- 1Department of Neurology, Shanghai Fourth People's Hospital Affiliated to Tongji University School of Medicine, Shanghai, China
- 2Department of Neurology, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People's Hospital Affiliated to Tongji University School of Medicine, Shanghai, China
- 3Department of Neurology, Tongji Hospital, Tongji University School of Medicine, Shanghai, China
- 4Department of Neurological Rehabilitation, Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center), Tongji University School of Medicine, Shanghai, China
Background: Transient ischemic attack (TIA) has a high incidence of recurrent vascular events. Hypoperfusion is one of the factors that are closely correlated with 7-day recurrence of TIA. This study aimed to evaluate the power of hypoperfusion shown on magnetic resonance (MR) perfusion imaging in predicting the incidence of 7-day recurrence of ischemic events after TIA.
Methods/Design: REATTACK is a prospective multi-centered cohort study on the correlation between MR perfusion and TIA recurrence. Ninety patients aged ≥18 years with recent (<7 days after onset) clinical TIA will be continuously included. All the patients will undergo diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI) assessments within 24 h after the onset of TIA. The subjects will then be divided into a PWI positive group and a PWI negative group according to the time-to-maximum of the residue function (Tmax). PWI will be repeated after 7 days and in 3 months. The primary clinical outcome will be the recurrence of TIA within 7 days after the onset of TIA. Secondary outcomes will be the recurrence of TIA in 3 months and modified Rankin scale (mRS) score. A chi-square test will be performed to compare the difference in the incidence of recurrent TIA between the two groups, and rank sum test in the mRS score. Multivariate logistic regression will be simultaneously performed to analyze the risk factors for the recurrence of TIA.
Discussion: The results of this study will confirm whether abnormal Tmax helps to identify the patients with TIA who have high risks of recurrent ischemic events. This would largely improve the prognosis of patients with TIA.
Trial Registration: www.chictr.org.cn, registration number: ChiCTR2000031863, registered on 12 April 2020.
Background
Transient ischemic attack (TIA) has been defined as a short-lasting episode of neurological dysfunction without evidence of acute infarction (Easton et al., 2009). Approximately 24 million people experience TIA every year in China (Wang et al., 2015). Patients with recent TIAs are at a high risk for recurrent strokes or other vascular events (Lavallee et al., 2007; Amarenco et al., 2016). For example, patients with TIA have a 4–10% possibility of developing a stroke within 7 days and a 10–20% (average 11%) possibility within 90 days, much higher than the risk of developing recurrent strokes (2–7%, average 4%) within 90 days after an acute ischemic stroke (Kelly et al., 2016). Urgent diagnosis and treatment can significantly reduce the risk of recurrent ischemic events after TIA (Amarenco et al., 2016; Kim et al., 2016). A cohort study in the UK showed that optimal secondary prevention decreased the incidence of recurrent ischemic events after TIA from 10.3 to 2.1% (Rothwell et al., 2007).
A recent study raised the hypothesis that patients with TIA have different degrees of recurrence, depending on clinical symptoms, pathological mechanisms, and treatments (Kelly et al., 2016). How to identify TIA patients at high risk of recurrence and provide timely treatment is a problem that needs to be solved urgently in the clinic. The age, blood pressure, clinical symptoms, duration, and diabetes score (ABCD2, 0–7 points) is a stratified approach based on clinical manifestations. It aims to facilitate the evaluation of patients with stroke/TIA by non-neurology medical staff in the emergency room. For neurology professionals, its specificity is low (Amarenco et al., 2009; Giles and Rothwell, 2010; Wardlaw et al., 2015).
