
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 16 March 2022
Sec. Cognitive Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.740195
This article is part of the Research Topic Neuroscience of Human Attachment: Volume II View all 7 articles
Ample research demonstrates that parents’ experience-based mental representations of attachment—cognitive models of close relationships—relate to their children’s social-emotional development. However, no research to date has examined how parents’ attachment representations relate to another crucial domain of children’s development: brain development. The present study is the first to integrate the separate literatures on attachment and developmental social cognitive neuroscience to examine the link between mothers’ attachment representations and 3- to 8-year-old children’s brain structure. We hypothesized that mothers’ attachment representations would relate to individual differences in children’s brain structures involved in stress regulation—specifically, amygdala and hippocampal volumes—in part via mothers’ responses to children’s distress. We assessed 52 mothers’ attachment representations (secure base script knowledge on the Attachment Script Assessment and self-reported attachment avoidance and anxiety on the Experiences in Close Relationships scale) and children’s brain structure. Mothers’ secure base script knowledge was significantly related to children’s smaller left amygdala volume but was unrelated to hippocampal volume; we found no indirect links via maternal responses to children’s distress. Exploratory analyses showed associations between mothers’ attachment representations and white matter and thalamus volumes. Together, these preliminary results suggest that mothers’ attachment representations may be linked to the development of children’s neural circuitry related to stress regulation.
Integral to attachment theory is the claim that individuals form experience-based mental representations of attachment—cognitive models of the nature of close relationships (Bowlby, 1969/1982). Those mental representations of attachment have consequences not only for individuals’ own development, but also for the development of their children (Steele et al., 2014; Jones et al., 2015). The vast majority of research on relations between caregivers’ attachment representations and children’s developmental outcomes examines how parents’ representations link to caregiving behaviors (Coppola et al., 2006; Jones et al., 2015) and their children’s attachment security (e.g., Steele et al., 2014). However, no research to date has examined how caregivers’ attachment representations relate to another crucial domain of child development: children’s brain development.
Some research indirectly indicates the possibility of a link between mothers’ attachment representations and children’s brain development. For example, abundant research demonstrates that mothers’ representations relate to children’s attachment security (e.g., van IJzendoorn, 1995; Steele et al., 2014), and an emerging body of research indicates that children’s attachment security is related to individual differences in brain structure (see Long et al., 2020, for a review; see Puhlmann et al., 2021, for evidence in adolescence, see also Ilyka et al., 2021). Furthermore, in addition to the large body of research demonstrating that mothers’ attachment representations guide caregiving behavior (e.g., Huth-Bocks et al., 2014; Jones et al., 2015), some research demonstrates that normative variation in caregiving relates to individual differences in children’s cortical and subcortical brain structures (Kok et al., 2015; Rifkin-Graboi et al., 2015; Farber et al., 2020; Ilyka et al., 2021; Richmond et al., 2021). Thus, although there is some indication of a possible indirect link between mothers’ attachment representations and children’s brain development, no research to date has examined the direct link or mechanisms through which this relation might occur.
The present study is the first to examine whether mothers’ attachment representations relate to their children’s brain structure and whether caregiving explains this link. We focus our examination of caregiving behaviors on mothers’ responses to children’s distress because such responses are a key form of co-regulation—external regulation of a child’s physiological rhythms and emotions by a sensitive caregiver (Spangler et al., 1994). Effective co-regulation is thought to support a child’s ability to self-regulate their own physiological and emotional responses to threat (Cassidy, 1994; Hofer, 1995; Luecken and Lemery, 2004; George and Solomon, 2008). For this reason, we focus our examination of children’s brain structure on two principal regions involved in circuits that facilitate stress regulation in times of threat: the hippocampus and the amygdala (McEwen, 2000; Callaghan and Tottenham, 2016a). Our focus on these two brain structures (in addition to their role in stress regulation) stems from an emerging body of evidence that normative variations in attachment security (e.g., Quirin et al., 2010; Lyons-Ruth et al., 2016) and caregiving (e.g., Rifkin-Graboi et al., 2015; Luby et al., 2016) relate to individual differences in both of these brain structures.
We begin with a discussion of attachment representations. Next, in order to support the expectations of a relation between mothers’ representations and children’s brain structure, we discuss the abundance of evidence that mothers’ attachment representations relate to their caregiving. Then, we discuss evidence that mothers’ caregiving relates to children’s brain structure and present the current study.
Mental representations of attachment are experience-based cognitive models that encompass individuals’ beliefs about the self and others—including expectations about how they themselves will be treated in close relationships (Bowlby, 1969/1982; Bretherton and Munholland, 2016). When individuals experience sensitive care from caregivers and other close relationship partners, they develop secure attachment representations. People with secure attachment representations hold beliefs that others will be available and sensitively responsive in times of need, that they themselves are worthy of such care, and that they are capable of and effective in eliciting care when needed. In contrast, experiences of rejection or neglect in close relationships can result in insecure mental representations, characterized by mistrust of others and negative beliefs about the self (Bowlby, 1969/1982).
There are multiple ways that researchers operationalize attachment representations. Two widely used constructs are secure base script knowledge and attachment style. The secure base script is a knowledge structure, or schema, consisting of a sequence of events wherein a person in distress seeks and receives care from a close other, and then the distress is resolved as a function of that care (Bretherton, 1985, 1987; Waters and Waters, 2006). The extent to which a person organizes information following this schema reflects their secure base script knowledge. The other widely used conceptualization of attachment representations is attachment style, consisting of two dimensions: attachment avoidance and attachment anxiety (Brennan et al., 1998). Attachment avoidance reflects the extent to which an individual is uncomfortable with intimacy and with depending on relationship partners for support. Attachment anxiety reflects the extent to which an individual is preoccupied with relationships and fears rejection and abandonment by relationship partners. High levels of attachment anxiety, attachment avoidance, or both indicate an insecure attachment style. Low levels of both anxiety and avoidance indicate attachment security, characterized by feelings that one is worthy of love and care, and that relationship partners will be available and responsive in times of need (Mikulincer and Shaver, 2007).
Although secure base script knowledge and attachment style capture subtly different aspects of mental representations, the two are closely intertwined. Secure base scripts are considered to be the raw building blocks of more complex mental representations (Bretherton, 1991). According to theory (Mikulincer and Shaver, 2007), a person who has access to a secure base script is unlikely to fear either rejection and abandonment (fears that are central to anxious attachment styles) or relying on relationship partners for support (fears central to avoidant attachment styles). Secure base script knowledge mitigates these fears because such scripts are characterized by a schema of consistently responsive care and alleviation of distress as a function of that care. As such, secure base scripts lay the foundation for an individual’s secure attachment style. Indeed, empirical data suggest that individuals with a secure attachment style demonstrate high secure base script knowledge (Dykas et al., 2006; Mikulincer et al., 2009).
Mothers’ attachment representations (both secure base script knowledge and attachment style) relate to the quality of care they provide to their children. Ample research reveals that mothers with greater access to secure base script knowledge provide more sensitive care to their children (e.g., Coppola et al., 2006; Huth-Bocks et al., 2014; Waters et al., 2017). For instance, one study demonstrated that mothers’ secure base script knowledge predicted greater positive parenting behaviors (e.g., sensitivity and positive affect) and fewer negative parenting behaviors (e.g., hostility and intrusiveness) during a free play task in the home (Huth-Bocks et al., 2014).
A separate body of evidence reveals links between parents’ insecure attachment style dimensions and a wide array of negative parenting behaviors (see Jones et al., 2015, for a review). Empirical work consistently demonstrates that attachment avoidance relates to low sensitivity and responsiveness (Rholes et al., 1995; Mills-Koonce et al., 2011), whereas links between attachment anxiety and sensitivity are more mixed (Jones et al., 2015). Nevertheless, both anxiety and avoidance are associated with mothers engaging in more frequent and harsh conflict with their children (e.g., Feeney, 2006; Selcuk et al., 2010; La Valley and Guerrero, 2012).
Examination of maternal caregiving is critical because early caregiving experiences become biologically embedded in the child and can have profound effects on development (McEwen, 2000; Meaney and Szyf, 2005; Belsky and de Haan, 2011; McLaughlin et al., 2019). Early experiences with caregivers predict the development of subcortical (e.g., Meaney and Szyf, 2005; Rifkin-Graboi et al., 2015; Gee, 2016; Sethna et al., 2017; Bernier et al., 2019) and cortical (e.g., Blaze et al., 2013; Kok et al., 2015) brain structures. Although abundant research demonstrates that early maltreatment and neglect relate to children’s brain development (see Belsky and de Haan, 2011; McLaughlin et al., 2019, for reviews), we focus here on normative variation in caregiving behaviors.
The hippocampus is sensitive to early stressful caregiving environments; elevated levels of stress hormones (e.g., cortisol) have particularly deleterious consequences for regions with an abundance of glucocorticoid receptors, such as the hippocampus (Sapolsky et al., 1986; McEwen, 2000; Conrad, 2009). As such, if caregivers cannot effectively regulate a child’s distress, then it is possible that the child may experience alterations in hippocampal development. For example, a series of studies has demonstrated that maternal support in early childhood predicts greater hippocampal volume and more rapid hippocampal development in mid to late childhood (Luby et al., 2012, 2016). Further, Blankenship et al. (2019) found that positive parenting of 4-year-olds predicted greater hippocampal head volumes 3 years later. There are, however, some null findings and findings in the opposite direction surrounding caregiving and the hippocampus. Some studies suggest positive caregiving experiences relate to smaller hippocampal volumes in infants (Rifkin-Graboi et al., 2015) and toddlers (Bernier et al., 2019), and other studies reveal no relation between maternal caregiving and hippocampal volume in early (Lee et al., 2019) and middle childhood (Kok et al., 2015). Inconsistent findings, combined with the crucial role of the hippocampus in children’s cognitive and emotional well-being (e.g., Barch et al., 2019), highlight the need to continue examining relations between caregiving and the hippocampus.
The amygdala is another structure involved in stress-regulation (Callaghan and Tottenham, 2016a). Specifically, research suggests that the amygdala is one of several forebrain regions that plays a critical role in upregulating the HPA axis following the onset of a stressful event (Honkaniemi et al., 1992). Research suggests that early exposure to negative environments is associated with larger amygdala volume (Tottenham et al., 2010; Roth et al., 2018)—a structural difference that has been attributed to accelerated amygdala maturation in threatening contexts (Tottenham et al., 2010). Although it is important to note that the vast majority of this research focuses on neglected and maltreated samples (Belsky and de Haan, 2011), some research corroborates these findings in typically developing community samples without histories of maltreatment or neglect. In one study, maternal sensitivity marginally related to smaller right and left amygdala volumes in infants (Rifkin-Graboi et al., 2015). Further, research found that mothers’ greater punishing responses predicted young adolescents’ larger amygdala volumes (Whittle et al., 2009). Moreover, one study demonstrated that maternal depression, often associated with deficits in caregiving (Lovejoy et al., 2000), was associated with larger amygdala volumes in late childhood (Lupien et al., 2011). Longitudinal research has demonstrated that maternal sensitivity in toddlers predicted smaller right amygdala volumes in late childhood (Bernier et al., 2019), maternal sensitivity in infancy predicted boys’ smaller amygdala volumes at age six (Lee et al., 2019), and positive parenting predicted less amygdala growth from early to mid-adolescence (Whittle et al., 2014). Some longitudinal work, however, has failed to demonstrate relations between parental sensitivity in early childhood and amygdala volume in middle childhood (Kok et al., 2015). Thus, further research is necessary to reconcile conflicting findings and strengthen confidence in the relations between normative variations in caregiving and children’s amygdala volume.
In addition to findings in subcortical structures, some research indicates that normative variations in maternal caregiving relate to variations in the cortex. It is important to note, however, that much of this limited literature involves adolescents and young adults. For example, maternal aggression predicted greater thickening of the superior frontal gyrus and the right parietal lobe in males from early adolescence to early adulthood (Whittle et al., 2016), and mothers’ punishing responses predicted greater dorsal anterior cingulate cortex and orbito-frontal cortex volumes in young adolescents (Whittle et al., 2009). Further, young adults’ self-reports of maternal warmth predicted greater gray matter volume (GMV) in the prefrontal cortex (Yang et al., 2018). Null findings exist as well. In one study, researchers failed to find relations between parental nurturance in early to middle childhood and cortical thickness in any brain structures in late adolescence (Avants et al., 2015).
