
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Hum. Neurosci. , 07 July 2020
Sec. Brain Imaging and Stimulation
Volume 14 - 2020 | https://doi.org/10.3389/fnhum.2020.00237
This article is part of the Research Topic Understanding the Link Between the Developing Brain and Behavior in Adolescents View all 9 articles
Adolescence is the time of onset of many psychiatric disorders. Half of pediatric patients present with comorbid psychiatric disorders that complicate both their medical and psychiatric care. Currently, diagnosis and treatment decisions are based on symptoms. The field urgently needs brain-based diagnosis and personalized care. Neuroimaging can shed light on how aberrations in brain circuits might underlie psychiatric disorders and their development in adolescents. In this perspective article, we summarize recent MRI literature that provides insights into development of psychiatric disorders in adolescents. We specifically focus on studies of brain structural and functional connectivity. Ninety-six included studies demonstrate the potential of MRI to assess psychiatrically relevant constructs, diagnose psychiatric disorders, predict their development or predict response to treatment. Limitations of the included studies are discussed, and recommendations for future research are offered. We also present a vision for the role that neuroimaging may play in pediatrics and primary care in the future: a routine neuropsychological and neuropsychiatric imaging (NPPI) protocol for adolescent patients, which would include a 30-min brain scan, a quality control and safety read of the scan, followed by computer-based calculation of the structural and functional brain network metrics that can be compared to the normative data by the pediatrician. We also perform a cost-benefit analysis to support this vision and provide a roadmap of the steps required for this vision to be implemented.
Adolescence is the time of onset of many psychiatric disorders. Half of pediatric patients present with comorbid psychiatric disorders that complicate both their medical and psychiatric care. Currently, diagnosis and treatment decisions are based on symptoms. The field urgently needs brain-based diagnosis and personalized care.
Neuroimaging can shed light on how aberrations in brain circuits might underlie psychiatric disorders and their development in adolescents. MRI has become the leading modality for mapping the human brain non-invasively. Apart from mapping individual brain regions, the importance of connections between these regions and their role within the brain network as a whole are becoming increasingly recognized and studied within the framework of connectomics (Sporns et al., 2005). Different MRI techniques can be applied to map the connections of the network and to quantify the connectivity strength. Most commonly, connections are derived from diffusion-weighted images (with tractography used to model white matter pathways) or from the functional MRI (fMRI) signal (using temporal correlation of the signal as a proxy for connectivity). Connections can be compared between subjects individually. However, one can also utilize graph theory, which offers new ways to perform network characterization and comparison. Graph theory operates with an abstracted notion of a graph, which is defined as a set of nodes (in our case, brain regions), connected by a set of edges (e.g., white matter tracts). Important network characteristics can be extracted, such as node degree, characteristic path length, average clustering coefficient and other quantifiable measures of network connectivity (Rubinov and Sporns, 2010). Studying the human connectome using graph theory offers a unique opportunity to better understand inter-individual differences in the neural circuitry.
MRI connectomics has been applied to both the adult and developing brain (Hagmann et al., 2010), including extensive work by our group (Tymofiyeva et al., 2012, 2013, 2014; Ziv et al., 2013). This framework also has been applied to study the neural signature of psychiatric disorders, for example, adult depression (Bai et al., 2012; Korgaonkar et al., 2014; Qin et al., 2014; Gong and He, 2015; Sacchet et al., 2016), as well as adolescent depression (Ellis et al., 2017; Tymofiyeva et al., 2017) and anxiety (Sharp and Telzer, 2017). In the next section, we systematically review both the structural and functional connectivity literature on psychiatric disorders and related symptoms in adolescents.
To perform a review of MRI literature over the last 5-years that provides insights into development of psychiatric disorders in adolescents, the electronic database PubMed was searched using the following Boolean search term, applied to titles and abstracts:
(MRI OR fMRI OR DTI) AND (adolescent OR youth) AND (psychiatric OR neurologic OR mental OR depression OR autism OR anxiety OR PTSD OR psychosis OR ADHD OR attention OR bipolar OR schizophrenia OR OCD) AND (connectivity OR connectome OR network OR circuit).
We thus focused on eight disorders: major depressive disorder (MDD), autism, anxiety, bipolar disorder, attention-deficit/hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), schizophrenia, and obsessive-compulsive disorder (OCD) or their relevant clinical and non-clinical symptoms in youth. We defined the age of adolescence as age between 10 and 19, whereas studies could also include older subjects in addition to those between 10 and 19.
The initial search resulted in 177 entries. After excluding articles that: (a) are not in English (1 article), (b) animal studies (5); (c) review articles (8), (d) focus on non-general populations such as Down syndrome (37), (e) do not include any pathology focus (11), (f) do not analyze brain connectivity (21), the resulting set comprised 94 articles. Two additional articles meeting the eligibility criteria were identified through other sources. The total number of articles included in qualitative synthesis was 96. The search results and main finding of the included articles are summarized in Table 1.
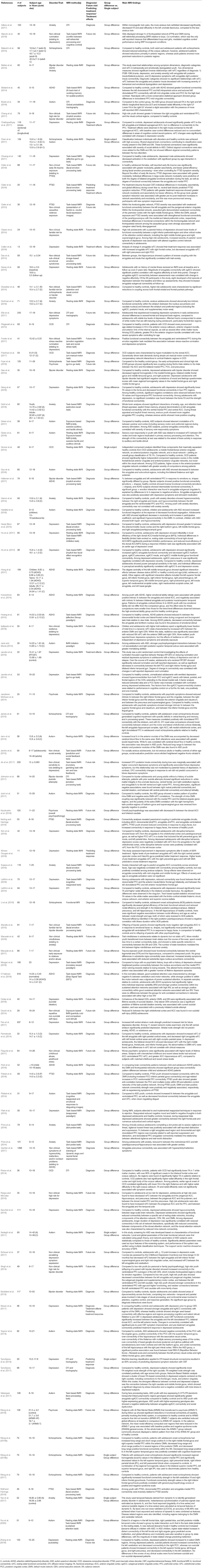
Table 1. Summary of the brain connectivity studies that provide insights into development of psychiatric disorders in adolescents published the last 5-years.
Of the 96 included articles (Table 1), 77 studied clinical populations (MDD, autism, anxiety, bipolar disorder, schizophrenia, ADHD, PTSD, OCD), whereas 19 assessed psychiatrically relevant constructs in non-clinical populations. For example, multiple studies demonstrated disruption in structural and functional connectivity in adolescents with MDD compared to controls in fronto-striatal, fronto-limbic, anterior cingulate cortical (ACC), insular, and amygdalar networks (Ho et al., 2014; LeWinn et al., 2014, 2018; Pannekoek et al., 2014; Davey et al., 2015; Henje Blom et al., 2015; Kim et al., 2016; Morgan et al., 2016; Chattopadhyay et al., 2017; Ellis et al., 2017; Straub et al., 2017; Tymofiyeva et al., 2017). Four studies identified circuitry predictive of treatment response in depressed teens (Jacobs et al., 2016; Straub et al., 2017; Klimes-Dougan et al., 2018; Tymofiyeva et al., 2019). These data suggest that MR imaging biomarkers based on connectivity between key brain regions may offer guidance for treatment selection for depressed adolescents.
Of the 96 included articles, 19 investigated constructs associated with mental illness such as increased rumination, decreased resilience, sensitivity to loss, increased MDD symptom expression, social anxiety, decreased mindfulness, hyperactivity, inattention, anhedonia, etc., without explicitly studying DSM-diagnoses in adolescents. We find the contribution of these studies relevant to the topic because of the limitation of the current diagnostic system and potential of alternative approaches such as the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC), as will be discussed in the following section. Arguably the most important implication of these neuroimaging findings is that they provide both the motivation and the rationale to pursue a much more explicitly preventative psychiatry approach in helping at-risk children before they present with a manifest psychiatric disorder (McCrory et al., 2017). Developing a neurocognitively informed screening tool capable of accurately indexing latent vulnerability is essential if we are to identify those children who are not yet overtly symptomatic but who are at most risk for the development of a future psychiatric disorder. More broadly, by establishing a better understanding of the specific neurocognitive mechanisms implicated in the pathogenesis of psychiatric disorders, we will be in a much better position to develop effective preventative interventions that increase the likelihood of resilient outcomes in children and adolescents (McCrory et al., 2017).
To graphically synthesize the findings of the reviewed literature, we have mapped the circuitry implicated in eight psychiatric disorders (MDD, autism, anxiety, bipolar disorder, schizophrenia, ADHD, PTSD, OCD) or their relevant non-clinical symptoms in youth onto a single brain model (Figure 1). Figure 1 illustrates both increased and decreased structural and functional connectivities as well as network properties that differentiate symptomatic subjects from controls. Similar to the model proposed by Williams (2017), connectivity aberrations associated with the disorders can be broadly classified as aberrations in six large-scale brain networks: default mode network (DMN), salience network (SN), threat network, reward network, attention network, and cognitive control network (CCN). Notably, this was observed not only for the mood disorders as proposed by Williams but also for other disorders, such as schizophrenia and autism (specifically associated with aberrations in DMN and SN) (Chen et al., 2017; Joshi et al., 2017; Wang et al., 2018a).
