
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 28 April 2021
Sec. Brain Imaging and Stimulation
Volume 15 - 2021 | https://doi.org/10.3389/fnhum.2021.655044
This article is part of the Research Topic Genetic and Environmental Influences on the Stressed Brain: A Neuroimaging Perspective View all 3 articles
 Yifeng Luo1,2†
Yifeng Luo1,2† Yu Liu3†
Yu Liu3† Zhao Qing4†
Zhao Qing4† Li Zhang5
Li Zhang5 Yifei Weng2
Yifei Weng2 Xiaojie Zhang1
Xiaojie Zhang1 Hairong Shan1
Hairong Shan1 Lingjiang Li5
Lingjiang Li5 Rongfeng Qi2*
Rongfeng Qi2* Zhihong Cao1*
Zhihong Cao1* Guangming Lu2*
Guangming Lu2*Background: Losing one’s only child may lead to post-traumatic stress disorder (PTSD), of which re-experiencing is the core symptom. However, neuroimaging studies of sex differences in re-experiencing in the context of the trauma of losing one’s only child and PTSD are scarce; comparisons of the functional networks from the hippocampal subfields to the thalamus might clarify the neural basis.
Methods: Thirty couples without any psychiatric disorder who lost their only child (non-PTSD group), 55 patients with PTSD, and 50 normal controls underwent resting-state functional magnetic resonance imaging. The functional connectivity (FC) from the hippocampal subregions to the thalamus and the correlations of FC with re-experiencing symptoms were analyzed within and between the sexes.
Results: Compared with husbands without PTSD, wives without PTSD had higher re-experiencing symptoms and weaker FC between the right hippocampal cornu ammonis 3 (RCA3) and the right thalamus (RT; RCA3-RT). Moreover, only the correlation between the RCA3-RT FC and re-experiencing in wives without PTSD was significant. Among the three groups, only the RCA3-RT FC in female subjects was markedly different. Additionally, the RCA3-RT FC in wives without PTSD was remarkably lower relative to female patients with PTSD.
Conclusion: Wives without PTSD who lost their only child had worse re-experiencing symptoms relative to their husbands, which was associated with the FC alteration between the hippocampal subregions and the thalamus. Importantly, the low level of the RCA3-RT FC may play a potentially protective role against the development of PTSD in wives who have lost their only child.
In China, the one-child policy, legislated in the 1970s, led to millions of single-child families (Hesketh et al., 2005). While this was an effort to curb the growing population, an unintended consequence was that the death of the only child placed parents at a high risk of post-traumatic stress disorder (PTSD) (Li and Wu, 2013; Yin et al., 2018); this included three symptom clusters: re-experiencing, avoidance, and hyperarousal (Association, 2013).
Re-experiencing symptoms, characterized by intrusions of unwanted memories of the traumatic event (Postel et al., 2019), are largely unique to PTSD and are central its diagnosis (Gelernter et al., 2019). Previous studies have reported that deficient context processing could lead to the emergence of recurrent intrusive memories (Liberzon and Abelson, 2016). Thus, the hippocampus, which is known to be involved in contextual memory (Maren et al., 2013), is associated with intrusions. Moreover, the thalamus plays a critical role in perceptual processing and fear conditioning in PTSD (Sherman and Guillery, 2006; Haber and Calzavara, 2009; Zhu et al., 2017). Additionally, there is existing evidence demonstrating that dysfunction within the interconnected context processing circuitry, which involves the hippocampus and the thalamus, plays a central role in the pathophysiology of PTSD (Liberzon and Abelson, 2016). Furthermore, the thalamus helps in driving hippocampal functions (Cassel and Pereira de Vasconcelos, 2015). However, despite evidence of functional connectivity (FC) between the hippocampus and thalamus in healthy individuals (Cunningham et al., 2017), few studies have conducted this examination in the context of PTSD, especially for the hippocampal subfields, which play different roles in context processing (Duvernoy, 2005; Carr et al., 2010). Evaluation of the thalamus and hippocampal subfields might further illuminate the hippocampal function in context processing in people with PTSD.
Neuroimaging studies of patients with PTSD have reported sex differences (Helpman et al., 2017), mainly through comparisons between males and females who had experienced different types of trauma or who lived in different environments. However, there are still two issues that need to be addressed. First, existing studies have not adequately clarified sex differences post-trauma but before PTSD onset. Second, it remains unclear whether the reported differences between male and female patients were induced by variations in trauma types or their post-trauma living environments. Thus, a neuroimaging study on couples without PTSD who live together and have experienced the same trauma—losing their only child—could provide valuable insight.
Previous studies have shown that female patients experienced more re-experiencing symptoms and were more likely to meet the criteria for PTSD than male patients (Zlotnick et al., 2001), and parents who lost their only child also showed significant sex differences in the incidence of PTSD and brain topological properties (Yin et al., 2018; Luo et al., 2019). However, sex differences in re-experiencing symptoms and context processing with regard to the trauma of losing one’s only child and PTSD have not been investigated, and the neural basis remains ambiguous.
