
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hum. Neurosci. , 15 December 2022
Sec. Brain Imaging and Stimulation
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.1065459
This article is part of the Research Topic Emerging Talents in Human Neuroscience: Cognitive Neuroscience 2022 View all 5 articles
 Satoshi Maesawa1*†
Satoshi Maesawa1*† Jun Torii1†
Jun Torii1† Daisuke Nakatsubo1,2
Daisuke Nakatsubo1,2 Hiroshi Noda3
Hiroshi Noda3 Manabu Mutoh1
Manabu Mutoh1 Yoshiki Ito1
Yoshiki Ito1 Tomotaka Ishizaki1
Tomotaka Ishizaki1 Takashi Tsuboi4
Takashi Tsuboi4 Masashi Suzuki4
Masashi Suzuki4 Takafumi Tanei1
Takafumi Tanei1 Masahisa Katsuno4
Masahisa Katsuno4 Ryuta Saito1
Ryuta Saito1Holmes tremor is a symptomatic tremor that develops secondary to central nervous system disorders. Stereotactic neuromodulation is considered when the tremors are intractable. Targeting the ventral intermediate nucleus (Vim) is common; however, the outcome is often unsatisfactory, and the posterior subthalamic area (PSA) is expected as alternative target. In this study, we report the case of a patient with intractable Holmes tremor who underwent dual-lead deep brain stimulation (DBS) to stimulate multiple locations in the PSA and thalamus. The patient was a 77-year-old female who complained of severe tremor in her left upper extremity that developed one year after her right thalamic infarction. Vim-thalamotomy using focused ultrasound therapy (FUS) was initially performed but failed to control tremor. Subsequently, we performed DBS using two leads to stimulate four different structures. Accordingly, one lead was implanted with the aim of targeting the ventral oralis nucleus (Vo)/zona incerta (Zi), and the other with the aim of targeting the Vim/prelemniscal radiation (Raprl). Electrode stimulation revealed that Raprl and Zi had obvious effects. Postoperatively, the patient achieved good tremor control without any side effects, which was maintained for two years. Considering that she demonstrated resting, postural, and intention/action tremor, and Vim-thalamotomy by FUS was insufficient for tremor control, complicated pathogenesis was presumed in her symptoms including both the cerebellothalamic and the pallidothalamic pathways. Using the dual-lead DBS technique, we have more choices to adjust the stimulation at multiple sites, where different functional networks are connected. Intractable tremors, such as Holmes tremor, may have complicated pathology, therefore, modulating multiple pathological networks is necessary. We suggest that the dual-lead DBS (Vo/Raprl and Vim/Zi) presented here is safe, technically feasible, and possibly effective for the control of Holmes tremor.
Holmes tremor is characterized as a symptomatic tremor, which develops secondary to central nervous system (CNS) diseases, including strokes, infections, tumors, trauma, and surgery (Hey et al., 2022). The consensus statement of movement disorder society defined Holmes tremor as resting, postural, and intentional tremor with low frequency and irregular amplitude (Krauss and Jankovic, 2002; Bhatia et al., 2018). Although the pathogenesis remains unclear, combined abnormalities including the dopaminergic nigrostriatal system, cerebello-thalamo-cortical pathway, and dentate-rubro-olivary pathway are considered to be associated with tremor development (Vidailhet et al., 1998; Akkus and Diramali, 2006).
Raina et al. (2016) reported that cerebrovascular disease (ischemia or hemorrhage) was the most common cause of Holmes tremor among the CNS diseases (48.3%). This symptom generally appeared late, and the median interval between the onset of CNS disease and tremor was reported to be 2 months (range, 7 days to 228 months). These lesions involve various subcortical structures, including the midbrain, thalamus, pons, and cerebellum. Some patients with Holmes tremors respond to L-dopa and dopamine agonists (Velez et al., 2002; Akkus and Diramali, 2006), whereas others are often medically refractory. In these cases, neuromodulation by stereotactic and functional techniques is considered, including deep brain stimulation (DBS), radiofrequency ablation (RF), and magnetic resonance-guided focused ultrasound ablation (MRgFUS). The ventral intermediate nucleus (Vim) is a common target site. Approximately two decades ago, the posterior subthalamic area (PSA) was also shown to be effective for the control of essential tremors and other intractable tremors (Velasco et al., 2001; Murata et al., 2003; Plaha et al., 2008; Fytagoridis et al., 2012). However, patients with Holmes tremor is often more intractable than in those with essential tremor.
We assume that neuromodulation of multiple targets is necessary to control such intractable tremors. Here, we report the case of a patient with intractable Holmes tremor, for whom MRgFUS targeting at the Vim was initially performed, but failed in tremor control, and thereafter dual-lead DBS targeting the PSA in addition to the thalamus was performed with implantation of two leads. The targeting sites were established to modify four different structures, the Vim to the prelemniscal radiation (Raprl) and the ventral oralis (Vo) to the zona incerta (Zi). The patient achieved good postoperative tremor control. Although several techniques for dual-lead DBS have been reported, to the best of our knowledge, this specific method has never been reported. Here, we present the techniques and outcomes of this surgery and discuss its clinical importance in intractable tremors.