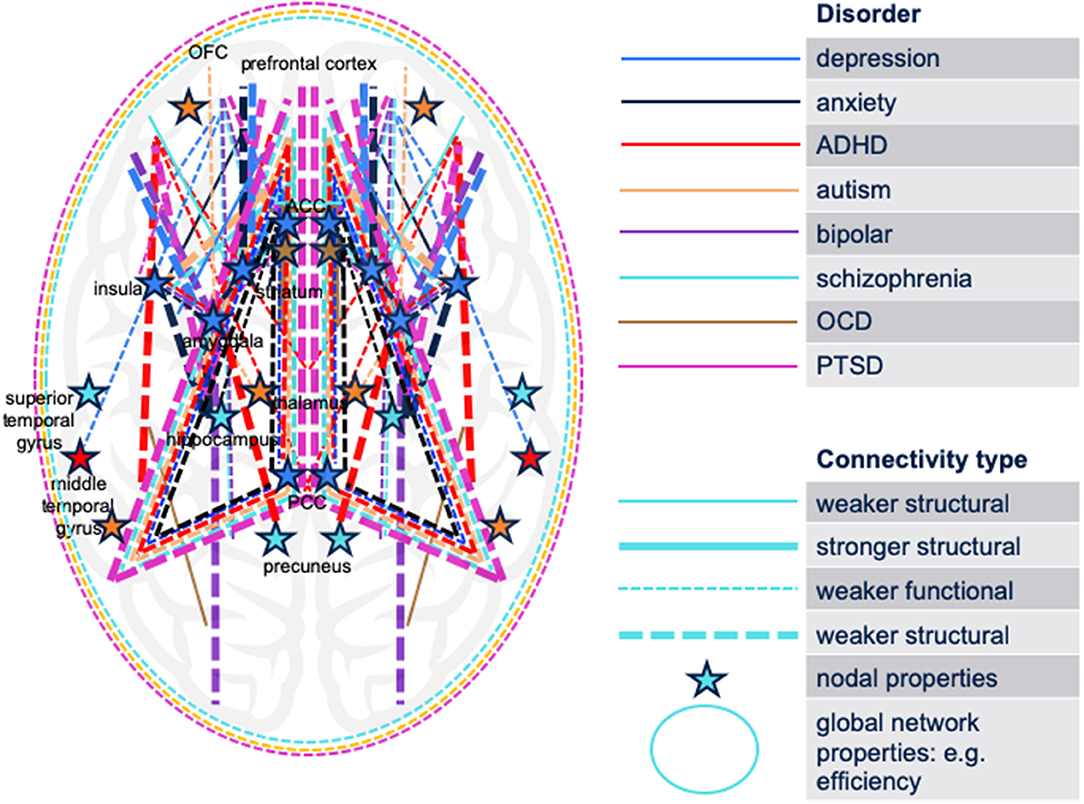
Figure 1. Schematic synthesis of the findings of the reviewed literature: a map of the brain circuitry implicated in eight psychiatric disorders (MDD, autism, anxiety, bipolar disorder, schizophrenia, ADHD, PTSD, OCD) or their relevant non-clinical symptoms in youth. Both increased and decreased structural and functional connectivities differentiate symptomatic subjects from controls. The cerebellum is not displayed. No differentiation between hemispheres is displayed. ADHD, attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder; ACC, anterior cingulate cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex.
The evidence from the reviewed literature requires further investigation of the predictive and diagnostic potential of MRI connectivity measurements as the number of studies and sample sizes are very small. For example, seven of the reviewed studies included under 30 subjects per group. While small studies may be instrumental in finding new candidate biomarkers, replication of the results is the key. Additional well-planned imaging studies with large sample sizes are required. Yet, the field is developing rapidly. Large-scale collaborative efforts by consortia such as ABCD (Casey et al., 2018), ENIGMA (Thompson et al., 2014, 2017), and IMAGEN (O'Halloran et al., 2018) will help identify robust brain connectivity signatures of psychopathologies in adolescents. Among the included study, one study (Kaczkurkin et al., 2018) reports functional connectivity analyses using resting-state functional MRI in 833 participants who received both arterial spin labeling (ASL) and resting-state imaging as part of the Philadelphia Neurodevelopmental Cohort. The results revealed that overall psychopathology was associated with decreased connectivity between the dorsal ACC and bilateral caudate.
Another limitation of the psychiatric imaging literature in general is a profusion of statistically significant, but minimally differentiating biological findings (Kapur et al., 2012). In other words, thousands of studies are published on different aspects of brain disorders to show aberrations of some features (structural or functional) in a patient group usually in comparison with a healthy cohort. While these studies are valuable in terms of finding relevant disease biomarkers, they are not sufficient for direct clinical diagnostic/predictive adoption (Arbabshirani et al., 2017). The main reason is that many of these findings are statistically significant at the group level, but the individual discrimination ability of the proposed biomarkers is not typically evaluated. Group analysis aims to estimate the probability of a certain biomarker given the group (e.g., healthy controls or a patient group): P[brain biomarker|group], and it is typically performed using general linear modeling (e.g., t-test or ANOVA). Single-subject prediction, on the other hand, predicts belonging to the group given the brain biomarker: P[group|brain biomarker], and it is typically performed using generalized linear model such as logistic regression or artificial intelligence (AI) approaches such as machine learning. As mentioned previously, only group analysis is commonly performed in neuroimaging studies. In such cases, effect size can serve as an important indicator of the individual discrimination ability of the biomarker, but it is often not reported—as it is with the majority of the studies reviewed in Table 1. For continuous data analysis, the correlation coefficient points at high or rather low discrimination ability, as in the example of the large study by Kaczkurkin and colleagues discussed above (Kaczkurkin et al., 2018), where Pearson r = −0.18 and r = −0.15. The effect size is low if the value of r varies around 0.1, medium if r varies around 0.3, and large if r varies more than 0.5 (Rosenthal and Rosnow, 1984; Cohen, 1988). Since classification provides information for each individual subject, it is considered a much harder task than reporting group differences. Nevertheless, recent extensive evidence shows the great potential of neuroimaging data for single subject prediction of various brain disorders in adults (Arbabshirani et al., 2017). Several of the studies in adolescents also performed single-subject prediction analysis. For example, functional brain connectivity developmental patterns were found to be a reliable biomarker of severe attention impairment in youth, with a peak receiver operating characteristic curve of 79.3%, measured by area under the curve (Kessler et al., 2016). In another example, machine learning was applied to structural connectivity data to predict symptom improvement in depressed adolescents in response to cognitive behavioral therapy (CBT), resulting in an accuracy of 83% (Tymofiyeva et al., 2019).
One fundamental challenge is that the reviewed clinical studies used the DSM-based diagnosis as the ground truth. The reliance on the categorical system of diagnosis has clear utility; however, its validity has been questioned and dimensional views of illness that incorporate continua of neurobiology and observable behavior have been proposed—i.e., NIMH RDoC (Insel et al., 2010). If one takes seriously the possibility of 1 day developing a neurobiologically-based diagnostic system that would replace the symptom-based DSM nosology, then one biases the results by using DSM diagnosis as the ground truth in research studies. Thus, the advancement of psychiatry appears to be hindered by circuitous reasoning (i.e., a “Catch-22”), and this circularity impedes the development of a clinically viable alternative system (Kapur et al., 2012). Importantly, a recent paper by Drysdale et al. reported promising results showing that adult depression can be subdivided into biological types (Drysdale et al., 2017). Specifically, functional MRI scans of more than 1,100 patients with clinical depression and healthy individuals enabled researchers to demonstrate that patients with depression can be divided into four subtypes based on distinct patterns of functional connectivity in limbic and fronto-striatal networks and different clinical symptoms (Drysdale et al., 2017). Notably, these four subtypes of depression were also associated with differences in clinical treatment outcome. While the study by Drysdale and colleagues demonstrated subtypes within one DSM category (adult MDD; Drysdale et al., 2017) neurobiologically-based diagnostic categories may easily span outside of the established DSM categories. Unsupervised clustering analyses in large datasets of community samples may help solve this problem and establish a classification system of connectome-based psychiatric disorders. In a study by Van Dam et al., data-driven approaches for identifying homogenous subgroups, spanning typical function to dysfunction, not only yielded clinically meaningful groups, but also captured behavioral and neurobiological variation among healthy individuals (Van Dam et al., 2017).
Here, we would like to present a vision of the role that neuroimaging may play in pediatrics and primary care in the future. We would like to start by presenting a case example of a clinical presentation of an adolescent patient to a primary care outpatient clinic.
A concerned parent brings to the primary care clinic a 15-year-old adolescent male with a recent history of increased mood lability, irritability and changes in his personality that has led to significant difficulties with the patient's relationship with his parents and siblings at home and the patient's teachers and classmates at school. The parent states that the patient's personal hygiene has declined at home and that he has started to withdraw from his relationships with his family and friends. The parent also reports that the patient at times loses his temper and has been oppositional with his family, teachers and friends. During the interview by the primary care provider, the adolescent patient complains of problems with feeling easily irritated by others, difficulties with sleep and concentration, and headaches. There are times that the patient also reports feeling “on top of the world” and that he can accomplish many great things. The parent informs the primary care provider that there is a history of mood disorders in the family including a parent with depression and a relative with possible bipolar disorder. The parent also states that there is a relative who has had significant problems with truancy and breaking rules whose behavioral problems started during adolescence. Finally, the parent reports that a great grandparent was hospitalized with a diagnosis of schizophrenia. The parent is concerned about the patient and has brought him to the primary care clinic for help with the son that the parent loves and cares for very deeply.
