- 1Division of Cognitive Neuroscience, New York State Psychiatric Institute, New York, NY, United States
- 2Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States
- 3Division of Translational Epidemiology, New York State Psychiatric Institute, New York, NY, United States
- 4Spirituality Mind Body Institute, Teachers College, Columbia University, New York, NY, United States
- 5Mailman School of Public Health, Columbia University, New York, NY, United States
The personal importance of religion or spirituality (R/S) has been associated with a lower risk for major depression (MDD), suicidal behavior, reduced cortical thinning and increased posterior EEG alpha, which has also been linked to antidepressant treatment response in MDD. Building on prior event-related potential (ERP) findings using an emotional hemifield paradigm, this study examined whether abnormal early (preconscious) responsivity to negative arousing stimuli, which is indicative of right parietotemporal dysfunction in both MDD patients and individuals at clinical high risk for MDD, is likewise moderated by R/S. We reanalyzed 72-channel ERP data from 127 individuals at high or low family risk for MDD (Kayser et al., 2017, NeuroImage Clin. 14, 692–707) after R/S stratification (low R/S importance, low/high risk, n = 38/61; high R/S importance, n = 15/13). ERPs were transformed to reference-free current source density (CSD) and quantified by temporal principal components analysis (tPCA). This report focused on N2 sink (peak latency 212 ms), the earliest prominent CSD-tPCA component previously found to be sensitive to emotional content. While overall N2 sink reflected activation of occipitotemporal cortex (prestriate/cuneus), as estimated via a distributed inverse solution, affective significance was marked by a relative (i.e., superimposed) positivity. Statistical analyses employed both non-parametric permutation tests and repeated measures ANOVA for mixed factorial designs with unstructured covariance matrix, including sex, age, and clinical covariates. Participants with low R/S importance, independent of risk status, showed greater ERP responsivity to negative than neutral stimuli, particularly over the right hemisphere. In contrast, early emotional ERP responsivity and asymmetry was substantially reduced for high risk individuals with high R/S importance, however, enhanced for low risk individuals with high R/S importance. Hemifield modulations of these effects (i.e., emotional ERP enhancements with left visual field/right hemisphere stimulus presentations) further corroborated these observations. Results suggest down-regulation of a right-lateralized network for salience detection at an early processing stage in high risk and high R/S importance individuals, presumably to prevent overactivation of ventral brain regions further downstream. These findings may point to a neurophysiological mechanism underlying resilience of families at risk for depression with high R/S prioritization.
Introduction
Religion (or religiousness) and spirituality (R/S) are widely considered core constituents of all human societies (e.g., Inzlicht et al., 2009, 2011; Crescentini et al., 2015). They can provide for many individuals purpose to human existence, as well as resources to cope with stressful life experiences which appear to have a beneficial impact on mental (e.g., depression) and physical (e.g., cardiovascular) health (Koenig et al., 2001, 2012; Hill and Pargament, 2003; Cobb et al., 2012). Despite the omnipresence of R/S and its putative health relevance, and a growing interest of relating R/S to brain mechanisms on a conceptual level (Fingelkurts and Fingelkurts, 2009; van Elk and Aleman, 2017), empirical neuroscientific research addressing these relations and the underlying neurobiological processes has been scarce.
Over the last 20 years, our group has focused on the protective mechanism afforded by the personal importance of R/S during an ongoing longitudinal study of families at high and low risk for depression (e.g., Weissman et al., 1997, 2005, 2006, 2016). Maternal R/S importance and mother-offspring concordance of R/S was associated with a 90% reduction of risk for incurring a depressive episode at 10-year follow-up (Miller et al., 1997), and offspring with high R/S importance had a 75% risk reduction of experiencing MDD 10 years later, particularly for those who were at high family risk because of a depressed parent (Miller et al., 2012). Parental R/S importance was associated with lower risk for suicidal behavior in offspring (Svob et al., 2018). High R/S importance was also associated with less cortical thinning (Miller et al., 2014), a putative morphologic endophenotype of familial risk for MDD (Peterson et al., 2009). Further implicating protective benefits of R/S importance for persons at high risk for depression, those who self-reported high R/S importance, when compared those who did not, had larger pial surface representing the outer boundary area between gray matter and cerebrospinal fluid (Liu et al., 2017). Moreover, posterior EEG alpha oscillations at rest, a putative biomarker of antidepressant treatment response (Tenke et al., 2011), was greater in individuals who rated R/S as highly important compared to those who did not, provided ratings were obtained during early stages of ontogenetic development (Tenke et al., 2013, 2017). Likewise, increased resting-state functional MRI default mode network connectivity in high compared to low risk individuals (Posner et al., 2016) was reported to be relatively decreased (i.e., lowered) with greater R/S importance (Svob et al., 2016).
Whereas the above cited reports investigated rather persistent, time-invariant neurobiological markers (i.e., structural MRI and posterior alpha; see Hao et al., 2017; Tenke et al., 2018), an even more critical question is how transient brain responses to salient stimuli are affected by R/S importance, as these processes reflect ongoing (state) mechanisms of coping with events in daily life. Dysfunctions in emotion processing and regulation are considered to be a core deficit of mood disorders (e.g., Gross and Munoz, 1995; Rive et al., 2013), and mindfulness meditation, which may be closely linked to R/S importance, has been claimed to exert beneficial effects on physical and mental health (Chiesa and Serretti, 2010; Hölzel et al., 2011; Lomas et al., 2015; Lutz et al., 2015; Tang et al., 2015). Over several decades, affective neuroscience has identified key modules for emotional processing and self-awareness, including amygdala, striatum, nucleus accumbens, anterior insula, orbitofrontal, ventromedial, prefrontal, anterior cingulate, and posterior cingulate cortex (e.g., Davidson et al., 2000; Phan et al., 2002; Phillips et al., 2003a, b; Pessoa, 2008; Price and Drevets, 2012).
The right occipitoparietotemporal cortex, specifically the rTPJ (e.g., Liotti and Tucker, 1995; Tucker, 2015), plays a critical role in the early detection of affective stimulus significance, a critical mechanism for survival guiding approach and withdrawal behavior (e.g., Lang et al., 1998; Bradley, 2009). These right hemisphere regions are embedded within a network involving cortical (anterior insula, anterior cingulate cortex) and subcortical (amygdala, striatum) structures for detecting emotional and reward saliency (Corbetta and Shulman, 2002; Lutz et al., 2015). Using rTMS, Crescentini et al. (2014, 2015) reported a direct link between R/S self-representations and neuronal activation of the right IPL, where increasing IPL excitability yielded a decrease of implicit R/S. Using fMRI, Johnson et al. (2014) found reduced BOLD activity in primary visual cortex in response to religious symbols with negative valence, and this activity was correlated with a measure of R/S importance, suggesting that a person’s R/S beliefs interact with salience detection and processing at an early stage in the processing hierarchy of the visual system. Interestingly, after reviewing the literature on beneficial clinical effects of mindfulness practices, Chiesa et al. (2013) considered the possibility that mindfulness meditation practices are, depending on the practitioner’s skill level, differentially linked to ‘top–down’ (short-term meditation practice) versus ‘bottom–up’ (long-term) control processes of emotion regulation (e.g., Ochsner and Gross, 2005; Ochsner et al., 2009).
Electrophysiological measures, such as ERPs, are particularly suited to study transient brain responses to motivationally salient stimuli because they (1) directly reflect neuronal activation and (2) allow characterization of consecutive processing stages with millisecond temporal resolution. However, few studies have examined ERP effects of religiosity or R/S importance. Inzlicht et al. (2009) found that stronger religious zeal and greater belief in God was associated with reduced ERN, considered a correlate of performance monitoring and self-regulation originating from anterior cingulate cortex (e.g., Gehring and Knight, 2000). The authors interpreted the reduced ERN as neurophysiological evidence for a protective buffer against anxiety (i.e., minimizing error experience) caused by a cognitive style associated with strong convictions. A follow-up study (Good et al., 2015) implied that decreased affective responses to errors are linked to focusing on God’s love and forgiveness rather than on God’s wrath and punishment. In another study, Thiruchselvam et al. (2017) reported that increased late ERP positivity to faces was less affected by peer ratings of attractiveness in non-religious as compared to religious undergraduates, suggesting higher social conformity in the latter group.
Given the overall scarcity of reports examining the effects of religiosity or R/S importance using ERP measures, the current study sought to build on a broad literature relying on automatic (bottom–up) ERP responses to affective stimuli (e.g., for reviews, see Olofsson et al., 2008; Hajcak et al., 2012). The most consistent finding is what is known as the LPP, an increased posterior positivity to emotional (pleasant or unpleasant) than neutral pictures, which emerges around 200 ms after stimulus onset and closely covaries with arousal (e.g., Kayser et al., 1997; Cuthbert et al., 2000; Schupp et al., 2000; Keil et al., 2008). It is assumed that the increase in positivity reflects an extra allocation of attentional resources to stimuli that intrinsically engage motivational brain circuits via re-entrant projections to and from brain structures outside the visual system, such as the lateral prefrontal cortex and amygdala (e.g., Vuilleumier and Driver, 2007; Pessoa, 2008; Bradley, 2009; Keil et al., 2009; Pourtois et al., 2013). Conversely, multiple prefrontal regions may exert inhibitory influences, allowing down-regulation of emotional processing (e.g., Tang et al., 2015). For example, active suppression of emotional arousal in response to unpleasant pictures was accompanied by reduced LPP amplitudes (Moser et al., 2006). Two ERP studies reported that experienced meditators, when compared to control participants without meditation experience, showed attenuated responses to negative pictures, either for the sustained LPP (500–1500 ms time interval, linked-mastoids EEG reference, frontal sites only; Sobolewski et al., 2011) or during an early LPP interval (140–400 ms, average reference; Reva et al., 2014). The latter study also found that the emotional ERP reductions were more prominent over the right than left hemisphere, suggesting that early, right-lateralized ‘bottom–up’ processes of salience detection are modulated by long-term meditators.
