- 1The Acupuncture and Tuina School/The 3rd Teaching Hospital, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2The First Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Brain imaging studies of tinnitus patients have revealed marked changes in brain structure and function, but there are inconsistencies in those findings. In this meta-analysis, we investigated concurrence across studies to clarify those abnormalities in brain structure and function in tinnitus. Neuroimaging studies published up to December 6, 2019 were searched in the PubMed, Web of Science, EMBASE, and Cochrane Library databases, Chinese Nation Knowledge Infrastructure, Chinese Biomedical Literature Database, the Chongqing VIP, and Wanfang Database. Study selection, quality assessment, and data extraction were performed by two independent researchers. Anisotropic effect size signed differential mapping (AES-SDM) was used to perform a multimodal analysis of available studies reporting whole-brain structural or functional data in tinnitus patients. There were 14 studies that met the inclusion criteria. The structural dataset comprised 242 tinnitus patients and 217 matched healthy subjects (HS), while the functional dataset included 130 tinnitus patients and 140 matched HS. Our analysis revealed structural alterations in the superior temporal gyrus, middle temporal gyrus (MTG), angular gyrus, caudate nucleus, superior frontal gyrus, and supplementary motor area, as well as functional differences in the MTG, middle occipital gyrus, precuneus, and right inferior parietal (excluding supramarginal and angular) gyri. The multimodal analysis revealed significant differences in the right MTG of tinnitus patients relative to HS. These findings suggest the involvement of the cortico-striatal circuits in the neuropathology of tinnitus.
Introduction
Tinnitus is characterized by the perception of sounds in the ears or head in the absence of any external stimulus (Wegger et al., 2017; Bauer, 2018) and affects ~10–15% of the population (Shargorodsky et al., 2010; Baguley et al., 2013; Bauer, 2018). It often occurs in association with hearing impairment, insomnia, depression, anxiety, and difficulty in concentration, and significantly impairs patients' quality of life (Reynolds et al., 2004; Langguth et al., 2013; Zeman et al., 2014). Although phantom sounds are thought to originate in the cochlea, tinnitus is also related to maladaptive neuroplastic changes in the central nervous system as it often persists even after the auditory nerve has been severed (Berliner et al., 1992; Silverstein, 2001).
Structural and functional alterations in multiple auditory and non-auditory brain regions have been detected in tinnitus patients (Adjamian et al., 2009; Husain and Schmidt, 2014). For example, increased volume of gray matter (GM) in the bilateral superior temporal gyrus (STG), right middle temporal gyrus (MTG), and right supramarginal gyrus and decreased GM volume in the bilateral hypothalamus, left superior frontal gyrus (SFG), and right occipital lobe were observed in tinnitus patients (Boyen et al., 2013). On the other hand, some studies have reported no changes in GM volume associated with tinnitus (Landgrebe et al., 2009; Melcher et al., 2013). Furthermore, one morphometric study showed an increased GM volume in the right SFG (Schmidt et al., 2018), whereas the opposite was demonstrated by another voxel-based morphometry (VBM) study (Boyen et al., 2013). Besides anatomical changes, functional alterations have been detected in tinnitus (Chen et al., 2014, 2015; Han et al., 2018a), as evidenced by increased amplitude of low-frequency fluctuations (ALFF) in the right MTG, right SFG, and right angular gyrus and decreased ALFF in the left cuneus, right middle occipital gyrus, and bilateral thalamus (Chen et al., 2014). However, increased regional homogeneity (ReHo) in the frontal cortex (Chen et al., 2015) and decreased ReHo in the frontal gyrus (Han et al., 2018a) have also been detected in two different studies.
Although tinnitus is thought to involve many brain areas, the evidence on regional alterations has been inconsistent across studies (Boyen et al., 2013; Chen et al., 2015; Han et al., 2018a; Schmidt et al., 2018), possibly due to differences in sample size, demographic and clinical characteristics of patients, imaging modality, and analytical approach (Boyen et al., 2013; Allan et al., 2016; Schmidt et al., 2018). At present, little is known about the association between structural and functional changes, as there have been few multimodal studies. A previous meta-analysis of functional magnetic resonance imaging (fMRI), positron emission tomography, and single-photon emission computed tomography studies revealed an increased activity in several brain regions including right SFG, MTG, bilateral insula, inferior frontal gyrus, parahippocampal gyrus, and posterior lobe of the cerebellum and a decreased activity in the left cuneus and right thalamus (Chen et al., 2017). Cross-validation from structural and functional brain alterations is still required to confirm the reliability of these findings and better understand the cerebral characters of tinnitus.
Anisotropic effect size signed differential mapping (AES-SDM) is a method used for the meta-analysis of neuroimaging data. Unlike activation likelihood estimate and multi-level kernel density analysis that are based on regional likelihood or frequency of reported peak locations of significant activation clusters (Wager et al., 2007; Eickhoff et al., 2009), AES-SDM combines peak coordinates and statistical parametric maps and uses standard effect size, which allows an exhaustive inclusion and accurate estimations of studies (Radua and Mataix-Cols, 2009; Radua et al., 2012a, 2014). AES-SDM has high sensitivity, consistency, and a low rate of false positives (Radua et al., 2012a) as well as higher accuracy than other coordinate-based methods owing to increased reliability and validity of the neuroimaging data (Radua et al., 2012a).
We speculated that there is an overlap in the structural and functional changes in patients with tinnitus compared to healthy subjects (HS). In this study, we used AES-SDM to test this hypothesis by comparing GM volume and spontaneous activity reported in whole-brain VBM and fMRI studies.
Materials and Methods
Search Strategies
A systematic search strategy was used to identify relevant studies. Two independent researchers (SC and JZ) performed a two-step literature search of the PubMed, Web of Science, EMBASE, and Cochrane Library databases, Chinese Nation Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), the Chongqing VIP (VIP), and the Wanfang Database (WF) to identify structural or functional imaging studies on tinnitus. The search was conducted up to December 6, 2019, with no time window specified for date of publication. The following English search terms were used: (“tinnitus” OR “acousma” OR “akoasm” OR “phantom sound” OR “auditory phantom sensation”) AND (“magnetic resonance imaging” OR “MRI” OR “functional MRI” OR “fMRI” OR “voxel-based morphometry” OR “VBM” OR “voxelwise” OR “gray matter” OR “regional homogeneity” OR “ReHo”) AND (“resting state”). This search strategy has been modified to be suitable for Chinese electronic databases. The reference lists of articles cited in reviews were manually checked for any studies not identified by our database searches. Corresponding authors were contacted by email or telephone if data were not available or if questions about the data arose.
