- 1School of Electrical Engineering, Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Brain Science and Brain-Computer Interface Technology, Zhengzhou, China
- 3The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Neurosurgery, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 5Department of Automation, Tsinghua University, Beijing, China
- 6Beijing National Research Center for Information Science and Technology, Beijing, China
Recently a positive treatment effect on disorders of consciousness (DOCs) with high-definition transcranial direct-current stimulation (HD-tDCS) has been reported; however, the neural modulation mechanisms of this treatment’s efficacy need further investigation. To better understand the processing of HD-tDCS interventions, a long-lasting HD-tDCS protocol was applied to 15 unresponsive wakefulness syndrome (UWS) patients and 20 minimally conscious states (MCS) patients in this study. Ten minutes of resting-state electroencephalograms (EEGs) were recorded from the patients, and the coma recovery scale-revised scores (CRS-Rs) were assessed for each patient from four time-points (T0, T1, T2, and T3). Brain networks were constructed by calculating the EEG spectral connectivity using the debiased weighted phase lag index (dwPLI) and then quantified the network information transmission efficiency by graph theory. We found that there was an increasing trend in local and global information processing of beta and gamma bands in resting-state functional brain networks during the 14 days of HD-tDCS modulation for MCS patients. Furthermore, the increased functional connectivity not only occurred in the local brain area surrounding the stimulation position but was also present across more global brain areas. Our results suggest that long-lasting HD-tDCS on the precuneus may facilitate information processing among neural populations in MCS patients.
Introduction
Understanding the neural mechanisms of recovery processes in patients with chronic disorders of consciousness (DOCs) is a daunting challenge for modern neuroscience (Chennu et al., 2016). DOCs can be subdivided into the following based neurobehavioral assessments: comas, unresponsive wakefulness syndrome (UWS; Laureys et al., 2010), minimally conscious state (MCS; Giacino et al., 2002), and emergence of the minimally conscious state (EMCS). The MCS can be further divided into MCS minus (MCS−) and MCS plus (MCS+; Bruno et al., 2012). Previous studies have used both pharmacological and non-pharmacological interventions—such as median nerve electrical stimulation, transcranial magnetic stimulation, spinal cord stimulation, and deep brain stimulation—to treat DOCs, but few outcome measurements have been assessed in such studies (Schiff et al., 2007; Giacino et al., 2012; Della Pepa et al., 2013; Yamamoto et al., 2013; Cossu, 2014; Thibaut et al., 2014; Tucker and Sandhu, 2016).
Recently, due to the use of invasive stimulation techniques have the ethical and technical limitations, the transcranial direct-current stimulation (tDCS) has been widely investigated to improve the consciousness level of DOC patients (Zhang and Song, 2018). This technique not only could avoid surgical risks but also is lower costs for patients compared to surgical interventions (Huang et al., 2019). Specifically, with a weak current that flows through the cerebral cortex from the anode to the cathode, the cortical excitability at stimulation sites were modulated by tDCS. Anodal tDCS increases neuronal activation by sub-threshold neuronal membrane polarization, while cathodal tDCS decreases cortical excitability (Lefaucheur et al., 2017). Several previous studies have confirmed the positive effect of employing tDCS on DOCs’ clinical improvement (Bai et al., 2017; Huang et al., 2017; Zhao et al., 2017). Currently, the most commonly used stimulation target is the left DLPFC (Angelakis et al., 2014; Estraneo et al., 2017), and these inspiring results indicate tDCS seems promising for the rehabilitation of DOC patients. Besides, the posterior parietal cortex, cerebellar cortex, and precuneus have also been selected in some studies (Huang et al., 2017; Cai et al., 2019). However, it is still a long way before tDCS becoming a formal clinical application for treating DOCs.
High-definition tDCS (HD-tDCS) is a new kind of neuromodulation technique that—instead of using two large pad electrodes as in traditional tDCS—uses small and compact scalp electrodes. Compared with tDCS, HD-tDCS is more precise and results in focal neural modulation and specific behavioral changes (Dmochowski et al., 2011; Villamar et al., 2013; Shekhawat and Vanneste, 2018). HD-tDCS has been proved to be effective to improve working memory, verbal learning, motor function, and control of pain and tinnitus (Borckardt et al., 2012; Kuo et al., 2013; Donnell et al., 2015; Nikolin et al., 2015; Shekhawat et al., 2016). Although these previous results suggest beneficial effects of HD-tDCS, the neural mechanisms of HD-tDCS on DOC have remained unclear.
Since electroencephalograms (EEGs) are convenient, high-cost performance, and comparatively suitable to work at the patient’s bedside, they play an irreplaceable role in DOC studies. Additionally, graph theory has also been widely used in the field of neuroscience recently (Bullmore and Sporns, 2009; Bullmore and Bassett, 2011; Fornito et al., 2013). Based on these approaches, recent studies have shown that both qualitative assessments by experts (Forgacs et al., 2014; Estraneo et al., 2016; Piarulli et al., 2016; Bagnato et al., 2017) and quantitative assessments using quasi-automated machine learning (Sitt et al., 2014) could be valid in evaluating the state of consciousness of patients by EEGs. Furthermore, some studies have reported that different resting-state functional networks characterize healthy and DOC subjects and that their consciousness levels are relevant to their network properties (Chennu et al., 2014, 2017; Malagurski et al., 2019). These findings suggest that using resting-state EEGs and graph theory to study DOC patients is feasible and may help to further understand and treat DOCs.
In the current study, resting-state EEGs and coma recovery scale-revised scores (CRS-Rs) were applied for assessing the treatment efficacy of long-lasting HD-tDCS in patients with DOC. We aimed to verify the efficacies of HD-tDCS treatment via electrophysiological evidence. Additionally, we investigated whether brain areas closer to the stimulus site were more likely functional recovery.
Materials and Methods
Patients
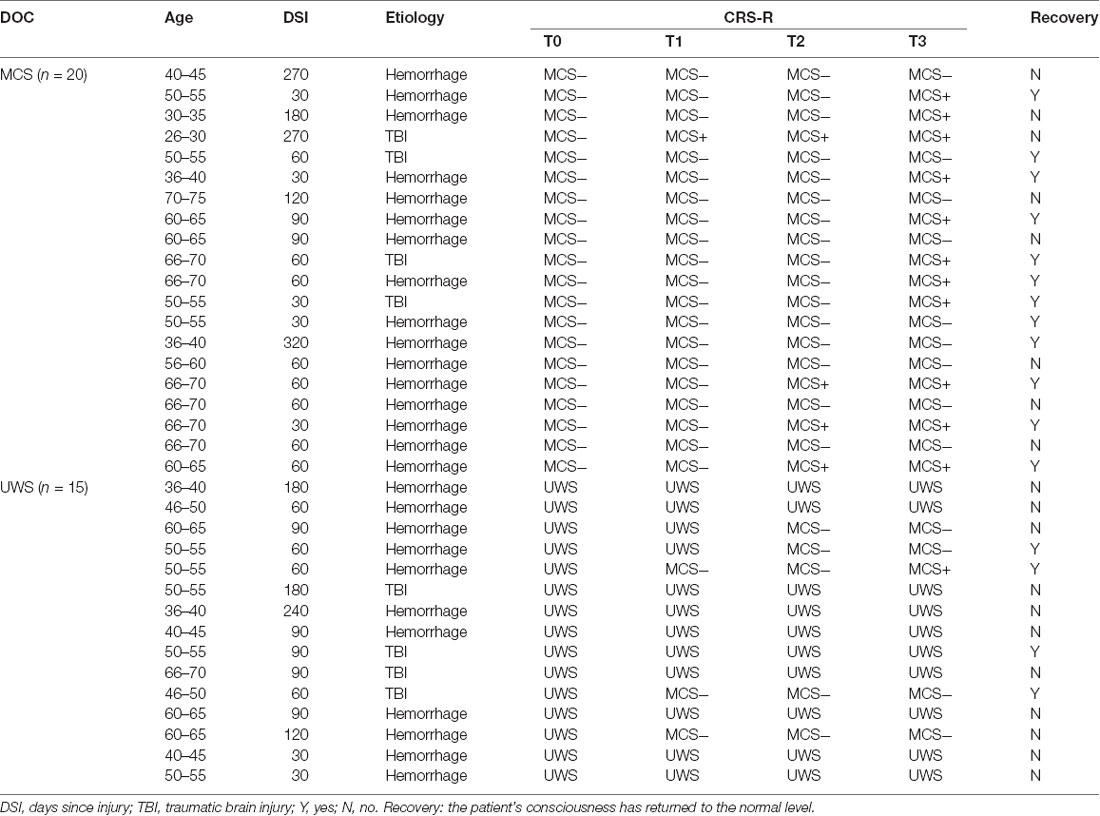
Thirty-five hospitalized DOC patients (13 females and 22 males, 51.7 ± 14.1 years old) were included in this study (Table 1). These patients did not show cortical lesions in the precuneus. Data collection was completed in the Zhengzhou Central Hospital Affiliated to Zhengzhou University. With the CRS-Rs, the score of the patient’s consciousness was assessed (Giacino et al., 2004) and all CRS-R were performed by trained physicians at T0, T1, T2, and T3. Each patient was evaluated by the same physician. In this study, we divided the MCS patients into MCS+ and MCS−.
