
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Hum. Neurosci. , 02 March 2021
Sec. Brain Imaging and Stimulation
Volume 15 - 2021 | https://doi.org/10.3389/fnhum.2021.612891
This article is part of the Research Topic Does Electrical Stimulation Map Brain Function? View all 14 articles
Direct electrical stimulation (DES) has been widely applied in both guidance of lesion resection and scientific research; however, the design and selection of intraoperative cognitive mapping tasks have not been updated in a very long time. We introduce updated mapping tasks for language and non-language functions and provide recommendations for optimal design and selection of intraoperative mapping tasks. In addition, with DES becoming more critical in current neuroscientific research, a task design that has not been widely used in DES yet (subtraction and conjunction paradigms) was introduced for more delicate mapping of brain functions especially for research purposes. We also illustrate the importance of designing a common task series for DES and other non-invasive mapping techniques. This review gives practical updated guidelines for advanced application of DES in clinical and neuroscientific research.
The persevering efforts of Penfield (Penfield and Boldrey, 1937; Penfield, 1958), Ojemann (Ojemann et al., 1989), Duffau (Tate et al., 2014; Rech et al., 2019), Berger (Sanai et al., 2008), and other neurosurgeons (Roux et al., 2003, 2004; Démonet et al., 2011; Wu et al., 2011) in awake surgery coupled with direct electrical stimulation (DES) led to the wide application of DES in identifying the “eloquent area” before lesion resection due to its high effectiveness and reliability. Accumulating evidence lent support to the importance of this technique, which aided in achieving more extensive resection with lower permanent neurological deficits (Sacko et al., 2011; De Witt Hamer et al., 2012; Gerritsen et al., 2019). Apart from clinical application, DES also provides a tremendous opportunity for neurosurgeons to step into neuroscientific research. Unlike traditional lesion studies, DES disturbs temporarily and reversibly the neural activity in a limited brain area or neural network. This method largely avoids the influence of functional reorganization after focal brain damage and provides a causal link between neural substrates and cognitive functions (Desmurget et al., 2013; Vaidya et al., 2019). Following in the footsteps of Penfield’s famous cortical homunculus research, other higher-order cognitive functions have been investigated using DES in the past decades pushing forward the frontiers of neuroscience (Sanai et al., 2008; Tate et al., 2014; Rech et al., 2019).
With the increasing popularity of DES in both clinical and scientific research, multiple teams have developed their own intraoperative cognitive mapping tasks, and the brain functions they mapped extend from sensorimotor to language and even non-language functions (Duffau, 2010). However, the details of task design and selection were rarely reported, leading to potential heterogeneity between institutions in mapping results and the efficacy of electrical stimulation. Therefore, a practical and standardized protocol for intraoperative task design and selection is needed in awake surgery. Moreover, with the frontier of neuroscience expanding, traditional tasks no longer fulfill the needs of current research; thus, the design of more complicated mapping tasks has become an important issue.
In this paper, we first summarize the electrical stimulation parameters (current intensity, stimulation duration, stimulation onset) used in DES. Next, we introduce popular tasks for the mapping of cognitive function and further propose a protocol for task selection to guide lesion resection. In addition, recent neuroscientific findings based on DES were reviewed, and an optimal way to design a delicate mapping task series for research purposes was demonstrated. With the increasing popularity of multimodal fusion in both clinical and scientific research, the importance of common task series for different mapping techniques was proposed.
Current intensity is one of the most important parameters that affects the sensitivity and specificity of DES. During cortical mapping, a bipolar electrode is used to deliver 1 ms (0.5 ms for anodal phase and another 0.5 ms for cathodal phase) biphasic square waves at 60 Hz, which facilitates the excitement of neural cells parallel to the bipolar axis. There are two main mapping strategies with distinct current intensities. Some researchers adopt a “negative mapping strategy” in which the amplitude for each cortical site is increased from 2 mA to a predetermined threshold (usually 6 mA) or until an after discharge is evoked (Sanai et al., 2008; Sarubbo et al., 2013). During cognitive mapping, the current intensity was 0.5–1 mA lower than the intensity determined in motor mapping (Sanai et al., 2008; Sarubbo et al., 2013; Hervey-Jumper et al., 2015). After brain mapping, tumor resection was guided by the “negative sites” that referred to the cortical areas without stimulation-induced motor or language function. Therefore, a minimal cortical exposure was allowed in this strategy. Another mapping strategy, which was used in our institute, focused more on the identification of positive cortical sites. The stimulation also began with the localization of primary sensory or motor area, but the current intensity increased gradually until the detection of sensory or motor changes. This intensity was then used for the cognitive mapping. Even though a larger cortical exposure was needed, more positive sites could be identified with this strategy, which made it more popular in current neuroscientific researches (Duffau et al., 2004; Khan et al., 2014; Tate et al., 2014; Sarubbo et al., 2020). Since the frequency was slightly different among institutes, the charge density/phase is a reliable parameter to describe the current intensity (Gordon et al., 1990). This parameter was widely used in animal studies (Agnew et al., 1983; Agnew and McCreery, 1987) or extraoperative stimulation (Elisevich et al., 2006; Cogan et al., 2016); however, it was rarely used in studies about awake surgery. Gordon et al. (1990) investigated the histological change within the proximity of electrode in the human brain, but the charge density/phase was lower than that commonly applied during intraoperative DES and the influence of electrical stimulation remained unknown. Therefore, further studies are needed to settle the safe threshold of charge density/phase.
Although numerous studies have used DES to reveal the functions of white matter tracts, rarely has a study provided detailed analysis of the current threshold of subcortical mapping (Bello et al., 2007; Duffau et al., 2008; Zemmoura et al., 2015; Puglisi et al., 2019). Most stated that the same intensity for cortical mapping was also used for subcortical mapping (Bello et al., 2007; Fujii et al., 2015; Puglisi et al., 2019). Even though the underlying neurophysiological mechanisms remains unknown and no clinical research is available to verify the efficacy, this strategy is now the only practical way to set current intensity for subcortical mapping and therefore is also the strategy adopted by our institute.
