- 1School of Psychological Sciences, Turner Institute for Brain and Mental Health, Monash University, Clayton, VIC, Australia
- 2Epworth Centre for Innovation in Mental Health, Epworth Healthcare, Camberwell, VIC, Australia
- 3Department of Psychiatry, Central Clinical School, Monash University, Melbourne, VIC, Australia
- 4Pragmatic Health Ethics Research Unit, Institut de Recherches Cliniques de Montréal, Montreal, QC, Canada
- 5Department of Medicine and Social and Preventive Medicine, Université de Montréal, Montreal, QC, Canada
- 6Medicine and Biomedical Ethics Unit, Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada
Background: How “success” is defined in clinical trials of deep brain stimulation (DBS) for refractory psychiatric conditions has come into question. Standard quantitative psychopathology measures are unable to capture all changes experienced by patients and may not reflect subjective beliefs about the benefit derived. The decision to undergo DBS for treatment-resistant depression (TRD) is often made in the context of high desperation and hopelessness that can challenge the informed consent process. Partners and family can observe important changes in DBS patients and play a key role in the recovery process. Their perspectives, however, have not been investigated in research to-date. The aim of this study was to qualitatively examine patient and caregivers’ understanding of DBS for TRD, their expectations of life with DBS, and how these compare with actual experiences and outcomes.
Methods: A prospective qualitative design was adopted. Semi-structured interviews were conducted with participants (six patients, five caregivers) before DBS-implantation and 9-months after stimulation initiation. All patients were enrolled in a clinical trial of DBS of the bed nucleus of the stria terminalis. Interviews were thematically analyzed with data saturation achieved at both timepoints.
Results: Two primary themes identified were: (1) anticipated vs. actual outcomes, and (2) trial decision-making and knowledge. The decision to undergo DBS was driven by the intolerability of life with severe depression coupled with the exhaustion of all available treatment options. Participants had greater awareness of surgical risks compared with stimulation-related risks. With DBS, patients described cognitive, emotional, behavioral and physical experiences associated with the stimulation, some of which were unexpected. Participants felt life with DBS was like “a roller coaster ride”—with positive, yet unsustained, mood states experienced. Many were surprised by the lengthy process of establishing optimum stimulation settings and felt the intervention was still a “work in progress.”
Conclusion: These findings support existing recommendations for iterative informed consent procedures in clinical trials involving long-term implantation of neurotechnology. These rich and descriptive findings hold value for researchers, clinicians, and individuals and families considering DBS. Narrative accounts capture patient and family needs and should routinely be collected to guide patient-centered approaches to DBS interventions.
Introduction
There is a pressing need for novel and effective treatments for people living with treatment-resistant depression (TRD). Approximately one-fifth of all people who experience depression will not respond to existing evidence-based therapies (Fava, 2003). Deep brain stimulation (DBS) is a potential treatment for depression currently being investigated. Primary outcome measures used in clinical trials of DBS for depression include the Hamilton Rating Scale for Depression (Hamilton, 1960) and Montgomery-Åsberg Depression Rating Scale (Montgomery and Asberg, 1979). While valuable for assessing subjective changes in depression symptoms as defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), these measures do not provide a comprehensive picture of the intervention’s overall impact and often do not fully capture participants’ beliefs about the benefit they have gained (de Haan et al., 2015; Mayberg, 2018). What “well” looks like and what is considered a “success” is also highly specific to each individual (Fins et al., 2017).
Qualitative investigations with those who undergo DBS is one method for gaining a more holistic and comprehensive understanding of intervention outcomes. Despite the growing recognition and need for patient-centered care and the elevation of patient voices within medical research and clinical practice (Greenhalgh et al., 2016; Sidhu et al., 2017; Braun and Clarke, 2019), few qualitative studies with this population have been conducted. Acquiring qualitative data from health care recipients and lived experience experts (e.g., patients, caregivers) is vital for improving the translation of clinical research outcomes into standard practice and health care (Institute of Medicine, 2001). In addition to highlighting an intervention’s successes and failures, qualitative data can reveal the meaning and significance of changes experienced by patients, for both themselves and those closest to them.
Patient expectations about the likely benefit of undergoing DBS can affect clinical outcomes (Okun and Foote, 2004; Maier et al., 2013), as well as raise challenging ethical questions when trialing DBS for mental illnesses, such as TRD (Bell and Racine, 2013). A handful of qualitative studies have explored the relevance of questions including whether individuals with severe and refractory depression have the capacity to consent to an experimental procedure and whether their decision to participate is motivated by unrealistic expectations of personal benefit (Christopher et al., 2012; Fisher et al., 2012). Fisher and colleagues interviewed 31 people enrolled in two DBS for depression trials and assessed their decision-making and capacity to consent using semi-structured interviews. All participants demonstrated intact capacity to consent; however, therapeutic misconception was present amongst some (i.e., participants viewed the study’s purpose as specifically helping their mental health rather than producing generalizable knowledge and underrated risks and overrated likelihood of personal benefit). The authors note that similar degrees of therapeutic misconception are represented in other clinical and non-clinical populations; therefore, they concluded that people with TRD do not appear uniquely susceptible to therapeutic misconception. Based on these results, informed consent processes for the two DBS trials were considered by the authors as sufficiently robust (Christopher et al., 2012).
Post-DBS qualitative data provides some different perspectives on these ethical issues. Klein et al. (2016) conducted focus groups and interviews with 15 recipients of DBS for either TRD and obsessive-compulsive disorder (OCD), exploring their attitudes toward emerging closed-loop DBS systems. In doing so, participants reflected on their own experiences of enrolling in an experimental DBS trial. Some perceived that depression-related cognitive and emotional vulnerabilities impacted their comprehension of information and how they evaluated the associated risks (“I could have cared less about the risks” p. 145). Some recalled a sense of desperation to rid themselves of depression with which high hopes and unrealistic expectations emerged. These findings suggest that nuanced consideration is needed when it comes to the process of conducting robust informed consent with this population.
The current study extends upon the existing research by exploring how these important pre-intervention ethical issues (e.g., decision-making capacity, awareness of risks/benefits, and expectations) are related to participants’ post-intervention outcomes and experiences. Caregivers (spouses, family members) were also included in the study. Caregivers have remained absent within psychiatric DBS research despite the fact they often play a significant role during all stages of clinical trial participation (e.g., decision-making, attending medical and research-related appointments, observing changes in the participant after DBS) (Klein et al., 2016; Thomson et al., 2019a). The purpose of the current study was to gain in-depth knowledge and insight into the experience of preparing for, and living with, DBS for TRD. To achieve this, semi-structured interviews were conducted with key stakeholders (e.g., patients and caregivers) before and after DBS. More specifically, the study aimed to examine: (1) factors influencing the decision to pursue DBS; (2) participants’ knowledge and understanding of DBS for TRD, including potential risks and benefits; (3) expectations held by patients and caregivers prior to DBS; (4) the subjective outcomes of the intervention; and (5) how outcomes compare with original expectations.
