- 1Musculoskeletal Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Department of Physiotherapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran
- 3Research Center for War-Affected People, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Human Movement Science, University of Zululand, Kwazulu-Natal, South Africa
Background: Lower limb spasticity after stroke is common that can affect the balance, increase the risk of falling, and reduces the quality of life.
Objective: First, evaluate the effects of spasticity severity of ankle plantar flexors on balance of patients after stroke. Second, to determine the relationship between the spasticity severity with ankle proprioception, passive ankle dorsiflexion range of motion (ROM), and balance confidence.
Methods: Twenty-eight patients with stroke based on the Modified Modified Ashworth Scale (MMAS) were divided into two groups: High Spasticity Group (HSG) (MMAS > 2) (n = 14) or a Low Spasticity Group (LSG) (MMAS ≤ 2) (n = 14). The MMAS scores, Activities-Specific Balance Confidence Questionnaire, postural sway of both affected and non-affected limbs under the eyes open and eyes closed conditions, timed up and go (TUG) test, passive ankle dorsiflexion ROM, and ankle joint proprioception were measured.
Results: The ankle joint proprioception was significantly better in the LSG compared to the HSG (p = 0.01). No significant differences were found between the LSG and HSG on all other outcome measures. There were no significant relationships between the spasticity severity and passive ankle dorsiflexion ROM, and balance confidence.
Conclusion: The severity of ankle plantar flexor spasticity had no effects on balance of patients with stroke. However, the ankle joint proprioception was better in patients with low spasticity. Our findings suggest that the balance is affected regardless of the severity of the ankle plantar flexor spasticity in this group of participants with stroke.
Introduction
Stroke is a common cause of disability and residual physical impairments following a stroke and can pose a significant threat to quality of life (World Health Organization, 2018). Every year, 3.7 million individuals globally suffer a hemorrhagic stroke, while 7.3 million suffer from an ischemic stroke (Feigin et al., 2017). Specifically, the sensorimotor and cognitive impairments following a stroke can have serious impacts on independence and activities of daily living (ADL) (Geurts et al., 2005). Of these stroke complications, impaired balance is critical for safe mobility, and any deficiencies in balance negatively affect gait, limit ADLs, and/or increases the risk of individuals falling (Paillex and So, 2005; Kollen et al., 2006; Van de Port et al., 2006).
Spasticity is one of common complications of stroke that negatively affects balance. Spasticity is a common sensorimotor disorder defined neurophysiologically as a velocity-dependent increase in muscle tone and stretch reflex hypersensitivity (Lance, 1980). It has been reported as many as 50% of patients with stroke have muscle spasticity (Dorňák et al., 2019). Spasticity contributes in balance dysfunction through various mechanisms (Sinkjær, 1996; Carpenter et al., 2004; Bensoussan et al., 2007; Trumbower et al., 2010). After stroke, lower limb spasticity decreases the range of motion (ROM) and increases the stiffness of the muscles and fascia around the joints (Gao et al., 2011). Further, balance control is further affected when inappropriate muscle and joint afferents and subsequent movement responses occur with inappropriate ankle strategies (Lee et al., 2010).
While lower limb spasticity affects balance, gait, and falling in post-stroke patients (Soyuer and Ozturk, 2007; Sommerfeld et al., 2012), the effects may vary according to the intensity of the muscle spasticity after stroke (Nardone et al., 2001; Cakar et al., 2010; Phadke et al., 2014). To these authors’ knowledge, only one study has investigated the relationship between the spasticity severity and the balance in patients with stroke (Rahimzadeh Khiabani et al., 2017). However, authors assessed only static balance using one force plate, and did not evaluate the ankle proprioception and ROM in patients with stroke. Further, they measured the severity of spasticity based on the Modified Ashworth Scale that its reliability and validity is questioned (Ansari et al., 2006) and caution had been expressed in using it for spasticity assessment (Fleuren et al., 2010). Thus, this study aimed to evaluate the effects of ankle plantar flexor spasticity severity on balance and to determine the relationship between the spasticity severity with ankle proprioception, passive ROM, and balance confidence in post-stroke patients. We hypothesized that stroke subjects with high spasticity would have greater balance impairment compared with stroke subjects with low spasticity.
Materials and Methods
Study Design
The protocol of this cross-sectional study has been previously reported (Mahmoudzadeh et al., 2020) with the exception of one modification made on posturography. The present study utilized two force plates to assess the both affected and less affected limbs separately. Modified Modified Ashworth Scale (MMAS) scores, Activities-Specific Balance Confidence Questionnaire, postural sway in the open and closed eyes conditions, timed up and go (TUG) test, ankle dorsiflexion passive ROM, and ankle joint proprioception were measured in two post-stroke patient groups based on the level of ankle plantar flexor spasticity [i.e., High Spasticity Group (HSG) (MMAS > 2) and a Low Spasticity Group (LSG) (MMAS ≤ 2)].
Setting
The measurements were taken at the Javad Movafaghian Research Center, Tehran, Iran.
Approval of Study Protocol
The study protocol was approved by the Review Board and the Ethical Committee of the Tehran University of Medical Sciences (IR.TUMS.FNM.REC.1397.012) in compliance with the Helsinki declaration. All participants provided their written informed consent prior to the assessments.
Informed Consent
All eligible participants provided a written formal consent after receiving information about the research procedure. Study details, risks, and outcome measures were explained to participants prior to giving the written informed consent and taking the measurements.
Participants
Patients with stroke were included from those who were referred to neurology and physiotherapy clinics in Tehran, Iran. The patients were included if they had the following criteria: (1) unilateral, first-ever Hemorrhagic/Ischemic stroke, (2) ankle plantar flexor spasticity ≥ 1 based on the MMAS, (3) walking ability, (4) no fixed contracture in the ankle, (5) independent standing with eyes open/closed, (6) ability to understand and follow the commands, and (7) no pain in the lower limbs. The exclusion criteria were: (1) vision problems, (2) depression, and (3) taking antispastic medications.