At present, psychiatric diagnoses are based on symptoms and classified as per the DSM criteria. One significant complicating factor is that most of the DSM diagnoses have been developed for adults, but these same DSM criteria are being applied to make diagnoses in children and adolescents. Based on the presenting symptoms and DSM criteria, the adolescent patient's differential diagnosis is broad. The patient's presenting symptoms of mood lability, irritability, sleep, and concentration problems, and relationship difficulties with family, friends and teachers could be due to a diagnosis of depression or bipolar disorder. The patient's loss of temper and oppositional behavior could be due to a mood disorder such as depression or oppositional defiant disorder that may be the beginning of a possible conduct disorder. The patient's report of feeling at times “on top of the world” and that he can accomplish many great things could be reflective of normative adolescent development or due to bipolar disorder. The patient's decrease in personal hygiene and withdrawal from his family and friends may be due to a mood disorder such as depression or the beginning of another possible psychiatric disorder such as schizoaffective disorder or schizophrenia. Finally, the patient's changes in personality such as his mood lability and complaints of headaches may be due to a mood disorder such as depression or a brain mass such as a tumor. Each of these possible diagnoses has a different clinical prognosis and treatment. Proper diagnosis is essential to ensure that the optimal clinical treatment is selected and to prevent the further worsening of the patient's condition due to either the incorrect diagnosis and treatment or the potential side effects of the use of the inappropriate treatment due to the incorrect diagnosis. For example, if the patient's symptoms lead the clinician to incorrectly make a diagnosis of bipolar disorder based on DSM criteria, then the patient will often be started on a mood stabilizer (e.g., lithium) or atypical antipsychotic medication (e.g., olanzapine) that have several potentially negative side effects including significant weight gain and increased risk for the development of type 2 diabetes. In addition to the potential negative consequences of an incorrect diagnosis to the patient, an incorrect diagnosis can also lead to great costs to the patient, patient's family, and society. For example, missing the diagnosis of depression can lead to significant present and future consequences since adolescent depression confers a strong risk for adult major depressive disorder, increased cardiovascular risk, medical illnesses, disability, premature death, academic and work problems, relationship problems with family and friends, substance abuse, and suicide (Rao et al., 1993, 1995, 1999; Lewinsohn et al., 1998; Pine et al., 1998; Armstrong and Costello, 2002; Lehrer et al., 2006; Copeland et al., 2007; Crum et al., 2008; Audrain-McGovern et al., 2009; Rao and Chen, 2009; Maughan et al., 2013; Liu et al., 2017). Depression is a highly prevalent, devastating, costly and frequently re-occurring chronic illness that the World Health Organization (WHO) ranks as the #1 leading cause of disability worldwide, affecting over 300 million people (WHO, 2017) at an estimated cost of over $210.5 billion per year in the U.S. alone (Greenberg et al., 2015).
Our vision for preventing many of the drastic consequences described in the case example is the introduction of a routine neuropsychological and neuropsychiatric imaging (NPPI) protocol for adolescent patients (Figure 2). The rationale for the presented vision is rooted in the research successes described above, with the expectation that the outlined limitations can be overcome with dedicated work of researchers around the world. The standardized protocol could consist of the following:
- A 30 min brain scan at a 3T MRI scanner that would include a localizer scan, a T1-weighted sequence, a 55-direction diffusion-weighted sequence and an eyes-closed resting-state fMRI sequence.
- A quality control and safety read of the scan by a radiologist.
- Network construction and a computer-based calculation of the structural and functional brain network metrics (e.g., using the Brain Connectivity Toolbox brain-connectivity-toolbox.net) (Rubinov and Sporns, 2010) that can be compared to the normative data by the pediatrician (e.g., using a platform like BRIDGE https://bridge.ucsf.edu).
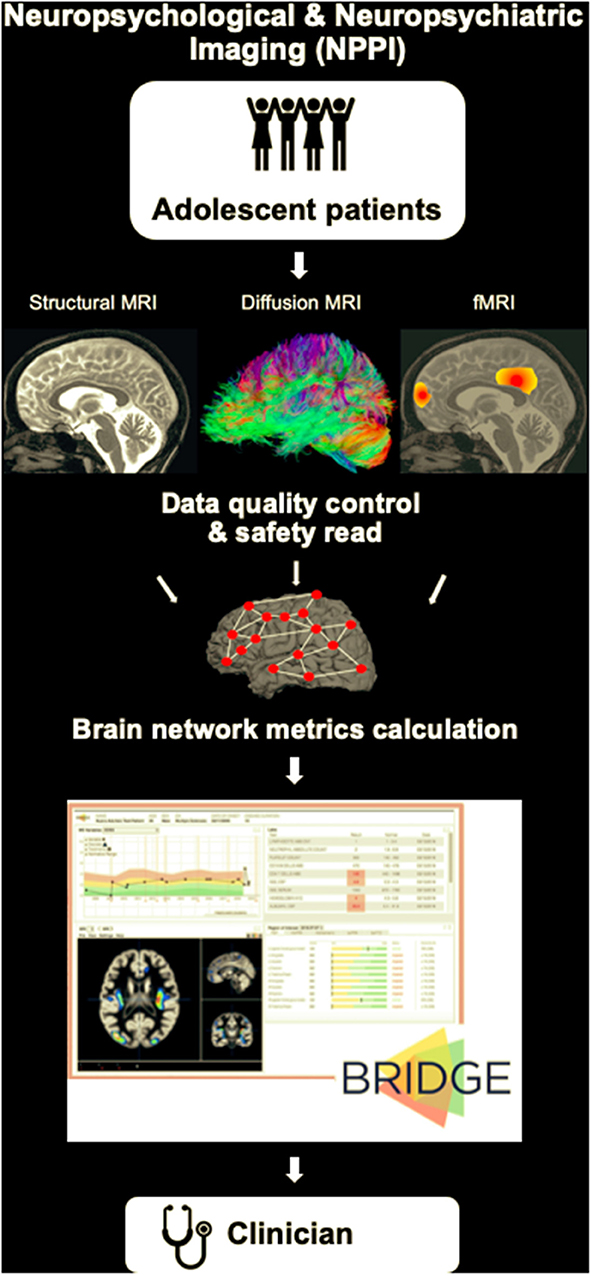
Figure 2. A schematic of our vision for a routine neuropsychological and neuropsychiatric imaging (NPPI) protocol for adolescent patients. The standardized MRI protocol could consist, for example, of a 30 min brain scan at a 3T MRI scanner that would include a localizer scan, a T1-weighted sequence, a 55-direction diffusion-weighted sequence and an eyes-closed resting-state fMRI sequence. The MRI scan will be followed by a quality control and safety read of the scan by a radiologist. Next, connectivity matrices will be derived, and structural and functional brain network metrics will be calculated. Platforms like BRIDGE (https://bridge.ucsf.edu) can be used to integrate the obtained data and provide the output to the clinician (pediatrician) by means of organized dashboards.
The chosen MRI sequences are an example of an easily standardizable set of sequences that in a short time could provide structural and functional connectivity data of sufficient quality. The choice of the 55-direction diffusion-weighted sequence is based on its suitability for high angular resolution diffusion imaging (HARDI) analyses that can help resolve crossing fibers (Tuch et al., 2002). The resting-state fMRI sequence can realistically be performed at any imaging center, and it can potentially allow for derivation of task-based information (Tavor et al., 2016).
The protocol can be offered to all adolescent patients without MRI contraindications (e.g., cardiac pacemakers, braces, etc.) to meet two goals:
a) To help generate the differential diagnosis for an individual patient or assess risk.
b) To personalize treatment (predict treatment outcome) for an individual patient.
For improved diagnostic/predictive accuracy, the scan can be potentially conducted at two different times or even once per year during the adolescent years: 14y.o., 15y.o., 16y.o., 17y.o. (Kessler et al., 2016), especially since the age of onset of different psychiatric disorders can vary. Normative modeling can provide a way to map deviations from an expected pattern at the individual level (Kessler et al., 2016; Marquand et al., 2019). Whereas identifying MRI-based “biotypes” as an alternative to DSM categories is one approach to make diagnosis using NPPI in the future, another approach would be to measure network aberration along several continuous NIMH RDoC dimensions and creating profiles that would inform risks similar to high blood pressure in cardiology, and inform clinical care by suggesting the optimal treatment choice for an individual patient (Williams, 2017).
While there are multiple steps necessary to implement this vision, the common concern that is raised when considering imaging is the cost of the MRI procedure, which we will address in the following section.
Here, we present a cost-benefit analysis of conducting routine NPPI in adolescents using only a single DSM diagnosis, MDD, as an example (Supplementary Figure 1).
Adolescent depression can be a significant economic burden to patients, families, and society. A cost-of-illness study on adolescent depression in the U.S. estimates direct costs of $1,120 and indirect costs of $310 per year, when accounting for the effects of school absences and parental lost days at work (Domino et al., 2009; Beecham, 2014). Furthermore, adolescents with depression are likely to have higher healthcare costs in other domains, due to more contacts with other healthcare providers (Lynch and Clarke, 2006). These ramifications often continue into adulthood as individuals who had depression in adolescence continue to be associated with higher healthcare utilization and increased work impairment in young adulthood (Keenan-Miller et al., 2007). Costs of adult depression in the U.S. are over $210.5 billion annually (Greenberg et al., 2015). This suggests an economic argument for timely identification and treatment of adolescent depression and for considering the costs of adult depression when weighing costs of MR imaging against benefits.
We estimate the costs of a NPPI scan at $400 per patient. This estimate is based on the University of California San Francisco (UCSF) 3T MRI external recharge rates: $350/30 min (https://radiology.ucsf.edu/research/core-services/7T-3T-MB) + $50–75 for reading for incidental findings (estimate provided by Research Radiology, San Francisco, USA). While scheduling short 30 min time slots at an MRI scanner may currently not be possible everywhere, streamlined procedures and even cost-reducing solutions such as dedicated head-only scanners (Foo et al., 2018) can be leveraged to increase throughput in the future. In contrast to a conventional MRI, the scan will not need to be read diagnostically by a neuroradiologist; instead a neuroradiologist will need to perform image quality control and a “safety read” for incidental findings. The brain network metrics will be calculated using a computer algorithm. In case of incidental findings, such as an indication of a tumor, further examination and/or treatment may be indicated. The effectiveness of the NPPI will depend on the sensitivity and specificity of the biomarkers. An accuracy of 80% (both sensitivity and specificity) is generally considered to characterize a clinically useful biomarker (Savitz et al., 2013). The adolescent brain connectivity papers reviewed above where single-subject prediction analysis was performed displayed biomarker accuracy in this range (80–83%) (Kessler et al., 2016; Tymofiyeva et al., 2019). Importantly, several single-subject prediction studies in adults that demonstrated MRI's potential to predict treatment response also studied for comparison the predictive value of demographic and clinical variables—as these variables are more readily available and would be a cheaper solution (Månsson et al., 2015; Thompson et al., 2015; Drysdale et al., 2017). These studies found that these demographic and clinical variables failed as predictors of clinical improvement after treatment (Månsson et al., 2015; Thompson et al., 2015; Drysdale et al., 2017).