Selection Criteria
The included studies met the following criteria: (1) original paper reported in English or Chinese viewed journal; (2) comprised patients diagnosed with subjective tinnitus and HS; (3) involved whole-brain structural or functional imaging of both groups; (4) results of x-y-z coordinates were reported in Montreal Neurological Institute (MNI) or Talairach coordination; and (5) a 1.5T or 3.0T MRI scanner was used.
The exclusion criteria were as follows: (1) it did not report a VBM comparison of GM volume, modulated GM concentration, ReHo between tinnitus patients and HS; (2) only reported region of interest (ROI) findings or small volume corrections in pre-selected ROIs; (3) data were already covered in one or more articles that were included in our analysis; (4) the patients had other uncontrolled diseases including liver or kidney or hematopoietic diseases, endocrine and immune diseases, mental disorders, malignancy, etc.; and (5) recruited participants were not adults. Studies of pulsatile tinnitus were also excluded owing to its unusual mechanism (Liu et al., 2018).
Authors of studies in which MNI or Talairach coordinates (necessary for voxel-level quantitative meta-analysis) were not explicitly reported were contacted to reduce the possibility of a biased sample set. In cases where the same or similar datasets were used in separate papers, only data from the analysis of the largest sample were included.
Recorded Variables
The following information was extracted from the selected studies: first author, year of publication, sample size, number of female participants, the mean age of participants, duration of tinnitus, type of MRI (1.5 or 3.0 T), image package, and full width at half maximum (FWHM). Statistically significant coordinates including the direction of GM volume or differential activity between tinnitus patients and HS were extracted. This information is shown in Table 1.
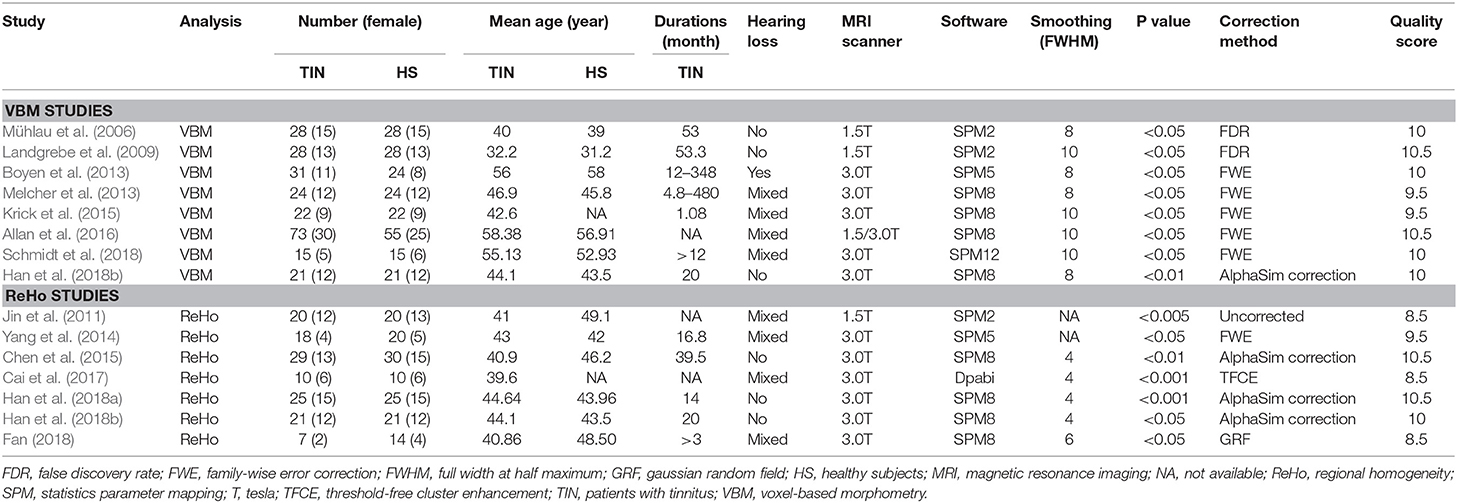
Table 1. Demographic and clinical characteristics of subjects in MRI datasets included in the meta-analysis.
Quality Assessment
The quality of all included studies was assessed using an 11-point checklist that was based on those used in previous meta-analyses (Shepherd et al., 2012; Du et al., 2014). The checklist focused on both clinical and demographic characteristics of individual study populations as well as important scanner parameters and methodological details (Table S1) (Shepherd et al., 2012; Du et al., 2014).
Study selection and data extraction and summarization were independently performed in a standardized manner by two investigators (SC and GX); any disagreements were resolved by a third investigator (RS). This meta-analysis adhered to Preferred Reporting Items for Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). This meta-analysis has been registered with the PROSPERO International Prospective Register of Systematic Reviews of the University of York (PROSPERO registration no. CRD42019123399).
Standard Meta-Analyses of Structural and Functional Abnormalities
Abnormalities in brain structure and spontaneous activity were subjected to the voxel-wise meta-analysis by AES-SDM (https://www.sdmproject.com/software) (Radua et al., 2012a,b).
The extracted peak information was combined to recreate effect-size and variance maps first by means of a Gaussian kernel, which assigned higher effect sizes to voxels closer to the peaks. The FWHM was set at 20 mm in the assignment in order to control for false-positive results (Radua et al., 2012a). Taking sample size, intra-study variability, and between-study heterogeneity into account, study maps were calculated voxel-wise to obtain the random effects mean. Studies with larger sample sizes made a larger contribution because the mean map was weighted by the square root of the sample size of each study. Thresholds were applied using default settings (voxel threshold P < 0.005, peak height threshold z > 1.00, and cluster size threshold >10 voxels) after calculating for the meta-analysis means (Radua et al., 2012a). Finally, we statistically analyzed the meta-analysis effect-size map by comparing to a null distribution created with a permutation algorithm. A leave-one-out jackknife sensitivity analysis was used to test the reproducibility of VBM and ReHo study findings, which consisted of repeating the mean analysis after systematically removing each study.
In order to exclude potential heterogeneity resulting from different VBM measurement methods, we carried out a subgroup analysis of VBM studies including a scanner (1.5 or 3.0 T scanner) and FWHM (8 and 10 mm). Funnel plots of the peaks of the main findings were performed to check whether the findings might have been driven by few or small studies. Meanwhile, the Egger test was also performed to detect the potential publication bias (Radua and Mataix-Cols, 2009).