Another stimulation parameter needed to consider is the time course. Normally, the stimulation lasts 1 s for language and other non-language mapping and is applied slightly earlier than stimulus onset. More recently, Morshed et al. (2020) proposed that prolonged stimulation duration resulted in higher sensitivity of DES. With the identification of time course of human cognitive activities, other studies have also attempted to change the temporal relation between electrical stimulation and stimulus to interfere a specific physiological stage (Nakai et al., 2019; Morshed et al., 2020). Further studies are needed on these new mapping protocols.
Currently, a majority of the DES carried out clinically are performed to locate language areas in the dominant hemisphere. A set of common language mapping tasks (counting, picture naming, reading) has been used since the era of Penfield (Penfield and Boldrey, 1937; Penfield, 1958), with the detailed design and selection of these tasks varying between institutes and countries.
Counting is an automatic speech task that is widely used in language assessment especially in patients who cannot complete complex cognitive tasks (Bookheimer et al., 2000). During language mapping, number counting is carried out first to identify cortical and subcortical structures related to speech output (Fernandez Coello et al., 2013; Mandonnet et al., 2017). The patients are asked to count from 1 to 50, and an interruption without movement (i.e., speech arrest) can be induced when stimulating the ventral premotor cortex (Sanai et al., 2008; Tate et al., 2014; Wu et al., 2015; Sarubbo et al., 2020), inferior frontal gyrus (Sanai et al., 2008; Wu et al., 2015), or area 55b (Rech et al., 2019), as well as white matter fibers including frontal aslant tract (Kinoshita et al., 2015) and arcuate fasciculus (Duffau, 2015; Sarubbo et al., 2020). Dysarthria is another disruption that is elicited during counting. Dysarthria-related sites are distributed within the lateral precentral and postcentral gyrus over the underlying white matter tracts (e.g., superior longitudinal fasciculus-III) (Tate et al., 2014; Duffau, 2015; Sarubbo et al., 2020) that participate in the articulatory network.
Picture naming is one of the most widely used tasks in language mapping. Most institutions follow the protocol that was first used byPenfield and Boldrey (1937) and Penfield (1958), and further described in detail by Ojemann and Mateer (1979). In this task, pictures of common objects are presented to patients one by one, and each is shown for 4 s. Patients need to name the object with a carrier phrase “This is a …” as soon as possible.
One major advantage of the picture naming task is that it is a “multifunctional” task that can evaluate multiple brain functions including visual recognition, conceptual formation, lexical retrieval, and the final speech output (Gleichgerrcht et al., 2015). Errors can be induced by the disruption of any single stage in naming processing. The most common language interference found in this task is anomia, which is defined as the DES-induced phenomenon in which the patient is able to speak out the carrier phrase but unable to name the object (Sanai et al., 2008) or name with incorrect words. Other errors [e.g., perseveration (Duffau et al., 2005, 2008; Gil Robles et al., 2005; Khan et al., 2014; Mandonnet et al., 2019b), hesitation (Hamberger et al., 2005), and tip-of-the-tongue (Hamberger et al., 2005)] are also reported in the literature. In addition, since speech output can also be assessed by this task, some researchers map speech arrest during picture naming instead of counting (Petrovich Brennan et al., 2007). DES-induced speech arrest can be distinguished from anomia through the inability of saying the carrier phase. Figure 1 shows an illustrative case of direct electrical mapping with picture naming task.
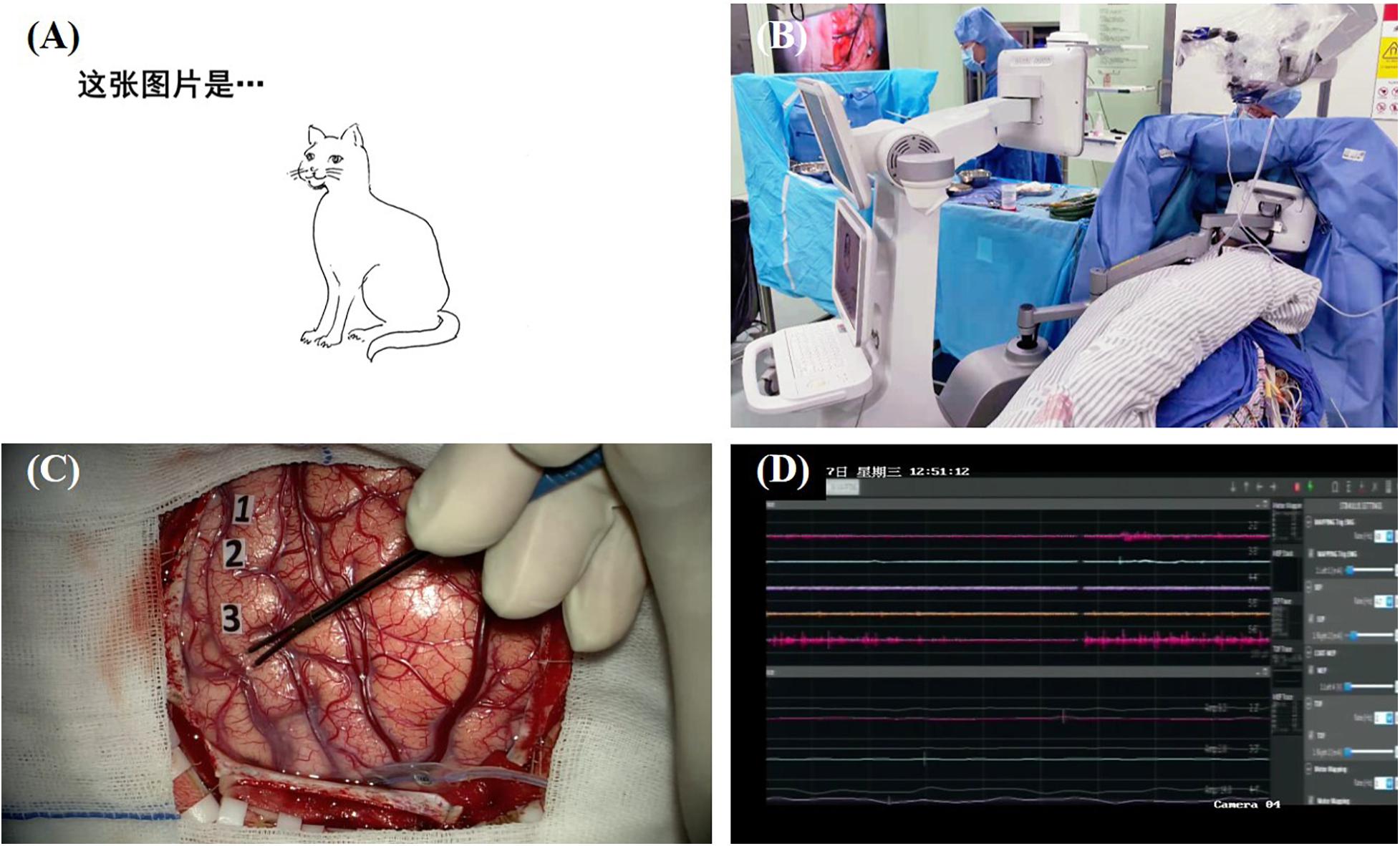
Figure 1. Illustration of direct electrical mapping with picture naming task. (A) A series of black-and-white line drawing of a common object is used as stimulating materials; (B) a specially designed machine is used to present the stimulus with a moveable screen [more details about this machine on our forthcoming article (Hameed et al., 2021)]; (C) neurosurgeon applies electrical stimulations with a bipolar electrode and uses sterile tags to mark the positive sites; (D) electrophysiological monitoring is used to record after-discharge activity.