Based on U.S. Census Bureau estimates, there were 4.1 million 14-year-olds in the U.S. in 2017 (US Census Bureau, 2017). If we performed a routine NPPI scan on every 14-year-old in the U.S. annually, we estimate a cost of $1.6 billion per year, based on an estimated cost of $400 per MRI scan. The estimated cost of adult MDD in the U.S. is over $210.5 billion annually (Greenberg et al., 2015). This means that NPPI would only have to help prevent (through early detection and personalized treatment) ~0.8% of adult MDD cases to completely cover its costs. Adolescence is the optimal time to intervene because it is when MDD often begins, and it increases the risk of adult depression by a factor of 2- to 3-fold (Pine et al., 1998).
In the calculation above we assumed that we would routinely scan every 14-year-old. This is an extreme scenario, whereas a more plausible scenario would be to scan only at-risk youth and youth presenting with clinically significant problems. This more targeted approach would reduce the estimated total cost of NPPI that we presented above. Given the significant costs of teen MDD to the patient, family and society, the cost of an MRI scan is well worth the benefit in terms of decreased prolonged emotional pain and suffering, lower risk of adult MDD and healthcare costs, and improved health and productivity of the patient. As discussed above, among many other problems, MDD elevates the risk for cardiovascular disease in both adolescents (Goldstein et al., 2015) and adults (Penninx, 2017), and MDD increases the medical costs of treating and managing primary care illnesses such as diabetes in adolescents (Stewart et al., 2005). Additionally, MRI scans are routinely done for medical conditions (e.g., lower back pain and knee injuries) that do not have the potentially devastating consequences of MDD (e.g., suicide). Another example is routine prenatal ultrasound imaging visits that are done with all, not just at-risk, pregnant women. Thus, once valid and reliable MRI biomarkers have been developed and tested for teen MDD, the cost of an MRI scan should not be a justifiable impediment since MRI scans are frequently performed for much less costly and devastating medical conditions. Medical insurance groups or Health Maintenance Organizations (HMOs) may consider this approach as a means of reducing overall healthcare costs, since early prevention could reduce the incidence of adult MDD, which is very costly to both the patient and healthcare system. We also wish to emphasize that our conservative estimate only focuses on MDD; however, many other psychiatric conditions can be potentially assessed using the same MRI scan.
What is needed for this vision to be implemented? We briefly present below a roadmap of the steps required for this vision to be implemented.
a) A large normative database. As discussed above, the field is moving toward collecting big data (that often include a replication sample). The ABCD study has already started to release MRI neuroimaging datasets for 10,000 youth who will be followed longitudinally and scanned every 2-years over a total duration of 10-years. As mentioned above, other initiatives such as ENIGMA and IMAGEN can also provide important big datasets.
b) A set of NPPI-derived metrics, their sensitivity/specificity, and guidelines on how to combine them with symptom-based information.
c) Consensus guidelines for when NPPI is indicated (expert consensus panel).
d) Required qualifications and pipeline for NPPI + data quality and safety read + metrics calculation. There is currently a high heterogeneity of analysis pipelines used by researchers to derive brain connectivity matrices, which can lead to large discrepancies between the resulting structural (Qi et al., 2015) and functional (Carp, 2012) network metrics. In addition, choice of brain parcellation and edge weights will also affect test-retest reliability of the resulting metrics (Cammoun et al., 2012; Yuan et al., 2019). Development of a standardized, objective, and publicly available pipeline is a necessary step. Once the connectivity matrices are derived, network metrics can be calculated, e.g., using the Brain Connectivity Toolbox (brain-connectivity-toolbox.net) (Rubinov and Sporns, 2010). The Brain Connectivity Toolbox is a MATLAB toolbox for complex-network analysis of structural and functional brain connectivity datasets, which is widely used by brain-imaging researchers and has been incorporated in many projects, including the Human Connectome Project. Platforms like BRIDGE (https://bridge.ucsf.edu) can be used to integrate the obtained data and provide the output to the clinician by means of organized dashboards. Required qualifications for data quality assessment and safety reads need to be specified.
e) CPT code. The Current Procedural Terminology (CPT) code set is a medical code set maintained by the American Medical Association through the CPT Editorial Panel. This step is required to enable insurance companies to pay for adolescent NPPI (e.g., similar to ultrasound for neonates).
At present, utilization of neuroimaging biomarkers for clinical practice is restricted to neurological conditions such as pre-surgical evaluation of epilepsy, differential diagnosis of coma, and brain-computer interfaces for locked-in patients (Arslan, 2018). For psychiatric conditions, this is yet to be established for routine clinical applications. Because adolescence is an especially vulnerable time for the development of many important psychiatric disorders, this period presents an especially important opportunity to clinically intervene. In this perspective article, we assess the MRI-based brain connectivity literature over the last 5-years that provides insights into development of psychiatric disorders in adolescents. While the subject numbers are still small and the focus on group differences as opposed to individual subject-based predictions prevails, the reviewed literature demonstrates the potential of MRI to diagnose psychiatric disorders, predict their development, and predict response to clinical treatment. We believe that the continuous progress in neuroimaging techniques together with the ongoing well-coordinated large-scale neuroimaging studies of adolescent brain development will help identify robust biomarkers for personalized/stratified medicine (Kapur et al., 2012) with a key focus on prevention. Inspired by this significant potential, we offer a vision for the role that neuroimaging may play in pediatrics and primary care in the future: a routine NPPI protocol for adolescent patients that could save significant costs to the patients, their families, and society, and significantly reduce suffering that often results from undiagnosed and misdiagnosed adolescent psychiatric disorders. The proposed vision can also help offer preventative measures to at-risk youth in a targeted manner—e.g., by recommending the Training for Awareness, Resilience and Action (TARA) (Henje Blom et al., 2014, 2017) to youth at risk for developing depression. Insurance companies are more likely to reimburse such preventative measures when numerical cut-offs are used, as will be provided by our proposed NPPI. The roadmap we provide in paper can help accomplish this endeavor.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author(s).
OT, DX, CH, and TY conceptualized the article. VZ performed literature search. OT, C-ML, and TY performed cost-benefit analysis. OT created figures. OT, VZ, C-ML, DX, CH, and TY wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Center for Complementary and Integrative Health (NCCIH) R21AT009173 and R61AT009864 to OT and TY; by the National Center for Advancing Translational Sciences (CTSI), National Institutes of Health, through UCSF-CTSI UL1TR001872; by the American Foundation for Suicide Prevention (AFSP) SRG-1-141-18 to OT and TY; by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) R01HD072074 to DX, CH, and OT; by UCSF Research Evaluation and Allocation Committee (REAC) and J. Jacobson Fund to OT, TY, and DX; by the Fahs-Beck Fund for Research and Experimentation at The New York Community Trust to OT; by the National Institute of Mental Health (NIMH) R01MH085734 and the Brain and Behavior Research Foundation (formerly NARSAD) to TY.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2020.00237/full#supplementary-material
Adluru, N., Luo, Z., Van Hulle, C. A., Schoen, A. J., Davidson, R. J., Alexander, A. L., et al. (2017). Anxiety-related experience-dependent white matter structural differences in adolescence: a monozygotic twin difference approach. Sci. Rep. 7:8749. doi: 10.1038/s41598-017-08107-6
Alarcón, G., Pfeifer, J. H., Fair, D. A., and Nagel, B. J. (2018). Adolescent gender differences in cognitive control performance and functional connectivity between default mode and fronto-parietal networks within a self-referential context. Front. Behav. Neurosci. 12:73. doi: 10.3389/fnbeh.2018.00073
Arbabshirani, M. R., Plis, S., Sui, J., and Calhoun, V. D. (2017). Single subject prediction of brain disorders in neuroimaging: promises and pitfalls. Neuroimage 145, 137–165. doi: 10.1016/j.neuroimage.2016.02.079
Armstrong, T. D., and Costello, E. J. (2002). Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J. Consult. Clin. Psychol. 70, 1224–1239. doi: 10.1037/0022-006X.70.6.1224
Arslan, A. (2018). “Application of neuroimaging in the diagnosis and treatment of depression,” in Understanding Depression: Clinical Manifestations, Diagnosis and Treatment, Vol. 2, ed Y. K. Kim (Singapore: Springer Singapore), 69–81.