The patients were divided into UWS (three females and 12 males, 51.0 ± 9.6 years old) and MCS (10 females and 10 males, 52.3 ± 16.9 years old) groups according to their CRS-R scores before treatment. DOC patients who had previously undergone tDCS treatment within the past month were excluded from the present study. The patients with aneurysm clips, pacemakers, other implanted devices, or other drugs that may influence the patients’ EEGs were also excluded from this study. This study was approved by the ethics committee of the Zhengzhou Central Hospital Affiliated to Zhengzhou University. Assessments on whether any of the included patients had been returned to the normal level were completed in June 2019.
Experimental and HD-tDCS
Experimental Process
As shown in Figure 1A, the experimental process consists of a consecutive 14 days HD-tDCS (position: anode centered over the precuneus; duration: 20 min; current: 2 mA) modulation. Every day each patient received two sessions stimulation in the morning and afternoon. We assessed the state of the patients’ consciousness with CRS-R at the following four time-points: the onset time of the experiment (T0), after 1 day of HD-tDCS (T1), after 7 days (T2), and the endpoint time of 14 days (T3). No adverse effect was found during the treatment.
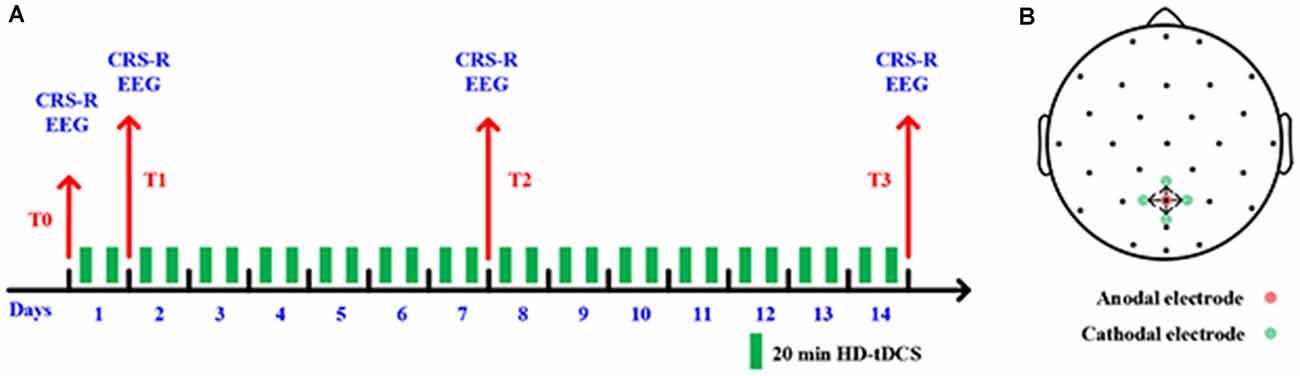
Figure 1. (A) Outline of the experimental design. (B) Positions of electrodes for high-definition transcranial direct-current stimulation (HD-tDCS).
HD-tDCS
In this study, five high-definition electrodes with an anode center electrode overlying the targeted brain area surrounded by four cathodal electrodes were used to transmit direct current to the brain (Model 4x1-C2: Soterix Medical Inc., New York, NY, USA). More restricted cortical neuromodulation and higher electric fields can be obtained by HD-tDCS (Villamar et al., 2013). Stimulating electrodes were arranged with specially designed plastic casings embedded in a 32-channel EEG recording cap. A previous study found that tDCS on the posterior parietal cortex could increase the CRS-R score for some DOC patients with MCS (Huang et al., 2017). As such, we placed the center anode at Pz position in line with the international 10-20 EEG system; the four cathodal electrodes were deployed approximately 3.5 cm radially from Pz position, approximately at Cz, P3, P4, and POz, as shown in Figure 1B.
Data Recording and Pre-processing
A 32-channel EEG recorder was used for data collection (Nicolet EEG V32, Natus, United States; Channel names: Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T7, T8, P7, P8, A1, A2, Fz, Cz, Pz, FC5, FC1, FC2, FC6, POz, CP1, CP2, CP6, Fpz, CP5, and Oz). Resting-state EEGs were recorded at four time-points (T0, T1, T2, and T3). The 32 channels EEG signals were continuously recorded covering the whole scalp consistent with the International 10/20 system with the Cz as reference. The signals were notch filtered at 50 Hz in the recorder. The sampling rate of EEG signals was set to 1,000 Hz. During the data collection process, the impedance of all electrodes was kept no more than 5 KΩ and the patients were behaviorally awake. The EEG recording lasted approximately 10 min for each session.
All EEG data from the 32 channels were retained for analysis using EEGLAB (Delorme and Makeig, 2004). EEG signals were bandpass filtered at 0.5 and 45 Hz with the zero-phase shift FIR filter and then were segmented into 10 s epochs (approximately 60 epochs). Relative to the mean voltage of the entire epoch, every epoch was baseline-corrected at each time point. To select the abnormal channels, the normalized variance of each channel was calculated and then manually interpolated with the surrounding electrodes. We used independent component analysis (ICA) to reject other artifacts (EMG, EOG, and ECG; Jung et al., 2000). The artifacts were firstly selected with an automatic EEG artifact detector (Mognon et al., 2011). The EEG signals were re-referenced to the common average reference (CAR; Nunez et al., 2001). The first 40 clean epochs from each patient were preserved for further analysis.
Construction of Brain Networks
The debiased weighted phase lag Index (dwPLI) was used for estimating spectral connectivity between pairs of channels. The dwPLI measuring of phase relationships is an estimator of scalp-level connectivity that is more robust and partially invariant to volume conduction in comparison to other estimators (Peraza et al., 2012). In the present study, the dwPLI measure was computed to estimate the functional connectivity between electrodes (Vinck et al., 2011) using the FieldTrip toolbox in MATLAB (Oostenveld et al., 2011).
