label study. Seizure 81, 304–309. doi: 10.1016/j.seizure.2020.08.028
Dalic, L. J., Warren, A. E. L., Bulluss, K. J., Thevathasan, W., Roten, A., Churilov, L., et al. (2022). DBS of thalamic centromedian nucleus for lennox-gastaut syndrome (estel trial). Ann Neurol 91, 253–267. doi: 10.1002/ana.26280
Danober, L., Deransart, C., Depaulis, A., Vergnes, M., and Marescaux, C. (1998). Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog. Neurob. 55, 27–57. doi: 10.1016/S0301-0082(97)00091-9
DeGiorgio, C. M., Schachter, S. C., Handforth, A., Salinsky, M., Thompson, J., Uthman, B., et al. (2000). Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 41, 1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x
Fischer-Williams, M., Poncet, M., Riche, D., and Naquet, R. (1968). Light-induced epilepsy in the baboon, Papio papio: cortical and depth recordings. Electroencephal. Clin. Neurophys. 25, 557–569. doi: 10.1016/0013-4694(68)90235-6
Fisher, R., Salanova, V., Witt, T., Worth, R., Henry, T., Gross, R., et al. (2010). Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908. doi: 10.1111/j.1528-1167.2010.02536.x
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55, 475–482. doi: 10.1111/epi.12550
Fisher, R. S., Uematsu, S., Krauss, G. L., Cysyk, B. J., McPherson, R., Lesser, R. P., et al. (1992). Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 33, 841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x
Gelisse, P., Genton, P., Thomas, P., Rey, M., Samuelian, J. C., and Dravet, C. (2001). Clinical factors of drug resistance in juvenile myoclonic epilepsy. J. Neurol. Neurosurg. Psychiatry 70, 240–243. doi: 10.1136/jnnp.70.2.240
Gibbs, F. A., Davis, H., and Lennox, W. G. (1935). The electro-encephalogram in epilepsy and in conditions of impaired consciousness. Archiv. Neurol. Psychiatry 34, 1133–1148.
Gloor, P. (1968). Generalized cortico-reticular epilepsies. some considerations on the pathophysiology of generalized bilaterally synchronous spike and wave discharge. Epilepsia 9, 249–263. doi: 10.1111/j.1528-1157.1968.tb04624.x
Hunter, J., and Jasper, H. H. (1949). Effects of thalamic stimulation in unanaesthetised animals: the arrest reaction and petit mal-like seizures, activation patterns and generalized convulsions. Electroencephal. Clin. Neurophys. 1, 305–324. doi: 10.1016/0013-4694(49)90043-7
Ilyas, A., Pizarro, D., Romeo, A. K., Riley, K. O., and Pati, S. (2019). The centromedian nucleus: Anatomy, physiology, and clinical implications. J. Clin. Neurosci. 63, 1–7. doi: 10.1016/j.jocn.2019.01.050
Ilyas, A., Snyder, K. M., Pati, S., and Tandon, N. (2022). Optimally targeting the centromedian nucleus of the thalamus for generalized epilepsy: a meta-analysis. Epilepsy Res. 184:106954. doi: 10.1016/j.eplepsyres.2022.106954
Jain, P., and Arya, R. (2021). Vagus nerve stimulation and seizure outcomes in pediatric refractory epilepsy: systematic review and meta-analysis. Neurology 2021:12030. doi: 10.1212/WNL.0000000000012030
Jasper, H., and Kershman, J. (1941). Electroencephalographic classification of the epilepsies. Archiv. Neurol. Psychiatry 45, 903–943. doi: 10.1001/archneurpsyc.1941.02280180015001
Jasper, H. H., and Drooglever-Fortuyn, J. (1947). Experimental studies on the functional anatomy of petit mal epilepsy. Res. Publ. Ass. Res. Nerv. Ment. Dis. 26, 272–298.