Although task protocol and the definition of DES-induced errors are relatively consistent, stimulating materials vary among different institutes, and only a few studies report the source of the pictures they used. Compared to image sets found randomly from the Internet, usage of batteries and image sets with normative data available is more recommended. Duffau et al. (2005); Mandonnet et al. (2017, 2019b), and Rech et al. (2019) select pictures from DO 80, which comprises of 80 black-and-white pictures with variables such as frequency, familiarity, and age of acquisition controlled. Some teams advocate the application of the Boston Naming test, which contains 60 line drawings with decreasing frequency. However, both our experience and previous literature (Chen et al., 2014) has revealed that this test contains several objects with relatively low frequency in Chinese (e.g., igloo, harp), which causes a higher probability of false positive. Therefore, cultural adjustment is needed in intraoperative task design. In our institute, the Snodgrass and Vanderwart picture set (Snodgrass and Vanderwart, 1980) is adopted, since the test contains 260 easily recognizable drawings of common objects from different categories and, more importantly, has norms for Mandarin Chinese available (Liu et al., 2011). For teams from other countries, the International Picture-Naming Project (IPNP) provides norms in seven different languages (American English, German, Mexican Spanish, Italian, Bulgarian, Hungarian, and Chinese) for a corpus of 520 drawings including those from the Snodgrass and Vanderwart picture set. Thus, this picture set showed a distinct advantage in language mapping.
In addition, another important point is the selection of drawings. If the difficulty of a naming task is too high, some intraoperative naming errors may not be directly caused by electrical stimulation and further lead to false positive readings. Thus, Little and Friedman (2004) proposed that patients should be trained preoperatively, and pictures that could not be named by most patients should be removed from the intraoperative task. However, no quantitative parameters like frequency, age of acquisition, and familiarity are used in their selection criteria, which deteriorates its practical value for other institutes. Previous psycholinguistic research have demonstrated that familiarity and age of acquisition are two major predictors for naming latencies (Weekes et al., 2007; Liu et al., 2011); thus, we recommend that only pictures with high concept familiarity and low age of acquisition should be included into intraoperative tasks to ensure the reliability of language mapping. Further studies are still needed to determine the cutoff of these parameters in our new selection criteria.
Besides these two major tasks (counting and picture naming), there are several other language mapping tasks reported in the literature. Several studies demonstrated that stimulating the posterior temporal lobe (Sanai et al., 2008) and inferior parietal lobule (Roux et al., 2004; Sanai et al., 2008; Wu et al., 2015) could induce alexia in word reading task. Tasks such as repetition (Fernandez Coello et al., 2013; Mandonnet et al., 2017; Sarubbo et al., 2020) and writing (Roux et al., 2003; Morrison et al., 2016) are also performed according to the tumor location (e.g., applying repetition task to identify arcuate fasciculus; Moritz-Gasser and Duffau, 2013). Moreover, for multilingual patients, a sentence translation task could be used to protect their function of switching between multiple languages.
In a recent review, Rofes and Miceli (2014) emphasized the importance of tasks with verbs and sentences in language mapping because the daily language activity poses far more demands on the cognitive network than simple automatic speech and picture naming tasks applied in awake surgery. More sophisticated tasks (e.g., auditory naming, sentence comprehension, completion, and reading) could be designed and added into language mapping procedure. These tasks could engage speech output, as well as conceptual formation and lexical retrieval in picture naming tasks, and in addition, higher level language processing such as syntax could also be tackled. Although these tasks are currently rarely applied in clinical setting due to their complexity, these tasks shed light on the sophisticated human language activity.
Despite the increasing application of DES by neurosurgeons in the past few decades (Sanai et al., 2008; De Witt Hamer et al., 2012; Tate et al., 2014; Rech et al., 2019), a majority of cases enrolled are those with lesions within or near the language areas in dominant hemisphere, and only language tasks are applied routinely during surgery. Recently, studies have started focusing on other non-language functions that also have a strong relation with postoperative quality of life (Duffau et al., 2004; Duffau, 2010; Gras-Combe et al., 2012; Fox et al., 2020).
Visual processing is another important neurological ability used for perceiving the surrounding environment. Previous studies based on non-invasive methods such as functional MRI (fMRI), lesion studies, and electroencephalography have proposed a dual stream network of visual processing. Like language deficits, disruptions in this network may lead to failure of sensory awareness of visual stimulus and interfere with daily activities. In order to protect such a critical function, tasks are added to detect the structures related to visual processing.