First, we calculated cross-spectral decompositions from cleaned EEG datasets. Then, to achieve the dwPLI measure, the cross-spectrum between the spectral decompositions of every pair of channels was calculated. Furthermore, we restricted the analysis to the delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–45 Hz) bands. Within each band, the dwPLI values across all epochs were averaged to represent the connectivity between channel pairs. For each patient’s dataset, the dwPLI values across all channel pairs were used to construct symmetric 32 × 32 × 4 dwPLI connectivity matrices for the delta, theta, alpha, beta, and gamma bands. Finally, each dwPLI matrix estimated above was proportionally thresholded. Values below this threshold were set to zero and other values were set to one, and 35% of the connectivity remained after thresholding. The threshold for each DOC patient was determined based on all four condition pools (T0, T1, T2, and T3) and was then applied to each condition separately. The binarized dwPLI connectivity matrices were used for further analysis. Due to the A1 and A2 electrodes being next to the face and vulnerable to noise, these two electrodes were excluded from the subsequent analysis. Finally, we obtained a symmetric 30 × 30 × 4 binarized dwPLI matrix for each band of each patient.
Brain Network Measures
Two network measures, clustering coefficient (Watts and Strogatz, 1998) and global efficiency (Latora and Marchiori, 2001) were used to explore the properties that refer to the information processing in the human brain network. The brain-network measurement algorithms were implemented by the Brain Connectivity Toolbox (Rubinov and Sporns, 2010).
The clustering coefficient was computed as follows:
in which i, j refers to the electrode number, vij is 1 if there is suprathreshold connectivity between electrodes i and j and 0 otherwise, and n is the number of electrodes with suprathreshold connections to electrode i. The clustering coefficient reflects the integrity and interconnectedness of a smaller network. Namely, it reflects local information processing. To get the average clustering coefficient of a network, the clustering coefficient values over all 30 channels were averaged for each patient.
Global network efficiency is closely related to the characteristic path length. The formula to compute global efficiency is as follows:
where lij indicates the shortest path length from the node j to the node i. Global network efficiency could reflect the efficiency of global information exchange in brain networks.
Statistical Analysis
To test whether the clustering coefficient and global efficiency were increased at T1, T2, and T3 compared to T0 in both VS and MCS groups, the single tail paired t-test was used. Ten repeated statistical tests (2 network properties × 5 EEG bands) were performed at each time point for the two groups. To reduce the false-positive rate for multiple comparisons, statistical tests were corrected with the BHFDR method (Benjamini and Hochberg, 1995). The similar statistical analysis method and corrected methods were then used to test whether the nodal dwPLI values were increased at T1, T2, and T3 compared to T0, only the repeated times were different, which was 30 in this test. When testing the significant changes of CRS-R scores, the beta/gamma-band average clustering coefficient, and global network efficiency between the two groups, a two-sample t-test, and BHFDR correction methods were used and the repeated times is five.
Results
Table 1 gives an account of the demographic and clinical data of the included patients. There are no significant differences between the UWS and MCS groups, except for CRS-Rs at the T0 phase. For the MCS group, there were 11 (55%) patients who showed improved consciousness states at the T3 phase and 12 (60%) patients had a recovery. Moreover, nine of 12 (75%) recovery patients had behaviorally improved. For the UWS group, there were five (33%) patients who showed improved consciousness states at the T3 phase and four (27%) patients had a recovery. Interestingly, three of four (75%) recovery patients had behaviorally improved.
Changes in Resting-State Brain Network Properties
For the UWS patients, as shown in Figure 2A, the average clustering coefficient values in the delta band decreased with increasing sessions of HD-tDCS; however, this trend was not significant (p > 0.05, FDR corrected). The average clustering coefficient values in theta, alpha, beta, and gamma bands did not show any significant change at the T3 phase compared with those of the T0 phase. The results in Figure 3A were the same as the above results.
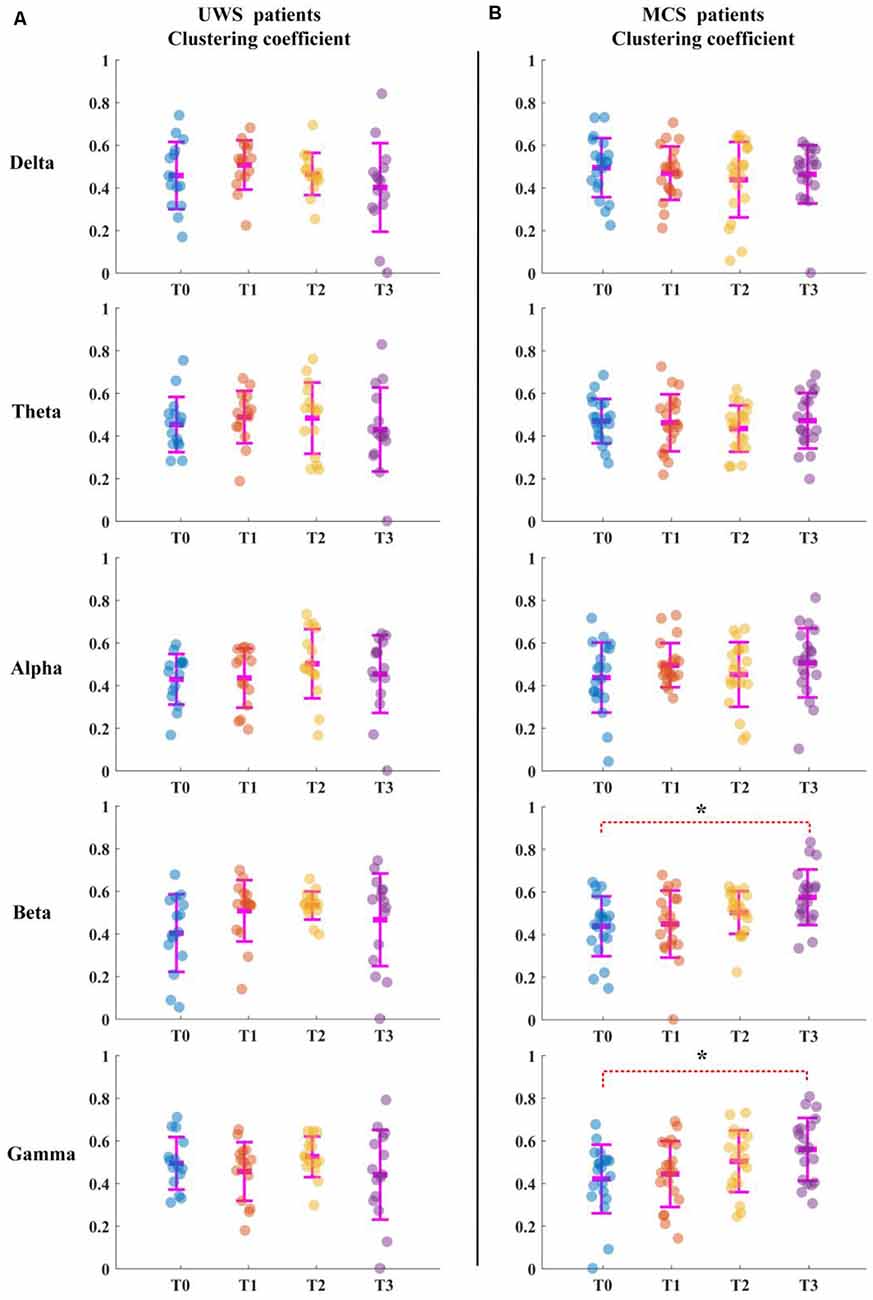
Figure 2. Average clustering coefficient for all patients in five bands. T0, T1, T2, and T3 represent four electroencephalograms (EEG) time points. The scatterplot shows the indicators of each patient. (A) Unresponsive wakefulness syndrome (UWS) patients. (B) Minimally conscious states (MCS) patients. *Denotes p < 0.05, FDR corrected.