We present the case of a right-handed 77–year-old female patient who developed a right thalamic infarction at the age of 74 years (Figure 1A). She developed numbness in the left upper and lower extremities, which gradually improved but did not completely dissipate. Approximately 1year after the onset of the infarction, she developed a tremor in her left upper extremity, which gradually worsened. She had no family history associated with tremor, and no medical history of diseases associated with tremor, such as hyperthyroidism. She was diagnosed with Holmes tremor and was subsequently treated with atenolol hydrochloride, L-dopa, and clonazepam. However, this combination therapy failed to control her tremor. Two years after the onset of the infarction, she was referred to our hospital for surgical intervention.
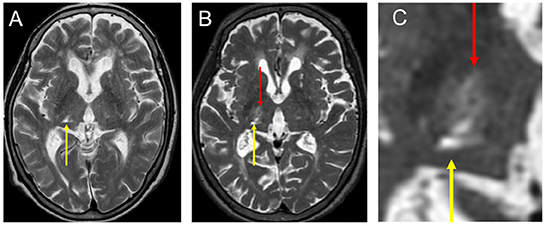
Figure 1. Preoperative images of her MRI. (A) T2 image taking before FUS—thalamotomy after right thalamic infarction (yellow arrow). The infarction was located in the right VC extending to the pulvinar nucleus. (B) T2 image taking a day after FUS—thalamotomy. Note that the lesion with super low intensity with surrounding high intensity was located in the Vim (red arrow). (C) High magnification of B. FUS, focused ultrasound surgery; MRI, magnetic resonance imaging; VC, ventral caudalis nucleus; Vim, ventral intermediate nucleus.
The patient had a rough and severe tremor in the left upper extremity, which was characterized by a frequency of approximately 3 Hz, and appeared in arm-stretching or wing-beating posture and goal-directed movement. Resting tremors were also observed. Dystonic postures and movements were accompanied by tremors, especially when writing letters. Additionally, she had mild and chronic numbness in her left upper and lower extremities, without other neurological deficits. Parkinsonism was not observed. Her cognition was within normal limits. Magnetic resonance imaging (MRI) revealed a small, old infarction in the ventral-posterolateral area of the thalamus, which was mainly consistent with the ventral caudalis (VC) and the pulvinar nucleus (Figure 1A). A dopamine transporter (DaT) scan showed a normal pattern. The skull density ratio (SDR) was 0.33. The Clinical Rating Scale for Tremor (CRST) score, which indicates tremor severity, was preoperatively examined. Briefly, the CRST part A score is related to tremors in nine parts of the body, whereas the CRST part B score is related to tremors that occur while writing and pouring liquids. The CRST part C score was used to assess the patients' quality of daily life. Her total CRST score was 46: 16 for part A; 16, for part B, and 14 for part C. The CRST in the diseased upper extremity (left) was 25, 9 for part A, and 16 for part B.
Three stereotactic options (DBS, RF, and MRgFUS) were presented, and the patient and her family were sufficiently informed about the general indications, risks, and benefits of the respective procedures. Although she understood that her SDR was less than optimal and the effectiveness of MRgFUS for Holmes tremor was not well established, she chose Vim-thalamotomy by MRgFUS because of the non-invasiveness of this treatment. MRgFUS was performed at the age of 76 (two and a half years after the onset of infarction) and showed no adverse events except for headache and nausea during the procedure. The maximum average temperature was 54°C, and the location of the lesion was considered appropriate (Figures 1B, C). Although she demonstrated slight improvement immediately after MRgFUS, her symptoms recurred at a follow-up of 1 month. The patient required another surgical intervention.