Materials and Methods
This exploratory study employed a prospective qualitative design and iterative thematic analysis approach. This article reports on the experiences of a subset of participants enrolled in a clinical trial of DBS for TRD (Australian New Zealand Clinical Trials Registry: ACTRN12613000412730)1. Separate and non-overlapping findings from the current sample examining personal and relational changes following DBS are reported elsewhere (Thomson et al., unpublished).
Participants
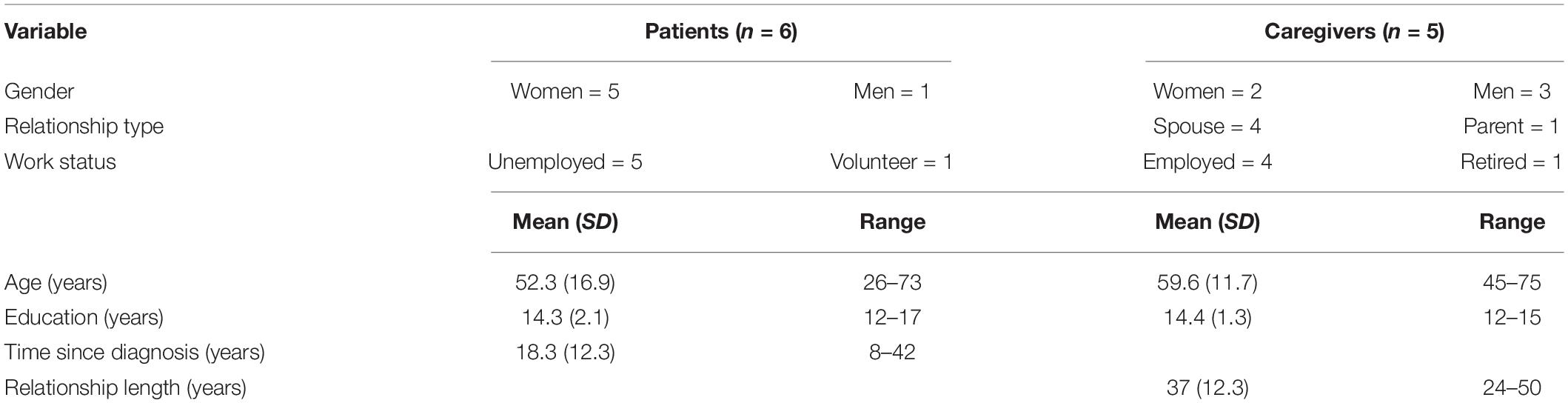
A consecutive sampling approach was used to recruit participants actively enrolled in the clinical trial who were awaiting surgery. These participants met Stage V criteria for TRD according to the Thase and Rush (1995) classification. This is the most severe classification with individuals failing to respond to adequate courses of all evidence-based therapies, including pharmacotherapy (all antidepressant classes and combination/augmentation strategies), psychotherapy (including but not limited to cognitive behavioral therapy) and non-invasive brain stimulation [electroconvulsive therapy (ECT), transcranial magnetic stimulation]. All participants had presented to the Victorian Mental Health Tribunal and received approval to undergo DBS for depression. The first-author (CT) recruited DBS candidates and their respective caregivers to the current study, providing them with verbal and written study information. All who were approached, agreed to participate in the study. The sample consisted of six DBS candidates and five caregivers (see Table 1). One candidate did not have a caregiver and participated independently.
Procedure
Semi-structured interviews were conducted either face-to-face (at participants’ home, research center) or via telephone/video-conference (for participants living interstate). Patients and caregivers were interviewed separately to allow for open discussion. Interviews were conducted by the first-author (CT), a female psychologist with training in qualitative methods and experience interviewing DBS patients, caregivers and clinicians (Thomson et al., 2019b, 2020). Pre-surgery interviews (n = 11, M = 46 min, range = 34–58) occurred 3–15-weeks prior to surgery. These interviews explored participants’ decision-making process, awareness and understanding of associated risks and benefits, and expectations and beliefs about potential outcomes (see Supplementary Material for interview schedules). Further probing questions were asked to elicit greater depth of information and responses were reflected back to participants to ensure interviewer understanding. Participants then underwent DBS surgery, with electrodes implanted in the bed nucleus of the stria terminalis. Surgery and recovery from surgery was medically uncomplicated for all participants. After a recovery period, participants commenced a randomized schedule of active and sham (control condition) stimulation settings, to which they were blinded. Over 5 months, five stimulation settings were trialed: one inactive, two low level (2 volts), and two moderate level (4 volts). Following this, stimulation continued in an open-label manner, with settings optimized according to each individual. Post-surgery interviews (n = 10, M = 55 min, range = 36–86) occurred 9–11-months after surgery and approximately 3-months into the optimization phase. These interviews explored participants’ experiences living with DBS, their subjectively perceived outcomes, and reflections on their pre-surgery beliefs and expectations. One candidate did not complete a postoperative interview as it was deemed too burdensome. Field notes were maintained and regular debriefing occurred between co-authors (CT, AC, RS). All interviews were digitally recorded and transcribed verbatim by a professional transcription service. Transcriptions were reviewed for accuracy and de-identified (by CT).
Qualitative Analysis
An iterative thematic analysis approach was chosen as it is suitable for exploring a single phenomenon (e.g., undergoing DBS) from different perspectives and can be used to highlight similarities, differences and inconsistencies in perspectives across time (Braun and Clarke, 2006). The analysis was conducted within a realist paradigm, which assumes a direct relationship between language and meaning or experiences. Therefore, participants’ language was assumed to represent the reality of their lives and reflect the meaning they assign to their experiences. The analysis and interpretation were conducted with a psychological lens, although the process of peer debriefing allowed input from different perspectives and disciplines (neuroethics, social science, neuropsychology) (Yilmaz, 2013).
