- 1Department of Neurology, The First Hospital of Jilin University, Changchun, China
- 2Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, College of Medicine, Rochester, MN, United States
Myelin oligodendrocyte glycoprotein antibody-associated disease is an immune-mediated demyelinating disease of the central nervous system that is present in both adults and children. The most common clinical manifestations are optic neuritis, myelitis, acute disseminated encephalomyelitis, and brainstem syndrome. Cerebral cortical encephalitis (CCE) is a rare clinical phenotype of myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD), which usually begins with seizures, headaches, and fever, and may be misdiagnosed as viral encephalitis in the early stages. Herein, we report two typical MOG antibody (MOG-Ab)-positive patients presenting with CCE, both of whom presented with headache, fever, seizures, and who recovered completely after immunotherapy. In addition, we performed a systematic review of the present literature from the perspectives of population characteristics, clinical symptoms, MRI abnormalities, treatments, and prognosis. Among the patients reported in 25 articles, 33 met our inclusion criteria, with the age of onset ranging from 4 to 52 years. Most of the patients had seizures, headache, fever, and unilateral cortical lesions on brain MRI. For acute CCE, 30 patients were treated with high-dose intravenous methylprednisolone, and the symptoms of most patients were completely relieved after immunotherapy. This study reported our experience and lessons learned in the diagnosis and treatment of MOG-Ab-positive CCE and provides a systematic review of the literature to analyse this rare clinical phenotype.
Introduction
Myelin oligodendrocyte glycoprotein is a membrane protein uniquely expressed on the surface of oligodendrocytes and myelin in the central nervous system of humans and other mammals (Ramanathan et al., 2016; Jain et al., 2021). Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) overlaps with multiple sclerosis, acute disseminated encephalomyelitis, and aquaporin 4 antibody (AQP4-Ab)-positive neuromyelitis optica spectrum disorders (NMOSD) in terms of clinical phenotype, and is now considered to be a new and independent entity in inflammatory demyelinating diseases of the central nervous system (Ramanathan et al., 2016; Cobo-Calvo et al., 2019). The demographic, clinical, and laboratory differences at the onset of MOGAD are usually age-related. Among children, the female-to-male ratio is similar (Jurynczyk et al., 2017; Cobo-Calvo et al., 2021), and presentation at onset is usually acute disseminated encephalomyelitis, especially under 10 years of age, followed by optic neuritis (ON), transverse myelitis (TM), and brainstem demyelination (Fernandez-Carbonell et al., 2016; Duignan et al., 2018). The overall prognosis is better in children than in adults, with less than 10% of motor disability and visual acuity disability after treatment, and a lower risk of relapse in children (Reindl and Waters, 2019; Cobo-Calvo et al., 2021). Compared with children, there are slightly more female patients among adults (Jurynczyk et al., 2017), and the first presentation is usually ON (as high as 50–70%) (Duignan et al., 2018). In acute attacks, high-dose intravenous methylprednisolone is used in both adults and children, and plasma exchange is preferred when recovery is incomplete. During maintenance therapy, intravenous immunoglobulin (IVIg) is the preferred first-line treatment for children, whereas azathioprine (AZA), mycophenolate mofetil (MMF), and rituximab (RTX) are the first-line treatments for adults (Whittam et al., 2020). Some studies have reported that the higher the antibody titre at the time of onset and the longer the duration of antibody positivity, the greater the possibility of relapse (Hennes et al., 2017; Jurynczyk et al., 2017). Some patients relapsed during or after the withdrawal of steroids, most of whom showed ON (Reindl and Waters, 2019); therefore, a previous study suggested that a prolonged steroid taper can reduce early relapse of MOGAD (Narayan et al., 2018).
Since MOG antibody (MOG-Ab)-related cerebral cortical encephalitis (CCE) was first reported by Ogawa et al. (2017), many cases of this rare clinical phenotype have been reported globally, which may have been diagnosed with unexplained steroid-responsive encephalitis in the early stages of the disease (Wang et al., 2021). CCE is a syndrome with an unclear clinical definition and is characterised by grey matter lesions on brain MRI, primarily involving the cerebral cortex and sulcus, but not the subcortical and deep white matter (Krupp et al., 2013; Ogawa et al., 2017; Hamid et al., 2018). In addition to fever, headache, and seizures, cerebral cortical symptoms, such as aphasia, dysarthria, paralysis, mental symptoms, and memory loss, also exist in patients with MOG-Ab-related CCE. A typical imaging feature is the hyperintensity of cortical lesions in fluid-attenuated inversion recovery (FLAIR). According to the above imaging characteristics, CCE is divided into two types: unilateral and bilateral. In this study, we report two cases of CCE with positive MOG-Ab and perform a systematic analysis of previously reported cases. The purpose of this study was to further describe the clinical features, imaging results, and prognosis of rare MOG-Ab-positive CCE.
Case Presentation
Patient 1
A previously healthy 24-year-old man presented with 2 days of fever and headache; he was diagnosed with viral encephalitis in another hospital a month ago because of “paroxysmal unconsciousness and convulsions,” which improved after antiviral and antiepileptic treatment. At the time of admission, his physical examination results were as follows: his muscle strength of the extremities was at stage V, and the pain sensation on the right side of his face was diminished. The brain MRI results showed FLAIR (Figure 1A) hyperintensity in the leptomeningeal areas of the bilateral frontal and parietal lobes without enhancement. Electroencephalogram (EEG) showed irregular sharp waves, and sharp slow waves in the left temporal region and right frontal region during the interictal period, which indicated focal discharge. The cerebrospinal fluid (CSF) displayed an elevated intracranial pressure (ICP, 295 mmH2O), white blood cell count (WBC, 436 × 106/l), and protein (1.16 g/l) levels. The titres of MOG-Ab in CSF and serum were 1:10 and 1:100, respectively. Extensive screening for infection (CSF culture, X-pert, and Torch virus) and systemic autoimmune conditions were all negative. Therefore, the patient was diagnosed with CCE with positive MOG-Ab (Supplementary Figure 1, clinical evolution of patient 1).