Multimodal Analysis of the Structural and Functional Response
To identify brain areas exhibiting structural and functional alterations in tinnitus patients, the structural and functional data were cross validated in a single meta-analysis map by computing the union of structural and functional p values (Radua et al., 2012a).
Meta-Regression Analysis
To examine any potential effects, a meta-regression analysis weighted by sample size and intra- and between-study variances (Radua et al., 2012a) was performed to evaluate associations between changes in the brain and subject characteristics (age and duration of tinnitus). The probability threshold was decreased to 0.005 to minimize the detection of false associations. Findings for the slope and one of the extremes of the regressor were considered, and those in regions not detected in the main analysis were discarded. Finally, fits obviously driven by an insufficient number of studies were also discarded by inspecting the regression plot (Radua et al., 2012a).
Results
Studies Included in the Meta-Analysis
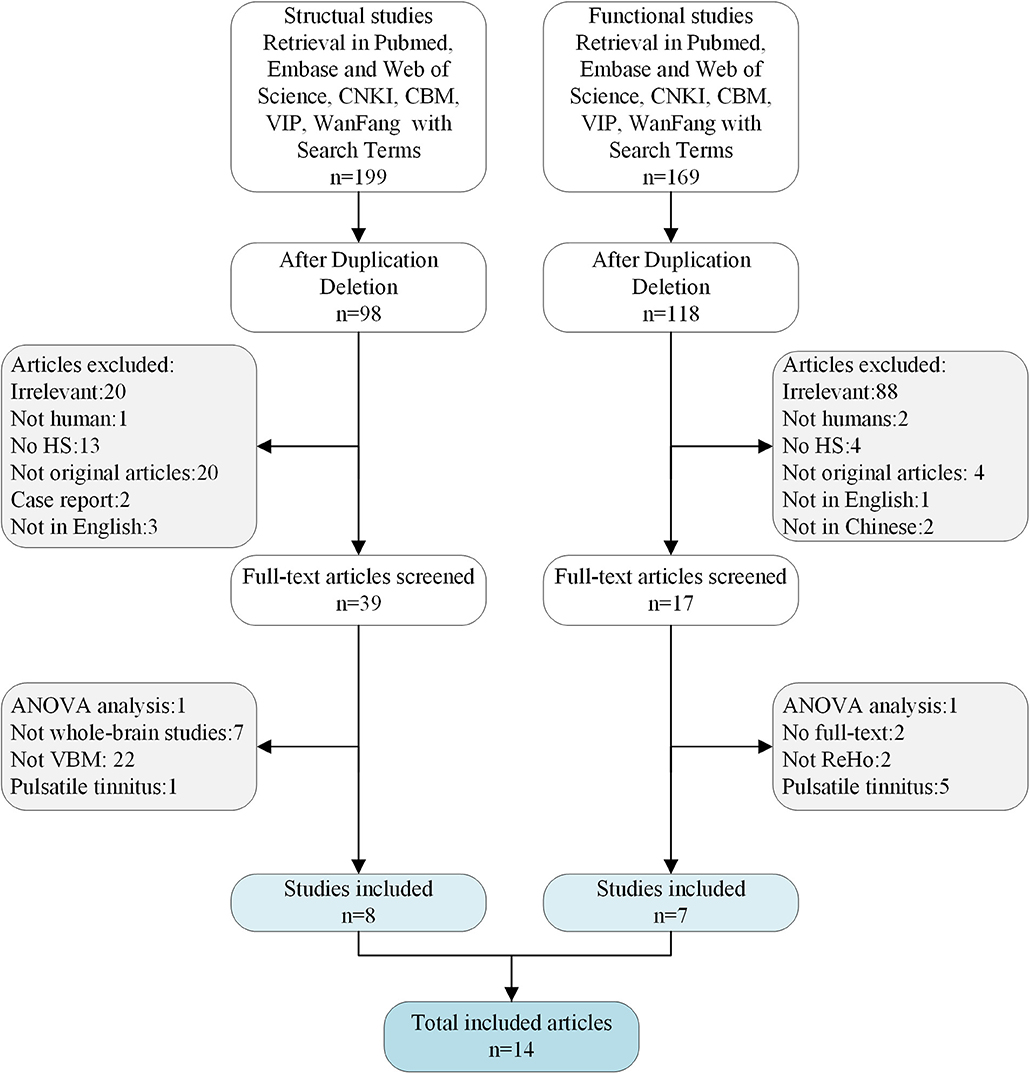
The meta-analysis included 14 studies that met the inclusion criteria (Figure 1). There were eight VBM datasets with a total of 242 patients with tinnitus (107 females; mean age: 48.91 years) and 217 HS (100 females; mean age: 47.66 years), and seven ReHo studies with a total of 130 patients with tinnitus (64 females; mean age: 36.03 years) and 140 HS (70 females; mean age: 37.83 years). All of the patients in these studies were drug naïve and had not undergone a “washout” period prior to MRI scanning. The studies had a mean quality score of 9.73 out of a total possible score of 11, indicating that they were of high quality. There were no significant differences in age or sex ratio between the two groups. Details of the literature search and criteria for article inclusion are shown in Figure 1. The demographic characteristics, clinical variables, scanning method, and significance level of inter-group comparisons in the included studies are shown in Table 1.