Audrain-McGovern, J., Rodriguez, D., and Kassel, J. D. (2009). Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction 104, 1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x
Bai, F., Shu, N., Yuan, Y., Shi, Y., Yu, H., Wu, D., et al. (2012). Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J. Neurosci. 32, 4307–4318. doi: 10.1523/JNEUROSCI.5061-11.2012
Balevich, E. C., Haznedar, M. M., Wang, E., Newmark, R. E., Bloom, R., Schneiderman, J. S., et al. (2015). Corpus callosum size and diffusion tensor anisotropy in adolescents and adults with schizophrenia. Psychiatry Res. 231, 244–251. doi: 10.1016/j.pscychresns.2014.12.005
Bebko, G., Bertocci, M., Chase, H., Dwojak, A., Bonar, L., Almeida, J., et al. (2015). Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res. 231, 77–86. doi: 10.1016/j.pscychresns.2014.10.015
Bédard, A.-C. V., Newcorn, J. H., Clerkin, S. M., Krone, B., Fan, J., Halperin, J. M., et al. (2014). Reduced prefrontal efficiency for visuospatial working memory in attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 53, 1020–1030.e6. doi: 10.1016/j.jaac.2014.05.011
Beecham, J. (2014). Annual research review: child and adolescent mental health interventions: a review of progress in economic studies across different disorders. J. Child Psychol. Psychiatry 55, 714–732. doi: 10.1111/jcpp.12216
Boets, B., Van Eylen, L., Sitek, K., Moors, P., Noens, I., Steyaert, J., et al. (2018). Alterations in the inferior longitudinal fasciculus in autism and associations with visual processing: a diffusion-weighted MRI study. Mol. Autism 9:10. doi: 10.1186/s13229-018-0188-6
Cammoun, L., Gigandet, X., Meskaldji, D., Thiran, J. P., Sporns, O., Do, K. Q., et al. (2012). Mapping the human connectome at multiple scales with diffusion spectrum MRI. J. Neurosci. Methods 203, 386–397. doi: 10.1016/j.jneumeth.2011.09.031
Carp, J. (2012). On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front. Neurosci. 6:149. doi: 10.3389/fnins.2012.00149
Casey, B. J., Cannonier, T., Conley, M. I., Cohen, A. O., Barch, D. M., Heitzeg, M. M., et al. (2018). The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32, 43–54. doi: 10.1016/j.dcn.2018.03.001
Chang, K., Garrett, A., Kelley, R., Howe, M., Sanders, E. M., Acquaye, T., et al. (2017). Anomalous prefrontal-limbic activation and connectivity in youth at high-risk for bipolar disorder. J. Affect. Disord. 222, 7–13. doi: 10.1016/j.jad.2017.05.051
Chattopadhyay, S., Tait, R., Simas, T., van Nieuwenhuizen, A., Hagan, C. C., Holt, R. J., et al. (2017). Cognitive behavioral therapy lowers elevated functional connectivity in depressed adolescents. EBioMedicine 17, 216–222. doi: 10.1016/j.ebiom.2017.02.010
Chen, H., Uddin, L. Q., Duan, X., Zheng, J., Long, Z., Zhang, Y., et al. (2017). Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism Res. 10, 1776–1786. doi: 10.1002/aur.1834
Chuang, J. -Y., Hagan, C. C., Murray, G. K., Graham, J. M. E., Ooi, C., Tait, R., et al. (2017). Adolescent major depressive disorder: neuroimaging evidence of sex difference during an affective Go/No-Go Task. Front. Psychiatry 8:119. doi: 10.3389/fpsyt.2017.00119
Cisler, J. M., Privratsky, A., Smitherman, S., Herringa, R. J., and Kilts, C. D. (2018). Large-scale brain organization during facial emotion processing as a function of early life trauma among adolescent girls. Neuroimage Clin. 17, 778–785. doi: 10.1016/j.nicl.2017.12.001
Cisler, J. M., Scott Steele, J., Smitherman, S., Lenow, J. K., and Kilts, C. D. (2013). Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiatry Res. 214, 238–246. doi: 10.1016/j.pscychresns.2013.06.003
Cisler, J. M., Sigel, B. A., Kramer, T. L., Smitherman, S., Vanderzee, K., Pemberton, J., et al. (2016). Modes of large-scale brain network organization during threat processing and posttraumatic stress disorder symptom reduction during TF-CBT among adolescent girls. PLoS ONE. 11:e0159620. doi: 10.1371/journal.pone.0159620
Clasen, P. C., Beevers, C. G., Mumford, J. A., and Schnyer, D. M. (2014). Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev. Cogn. Neurosci. 7, 13–22. doi: 10.1016/j.dcn.2013.10.008
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Routledge.
Copeland, W. E., Miller-Johnson, S., Keeler, G., Angold, A., and Costello, E. J. (2007). Childhood psychiatric disorders and young adult crime: a prospective, population-based study. Am. J. Psychiatry 164, 1668–1675. doi: 10.1176/appi.ajp.2007.06122026
Crum, R. M., Green, K. M., Storr, C. L., Chan, Y.-F., Ialongo, N., Stuart, E. A., et al. (2008). Depressed mood in childhood and subsequent alcohol use through adolescence and young adulthood. Arch. Gen. Psychiatry 65, 702–712. doi: 10.1001/archpsyc.65.6.702
Cullen, K. R., Klimes-Dougan, B., Vu, D. P., Westlund Schreiner, M., Mueller, B. A., Eberly, L. E., et al. (2016). Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. J. Child Adolesc. Psychopharmacol. 26, 705–712. doi: 10.1089/cap.2015.0232
Das, P., Coulston, C. M., Bargh, D. M., Tanious, M., Phan, K. L., Calhoun, V. D., et al. (2013). Neural antecedents of emotional disorders: a functional magnetic resonance imaging study of subsyndromal emotional symptoms in adolescent girls. Biol. Psychiatry 74, 265–272. doi: 10.1016/j.biopsych.2013.01.030
Davey, C. G., Whittle, S., Harrison, B. J., Simmons, J. G., Byrne, M. L., Schwartz, O. S., et al. (2015). Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol. Med. 45, 1001–1009. doi: 10.1017/S0033291714002001
Diwadkar, V. A., Bakshi, N., Gupta, G., Pruitt, P., White, R., and Eickhoff, S. B. (2014). Dysfunction and dysconnection in cortical-striatal networks during sustained attention: genetic risk for schizophrenia or bipolar disorder and its impact on brain network function. Front. Psychiatry 5:50. doi: 10.3389/fpsyt.2014.00050
Domino, M. E., Burns, B. J., Mario, J., Reinecke, M. A., Vitiello, B., Weller, E. B., et al. (2009). Service use and costs of care for depressed adolescents: who uses and who pays? J. Clin. Child Adolesc. Psychol. 38, 826–836. doi: 10.1080/15374410903259023
Dorfman, J., Benson, B., Farber, M., Pine, D., and Ernst, M. (2016). Altered striatal intrinsic functional connectivity in pediatric anxiety. Neuropsychologia 85, 159–168. doi: 10.1016/j.neuropsychologia.2016.03.019
Drysdale, A. T., Grosenick, L., Downar, J., Dunlop, K., Mansouri, F., Meng, Y., et al. (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23, 28–38. doi: 10.1038/nm.4246
Ellis, R., Seal, M. L., Adamson, C., Beare, R., Simmons, J. G., Whittle, S., et al. (2017). Brain connectivity networks and longitudinal trajectories of depression symptoms in adolescence. Psychiatry Res. Neuroimag. 260, 62–69. doi: 10.1016/j.pscychresns.2016.12.010
Fitzgerald, K. D., Liu, Y., Reamer, E. N., Taylor, S. F., and Welsh, R. C. (2014). Atypical frontal-striatal-thalamic circuit white matter development in pediatric obsessive-compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry 53, 1225–1233 e1-e9. doi: 10.1016/j.jaac.2014.08.010
Foo, T. K. F., Laskaris, E., Vermilyea, M., Xu, M., Thompson, P., Conte, G., et al. (2018). Lightweight, compact, and high-performance 3T MR system for imaging the brain and extremities. Magn. Reson. Med. 80, 2232–2245. doi: 10.1002/mrm.27175
Fowler, C. H., Miernicki, M. E., Rudolph, K. D., and Telzer, E. H. (2017). Disrupted amygdala-prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Dev. Cogn. Neurosci. 27, 99–106. doi: 10.1016/j.dcn.2017.09.002
Friedman, A. L., Burgess, A., Ramaseshan, K., Easter, P., Khatib, D., Chowdury, A., et al. (2017). Brain network dysfunction in youth with obsessive-compulsive disorder induced by simple uni-manual behavior: the role of the dorsal anterior cingulate cortex. Psychiatry Res. 260, 6–15. doi: 10.1016/j.pscychresns.2016.12.005
Fryer, S. L., Roach, B. J., Ford, J. M., Donaldson, K. R., Calhoun, V. D., Pearlson, G. D., et al. (2019). Should i stay or should i go? fmri study of response inhibition in early illness schizophrenia and risk for psychosis. Schizophr Bull. 45, 158–68. doi: 10.1093/schbul/sbx198
Gao, W., Jiao, Q., Lu, S., Zhong, Y., Qi, R., Lu, D., et al. (2014). Alterations of regional homogeneity in pediatric bipolar depression: a resting-state fMRI study. BMC Psychiatry 14:222. doi: 10.1186/s12888-014-0222-y
Geng, H., Wu, F., Kong, L., Tang, Y., Zhou, Q., Chang, M., et al. (2016). Disrupted structural and functional connectivity in prefrontal-hippocampus circuitry in first-episode medication-naïve adolescent depression. PLoS ONE 11:e0148345. doi: 10.1371/journal.pone.0148345
Gold, A. L., Shechner, T., Farber, M. J., Spiro, C. N., Leibenluft, E., Pine, D. S., et al. (2016). Amygdala-cortical connectivity: associations with anxiety, development, and threat. Depress Anxiety 33, 917–926. doi: 10.1002/da.22470
Goldstein, B. I., Carnethon, M. R., Matthews, K. A., McIntyre, R. S., Miller, G. E., Raghuveer, G., et al. (2015). Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the american heart association. Circulation 132, 965–986. doi: 10.1161/CIR.0000000000000229
Gong, Q., and He, Y. (2015). Depression, neuroimaging and connectomics: a selective overview. Biol. Psychiatry 77, 223–235. doi: 10.1016/j.biopsych.2014.08.009
Green, S. A., Hernandez, L., Bookheimer, S. Y., and Dapretto, M. (2016). Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J. Am. Acad. Child Adolesc. Psychiatry 55, 618–626.e1. doi: 10.1016/j.jaac.2016.04.013
Green, S. A., Hernandez, L., Bookheimer, S. Y., and Dapretto, M. (2017). Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res. 10, 801–809. doi: 10.1002/aur.1726
