- 1Department of Psychology, University of Tehran, Tehran, Iran
- 2Department of Cognitive Psychology, Institute for Cognitive Science Studies, Tehran, Iran
- 3Convergent Technologies Research Center, University of Tehran, Tehran, Iran
- 4Department of Neuropsychology, Faculty of Psychology, Institute of Cognitive Neuroscience, Ruhr-University Bochum, Bochum, Germany
- 5Department of Cognitive Sciences, University of Messina, Messina, Italy
- 6Unité de Recherche Clinique Intersectorielle en Psychiatrie Pierre Deniker, Centre Hospitalier Henri Laborit, Poitiers, France
- 7University Poitiers & CHU Poitiers, INSERM U1084, Laboratoire Expérimental et Clinique en Neurosciences, Poitiers, France
Background: Cognitive impairments are prevalent in patients with unipolar and bipolar depressive disorder (UDD and BDD, respectively). Considering the fact assessing cognitive functions is increasingly feasible for clinicians and researchers, targeting these problems in treatment and using them at baseline as predictors of response to treatment can be very informative.
Method: In a naturalistic, retrospective study, data from 120 patients (Mean age: 33.58) with UDD (n = 56) and BDD (n = 64) were analyzed. Patients received 20 sessions of bilateral rTMS (10 Hz over LDLPFC and 1 HZ over RDLPFC) and were assessed regarding their depressive symptoms, sustained attention, working memory, and executive functions, using the Beck Depression Inventory (BDI-II) and Neuropsychological Test Automated Battery Cambridge, at baseline and after the end of rTMS treatment course. Generalized estimating equations (GEE) and logistic regression were used as the main statistical methods to test the hypotheses.
Results: Fifty-three percentage of all patients (n = 64) responded to treatment. In particular, 53.1% of UDD patients (n = 34) and 46.9% of BDD patients (n = 30) responded to treatment. Bilateral rTMS improved all cognitive functions (attention, working memory, and executive function) except for visual memory and resulted in more modulations in the working memory of UDD compared to BDD patients. More improvements in working memory were observed in responded patients and visual memory, age, and sex were determined as treatment response predictors. Working memory, visual memory, and age were identified as treatment response predictors in BDD and UDD patients, respectively.
Conclusion: Bilateral rTMS improved cold cognition and depressive symptoms in UDD and BDD patients, possibly by altering cognitive control mechanisms (top-down), and processing negative emotional bias.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) has been increasingly used as a therapeutic solution for depression for more than a decade and evidence regarding its efficacy has been reported in several meta-analyses (Brunoni et al., 2017; Cao et al., 2018; Mutz et al., 2018). Worth noting is that the United States Food and Drug Administration (FDA) has approved certain rTMS protocols to treat major depressive disorder (MDD) (Berlim et al., 2017; Hung et al., 2020), however, the question remains on how to improve response to this treatment by optimizing stimulation protocols (Cash et al., 2017; Pitkänen et al., 2017; Miron et al., 2021) and developing markers to enable prediction of its therapeutic effects on patients. In that light, predictors of treatment response to rTMS, potentially identifying patients who will likely respond to the intervention, play an essential role in the pursuit to deliver personalized rTMS treatment.
In a recent investigation, response rates of MDD patients have been reported to be somewhere between 58 and 83 percent (Sackeim et al., 2020), while it has been reported to be 41% in a sample of patients with bipolar depressive disorder (Rostami et al., 2017). Previously, studies on predictors of response to rTMS treatment have detected several markers such as demographic (Fregni et al., 2006; Aguirre et al., 2011; Pallanti et al., 2012; Kedzior et al., 2014), clinical (Brakemeier et al., 2008; Grammer et al., 2015; Fitzgerald et al., 2016; Rostami et al., 2017; Trevizol et al., 2020), electrophysiological (Arns et al., 2010; Narushima et al., 2010; Micoulaud-Franchi et al., 2012; Kazemi et al., 2016, 2018), and neuroimaging (Avissar et al., 2017; Corlier et al., 2019; Williams et al., 2021).
Although cognitive functions have been considered as predictors of response in the context of other interventions (Park et al., 2018) its potential as a predictor of response to rTMS, to the best of our knowledge, have thus far been considered by only two studies (Furtado et al., 2012; Hoy et al., 2012); identifying visuo-spatial working memory as predictor (Hoy et al., 2012). Apart from the investigations which have reported significant modulations of rTMS on cognitive functions in healthy populations (Kazemi et al., 2020; Patel et al., 2020; Rostami et al., 2021), various studies have considered cognitive functions as predictors of response to antidepressants. For example, early changes in facial emotion recognition has been identified as a predictor of response to antidepressants in a systematic review (Park et al., 2018). Furthermore, performance level in a verbal memory task has also been proposed as a cognitive marker (Spronk et al., 2011). However, the results are heterogeneous and some studies did not report a significant relationship between cognitive functions and treatment response (Groot et al., 1996; Doraiswamy et al., 2003; Alexopoulos et al., 2007; Lin et al., 2014; Bingham et al., 2015), and others did (Potter et al., 2004; Story et al., 2008; Bruder et al., 2014). Nevertheless, executive functions are recognized as a potential cognitive marker in most studies (Groves et al., 2018). Given that some cognitive functions have already been identified as predictors of treatment response to other modalities of interventions (Park et al., 2018) to, there is a rationale to investigate the possibility of considering them as a potential cognitive predictor of rTMS treatment response. Thus a group of cognitive functions, commonly called cold cognition, referring specifically to an absence of any emotional influence during the processing of the information, i.e., with emotionally neutral stimuli and without motivational relevance (Roiser and Sahakian, 2013).
In the study of Hoy et al. (2012), subjects received four different TMS protocols collectively. However, since the rTMS target region can significantly impact the outcome in question, focusing on a single protocol seems necessary to reliably draw conclusions on the involvement of a specific brain region in the variable of interest. Although a few studies have investigated the efficacy of rTMS to improve cognitive functioning BDD patients (Hu et al., 2016; Myczkowski et al., 2018; Yang et al., 2019), one aim of the current work has been on the potential of baseline cognitive functioning to predict the response to rTMS treatment in both BDD and UDD patients, which has been less explored in general, even less in the context of rTMS treatment, and least in the context of UDD and BDD. Therefore, using a bilateral rTMS protocol, we aimed to (1) examine and compare rTMS efficacy in two groups of patients (BDD & UDD) and (2) identify cognitive markers of treatment response to rTMS in the two subgroups and in all the depressed patients as a whole.
Materials and Methods
Participants
In total, out of 135 MDD patients (71 female), 120 (Mean age = 33.58) who received rTMS treatment in Atieh Clinical Neuroscience Center, Tehran, Iran, were analyzed (56 UDD and 64 BDD), in a retrospective naturalistic investigation. The diagnosis of depression was made by a psychiatrist according to DSM-V criteria through clinical interviews. Patients with BDI-II scores equal and above 18 were included in the study. All patients had received 20 sessions of rTMS treatment and provided informed written consent forms.
rTMS Treatment Parameters
The 20 sessions of rTMS was performed by a Neuro MS stimulator with a 70 mm, air-cooled figure of 8 coil (Neurosoft, Russia). A bilateral stimulation protocol was used for all the patients. In every session, rTMS was applied on the right DLPFC and the left DLPFC. Resting motor threshold (RMT) was defined as the lowest stimulation intensity required for a visible muscle reflex in Abductor Policies Brevis (APB) after a minimum of 5 out of 10 single TMS pulses. The 10-HZ rTMS protocol on left DLPFC consisted of 75 trains each of which lasting for 5 s, inter-train intervals of 10 s, and stimulation intensity of 110% of the individual RMT, meaning 3,750 pulses in each session (75,000 total pulses during the course of treatment). The right DLPFC stimulation protocol however included 1 Hz 10 s trains, with an inter-train interval of 2 s. A total of 150 trains were delivered at an intensity of 120% of individual RMT, making it 1,500 pulses in each treatment session (30,000 pulses in total).
Outcome Measures
The Beck Depression Inventory—Second Edition (BDI-II) was used to assess the primary outcomes. The criterion for response to treatment was considered minimum 50 percent decrease in BDI-II scores after the end of the treatment course, and remission was defined as BDI-II scores of <8 at the end of the treatment course. Cambridge Neuropsychological Test Automated Battery (CANTAB®, Cambridge Cognition Ltd., United Kingdom) has been applied for cognitive assessments. More details regarding CANTAB tasks and specifications can be found in the literature (Lawrence et al., 1996), however, a brief description will follow.