Meta-Analyses of GM Volume Changes
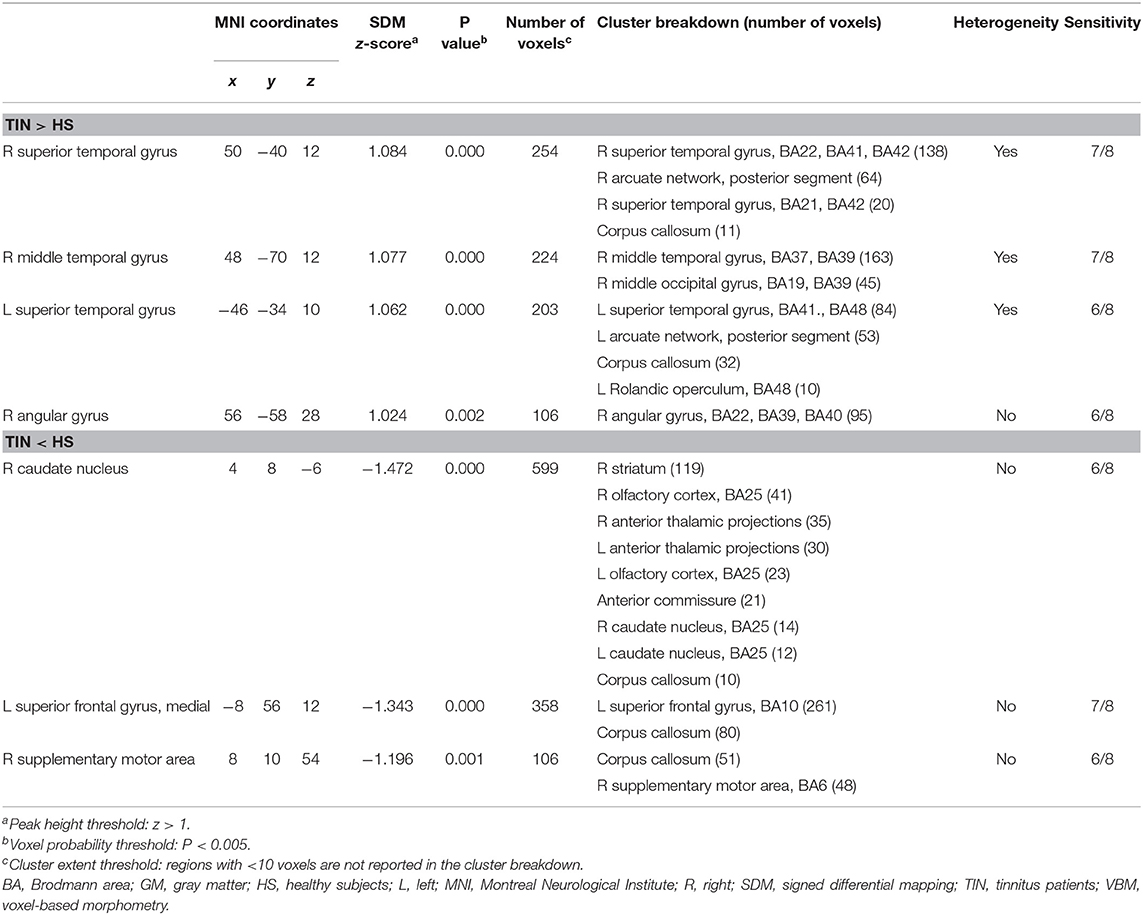
Seven peak foci were reported in this VBM meta-analysis. Patients with tinnitus showed increased GM volume in the right STG (P = 0.000, z = 1.084), right MTG (P = 0.000, z = 1.077), left STG (P = 0.000, z = 1.062), and right angular gyrus (P = 0.002, z = 1.024), and a decreased volume in the right caudate nucleus (P = 0.000, z = −1.472), left SFG (P = 0.000, z = −1.343), and right supplementary motor are (P = 0.001, z = −1.196) relative to HS (Figure 2A and Table 2). The sensitivity analysis showed that all results above were highly reproducible, as most were preserved in combinations of the dataset (Table S2A).
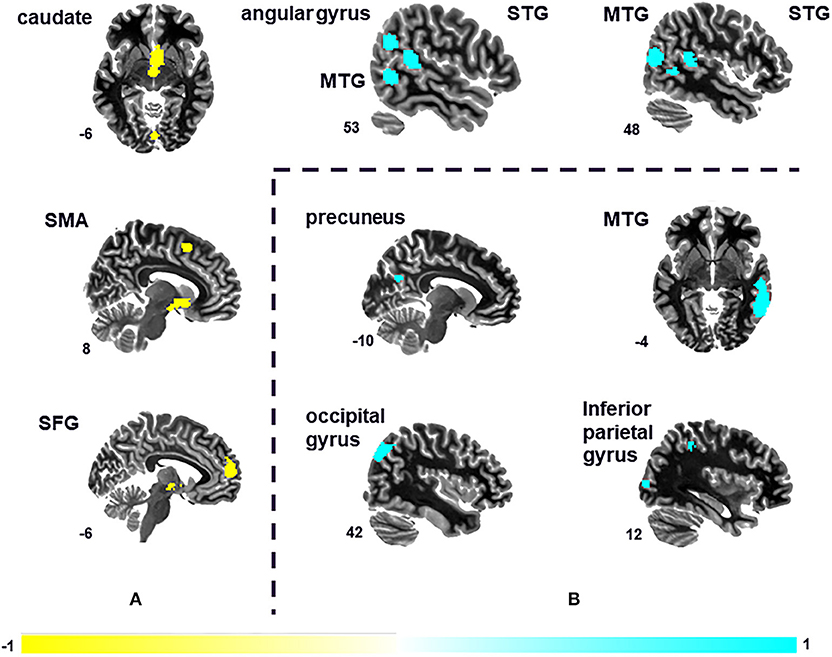
Figure 2. Differences in the brain regions between tinnitus patients and HS. (A) Differences in gray matter (GM) volume between tinnitus patients and HS. (B) Changes in spontaneous brain activity of tinnitus patients compared with HS. MTG, middle temporal gyrus; SFG, superior frontal gyrus; SMA, supplementary motor area; STG, superior temporal gyrus.
Meta-Analyses of ReHo Abnormalities
A total of four peak foci were reported in the ReHo studies. AES-SDM of the fMRI data revealed hyperactivation in the right MTG (P = 0.000, z = 1.775), right middle occipital gyrus (P = 0.002, z = 1.422), left precuneus (P = 0.002, z = 1.460), and right inferior parietal (excluding supramarginal and angular) gyri (P = 0.003, z = 1.406) of tinnitus patients relative to HS (Figure 2B and Table 3). The jackknife sensitivity analyses showed that these results were highly reproducible (Table S2B).