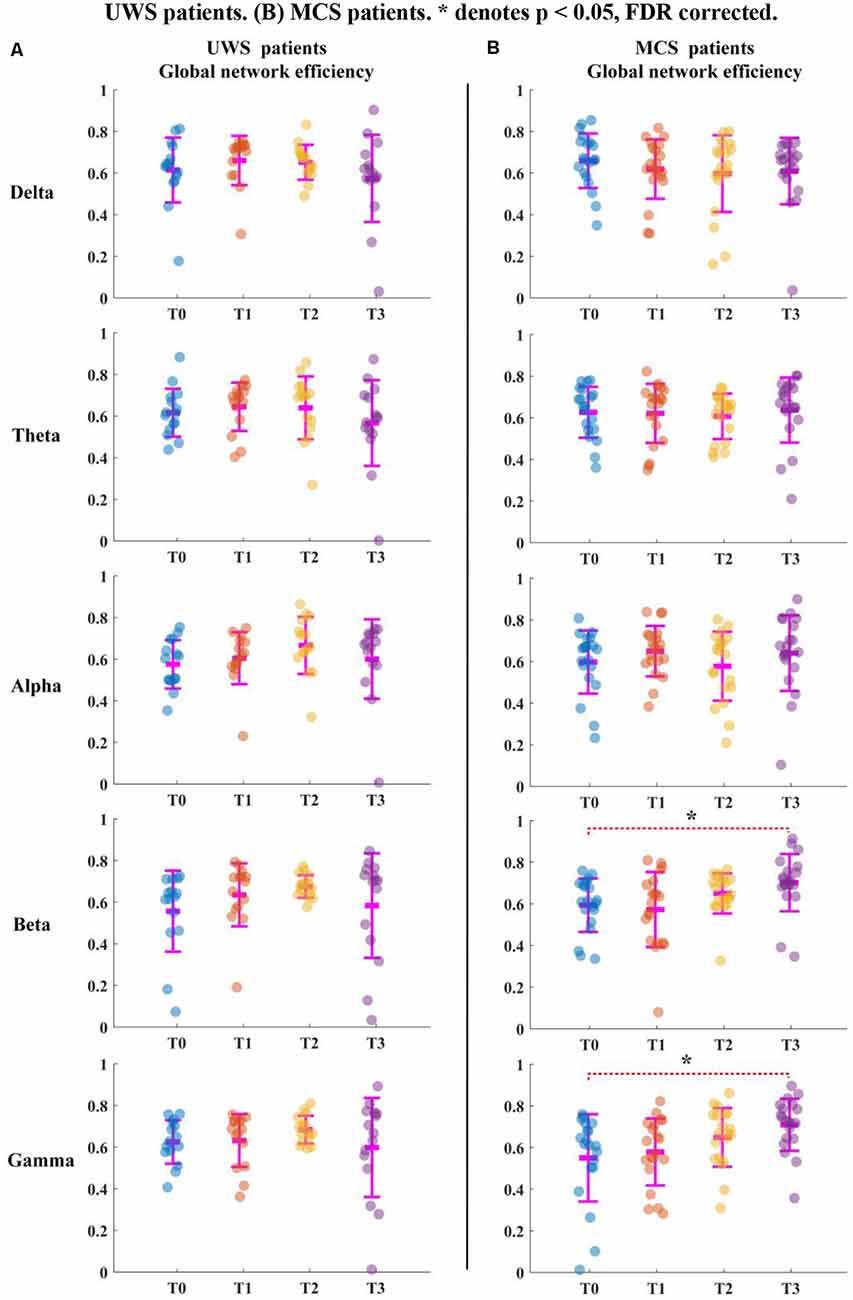
Figure 3. Global network efficiency for all patients in five bands. T0, T1, T2, and T3 represent four EEG time points. The scatterplot shows the indicators of each patient. (A) UWS patients. (B) MCS patients. *Denotes p < 0.05, FDR corrected.
For the MCS patients, as shown in Figure 2B, the average clustering coefficient values in beta (p < 0.05, FDR corrected) and gamma (p < 0.05, FDR corrected) bands increased with sessions of HD-tDCS. The average clustering coefficient values in the delta band decreased at the T3 phase; however, this trend was not significant (p > 0.05, FDR corrected). The average clustering coefficient values in alpha and beta bands did not show a significant change at the T3 phase compared with those at the T0 phase. As shown in Figure 3B, the global efficiency values in the delta band decreased (p > 0.05, FDR corrected). In the beta and gamma bands, the global efficiency values increased with increasing sessions of HD-tDCS (p < 0.05, FDR corrected). In the delta and alpha bands, the global efficiency values did not show a significant change.
We analyzed the changes in CRS-R scores, average clustering coefficient, and global network efficiency at T1, T2, and T3 for UWS and MCS groups. As showed in Figure 4A, although the two groups both showed a score increase, the MCS patients had a higher score increase. The increased CRS-R scores in the MCS group significantly higher than in the UWS group at the T3 phase (p < 0.05, FDR corrected). In Figures 4B,C, the beta band changes of the average clustering coefficient and global network efficiency increased in T1 and T2 phases but decreased in T3 for the UWS group. For the MCS group, although it didn’t show a significant difference at the T3 phase between the two groups, the changes increased steadily with the HD-tDCS treatment. In Figures 4D,E, the gamma band changes of average clustering coefficient and global network efficiency were similar to above beta band results, but it showed significant difference (p < 0.05, FDR corrected) at T3 between the two groups.