Rapid Visual Information Processing
RVIP is a visual task used for evaluating sustained attention (Hilti et al., 2010). The variables in RVIP were as follows: (1) A′ which quantifies the subject's tendency to respond, (2) B" which is a measure of the response tendency of the subject, (3) mean latency which is simply the average time it took the participant to respond correctly, (4) probability of hits which is calculated by dividing total hits over total misses plus total hits, (5) total correct rejections being the number of times when the examinee does not respond when they are not supposed to respond, and (6) total hits which is simply the number of correct responses.
Spatial Working Memory
SWM is a self-ordered search task evaluating non-verbal working memory (Owen et al., 1990, 1995). There are three types of errors in this task: (I) Within errors (Searching a box that has already been found to be empty; (II) between errors (revisiting a box where a token had previously been found); and (III) Double errors that contain both a within error and between error. There are two significant indices: (1) strategy utilization which is the number of search sequences starting with a novel box in both 6- and 8-box problems, and (2) errors in total, which is calculated based on the between errors, within errors, and double errors of particular box problems (i.e., between errors + within errors–double errors). This test evaluates working memory and utilization of search strategies and is a relatively accurate tool for measuring working memory, frontal lobe function, and executive functions (Oi et al., 2017; Beattie et al., 2018).
Delayed Matching to Sample
This task evaluates the capacity to identify complicated visual patterns after different durations of delay between stimuli (e.g., 0, 4, or 12 s) (Cambridge Cognition Ltd., 2012; Toornstra et al., 2020). In another version of the DMS test, participants are required to respond to one of 1, 2, 4, or 8 peripheral shapes that match the one present on the center of the screen simultaneously. To perform a recognition memory test, participants must memorize a visual pattern and then determine which of the four presented patterns is identical to the memorized one (the delay between the target stimulus and response differ between 0, 4, 8, and 12 s).
One Touch Stockings of Cambridge
OTS is an executive function test to assess spatial planning and working memory, developed based on the Tower of London test (Shallice, 1982; Sahakian et al., 1988; Owen et al., 1990). In this test, the aim is to determine how many moves are required to make a display look like the other (each moved ball equals one move). The outcome measures include “Problems solved on first choice,” “Mean choices to correct,” “Mean latency (speed of response) to first choice,” and “Mean latency to correct.” Each of these measures may be calculated for all problems or problems with a specified number of moves (1-move to 5 or 6).
Statistical Analysis
All data analyses were performed using the SPSS software, version 19.0 (IBM, SPSS, Inc., Chicago, IL), with p-values below 0.05 as statistically significant. Considering the fact that no outliers were present in the data and based on relevant statistical tests, data distribution was considered normal thus parametric tests were used all throughout the analyses. To describe CANTAB scores, means ± SD were reported. To investigate the overall efficacy of rTMS treatment and pre and post-treatment differences (before and after 20 rTMS sessions), paired t-tests were first used for all patients irrespective of their specific diagnosis, and then used separately for UDD and BDD patients. Furthermore, a marginal model using generalized estimating equations (GEE) was used to investigate the effect of depression type (i.e., UDD and BDD) on performance in CANTAB tasks (model 1; 2 groups). A second marginal model using GEE was also used to test the effect of depression types on cognitive functions as a result of the intervention, by considering treatment response or remission (model 2; 4 groups, i.e., UDD responders, UDD non-responders, BDD responders, BDD non-responders, UDD remitters, UDD non-remitters, BDD remitters, and BDD non-remitters). All post versus pre comparisons were performed separately in each test for each variable in order to avoid the problem of multiple comparisons. For example, A′ scores of RVP have been considered as the response variable and the effect of time (pre and post treatment) have been investigated in the form of a covariate by selecting the independent working correlation matrix and a linear model in the in the GEE analysis.
Next, to investigate potential demographic and/or cognitive rTMS response predictors in responded vs. non-responded and remitted vs. non-remitted patients among all patients, UDD patients, and BDD patients, binary logistic regressions with a stepwise backward selection of variables were performed. Demographic variables, namely age and sex, and the related CANTAB test scores were considered as independent variables. A binary treatment response classification was considered as outcome meaning that “responder” and “non-responder” data were represented by one and zero. Similarly, a binary response classification was used as an outcome variable meaning that the data associated with “remitted” vs. “non-remitted” groups as one and zero, respectively. Due to internal correlations among test scores, separate regression models were used for each CANTAB test. Also, response and remission values were used as dependent variables separately in each model for every individual CANTAB test.
Results
Two Supplementary Materials containing figures and statistical values regarding all the cognitive assessments are provided in the supplementary section of the paper.
Data Overview
From the original sample of patients, 15 cases were excluded because they had not completed 20 sessions of rTMS treatment, thus 120 patients (age: 33.58 ± 11.1) were considered for further analysis, consisting of 71 women (age: 33.18 ± 10.19) and 49 men (age: 34.16 ± 12.31). Out of the 120 patients, 56 had a diagnosis of UDD and 64 of BDD. The treatment was well-tolerated in all patients. Aside from a mild headache after the rTMS session, usually disappearing within 1–2 days after, no adverse effects were reported. After the completion of the treatment course, 64 patients (53%) experienced at least 50% reduction in depressive symptoms (responded), and 41 patients (34%) achieved remission (Supplementary Table 1). More specifically, of all patients who responded, 34 were UDD (53.1%), and 30 (46.9%) were BDD, and of all patients who experienced remission, 22 (53.7%) were UDD, and 19 (46.3%) were BDD. The mean and standard deviation of pre and post treatment CANTAB scores are presented in Supplementary Table 1.
Effectiveness of rTMS Treatment
Overall Efficacy
The first aim was to see if there were differences between pre and post stimulation performance in RVP, SWM, DMS, and OTS. The results showed a significant increase in RVP subscales including “A′,” “B”,” “probability of hit,” “Total correct rejection,” and “Total hits” if all patients were considered as one group. Moreover, an overall significant decrease in “Mean latency” was observed in the combined group and UDD patients. Furthermore, a significant decrease in the “Probability of false alarms” and “Total false alarms” was also observed. Among all the SWM subscales, “Within-errors,” “Between-errors,” “Total errors,” “First response time (4 boxes),” “Last response time (4 boxes),” “Last response time (6 boxes),” and “Last response time (8 boxes)” significantly decreased after treatment. In DSM subscales, only “Mean correct latency (simultaneous)” showed significant decrease both in the whole group as well as in BDD patients. Finally, “Mean latency to correct,” “Mean choices to correct,” and “Mean latency to first choice” among OTS subscales significantly decreased and “Problems solved on first choice” showed significant increase in all study groups. The trends of changes in all the scores and subscores of the cognitive measures are presented in Supplementary Figures 1-7.
Depression Type
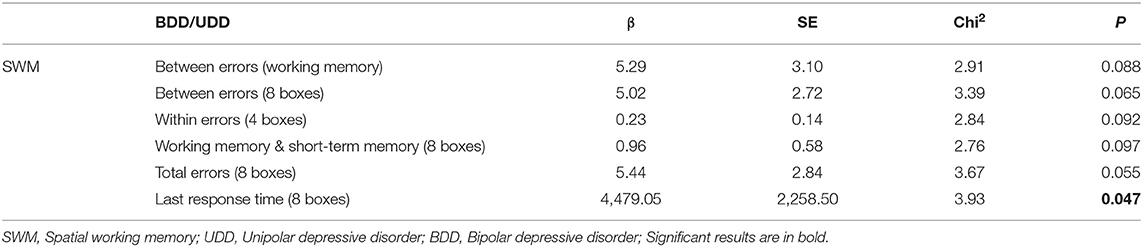
Marginal model 1 was used to compare the efficacy of rTMS in BDD and UDD patients and the results showed that among SWM measures, “Last response time (8 boxes)” was ~51% higher in BDD compared to UDD patients (Table 1).
Marginal model 2 was used to test if depression type affects performance in CANTAB scores based on response (yes, no) or remission (yes, no). The dependent variable in each model was each subset of test scores with time as a covariate in the model. Interaction of time and group can show potential differences between the effects of rTMS in UDD and BDD patients during the course of the treatment, i.e., pre and post. The significant interactions are provided in Table 1.
In marginal model 2, the interaction effects of time were calculated in UDD responded vs. BDD responded, UDD responded vs. UDD non-responded patients, and BDD responded vs. BDD non-responded patients (Tables 2–4). Among all responded patients, UDD patients showed a greater decrease in B" as well as in SWM subscales including “Between-errors (working memory)”, “Between- errors (4 boxes),” “Between-errors (8 boxes),” “Total errors (between errors + within errors—double errors),” “Total errors (4 boxes),” “Total errors (8 boxes),” and “Last response time (8 boxes)” compared to BDD patients. The data for each variable is presented in Table 2. Furthermore, UDD responded patients exhibited 89 percent decrease in “Between-errors (4 boxes),” among SWM subscales, whereas “Between-errors (6 boxes)” and “Total errors (6 boxes)” had a 99% increase in UDD non-responded patients. This indicates that UDD responded patients showed significant improvements in working memory compared to UDD non-responded patients. Lastly, BDD responded patients showed a significant increase in “Between errors (working memory),” and “Total errors (working memory + short-term memory)” compared to BDD non-responded patients. Among OTS subscales, BDD responded patients showed a significant increase in “Problems solved on first choice” compared to BDD non-responded patients, indicating that executive functions improved more in BDD responded compared to BDD non-responded patients.