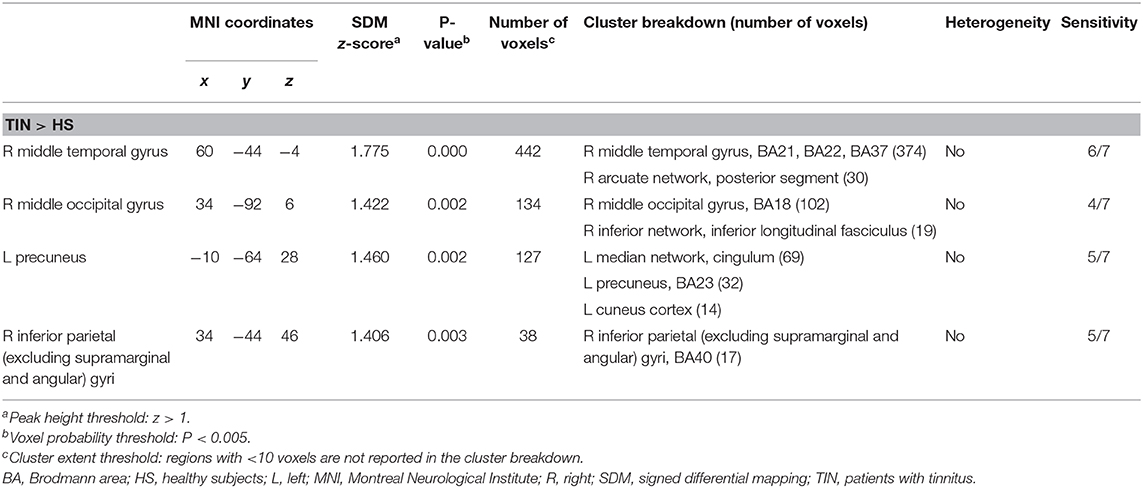
Table 3. Brain regions showing spontaneous brain activity differences between patients with tinnitus and healthy subjects.
Multimodal Analysis of GM Volume and Brain Response
We summarized our findings into a single meta-analytic map in order to identify regions showing abnormalities in both VBM and ReHo studies. The multimodal analysis revealed significant differences in the right MTG (P < 0.0025) of patients with tinnitus relative to HS.
Heterogeneity Analysis and Publication Bias
The heterogeneity analysis indicated significant heterogeneity among the VBM studies with altered GM volume in the right STG, right MTG, and left STG (P < 0.005) (Table S3) and showed insignificant heterogeneity among the ReHo studies. We conducted the funnel plots and Egger tests, although there were too few VBM and ReHo studies for evaluation of publication bias in the meta-analysis, which requires at least 10 studies. The funnel plots demonstrated that the main findings were driven by at least seven VBM studies and six ReHo studies, respectively (Figure S1). Analysis of publication bias revealed that the Egger tests were insignificant in the peaks of the altered brain regions in the VBM meta-analysis (P = 0.715) and the ReHo meta-analysis (P = 0.990).
Subgroup Analysis
A subgroup analysis of VBM studies using different FWHM values (8 mm/10 mm) yielded no significant differences. The subgroup analysis of VBM studies using different scanners (1.5/3.0 T) revealed structural abnormality in the left medial SFG and right supplementary motor area of patients with tinnitus (Table S4).
Meta-Regression
Although it is not recommended for fewer than nine studies (Radua and Mataix-Cols, 2009), we carried out a meta-regression analysis to examine potential confounding variables (mean age and disease duration). The mean age of patients was associated with increased GM volume in the bilateral STG, right MTG, right angular gyrus in VBM studies (Table S5A). Moreover, the mean age of patients was associated with hyperactivation in the right MTG and right middle occipital gyrus in ReHo studies (Table S5B). The meta-regression of disease duration showed insignificance among the brain regions in VBM studies and ReHo studies.
Discussion
This is the first multimodal neuroimaging meta-analysis investigating the neural substrates of tinnitus by combining information from whole-brain VBM studies of GM volume and ReHo studies of spontaneous brain activity.
The main findings were that GM volume was increased in bilateral STG, the right MTG, and right angular gyrus and decreased in the right caudate nucleus, left medial SFG, and right supplementary motor area of patients with tinnitus compared with HS (Figure 2A and Table 2). Additionally, tinnitus was associated with hyperactivation in the right MTG, right middle occipital gyrus, left precuneus, and right inferior parietal (excluding supramarginal and angular) gyri (Figure 2B and Table 3). These results remained unchanged when each study was removed in the jackknife sensitivity analysis.
Abnormalities are more frequently reported in the STG and MTG than in other parts of the auditory cortex in neuroimaging studies of tinnitus (Boyen et al., 2013; Chen et al., 2014; Han et al., 2018a). The temporal gyrus is critical for auditory processing that includes simple auditory stimulus processing and semantic memory (May and Tiitinen, 2010; Recasens et al., 2014), and is a connection in the hierarchical structure of the primary auditory cortex and frontal lobe (Ishishita et al., 2019). Our results revealed abnormal brain responses in this region, suggesting their involvement in tinnitus. Prolonged exposure to an internal sound such as a tone and/or noise is associated with increased GM volume in cortical regions of auditory processing (Boyen et al., 2013).
Tinnitus patients often exhibit varying degrees of hearing impairment. Hearing loss patients with or without tinnitus use more contextual information from semantic memory to maintain normal communication, which may result in increased GM volume of the auditory association area (Boyen et al., 2013). However, a recent neuroimaging study did not find any differences in the temporal gyrus between recent-onset patients with tinnitus with mild hearing impairment and HS (Krick et al., 2015). As the present meta-analysis included patients with tinnitus as well as those without hearing loss, we were unable to perform a regression analysis of hearing loss severity. In addition, differences in the temporal gyrus have been reported between long-term and recent-onset patients with tinnitus (Schmidt et al., 2018). Although this implies that the duration of tinnitus alters the temporal gyrus, we did not find any correlation between these two variables in this meta-analysis. Furthermore, some studies have shown increased neuronal activity in the right hemisphere—especially the right MTG—in tinnitus (Mirz et al., 1999; Chen et al., 2014), whereas others have reported left lateralization (Langguth et al., 2006; Geven et al., 2014). Therefore, although the right MTG involvement found in this study suggests right lateralization, most patients exhibited unilateral or bilateral tinnitus, making it difficult to conclude that tinnitus has a unilateral tendency.