Although limited, some research has demonstrated relations between infants’ and children’s cortical brain structure and normative caregiving. For example, evidence suggests that mothers’ negative affect relates to infants’ smaller total gray and white matter volumes (Sethna et al., 2017), and parental praise in middle childhood relates to greater GMV in the insula (Matsudaira et al., 2016). In addition, parental sensitivity in early childhood predicted greater brain volume and total GMV in middle childhood (Kok et al., 2015). An intervention study, however, failed to find any effects of a parent sensitivity program on infants’ total and regional GMV s (Milgrom et al., 2010). Promising early research indicates that some relations between caregiving and children’s cortical structures exist. Yet findings about which cortical structures relate to caregiving and the exact nature of these relations have not yet converged, possibly due to the limited number of studies. Thus, the present study aimed to help the field better understand how parenting becomes biologically embedded in the developing brain.
The present study emerged from the following: (a) compelling evidence that mothers’ attachment representations relate to their caregiving behaviors, (b) sparse and somewhat inconsistent evidence that normative variation in mothers’ caregiving behaviors relates to individual differences in children’s brain structure, and (c) the lack of direct examination of links between mothers’ attachment representations and children’s brain structure. As such, the present study had two principal goals: (1) To examine relations between mothers’ attachment representations and children’s brain structure, and (2) to examine the indirect effect of mothers’ attachment representations on children’s brain structures through one aspect of mothers’ caregiving, responses to children’s distress. We included data from 52 children (ranging in age from 3 to 8 years) and their mothers. Mothers completed the Attachment Script Assessment (ASA; Waters and Rodrigues-Doolabh, 2001) and the Experiences in Close Relationships Scale (ECR; Brennan et al., 1998) to assess attachment representations. Mothers also completed the Coping with Children’s/Toddlers’ Negative Emotions Scales to assess responses to distress and children completed an magnetic resonance imaging (MRI) scan.
Our pre-registered hypotheses1 were that: (1) mothers’ secure base script knowledge would relate positively to their children’s hippocampal volumes and negatively to their children’s amygdala volumes, (2) mothers’ attachment avoidance and attachment anxiety would relate negatively to their children’s hippocampal volumes and positively to their children’s amygdala volumes, and (3) mothers’ unsupportive responses to children’s distress would facilitate indirect links between mothers’ attachment representations (secure base script knowledge, attachment avoidance, and attachment anxiety) and amygdala and hippocampal volumes. Our pre-registered exploratory analyses examined whether: (1) mothers’ attachment representations would relate to global metrics of brain development, including children’s intracranial volume, total gray and white matter volume, subcortical GMV, and patterns of regional cortical thickness and surface area, and (2) mothers’ supportive responses toward children’s distress would facilitate indirect relations between mothers’ attachment representations and children’s brain structure. We had no a priori hypotheses about whole brain metrics or regional cortical thickness and surface area due to the paucity of research and inconsistent findings on early normative caregiving experiences and children’s cortical structure. We focused our confirmatory analyses on unsupportive, as opposed to supportive, responses toward distress. This was due to research demonstrating the relatively greater link between unsupportive responses and mothers’ insecure attachment (Jones et al., 2014), and between unsupportive responses and children’s poor social-emotional functioning (e.g., Eisenberg et al., 1999; Fabes et al., 2001; Perry et al., 2012; Shewark and Blandon, 2015), both aspects of functioning that relate to differences in brain structure (Gee, 2016). However, supportive responses were also examined in exploratory analyses.
Participants were drawn from two larger longitudinal studies investigating memory and brain development (see Botdorf and Riggins, 2018; Allard et al., 2019; Canada et al., 2019). Dyads were included in the present study if the mother had completed the Attachment Script Assessment (Waters and Rodrigues-Doolabh, 2001).
Participants were 36 mothers (Mage = 33.23, SD = 5.03) and their 4- to 8-year-old children (20 males; Mage = 6.66, SD = 1.29) taken from a larger longitudinal study examining behavioral and brain development during early childhood. The majority of children (77%) identified as White, 11.5% identified as African American, and 11.5% identified as multiracial. Regarding ethnicity, 9.1% were Latinx/Hispanic. The majority of families were affluent: 79% of families had an average household income of more than $95,000/year; 86.1% of participants had a parent with at least a college degree, and 50% of participants had a parent with a post-graduate degree (see Table 1 for more demographic information). Participants were recruited from the Baltimore-Washington metropolitan area through flyers, online advertisements, and a University-maintained database of families interested in participating in research.
Exclusionary criteria were: children’s history of head trauma or brain abnormality, abnormal circadian function, neurological disorders, premature birth, diagnosis of ADHD or other learning disability, diagnosis of psychiatric disorders, history of developmental delays or disorders, family history of autism spectrum disorder, child or parent was not English-speaking, and contraindications for MRI (e.g., metal in the body).
Mothers were provided with a personal link to complete the online questionnaires about their attachment style (anxiety and avoidance), their responses to children’s distress, and demographic information. Mothers were allowed to complete this survey at home or in the lab while their child was participating in the study. Participants visited the university for two visits, typically within 1 week of each other. During the first visit, children participated in an MRI scan. During the second visit, mothers completed the Attachment Script Assessment (ASA; Waters and Rodrigues-Doolabh, 2001; Waters and Waters, 2006) to assess secure base script knowledge while their children participated in memory tasks unrelated to the present study. Mothers were compensated with cash and children were given a small prize for their participation.
To prepare for MR data acquisition, participants were familiarized with the scanner environment using a mock MRI scanner. Children were provided motion feedback and practiced lying still during the mock scan. Following practice in the mock scanner, children were scanned using a Siemens 3.0T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel coil. Given the high possibility of movement in younger populations, additional padding was placed around each participant’s head to reduce head movement. Children also watched a movie during the scan to enhance compliance. A high resolution (0.9 mm3) T1-weighted whole brain structural scan consisting of 176 contiguous sagittal slices (1900 ms TR; 2.32 ms TE; 900 ms inversion time; 9° flip angle; pixel matrix = 256 × 256) was acquired during imaging. To ensure high image quality, images were visually inspected following the scan. If the image quality was deemed low, the scan was repeated.
Preprocessing of structural T1-weighted images consisted of image registration, skull stripping, smoothing, motion correction, and subcortical segmentation via Freesurfer (v5.12). Hippocampal and amygdala volumes were obtained and adjusted via the automated segmentation adapter tool3 (Wang et al., 2011) and split into subregions using standard anatomical landmarks (Riggins et al., 2015). To obtain quality measures of cortical thickness and surface area, two trained coders checked the boundary lines separating white and pial surfaces. In the case of errors, such as slices where the gray/white matter boundary extended past the skull or the pial boundary encapsulated portions of the skull, editors corrected these boundaries. Corrections were only made if the error persisted for more than 7 contiguous slices (Botdorf and Riggins, 2018). Alterations were first made by changing the watershed value in FreeSurfer. If this step did not eliminate the error, then manual edits were made (Ducharme et al., 2014). Edits were made for approximately 58% of the sample and involved an average of 14.6 slices (range 9–100). After all corrections were made, cortical thickness and surface area were calculated using FreeSurfer (Fischl and Dale, 2000). Freesurfer was also used to extract total and subcortical GMV (Fischl et al., 2002).
Mothers were instructed to create a story, using a 12-to-14-word list as a guide (Waters and Rodrigues-Doolabh, 2001; Waters and Waters, 2006). The procedure includes two stories about parent–child relationships (e.g., a parent and child at the doctor’s office) and two stories about adult–adult relationships (e.g., a couple on a camping trip).
Stories were coded on a 7-point scale indicating the extent to which the story demonstrates secure base script knowledge, from 7 (extensive secure base script knowledge) to 1 (absence of secure base script knowledge). High scores reflect stories wherein an individual becomes distressed, seeks and receives care from a supportive caregiver (in the parent–child stories) or a supportive partner (in the adult–adult stories), is comforted as a function of that care, and is able to resume activities. The four scores from each participant were averaged to create a mean score of secure base script knowledge. All stories were coded by two masked coders who were trained to reliability by a developer of the task (Harriet Waters) and demonstrated strong interrater reliability (α = 0.88).
Mothers completed the general form of the widely used 36-item self-report measure of adult attachment anxiety and avoidance; this version assesses these dimensions with respect to close relationships generally, rather than asking specifically about romantic relationships (Brennan et al., 1998). The avoidance dimension reflects individuals’ feelings of discomfort with close relationships and avoidance of intimacy or reliance on others (e.g., “I get uncomfortable when someone wants to be very close to me”), whereas the anxiety dimension reflects individuals’ fear of interpersonal rejection and abandonment (e.g., “I worry about being rejected or abandoned”). Each item is rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree), and some items are reverse-scored so that higher scores represent greater avoidance or anxiety. Mothers’ attachment anxiety and avoidance were calculated by averaging responses across subscale items. Good internal consistency was evident (anxiety ω = 0.93, avoidance, ω = 0.94).
This questionnaire was used to measure mothers’ unsupportive responses to child distress. Participants rated their likelihood of engaging in each of 6 possible responses to their children’s negative emotions in 12 hypothetical scenarios about a child in distress (e.g., “If my child falls off his bike and breaks it, and then gets upset and cries, I would…”) (Fabes et al., 1990). For each scenario, caregivers rated each possible response from 1 (very unlikely) to 7 (very likely). Following Fabes et al. (2002), we created two indices: supportive and unsupportive responses to distress.
For each scenario, unsupportive responses include the following: (1) distress reactions (e.g., “Remain calm and not let myself get anxious” [reverse-scored]); (2) punitive reactions (e.g., “Tell my child that if he doesn’t stop crying, he won’t be allowed to ride his bike anytime soon”); and (3) minimizing reactions (e.g., “Tell my child that he is over-reacting”).
For each scenario, supportive responses include the following: (1) expressive encouragement (e.g., “Tell my child it’s OK to cry”); (2) emotion-focused reactions (e.g., “Comfort my child and try to get him to forget about the accident”); and (3) problem-focused reactions (e.g., “Help my child figure out how to get the bike fixed”). The subscales demonstrated strong reliability and construct validity in previous research (Fabes et al., 2002; Shewark and Blandon, 2015) and strong reliability in the present sample (unsupportive ω = 0.92, supportive ω = 0.95). Reliability reported for the unsupportive subscale does not include one item from the punitive subscale (“Send my child to his or her room to cool off”) because a reliability statistic could not be computed due to missingness. Subscale reliability without this item is very strong (ω = 0.92); as we did throughout all analyses, we utilized participant mean imputation for missing values for this item.
Participants were 16 mothers (Mage = 38, SD = 5.21) and their 3- to 5-year-old children (8 males; Mage = 4.81, SD = 0.49) taken from a larger longitudinal study examining behavioral and brain development during early childhood. All parents identified their children as White. Regarding ethnicity, 27% were Latinx/Hispanic. The majority of families were affluent. All had an average household income of more than $95,000/year, all had a parent with at least a college degree, and 90.1% had a parent with a post-graduate degree (see Table 1 for additional demographic information). Recruitment procedures and inclusion and exclusion criteria were the same as in Study 1.
The measures and procedures in Study 2 were identical to those in Study 1 with the following exceptions: (a) Study 2 participants completed the Coping with Toddlers’ Negative Emotions Scale (CTNES; Fabes et al., 1990) due to differences in children’s ages between Studies 1 and 2; (b) Study 2 participants completed the short form of the Experiences in Close Relationships scale (ECR-S, Wei et al., 2007); (c) Study 2 research sessions typically took place within 2 weeks of one another; (d) Study 2 mothers always completed the ASA at home, never in the lab because the child participated in the study from the home; (e) Study 2 participants received additional instructions and practice to prepare them for the scan due to their younger ages; (f) Study 2 MRI Images were analyzed using Freesurfer version 6.0.0 (see footnote 2; Fischl et al., 2002; Fischl, 2012).
In Study 2, participants completed the same MR acclimation task and data acquisition procedures as in Study 1 with one additional step. Prior to their visit, Study 2 children completed an additional at-home MR acclimation task to increase scan success rate: Children lay in a fabric tunnel while they listened to MR noises and motion feedback from an experimenter.