Sample Size
Considering a previous study and β = Zβ = 0.842, α = 0.05, α = Zα = 1.96 (Rahimzadeh Khiabani et al., 2017; Mahmoudzadeh et al., 2020), the sample size was calculated at 28 (n = 14 in each group).
Procedures
The study procedures and measurements utilized in this study have been published previously (Mahmoudzadeh et al., 2020). Demographic data of the all patients were collected prior to the initiation of assessments. All tests were performed by an experienced physiotherapist. Spasticity severity of ankle plantar flexor muscle was evaluated using the MMAS (Ghotbi et al., 2011; Nakhostin Ansari et al., 2012). Patients were classified as High (MMAS ≥ 2) (HSG n = 14) and Low (MMAS < 2) (LSG n = 14) spasticity. The Activities-Specific Balance Confidence (ABC) questionnaire was used to assess the balance confidence (Salbach et al., 2006; Azad et al., 2016) and includes16 questions asking subjects to score their confidence in performing their activities in daily living from 0% (no confidence) to 100% (complete confidence). Posturography was used to assess the static balance (Sawacha et al., 2013; Lendraitienë et al., 2017) using two force plates which were placed together without spaces between to measure the postural sway of affected and less affected limbs independently. The examiner asked each patient to stand on the force plate with bare feet, heel spacing to be 9 cm, the angle between the two feet being 30 degrees, and upper limbs alongside the body. The patient was asked to look at a point on the wall at a distance of 3 m during the test with an open eye and closed eye. The open or closed eye conditions were randomly applied and a 2-min rest was considered between these two conditions. Each condition was repeated for three times (with intervals of 20 s) and the duration of each repetition was 20 s (Rahimzadeh Khiabani et al., 2017). The dynamic balance of patients was measured using the TUG test (Ng and Hui-Chan, 2005). Ankle passive ROM was measured using a standard goniometer (Radinmehr et al., 2019). The ankle joint proprioception was measured using electrogonometer as reconstruction errors. To assess the proprioception, the average of three repetitions of reconstruction angles measured at the angles of 5° and 15° plantar flexion as well as 15° dorsi flexion were calculated as the angles of reconstruction errors.
Outcome Measures
The primary outcome measures were the MMAS scores, ABC questionnaire, posturography measures in open- and closed-eyes conditions, and TUG test. The secondary outcome measures were the ankle passive ROM and ankle joint proprioception. BioWare software (Bioware 5.3.2.9-2.0, Kistler Bioware.msi, Kistler Instrument Group) was used for transforming the force plate data numerical mode. Medio-lateral (ML) and antrio-posterior (AP) displacement, average and instant velocity of the center of pressure (COP) and area were calculated using Excel (Excel, 2010, Ink) and MATLAB (MATLAB, R2018b, Ink) softwares.
Statistical Analysis
Normal distribution of the data was assessed by the Kolmogrov-Smirnov test. T-tests were used to compare the clinical data between two groups. A mixed model Repeated Measures ANOVA of 2 × 2 × 2 was performed to analyze the “Group effect” (High spasticity vs. Low spasticity), “Limb effect” (affected and unaffected limbs), “Eye effect” (eyes open and closed conditions), and interactions between variables. The relationships between the severity of spasticity and outcome variables were analyzed using the Spearman’s correlation test. SPSS software (SPSS, version 22, SPSS Inc., Chicago, IL, United States) was used for the data analysis. Statistical significance was defined at α = 0.05.
Results
Participant Demographics
Twenty-eight post-stroke patients were included in the current study. High and Low spasticity groups were similar in terms of height, weight, age, the time since the stroke onset, etiology (i.e., ischemic or hemorrhage) and affected side (P > 0.05). Demographic characteristics of the two study groups are illustrated in Table 1.
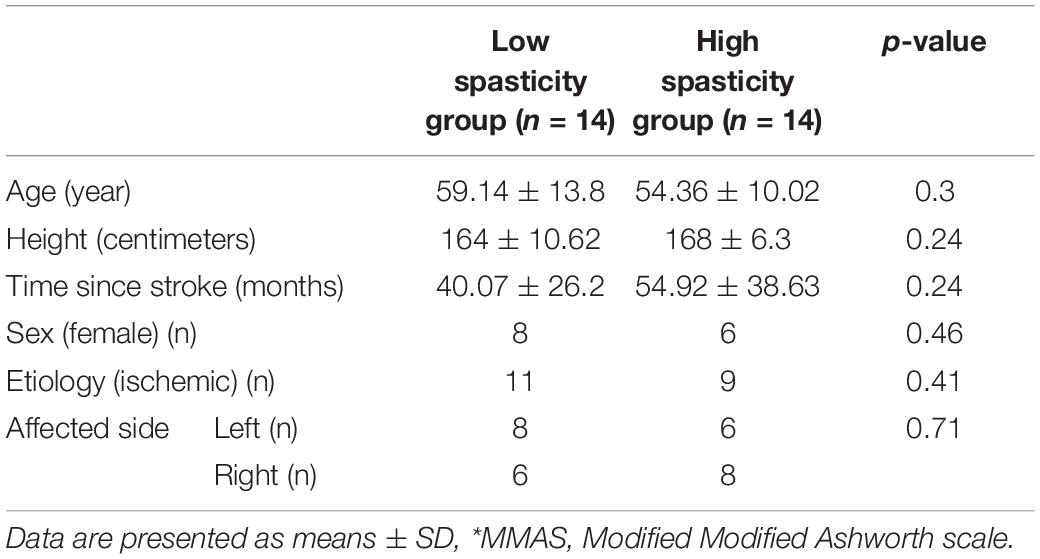
Table 1. Demographic characteristics of the High (MMAS* ≥ 2) (HSG n = 14) and Low (MMAS < 2) (LSG n = 14) spasticity study groups.
