
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 11 May 2022
Sec. Brain Imaging and Stimulation
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.888701
This article is part of the Research TopicDeep Brain Stimulation Think Tank: Updates in Neurotechnology and Neuromodulation, Volume IIIView all 15 articles
 Yuka Hayashi1
Yuka Hayashi1 Takayasu Mishima1*
Takayasu Mishima1* Shinsuke Fujioka1
Shinsuke Fujioka1 Takashi Morishita2
Takashi Morishita2 Tooru Inoue2
Tooru Inoue2 Shigeki Nagamachi3
Shigeki Nagamachi3 Yoshio Tsuboi1*
Yoshio Tsuboi1*
Introduction: Deep brain stimulation (DBS) is an effective treatment for advanced Parkinson’s disease (PD) with the targeting bilateral subthalamic nucleus or globus pallidus internus (STN or GPi-DBS). So far, detailed studies on the efficacy of unilateral STN-DBS for motor symptoms have been reported, but few studies have been conducted on unilateral GPi-DBS.
Materials and Methods: Seventeen patients with Parkinson’s disease (PwPD) who underwent unilateral GPi-DBS were selected. We conducted comparison analyses between scores obtained 6–42 months pre- and postoperatively using the following measurement tools: the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III, the Hoehn and Yahr stage, the presence/absence of dyskinesia, Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), Geriatric Depression Scale (GDS), levodopa equivalent dose (LED), and cerebral blood flow by single photon emission computed tomography (SPECT). Patient backgrounds were compared between four cohorts with favorable (good responders, ≥50% improvement) and unfavorable (poor responders, <50% improvement) postoperative outcome.
Results: Significant improvement was observed postoperatively in the following: total MDS-UPDRS Part III scores during the off period, contralateral scores, ipsilateral scores, and axial scores. Similarly, the Hoehn and Yahr stages during the off period, and GDS also showed significant decrease. In contrast, LED, MMSE, and FAB remained unchanged while the number of patients who scored positive for dyskinesia decreased by 40%. Abnormal cerebral blood flow preoperatively seen in the cerebral cortex had normalized in the total score-based good responder cohort. In the ipsilateral score-based good responder cohort, cerebral blood flow increased in the contralateral frontal lobe including in the premotor cortex, contralateral to the DBS. Compared to the poor responders, postoperative good responders demonstrated significantly higher preoperative MMSE scores.
Discussion: Unilateral GPi-DBS therapy was effective in improving contralateral, ipsilateral, and axial motor symptoms of patients with advanced PD; in particular, it was found to be especially beneficial in PwPD whose cognitive function was unimpaired; the treatment efficacy rivaled that of bilateral counterparts up till at least 6 months postoperatively. Finally, normalization of preoperative abnormalities in cerebral blood flow and increased cerebral blood flow in the contralateral frontal lobe indicated the beneficial potential of this therapy on ipsilateral motor symptoms.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by both motor and non-motor symptoms such as tremor, muscle rigidity, bradykinesia, and inability to retain a suitable posture, in addition to dysosmia, dysautonomia, cognitive impairment, psychosis, and sleep disturbance. The effectiveness of dopamine replacement therapy (DRT) at the early stage of PD has been well-documented; DRT in combination with a routine exercise regimen enables long-term preservation of activity of daily living (ADL). Nevertheless, levodopa-induced motor complications, such as wearing off or dyskinesia, eventually emerge as the disease progresses and necessitate a dosage increase. Inevitably, ADL and quality of life (QOL) deteriorate as it becomes increasingly harder for patients to move freely (Berganzo et al., 2016; Gökçal et al., 2017). Upon reaching this stage, maintaining regular motor functions while controlling motor complications becomes an insurmountable challenge for patients with Parkinson’s disease (PwPD) even with the appropriate prescription of drug therapy and a routine exercise regimen. Fortunately, device-assisted therapies such as deep brain stimulation (DBS) and levodopa-carbidopa intestinal gel (LCIG) are available and have been shown to be highly effective for a carefully selected group of PwPD who reached such a plateau at advanced stages (Deep-Brain Stimulation for Parkinson’s Disease Study Group et al., 2001; Antonini et al., 2017).
By frequently stimulating two electrode targets implanted in the subthalamic nucleus (STN) and the internal segment of the globus pallidus (GPi), advanced PD symptoms improve under DBS therapy. Although differences in treatment effects do exist between STN and GPi, both therapies are equally useful in reducing both core motor symptoms and motor complications (Deep-Brain Stimulation for Parkinson’s Disease Study Group et al., 2001). While bilateral STN and GPi-DBS therapies have recently been established as standard treatment, there are also promising reports on the effectiveness of unilateral DBS therapies (Merello et al., 1999; Loher et al., 2002; Linazasoro et al., 2003; Germano et al., 2004; Chung et al., 2006; Kim et al., 2009; Okun et al., 2009, 2014; Walker et al., 2009; Zahodne et al., 2009). A steadily accumulating body of evidence (Kumar et al., 1999; Merello et al., 1999; Loher et al., 2002; Linazasoro et al., 2003; Germano et al., 2004; Chung et al., 2006; Nakamura et al., 2007; Kim et al., 2009; Okun et al., 2009, 2014; Walker et al., 2009; Zahodne et al., 2009) suggests that unilateral STN-DBS therapy improves motor symptoms even in research that evaluated motor symptoms separately from the contralateral, ipsilateral, and axial aspects (Kumar et al., 1999; Chung et al., 2006; Nakamura et al., 2007; Agostino et al., 2008; Tabbal et al., 2008; Walker et al., 2009; Hasegawa et al., 2020). Moreover, unilateral STN-DBS therapy is capable of improving ipsilateral symptoms in other movement disorders such as essential tremor (Peng-Chen et al., 2013). Other noteworthy benefits of unilateral STN-DBS therapy for PwPD include reducing dyskinesia, depression, and the levodopa equivalent dose (LED) (Linazasoro et al., 2003; Chung et al., 2006; Kim et al., 2009; Walker et al., 2009) as well as improving ADL and QOL (Kumar et al., 1999; Linazasoro et al., 2003; Chung et al., 2006; Walker et al., 2009; Zahodne et al., 2009; Okun et al., 2014). While there is plenty of evidence in the literature that supports the overall effectiveness of unilateral GPi-DBS on PD (Merello et al., 1999; Vingerhoets et al., 1999; Loher et al., 2002; Nakamura et al., 2007; Rodrigues et al., 2007; Okun et al., 2009, 2014; Zahodne et al., 2009), studies that examined the effect of unilateral GPi-DBS on motor symptoms of PD in the contralateral, ipsilateral, and axial sides are scarce (Merello et al., 1999; Loher et al., 2002). Thus, in order to clarify the effectiveness of unilateral GPi-DBS from a different angle and identify suitable patient candidates, this study conducted a detailed comparative analyses of the pre- and postoperative changes in motor and non-motor symptoms of PwPD who underwent unilateral GPi-DBS.