Therefore, in this study, conducted to explore the neural mechanism of sex differences in re-experiencing symptoms, we recruited relatively homogeneous couples without any psychiatric disorder who experienced the same trauma—the death of their only child—and analyzed FC between the hippocampal subregions and thalamus and the correlations of FC with re-experiencing symptoms. Moreover, patients with PTSD and normal controls (NCs) were recruited to explore the changing trend in FC between the sexes, to further elaborate its role between the hippocampal subregions and thalamus in couples without PTSD.
This study was approved by the Ethics Committee of Jiangsu University, and written informed consent was obtained from all participants, in keeping with the Declaration of Helsinki. In 2017, a small PTSD survey of Han Chinese adults who lost their only child was performed in China. The survey included 237 such parents among whom 30 couples without any psychiatric disorder were selected (non-PTSD group). The comparison between husbands and wives was conducted using the paired t-test, which is considered ideal for analyzing sex differences. The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition was used to ensure that these couples did not have any psychiatric disorder (First et al., 1998). The severity of symptoms, especially re-experiencing symptoms (Criterion B and items B1-5), was evaluated by the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995).
To further illuminate sex differences, we selected 55 patients with PTSD who had also lost their only child (39 females and 16 males; PTSD group) from the total sample of 237 parents. Owing to the difficulty in recruiting enough couples where both partners had PTSD (there were only eight such couples in our database), we could only individually analyze female and male patients with PTSD to further explore the sex effect in comparison with couples without PTSD. Moreover, 50 local healthy adults (30 women and 20 men) without any trauma were chosen as the NC group.
The exclusion criteria were as follows: (1) any history of or current brain injury or other major medical or neurological conditions, (2) contraindications to magnetic resonance imaging (MRI), (3) left-handedness, (4) unavailable data, and (5) head translation of more than 1.5 mm or rotation of more than 1.5° during the MRI.
Besides the CAPS, the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960), the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959), and the Mini-Mental State Examination (MMSE) (Folstein et al., 1983) were used to assess the neuropsychological situation of each participant. In addition, the Chinese Social Support Rating Scale (SSRS) (Cheng et al., 2008) and the Simple Coping Style Questionnaire (SCSQ) (Jiang et al., 2017) were used to evaluate the social support level and individual coping ability, respectively. Detailed descriptions are available in Note 1 of the Supplementary material (Supplementary Material Note 1).
MRI data for all participants were obtained using a Philips Achieva 3.0T MRI device (Philips, Amsterdam, Netherlands). Participants were instructed to keep their eyes closed and refrain from thinking about anything during the procedure. Additionally, foam padding was used to minimize head motion. The parameters of the MRI sequences were set as follows: (1) sagittal high-resolution three-dimensional T1-weighted turbo fast echo sequence for structural data: repetition time (TR) = 9.7 ms, echo time (TE) = 4.6 ms, flip angle 9°, field of view (FOV) = 256 × 256 mm2, matrix size = 256 × 256, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3, 160 slices and (2) echo planar imaging sequence for resting-state functional MRI data: TR = 2000 ms, TE = 30 ms, flip angle 90°, FOV = 192 × 192, voxel size = 3 × 3 × 4 mm3, image volumes = 230, number of axial sections per volume = 35.
The image preprocessing was performed using a toolbox for Data Processing and Analysis for Brain Imaging (DPABI) (Yan et al., 2016) based on the MATLAB platform (MathWorks, Natick, MA, United States). The initial ten volumes were excluded for steady state longitudinal magnetization; the slice timing and head motion correction were conducted on all the remaining volumes. Individual T1-weighted images were co-registered to the functional images and then segmented into gray matter, white matter, and cerebrospinal fluid, and transformed into the standard Montreal Neurological Institute (MNI) space. The functional images were transformed into the MNI stereotaxic space (3 × 3 × 3 mm3), using the parameters of the T1-weighted image normalization, and then smoothed with an 8mm full width at half maximum (FWHM) isotropic Gaussian kernel. The mean signals from cerebrospinal fluid and white matter were regressed out. The imaging data were also temporally filtered (bandpass: 0.01–0.1 Hz) (Qi et al., 2020).
After preprocessing, the FC between the hippocampal subfields (four regions in each hemisphere: the cornu ammonis (CA1, CA2, and CA3) and dentate gyrus) and the thalamus was analyzed within each group (female vs. male) and between the three groups (non-PTSD, PTSD, and NC). The cytoarchitectonically probabilistic maps from the JuBrain Cytoarchitectonic Atlas in the SPM Anatomy Toolbox (Eickhoff et al., 2005) were used to define the hippocampal subregions in each hemisphere (Supplementary Figure 1). The process of analyzing the FC between the hippocampal subfields and thalamus included the computation of the average time series, the correlation coefficients, and the resting-state fc map (Qi et al., 2020) (Supplementary Material Note 2 in detail).
The data were analyzed using SPSS version 25 (IBM Corp., Armonk, NY, United States). Between-group comparisons of the demographic characteristics and neuropsychological scores were performed using analysis of variance (ANOVA) statistics for the continuous variables.