Tinnitus sensation representations generated or expressed in auditory cortex are necessary (Lanting et al., 2009) but not sufficient to account for suffering from tinnitus. Central auditory system hypotheses of tinnitus genesis have been proposed to account for the discrepancy between audiometric profile and tinnitus perceptual attributes, including thalamocortical dysrhythmia in frequencies (Llinas et al., 1999; Weisz et al., 2007), the striatal gating model (Larson and Cheung, 2012), etc. Decreased GM volume of caudate nucleus has been found in patients with tinnitus in this meta-analysis, supporting that the striatal gating model might be involved in tinnitus genesis. The caudate nucleus has been found be projected from the superior temporal cortex and rostral and midportion aspects of association cortex on the superior temporal gyrus in monkeys and cats (Reale and Imig, 1983; Yeterian and Pandya, 1998). This nucleus is hypothesized to act as a gating mechanism for auditory phantom percepts, which can suppress the enduring tinnitus loudness (Larson and Cheung, 2013). The requisite cortico-striatal neural circuitry is in place for dysfunction striatal connectivity to enable perception of auditory phantoms (Larson and Cheung, 2012; Hinkley et al., 2015).
We also found decreased GM volume of SFG in patients with tinnitus. This brain region plays an important role in executive function and emotion processing and attention, in contrast to the sensory processing that is affected in chronic tinnitus (Mirz et al., 2000; Weisz et al., 2004). Results from resting-state fMRI studies indicate that SFG is the main cortical area affected by tinnitus (Chen et al., 2014, 2016). Moreover, disrupted functional connectivity between the SFG and amygdala has been observed in patients, suggesting that executive control of attention network in the SFG enhances the negative attributes of tinnitus contributed by the amygdala (Chen et al., 2017). A decreased SFG GM volume has been attributed to peripheral hearing loss (Husain et al., 2011; Boyen et al., 2013). In our meta-analysis, there was no available uniform audiometric data reported in the included studies, making it difficult to distinguish between the effects of hearing impairment and tinnitus on SFG alteration.
Besides auditory brain regions, auditory spatial information is also processed by the visual centers of the brain (Bedny et al., 2010; Fiehler and Rosler, 2010). Increased activity in the right middle occipital gyrus of patients with tinnitus were observed in the current meta-analysis. This brain region participates in visual processing, which may be processed cross-modally in phantom auditory perception (Murray et al., 2005). Furthermore, the increased GM volume of angular and hyperactivation of precuneus were revealed in this meta-analysis. Interestingly, the MTG, angular gyrus, and precuneus also belong to the default mode network (DMN), which is most active at rest (Raichle et al., 2001; Mantini et al., 2007). These aberrant neural activities may be responsible for the dysfunction of DMN in patients with tinnitus, although the source or their type within specific DMN regions due to tinnitus remains unclear.
The analysis of heterogeneity showed that findings from structural neuroimaging studies were inconsistent, while the subgroup analysis using a different scanner revealed different structural abnormalities in the left medial SFG and right supplementary motor area among patients with tinnitus. In addition, the mean age of patients was associated with increased GM volume in the bilateral STG, right MTG in VBM studies. This variability could be due to small sample sizes, demographic characteristics, and methodological differences (e.g., in the scanner) among studies. One study reported differences in GM of various brain regions between patients with mild and more severe long-term tinnitus (Schmidt et al., 2018). We did not perform meta-regression of tinnitus severity in this study as the different questionnaires used to assess tinnitus symptoms were not uniform; however, the severity of tinnitus may also contribute to the observed heterogeneity.
The multimodal analysis revealed significant differences in the right MTG of patients with tinnitus and HS. The temporal cortex—specifically the MTG—was found to be altered in patients with tinnitus (Mirz et al., 1999; May and Tiitinen, 2010; Chen et al., 2014; Recasens et al., 2014), suggesting that this brain area is closely associated with tinnitus development (Farhadi et al., 2010).
The present study had some limitations. First, a relatively limited number of datasets were analyzed owing to the exclusion criteria. There were only 14 studies included totally; the results of this meta-analyses were preliminary. Second, patients with tinnitus, both with and without hearing loss, were included, which precluded a regression analysis of hearing loss severity.
In conclusion, the results of our meta-analysis revealed abnormalities not only in auditory areas but also in the caudate nucleus, which suggests the involvement of the cortico-striatal circuits in the neuropathology of tinnitus.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Author Contributions
RS, SC, and FL contributed to study conception and design and conceived the data analysis strategy. SC, GX, and JZ acquired the data. SC, GX, and RS collated and analyzed the data. SC, GX, and JZ drafted the manuscript. YQ, ZL, ZH, TY, PM, RS, and FL discussed, read, and revised the manuscript. All authors approved the publication of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NO. 81590950, 81590951, 81904096), the China Postdoctoral Science Foundation (NO. 2019M653361), and Research Open Project of Zhejiang Chinese Medical University (ZYX2018003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Prof. Fang Zeng for assistance and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2020.00028/full#supplementary-material
References
Adjamian, P., Sereda, M., and Hall, D. A. (2009). The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear. Res. 253, 15–31. doi: 10.1016/j.heares.2009.04.001
Allan, T. W., Besle, J., Langers, D. R. M., Davies, J., Hall, D. A., Palmer, A. R., et al. (2016). Neuroanatomical alterations in tinnitus assessed with magnetic resonance imaging. Front. Aging Neurosci. 8:221. doi: 10.3389/fnagi.2016.00221
Baguley, D., McFerran, D., and Hall, D. (2013). Tinnitus. Lancet 382, 1600–1607. doi: 10.1016/S0140-6736(13)60142-7
Bedny, M., Konkle, T., Pelphrey, K., Saxe, R., and Pascual-Leone, A. (2010). Sensitive period for a multimodal response in human visual motion area MT/MST. Curr. Biol. 20, 1900–1906. doi: 10.1016/j.cub.2010.09.044
Berliner, K. I., Shelton, C., Hitselberger, W. E., and Luxford, W. M. (1992). Acoustic tumors: effect of surgical removal on tinnitus. Am. J. Otol. 13, 13–17. doi: 10.1097/00129492-199201000-00005
Boyen, K., Langers, D. R., de Kleine, E., and van Dijk, P. (2013). Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 295, 67–78. doi: 10.1016/j.heares.2012.02.010
Cai, W., Ou, J., Liang, J. G., and Zhang, T. (2017). Resting state functiona magnetic resonance imaging of tinnitus: regional homogeneity and functional connectivity. J. Pract. Med. 33, 2329–2332. doi: 10.3389/fnhum.2017.00022
Chen, Y. C., Feng, Y., Xu, J. J., Mao, C. N., Xia, W., Ren, J., et al. (2016). Disrupted brain functional network architecture in chronic tinnitus patients. Front. Aging Neurosci. 8:174. doi: 10.3389/fnagi.2016.00174
Chen, Y. C., Wang, F., Wang, J., Bo, F., Xia, W., Gu, J. P., et al. (2017). Resting-state brain abnormalities in chronic subjective tinnitus: a meta-analysis. Front. Hum. Neurosci. 11:22. doi: 10.3389/fnhum.2017.00022
Chen, Y. C., Zhang, J., Li, X. W., Xia, W., Feng, X., Gao, B., et al. (2014). Aberrant spontaneous brain activity in chronic tinnitus patients revealed by resting-state functional MRI. NeuroImage Clin. 6, 222–228. doi: 10.1016/j.nicl.2014.09.011
Chen, Y. C., Zhang, J., Li, X. W., Xia, W., Feng, X., Qian, C., et al. (2015). Altered intra- and interregional synchronization in resting-state cerebral networks associated with chronic tinnitus. Neural Plast. 2015:475382. doi: 10.1155/2015/475382
Du, M., Liu, J., Chen, Z., Huang, X., Li, J., Kuang, W., et al. (2014). Brain grey matter volume alterations in late-life depression. J. Psychiatr. Neurosci. 39, 397–406. doi: 10.1503/jpn.130275
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Fan, K. M. (2018). A Resting-State Functional MRI Study of Abnormal Spontaneous Brain Function in Tinnitus Patients. Hangzhou Normal University.