Table 2. Results of GEE analysis regarding the effects of depression types and response to treatment on CANTAB scores (UDD responders vs. BDD responders).
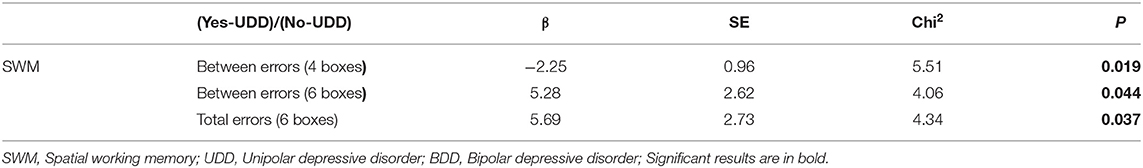
Table 3. Results of GEE analysis regarding the effects of depression types and response to treatment on CANTAB scores (UDD responders vs. UDD Non-responders).

Table 4. Results of GEE analysis regarding the effects of depression types and response to treatment on CANTAB scores (BDD responders vs. BDD Non-responders).
Predictors of rTMS Treatment
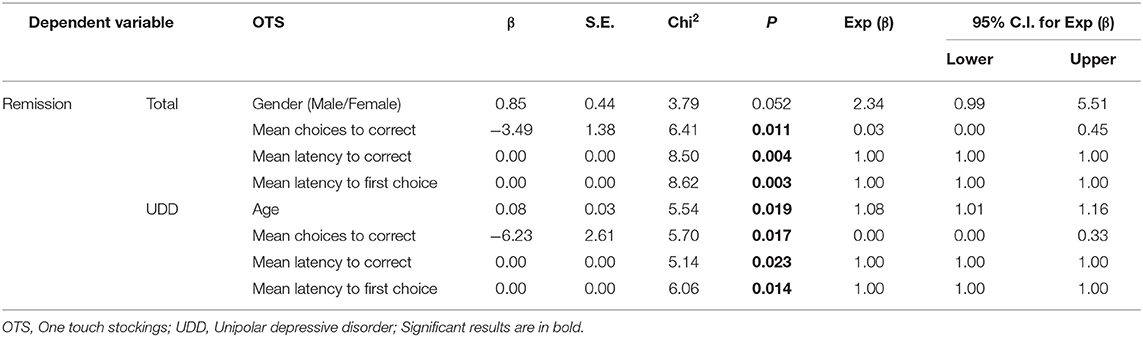
Logistic regression (Backward Wald method) was used to find predictors of treatment response or remission. Age and sex were entered into each model as covariates. First, the model was performed on all patients as a whole and then separately on UDD and BDD groups. The dependent variable was either remission or response, and CANTAB scores were considered as independent variables separately. For example, in DMS, which included 13 sub-scores thus 13 independent variables plus two covariates, namely age and sex were entered into the model. The results of this statistical model is reported in Tables 5–7.
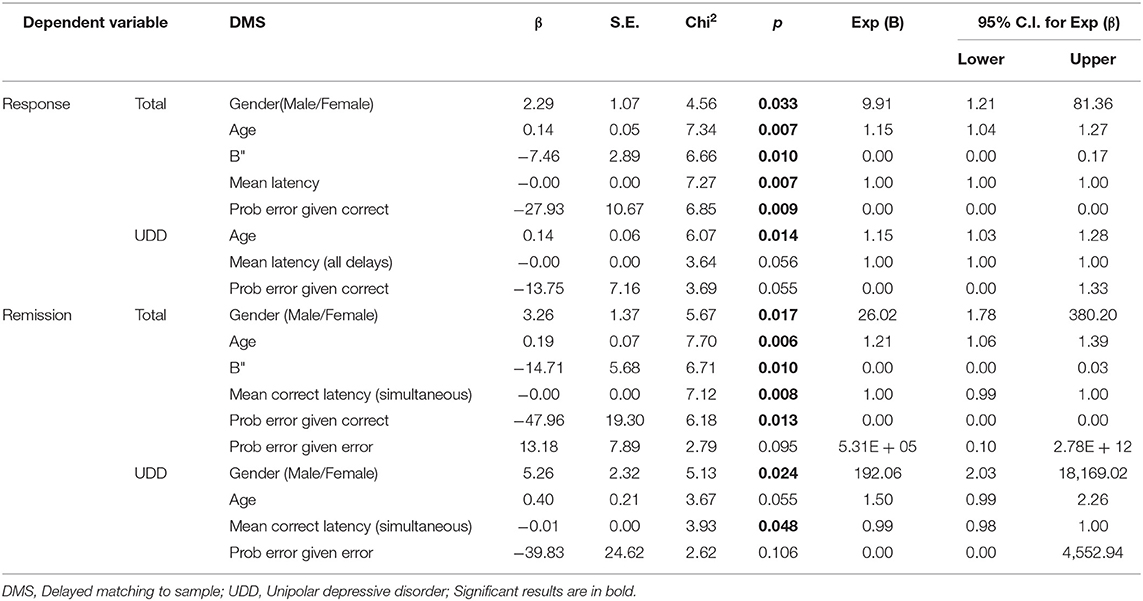
Table 5. Binary logistic regression analysis: demographic and DMS predictors of response or remission.
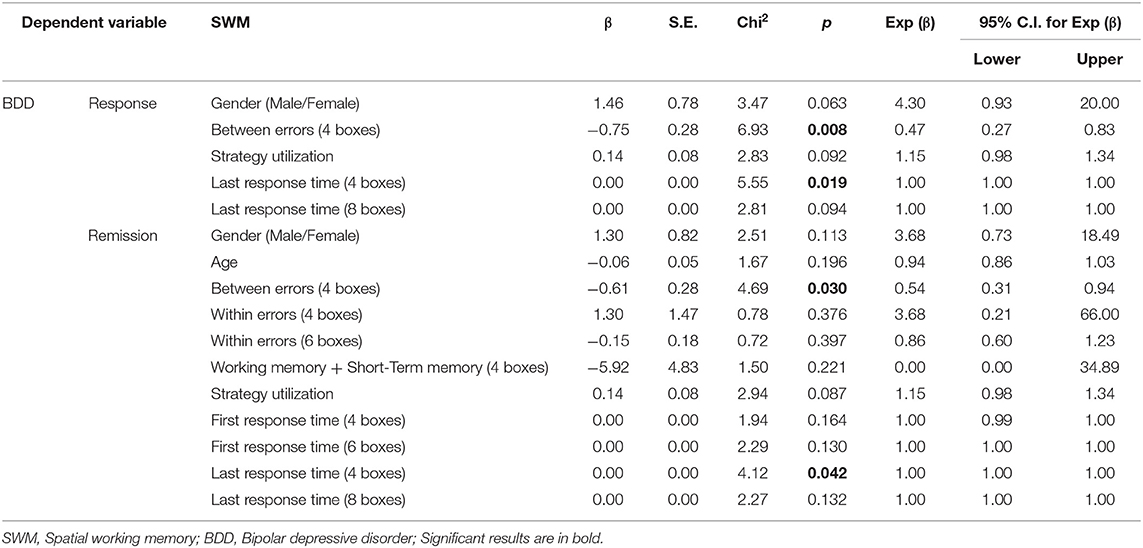
Table 6. Binary logistic regression analysis: demographic and SWM predictors of response or remission.
For DMS scores, when response was used as an independent variable, the odds of the “B” (β = −7.5, p ~ 0.010), “Mean latency” (β = −0.003, p ~ 0.007), and “Prob error given correct” (β = −27.9, p ~ 0.009) were lower among responded compared to non-responded patients as a whole (i.e., without dividing them into BDD and UDD).Also, males were 9.91 times more likely to respond than females. If age increases by one, the odds ratio increases by 15 percent. In addition, we observed that in UDD patients, the odds ratio of “Mean latency (all delays) ” (β = −0.002, p ~ 0.056), and “Prob error given correct” (β = −13.7, p ~ 0.055) were lower among responded compared to non-responded patients in a marginally significant manner (p < 0.1). Also, if age increases by one, the odds increases by 15 percent.