Clinical Measures
No significant difference was found between the two groups for TUG, ABC scores, and ankle passive dorsiflexion ROM (p > 0.05). However, ankle joint proprioception was found to be significantly different between the groups (p = 0.01) (Table 2).
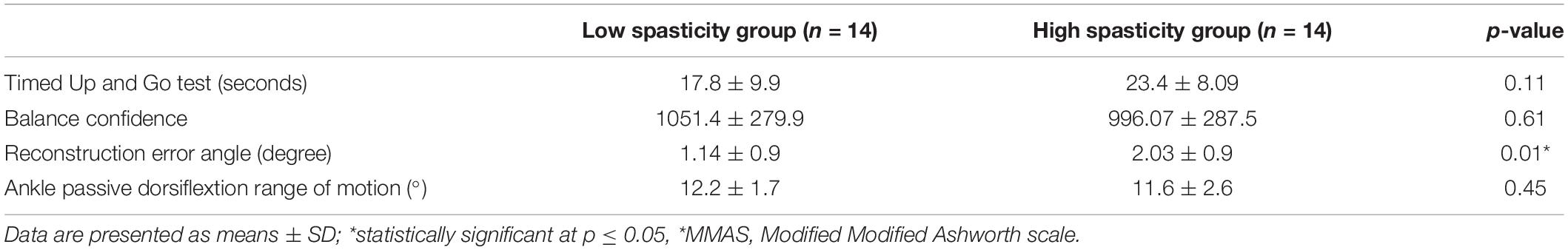
Table 2. Clinical characteristics of the High (MMAS* ≥ 2) (HSG n = 14) and Low (MMAS < 2) (LSG n = 14) spasticity study groups.
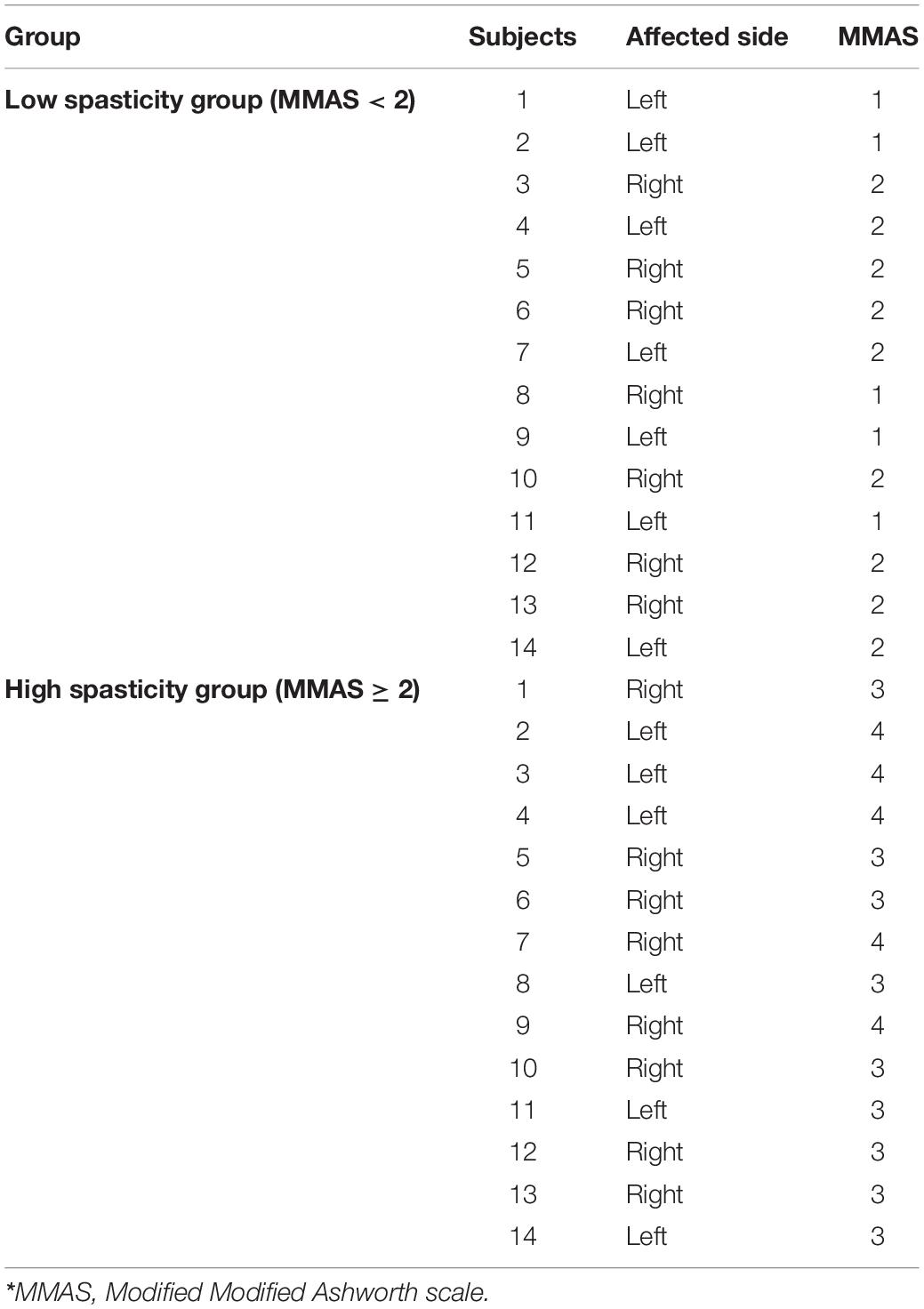
The distribution of spasticity severity based on MMAS in low and high spasticity groups is presented in Table 3. In the low spasticity group, spasticity severity of five patients were MMAS = 1 and spasticity severity of nine patients were MMAS = 2. In the high spasticity group, spasticity severity of nine patient were MMAS = 3 and spasticity severity of other five patients were MMAS = 4.