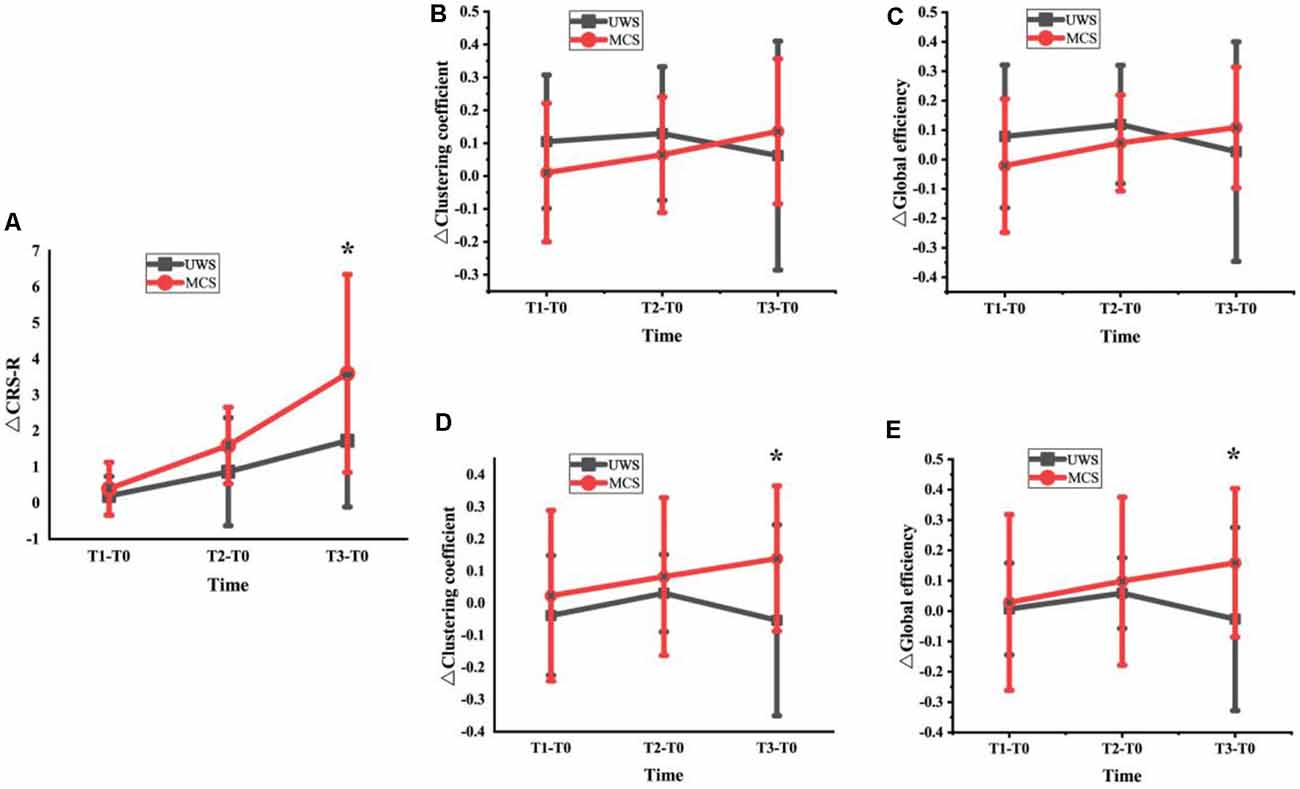
Figure 4. Changes of CRS-R scores (A), average clustering coefficient and global network efficiency at T1, T2 and T3 for UWS and MCS groups [Beta band: (B) and (C); Gamma band: (D) and (E); *denotes a significant difference (*denotes p < 0.05, FDR corrected)].
Changes in Average Nodal Connection Strength
The average clustering coefficient and global efficiency values could not estimate which brain regions function increased or decreased after 14 days of HD-tDCS therapy. Therefore, the average nodal ΔdwPLI topographs of UWS and MCS patients in the five frequency bands were plotted. To obtain the average nodal ΔdwPLI of each node, the symmetric 30 × 30 × 4 binarized dwPLI matrix was averaged by rows; then, we obtained the average nodal connection-strength matrix (30 × 4). Finally, the nodal ΔdwPLI matrix (30 × 1) was obtained by calculating the difference between the four phases (T1-T0, T2-T0, and T3-T0).
For the UWS patients, as shown in Figure 5A, there were no significantly increased nodal dwPLI values in the delta, theta, alpha, beta, or gamma bands between the T0 and T3 phase. Although the nodal dwPLI values increased in some regions in terms of theta, alpha, beta, and gamma bands for the T2 phase. But the results didn’t show statistical significance.
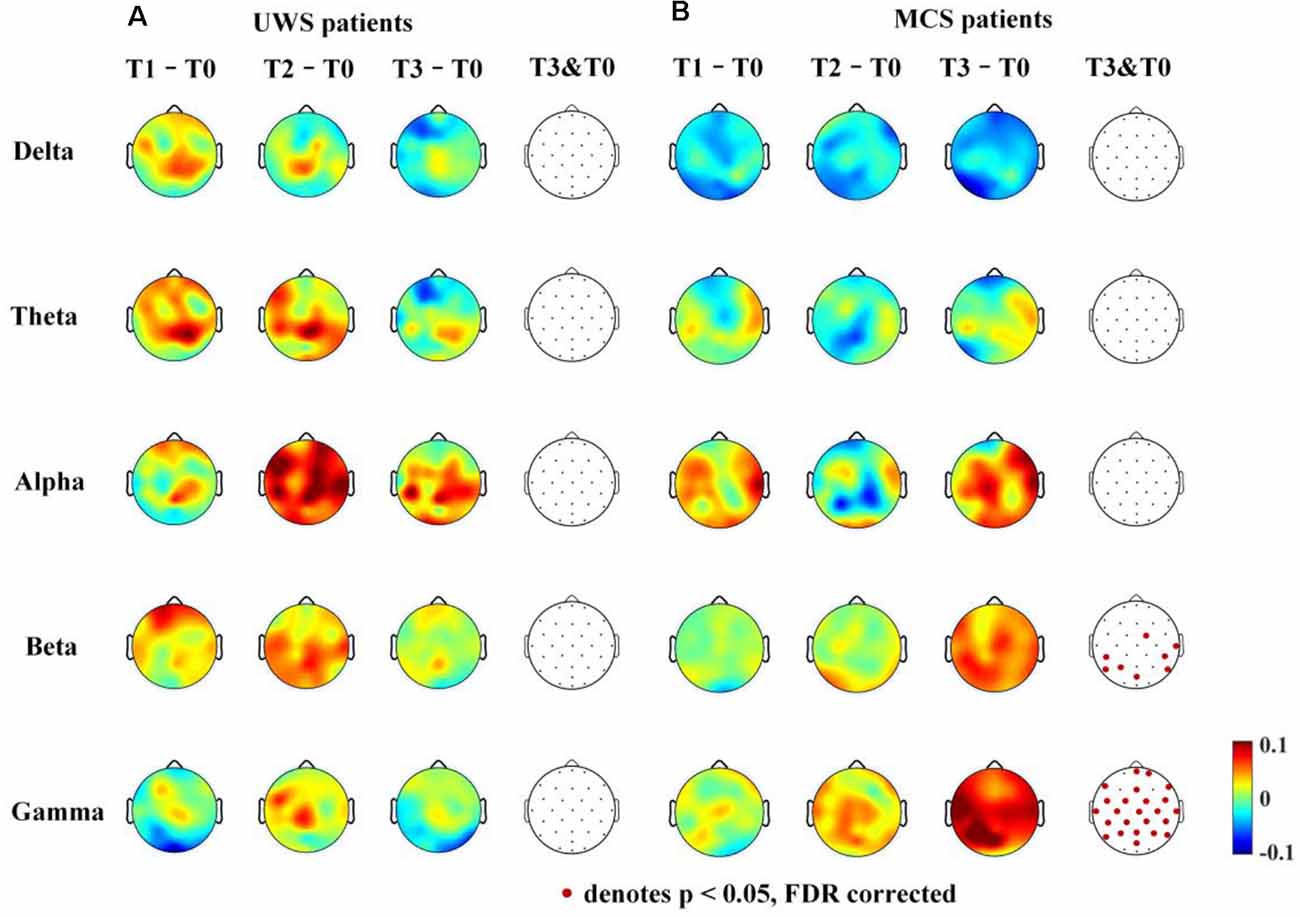
Figure 5. Average nodal ΔdwPLI (debiased weighted phase lag index) topographs (T1 – T0, T2 – T0 and T3 – T0) in UWS and MCS patients in terms of delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–45 Hz) bands. The electrodes with significantly increased dwPLI (T0 →T3) are denoted by colored solid circles (A, UWS patients; B, MCS patients).
For the MCS patients, as shown in Figure 5B, the mean nodal dwPLI values decreased in the delta band. In the theta and alpha band, there were no significant changes in the mean nodal dwPLI values. In the beta band, the mean nodal dwPLI values increased in FC2, CP5, CP6, T8, P3, P7, P8, and POz (p < 0.05, FDR corrected). In the gamma band, the mean nodal dwPLI values increased in all electrodes (p < 0.05, FDR corrected), except Fp1, F3, F4, O1, O2, and Oz.