For DMS scores, when remission was used as an independent variable, the odds ratio of the “B"” (β = −14.7, p ~ 0.010), “Mean correct latency (simultaneous)” (β = −0.005, p ~ 0.008) and “Prob error given correct” (β = −48.0, p ~ 0.013) were lower among remitted compared to non-remitted patients as a whole. Furthermore, males were 26 times more likely to reach remission compared to females. If age increases by one, the odds ratio increases by 21 percent. Moreover, in UDD patients, the odds ratio of “Mean correct latency (simultaneous)” was lower among remitted compared to non-remitted patients (β = −0.01, p ~ 0.048) and males were more likely to reach remission than females.
For SWM scores, when response to treatment was used as an independent variable, in BDD patient, the odds ratio of the “Between-errors (4 boxes)” (β = −0.75, p ~ 0.008) and “Last response time (4 boxes)” (β = 0.0003, p ~ 0.019) were lower among responded compared to non-responded patients. In BDD patients, when considering SWM scores with remission as the independent variable, the odds ratio of the “Between-errors (4 boxes)” (β = −0.61, p ~ 0.030) and “Last response time” (β = −0.0004, p ~ 0.042) were lower among remitted compared to non-remitted patients.
Considering OTS scores with treatment remission as the independent variable, the odds of the “Mean choices to correct” (β = −3.5, p ~ 0.011), “Mean latency to correct” (β = 0.0007, p ~ 0.004), and “Mean latency to first choice” (β = −0.0008, p ~ 0.003) were lower among remitted compared to non-remitted patients as a whole. Lastly, in UDD patients, the odds of the “Mean choices to correct” (β = −6.23, p ~ 0.017), “Mean latency to correct” (β = 0.001, p ~ 0.023), and “Mean latency to first choice” (−0.001, p ~ 0.014) were lower among remitted compared to non-remitted patients. If age increases by one, the odds ratio increases by 8%.
Discussion
The results of this study showed no significant difference in the efficacy of bilateral rTMS on cognitive function in unipolar and bipolar depression. Considering response rates (responded vs. non-responded), the efficacy of rTMS in improving working memory in UDD and BDD patients was significantly different. Considering remission rates (remitted vs. non-remitted), the efficacy of rTMS in improving working memory and visual memory was different among UDD and BDD patients as well. Cognitive predictors of response and remission in both patient groups (UDD and BDD) were determined. Taking all the patients as a whole, visual memory, age, and sex were determined as the predictors of response to rTMS. However, visual memory and age in UDD patients and working memory performance in BDD patients were identified as predictors of treatment response. Regarding remission, when considering all patients as one group, visual memory, age, sex, and executive functions were identified as remission predictors. Executive functions and age; and working memory were identified as remission predictors in UDD and BDD patients, respectively.
rTMS Efficacy on Cognitive Functions
rTMS Outcome Regarding Cognitive Functioning of UDD and BDD Patients
Our findings showed that bilateral rTMS improved cognitive functioning in general and sustained attention, working memory, and executive functions in particular, in depressed patients. Taking depression types into account, a significant difference was observed between the two types of depression in working memory performance, before and after treatment. UDD patients showed more improvement in working memory than BDD patients, but no significant difference was observed in other cognitive functions. It should be noted that in the absence of a more sophisticated control condition, it would not be possible to dissociate the effect of the improvement in cognitive function on mood, or vice versa thus reliably concluding that one is the cause and the other the effect.
However, previous studies which investigated efficacy of rTMS in improving cognitive functions in both clinical and healthy population report seemingly diverging results. A meta-analysis including 18 studies, on the effects of rTMS on cognitive functions in MDD patients reported no significant effect on cognitive functions (Martin et al., 2017) in accordance with another meta-analysis on healthy population (Patel et al., 2020). However, some promising results have also been observed (Patel et al., 2020) suggesting that high-frequency rTMS of DLPFC could improve executive functioning, and low-frequency rTMS could enhance episodic memory and visual perception. However, another meta-analysis showed that rTMS improved working memory in both healthy and clinical populations in all indexes and tDCS only improved reaction time (Brunoni and Vanderhasselt, 2014).
Similar to the context of rTMS effects on clinical symptoms, it is possible to attribute the variability in rTMS outcomes regarding cognitive symptoms to non-homogeneity in stimulation parameters in different studies since for example, frequency of stimulation has been shown to play a significant role (Patel et al., 2020). Thus, it is safe to assume that other stimulation parameters such as the total number of pulses or the intensity of the stimulation can potentially impact the rTMS outcome. Furthermore, number of pulses in each session and total number of sessions are other points of difference with similar studies in this context, that may explain our different results (Koren et al., 2001; Mottaghy et al., 2002; Rami et al., 2003; Huang et al., 2004; Vanderhasselt et al., 2007; Viggiano et al., 2008; Barr et al., 2009; Upton et al., 2010; Kim et al., 2012; Gaudeau-Bosma et al., 2013; Fried et al., 2014; Pearce et al., 2014). In the current study, a relatively higher number of pulses were administered compared to the studies included in both of the meta-analyses mentioned previously and it is noteworthy that a previous study on the effects of different durations of rTMS on cortical inhibition and excitability showed that longer sessions with a greater number of pulses could result in more pronounced changes in cortical inhibition followed by rTMS (de Jesus et al., 2014), which can significantly contribute to a significant modulation in the behavior or cognitive function in question. It seems that concerns regarding rTMS safety parameters generally lead many researchers to use protocols with minimum suggested values in each stimulation parameter (e.g., minimum number of total pulses per session, minimum number of sessions, and longer inter-train intervals) although the latest clinical safety guideline emphasizes that with a proper use of RMT, it is not necessary to use the suggested parameters in the previous two guidelines (Rossi et al., 2021). Furthermore, there is also evidence regarding the possibility of using shorter inter-train intervals (Cash et al., 2017) which is also a potential candidate to maximize the possibility of observing an outcome. There are studies that have corroborated this finding in different clinical population (e.g., stroke) (Ke et al., 2020) and also the results of two of our previous investigations using this protocol revealed a very acceptable response rate (Kazemi et al., 2016, 2018). The stimulation intensity is another parameter that has a decisive role in the response rate to rTMS in patients with depression (Fitzgerald et al., 2016) and it has been shown that higher stimulation intensities are associated with better treatment response (Padberg et al., 2002; Fitzgerald et al., 2016). Altogether, the use of a higher stimulation intensity and higher number of pulses in the current study could have contributed to observed effects of rTMS effects on cognitive function.
rTMS Outcome Regarding Cognitive Functioning in Responders vs. Non-responders
In general, responded patients showed more improvements in cognitive functioning than non-responded patients. In particular, more improvements were observed in working memory performance in responded BDD compared to responded UDD patients (i.e., bipolar responded vs. unipolar responded). These results are in line with previous studies (Bailey et al., 2018) according to which, MDD patients who responded to treatment showed significant improvements in working memory compared to non-responding patients (Bailey et al., 2018). There is evidence regarding more pronounced reduction in DLPFC activity in MDD compared to BDD patients while performing a working memory task (Zhu et al., 2018; Manelis et al., 2020) which can be one of the reasons behind the finding that BDD patients showed more improvements in working memory in our study. Moreover, there are reports showing that UDD patients exhibit more gray matter reduction in DLPFC in both hemispheres compared to BDD patients (de Azevedo-Marques Périco et al., 2011) thus possibly contributing to the relatively less improvement in working memory in UDD patients observed in this study. This pattern seems consistent with the findings of another study in which BDD patients showed more improvements in working memory compared to UDD patients (Xu et al., 2012).
Predictors of Treatment Response and Remission
Treatment Response Prediction in UDD and BDD Patients
Compared to studies using hot cognition as predictor of treatment response to anti-depressants, fewer studies have focused on cold cognition (~26 vs. 3 papers) (Seeberg et al., 2018). In the context of rTMS treatment, to the best of our knowledge, three studies focused on cognitive predictors (hot cognition), among which one has only used cognitive assessments (Hoy et al., 2012) and others also took advantage of the fMRI methodology (Furtado et al., 2012; Hernández-Ribas et al., 2013). In the first study, involving 137 participants, immediate visual-spatial memory was considered as predictor of response to rTMS (Hoy et al., 2012). The second study found that while performing an executive function task (i.e., word generation), responders show lower activity in perigenual, medial OFC and middle frontal cortices as well as higher activity in ventral- caudal putamen in baseline (Hernández-Ribas et al., 2013). Finally, the third study found no significant differences between responders and non-responders in cognitive performance. All of these studies were performed on MDD patients, but in the study of Hoy et al., 4 out of 137 and in the study of Furtado et al., 6 out of 21 patients were diagnosed with bipolar disorder. Furthermore, different numbers of protocols were used in each study (Furtado et al., 2012; Hoy et al., 2012; Hernández-Ribas et al., 2013). Regardless of depression types, we found visual memory, age, and sex to act as predictors of response to rTMS. This is in line with the previous findings using cognitive performance measures (Hoy et al., 2012) and a study used combined approach (Hernández-Ribas et al., 2013).