Farhadi, M., Mahmoudian, S., Saddadi, F., Karimian, A. R., Mirzaee, M., Ahmadizadeh, M., et al. (2010). Functional brain abnormalities localized in 55 chronic tinnitus patients: fusion of SPECT coincidence imaging and MRI. J. Cereb. Blood Flow Metab. 30, 864–870. doi: 10.1038/jcbfm.2009.254
Fiehler, K., and Rosler, F. (2010). Plasticity of multisensory dorsal stream functions: evidence from congenitally blind and sighted adults. Restor. Neurol. Neurosci. 28, 193–205. doi: 10.3233/RNN-2010-0500
Geven, L. I., de Kleine, E., Willemsen, A. T., and van Dijk, P. (2014). Asymmetry in primary auditory cortex activity in tinnitus patients and controls. Neuroscience 256, 117–125. doi: 10.1016/j.neuroscience.2013.10.015
Han, Q., Feng, Y., Liu, D., Yin, X., Zuo, Z., and Wang, J. (2018b). MRI analysis of regional homogeneity and gray matter structure in chronic subjective tinnitus. Chin. J. Med. Imaging Technol. 34, 30–34. doi: 10.13929/j.1003-3289.201704046
Han, Q., Zhang, Y., Liu, D., Wang, Y., Feng, Y., Yin, X., et al. (2018a). Disrupted local neural activity and functional connectivity in subjective tinnitus patients: evidence from resting-state fMRI study. Neuroradiology 60, 1193–1201. doi: 10.1007/s00234-018-2087-0
Hinkley, L. B., Mizuiri, D., Hong, O., Nagarajan, S. S., and Cheung, S. W. (2015). Increased striatal functional connectivity with auditory cortex in tinnitus. Front. Hum. Neurosci. 9:568. doi: 10.3389/fnhum.2015.00568
Husain, F. T., Medina, R. E., Davis, C. W., Szymko-Bennett, Y., Simonyan, K., Pajor, N. M., et al. (2011). Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 1369, 74–88. doi: 10.1016/j.brainres.2010.10.095
Husain, F. T., and Schmidt, S. A. (2014). Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 307, 153–162. doi: 10.1016/j.heares.2013.07.010
Ishishita, Y., Kunii, N., Shimada, S., Ibayashi, K., Tada, M., Kirihara, K., et al. (2019). Deviance detection is the dominant component of auditory contextual processing in the lateral superior temporal gyrus: a human ECoG study. Human Brain Mapp. 40, 1184–1194. doi: 10.1002/hbm.24438
Jin, L., Zhao, X., Wang, P., and Ge, R. (2011). Functional magnetic responance imaging in tinnitus patients. Chin. Arch. Otolaryngol. Head Neck Surg. 18, 174–177. doi: 10.16066/j.1672-7002.2011.04.001
Krick, C. M., Grapp, M., Daneshvar-Talebi, J., Reith, W., Plinkert, P. K., and Bolay, H. V. (2015). Cortical reorganization in recent-onset tinnitus patients by the Heidelberg Model of Music Therapy. Front. Neurosci. 9:49. doi: 10.3389/fnins.2015.00049
Landgrebe, M., Langguth, B., Rosengarth, K., Braun, S., Koch, A., Kleinjung, T., et al. (2009). Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. NeuroImage 46, 213–218. doi: 10.1016/j.neuroimage.2009.01.069
Langguth, B., Eichhammer, P., Kreutzer, A., Maenner, P., Marienhagen, J., Kleinjung, T., et al. (2006). The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus–first results from a PET study. Acta Otolaryngol. 84–88. doi: 10.1080/03655230600895317
Langguth, B., Kreuzer, P. M., Kleinjung, T., and De Ridder, D. (2013). Tinnitus: causes and clinical management. Lancet Neurol. 12, 920–930. doi: 10.1016/S1474-4422(13)70160-1
Lanting, C. P., de Kleine, E., and van Dijk, P. (2009). Neural activity underlying tinnitus generation: results from PET and fMRI. Hear. Res. 255, 1–13. doi: 10.1016/j.heares.2009.06.009
Larson, P. S., and Cheung, S. W. (2012). Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery 70, 398–406. doi: 10.1227/NEU.0b013e3182320ab5
Larson, P. S., and Cheung, S. W. (2013). A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J. Neurosurg. 118, 192–194. doi: 10.3171/2012.9.JNS12594
Liu, Y., Lv, H., Zhao, P., Liu, Z., Chen, W., Gong, S., et al. (2018). Neuroanatomical alterations in patients with early stage of unilateral pulsatile tinnitus: a voxel-based morphometry study. Neural Plast. 2018:4756471. doi: 10.1155/2018/4756471
Llinas, R. R., Riary, U., Jeanmonod, D., Kronberg, E., and Mitra, P. P. (1999). Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U.S.A. 96, 15222–152277. doi: 10.1073/pnas.96.26.15222
Mantini, D., Perrucci, M. G., Del, G., Romani, G. L., and Corbetta, M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U.S.A. 104, 13170–13175. doi: 10.1073/pnas.0700668104
May, P. J., and Tiitinen, H. (2010). Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology 47, 66–122. doi: 10.1111/j.1469-8986.2009.00856.x
Melcher, J. R., Knudson, I. M., and Levine, R. A. (2013). Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear. Res. 295, 79–86. doi: 10.1016/j.heares.2012.03.013
Mirz, F., Gjedde, A., Sodkilde-Jrgensen, H., and Pedersen, C. B. (2000). Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport 11, 633–637. doi: 10.1097/00001756-200002280-00039
Mirz, F., Pedersen, B., Ishizu, K., Johannsen, P., Ovesen, T., Stodkilde-Jorgensen, H., et al. (1999). Positron emission tomography of cortical centers of tinnitus. Hear. Res. 134, 133–144. doi: 10.1016/S0378-5955(99)00075-1