This is a retrospective observational study conducted at a single institution, the Fukuoka University Hospital. Of the database of 343 PwPD who visited our hospital during the period of 1 December 2014 to 30 September 2019, 17 patients, who received unilateral GPi-DBS stimulation to either right or left side and who were available for a 6 month postoperative assessment, were selected (Figure 1). All procedures were performed by a fellowship-trained functional neurosurgeon (T.M.), and the same DBS system was used in all study subjects (model 3387 DBS lead and Activa SC pulse generator, Medtronic, Minneapolis, MN, United States). Although our hospital is primarily focused on the staged GPi-DBS approach (Samii et al., 2007), we selected patients who requested either a unilateral or a staged DBS therapy when presented with the choice of bilateral, unilateral, or staged DBS therapy. These 17 patients all met the United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (UKBBC) and had been diagnosed with sporadic PD by a specialized neurologist (Hughes et al., 1992). Furthermore, all patients were preoperatively suffering from motor complications that: were difficult to control with a combined drug and exercise regimen; had shown >33% improvement in a total score obtained in the Movement Disorder Society Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS Part III) in response to the levodopa challenge test (LDCT) that compared assessment during the off-stage and after taking levodopa; and were without prominent cognitive impairment [Mini-Mental State Examination (MMSE) score < 24] (Mishima et al., 2021). Concerning the programming, we follow the basic programming concept (Volkmann et al., 2002). Following testing the threshold levels of stimulation-induced side effects and therapeutic windows of monopolar stimulation at each contact, we usually select contact 1 or 2 as the active contact as the initial setting. Patients were instructed to regularly visit the programming clinic once a month for the stimulation adjustment performed by neurologists specializing movement disorders (Y.H., T.M., S.F., and Y.T.). The stimulation intensity and pulse width were gradually increased or decreased, and bipolar setting was selected when the intensity of monopolar stimulation reached the threshold level of side effects.
Measurements used for the assessment of motor and non-motor function respectively were as follows: MDS-UPDRS Part III, Hoehn and Yahr stages, presence/absence of dyskinesia; MMSE, Frontal Assessment Battery (FAB), and Geriatric Depression Scale (GDS). In addition, oral treatment was assessed with the levodopa equivalent dose (LED) (Tomlinson et al., 2010). A comparison analysis between pre- and approximately 6-month postoperative scores (mean, 6.6 ± 0.7 months; range, 6–7 months) was performed for each item. Both pre- and postoperative assessment with MDS-UPDRS Part III was undertaken during the off state. The assessment was conducted not only with total MDS-UPDRS Part III score, but also considered the ipsilateral and contralateral (total unilateral MDS-UPDRS Part III score from the sub-item 20–26) and axial scores (Kotagal et al., 2014) (total MDS-UPDRS Part III score from the sub-item 1, 9, 10, 12, 13) independently. In addition, two cohorts (Table 1) were created for comparison consisting of PwPD with favorable (good responder, total MDS-UPDRS Part III score ≥ 50%) and unfavorable (poor responder, total MDS-UPDRS Part III score < 50%) postoperative outcome. Comparisons were made in the following item categories obtained preoperatively: age, sex, disease duration, total MDS-UPDRS Part III score, Hoehn and Yahr stage (each on/off period), MMSE, FAB, GDS, LED, and presence/absence of dyskinesia.

Table 1. Comparison of each item at baseline between good responders (changes of Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III total score after surgery ≧50%) and poor responders (<50%).
Changes in pre- and approximately 11-month postoperative distribution of cerebral blood flow (mean, 11.1 ± 9.7 months; range, 6 months–3.5 years) were examined with 99mTc-ECD single photon emission computed tomography (SPECT). SPECT data was missing from 3 patients (Case No. 4, 11, 17); therefore, the SPECT examination was based on available data from 14 patients. A SPECT examination was performed with patients’ eyes closed at resting state using a Technetium-99m ethyl cysteinate dimer (99mTc-ECD) 600–900 MBq. A triple-detector gamma camera system (GCA-9300R; Cannon Medical Systems, Tokyo, Japan) was used for imaging. Data were collected during the period 5–16 min after a radioisotope (RI) was administered under the following conditions: 120°, 30 locations × 3, 120 s, a main energy window (20% of 141 KeV), a sub window (<7%). A high-resolution fan beam collimator was selected. SPECT images were corrected with 3D-OSEM reconstruction using absorptive correction (+), scatter−correction (+), μ value 0.15, and the Butterworth filter (BW); it was performed at order 4 (cut-off frequency, 0.13 cy/pixel), repetition time 10, and adding frequency of 10 times. The image analysis software eZIS (Fujifilm RI Pharma., Tokyo, Japan) was used to conduct image statistical analysis; this could be performed on a personal computer and was concordant with the patient SPECT image. Specifically, anatomically standardized SPECT images were compared with the images stored in the database of a standard brain of the corresponding age, for each patient’s data. Z-score for a region of interest (ROI) for each of 52 regions was determined with a fine stereotaxic region of interest template (Fine SRT) (Fuji Film, Tokyo, Japan) (Figure 2; Takeuchi, 2005). A total of four cohorts (Tables 2A,B) were created based on: (a) the total MDS-UPDRS Part III score, with favorable (good responder, ≥50%) and unfavorable (poor responder, <50%) postoperative outcome; (b) ipsilateral score, with favorable (good responder, improvement rate ≥ 50%) and unfavorable (poor responder, improvement rate < 50%) postoperative outcome (Antonini et al., 2003a). A comparison was made between pre- and postoperative Z-scores for each item category. For all item categories, paired t-tests were used for pre- and postoperative comparison and the Mann-Whitney test and the chi−square test were applied in the comparative analysis of good vs. poor postoperative responders. The threshold of statistical significance was established at p < 0.05 with a two-sided testing procedure. This study was approved by the Ethics Committee of the Fukuoka University Hospital (number, U20-04-001).
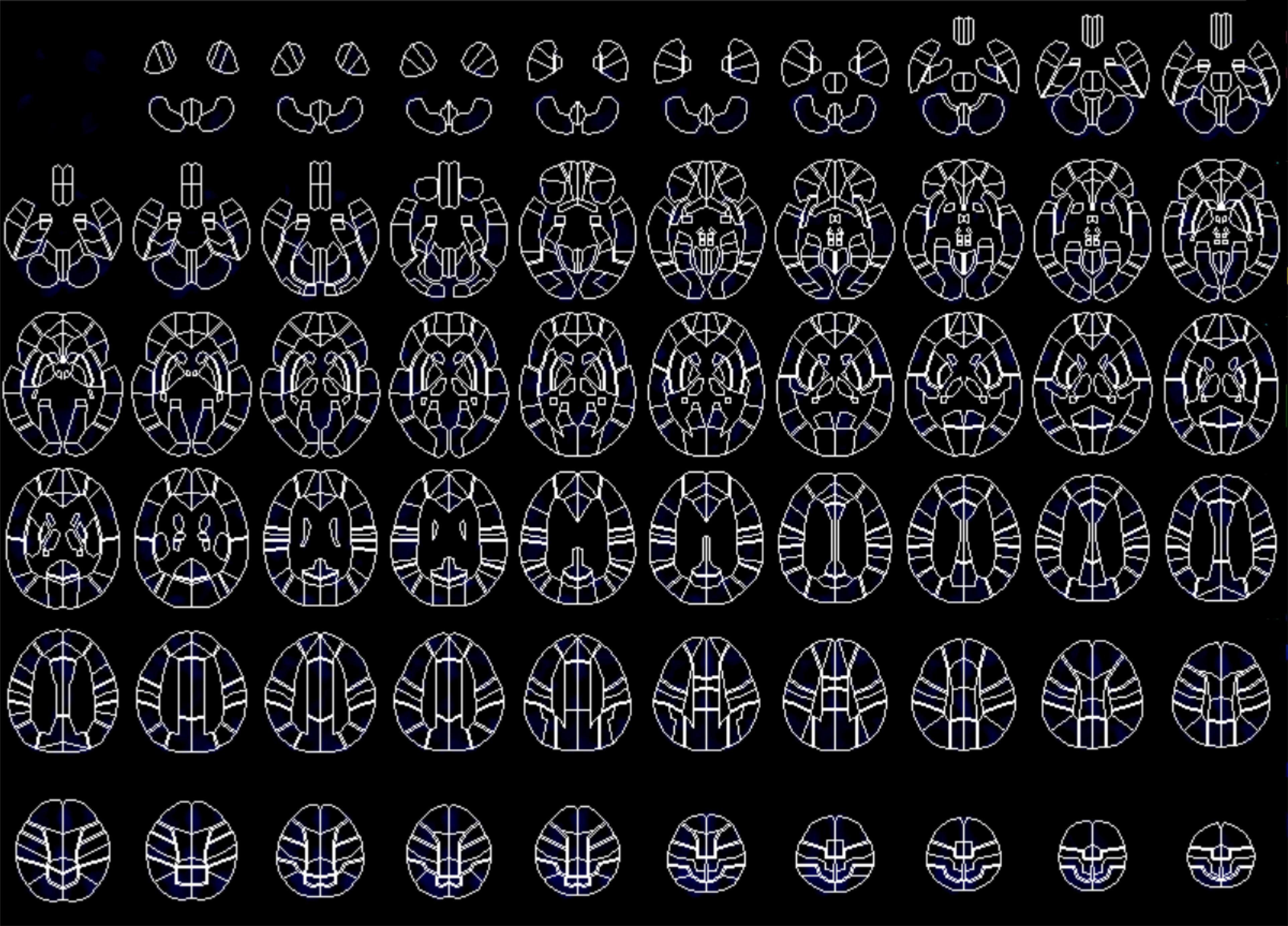
Figure 2. Fine stereotactic region of interest (ROI) template (SRT) image composed of 52 areas of ROI in Single Photon Emission Computed Tomography (SPECT) study.