Hippocampal and amygdala volumes were obtained via Freesurfer 6.0 (see footnote 2) and adjusted via the automated segmentation adapter tool (see footnote 3; Wang et al., 2011). Previous research has suggested that although different versions of Freesurfer produce nominally different values for subcortical volumes and cortical thickness (e.g., Gronenschild et al., 2012), ultimately these variations do not change outcomes in correlational research (Chepkoech et al., 2016; Bigler et al., 2020). We explored the potential effects of using different versions of Freesurfer in Study 1 and 2 by re-processing the cases from Study 2 in version 5.1. We then ran correlational analyses between volumes obtained from version 5.1 and 6.0 (rs ranged from 0.84 to 0.99) and replaced values obtained from version 6.0 with values from 5.1 in analyses that yielded significant effects (i.e., thalamus and amygdala). All results were similar, supporting our conclusion that the variation in Freesurfer version was not driving the observed effects. We retained the values from 5.1 as they were subjected to a more rigorous quality control procedure that was similar across the two studies.
Mothers completed a self-report measure of adult attachment anxiety and avoidance in close relationships (six anxiety items, five avoidance items; mean scores calculated for each subscale) (Wei et al., 2007). Each item is rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). ECR-S yields reliable and valid scores, with high correlations between the short and original versions for both anxiety (r = 0.94) and avoidance (r = 0.95) subscales (Wei et al., 2007). Although the ECR-S is a 12-item measure, one avoidance item was accidentally omitted (“I find that my partners don’t want to get as close as I would like”); however, the measure still demonstrated good internal consistency in the present study (anxiety, ω = 0.78, avoidance, ω = 0.90).
This questionnaire mirrors the Coping with Children’s Negative Emotions Scale (CCNES) and is adapted to reflect scenarios with toddlers (Spinrad et al., 2004). Following Gudmundson and Leerkes (2012), we created two indices: supportive and unsupportive responses to distress. Studies support the reliability and construct validity of the CTNES in preschool children (e.g., Eisenberg et al., 2010; Gudmundson and Leerkes, 2012), and both subscales demonstrated strong reliability in the present study (unsupportive ω = 0.93, supportive ω = 0.96).
Our full data analysis plan was pre-registered (see footnote 1). Consistent with this plan, we first examined race (coded as White and non-White) and household income as possible empirically derived covariates. We used Kendall’s Tau to explore whether household income related to amygdala or hippocampal volumes. We used independent samples t-tests to determine whether race (dichotomized as White or non-White) related to amygdala, and hippocampal volumes. None of the bivariate relations was significant (all ps > 0.05); thus, race and household income were not utilized as covariates. We included the a priori covariates of child age and sex in all of the following analyses because we expected them to relate to amygdala and hippocampal volumes; as predicted, both age and sex were significantly related to the brain measures of interest (see Table 2). Next, we used Pearson’s correlations to examine whether any of our three attachment representation variables (anxiety, avoidance, and secure base script knowledge) related to intracranial volume (referred to as estimated Total Intracranial Volume or eTIV), total GMV, subcortical GMV, or white matter volume (WMV). None of the bivariate correlations was significant (all ps > 0.05); thus eTIV, total gray matter, subcortical gray matter, and white matter volume were not utilized as covariates in the primary analyses.
Second, we conducted multiple regression analyses using Mplus version 5.2 (Muthén and Muthén, 1998-2007) to test our pre-registered hypotheses that mothers’ attachment representations would relate to bilateral amygdala and hippocampal volumes, and to test our follow-up exploratory questions about relations among attachment representations and unilateral amygdala volumes and hippocampal subregions. Following Mueller and Hancock (2010), we utilized full information maximum likelihood estimation (FIML) to handle missing data. We used participant mean imputation to estimate scores for participants with missing item responses on a given scale, a statistically sound technique when less than 10% of item scores are missing (Schafer and Graham, 2002; Parent, 2013). To control for the potential of type 1 error, we conducted sensitivity analyses utilizing certain regions of the brain we did not expect to relate to caregiving/attachment representations (the thalamus and occipital cortex, see Humphreys et al., 2018).
Third, we conducted analyses of indirect effects using Mplus’ MODEL INDIRECT procedure (Stride et al., 2015). This procedure utilizes bootstrapping methods to estimate confidence intervals around the indirect effect. As recommended by Preacher and Hayes (2008) analyses were conducted with 5,000 bootstrapped samples. We used this procedure to test our pre-registered hypotheses regarding indirect effects of mothers’ attachment representations on children’s bilateral hippocampal and amygdala volumes through unsupportive responses to children’s distress, and to test our follow-up exploratory questions about the above indirect effects through supportive responses.
Finally, we conducted an exploratory vertex-wise whole-brain analysis in Freesurfer’s Qdec application to test links between mothers’ attachment representations, cortical thickness, and surface area. Monte Carlo simulations were used to correct for multiple comparisons. The final whole-brain significance threshold was set at p < 0.05.
Table 2 displays bivariate correlations among all major study variables and Table 3 presents descriptive statistics for all major study variables. Out of the 52 participants, three were missing MRI data, seven were missing attachment avoidance and anxiety, and six were missing supportive and unsupportive responses to children’s distress. Missing values were handled with FIML.
After controlling for child age and sex, mothers’ secure base script knowledge marginally predicted smaller bilateral amygdala volumes (β = –0.19, p = 0.060). To explore this finding further, we examined unilateral amygdala volumes. Greater secure base script knowledge predicted smaller left (β = –0.21, p = 0.031; see Figure 1) but not right (β = –0.13, p = 0.229) amygdala volumes. Neither attachment anxiety (β = –0.14, p = 0.252) nor attachment avoidance (β = –0.05, p = 0.718) predicted bilateral amygdala volumes. There were no significant relations between secure base script knowledge, anxiety, or avoidance and bilateral hippocampal volumes (all ps > 0.295). To explore possible associations between mothers’ attachment representations and hippocampal subregions, we examined whether secure base script knowledge, anxiety, and avoidance related to hippocampal head, body, and tail volumes. There were no significant relations between any measures of attachment representations and hippocampal head, body, or tail volumes; however, secure base scripts knowledge marginally predicted smaller hippocampal head volumes (β = –0.21, p = 0.078), but anxiety (β = –0.18, p = 0.243) and avoidance (β = 0.03, p = 0.876) did not.
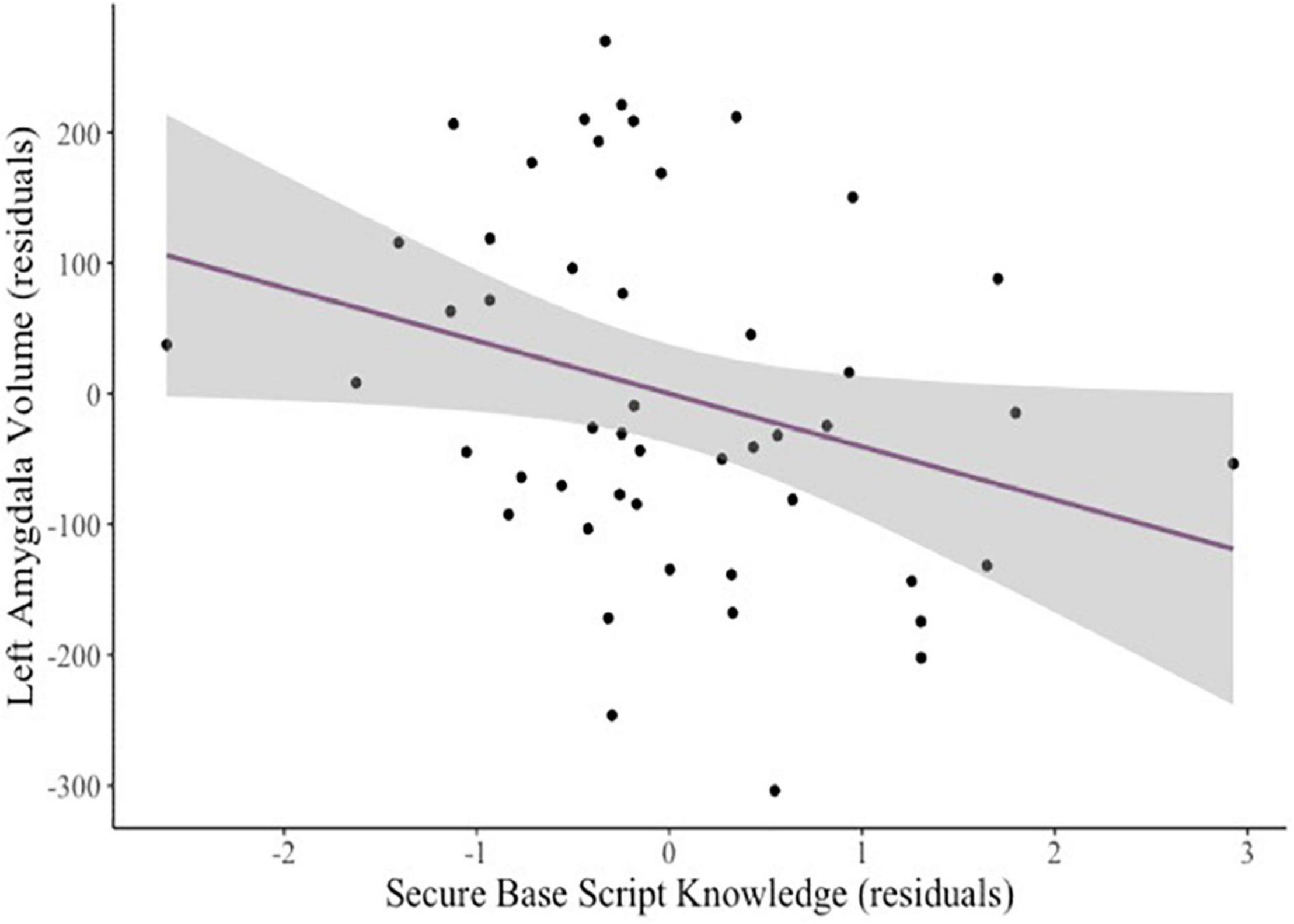
Figure 1. Added variable plot displaying the partial correlation between mothers’ secure base script knowledge and children’s left amygdala volume controlling for covariates (sex and age). The shaded region reflects the 95% confidence interval around the regression line.
In a pre-registered follow up sensitivity analysis to explore specificity of the associations of secure base script knowledge and amygdala volume, we examined two regions we did not expect to relate to secure base script knowledge: The occipital cortex and the thalamus (Humphreys et al., 2018). As expected, there was no relation between secure base script knowledge and occipital cortex volume (β = –0.08, p = 0.548). However, contrary to our expectations, greater secure base script knowledge (β = –0.37, p = 0.001; see Figure 2) and greater attachment anxiety (β = –0.30, p = 0.039) predicted smaller thalamus volume.
