
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci., 06 December 2022
Sec. Brain Health and Clinical Neuroscience
Volume 16 - 2022 | https://doi.org/10.3389/fnhum.2022.1022791
This article is part of the Research TopicLong-Lasting Neurobehavioral and Psychiatric Implications of Early Life AdversityView all 9 articles
Introduction: Childhood trauma is known to have dramatic effects on the risks for developing psychiatric disorders and increased suicidality. We conducted a meta-analysis of whole brain voxel-based morphometry (VBM) correlates of childhood trauma in adolescents exposed to childhood maltreatment (N = 379) and unexposed controls (N = 348).
Methods: Anisotropic effect size-signed differential mapping (AES-SDM) was utilized to synthesize the studies.
Results: We observed increased volume amongst adolescents with a history of childhood trauma in regions that are involved in motor functions and language production: left precentral gyrus, including part of the left inferior frontal gyrus, left fibers of the body of corpus callosum, and left postcentral gyrus. We observed decreased volume amongst adolescents with a history of childhood trauma in regions that are involved in language processing and/or sensory processing: bilateral cerebellum, bilateral middle temporal gyrus, left rostrum of corpus callosum, and bilateral supramarginal gyrus.
Discussion: We suggest that these morphometric differences may be reflective of impaired motor development and increased sensory sensitivity and hypervigilance in adolescents with experiences of childhood trauma. Our results differ from meta-analytical findings in adults with history of childhood trauma and may contribute to a better understanding of neural mechanisms of childhood trauma, prediction of neurodevelopmental outcomes, and development of more effective and personalized therapies.
Trauma experienced during childhood is known to be strongly associated with risks for developing psychiatric disorders and increased suicidality (Angelakis et al., 2019; LeMoult et al., 2020; McKay et al., 2021). Understanding the neural mechanisms of childhood trauma may help explain the increased risk of suicidality, predict health outcomes, and offer better and more personalized treatments. Brain regions involved in emotion regulation are often implicated in both psychiatric disorders and childhood trauma. Specifically, meta-analyses of brain morphometry associated with childhood trauma indicate that aberrations in the prefrontal-limbic system, which encompasses the prefrontal cortex, hippocampus, and amygdala, could be a result of childhood trauma and an underlying cause for the subsequent development of mental disorders (Paquola et al., 2016).
Most of the morphometric studies of childhood trauma correlates, however, focused on adults. For a better understanding of a potential deviation from the normal neurodevelopmental trajectory after experiencing childhood trauma, neuroimaging studies need to be conducted earlier in life. Measuring childhood trauma correlates in adulthood may be confounded by the effects of aging and adult trauma exposure, as well as the accumulated effects that physical and mental illness can reciprocally have on brain structure. Mood disorders linked to childhood trauma such as Major Depressive Disorder (MDD) are especially likely to appear during adolescence (Di Martino et al., 2014). This warrants a special research focus on adolescent brains.
Indeed, adolescent brains differ from adult brains and may show different aberrations linked to childhood trauma compared to adults. For example, a meta-analysis by Paquola et al. (2016) reported that trauma cohorts (from 38 studies) exhibited smaller hippocampus and amygdala volumes bilaterally. The most robust findings of the whole brain voxel-based morphometry (VBM) meta-analysis were reduced gray matter in the right dorsolateral prefrontal cortex and right hippocampus amongst adults with a history of childhood trauma. Interestingly, meta-regression analysis showed that age did moderate results such that larger differences in amygdala gray matter were present in older samples (Paquola et al., 2016). The exclusion of child studies also altered the main findings in four other meta-analyses of gray matter correlates of childhood trauma (Woon and Hedges, 2008; Lim et al., 2014; Riem et al., 2015; Pollok et al., 2022). A recent meta-analysis by Pollok et al. (2022) uncovered gray matter volume effects associated with a wide range of early life adversities (including low socio-economic status, urban upbringing, maltreatment, prenatal selective serotonin reuptake inhibitors (SSRI) exposure, very low birth weight, family history of alcohol dependence, etc.) in the right hippocampus, right amygdala, and the left inferior frontal gyrus. In their sub-analysis of the combined young children and adolescent group (age range: 3.51–17.09 years) the findings were preserved in the right amygdala and hippocampus, but no result was found for adults (age range: 18.40–56.89 years) (Pollok et al., 2022).
It can be expected that findings in the adolescent brain would differ from those in young children, as well as adults. The expected differences can, as discussed above, be linked to a different neurodevelopmental stage and to potential confounding by the effects of aging and mental illness. An additional reason for expected differences is that adolescents may give a more accurate account of their childhood experiences (Newbury et al., 2018) than when they become adults (Widom and Shepard, 1996; Widom and Morris, 1997). Finally, knowledge of brain morphometric aberrations in adolescents who have experienced childhood trauma may be critical for development of new interventions, since adolescence is a second peak of neuroplasticity when language is well developed, and interventions can be especially effective (UNICEF Office of Research - Innocenti, 2017). All these factors warrant a special focus on adolescents in studying effects of childhood trauma.
Given the importance of the developmental aspect of childhood trauma effects, the goal of this study was to conduct a meta-analysis of brain morphometric aberrations in adolescents who have experienced childhood trauma.