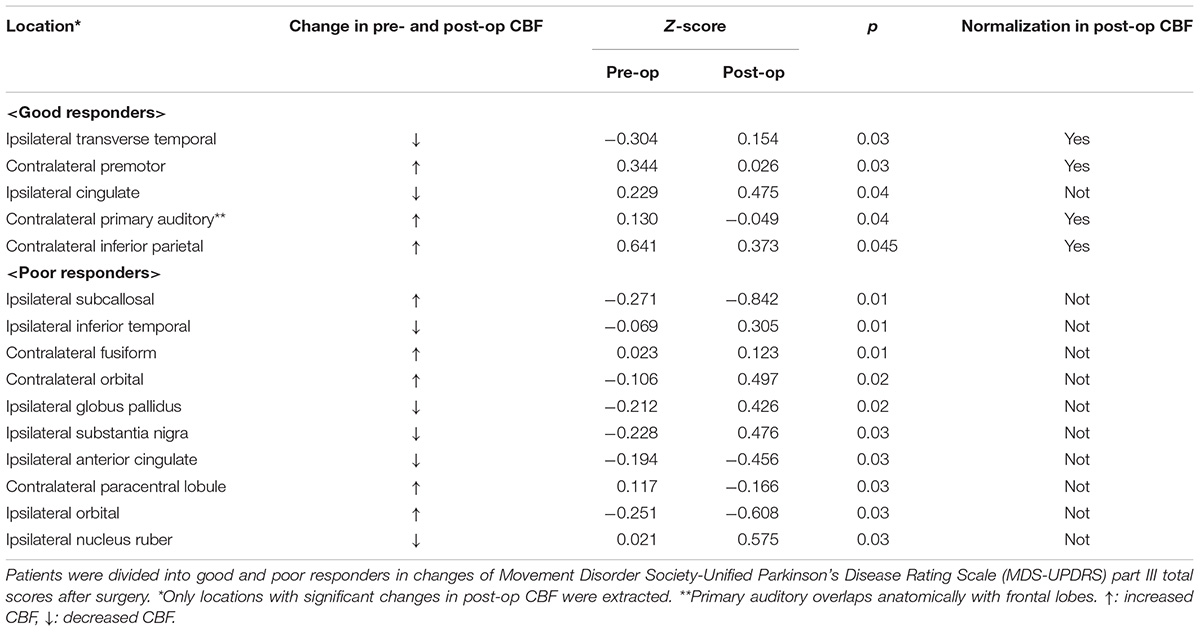
Table 2A. Comparison of Z-score between baseline and after unilateral deep brain stimulation of the globus pallidus internus (GPi-DBS) by brain perfusion Single Photon Emission Computed Tomography (SPECT).
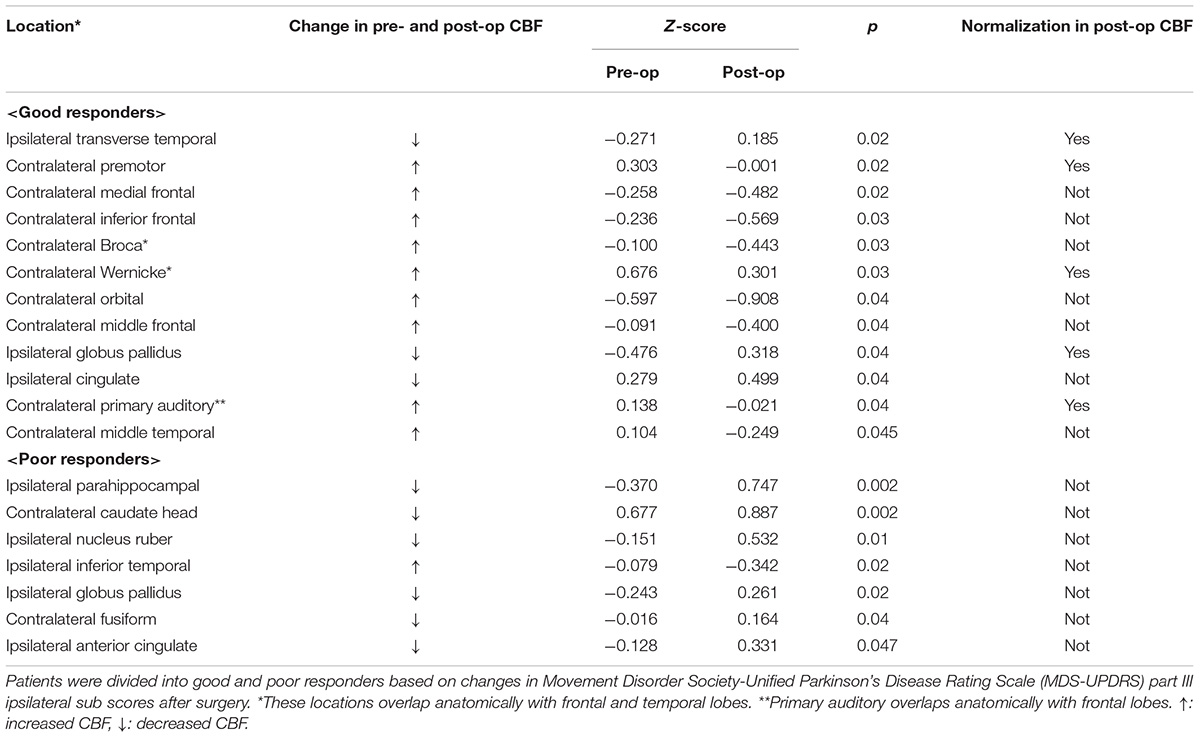
Table 2B. Comparison of Z-score between baseline and after unilateral deep brain stimulation of the globus pallidus internus (GPi-DBS) by brain perfusion single photon emission computed tomography (SPECT).