Transcript data was imported into and organized using NVivo 12 software (QSR International Pty Ltd., Doncaster, Australia). Thematic analysis was conducted according to the six-step process outlined by Braun and Clarke (2006). This involved: familiarization through repeating listening and reading of interviews, initial generation of codes, searching for themes, reviewing and refining themes, defining and naming themes, and reporting using representative quotes with pseudonyms to protect confidentiality. The analysis process was iterative and inductive (data-driven), aligning with the “codebook” approach to thematic analysis (Braun and Clarke, 2020). Cross-coding was conducted on a subset (6) of interviews (by CT, AC, RS), with discussions held amongst the coding team to develop and refine a coding structure. All interviews were subsequently coded by the first-author and interviewer (CT). Data saturation, the point at which no novel themes were identified in analysis, was reached at interview 9 of the pre-DBS interviews (total of 11) and 9 of the post-DBS interviews (total of 10). The “Consolidated Criteria for Reporting Qualitative Research” (COREQ) was used to support transparent and comprehensive methodological reporting (Tong et al., 2007).
Results
The thematic analysis revealed two primary themes that are presented within the current article (Table 2). Primary themes developed longitudinally with secondary themes reflecting specific timepoints (pre-/post-DBS). Patient and caregiver perspectives are represented across all themes, with example quotes presented in Tables 3, 4.
Anticipated vs. Actual Outcomes
In anticipation of DBS, participants shared beliefs about potential responses to stimulation. Most recognized that responses could vary considerably person-to-person and rather than being a one-off procedure, much testing and talking was needed to optimize settings. Some patients felt they would perceive no difference in stimulation settings unless one was exerting a beneficial effect. Others flagged potential “strange problems” e.g., hypomania, impatience or becoming “too over-reactive.” The prospect of the inactive (control) setting was particularly worrying for some, but was understood as a research requirement. The anticipated time it would take for patients to detect a beneficial setting varied from fairly immediate (as heard in “miracle” stories) (Racine et al., 2007), to a couple of weeks or months. One patient felt confident they would know if the treatment was working, having experienced distinct, albeit brief, periods of wellness in response to other treatments. In contrast, one caregiver was concerned their loved-one may not recognize improvements they were experiencing, as had occurred with another experimental treatment.
Participants distinguished between what they were expecting from the procedure and what they hoped the outcome would be. As an experimental trial, some held no expectations at all or considered their probability of remission in light of outcomes from other trials. With extensive histories of non-response to standard depression treatments, some were inclined to consider “no benefit” the most likely outcome and entertained few hopes to avoid later disappointment. In contrast, one patient described their expectations in positive, absolute terms (“I expect to recover”), explaining optimism was necessary to give them determination to proceed. This affirming mindset appeared balanced by a realistic understanding of the trial’s experimental nature. One patient and caregiver had held initially high expectations after seeing a positive case study in the media; however, these were tempered after discussions with the clinical trial team and receiving further information. Many emphasized how meaningful even a small or partial improvement would be for their quality of life.
In addition to DBS, participants were aware that engaging in additional therapies and practices would likely be required to aid recovery. Continuation of current approaches e.g., medication, psychotherapy, routine exercise etc. was still considered necessary to maximize rehabilitation and recovery. Some felt they would need to re-focus on lifestyle factors, including sleep hygiene, diet, exercise, and social connection. New input from counseling or social work services was recognized by participants for supporting psychosocial rehabilitation, e.g., regaining life-skills, re-establishing spousal relationships.
At the follow-up interview, majority of participants indicated their life after DBS had felt like “a roller-coaster ride.” This was regardless of their subjectively perceived outcome; meaningful improvement (n = 2), little (transient/subtle) to no benefit (n = 3), or increased depression (n = 1). Participants described a variety of responses they thought were due to stimulation settings. Positive changes included: a lift in mood (subtle to substantial), expressions of joy (tears/laughter), improved sleep, increased energy, more interest in people and surroundings, more talkative and engaged, and increased motivation to do things (shopping, create things, see people). In two cases, positive stimulation effects were sustained, while others only experienced transient benefits (2–3 days). Undesirable responses that participants attributed to stimulation settings were: increased irritability, anxiety, urges to self-harm, cognitive effects (confusion, poor memory and problem-solving), manic episodes, disturbed sleep, acne, and further decreases in mood, energy, motivation, and confidence. A general sense of unease (“off kilter,” “really crook”) was also common. These experiences were mostly transient and remitted with stimulation parameter adjustments. Turning the device on and off (in order to conduct medical procedures or patient self-experimentation with the device) was associated with: panic attacks, dissociative experiences, sensations of a childhood memory, and manic episodes. Other experiences, some related and others unrelated to DBS, also contributed to the roller-coaster experience, including surgery-related anxiety and trauma, adjusting to new emotions (joy, anger, pride), suicide attempts (n = 2), managing social reintegration, relationship difficulties (with caregiver and non-caregivers), medical issues, and family bereavement.
When reflecting how outcomes compared with initial expectations, most felt their situation was still a “work in progress.” Some who had only experienced glimpses of a positive effect remained hopeful that effective device settings could be identified. However, the question of how long to persist was raised. One patient who had no existing expectations was “neither disappointed nor surprised” with their apparent lack of benefit. Those who had experienced benefit were in the infant stages of wellness, navigating various new experiences. In one case psychological support was important for guiding the patient’s adjustment. Some participants offered percentage indicators of recovery (varying from 40 to 70% improvement). While these exceeded prior comments that “any improvement” would do, a desire for more to be gained post-trial was present. One patient who became interested in DBS after viewing a positive media story expressed disappointment that they had not experienced such an immediate or dramatic effect.
Trial Decision-Making and Knowledge
In anticipation of surgery, candidates discussed their decision to pursue DBS. Most were introduced to DBS by their treating psychiatrist, where it was raised as an experimental approach being trialed for treatment-resistant individuals. Others encountered it while researching alternative treatments online. All had treating clinicians willing to support their application into the clinical trial. All patients had conducted some personal research into DBS, including reviewing the peer-reviewed literature (those with higher education, academic experience) to searching Google and YouTube (case studies, footage of surgery). This research was mostly driven by patients themselves, but occasionally by caregivers with patients then viewing their findings. Some caregivers conducted limited research, as they trusted the patient’s judgment and the experts supporting them. While independent research and discussions with medical professionals were influential, the lack of treatment alternatives was the driving force behind most patients’ decision. All felt confident they had exhausted all alternative treatment options and while DBS was considered “pretty hard core,” as the only option left, patients were willing to pursue something “extreme.” Being approved for DBS provided hope some caregivers felt was keeping their loved one from suicide.
Participants recalled many surgical risks associated with DBS (e.g., stroke, brain bleed, infection, seizure, general anesthetic complications, death). While concerning, most were comforted knowing it was a routine surgery for movements disorders (i.e., Parkinson’s disease, essential tremor) with low chance of adverse events, and had trust in the surgeon’s skill. The surgical risks were often compared with those associated with undergoing ECT, with hope DBS would reduce future need for ECT. Awareness of stimulation-related adverse events appeared less well known, although mania/hypomania, sleep disturbances, and adverse mood effects (anxiety, agitation) were raised by some. One caregiver considered these a part of the research process for finding the best settings, while another expressed concern their loved one would not report adverse effects and attempt to endure them.