When taking depression types into account, we observed some differences that previous studies have not reported, possibly due to smaller sample sizes. While there is evidence that BDD patients have difficulties in both cold and hot cognition (Roiser et al., 2009), to the best of our knowledge, most studies have focused on cold cognition in UDD patients, and thus far, there has been no study on BDD patients in that respect (Seeberg et al., 2018). The results of the current investigation showed that working memory; and visual memory and age could be used as predictors of response to rTMS in patients with BDD and UDD, respectively.
Visual Memory
In general, we found that older men with better visual memory performance responded better to treatment. Also, individuals with UDD with a better performance in visual memory tasks showed better response to the treatment. Furthermore, older men who had better scores in visual memory tests at baseline were more likely to reach remission. By older age, middle-age range and not elderly is meant. However, it is noteworthy that the older the age, the more critical the role of visual memory would be in predicting treatment response. Based on the neuroimaging evidence, different areas of the brain are activated in older vs. younger subjects while performing visual memory tasks (Bennett et al., 2001). Although the DLPFC and other frontal areas are not among the above mentioned brain regions, there is evidence showing that older adults recruit brain areas including the DLPFC and middle temporal areas while performing visual memory tasks (McIntosh et al., 1999). Therefore, considering the normal activity of these areas in older adults, the effect of rTMS on bilateral DLPFC can potentially be an indicator for predicting response to rTMS treatment.
Consistent with Hoy et al. (2012), our study revealed that visual memory could be considered as a predictor of response to rTMS (Hoy et al., 2012). Moreover, our results share similarities with another pharmacological study (Herrera-Guzmán et al., 2008) although in general visual memory has not been replicated as a response predictor to the extent that working memory and executive functions, in pharmacological studies. In our study, response to bilateral rTMS was associated with visual memory, age and sex. Age (Malik et al., 2016; Rostami et al., 2017) and sex (Huang et al., 2008; Malik et al., 2016) have previously been recognized as demographic response predictors. On the other hand, age and sex are two potential factors affecting memory performance, and in particular, visual memory performance (Pauls et al., 2013; Piccardi et al., 2016; Garg et al., 2017; Voyer et al., 2017).
Working Memory and Executive Function
When all the patients are considered, individuals with a better performance in executive functions at the baseline were more likely to experience remission. Among BDD patients, individuals who had better working memory performance responded better to treatment and reached remission, and in UDD patients, individuals who had better performances in executive functions at the baseline had a better chance for remission.
In line with Hernández-Ribas et al. our study also showed executive functions as a potential candidate for response prediction (Hernández-Ribas et al., 2013). Moreover, our findings are consistent with pharmacological (Dunkin et al., 2000; Alexopoulos et al., 2005; Sneed et al., 2007; Herrera-Guzmán et al., 2008; Shiroma et al., 2014; Etkin et al., 2015; Murrough et al., 2015; Bastos et al., 2017) and psychological studies (Beaudreau et al., 2015; Kundermann et al., 2015; Morimoto et al., 2016; Bastos et al., 2017). Executive functions have been recognized as one of the most important predictors of response to anti-depressants (Groves et al., 2018).
Other than pharmacological studies, few studies investigated cognitive predictors of response to psychotherapy and better executive function was associated with better response to CBT (Kundermann et al., 2015). In addition, in cognitive remediation, executive dysfunction has been shown to be associated with predicting treatment response (Morimoto et al., 2016). Discrepancies in these findings might be due to the different nature of psychological therapies. In general, regarding executive function, similar to cognitive markers of visual memory, better test performance is an indicator of normal activity in prefrontal brain regions, which is a predictor of favorable response to treatment.
Limitations
The reported results in the current study should be cautiously interpreted as this has been a naturalistic retrospective study which means that although it mimics the real life and treatment course of patients without the active interference of researchers thus producing relatively more ecologically valid results, however, patient activities are not standardized and do not strictly follow a specific study protocol, which may introduce confounding variables affecting the results. The absence of a sham group in this study, as dictated by its design can also limit the extent to which the results can be generalized and more reliably interpreted.
Conclusion
The cognitive neuropsychologic model for depression (Roiser et al., 2012) implies that negative emotional processing biases depend on both bottom- up responses to salient emotional stimulus and weak top-down cognitive control mechanisms which are required for responding to task-irrelevant emotional information and as decreased response of lateral frontal and dorsal ACC in situations where the response to task-irrelevant emotional information must be suppressed indicates better cognitive control and reflects normal brain activity and better adaptability, rTMS treatment outcome on brain regions involved in cognitive control can result in altered cold cognition and subsequently reduce negative biases impacting depression symptoms. In this study, cognitive predictors of response to bilateral rTMS in patients with unipolar and bipolar depression were identified. Considering their relative accessibility, using cognitive tests at baseline and before the start of the treatment can be a useful and informative approach for the prediction of treatment response and pave the way toward a more personalized and effective brain stimulation treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
RR and RK: designed the study. AH, SA, and RK: carried out the study. ZN: analyzed the data. RR, RK, AH, and SA: interpreted the results and wrote the manuscript. RR, RK, and NJ: reviewed the final draft. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.888472/full#supplementary-material
References
Aguirre, I., Carretero, B., Ibarra, O., Kuhalainen, J., Martínez, J., Ferrer, A., et al. (2011). Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. J. Affect. Disord. 130, 466–469. doi: 10.1016/j.jad.2010.10.038
Alexopoulos, G. S., Kiosses, D. N., Heo, M., Murphy, C. F., Shanmugham, B., Gunning-Dixon, F., et al. (2005). Executive dysfunction and the course of geriatric depression. Biol. Psychiatry 58, 204–210. doi: 10.1016/j.biopsych.2005.04.024
Alexopoulos, G. S., Murphy, C. F., Gunning-Dixon, F. M., Kalayam, B., Katz, R., Kanellopoulos, D., et al. (2007). Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport 18, 217–221. doi: 10.1097/WNR.0b013e328013ceda
Arns, M., Spronk, D., and Fitzgerald, P. B. (2010). Potential differential effects of 9 Hz rTMS and 10 Hz rTMS in the treatment of depression. Brain Stimul. 3, 124–126. doi: 10.1016/j.brs.2009.07.005
Avissar, M., Powell, F., Ilieva, I., Respino, M., Gunning, F. M., Liston, C., et al. (2017). Functional connectivity of the left DLPFC to striatum predicts treatment response of depression to TMS. Brain Stimul. 10, 919–925. doi: 10.1016/j.brs.2017.07.002
Bailey, N., Hoy, K., Rogasch, N., Thomson, R., McQueen, S., Elliot, D., et al. (2018). Responders to rTMS for depression show increased fronto-midline theta and theta connectivity compared to non-responders. Brain Stimul. 11, 190–203. doi: 10.1016/j.brs.2017.10.015
Barr, M. S., Farzan, F., Rusjan, P. M., Chen, R., Fitzgerald, P. B., Daskalakis, Z. J., et al. (2009). Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology 34, 2359–2367. doi: 10.1038/npp.2009.79
Bastos, A. G., Guimaraes, L. S., and Trentini, C. M. (2017). Predictors of response in the treatment of moderate depression. Braz. J Psychiatry 39, 12–20. doi: 10.1590/1516-4446-2016-1976
Beattie, H. L., Schutte, A. R., and Cortesa, C. S. (2018). The relationship between spatial working memory precision and attention and inhibitory control in young children. Cogn. Dev. 47, 32–45. doi: 10.1016/j.cogdev.2018.02.002
Beaudreau, S. A., Rideaux, T., O'Hara, R., and Arean, P. (2015). Does cognition predict treatment response and remission in psychotherapy for late-life depression? Am. J. Geriatr. Psychiatry 23, 215–219. doi: 10.1016/j.jagp.2014.09.003
Bennett, P. J., Sekuler, A. B., McIntosh, A. R., and Della-Maggiore, V. (2001). The effects of aging on visual memory: evidence for functional reorganization of cortical networks. Acta Psychol. 107, 249–273. doi: 10.1016/S0001-6918(01)00037-3
Berlim, M. T., McGirr, A., Dos Santos, N. R., Tremblay, S., and Martins, R. (2017). Efficacy of theta burst stimulation (TBS) for major depression: an exploratory meta-analysis of randomized and sham-controlled trials. J. Psychiatr. Res. 90, 102–109. doi: 10.1016/j.jpsychires.2017.02.015
Bingham, K. S., Whyte, E. M., Meyers, B. S., Mulsant, B. H., Rothschild, A. J., Banerjee, S., et al. (2015). Relationship between cerebrovascular risk, cognition, and treatment outcome in late-life psychotic depression. Am. J. Geriatr. Psychiatry 23, 1270–1275. doi: 10.1016/j.jagp.2015.08.002
Brakemeier, E. L., Wilbertz, G., Rodax, S., Danker-Hopfe, H., Zinka, B., Zwanzger, P., et al. (2008). Patterns of response to repetitive transcranial magnetic stimulation (rTMS) in major depression: replication study in drug-free patients. J. Affect. Disord. 108, 59–70. doi: 10.1016/j.jad.2007.09.007
Bruder, G. E., Alvarenga, J. E., Alschuler, D., Abraham, K., Keilp, J. G., Hellerstein, D. J., et al. (2014). Neurocognitive predictors of antidepressant clinical response. J. Affect. Disord. 166, 108–114. doi: 10.1016/j.jad.2014.04.057
Brunoni, A. R., Chaimani, A., Moffa, A. H., Razza, L. B., Gattaz, W. F., Daskalakis, Z. J., et al. (2017). Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry 74, 143–152. doi: 10.1001/jamapsychiatry.2016.3644
Brunoni, A. R., and Vanderhasselt, M. A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 86, 1–9. doi: 10.1016/j.bandc.2014.01.008
Cambridge Cognition Ltd. (2012). CANTAB Eclipse Test Administration Guide. Bottisham, CA: Cambridge Cognition Ltd.