Table 3 gives the background data of the 17 patients who participated in our study: male to female sex ratio, 7:10; average age, 62.2 ± 5.8 years old; average disease duration, 11.2 ± 5.2 years, mean preoperative LED 1,033.7 ± 284.6 mg/day; average pre- and postoperative differences in Hoehn and Yahr stages during off period, 4.1 ± 0.2/2.8 ± 0.8. Only two patients met the EARLYSTIM criteria (Schuepbach et al., 2013). No adverse events such as intra- and postoperative bleeding or infection occurred during our study. All patients underwent CT postoperative day 9 to evaluate the electrode position in the GPi, and a board-certified neurosurgeon (T.M.) confirmed that there was no lead misplacement. Figures 3A,B show MDS-UPDRS Part III score, Hoehn and Yahr stage, MMSE, FAB, GDS, and LED. Compared to preoperative data, significant postoperative improvement was identified in the following item categories: total MDS-UPDRS Part III score, 50.2 ± 12.3 vs. 25.5 ± 12.2, improvement rate 50.7%, p < 0.0001; contralateral score, 15.0 ± 4.8 vs. 7.4 ± 4.1, improvement rate 48.1%, p < 0.0001; ipsilateral score, 14.4 ± 5.5 vs. 7.1 ± 4.9, improvement rate 50.6%, p < 0.0001; and axial score 10.5 ± 3.1 vs. 4.9 ± 3.4, improvement rate 53.6%, p < 0.0001. In all cases, postoperative total MDS-UPDRS part III score during the off period improved compared to preoperative (Supplementary Table 1). The Hoehn and Yahr stage for both on period (p < 0.0001) and off period (p < 0.04) were significantly decreased postoperatively. Although not significant, the number of patients with dyskinesia decreased from 15 (88.2%) to 9 (52.9%) after GPi-DBS therapy, while no changes were detected in LED. Similarly, MMSE and FAB were unchanged whereas GDS (4.5 ± 2.6 vs. 3.2 ± 2.1, p = 0.04) showed improvement. The postoperative good responder cohort (n = 9, including two patients who met the EARLYSTIM criteria) demonstrated significantly higher MMSE scores (28.2 ± 0.8 vs. 26.9 ± 1.5, p < 0.05) compared to postoperative poor responder counterparts even before undergoing GPi-DBS therapy (Table 1). No patient has displayed serious adverse events at the 6-month postoperative period, to date. Pre- and postoperative Z-scores for 14 patients who experienced 99m-Tc-ECD SPECT are presented in Table 2A (Comparisons were made between good and poor responders based on total MDS-UPDRS Part III score) and Table 2B (Comparisons were made between good and poor responders based on ipsilateral score). Two noteworthy changes were detected postoperatively. First, abnormalities in cerebral blood flow observed in the bilateral cerebral cortex before GPi-DBS had normalized postoperatively in the total MDS-UPDRS Part III score good responder cohort (n = 8, mean age 62.0 ± 3.2 years old, postoperative improvement rate 69.2 ± 14.5%). In contrast, the poor responder cohort (n = 6, 62.7 ± 4.3 years old 27.3 ± 8.3%) showed scores that further deviated from the normal value in the bilateral cerebral cortex postoperatively (Table 2A). The second noticeable change was the significant postoperative increase in blood flow in the contralateral frontal lobe of the ipsilateral good responder cohort (n = 9, 61.8 ± 3.2 years old, 74.0 ± 13.9%). However, scores for the bilateral cerebral cortex showed greater deviation from the normal value or baseline in the poor responder counterparts (n = 5, 63.0 ± 4.6 years old, 5.9 ± 36.0%) after GPi-DBS therapy (Table 2B).
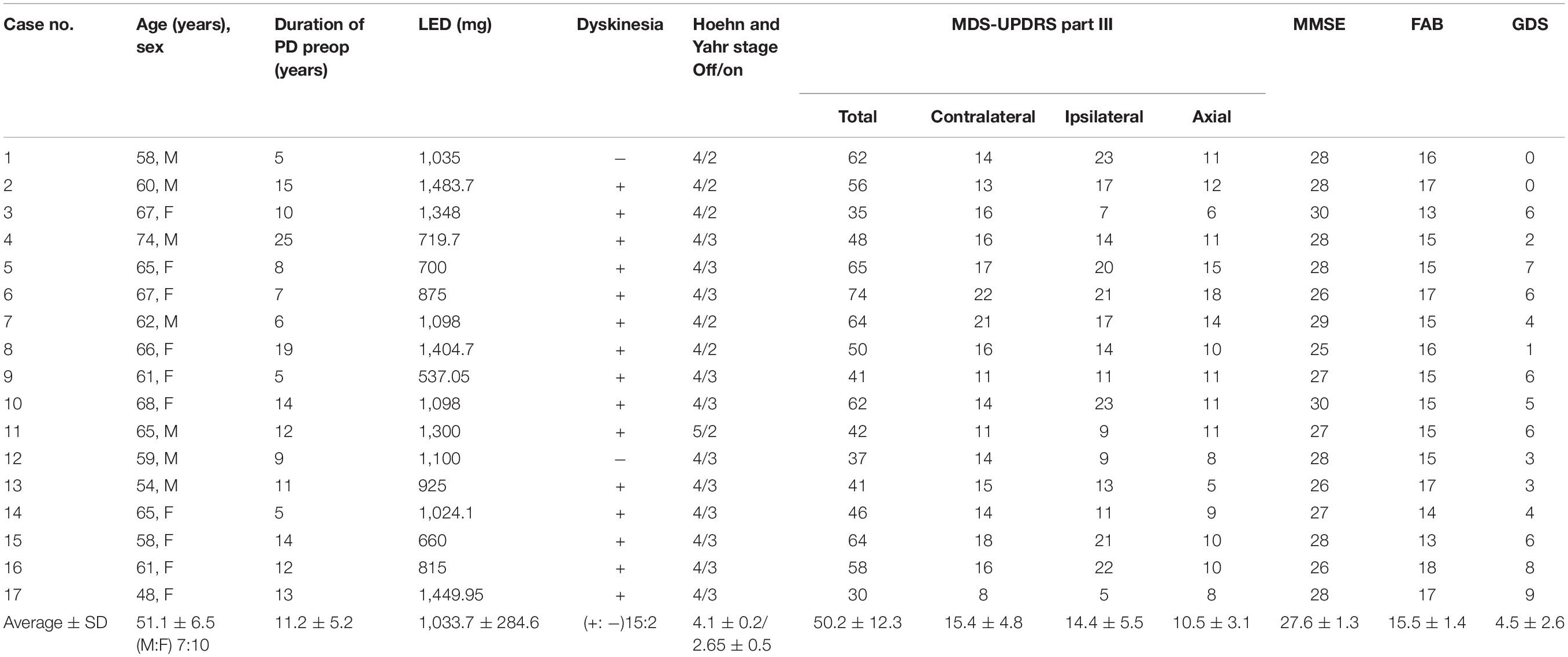
Table 3. Baseline characteristics of 17 patients with advanced Parkinson’s disease received unilateral deep brain stimulation of the globus pallidus internus (GPi-DBS).