Several ERP studies have previously reported evidence of right-lateralized brain activation during an early (preconscious) stage of affective processing (e.g., Kayser et al., 1997, 2000; Junghöfer et al., 2001; Keil et al., 2001, 2002). To specifically probe hemispheric asymmetries of emotional processing in an ongoing study of families at high and low risk for depression (Kayser et al., 2016, 2017), we have employed the visual half-field technique, which exploits the functional neuroanatomy of the visual system. Brief, lateralized stimulus presentations to one hemifield are exclusively projected to the contralateral visual cortex (e.g., left hemifield to right hemisphere), providing direct (immediate) access to the presented information, whereas the ipsilateral hemisphere has only indirect (secondary) access to this information after commissural transfer (e.g., Young, 1982; Springer and Deutsch, 1989). Pairs of pictures depicting facial areas of patients with skin diseases before (negative valence) and after (neutral) surgical treatment were used as stimulus material during this hemifield paradigm (Kayser et al., 1997, 2000, 2016, 2017). While most studies have manipulated emotional content by using stimuli of the International Affective Picture System (IAPS; e.g., Lang et al., 2005; Bradley and Lang, 2007), the advantage and main purpose of this paired stimulus set is isolating the emotional content construct (negative valence, high arousal) from other stimulus characteristics (e.g., content, complexity, luminance, contrast, color), as changes in these properties will also affect early and late ERP components (e.g., Delplanque et al., 2007; Wiens et al., 2011). Self-report ratings of valence and arousal (Bradley and Lang, 1994) for these stimuli indicated a near optimal characterization of the evaluative space for affective stimuli along a negativity dimension (see Figure 1 in Kayser et al., 2016). In support of our hypotheses, we found right-lateralized emotional effects (i.e., a relative positivity for negative than neutral stimuli) for an early ERP component, termed an N2 sink peaking at about 200 ms, which could be attributed to neuronal generator sources within right occipitotemporal cortex using a distributed inverse solution (Kayser et al., 2016). These early emotional ERP asymmetries were further modulated by hemifield, with larger amplitude and asymmetry of emotional effects for left hemifield (right hemisphere) presentations. A follow-up report (Kayser et al., 2017) showed that these early emotional ERP asymmetries were markedly reduced in individuals at high than low family risk for depression, which is in agreement with evidence showing reduced electrophysiological responsivity to affective signals over right temporoparietal regions in MDD patients (e.g., Kayser et al., 2000; Moratti et al., 2008, 2015; Domschke et al., 2015) and individuals at increased risk for MDD (e.g., Kujawa et al., 2015; Nelson et al., 2015, 2016; Weinberg et al., 2015; Gibb et al., 2016); however, hemifield modulations did not differ between risk groups, suggesting top–down rather than bottom-up effects of risk.
If personal importance of R/S is related to or represents a protective mechanism against familial risk for depression (Miller et al., 1997, 2012), one possible means to accomplish a beneficial effect on well-being and mental health would be to modulate or down-regulate (negative) arousal linked to salience detection at an early stage of processing, that is, even before the information reaches conscious awareness (e.g., LeDoux, 2015), as implicated by the ERP findings of Reva et al. (2014). Accordingly, we would predict that activity within the right-lateralized network for salience detection (Corbetta and Shulman, 2002) is disrupted or inhibited in high risk individuals with high importance of R/S, thereby exhibiting reduced early emotional ERP asymmetries and/or reduced hemifield modulations of these effects. The purpose of the present exploratory study was to test these predictions by taking advantage of an existing and already processed 72-channel ERP data set obtained during our emotional hemifield paradigm in a large sample (N = 127) of high and low risk individuals (Kayser et al., 2017), who had also provided ratings of R/S importance. Following our prior reports (Miller et al., 1997, 2012, 2014; Tenke et al., 2013, 2017), we stratified the sample into participants who had rated their personal R/S importance as high versus those who did not. This dichotomous R/S variable was then added to the split-plot repeated measures design for N2 sink, that is, the earliest ERP component that had yielded prominent emotional content effects in this hemifield paradigm (Kayser et al., 2016).
Materials and Methods
Participants
The sample has been described in detail in our prior report (Kayser et al., 2017). Briefly, it consisted of 127 Caucasian individuals (58 male) between 13 and 59 years of age (Mean ± SD = 33.2 ± 13.9) who were enrolled in a multi-generation, 35-year longitudinal study of families at high and low risk for major depression (Talati et al., 2013; Weissman et al., 2016). Probands were initially selected for the presence or absence of a lifetime history of MDD from outpatient psychiatric clinics and their urban community in New Haven, CT, United States (Weissman et al., 1982, 1992). The current participants were biological descendants (i.e., the second and third generation) of the original probands (i.e., the first generation). Participants with at least one depressed parent or grandparent were considered at high family risk for MDD (n = 74, 32 male, age: 35.1 ± 14.2 years), whereas all others were considered at low family risk for MDD (n = 53, 26 male, age: 30.4 ± 13.0). Most participants (n = 114, 89.8%) were right-handed (Oldfield, 1971; for further details, see Kayser et al., 2017). Participants had to be older than 12 years and without a history of seizures, head trauma or psychosis to be eligible for the emotional hemifield task.
A comprehensive summary of all relevant clinical assessments has been given in our previous report (Kayser et al., 2017). Briefly, clinicaln assessments, beginning as early as age 6, were obtained from probands, their spouses, offspring and grandchildren at six longitudinal waves using semi-structured interviews. Diagnoses were based on age-appropriate versions of the Schedule for Affective Disorders and Schizophrenia-Lifetime Version (Kaufman et al., 1997) using a best estimate procedure (Leckman et al., 1982), allowing lifetime diagnoses of MDD and anxiety disorder (AD). Current depressive and anxiety symptoms were also assessed on all but eight participants using the Hamilton Rating Scales for Depression (Hamilton, 1967) and Anxiety (Hamilton, 1959) for adults and the Children’s Depression Rating Scale (Poznanski et al., 1985) and Revised Child Manifest Anxiety Scale (Perrin and Last, 1992) for children 17 years and under. These data were converted to standard scores (CurrDepz, CurrAnxz) and the missing data were imputed from the existing data of current depressive and anxiety symptoms (Kayser et al., 2017).
All participants had normal or corrected-to-normal visual acuity. EEG testing was performed at the Psychophysiology Laboratory at New York State Psychiatric Institute (NYSPI) during the sixth wave of assessments. All procedures were approved by the Institutional Review Boards at Yale University and at NYSPI/Columbia University. All participants gave written informed consent (≥18 years) or provided written assent (<18 years; written informed consent from parents) in accordance with the ethical standards specified in the 1964 Declaration of Helsinki.
Importance of Religion or Spirituality
While most of the original families (Generation 1) were Roman Catholic, the self-identified denomination of the 127 participants (Generations 2 and 3) at the time of data collection was more diverse, including Catholic (n = 66, 52.0%), Protestant (n = 17, 13.4%), Jewish (n = 5, 3.9%), personal beliefs not affiliated with any institutional religion (n = 15, 11.8%), Agnostic or Atheist (n = 13, 10.2%), and other or refused to answer (n = 11, 8.7%).
Data collection in this ongoing longitudinal study (now in Year 40) has typically been separated by approximately 5–10 years increments since Year 10 (i.e., at Years 0, 2, 10, 20, 25, 30, 35, and 40). Beginning with Year 10, participants also rated their personal importance of religion or spirituality (R/S) on a four-level Likert scale, with response options to the question “How important is religion or spirituality to you?” ranging from “not important at all” to “highly important.” This item has been found to show robust correlations with the Fetzer Institute full-scale measure of personal spirituality (see Miller et al., 2014). The terms “religion” and “spirituality” were both included in this question because they are frequently linked together in studies on health (e.g., Koenig et al., 2001). To estimate test-retest reliability of R/S importance, an intraclass correlation coefficient (ICC) was calculated from ratings obtained since Year 10, which were each available for n = 62 participants (24% missing data across all waves), yielding an ICC of 0.828, which is considered excellent (Cicchetti, 1994) or good (Koo and Li, 2016).
In accordance with prior reports (Miller et al., 1997, 2012, 2014; Tenke et al., 2013, 2017), the sample was stratified by each participant’s response to this item as either “highly important” (R/S+) or any other response (R/S−). Given our recent findings suggesting that an early stage in the ontogenesis of R/S is critical for its link to posterior resting alpha (Tenke et al., 2017), both R/S importance and EEG alpha being putative markers of resilience against MDD (Tenke et al., 2013), the earliest available importance rating was used for this classification (mean age at R/S importance rating: 19.4 ± 9.9 years). Table 1 shows the resulting stratification of risk status by R/S importance along with core demographics variables.