With regards to the wives and husbands in the non-PTSD group, the paired t-test was used to compare differences in FC between hippocampal subfields and the thalamus using DPABI. Significant clusters were identified using the Gaussian Random Field at corrected p < 0.05, which corresponded to a voxel p < 0.01 and a cluster level with p < 0.05. Then, the correlations of the FC with the CAPS scores in the non-PTSD group were assessed using partial correlations, accounting for age and educational level.
For the NC and PTSD groups, based on the significant results in the paired t-test and correlation conducted with the non-PTSD group, FC was reprocessed in the DPABI using the mask, after which the values were extracted from single-subject FC maps in all participants. Then the 2 (male and female) × 3 (HC, non-PTSD, and PTSD) ANOVA investigating the interaction between sex and diagnosis was quoted. Additionally, the post hoc analysis (sex differences in FC within the NC and PTSD groups, as well as between the three groups) was performed. Results were considered significant at corrected p < 0.05 (using the false discovery rate correction for multiple comparisons).
Between the wives/women and husbands/men in every group, there were no significant differences in age, educational level, duration of trauma, and scores on the SSRS, SCSQ, MMSE, and total CAPS (p > 0.05). However, compared with husbands without PTSD, wives without PTSD had higher scores on the HAM-A and Criterion B and items B1 and B4 on the CAPS (p < 0.05). Additionally, HAM-D scores in female patients with PTSD were higher than in male patients with PTSD (Table 1). Among the NC, non-PTSD, and PTSD groups, no significant differences were found in age, educational level, and MMSE scores in male or female participants. SSRS and SCSQ scores were not significantly different between the PTSD and non-PTSD groups for both female and male participants (Supplementary Table 1).
Compared with husbands without PTSD, wives without PTSD had weaker FC between the right hippocampal CA3 and the right thalamus (RCA3-RT), as well as FC between the right hippocampal CA3 and the left thalamus (Figure 1 and Table 2). Based on the clinical comparisons in Table 1, the correlations between the B, B1, and B4 scores and the abnormal FC were investigated in the group without PTSD. Only the correlation between the RCA3-RT FC and B1 score was significant in wives without PTSD (r = 0.41, p = 0.03), but not in husbands (Figure 2).
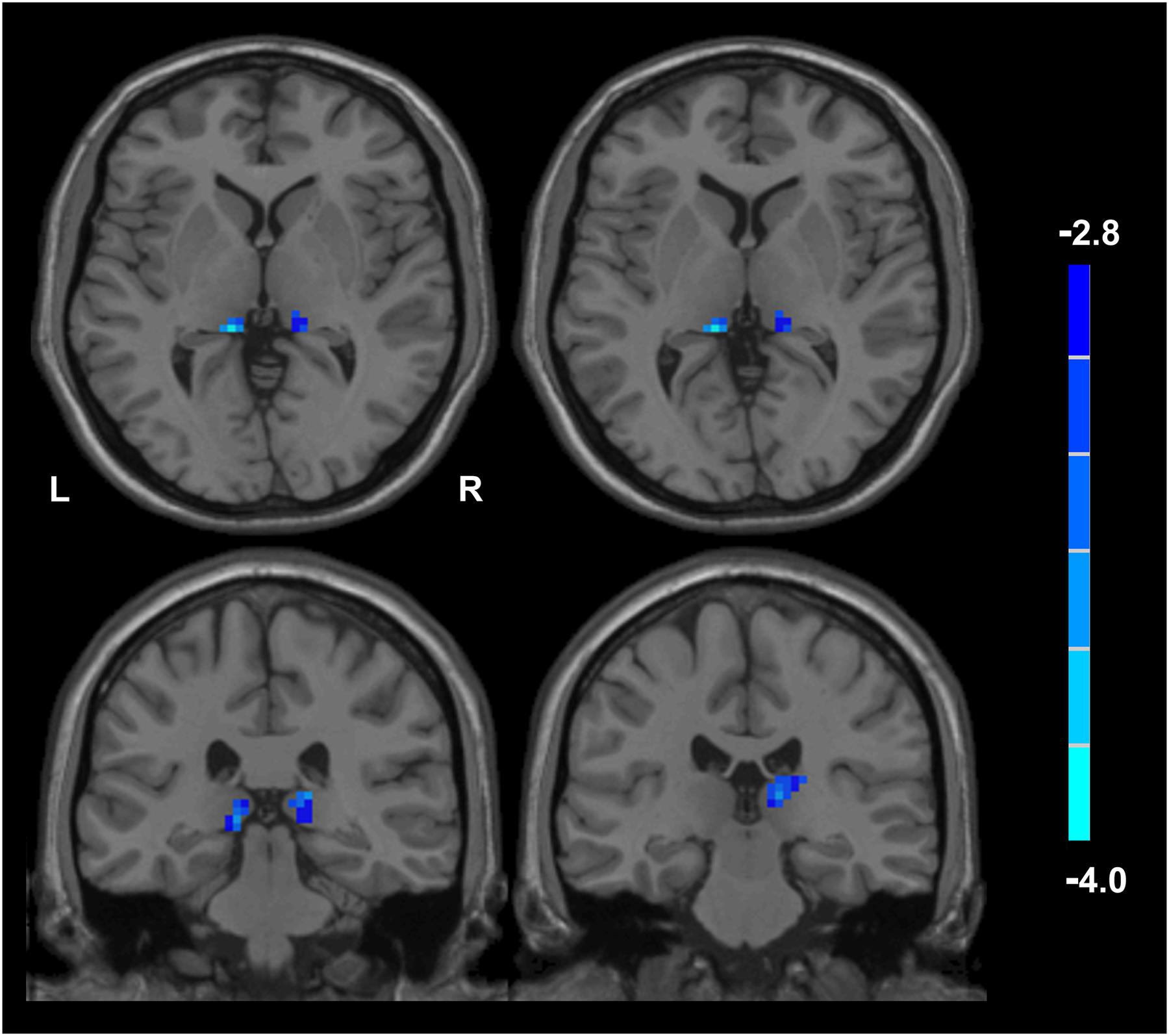
Figure 1. Functional connectivity (FC) between the wives and husbands in the non-PTSD group. Compared with the non-PTSD husbands, the non-PTSD wives had weaker FC between the right hippocampal CA3 and the bilateral thalamus (p < 0.05, corrected). PTSD, post-traumatic stress disorder; CA, Cornu Ammonis.