The literature search was conducted through December 2021 in PubMed using the following search string within title/abstract: (“childhood maltreatment” OR “child abuse” OR “early stress” OR “childhood adversities” OR “childhood trauma”) AND (“structural gray matter” OR “voxel-based morphometry” OR “whole-brain” OR “whole brain” OR “voxel based morphometry” OR “structural grey matter” OR “gray matter volume” OR “grey matter volume.” Additional papers identified through citation searching were added to the screening step. Titles and abstracts were screened to determine whether articles met the following overarching inclusion criteria: original empirical research articles with measures of whole-brain gray matter and childhood trauma, conducted in a human adolescent sample (10 < age < 23). Additionally, the following exclusion criteria were used: fewer than 10 subjects, no control, no whole-brain VBM analysis, or peak coordinates of significant clusters in MNI or Talairach space were not reported and could not be obtained from the authors. Records were screened by two reviewers independently.
For the purposes of this review, we defined trauma as witnessing or experiencing emotional, physical or sexual abuse or emotional or physical neglect. We used an expanded definition of adolescence as an age range between 10 years (when puberty begins) and 23 years (Sawyer et al., 2018).
From each included study, we extracted participants’ mean age, sex ratio, psychiatric diagnosis, and medications separately for subjects with and without exposure to childhood maltreatment/adversity, as well as maltreatment/adversity types, and peak voxel coordinates. Data were screened by two reviewers (authors RK and CN) independently. Any disagreement between the two reviewers was resolved by a third, independent reviewer (OT).
The analysis was conducted using anisotropic effect size-signed differential mapping (AES-SDM version 5.142). This choice of methodology allowed us to compare our results to the results of the meta-analysis conducted in adult subjects by Paquola et al. (2016). AES-SDM combines peak coordinates and statistical parametric maps by using standard effect size and variance-based meta-analytic calculations (Radua et al., 2012, 2014). Random-effects models are used in which each study is weighted according to its sample size and variability. This method enables conjunction analyses to compare abnormalities between the study groups (subjects with childhood trauma compared to those without childhood trauma) based on the evaluation of effect sizes.