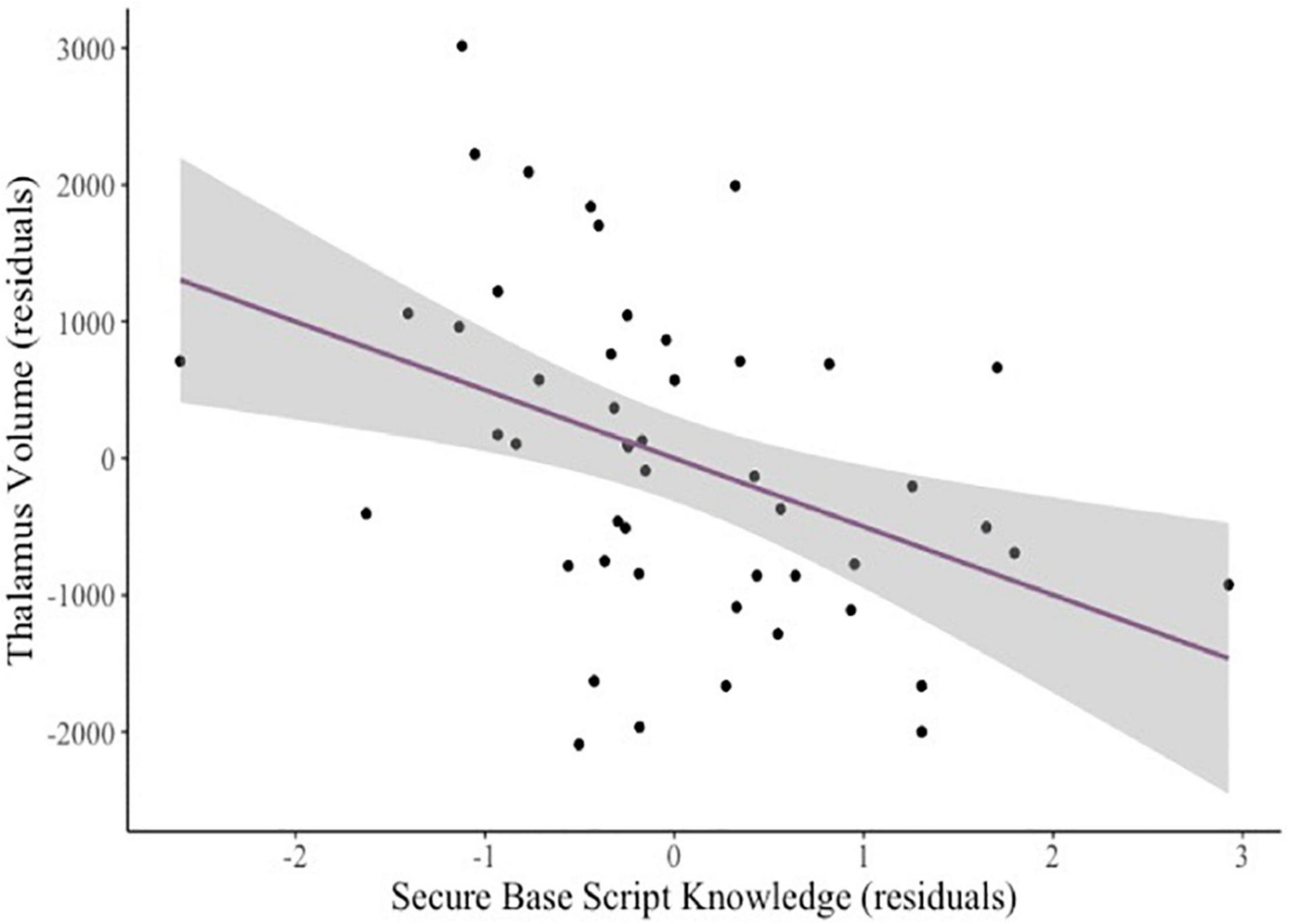
Figure 2. Added variable plot displaying the partial correlation between mothers’ secure base script knowledge and children’s thalamus volume controlling for covariates (sex and age). The shaded region reflects the 95% confidence interval around the regression line.
We tested whether mothers’ self-reported caregiving in response to children’s distress explained indirect relations between attachment representations and bilateral hippocampal and amygdala volumes. There were no significant indirect effects of secure base script knowledge, attachment avoidance, or attachment anxiety on bilateral amygdala volumes or hippocampal volumes through unsupportive responses to distress (all ps > 0.649). The same pattern held when exploring the role of supportive responses to children’s distress (all ps > 0.232). There were also no significant direct links between bilateral hippocampal and amygdala volume and either supportive or unsupportive responses to distress (all ps > 0.402). Mothers’ attachment anxiety predicted less supportive responses to distress (β = –0.45, p = 0.001). Contrary to our predictions, neither secure base script knowledge nor attachment avoidance predicted supportive or unsupportive responses to distress (all ps > 0.271).
To explore whether mothers’ attachment representations related to whole brain metrics, we tested relations among our attachment predictors and intracranial volume (eTIV), total GMV, subcortical GMV, and WMV controlling for age and sex. There were no significant relations between eTIV and secure base script knowledge (β = –0.19, p = 0.108), attachment anxiety (β = –0.02, p = 0.904), or attachment avoidance (β = –0.16, p = 0.264). There were also no significant relations between total GMV and secure base script knowledge (β = –0.19, p = 0.106), attachment anxiety (β = –0.16, p = 0.266) or attachment avoidance (β = 0, p = 1.00). No significant relations between attachment predictors and subcortical GMV emerged (all ps > 0.409). There was a significant negative relation between WMV and secure base script knowledge (β = –0.27, p < 0.009; see Figure 3) but not attachment anxiety (β = –0.03, p = 0.788) or avoidance (β = –0.12, p = 0.384).
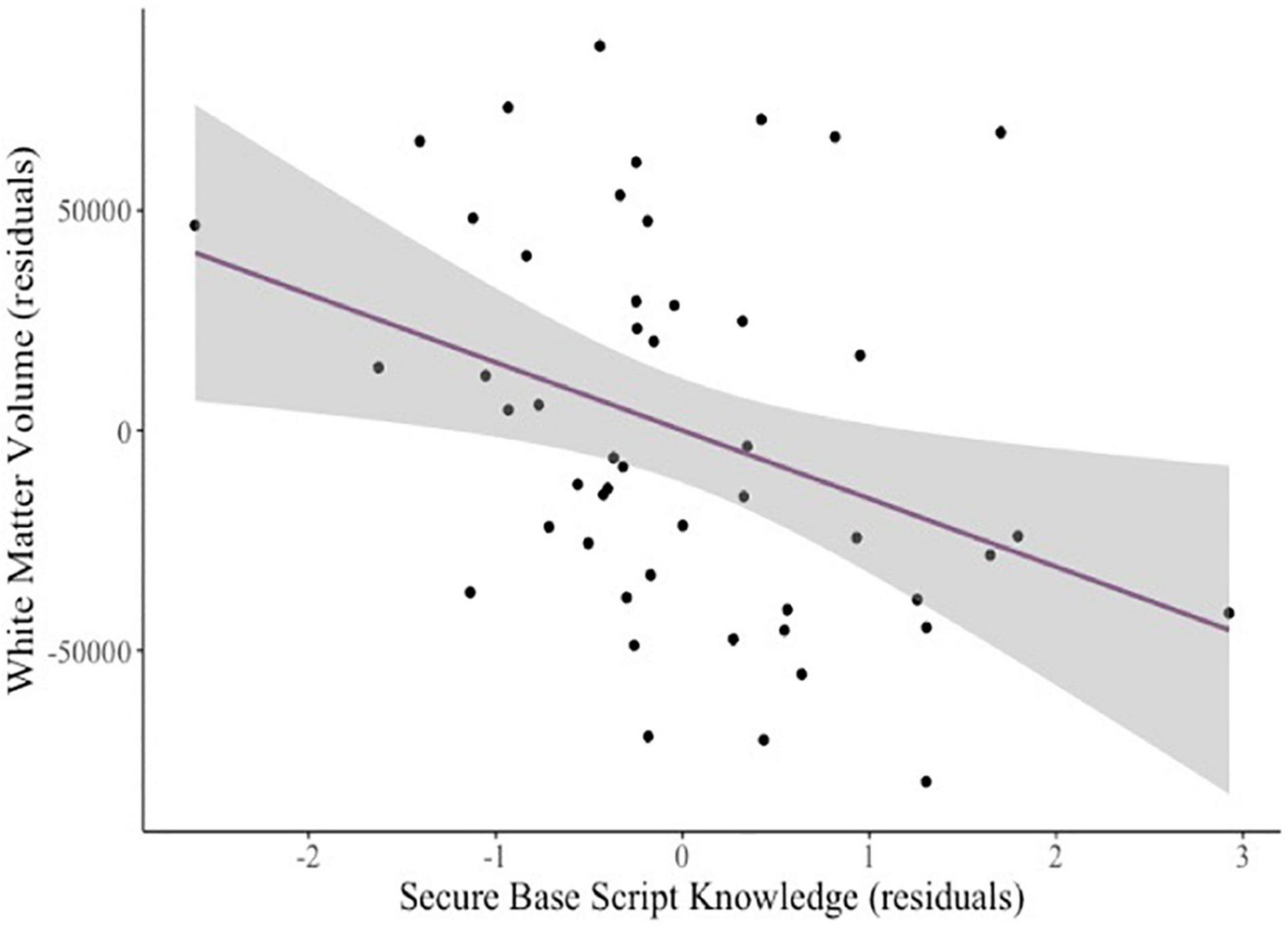
Figure 3. Added variable plot displaying the partial correlation between mothers’ secure base script knowledge and children’s white matter volume controlling for covariates (sex and age). The shaded region reflects the 95% confidence interval around the regression line.
To explore whether a whole brain effect may be contributing to the relation between secure base script knowledge and left amygdala volume and thalamus volume, we added eTIV to the models predicting left amygdala volume and thalamus volume, controlling for child sex and age. When including eTIV in the model, the relation between secure base script knowledge and the left amygdala became non-significant (β = –0.15, p = 0.108). The relation between secure base script knowledge and thalamus volume, however, remained significant when controlling for eTIV (β = –0.29, p = 0.006).
Finally, consistent with our pre-registered approach, we utilized a vertex-by-vertex analysis to determine whether mothers’ attachment representations related to children’s cortical thickness and surface area. The vertex-by-vertex whole brain analysis did not demonstrate any relations among our attachment predictors of interest and cortical thickness. However, greater secure base script knowledge predicted greater surface area of the right pericalcarine cortex (p < 0.05). This pattern of results remained the same when the 14 subjects who had the largest numbers of edits (i.e., greater than the mean or more than 15) were removed.
The present study is first to our knowledge to examine links between mothers’ attachment representations and children’s brain structure, and to explore potential indirect effects via caregiving behavior in response to child distress. In a non-clinical sample, we examined two conceptualizations of mothers’ attachment representations: self-reported attachment style and secure base script knowledge (i.e., mothers’ procedural knowledge of how a secure base is provided in times of need; Waters and Waters, 2006). Mothers’ greater secure base script knowledge, but not attachment style, was associated with smaller left amygdala volume in early childhood. In contrast, mothers’ attachment representations were not significantly related to children’s hippocampal volume. Pre-registered exploratory analyses revealed that mothers’ greater secure base script knowledge was also associated with children’s smaller WMV, smaller thalamus volume, and larger surface area of the right pericalcarine cortex, whereas maternal representations were unrelated to cortical thickness (see Figures 4, 5 for visualizations of cortical and subcortical structures). Contrary to predictions, we observed no indirect effects via caregiving behavior in response to child distress. Findings highlight the role that parental attachment representations—particularly secure base script knowledge—may play in children’s brain structure and add to a growing literature on the ways in which parental factors shape the developing brain in childhood (Belsky and de Haan, 2011; Farber et al., 2020). We discuss each finding in turn and outline directions for future research.