Posturography
Posturography data for the groups are presented in Table 4. There were no significant differences in the medio-lateral (ML) and anterio-posterior (AP) displacements, velocity, and area between open and close eyes conditions, between the affected and unaffected limbs within groups and between groups. The interactions between the groups and the limbs or eyes conditions were not significant (p > 0.05).
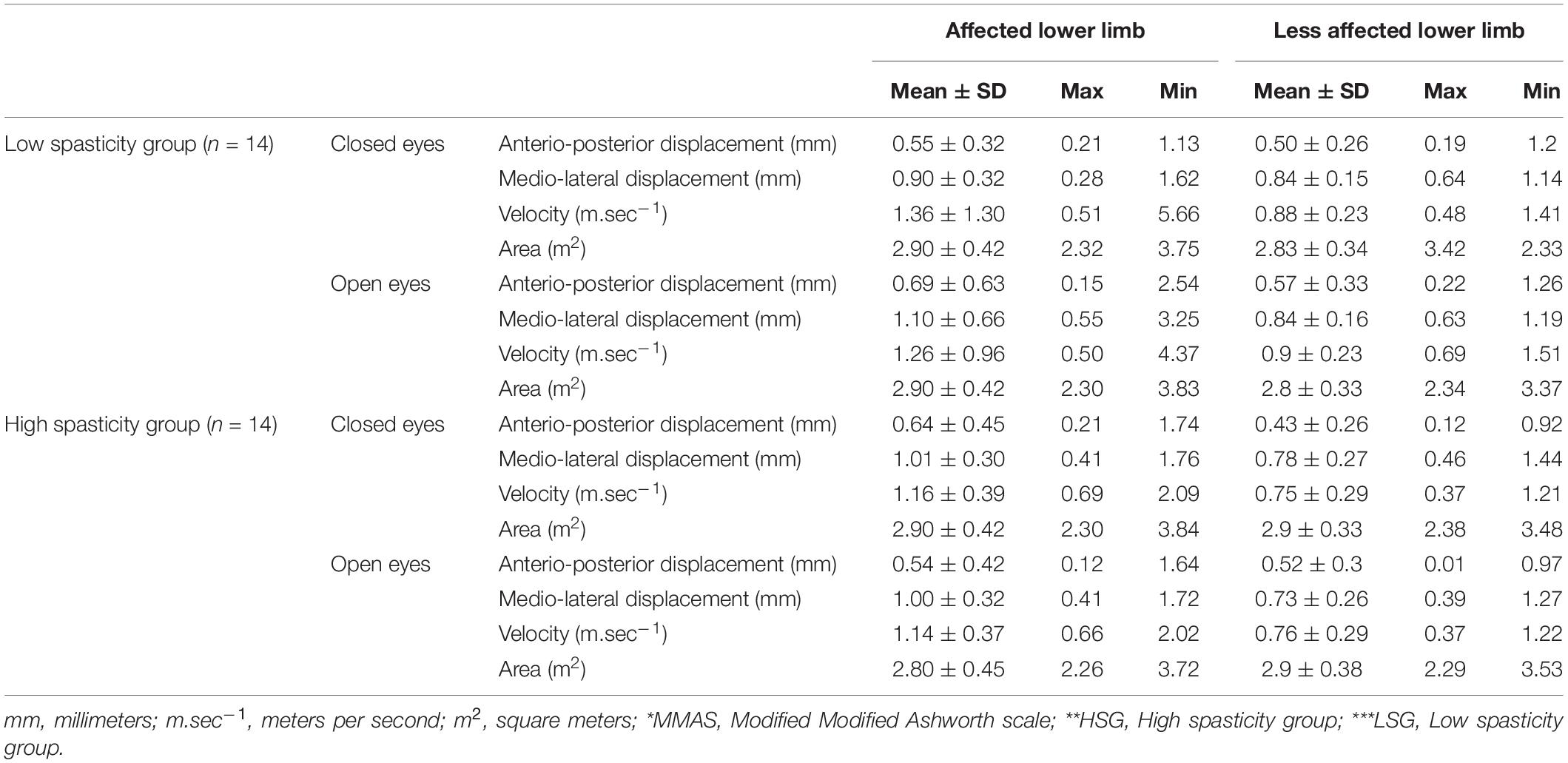
Table 4. Posturography data of the High (MMAS* ≥ 2) (HSG** n = 14) and Low (MMAS < 2) (LSG*** n = 14) spasticity study groups.
Correlations
There was a significant correlation between the ABS scores of balance confidence and the TUG test in the HSG (r = −0.55, p = 0.04) (Table 5). There were no other significant correlations between the variables.