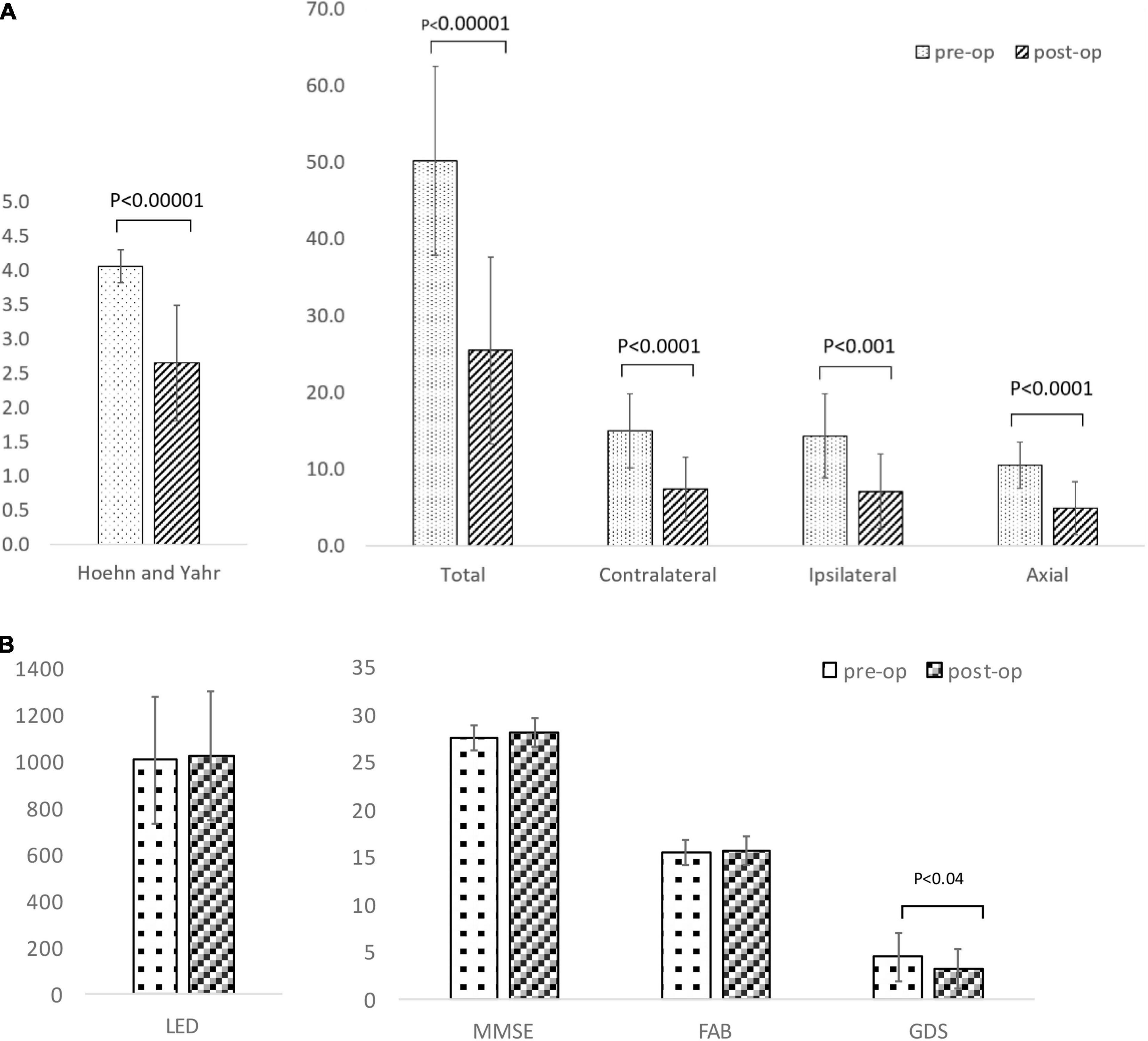
Figure 3. (A) Comparison of Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) motor scores and Hoehn and Yahr stage between baseline and after unilateral deep brain stimulation of the globus pallidus internus (GPi-DBS). (B) Comparison of levodopa equivalent dose (LED), Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), Geriatric depression scale (GDS) between baseline and after unilateral deep brain stimulation of globus pallidus internus (GPi-DBS).
At postoperative 6 months, motor symptoms of PwPD significantly improved in the axis and sides contralateral or ipsilateral to the target area treated with DBS. Given the 50.7% improvement rates of the total UPDRS part III scores obtained in this study, and the 24–67% (postoperative 6 months) (Kumar et al., 1999; Houeto et al., 2000; Molinuevo et al., 2000; Deep-Brain Stimulation for Parkinson’s Disease Study Group et al., 2001; Volkmann et al., 2001, 2009; Simuni et al., 2002; Thobois et al., 2002; Schupbach et al., 2005; Tanei et al., 2009a; Mei et al., 2020) or 38–56% (postoperative 3–7 months) (Vingerhoets et al., 1999; Deep-Brain Stimulation for Parkinson’s Disease Study Group et al., 2001; Volkmann et al., 2001; Loher et al., 2002; Tanei et al., 2009a) improvement rates from the bilateral STN or GPi-DBS in previous studies, it is reasonable to conclude that unilateral GPi-DBS therapy is equally as effective as bilateral DBS therapy. Improvement rates in our study (50.7%) were similar to the rates obtained in other unilateral GPi-DBS studies: 16.0–48.5% (Merello et al., 1999; Vingerhoets et al., 1999; Loher et al., 2002; Rodrigues et al., 2007; Okun et al., 2009, 2014; Zahodne et al., 2009). Contralateral scores also improved in similar rates to other studies (28.8–50.0%) (Merello et al., 1999; Loher et al., 2002) in the current study (48.1%). On the other hand, while improvement rates (23–24%) for the ipsilateral scores did not reach significance in other studies (Loher et al., 2002), our improvement rate was significant at 50.6%. There is only one study that previously showed a significant improvement in ipsilateral scores in unilateral GPi-DBS therapy; it evaluated the improvement solely on fingertip mobility (Nakamura et al., 2007). It is not clear what contributed to the remarkable improvement rates of the ipsilateral scores in this study; however, one might be the difference in measurement methods as MDS-UPDRS part III has not been used to assess motor symptoms of the ipsilateral side before this study. Interestingly, an equally remarkable improvement to ipsilateral scores was observed in the contralateral scores. Furthermore, improvement rate for axial score was notably high (56.7%) and rivaled improvements in contralateral and ipsilateral scores. This warrants further study since results concerning improvement rates for axial score after unilateral GPi-DBS therapy have been inconsistent: while Loher et al. (2002) reports a significant rate of improvement (41%), no improvement was identified in two other studies (Merello et al., 1999; Rodrigues et al., 2007). The current study assessed the significant differences between gait and postural stability scales of the MDS-UPDRS Part III; and freezing, walking, and balance scales in the Part II section of the same scale. The fact that we removed a total sum of item 1, 9, 10, 12, 13 (Takeuchi, 2005) in the MDS-UPDRS Part III of the motor examination section and defined them as an “axial score” may have contributed to the incomparably remarkable improvement in our study.
Studies (Merello et al., 1999; Loher et al., 2002; Volkmann et al., 2009) that assessed severity of dyskinesia after bilateral or unilateral GPi-DBS therapy, with the Rush Dyskinesia Rating Scale and the UPDRS part IV, have demonstrated a significant postoperative improvement. Although results were not significant, the number of patients who scored positive for dyskinesia (or who reported the presence of dyskinesia) during the on period decreased by 40%: from 15/17 preoperatively to 9/15 postoperatively. Caution must be exercised when interpreting this result; rather than the severity of dyskinesia, this analysis only focused on the presence or absence of dyskinesia.