The morphological definition of TIA, that is, diffusion-weighted imaging (DWI)-negative TIA, did not clearly distinguish the tissue perfusion status of all patients with TIA. Low perfusion may be one of the mechanisms of TIA recurrence. Perfusion weighted imaging (PWI) is positive in 20–40% of patients with clinical TIA, while DWI is negative (Mlynash et al., 2009; Kleinman et al., 2012; Asdaghi et al., 2013; Grams et al., 2016; Lee et al., 2017). Previous studies have suggested that hypoperfusion might be associated with recurrent TIAs, stroke, and continued deterioration of neurological symptoms (Olivot et al., 2008; Asdaghi et al., 2011, 2013; Lee et al., 2017). The previous study conducted by the authors has assessed 66 patients with TIA with anterior circulation symptoms and found that hypoperfusion is present in 50% of the patients (33/66), with 31.8% (21/66) showing PWI-positive and DWI-negative findings (Wang et al., 2019b). In order to further explore whether the recurrence of TIA in patients with no lesions shown on DWI is related to hypoperfusion, 46 patients with normal DWI were followed up for 3 months in the study. The incidence of recurrent TIA was 41.2% (14/34) in the hypoperfusion group and 16.7% (2/12) in the normal perfusion group. There was a significant statistical difference between the two groups.
There are many modalities for assessing hypoperfusion. Although mean transit time (MTT) and time to peak (TTP) can be used to observe abnormal changes in perfusion in patients with TIA (Kleinman et al., 2012), time-to-maximum of residue function (Tmax) ≥ 4 s is currently the best one for evaluating perfusion abnormalities in the early stage (Olivot et al., 2009; Wang et al., 2019a,b). The previous results showed that the prevalence of Tmax ≥ 4 s > 10 ml was 50% (33/66). Limb fatigue combined with Tmax ≥ 4 s > 10 ml was an independent risk factor for recurrence (adjusted OR = 3.74, 95% CI = 1.02–13.64, P = 0.046). Therefore, it is feasible and clinically significant to use Tmax ≥ 4 s to evaluate the perfusion status of patients with TIA.
The Prospective Cohort Study of Hypoperfusion in Predicting Recurrent Transient Ischemic Attacks (REATTACK) trial is, therefore, designed to explore the correlation between hypoperfusion and TIA recurrence as well as prognosis. By completing this prospective cohort study of patients with TIA with low perfusion (Tmax ≥ 4 s > 10 ml), it is likely to further the insight into the pathological mechanism of TIA recurrence.
Methods
Study Design
REATTACK is a prospective cohort study with a 3-month follow-up period. The participants are patients who had TIA within 7 days of onset diagnosed by two neurologists. Brain DWI and PWI assessments will be completed within 24 h of admission, and Tmax > 4 s > 10 ml is used to determine the presence of hypoperfusion. According to baseline MRI-PWI examination, the patients are divided into the PWI positive and PWI negative groups. DWI, PWI, fluid-attenuated inversion recovery (FLAIR), and magnetic resonance angiography (MRA) examinations will be repeated 7 days later. The main outcomes are the incidence of recurrent TIA within 7 days and in 3 months, and the mRS score in 3 months.
Objectives
Primary Objective
▪ To assess the added diagnostic value of hypoperfusion demonstrated on PWI in predicting recurrent vascular events of patients with TIA within 7 days.
Secondary Objective
▪ To determine the prevalence and course of hypoperfusion and its effect on recurrent vascular events in patients with TIA 3 months after onset.
Study Population and Setting
All patients will be recruited from the outpatient of the Neurology Department of Shanghai Fourth People's Hospital and Tongji Hospital; both are affiliated to Tongji University School of Medicine. TIA will be diagnosed by two experienced attending physicians in the Neurology Department.
Inclusion Criteria
• Aged 18 years or older
• No intracerebral hemorrhage
• Patients or their relatives are willing to participate in the study and provide written informed consent
• Meet the diagnostic criteria for transient ischemic attack
• Present within 7 days of onset.
Exclusion Criteria
• The recent episode lasted more than 1 h but <4.5 h upon arrival at the hospital
• Pre-stroke mRS ≥ 2
• History of severe bleeding or major surgery within 30 days
• Concomitant terminal illness with a life expectancy ≤12 months
• Any known hereditary or acquired hemorrhagic diathesis, coagulation factor deficiency, or intake of oral anticoagulants with INR > 1.7
• Platelet count <100,000/mm3
• Hemoglobin <10 g/dl
• Serum glucose level ≤50 mg/dl
• Severe renal insufficiency with creatinine clearance rate <30 ml/min
• Patients or their relatives refuse to or not available for MRI perfusion test
• Uncooperative patients in 3-month follow-up.