To conduct statistical inference, we used the recommended threshold of p = 0.005 with peak z > 1 and cluster extent of > 20 voxels (Radua et al., 2012). We used jackknife sensitivity analysis to assess the contribution of each study to the overall results and thus explore the robustness of the results. We considered results that lost significance in > 10% of iterations as non-robust. Publication bias was assessed using Egger’s test for asymmetry of the funnel plot for each significant peak voxel derived from the AES-SDM meta-analysis.
The initial literature search identified 123 studies (Figure 1). Three additional papers were identified through citation searching. After screening based on title and abstract review, 29 studies were selected for full text assessment for eligibility. After the assessment, 11 papers were included in the final analysis (Tomoda et al., 2009a,b, 2011, 2012; Liao et al., 2013; Lu et al., 2013; Walsh et al., 2014; Kelly et al., 2015; Fujisawa et al., 2018; Lim et al., 2018; Gao et al., 2021).
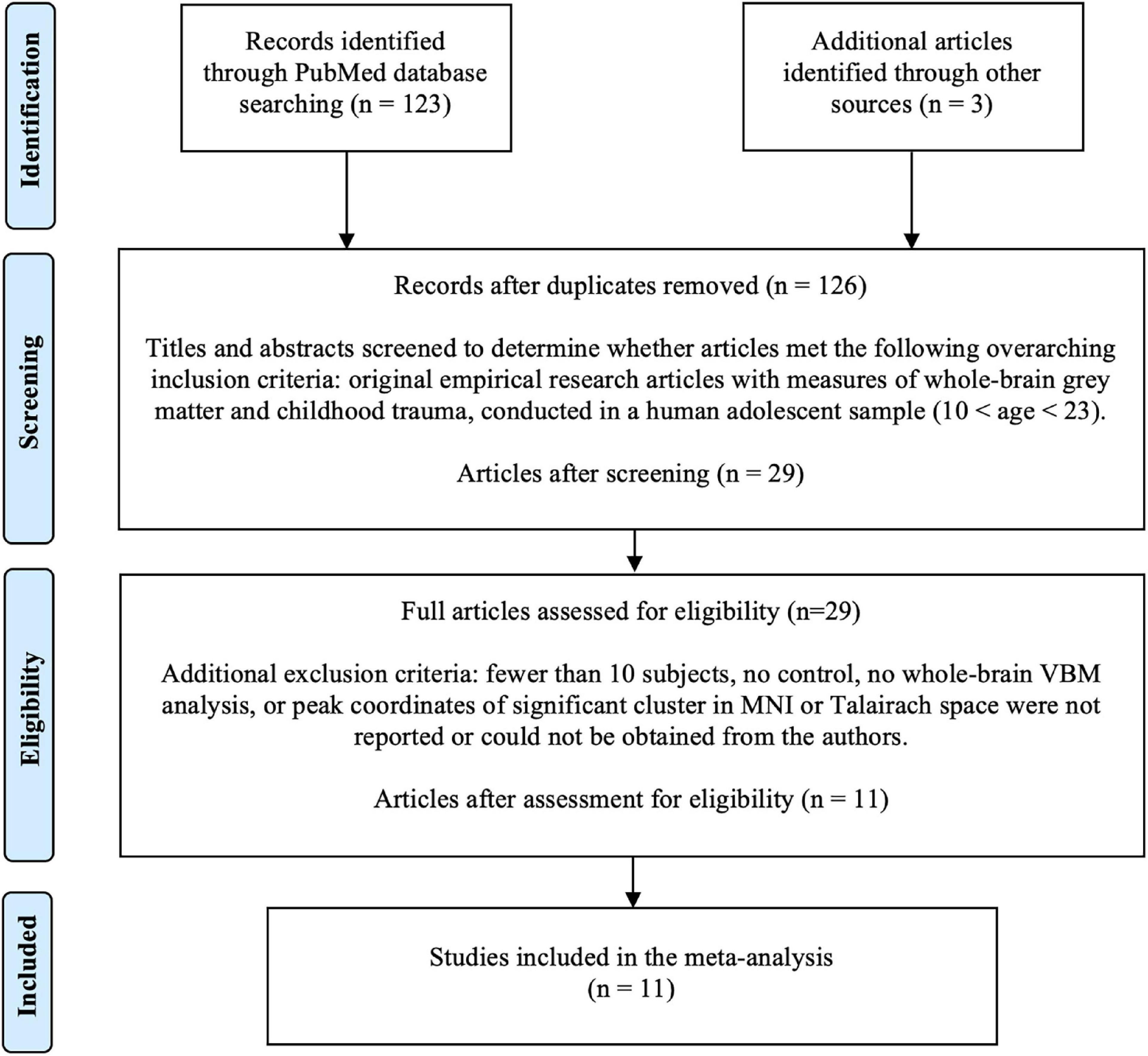
Figure 1. Literature search flow using PRISMA guidelines (Moher et al., 2009). VBM, voxel-based morphometry.
Summaries of sample characteristics of the included studies are described in Table 1. The total number of adolescents exposed to childhood maltreatment was 379. The total number of unexposed controls was 348.
The results of the meta-analysis are presented in Table 2 and in Figure 2. We obtained one cluster with significantly larger volume in adolescents with childhood trauma (Cluster A) and six clusters with significantly smaller volume in adolescents with childhood trauma (Clusters B–G). Cluster A included parts of the left precentral gyrus (including left inferior frontal gyrus, left fibers of the body of corpus callosum, left postcentral gyrus), and Clusters B–G included the bilateral cerebellum (including right lingual gyrus), bilateral middle temporal gyrus, left rostrum of corpus callosum, and bilateral supramarginal gyrus (including left inferior parietal gyrus) amongst adolescents with a history of childhood trauma. According to Jackknife analysis and Egger’s test, no single study was driving the reported endophenotype effect.
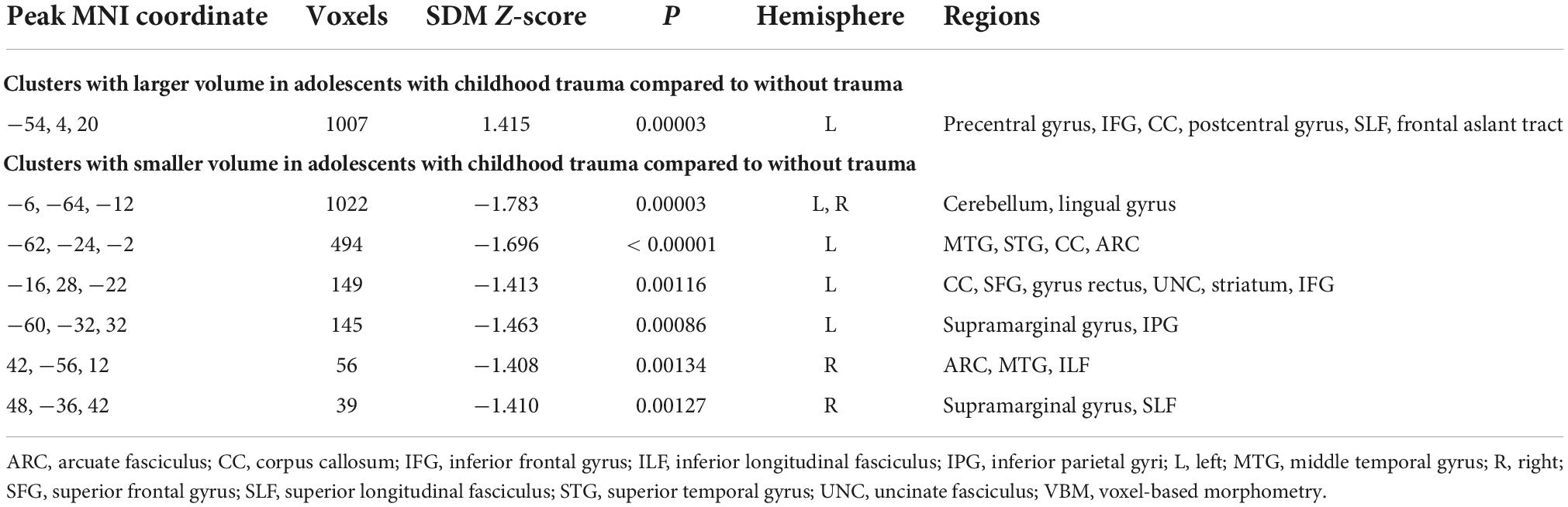
Table 2. Results of whole brain voxel-based morphometry (VBM) meta-analysis of childhood trauma in adolescents.