Participants generally felt well-informed of the risks and benefits, having held multiple discussions with the clinical trial team, the surgical team, and their treating clinician over many months and years. One patient noted they “had to be” well-informed in preparation to sit before the Victorian Mental Health Tribunal. Some patients felt having an opportunity to talk to others with a DBS implant (not necessarily for depression), would be beneficial to understand more about the physical aspect of having the device.
After DBS, participants reflected on how informed and prepared they had been prior to surgery. Some patients were surprised by the physical discomfort resulting from the implanted device e.g., tenderness behind ear where wire runs, pain in chest where implantable pulse generator (IPG) rests when sleeping on side and while driving due to seatbelt rubbing against IPG. While acknowledging the difficulty in pre-empting such outcomes, one patient felt they would have preferred the IPG on the opposite side had they been given a choice. And while prepared for surgery from a procedural perspective, one patient felt unprepared for how personally confronting the experience was, and highlighted the importance of comfort and reassurance from medical staff during the procedure. Participants generally felt well-informed regarding risks and procedures, but were surprised by how long the whole process was (e.g., getting Tribunal approval, scheduling surgery, conducting clinical trial tests, and regularly adjusting parameters). The number of research center visits required had also been a surprise to some, with both caregivers and patients acknowledging they may have not fully absorbed this information prior.
Participants were asked what advice they would give others considering DBS for depression based on their experience. The most common perspective was that it must be a last resort and all less invasive options need to be tried first. Being well-informed and prepared was considered essential, while remembering it is a research trial and a positive outcome, like you might read or hear about, is unlikely. Being prepared for a “long, drawn-out process” was also emphasized. Many felt that if someone (patient or caregiver) were “in the same boat” as they had been and were considering DBS, they would recommend it, even if their outcome had not been overly positive.
Participants were also asked to consider if they had their time again, would they still decide to undergo DBS (or support their loved-one to)? Most participants felt they would, regardless of their experience and present outcome. The lack of alternatives was again sighted as a worthy reason to attempt it, and those who had experienced no benefit felt their situation could change, so it was yet to be determined whether it was worthwhile.
Discussion
Through prospective semi-structured interviews with key stakeholders, this study sought to gain in-depth knowledge and insight into the lived experience of DBS for TRD. Through this process, key ethical issues were explored including: informed consent, decision-making capacity, intervention expectations, and subjective outcomes. The relevant findings and implications of each are discussed below.
Informed Consent and Decision-Making Capacity
In most instances, participants demonstrated reasonable knowledge and understanding of DBS for TRD, including awareness of potential risks and benefits. Patients reported feeling both well-informed and prepared, having participated in extensive consenting discussions. All had been evaluated by the Victorian Mental Health Tribunal in order to receive permission to undergo DBS. This process involves a hearing held between three tribunal members, the DBS candidate, a support person (if needed), and the clinical/research team. The panel assesses the candidate’s suitability for the procedure and capacity to consent to it. The majority of the candidates indicated that they found this experience anxiety-provoking and onerous. It could be argued that this requirement stigmatizes those with mental illness compared with other neurological indications for DBS where neurocognitive impairment is common (i.e., Parkinson’s disease) (Thomson et al., 2019a). While the efficacy of DBS for depression remains under investigation, however, this safeguarding procedure should ensure that only people with acceptable levels of understanding and preparedness who are well supported by an appropriately knowledgeable and experienced clinical team proceed to surgery.
Despite demonstrating comprehension and retention of information required to make an informed decision, some post-DBS comments indicated that this had little bearing on the candidates’ decision and acknowledged the impact depression had on their engagement with this information (e.g., impaired memory, concentration difficulties, challenges making independent decisions, hopelessness/nihilism). Hopelessness, suicidal ideation and reduced concern for preservation of ones’ life (“I want to die anyway” Patient 4) affected appraisals of surgical risks and desperation to be relieved from persistent depression in the absence of alternatives meant participants were willing to take a chance. Others have noted the difficulties involved in establishing meaningful informed consent for DBS in the midst of extreme hopelessness, desperation, and a lack of alternatives (Bell et al., 2010; Klein et al., 2016). Given the severity of mental illness, poor quality of life, lack of treatment prospects, and risk of dying by suicide possessed by people with TRD, it is reasonable and expected that their appraisals of risk and decision-making will be influenced by hopelessness and nihilism. These inherent features of TRD should be recognized, acknowledged and balanced alongside persistent efforts to conduct thorough and comprehensive consent. Further patient-led research is needed to understand how best to provide information about DBS and maximize participant comprehension and appreciation, particularly of long-term risks and outcomes.
Participants were aware of the short-term risks (i.e., risks posed by undergoing surgery). DBS surgical risks are well-documented, and given their life-threatening potential, it is unsurprising that these were in the forefront of participants’ minds during pre-DBS interviews. Less certainty was expressed regarding stimulation and device-related risks. This aligns with findings in Parkinson’s disease, where patient and caregiver awareness of surgical risks was superior to stimulation-related risks such as transient personality and behavioral changes (Thomson et al., 2020). Patients in the current study experienced various stimulation-related effects that remitted following adjustment (e.g., anxiety, irritability, disturbed sleep, mania, self-harm urges). While transient, some of these were unsettling and distressing for participants, contributing to their “rollercoaster” experience. Uncharacteristic and problematic stimulation-dependent behaviors (e.g., impulsive and reckless decision-making while manic) also have the potential to impact the individual and their relationships in the long-term (Agid et al., 2006; Mosley et al., 2019; Thomson et al., 2019b).
Participants demonstrated some awareness, but ultimately an underappreciation of, long-term risks and consequences of DBS. Such consequences included the time-burden associated with regularly recharging the DBS device (discussed at length in Thomson et al., unpublished) and the travel/time-burden associated with frequent visits to the research center (to complete clinical tests, stimulator adjustments and monitor effects). This was particularly the case for those living interstate and caregivers with work commitments. Full pre-surgical appreciation of these long-term implications appeared inhibited by the urgency to pursue a novel treatment. When people express their situation “can’t get any worse” or they “have nothing to lose” (Caregiver 6), emphasizing how participation in a clinical trial and receiving DBS could alter and complicate daily life is particularly important (de Haan et al., 2015; Thomson and Carter, 2020). As one caregiver described it—DBS is “a lifestyle change.” While patient and caregiver journeys with DBS were rarely straightforward, all indicated they would make the same decision if they had their time again. For the reasons that: (1) they had derived some benefit from DBS, (2) they felt hopeful they may yet still, or (3) in order to have the question “will it work?” answered.