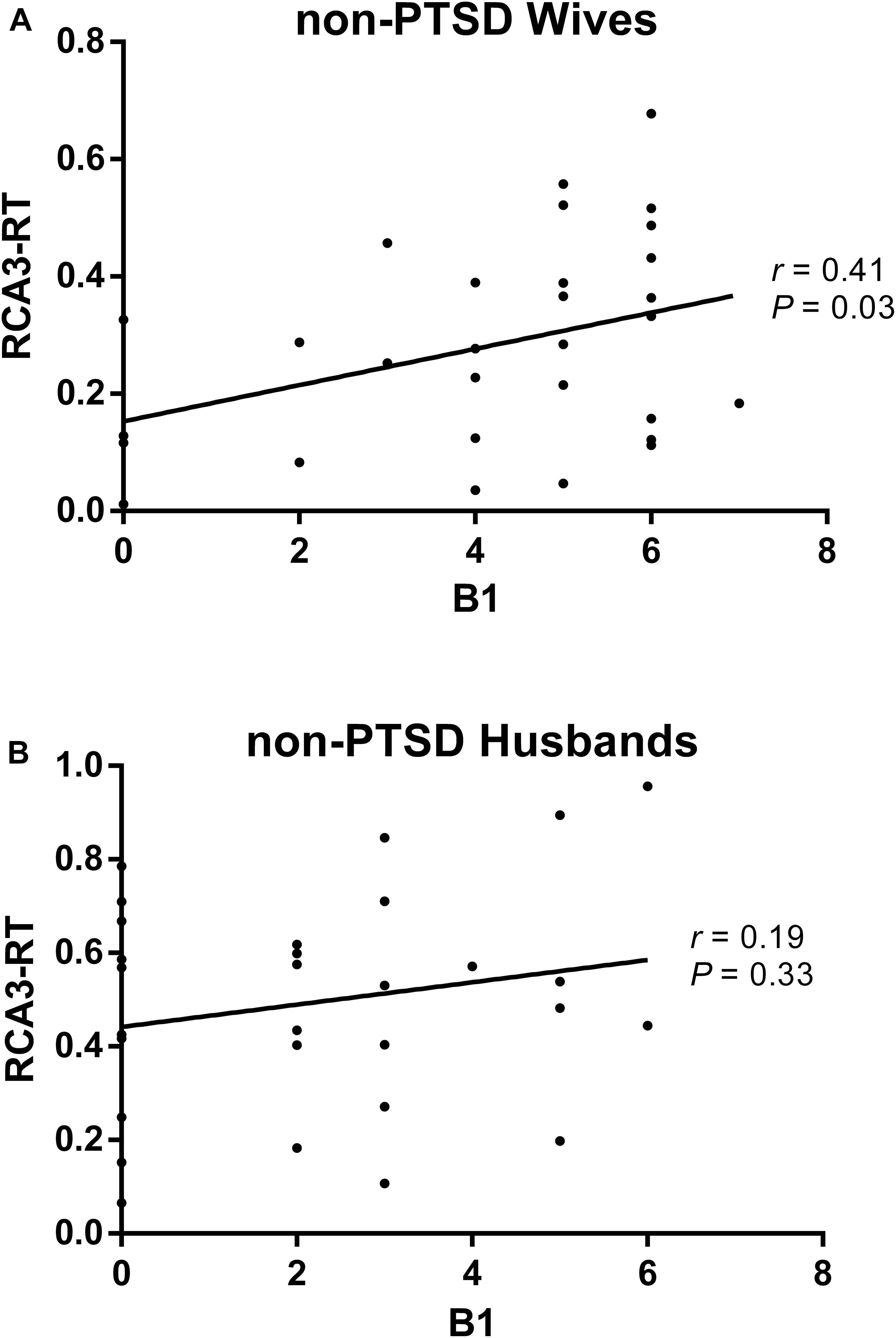
Figure 2. The correlation between the re-experiencing symptom and the abnormal functional connectivity (FC) in the non-PTSD wives (A) and husbands (B). Only the correlation between the RCA3-RT FC and B1 score was significant (r = 0.41, P = 0.03) in the non-PTSD wives, but not in the non-PTSD husbands. RCA3-RT FC, the functional connectivity between right hippocampal CA3 and right thalamus; CA, Cornu Ammonis.