Greenberg, P. E., Fournier, A.-A., Sisitsky, T., Pike, C. T., and Kessler, R. C. (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 76, 155–162. doi: 10.4088/JCP.14m09298
Gruner, P., Vo, A., Argyelan, M., Ikuta, T., Degnan, A. J., John, M., et al. (2014). Independent component analysis of resting state activity in pediatric obsessive-compulsive disorder. Hum. Brain Mapp. 35, 5306–5315. doi: 10.1002/hbm.22551
Guo, X., Duan, X., Long, Z., Chen, H., Wang, Y., Zheng, J., et al. (2016). Decreased amygdala functional connectivity in adolescents with autism: a resting-state fMRI study. Psychiatry Res. Neuroimag. 257, 47–56. doi: 10.1016/j.pscychresns.2016.10.005
Hafeman, D., Bebko, G., Bertocci, M. A., Fournier, J. C., Chase, H. W., Bonar, L., et al. (2017). Amygdala-prefrontal cortical functional connectivity during implicit emotion processing differentiates youth with bipolar spectrum from youth with externalizing disorders. J. Affect. Disord. 208, 94–100. doi: 10.1016/j.jad.2016.09.064
Hagmann, P., Cammoun, L., Gigandet, X., Gerhard, S., Grant, P. E., Wedeen, V., et al. (2010). MR connectomics: principles and challenges. J. Neurosci. Methods 194, 34–45. doi: 10.1016/j.jneumeth.2010.01.014
Hamm, L. L., Jacobs, R. H., Johnson, M. W., Fitzgerald, D. A., Fitzgerald, K. D., Langenecker, S. A., et al. (2014). Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol. Mood Anxiety Disord. 4:15. doi: 10.1186/s13587-014-0015-4
Harlalka, V., Bapi, R. S., Vinod, P. K., and Roy, D. (2018). Age, disease and their interaction effects on intrinsic connectivity of children and adolescents in autism spectrum disorder using functional connectomics. Brain Connect. 8, 407–419. doi: 10.1089/brain.2018.0616
Henje Blom, E., Connolly, C. G., Ho, T. C., LeWinn, K. Z., Mobayed, N., Han, L., et al. (2015). Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. J. Affect Disord. 178, 215–223. doi: 10.1016/j.jad.2015.03.012
Henje Blom, E., Duncan, L. G., Ho, T. C., Connolly, C. G., LeWinn, K. Z., Chesney, M., et al. (2014). The development of an RDoC-based treatment program for adolescent depression: “Training for Awareness, Resilience, and Action” (TARA). Front. Hum. Neurosci. 8:630. doi: 10.3389/fnhum.2014.00630
Henje Blom, E., Tymofiyeva, O., Chesney, M. A., Ho, T. C., Moran, P., Connolly, C. G., et al. (2017). Feasibility and preliminary efficacy of a novel RDoC-based treatment program for adolescent depression: “Training for Awareness Resilience and Action” (TARA)—a pilot study. Front Psychiatry 7:208. doi: 10.3389/fpsyt.2016.00208
Ho, T. C., Sacchet, M. D., Connolly, C. G., Margulies, D. S., Tymofiyeva, O., Paulus, M. P., et al. (2017). Inflexible functional connectivity of the dorsal anterior cingulate cortex in adolescent major depressive disorder. Neuropsychopharmacology 42, 2434–2445. doi: 10.1038/npp.2017.103
Ho, T. C., Yang, G., Wu, J., Cassey, P., Brown, S. D., Hoang, N., et al. (2014). Functional connectivity of negative emotional processing in adolescent depression. J. Affect Disord. 155, 65–74. doi: 10.1016/j.jad.2013.10.025
Hong, J., Park, B.-Y., Cho, H.-H., and Park, H. (2017). Age-related connectivity differences between attention deficit and hyperactivity disorder patients and typically developing subjects: a resting-state functional MRI study. Neural. Regen. Res. 12, 1640–1647. doi: 10.4103/1673-5374.217339
Hulvershorn, L. A., Mennes, M., Castellanos, F. X., Di Martino, A., Milham, M. P., Hummer, T. A., et al. (2014). Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 53, 351–361.e1. doi: 10.1016/j.jaac.2013.11.012
Hwang, S., White, S. F., Nolan, Z. T., Craig Williams, W., Sinclair, S., and Blair, R. J. R. (2015). Executive attention control and emotional responding in attention-deficit/hyperactivity disorder–a functional MRI study. Neuroimage Clin. 9, 545–554. doi: 10.1016/j.nicl.2015.10.005
Iadipaolo, A. S., Marusak, H. A., Paulisin, S. M., Sala-Hamrick, K., Crespo, L. M., Elrahal, F., et al. (2018). Distinct neural correlates of trait resilience within core neurocognitive networks in at-risk children and adolescents. Neuroimage Clin. 20, 24–34. doi: 10.1016/j.nicl.2018.06.026
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379
Jack, A., and Morris, J. P. (2014). Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev. Cogn. Neurosci. 10, 77–92. doi: 10.1016/j.dcn.2014.08.001
Jacobs, R. H., Jenkins, L. M., Gabriel, L. B., Barba, A., Ryan, K. A., Weisenbach, S. L., et al. (2014). Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS ONE 9:e104366. doi: 10.1371/journal.pone.0104366
Jacobs, R. H., Watkins, E. R., Peters, A. T., Feldhaus, C. G., Barba, A., Carbray, J., et al. (2016). Targeting ruminative thinking in adolescents at risk for depressive relapse: rumination-focused cognitive behavior therapy in a pilot randomized controlled trial with resting state fMRI. PLoS ONE 11:e0163952. doi: 10.1371/journal.pone.0163952
Jacobson McEwen, S. C., Connolly, C. G., Kelly, A. M. C., Kelleher, I., O'Hanlon, E., Clarke, M., et al. (2014). Resting-state connectivity deficits associated with impaired inhibitory control in non-treatment-seeking adolescents with psychotic symptoms. Acta Psychiatr Scand. 129, 134–142. doi: 10.1111/acps.12141
James, A., Joyce, E., Lunn, D., Hough, M., Kenny, L., Ghataorhe, P., et al. (2016). Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. Eur. Psychiatry 35, 32–8. doi: 10.1016/j.eurpsy.2016.01.2426
Jann, K., Hernandez, L. M., Beck-Pancer, D., McCarron, R., Smith, R. X., Dapretto, M., et al. (2015). Altered resting perfusion and functional connectivity of default mode network in youth with autism spectrum disorder. Brain Behav. 5:e00358. doi: 10.1002/brb3.358
Jarcho, J. M., Romer, A. L., Shechner, T., Galvan, A., Guyer, A. E., Leibenluft, E., et al. (2015). Forgetting the best when predicting the worst: preliminary observations on neural circuit function in adolescent social anxiety. Dev. Cogn. Neurosci. 13, 21–31. doi: 10.1016/j.dcn.2015.03.002
Jin, J., Narayanan, A., Perlman, G., Luking, K., DeLorenzo, C., Hajcak, G., et al. (2017). Orbitofrontal cortex activity and connectivity predict future depression symptoms in adolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2, 610–618. doi: 10.1016/j.bpsc.2017.02.002
Johnston, J. A. Y., Wang, F., Liu, J., Blond, B. N., Wallace, A., Liu, J., et al. (2017). Multimodal Neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am. J. Psychiatry 174, 667–675. doi: 10.1176/appi.ajp.2016.15050652
Joshi, G., Arnold Anteraper, S., Patil, K. R., Semwal, M., Goldin, R. L., Furtak, S. L., et al. (2017). Integration and segregation of default mode network resting-state functional connectivity in transition-age males with high-functioning autism spectrum disorder: a proof-of-concept study. Brain Connect 7, 558–573. doi: 10.1089/brain.2016.0483
Kaczkurkin, A. N., Moore, T. M., Calkins, M. E., Ciric, R., Detre, J. A., Elliott, M. A., et al. (2018). Common and dissociable regional cerebral blood flow differences associate with dimensions of psychopathology across categorical diagnoses. Mol. Psychiatry 23, 1981–1989. doi: 10.1038/mp.2017.174
Kapur, S., Phillips, A. G., and Insel, T. R. (2012). Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol. Psychiatry 17, 1174–1179. doi: 10.1038/mp.2012.105
Keding, T. J., and Herringa, R. J. (2016). Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 2903–2912. doi: 10.1038/npp.2016.104
Keenan-Miller, D., Hammen, C. L., and Brennan, P. A. (2007). Health outcomes related to early adolescent depression. J. Adolesc. Health 41, 256–262. doi: 10.1016/j.jadohealth.2007.03.015
Kessler, D., Angstadt, M., and Sripada, C. (2016). Growth charting of brain connectivity networks and the identification of attention impairment in youth, JAMA Psychiatry 73, 481–489. doi: 10.1001/jamapsychiatry.2016.0088
Kim, S. M., Park, S. Y., Kim, Y. I., Son, Y. D., Chung, U.-S., Min, K. J., et al. (2016). Affective network and default mode network in depressive adolescents with disruptive behaviors. Neuropsychiatr. Dis. Treat. 12, 49–56. doi: 10.2147/NDT.S95541
Klimes-Dougan, B., Westlund Schreiner, M., Thai, M., Gunlicks-Stoessel, M., Reigstad, K., and Cullen, K. R. (2018). Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 194–202. doi: 10.1016/j.pnpbp.2017.10.015
Korgaonkar, M. S., Fornito, A., Williams, L. M., and Grieve, S. M. (2014). Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol. Psychiatry 76, 567–574. doi: 10.1016/j.biopsych.2014.02.018
Kujawa, A., Wu, M., Klumpp, H., Pine, D. S., Swain, J. E., Fitzgerald, K. D., et al. (2016). Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biol. Psychiatry Cogn. Neurosci Neuroimag. 1, 345–352. doi: 10.1016/j.bpsc.2016.01.006
Lehrer, J. A., Shrier, L. A., Gortmaker, S., and Buka, S. (2006). Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics 118, 189–200. doi: 10.1542/peds.2005-1320
LeWinn, K. Z., Connolly, C. G., Wu, J., Drahos, M., Hoeft, F., Ho, T. C., et al. (2014). White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J. Am. Acad. Child Adolesc. Psychiatry 53, 899–909. doi: 10.1016/j.jaac.2014.04.021
LeWinn, K. Z., Strigo, I. A., Connolly, C. G., Ho, T. C., Tymofiyeva, O., Sacchet, M. D., et al. (2018). An exploratory examination of reappraisal success in depressed adolescents: preliminary evidence of functional differences in cognitive control brain regions. J. Affect Disord. 240, 155–164. doi: 10.1016/j.jad.2018.07.020
Lewinsohn, P. M., Rohde, P., and Seeley, J. R. (1998). Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin. Psychol. Rev. 18, 765–794.