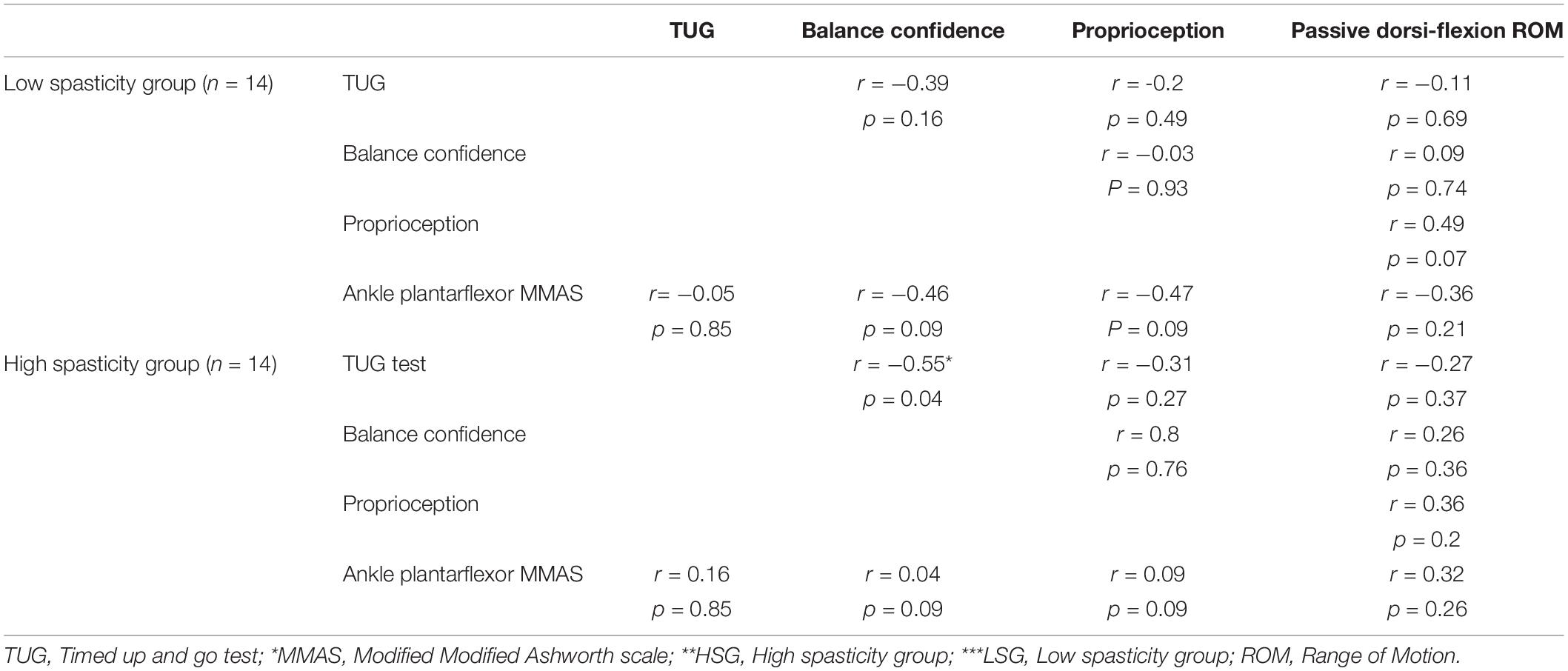
Table 5. Correlation between variables in the High (MMAS* ≥ 2) (HSG** n = 14) and Low (MMAS* < 2) (LSG*** n = 14) spasticity study groups low and high spasticity groups.
Discussion
To these authors’ knowledge, this is the first study that evaluated the effects of ankle plantar flexor spasticity severity on balance and determined the relationship between the spasticity severity with ankle proprioception, passive ROM, and balance confidence in post-stroke patients. We found no differences between the LSG and HSG groups in terms of balance confidence, dynamic balance, and ankle dorsiflexion ROM. In addition, postural sway in the open and closed eye conditions was not different in both the LSG and HSG groups for both the less affected and affected limbs. However, ankle joint proprioception in terms of repositioning error angle was better in the LSG compared to the HSG. A relationship was found between TUG scores and balance confidence in the HSG.
In the standing position, the central nervous system keeps an individual’s center of pressure within the base of support (Shumway-Cook and Woollacott, 2007). Therefore, the amount of sway of the pressure center is considered as an indicator of the balance control such that less sways indicate more stability or better balance control (Inness et al., 2015). The findings of the present study showed that there was no difference between ML and AP displacements between the low and high spasticity groups. This finding is similar to that of Rahimzadeh Khiabani et al. (2017) that revealed no differences in ML and AP displacements between the two groups of patients with low and high ankle plantar flexor spasticity. However, Rahimzadeh Khiabani et al. (2017), used only a single force plate and the amount of displacement was the sum of the displacements of both affected and less affected legs. A study used two force plates in patients post-stroke and found no differences in ML and AP displacements of posturography (Singer et al., 2013).
Various investigations have examined the balance control of patients post-stroke in the frontal plane (Geurts et al., 2005; Marigold and Eng, 2006). A review of standing balance in patients with stroke found increased ML sway and impaired balance with asymmetric weight bearing toward the less affected limb (Geurts et al., 2005). In the present study, the absence of a difference in ML displacement between the low and high spasticity groups might have been due to the lack of a difference in the severity of hip and knee spasticity between groups. It may be that ML sway occurs primarily due to the activity of hip adductors and abductors (Winter et al., 1996).
In this study, in line with previous studies (Sosnoff et al., 2010; Rahimzadeh Khiabani et al., 2017), there was no difference in the AP displacement of affected and less affected limbs between the low and high spasticity groups. This may be explained by the fact that the hip and knee movement strategies could be utilized to minimize the ankle movements (Sosnoff et al., 2010). In addition, the compensatory activity of the less affected limb may have played a role in limiting AP displacements of both affected limb and less affected limb observed in this study (Rahimzadeh Khiabani et al., 2017). The differences reported in the AP displacements of patients post-stroke and healthy individuals point to the role of spasticity, regardless of its level, adapted strategies to maintain the balance, and posture stabilization through minimizing the displacements in the frontal and sagittal planes (Marigold et al., 2004; Genthon et al., 2008).
Interestingly, no difference was found in the postural sway between the low and high spasticity groups and between the affected and less affected limbs after the removal of the vision. This was unexpected in that patients post-stroke typically rely on the vision for maintaining their balance (Bonan et al., 2004; Marigold and Eng, 2006). A possible reason for this finding could be that both the low and high spasticity groups used a stiffening strategy by maintaining the knees in extension to increase the stability and balance, regardless of their eyes being open or closed (Mansfield et al., 2013). Further, both groups could also have shifted more weight onto the affected limb in both the eyes open and closed conditions as a further strategy to minimize the AP displacements and thus improving the postural balance (Mansfield et al., 2013).