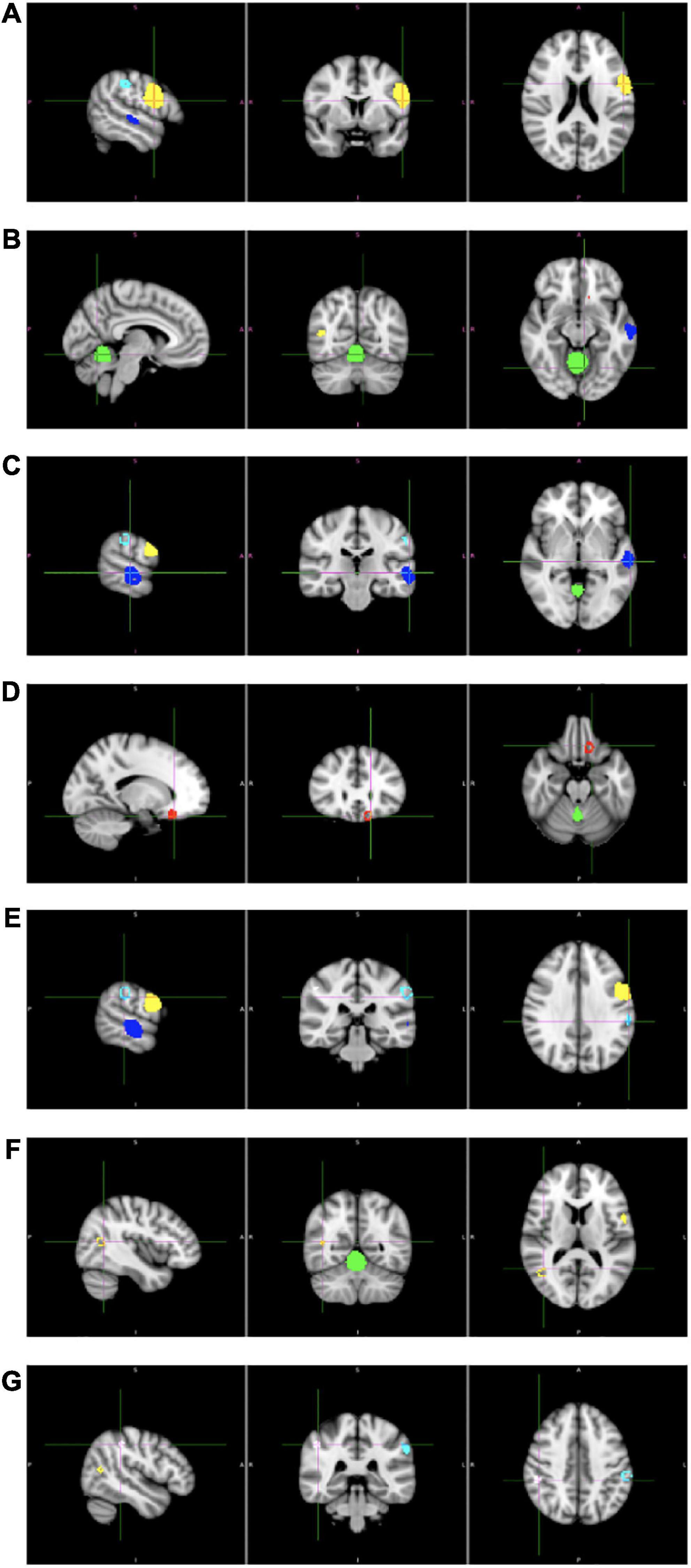
Figure 2. Results of whole brain voxel-based morphometry (VBM) meta-analysis of childhood trauma in adolescents. Clusters (A–G) corresponds to the clusters in Table 1.
In the present study, we conducted a meta-analysis of brain morphometric correlates of childhood trauma in adolescents. Only studies with a whole-brain VBM analysis of differences between adolescents with and without history of childhood trauma were included, which resulted in 11 studies. The results indicate increased volume in the left precentral gyrus (including left inferior frontal gyrus, left fibers of the body of corpus callosum, left postcentral gyrus), and reduced volume in the bilateral cerebellum (including right lingual gyrus), bilateral middle temporal gyrus, left rostrum of corpus callosum, and bilateral supramarginal gyrus (including left inferior parietal gyrus) amongst adolescents with a history of childhood trauma.
Before offering any possible interpretation of the obtained results, we would like to discuss the methodological issue linked to VBM result interpretation in general. Decreased volume or thickness of gray matter obtained as a result of VBM is often interpreted as an actual thinning due to synaptic pruning and cell loss (Gogtay et al., 2004; Tamnes et al., 2010). However, cortical thickness estimates from MRI are based on the definition of the gray–white boundary. This boundary depends on the difference in T1 of white and gray matter, which is coupled with myelin content. Any misestimates of this boundary will lead to inaccuracies in estimating cortical thickness from MRI measurements. A recent study supports that, for example, the key source of apparent thinning of the human visual cortex during childhood is increased myelination of axons (Natu et al., 2019). By combining multiple quantitative neuroimaging methods and histology in postmortem data, Natu et al. (2019) provide evidence that the cortex does not thin during childhood but instead becomes more myelinated. Their results suggest that increased myelination during childhood changes the intensity of voxels on T1-weighted MRI and thus shifts the apparent gray–white matter boundary toward the cortical surface (Natu et al., 2019). This new evidence contradicts conclusions of previous research (Tamnes et al., 2010) but it has an improved methodology and validation.