Based on the fact that a lesion has already been created in the Vim but failed to control tremor by MRgFUS, we suggested DBS dually targeting the PSA in addition to the thalamus. Considering the possibility that stimulation of the Vo may also be effective based on reports from other facilities (Foote and Okun, 2005; Gallay et al., 2008; Oliveria et al., 2017), we also included Vo as a target. Accordingly, we planned dual-lead DBS; one target was the Vo + Zi (Figure 2), and the other target was Vim + Raprl (Figure 3). The lead for the Vo and the Zi was placed laterally and anteriorly, and the initial target coordination was calculated with reference to the Schaltebrand atlas (Schaltenbrand and Wahren, 1977). The X coordinate was 14.0 mm right from the midline, the Y coordinate was 3.0 mm posterior from the midpoint of the anterior-posterior commissure (AC-PC) line, and the Z coordinate was just at the AC-PC plane. The initial target was set at the border of the thalamus and Zi, and we intended to insert it deeper from the target with microelectrode recording guidance. The angle to the AC-PC plane was 46° and the angle from the midline was 26° (Figure 2). On the other hand, the other lead for the Vim and the Raprl was placed medially and posteriorly, the initial target was set at the bottom of the Raprl; X coordinate was 10.5 mm right from the midline, Y coordinate was 6.7 mm posterior from the midpoint of AC-PC line, and Z coordinate was 4 mm inferior from the AC-PC plane. The angle to the AC-PC plane was 65°, and the angle from the midline was 19° (Figure 3). Surgical planning was performed using a planning workstation, Voxim (Renishaw, New Mills, UK) (Supplementary Figures S1A–D). During the surgical procedure, the cranial part was subjected to local anesthesia. A Leksell stereotactic frame (Elekta, Stockholm, Sweden) was used, and electrodes were inserted using Neuromate (Renishaw, New Mills, UK). The microelectrode recording (MER) was performed. We found irregular and hyperactive activity in the Vim, with some sensorimotor responses in the upper limb, but the neuronal activities in the Raprl were not identifiable from the background. We also found some neuronal activity in the Vo, but the activity in the Zi was not obvious. However, 3–5 mm deep from the presumed location of the Zi, we obtained hyper-neuronal activity, consistent with the subthalamic nucleus (STN). Macro-stimulation of 1–3 mA was performed at these four targets, and no side effects were confirmed. Finally, two stimulation leads were implanted. In this case, the directional lead, which consisted of the deepest ring (contact 1), the second deepest ring with three different directed parts (contacts 2, 3, and 4), the third ring with three different directed parts (contacts 5, 6, and 7), and the superficial ring (contact 8) (Boston Scientific, USA), was utilized for the electrode targeting at Vo + Zi. This was because we attempted to prevent anterior stimulation of the internal capsule by using its directivity. The contacts 1 to 4 were in the Zi, and 5 to 8 were in the Vo (Figure 2). For the Vim + Raprl targeting lead, a twisting lead (Boston Scientific, USA), which has eight contacts (contacts 1 to 8) and enables stimulation over a relatively long distance along the axis, was utilized. The contacts 1 to 4 were in the Raprl, and 5 to 8 were in the Vim (Figure 3). For the pulse-generator, Genus P16 (Boston Scientific, USA) was implanted into the subcutaneous space of the left chest under general anesthesia. All the procedures were completed without complications.
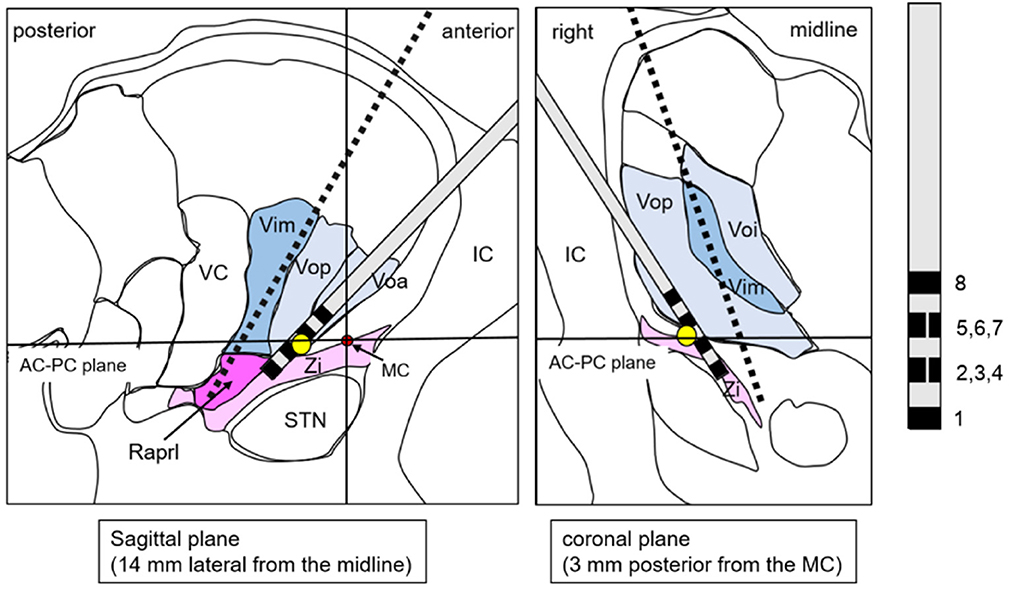
Figure 2. A schema shows the lead targeting the Vo/Zi superimposed on the atlas, which is adopted from the stereotaxy atlas (14). The yellow circle represents the initial target. Red circle represents the location of the MC. Black broken line is transparent image of the other lead. AC, anterior commissure; IC, internal capsule; MC, midpoint of AC-PC line; PC, posterior commissure; Raprl, prelemniscal radiation; STN, subthalamic nucleus; VC, ventral caudalis; Vop (a, i), ventral oralis nucleus posterior (anterior, interna); Zi, zona incerta.