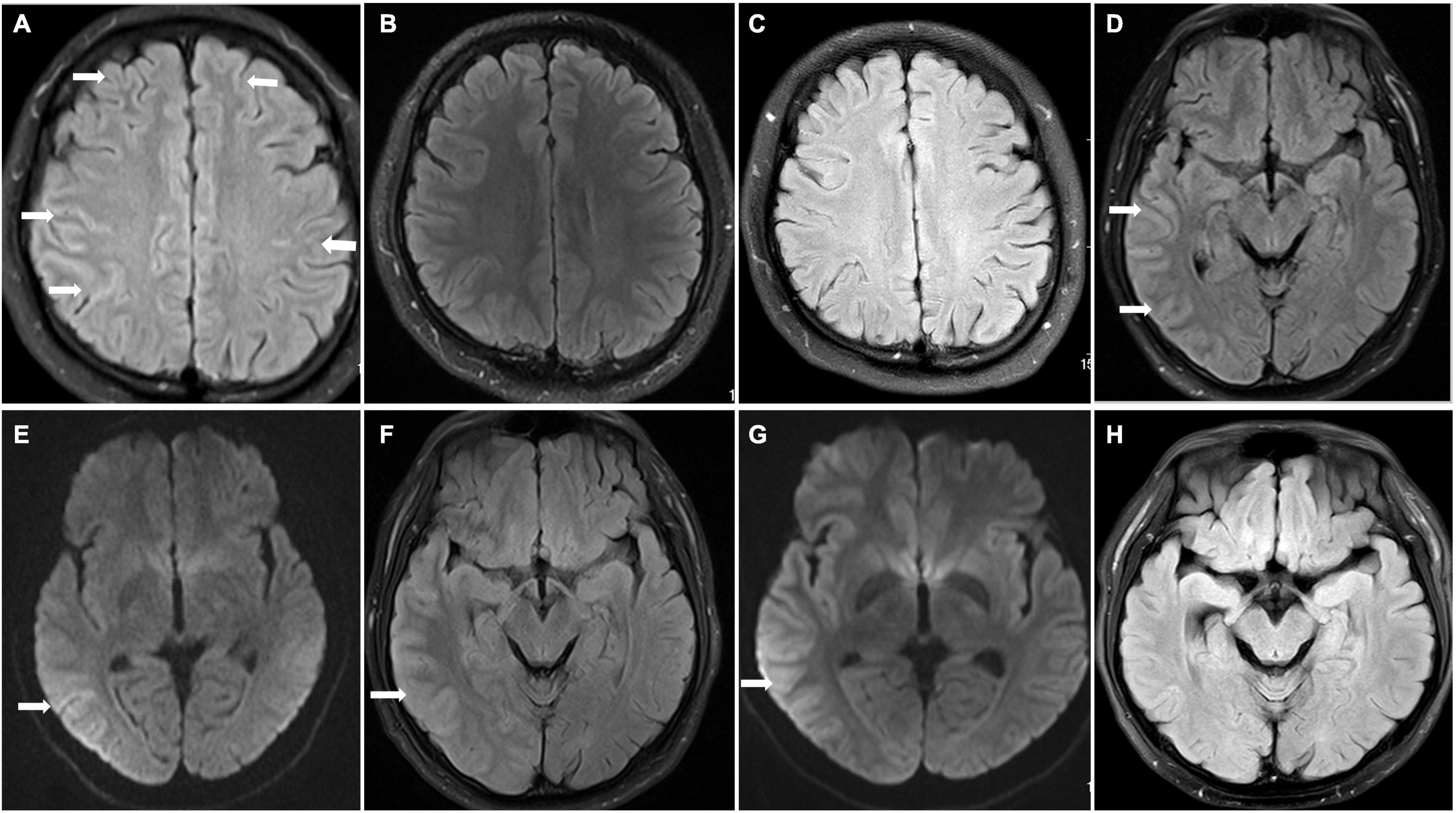
Figure 1. Brain magnetic resonance imaging (MRI) findings in patients 1 and 2. Patient 1 (A–C) On admission, FLAIR hyperintensity was seen in the leptomeningeal of bilateral frontal lobe and parietal lobe in patient 1 (A) (arrowhead); repeat brain MRI FLAIR result returned to normal at discharge and 8 months after discharge (B,C). Patient 2 (D–G) Brain MRI results showed T2 FLAIR and DWI hyperintensity (D,E) (arrowhead) with swelling of brain tissue and narrowing of sulcus; the swelling of brain tissue was alleviated, and hyperintensity of FLAIR and DWI was improved after immunotherapy (F,G) (arrowhead); brain MRI normalized at 1 month after discharge (H).
The patient received immunoglobulin (0.4 g/Kg/d) for 5 days and methylprednisolone for 15 days followed by oral prednisolone (PSL). His symptoms were completely relieved after immunotherapy, and a repeat brain MRI was normal (Figure 1B). Considering the patient’s economic conditions, he was not treated with MMF, RTX, or AZA after discharge. Follow-up continued for 8 months, and no further relapse was recorded, and a repeat brain MRI showed normal results (Figure 1C).
Patient 2
A 25-year-old man presented with headache, fever, seizures, and dysarthria without previous medical, family, or genetic history. Physical examination on admission showed that the muscle strength of the left upper limb was grade V, and the bilateral pathological signs were positive. Brain MRI results showed FLAIR (Figure 1D) hyperintensity and diffusion-weighted images (DWI; Figure 1E) showed diffusion restriction in the right temporal lobe, parietal lobe, and occipital lobe without enhancement (Supplementary Figure 2, clinical evolution of the patient 2).
After admission, he was treated with latamoxef, penciclovir, and levetiracetam. CSF analysis revealed a WBC count of 219 × 106/L and elevated protein levels of 0.68 g/l. CSF autoimmune encephalopathy antibody testing showed negative results, including anti-N-methyl-D-aspartate receptor (NMDAR) IgG, leucine-rich glioma inactivated 1 IgG, and contactin-associated protein 2 IgG. Therefore, we considered the diagnosis of viral encephalitis and continued the treatment. However, the patient’s symptoms worsened with acute type II respiratory failure, which was treated with tracheal intubation, followed by a decrease in the left upper limb muscle strength (grade 0) on the ninth day of admission. Lumbar puncture was performed again, showing that the MOG-Ab was positive in both the CSF and serum, with a titre of 1:10 in CSF and 1:100 in serum. The patient received high-dose intravenous methylprednisolone and IVIg, which was followed by complete resolution of his symptoms. Repeat brain MRI results showed lower hyperintensity of FLAIR (Figure 1F) and DWI (Figure 1G) than before. His dose of PSL was gradually decreased after discharge. During the follow-up, repeated brain MRI normalised at 1 month without relapse (Figure 1H).