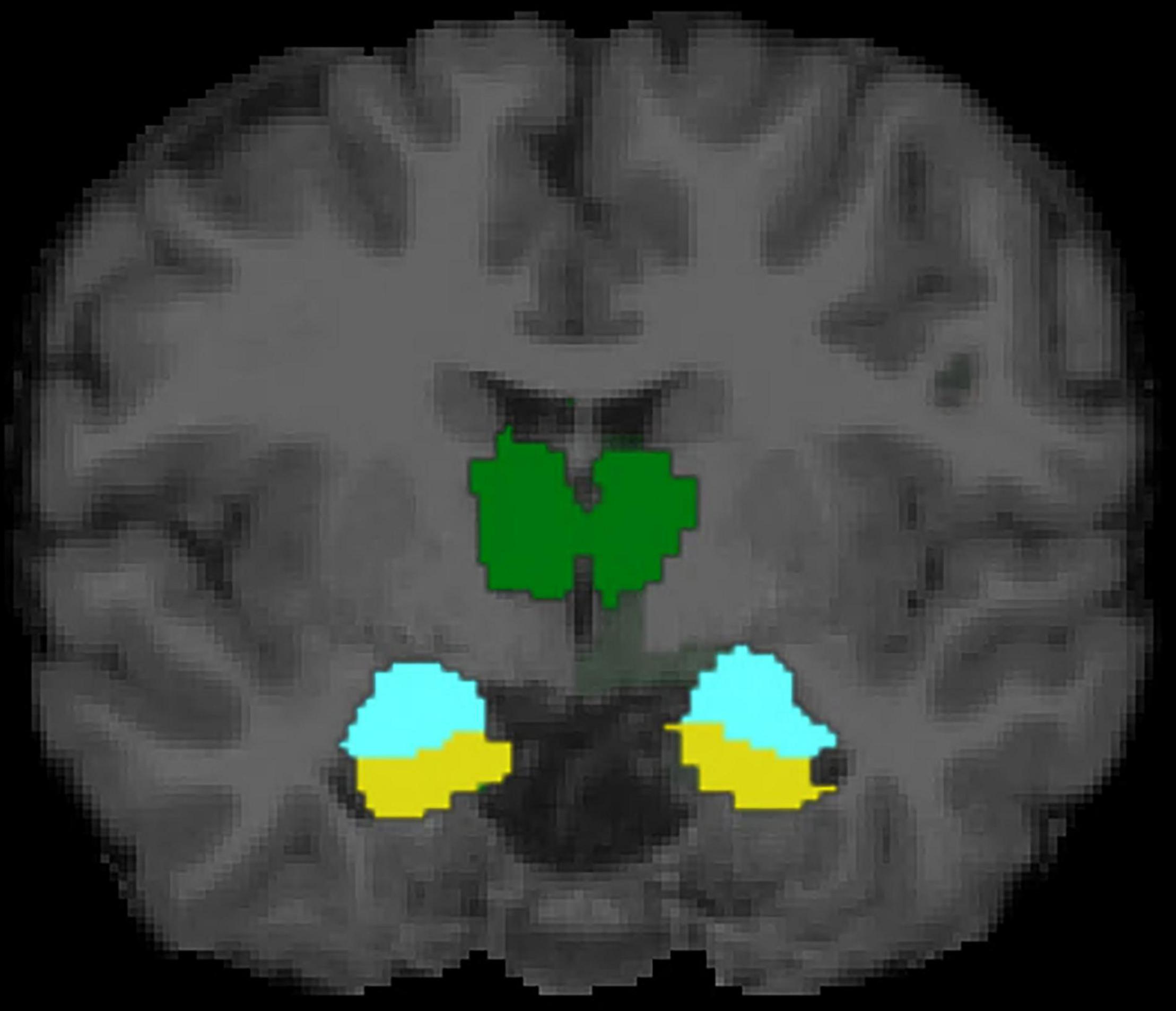
Figure 4. Subcortical structures of interest in mid-coronal view. Hippocampus depicted in yellow, amygdala depicted in turquoise, and thalamus depicted in green.
The finding that mothers’ secure base script knowledge was associated with children’s smaller left amygdala volume (and marginally to smaller bilateral volume) provides partial support for our hypotheses. Mothers’ secure base script knowledge involves a schema for the effective co-regulation of distress and protection from potential threats in the child’s environment (Waters and Waters, 2006). Children’s experiences of sensitive care to help manage distress, particularly in times of threat, help them learn to self-regulate (Hofer, 1995; Luecken and Lemery, 2004; Köhler-Dauner et al., 2019). Indeed, the function of a secure base is to protect children from threat (Bowlby, 1969/1982), and experiences of secure base provision (guided by mothers’ scripts) may be especially important for the development of neurobiological systems involved in threat responding, including the amygdala (Cassidy et al., 2013). In addition, mothers’ secure base script knowledge has been linked to child secure attachment (e.g., Bost et al., 2006), and secure attachment is a robust predictor of self-regulation (see Cassidy, 1994; Calkins and Leerkes, 2011). Thus, maternal secure base script knowledge may be particularly important for the development of neural circuits involved in stress reactivity and self-regulation. This aligns with the theory that the extent to which sensitive caregivers can co-regulate children’s distress shapes amygdala development (Callaghan and Tottenham, 2016a) and children’s physiological responses to stressful situations (Köhler-Dauner et al., 2019), and with research linking negative parenting to accelerated maturation and larger amygdala volume on the one hand (Tottenham et al., 2010) and positive parenting to smaller amygdala volume on the other hand (Whittle et al., 2014; Rifkin-Graboi et al., 2015). Together, findings suggest that the full spectrum of parenting experiences—not only adversity but also normative variation in parental characteristics—merit attention to understand ways in which the social environment is reflected in brain structure (Belsky and de Haan, 2011; Farber et al., 2020).
Although we had a priori hypotheses regarding links between amygdala volume and mothers’ secure base script knowledge driven by theory (Cassidy, 1994; George and Solomon, 2008; Callaghan and Tottenham, 2016a) and empirical research (Tottenham et al., 2010; Rifkin-Graboi et al., 2015), it is important to note that our hypotheses involved bilateral amygdala volume; our examination of unilateral volumes arose from a data-driven decision to probe the marginal effect of secure base script knowledge on bilateral amygdala volume (p = 0.060) that was not included in our pre-registration. The exploratory nature and small effect size of secure base script knowledge on left amygdala volume led us to interpret these findings as preliminary. We encourage efforts to replicate these initial findings in larger samples.
It is also important to note that the observed association with amygdala volume became non-significant when adjusting for intracranial volume (eTIV), suggesting that effects may not be localized to the amygdala but may have more widespread effects; however, direct associations with eTIV and total GMV were not significant in the present study. Future research should consider effects on additional regions of interest, as well as metrics of global brain development and change in these measures over time.
What is not yet clear is exactly how parental secure base script knowledge is linked to child amygdala volume. Previous work shows that secure base script knowledge predicts greater parental sensitivity, positive affect, emotional support, and lower hostility and intrusiveness (Coppola et al., 2006; Huth-Bocks et al., 2014; Waters et al., 2017). In this study, however, secure base script knowledge was unrelated to mothers’ responses to child distress, and these caregiving behaviors in turn were unrelated to amygdala volume. One explanation is that the specific dimensions of caregiving assessed in present study—namely, mother-reported supportive and unsupportive responses to distress—may not be relevant mediators. Instead, observed parental sensitivity, secure base provision, autonomy support, positive affect, emotion regulation, emotional availability, sensitive touch, and engagement (vs. disengagement or neglect), as well as child factors such as attachment and emotion regulation, merit examination in future studies. Relatedly, it is possible that social desirability limited the extent to which true variation in maternal supportive and unsupportive responses to distress could be adequately captured via self-report, although ample previous work has linked this caregiving measure to maternal attachment representations (Jones et al., 2014) and observed caregiving behavior (e.g., Spinrad et al., 2007). In the present study, however, mother-reported supportive responses were highly negatively skewed, limiting variability. Some previous studies that find significant links between parenting and hippocampal volume have used observational measures of lab-based parent–child interactions (e.g., Blankenship et al., 2019); future work using observational assessments of parents’ response to distress may reveal greater variability, with meaningful links to both parent representations and child brain development. Further, future work using observational assessments with clinical samples could leverage greater variability to assess the possibility that such links manifest only at the extreme ends of the caregiving spectrum.4
An additional explanation for our results may be developmental timing: It is possible that caregiver responses to child distress are more salient mediators during sensitive periods for the development of attachment and for brain development, such as infancy; indeed, much of the previous work linking parental sensitivity to amygdala volume has focused on caregiving during infancy (e.g., Rifkin-Graboi et al., 2015; Bernier et al., 2019) or beginning in infancy (e.g., Tottenham et al., 2010; Lupien et al., 2011). It is also possible that the indirect effects of mothers’ representations unfold over time, such that parental secure base script knowledge predicts relative changes in caregiving, or that positive caregiving predicts relative changes in amygdala development that are best captured longitudinally, similar to findings that positive parenting predicts less relative growth of the amygdala in adolescence (Whittle et al., 2014).
Contrary to hypotheses, mothers’ attachment representations were not significantly related to hippocampal volume; only a marginal association was observed between secure base scripts and smaller hippocampal head volumes. To the extent that more secure representations forecast positive parenting (e.g., Coppola et al., 2006; Huth-Bocks et al., 2014), these findings contrast with some previous work linking positive parenting in early childhood to larger hippocampal volume among school-age children with and without depressive symptoms (e.g., Luby et al., 2012), and are instead more consistent with the null results reported in two studies with non-clinical samples in early childhood (Kok et al., 2015; Lee et al., 2019). Importantly, however, there was also no indirect effect of parent representations on hippocampal volume via mother-reported caregiving in response to distress, in part because response to distress was unrelated to hippocampal volume in the present sample. One possibility is that our focus on hippocampal structure is too coarse, as we did not have resolution to look at functional subfields. Future work could examine potential associations between parental mental representations and hippocampal subfields such as the dentate gyrus, building on previous work linking parental maltreatment with smaller left CA4-DG subfield volumes (Teicher et al., 2012). It may be that normative variations in caregiving are not enough to produce substantial change in the hippocampus. Perhaps the hippocampus is only sensitive to particularly high levels of stress. This is plausible, given the other null results in normative samples in early childhood (Kok et al., 2015; Lee et al., 2019) and the possible issue of publication bias. Or perhaps no direct link exists, though there may be indirect effects via additional caregiving and child mechanisms (e.g., observed parental sensitivity, child attachment), which warrant future research.
In follow-up exploratory analyses, mothers’ greater secure base script knowledge was associated with children’s smaller thalamus volume, smaller WMV, and larger surface area of the right pericalcarine cortex; mothers’ attachment anxiety was associated with children’s smaller thalamus volume; and maternal representations were not associated with children’s cortical thickness in any brain regions. Given their exploratory nature, we interpret them with caution.
Although not hypothesized a priori, findings regarding the thalamus are consistent with some previous work. Findings could be due to the fact that the thalamus and amygdala work together in stress-response circuitry, as demonstrated in research with animals (Spencer et al., 2004; Penzo et al., 2015; Wei et al., 2015) and humans (Öhman, 2005). For instance, one animal study found that the paraventricular nucleus of the thalamus (PVT) and the amygdala worked together in a circuit that established fear memory and facilitated fear responses during a fear conditioning task in mice (Penzo et al., 2015). Research in humans has demonstrated a link between children’s disorganized attachment (a correlate of insensitive care; Moran et al., 2008) and smaller gangliothalamic ovoid (comprised of the thalamus and the basal ganglia) diameter in infancy (Tharner et al., 2011). Maternal sensitivity has also been linked to smaller subcortical GMV (which includes the thalamus, as well as the caudate, putamen, and globus pallidus) in infancy (Sethna et al., 2017). It is surprising, however, that greater secure base script knowledge (indicating secure representations) and greater attachment anxiety (indicating insecure representations) predicted thalamus volume in the same direction. This region warrants further investigation in studies of parenting and child brain structure.
Findings regarding WMV contrast with research suggesting that smaller WMV is associated with more negative parenting experiences (Belsky and de Haan, 2011). Notably, however, the majority of these studies focus on neglected and maltreated samples (but see Sethna et al., 2017 for evidence in infants from a community sample). One possibility is that a curvilinear relation exists across the spectrum of caregiving experiences, with experiences of extreme caregiving adversity linked to abnormally small volumes across a number of brain structures (e.g., McLaughlin et al., 2019), but normative variation in caregiving quality associated with volumes in the opposite direction (although still within typical ranges, e.g., Rifkin-Graboi et al., 2015; Bernier et al., 2019). Findings regarding the right pericalcarine cortex contrast with the broader literature about structures sensitive to caregiving experiences (Belsky and de Haan, 2011; Farber et al., 2020), and given our small sample, underscore the need for future research.
That there were no significant associations between secure base script knowledge and total GMV, subcortical GMV, or intracranial volume may similarly reflect different patterns of association in non-clinical samples, compared to what we know about caregiving adversity, and/or possible non-linear associations. Although eTIV and GMV did not meet conventional thresholds for statistical significance (ps ∼0.10), given the exploratory nature of the work, we point out these relations as an area for future investigation with a larger sample to increase power to detect potential small effects. Another interpretation is that effects may be specific to structures involved in stress regulation and are not driven by a whole brain effect. An additional possibility is that genotype plays a role in how parental characteristics relate to child brain structure. For example, one study found that maternal acceptance was positively associated with regional GMV of the left thalamus for adolescent carriers of the FKBP5-T allele, but negatively associated for those without this allele (Matsudaira et al., 2019). Thus, these preliminary findings may be better understood by leveraging genetic research to examine gene by caregiving interactions.