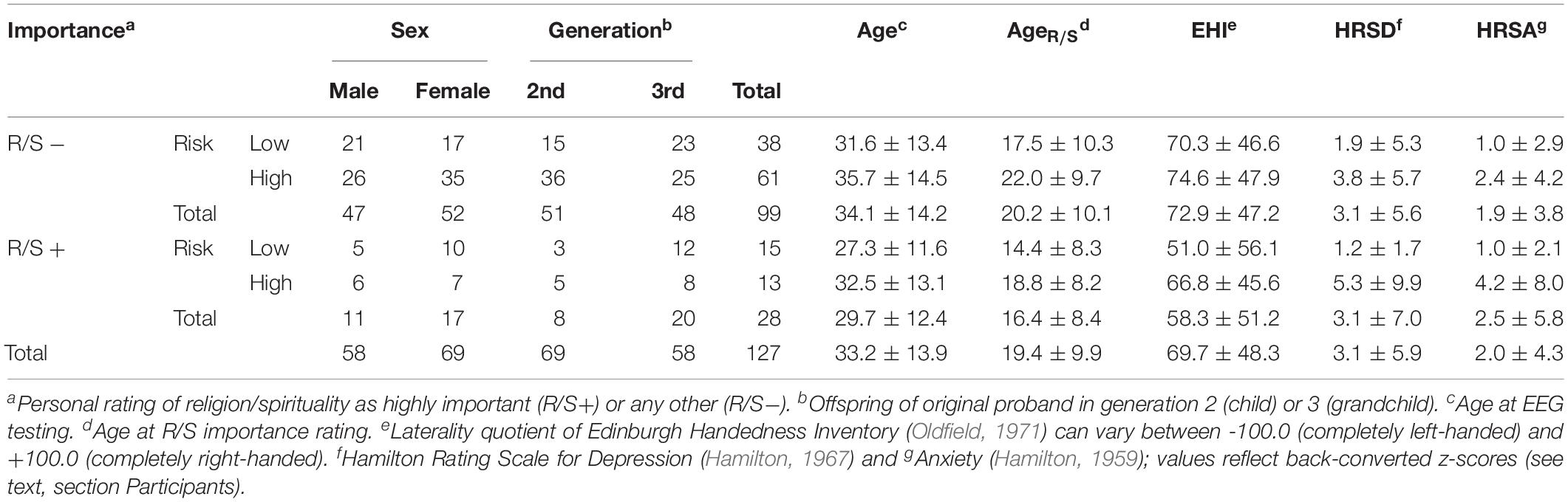
Table 1. Crosstabulation of religion/spirituality importance and family risk of MDD with sex and generation, and corresponding means (±SD) of core demographic and clinical variables.
While there were no significant differences in sex (Fisher’s Exact Test, p = 0.59), age, age at R/S importance rating, or handedness with generation included as a covariate (all F[1,122] < 1.3), the sample included more high than low risk participants from the second generation (Fisher’s Exact Test, p = 0.02). High compared to low risk participants had more current symptoms of depression (F[1,122] = 4.85, p = 0.03) and anxiety (F[1,122] = 4.30, p = 0.04), but there were no significant effects involving R/S importance (all F[1,122] ≤ 2.0, all p ≥ 0.16). In the R/S low importance group, there was a significantly greater number of high than low risk participants with a lifetime history of MDD (35 vs. 11) compared to those without (27 vs. 26; Fisher’s Exact Test, p = 0.007). However, lifetime history of AD was not significantly different for the groups stratified by family risk and R/S importance (Fisher’s Exact Test, p = 0.15).
A total of 41 participants (32%) had a lifetime history of substance use disorder, including alcohol, however, there were no significant subgroup differences (R/S−, low/high risk, n = 11/21; R/S+, n = 4/5; Fisher’s Exact Test, p = 0.70). This was also the case when data were limited to current substance use disorder (i.e., within the last 18 months; R/S−, low/high risk, n = 2/7; R/S+, n = 1/1; Fisher’s Exact Test, p = 0.49) or when considering only substance or alcohol use (i.e., without meeting criteria for dependence; R/S−, low/high risk, n = 5/10; R/S+, n = 2/0; Fisher’s Exact Test, p = 0.15). A total of 23 participants (18%) had a lifetime history of nicotine dependence, and there were also no significant subgroup differences (R/S−, low/high risk, n = 3/17; R/S+, n = 1/2; Fisher’s Exact Test, p = 0.45), including when considering only current nicotine dependence (R/S−, low/high risk, n = 2/9; R/S+, n = 1/1; Fisher’s Exact Test, p = 0.42).
Current use of psychotropic medications (i.e., within the last 3 months, including sedatives, stimulants, antidepressants, anticonvulsants, and lithium, but excluding over the counter medications) was observed for 24 participants (19%), revealing no significant subgroup differences (R/S−, low/high risk, n = 4/17; R/S+, n = 0/3; Fisher’s Exact Test, p = 1.0). Current use of non-prescribed substances (i.e., alcohol and illicit substances, including cannabis, cocaine, heroin, etc.) was reported by 11 participants (9%), also revealing no significant subgroup differences (R/S−, low/high risk, n = 2/8; R/S+, n = 0/1; Fisher’s Exact Test, p = 1.0). Finally, current use of medications to treat physical problems (i.e., including cancer, cardiovascular disease, thyroid disorder, arthritis, autoimmune disorder, skin conditions, liver, kidney, allergies or respiratory disease, among others) was reported for 69 participants (54%), which also did not differ between subgroups (R/S−, low/high risk, n = 19/38; R/S+, n = 5/7; Fisher’s Exact Test, p = 0.74).
There were also no differences between participants at high vs. low risk for depression or high vs. low R/S importance in current medication use to treat physical ailings or in use of non-prescribed substances (all p ≥ 0.11). As expected, high risk individuals were more likely than those at low risk to use psychotropic medications (Fisher’s Exact Test, p = 0.006), however, there were no differences between R/S subgroups (p = 0.28).
Stimuli and Procedure
All considerations pertaining to stimulus selection, characteristics, ratings, underlying rationale and presentation procedure have been detailed previously (Kayser et al., 1997, 2000, 2016). Briefly, stimuli consisted of 16 closely matched pairs of pictures depicting facial areas of patients with dermatological diseases before (negative) and after (neutral) surgical treatment. Neutral stimuli differed from their negative counterpart only in the emotionally relevant feature but were almost identical in all other aspects. Stimulus ratings via self-assessment manikin (Bradley and Lang, 1994) indicated that negative stimuli were perceived as moderately unpleasant and arousing whereas neutral stimuli were seen as neither pleasant or unpleasant and not arousing (Kayser et al., 2016). Stimuli were briefly presented for 250 ms on a monitor to the left or right hemifield (NeuroScan Inc., 2003) using a pseudo-randomized sequence (i.e., four blocks of 32 trials) with variable intertrial intervals (8–13 s). Participants attended to the stimulus presentations while maintaining fixation but did not respond manually. Trials with horizontal eye movements (saccades) exceeding 2° from fixation during stimulus exposure were rejected.
Data Acquisition and Processing
ERP acquisition and processing procedures have been described in detail (Kayser et al., 2016). Briefly, 72-channel EEGs were obtained at 1024 samples/s (BioSemi Inc., 2001), followed by identification and elimination or reduction of typical recording artifacts (i.e., blinks, electrolyte bridges, drifts, movements, muscle, etc.). ERP waveforms were computed for all four conditions (i.e., emotional content [negative, neutral] × hemifield [left, right]), low-pass filtered at 12.5 Hz (-24 dB/octave), and transformed into CSD estimates (μV/cm2 units; spline flexibility m = 4; smoothing constant λ = 2.5 ∗ 10–5; Kayser and Tenke, 2006a, b, 2015) using spherical splines (Perrin et al., 1989, 1990). CSDs represent reference-free estimates of radial current flow at scalp (i.e., negative [sink] or positive [source] values represent current flow exiting or toward the scalp, respectively) that avoid several pitfalls of volume-conducted surface potentials while also providing sharper topographies and a more focused component structure (i.e., higher temporal resolution; Tenke and Kayser, 2012; Burle et al., 2015; Carvalhaes and de Barros, 2015; Kayser and Tenke, 2015). CSDs were submitted to temporal PCA (tPCA; Kayser and Tenke, 2003) to obtain data-driven summaries of radial current flow, or reference-free ERP components reflecting neuronal generator patterns at scalp (Kayser and Tenke, 2015). While our prior report identified and analyzed three consecutive CSD-tPCA factors peaking between 200 and 700 ms that were robustly linked to emotional content, the present report focuses on the first of these factors that corresponded to a temporoparietal N2 sink (peak latency 212 ms). The superimposed emotional effects (increased sources [positivity] for negative than neutral stimuli) were strongly lateralized to the right hemisphere, and their distributed inverses (sLORETA; Pascual-Marqui, 2002; Tadel et al., 2011; for computational details using CSD-tPCA factor scores, see Supplementary Appendix and Kayser et al., 2016) revealed maximal activations in right occipitotemporal cortex.
Statistical Analysis
To assess the combined effects of family risk for depression and R/S importance in the context of the emotional hemifield paradigm, the present report adopted the two-pronged analytical approach employed by Kayser et al. (2017). First, differences in emotional content were evaluated for N2 sink factor scores via non-parametric randomization tests (Maris, 2004; Kayser et al., 2007) to probe the entire topography for each subgroup (i.e., R/S importance × risk). Second, N2 sink factor scores were pooled across three lateral-inferior parietooccipital sites over each hemisphere (PO9/10, PO7/8, P7/8) where emotional content effects were most robust (Kayser et al., 2016). Emotional content, visual field, and group effects were then evaluated with repeated measures analysis of variance (ANOVA) for mixed factorial designs (including between- and within-subjects variables as required), using an unstructured covariance matrix (BMDP-5V; Dixon, 1992) and adding sex, age, lifetime history of MDD and AD, and current severity of symptoms for depression and anxiety as covariates. Unlike conventional F statistics, this analytical model is based on maximum likelihood estimates and χ2 statistics within a linear regression model, which allows the exploration of interaction sources via linear combinations of regression parameters. We employed a conventional level of significance (p < 0.05) and report Cohen’s w for effect sizes (small = 0.1, medium = 0.3, large = 0.5; Cohen, 1988).