Kabay, S. C., Gumustas, O. G., Karaman, H. O., Ozden, H., and Erdinc, O. (2010). A proton magnetic resonance spectroscopic study in juvenile absence epilepsy in early stages. Eur. J. Paediatr. Neurol. 14, 224–228. doi: 10.1016/j.ejpn.2009.06.004
Kamitaki, B. K., Janmohamed, M., Kandula, P., Elder, C., Mani, R., Wong, S., et al. (2022). Clinical and EEG factors associated with antiseizure medication resistance in idiopathic generalized epilepsy. Epilepsia 63, 150–161. doi: 10.1111/epi.17104
Kim, J. B., Suh, S. I., Seo, W. K., Oh, K., Koh, S. B., and Kim, J. H. (2014). Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia 55, 592–600. doi: 10.1111/epi.12580
Kim, S. H., Lim, S. C., Yang, D. W., Cho, J. H., Son, B. C., Kim, J., et al. (2017). Thalamo-cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr. Dis. Treat. 13, 2607–2619. doi: 10.2147/NDT.S148617
Kokkinos, V., Urban, A., Sisterson, N. D., Li, N., Corson, D., and Richardson, R. M. (2020). Responsive neurostimulation of the thalamus improves seizure control in idiopathic generalized epilepsy: a case report. Neurosurgery 87, E578–E583. doi: 10.1093/neuros/nyaa001
Kostov, H., Larsson, P. G., and Roste, G. K. (2007). Is vagus nerve stimulation a treatment option for patients with drug-resistant idiopathic generalized epilepsy? Acta Neurol. Scand. Suppl. 187, 55–58. doi: 10.1111/j.1600-0404.2007.00848.x
Kwan, P., Arzimanoglou, A., Berg, A. T., Brodie, M. J., Allen Hauser, W., Mathern, G., et al. (2010). Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51, 1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x
Kwan, P., and Brodie, M. J. (2000). Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319. doi: 10.1056/NEJM200002033420503
Kwon, C. S., Schupper, A. J., Fields, M. C., Marcuse, L. V., La Vega-Talbott, M., Panov, F., et al. (2020). Centromedian thalamic responsive neurostimulation for Lennox-Gastaut epilepsy and autism. Ann. Clin. Transl. Neurol. 7, 2035–2040. doi: 10.1002/acn3.51173
Lewy, F., and Gammon, G. D. (1940). Influence of sensory systems on spontaneous activity of cerebral cortex. J. Neurophys. 3, 388–395. doi: 10.1152/jn.1940.3.5.388
Marcus, E. M., and Watson, C. W. (1966). Bilateral synchronous spike wave electrographic patterns in the cat: interaction of bilateral cortical foci in the intact, the bilateral cortical-callosal, and adiencephalic preparation. Archiv. Neurol. 14, 601–610. doi: 10.1001/archneur.1966.00470120033006
Middlebrooks, E. H., Okromelidze, L., Lin, C., Jain, A., Westerhold, E., Ritaccio, A., et al. (2021). Edge-enhancing gradient echo with multi-image co-registration and averaging (EDGE-MICRA) for targeting thalamic centromedian and parafascicular nuclei. Neuroradiol. J. 34, 667–675. doi: 10.1177/19714009211021781
Moeller, F., Siebner, H. R., Wolff, S., Muhle, H., Granert, O., Jansen, O., et al. (2008). Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia 49, 1510–1519.
Morrell, M. J. (2011). Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77, 1295–1304. doi: 10.1212/WNL.0b013e3182302056
Mory, S. B., Li, L. M., Guerreiro, C. A., and Cendes, F. (2003). Thalamic dysfunction in juvenile myoclonic epilepsy: a proton MRS study. Epilepsia 44, 1402–1405. doi: 10.1046/j.1528-1157.2003.67702.x
Pellegrini, A., Musgrave, J., and Gloor, P. (1979). Role of afferent input of subcortical origin in the genesis of bilaterally synchronous epileptic discharges of feline generalized penicillin epilepsy. Exp. Neurol. 64, 155–173. doi: 10.1016/0014-4886(79)90012-8
Penfield, W., and Erickson, T. C. (1941). Epilepsy and Cerebral Localization: A Study of the Mechanism, Treatment and Prevention of Epileptic Seizures. Springfield, IL: C. C. Thomas. doi: 10.1097/00007611-194202000-00031
Penfield, W., and Jasper, H. (1954). Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown and Company.
Pietrafusa, N., La Neve, A., de Palma, L., Boero, G., Luisi, C., Vigevano, F., et al. (2021). Juvenile myoclonic epilepsy: long-term prognosis and risk factors. Brain Dev. 43, 688–697. doi: 10.1016/j.braindev.2021.02.005
Ryvlin, P., and Jehi, L. E. (2022). Neuromodulation for Refractory Epilepsy. Epilepsy Curr. 22, 11–17. doi: 10.1177/15357597211065587
Salek-Haddadi, A., Lemieux, L., Merschhemke, M., Friston, K. J., Duncan, J. S., and Fish, D. R. (2003). Functional magnetic resonance imaging of human absence seizures. Ann. Neurol. 53, 663–667. doi: 10.1002/ana.10586
Seneviratne, U., Cook, M., and D’Souza, W. (2012). The prognosis of idiopathic generalized epilepsy. Epilepsia 53, 2079–2090. doi: 10.1111/j.1528-1167.2012.03723.x
Sisterson, N. D., Kokkinos, V., Urban, A., Li, N., and Richardson, R. M. (2022). Responsive neurostimulation of the thalamus improves seizure control in idiopathic generalised epilepsy: initial case series. J. Neurol. Neurosurg. Psychiatry 2022:327512. doi: 10.1136/jnnp-2021-327512
Son, B. C., Shon, Y. M., Choi, J. G., Kim, J., Ha, S. W., Kim, S. H., et al. (2016). Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact. Funct. Neurosurg. 94, 187–197. doi: 10.1159/000446611
Spiegel, E., and Wycis, H. (1950). Thalamic recordings in man with special reference to seizure discharges. Electroencephalogr. Clin. Neurophys. 2, 23–27.