The changes in the nodal connection strength for MCS patients had a consistent increasing/decreasing trend during the HD-tDCS intervention. In contrast, for UWS patients, the changes in the node connection strength were not consistent. Additionally, the connection strength may have increased at the T2 phase, and then decreased at the T3 phase.
Discussion
In this study, based on scalp EEGs, we probed the changes in resting-state functional networks for the UWS and MCS patients who received 14 days of HD-tDCS interventions. Additionally, we investigated whether there was any improved functional connectivity in different brain regions of these UWS and MCS patients. We found that local and global efficiency increased in beta and gamma bands after intervention for MCS patients. In contrast, UWS patients did not show any significant change in these two bands. Furthermore, for MCS patients, the increase of functional connectivity was distributed across nearly all brain regions, which is inconsistent with our experience that the sequence of functional rehabilitation of brain regions is in accordance with the distance of the stimulus area.
Studies had shown that anodal tDCS of the left DLPFC leads to cognitive improvement in healthy volunteers (Fregni et al., 2005; Ohn et al., 2008), hence, most studies selected the left DLPFC as stimulation target of DOC patients and verified that it is safe and can improve the state of consciousness for some chronic patients with MCS (Thibaut et al., 2017). While the precuneus was used in our study and the reasons are as follows. On the one hand, some studies’ results demonstrated that the precuneus may play a central role in the neural network correlates of consciousness (Laureys et al., 2006; Cavanna, 2007). On the other hand, the left DLPFC is common in traumatic brain injury, so an alternative stimulation target is needed for the patients with DLPFC injury. Our results proved that the stimulation on precuneus with HD-tDCS could benefit the recovery of MCS patients.
Increased Network Local and Global Information Processing After HD-tDCS Intervention
In this study, we found that there was an increasing trend in local and global information processing of resting-state functional brain networks during the 14 days of HD-tDCS interventions. Although UWS patients did not show any significant change from the T0 phase to the T3 phase, we still observed that information processing was increased. The electrophysiological findings were consistent with clinical statistical results (Table 1). For the MCS group, there were more patients exhibited significant improvements at T3 compared with the UWS group, and most of the patients with improved consciousness states had behaviorally improved. This finding was consistent with conclusions from previous studies. A previous study showed that the brain networks of DOC patients had reduced local and global efficiencies compared with those of healthy controls (Chennu et al., 2014). The increased local and global efficiencies after 14 days of HD-tDCS interventions suggested that HD-tDCS may be useful for treating DOC patients. Our recent study also demonstrated that long-lasting HD-tDCS over the precuneus is promising for the treatment of DOC patients (Guo et al., 2019). Rizkallah et al. (2019) reported that resting-state brain networks of DOC patients showed significantly increased local information processing when compared to that of controls. Similar results were reported by Chennu et al. (2017), in which they found that there was a linear trend with an increasing level of consciousness in local information processing of alpha-band brain networks.
The reasons why HD-tDCS interventions had better effects on the MCS group compared to the UWS group need to be further investigated. One possible reason is that the MCS patients have more residual functional connectivity of the default mode network (DMN; Naro et al., 2017). Such functional connectivity is an indicator of covert consciousness, and the HD-tDCS may indirectly modulate other brain networks through the residual DMN.
Surprisingly, our findings mainly revealed significant differences via HD-tDCS interventions in terms of beta and gamma bands. The beta frequency range is related to selective attention, large-scale neuronal integration, states of the motor system, and environmental stimuli (Gilbertson et al., 2005; Engel and Fries, 2010; Donner and Siegel, 2011; Bonfiglio et al., 2014); gamma oscillatory activity is correlated with the level of awareness (Naro et al., 2016; Koch et al., 2016; Siclari et al., 2017). In our present study, the increased network of local and global information processing in beta and gamma bands suggests that there was the amelioration of symptoms in the included MCS patients.
Increased Functional Connections Were Not Limited to the Stimulation Area
Another important finding was that the recovered functional connections in the beta and gamma bands were not only in the local brain area surrounding the stimulation position but were distributed across a more global brain area. This is not entirely inconsistent with our expected results; we originally hypothesized that the brain areas close to the stimulus position would be more likely to recover. Such results may be caused by the following two reasons. First, coma patients have been found to have a lower number of significant functional connections (Chennu et al., 2014; Malagurski et al., 2019), whereas MCS patients have been demonstrated to retain whole-brain functional connectivity (van den Heuvel et al., 2017; Malagurski et al., 2019). As such, stimulus information may be transmitted to other brain regions through these reserved connections. Additionally, although some specific brain regions or networks are specialized for performing specific tasks (He et al., 2018), integration of information cannot be done without whole-brain functional connectivity for complex cognition and behavior. Hence, the recovery of consciousness relies on strengthening of whole-brain functional connectivity.
Limitations
Our study has two limitations. On the one hand, due to the patient’s family’s strong expectations for the recovery of consciousness, the sham stimulation group that lasted for 2 weeks did not recruit enough volunteers. So this study is not a random double-blind sham-controlled trial, thus the evidence obtained in the current study is not strong enough. For future HD-tDCS studies, adding a control group is necessary. On the other hand, although we have already recruited a lot of volunteers, the number is not big enough, more volunteers should be recruited for future study for making the results more convincing.
Conclusion
Long-lasting HD-tDCS over the precuneus facilitated the recovery of consciousness for MCS patients. The increased clustering coefficient and global efficiency of resting-state brain networks within beta and gamma bands further supported the positive efficacy of HD-tDCS interventions. Additionally, we found that recovery of brain connectivity not only occurred in the local brain area surrounding the stimulation position but also occurred in more global brain areas. The results suggest that long-lasting HD-tDCS on the precuneus may facilitate information processing among neural populations in MCS patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of the Zhengzhou Central Hospital Affiliated to Zhengzhou University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of any potentially identifiable data included in this article.
Author Contributions
RZ: conceptualization, methodology, software, and writing—reviewing and editing. LZ: conceptualization, methodology, software, and writing—original draft preparation. YG: conceptualization, methodology, and data curation. LS: investigation, funding acquisition, and supervision. JG: investigation, supervision, and validation. XW: investigation and supervision. YH: conceptualization, project administration, funding acquisition, investigation, and supervision.