Results
Electrophysiological Data
Figure 1 provides a detailed overview of the emotional content effects related to a distinct N2 sink peaking at about 200 ms, followed by a subsequent P3 source at about 300 ms, as can be seen in the mean CSD waveforms for negative and neutral stimuli at selected lateral parietal sites (P7/8). Furthermore, differences of emotional content emerged around 150 ms, revealing more positive-going CSDs for negative than neutral stimuli that persisted throughout the recording epoch. Notably, this basic CSD component structure and the superimposed emotional effects were present in all subgroups, although to a different degree.
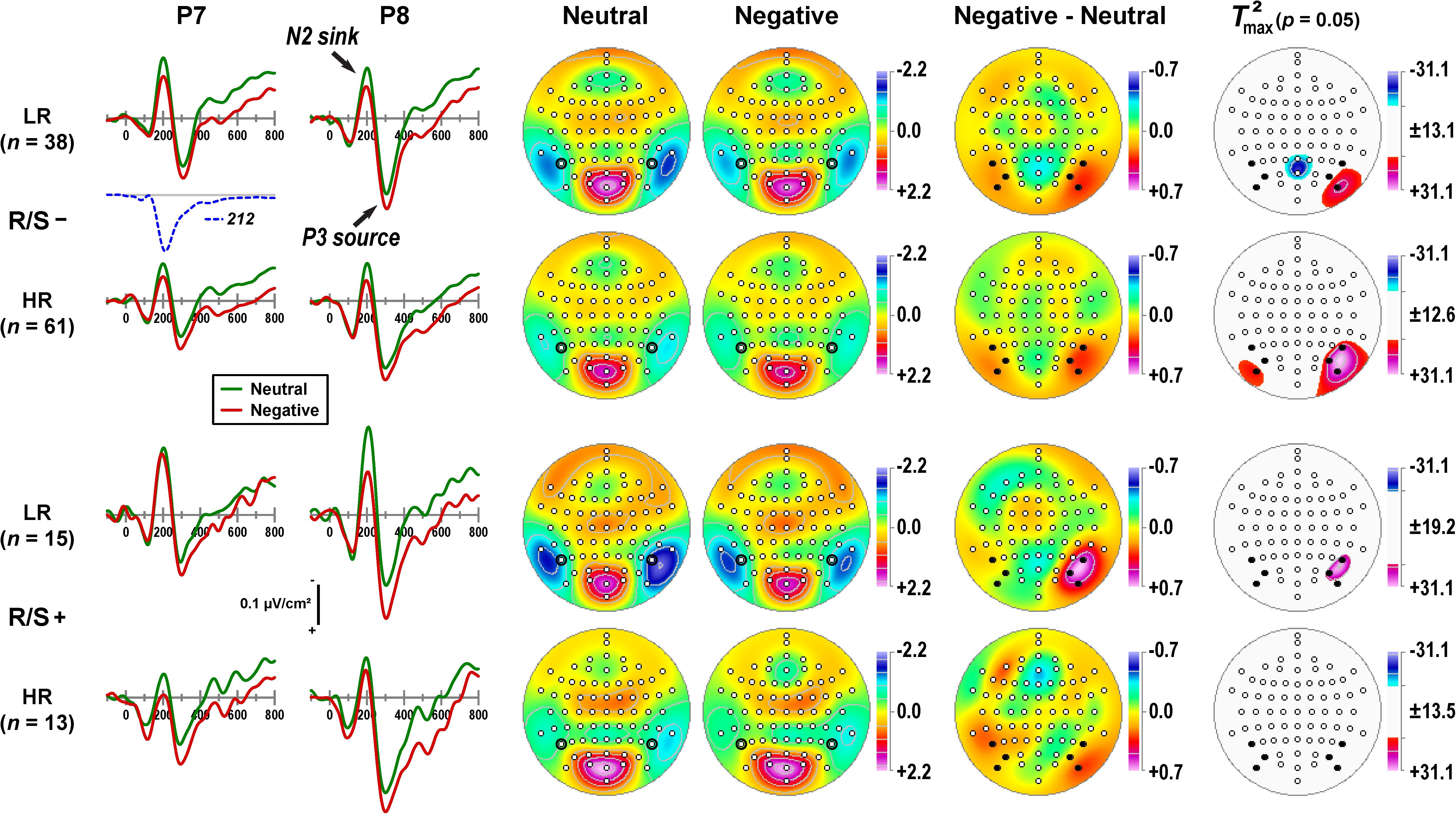
Figure 1. Columns 1–2: Current source density (CSD) [μV/cm2] waveforms (–100 to 800 ms, 100 ms pre-stimulus baseline) for negative and neutral stimuli (pooled across hemifield) at selected left and right lateral-parietal sites (P7, P8; black circles in columns 3–4) for subgroups stratified by personal importance of religion/spirituality (R/S–: less than highly important; R/S+: highly important) and MDD family risk (LR/HR: low/high risk). Distinct CSD components (N2 sink, P3 source) are labeled in italics at P8 for R/S– individuals at low risk. Factor loadings of the targeted temporal PCA factor corresponding to N2 sink (peak latency 212 ms; Kayser et al., 2016) are shown for comparison (dashed line). Columns 3–6: Statistical evaluation of topographic emotional content effects for the corresponding CSD-tPCA factor scores using randomization tests for paired samples (10,000 repetitions) for each subgroup. Shown are the mean factor score topographies for neutral and negative stimuli, the emotional content net effect (negative-minus-neutral), and squared univariate (channel-specific) paired samples T statistics thresholded at the 95th quantile (p = 0.05) of the corresponding randomization distribution derived from the full sample (maximum of all 72-channel squared univariate paired samples T statistics). To facilitate comparisons of the max(T2) topographies with the underlying difference topographies, the sign of the difference at each site was applied to the respective T2 value, which is otherwise always positive. Symmetric scales were optimized for score ranges across neutral and negative stimuli and all subgroups. All topographies are two-dimensional representations of spherical spline interpolations (m = 2; λ = 0) derived from the mean factors scores or T2 statistics available for each recording site. Sites marked as black dots (Columns 5–6) were used in repeated measures ANOVA.
The common variance associated with N2 sink was captured by a CSD-tPCA factor peaking at 212 ms (dashed line in Figure 1; 5.7% total variance; Kayser et al., 2016). The corresponding factor score topographies consisted of prominent lateral temporoparietal sinks that were paired with a mid-parietooccipital P2 source (Figure 1, columns 3-4), implicating a dipolar generator involving secondary visual (prestriate) cortex and cuneus. The topography representing the corresponding emotional content net effect (i.e., negative-minus-neutral) revealed maximum differences at lateral-inferior parietooccipital sites (PO9/10, PO7/8, P7/8) that were larger over the right than left hemisphere (Figure 1, column 5). The non-parametric evaluations of these differences (see permutation tests in Figure 1, column 6) confirmed robust right-greater-than-left asymmetries at this region except for high risk participants with high R/S importance.
Further clarification of the putative generators underlying the emotional content net effects in each subgroup was provided by their distributed inverses (sLORETA solutions) shown in Figure 2, which utilize the entire CSD-tPCA-based component topography (for computational details, see Supplementary Appendix and Kayser et al., 2016). While robust asymmetric (i.e., right-greater-than-left) sources in extrastriate cortex can be seen for low risk individuals, these asymmetries were more subtle or absent in high risk individuals (compare rows 1 and 3 vs. rows 2 and 4 in Figure 2). Moreover, these early posterior (i.e., extrastriate cortex) activations were accompanied by strong inferior temporal activations for low risk participants with high R/S importance.