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Mühlau, M., Rauschecker, J. P., Oestreicher, E., Gaser, C., Rottinger, M., Wohlschlager, A. M., et al. (2006). Structural brain changes in tinnitus. Cereb. Cortex 16, 1283–1288. doi: 10.1093/cercor/bhj070
Murray, M. M., Molholm, S., Michel, C. M., Heslenfeld, D. J., Ritter, W., Javitt, D. C., et al. (2005). Grabbing your ear: rapid auditory-somatosensory multisensory interactions in low-level sensory cortices are not constrained by stimulus alignment. Cereb. Cortex 15, 963–974. doi: 10.1093/cercor/bhh197
Radua, J., Borgwardt, S., Crescini, A., Mataix-Cols, D., Meyer-Lindenberg, A., McGuire, P. K., et al. (2012b). Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 36, 2325–2333. doi: 10.1016/j.neubiorev.2012.07.012
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Radua, J., Mataix-Cols, D., Phillips, M. L., El-Hage, W., Kronhaus, D. M., Cardoner, N., et al. (2012a). A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001
Radua, J., Rubia, K., Canales-Rodriguez, E. J., Pomarol-Clotet, E., Fusar-Poli, P., and Mataix-Cols, D. (2014). Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 5:13. doi: 10.3389/fpsyt.2014.00013
Raichle, M. E., Macleod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., and Shulman, G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682. doi: 10.1073/pnas.98.2.676
Reale, R. A., and Imig, T. J. (1983). Auditory cortical field projections to the basal gangila of the cat. Neuroscience 8, 67–86. doi: 10.1016/0306-4522(83)90026-X
Recasens, M., Grimm, S., Capilla, A., Nowak, R., and Escera, C. (2014). Two sequential processes of change detection in hierarchically ordered areas of the human auditory cortex. Cereb. Cortex 24, 143–153. doi: 10.1093/cercor/bhs295
Reynolds, P., Gardner, D., and Lee, R. (2004). Tinnitus and psychological morbidity: a cross-sectional study to investigate psychological morbidity in tinnitus patients and its relationship with severity of symptoms and illness perceptions. Clin. Otolaryngol. Allied Sci. 29, 628–634. doi: 10.1111/j.1365-2273.2004.00879.x
Schmidt, S. A., Zimmerman, B., Bido Medina, R. O., Carpenter-Thompson, J. R., and Husain, F. T. (2018). Changes in gray and white matter in subgroups within the tinnitus population. Brain Res. 1679, 64–74. doi: 10.1016/j.brainres.2017.11.012
Shargorodsky, J., Curhan, G. C., and Farwell, W. R. (2010). Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 123, 711–718. doi: 10.1016/j.amjmed.2010.02.015
Shepherd, A. M., Matheson, S. L., Laurens, K. R., Carr, V. J., and Green, M. J. (2012). Systematic meta-analysis of insula volume in schizophrenia. Biol. Psychiatry 72, 775–784. doi: 10.1016/j.biopsych.2012.04.020
Silverstein, H. (2001). A century of eighth nerve surgery. Otol. Neurotol. 22:401–416. doi: 10.1097/00129492-200105000-00023
Wager, T. D., Lindquist, M., and Kaplan, L. (2007). Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci. 2, 150–158. doi: 10.1093/scan/nsm015
Wegger, M., Ovesen, T., and Larsen, D. G. (2017). Acoustic coordinated reset neuromodulation: a systematic review of a novel therapy for tinnitus. Front. Neurol. 8:36. doi: 10.3389/fneur.2017.00036
Weisz, N., Muller, S., Schlee, W., Dohrmann, K., Hartmann, T., and Elbert, T. (2007). The neural code of auditory phantom perception. J. Neurosci. 27, 1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007
Weisz, N., Voss, S., Berg, P., and Elbert, T. (2004). Abnormal auditory mismatch response in tinnitus sufferers with high-frequency hearing loss is associated with subjective distress level. BMC Neurosci. 5:8. doi: 10.1186/1471-2202-5-8
Yang, H., Zheng, Y., Ou, Y., and Huang, X. (2014). Regional homogeneity on resting state fMRI in patients with tinnitus. J. Otol. 9, 173–178. doi: 10.1016/j.joto.2014.10.001
Yeterian, E. H., and Pandya, D. N. (1998). Corticostriatal connections of the superior temporal region in rhesus monkeys. J. Comp. Neurol. 399, 384–402. doi: 10.1002/(SICI)1096-9861(19980928)399:3<384::AID-CNE7>3.0.CO;2-X
Keywords: meta-analysis, tinnitus, multimodal, neuroimaging, signed differential mapping, voxel-based morphometry
Citation: Cheng S, Xu G, Zhou J, Qu Y, Li Z, He Z, Yin T, Ma P, Sun R and Liang F (2020) A Multimodal Meta-Analysis of Structural and Functional Changes in the Brain of Tinnitus. Front. Hum. Neurosci. 14:28. doi: 10.3389/fnhum.2020.00028
Received: 13 August 2019; Accepted: 21 January 2020;
Published: 25 February 2020.
Edited by:
Yale Cohen, University of Pennsylvania, United StatesReviewed by:
Elliott D. Kozin, Harvard Medical School, United StatesPingLei Pan, Southeast University, China
Copyright © 2020 Cheng, Xu, Zhou, Qu, Li, He, Yin, Ma, Sun and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanrong Liang, YWN1cmVzZWFyY2hAMTI2LmNvbQ==; Ruirui Sun, c3VucnVpcnVpQGNkdXRjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Shirui Cheng
Shirui Cheng Guixing Xu1†
Guixing Xu1† Zhengjie Li
Zhengjie Li Fanrong Liang
Fanrong Liang