Through comparisons of the values extracted from single-subject FC maps, the main effect of sex was significant (F = 5.76, p = 0.018), as well as the diagnosis-by-sex interaction effect (F = 4.49, p = 0.013). Additionally, only the RCA3-RT FC in female participants was markedly different among the NC, non-PTSD, and PTSD groups (F = 3.61, p = 0.031), and the RCA3-RT FC between wives without PTSD and female patients with PTSD was remarkably different (p = 0.009). However, the RCA3-RT FC was not significantly different between men and women in the NC or PTSD groups (Figure 3A; NC: F = 0.44, p = 0.51; PTSD: F = 0.27, p = 0.61). Moreover, the correlation between the RCA3-RT FC and B1 score was not significant in male and female participants with PTSD (p > 0.05).
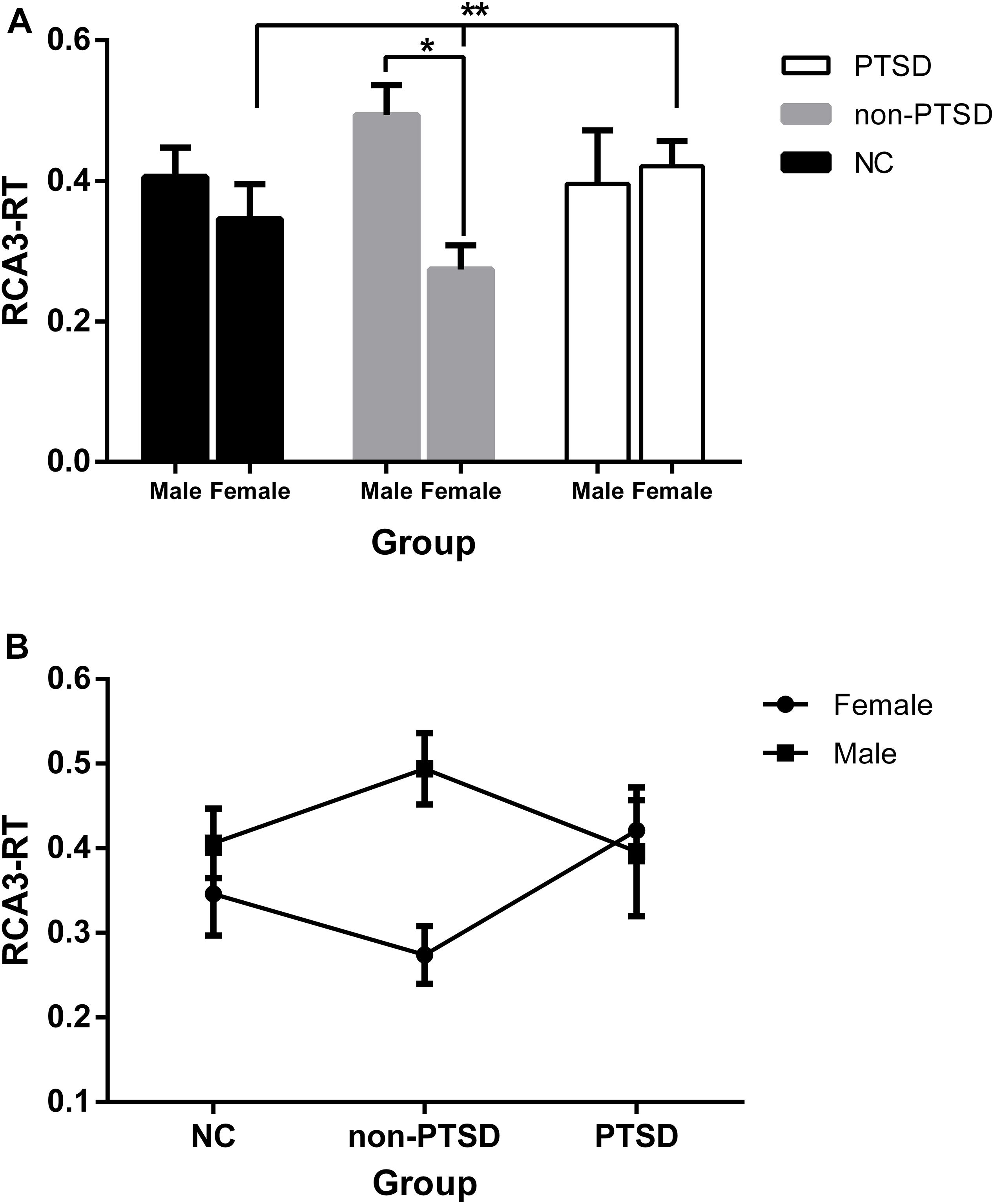
Figure 3. Functional connectivity (FC) between the female and male within and among the groups adjusting for the effects of age and education levels. (A) Except the non-PTSD group (*), neither the NC nor the PTSD group, the RCA3-RT FC was not significantly different between the male and female. Additionally, in the female participants, the RCA3-RT FC was significantly different among the NC, non-PTSD, and PTSD groups (**); (B) the dynamic changes in the three groups in the male or female. RCA3-RT, right hippocampal CA3 and the right thalamus; CA, Cornu Ammonis; PTSD, post-traumatic stress disorder; NC, normal control; *, **P < 0.05.