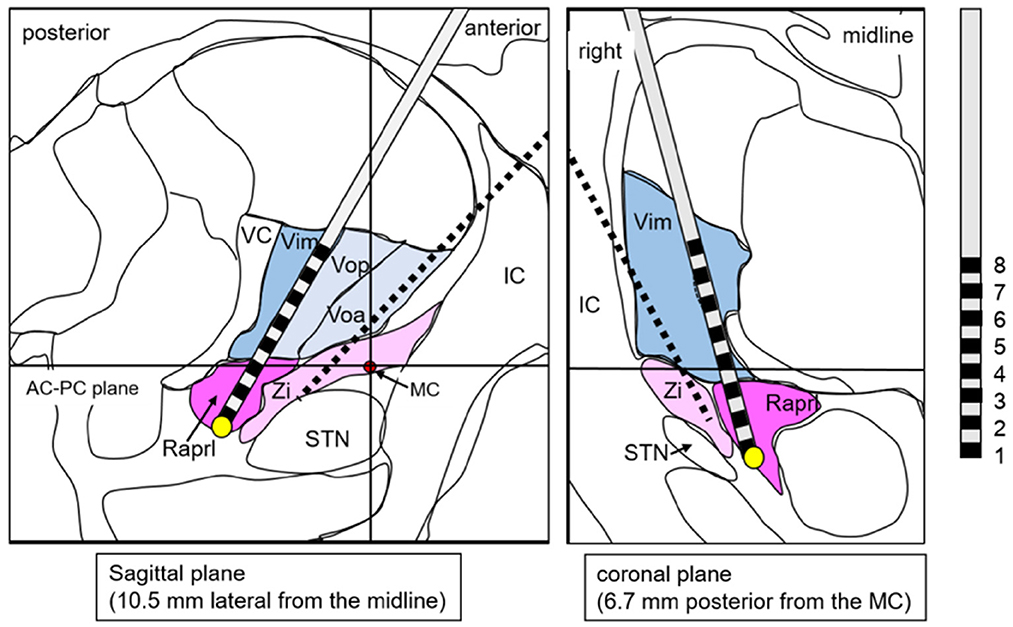
Figure 3. A schema shows the lead targeting the Vim/Raprl superimposed on the atlas, which is adopted from the stereotaxy atlas (Schaltenbrand and Wahren, 1977). The yellow circle is the initial target. Red circle is the location of the MC. Black broken line is transparent image of the other lead. AC, anterior commissure; IC, internal capsule; MC, midpoint of AC-PC line; PC, posterior commissure; Raprl, prelemniscal radiation; STN, subthalamic nucleus; VC, ventral caudalis; Vop (a), ventral oralis nucleus posterior (anterior); Zi, zona incerta.
The postoperative course was uneventful, and imaging studies, including radiography (Supplementary Figures S1E, F) and computed tomography, demonstrated the optimal location of leads without complications. Electrostimulation screening revealed that Vim and Vo had weak effects in this case, while Raprl and Zi had obvious effects on the tremor. By performing dual-lead DBS combined with Raprl and Zi stimulation, the tremor suppression effect was greater than that of a single stimulation. Numbness was likely induced by stimulation in the posterior area, where the VC was located, and we assumed some post-infarction effects. Dysarthria was also likely to be induced by stimulation extending to a wide area with high current intensity; therefore, we attempted to limit the stimulated area by bipolar stimulation. Finally, the stimulation conditions were as follows: Raprl: the contact setting was 1 (-) and 2 (+), the intensity was 3.0 mA, duration was 50 μs, and the frequency was 149 Hz; and Zi: the contact setting was 1 (-), 2, 3, and 4 [ring-mode stimulation at the second deepest contacts (+)], the intensity was 3.0 mA, the duration was 50 μs, and the frequency was 149 Hz. The summary of electrostimulation screening is shown in the Table 1. Although the tremor did not completely disappear, she had good tremor control without any complications, and her quality of daily life significantly improved. Postoperative CRST score at the 6-month follow-up totaled 32 (30 % reduction): 6 for part A (62.5% reduction), 13 for part B (18.7 % reduction), and 10 for part C (28.6 % reduction). CRST in the diseased upper extremity (left) totaled 17 (32 % reduction): 4 for part A (55.5% reduction), and 13 for part B (18.7 % reduction). Good tremor control was maintained for 2 years after surgery.