Funding
This research was supported by grants from the National Natural Science Foundation of China projects (NSFC, Nos. 61603344 and 61803342); Science and Technology Project of Henan Province (202102310210); Key Project of Discipline Construction of Zhengzhou University (XKZDQY201905); the Key Research Projects of Henan Higher Education Institutions (No. 16A120008); the Key Scientific Research Projects of Henan Province Universities and Colleges (No. 19A320041); and the Key R&D Program of Guangdong Province (No. 2018B030339001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
Angelakis, E., Liouta, E., Andreadis, N., Korfias, S., Ktonas, P., Stranjalis, G., et al. (2014). Transcranial direct current stimulation effects in disorders of consciousness. Arch. Phys. Med. Rehab. 95, 283–289. doi: 10.1016/j.apmr.2013.09.002
Bagnato, S., Boccagni, C., Prestandrea, C., Fingelkurts, A. A., Fingelkurts, A. A., and Galardi, G. (2017). Changes in standard electroencephalograms parallel consciousness improvements in patients with unresponsive wakefulness syndrome. Arch. Phys. Med. Rehab. 98, 665–672. doi: 10.1016/j.apmr.2016.09.132
Bai, Y., Xia, X., Kang, J., Yang, Y., He, J., and Li, X. (2017). TDCS modulates cortical excitability in patients with disorders of consciousness. Neuroimage Clin. 15, 702–709. doi: 10.1016/j.nicl.2017.01.025
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bonfiglio, L., Piarulli, A., Olcese, U., Andre, P., Arrighi, P., Frisoli, A., et al. (2014). Spectral parameters modulation and source localization of blink-related alpha and low-beta oscillations differentiate minimally conscious state from vegetative state/unresponsive wakefulness syndrome. PLoS One 9. doi: 10.1371/journal.pone.0095948
Borckardt, J. J., Bikson, M., Frohman, H., Reeves, S. T., Datta, A., Bansal, V., et al. (2012). A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J. Pain 13, 112–120. doi: 10.1016/j.jpain.2011.07.001
Bruno, M. A., Majerus, S., Boly, M., Vanhaudenhuyse, A., Schnakers, C., Gosseries, O., et al. (2012). Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J. Neurol. 259, 1087–1098. doi: 10.1007/s00415-011-6303-7
Bullmore, E. T., and Bassett, D. S. (2011). Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 7, 113–140. doi: 10.1146/annurev-clinpsy-040510-143934
Bullmore, E. T., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575
Cai, T. T., Xia, X. Y., Zhang, H., Guo, Y. K., and Bai, Y. (2019). High-definition transcranial direct current stimulation modulates neural activities in patients with prolonged disorders of consciousness. Brain Stimul. 12, 1619–1621. doi: 10.1016/j.brs.2019.08.017
Cavanna, A. E. (2007). The precuneus and consciousness. CNS Spectr. 12, 545–552. doi: 10.1017/s1092852900021295
Chennu, S., Annen, J., Wannez, S., Thibaut, A., Chatelle, C., Cassoi, H., et al. (2017). Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain 140, 2120–2132. doi: 10.1093/brain/awx163
Chennu, S., Finoia, P., Kamau, E., Allanson, J., Williams, G. B., Monti, M. M., et al. (2014). Spectral signatures of reorganised brain networks in disorders of consciousness. PLoS Comput. Biol. 10:e1003887. doi: 10.1371/journal.pcbi.1003887
Chennu, S., O’Connor, S., Adapa, R., Menon, D. K., and Bekinschtein, T. A. (2016). Brain connectivity dissociates responsiveness from drug exposure during propofol-induced transitions of consciousness. PLoS Comput. Biol. 12. doi: 10.1371/journal.pcbi.1004669
Cossu, G. (2014). Therapeutic options to enhance coma arousal after traumatic brain injury: state of the art of current treatments to improve coma recovery. Br. J. Neurosurg. 28, 187–198. doi: 10.3109/02688697.2013.841845
Della Pepa, G. M., Fukaya, C., La Rocca, G., Zhong, J., and Visocchi, M. (2013). Neuromodulation of vegetative state through spinal cord stimulation: where are we now and where are we going? Stereotact. Funct. Neurosurg. 91, 275–287. doi: 10.1159/000348271
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Dmochowski, J. P., Datta, A., Bikson, M., Su, Y., and Parra, L. C. (2011). Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 8:046011. doi: 10.1088/1741-2560/8/4/046011
Donnell, A., Nascimento, T. D., Lawrence, M., Gupta, V., Zieba, T., Truong, D. Q., et al. (2015). High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimul. 8, 1085–1092. doi: 10.1016/j.brs.2015.06.008
Donner, T. H., and Siegel, M. (2011). A framework for local cortical oscillation patterns. Trends Cogn. Sci. 15, 191–199. doi: 10.1016/j.tics.2011.03.007
Engel, A. K., and Fries, P. (2010). Beta-band oscillations-signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Estraneo, A., Loreto, V., Guarino, I., Boemia, V., Paone, G., Moretta, P., et al. (2016). Standard EEG in diagnostic process of prolonged disorders of consciousness. Clin. Neurophysiol. 127, 2379–2385. doi: 10.1016/j.clinph.2016.03.021
Estraneo, A., Pascarella, A., Moretta, P., Masotta, O., Fiorenza, S., Chirico, G., et al. (2017). Repeated transcranial direct current stimulation in prolonged disorders of consciousness: a double-blind cross-over study. J. Neurol. Sci. 375, 464–470. doi: 10.1016/j.jns.2017.02.036
Forgacs, P. B., Conte, M. M., Fridman, E. A., Voss, H. U., Victor, J. D., and Schiff, N. D. (2014). Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 76, 869–879. doi: 10.1002/ana.24283
Fornito, A., Zalesky, A., and Breakspear, M. (2013). Graph analysis of the human connectome: promise, progress, and pitfalls. NeuroImage 80, 426–444. doi: 10.1016/j.neuroimage.2013.04.087
Fregni, F., Boggio, P. S., Nitsche, M., Bermpohl, F., Antal, A., Feredoes, E., et al. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 166, 23–30. doi: 10.1007/s00221-005-2334-6
Giacino, J. T., Ashwal, S., Childs, N., Cranford, R., Jennett, B., Katz, D. I., et al. (2002). The minimally conscious state: definition and diagnostic criteria. Neurology 58, 349–353. doi: 10.1212/wnl.58.3.506
Giacino, J. T., Kalmar, K., and Whyte, J. (2004). The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehab. 85, 2020–2029. doi: 10.1016/j.apmr.2004.02.033
Giacino, J. T., Whyte, J., Bagiella, E., Kalmar, K., and Mark, S. (2012). Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 366, 819–826. doi: 10.1056/NEJMoa1102609
Gilbertson, T., Lalo, E., Doyle, L., Di Lazzaro, V., Cioni, B., and Brown, P. (2005). Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J. Neurosci. 25, 7771–7779. doi: 10.1523/jneurosci.1762-05.2005
Guo, Y. K., Bai, Y., Xia, X. Y., Li, J. J., Wang, X. L., Dai, Y. W., et al. (2019). Effects of long-lasting high-definition transcranial direct current stimulation in chronic disorders of consciousness: a pilot study. Front. Neurosci. 13:412. doi: 10.3389/fnins.2019.00412
He, B., Sohrabpour, A., Brown, E., and Liu, Z. (2018). Electrophysiological source imaging: a noninvasive window to brain dynamics. Annu. Rev. Biomed. Eng. 20, 171–196. doi: 10.1146/annurev-bioeng-062117-120853
Huang, H., Sharma, H., Chen, L., Saberi, H., and Mao, G. (2019). 2018 yearbook of neurorestoratology. J. Neurorest. 5, 111–115. doi: 10.26599/JNR.2019.9040003
Huang, W., Wannez, S., Fregni, F., Hu, X., Jing, S., Martens, G., et al. (2017). Repeated stimulation of the posterior parietal cortex in patients in minimally conscious state: a sham-controlled randomized clinical trial. Brain Stimul. 10, 718–720. doi: 10.1016/j.brs.2017.02.001