Intervention Expectations
High expectations have been highlighted as another aspect of informed consent that require careful management given the potential for disappointment and negative postoperative outcomes (Bell et al., 2009, 2010). In other populations (Parkinson’s disease, epilepsy), unrealistic expectations have been associated with poorer psychosocial adjustment and subjective, psychometric and functional outcomes (Wilson et al., 2001; Haahr et al., 2010; Maier et al., 2013; Hasegawa et al., 2014; Baertschi et al., 2019). Media stories are often implicated in the development of unrealistic expectations. Media portrayals of DBS are overwhelmingly positive, depicting best-case scenarios with limited reporting of associated risks (Racine et al., 2007; Gilbert and Ovadia, 2011). Media coverage of DBS for depression generally presents the “miracle cure” narrative (Dobbs, 2006; Talan, 2008; CNN, 2014; PBS, 2016). The other extreme, the “horror story,” is depicted to a lesser extent (Egan, 2015). Such coverage can result in blind optimism or unfounded fears of DBS, both of which have the potential to damage the scientific development of the emerging intervention (Johansson et al., 2011). In one couple’s case, their decision to pursue DBS had been influenced by a positive media story. Despite having gone through a comprehensive consent process about the procedure’s experimental nature, they still experienced disappointment with an inadequate outcome. This demonstrates the potency of hope elicited by such narratives, and reflects what Baertschi et al. (2019) refer to as the emotional facet of expectations.
Some participants expressed strong hopes for DBS that were held in balance with knowledge and understanding of the intervention’s experimental nature and uncertain outcome. Based on interviews conducted with a sample of DBS recipients with Parkinson’s disease, Baertschi et al. (2019) identified participant expectations consisting of two distinct components. Some patients could intellectually acknowledge the science and research-based information reinforced by medical professionals (cognitive facet), while still holding hopes the treatment would lead to something extraordinarily positive (emotional facet). The authors report that these “secret hopes” were not revealed to medical staff pre-operatively. In the current study, hope appeared to be a powerful motivator to proceed with the experimental procedure and intensive clinical trial, regardless of how small or unspoken it was. The degree of hope present in participants’ pre-DBS mindsets varied and appeared to serve a protective function. Some participants maintained an optimistic and hopeful mindset in order to have the courage and motivation to follow through with the intervention. While others maintained a rational mindset with minimal acknowledgment of their hopes, for fear of later disappointment (as had been experienced numerous times before). A hopeful mindset is not necessarily problematic (Sotsky et al., 2006), unless of course it reflects a fundamental misunderstanding of the research purpose or prospect of benefit (Horng and Grady, 2003). Indeed, hopelessness (a common symptom of depression itself), has the potential to obscure perceptions of benefit and intervention outcomes (Brent et al., 1998).
Subjective Outcomes
Participants reflected extensively on their experience of living with DBS, including the perceived benefits of the procedure (or lack thereof). The significance of small changes was evident. For example, a patient was relieved to spend minutes rather than hours crying upon waking each morning, and a caregiver was thankful to be able to engage in small conversations with their partner rather than sitting in silence. Such changes were considered meaningful and while many pre-DBS comments suggested that any improvement would do, a desire to gain more from DBS was common. In other DBS samples (movement and psychiatric disorders), there is evidence that patients and caregivers shift their expectations for DBS based on their postoperative experiences. This includes patients wanting to achieve goals that were not discussed preoperatively or that medical professionals indicated were unattainable [e.g., “(The patients) shift the goalposts a little” DBS Nurse] (Thomson, 2020). Patients’ desired level of control over their DBS stimulator can also alter as they adjust to living with the device (Merner et al., 2021). Families can also develop an increased expectation that adjustment of the DBS settings will resolve any issue they observe in the patient (Klein et al., 2016), a perception that can be frustrating and invalidating for the patient themselves.
Another common reflection from participants was the length and uncertainty of the process of establishing whether DBS had “worked.” DBS for any treatment indication requires extensive testing and trialing of stimulation parameters, a process that can expose patients and families to a variety of desirable and undesirable changes. This “rollercoaster” experience of encouraging and disappointing responses was a surprise to most participants, as was the length of the optimization process. In comparison with movement disorders, the process of optimizing settings in depression is complicated by the lack of consistent acute behavioral and clinical effects (Ramasubbu et al., 2013), with a lag of 2 weeks between adjustment and detectable effects common (Holtzheimer et al., 2012). Regardless of clinical trial protocols, optimization is a complex, time-consuming, and at times imprecise process that can take 12-months or more to complete (Dougherty et al., 2015; Bergfeld et al., 2016; Ramasubbu et al., 2018; van Westen et al., 2021b). While participants were prepared for this, the reality of the process was challenging. Adjusting to the observed changes is rarely straightforward either. In OCD, DBS patients often take time to recognize changes, before gradually making sense of them and integrating them successfully into their lives (de Haan et al., 2015; van Westen et al., 2021a). As one patient with OCD remarked: “DBS is no ON/OFF switch” (p. 12) (van Westen et al., 2021b). It is therefore unsurprising that at 9-months post-DBS many of the current sample considered DBS “still a work in progress” (Caregiver 3).
Implications and Recommendations
The current findings hold a number of implications for informing and consenting participants to clinical DBS research. There have been calls from both neuroethics and scientific communities for the long-term risks and consequences of participation in psychiatric DBS trials to be robustly outlined for potential participants (Hendriks et al., 2019; Goering et al., 2021; Vedam-Mai et al., 2021). This would include thoroughly addressing the burden associated with participation (regular travel to research center, clinical tests), information on the day-to-day impact of DBS (e.g., recharging, stimulation side-effects, psychosocial adjustment), and providing clear guidance on post-trial continuity of care (given DBS can be a life-long intervention that is dependent on specialist care and requires maintenance) (Thomson and Carter, 2020). There are limitations to contractual “disclose and sign” informed consent processes, notably that information presented preoperatively is later forgotten or goes unappreciated. There have been recommendations for more experiential and interactive forms of informed consent in DBS (Bell et al., 2010; Liddle et al., 2019), that draw upon the knowledge of lived experience experts. Such a process could involve corrective feedback and use of narrative accounts from DBS recipients and their families (e.g., videos, written accounts). Those with lived experience can answer questions and provide perspectives that clinical research teams cannot. Information delivered in narrative form is also often well-retained (Mazor et al., 2007; Thomson et al., 2020). Further research is required to establish what forms of narrative evidence are most effective; however, a range of different outcomes and experiences must be represented to ensure personal stories do not set an expectation for a single, best-case scenario (as occurs with media stories). The current findings also demonstrate that participants’ desires and expectations for DBS adjust based on their personal experience with the device. As such, informed consent for this long-term and adaptable neurotechnology requires an iterative and ongoing process. This recommendation has previously been made for DBS in Parkinson’s disease (Kubu et al., 2018; Liddle et al., 2019; Mosley et al., 2019), and is potentially more relevant in clinical trials of DBS for psychiatric conditions where the risks and benefits are less established.