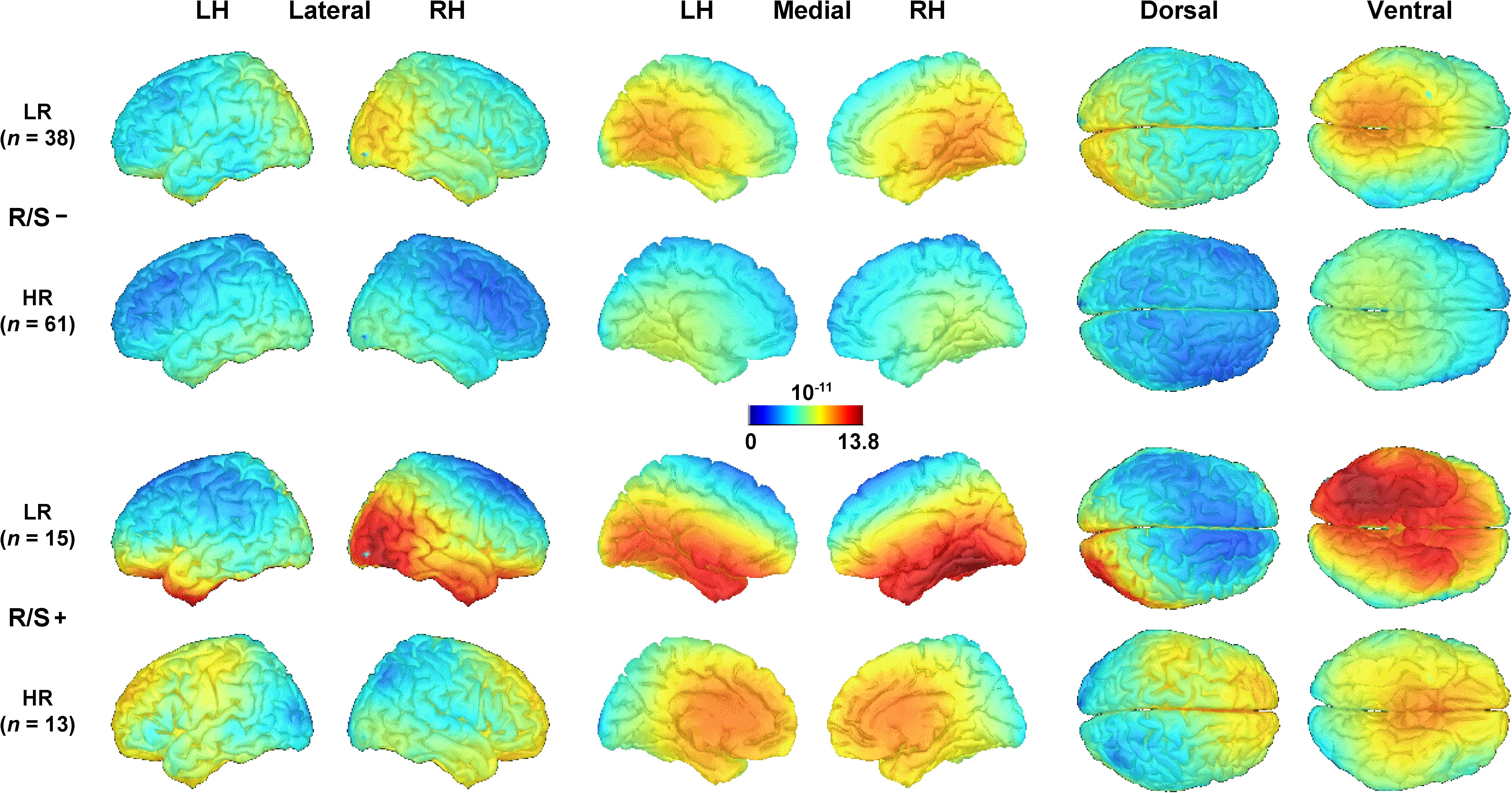
Figure 2. Distributed inverse solutions (sLORETA; Pascual-Marqui, 2002; Tadel et al., 2011) of emotional content net effects (negative-minus-neutral; see Figure 1, column 5) for N2 sink (see Supplementary Appendix for back-projection of CSD-tPCA factors to surface potential data space). An optimized scale range [(pA/m)1/2] was used across all subgroups. LH/RH, left/right hemisphere; LR/HR, low/high risk; R/S, religion/spirituality less than highly (–) or highly (+) important.
Figure 3 shows the mean emotional content net effects at lateral-inferior parietooccipital sites as revealed by the parametric analyses. Most importantly, there was a significant four-way interaction of risk status × R/S importance × emotional content × hemisphere, χ2[1] = 6.40, p = 0.01, w = 0.22.1 Follow-up analyses revealed a significant emotional content × hemisphere interaction for low risk participants with high R/S importance, χ2[1] = 16.1, p = 0.0001, w = 0.36, originating from a robust emotional content effect at the right hemisphere, χ2[1] = 30.2, p < 0.0001, w = 0.49, but absence thereof at the left hemisphere. Participants with low R/S importance, and independent of risk status, had significant or marginally significant emotional content effects over both hemispheres, all χ2[1] ≥ 3.62, all p < 0.06, 0.17 ≤ w ≤ 0.45, although nevertheless stronger over the right hemisphere, as supported by a marginally significant emotional content × hemisphere interaction for high risk participants with low R/S importance, χ2[1] = 2.93, p = 0.07, w = 0.15. In contrast, high risk participants with high R/S importance showed no significant emotional content effects at either hemisphere, both χ2[1] ≤ 1.64, p ≥ 0.20, w ≤ 0.11.2
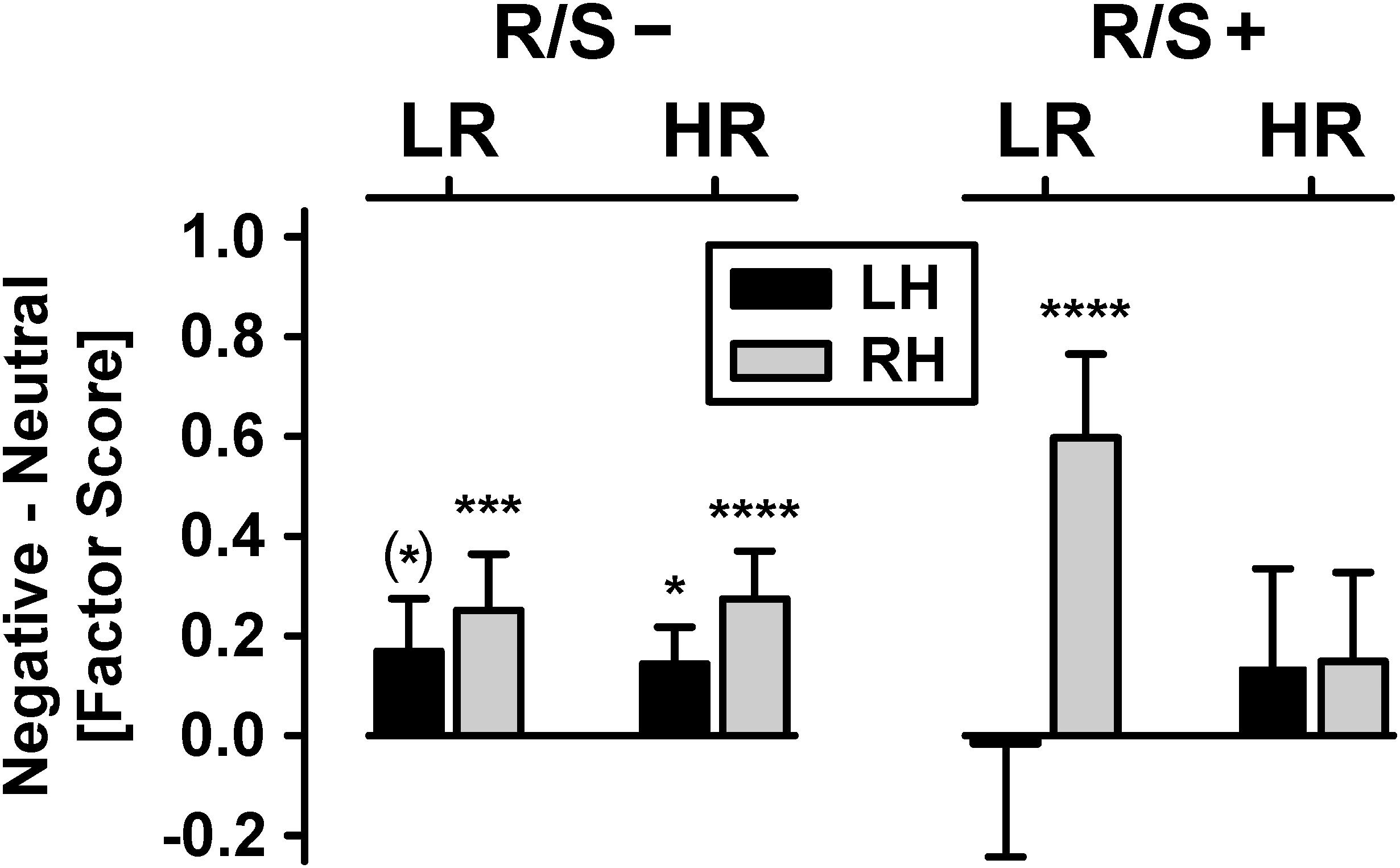
Figure 3. Emotional content net effects (negative-minus-neutral) for each hemisphere (LH/RH: left/right hemisphere), pooled across visual field. Shown are mean (±SEM) differences of N2 sink amplitudes [factor scores] over parietooccipital regions for subgroups stratified by personal importance of religion/spirituality (R/S–: less than highly important; R/S+: highly important) and MDD family risk (LR/HR: low/high risk). Significant simple effects of emotional content (i.e., differences between negative and neutral), corresponding to small to large effects (0.18 ≤ w ≤ 0.49), are marked as: (∗) p < 0.10; ∗p < 0.05; ∗∗∗p < 0.001; ****p < 0.0001.
Figure 4 shows the hemifield modulations of these emotional content × hemisphere effects separately for each subgroup, which revealed enhanced emotional content effects over the right hemisphere for left but not right visual field presentations (Kayser et al., 2016, 2017). Left hemifield presentations showed right-greater-than-left asymmetries of emotional content for participants at low risk with high R/S importance, χ2[1] = 21.2, p < 0.0001, w = 0.41 (Figure 4, inverted triangles, column 3), at high risk with low R/S importance, χ2[1] = 10.6, p = 0.001, w = 0.29 (column 2), and at low risk with low R/S importance, χ2[1] = 3.06, p = 0.08, w = 0.16 (column 1), but not for those at high risk with high R/S, χ2[1] = 0.58, p = 0.45, w = 0.07 (column 4). In contrast, right hemifield presentations failed to reveal any significant asymmetric emotional content effects for any subgroup (Figure 4, upright triangles). These group-dependent hemifield modulations of asymmetric emotional content effects were further supported by simple interactions of emotional content, hemisphere, and visual field for individuals at low risk (R/S importance: low, χ2[1] = 3.17, p = 0.08, w = 0.16; high, χ2[1] = 6.87, p = 0.009, w = 0.23) and high risk with low R/S importance, χ2[1] = 10.1, p = 0.002, w = 0.28, but not for those at high risk with high R/S importance, χ2[1] = 1.22, p = 0.27, w = 0.1.