Across our analyses, mothers’ secure base script knowledge appeared to be a more salient predictor of child brain structure, whereas maternal attachment style was largely not significant. One explanation for this difference in the predictive strength of these aspects of maternal representations is that secure base script knowledge taps scripts that reflect mothers’ own early experience receiving care, whereas self-reported adult attachment style dimensions tap experiences in current close relationships—evidenced by the relatively greater link of early sensitive care to secure base script knowledge than to attachment style (Steele et al., 2014). A substantial body of research demonstrates that narrative assessments of attachment representations such as the Adult Attachment Interview and secure base script knowledge predict child outcomes such as attachment security (e.g., Verhage et al., 2016), compared to a much smaller but growing literature on parental attachment style (Jones et al., 2015). Thus, although both attachment styles and secure base script knowledge may guide parenting behavior, parents’ scripts of how distress is met with sensitive care and co-regulation may be more important for child attachment security and in turn, the associated development of brain circuits that support the regulation of stress. Another reason may be that attachment style reflects emotion regulation strategies in close relationships broadly, whereas half of the secure base script stories refer specifically to parent–child relationships and may therefore tap into the caregiving system in ways more directly related to child brain development. More specifically, it is possible that secure base script knowledge is a better indicator of the extent to which mothers are able to effectively co-regulate their child’s distress, which in turn is linked to the development of neural circuits involved in children’s self-regulation. We cautiously suggest that mothers’ secure base script knowledge is a more relevant attachment representation than attachment style for predicting brain structure in early childhood.
Strengths of the study include its novel examination of parents’ attachment representations as a potential source of individual differences in child brain structure; its assessment of multiple conceptualizations of parents’ representations (attachment style and secure base script knowledge), including a gold standard task-based measure of secure base script knowledge; and its focus on an important period of neurodevelopment spanning preschool to school-age.
These findings, however, should be considered along with the study’s limitations. First, the small sample size and its lack of diversity limit the strength of the conclusions that can be drawn from this study, and results should be regarded as preliminary. Relatedly, we did not have adequate power to test potential moderators, such as child age, gender, race/ethnicity and exposure to discrimination, SES, or temperament. It is possible that maternal representations and/or caregiving behaviors are more strongly related to brain development among specific groups of children, such as younger children, temperamentally fearful children (who may be particularly susceptible to their caregiving environment; Belsky and Pluess, 2009), or children exposed to poverty and other stressors (i.e., reflecting a caregiving stress-buffering effect; Luby et al., 2013). Future work in larger samples across a wider age range and demographic profile could test these moderators to better characterize how factors within the child, parent, and broader bioecological context relate to individual differences in brain development. Further, effect sizes were small and research in larger samples is crucial for testing the robustness and replicability of the findings.
Second, the design was cross-sectional and correlational. Thus, although our theoretical model was based on theory and previous research, full criteria for mediation were not met because all variables were assessed at the same time point. Relatedly, we cannot establish directionality or causality; it is possible that aspects of child brain development give rise to child behaviors that elicit certain caregiving responses, or that underlying shared genetic factors inform both parental characteristics and child brain development. Thus, an important direction for future longitudinal work is to examine how parental representations, caregiving, and child brain development unfold over time (e.g., using cross-lagged analyses to establish the direction of effects), whether associations change with development, and whether sensitive periods exist for parental representations and behaviors to demonstrate links with specific brain structures. Further, experimental work could examine whether parenting interventions to shift parental attachment representations have downstream effects on child brain development. One study shows that attachment-based parenting interventions such as Attachment and Biobehavioral Catch-up (ABC; Dozier and Bernard, 2019) in infancy impact brain function in middle childhood (Valadez et al., 2020). Whether such effects are predicted by intervention-related changes in maternal representations, and whether effects extend to child brain structure, are open questions.
Third, caregiving behavior was assessed via self-report, subject to reporter bias and social desirability. Thus, it will be important to incorporate observational measures of caregiving to better understand the mechanisms by which parental representations may relate to brain structure.
Finally, although we focused on brain structure as a starting point for future research on this topic, it is possible that mothers’ attachment representations link more strongly to brain function (see Cruciani et al., 2021, for evidence of attachment-related differences in functional connectivity in the amygdala and the hippocampus). For example, research has indicated that adverse caregiving experiences can hinder the development of neural circuitry critical for stress-regulation, specifically, connectivity between the amygdala and PFC (Burghy et al., 2012; Tottenham, 2015). The amygdala is particularly sensitive to threatening stimuli, and as the PFC matures, it couples with the amygdala to modulate emotional responses to threat and reduce distress (Banks et al., 2007; Tottenham, 2015). The presence of a supportive caregiver to co-regulate a child’s distress during sensitive periods of brain development provides the scaffolding for optimal amygdala-PFC connectivity (Tottenham, 2015; Callaghan and Tottenham, 2016b). Such scaffolding can foster the amygdala-PFC coupling necessary for children’s self-regulation when a caregiver is not present (Banks et al., 2007; Dougherty et al., 2015). To the extent that mothers’ attachment representations link to caregiving behaviors that co-regulate stress (Jones et al., 2014, 2015), children’s amygdala-PFC connectivity could be a mechanism through which mothers’ attachment representations “get under the skin” and shape the development of children’s self-regulatory capacities. Studies examining links of parental representations to child brain function (e.g., amygdala–PFC connectivity, amygdala reactivity to stressors; Callaghan and Tottenham, 2016a) could be a fruitful next step.
Central to attachment theory is the idea that mental representations of close relationships guide caregiving behavior and help organize the social-emotional development of the next generation (Main et al., 1985; Steele et al., 2014). The present study integrates two caregiving literatures—one demonstrating that parents’ secure mental representations support sensitive caregiving and child social-emotional adaptation (e.g., Raby et al., 2021), particularly self-regulation (e.g., Madigan et al., 2007; Waters et al., 2015), and another demonstrating that parenting behaviors meaningfully shape child brain development, particularly neural structures involved in stress regulation circuitry (e.g., the amygdala; Callaghan and Tottenham, 2016a). Our preliminary findings highlight a possible role of parents’ secure base script knowledge specifically in predicting amygdala development in early childhood; however, substantial questions remain regarding potential mechanisms, the role of developmental timing of caregiving experiences, and the generalizability of these findings to other populations (e.g., non-WEIRD samples) and ages (e.g., adolescents). Results extend findings linking mothers’ secure base script knowledge to child outcomes (e.g., Steele et al., 2014) and point to parental mental representations as an important factor to enrich research on the caregiving correlates of child brain development.
The datasets presented in this article are not readily available because of concerns related to participant confidentiality. Requests to access the datasets should be directed to TR, cmlnZ2luc0B1bWQuZWR1.
The studies involving human participants were reviewed and approved by University of Maryland Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
MF: coordinating the project, generating research questions, conducting statistical analyses, writing, and editing. JS: generating research questions, coding the Attachment Script Assessment, writing, and editing. MS: coding the Attachment Script Assessment, writing, editing, and generating research questions. TA: recruiting participants, collecting data, generating research questions, consulting, processing and analyzing MRI data, assisting with statistical analyses, and editing. JC: generating research questions, consulting, writing, and editing. TR: designing and overseeing Study 1 and Study 2, recruiting participants, collecting data, generating research questions, consulting, writing, and editing. All authors: contributed to the article and approved the submitted version.
Preparation of this manuscript was supported by a University of Maryland College of Behavioral and Social Sciences Dean’s Fellowship and by summer support from the University of Maryland’s Janet Johnson Fund to MF. The Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) provided support to JS (Award Number F32HD102119) and TR (Award Numbers R01HD079518 and R21HD094758). In addition, the National Science Foundation provided support to TR (Award Number BCS1749280).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Harriet Waters for providing training in coding the Attachment Script Assessment. We also want to acknowledge members of the Neurocognitive Development Lab for helping with data collection. Finally, we wish to express their gratitude to participating families.
Allard, T., Riggins, T., Ewell, A., Weinberg, B., Lokhandwala, S., and Spencer, R. M. C. (2019). Measuring neural mechanisms underlying sleep-dependent memory consolidation during naps in early childhood. J. Vis. Exp. 152:e60200. doi: 10.3791/60200
Avants, B. B., Hackman, D. A., Betancourt, L. M., Lawson, G. M., Hurt, H., and Farah, M. J. (2015). Relation of childhood home environment to cortical thickness in late adolescence: specificity of experience and timing. PLoS One 10:e0138217. doi: 10.1371/journal.pone.0138217
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., and Phan, K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2, 303–312. doi: 10.1093/scan/nsm029
Barch, D. M., Harms, M. P., Tillman, R., Hawkey, E., and Luby, J. L. (2019). Early childhood depression, emotion regulation, episodic memory, and hippocampal development. J. Abnorm. Psychol. 128, 81–95. doi: 10.1037/abn0000392
Belsky, J., and de Haan, M. (2011). Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry 52, 409–428. doi: 10.1111/j.1469-7610.2010.02281.x
Belsky, J., and Pluess, M. (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 135, 885–908. doi: 10.1037/a0017376
Bernier, A., Dégeilh, F., Leblanc, É, Daneault, V., Bailey, H. N., and Beauchamp, M. H. (2019). Mother–infant interaction and child brain morphology: a multidimensional approach to maternal sensitivity. Infancy 24, 120–138. doi: 10.1111/infa.12270
Bigler, E. D., Skiles, M., Wade, B. S. C., Abildskov, T. J., Tustison, N. J., Scheibel, R. S., et al. (2020). FreeSurfer 5.3 versus 6.0: are volumes comparable? A Chronic Effects of Neurotrauma Consortium study. Brain Imaging Behav. 14, 1318–1327. doi: 10.1007/s11682-018-9994-x
Blankenship, S., Chad-Friedman, E., Riggins, T., and Dougherty, L. R. (2019). Early parenting predicts hippocampal subregion volume via stress reactivity in childhood. Dev. Psychobiol. 61, 125–140. doi: 10.1002/dev.21788
Blaze, J., Scheuing, L., and Roth, T. L. (2013). Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev. Neurosci. 35, 306–316. doi: 10.1159/000350716
Bost, K. K., Shin, N., Mcbride, B. A., Brown, G. L., Vaughn, B. E., Coppola, G., et al. (2006). Maternal secure base scripts, children’s attachment security, and mother–child narrative styles. Attach. Hum. Dev. 8, 241–260. doi: 10.1080/14616730600856131
Botdorf, M., and Riggins, T. (2018). When less is more: thinner fronto-parietal cortices are associated with better forward digit span performance during early childhood. Neuropsychologia 121, 11–18. doi: 10.1016/j.neuropsychologia.2018.10.020
Bowlby, J. (1969/1982). Attachment and Loss: Attachment, 2nd Edn, Vol. 1. New York, NY: Basic Books. (Original work published 1969).
Brennan, K. A., Clark, C. L., and Shaver, P. R. (1998). “Self-report measurement of adult attachment: an integrative overview,” in Attachment Theory and Close Relationships, eds J. A. Simpson and W. S. Rholes (New York, NY: Guilford Press), 46–76.
Bretherton, I. (1985). Attachment theory: retrospect and prospect. Monogr. Soc. Res. Child Dev. 50, 3–38.
Bretherton, I. (1987). “New perspectives on attachment relations: security, communication, and internal working models,” in Handbook of Infant Development, ed. J. D. Osofsky (New York, NY: John Wiley & Sons), 1061–1100.
Bretherton, I. (1991). “Pouring new wine into old bottles: the social self as internal working model,” in Minnesota Symposium on Child Psychology: Self Processes in Development, Vol. 23, eds M. Gunnar and L. A. Sroufe (Hillsdale, NJ: Erlbaum), 1–41.
Bretherton, I., and Munholland, K. A. (2016). “The internal working model construct in light of contemporary neuroimaging research,” in Handbook of Attachment: Theory, Research, and Clinical Applications, 2nd Edn, eds J. Cassidy and P. R. Shaver (New York, NY: Guilford Press), 63–88.
Burghy, C. A., Stodola, D. E., Ruttle, P. L., Molloy, E. K., Armstrong, J. M., Oler, J. A., et al. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 15, 1736–1741. doi: 10.1038/nn.3257
Calkins, S. D., and Leerkes, E. M. (2011). “Early attachment processes and the development of emotional self-regulation,” in Handbook of Self-Regulation: Research, Theory, and Applications, eds K. D. Vohs and R. F. Baumeister (New York, NY: Guilford Press), 355–373.