Permanent visual field deficit due to disruption of optic radiation is a common postoperative complication of patients with lesions in temporal or occipital lobe. Despite the application of multimodal neuroimaging in visual protection, direct mapping of optic radiation was not achieved by these non-invasive methods. Duffau et al. (2004) first reported that electrical stimulation of optic radiations induced visual disorder during picture naming task in a patient with lesions invading the temporal lobe and the temporo-occipital junction. An intraoperative visual task was later introduced in Gras-Combe et al. (2012). In this task, patients are asked to name the two pictures located diagonally on the screen with their eyes fixed on the center. They reported that all the 14 patients experienced visual symptoms during awake surgery; thus, optic radiations were identified and protected with only one patient having permanent postoperative hemianopia. Recently, an integrated method of visual pathway tracking using both diffusion tensor imaging and DES was reported, indicating the potential advantages of multimodal brain mapping (Mazerand et al., 2017).
Another deficit in visual processing is spatial neglect. Unlike visual field deficit, spatial neglect is an attentional deficit in which the patient has an intact visual field but ignores part of the visual space. Even though this phenomenon has gained the attention of neurologists and there are extensive number of assessments available, few studies report on a method to protect visuospatial function during neurosurgery. The main reason for this is that visuospatial function is related to non-dominant hemisphere whose cognitive role has long been ignored. Some pioneers have proposed the line bisection task, which could be used to map visuospatial function. In this task, patients are asked to mark the midpoint of a line with a pen or finger. Some researchers have successfully identified that spatial neglect in line bisection task could be induced when electrically stimulating the inferior parietal lobule and the posterior temporal lobe (Thiebaut de Schotten et al., 2005; Démonet et al., 2011; Velasquez et al., 2018), which is consistent with fMRI results. Démonet et al. (2011) reported that no postoperative spatial neglect was found in all 20 cases whose positive sites of line bisection errors were spared, indicating the clinical value of line bisection task. Other neuropsychological tests including clock test and judgment of line orientation for visuospatial function could also be used in awake surgery. Compared to the line bisection task, these tasks are easier to complete during surgery since no hand movement is needed. However, these new tasks have not been widely applied yet, and further investigation is needed to illustrate the reliability of these tasks in DES for minimizing the postoperative spatial neglect.
Compared to visual field and visuospatial function, other higher cognitive functions (e.g., calculation, working memory, music) have received lesser attention during surgery. However, these functions are also closely related to patients’ quality of life. For example, arithmetic processing is an indispensable brain function for a mass of daily activities, and recently, a few teams have begun using calculation task to prevent permeant acalculia (Duffau et al., 2002; Della Puppa et al., 2013; Della Puppa et al., 2015; Matsuda et al., 2019). Another important issue in mapping higher cognitive functions is that patients have differing demands of their postoperative functions based on their jobs and lifestyles. We have previously reported on a music student with glioma in Broca’s area (Zhang et al., 2013). In order to protect her musical ability, two music tasks including humming a popular Chinese song and reading a piece of musical note were performed after routine language mapping. During surgery, two music interference sites were identified and spared. Postoperative evaluation showed that her music function had surprisingly improved slightly relative to the preoperative level.
In addition to the cognitive functions mentioned above, studies have also attempted to locate domain-general cognitive networks involved in emotion and working memory. Thus, more complex tasks like emotion (Gordon et al., 1996; Fox et al., 2020), Stroop test (Wager et al., 2013; Puglisi et al., 2019), and N-back test (Motomura et al., 2018, 2019) were introduced into awake surgery. Fox et al. (2020) showed that stimulation in the limbic system could elicit different emotion responses (e.g., joy, nervousness). For the N-back task, errors could be elicited when stimulating the dorsolateral prefrontal cortex (Motomura et al., 2018, 2019). It is important to note that in most studies (Démonet et al., 2011; Fox et al., 2020), these higher cognitive function tasks are performed solely for scientific purpose instead of function preservation, and the identified neural substrates were resected even though positive sites existed. Therefore, further studies, especially those with large cohorts, are needed to settle their practicability in clinical contexts.
Varying cognitive mapping tasks available for different cognitive domains are accumulating with the increasing application of DES. However, the maximum duration that patients can stay conscious and complete mapping tasks is about 1 h based on our and others experience (Mandonnet et al., 2019a), which means that tasks used in surgery is tightly limited. Under these circumstances, tasks that integrate multiple cognitive domains are recommended more since several cerebral functions can be evaluated simultaneously.
During tasks selection, the first thing to take into account is the patient’s basic information, including job, hobbies, lifestyles, and, more importantly, their preoperative cognitive function. Thus, a set of detailed neuropsychological assessments should be performed to set a baseline for each patient. In our institute, every patient is assessed pre- and postoperatively using a series of comprehensive batteries, including a questionnaire for personal information, the Edinburgh Handedness Inventory for handedness, the Mini-Mental State Examination for potential cognitive impairments, as well as the Boston Naming Test and the Aphasia Battery of Chinese for language function. Calculation and visuospatial function are also evaluated in the Aphasia Battery of Chinese. Based on the assessment results of different cognitive functions, tasks are chosen based on the preoperatively intact or mildly compromised functions deserving more attention.
Characteristics of a tumor, especially its location, is another important factor in task selection. Figure 2 illustrates the tasks recommended for lesions in different regions. In addition, the role of white matter tracts in cognitive function has been confirmed by multiple studies and Fernandez Coello et al. (2013) proposed that plasticity of white matter tracts made them more crucial in brain function protection. In neurosurgery, these tracts mark the boundary of lesion resection; thus, applying subcortical electrical stimulation to identify these fiber tracts is as important as cortical mapping. Based on the available neuroscientific research results, we present our proposed intraoperative tasks for tumors invading different white mater tracts in Figure 3.
Table 1 summarizes common language and non-language tasks that we recommend for clinical practice. Information including hemisphere, lesion location, task design, and responses are also given.
In addition to its application as a clinical tool, DES is currently a popular method in neuroscientific research due to its ability to probe the causal relation between brain functions and structures.