This study demonstrated that the high spasticity group displayed worse ankle proprioception when compared to the low spasticity group. This finding is in agreement with a previous study that showed proprioception impairment in patients in post-stroke (Carey et al., 1993). This finding could be expected as the high spasticity has been shown to impair the accuracy of deep position sense input (Lee et al., 2010; Gao et al., 2011). While a decreased ankle proprioception in post-stroke patients with spasticity may be postulated that can impair the balance function (Horak et al., 1997; Niam et al., 1999; Tyson et al., 2006), this unexpectedly was not found in the present study. This could be due to the use of an ankle strategy depending on the type of somatosensory input for balance control.
In the present study, in line with a previous study (Rahimzadeh Khiabani et al., 2017), no difference was found in the ABC scores between the low and high spasticity groups. We expected with increasing the severity of muscle spasticity, balance confidence would decrease in post-stroke patients. Nevertheless, a negative relationship between balance confidence and static balance as well as gait in patients post-stroke has been demonstrated (Schinkel-Ivy et al., 2016, 2017). In addition, a higher spasticity level has been associate with recurrent falling in patients post-stroke (Wei et al., 2017).
In this study, dynamic balance, as measured by the TUG, was not different between the low and high spasticity groups. This indicated that regardless of spasticity level, the dynamic balance was impaired in this sample of patients with stroke. However, the scores on the TUG test were worse in the High spasticity group than those in the Low spasticity group (23.4 vs. 17.8). A difference of ∼6 s between the two groups was clinically relevant that indicated that with increasing spasticity level severity the dynamic balance would worsen as demonstrated in previous studies with stroke (Lin et al., 2006; Soyuer and Ozturk, 2007; Sommerfeld et al., 2012). Replicating the study with more patients in the groups will clarify it.
Passive ankle ROM was not different between low and high spasticity groups. A previous study has reported that a higher spasticity was associated with higher limitations in the passive ROM (Li, 2020). In this study, both the low and high spasticity group had significant restricted ankle passive ROM. This suggests that the ankle passive ROM is influenced by ankle muscle spasticity regardless of spasticity intensity. Spasticity, weakness of ankle muscles, and muscle contracture may explain the limited ankle passive ROM in both groups (Mecagni et al., 2000; Li, 2020).
There was a significant negative correlation between the balance confidence and TUG test in the High spasticity group. This finding indicates that the time for the TUG test will be lower with more confidence on balance. However, there was no significant correlation between spasticity and proprioception and passive ROM of the ankle, balance confidence, TUG test, and postural sway in each group. The sample size is very important to examine the correlation in cross-sectional studies. As the number of variables increases, more samples are needed (Schönbrodt and Perugini, 2013). The non-significant correlations obtained for the variables might be due to the small sample size. A study with larger sample of stroke patients of different level of spasticity is required to clarify the size of correlations.
Limitations
Although the sample size was determined, the number of samples may have been too small to investigate the relationship between clinical data and static and dynamic balance (Schönbrodt and Perugini, 2013). In the present study, both groups of stroke patients had spasticity and there was also no control group. A control group consisting of neurologically healthy people or stroke patients without spasticity will help to more closely examine the effect of spasticity on balance.
Conclusion
These results show that although stroke patients with spasticity have impaired static and dynamic balance, the severity of spasticity has no effect on the exacerbation of balance control. Therefore, the spasticity post stroke must be considered for management regardless of its severity.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.FNM.REC.1397.012). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
NN, SN, and AM presented the study conception and idea. NN, SN, AM, OM, EG, BS, and IS designed the study protocol. AM drafted the first version of this manuscript that was reviewed and revised critically for intellectual content by NN. AM, EG, and OM collected the data. NN, SN, BS, and IS revised the manuscript. All authors read, commented, and approved the final manuscript for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Research Deputy of Tehran University of Medical Sciences for supporting our work. We express our gratitude to the Non-Communicable Disease Intervention Research Unit (NCDIRU) for its assistance on this study.
References
Ansari, N. N., Naghdi, S., Moammeri, H., and Jalaie, S. (2006). Ashworth Scales are unreliable for the assessment of muscle spasticity. Physiother. Theory Pract. 22, 119–125.
Azad, A., Taghizadeh, G., Mohammadian, E., Mohammadinezhad, T., and Lajevardi, L. (2016). Persian translation and test-retest reliability of the activities-specific balance confidence scale in iranian chronic stroke. J. Modern Rehabil. 10, 74–79.
Bensoussan, L., Viton, J.-M., Schieppati, M., Collado, H., de Bovis, V. M., Mesure, S., et al. (2007). Changes in postural control in hemiplegic patients after stroke performing a dual task. Arch. Phys. Med. Rehabil. 88, 1009–1015. doi: 10.1016/j.apmr.2007.05.009
Bonan, I. V., Colle, F. M., Guichard, J. P., Vicaut, E., Eisenfisz, M., Huy, P. T. B., et al. (2004). Reliance on visual information after stroke. Part I: balance on dynamic posturography. Arch. Phys. Med. Rehabil. 85, 268–273. doi: 10.1016/j.apmr.2003.06.017
Cakar, E., Durmus, O., Tekin, L., Dincer, U., and Kiralp, M. (2010). The ankle-foot orthosis improves balance and reduces fall risk of chronic spastic hemiparetic patients. Eur. J. Phys. Rehabil. Med. 46, 363–368.
Carey, L. M., Matyas, T. A., and Oke, L. E. (1993). Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch. Phys. Med. Rehabil. 74, 602–611. doi: 10.1016/0003-9993(93)90158-7
Carpenter, M., Allum, J., Honegger, F., Adkin, A., and Bloem, B. (2004). Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 75, 1245–1254.
Dorňák, T., Justanová, M., Konvalinková, R., Øíha, M., Mužík, J., Hoskovcová, M., et al. (2019). Prevalence and evolution of spasticity in patients suffering from first-ever stroke with carotid origin: a prospective, longitudinal study. Eur. J. Neurol. 26, 880–886. doi: 10.1111/ene.13902
Feigin, V. L., Norrving, B., and Mensah, G. A. (2017). Global burden of stroke. Circ. Res. 120, 439–448.