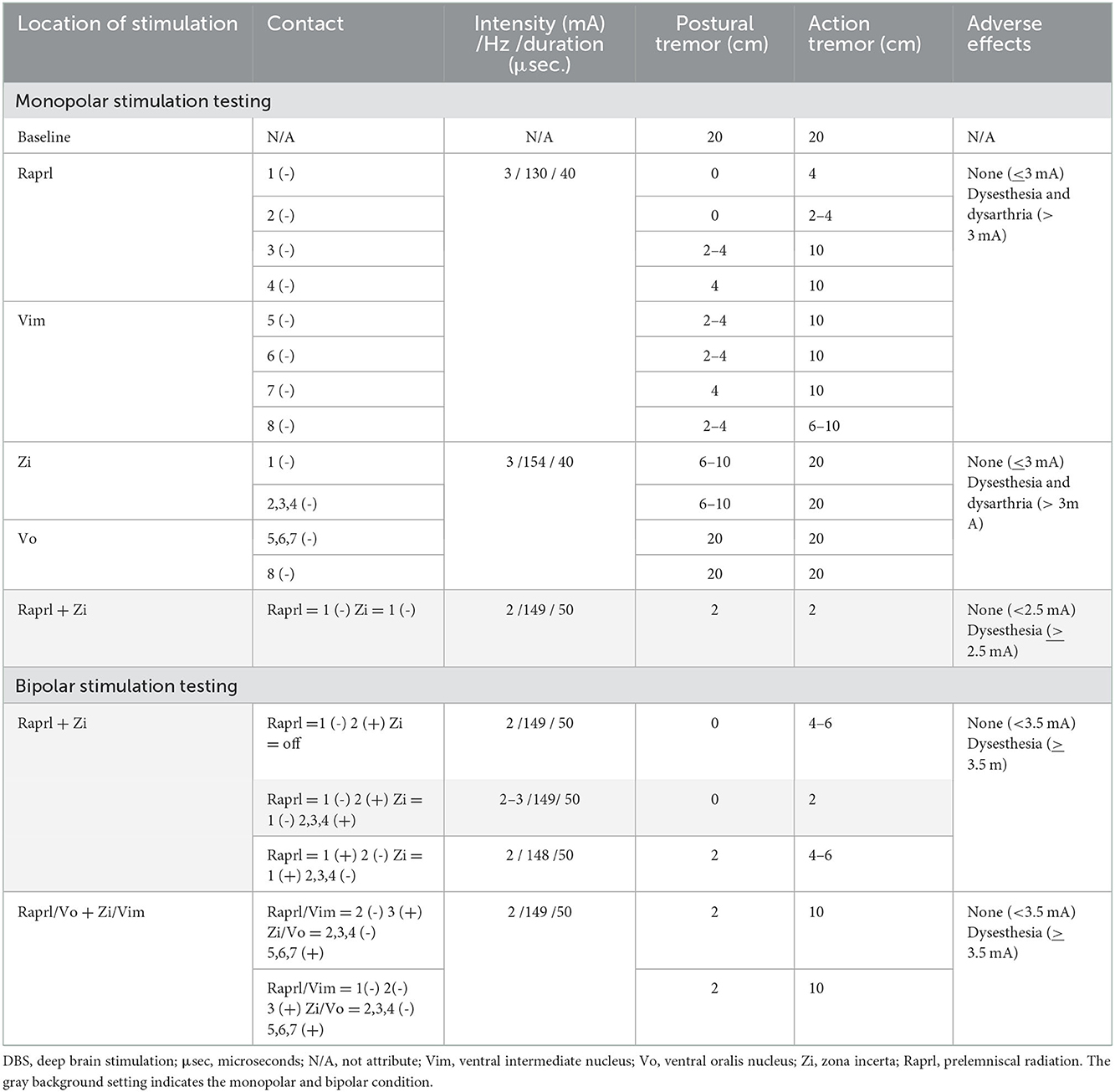
Table 1. Summary of electrostimulation screening with dual-lead DBS for Holmes tremor (gray background shows optimal setting in monopolar and bipolar condition).
Holmes tremor occurs after various subcortical lesions and presents with complex symptoms. When performing neuromodulatory therapies, it is necessary to consider which functional networks are affected. Mendonça et al. reported a review and meta-analysis of DBS outcomes for Holmes and lesion-related tremors, summarizing 35 papers on 82 patients (Mendonça et al., 2018). They reported that Vim DBS followed by the globus pallidum interna (GPi) DBS were preferred, and tremor improvement after DBS in patients with post-stroke Holmes tremor was 77.5%. Among them, 13.5% of the patients had < 50% tremor improvement. More recently, Wang et al. conducted a review and meta-analysis of tremor outcomes for Holmes tremor, emphasizing that DBS targeting the GPi had greater benefits than the Vim (Wang et al., 2022). Interestingly, there were a few patients successfully treated by dual-lead stimulation with the Vo and other targets such as the PSA. This finding suggests heterogenicity among the patients with Holmes tremor.