In light of this understanding of VBM results in general, we would like to discuss the findings of our meta-analysis. All of our gray matter findings are located in brain regions that previously demonstrated apparent thinning in healthy youth aged 8–30 years (Tamnes et al., 2010). The finding of a thicker gray matter in youth with childhood trauma compared to those without childhood trauma may therefore reflect less myelination of axons adjacent to the regions, whereas thinner gray matter may reflect more myelination. We observed larger volume (potentially less myelination of axons adjacent to the regions) amongst adolescents with a history of childhood trauma in the left precentral gyrus (including left inferior frontal gyrus, left fibers of the body of corpus callosum, left postcentral gyrus). The precentral gyrus is associated with motor functions, whereas the opercular part of inferior frontal gyrus adjacent to the precentral gyrus in the left hemisphere is most likely associated with language production. Gvozdanovic et al. (2017) reported larger inferior frontal gyrus/precentral gyrus volumes were also related to an increased number of early intrusive film memories in healthy young females, as well as subjective distress and vividness of the intrusions. Changes in functional connectivity between the precentral gyrus and limbic regions have been reported in a trauma film group compared to a control film group during intrusive film picture presentation (Gvozdanovic et al., 2017). In another study, functional connectivity strength between bilateral precentral gyri and left amygdala positively correlated with the magnitude of reported physical abuse (Gvozdanovic et al., 2020). Finally, in an Affective Stroop task study, increased maltreatment (in particular abuse) was associated with decreased differential responsiveness of the precentral gyrus to incongruent task trials compared with view trials (Blair et al., 2019). On the behavioral level, the finding obtained in our study aligns with the recent call to consider physical developmental deficits in addition to cognitive, emotional, and social deficits when linking adverse childhood experiences to school-readiness (Wade et al., 2017). In a study by Wade et al. (2017), children with maltreatment showed rates of impaired motoric development five to seven times higher than expected, with those exposed to sexual or physical abuse having the highest rates. It has even been suggested that poor motor coordination may be causal in relation to emotional deficits such as anxiety, mediated by negative self-concept and low social support (Cairney et al., 2010). In addition to the primary motor cortex, the precentral gyrus also contains a portion of the supplementary motor cortex, which is involved in the planning of voluntary limb movement (Schott, 1993). A more speculative interpretation of the observed differences in our study is that they are related to tonic immobility—an involuntary motor and vocal inhibition reaction, considered the last-ditch response of the defensive cascade model, that can be induced by various types of traumatic events (Kalaf et al., 2017).
This meta-analysis also showed reduced volume amongst adolescents with a history of childhood trauma: in the bilateral cerebellum (including right lingual gyrus), bilateral middle temporal gyrus, left rostrum of corpus callosum, and bilateral supramarginal gyrus (including left inferior parietal gyrus). Almost all of these regions are involved in language processing and/or sensory processing. Since reduced apparent gray matter volume may be reflective of increased myelination of the adjacent white matter as described above, these results may be reflective of adaptive strategies in adolescents with experience of childhood trauma. In the case of abuse, children may become highly attuned to the environment and overly reliant on external cues (Mackiewicz Seghete et al., 2017). In the case of neglect sensory hypersensitivity is present less frequently than in the case of abuse and the opposite pattern of underresponsiveness is also observed (Howard et al., 2020), but an association with a heightened responsiveness to environmental stimuli, such as salient visual stimuli within visual cortices, has also been shown (Blair et al., 2019).
In summary, we suggest that the morphometric differences obtained in our study may be reflective of impaired motor development and increased sensory sensitivity and hypervigilance in adolescents who have experienced childhood trauma.
Interestingly, our results differ from the most robust findings of the whole brain VBM meta-analysis in adults with a history of childhood trauma by Paquola et al. (2016), who observed reduced gray matter in the right dorsolateral prefrontal cortex and right hippocampus. Their meta-regression analysis showed that age did moderate results such that larger differences in amygdala gray matter were present in older samples (Paquola et al., 2016). One possible interpretation is that, at first, the brain of a child experiencing trauma adapts to monitor threat, and over time the experienced childhood trauma takes a toll on other systems, creating more pronounced changes in the subcortical limbic structures and prefrontal cortex.
The interpretation of our results only partially aligns with a recent diffusion tensor imaging (DTI) meta-analysis that included child and adolescent samples along with adult samples and showed aberrations in structural connectivity associated with childhood trauma (Lim et al., 2020). Lim et al. (2020) found that maltreated individuals had significantly reduced fractional anisotropy (FA) in the left anterior thalamic radiation and bilateral fornix, optic radiations, inferior longitudinal fasciculus, and inferior frontal-occipital fasciculus, along with the anterior portions of the corpus callosum. There were no regions with increased FA. This lack of alignment can be due to the differences in demographics, age, and comorbidities. It can also be linked to the fact that white matter myelination close to the gray matter boundary might not be well-captured by FA, which gets closer to zero in the vicinity of the gray matter.