As mentioned above, DES is mostly performed to identify language areas; therefore, most significant results have been obtained in neurolinguistic research. In the past 20 years, tasks such as counting, picture naming, and reading have been used by different teams to construct the probabilistic maps of language areas. A preliminary consensus had been achieved that key regions for language processing include the pars triangularis, pars opercularis, and ventral precentral gyrus in frontal lobe, as well as the posterior part of temporal lobe (Sanai et al., 2008; Wu et al., 2015; Sarubbo et al., 2020). Language-related white matter tracts were also revealed for the first time using subcortical stimulation, demonstrating the structural connectivity between key language areas. Furthermore, Duffau (2015) proposed a language processing model, which illustrates the anatomical basis of dual stream model of Hickok and Poeppel (Hickok and Poeppel, 2007; Hickok, 2012).
While the general framework of language processing has been constructed, there are still controversies on the exact role that a certain brain region play. Therefore, current neurolinguistic studies focus more on a specific stage in language processing rather than the whole stream. However, traditional tasks, like picture naming, engage plenty of processing stages, and the interruption of each stage could cause dysfunctions. In most studies, despite the generation of spatial map of anomia, it is nearly impossible to identify which stage in language processing was blocked in stimulation-induced anomia. Thus, the functional role of each structure is illustrated by evidence from other modality [e.g., fMRI, electrocorticography (ECoG), lesion studies] rather than direct evidence from DES. To address this issue, some researchers adopted the cognitive subtraction and conjunction paradigms in fMRI studies. These two paradigms usually contain a series of tasks, so, as their names suggest, the subtraction paradigm aims to locate the different processing stage between tasks and the conjunction paradigm is designed to locate the shared one (Price and Friston, 1997). Forseth et al. (2018) combined these two paradigms and compared the disruption of positive sites for a series of tasks including picture naming, naming to definition, sentence repetition, and sensorimotor effects. For the first time, a solid evidence derived from DES was provided to support the role of middle fusiform gyrus as the lexical semantic hub. Another study from Lee and his colleagues also applied the same paradigm and successfully demonstrated that the morphological characteristic in syntax is attributed to a small region within the posterior part of superior temporal gyrus (Lee et al., 2018). Even though the application of this task design strategy is still in its initial stage, an increasing amount of literature using this strategy could be expected since there are abundant task series available for adaptation in previous fMRI studies.
Despite its irreplaceable role as the sole reliable method to identify the eloquent neural substrates during surgery, DES, as an invasive mapping method, cannot be applied pre- and postoperatively. Except for the rare studies based on repeated brain mapping in cases with glioma recurrence (Krieg et al., 2014; Southwell et al., 2016), findings on brain plasticity are mainly derived from non-invasive techniques including fMRI, transcranial magnetic stimulation (TMS), and positron emission tomography (PET). Several case reports have tried to investigate the perioperative reorganization of cognitive networks with both DES and other techniques (Krieg et al., 2014; Spena et al., 2015). However, the task variation between techniques in studies affects the reliability of the findings. Therefore, a common task series that could be applied for different modalities is needed for the direct comparison of eloquent structures pre-, intra-, and postoperatively. In our institute, a set of language tasks, including counting, reading, and picture naming, is used in task-fMRI, DES, as well TMS for perioperative language mapping. More tasks for other cognitive domains could also be added into this common task series in our future work. This common task series can be used to not only investigate brain plasticity but also compare the reliability of DES and other non-invasive techniques in brain mapping.
In spite of the increasing number of institutes applying DES in the clinical context, the design and selection of mapping tasks vary among institutions. In this review, we proposed a practical method for the optimization of existing mapping tasks and personalized selection of tasks based on patients’ situation. Such a universal principle in task design and selection may provide guidance for other teams and further eliminate the heterogeneity caused by tasks difference between institutions. Additionally, to match the advances in neuroscientific research, direction of task design (i.e., subtraction and conjunction paradigms) for the next era of DES studies is discussed, and the importance of combing DES and other techniques is also illustrated.
JW and LB were responsible for the execution, data collection, and manuscript writing. JZ and JL involved in data collection. JW supervised the study and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (project no. 81701289), the Shanghai Rising-Star Program (project no. 19QA1401700), the Shanghai Shenkang Hospital Development Center (SHDC12018114), the Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX01), and ZJLab.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agnew, W. F., and McCreery, D. B. (1987). Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery 20, 143–147. doi: 10.1097/00006123-198701000-00030