Cao, X., Deng, C., Su, X., and Guo, Y. (2018). Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front. Psychiatry 9, 413. doi: 10.3389/fpsyt.2018.00413
Cash, R. F., Dar, A., Hui, J., De Ruiter, L., Baarbé, J., Fettes, P., et al. (2017). Influence of inter-train interval on the plastic effects of rTMS. Brain Stimul. 10, 630–636. doi: 10.1016/j.brs.2017.02.012
Corlier, J., Wilson, A., Hunter, A. M., Vince-Cruz, N., Krantz, D., Levitt, J., et al. (2019). Changes in functional connectivity predict outcome of repetitive transcranial magnetic stimulation treatment of major depressive disorder. Cereb. Cortex 29, 4958–4967. doi: 10.1093/cercor/bhz035
de Azevedo-Marques Périco, C., Duran, F. L., Zanetti, M. V., Santos, L. C., Murray, R. M., Scazufca, M., et al. (2011). A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar Disord. 13, 28–40. doi: 10.1111/j.1399-5618.2011.00896.x
de Jesus, D. R., de Souza Favalli, G. P., Hoppenbrouwers, S. S., Barr, M. S., Chen, R., Fitzgerald, P. B., et al. (2014). Determining optimal rTMS parameters through changes in cortical inhibition. Clin. Neurophysiol. 125, 755–762. doi: 10.1016/j.clinph.2013.09.011
Doraiswamy, P. M., Krishnan, K. R. R., Oxman, T., Jenkyn, L. R., Coffey, D. J., Burt, T., et al. (2003). Does antidepressant therapy improve cognition in elderly depressed patients? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 58, 1137–1144. doi: 10.1093/gerona/58.12.M1137
Dunkin, J. J., Leuchter, A. F., Cook, I. A., Kasl-Godley, J. E., Abrams, M., Rosenberg-Thompson, S., et al. (2000). Executive dysfunction predicts nonresponse to fluoxetine in major depression. J. Affect. Disord. 60, 13–23. doi: 10.1016/S0165-0327(99)00157-3
Etkin, A., Patenaude, B., Song, Y. J. C., Usherwood, T., Rekshan, W., Schatzberg, A. F., et al. (2015). A cognitive–emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology 40, 1332–1342. doi: 10.1038/npp.2014.333
Fitzgerald, P. B., Hoy, K. E., Anderson, R. J., and Daskalakis, Z. J. (2016). A study of the pattern of response to rTMS treatment in depression. Depress. Anxiety 33, 746–753. doi: 10.1002/da.22503
Fregni, F., Marcolin, M. A., Myczkowski, M., Amiaz, R., Hasey, G., Rumi, D. O., et al. (2006). Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 9, 641–654. doi: 10.1017/S1461145705006280
Fried, P. J., Rushmore, R. J. III., Moss, M. B., Valero-Cabré, A., and Pascual-Leone, A. (2014). Causal evidence supporting functional dissociation of verbal and spatial working memory in the human dorsolateral prefrontal cortex. Euro. J. Neurosci. 39, 1973–1981. doi: 10.1111/ejn.12584
Furtado, C. P., Hoy, K. E., Maller, J. J., Savage, G., Daskalakis, Z. J., Fitzgerald, P. B., et al. (2012). Cognitive and volumetric predictors of response to repetitive transcranial magnetic stimulation (rTMS)—a prospective follow-up study. Psychiatry Res. Neuroimaging 202, 12–19. doi: 10.1016/j.pscychresns.2012.02.004
Garg, N., Jain, N., Mittal, S., and Verma, P. (2017). Gender variation in short term auditory and visual memory. Int. J. Physiol. 5, 171–175. doi: 10.5958/2320-608X.2017.00038.5
Gaudeau-Bosma, C., Moulier, V., Allard, A. C., Sidhoumi, D., Bouaziz, N., Braha, S., et al. (2013). Effect of two weeks of rTMS on brain activity in healthy subjects during an n-back task: a randomized double blind study. Brain Stimul. 6, 569–575. doi: 10.1016/j.brs.2012.10.009
Grammer, G. G., Kuhle, A. R., Clark, C. C., Dretsch, M. N., Williams, K. A., Cole, J. T., et al. (2015). Severity of depression predicts remission rates using transcranial magnetic stimulation. Front. Psychiatry 6, 114. doi: 10.3389/fpsyt.2015.00114
Groot, M. H., de Nolen, W. A, Huijsman, A. M., and Bouvy, P. F. (1996). Lateralized neuropsychological functioning in depressive patients before and after drug therapy. Biol. Psychiatry 40, 1282–1287. doi: 10.1016/0006-3223(95)00654-0
Groves, S. J., Douglas, K. M., and Porter, R. J. (2018). A systematic review of cognitive predictors of treatment outcome in major depression. Front. Psychiatry 9, 382. doi: 10.3389/fpsyt.2018.00382
Hernández-Ribas, R., Deus, J., Pujol, J., Segalàs, C., Vallejo, J., Menchón, J. M., et al. (2013). Identifying brain imaging correlates of clinical response to repetitive transcranial magnetic stimulation (rTMS) in major depression. Brain Stimul. 6, 54–61. doi: 10.1016/j.brs.2012.01.001
Herrera-Guzmán, I., Gudayol-Ferré, E., Lira-Mandujano, J., Herrera-Abarca, J., Herrera-Guzmán, D., Montoya-Pérez, K., et al. (2008). Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res. 160, 72–82. doi: 10.1016/j.psychres.2007.04.012
Hilti, C. C., Hilti, L. M., Heinemann, D., Robbins, T., Seifritz, E., Cattapan-Ludewig, K., et al. (2010). Impaired performance on the rapid visual information processing task (RVIP) could be an endophenotype of schizophrenia. Psychiatry Res. 177, 60–64. doi: 10.1016/j.psychres.2009.12.012
Hoy, K. E., Segrave, R. A., Daskalakis, Z. J., and Fitzgerald, P. B. (2012). Investigating the relationship between cognitive change and antidepressant response following rTMS: a large scale retrospective study. Brain Stimul. 5, 539–546. doi: 10.1016/j.brs.2011.08.010
Hu, S. H., Lai, J. B., Xu, D. R., Qi, H. L., Peterson, B. S., Bao, A. M., et al. (2016). Efficacy of repetitive transcranial magnetic stimulation with quetiapine in treating bipolar II depression: a randomized, double-blinded, control study. Sci. Rep. 6, 30537. doi: 10.1038/srep30537
Huang, C. C., Su, T. P., Shan, I. K., and Wei, I. H. (2004). Effect of 5 Hz repetitive transcranial magnetic stimulation on cognition during a Go/NoGo task. J. Psychiatr. Res. 38, 513–520. doi: 10.1016/j.jpsychires.2004.01.006
Huang, C. C., Wei, I. H., Chou, Y. H., and Su, T. P. (2008). Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology 33, 821–831. doi: 10.1016/j.psyneuen.2008.03.006
Hung, Y. Y., Yang, L. H., Stubbs, B., Li, D. J., Tseng, P. T., Yeh, T. C., et al. (2020). Efficacy and tolerability of deep transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. Prog. Neuro Psychopharmacol. Biol. Psychiatry 99, 109850. doi: 10.1016/j.pnpbp.2019.109850
Kazemi, R., Rostami, R., Dehghan, S., Nasiri, Z., Lotfollahzadeh, S., Hadipour, A. L., et al. (2020). Alpha frequency rTMS modulates theta lagged nonlinear connectivity in dorsal attention network. Brain Res. Bull. 162, 271–281. doi: 10.1016/j.brainresbull.2020.06.018
Kazemi, R., Rostami, R., Khomami, S., Baghdadi, G., Rezaei, M., Hata, M., et al. (2018). Bilateral transcranial magnetic stimulation on DLPFC changes resting state networks and cognitive function in patients with bipolar depression. Front. Hum. Neurosci. 12, 356. doi: 10.3389/fnhum.2018.00356
Kazemi, R., Rostami, R., Khomami, S., Horacek, J., Brunovsky, M., Novak, T., et al. (2016). Electrophysiological correlates of bilateral and unilateral repetitive transcranial magnetic stimulation in patients with bipolar depression. Psychiatry Res. 240, 364–375. doi: 10.1016/j.psychres.2016.04.061
Ke, J., Zou, X., Huang, M., Huang, Q., Li, H., Zhou, X., et al. (2020). High-frequency rTMS with two different inter-train intervals improves upper limb motor function at the early stage of stroke. J. Int. Med. Res. 48, 0300060520928737. doi: 10.1177/0300060520928737