Recruitment and Consent
Patients suspected of clinical TIA will be recruited by attending general practitioners or neurologists at the time they present to the neurology clinic. They and/or their relatives will be asked whether they agree to be contacted by researchers regarding the possibility of participating in this study. If the patients and/or their relatives agree to do so, their eligibility will be assessed, and the detailed procedures of this study will be explained to them. Lastly, written informed consent will be obtained from them if they are eligible for this study.
Procedures
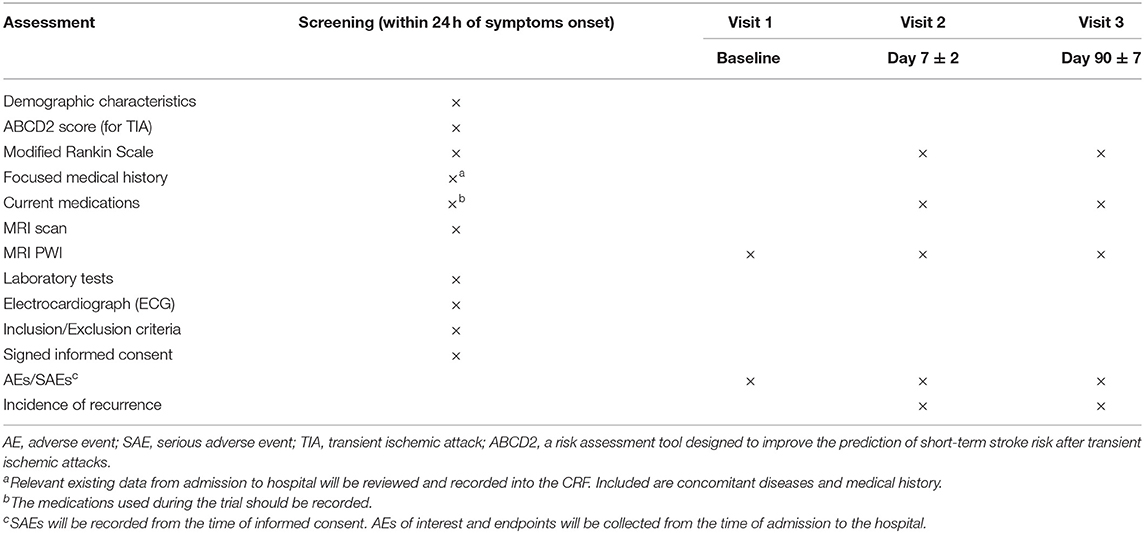
Ninety patients will be recruited in the next 1.5 years based on the calculation of the authors. Baseline assessments will be completed when the participants visit the neurology clinic of Shanghai Fourth People's Hospital or Tongji Hospital. The first PWI will be completed within 24 h after onset, and the subsequent PWI will be completed on the 7th day ± 2. Three visits to the hospital are required, which include the first visit for randomization (baseline) and the next two for repeat PWI on day 7 ± 2 and follow-up on 90 ± 7 days. The incidence of recurrent vascular events will be collected from all the patients on the second and third visits. Meanwhile, etiological subtypes (large artery occlusive disease, small vessel disease, cardioembolism, etc.) should also be considered for reasons of early recurrent stroke (Purroy et al., 2007). Baseline and follow-up assessments are summarized in Table 1.