Li, M., Becker, B., Zheng, J., Zhang, Y., Chen, H., Liao, W., et al. (2019). Dysregulated maturation of the functional connectome in antipsychotic-naïve, first-episode patients with adolescent-onset schizophrenia. Schizophr. Bull. 45, 689–697. doi: 10.1093/schbul/sby063
Liu, R. T., Hernandez, E. M., Trout, Z. M., Kleiman, E. M., and Bozzay, M. L. (2017). Depression, social support, and long-term risk for coronary heart disease in a 13-year longitudinal epidemiological study. Psychiatry Res. 251, 36–40. doi: 10.1016/j.psychres.2017.02.010
Lynch, F. L., and Clarke, G. N. (2006). Estimating the economic burden of depression in children and adolescents. Am. J. Prev. Med. 31(Suppl. 1), S143–S151. doi: 10.1016/j.amepre.2006.07.001
Månsson, K. N. T., Frick, A., Boraxbekk, C.-J., Marquand, A. F., Williams, S. C. R., Carlbring, P., et al. (2015). Predicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Transl. Psychiatry 5:e530. doi: 10.1038/tp.2015.22
Manelis, A., Ladouceur, C. D., Graur, S., Monk, K., Bonar, L. K., Hickey, M. B., et al. (2015). Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain 138, 2777–2790. doi: 10.1093/brain/awv176
Marquand, A. F., Kia, S. M., Zabihi, M., Wolfers, T., Buitelaar, J. K., and Beckmann, C. F. (2019). Conceptualizing mental disorders as deviations from normative functioning. Mol. Psychiatry. 24, 1415–1424. doi: 10.1038/s41380-019-0441-1
Marusak, H. A., Elrahal, F., Peters, C. A., Kundu, P., Lombardo, M. V., Calhoun, V. D., et al. (2018). Mindfulness and dynamic functional neural connectivity in children and adolescents. Behav. Brain Res. 15, 211–218. doi: 10.1016/j.bbr.2017.09.010
Marusak, H. A., Hatfield, J. R. B., Thomason, M. E., and Rabinak, C. A. (2017). Reduced ventral tegmental area-hippocampal connectivity in children and adolescents exposed to early Threat. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2, 130–137. doi: 10.1016/j.bpsc.2016.11.002
Maughan, B., Collishaw, S., and Stringaris, A. (2013). Depression in childhood and adolescence. J. Can. Acad. Child Adolesc. Psychiatry. 22, 35–40.
McCrory, E. J., Gerin, M. I., and Viding, E. (2017). Annual research review: childhood maltreatment, latent vulnerability the shift to preventative psychiatry – the contribution of functional brain imaging. J. Child Psychol. Psychiatry 58, 338–357. doi: 10.1111/jcpp.12713
Morgan, J. K., Shaw, D. S., Olino, T. M., Musselman, S. C., Kurapati, N. T., and Forbes, E. E. (2016). History of depression and frontostriatal connectivity during reward processing in late adolescent boys. J. Clin. Child Adolesc. Psychol. 45, 59–68. doi: 10.1080/15374416.2015.1030753
O'Halloran, L., Cao, Z., Ruddy, K., Jollans, L., Albaugh, M. D., Aleni, A., et al. (2018). Neural circuitry underlying sustained attention in healthy adolescents and in ADHD symptomatology. Neuroimage 169, 395–406. doi: 10.1016/j.neuroimage.2017.12.030
Ordaz, S. J., Goyer, M. S., Ho, T. C., Singh, M. K., and Gotlib, I. H. (2018). Network basis of suicidal ideation in depressed adolescents. J. Affect Disord. 226, 92–99. doi: 10.1016/j.jad.2017.09.021
Osuch, E., Ford, K., Wrath, A., Bartha, R., Neufeld, R., et al. (2014). Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 223, 104–112. doi: 10.1016/j.pscychresns.2014.05.003
Pan, P. M., Sato, J. R., Salum, G. A., Rohde, L. A., Gadelha, A., Zugman, A., et al. (2017). Ventral striatum functional connectivity as a predictor of adolescent depressive disorder in a longitudinal community-based sample. Am. J. Psychiatry 174, 1112–1119. doi: 10.1176/appi.ajp.2017.17040430
Pannekoek, J. N., van der Werff, S. J. A., Meens, P. H. F., van den Bulk, B. G., Jolles, D. D., Veer, I. M., et al. (2014). Aberrant resting-state functional connectivity in limbic and salience networks in treatment–naïve clinically depressed adolescents. J. Child Psychol. Psychiatry. 55, 1317–1327. doi: 10.1111/jcpp.12266
Paquola, C., Bennett, M. R., Hatton, S. N., Hermens, D. F., and Lagopoulos, J. (2017). Utility of the cumulative stress and mismatch hypotheses in understanding the neurobiological impacts of childhood abuse and recent stress in youth with emerging mental disorder. Hum. Brain Mapp. 38, 2709–2721. doi: 10.1002/hbm.23554
Park, B., Kim, J., and Park, H. (2016). Differences in connectivity patterns between child and adolescent attention deficit hyperactivity disorder patients. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 1127–1130. doi: 10.1109/EMBC.2016.7590902
Patriat, R., Birn, R. M., Keding, T. J., and Herringa, R. J. (2016). Default-mode network abnormalities in pediatric posttraumatic stress disorder, J. Am. Acad. Child Adolesc. Psychiatry 55, 319–327. doi: 10.1016/j.jaac.2016.01.010
Penninx, B. W. J. H. (2017). Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 74, 277–286. doi: 10.1016/j.neubiorev.2016.07.003
Pine, D. S., Cohen, P., Gurley, D., Brook, J., and Ma, Y. (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry 55, 56–64. doi: 10.1001/archpsyc.55.1.56
Pitskel, N. B., Bolling, D. Z., Kaiser, M. D., Pelphrey, K. A., and Crowley, M. J. (2014). Neural systems for cognitive reappraisal in children and adolescents with autism spectrum disorder. Dev Cogn Neurosci. 10, 117–128. doi: 10.1016/j.dcn.2014.08.007
Platt, B., Campbell, C. A., James, A. C., Murphy, S. E., Cooper, M. J., and Lau, J. Y. F. (2015). Cognitive reappraisal of peer rejection in depressed versus non-depressed adolescents: functional connectivity differences. J Psychiatr Res. 61, 73–80. doi: 10.1016/j.jpsychires.2014.11.016
Price, R. B., Allen, K. B., Silk, J. S., Ladouceur, C. D., Ryan, N. D., Dahl, R. E., et al. (2016). Vigilance in the laboratory predicts avoidance in the real world: a dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev. Cogn. Neurosci. 19, 128–136. doi: 10.1016/j.dcn.2016.03.001
Price, R. B., Siegle, G. J., Silk, J. S., Ladouceur, C. D., McFarland, A., Dahl, R. E., et al. (2014). Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depress Anxiety 31, 178–87. doi: 10.1002/da.22255
Qi, S., Meesters, S., Nicolay, K., Romeny BM ter, H., and Ossenblok, P. (2015). The influence of construction methodology on structural brain network measures: a review. J Neurosci. Methods 253, 170–182. doi: 10.1016/j.jneumeth.2015.06.016
Qin, J., Wei, M., Liu, H., Yan, R., Luo, G., Yao, Z., et al. (2014). Abnormal brain anatomical topological organization of the cognitive-emotional and the frontoparietal circuitry in major depressive disorder. Magn. Reson. Med. 72, 1397–1407. doi: 10.1002/mrm.25036
Quinlan, E. B., Cattrell, A., Jia, T., Artiges, E., Banaschewski, T., Barker, G., et al. (2017). Psychosocial stress and brain function in adolescent psychopathology. Am. J. Psychiatry 174, 785–94. doi: 10.1176/appi.ajp.2017.16040464
Rao, U., and Chen, L. A. (2009). Characteristics, correlates, and outcomes of childhood and adolescent depressive disorders. Dialog. Clin. Neurosci, 11, 45–62.
Rao, U., Ryan, N. D., Birmaher, B., Dahl, R. E., Williamson, D. E., Kaufman, J., et al. (1995). Unipolar depression in adolescents: clinical outcome in adulthood. J. Am. Acad. Child Adolesc Psychiatry 34, 566–578. doi: 10.1097/00004583-199505000-00009
Rao, U., Ryan, N. D., Dahl, R. E., Birmaher, B., Rao, R., Williamson, D. E., et al. (1999). Factors associated with the development of substance use disorder in depressed adolescents. J. Am. Acad. Child Adolesc. Psychiatry 38, 1109–1117. doi: 10.1097/00004583-199909000-00014
Rao, U., Weissman, M. M., Martin, J. A., and Hammond, R. W. (1993). Childhood depression and risk of suicide: a preliminary report of a longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 32, 21–27. doi: 10.1097/00004583-199301000-00004
Rosenthal, R., and Rosnow, R. L. (1984). Essentials of Behavioral Research: Methods and Data Analysis. New York, NY: McGraw-Hill.