As depicted in Figure 3B, the change tendency of the RCA3-RT FC in female participants showed that compared with their NC counterparts, FC in wives without PTSD declined, and significantly rose in female patients with PTSD. Among male participants, compared with their NC counterparts, FC in husbands without PTSD increased, and declined in male patients with PTSD.
The HAM-D and HAM-A scores were significantly different between some groups (Table 1), along with age and educational level. Thus, they were added as covariates in the correlation analysis of the non-PTSD group. Like the above results, the correlation between the RCA3-RT FC and B1 score was significant (r = 0.43, p = 0.027) only in wives without PTSD (Supplementary Figure 2).
Additionally, based on the FC result, to eliminate the possible confounding effect of hippocampal structural alteration on functional abnormality, voxel-based morphometry analysis was used to analyze the CA3 volume in a manner similar to prior PTSD studies (Chen and Etkin, 2013; Qi et al., 2020). We found that CA3 volume was not significantly different between the male and female participants within and across the three groups (Supplementary Tables 2, 3).
To minimize the confounding effect of head motion (Yan C. et al., 2013; Yan C. G. et al., 2013), the Friston 24-parameter model was used to regress out head motion effects (Friston et al., 1996). The mean framewise displacement was also added as a covariate. The RCA3-RT FC was also significantly different among the NC, non-PTSD, and PTSD groups only in female participants (F = 3.73, p = 0.028). The correlation between the RCA3-RT FC and B1 score was also significant only in wives without PTSD (p = 0.04).
This is the first neuroimaging study to explore the sex effect of the trauma by using the couples who live together and have experience of the same trauma, which was the important advantage of this study. In the present study, we investigated the FC between the hippocampal subfields and thalamus and the association between FC and re-experiencing symptoms in wives and husbands without PTSD who lost their only child, as well as in parents with PTSD. We found that wives who had lost their only child but did not have PTSD showed worse re-experiencing symptoms (B1), which was associated with lower FC between the right CA3 and the thalamus, than their husbands. Importantly, lower FC between CA3 and the thalamus in wives may reflect a protective factor against the development of PTSD.
Re-experiencing symptoms include sudden, unwanted memories of the trauma, nightmares, and even distortions of experience that can make an individual feel as if the event is recurring (Schnurr et al., 2009). Thus, long-term and higher re-experiencing symptoms may result in a higher likelihood of trauma-exposed individuals developing PTSD. In the present study, despite similar social support levels and individual coping ability, wives who had lost their only child but did not have PTSD showed more serious re-experiencing symptoms than their husbands did. Thus, these wives might be at greater risk for the development of PTSD. However, many wives had not developed PTSD despite the presence of re-experiencing symptoms for several years (the average duration since the loss of the only child was more than 10 years).
The hippocampus plays a critical role in contextual learning and memory (Holland and Bouton, 1999). It establishes new memory representations, minimizes overlapping of previous memories, and also helps retrieve old memories based on partial information (Carr et al., 2010; Liberzon and Abelson, 2016). These processes include two abilities: (1) pattern separation: diminishing the similarity between two similar memories and (2) pattern completion: recalling a memory based on a partial cue (Yassa and Stark, 2011). The CA3 is thought to be involved in both processes. Compared with their husbands, wives without PTSD showed lower FC between the right CA3 and the thalamus. Moreover, the correlational analysis revealed that the RCA3-RT FC was positively associated with item B1 (recurrent and intrusive distressing recollections of the event) only in wives without PTSD. This suggested that the alteration of the hippocampal subfield FC is related to worse re-experiencing symptoms in wives who have lost their only child.