MRI Protocol
MRI is performed using a 1.5- or 3.0-T Avanto scanner (Siemens, Erlangen, Germany). The imaging protocol consists of DWI, PWI, FLAIR, and MRA. Imaging parameters are listed below. Axial EPI-DWI: TR = 3,600 ms, TE = 102 ms; FOV = 2.3 cm2, b value = 0 and 1,000 s/mm2; EPI factor = 192; matrix size = 192 × 192; bandwidth = 964 Hz/pixel; 19 slices, slice thickness = 5.5 mm. Axial FLAIR: TR = 4,000 ms, TE = 92 ms; FOV = 2.3 cm2; TI = 1532.6 ms; matrix size = 256 × 190; bandwidth = 190 Hz/Px; 18 slices, slice thickness = 5.5 mm. Axial EPI-PWI: TR = 1,590, TE = 32 ms; measurements = 50; FOV = 2.3 cm2; matrix size = 128 × 128; band width = 1,346 Hz/pixel; 19 slices, slice thickness = 5.5 mm. Gd-DTPA contrast agent (gadopentetate dimeglumine; Shanghai Pharmaceutical Corporation, Shanghai, China) is intravenously injected (0.2 mmol/kg body weight) at a rate of 4 ml/s after flushing with 30 ml saline. Time-of-flight MRA: TR = 25 ms, TE = 7 ms; FOV = 1.8 cm2; matrix size = 241 × 256; bandwidth = 100 Hz/PX; slice thickness =0.7 mm. Ischemic lesion was localized by taking clinical manifestations of patients with TIA into consideration.
Estimates of the volume of hypoperfusion on MRI perfusion scans are performed using the RAPID software, which is an automated image post-processing software (Rothwell et al., 2007; Wang et al., 2019a). Lesion volume of Tmax ≥ 4 s ≥ 10 ml is used for determining perfusion deficits in patients with TIA (Liu et al., 2007; Najib et al., 2019). Two experienced radiologists independently evaluated the presence of acute ischemic lesions on DWI/PWI.
Sample Size and Power Calculation
Two-sided chi-square test is performed at P = 0.05 (alpha) to compare the incidence of recurrent vascular events between patients with TIA with PWI-positive and PWI-negative findings. Previous studies have shown that 10% of patients with TIA will relapse; however, the pilot study showed that 34.8% of PWI-positive patients with TIA had recurrent TIA. With the testing power of 80%, the significance level of 0.05 (two-sided), and a 1:1 ratio, with a dropout rate of 5%, a total of 90 patients are needed to detect the difference in relative risks between patients with TIA with PWI negative and PWI positive findings.
Statistical Analysis
The proportion of patients with recurrent vascular events on days 7 and 90 will be presented as frequency (percentage). Continuous variables will be presented as mean ± SD or median with interquartile range (IQR). Categorical parameters are presented as independent proportions. Baseline information of patients with or without hypoperfusion abnormalities is compared using the Mann-Whitney U-test or t-test for continuous variables and Fisher's exact test or χ2 for categorical variables. Logistic regression analysis will be performed to compare the proportion of recurrence between the two groups. P < 0.05 (two-sided) is considered statistically significant. Data analysis will be performed using IBM SPSS (version 20.0) for Windows (SPSS Inc., Chicago, IL, USA). The Data and Safety Monitoring Board will conduct an interim analysis on 45 patients (half of the required sample size). P < 0.05 is considered statistically significant in the interim and final analyses.
Discussion
Based on a multi-centered community epidemiological study on TIA in Chinese adults, the prevalence of TIA in the Chinese population is as high as 2.4%. The number of patients with TIA in China is as high as 10–12 million (Liu et al., 2007). MRI-based hypoperfusion in patients with TIA is closely related to the time of completing MR imaging. A recent study has shown that hypoperfusion, detected by MRI, is present in 42.4% (25/59) of patients with TIA, and the median (IQR) time of symptom onset to baseline MR-PWI was 5 (4–9) days (Wang et al., 2019a). This is similar to the results of a previous study that has demonstrated the presence of hypoperfusion in 42% (57/137) of patients with TIA, with a median time of 9.2 h from symptom onset to completing MR-PWI (Asdaghi et al., 2013). When the time from symptom onset to the first PWI was shortened, such as a median time of 287 (136–591) min, the presence of hypoperfusion decreased to 25% (16/64) (Lee et al., 2017).
A recent study has shown an incidence of 7.4% of 90-day recurrence of stroke after TIA (Najib et al., 2019). There are multiple potential clinical risk factors for MR perfusion abnormality in the context of TIA. Insufficient local tissue perfusion may be the cause of recurrent TIA (Wang et al., 2019a,b).
This study has its own limitations. First, all the patients were recruited from the same city (Shanghai), and there may be bias in-patient selection. Second, the number of participants is relatively small. Third, treatments for patients with TIA might be slightly different, which will influence the likelihood of TIA recurrence.