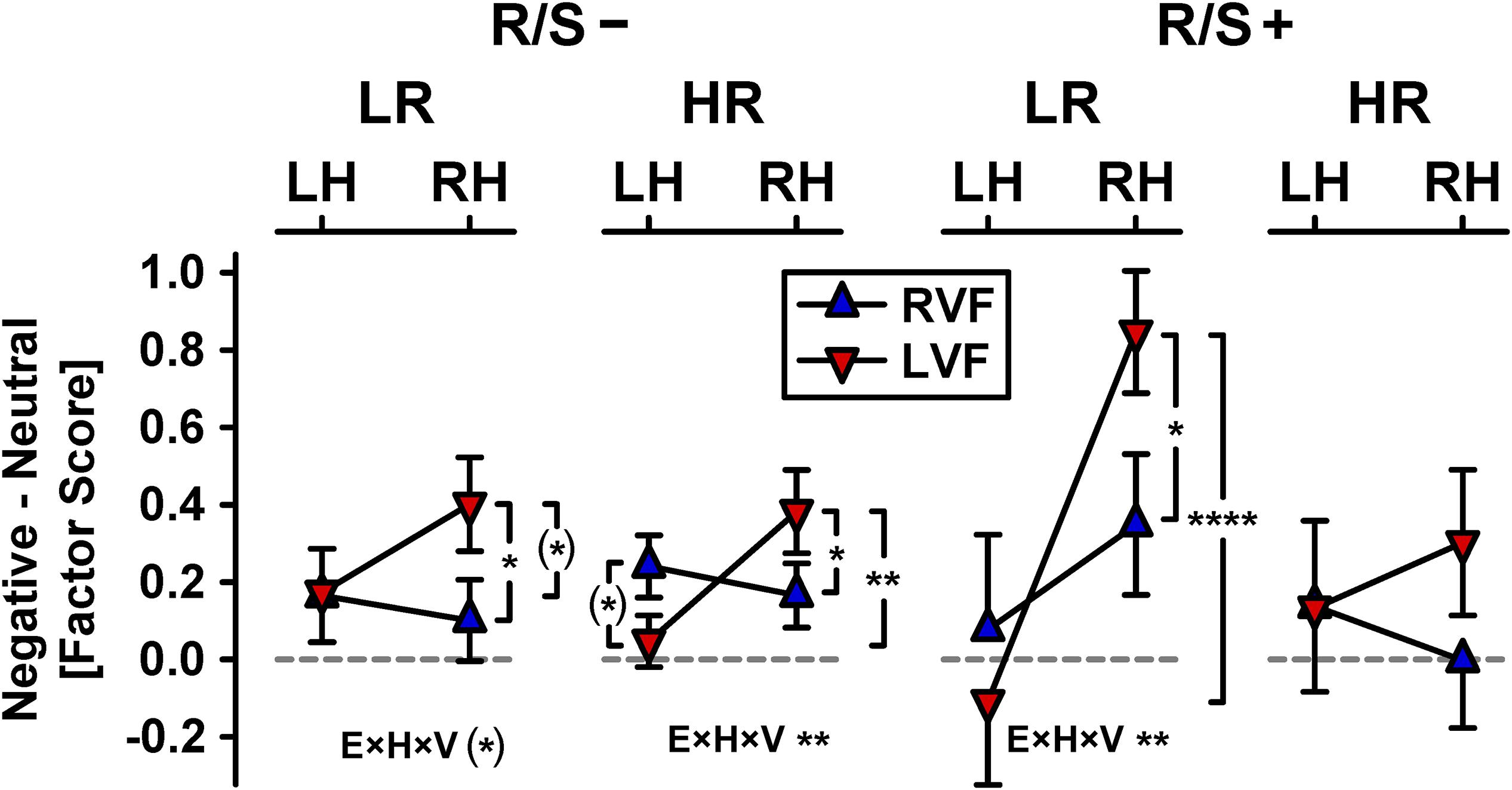
Figure 4. Hemifield modulations of emotional content net effects shown in Figure 3 (RVF/LVF: right/left visual field; all other abbreviations as in Figure 3). Significant pairwise simple effects (indicated by vertical brackets) and emotional content × hemisphere × visual field interactions (E×H×V), the latter corresponding to small to medium effects (0.16 ≤ w ≤ 0.28), are marked as: (∗)p < 0.10; ∗p < 0.05; ∗∗p < 0.01; ****p < 0.0001.
Discussion
The present report evaluated whether individuals at high familial risk for major depression, who rated religion and spirituality as highly important during adolescence and young adulthood, differed in their early, bottom–up processing of unpleasant pictures during an emotional hemifield paradigm (Kayser et al., 2016, 2017). In agreement with prior studies (Miller et al., 2012, 2014; Svob et al., 2016; Liu et al., 2017), R/S importance significantly modulated outcome measures dependent on risk status. Here, overall early emotional ERP responsivity and asymmetry, which was observed for low R/S importance participants independent of risk status, was substantially diminished or absent for high risk individuals with high R/S importance; however, it was enhanced for low risk individuals with high R/S importance. This is in line with the idea that neuronal activation within the right-lateralized network for salience detection (Corbetta and Shulman, 2002) is down-regulated at an early processing stage in prestriate cortex and cuneus in high risk individuals with high R/S importance. The putative protective function is to prevent or weaken the downstream production of an affective state in response to unpleasant, arousing stimuli involving ventral brain regions (Phillips et al., 2003a, b), which involve inferior temporal and also posterior cingulate cortex for the present emotional hemifield paradigm (Kayser et al., 2016). By contrast, increased allocation of attention to motivationally significant pictures was seen for low risk individuals with high R/S importance, who supposedly would not suffer from experiencing negative arousal; rather, these individuals may enhance processing of motivationally salient stimuli consistent with the idea that mindfulness is associated with a state of relaxed alertness that may promote mental health (e.g., Lomas et al., 2015; Lutz et al., 2015). Interestingly, recent fMRI findings by Miller et al. (2018) implicated reduced activity in left inferior parietal cortex when participants recalled personalized spiritual compared to neutral-relaxing experiences, suggesting prolonged lateralized effects of modulatory mechanisms related to R/S importance.
Our prior report using these data indicated that risk status did not alter hemifield-dependent modulations of early emotional ERP effects (Kayser et al., 2017). The present analysis qualifies this finding because high R/S importance differentially affected risk status, revealing enhanced visual field effects in the low risk group, particularly for left hemifield (right hemisphere) stimulations, whereas hemifield effects were weaker and non-significant in the high risk group (Figure 4). This indicates that R/S importance affects allocation of attentional resources to stimuli that intrinsically engage motivational brain circuits (i.e., motivated attention; Bradley et al., 2003; Vuilleumier and Driver, 2007; Bradley, 2009) at an early processing stage, that is, during the stimulus-driven (bottom–up) visual stream that precedes conscious awareness (e.g., LeDoux, 1989, 2015; Tamietto and de Gelder, 2010). Although speculative, this interpretation of our findings is in accordance with the idea that ‘bottom–up’ control processes of emotion regulation are linked to experienced mindfulness meditation (Chiesa et al., 2013), assuming that high R/S importance may reflect a trait aspect or rather stable characteristic of mindfulness. In any case, we note that these conclusions regarding the early onset of these modulatory R/S importance effects are firmly grounded in the temporal sequence of brain activation uniquely afforded by ERPs. This favorable characteristic of ERPs is further improved by their CSD transformations, which provide a more distinct time course than their surface potentials counterparts (Kayser and Tenke, 2006a; Burle et al., 2015), where the signal is impeded (i.e., spatially smeared) by volume conduction (e.g., Nunez and Srinivasan, 2006; Tenke and Kayser, 2012).
Due to the uniqueness of the emotional hemifield paradigm and given the scarceness of prior ERP research with religiosity or the R/S importance construct, it is difficult to integrate the present findings within the broader literature. The association between reduced ERN and firmness of religious belief by Inzlicht et al. (2009) and Good et al. (2015) are consistent with the present results if interpreted as R/S importance affording a protective buffer (i.e., against anxiety or negative affective arousal). In a neuroscience-based account, Inzlicht et al. (2011) suggested that religion is widespread among humans because it fulfills the need for meaning (i.e., the perceived coherence between beliefs, salient goals, and perceptions of the environment), making the world appear ordered, controlled, and understandable. Although these researchers have focused on brain activity attributed to anterior cingulate cortex and processes linked to cognitive (self-)control, their “motivated meaning-making” account stresses that religion is a motivated process – this aligns with the idea that differences in personal R/S importance are associated with bottom-up processes of motivated attention (Bradley, 2009), as studied here. Our findings are also in agreement with fMRI findings by Johnson et al. (2014) suggesting that R/S importance affects salience detection at an early stage of the visual system processing hierarchy. Moreover, our findings appear to be in line with rTMS findings by Crescentini et al. (2014, 2015) that suggest a link between the R/S construct and differential activation of right inferior parietal cortex. Clearly, more hypothesis-driven research is needed in the fields of social and affective neuroscience to help deepen our understanding of the relationship between R/S importance, brain activation, and health benefits. However, the present findings allow for the generation of specific testable predictions, namely up- versus down-regulation of early, right-lateralized activity associated with salience detection in individuals with high personal importance of religion or spirituality, depending on their family risk status for depression.