Recently, Joutsa et al. performed mapping for Holmes tremor network using Human Brain Connectome (Joutsa et al., 2019), and found that there was a common circuit consisted of nodes in the red nucleus, thalamus (Vo), GPi, and cerebellum. They noted that network disturbance in Holmes tremor may be more complicated compared to that in essential tremor. The pallidothalamic pathway may play an essential role in the pathogenesis of Holmes tremor, and thereby be the preferred surgical targets for patients with Holmes tremor. In our case, Vim-thalamotomy using MRgFUS was ineffective. This result indicates the involvement of other functional networks aside from the cerebellothalamic pathway.
PSA DBS has been shown to be efficacious for patients with intractable tremors, even with DBS or ablation targeting the Vim (Velasco et al., 2001; Murata et al., 2003; Plaha et al., 2008; Fytagoridis et al., 2012; Barbe et al., 2018). The PSA is an anatomical region consisting of Raprl and Zi. The Raprl is a white matter structure that contains the cerebellothalamic tracts [alternatively called as the dentato-rubro-thalamic tract (DRTT)] and mesencephalic reticular thalamic tracts, while the Zi relays the cerebellar-thalamic-cortical and pallidothalamic pathways (Watson et al., 2014). While both are known to be effective in suppressing tremors, possible side effects, such as balance dysfunction, ataxia, dysarthria, and diplopia, are likely to be induced by relatively lower stimulation than the Vim (Barbe et al., 2018; Hidding et al., 2019). The PSA covers a fairly wide area, and the optimal target for tremor control with minimal adverse effects differs among studies (Velasco et al., 2001; Murata et al., 2003; Plaha et al., 2008; Fytagoridis et al., 2012; Barbe et al., 2018; Hidding et al., 2019). As described above, the Zi and Raprl play different roles in functional networks, and their locations are geographically distant. A large volume of tissue activated covering both structures using a single lead may readily cause adverse effects. Therefore, we decided to place two electrodes independently in the Zi and Raprl to stimulate both structures with minimal risk of adverse effects.
In previous reports, various methods have been introduced for dual-targeting DBS, including Raprl following Vim, Raprl + Zi following Vim (Kobayashi et al., 2014; Bot et al., 2018; Dos Santos Ghilardi et al., 2018), and PSA + STN following Vim (Coenen et al., 2016; Neudorfer et al., 2019). Notably, simultaneous stimulation of the Vim/Raprl and the Vo/Zi using two leads, which we employed for our case, was also described as a theoretically feasible model (Iorio-Morin et al., 2020). However, there have been no reports about actual clinical use.
The current DBS systems enable stimulation adjustment of two leads using a single pulse-generator device. Therefore, in unilateral Holmes tremor, multiple targeting methods using dual electrodes may be technically feasible and more effective, as in the present case. Patients with Holmes tremor may have complicated pathology affecting multiple functional networks, including the cerebellothalamic pathway and the pallidothalamic pathway. Therefore, stimulation using dual electrodes can modify these networks, which may lead to better outcomes. In our case, electrodes were placed dorsally in the Vim and the Vo separately, and ventrally in both the Zi and Raprl in the PSA. A single tract could anatomically cover both the Vo and Zi or both the Vo and Raprl; therefore, this method is technically reasonable.
As future steps to validate the effectiveness of our dual-lead DBS technique, imaging-based studies on multiple cases should be conducted. More specifically, tractography methods, visualizing the Raprl (Lefranc et al., 2015), DRTT (Fenoy and Schiess, 2018), and other tracts, may shed more light on the pathogenesis of Holmes tremor. In addition, the effects of different stimulation settings, such as amplitude, stimulation frequency, and pulse width, should be tested (Hidding et al., 2022). These studies may contribute to explain our hypothesis that Holmes tremor involve multiple networks.
Our case study had the following limitations. First, the stimulation of the Vim and Vo was ineffective for tremor control, which indicated that the contacts may not be optimally located in these structures. The lead for Vim/Raprl could have been more effective if it passed more laterally in the Vim. Second, we did not use stages approach in this case, which could be a good option as it is less invasive. Thirdly, it should be noted that the more electrodes implanted, the greater the risk of intracranial hemorrhage or postoperative infection.
In conclusion, our method using dual electrodes could potentially provide us with more options to adjust the stimulation at multiple regions. As a result, we could achieve optimal tremor control while minimizing potential adverse effects. We confidently suggest that dual-targeting DBS for Vo/Zi and Vim/Raprl is a feasible and effective method for patients with intractable tremors who have complicated pathologies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Local Ethics Committee of Nagoya University (No. 2017-0147). The patients/participants provided their written informed consent to participate in this study.
Substantial contributions to the conception and design of the work: SM, JT, DN, TTs, MK, and RS. Acquisition and analysis of the data: SM, JT, DN, MM, HN, YI, TI, MS, and TTa. Interpretation of data: SM, DN, TTs, MK, and RS. Drafting the work and revising it critically for important intellectual content: SM and JT. All authors approved the final version of the article.