A further recommendation is for an expansion of DBS clinical trial protocols to include more in-depth, qualitative studies of this kind. Data collected in clinical trials provides important indicators of intervention efficacy and safety; whereas qualitative data provides insight into the experiential effects of DBS and can elucidate unexpected or paradoxical outcomes (e.g., difficulties with psychosocial adjustment). Thus, adopting both approaches will give the most complete picture of the impacts of DBS. The variety of experiential information derived from qualitative studies can be used to better inform prospective patients and caregivers on what to expect. Qualitative research strives for transferability rather than generalizability, and an overview of patient experiences can assist in preparing patients and families for DBS.
A final recommendation is for increased inclusion of caregivers in the research process. This includes informed consent procedures in order for caregivers to have a full understanding of what their loved one is agreeing to and what impact it is likely to have on both of their lives. Research teams often seek informal feedback from caregivers about what they are observing in the patient, whether it be subtle improvements (e.g., increase in energy, activity) or excessive adverse effects (e.g., mania). There is, however, much potential in examining how caregivers themselves are affected by their loved one’s participation in a DBS clinical trial (e.g., quality of life, mood and anxiety).
Limitations
The patient sample was small (n = 6) and reflects the small numbers undergoing the procedure for depression in Australia. This sample represents the entirety of those who have received DBS for TRD in Australia since December 2016. For the purpose of deriving in-depth, qualitative information, samples of this size can be sufficient (Crouch and McKenzie, 2006; Guest et al., 2006) and data saturation (commonly used to determine adequate sample size) was reached. This rich and in-depth data has high transferability; however, the specific context in which this data was derived should be held in mind (e.g., target lead location, patient characteristics, geographical location, clinical trial protocol, available psychosocial supports etc.) given the potential for such factors to influence experiences and outcomes.
Conclusion
This is the first prospective qualitative study to be conducted with individuals undergoing DBS for TRD, with the added perspective of caregivers. The prospective design ensured participants’ knowledge, expectations and beliefs accurately reflected their pre-DBS circumstances and allowed for contrast with actual outcomes and experiences. Caregivers played an important role throughout the DBS process and were impacted by their loved one’s participation in various ways. The progress and development of psychiatric DBS clinical research depends on knowledge acquired through both large-scale, robust clinical trials as well as small, in-depth qualitative studies such as this one.
Data Availability Statement
The datasets presented in this article are not readily available because of the personal nature of the information included within the interview transcripts and in order to maintain participant confidentiality. All relevant data are presented within the manuscript. Requests to access the datasets should be directed to corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Monash University Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CT, RS, ER, and AC designed the study and research protocol. CT recruited study participants, collected the interview data, and analyzed the interview data. AC and RS assisted with data analysis interpretation. CT wrote the manuscript. All authors provided critical feedback and contributed to the article and approved the submitted version.
Funding
This work was supported by an NHMRC grant (APP1077859). AC was supported by an Australian National Health and Medical Research Council Career Development Fellowship (1123311). RS was supported by the David W. Turner Endowment Fund. ER was supported by a Fonds de recherche du Québec—Santé career award. CT received an Australian Postgraduate Award scholarship to support her during her doctoral studies.
Conflict of Interest
PF has received equipment for research from MagVenture A/S, Nexstim, Neuronetics and Brainsway Ltd., and funding for research from Neuronetics. He is a founder of TMS Clinics Australia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank all the participants for generously sharing their experiences.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.755276/full#supplementary-material
Footnotes
- ^ Inclusion/exclusion criteria, extensive demographic data, surgical information (including lead placement), and full psychometric outcomes from the clinical trial will be reported in a subsequent publication (in preparation). Any correspondence regarding this efficacy study should be directed to cGF1bC5maXR6Z2VyYWxkQG1vbmFzaC5lZHU=
References
Agid, Y., Schupbach, M., Gargiulo, M., Mallet, L., Houeto, J. L., Behar, C., et al. (2006). Neurosurgery in Parkinson’s disease: the doctor is happy, the patient less so?. J. Neural Trans. 70, 409–414.
Baertschi, M., Favez, N., Radomska, M., Herrmann, F., Burkhard, R. P., Weber, K., et al. (2019). An empirical study on the application of the burden of normality to patients undergoing deep brain stimulation for Parkinson’s disease. J. Psychos. Rehabil. Ment. Health 6, 175–186. doi: 10.1007/s40737-019-00149-5
Bell, E., Mathieu, G., and Racine, E. (2009). Preparing the ethical future of deep brain stimulation. Surg. Neurol. 72, 577–586. doi: 10.1016/j.surneu.2009.03.029
Bell, E., Maxwell, B., McAndrews, M. P., Sadikot, A., and Racine, E. (2010). Hope and patients’ expectations in deep brain stimulation: healthcare providers’ perspectives and approaches. J. Clin. Ethics 21, 112–124.
Bell, E., and Racine, E. (2013). Clinical and ethical dimensions of an innovative approach for treating mental illness: a qualitative study of health care trainee perspectives on deep brain stimulation. Can. J. Neurosci. Nurs. 35, 23–32.
Bergfeld, I. O., Mantione, M., Hoogendoorn, M. L., Ruhe, H. G., Notten, P., van Laarhoven, J., et al. (2016). Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 73, 456–464. doi: 10.1001/jamapsychiatry.2016.0152
Braun, V., and Clarke, V. (2006). Using thematic analysis in psychology. Qual. Res. Psychol. 3, 77–101.
Braun, V., and Clarke, V. (2019). Novel insights into patients’ life-worlds: the value of qualitative research. Lancet Psychiatry 6, 720–721. doi: 10.1016/s2215-0366(19)30296-2
Braun, V., and Clarke, V. (2020). One size fits all? What counts as quality practice in (reflexive) thematic analysis?. Qual. Res. Psychol. [Epub ahead of print]. doi: 10.1080/14780887.2020.1769238
Brent, D. A., Kolko, D. J., Birmaher, B., Baugher, M., Bridge, J., Roth, C., et al. (1998). Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J. Am. Acad. Child Adolesc. Psychiatry 37, 906–914. doi: 10.1097/00004583-199809000-00010
Christopher, P. P., Leykin, Y., Appelbaum, P. S., Holtzheimer, P. E., Mayberg, H. S., and Dunn, L. B. (2012). Enrolling in deep brain stimulation research for depression: influences on potential subjects’ decision making. Depress. Anxiety 29, 139–146. doi: 10.1002/da.20916
CNN (2014). Deep Brain Stimulation Treats Depression. Available online at: https://www.youtube.com/watch?v=Jk0TGTdCXgQ (accessed May 01, 2021).