Agnew, W. F., Yuen, T. G., and McCreery, D. B. (1983). Morphologic changes after prolonged electrical stimulation of the cat’s cortex at defined charge densities. Exp. Neurol. 79, 397–411. doi: 10.1016/0014-4886(83)90221-2
Bello, L., Gallucci, M., Fava, M., Carrabba, G., Giussani, C., Acerbi, F., et al. (2007). Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 60, 67–80; discussion 80-62. doi: 10.1227/01.NEU.0000249206.58601.DE
Bookheimer, S. Y., Zeffiro, T. A., Blaxton, T. A., Gaillard, P. W., and Theodore, W. H. (2000). Activation of language cortex with automatic speech tasks. Neurology 55, 1151–1157. doi: 10.1212/wnl.55.8.1151
Chen, T. B., Lin, C. Y., Lin, K. N., Yeh, Y. C., Chen, W. T., Wang, K. S., et al. (2014). Culture qualitatively but not quantitatively influences performance in the Boston naming test in a chinese-speaking population. Dement. Geriatr. Cogn. Dis. Extra 4, 86–94. doi: 10.1159/000360695
Cogan, S. F., Ludwig, K. A., Welle, C. G., and Takmakov, P. (2016). Tissue damage thresholds during therapeutic electrical stimulation. J. Neural. Eng. 13:021001. doi: 10.1088/1741-2560/13/2/021001
De Witt Hamer, P. C., Robles, S. G., Zwinderman, A. H., Duffau, H., and Berger, M. S. (2012). Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 30, 2559–2565. doi: 10.1200/JCO.2011.38.4818
Della Puppa, A., De Pellegrin, S., d’Avella, E., Gioffre, G., Munari, M., Saladini, M., et al. (2013). Right parietal cortex and calculation processing: intraoperative functional mapping of multiplication and addition in patients affected by a brain tumor. J. Neurosurg. 119, 1107–1111. doi: 10.3171/2013.6.JNS122445
Della Puppa, A., De Pellegrin, S., Lazzarini, A., Gioffre, G., Rustemi, O., Cagnin, A., et al. (2015). Subcortical mapping of calculation processing in the right parietal lobe. J. Neurosurg. 122, 1038–1041. doi: 10.3171/2014.10.JNS14261
Démonet, J.-F., Lotterie, J.-A., Draper, L., Brauge, D., Boukhatem, L., Lauwers-Cances, V., et al. (2011). Electrostimulation mapping of spatial neglect. Neurosurgery 69, 1218–1231. doi: 10.1227/NEU.0b013e31822aefd2
Desmurget, M., Song, Z., Mottolese, C., and Sirigu, A. (2013). Re-establishing the merits of electrical brain stimulation. Trends Cogn. Sci. 17, 442–449. doi: 10.1016/j.tics.2013.07.002
Duffau, H. (2010). Awake surgery for nonlanguage mapping. Neurosurgery 66, 523–528; discussion 528-529. doi: 10.1227/01.NEU.0000364996.97762.73
Duffau, H. (2015). Stimulation mapping of white matter tracts to study brain functional connectivity. Nat. Rev. Neurol. 11, 255–265. doi: 10.1038/nrneurol.2015.51
Duffau, H., Denvil, D., Lopes, M., Gasparini, F., Cohen, L., Capelle, L., et al. (2002). Intraoperative mapping of the cortical areas involved in multiplication and subtraction: an electrostimulation study in a patient with a left parietal glioma. J. Neurol. Neurosurg. Psychiatry 73, 733–738. doi: 10.1136/jnnp.73.6.733
Duffau, H., Gatignol, P., Mandonnet, E., Peruzzi, P., Tzourio-Mazoyer, N., and Capelle, L. (2005). New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128(Pt 4), 797–810. doi: 10.1093/brain/awh423
Duffau, H., Peggy Gatignol, S. T., Mandonnet, E., Capelle, L., and Taillandier, L. (2008). Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J. Neurosurg. 109, 461–471. doi: 10.3171/JNS/2008/109/9/0461
Duffau, H., Velut, S., Mitchell, M. C., Gatignol, P., and Capelle, L. (2004). Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir. (Wien) 146, 265–269; discussion 269-270. doi: 10.1007/s00701-003-0199-7
Elisevich, K., Jenrow, K., Schuh, L., and Smith, B. (2006). Long-term electrical stimulation-induced inhibition of partial epilepsy. Case report. J. Neurosurg. 105, 894–897. doi: 10.3171/jns.2006.105.6.894
Fernandez Coello, A., Moritz-Gasser, S., Martino, J., Martinoni, M., Matsuda, R., and Duffau, H. (2013). Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J. Neurosurg. 119, 1380–1394. doi: 10.3171/2013.6.JNS122470
Forseth, K. J., Kadipasaoglu, C. M., Conner, C. R., Hickok, G., Knight, R. T., and Tandon, N. (2018). A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain 141, 2112–2126. doi: 10.1093/brain/awy120
Fox, K. C. R., Shi, L., Baek, S., Raccah, O., Foster, B. L., Saha, S., et al. (2020). Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain. Nat. Hum. Behav. 4, 1039–1052. doi: 10.1038/s41562-020-0910-1
Fujii, M., Maesawa, S., Motomura, K., Futamura, M., Hayashi, Y., Koba, I., et al. (2015). Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca’s area in the dominant hemisphere of patients with glioma. J. Neurosurg. 122, 1390–1396. doi: 10.3171/2014.10.JNS14945
Gerritsen, J. K. W., Vietor, C. L., Rizopoulos, D., Schouten, J. W., Klimek, M., Dirven, C. M. F., et al. (2019). Awake craniotomy versus craniotomy under general anesthesia without surgery adjuncts for supratentorial glioblastoma in eloquent areas: a retrospective matched case-control study. Acta Neurochir. 161, 307–315.
Gil Robles, S., Gatignol, P., Capelle, L., Mitchell, M. C., and Duffau, H. (2005). The role of dominant striatum in language: a study using intraoperative electrical stimulations. J. Neurol. Neurosurg. Psychiatry 76, 940–946. doi: 10.1136/jnnp.2004.045948
Gleichgerrcht, E., Fridriksson, J., and Bonilha, L. (2015). Neuroanatomical foundations of naming impairments across different neurologic conditions. Neurology 85, 284–292. doi: 10.1212/WNL.0000000000001765
Gordon, B., Hart, J., Lesser, R. P., and Arroyo, S. (1996). “Chapter 37 Mapping cerebral sites for emotion and emotional expression with direct cortical electrical stimulation and seizure discharges,” in The Emotional Motor System, eds G. Holstege, R. Bandler, and C. B. Saper (Amsterdam: Elsevier), 617–622.