After the unilateral GPi-DBS therapy, PwPD showed reduced depression; however, no change was detected in their cognitive function during the evaluation of non-motor symptoms. Consistent with other unilateral GPi-DBS studies (Loher et al., 2002; Simuni et al., 2002; Rothlind et al., 2007; Zahodne et al., 2009), this positive effect on depression found in the present study further increased confidence in GPi-DBS’s ability to ease depression in PwPD. Compared to the poor responder counterparts, the good responder cohort, who showed favorable outcomes in motor symptoms after unilateral GPi-DBS therapy, scored significantly higher in MMSE, indicating a better cognitive function. Thus, it is speculated that unilateral GPi-DBS is most effective for PwPD with preserved cognitive functions. Furthermore, the fact that the two cases that met the EARLYSTIM criteria in this study belonged to the good responder cohort, also suggests that the use of the unilateral GPi-DBS in the early stages of PD can be especially beneficial. Nevertheless, the small sample size warrants caution and further replication.
This study examined 99mECD-SPECT pre- and postoperatively and noted that abnormal cerebral blood flow, preoperatively observed in the bilateral cortex, normalized after unilateral GPi-DBS in PwPD who showed improvement in motor symptoms. In addition, cerebral blood flow increased in the frontal lobe including in the premotor cortex contralateral to the side stimulated with DBS. The majority of studies that investigated correlations to improvement in motor symptoms with cerebral blood flow tomography or SPECT, concerned patients who underwent STN-DBS therapy. A potential link between the increase in cerebral blood flow in the motor-related areas of the frontal lobe (e.g., premotor cortex, pre-SMA, SMA, and anterior cingulate) and reduced motor symptoms after unilateral STN-DBS therapy has been suggested in 99mTc-ECD SPECT studies (Sestini et al., 2002, 2005; Antonini et al., 2003a; Paschali et al., 2013). In addition, potential links between the postoperative normalization of abnormal blood flow (Cilia et al., 2009) in the bilateral cerebral cortex, nucleus basalis, or hypothalamic loop, and improvement in motor symptoms have been documented in the literature (Antonini et al., 2003b). Only a single assessment study (Tanei et al., 2009b) exists regarding the postoperative effect of unilateral STN-DBS; a significant vascular flow increase within the bilateral cingulate gyrus and cerebellum was identified, whereas vascular flow significantly decreased in both the bilateral medial frontal and superior temporal lobes. A significant correlation has also been found in the literature (van Laere et al., 2000) concerning GPi-DBS and 99mECD-SPECT between decreased vascular flow in the ipsilateral thalamus and corpus striatum in relation to improvement in motor symptoms. However, comparison of the latter study to the current study is not relevant since the lesion effect of inserting electrodes serves as a confounding factor. Revitalized cortical activity was detected postoperatively in the motor-related areas of the frontal lobe, including in the ipsilateral side, in a unilateral GPi-DBS study using near-infrared spectroscopy (NIRS) (Morishita et al., 2016).
Insights are offered in the unilateral STN-DBS study, regarding the potential improvement mechanism in the ipsilateral symptoms. Motor symptoms of the ipsilateral side may be positively affected by the stimulation received by the ipsilateral pedunculopontine tegmental nucleus (PPN) (Nakano, 2000) transmitted through the contralateral side of the brain; this takes place via input from the ipsilateral supplementary motor area including the neuronal network of the cortex-basal ganglia-thalamus loop to the bilateral basal ganglia (Parent and Hazrati, 1995a,b; Chung et al., 2006; Tabbal et al., 2008) and input from the bilateral GPi and substantia nigra compacta into the bilateral thalamus and brain stem (Parent and Hazrati, 1995a,b; Levy et al., 1997; Chung et al., 2006; Tabbal et al., 2008; Peng-Chen et al., 2013). Based on our findings that showed a significant increase in cerebral blood flow in the contralateral frontal lobe, including in the premotor cortex, it is speculated that ipsilateral symptoms might be improved by using unilateral GPi-DBS therapy that stimulates the area contralateral to the side being stimulated in the cortex-basal ganglia-thalamus loop. The unilateral pyramidal tracts are involved in about 20% of control in motor functions of the body axis on the ipsilateral side (Germano et al., 2004). Thus, axial symptoms may be mitigated by improvement in blood flow of the unilateral premotor cortex areas. Previous study indicates that a deterioration in blood flow is noticeable in the frontal cingulate gyrus of PwPD who had dominant axial symptoms (Mito et al., 2006). Therefore, a significant increase in blood flow in areas that affect motor symptoms after unilateral GPi-DBS therapy, such as the frontal cingulate gyrus and frontal lobe areas including the premotor cortex, might contribute to the improvement in axial symptoms.
Despite being one of the few detailed studies on the effectiveness of unilateral GPi-DBS therapy, this study has several limitations. Firstly, readers should be reminded that this was a retrospective observational study based on data from a single institution and the period of observation only lasted for 6 months with a small sample size of 17 patients. Secondly, this study attempted to offer insights by comparing the analyses of patients who underwent unilateral GPi-DBS in the current study to similar previous studies. Because it is impossible to fully control the conditions of studies that have already been conducted, our interpretations may be confounded. Thirdly, the period when 99mTc-ECD SPECT was performed postoperatively ranged widely; therefore, our assessment of cerebral blood flow may have been affected by other factors, such as the rate of progression in PD. Finally, this study examined the cerebral hemisphere ipsilateral or contralateral to the side where the DBS lead was inserted, without any clear knowledge of which side should be prioritized for which symptoms. In addition, this study did not evaluate the relationships between stimulation fields and the clinical responses/SPECT findings. Further studies are warranted to address these limitations.
Regardless of these limitations, this 6-month postoperative assessment was valuable in that it underscored the potential of unilateral GPi-DBS therapy, in improving both motor- and non-motor symptoms including depression and in maintaining speech and cognitive function of PwPD, that does not pale in comparison to bilateral GPi-DBS therapy. The unilateral GPS-DBS therapy performed on PwPD, whose cognitive function remains unimpaired at relatively early stages of the disease, demonstrated therapeutic benefit equivalent to that of the bilateral counterparts, at over 6 months postoperatively. However, the results of this study constitute only a snapshot of information regarding the effect of unilateral GPi-DBS therapy and warrant a further investigation into long-term effects of the therapy and mechanisms responsible for mitigating various PD symptoms. Given the fact that unilateral GPi-DBS is less invasive and requires less battery energy than bilateral counterparts, the former could prove economically advantageous if it could systematically be shown that its effects on debilitating PD symptoms are long-lasting.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Fukuoka Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YT, TMi, and YH: conception and design. YT, TMi, SN, and YH: analysis and interpretation of data. YH: drafting the manuscript. YT, TMi, SF, TI, TMo, and SN: revising manuscript critically for important intellectual content. YT: final approval of the version to be submitted. All authors contributed substantially to this study.
This study was partly supported by a Japan Society for the Promotion of Science grant-in-aid for scientific research (C) (Grant No. 18K08956).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the staff at Fukuoka University Hospital for their kind support and for the management of patients.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.888701/full#supplementary-material
ADL, activity of daily living; BW, Butterworth filter; DBS, deep brain stimulation; DRT, dopamine replacement therapy; Fine SRT, fine stereotaxic region of interest template; FAB, Frontal Assessment Battery; GDS, Geriatric Depression Scale; GPi, globus pallidus internus; LDCT, levodopa challenge test; LED, levodopa equivalent dose; LCIG, levodopa-carbidopa intestinal gel; MMSE, Mini-Mental State Examination; MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; NIRS, near-infrared spectroscopy; PD, Parkinson’s disease; PwPD, patients with Parkinson’s disease; PPN, pedunculopontine tegmental nucleus; QOL, quality of life; RI, radioisotope; ROI, region of interest; SPECT, single photon emission computed tomography; STN, subthalamic nucleus; STN-DBS, subthalamic nucleus deep brain stimulation; UKBBC, United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria.