The study has several limitations. First, the assessment of personal R/S importance relies on a single self-report item, which may be an inadequate representation of what comprises religiosity and spirituality (e.g., Hill and Pargament, 2003). However, a more recent wave of this longitudinal study (N = 282) employed a comprehensive survey that included several validated scales assessing religious beliefs and experiences and used an exploratory factor analysis to uncover latent R/S constructs that would covary with the R/S importance item (Svob et al., 2019). The first factor (15.8% explained variance) was directly related to the R/S importance item (r = 0.82), as well as personal relationship with the Divine, forgiveness by God, religious activities, and religious coping. At the same time, this R/S factor precluded other R/S aspects, including gratitude, altruism, and social support. The R/S factor was reliability reproduced for key subgroups, that is, for generations 2 (n = 140) and 3 (n = 99), and for high (n = 150) and low (n = 89) risk individuals, thereby affirming adequate single-item construct validity. The R/S factor observed in this study also closely matched one of five invariant factors identified across three diverse cultures (China, India, United States) by McClintock et al. (2016). That factor, termed Religious and Spiritual Reflection and Commitment, likewise correlated robustly (r = 0.79) with McClintock et al.’s (2016) measure of R/S importance, further suggesting that this single self-report item may be adequate for use in health studies lacking the resources for more extensive measures.
Second, due to the cross-sectional design of the current report (i.e., ERP recordings for the emotional hemifield task were only obtained at Year 30; e.g., Talati et al., 2013), it is unclear whether the differential electrophysiological effects observed for high versus low R/S importance are directly linked to specific functional outcomes (e.g., suicidal ideation, cardiovascular health), as such relationships are only implied by familial risk status. Third, the generalizability of the findings is limited by the lack of ethnic, socioeconomic and religious diversity (i.e., the sample was predominantly Caucasian, middle class and Catholic), as all participants were part of a three-generation longitudinal, cohort study that started over 30 years ago (Weissman et al., 1982); still, the sample’s homogeneity also constitutes a strength. Fourth, given that loss of pleasure (anhedonia) is a distinct feature of depression (e.g., Watson et al., 1988), resulting in blunted positive affect and responsivity to reward, the lack of pleasant stimuli may be viewed as a limitation. However, the inclusion of appetitive stimuli will also require the inclusion of corresponding ‘neutral’ stimuli matched for content, as discussed previously (Kayser et al., 2000), which is a significant advantage of the present negative/neutral stimulus pairs.
Conclusion
Personal importance of religion or spirituality was found to be differentially associated with automatic, preconscious processing of unpleasant stimuli (motivated attention) in individuals at high and low familial risk for depression. For participants indicating high personal importance of R/S in adolescence or young adulthood, emotional ERPs characterizing early (200 ms), right-lateralized emotional arousal of occipitotemporal (extrastriate) cortex (Kayser et al., 2016) were enhanced for individuals at low risk but were reduced for individuals at high risk. These findings are consistent with prior clinical and neurophysiological evidence suggesting that personal R/S importance may function as a protective buffer against stressful (lifetime) events (i.e., resilience against MDD; e.g., Miller et al., 1997; Svob et al., 2018), presumably by preventing harmful, affective (over-)arousal downstream. Furthermore, the findings implicate a specific neurofunctional mechanism of emotion regulation (Rive et al., 2013; Tang et al., 2015) underlying certain health benefits linked to personal importance of religion and spirituality.
Data Availability Statement
The datasets for this study will not be made publicly available because data were obtained as part of an ongoing, multi-generational study of families at risk for depression that started in 1982 before there was data sharing; therefore, consent was not obtained. Public data sharing, even anonymously, is restricted by participants’ informed consent. We are in the process of obtaining consent. This will take several years as we enroll subjects into our new study. Interested readers may contact the Principal Investigator, MW, for more information, or by visiting the study’s website at: http://highriskdepression.org.
Ethics Statement
All procedures of this research were approved by the Institutional Review Boards at Yale University and at New York State Psychiatric Institute/Columbia University. All participants gave written informed consent (≥18 years) or provided written assent (<18 years; written informed consent from parents) in accordance with the ethical standards specified in the 1964 Declaration of Helsinki.
Author Contributions
JK and CT: design and conceptual idea for reanalysis of existing data. JK: conceptualization and development of emotional hemifield paradigm, data analysis, figures, tables, and supplementary appendix, and drafting and revising the manuscript. MG, JS, and VW: maintenance of clinical database. CS, LM, PW, and MW: conceptualization of single-item measure for assessing personal importance of religion and spirituality. All living authors have critically reviewed the work and provided their final approval for publication.
Funding
This work was supported by grants from the National Institute of Mental Health (MH36197) and the John F. Templeton Foundation (#54679).
Conflict of Interest
For activities unrelated to the current research, the authors report the following financial disclosures during the past 3 years. JK: funding from NIMH and John Templeton Foundation. LM: funding and royalties from American Psychological Association Press, Oxford University Press, Picadore Press, John Templeton Foundation, and Rockefeller Philanthropic Associates. MW: funding from NIMH, the Templeton Foundation and royalties for publications from Perseus Press, Oxford University Press, American Psychiatric Association Press and for the Social Adjustment Scale from Multihealth Systems Inc. None of these pose a conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We dedicate this report to our colleagues and friends, Craig E. Tenke and Virginia Warner, who both passed away unexpectedly in 2017.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2019.00436/full#supplementary-material
Abbreviations
CSD, current source density; EEG, electroencephalogram; ERN, error-related negativity; ERP, event-related potentials; IPL, inferior parietal lobe; LPP, late positive potential; MDD, major depressive disorder; PCA, principal components analysis; rTPJ, right temporoparietal junction; R/S, religion/spirituality; rTMS, repetitive transcranial magnetic stimulation; sLORETA, standardized low resolution brain electromagnetic tomography; tPCA, temporal principal components analysis.
Footnotes
- ^ To address the issue of multiple comparisons in multiway ANOVA designs (Cramer et al., 2016), the false discovery rate (FDR; Benjamini and Hochberg, 1995) was calculated for the list of p-values (all main effects and interactions). Critically, the four-way interaction of risk status × R/S importance × emotional content × hemisphere remained significant after FDR correction (p = 0.04).
- ^ Despite concerns about small cell sizes (Table 1), given the higher prevalence of MDD in women than men (Weissman and Klerman, 1977; Weissman et al., 1984), a repeated measures ANOVA was computed with sex as an additional between-subjects factor. First, the strength of the risk status × R/S importance × emotional content × hemisphere interaction increased substantially, χ2[1] = 10.25, p = 0.001, suggesting that variance associated with sex differences weakened this effect. Second, this analysis yielded three significant effects involving sex: sex × emotional content × hemisphere, χ2[1] = 4.99, p = 0.03; sex × R/S importance × emotional content × hemisphere, χ2[1] = 4.70, p = 0.03; and risk status × R/S importance × sex × hemisphere, χ2[1] = 10.0, p = 0.001. Only the first two interactions, which include emotional content and hemisphere, are relevant to this study. The emotional content × hemisphere interaction, originating from larger emotional content effects (i.e., negative being more positive than neutral) over the right than left hemisphere, was seen for each sex, however, more robust for males than females. R/S importance further qualified this three-way interaction in that these effects were primarily driven by male participants with high R/S importance.
References
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
BioSemi Inc. (2001). ActiveTwo - Multichannel, DC amplifier, 24-bit resolution, biopotential measurement system with active electrodes. Amsterdam: Author.
Bradley, M. M. (2009). Natural selective attention: orienting and emotion. Psychophysiology 46, 1–11. doi: 10.1111/j.1469-8986.2008.00702.x
Bradley, M. M., and Lang, P. J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 25, 49–59. doi: 10.1016/0005-7916(94)90063-9
Bradley, M. M., and Lang, P. J. (2007). “The international affective picture system (IAPS) in the study of emotion and attention,” in Handbook of Emotion Elicitation and Assessment, eds J. A. Coan and J. J. B. Allen, (New York, NY: Oxford University Press), 29–46.
Bradley, M. M., Sabatinelli, D., Lang, P. J., Fitzsimmons, J. R., King, W., and Desai, P. (2003). Activation of the visual cortex in motivated attention. Behav. Neurosci. 117, 369–380. doi: 10.1037/0735-7044.117.2.369
Burle, B., Spieser, L., Roger, C., Casini, L., Hasbroucq, T., and Vidal, F. (2015). Spatial and temporal resolutions of EEG: is it really black and white? A scalp current density view. Int. J. Psychophysiol. 97, 210–220. doi: 10.1016/j.ijpsycho.2015.05.004
Carvalhaes, C., and de Barros, J. A. (2015). The surface Laplacian technique in EEG: theory and methods. Int. J. Psychophysiol. 97, 174–188. doi: 10.1016/j.ijpsycho.2015.04.023
Chiesa, A., and Serretti, A. (2010). A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol. Med. 40, 1239–1252. doi: 10.1017/S0033291709991747
Chiesa, A., Serretti, A., and Jakobsen, J. C. (2013). Mindfulness: top-down or bottom-up emotion regulation strategy? Clin. Psychol. Rev. 33, 82–96. doi: 10.1016/j.cpr.2012.10.006
Cicchetti, D. V. (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 6, 284–290. doi: 10.1037/1040-3590.6.4.284
Cobb, M., Puchalski, C. M., and Rumbold, B. (2012). Oxford Textbook of Spirituality in Healthcare. Oxford: Oxford University Press.
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Erlbaum.