Crouch, M., and McKenzie, H. (2006). The logic of small samples in interview-based qualitative research. Soc. Sci. Inform. 45, 483–499. doi: 10.1177/0539018406069584
de Haan, S., Rietveld, E., Stokhof, M., and Denys, D. (2015). Effects of deep brain stimulation on the lived experience of obsessive-compulsive disorder patients: in-depth interviews with 18 patients. PLoS One 10:e0135524. doi: 10.1371/journal.pone.0135524
Dougherty, D. D., Rezai, A. R., Carpenter, L. L., Howland, R. H., Bhati, M. T., O’Reardon, J. P., et al. (2015). A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78, 240–248. doi: 10.1016/j.biopsych.2014.11.023
Egan, D. (2015). Adverse Effects: The Perils of Deep Brain Stimulation for Depression. Available online at: https://www.madinamerica.com/2015/09/adverse-effects-perils-deep-brain-stimulation-depression/ (accessed May 1, 2021).
Fava, M. (2003). Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53, 649–659.
Fins, J. J., Kubu, C. S., Mayberg, H. S., Merkel, R., Nuttin, B., and Schlaepfer, T. E. (2017). Being open minded about neuromodulation trials: finding success in our “failures”. Brain Stimul. 10, 181–186. doi: 10.1016/j.brs.2016.12.012
Fisher, C. E., Dunn, L. B., Christopher, P. P., Holtzheimer, P. E., Leykin, Y., Mayberg, H. S., et al. (2012). The ethics of research on deep brain stimulation for depression: decisional capacity and therapeutic misconception. Ann. N. Y. Acad. Sci. 1265, 69–79. doi: 10.1111/j.1749-6632.2012.06596.x
Gilbert, F., and Ovadia, D. (2011). Deep brain stimulation in the media: over-optimistic portrayals call for a new strategy involving journalists and scientists in ethical debates. Front. Integr. Neurosci. 5:16. doi: 10.3389/fnint.2011.00016
Goering, S., Klein, E., Specker Sullivan, L., Wexler, A., Aguera, Y. A. B., Bi, G., et al. (2021). Recommendations for responsible development and application of neurotechnologies. Neuroethics [Epub ahead of print]. doi: 10.1007/s12152-021-09468-6
Greenhalgh, T., Annandale, E., Ashcroft, R., Barlow, J., Black, N., Bleakley, A., et al. (2016). An open letter to The BMJ editors on qualitative research. BMJ 352:i563. doi: 10.1136/bmj.i563
Guest, G., Bunce, A., and Johnson, L. (2006). How many interviews are enough? An experiment with data saturation and variability. Field Methods 18, 59–82.
Haahr, A., Kirkevold, M., Hall, E. O., and Ostergaard, K. (2010). From miracle to reconciliation: a hermeneutic phenomenological study exploring the experience of living with Parkinson’s disease following deep brain stimulation. Int. J. Nurs. Stud. 47, 1228–1236. doi: 10.1016/j.ijnurstu.2010.03.006
Hasegawa, H., Samuel, M., Douiri, A., and Ashkan, K. (2014). Patients’ expectations in subthalamic nucleus deep brain stimulation surgery for parkinson disease. World Neurosurg. 82, 1295.e2–1299.e2. doi: 10.1016/j.wneu.2014.02.001
Hendriks, S., Grady, C., Ramos, K. M., Chiong, W., Fins, J. J., Ford, P., et al. (2019). Ethical challenges of risk, informed consent, and posttrial responsibilities in human research with neural devices: a review. JAMA Neurol. [Epub ahead of print]. doi: 10.1001/jamaneurol.2019.3523
Holtzheimer, P. E., Kelley, M. E., Gross, R. E., Filkowski, M. M., Garlow, S. J., Barrocas, A., et al. (2012). Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158. doi: 10.1001/archgenpsychiatry.2011.1456
Horng, S., and Grady, C. (2003). Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, & therapeutic optimism. IRB Ethics Hum. Res. 25, 11–16.
Institute of Medicine. (2001). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press.
Johansson, V., Garwicz, M., Kanje, M., Röcklinsberg, H., Schouenborg, J., Tingström, A., et al. (2011). Beyond blind optimism and unfounded fears: deep brain stimulation for treatment resistant depression. Neuroethics 6, 457–471. doi: 10.1007/s12152-011-9112-x
Klein, E., Goering, S., Gagne, J., Shea, C. V., Franklin, R., Zorowitz, S., et al. (2016). Brain-computer interface-based control of closed-loop brain stimulation: attitudes and ethical considerations. Brain Comput. Interfaces 3, 140–148. doi: 10.1080/2326263x.2016.1207497
Kubu, C. S., Frazier, T., Cooper, S. E., Machado, A., Vitek, J., and Ford, P. J. (2018). Patients’ shifting goals for deep brain stimulation and informed consent. Neurology 91, e472–e478. doi: 10.1212/WNL.0000000000005917
Liddle, J., Beazley, G., Gustafsson, L., and Silburn, P. (2019). Mapping the experiences and needs of deep brain stimulation for people with Parkinson’s disease and their family members. Brain Impairment 20, 211–225. doi: 10.1017/BrImp.2019.3
Maier, F., Lewis, C. J., Horstkoetter, N., Eggers, C., Kalbe, E., Maarouf, M., et al. (2013). Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. J. Neurol. Neurosurg. Psychiatry 84, 1273–1281. doi: 10.1136/jnnp-2012-303670
Mayberg, H. S. (2018). What is well? Reconciling first- and third- person perspectives on depression recovery with DBS. Paper presented at the Federation of European Neuroscience Societies 11th Forum of Neuroscience (Berlin: Springer).
Mazor, K. M., Baril, J., Dugan, E., Spencer, F., Burgwinkle, P., and Gurwitz, J. H. (2007). Patient education about anticoagulant medication: is narrative evidence or statistical evidence more effective? Patient Educ. Counsel. 69, 145–157.
Merner, A. R., Frazier, T., Ford, P. J., Cooper, S. E., Machado, A., Lapin, B., et al. (2021). Changes in Patients’ desired control of their deep brain stimulation and subjective global control over the course of deep brain stimulation. Front. Hum. Neurosci. 15:642195. doi: 10.3389/fnhum.2021.642195
Montgomery, S. A., and Asberg, M. (1979). A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389.