Agostino, R., Dinapoli, L., Modugno, N., Iezzi, E., Gregori, B., Esposito, V., et al. (2008). Ipsilateral sequential arm movements after unilateral subthalamic deep-brain stimulation in patients with Parkinson’s disease. Mov. Disord. 23, 1718–1724. doi: 10.1002/mds.22203
Antonini, A., Landi, A., Benti, R., Mariani, C., De Notaris, R., Marotta, G., et al. (2003a). Functional neuroimaging (PET and SPECT) in the selection and assessment of patients with Parkinson’s disease undergoing deep brain stimulation. J. Neurosurg. Sci. 47, 40–46.
Antonini, A., Marotta, G., Benti, R., Landi, A., De Notaris, R., Mariani, C., et al. (2003b). Brain flow changes before and after deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. Neurol. Sci. 24, 151–152. doi: 10.1007/s10072-003-0104-4
Antonini, A., Poewe, W., Chaudhuri, K. R., Jech, R., Pickut, B., Pirtošek, Z., et al. (2017). Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat. Disord. 45, 13–20. doi: 10.1016/j.parkreldis.2017.09.018
Berganzo, K., Tijero, B., González-Eizaguirre, A., Somme, J., Lezcano, E., Gabilondo, I., et al. (2016). Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurologia 31, 585–591. doi: 10.1016/j.nrl.2014.10.010
Chung, S. J., Jeon, S. R., Kim, S. R., Sung, Y. H., and Lee, M. C. (2006). Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur. Neurol. 56, 127–132. doi: 10.1159/000095704
Cilia, R., Marotta, G., Landi, A., Isaias, I. U., Mariani, C. B., Vergani, F., et al. (2009). Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson’s disease: a prospective case-control study. Clin. Neurol. Neurosurg. 111, 140–146. doi: 10.1016/j.clineuro.2008.09.018
Deep-Brain Stimulation for Parkinson’s Disease Study Group, Obeso, J. A., Olanow, C. W., Rodriguez-Oroz, M. C., Krack, P., Kumar, R., et al. (2001). Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N. Engl. J. Med. 345, 956–963. doi: 10.1056/NEJMoa000827
Germano, I. M., Gracies, J. M., Weisz, D. J., Tse, W., Koller, W. C., and Olanow, C. W. (2004). Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J. Neurosurg. 101, 36–42. doi: 10.3171/jns.2004.101.1.0036
Gökçal, E., Gür, V. E., Selvitop, R., Babacan Yildiz, G., and Asil, T. (2017). Motor and non-motor symptoms in Parkinson’s disease: effects on quality of life. Noro Psikiyatr. Ars. 54, 143–148. doi: 10.5152/npa.2016.12758
Hasegawa, H., Fischer, P., Tan, H., Pogosyan, A., Samuel, M., Brown, P., et al. (2020). The effect of unilateral subthalamic nucleus deep brain stimulation on contralateral subthalamic nucleus local field potentials. Neuromodulation 23, 509–514. doi: 10.1111/ner.13155
Houeto, J. L., Damier, P., Bejjani, P. B., Staedler, C., Bonnet, A. M., Arnulf, I., et al. (2000). Subthalamic stimulation in Parkinson disease: a multidisciplinary approach. Arch. Neurol. 57, 461–465. doi: 10.1001/archneur.57.4.461
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Kim, H. J., Paek, S. H., Kim, J. Y., Lee, J. Y., Lim, Y. H., Kim, D. G., et al. (2009). Two-year follow-up on the effect of unilateral subthalamic deep brain stimulation in highly asymmetric Parkinson’s disease. Mov. Disord. 24, 329–335. doi: 10.1002/mds.22211
Kotagal, V., Albin, R. L., Müller, M. L., Koeppe, R. A., Frey, K. A., and Bohnen, N. I. (2014). Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology 82, 1514–1520. doi: 10.1212/WNL.0000000000000356
Kumar, R., Lozano, A. M., Sime, E., Halket, E., and Lang, A. E. (1999). Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology 53, 561–566. doi: 10.1212/wnl.53.3.561
Levy, R., Hazrati, L. N., Herrero, M. T., Vila, M., Hassani, O. K., Mouroux, M., et al. (1997). Re-evaluation of the functional anatomy of the basal ganglia in normal and Parkinsonian states. Neuroscience 75, 335–343. doi: 10.1016/s0306-4522(96)00409-5
Linazasoro, G., Van Blercom, N., and Lasa, A. (2003). Unilateral subthalamic deep brain stimulation in advanced Parkinson’s disease. Mov. Disord. 18, 713–716. doi: 10.1002/mds.10407
Loher, T. J., Burgunder, J. M., Pohle, T., Weber, S., Sommerhalder, R., and Krauss, J. K. (2002). Long-term pallidal deep brain stimulation in patients with advanced Parkinson disease: 1-year follow-up study. J. Neurosurg. 96, 844–853. doi: 10.3171/jns.2002.96.5.0844
Mei, S., Eisinger, R. S., Hu, W., Tsuboi, T., Foote, K. D., Hass, C. J., et al. (2020). Three-year gait and axial outcomes of bilateral STN and GPi Parkinson’s disease deep brain stimulation. Front. Hum. Neurosci. 14:1. doi: 10.3389/fnhum.2020.00001
Merello, M., Nouzeilles, M. I., Kuzis, G., Cammarota, A., Sabe, L., Betti, O., et al. (1999). Unilateral radiofrequency lesion versus electrostimulation of posteroventral pallidum: a prospective randomized comparison. Mov. Disord. 14, 50–56. doi: 10.1002/1531-8257(199901)14:1<50:aid-mds1010<3.0.co;2-6
Mishima, T., Fujioka, S., Morishita, T., Inoue, T., and Tsuboi, Y. (2021). Personalized medicine in Parkinson’s Disease: new options for advanced trearments. J. Pers. Med. 11:650. doi: 10.3390/jpm11070650
Mito, Y., Yoshida, K., Yabe, I., Makino, K., Tashiro, K., Kikuchi, S., et al. (2006). Brain SPECT analysis by 3D-SSP and phenotype of Parkinson’s disease. J. Neurol. Sci. 241, 67–72. doi: 10.1016/j.jns.2005.10.017
Molinuevo, J. L., Valldeoriola Tolosa, E., Tolosa, E., Rumia, J., Valls-Sole, J., Roldan, H., et al. (2000). Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch. Neurol. 57, 983–988. doi: 10.1001/archneur.57.7.983
Morishita, T., Higuchi, M. A., Saita, K., Tsuboi, Y., Abe, H., and Inoue, T. (2016). Changes in motor-related cortical activity following deep brain stimulation for parkinson’s disease detected by functional near infrared spectroscopy: a pilot study. Front. Hum. Neurosci. 10:629. doi: 10.3389/fnhum.2016.00629
Nakamura, K., Christine, C. W., Starr, P. A., and Marks, W. J. Jr. (2007). Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov. Disord. 22, 619–626. doi: 10.1002/mds.21300
Nakano, K. (2000). Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 22, S5–S16. doi: 10.1016/s0387-7604(00)00139-x
Okun, M. S., Fernandez, H. H., Wu, S. S., Kirsch-Darrow, L., Bowers, D., Bova, F., et al. (2009). Cognition and mood in Parkinson disease in STN versus GPi DBS: the COMPARE Trial. Ann. Neurol. 65, 586–595. doi: 10.1002/ana.21596
Okun, M. S., Wu, S. S., Fayad, S., Ward, H., Bowers, D., Rosado, C., et al. (2014). Acute and chronic mood and apathy outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One 9:e1141404. doi: 10.1371/journal.pone.0114140