Kedzior, K. K., Azorina, V., and Reitz, S. K. (2014). More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of 54 sham-controlled studies published between 1997–2013. Neuropsychiatr. Dis. Treat. 10, 727. doi: 10.2147/NDT.S58405
Kim, S. H., Han, H. J., Ahn, H. M., Kim, S. A., and Kim, S. E. (2012). Effects of five daily high-frequency rTMS on stroop task performance in aging individuals. Neurosci. Res. 74, 256–260. doi: 10.1016/j.neures.2012.08.008
Koren, D., Shefer, O., Chistyakov, A., Kaplan, B., Feinsod, M., Klein, E., et al. (2001). Neuropsychological effects of prefrontal slow rTMS in normal volunteers: a double-blind sham-controlled study. J. Clin. Exp. Neuropsychol. 23, 424–430. doi: 10.1076/jcen.23.4.424.1225
Kundermann, B., Hemmeter-Spernal, J., Strate, P., Gebhardt, S., Huber, M. T., Krieg, J. C., et al. (2015). Neuropsychological predictors of the clinical response to cognitive-behavioral therapy in patients with major depression. Zeitschrift für Neuropsychol. 26, 87–97. doi: 10.1024/1016-264X/a000130
Lawrence, A. D., Sahakian, B. J., Hodges, J. R., Rosser, A. E., Lange, K. W., Robbins, T. W., et al. (1996). Executive and mnemonic functions in early Huntington's disease. Brain 119, 1633–1645. doi: 10.1093/brain/119.5.1633
Lin, K., Xu, G., Lu, W., Ouyang, H., Dang, Y., Lorenzo-Seva, U., et al. (2014). Neuropsychological performance in melancholic, atypical and undifferentiated major depression during depressed and remitted states: a prospective longitudinal study. J. Affect. Disord. 168, 184–191. doi: 10.1016/j.jad.2014.06.032
Malik, A. M., Haque, Z., Ide, G., and Farley, A. (2016). Gender and age as factors in response and remission of depression treated with transcranial magnetic stimulation. Brain Stimul. 9, e7. doi: 10.1016/j.brs.2016.06.022
Manelis, A., Iyengar, S., Swartz, H. A., and Phillips, M. L. (2020). Prefrontal cortical activation during working memory task anticipation contributes to discrimination between bipolar and unipolar depression. Neuropsychopharmacology 45, 956–963. doi: 10.1038/s41386-020-0638-7
Martin, D. M., McClintock, S. M., Forster, J. J., Lo, T. Y., and Loo, C. K. (2017). Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress. Anxiety 34, 1029–1039. doi: 10.1002/da.22658
McIntosh, A., Sekuler, A., Penpeci, C., Rajah, M., Grady, C., Sekuler, R., et al. (1999). Recruitment of unique neural systems to support visual memory in normal aging. Curr. Biol. 9, 1275–1278. doi: 10.1016/S0960-9822(99)80512-0
Micoulaud-Franchi, J. A., Richieri, R., Cermolacce, M., Loundou, A., Lancon, C., Vion-Dury, J., et al. (2012). Parieto-temporal alpha EEG band power at baseline as a predictor of antidepressant treatment response with repetitive transcranial magnetic stimulation: a preliminary study. J. Affect. Disord. 137, 156–160. doi: 10.1016/j.jad.2011.12.030
Miron, J. P., Voetterl, H., Fox, L., Hyde, M., Mansouri, F., Dees, S., et al. (2021). Optimized repetitive transcranial magnetic stimulation techniques for the treatment of major depression: a proof of concept study. Psychiatry Res. 298, 113790. doi: 10.1016/j.psychres.2021.113790
Morimoto, S. S., Gunning, F. M., Wexler, B. E., Hu, W., Ilieva, I., Liu, J., et al. (2016). Executive dysfunction predicts treatment response to neuroplasticity-based computerized cognitive remediation (nCCR-GD) in elderly patients with major depression. Am. J. Geriatr. Psychiatry 24, 816–820. doi: 10.1016/j.jagp.2016.06.010
Mottaghy, F., Gangitano, M., Sparing, R., Krause, B., and Pascual-Leone, A. (2002). Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb. Cortex 12, 369–375. doi: 10.1093/cercor/12.4.369
Murrough, J. W., Burdick, K. E., Levitch, C. F., Perez, A. M., Brallier, J. W., Chang, L. C., et al. (2015). Neurocognitive effects of ketamine and association with antidepressant response in individuals with treatment-resistant depression: a randomized controlled trial. Neuropsychopharmacology 40, 1084–1090. doi: 10.1038/npp.2014.298
Mutz, J., Edgcumbe, D. R., Brunoni, A. R., and Fu, C. H. Y. (2018). Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci. Biobehav. Rev. 92, 291–303. doi: 10.1016/j.neubiorev.2018.05.015
Myczkowski, M. L., Fernandes, A., Moreno, M., Valiengo, L., Lafer, B., Moreno, R. A., et al. (2018). Cognitive outcomes of TMS treatment in bipolar depression: safety data from a randomized controlled trial. J. Affect. Disord. 235, 20–26. doi: 10.1016/j.jad.2018.04.022
Narushima, K., McCormick, L. M., Yamada, T., Thatcher, R. W., and Robinson, R. G. (2010). Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J. Neuropsychiatry Clin. Neurosci. 22, 75–84. doi: 10.1176/jnp.2010.22.1.75
Oi, Y., Kita, Y., Suzuki, K., Okumura, Y., Okuzumi, H., Shinoda, H., et al. (2017). Spatial working memory encoding type modulates prefrontal cortical activity. Neuroreport 28, 391–396. doi: 10.1097/WNR.0000000000000761
Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E., and Robbins, T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28, 1021–1034. doi: 10.1016/0028-3932(90)90137-D
Owen, A. M., Sahakian, B. J., Semple, J., Polkey, C. E., and Robbins, T. W. (1995). Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33, 1–24. doi: 10.1016/0028-3932(94)00098-A
Padberg, F., Zwanzger, P., Keck, M. E., Kathmann, N., Mikhaiel, P., Ella, R., et al. (2002). Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology 27, 638–645. doi: 10.1016/S0893-133X(02)00355-X
Pallanti, S., Cantisani, A., Grassi, G., Antonini, S., Cecchelli, C., Burian, J., et al. (2012). rTMS age-dependent response in treatment-resistant depressed subjects: a mini-review. CNS Spectr. 17, 24–30. doi: 10.1017/S1092852912000417
Park, C., Pan, Z., Brietzke, E., Subramaniapillai, M., Rosenblat, J. D., Zuckerman, H., et al. (2018). Predicting antidepressant response using early changes in cognition: a systematic review. Behav. Brain Res. 35, 154–160. doi: 10.1016/j.bbr.2018.07.011
Patel, R., Silla, F., Pierce, S., Theule, J., and Girard, T. A. (2020). Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): a quantitative meta-analysis in healthy adults. Neuropsychologia 141, 107395. doi: 10.1016/j.neuropsychologia.2020.107395
Pauls, F., Petermann, F., and Lepach, A. C. (2013). Gender differences in episodic memory and visual working memory including the effects of age. Memory 21, 857–874. doi: 10.1080/09658211.2013.765892
Pearce, A. J., Lum, J. A., Seth, S., Rafael, O., Hsu, C. M. K., Drury, H. G., et al. (2014). Multiple bout rTMS on spatial working memory: a comparison study of two cortical areas. Biol. Psychol. 100, 56–59. doi: 10.1016/j.biopsycho.2014.05.002
Piccardi, L., Matano, A., D'Antuono, G., Marin, D., Ciurli, P., Incoccia, C., et al. (2016). Persistence of gender related-effects on visuo-spatial and verbal working memory in right brain-damaged patients. Front. Behav. Neurosci. 10, 139. doi: 10.3389/fnbeh.2016.00139
Pitkänen, M., Kallioniemi, E., and Julkunen, P. (2017). Effect of inter-train interval on the induction of repetition suppression of motor-evoked potentials using transcranial magnetic stimulation. PLoS ONE 12, e0181663. doi: 10.1371/journal.pone.0181663