Fleuren, J. F., Voerman, G. E., Erren-Wolters, C. V., Snoek, G. J., Rietman, J. S., Hermens, H. J., et al. (2010). Stop using the Ashworth Scale for the assessment of spasticity. J. Neurol. Neurosurg. Psychiatry 81, 46–52. doi: 10.1136/jnnp.2009.177071
Gao, F., Ren, Y., Roth, E. J., Harvey, R., and Zhang, L. Q. (2011). Effects of repeated ankle stretching on calf muscle-tendon and ankle biomechanical properties in stroke survivors. Clin. Biomech. 26, 516–522. doi: 10.1016/j.clinbiomech.2010.12.003
Genthon, N., Rougier, P., Gissot, A.-S., Froger, J., Pélissier, J., and Pérennou, D. (2008). Contribution of each lower limb to upright standing in stroke patients. Stroke 39, 1793–1799. doi: 10.1161/strokeaha.107.497701
Geurts, A. C., de Haart, M., van Nes, I. J., and Duysens, J. (2005). A review of standing balance recovery from stroke. Gait Posture 22, 267–281.
Ghotbi, N., Nakhostin Ansari, N., Naghdi, S., and Hasson, S. (2011). Measurement of lower-limb muscle spasticity: intrarater reliability of modified modified ashworth scale. J. Rehabil. Res. Dev. 48:83. doi: 10.1682/jrrd.2010.02.0020
Horak, F. B., Henry, S. M., and Shumway-Cook, A. (1997). Postural perturbations: new insights for treatment of balance disorders. Phys. Ther. 77, 517–533. doi: 10.1093/ptj/77.5.517
Inness, E. L., Mansfield, A., Biasin, L., Brunton, K., Bayley, M., and McIlroy, W. E. (2015). Clinical implementation of a reactive balance control assessment in a sub-acute stroke patient population using a ‘lean-and-release’methodology. Gait Posture 41, 529–534. doi: 10.1016/j.gaitpost.2014.12.005
Kollen, B., Kwakkel, G., and Lindeman, E. (2006). Functional recovery after stroke: a review of current developments in stroke rehabilitation research. Rev. Recent Clin. Trials 1, 75–80.
Lance, J. W. (1980). “Pathophysiology of spasticity and clinical experience with baclofen,” in Spasticity: Disordered Motor Control, eds J. W. Lance, R. G. Feldman, R. R. Young, and W. P. Koella (Chicago, IL: Year Book), 185–204.
Lee, K. B., Park, Y. H., Song, E. K., Yoon, T. R., and Jung, K. I. (2010). Static and dynamic postural balance after successful mobile-bearing total ankle arthroplasty. Arch. Phys. Med. Rehabil. 91, 519–522. doi: 10.1016/j.apmr.2009.12.017
Lendraitienë, E., Tamošauskaitë, A., Petruševičienë, D., and Savickas, R. (2017). Balance evaluation techniques and physical therapy in post-stroke patients: a literature review. Neurol. Neurochir. Pol. 51, 92–100. doi: 10.1016/j.pjnns.2016.11.003
Li, S. (2020). Ankle and foot spasticity patterns in chronic stroke survivors with abnormal gait. Toxins 12:646.
Lin, P.-Y., Yang, Y.-R., Cheng, S.-J., and Wang, R.-Y. (2006). The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 87, 562–568. doi: 10.1016/j.apmr.2005.12.042
Mahmoudzadeh, A., Ansari, N. N., Naghdi, S., Sadeghi-Demneh, E., Motamedzadeh, O., Shaw, B. S., et al. (2020). Effect of ankle plantar flexor spasticity level on balance in patients with stroke: protocol for a cross-sectional study. JMIR Res. Protoc. 9:e16045. doi: 10.2196/16045
Mansfield, A., Danells, C. J., Zettel, J. L., Black, S. E., and McIlroy, W. E. (2013). Determinants and consequences for standing balance of spontaneous weight-bearing on the paretic side among individuals with chronic stroke. Gait Posture 38, 428–432. doi: 10.1016/j.gaitpost.2013.01.005
Marigold, D. S., and Eng, J. J. (2006). The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture 23, 249–255. doi: 10.1016/j.gaitpost.2005.03.001
Marigold, D. S., Eng, J. J., Tokuno, C. D., and Donnelly, C. A. (2004). Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil. Neural Repair 18, 222–229. doi: 10.1177/1545968304271171
Mecagni, C., Smith, J. P., Roberts, K. E., and O’Sullivan, S. B. (2000). Balance and ankle range of motion in community-dwelling women aged 64 to 87 years: a correlational study. Phys. Ther. 80, 1004–1011. doi: 10.1093/ptj/80.10.1004
Nakhostin Ansari, N., Naghdi, S., Forogh, B., Hasson, S., Atashband, M., and Lashgari, E. (2012). Development of the persian version of the modified modified ashworth scale: translation, adaptation, and examination of interrater and intrarater reliability in patients with poststroke elbow flexor spasticity. Disabil. Rehabil. 34, 1843–1847. doi: 10.3109/09638288.2012.665133
Nardone, A., Galante, M., Lucas, B., and Schieppati, M. (2001). Stance control is not affected by paresis and reflex hyperexcitability: the case of spastic patients. J. Neurol. Neurosurg. Psychiatry 70, 635–643. doi: 10.1136/jnnp.70.5.635
Ng, S. S., and Hui-Chan, C. W. (2005). The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 86, 1641–1647.
Niam, S., Cheung, W., Sullivan, P. E., Kent, S., and Gu, X. (1999). Balance and physical impairments after stroke. Arch. Phys. Med. Rehabil. 80, 1227–1233.
Paillex, R., and So, A. (2005). Changes in the standing posture of stroke patients during rehabilitation. Gait Posture 21, 403–409.