Materials and Methods
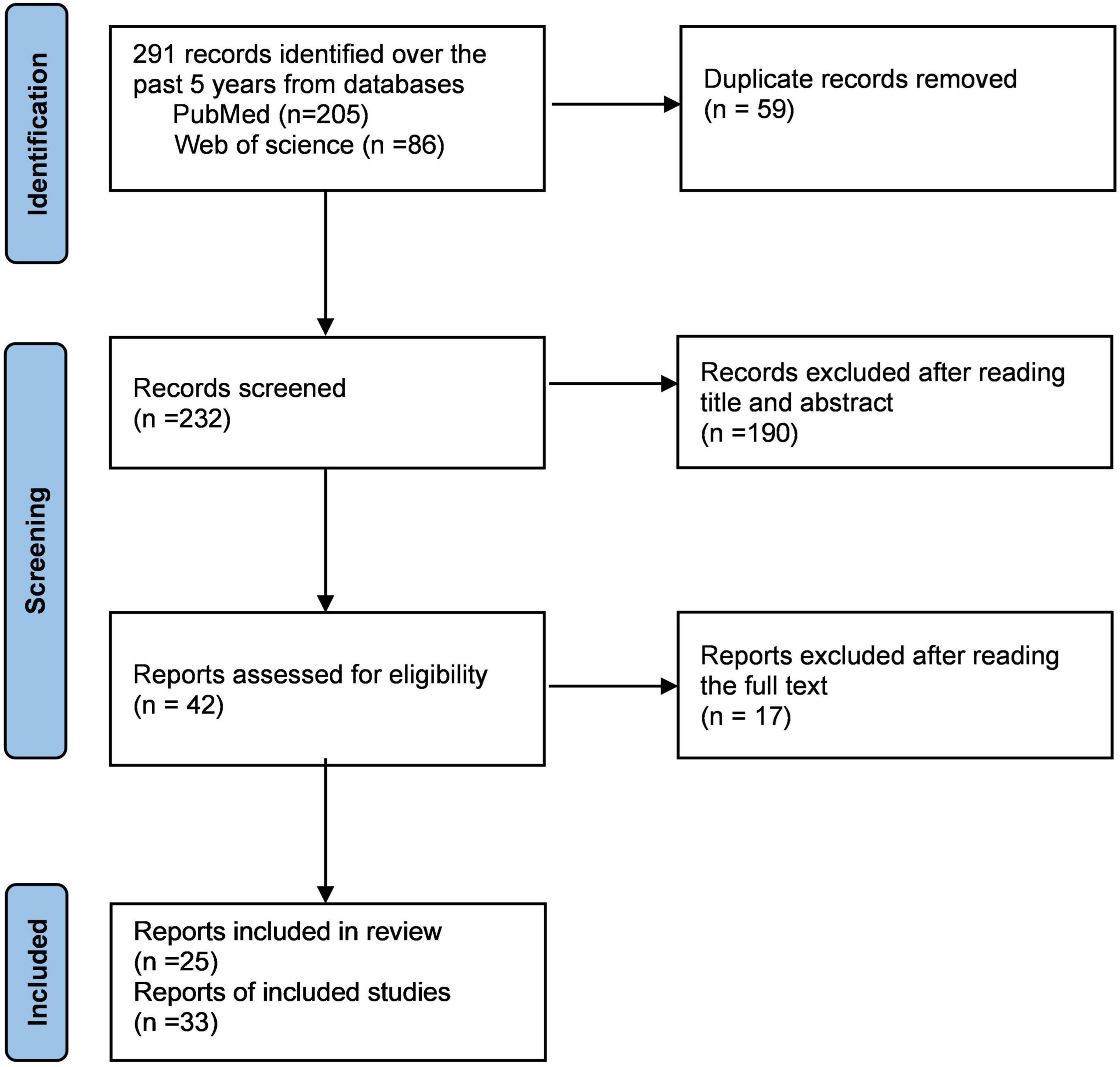
To obtain a more thorough understanding of MOG-Ab-associated CCE, we searched and screened studies in PubMed and Web of Science until August 2021 to identify patients according to the following process (Figure 2, review flowchart). The inclusion criteria were as follows: (1) Patients who tested positive only for serum MOG-Ab during the course of the disease, and no other antibodies such as AQP4-Ab, NMDAR-Ab, and contactin-associated protein 2-Ab that may indicate other diseases. (2) Patients explicitly diagnosed with MOG-Ab-related CCE for the first time. (3) Presence of abnormal hyperintensity on brain MRI, mainly in the cerebral cortex or sulcus. (4) Availability of complete data on clinical symptoms, imaging, and treatment.
Results
We finally obtained 33 cases from 25 articles and combined with our two cases, a total of 35 cases were reviewed systematically (Ogawa et al., 2017; Hamid et al., 2018; Ikeda et al., 2018; Budhram et al., 2019, 2020; Haddad et al., 2019; Patterson et al., 2019; Fujimori et al., 2020b; Hochmeister et al., 2020; Katsuse et al., 2020; Matoba et al., 2020; Otani et al., 2020; Russ et al., 2020; Takamatsu et al., 2020; Tao et al., 2020; Ahsan et al., 2021; Chang et al., 2021; Doig et al., 2021; Jain et al., 2021; Kim et al., 2021; Maniscalco et al., 2021; Nie et al., 2021; Stamenova et al., 2021; Tian et al., 2021; Wang et al., 2021; Table 1). The main demographic data, clinical characteristics, laboratory results, and imaging results are presented in Table 2.