Our study should be interpreted in light of its limitations. The timing and the type of childhood trauma experienced (e.g., abuse vs. neglect) can be important (McLaughlin et al., 2019), as well as the accompanying psychiatric diagnosis (Table 1). Due to the limited sample size, we could not explore these variables —as well as other important variables such as sex and age—as moderators. In some of the included studies subjects who experienced childhood trauma had a psychiatric diagnosis, whereas the controls did not (Table 1). With small sample sizes and varied diagnoses within and across the included studies, it is difficult to disentangle brain differences related to childhood trauma and those related to specific psychiatric disorders. Interestingly, in the study by Lim et al. (2018), although there were no significant differences in the regions of interest (ROIs) between the abuse group and psychiatric controls or between the psychiatric and healthy controls, the brain measurements of the psychiatric controls were in between those of the abuse group and healthy controls. This suggests that the abuse group, by nature of the abuse experience combined with the psychiatric comorbidities, was more adversely affected than the psychiatric controls (Lim et al., 2018). The results of our meta-analysis also show a significant overlap with the results of a VBM-focused meta-analysis by Serra-Blasco et al. (2021), showing correlates of depression, anxiety, and post-traumatic stress disorder (PTSD) in regions such as left inferior frontal gyrus (L IFG), left superior temporal gyrus (L STG), superior frontal gyrus (SFG), fusiform gyrus [we found inferior longitudinal fasciculus (ILF) which connects to fusiform], and cerebellum, which all coincide with our findings. Although the meta-analysis by Serra-Blasco et al. (2021) was performed in a mixed population, the same group has published a protocol for a planned VBM-based meta-analysis of all major mental disorders and their comorbidities, in which they will have separate single linear models, one for children/adolescents and one for adults (Fortea et al., 2022). Our results combined with the knowledge that will be gained in this future meta-analysis could help shed light on the differences between manifestations of childhood trauma vs. specific psychiatric disorders in the adolescent brain.
An important strength of our study is its focus on adolescents. Previous meta-analysis excluded studies with individuals younger than 18 years (Paquola et al., 2016). Adolescent brains differ from adult brains and may show different aberrations linked to childhood trauma compared to adults. Moreover, adolescents may give a more accurate account of their childhood experiences than after they become adults. By restricting the age of studied subjects (age < 23 years) we addressed the potential confounding effects of aging and adult trauma exposure, and we addressed the accumulated effects physical and mental illness can have on brain structure.
The difference in findings between this meta-analysis in adolescents and prior meta-analytical findings in adults with a history of childhood trauma underlines the importance of examining adolescents and not assuming that adults and adolescents are the same. In mental health, data from adults are often applied to teens because of a critical lack of data in teens. Our results have important implications for the development of novel treatments for adolescents with psychiatric disorders (e.g., adolescent MDD), that could target brain regions associated with trauma and thus address a root cause potentially underlying psychiatric disorders and key transdiagnostic conditions such as suicidality. A better understanding of neural mechanisms of childhood trauma in adolescents may help develop such brain targets, offer more personalized therapies, monitor treatment effects, and predict neurodevelopmental outcomes.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
OT, RH, RK, CN, and TY conceived the work. OT, RH, RK, and CN conducted data collection. RH conducted data analyses. OT prepared the figures. OT and RH drafted the work. OT, RH, RK, CN, JM, and TY interpreted the data. All authors co-wrote the manuscript.
This study was supported by the National Center for Complementary and Integrative Health (NCCIH) R21AT009173, R61AT009864, and R33AT009864 to OT and TY, the UCSF Research Resident Training Program R25 grant to RH, the National Center for Advancing Translational Sciences (CTSI), National Institutes of Health, through UCSF-CTSI UL1TR001872 to OT and TY, the American Foundation for Suicide Prevention (AFSP) SRG-1-141-18 to OT and TY, UCSF Research Evaluation and Allocation Committee (REAC) and J. Jacobson Fund to OT and TY, the Fahs-Beck Fund for Research and Experimentation at The New York Community Trust to OT, the National Institute of Mental Health (NIMH) R01MH085734 and the Brain and Behavior Research Foundation (formerly NARSAD) to TY, and the NICHD R01HD088438 to JM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Angelakis, I., Gillespie, E. L., and Panagioti, M. (2019). Childhood maltreatment and adult suicidality: a comprehensive systematic review with meta-analysis. Psychol. Med. 49, 1057–1078. doi: 10.1017/S0033291718003823