Potter, G. G., Kittinger, J. D., Wagner, H. R., Steffens, D. C., and Krishnan, K. R. R. (2004). Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology 29, 2266–2271. doi: 10.1038/sj.npp.1300551
Rami, L., Gironell, A., Kulisevsky, J., Garcia-Sánchez, C., Berthier, M., and Estevez-Gonzalez, A. (2003). Effects of repetitive transcranial magnetic stimulation on memory subtypes: a controlled study. Neuropsychologia 41, 1877–1883. doi: 10.1016/S0028-3932(03)00131-3
Roiser, J. P., Cannon, D. M., Gandhi, S. K., Tavares, J. T., Erickson, K., Wood, S., et al. (2009). Hot and cold cognition in unmedicated depressed subjects with bipolar disorder. Bipolar Disord. 11, 178–189. doi: 10.1111/j.1399-5618.2009.00669.x
Roiser, J. P., Elliott, R., and Sahakian, B. J. (2012). Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37, 117–136. doi: 10.1038/npp.2011.183
Roiser, J. P., and Sahakian, B. J. (2013). Hot and cold cognition in depression. CNS Spectr. 18, 139–149. doi: 10.1017/S1092852913000072
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmöller, J., et al. (2021). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert guidelines. Clin. Neurophysiol. 132, 269–306. doi: 10.1016/j.clinph.2020.10.003
Rostami, R., Kazemi, R., Mozaffarinejad, F., Nasiri, Z., Rostami, M., Hadipour, A. L., et al. (2021). 6 Hz transcranial alternating current stimulation of mPFC improves sustained attention and modulates alpha phase synchronization and power in dorsal attention network. Cogn. Neurosci. 12, 1–13. doi: 10.1080/17588928.2020.1817881
Rostami, R., Kazemi, R., Nitsche, M. A., Gholipour, F., and Salehinejad, M. (2017). Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin. Neurophysiol. 128, 1961–1970. doi: 10.1016/j.clinph.2017.07.395
Sackeim, H. A., Aaronson, S. T., Carpenter, L. L., Hutton, T. M., Mina, M., Pages, K., et al. (2020). Clinical outcomes in a large registry of patients with major depressive disorder treated with transcranial magnetic stimulation. J. Affect. Disord. 277, 65–74. doi: 10.1016/j.jad.2020.08.005
Sahakian, B. J., Morris, R. G., Evenden, J. L., Heald, A., Levy, R., Philpot, M., et al. (1988). A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain 111, 695–718. doi: 10.1093/brain/111.3.695
Seeberg, I., Kjaerstad, H. L., and Miskowiak, K. W. (2018). Neural and behavioral predictors of treatment efficacy on mood symptoms and cognition in mood disorders: a systematic review. Front. Psychiatry 9, 337. doi: 10.3389/fpsyt.2018.00337
Shallice, T. (1982). Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 298, 199–209. doi: 10.1098/rstb.1982.0082
Shiroma, P. R., Albott, C. S., Johns, B., Thuras, P., Wels, J., Lim, K. O., et al. (2014). Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol. 17, 1805–1813. doi: 10.1017/S1461145714001011
Sneed, J. R., Roose, S. P., Keilp, J. G., Krishnan, K. R. R., Alexopoulos, G. S., Sackeim, H. A., et al. (2007). Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am. J. Geriatr. Psychiatry 15, 553–563. doi: 10.1097/JGP.0b013e3180302513
Spronk, D., Arns, M., Barnett, K., Cooper, N., and Gordon, E. (2011). An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: a pilot study. J. Affect. Disord. 128, 41–48. doi: 10.1016/j.jad.2010.06.021
Story, T. J., Potter, G. G., Attix, D. K., Welsh-Bohmer, K. A., and Steffens, D. C. (2008). Neurocognitive correlates of response to treatment in late-life depression. Am. J. Geriatr. Psychiatry 16, 752–759. doi: 10.1097/JGP.0b013e31817e739a
Toornstra, A., Hurks, P. P., Van der Elst, W., Kok, G., and Curfs, L. M. (2020). Measuring visual matching and short-term recognition memory with the CANTAB® delayed matching to sample task in schoolchildren: effects of demographic influences, multiple outcome measures and regression-based normative data. Child Neuropsychol. 26, 189–218. doi: 10.1080/09297049.2019.1642316
Trevizol, A. P., Downar, J., Vila-Rodriguez, F., Thorpe, K. E., and Blumberger, D. M. (2020). Predictors of remission after repetitive transcranial magnetic stimulation for the treatment of major depressive disorder: an analysis from the randomised non-inferiority THREE-D trial. EClinicalMedicine 22, 100349. doi: 10.1016/j.eclinm.2020.100349
Upton, D. J., Cooper, N. R., Laycock, R., Croft, R. J., and Fitzgerald, P. B. (2010). A combined rTMS and ERP investigation of dorsolateral prefrontal cortex involvement in response inhibition. Clin. EEG Neurosci. 41, 127–131. doi: 10.1177/155005941004100304
Vanderhasselt, M. A., De Raedt, R., Baeken, C., Leyman, L., Clerinx, P., and D'haenen, H. (2007). The influence of rTMS over the right dorsolateral prefrontal cortex on top-down attentional processes. Brain Res. 1137, 111–116. doi: 10.1016/j.brainres.2006.12.050
Viggiano, M. P., Giovannelli, F., Borgheresi, A., Feurra, M., Berardi, N., Pizzorusso, T., et al. (2008). Disruption of the prefrontal cortex function by rTMS produces a category-specific enhancement of the reaction times during visual object identification. Neuropsychologia 46, 2725–2731. doi: 10.1016/j.neuropsychologia.2008.05.004
Voyer, D., Voyer, S. D., and Saint-Aubin, J. (2017). Sex differences in visual-spatial working memory: a meta-analysis. Psychon. Bull. Rev. 24, 307–334. doi: 10.3758/s13423-016-1085-7
Williams, L. M., Coman, J. T., Stetz, P. C., Walker, N. C., Kozel, F. A., George, M. S., et al. (2021). Identifying response and predictive biomarkers for transcranial magnetic stimulation outcomes: protocol and rationale for a mechanistic study of functional neuroimaging and behavioral biomarkers in veterans with pharmacoresistant depression. BMC Psychiatry 21, 35. doi: 10.1186/s12888-020-03030-z
Xu, G., Lin, K., Rao, D., Dang, Y., Ouyang, H., Guo, Y., et al. (2012). Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J. Affect. Disord. 136, 328–339. doi: 10.1016/j.jad.2011.11.029
Yang, L. L., Zhao, D., Kong, L. L., Sun, Y. Q., Wang, Z. Y., Gao, Y. Y., et al. (2019). High-frequency repetitive transcranial magnetic stimulation (rTMS) improves neurocognitive function in bipolar disorder. J. Affect. Disord. 246, 851–856. doi: 10.1016/j.jad.2018.12.102
Keywords: bipolar depression, unipolar depression, transcranial magnetic stimulation (TMS), cognitive predictors, cold cognition, predictors of treatment response
Citation: Rostami R, Kazemi R, Nasiri Z, Ataei S, Hadipour AL and Jaafari N (2022) Cold Cognition as Predictor of Treatment Response to rTMS; A Retrospective Study on Patients With Unipolar and Bipolar Depression. Front. Hum. Neurosci. 16:888472. doi: 10.3389/fnhum.2022.888472
Received: 02 March 2022; Accepted: 06 June 2022;
Published: 25 July 2022.
Edited by:
Ryouhei Ishii, Osaka Prefecture University, JapanReviewed by:
Ioana Miclutia, Iuliu Haţieganu University of Medicine and Pharmacy, RomaniaTihomir V. Ilić, Military Medical Academy, Serbia
Copyright © 2022 Rostami, Kazemi, Nasiri, Ataei, Hadipour and Jaafari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reza Rostami, cnJvc3RhbWlAdXQuYWMuaXI=
 Reza Rostami
Reza Rostami Reza Kazemi
Reza Kazemi Zahra Nasiri
Zahra Nasiri Somayeh Ataei4
Somayeh Ataei4 Abed L. Hadipour
Abed L. Hadipour