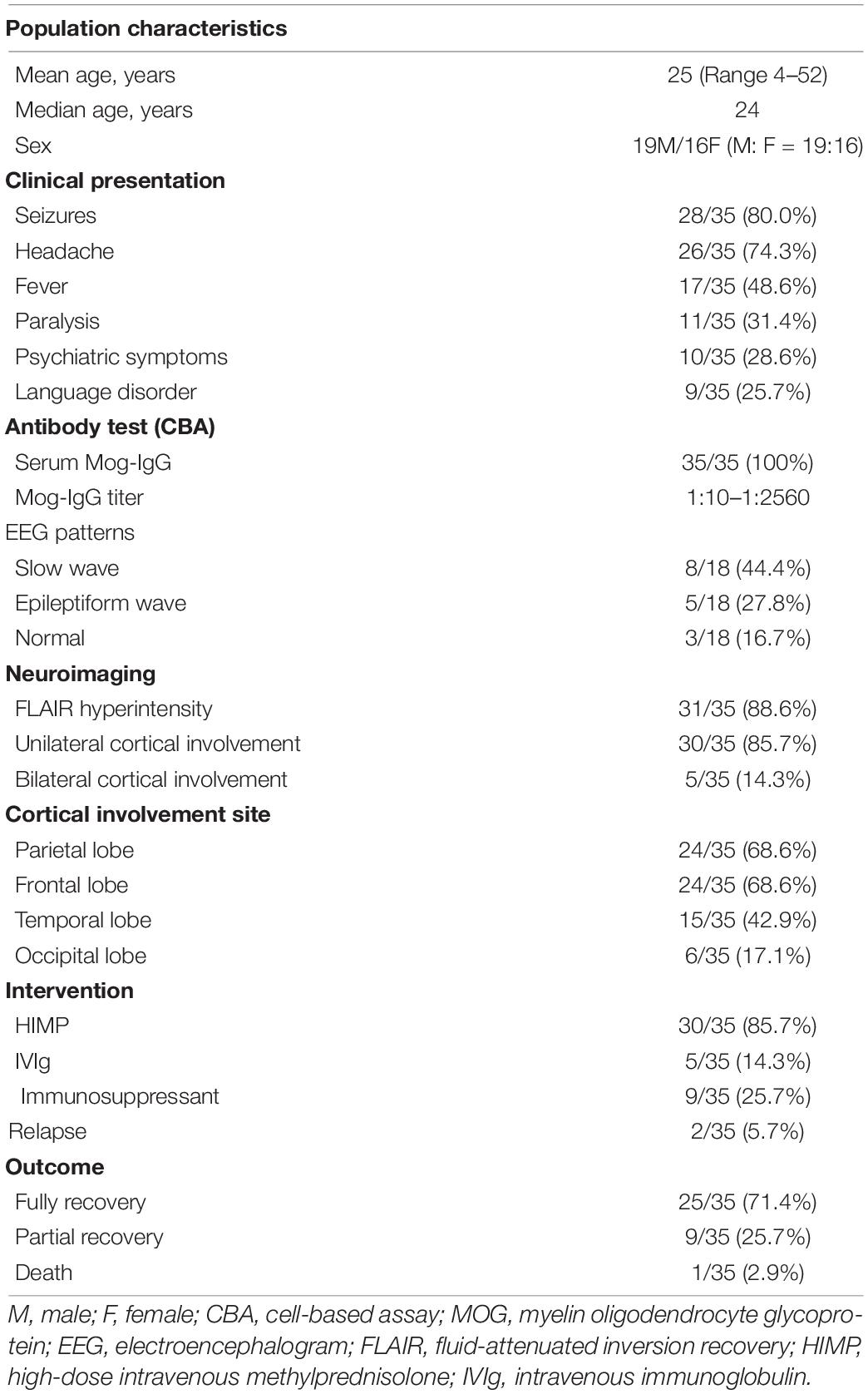
Table 2. Main demographic data, clinical characteristics, laboratory, and imaging results in described MOG-Ab associated cerebral cortical encephalitis.
Demographic Data
Among the 35 patients, 16 were women and 10 were men. The average age of onset of the patients was 25 years (range 4–52 years), and the median age for disease onset was 24 years.
Clinical Symptoms
The clinical symptoms of the disease included seizures, headache, fever, paralysis, mental disorders, language disorders, disorientation, memory decline, and autonomic nervous system dysfunction. The most prominent clinical manifestation was a seizure, which occurred in 28 (82.6%) patients during the course of the disease. Among the 28 cases with seizures, 23 described the types of seizures: 19 patients presented with generalised tonic-clonic seizures (GTCS) at the time of onset, and seven patients initially presented with focal motor seizures, three of whom eventually developed GTCS.
Magnetic Resonance Imaging Patterns
The study found that 21 patients showed cortical hyperintensity on the MRI FLAIR sequence, while the others showed swelling of the cortex and narrowing or disappearance of the sulcus. Thirty patients showed unilateral cortical involvement, of which the frontal lobe and parietal lobe were most likely to be involved, while the occipital lobe was rarely involved. Of the five patients with bilateral CCE, four had bilateral frontal lobes involved. Twenty-one patients received brain MRI DWI sequence in the acute setting, nine of whom had diffusion limitation.
Myelin Oligodendrocyte Glycoprotein Antibody Titre
Serum MOG-Abs were detected by a cell-based assay in all patients at the onset or during the course of the disease. The titres of MOG-Abs vary greatly, ranging from 1:10 to 1:2,560. In general, a higher titre value results in a longer duration of headache and fever, a higher frequency of seizures, and a greater degree of visual impairment and paralysis. After immunotherapy, the antibody titre decreased significantly and could even be negative.
Electroencephalogram Patterns
Since most patients exhibit seizures as a first symptom, EEG is necessary for differential diagnosis and further treatment. Eighteen patients underwent EEG, and 15 presented with abnormal EEG, which can present as slow waves, epilepsy waves, or other abnormalities.
Treatments
In the acute setting of CCE, 30 patients received high-dose intravenous methylprednisolone, four of whom received it combined with IVIg. During the remission period of maintenance therapy, nine patients received immunosuppressants to prevent relapse, including MMF, RTX, AZA, and cyclophosphamide.
Prognosis
Among all the patients, 25 recovered completely, as depicted by both radiodiagnosis and symptoms. Additionally, nine partially recovered, and one died. During the follow-up period, there were two patients with relapse, one presented with ON, and the other presented with headache and seizures, both of whom partially recovered eventually.
Discussion
Compared with the previously reported CCE, our cases were generally consistent with the previous cases in terms of clinical manifestation, imaging, and treatment. However, the rapid deterioration of patient 2 emphasizes the importance and necessity of early diagnosis and treatment. MOG-Ab-associated CCE may produce severe clinical symptoms, and even deaths have been reported. Therefore, early diagnosis and treatment are needed, which will greatly improve the prognosis of patients; otherwise, serious complications may occur. Since the most common symptoms are seizures, headache, and fever, it is easily misdiagnosed as intracranial infection, such as viral encephalitis and meningeal inflammation (Budhram et al., 2019). At present, as a new method for the diagnosis of pathogen infection, metagenomic next-generation sequencing can help improve the detection rate of encephalitis caused by pathogen infection (Wilson et al., 2019).