The REATTACK trial will provide the power of predicting recurrent vascular events on days 7 and 90 based on hypoperfusion observed on PWI, which is completed within 24 h after symptom onset. This will guide the implementation of effective therapeutics for secondary prevention of TIA and/or stroke.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of Shanghai Fourth People's Hospital and Tongji Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YW and HL participated in the design and coordination of the study and drafted the manuscript. LJ designed the study and revised the manuscript for important intellectual content. SW conceived and designed the study and supervised the entire process. All the authors read and approved the final manuscript.
Funding
This study was supported by a grant from the Health and Family Planning Commission of Hongkou District, Shanghai (Hongkou Health 1902-05). The funder did not participate in the design of the study, collection, analysis, and interpretation of data, and writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the general practitioners of community hospitals and neurologists of Shanghai Fourth People's Hospital and Tongji Hospital for their willingness to help with patient recruitment.
Abbreviations
CIs, confidence intervals; CTA, computed tomography angiography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; IQR, interquartile range; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; mRS, modified ranking scale; MTT, mean transit time; PWI, perfusion-weighted imaging; TIA, transient ischemic attack; Tmax, time-to-maximum of residue function; TTP, time to peak.
References
Amarenco, P., Labreuche, J., Lavallee, P. C., Meseguer, E., Cabrejo, L., Slaoui, T., et al. (2009). Does ABCD2 score below 4 allow more time to evaluate patients with a transient ischemic attack? Stroke 40, 3091–3095. doi: 10.1161/STROKEAHA.109.552042
Amarenco, P., Lavallee, P. C., Labreuche, J., Albers, G. W., Bornstein, N. M., Canhao, P., et al. (2016). One-year risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 374, 1533–1542. doi: 10.1056/NEJMoa1412981
Asdaghi, N., Hameed, B., Saini, M., Jeerakathil, T., Emery, D., and Butcher, K. (2011). Acute perfusion and diffusion abnormalities predict early new MRI lesions 1 week after minor stroke and transient ischemic attack. Stroke 42, 2191–2195. doi: 10.1161/STROKEAHA.110.611376
Asdaghi, N., Hill, M. D., Coulter, J. I., Butcher, K. S., Modi, J., Qazi, A., et al. (2013). Perfusion MR predicts outcome in high-risk transient ischemic attack/minor stroke: a derivation-validation study. Stroke 44, 2486–2492. doi: 10.1161/STROKEAHA.111.000208
Easton, J. D., Saver, J. L., Albers, G. W., Alberts, M. J., Chaturvedi, S., Feldmann, E., et al. (2009). Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40, 2276–2293. doi: 10.1161/STROKEAHA.108.192218
Giles, M. F., and Rothwell, P. M. (2010). Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke 41, 667–673. doi: 10.1161/STROKEAHA.109.571174
Grams, R. W., Kidwell, C. S., Doshi, A. H., Drake, K., Becker, J., Coull, B. M., et al. (2016). Tissue-negative transient ischemic attack: is there a role for perfusion MRI? AJR Am. J. Roentgenol. 207, 157–162. doi: 10.2214/AJR.15.15447
Kelly, P. J., Albers, G. W., Chatzikonstantinou, A., De Marchis, G. M., Ferrari, J., George, P., et al. (2016). Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol. 15, 1238–1247. doi: 10.1016/S1474-4422(16)30236-8
Kim, A. S., Easton, J. D., and Johnston, S. C. (2016). Risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 375, 386–387. doi: 10.1056/NEJMc1606657