Blair, K. S., Aloi, J., Crum, K., Meffert, H., White, S. F., Taylor, B. K., et al. (2019). Association of different types of childhood maltreatment with emotional responding and response control among youths. JAMA Netw. Open 2:e194604. doi: 10.1001/jamanetworkopen.2019.4604
Cairney, J., Veldhuizen, S., and Szatmari, P. (2010). Motor coordination and emotional–behavioral problems in children. Curr. Opin. Psychiatry 23, 324–329. doi: 10.1097/YCO.0b013e32833aa0aa
Di Martino, A., Fair, D. A., Kelly, C., Satterthwaite, T. D., Castellanos, F. X., Thomason, M. E., et al. (2014). Unraveling the miswired connectome: a developmental perspective. Neuron 83, 1335–1353. doi: 10.1016/j.neuron.2014.08.050
Fortea, L., Albajes-Eizagirre, A., Yao, Y.-W., Soler, E., Verdolini, N., Hauson, A. O., et al. (2022). Focusing on comorbidity—a novel meta-analytic approach and protocol to disentangle the specific neuroanatomy of co-occurring mental disorders. Front. Psychiatry 12:807839. doi: 10.3389/fpsyt.2021.807839
Fujisawa, T. X., Shimada, K., Takiguchi, S., Mizushima, S., Kosaka, H., Teicher, M. H., et al. (2018). Type and timing of childhood maltreatment and reduced visual cortex volume in children and adolescents with reactive attachment disorder. NeuroImage Clin. 20, 216–221. doi: 10.1016/j.nicl.2018.07.018
Gao, Y., Jiang, Y., Ming, Q., Zhang, J., Ma, R., Wu, Q., et al. (2021). Neuroanatomical changes associated with conduct disorder in boys: influence of childhood maltreatment. Eur. Child Adolesc. Psychiatry 31, 601–613. doi: 10.1007/s00787-020-01697-z
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174–8179. doi: 10.1073/pnas.0402680101
Gvozdanovic, G., Stämpfli, P., Seifritz, E., and Rasch, B. (2020). Structural brain differences predict early traumatic memory processing. Psychophysiology 57:e13354. doi: 10.1111/psyp.13354
Gvozdanovic, G. A., Stämpfli, P., Seifritz, E., and Rasch, B. (2017). Neural correlates of experimental trauma memory retrieval. Hum. Brain Mapp. 38, 3592–3602. doi: 10.1002/hbm.23613
Howard, A. R. H., Lynch, A. K., Call, C. D., and Cross, D. R. (2020). Sensory processing in children with a history of maltreatment: an occupational therapy perspective. Vulnerable Child. Youth Stud. 15, 60–67. doi: 10.1080/17450128.2019.1687963
Kalaf, J., Coutinho, E. S. F., Vilete, L. M. P., Luz, M. P., Berger, W., Mendlowicz, M., et al. (2017). Sexual trauma is more strongly associated with tonic immobility than other types of trauma – A population based study. J. Affect. Disord. 215, 71–76. doi: 10.1016/j.jad.2017.03.009
Kelly, P. A., Viding, E., Puetz, V. B., Palmer, A. L., Mechelli, A., Pingault, J. B., et al. (2015). Sex differences in socioemotional functioning, attentional bias, and gray matter volume in maltreated children: a multilevel investigation. Dev. Psychopathol. 27, 1591–1609. doi: 10.1017/S0954579415000966
LeMoult, J., Humphreys, K. L., Tracy, A., Hoffmeister, J. A., Ip, E., and Gotlib, I. H. (2020). Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry 59, 842–855. doi: 10.1016/j.jaac.2019.10.011
Liao, M., Yang, F., Zhang, Y., He, Z., Song, M., Jiang, T., et al. (2013). Childhood maltreatment is associated with larger left thalamic gray matter volume in adolescents with generalized anxiety disorder. PLoS One 8:e71898. doi: 10.1371/journal.pone.0071898
Lim, L., Hart, H., Mehta, M., Worker, A., Simmons, A., Mirza, K., et al. (2018). Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol. Med. 48, 1034–1046. doi: 10.1017/S0033291717002392
Lim, L., Howells, H., Radua, J., and Rubia, K. (2020). Aberrant structural connectivity in childhood maltreatment: a meta-analysis. Neurosci. Biobehav. Rev. 116, 406–414. doi: 10.1016/j.neubiorev.2020.07.004
Lim, L., Radua, J., and Rubia, K. (2014). Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am. J. Psychiatry 171, 854–863. doi: 10.1176/appi.ajp.2014.13101427
Lu, S., Gao, W., Wei, Z., Wu, W., Liao, M., Ding, Y., et al. (2013). Reduced cingulate gyrus volume associated with enhanced cortisol awakening response in young healthy adults reporting childhood trauma. PLoS One 8:e69350. doi: 10.1371/journal.pone.0069350
Mackiewicz Seghete, K. L., Kaiser, R. H., DePrince, A. P., and Banich, M. T. (2017). General and emotion-specific alterations to cognitive control in women with a history of childhood abuse. NeuroImage Clin. 16, 151–164. doi: 10.1016/j.nicl.2017.06.030
McKay, M. T., Cannon, M., Chambers, D., Conroy, R. M., Coughlan, H., Dodd, P., et al. (2021). Childhood trauma and adult mental disorder: a systematic review and meta-analysis of longitudinal cohort studies. Acta Psychiatr. Scand. 143, 189–205. doi: 10.1111/acps.13268