Gordon, B., Lesser, R. P., Rance, N. E., Hart, J. Jr., Webber, R., Uematsu, S., et al. (1990). Parameters for direct cortical electrical stimulation in the human: histopathologic confirmation. Electroencephalogr. Clin. Neurophysiol. 75, 371–377. doi: 10.1016/0013-4694(90)90082-u
Gras-Combe, G., Moritz-Gasser, S., Herbet, G., and Duffau, H. (2012). Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J. Neurosurg. 117, 466–473. doi: 10.3171/2012.6.JNS111981
Hamberger, M. J., Seidel, W. T., McKhann, G. M. II, Perrine, K., and Goodman, R. R. (2005). Brain stimulation reveals critical auditory naming cortex. Brain 128(Pt 11), 2742–2749. doi: 10.1093/brain/awh621
Hameed, N. U. F., Zhao, Z., Zhang, J., Bu, L., Zhou, Y., Jin, L., et al. (2021). A novel intraoperative brain mapping integrated task-presentation platform. Oper. Neurosurg. doi: 10.1093/ons/opaa476
Hervey-Jumper, S. L., Li, J., Lau, D., Molinaro, A. M., Perry, D. W., Meng, L., et al. (2015). Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J. Neurosurg. 123, 325–339. doi: 10.3171/2014.10.JNS141520
Hickok, G. (2012). Computational neuroanatomy of speech production. Nat. Rev. Neurosci. 13, 135–145. doi: 10.1038/nrn3158
Hickok, G., and Poeppel, D. (2007). The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402. doi: 10.1038/nrn2113
Khan, O. H., Herbet, G., Moritz-Gasser, S., and Duffau, H. (2014). The role of left inferior fronto-occipital fascicle in verbal perseveration: a brain electrostimulation mapping study. Brain Topogr. 27, 403–411. doi: 10.1007/s10548-013-0343-5
Kinoshita, M., de Champfleur, N. M., Deverdun, J., Moritz-Gasser, S., Herbet, G., and Duffau, H. (2015). Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct. Funct. 220, 3399–3412. doi: 10.1007/s00429-014-0863-0
Krieg, S. M., Sollmann, N., Hauck, T., Ille, S., Meyer, B., and Ringel, F. (2014). Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neurosci. 15:20. doi: 10.1186/1471-2202-15-20
Lee, D. K., Fedorenko, E., Simon, M. V., Curry, W. T., Nahed, B. V., Cahill, D. P., et al. (2018). Neural encoding and production of functional morphemes in the posterior temporal lobe. Nat. Commun. 9:1877. doi: 10.1038/s41467-018-04235-3
Little, K. M., and Friedman, A. H. (2004). Awake craniotomy for malignant glioma resection. Int. Congress Series 1259, 409–414. doi: 10.1016/s0531-5131(03)01727-8
Liu, Y., Hao, M., Li, P., and Shu, H. (2011). Timed picture naming norms for Mandarin Chinese. PLoS One 6:e16505. doi: 10.1371/journal.pone.0016505
Mandonnet, E., Herbet, G., and Duffau, H. (2019a). Letter: introducing new tasks for intraoperative mapping in awake glioma surgery: clearing the line between patient care and scientific research. Neurosurgery 86, E256–E257. doi: 10.1093/neuros/nyz447
Mandonnet, E., Herbet, G., Moritz-Gasser, S., Poisson, I., Rheault, F., and Duffau, H. (2019b). Electrically induced verbal perseveration: a striatal deafferentation model. Neurology 92, e613–e621. doi: 10.1212/WNL.0000000000006880
Mandonnet, E., Sarubbo, S., and Duffau, H. (2017). Proposal of an optimized strategy for intraoperative testing of speech and language during awake mapping. Neurosurg. Rev. 40, 29–35. doi: 10.1007/s10143-016-0723-x
Matsuda, R., Tamura, K., Nishimura, F., Nakagawa, I., and Motoyama, Y. (2019). Subcortical calculation mapping during parietal glioma surgery in the dominant hemisphere: a case report. World Neurosurg. 121, 205–210. doi: 10.1016/j.wneu.2018.10.046
Mazerand, E., Le Renard, M., Hue, S., Lemee, J. M., Klinger, E., and Menei, P. (2017). Intraoperative subcortical electrical mapping of the optic tract in awake surgery using a virtual reality headset. World Neurosurg. 97, 424–430. doi: 10.1016/j.wneu.2016.10.031
Moritz-Gasser, S., and Duffau, H. (2013). The anatomo-functional connectivity of word repetition: insights provided by awake brain tumor surgery. Front. Hum. Neurosci. 7:405. doi: 10.3389/fnhum.2013.00405
Morrison, M. A., Tam, F., Garavaglia, M. M., Golestanirad, L., Hare, G. M., Cusimano, M. D., et al. (2016). A novel tablet computer platform for advanced language mapping during awake craniotomy procedures. J. Neurosurg. 124, 938–944. doi: 10.3171/2015.4.JNS15312
Morshed, R. A., Young, J. S., Lee, A. T., Berger, M. S., and Hervey-Jumper, S. L. (2020). Clinical pearls and methods for intraoperative awake language mapping. Neurosurgery [Epub ahead of print], nyaa440. doi: 10.1093/neuros/nyaa440
Motomura, K., Chalise, L., Ohka, F., Aoki, K., Tanahashi, K., Hirano, M., et al. (2018). Supratotal resection of diffuse frontal lower grade gliomas with awake brain mapping, preserving motor, language, and neurocognitive functions. World Neurosurg. 119, 30–39. doi: 10.1016/j.wneu.2018.07.193
Motomura, K., Chalise, L., Ohka, F., Aoki, K., Tanahashi, K., Hirano, M., et al. (2019). Neurocognitive and functional outcomes in patients with diffuse frontal lower-grade gliomas undergoing intraoperative awake brain mapping. J. Neurosurg. 132, 1683–1691. doi: 10.3171/2019.3.JNS19211
Nakai, Y., Sugiura, A., Brown, E. C., Sonoda, M., Jeong, J. W., Rothermel, R., et al. (2019). Four-dimensional functional cortical maps of visual and auditory language: intracranial recording. Epilepsia 60, 255–267. doi: 10.1111/epi.14648
Ojemann, G., and Mateer, C. (1979). Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science 205, 1401–1403. doi: 10.1126/science.472757
Ojemann, G., Ojemann, J., Lettich, E., and Berger, M. (1989). Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J. Neurosurg. 71, 316–326. doi: 10.3171/jns.1989.71.3.0316
Penfield, W. (1958). Some mechanisms of consciousness discovered during electrical stimulation of the brain. Proc. Natl. Acad. Sci. U.S.A. 44, 51–66. doi: 10.1073/pnas.44.2.51
Penfield, W., and Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443. doi: 10.1093/brain/60.4.389
Petrovich Brennan, N. M., Whalen, S., de Morales Branco, D., O’Shea, J. P., Norton, I. H., and Golby, A. J. (2007). Object naming is a more sensitive measure of speech localization than number counting: converging evidence from direct cortical stimulation and fMRI. Neuroimage 37(Suppl. 1), S100–S108. doi: 10.1016/j.neuroimage.2007.04.052
Price, C. J., and Friston, K. J. (1997). Cognitive conjunction: a new approach to brain activation experiments. Neuroimage 5(4 Pt 1), 261–270. doi: 10.1006/nimg.1997.0269
Puglisi, G., Howells, H., Sciortino, T., Leonetti, A., Rossi, M., Conti Nibali, M., et al. (2019). Frontal pathways in cognitive control: direct evidence from intraoperative stimulation and diffusion tractography. Brain 142, 2451–2465. doi: 10.1093/brain/awz178
Rech, F., Herbet, G., Gaudeau, Y., Mezieres, S., Moureau, J. M., Moritz-Gasser, S., et al. (2019). A probabilistic map of negative motor areas of the upper limb and face: a brain stimulation study. Brain 142, 952–965. doi: 10.1093/brain/awz021
Rofes, A., and Miceli, G. (2014). Language mapping with verbs and sentences in awake surgery: a review. Neuropsychol. Rev. 24, 185–199. doi: 10.1007/s11065-014-9258-5
Roux, F. E., Boetto, S., Sacko, O., Chollet, F., and Tremoulet, M. (2003). Writing, calculating, and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome. J. Neurosurg. 99, 716–727. doi: 10.3171/jns.2003.99.4.0716
Roux, F. E., Lubrano, V., Lauwers-Cances, V., Tremoulet, M., Mascott, C. R., and Demonet, J. F. (2004). Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain 127(Pt 8), 1796–1810. doi: 10.1093/brain/awh204
Sacko, O., Lauwers-Cances, V., Brauge, D., Sesay, M., Brenner, A., and Roux, F. E. (2011). Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery 68, 1192–1198.