Parent, A., and Hazrati, L. N. (1995a). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 20, 91–127. doi: 10.1016/0165-0173(94)00007-c
Parent, A., and Hazrati, L. N. (1995b). Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 20, 128–154. doi: 10.1016/0165-0173(94)00008-d
Paschali, A., Constantoyannis, C., Angelatou, F., and Vassilakos, P. (2013). Perfusion brain SPECT in assessing motor improvement after deep brain stimulation in Parkinson’s disease. Acta Neurochir. 155, 497–505. doi: 10.1007/s00701-012-1610-z
Peng-Chen, Z., Morishita, T., Vaillancourt, D., Favilla, C., Foote, K. D., Okun, M. S., et al. (2013). Unilateral thalamic deep brain stimulation in essential tremor demonstrates long-term ipsilateral effects. Parkinsonism Relat. Disord. 19, 1113–1117. doi: 10.1016/j.parkreldis.2013.08.001
Rodrigues, J. P., Walters, S. E., Watson, P., Stell, R., and Mastaglia, F. L. (2007). Globus pallidus stimulation in advanced Parkinson’s disease. J. Clin. Neurosci. 14, 208–215. doi: 10.1016/j.jocn.2005.11.023
Rothlind, J. C., Cockshott, R. W., Starr, P. A., and Marks, W. J. (2007). Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J. Int. Neuropsychol. Soc. 13, 68–79. doi: 10.1017/S1355617707070105
Samii, A., Kelly, V. E., Slimp, J. C., Shumway-Cook, A., and Goodkin, R. (2007). Staged unilateral versus bilateral subthalamic nucleus stimulator implantation in Parkinson disease. Mov. Disord. 22, 1476–1481. doi: 10.1002/mds.21554
Schuepbach, W. M., Rau, J., Knudsen, K., Volkmann, J., Krack, P., Timmermann, L., et al. (2013). Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 368, 610–622. doi: 10.1056/NEJMoa1205158
Schupbach, W. M., Chastan, N., Welter, M. L., Houeto, J. L., Mesnage, V., Bonnet, A. M., et al. (2005). Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J. Neurol. Neurosurg. Psychiatry 76, 1640–1644. doi: 10.1136/jnnp.2005.063206
Sestini, S., Ramat, S., Formiconi, A. R., Ammannati, F., Sorbi, S., and Pupi, A. (2005). Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: a 4-year follow-up study with rCBF SPECT. J. Nucl. Med. 46, 1444–1454.
Sestini, S., Scotto, di Luzio, A., Ammannati, F., De Cristofaro, M. T., Passeri, A., et al. (2002). Changes in regional cerebral blood flow caused by deep-brain stimulation of the subthalamic nucleus in Parkinson’s disease. J. Nucl. Med. 43, 725–732.
Simuni, T., Jaggi, J. L., Mulholland, H., Hurtig, H. I., Colcher, A., Siderowf, A. D., et al. (2002). Bilateral stimulation of the subthalamic nucleus in patients with Parkinson disease: a study of efficacy and safety. J. Neurosurg. 96, 666–672. doi: 10.3171/jns.2002.96.4.0666
Tabbal, S. D., Ushe, M., Mink, J. W., Revilla, F. J., Wernle, A. R., Hong, M., et al. (2008). Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp. Neurol. 211, 234–242. doi: 10.1016/j.expneurol.2008.01.024
Takeuchi, R. (2005). Atlas of the Constant Regions of Interest on the Brain Anatomically Standardized by SPM. La Mesa, CA: Kyouritsu.
Tanei, T., Kajita, Y., Kaneoke, Y., Takebayashi, S., Nakatsubo, D., and Wakabayashi, T. (2009a). Staged bilateral deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Acta Neurochir. 151, 589–594. doi: 10.1007/s00701-009-0293-6
Tanei, T., Kajita, Y., Nihashi, T., Kaneoke, Y., Takebayashi, S., Nakatsubo, D., et al. (2009b). Changes in regional blood flow induced by unilateral subthalamic nucleus stimulation in patients with Parkinson’s disease. Neurol. Med. Chir. 49, 507–513. doi: 10.2176/nmc.49.507
Thobois, S., Metens, P., Guenot, M., Hermier, M., Mollion, H., Bouvard, M., et al. (2002). Subthalamic nucleus stimulation in Parkinson’s disease: clinical evaluation of 18 patients. J. Neurol. 249, 529–534. doi: 10.1007/s004150200059
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653.
van Laere, K., van der Linden, C., Santens, P., Vandewalle, V., Caemaert, J., Ir, P. L., et al. (2000). 99Tc(m)-ECD SPET perfusion changes by internal pallidum stimulation in Parkinson’s disease. Nucl. Med. Commun. 21, 1103–1112. doi: 10.1097/00006231-200012000-00003
Vingerhoets, G., van der Linden, C., Lannoo, E., Vandewalle, V., Caemaert, J., Wolters, M., et al. (1999). Cognitive outcome after unilateral pallidal stimulation in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 66, 297–304. doi: 10.1136/jnnp.66.3.297
Volkmann, J., Albanese, A., Kulisevsky, J., Tornqvist, A. L., Houeto, J. L., Pidoux, B., et al. (2009). Long-term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson’s disease. Mov. Disord. 24, 1154–1161. doi: 10.1002/mds.22496
Volkmann, J., Allert, N., Voges, J., Weiss, P. H., Freund, H. J., and Sturm, V. (2001). Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology 56, 548–551. doi: 10.1212/wnl.56.4.548
Volkmann, J., Herzog, J., Kopper, F., and Deuschl, G. (2002). Introduction to the programming of deep brain stimulators. Mov. Disord. 17, S181–S187. doi: 10.1002/mds.10162
Walker, H. C., Watts, R. L., Guthrie, S., Wang, D., and Guthrie, B. L. (2009). Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson’s disease at 1 year. Neurosurgery 65, 302–309. doi: 10.1227/01.NEU.0000349764.34211.74
Zahodne, L. B., Okun, M. S., Foote, K. D., Fernandez, H. H., Rodriguez, R. L., Wu, S. S., et al. (2009). Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J. Neurol. 256, 1321–1329. doi: 10.1007/s00415-009-5121-7
Keywords: Parkinson’s disease (PD), unilateral, deep brain stimuation, globus pallidum internus (Gpi), subthalamic nucleus (STN), ipsilateral, axial, single photon emission computed tomography (SPECT)
Citation: Hayashi Y, Mishima T, Fujioka S, Morishita T, Inoue T, Nagamachi S and Tsuboi Y (2022) Unilateral GPi-DBS Improves Ipsilateral and Axial Motor Symptoms in Parkinson’s Disease as Evidenced by a Brain Perfusion Single Photon Emission Computed Tomography Study. Front. Hum. Neurosci. 16:888701. doi: 10.3389/fnhum.2022.888701
Received: 03 March 2022; Accepted: 07 April 2022;
Published: 11 May 2022.
Edited by:
Adolfo Ramirez-Zamora, University of Florida, United StatesReviewed by:
Jun Yu, University of Florida, United StatesCopyright © 2022 Hayashi, Mishima, Fujioka, Morishita, Inoue, Nagamachi and Tsuboi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takayasu Mishima, bWlzaGltYTEwMDZAZnVrdW9rYS11LmFjLmpw; Yoshio Tsuboi, dHN1Ym9pQGNpcy5mdWt1b2thLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.