Callaghan, B. L., and Tottenham, N. (2016a). The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 7, 76–81. doi: 10.1016/j.cobeha.2015.11.018
Callaghan, B. L., and Tottenham, N. (2016b). The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41, 163–176. doi: 10.1038/npp.2015.204
Canada, K., Ngo, C. T., Newcombe, N. S., Geng, F., and Riggins, T. (2019). It’s all in the details: relations between young children’s developing pattern separation abilities and hippocampal subfield volumes. Cereb. Cortex 29, 3427–3433. doi: 10.1093/cercor/bhy211
Cassidy, J. (1994). Emotion regulation: influences of attachment relationships. Monogr. Soc. Res. Child Dev. 59, 228–249. doi: 10.1111/j.1540-5834.1994.tb01287.x
Cassidy, J., Ehrlich, K. B., and Sherman, L. J. (2013). “Child-parent attachment and response to threat: a move from the level of representation,” in Nature and Development of Social Connections: From Brain to Group, eds M. Mikulincer and Shaver, P. R. (American Psychological Association), 125–144.
Chepkoech, J. L., Walhovd, K. B., Grydeland, H., Fjell, A. M., and Alzheimer’s Disease Neuroimaging Initiative (2016). Effects of change in FreeSurfer version on classification accuracy of patients with Alzheimer’s disease and mild cognitive impairment. Hum. Brain Mapp. 37, 1831–1841. doi: 10.1002/hbm.23139
Conrad, C. D. (2009). Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 19, 395–411. doi: 10.1515/revneuro.2008.19.6.395
Coppola, G., Vaughn, B. E., Cassibba, R., and Costantini, A. (2006). The attachment script representation procedure in an Italian sample: associations with Adult Attachment Interview scales and with maternal sensitivity. Attach. Hum. Dev. 8, 209–219. doi: 10.1080/14616730600856065
Cruciani, G., Boccia, M., Lingiardi, V., Giovanardi, G., Zingaretti, P., and Spitoni, G. F. (2021). An exploratory study on resting-state functional connectivity in individuals with disorganized attachment: evidence for key regions in amygdala and hippocampus. Brain Sci. 11:1539.
Dougherty, L. R., Blankenship, S. L., Spechler, P. A., Padmala, S., and Pessoa, L. (2015). An fMRI pilot study of cognitive reappraisal in children: divergent effects on brain and behavior. J. Psychopathol. Behav. Assess. 37, 634–644. doi: 10.1007/s10862-015-9492-z
Dozier, M., and Bernard, K. (2019). Coaching Parents of Vulnerable Infants: The Attachment and Biobehavioral Catch-Up Approach. New York, NY: Guilford Press.
Ducharme, S., Albaugh, M. D., Hudziak, J. J., Botteron, K. N., Nguyen, T. V., Truong, C., et al. (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex 24, 2941–2950. doi: 10.1093/cercor/bht151
Dykas, M. J., Woodhouse, S. S., Cassidy, J., and Waters, H. S. (2006). Narrative assessment of attachment representations: links between secure base scripts and adolescent attachment. Attach. Hum. Dev. 8, 221–240. doi: 10.1080/14616730600856099
Eisenberg, N., Fabes, R. A., Shepard, S. A., Guthrie, I. K., Murphy, B. C., and Reiser, M. (1999). Parental reactions to children’s negative emotions: longitudinal relations to quality of children’s social functioning. Child Dev. 70, 513–534. doi: 10.1111/1467-8624.00037
Eisenberg, N., Spinrad, T. L., Eggum, N. D., Silva, K. M., Reiser, M., Hofer, C., et al. (2010). Relations among maternal socialization, effortful control, and maladjustment in early childhood. Dev. Psychopathol. 22:507. doi: 10.1017/S0954579410000246
Fabes, R. A., Eisenberg, N., and Bernzweig, J. (1990). Coping with Children’s Negative Emotions Scale (CCNES): Description and Scoring. Tempe, AZ: Arizona State University.
Fabes, R. A., Leonard, S. A., Kupanoff, K., and Martin, C. L. (2001). Parental coping with children’s negative emotions: relations with children’s emotional and social responding. Child Dev. 72, 907–920. doi: 10.1111/1467-8624.00323
Fabes, R. A., Poulin, R. E., Eisenberg, N., and Madden-Derdich, D. A. (2002). The Coping with Children’s Negative Emotions Scale (CCNES): psychometric properties and relations with children’s emotional competence. Marriage Fam. Rev. 34, 285–310. doi: 10.1300/J002v34n03_05
Farber, M. J., Gee, D. G., and Hariri, A. R. (2020). Normative range parenting and the developing brain: a scoping review and recommendations for future research. Eur. J. Neurosci. doi: 10.1111/ejn.15003
Feeney, J. A. (2006). Parental attachment and conflict behavior: implications for offspring’s attachment, loneliness, and relationship satisfaction. Pers. Relationsh. 13, 19–36. doi: 10.1111/j.1475-6811.2006.00102.x
Fischl, B., and Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 97, 11050–11055. doi: 10.1073/pnas.200033797
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/S0896-6273(02)00569-X
Gee, D. G. (2016). “Sensitive periods of emotion regulation: influences of parental care on frontoamygdala circuitry and plasticity,” in Maternal Brain Plasticity: Preclinical and Human Research and Implications for Intervention. New Directions for Child and Adolescent Development, Vol. 153, eds H. J. V. Rutherford and L. C. Mayes (Hoboken, NJ: Wiley), 87–110. doi: 10.1002/cad.20166
George, C., and Solomon, J. (2008). “The caregiving system: a behavioral systems approach to parenting,” in Handbook of Attachment: Theory, Research, and Clinical Applications, 2nd Edn, eds J. Cassidy and P. R. Shaver (New York, NY: Guilford Press), 833–856.
Gronenschild, E. H., Habets, P., Jacobs, H. I., Mengelers, R., Rozendaal, N., Van Os, J., et al. (2012). The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One 7:e38234. doi: 10.1371/journal.pone.0038234
Gudmundson, J. A., and Leerkes, E. M. (2012). Links between mothers’ coping styles, toddler reactivity, and sensitivity to toddler’s negative emotions. Infant Behav. Dev. 35, 158–166. doi: 10.1016/j.infbeh.2011.07.004
Hofer, M. A. (1995). “Hidden regulators: implications for a new understanding of attachment, separation, and loss,” in Attachment Theory: Social, Development and Clinical Perspectives, eds S. Goldberg, R. Muir, and J. Kerr (New York, NY: Analytic Press), 203–230.
Honkaniemi, J., Kainu, T., Ceccatelli, S., Rechardt, L., Hökfelt, T., and Pelto-Huikko, M. (1992). Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. Neuroreport 3, 849–852. doi: 10.1097/00001756-199210000-00007
Humphreys, K. L., King, L. S., Sacchet, M. D., Camacho, M. C., Colich, N. L., Ordaz, S. J., et al. (2018). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev. Sci. 22:e12775. doi: 10.1111/desc.12775
Huth-Bocks, A. C., Muzik, M., Beeghly, M., Earls, L., and Stacks, A. M. (2014). Secure base scripts are associated with maternal parenting behavior across contexts and reflective functioning among trauma-exposed mothers. Attach. Hum. Dev. 16, 535–556. doi: 10.1080/14616734.2014.967787
Ilyka, D., Johnson, M. H., and Lloyd-Fox, S. (2021). Infant social interactions and brain development: a systematic review. Neurosci. Biobehav. Rev. 130, 448–469. doi: 10.1016/j.neubiorev.2021.09.001
Jones, J. D., Brett, B. E., Ehrlich, K. B., Lejuez, C. W., and Cassidy, J. (2014). Maternal attachment style and responses to adolescents’ negative emotions: the mediating role of maternal emotion regulation. Parenting 14, 235–257. doi: 10.1080/15295192.2014.972760
Jones, J. D., Cassidy, J., and Shaver, P. R. (2015). Parents’ self-reported attachment styles: a review of links with parenting behaviors, emotions, and cognitions. Pers. Soc. Psychol. Rev. 19, 44–76. doi: 10.1177/1088868314541858
Köhler-Dauner, F., Roder, E., Krause, S., Buchheim, A., Gündel, H., Fegert, J. M., et al. (2019). Reduced caregiving quality measured during the strange situation procedure increases child’s autonomic nervous system stress response. Child Adolesc. Psychiatry Mental Health 13, 1–12. doi: 10.1186/s13034-019-0302-3
Kok, R., Thijssen, S., Bakermans-Kranenburg, M. J., Jaddoe, V. W., Verhulst, F. C., White, T., et al. (2015). Normal variation in early parental sensitivity predicts child structural brain development. J. Am. Acad. Child Adolesc. Psychiatry 54, 824–831. doi: 10.1016/j.jaac.2015.07.009
La Valley, A. G., and Guerrero, L. K. (2012). Perceptions of conflict behavior and relational satisfaction in adult parent–child relationships: a dyadic analysis from an attachment perspective. Commun. Res. 39, 48–78. doi: 10.1177/0093650210391655
Lee, A., Poh, J. S., Wen, D. J., Tan, H. M., Chong, Y. S., Tan, K. H., et al. (2019). Maternal care in infancy and the course of limbic development. Dev. Cogn. Neurosci. 40, 100714. doi: 10.1016/j.dcn.2019.100714
Long, M., Verbeke, W., Ein-Dor, T., and Vrtička, P. (2020). A functional neuro-anatomical model of human attachment (NAMA): insights from first-and second-person social neuroscience. Cortex 126, 281–321. doi: 10.1016/j.cortex.2020.01.010
Lovejoy, M. C., Graczyk, P. A., O’Hare, E., and Neuman, G. (2000). Maternal depression and parenting behavior: a meta-analytic review. Clin. Psychol. Rev. 20, 561–592. doi: 10.1016/S0272-7358(98)00100-7
Luby, J. L., Barch, D. M., Belden, A., Gaffrey, M. S., Tillman, R., Babb, C., et al. (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. U.S.A. 109, 2854–2859. doi: 10.1073/pnas.1118003109
Luby, J. L., Belden, A., Harms, M. P., Tillman, R., and Barch, D. M. (2016). Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc. Natl. Acad. Sci. U.S.A. 113, 5742–5747. doi: 10.1073/pnas.1601443113
Luby, J., Belden, A., Botteron, K., Marrus, N., Harms, M. P., Babb, C., et al. (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142. doi: 10.1001/jamapediatrics.2013.3139
Luecken, L. J., and Lemery, K. S. (2004). Early caregiving and physiological stress responses. Clin. Psychol. Rev. 24, 171–191. doi: 10.1016/j.cpr.2004.01.003
Lupien, S. J., Parent, S., Evans, A. C., Tremblay, R. E., Zelazo, P. D., Corbo, V., et al. (2011). Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl. Acad. Sci. U.S.A. 108, 14324–14329. doi: 10.1073/pnas.1105371108
Lyons-Ruth, K., Pechtel, P., Yoon, S. A., Anderson, C. M., and Teicher, M. H. (2016). Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 308, 83–93. doi: 10.1016/j.bbr.2016.03.050
Madigan, S., Moran, G., Schuengel, C., Pederson, D. R., and Otten, R. (2007). Unresolved maternal attachment representations, disrupted maternal behavior and disorganized attachment in infancy: links to toddler behavior problems. J. Child Psychol. Psychiatry 48, 1042–1050. doi: 10.1111/j.1469-7610.2007.01805.x
Main, M., Kaplan, N., and Cassidy, J. (1985). Security in infancy, childhood, and adulthood: a move to the level of representation. Monogr. Soc. Res. Child Dev. 50, 66–104. doi: 10.2307/3333827
Matsudaira, I., Oba, K., Takeuchi, H., Sekiguchi, A., Tomita, H., Taki, Y., et al. (2019). rs1360780 of the FKBP5 gene modulates the association between maternal acceptance and regional gray matter volume in the thalamus in children and adolescents. PLoS One 14:e0221768. doi: 10.1371/journal.pone.0221768
Matsudaira, I., Yokota, S., Hashimoto, T., Takeuchi, H., Asano, K., Asano, M., et al. (2016). Parental praise correlates with posterior insular cortex gray matter volume in children and adolescents. PLoS One 11:e0154220. doi: 10.1371/journal.pone.0154220
McEwen, B. S. (2000). Effects of adverse experiences for brain structure and function. Biol. Psychiatry 48, 721–731. doi: 10.1016/S0006-3223(00)00964-1
McLaughlin, K. A., Weissman, D., and Bitrán, D. (2019). Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 1, 277–312. doi: 10.1146/annurev-devpsych-121318-084950
Meaney, M. J., and Szyf, M. (2005). Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 7, 103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney
Mikulincer, M., and Shaver, P. R. (2007). Attachment in Adulthood: Structure, Dynamics, and Change. New York, NY: Guilford Press.