Although our study did not include cortical encephalitis caused by other pathogens, during the screening of the literature we found that MOG-Ab can coexist with other antibodies, especially anti-NMDAR-Ab (Zhou et al., 2017; Sinani et al., 2020; Nan et al., 2021). The new data show that NMDARs exist on the myelin sheath formed by oligodendrocytes (Lipton, 2006); therefore, NMDAR may be positive and can cause demyelination when an immune response occurs. In an analysis of 691 cases of anti-NMDAR encephalitis, 12 cases were found to be MOG-Ab-positive at the same time (Titulaer et al., 2014). When a patient has symptoms of CCE, it is difficult to identify whether it is caused by MOG-Ab or anti-NMDAR-Ab. Fortunately, their treatment principles are generally similar, consisting of HIMP or IVIg. Notably, RTX is more likely to be used to treat anti-NMDAR encephalitis with an acute onset (Dalmau et al., 2011; Dou et al., 2020; Thaler et al., 2021), but only in remission periods for maintenance treatment in MOGAD. We recommend that patients with MOGAD should be tracked and simultaneously tested for MOG-Ab and other antibodies in the serum and CSF for the early detection of an overlapping syndrome.
In a single-centre cohort study from China, 20.7% of MOG-Ab-positive patients had typical encephalitis symptoms, and 72.2% had cortical changes during the course of encephalitis (Wang et al., 2019). Seizures are the most common symptom of CCE, which may be caused by immune-mediated demyelination. Immunotherapy may be more effective than conventional antiepileptic therapy (Cobo-Calvo et al., 2018). In general, cortical damage caused by epilepsy is characterised by hyperintensity on DWI, which indicates cytotoxic oedema (Milligan et al., 2009; Chatzikonstantinou et al., 2011), while cortical damage caused by MOG-Ab-related CCE is usually indicated by abnormal hyperintensity on FLAIR. “FLAMES” is a new term used to characterise the clinical and radiological syndromes of cortical FLAIR-hyperintense lesions in anti-MOG-Ab-associated encephalitis with seizures (Budhram et al., 2019). A study has pointed out that long-term oral anti-epileptic drugs (AEDs) have no significant effect on the control of seizures, while immunosuppressant MMF can significantly reduce the occurrence of acute seizures (Yao et al., 2019). In our systematic review, since most patients received steroid therapy and AEDs at the same time, the effect of AEDs alone was unknown. Therefore, it is still not clear whether AEDs should be used for extended periods, and it should be evaluated comprehensively according to the effect of steroid application, along with the type, duration, and frequency of seizures.
In our study, 31 patients showed cortical hyperintensity on brain MRI FLAIR, which was relatively unique but not specific. In the case of atypical symptoms, they should be distinguished from other cortical diseases, such as seizures, Creutzfeldt-Jakob disease, subarachnoid haemorrhage, meningeal carcinoma, and reversible posterior leukoencephalopathy syndrome (Renard et al., 2015). Several researchers have reported seizure-induced reversible MRI abnormalities (SRMA) that are characterised by MRI abnormalities, which resolve completely at follow-up (Cole, 2004; Briellmann et al., 2005). In a systematic review from the University of Melbourne in Australia, which involved 27 patients with SRMA, 12 (44%) patients demonstrated SRMA in the cortical layer on brain MRI and 21 (78%) patients showed hyperintensity on MRI FLAIR (Mariajoseph et al., 2021). Based on the similarity between SRMA and MOG-Ab-mediated CCE, the detection of MOG antibodies is vital for accurate diagnosis.
According to the results of our review, involvement of the cortex was mostly limited to the unilateral cerebral cortex. In a systematic review, brain MRI lesions with bilateral medial and frontal cerebral cortical encephalitis were superimposed with a computer software program, showing that most of the lesions were distributed in the supply area of the anterior cerebral artery, while the lesions with unilateral frontal cerebral cortical encephalitis were distributed in the middle cerebral artery region (Fujimori et al., 2020a). Therefore, we speculated that the lesions of the cortex are involved in vascular distribution. Reviewing the cases in our study, we observed that in the 11 patients who underwent cerebrovascular perfusion tests, including MRI, arterial spin labelling imaging, and single-photon emission computed tomography, 10 patients displayed vasodilation of the cerebrovascular branch, indicating high cerebrovascular perfusion (Ogawa et al., 2017; Ikeda et al., 2018; Fujimori et al., 2020b; Katsuse et al., 2020; Matoba et al., 2020; Otani et al., 2020; Jain et al., 2021; Nie et al., 2021), which usually can be completely restored to normal after steroid therapy. Although there is no clear conclusion about the relationship between vascular distribution and lesions, we speculate that peripheral vessels of the middle cerebral artery or anterior cerebral artery may also be involved in the pathogenesis of the disease after cortical inflammation occurs.
It was found that the titre value of MOG-Ab in the serum of the patients varied greatly, which can be used to predict relapse (Tea et al., 2019; Marchionatti et al., 2021), supporting the pathogenic role of MOG-Ab. However, the MOG-Ab titre value was not entirely positively correlated with the severity and recurrence of the patient’s disease in our study, which may be related to the timing of serum collection, meaning that the titre value will decrease significantly after steroid therapy. In a patient with case 30, although the patient’s MOG-Ab titre was 1:320, it manifested itself with fulminant phenotype, and the patient died shortly due to older age and extensive cortical involvement. Therefore, for each individual, we should make a comprehensive judgment of the patient’s prognosis according to age, past medical history, and clinical characteristics.
The first-line treatment of patients includes HIMP and IVIg in the acute setting, and significant improvements in clinical symptoms and imaging can usually be observed after steroid treatment. By reviewing the patients’ prognosis, we found that the lesions resolved completely in most patients, which is significantly different from that in multiple sclerosis and NMOSD; the underlying mechanisms of which are not yet fully understood. In a retrospective cohort study from the Mayo Clinic, comparing the evolution of MRI lesions in different central nervous system demyelinating disorders, MRI normalisation of brain T2 signal abnormality was more common with MOGAD for brain attacks (MOGAD, 39%; AQP4-IgG-NMOSD, 10%; Multiple sclerosis, 5%), and the greater remyelination capacity, less neuronal loss, or a predominant functional vs. structural damage in MOGAD might account for this difference (Sechi et al., 2021).