Rosso, I. M., Olson, E. A., Britton, J. C., Stewart, S. E., Papadimitriou, G., Killgore, W. D., et al. (2014). Brain white matter integrity and association with age at onset in pediatric obsessive-compulsive disorder. Biol. Mood Anxiety Disord. 4:13. doi: 10.1186/s13587-014-0013-6
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Rzepa, E., and McCabe, C. (2016). Decreased anticipated pleasure correlates with increased salience network resting state functional connectivity in adolescents with depressive symptomatology. J. Psychiatr. Res. 82, 40–47. doi: 10.1016/j.jpsychires.2016.07.013
Sacchet, M. D., Ho, T. C., Connolly, C. G., Tymofiyeva, O., Lewinn, K. Z., Han, L. K., et al. (2016). Large-scale hypoconnectivity between resting-state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology 41, 2951–2960. doi: 10.1038/npp.2016.76
Sadeghi, M., Khosrowabadi, R., Bakouie, F., Mahdavi, H., Eslahchi, C., and Pouretemad, H. (2017). Screening of autism based on task-free fMRI using graph theoretical approach. Psychiatry Res. Neuroimaging 263, 48–56. doi: 10.1016/j.pscychresns.2017.02.004
Savitz, J. B., Rauch, S. L., and Drevets, W. C. (2013). Clinical application of brain imaging for the diagnosis of mood disorders: the current state of play. Mol. Psychiatry 18, 528–539. doi: 10.1038/mp.2013.25
Scheuer, H., Alarcón, G., Demeter, D. V., Earl, E., Fair, D. A., and Nagel, B. J. (2017). Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Res. Neuroimaging 266, 35–41. doi: 10.1016/j.pscychresns.2017.05.012
Sharp, P. B., and Telzer, E. H. (2017). Structural connectomics of anxious arousal in early adolescence: translating clinical and ethological findings. NeuroImage Clin. 16, 604–609. doi: 10.1016/j.nicl.2017.09.012
Singh, M. K., Chang, K. D., Kelley, R. G., Saggar, M., Reiss, A. L., and Gotlib, I. H. (2014). Early signs of anomalous neural functional connectivity in healthy offspring of parents with bipolar disorder. Bipolar Disord. 16, 678–689. doi: 10.1111/bdi.12221
Sporns, O., Tononi, G., and Kotter, R. (2005). The human connectome: a structural description of the human brain. PLoS Comput. Biol. 1:e42. doi: 10.1371/journal.pcbi.0010042
Stewart, S. M., Rao, U., Emslie, G. J., Klein, D., and White, P. C. (2005). Depressive symptoms predict hospitalization for adolescents with type 1 diabetes mellitus. Pediatrics 115, 1315–1319. doi: 10.1542/peds.2004-1717
Stoddard, J., Gotts, S. J., Brotman, M. A., Lever, S., Hsu, D., Zarate, C., et al. (2016). Aberrant intrinsic functional connectivity within and between corticostriatal and temporal-parietal networks in adults and youth with bipolar disorder. Psychol. Med. 46, 1509–1522. doi: 10.1017/S0033291716000143
Straub, J., Metzger, C. D., Plener, P. L., Koelch, M. G., Groen, G., and Abler, B. (2017). Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. J. Affect Disord. 209, 135–139. doi: 10.1016/j.jad.2016.11.024
Tavor, I., Jones, O. P., Mars, R. B., Smith, S. M., Behrens, T. E., and Jbabdi, S. (2016). Task-free, M. R. I., predicts individual differences in brain activity during task performance. Science 352, 216–220. doi: 10.1126/science.aad8127
Thompson, D. G., Kesler, S. R., Sudheimer, K., Mehta, K. M., Thompson, L. W., Marquett, R. M., et al. (2015). FMRI activation during executive function predicts response to cognitive behavioral therapy in older, depressed adults. Am. J. Geriatr. Psychiatry 23. 13–22. doi: 10.1016/j.jagp.2014.02.001
Thompson, P. M., Andreassen, O. A., Arias-Vasquez, A., Bearden, C. E., Boedhoe, P. S., Brouwer, R. M., et al. (2017). ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage 145, 389–408. doi: 10.1016/j.neuroimage.2015.11.057
Thompson, P. M., Stein, J. L., Medland, S. E., Hibar, D. P., Vasquez, A. A., Renteria, M. E., et al. (2014). The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182. doi: 10.1007/s11682-013-9269-5
Traynor, J. M., Doyle-Thomas, K a. R., Hanford, L. C., Foster, N. E., Tryfon, A., Hyde, K. L., et al. (2018). Indices of repetitive behaviour are correlated with patterns of intrinsic functional connectivity in youth with autism spectrum disorder. Brain Res. 1685, 79–90. doi: 10.1016/j.brainres.2018.02.009
Tuch, D. S., Reese, T. G., Wiegell, M. R., Makris, N., Belliveau, J. W., and Wedeen, V. J. (2002). High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn. Resonance Med. 48, 577–582. doi: 10.1002/mrm.10268
Tymofiyeva, O., Connolly, C. G., Ho, T. C., Sacchet, M. D., Henje Blom, E., LeWinn, K. Z., et al. (2017). DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J. Affect Disord. 207, 18–25. doi: 10.1016/j.jad.2016.09.013
Tymofiyeva, O., Hess, C. P., Ziv, E., Lee, P. N., Glass, H. C., Ferriero, D. M., et al. (2013). A DTI-Based Template-Free Cortical Connectome Study of Brain Maturation. PLoS ONE 8:e63310. doi: 10.1371/journal.pone.0063310
Tymofiyeva, O., Hess, C. P., Ziv, E., Tian, N., Bonifacio, S. L., McQuillen, P. S., et al. (2012). Towards the “Baby Connectome”: mapping the structural connectivity of the newborn brain. PLoS ONE 7:e31029. doi: 10.1371/journal.pone.0031029
Tymofiyeva, O., Yuan, J. P., Huang, C.-Y., Connolly, C. G., Henje Blom, E., Xu, D., et al. (2019). Application of machine learning to structural connectome to predict symptom reduction in depressed adolescents with cognitive behavioral therapy (CBT). Neuroimage Clin. 23:101914. doi: 10.1016/j.nicl.2019.101914
Tymofiyeva, O., Ziv, E., Barkovich, A. J., Hess, C. P., and Xu, D. (2014). Brain without anatomy: construction and comparison of fully network-driven structural MRI connectomes. PLoS ONE 9:e96196. doi: 10.1371/journal.pone.0096196
US Census Bureau (2017). Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States April 1, 2010 to July 1, 2017. Population Estimates. Available online at: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk (accessed September 26, 2018).
Van Dam, N. T., O'Connor, D., Marcelle, E. T., Ho, E. J., Cameron Craddock, R., Tobe, R. H., et al. (2017). Data-driven phenotypic categorization for neurobiological analyses: beyond DSM-5 Labels. Biol. Psychiatry 81, 484–494. doi: 10.1016/j.biopsych.2016.06.027
Velasquez, F., Wiggins, J. L., Mattson, W. I., Martin, D. M., Lord, C., and Monk, C. S. (2017). The influence of 5-HTTLPR transporter genotype on amygdala-subgenual anterior cingulate cortex connectivity in autism spectrum disorder. Dev. Cogn. Neurosci. 24, 12–20. doi: 10.1016/j.dcn.2016.12.002
Wang, C., Lee, J., Ho, N. F., Lim, J. K. W., Poh, J. S., Rekhi, G., et al. (2018). Large-scale network topology reveals heterogeneity in individuals with at risk mental state for psychosis: findings from the longitudinal youth-at-risk study. Cereb. Cortex 28, 4234–4243. doi: 10.1093/cercor/bhx278
Wang, S., Zhan, Y., Zhang, Y., Lv, L., Wu, R., Zhao, J., et al. (2017). Abnormal functional connectivity strength in patients with adolescent-onset schizophrenia: a resting-state fMRI study. Eur. Child Adolesc. Psychiatry 26, 839–845. doi: 10.1007/s00787-017-0958-2
Wang, S., Zhan, Y., Zhang, Y., Lyu, L., Lyu, H., Wang, G., et al. (2018a). Abnormal long- and short-range functional connectivity in adolescent-onset schizophrenia patients: a resting-state fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 445–451. doi: 10.1016/j.pnpbp.2017.08.012
Wang, S., Zhang, Y., Lv, L., Wu, R., Fan, X., Zhao, J., et al. (2018b). Abnormal regional homogeneity as a potential imaging biomarker for adolescent-onset schizophrenia: a resting-state fMRI study and support vector machine analysis. Schizophr Res. 192, 179–184. doi: 10.1016/j.schres.2017.05.038
WHO (2017). Depression. Available online at: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed March 24, 2017).
Williams, L. M. (2017). Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety 34, 9–24. doi: 10.1002/da.22556
Wolf, R. C., and Herringa, R. J. (2016). Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 822–831. doi: 10.1038/npp.2015.209
Yao, Z., Liao, M., Hu, T., Zhang, Z., Zhao, Y., Zheng, F., et al. (2017). An effective method to identify adolescent generalized anxiety disorder by temporal features of dynamic functional connectivity. Front. Hum. Neurosci. 11:492. doi: 10.3389/fnhum.2017.00492
You, X., Norr, M., Murphy, E., Kuschner, E., Bal, E., Gaillard, W., et al. (2020). Atypical modulation of distant functional connectivity by cognitive state in children with autism spectrum disorders. Front. Hum. Neurosci. 7:482. doi: 10.3389/fnhum.2013.00482
Yuan, J. P., Henje Blom, E., Flynn, T., Chen, Y., Ho, T. C., Connolly, C. G., et al. (2019). Test-retest reliability of graph theoretic metrics in adolescent brains. Brain Connect 9, 144–154. doi: 10.1089/brain.2018.0580
Zhang, S., Chen, J. -M., Kuang, L., Cao, J., Zhang, H., Ai, M., et al. (2016). Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry 16:337. doi: 10.1186/s12888-016-1047-7
Keywords: MRI, adolescence, brain connectivity, psychiatric disorders, depression
Citation: Tymofiyeva O, Zhou VX, Lee C-M, Xu D, Hess CP and Yang TT (2020) MRI Insights Into Adolescent Neurocircuitry—A Vision for the Future. Front. Hum. Neurosci. 14:237. doi: 10.3389/fnhum.2020.00237
Received: 12 December 2019; Accepted: 29 May 2020;
Published: 07 July 2020.
Edited by:
Tim J. Silk, Deakin University, AustraliaReviewed by:
Miao Cao, Fudan University, ChinaCopyright © 2020 Tymofiyeva, Zhou, Lee, Xu, Hess and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Tymofiyeva, b2xnYS50eW1vZml5ZXZhQHVjc2YuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.