Jung, T. P., Makeig, S., Westerfield, M., Townsend, J., Courchesne, E., and Sejnowski, T. J. (2000). Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 111, 1745–1758. doi: 10.1016/s1388-2457(00)00386-2
Koch, C., Massimini, M., Boly, M., and Tononi, G. (2016). Neural correlates of consciousness: progress and problems. Nat. Rev. Neurosci. 17, 307–321. doi: 10.1038/nrn.2016.22
Kuo, H. I., Bikson, M., Datta, A., Minhas, P., Paulus, W., Kuo, M. F., et al. (2013). Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 6, 644–648. doi: 10.1016/j.brs.2012.09.010
Latora, V., and Marchiori, M. (2001). Efficient behavior of small-world networks. Phys. Rev. Lett. 87:198701. doi: 10.1103/physrevlett.87.198701
Laureys, S., Boly, M., and Maquet, P. (2006). Tracking the recovery of consciousness from coma. J. Clin. Invest. 116, 1823–1825. doi: 10.1172/jci29172
Laureys, S., Celesia, G. G., Cohadon, F., Lavrijsen, J., Leon-Carrion, J., Sannita, W. G., et al. (2010). Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 8:68. doi: 10.1186/1741-7015-8-68
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92. doi: 10.1016/j.brs.2017.01.196
Malagurski, B., Peran, P., Sarton, B., Vinour, H., Naboulsi, E., Riu, B., et al. (2019). Topological disintegration of resting state functional connectomes in coma. NeuroImage 195, 354–361. doi: 10.1016/j.neuroimage.2019.03.012
Mognon, A., Jovicich, J., Bruzzone, L., and Buiatti, M. (2011). ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 48, 229–240. doi: 10.1111/j.1469-8986.2010.01061.x
Naro, A., Bramanti, P., Leo, A., Russo, M., and Calabro, R. S. (2016). Transcranial alternating current stimulation in patients with chronic disorder of consciousness: a possible way to cut the diagnostic gordian knot? Brain Topogr. 29, 623–644. doi: 10.1007/s10548-016-0489-z
Naro, A., Leo, A., Manuli, A., Cannavò, A., Bramanti, A., Bramanti, P., et al. (2017). How far can we go in chronic disorders of consciousness differential diagnosis? The use of neuromodulation in detecting internal and external awareness. Neuroscience 349:165. doi: 10.1016/j.neuroscience.2017.02.053
Nikolin, S., Loo, C. K., Bai, S. W., Dokos, S., and Martin, D. M. (2015). Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. NeuroImage 117, 11–19. doi: 10.1016/j.neuroimage.2015.05.019
Nunez, P. L., Wingeier, B. M., and Silberstein, R. B. (2001). Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum. Brain Mapp. 13, 125–164. doi: 10.1002/hbm.1030
Ohn, S. H., Park, C. I., Yoo, W. K., Ko, M. H., Choi, K. P., Kim, G. M., et al. (2008). Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 19, 43–47. doi: 10.1097/WNR.0b013e3282f2adfd
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011:156869. doi: 10.1155/2011/156869
Peraza, L. R., Aziz, U. R. A., Gary, G., and David, M. H. (2012). Volume conduction effects in brain network inference from electroencephalographic recordings using phase lag index. J. Neurosci. Methods 207, 189–199. doi: 10.1016/j.jneumeth.2012.04.007
Piarulli, A., Bergamasco, M., Thibaut, A., Cologan, V., Gosseries, O., and Laureys, S. (2016). EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 263, 1746–1760. doi: 10.1007/s00415-016-8196-y
Rizkallah, J., Annen, J., Modolo, J., Gosseries, O., Benquet, P., Mortaheb, S., et al. (2019). Decreased integration of EEG source-space networks in disorders of consciousness. Neuroimage Clin. 23:101841. doi: 10.1016/j.nicl.2019.101841
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Schiff, N. D., Giacino, J. T., Kalmar, K., Victor, J. D., Baker, K., Gerber, M., et al. (2007). Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 448:600. doi: 10.3410/f.1089473.543595
Shekhawat, G. S., Sundram, F., Bikson, M., Truong, D., De Ridder, D., Stinear, C. M., et al. (2016). Intensity, duration, and location of high-definition transcranial direct current stimulation for tinnitus relief. Neurorehabil. Neural Repair 30, 349–359. doi: 10.1177/1545968315595286
Shekhawat, G. S., and Vanneste, S. (2018). High-definition transcranial direct current stimulation of the dorsolateral prefrontal cortex for tinnitus modulation: a preliminary trial. J. Neural Transm. 125, 163–171. doi: 10.1007/s00702-017-1808-6
Siclari, F., Baird, B., Perogamvros, L., Bernardi, G., LaRocque, J. J., Riedner, B., et al. (2017). The neural correlates of dreaming. Nat. Neurosci. 20, 872–878. doi: 10.1038/nn.4545
Sitt, J. D., King, J. R., El Karoui, I., Rohaut, B., Faugeras, F., Gramfort, A., et al. (2014). Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 137, 2258–2270. doi: 10.1093/brain/awu141
Thibaut, A., Bruno, M. A., Ledoux, D., Demertzi, A., and Laureys, S. (2014). tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology 82, 1112–1118. doi: 10.1212/wnl.0000000000000260
Thibaut, A., Wannez, S., Donneau, A. F., Chatelle, C., Gosseries, O., Bruno, M. A., et al. (2017). Controlled clinical trial of repeated prefrontal tDCS in patients with chronic minimally conscious state. Brain Inj. 31, 466–474. doi: 10.1080/02699052.2016.1274776
Tucker, C., and Sandhu, K. (2016). The effectiveness of zolpidem for the treatment of disorders of consciousness. Neurocrit. Care 24, 488–493. doi: 10.1007/s12028-015-0227-5
van den Heuvel, M. P., de Lange, S. C., Zalesky, A., Seguin, C., Yeo, B. T. T., and Schmidt, R. (2017). Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: issues and recommendations. NeuroImage 152, 437–449. doi: 10.1016/j.neuroimage.2017.02.005
Villamar, M. F., Volz, M. S., Bikson, M., Datta, A., DaSilva, A. F., and Fregni, F. (2013). Technique and considerations in the use of 4·1 ring high-definition transcranial direct current stimulation (HD-tDCS). J. Vis. Exp. 14:e50309. doi: 10.3791/50309
Vinck, M., Oostenveld, R., van Wingerden, M., Battaglia, F., and Pennartz, C. M. A. (2011). An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage 55, 1548–1565. doi: 10.1016/j.neuroimage.2011.01.055
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of “small-world” networks. Nature 393:440. doi: 10.14293/s2199-1006.1.sor-phys.cl4jpct.v1
Yamamoto, T., Katayama, Y., Obuchi, T., Kobayashi, K., Oshima, H., and Fukaya, C. (2013). Deep brain stimulation and spinal cord stimulation for vegetative state and minimally conscious state. World Neurosurg. 80. doi: 10.1016/j.wneu.2012.04.010
Zhang, Y., and Song, W. Q. (2018). Transcranial direct current stimulation in disorders of consciousness: a review. Int. J. Neurosci. 128, 255–261. doi: 10.1080/00207454.2017.1381094
Keywords: disorders of consciousness, high-definition transcranial direct-current stimulation, electroencephalography, coma recovery scale-revised scores, resting-state brain network
Citation: Zhang R, Zhang L, Guo Y, Shi L, Gao J, Wang X and Hu Y (2020) Effects of High-Definition Transcranial Direct-Current Stimulation on Resting-State Functional Connectivity in Patients With Disorders of Consciousness. Front. Hum. Neurosci. 14:560586. doi: 10.3389/fnhum.2020.560586
Received: 09 May 2020; Accepted: 26 August 2020;
Published: 23 September 2020.
Edited by:
Feng Liu, Tianjin Medical University General Hospital, ChinaReviewed by:
Yunfa Fu, Kunming University of Science and Technology, ChinaAntonino Naro, Centro Neurolesi Bonino Pulejo (IRCCS), Italy
Copyright © 2020 Zhang, Zhang, Guo, Shi, Gao, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxia Hu, aHV5dXhpYUB6enUuZWR1LmNu
† These authors have contributed equally to this work
 Rui Zhang
Rui Zhang Lipeng Zhang
Lipeng Zhang Yongkun Guo
Yongkun Guo Li Shi5,6
Li Shi5,6 Yuxia Hu
Yuxia Hu