However, some patients relapse after withdrawal therapy (Cobo-Calvo et al., 2019), and therefore, patients may be treated with immunosuppressive therapy, such as MMF, RTX, and cyclophosphamide, to prevent relapse. The patient may relapse with CCE again, or any type of MOGAD. Although the MOG-Ab titre value can be used to predict relapse, it cannot be relied upon alone. New research suggests that FNFAIP3 levels decreased at relapse compared to remission, which has clinical utility as a relapse biomarker and potential therapeutic target (Saxena et al., 2020). Steps are being undertaken toward its potential use in clinical settings.
Our study was retrospective and had certain limitations. Currently, MOG-Ab-associated CCE is still rare, the sample size is not large enough, and the results may be biased. Moreover, the follow-up time of each case varied, which may have affected the prognosis.
Conclusion
In conclusion, MOG-Ab-associated CCE is still a rare clinical type of MOGAD. In patients with headaches, fever, and seizures, the detection of serum MOG-Ab should be performed at the earliest, in order to make a clear diagnosis and administer timely treatment. Moreover, the regular detection of MOG-Ab titres during follow-up contributes to the early detection of disease relapse.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were approved by the Human and Research Ethics committees of the first Bethune Hospital of Jilin University. The patients/participants provided written informed consent for the publication of any identifiable data/images presented in the manuscript.
Author Contributions
MD and HS conceived the topic, designed the outline of this review, and wrote the manuscript. YL and JS prepared the figures and tables. LC and PS critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (82071351).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.782490/full#supplementary-material
Abbreviations
AED, anti-epileptic drugs; AQP4-Ab, aquaporin 4 antibody; CCE, cerebral cortical encephalitis; DWI, diffusion-weighted images; EEG, electroencephalogram; FLAIR, fluid-attenuated inversion recovery; MOG, myelin oligodendrocyte glycoprotein; MOGAD, myelin-associated disease; MOG-Ab, myelin oligodendrocyte glycoprotein antibody; NMOSD: neuromyelitis optica spectrum disorders; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; RTX, rituximab; NMDR, anti-N-methyl-D-aspartate receptor; SRMA, seizure-induced reversible MRI abnormalities.
References
Ahsan, N., Jafarpour, S., and Santoro, J. D. (2021). Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin. Exp. Pediatr. 64, 310–312. doi: 10.3345/cep.2020.01963
Briellmann, R. S., Wellard, R. M., and Jackson, G. D. (2005). Seizure-associated abnormalities in epilepsy: evidence from MR imaging. Epilepsia 46, 760–766.
Budhram, A., Mirian, A., Le, C., Hosseini-Moghaddam, S. M., Sharma, M., and Nicolle, M. W. (2019). Unilateral cortical FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J. Neurol. 266, 2481–2487. doi: 10.1007/s00415-019-09440-8
Budhram, A., Sechi, E., Nguyen, A., Lopez-Chiriboga, A. S., and Flanagan, E. P. (2020). FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES): is immunotherapy always needed to put out the fire? Mult. Scler. Relat. Dis. 44:102283. doi: 10.1016/j.msard.2020.102283
Chang, Y. C., Sharma, M., and Budhram, A. (2021). Unilateral cortical FLAIR-hyperintense lesion in anti-MOG-associated encephalitis with seizures (FLAMES) on TNF inhibitor therapy. J. Neurol. Neurosur. Psychiatry 92, 678–679. doi: 10.1136/jnnp-2021-326401
Chatzikonstantinou, A., Gass, A., Förster, A., Hennerici, M. G., and Szabo, K. (2011). Features of acute DWI abnormalities related to status epilepticus. Epilepsy Res. 97, 45–51. doi: 10.1016/j.eplepsyres.2011.07.002
Cobo-Calvo, A., Ruiz, A., Maillart, E., Audoin, B., Zephir, H., Bourre, B., et al. (2018). Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 90, e1858–e1869. doi: 10.1212/WNL.0000000000005560
Cobo-Calvo, A., Ruiz, A., Rollot, F., Arrambide, G., Deschamps, R., Maillart, E., et al. (2021). Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann. Neurol. 89, 30–41. doi: 10.1002/ana.25909
Cobo-Calvo, A., Vukusic, S., and Marignier, R. (2019). Clinical spectrum of central nervous system myelin oligodendrocyte glycoprotein autoimmunity in adults. Curr. Opin. Neurol. 32, 459–466. doi: 10.1097/WCO.0000000000000681
Cole, A. J. (2004). Status epilepticus and periictal imaging. Epilepsia 45, (Suppl. 4), 72–77. doi: 10.1111/j.0013-9580.2004.04014.x
Dalmau, J., Lancaster, E., Martinez-Hernandez, E., Rosenfeld, M. R., and Balice-Gordon, R. (2011). Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 10, 63–74. doi: 10.1016/S1474-4422(10)70253-2
Doig, D., McNamara, C., Mewasingh, L., Beri, S., Jones, B., Kachramanoglou, C., et al. (2021). Autoimmune cortical encephalitis in two children with anti-myelin oligodendrocyte glycoprotein (MOG) antibody. J. Neurol. 268, 1096–1101. doi: 10.1007/s00415-020-10260-4
Dou, X., Li, D., Wu, Y., Wang, Z., Yang, L., Ma, N., et al. (2020). Efficacy and safety of rituximab in chinese children with refractory anti-NMDAR encephalitis. Front. Neurol. 11:606923. doi: 10.3389/fneur.2020.606923