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI, No. 22H03184).
We would like to extend our gratitude to Ms. Satomi Mizuno, who assisted us in the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.1065459/full#supplementary-material
Supplementary Figure S1. Surgical plan of the dual-lead DBS; the Vo/Zi, and the Vim/Raprl. (A) Axial, (B) Coronal, (C) Sagittal image: The lead for the Vo/Zi shows green line, and the other for the Vim / Raprl shows yellow line. (D) An overview of two leads (the Vo/ZI: red, the Vim/Raprl: yellow). (E,F) Were postoperative X–ray images showing two-lead DBS. AC, anterior commissure; MC, midpoint of AC-PC line; PC, posterior commissure; Raprl, prelemniscal radiation; Vim, ventral intermediate nucleus; Vo, ventral oralis nucleus; Zi, zona incerta.
Akkus, D. E., and Diramali, A. B. (2006). Postischemic delayed Holmes' tremor responding to low-dose cabergoline. Mov Disord. 21, 733–4. doi: 10.1002/mds.20862
Barbe, M. T., Reker, P., Hamacher, S., Franklin, J., Kraus, D., Dembek, T. A., et al. (2018). DBS of the PSA and the VIM in essential tremor: a randomized, double-blind, crossover trial. Neurology 91, e543–550. doi: 10.1212/WNL.0000000000005956
Bhatia, K. P., Bain, P., Bajaj, N., Elble, R. J., Hallett, M., Louis, E. D., et al. (2018). Tremor task force of the international parkinson and movement disorder society. consensus statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33, 75–87. doi: 10.1002/mds.27121
Bot, M., van Rootselaar, F., Contarino, M. F., Odekerken, V., Dijk, J., et al. (2018). Deep brain stimulation for essential tremor: aligning thalamic and posterior subthalamic targets in 1 surgical trajectory. Oper Neurosurg (Hagerstown). 15, 144–52. doi: 10.1093/ons/opx232
Coenen, V. A., Rijntjes, M., Prokop, T., Piroth, T., Amtage, F., Urbach, H., et al. (2016). One-pass deep brain stimulation of dentato-rubro-thalamic tract and subthalamic nucleus for tremor-dominant or equivalent type Parkinson's disease. Acta Neurochir (Wien). 158, 773–81. doi: 10.1007/s00701-016-2725-4
Dos Santos Ghilardi, M. G., Ibarra, M., Alho, E. J. L., Reis, P. R., Lopez Contreras, W. O., Hamani, C., et al. (2018). Double-target DBS for essential tremor: 8-contact lead for cZI and Vim aligned in the same trajectory. Neurology 90, 476–8. doi: 10.1212/WNL.0000000000005076
Fenoy, A. J., and Schiess, M. C. (2018). Comparison of tractography-assisted to atlas-based targeting for deep brain stimulation in essential tremor. Mov. Disord. 33, 1895–901. doi: 10.1002/mds.27463
Foote, K. D., and Okun, M. S. (2005). Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: two leads may be better than one: technical note. Neurosurgery 56, E445. doi: 10.1227/01.NEU.0000157104.87448.78
Fytagoridis, A., Sandvik, U., Aström, M., Bergenheim, T., and Blomstedt, P. (2012). Long term follow-up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry 83, 258–62. doi: 10.1136/jnnp-2011-300765
Gallay, M. N., Jeanmonod, D., Liu, J., and Morel, A. (2008). Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct. 212, 443–63. doi: 10.1007/s00429-007-0170-0
Hey, G., Hu, W., Wong, J., Tsuboi, T., Burns, M. R., Ramirez-Zamora, A., et al. (2022). Evolving concepts in our understanding and treatment of holmes tremor, over 100 years in the making. Tremor Other Hyperkinet Mov (NY) 12, 18. doi: 10.5334/tohm.683
Hidding, U., Lezius, S., Schaper, M., Buhmann, C., Gerloff, C., Pötter-Nerger, M., et al. (2022). Combined short-pulse and directional deep brain stimulation of the thalamic ventral intermediate area for essential tremor. Neuromodulation 22, 01286–7. doi: 10.1016/j.neurom.2022.09.009
Hidding, U., Schaper, M., Moll, C. K. E., Gulberti, A., Köppen, J., Buhmann, C., et al. (2019). Mapping stimulation-induced beneficial and adverse effects in the subthalamic area of essential tremor patients. Parkinsonism Relat Disord. 64, 150–55. doi: 10.1016/j.parkreldis.2019.03.028
Iorio-Morin, C., Fomenko, A., and Kalia, S. K. (2020). Deep-brain stimulation for essential tremor and other tremor syndromes: a narrative review of current targets and clinical outcomes. Brain Sci. 10, 925. doi: 10.3390/brainsci10120925