Phadke, C. P., Moody, T., Mochizuki, G., Gage, W., Ismail, F., and Boulias, C. (2014). Relationship between spasticity and balance confidence in persons post-stroke. Arch. Phys. Med. Rehabil. 95:e15. doi: 10.1016/j.apmr.2014.07.024
Radinmehr, H., Ansari, N. N., Naghdi, S., Tabatabaei, A., and Moghimi, E. (2019). Comparison of Therapeutic ultrasound and radial shock wave therapy in the treatment of plantar flexor spasticity after stroke: a prospective, single-blind, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 28, 1546–1554. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.008
Rahimzadeh Khiabani, R., Mochizuki, G., Ismail, F., Boulias, C., Phadke, C. P., and Gage, W. H. (2017). Impact of spasticity on balance control during quiet standing in persons after stroke. Stroke Res. Treat. 2017:6153714. doi: 10.1155/2017/6153714
Salbach, N. M., Mayo, N. E., Hanley, J. A., Richards, C. L., and Wood-Dauphinee, S. (2006). Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Arch. Phys. Med. Rehabil. 87, 1597–1604. doi: 10.1016/j.apmr.2006.08.336
Sawacha, Z., Carraro, E., Contessa, P., Guiotto, A., Masiero, S., and Cobelli, C. (2013). Relationship between clinical and instrumental balance assessments in chronic post-stroke hemiparesis subjects. J. NeuroEng. Rehabil. 10:95. doi: 10.1186/1743-0003-10-95
Schinkel-Ivy, A., Inness, E. L., and Mansfield, A. (2016). Relationships between fear of falling, balance confidence, and control of balance, gait, and reactive stepping in individuals with sub-acute stroke. Gait Posture 43, 154–159. doi: 10.1016/j.gaitpost.2015.09.015
Schinkel-Ivy, A., Wong, J. S., and Mansfield, A. (2017). Balance confidence is related to features of balance and gait in individuals with chronic stroke. J. Stroke Cerebrovasc. Dis. 26, 237–245. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.022
Schönbrodt, F. D., and Perugini, M. (2013). At what sample size do correlations stabilize? J. Res. Pers. 47, 609–612. doi: 10.1016/j.jrp.2013.05.009
Singer, J. C., Mansfield, A., Danells, C. J., McIlroy, W. E., and Mochizuki, G. (2013). The effect of post-stroke lower-limb spasticity on the control of standing balance: inter-limb spatial and temporal synchronisation of centres of pressure. Clin. Biomech. 28, 921–926. doi: 10.1016/j.clinbiomech.2013.07.010
Sinkjær, T. (1996). Muscle, reflex and central components in the control of the ankle joint in healthy and spastic man. Acta Neurol. Scand. Suppl. 170, 1–28.
Sommerfeld, D. K., Gripenstedt, U., and Welmer, A.-K. (2012). Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am. J. Phys. Med. Rehabil. 91, 814–820. doi: 10.1097/PHM.0b013e31825f13a3
Sosnoff, J. J., Shin, S., and Motl, R. W. (2010). Multiple sclerosis and postural control: the role of spasticity. Arch. Phys. Med. Rehabil. 91, 93–99. doi: 10.1016/j.apmr.2009.09.013
Soyuer, F., and Ozturk, A. (2007). The effect of spasticity, sense and walking aids in falls of people after chronic stroke. Disabil. Rehabil. 29, 679–687. doi: 10.1080/09638280600925860
Trumbower, R. D., Ravichandran, V. J., Krutky, M. A., and Perreault, E. J. (2010). Contributions of altered stretch reflex coordination to arm impairments following stroke. J. Neurophysiol. 104, 3612–3624. doi: 10.1152/jn.00804.2009
Tyson, S. F., Hanley, M., Chillala, J., Selley, A., and Tallis, R. C. (2006). Balance disability after stroke. Phys. Ther. 86, 30–38. doi: 10.1093/ptj/86.1.30
Van de Port, I., Kwakkel, G., Schepers, V., and Lindeman, E. (2006). Predicting mobility outcome one year after stroke: a prospective cohort study. J. Rehabil. Med. 38, 218–223. doi: 10.1080/16501970600582930
Wei, T. S., Liu, P. T., Chang, L. W., and Liu, S. Y. (2017). Gait asymmetry, ankle spasticity, and depression as independent predictors of falls in ambulatory stroke patients. PLoS One 12:e0177136. doi: 10.1371/journal.pone.0177136
Winter, D. A., Prince, F., Frank, J., Powell, C., and Zabjek, K. F. (1996). Unified theory regarding A/P and M/L balance in quiet stance. J. Neurophysiol. 75, 2334–2343. doi: 10.1152/jn.1996.75.6.2334
Keywords: stroke, dynamic balance, postural sway, spasticity severity, balance confidence
Citation: Mahmoudzadeh A, Nakhostin Ansari N, Naghdi S, Ghasemi E, Motamedzadeh O, Shaw BS and Shaw I (2021) Role of Spasticity Severity in the Balance of Post-stroke Patients. Front. Hum. Neurosci. 15:783093. doi: 10.3389/fnhum.2021.783093
Received: 25 September 2021; Accepted: 24 November 2021;
Published: 10 December 2021.
Edited by:
Nejc Sarabon, University of Primorska, SloveniaReviewed by:
Daniela De Bartolo, Santa Lucia Foundation, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyJanina Manzieri Prado-Rico, Universidade Cidade de São Paulo, Brazil
Copyright © 2021 Mahmoudzadeh, Nakhostin Ansari, Naghdi, Ghasemi, Motamedzadeh, Shaw and Shaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashraf Mahmoudzadeh, YW1haG1vdWR6YWRlaDU4QGdtYWlsLmNvbQ==
 Ashraf Mahmoudzadeh
Ashraf Mahmoudzadeh Soofia Naghdi
Soofia Naghdi Ehsan Ghasemi1
Ehsan Ghasemi1