Kleinman, J. T., Zaharchuk, G., Mlynash, M., Ogdie, A. A., Straka, M., Lansberg, M. G., et al. (2012). Automated perfusion imaging for the evaluation of transient ischemic attack. Stroke 43, 1556–1560. doi: 10.1161/STROKEAHA.111.644971
Lavallee, P. C., Meseguer, E., Abboud, H., Cabrejo, L., Olivot, J. M., Simon, O., et al. (2007). A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 6, 953–960. doi: 10.1016/S1474-4422(07)70248-X
Lee, J., Inoue, M., Mlynash, M., Mann, S. K., Cereda, C. W., Ke, M., et al. (2017). MR perfusion lesions after TIA or minor stroke are associated with new infarction at 7 days. Neurology 88, 2254–2259. doi: 10.1212/WNL.0000000000004039
Liu, M., Wu, B., Wang, W. Z., Lee, L. M., Zhang, S. H., and Kong, L. Z. (2007). Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 6, 456–464. doi: 10.1016/S1474-4422(07)70004-2
Mlynash, M., Olivot, J. M., Tong, D. C., Lansberg, M. G., Eyngorn, I., Kemp, S., et al. (2009). Yield of combined perfusion and diffusion MR imaging in hemispheric TIA. Neurology 72, 1127–1133. doi: 10.1212/01.wnl.0000340983.00152.69
Najib, N., Magin, P., Lasserson, D., Quain, D., Attia, J., Oldmeadow, C., et al. (2019). Contemporary prognosis of transient ischemic attack patients: a systematic review and meta-analysis. Int. J. Stroke. 14, 460–467. doi: 10.1177/1747493018823568
Olivot, J. M., Mlynash, M., Thijs, V. N., Kemp, S., Lansberg, M. G., Wechsler, L., et al. (2008). Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE). Stroke 39, 2257–2263. doi: 10.1161/STROKEAHA.107.511535
Olivot, J. M., Mlynash, M., Thijs, V. N., Kemp, S., Lansberg, M. G., Wechsler, L., et al. (2009). Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke 40, 469–475. doi: 10.1161/STROKEAHA.108.526954
Purroy, F., Montaner, J., Molina, C. A., Delgado, P., Ribo, M., and Alvarez-Sabín, J. (2007). Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 38, 3225–3229. doi: 10.1161/STROKEAHA.107.488833
Rothwell, P. M., Giles, M. F., Chandratheva, A., Marquardt, L., Geraghty, O., Redgrave, J. N., et al. (2007). Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 370, 1432–1442. doi: 10.1016/S0140-6736(07)61448-2
Wang, Y., Liang, H., Luo, Y., Zhou, Y., Jin, L., Wang, S., et al. (2019a). History of Hypertension Is Associated With MR Hypoperfusion in Chinese Inpatients with DWI-Negative TIA. Front. Neurol. 10:867. doi: 10.3389/fneur.2019.00867
Wang, Y., Xiao, J., Luo, Y., Wang, S., Liang, H., and Jin, L. (2019b). Risk factors of perfusion and diffusion abnormalities on MRI in hemispheric TIA: a case-control study. Ann. Transl. Med. 7:808. doi: 10.21037/atm.2019.12.69
Wang, Y., Zhao, X., Jiang, Y., Li, H., Wang, L., Johnston, S. C., et al. (2015). Prevalence, knowledge, and treatment of transient ischemic attacks in China. Neurology 84, 2354–2361. doi: 10.1212/WNL.0000000000001665
Keywords: transient ischemic attack, hypoperfusion, magnetic resonance perfusion, prospective, cohort, recurrence
Citation: Wang Y, Liang H, Jin L and Wang S (2021) Power of Hypoperfusion in Predicting Recurrent Transient Ischemic Attacks: Protocol of a Prospective Cohort Study. Front. Hum. Neurosci. 15:654383. doi: 10.3389/fnhum.2021.654383
Received: 16 January 2021; Accepted: 19 May 2021;
Published: 24 June 2021.
Edited by:
Teresa Liu-Ambrose, University of British Columbia, CanadaReviewed by:
Hüseyin Akan, Ondokuz Mayis University, TurkeyYorito Hattori, National Cerebral and Cardiovascular Center, Japan
Copyright © 2021 Wang, Liang, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingjing Jin, bGluZ2ppbmdqaW5AMTYzLmNvbQ==; Shaoshi Wang, d2FuZ3NoYW9zaGlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Yue Wang
Yue Wang Huazheng Liang
Huazheng Liang Lingjing Jin
Lingjing Jin Shaoshi Wang
Shaoshi Wang