McLaughlin, K. A., Weissman, D., and Bitrán, D. (2019). Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 1, 277–312. doi: 10.1146/annurev-devpsych-121318-084950
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6, doi: 10.1371/journal.pmed.1000097
Natu, V. S., Gomez, J., Barnett, M., Jeska, B., Kirilina, E., Jaeger, C., et al. (2019). Apparent thinning of human visual cortex during childhood is associated with myelination. Proc. Natl. Acad. Sci. U.S.A. 116, 20750–20759. doi: 10.1073/pnas.1904931116
Newbury, J. B., Arseneault, L., Moffitt, T. E., Caspi, A., Danese, A., Baldwin, J. R., et al. (2018). Measuring childhood maltreatment to predict early-adult psychopathology: comparison of prospective informant-reports and retrospective self-reports. J. Psychiatr. Res. 96, 57–64. doi: 10.1016/j.jpsychires.2017.09.020
Paquola, C., Bennett, M. R., and Lagopoulos, J. (2016). Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neurosci. Biobehav. Rev. 69, 299–312. doi: 10.1016/j.neubiorev.2016.08.011
Pollok, T. M., Kaiser, A., Kraaijenvanger, E. J., Monninger, M., Brandeis, D., Banaschewski, T., et al. (2022). Neurostructural traces of early life adversities: a meta-analysis exploring age- and adversity-specific effects. Neurosci. Biobehav. Rev. 135:104589. doi: 10.1016/j.neubiorev.2022.104589
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Rubia, K., Canales-Rodríguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 5:13. doi: 10.3389/fpsyt.2014.00013
Riem, M. M. E., Alink, L. R. A., Out, D., Ijzendoorn, M. H. V., and Bakermans-Kranenburg, M. J. (2015). Beating the brain about abuse: empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 27, 507–520. doi: 10.1017/S0954579415000127
Sawyer, S. M., Azzopardi, P. S., Wickremarathne, D., and Patton, G. C. (2018). The age of adolescence. Lancet Child Adolesc. Health 2, 223–228. doi: 10.1016/S2352-4642(18)30022-1
Schott, G. D. (1993). Penfield’s homunculus: a note on cerebral cartography. J. Neurol. Neurosurg. Psychiatry 56, 329–333. doi: 10.1136/jnnp.56.4.329
Serra-Blasco, M., Radua, J., Soriano-Mas, C., Gómez-Benlloch, A., Porta-Casteràs, D., Carulla-Roig, M., et al. (2021). Structural brain correlates in major depression, anxiety disorders and post-traumatic stress disorder: a voxel-based morphometry meta-analysis. Neurosci. Biobehav. Rev. 129, 269–281. doi: 10.1016/j.neubiorev.2021.07.002
Tamnes, C. K., Ostby, Y., Fjell, A. M., Westlye, L. T., Due-Tønnessen, P., and Walhovd, K. B. (2010). Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex N.Y. 1991, 534–548. doi: 10.1093/cercor/bhp118
Tomoda, A., Navalta, C. P., Polcari, A., Sadato, N., and Teicher, M. H. (2009a). Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol. Psychiatry 66, 642–648. doi: 10.1016/j.biopsych.2009.04.021
Tomoda, A., Suzuki, H., Rabi, K., Sheu, Y. S., Polcari, A., and Teicher, M. H. (2009b). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage 47(Suppl. 2), T66–T71. doi: 10.1016/j.neuroimage.2009.03.005
Tomoda, A., Polcari, A., Anderson, C. M., and Teicher, M. H. (2012). Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PLoS One 7:e52528. doi: 10.1371/journal.pone.0052528
Tomoda, A., Sheu, Y. S., Rabi, K., Suzuki, H., Navalta, C. P., Polcari, A., et al. (2011). Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. NeuroImage 54(Suppl. 1), S280–S286. doi: 10.1016/j.neuroimage.2010.05.027
UNICEF Office of Research - Innocenti (2017). The Adolescent Brain: A Second Window of Opportunity. Florence: UNICEF Office of Research - Innocenti.
Wade, T. J., Bowden, J., and Jane Sites, H. (2017). Child maltreatment and motor coordination deficits among preschool children. J. Child Adolesc. Trauma 11, 159–162. doi: 10.1007/s40653-017-0186-4
Walsh, N. D., Dalgleish, T., Lombardo, M. V., Dunn, V. J., Van Harmelen, A. L., Ban, M., et al. (2014). General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. NeuroImage Clin. 4, 308–318. doi: 10.1016/j.nicl.2014.01.001
Widom, C. S., and Morris, S. (1997). Accuracy of adult recollections of childhood victimization, Part 2: childhood sexual abuse. Psychol. Assess. 9, 34–46. doi: 10.1037/1040-3590.9.1.34
Widom, C. S., and Shepard, R. L. (1996). Accuracy of adult recollections of childhood victimization: part 1. Childhood physical abuse. Psychol. Assess. 8, 412–421. doi: 10.1037/1040-3590.8.4.412
Keywords: childhood trauma, child maltreatment, adolescent brain, VBM, brain development
Citation: Tymofiyeva O, Hu R, Kidambi R, Nguyen C, Max JE and Yang TT (2022) A meta-analysis of brain morphometric aberrations in adolescents who experienced childhood trauma. Front. Hum. Neurosci. 16:1022791. doi: 10.3389/fnhum.2022.1022791
Received: 19 August 2022; Accepted: 09 November 2022;
Published: 06 December 2022.
Edited by:
Carina Soares-Cunha, University of Minho, PortugalReviewed by:
Xing-Da Ju, Northeast Normal University, ChinaCopyright © 2022 Tymofiyeva, Hu, Kidambi, Nguyen, Max and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Tymofiyeva, T2xnYS5UeW1vZml5ZXZhQHVjc2YuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.