To further explore the role of the abnormal RCA3-RT FC in wives who had lost their only child but did not have PTSD, both male and female NC, non-PTSD, and PTSD groups were investigated. The differences in RCA3-RT FC between healthy female and male participants were not significant. This implied that the differences in RCA3-RT FC between wives and husbands without PTSD resulted from re-experiencing symptoms and not sex. Additionally, from the analysis of the female participants in the three groups, we found that RCA3-RT FC in wives without PTSD was lower than in healthy individuals and female patients with PTSD. This suggested that the decrease in the RCA3-RT FC may be a protective factor against the development of PTSD in wives who have lost their only child. Furthermore, the positive association between re-experiencing symptoms (B1) and RCA3-RT FC implied that lower RCA3-RT FC might be an indication of more robust protective ability in wives without PTSD. The neurocircuitry models of PTSD showed that the hippocampus and prefrontal cortex could inhibit hyperactivity in the amygdala to prevent the development of PTSD (Pitman et al., 2012), and the hippocampus and prefrontal cortex are also included in the context processing circuitry (Liberzon and Abelson, 2016). Furthermore, the hippocampal-prefrontal communication involves the thalamus, which participates in the consolidation of enduring memories at the system level (Cassel and Pereira de Vasconcelos, 2015). Thus, a lower RCA3-RT FC, which implied a reduction in the communication between the hippocampal subfield-CA3 and thalamus, suggested that after the severe trauma of losing their only child, the consolidation of traumatic enduring memories was weakened in wives without PTSD; this, in turn, reduced re-experiencing symptoms and protected them from PTSD.
Our study had several limitations. First, based on the key role and the interrelation of the hippocampal subfields and the thalamus in the context processing, we focused only on the FC between the hippocampal subfields and the thalamus in the present study. However, a deeper analysis of brain network alteration during the development of PTSD may be beyond the scope of the current study. Other aspects of FC, such as the default mode and executive control networks, would be examined in future studies. Second, the cross-sectional design of the study prevented us from ascertaining how the RCA3-RT FC changed over time in couples without PTSD. Third, although it is beneficial to control other variances, the trauma examined in this study was very specific. Hence, any attempt to generalize the results to other traumatic events must be undertaken with caution. Fourth, the sample size of the non-PTSD couples was small, and the samples of the PTSD and NC groups were not couples. Thus, future studies must recruit more couples to enhance the reliability of our study.
In conclusion, our study revealed that wives who lost their only child but did not develop PTSD had more serious re-experiencing symptoms than their husbands. The severity of the re-experiencing symptoms was associated with the FC changes between the hippocampal subregions and the thalamus only in these wives. Importantly, our results suggest a potential protective role of the low level of the RCA3-RT FC in wives without PTSD.
The datasets generated for this study are available on request to the corresponding authors.
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee of Jiangsu University. The patients/participants provided their written informed consent to participate in this study.
ZC, GL, and RQ: experiment design. YFL and ZQ: manuscript writing. YL: image processing and statistical analyses. YW and HS: image acquisition. LZ, LL, and XZ: participant recruitment. All authors read and approved the final manuscript.
This work was supported by the Grants from the National Natural Science Foundation of China (Grant Nos. 81801678, 81671672, and 81301209), the Jiangsu Provincial Medical Youth Talent (Grant Nos. QNRC2016207 and QNRC2016888), the Chinese Key Grant (Grant No. BWS11J063), the Youth Nature Science Foundation of Jiangsu Province (Grant No. BK20170223), the Commission of Health and Family Planning of Wuxi (Grant No. Z201908), and Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (Grant No. BJ2020109).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all subjects for their willingness to participate in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2021.655044/full#supplementary-material
Association, D. S. M. A. P. (2013). Diagnostic and Statistical Manual of Mental Disorders. Arlington: American Psychiatric Publishing.
Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., et al. (1995). The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress 8, 75–90. doi: 10.1007/BF02105408
Carr, V. A., Rissman, J., and Wagner, A. D. (2010). Imaging the human medial temporal lobe with high-resolution fMRI. Neuron 65, 298–308. doi: 10.1016/j.neuron.2009.12.022
Cassel, J. C., and Pereira de Vasconcelos, A. (2015). Importance of the ventral midline thalamus in driving hippocampal functions. Prog. Brain Res. 219, 145–161. doi: 10.1016/bs.pbr.2015.03.005
Chen, A. C., and Etkin, A. (2013). Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38, 1889–1898. doi: 10.1038/npp.2013.122
Cheng, Y., Liu, C., Mao, C., Qian, J., Liu, K., and Ke, G. (2008). Social support plays a role in depression in Parkinson’s disease: a cross-section study in a Chinese cohort. Parkinsonism Relat. Disord. 14, 43–45. doi: 10.1016/j.parkreldis.2007.05.011
Cunningham, S. I., Tomasi, D., and Volkow, N. D. (2017). Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum. Brain Mapp. 38, 938–956. doi: 10.1002/hbm.23429
Duvernoy, H. M. (2005). The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Berlin: Springer Science & Business Media.
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25, 1325–1335. doi: 10.1016/j.neuroimage.2004.12.034
First, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. (1998). Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (February 1996 Final), SCID-I/P. New York: Biometrics Research Department, New York State Psychiatric Institute.