Mosley, P. E., Robinson, K., Coyne, T., Silburn, P., Breakspear, M., and Carter, A. (2019). ‘Woe betides anybody who tries to turn me down.’ a qualitative analysis of neuropsychiatric symptoms following subthalamic deep brain stimulation for Parkinson’s disease. Neuroethics [Epub ahead of print]. doi: 10.1007/s12152-019-09410-x
Okun, M. S., and Foote, K. D. (2004). A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist 10:290.
PBS (2016). Deep Brain Stimulation (DBS). Available online at: https://www.youtube.com/watch?v=hki3lR_Ysvo (accessed May 01, 2021)
Racine, E., Waldman, S., Palmour, N., Risse, D., and Illes, J. (2007). “Currents of Hope”: neurostimulation techniques in U.S. and U.K. Print Media. Camb. Q. Healthc. Ethics 16, 312–316. doi: 10.1017/s0963180107070351
Ramasubbu, R., Anderson, S., Haffenden, A., Chavda, S., and Kiss, Z. H. (2013). Double-blind optimization of subcallosal cingulate deep brain stimulation for treatment-resistant depression: a pilot study. J. Psychiatry Neurosci. 38, 325–332. doi: 10.1503/jpn.120160
Ramasubbu, R., Lang, S., and Kiss, Z. H. T. (2018). Dosing of electrical parameters in Deep Brain Stimulation (DBS) for intractable depression: a review of clinical studies. Front. Psychiatry 9:302. doi: 10.3389/fpsyt.2018.00302
Sidhu, K., Jones, R., and Stevenson, F. (2017). Publishing qualitative research in medical journals. Br. J. Gen. Pract. 67, 229–230. doi: 10.3399/bjgp17X690821
Sotsky, S. M., Glass, D. R., Shea, M. T., Pilkonis, P. A., Collins, F., Elkin, I., et al. (2006). Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH treatment of depression collaborative research program. Focus 4, 278–290. doi: 10.1176/foc.4.2.278
Talan, J. (2008). Deep Brain Stimulation Offers Hope in Depression. Available online at: https://www.dana.org/article/deep-brain-stimulation-offers-hope-in-depression/ (accessed May 1, 2021).
Thase, M. E., and Rush, A. J. (1995). “Treatment-resistant depression,” in Psychopharmacology, the Fourth Generation of Progress, eds F. E. Bloom and D. J. Kupfer (New York, NY: Raven Press), 1081–1098.
Thomson, C. J. (2020). The Impact of Deep Brain Stimulation on Personality, Self and Relationships: A Qualitative Exploration in A Neurological and Psychiatric Population. Doctoral dissertation. Clayton: Monash University.
Thomson, C. J., and Carter, A. (2020). Ethical issues in experimental treatments for psychiatric disorders: lessons from deep brain stimulation. Transl. Issues Psychol. Sci. 6, 240–246. doi: 10.1037/tps0000267
Thomson, C. J., Segrave, R., Gardner, J., and Carter, A. (2019a). Patients’ weighing of the long-term risks and consequences associated with deep brain stimulation in treatment-resistant depression. AJOB Neurosci. 9, 243–245. doi: 10.1080/21507740.2018.1561542
Thomson, C. J., Segrave, R. A., and Carter, A. (2019b). Changes in personality associated with deep brain stimulation: a qualitative evaluation of clinician perspectives. Neuroethics [Epub ahead of print]. doi: 10.1007/s12152-019-09419-2
Thomson, C. J., Segrave, R. A., Racine, E., Warren, N., Thyagarajan, D., and Carter, A. (2020). “He’s Back so I’m Not Alone”: the impact of deep brain stimulation on personality, self, and relationships in Parkinson’s disease. Qual. Health Res. 30, 2217–2233. doi: 10.1177/1049732320951144
Tong, A., Sainsbury, P., and Craig, J. (2007). Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 19, 349–357. doi: 10.1093/intqhc/mzm042
van Westen, M., Rietveld, E., van Hout, A., and Denys, D. (2021b). ‘Deep brain stimulation is no ON/OFF-switch’: an ethnography of clinical expertise in psychiatric practice. Phenomenol. Cogn. Sci. [Epub ahead of print]. doi: 10.1007/s11097-021-09732-3
van Westen, M., Rietveld, E., Bergfeld, I. O., de Koning, P., Vullink, N., Ooms, P., et al. (2021a). Optimizing deep brain stimulation parameters in obsessive-compulsive disorder. Neuromodulation 24, 307–315. doi: 10.1111/ner.13243
Vedam-Mai, V., Deisseroth, K., Giordano, J., Lazaro-Munoz, G., Chiong, W., Suthana, N., et al. (2021). Proceedings of the eighth annual deep brain stimulation think tank: advances in optogenetics, ethical issues affecting dbs research, neuromodulatory approaches for depression, adaptive neurostimulation, and emerging DBS technologies. Front. Hum. Neurosci. 15:644593. doi: 10.3389/fnhum.2021.644593
Wilson, S., Bladin, P., and Saling, M. (2001). The “burden of normality”: concepts of adjustment after surgery for seizures. J. Neurol. Neurosurg. Psychiatry 70, 649–656.
Keywords: deep brain stimulation (DBS), neuromodulation, depression, informed consent, expectations, subjective outcomes, ethics, neurotechnology
Citation: Thomson CJ, Segrave RA, Fitzgerald PB, Richardson KE, Racine E and Carter A (2021) “Nothing to Lose, Absolutely Everything to Gain”: Patient and Caregiver Expectations and Subjective Outcomes of Deep Brain Stimulation for Treatment-Resistant Depression. Front. Hum. Neurosci. 15:755276. doi: 10.3389/fnhum.2021.755276
Received: 08 August 2021; Accepted: 06 September 2021;
Published: 29 September 2021.
Edited by:
Michael S. Okun, University of Florida Health, United StatesReviewed by:
Bhavana Patel, University of Florida, United StatesTerry Coyne, University of Queensland, Australia
Aparna Wagle Shukla, University of Florida, United States
Copyright © 2021 Thomson, Segrave, Fitzgerald, Richardson, Racine and Carter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cassandra J. Thomson, Y2Fzc2FuZHJhLnRob21zb25AbW9uYXNoLmVkdQ==
 Cassandra J. Thomson
Cassandra J. Thomson Rebecca A. Segrave
Rebecca A. Segrave Paul B. Fitzgerald
Paul B. Fitzgerald Karyn E. Richardson
Karyn E. Richardson Eric Racine
Eric Racine Adrian Carter
Adrian Carter