Stefan, H., Paulini-Ruf, A., Hopfengartner, R., and Rampp, S. (2009). Network characteristics of idiopathic generalized epilepsies in combined MEG/EEG. Epilepsy Res. 85, 187–198. doi: 10.1016/j.eplepsyres.2009.03.015
Steriade, M. (2006). “Spike-wave seizures in corticothalamic systems,” in Generalized Seizures: From Clinical Phenomenology to Underlying Systems and Networks, eds E. Hirsch, F. Andermann, P. Chauvel, J. Engel, F. Lopes, and H. Luders (Montrouge: John Libbey Eurotext), 127–146.
Tenney, J. R., Fujiwara, H., Horn, P. S., Jacobson, S. E., Glauser, T. A., and Rose, D. F. (2013). Focal corticothalamic sources during generalized absence seizures: a MEG study. Epilep. Res. 106, 113–122.
Tyvaert, L., Chassagnon, S., Sadikot, A., LeVan, P., Dubeau, F., and Gotman, J. (2009). Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology 73, 2018–2022. doi: 10.1212/WNL.0b013e3181c55d02
Valentin, A., Garcia Navarrete, E., Chelvarajah, R., Torres, C., Navas, M., Vico, L., et al. (2013). Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54, 1823–1833. doi: 10.1111/epi.12352
Velasco, A. L., Velasco, F., Jimenez, F., Velasco, M., Castro, G., Carrillo-Ruiz, J. D., et al. (2006). Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia 47, 1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x
Velasco, F., Velasco, A. L., Velasco, M., Jimenez, F., Carrillo-Ruiz, J. D., and Castro, G. (2007). Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. Acta Neurochir. Suppl. 97(Pt 2), 337–342. doi: 10.1007/978-3-211-33081-4_38
Velasco, F., Velasco, M., Jimenez, F., Velasco, A. L., Brito, F., Rise, M., et al. (2000). Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery 47, 295–304. doi: 10.1097/00006123-200008000-00007
Velasco, F., Velasco, M., Ogarrio, C., and Fanghanel, G. (1987). Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: a preliminary report. Epilepsia 28, 421–430. doi: 10.1111/j.1528-1157.1987.tb03668.x
Velasco, F., Velasco, M., Velasco, A. L., Jimenez, F., Marquez, I., and Rise, M. (1995). Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia 36, 63–71. doi: 10.1111/j.1528-1157.1995.tb01667.x
Velasco, M., Velasco, F., Velasco, A. L., Jimenez, F., Brito, F., and Marquez, I. (2000). Acute and chronic electrical stimulation of the centromedian thalamic nucleus: modulation of reticulo-cortical systems and predictor factors for generalized seizure control. Arch. Med. Res. 31, 304–315. doi: 10.1016/s0188-4409(00)00085-0
Verrotti, A., Striano, P., Iapadre, G., Zagaroli, L., Bonanni, P., Coppola, G., et al. (2018). The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure 63, 17–25. doi: 10.1016/j.seizure.2018.10.016
Vetkas, A., Fomenko, A., Germann, J., Sarica, C., Iorio-Morin, C., Samuel, N., et al. (2022). Deep brain stimulation targets in epilepsy: systematic review and meta-analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia 63, 513–524. doi: 10.1111/epi.17157
Warren, A. E. L., Dalic, L. J., Thevathasan, W., Roten, A., Bulluss, K. J., and Archer, J. (2020). Targeting the centromedian thalamic nucleus for deep brain stimulation. J. Neurol. Neurosurg. Psychiatry 91, 339–349. doi: 10.1136/jnnp-2019-322030
Welch, W. P., Hect, J. L., and Abel, T. J. (2021). Case report: responsive neurostimulation of the centromedian thalamic nucleus for the detection and treatment of seizures in pediatric primary generalized epilepsy. Front. Neurol. 12:656585. doi: 10.3389/fneur.2021.656585
Williams, D. (1953). A study of thalamic and cortical rhythms in petit mal. Brain 76, 50–69. doi: 10.1093/brain/76.1.50
Keywords: anterior thalamic nucleus, centromedian nucleus, deep brain stimulation, epilepsy, idiopathic generalized epilepsy, responsive neurostimulation
Citation: Zillgitt AJ, Haykal MA, Chehab A and Staudt MD (2022) Centromedian thalamic neuromodulation for the treatment of idiopathic generalized epilepsy. Front. Hum. Neurosci. 16:907716. doi: 10.3389/fnhum.2022.907716
Received: 30 March 2022; Accepted: 13 July 2022;
Published: 03 August 2022.
Edited by:
Glenn D. R. Watson, Duke University, United StatesReviewed by:
Imran H. Quraishi, Yale University, United StatesCopyright © 2022 Zillgitt, Haykal, Chehab and Staudt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael D. Staudt, TWljaGFlbC5TdGF1ZHRAYmVhdW1vbnQub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.