Mikulincer, M., Shaver, P. R., Sapir-Lavid, Y., and Avihou-Kanza, N. (2009). What’s inside the minds of securely and insecurely attached people? The secure-base script and its associations with attachment-style dimensions. J. Pers. Soc. Psychol. 97, 615–633. doi: 10.1037/a0015649
Milgrom, J., Newnham, C., Anderson, P. J., Doyle, L. W., Gemmill, A. W., Lee, K., et al. (2010). Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr. Res. 67, 330–335. doi: 10.1203/PDR.0b013e3181cb8e2f
Mills-Koonce, W. R., Appleyard, K., Barnett, M., Deng, M., Putallaz, M., and Cox, M. (2011). Adult attachment style and stress as risk factors for early maternal sensitivity and negativity. Infant Ment. Health J. 32, 277–285. doi: 10.1002/imhj.20296
Moran, G., Forbes, L., Evans, E., Tarabulsy, G. M., and Madigan, S. (2008). Both maternal sensitivity and atypical maternal behavior independently predict attachment security and disorganization in adolescent mother–infant relationships. Infant Behav. Dev. 31, 321–325. doi: 10.1016/j.infbeh.2007.12.012
Mueller, R. O., and Hancock, G. R. (2010). “Structural equation modeling,” in The Reviewer’s Guide to Quantitative Methods in the Social Sciences, eds G. R. Hancock and R. O. Mueller (New York, NY: Routledge), 371–383.
Muthén, L. K., and Muthén, B. O. (1998–2007). Mplus User’s Guide, Fifth Edn. Los Angeles, CA: Muthén & Muthén.
Öhman, A. (2005). The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology 30, 953–958. doi: 10.1016/j.psyneuen.2005.03.019
Parent, M. C. (2013). Handling item-level missing data: simpler is just as good. Couns. Psychol. 41, 568–600. doi: 10.1177/0011000012445176
Penzo, M. A., Robert, V., Tucciarone, J., De Bundel, D., Wang, M., Van Aelst, L., et al. (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459. doi: 10.1038/nature13978
Perry, N. B., Calkins, S. D., Nelson, J. A., Leerkes, E. M., and Marcovitch, S. (2012). Mothers’ responses to children’s negative emotions and child emotion regulation: the moderating role of vagal suppression. Dev. Psychobiol. 54, 503–513. doi: 10.1002/dev.20608
Preacher, K. J., and Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879–891. doi: 10.3758/BRM.40.3.879
Puhlmann, L. M. C., Derome, M., Morosan, L., Kilicel, D., Vrtička, P., and Debbané, M. (2021). Longitudinal associations between self-reported attachment dimensions and neurostructural development from adolescence to early adulthood. Attach. Hum. Dev. 1–19. doi: 10.1080/14616734.2021.1993628
Quirin, M., Gillath, O., Pruessner, J. C., and Eggert, L. D. (2010). Adult attachment insecurity and hippocampal cell density. Soc. Cogn. Affect. Neurosci. 5, 39–47. doi: 10.1093/scan/nsp042
Raby, K., Waters, T., Tabachnick, A., Zajac, L., and Dozier, M. (2021). Increasing secure base script knowledge among parents with Attachment and Biobehavioral Catch-up. Dev. Psychopathol. 33, 554–564. doi: 10.1017/S0954579420001765
Rholes, W. S., Simpson, J. A., and Blakely, B. S. (1995). Adult attachment styles and mothers’ relationships with their young children. Pers. Relationsh. 2, 35–54. doi: 10.1111/j.1475-6811.1995.tb00076.x
Richmond, S., Beare, R., Johnson, K. A., Allen, N. B., Seal, M. L., and Whittle, S. (2021). Towards understanding neurocognitive mechanisms of parenting: maternal behaviors and structural brain network organization in late childhood. Hum. Brain Mapp. 42, 1845–1862. doi: 10.1002/hbm.25334
Rifkin-Graboi, A., Kong, L., Sim, L. W., Sanmugam, S., Broekman, B. F. P., Chen, H., et al. (2015). Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry 5:e668. doi: 10.1038/tp.2015.133
Riggins, T., Blankenship, S. L., Mulligan, E., Rice, K., and Redcay, E. (2015). Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Dev. 86, 1710–1718. doi: 10.1111/cdev.12445
Roth, M. C., Humphreys, K. L., King, L. S., and Gotlib, I. H. (2018). Self-reported neglect, amygdala volume, and symptoms of anxiety in adolescent boys. Child Abuse Neglect 80, 80–89. doi: 10.1016/j.chiabu.2018.03.016
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 7, 284–301. doi: 10.1210/edrv-7-3-284
Schafer, J. L., and Graham, J. W. (2002). Missing data: our view of the state of the art. Psychol. Methods 7, 147–177. doi: 10.1037/1082-989X.7.2.147
Selcuk, E., Günaydin, G., Sumer, N., Harma, M., Salman, S., Hazan, C., et al. (2010). Self-reported romantic attachment style predicts everyday maternal caregiving behavior at home. J. Res. Pers. 44, 544–549. doi: 10.1016/j.jrp.2010.05.007
Sethna, V., Pote, I., Wang, S., Gudbrandsen, M., Blasi, A., McCusker, C., et al. (2017). Mother–infant interactions and regional brain volumes in infancy: an MRI study. Brain Struct. Funct. 222, 2379–2388. doi: 10.1007/s00429-016-1347-1
Shewark, E. A., and Blandon, A. Y. (2015). Mothers’ and fathers’ emotion socialization and children’s emotion regulation: a within-family model. Soc. Dev. 24, 266–284. doi: 10.1111/sode.12095
Spangler, G., Schieche, M., Ilg, U., Maier, U., and Ackermann, C. (1994). Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Dev. Psychobiol. 27, 425–437. doi: 10.1002/dev.420270702
Spencer, S. J., Fox, J. C., and Day, T. A. (2004). Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res. 997, 234–237. doi: 10.1016/j.brainres.2003.10.054
Spinrad, T. L., Eisenberg, N., Gaertner, B., Popp, T., Smith, C. L., Kupfer, A., et al. (2007). Relations of maternal socialization and toddlers’ effortful control to children’s adjustment and social competence. Dev. Psychol. 43, 1170–1186. doi: 10.1037/0012-1649.43.5.1170
Spinrad, T. L., Eisenberg, N., Kupfer, A., Gaertner, B., and Michalik, N. (2004). “The coping with toddlers’ negative emotions scale,” in Paper Presented at the Biennial International Conference on Infant Studies, Chicago, IL.
Steele, R. D., Waters, T. E., Bost, K. K., Vaughn, B. E., Truitt, W., Waters, H. S., et al. (2014). Caregiving antecedents of secure base script knowledge: a comparative analysis of young adult attachment representations. Dev. Psychol. 50, 2526–2538. doi: 10.1037/a0037992
Stride, C. B., Gardner, S., Catley, N., and Thomas, F. (2015). Mplus Code for Mediation, Moderation, and Moderated Mediation Models. Available online at: http://offbeat.group.shef.ac.uk/FIO/index.htm (accessed August 17, 2020).
Teicher, M. H., Anderson, C. M., and Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U.S.A. 109, E563–E572. doi: 10.1073/pnas.1115396109
Tharner, A., Herba, C. M., Luijk, M. P. C. M., Van IJzendoorn, M. H., Bakermans-Kranenburg, M. J., and Govaert, P. P. (2011). Subcortical structures and the neurobiology of infant attachment disorganization: a longitudinal ultrasound imaging study. Soc. Neurosci. 6, 336–347. doi: 10.1080/17470919.2010.538219
Tottenham, N. (2015). Social scaffolding of human amygdala-mPFC circuit development. Soc. Neurosci. 10, 489–499. doi: 10.1080/17470919.2015.1087424
Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., et al. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61. doi: 10.1111/j.1467-7687.2009.00852.x
Valadez, E. A., Tottenham, N., Tabachnick, A. R., and Dozier, M. (2020). Early parenting intervention effects on brain responses to maternal cues among high-risk children. Am. J. Psychiatry 177, 818–826. doi: 10.1176/appi.ajp.2020.20010011
van IJzendoorn, M. H. (1995). Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol. Bull. 117, 387–403. doi: 10.1037/0033-2909.117.3.387
Verhage, M. L., Schuengel, C., Madigan, S., Fearon, R. M. P., Oosterman, M., Cassibba, R., et al. (2016). Narrowing the transmission gap: a synthesis of three decades of research on intergenerational transmission of attachment. Psychol. Bull. 142, 337–366. doi: 10.1037/bul0000038
Wang, H., Das, S. R., Suh, J. W., Altinay, M., Pluta, J., Craige, C., et al. (2011). A learning-based wrapper method to correct systematic errors in automatic image segmentation: consistently improved performance in hippocampus, cortex and brain segmentation. NeuroImage 55, 968–985. doi: 10.1016/j.neuroimage.2011.01.006
Waters, H. S., and Rodrigues-Doolabh, L. (2001). “Are attachment scripts the building blocks of attachment representations,” in Paper Presented at the Biennial Meeting of the Society for Research in Child Development, Washington, DC.
Waters, H. S., and Waters, E. (2006). The attachment working models concept: among other things, we build script-like representations of secure base experiences. Attach. Hum. Dev. 8, 185–197. doi: 10.1080/14616730600856016
Waters, T. E. A., Ruiz, S. K., and Roisman, G. I. (2017). Origins of secure base script knowledge and the developmental construction of attachment representations. Child Dev. 88, 198–209. doi: 10.1111/cdev.12571
Waters, T. E., Bosmans, G., Vandevivere, E., Dujardin, A., and Waters, H. S. (2015). Secure base representations in middle childhood across two Western cultures: associations with parental attachment representations and maternal reports of behavior problems. Dev. Psychol. 51:1013. doi: 10.1037/a0039375
Wei, M., Russell, D. W., Mallinckrodt, B., and Vogel, D. L. (2007). The Experiences in Close Relationship Scale (ECR)-short form: reliability, validity, and factor structure. J. Pers. Assess. 88, 187–204. doi: 10.1080/00223890701268041
Wei, P., Liu, N., Zhang, Z., Liu, X., Tang, Y., He, X., et al. (2015). Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6:6756. doi: 10.1038/ncomms7756
Whittle, S., Simmons, J. G., Dennison, M., Vijayakumar, N., Schwartz, O., Yap, M. B., et al. (2014). Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev. Cogn. Neurosci. 8, 7–17. doi: 10.1016/j.dcn.2013.10.006
Whittle, S., Vijayakumar, N., Dennison, M., Schwartz, O., Simmons, J. G., Sheeber, L., et al. (2016). Observed measures of negative parenting predict brain development during adolescence. PLoS One 11:e0147774.
Whittle, S., Yap, M. B., Yücel, M., Sheeber, L., Simmons, J. G., Pantelis, C., et al. (2009). Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc. Cogn. Affect. Neurosci. 4, 247–256. doi: 10.1093/scan/nsp012
Keywords: attachment, secure base scripts, amygdala, hippocampus, parenting, early childhood, brain structure
Citation: Fitter MH, Stern JA, Straske MD, Allard T, Cassidy J and Riggins T (2022) Mothers’ Attachment Representations and Children’s Brain Structure. Front. Hum. Neurosci. 16:740195. doi: 10.3389/fnhum.2022.740195
Received: 12 July 2021; Accepted: 24 January 2022;
Published: 16 March 2022.
Edited by:
Anna Buchheim, University of Innsbruck, AustriaReviewed by:
Christine Stelzel, International Psychoanalytic University Berlin, GermanyCopyright © 2022 Fitter, Stern, Straske, Allard, Cassidy and Riggins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan H. Fitter, bWhmaXR0ZXJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.