Duignan, S., Wright, S., Rossor, T., Cazabon, J., Gilmour, K., Ciccarelli, O., et al. (2018). Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev. Med. Child Neurol. 60, 958–962. doi: 10.1111/dmcn.13703
Fernandez-Carbonell, C., Vargas-Lowy, D., Musallam, A., Healy, B., McLaughlin, K., Wucherpfennig, K. W., et al. (2016). Clinical and MRI phenotype of children with MOG antibodies. Mult. Scler. 22, 174–184. doi: 10.1177/1352458515587751
Fujimori, J., Nakamura, M., Yagihashi, T., and Nakashima, I. (2020a). Clinical and radiological features of adult onset bilateral medial frontal cerebral cortical encephalitis with anti-myelin oligodendrocyte glycoprotein antibody. Front. Neurol. 11:600169. doi: 10.3389/fneur.2020.600169
Fujimori, J., Ogawa, R., Murata, T., Jin, K., Yazawa, Y., and Nakashima, I. (2020b). Unilateral chronic pulsatile headache as the single manifestation of anti-MOG antibody-associated unilateral cerebral cortical encephalitis. J. Neuroimmunol. 346:577322. doi: 10.1016/j.jneuroim.2020.577322
Haddad, N., Roussel, B., Pelcovits, A., and Rizvi, S. (2019). Optic neuritis, encephalitis and leptomeningeal enhancement in a patient with anti-MOG antibodies: a case study. Mult. Scler. Relat. Dis. 34, 14–16. doi: 10.1016/j.msard.2019.06.010
Hamid, S. H. M., Whittam, D., Saviour, M., Alorainy, A., Mutch, K., Linaker, S., et al. (2018). Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol. 75, 65–71. doi: 10.1001/jamaneurol.2017.3196
Hennes, E. M., Baumann, M., Schanda, K., Anlar, B., Bajer-Kornek, B., Blaschek, A., et al. (2017). Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89, 900–908. doi: 10.1212/WNL.0000000000004312
Hochmeister, S., Gattringer, T., Asslaber, M., Stangl, V., Haindl, M. T., Enzinger, C., et al. (2020). A fulminant case of demyelinating encephalitis with extensive cortical involvement associated with anti-MOG antibodies. Front. Neurol. 11:31. doi: 10.3389/fneur.2020.00031
Ikeda, T., Yamada, K., Ogawa, R., Takai, Y., Kaneko, K., Misu, T., et al. (2018). The pathological features of MOG antibody-positive cerebral cortical encephalitis as a new spectrum associated with MOG antibodies: a case report. J. Neurol. Sci. 392, 113–115. doi: 10.1016/j.jns.2018.06.028
Jain, K., Cherian, A., Divya, K. P. D., Rajalakshmi, P., Thomas, B., and Nandana, J. (2021). FLAMES: a novel burning entity in MOG IgG associated disease. Mult. Scler. Relat. Dis. 49:102759. doi: 10.1016/j.msard.2021.102759
Jurynczyk, M., Messina, S., Woodhall, M. R., Raza, N., Everett, R., Roca-Fernandez, A., et al. (2017). Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140, 3128–3138. doi: 10.1093/brain/awx276
Katsuse, K., Shimizu, G., Saito Sato, N., Hatano, K., Yagi, S., Kimura, T., et al. (2020). Epilepsia partialis continua as an early sign of anti-myelin oligodendrocyte glycoprotein antibody-positive encephalitis. Intern. Med. 59, 1445–1449. doi: 10.2169/internalmedicine.3076-19
Kim, K. H., Cho, J., Cho, K. H., Shin, H. Y., and Kim, S. W. (2021). Anti-myelin oligodendrocyte glycoprotein antibody-positive encephalitis with seizure and unilateral cortical fluid-attenuated inversion recovery-hyperintense lesions. J. Clin. Neurol. 17, 481–483. doi: 10.3988/jcn.2021.17.3.481
Krupp, L. B., Tardieu, M., Amato, M. P., Banwell, B., Chitnis, T., Dale, R. C., et al. (2013). International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult. Scler. 19, 1261–1267. doi: 10.1177/1352458513484547
Lipton, S. A. (2006). NMDA receptors, glial cells, and clinical medicine. Neuron 50, 9–11. doi: 10.1016/j.neuron.2006.03.026
Maniscalco, G. T., Allegorico, L., Alfieri, G., Napolitano, M., Ranieri, A., Renna, R., et al. (2021). Anti-MOG-associated demyelinating disorders: two sides of the same coin. Neurol. Sci. 42, 1531–1534. doi: 10.1007/s10072-020-04892-7
Marchionatti, A., Hansel, G., Avila, G. U., and Sato, D. K. (2021). Detection of MOG-IgG in clinical samples by live cell-based assays: performance of immunofluorescence microscopy and flow cytometry. Front. Immunol. 12:642272. doi: 10.3389/fimmu.2021.642272
Mariajoseph, F. P., Muthusamy, S., Amukotuwa, S., and Seneviratne, U. (2021). Seizure-induced reversible MRI abnormalities in patients with single seizures: a systematic review. Epileptic Dis. 23, 552–562. doi: 10.1684/epd.2021.1300
Matoba, S., Inoue, M., Morihata, H., and Takeshima, T. (2020). Case report of myelin oligodendrocyte glycoprotein antibody-positive encephalitis mimicking hemiplegic migraine. Neurol. Clin. Neurosci. 8, 323–325. doi: 10.1111/ncn3.12420
Milligan, T. A., Zamani, A., and Bromfield, E. (2009). Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure 18, 104–108. doi: 10.1016/j.seizure.2008.07.004
Nan, D., Zhang, Y., Han, J., and Jin, T. (2021). Clinical features and management of coexisting anti-N-methyl-D-aspartate receptor encephalitis and myelin oligodendrocyte glycoprotein antibody-associated encephalomyelitis: a case report and review of the literature. Neurol. Sci. 42, 847–855. doi: 10.1007/s10072-020-04942-0
Narayan, R., Simpson, A., Fritsche, K., Salama, S., Pardo, S., Mealy, M., et al. (2018). MOG antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Dis. 25, 66–72.