Sanai, N., Mirzadeh, Z., and Berger, M. S. (2008). Functional outcome after language mapping for glioma resection. N. Engl. J. Med. 358, 18–27. doi: 10.1056/NEJMoa067819
Sarubbo, S., De Benedictis, A., Maldonado, I. L., Basso, G., and Duffau, H. (2013). Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct. Funct. 218, 21–37. doi: 10.1007/s00429-011-0372-3
Sarubbo, S., Tate, M., De Benedictis, A., Merler, S., Moritz-Gasser, S., Herbet, G., et al. (2020). Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: an original functional atlas of the human brain. Neuroimage 205:116237. doi: 10.1016/j.neuroimage.2019.116237
Snodgrass, J. G., and Vanderwart, M. (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 6, 174–215. doi: 10.1037//0278-7393.6.2.174
Southwell, D. G., Hervey-Jumper, S. L., Perry, D. W., and Berger, M. S. (2016). Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J. Neurosurg. 124, 1460–1469. doi: 10.3171/2015.5.Jns142833
Spena, G., Costi, E., Panciani, P. P., Roca, E., Migliorati, K., and Fontanella, M. M. (2015). Acute functional reactivation of the language network during awake intraoperative brain mapping. Neurocase 21, 403–407. doi: 10.1080/13554794.2014.910306
Tate, M. C., Herbet, G., Moritz-Gasser, S., Tate, J. E., and Duffau, H. (2014). Probabilistic map of critical functional regions of the human cerebral cortex: broca’s area revisited. Brain 137(Pt 10), 2773–2782. doi: 10.1093/brain/awu168
Thiebaut de Schotten, M., Urbanski, M., Duffau, H., Volle, E., Levy, R., Dubois, B., et al. (2005). Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309, 2226–2228. doi: 10.1126/science.1116251
Vaidya, A. R., Pujara, M. S., Petrides, M., Murray, E. A., and Fellows, L. K. (2019). Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 23, 653–671. doi: 10.1016/j.tics.2019.05.009
Velasquez, C., Gomez, E., and Martino, J. (2018). Mapping visuospatial and self-motion perception functions in the left parietal lobe. Neurosurg. Focus 45(VideoSuppl. 2), V8. doi: 10.3171/2018.10.FocusVid.18286
Wager, M., Du Boisgueheneuc, F., Pluchon, C., Bouyer, C., Stal, V., Bataille, B., et al. (2013). Intraoperative monitoring of an aspect of executive functions: administration of the Stroop test in 9 adult patients during awake surgery for resection of frontal glioma. Neurosurgery 72(2 Suppl. Operative), ons169–ons180; discussion ons180-161. doi: 10.1227/NEU.0b013e31827bf1d6
Weekes, B. S., Shu, H., Hao, M., Liu, Y., and Tan, L. H. (2007). Predictors of timed picture naming in Chinese. Behav. Res. Methods 39, 335–342. doi: 10.3758/bf03193165
Wu, J., Lu, J., Zhang, H., Zhang, J., Yao, C., Zhuang, D., et al. (2015). Direct evidence from intraoperative electrocortical stimulation indicates shared and distinct speech production center between Chinese and English languages. Hum. Brain Mapp. 36, 4972–4985. doi: 10.1002/hbm.22991
Wu, J. S., Zhang, J., Zhuang, D. X., Yao, C. J., Qiu, T. M., Lu, J. F., et al. (2011). Current status of cerebral glioma surgery in China. Chin. Med. J. (Engl.) 124, 2569–2577. doi: 10.3760/cma.j.issn.0366-6999.2011.17.002
Zemmoura, I., Herbet, G., Moritz-Gasser, S., and Duffau, H. (2015). New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum. Brain Mapp. 36, 2215–2230. doi: 10.1002/hbm.22766
Zhang, J., Lu, J. F., Wu, J. S., Yao, C. J., Zhuang, D. X., Qiu, T. M., et al. (2013). A unique case of Chinese language and music dissociation with tumor located in Broca’s area: multimodal mapping for tumor resection and functional preservation. Clin. Neurol. Neurosurg. 115, 2230–2233. doi: 10.1016/j.clineuro.2013.07.011
Keywords: direct electrical stimulation, cognitive function, awake surgery, neuroscience, neurophysiology
Citation: Bu L, Lu J, Zhang J and Wu J (2021) Intraoperative Cognitive Mapping Tasks for Direct Electrical Stimulation in Clinical and Neuroscientific Contexts. Front. Hum. Neurosci. 15:612891. doi: 10.3389/fnhum.2021.612891
Received: 01 October 2020; Accepted: 25 January 2021;
Published: 02 March 2021.
Edited by:
Riki Matsumoto, Kobe University, JapanReviewed by:
Tracy L. Luks, University of California, San Francisco, United StatesCopyright © 2021 Bu, Lu, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junfeng Lu, anVuZmVuZ19sdUBmdWRhbi5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.