Folstein, M. F., Robins, L. N., and Helzer, J. E. (1983). The Mini-Mental State Examination. Arch. Gen. Psychiatry 40:812. doi: 10.1001/archpsyc.1983.01790060110016
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., and Turner, R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. doi: 10.1002/mrm.1910350312
Gelernter, J., Sun, N., Polimanti, R., Pietrzak, R., Levey, D. F., Bryois, J., et al. (2019). Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 22, 1394–1401. doi: 10.1038/s41593-019-0447-7
Haber, S. N., and Calzavara, R. (2009). The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res. Bull. 78, 69–74. doi: 10.1016/j.brainresbull.2008.09.013
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Helpman, L., Zhu, X., Suarez-Jimenez, B., Lazarov, A., Monk, C., and Neria, Y. (2017). Sex Differences in Trauma-Related Psychopathology: a Critical Review of Neuroimaging Literature (2014-2017). Curr. Psychiatry Rep. 19:104. doi: 10.1007/s11920-017-0854-y
Hesketh, T., Lu, L., and Xing, Z. W. (2005). The effect of China’s one-child family policy after 25 years. N. Engl. J. Med. 353, 1171–1176. doi: 10.1056/NEJMhpr051833
Holland, P. C., and Bouton, M. E. (1999). Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 9, 195–202. doi: 10.1016/s0959-4388(99)80027-0
Jiang, X. R., Du, J. J., and Dong, R. Y. (2017). Coping Style, Job Burnout and Mental Health of University Teachers of the Millennial Generation. Eurasia J. Math. Sci. Technol. Educ. 13, 3379–3392. doi: 10.12973/eurasia.2017.00734a
Li, Y., and Wu, S. (2013). Health care for older Chinese people who lose their only child. Lancet 381:536. doi: 10.1016/S0140-6736(13)60280-9
Liberzon, I., and Abelson, J. L. (2016). Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92, 14–30. doi: 10.1016/j.neuron.2016.09.039
Luo, Y., Qi, R., Zhang, L., Qing, Z., Weng, Y., Wang, W., et al. (2019). Functional brain network topology in parents who lost their only child in China: post-traumatic stress disorder and sex effects. J. Affect. Disord. 257, 632–639. doi: 10.1016/j.jad.2019.07.004
Maren, S., Phan, K. L., and Liberzon, I. (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428. doi: 10.1038/nrn3492
Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., et al. (2012). Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787. doi: 10.1038/nrn3339
Postel, C., Viard, A., Andre, C., Guenole, F., de Flores, R., Baleyte, J. M., et al. (2019). Hippocampal subfields alterations in adolescents with post- traumatic stress disorder. Hum. Brain Mapp. 40, 1244–1252. doi: 10.1002/hbm.24443
Qi, R., Luo, Y., Zhang, L., Weng, Y., Surento, W., Xu, Q., et al. (2020). Decreased functional connectivity of hippocampal subregions and methylation of the NR3C1 gene in Han Chinese adults who lost their only child. Psychol. Med. 2020, 1–10. doi: 10.1017/S0033291720000045
Schnurr, P. P., Lunney, C. A., Forshay, E., Thurston, V. L., Chow, B. K., Resick, P. A., et al. (2009). Sexual function outcomes in women treated for posttraumatic stress disorder. J. Womens Health 18, 1549–1557. doi: 10.1089/jwh.2008.1165
Sherman, S. M., and Guillery, R. W. (2006). Exploring the Thalamus and Its Role in Cortical Function. Cambridge: MIT press.
Yan, C., Craddock, R. C., Zuo, X., Zang, Y., and Milham, M. P. (2013). Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage 80, 246–262. doi: 10.1016/j.neuroimage.2013.04.081
Yan, C. G., Cheung, B., Kelly, C., Colcombe, S., Craddock, R. C., Di Martino, A., et al. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. doi: 10.1016/j.neuroimage.2013.03.004
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yassa, M. A., and Stark, C. E. L. (2011). Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525.
Yin, Q., Shang, Z., Zhou, N., Wu, L., Liu, G., Yu, X., et al. (2018). An investigation of physical and mental health consequences among Chinese parents who lost their only child. BMC Psychiatry 18:45. doi: 10.1186/s12888-018-1621-2
Zhu, X., Helpman, L., Papini, S., Schneier, F., Markowitz, J. C., Van Meter, P. E., et al. (2017). Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety 34, 641–650. doi: 10.1002/da.22594
Keywords: lost only child, sex differences, couple, functional connectivity, hippocampal subfields, re-experiencing symptom
Citation: Luo Y, Liu Y, Qing Z, Zhang L, Weng Y, Zhang X, Shan H, Li L, Qi R, Cao Z and Lu G (2021) Sex Differences in Re-experiencing Symptoms Between Husbands and Wives Who Lost Their Only Child in China: A Resting-State Functional Connectivity Study of Hippocampal Subfields. Front. Hum. Neurosci. 15:655044. doi: 10.3389/fnhum.2021.655044
Received: 18 January 2021; Accepted: 01 April 2021;
Published: 28 April 2021.
Edited by:
Feng Liu, Tianjin Medical University General Hospital, ChinaReviewed by:
Mingrui Xia, Beijing Normal University, ChinaCopyright © 2021 Luo, Liu, Qing, Zhang, Weng, Zhang, Shan, Li, Qi, Cao and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongfeng Qi, cWlyb25nZmVuZ0AxNjMuY29t; Zhihong Cao, bHVveWlmZW5nMTIwN0AxNjMuY29t; Guangming Lu, Y2pyLmx1Z3VhbmdtaW5nQHZpcC4xNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.