Nie, H., Gao, H., Li, Y., and Shen, Y. (2021). Unilateral cerebral cortical encephalitis (CCE) with positive anti-MOG antibodies: a case report. Medicine 100:e26087. doi: 10.1097/MD.0000000000026087
Ogawa, R., Nakashima, I., Takahashi, T., Kaneko, K., Akaishi, T., Takai, Y., et al. (2017). MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol. Neuroimmunol. Neuroinf. 4:e322. doi: 10.1212/NXI.0000000000000322
Otani, T., Irioka, T., Igarashi, S., Kaneko, K., Takahashi, T., and Yokota, T. (2020). Self-remitting cerebral cortical encephalitis associated with myelin oligodendrocyte glycoprotein antibody mimicking acute viral encephalitis: a case report. Mult. Scler. Relat. Dis. 41:102033. doi: 10.1016/j.msard.2020.102033
Patterson, K., Iglesias, E., Nasrallah, M., González-Álvarez, V., Suñol, M., Anton, J., et al. (2019). Anti-MOG encephalitis mimicking small vessel CNS vasculitis. Neurol. Neuroimmunol. Neuroinf. 6:e538. doi: 10.1212/NXI.0000000000000538
Ramanathan, S., Dale, R. C., and Brilot, F. (2016). Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun. Rev. 15, 307–324. doi: 10.1016/j.autrev.2015.12.004
Reindl, M., and Waters, P. (2019). Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 15, 89–102. doi: 10.1038/s41582-018-0112-x
Renard, D., Castelnovo, G., Bouly, S., Le Floch, A., Waconge, A., De Verdal, M., et al. (2015). Cortical abnormalities on MRI: what a neurologist should know. Pract. Neurol. 15, 257–265. doi: 10.1136/practneurol-2015-001113
Russ, J. B., Timbie, C. M., Li, Y., and Gonzalez-Giraldo, E. (2020). Clinical reasoning: an 11-year-old girl with focal seizures, fevers, and unilateral, enhancing cortical lesions. Neurology 95, e3153–e3159. doi: 10.1212/WNL.0000000000010655
Saxena, S., Lokhande, H., Gombolay, G., Raheja, R., Rooney, T., and Chitnis, T. (2020). Identification of TNFAIP3 as relapse biomarker and potential therapeutic target for MOG antibody associated diseases. Sci. Rep. 10:12405. doi: 10.1038/s41598-020-69182-w
Sechi, E., Krecke, K. N., Messina, S. A., Buciuc, M., Pittock, S. J., Chen, J. J., et al. (2021). Comparison of MRI lesion evolution in different central nervous system demyelinating disorders. Neurology 97, e1097–e1109. doi: 10.1212/WNL.0000000000012467
Sinani, A. A., Maawali, S. A., Alshekaili, J., Kindi, M. A., Ramadhani, K. A., Khabouri, J. A., et al. (2020). Overlapping demyelinating syndrome (neuromyelitis optica spectrum disorders NMOSD with anti-NMDA receptor encephalitis). A case report. Mult. Scler. Relat. Dis. 42:102153. doi: 10.1016/j.msard.2020.102153
Stamenova, S., Redha, I., Schmierer, K., and Garcia, M. E. (2021). FLAIR-hyperintense lesions in anti-MOG-associated encephalitis with seizures (FLAMES) unmasked by withdrawal of immunosuppression for Crohn’s disease? Mult. Scler. Relat. Dis. 48:102729. doi: 10.1016/j.msard.2020.102729
Takamatsu, T., Yamanaka, G., Uryu, H., Takeshita, M., Morishita, N., Morichi, S., et al. (2020). Improvement in recurrent anti-myelin oligodendrocyte glycoprotein antibodypositive cerebral cortical encephalitis not requiring antiinflammatory therapy following the decrease in cytokine/chemokine levels. Mult. Scler. Relat. Dis. 43:102168. doi: 10.1016/j.msard.2020.102168
Tao, R., Qin, C., Chen, M., Yu, H. H., Wu, L. J., Bu, B. T., et al. (2020). Unilateral cerebral cortical encephalitis with epilepsy: a possible special phenotype of MOG antibody-associated disorders. Int. J. Neurosci. 130, 1161–1165. doi: 10.1080/00207454.2020.1720676
Tea, F., Lopez, J. A., Ramanathan, S., Merheb, V., Lee, F. X. Z., Zou, A., et al. (2019). Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol. Commun. 7:145. doi: 10.1186/s40478-019-0786-3
Thaler, F. S., Zimmermann, L., Kammermeier, S., Strippel, C., Ringelstein, M., Kraft, A., et al. (2021). Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: real-world evidence from the GENERATE registry. Neurol. Neuroim. Neuroinf. 8:e1088. doi: 10.1212/NXI.0000000000001088
Tian, F., Liu, X., Yang, C., Wang, B., Song, Z., and Zhang, Y. (2021). MOG antibody-positive cerebral cortical encephalitis: two case reports and literature review. Int. J. Dev. Neurosci. 81, 342–351. doi: 10.1002/jdn.10106
Titulaer, M. J., Höftberger, R., Iizuka, T., Leypoldt, F., McCracken, L., Cellucci, T., et al. (2014). Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 75, 411–428. doi: 10.1002/ana.24117
Wang, L., ZhangBao, J., Zhou, L., Zhang, Y., Li, H., Li, Y., et al. (2019). Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai. China. Eur. J. Neurol. 26, 168–174. doi: 10.1111/ene.13790
Wang, Y. F., Liu, X. W., Lin, J. M., Liang, J. Y., Zhao, X. H., and Wang, S. J. (2021). The clinical features of FLAIR-hyperintense lesions in anti-mog antibody associated cerebral cortical encephalitis with seizures: case reports and literature review. Front. Immunol. 12:582768. doi: 10.3389/fimmu.2021.582768
Whittam, D. H., Karthikeayan, V., Gibbons, E., Kneen, R., Chandratre, S., Ciccarelli, O., et al. (2020). Treatment of MOG antibody associated disorders: results of an international survey. J. Neurol. 267, 3565–3577. doi: 10.1007/s00415-020-10026-y
Wilson, M. R., Sample, H. A., Zorn, K. C., Arevalo, S., Yu, G., Neuhaus, J., et al. (2019). Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N. Engl. J. Med. 380, 2327–2340. doi: 10.1056/NEJMoa1803396
Yao, Y., Xu, Y., Ren, H., Zhou, X., Jin, L., Huang, Y., et al. (2019). Acute epileptic seizures in myelin oligodendrocyte glycoprotein encephalomyelitis and neuromyelitis optica spectrum disorder: a comparative cohort study. Mult. Scler. Relat. Dis. 27, 281–288. doi: 10.1016/j.msard.2018.11.007
Keywords: cortical, encephalitis, autoimmune, seizures, MOG
Citation: Shu H, Ding M, Shang P, Song J, Lang Y and Cui L (2022) Myelin Oligodendrocyte Glycoprotein Antibody Associated Cerebral Cortical Encephalitis: Case Reports and Review of Literature. Front. Hum. Neurosci. 15:782490. doi: 10.3389/fnhum.2021.782490
Received: 24 September 2021; Accepted: 22 November 2021;
Published: 03 January 2022.
Edited by:
Bin Qiu, Yale University, United StatesReviewed by:
Eoin Flanagan, Mayo Clinic, United StatesYukihiro Yoneda, Hyogo Prefectural Amagasaki General Medical Center, Japan
Mo Yang, People’s Liberation Army General Hospital, China
Chao Zhang, Tianjin Medical University General Hospital, China
Copyright © 2022 Shu, Ding, Shang, Song, Lang and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Cui, bGN1aUBqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Hang Shu
Hang Shu Manqiu Ding1†
Manqiu Ding1† Pei Shang
Pei Shang Jia Song
Jia Song Yue Lang
Yue Lang Li Cui
Li Cui