Joutsa, J., Shih, L. C., and Fox, M. D. (2019). Mapping Holmes tremor circuit using the human brain connectome. Ann Neurol. 86, 812–820. doi: 10.1002/ana.25618
Kobayashi, K., Katayama, Y., Oshima, H., Watanabe, M., Sumi, K., Obuchi, T., et al. (2014). Multitarget, dual-electrode deep brain stimulation of the thalamus and subthalamic area for treatment of Holmes' tremor. J Neurosurg. 120, 1025–1032. doi: 10.3171/2014.1.JNS12392
Krauss, J. K., and Jankovic, J. (2002). Head injury and posttraumatic movement disorders. Neurosurgery 50, 927–39. doi: 10.1227/00006123-200205000-00003
Lefranc, M., Carron, R., and Régis, J. (2015). Prelemniscal radiations: a new reliable landmark of the thalamic nucleus ventralis intermedius location. Stereotact. Funct. Neurosurg. 93, 400–6. doi: 10.1159/000441393
Mendonça, M. D., Meira, B., Fernandes, M., Barbosa, R., and Bugalho, P. (2018). Deep brain stimulation for lesion-related tremors: a systematic review and meta-analysis. Parkinsonism Relat Disord. 47, 8–14. doi: 10.1016/j.parkreldis.2017.12.014
Murata, J., Kitagawa, M., Uesugi, H., Saito, H., Iwasaki, Y., Kikuchi, S., et al. (2003). Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg 99, 708–15. doi: 10.3171/jns.2003.99.4.0708
Neudorfer, C., Hinzke, M., Hunsche, S., El Majdoub, F., Lozano, A., Maarouf, M., et al. (2019). Combined deep brain stimulation of subthalamic nucleus and ventral intermediate thalamic nucleus in tremor-dominant parkinson's disease using a parietal approach. Neuromodulation 22, 493–502. doi: 10.1111/ner.12943
Oliveria, S. F., Rodriguez, R. L., Bowers, D., Kantor, D., Hilliard, J. D., Monari, E. H., et al. (2017). Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 16, 691–700. doi: 10.1016/S1474-4422(17)30166-7
Plaha, P., Khan, S., and Gill, S. S. (2008). Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry 79, 504–13. doi: 10.1136/jnnp.2006.112334
Raina, G. B., Cersosimo, M. G., Folgar, S. S., Giugni, J. C., Calandra, C., Paviolo, J. P., et al. (2016). Holmes tremor: clinical description, lesion localization, and treatment in a series of 29 cases. Neurology 86, 931–38. doi: 10.1212/WNL.0000000000002440
Velasco, F., Jiménez, F., Pérez, M. L., Carrillo-Ruiz, J. D., Velasco, A. L., Ceballos, J., et al. (2001). Electrical stimulation of the prelemniscal radiation in the treatment of Parkinson's disease: an old target revised with new techniques. Neurosurgery 49, 293–308. doi: 10.1227/00006123-200108000-00009
Velez, M., Cosentino, C., and Torres, L. (2002). Levodopa responsive rubral (Holmes) tremor. Mov Disord. 17, 741–742. doi: 10.1002/mds.10224
Vidailhet, M., Jedynak, C. P., Pollak, P., and Agid, Y. (1998). Pathology of symptomatic tremors. Mov Disord. 13(Suppl. 3), 49–54. doi: 10.1002/mds.870131309
Wang, K. L., Wong, J. K., Eisinger, R. S., Carbunaru, S., Smith, C., Hu, W., et al. (2022). Therapeutic advances in the treatment of holmes tremor: systematic review. Neuromodulation 25, 796–803. doi: 10.1111/ner.13220
Keywords: Holmes tremor, deep brain stimulation, thalamus, posterior subthalamic area, dual-lead
Citation: Maesawa S, Torii J, Nakatsubo D, Noda H, Mutoh M, Ito Y, Ishizaki T, Tsuboi T, Suzuki M, Tanei T, Katsuno M and Saito R (2022) A case report: Dual-lead deep brain stimulation of the posterior subthalamic area and the thalamus was effective for Holmes tremor after unsuccessful focused ultrasound thalamotomy. Front. Hum. Neurosci. 16:1065459. doi: 10.3389/fnhum.2022.1065459
Received: 09 October 2022; Accepted: 05 December 2022;
Published: 15 December 2022.
Edited by:
Umer Akbar, Brown University, United StatesReviewed by:
Leonardo Almeida, University of Florida, United StatesCopyright © 2022 Maesawa, Torii, Nakatsubo, Noda, Mutoh, Ito, Ishizaki, Tsuboi, Suzuki, Tanei, Katsuno and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Maesawa,  c21hZXNhd2FAbWVkLm5hZ295YS11LmFjLmpw
c21hZXNhd2FAbWVkLm5hZ295YS11LmFjLmpw
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.