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. doi: 10.1038/nrn755
Cramer, A. O., van Ravenzwaaij, D., Matzke, D., Steingroever, H., Wetzels, R., Grasman, R. P., et al. (2016). Hidden multiplicity in exploratory multiway ANOVA: prevalence and remedies. Psychon. Bull. Rev. 23, 640–647. doi: 10.3758/s13423-015-0913-5
Crescentini, C., Aglioti, S. M., Fabbro, F., and Urgesi, C. (2014). Virtual lesions of the inferior parietal cortex induce fast changes of implicit religiousness/spirituality. Cortex 54, 1–15. doi: 10.1016/j.cortex.2014.01.023
Crescentini, C., Di Bucchianico, M., Fabbro, F., and Urgesi, C. (2015). Excitatory stimulation of the right inferior parietal cortex lessens implicit religiousness/spirituality. Neuropsychologia 70, 71–79. doi: 10.1016/j.neuropsychologia.2015.02.016
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., and Lang, P. J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 52, 95–111. doi: 10.1016/s0301-0511(99)00044-7
Davidson, R. J., Jackson, D. C., and Kalin, N. H. (2000). Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull. 126, 890–909. doi: 10.1037/0033-2909.126.6.890
Delplanque, S., N’diaye, K., Scherer, K., and Grandjean, D. (2007). Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J. Neurosci. Methods 165, 144–150. doi: 10.1016/j.jneumeth.2007.05.030
Dixon, W. J. (ed.) (1992). (BMDP)Statistical Software Manual: To Accompany the 7.0 Software Release. Berkeley, CA: University of California Press.
Domschke, K., Zwanzger, P., Rehbein, M. A., Steinberg, C., Knoke, K., Dobel, C., et al. (2015). Magnetoencephalographic correlates of emotional processing in major depression before and after pharmacological treatment. Int. J. Neuropsychopharmcol. 9:yv093. doi: 10.1093/ijnp/pyv093
Fingelkurts, A. A., and Fingelkurts, A. A. (2009). Is our brain hardwired to produce God, or is our brain hardwired to perceive God? A systematic review on the role of the brain in mediating religious experience. Cogn. Process. 10, 293–326. doi: 10.1007/s10339-009-0261-3
Gehring, W. J., and Knight, R. T. (2000). Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 3, 516–520. doi: 10.1038/74899
Gibb, B. E., Pollak, S. D., Hajcak, G., and Owens, M. (2016). Attentional biases in children of depressed mothers: an event-related potential (ERP) study. J. Abnorm. Psychol. 125, 1166–1178. doi: 10.1037/abn0000216
Good, M., Inzlicht, M., and Larson, M. J. (2015). God will forgive: reflecting on God’s love decreases neurophysiological responses to errors. Soc. Cogn. Affect. Neurosci. 10, 357–363. doi: 10.1093/scan/nsu096
Gross, J. J., and Munoz, R. F. (1995). Emotion regulation and mental health. Clin. Psychol. Sci. Pract. 2, 151–164.
Hajcak, G., Weinberg, A., MacNamara, A., and Foti, D. (2012). “ERPs and the study of emotion,” in The Oxford Handbook of Event-Related Potential Components, eds S. J. Luck and E. S. Kappenman, (New York, NY: Oxford University Press), 441–472.
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x
Hao, X., Talati, A., Shankman, S. A., Liu, J., Kayser, J., Tenke, C., et al. (2017). Stability of cortical thinning in persons at increased familial risk for major depression across eight years. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 619–625. doi: 10.1016/j.bpsc.2017.04.009
Hill, P. C., and Pargament, K. I. (2003). Advances in the conceptualization and measurement of religion and spirituality: Implications for physical and mental health research. Am. Psychol. 58, 64–74. doi: 10.1037/0003-066x.58.1.64
Hölzel, B. K., Lazar, S. W., Gard, T., Schuman Olivier, Z., Vago, D. R., and Ott, U. (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 6, 537–559. doi: 10.1177/1745691611419671
Inzlicht, M., McGregor, I., Hirsh, J. B., and Nash, K. (2009). Neural markers of religious conviction. Psychol. Sci. 20, 385–392. doi: 10.1111/j.1467-9280.2009.02305.x
Inzlicht, M., Tullett, A. M., and Good, M. (2011). The need to believe: a neuroscience account of religion as a motivated process. Rel. Brain Behav. 1, 192–251.
Johnson, K. D., Rao, H., Wintering, N., Dhillon, N., Hu, S., Zhu, S., et al. (2014). Pilot study of the effect of religious symbols on brain function: association with measures of religiosity. Spiritual. Clin. Pract. 1, 82–98. doi: 10.1037/scp0000015
Junghöfer, M., Bradley, M. M., Elbert, T. R., and Lang, P. J. (2001). Fleeting images: a new look at early emotion discrimination. Psychophysiology 38, 175–178. doi: 10.1017/s0048577201000762
Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., et al. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. doi: 10.1097/00004583-199707000-00021
Kayser, J., Bruder, G. E., Tenke, C. E., Stewart, J. E., and Quitkin, F. M. (2000). Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int. J. Psychophysiol. 36, 211–236. doi: 10.1016/s0167-8760(00)00078-7
Kayser, J., Tenke, C., Nordby, H., Hammerborg, D., Hugdahl, K., and Erdmann, G. (1997). Event-related potential (ERP) asymmetries to emotional stimuli in a visual half-field paradigm. Psychophysiology 34, 414–426. doi: 10.1111/j.1469-8986.1997.tb02385.x
Kayser, J., and Tenke, C. E. (2003). Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin. Neurophysiol. 114, 2307–2325. doi: 10.1016/s1388-2457(03)00241-4
Kayser, J., and Tenke, C. E. (2006a). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 117, 348–368. doi: 10.1016/j.clinph.2005.08.034
Kayser, J., and Tenke, C. E. (2006b). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin. Neurophysiol. 117, 369–380. doi: 10.1016/j.clinph.2005.08.033
Kayser, J., and Tenke, C. E. (2015). Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: a tutorial review. Int. J. Psychophysiol. 97, 189–209. doi: 10.1016/j.ijpsycho.2015.04.012
Kayser, J., Tenke, C. E., Abraham, K. S., Alschuler, D. M., Alvarenga, J. E., Skipper, J., et al. (2016). Neuronal generator patterns at scalp elicited by lateralized aversive pictures reveal consecutive stages of motivated attention. Neuroimage 142, 337–350. doi: 10.1016/j.neuroimage.2016.05.059
Kayser, J., Tenke, C. E., Abraham, K. S., Alschuler, D. M., Alvarenga, J. E., Skipper, J., et al. (2017). Motivated attention and family risk for depression: neuronal generator patterns at scalp elicited by lateralized aversive pictures reveal blunted emotional responsivity. Neuroimage Clin. 14, 692–707. doi: 10.1016/j.nicl.2017.03.007
Kayser, J., Tenke, C. E., Gates, N. A., and Bruder, G. E. (2007). Reference-independent ERP old/new effects of auditory and visual word recognition memory: joint extraction of stimulus- and response-locked neuronal generator patterns. Psychophysiology 44, 949–967. doi: 10.1111/j.1469-8986.2007.00562.x
Keil, A., Bradley, M. M., Hauk, O., Rockstroh, B., Elbert, T., and Lang, P. J. (2002). Large-scale neural correlates of affective picture processing. Psychophysiology 39, 641–649. doi: 10.1017/s0048577202394162
Keil, A., Müller, M. M., Gruber, T., Wienbruch, C., Stolarova, M., and Elbert, T. (2001). Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin. Neurophysiol. 112, 2057–2068. doi: 10.1016/s1388-2457(01)00654-x
Keil, A., Sabatinelli, D., Ding, M., Lang, P. J., Ihssen, N., and Heim, S. (2009). Re-entrant projections modulate visual cortex in affective perception: evidence from Granger causality analysis. Hum. Brain Mapp. 30, 532–540. doi: 10.1002/hbm.20521
Keil, A., Smith, J. C., Wangelin, B. C., Sabatinelli, D., Bradley, M. M., and Lang, P. J. (2008). Electrocortical and electrodermal responses covary as a function of emotional arousal: a single-trial analysis. Psychophysiology 45, 516–523. doi: 10.1111/j.1469-8986.2008.00667.x
Koenig, H., King, D. A., and Carson, V. B. (2012). Handbook of Religion and Health, 2nd Edn. New York, NY: Oxford University Press.
Koenig, H., McCullough, M. E., and Larson, D. B. (2001). Handbook of Religion and Health. New York, NY: Oxford University Press.
Koo, T. K., and Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. doi: 10.1016/j.jcm.2016.02.012
Kujawa, A., Proudfit, G. H., Laptook, R., and Klein, D. N. (2015). Early parenting moderates the association between parental depression and neural reactivity to rewards and losses in offspring. Clin. Psychol. Sci. 3, 503–515. doi: 10.1177/2167702614542464
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (1998). Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol. Psychiatry 44, 1248–1263. doi: 10.1016/s0006-3223(98)00275-3
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (2005). International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report A-6). Gainesville, FL: University of Florida.
Leckman, J. F., Sholomskas, D., Thompson, W. D., Belanger, A., and Weissman, M. M. (1982). Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch. Gen. Psychiatry 39, 879–883.
LeDoux, J. E. (2015). Anxious: Using the brain to Understand and Treat Fear and Anxiety. New York, NY: Penguin Books.
Liotti, M., and Tucker, D. M. (1995). “Emotion in asymmetric corticolimbic networks,” in Brain Asymmetry, eds R. J. Davidson and K. Hugdahl, (Cambridge, MA: MIT Press), 389–423.
Liu, J., Svob, C., Wickramaratne, P., Hao, X., Talati, A., Kayser, J., et al. (2017). Neuroanatomical correlates of familial risk-for-depression and religiosity/spirituality. Spiritual. Clin. Pract. 4, 32–42. doi: 10.1037/scp0000123
Lomas, T., Ivtzan, I., and Fu, C. H. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci. Biobehav. Rev. 57, 401–410. doi: 10.1016/j.neubiorev.2015.09.018
Lutz, A., Jha, A. P., Dunne, J. D., and Saron, C. D. (2015). Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 70, 632–658. doi: 10.1037/a0039585
Maris, E. (2004). Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology 41, 142–151. doi: 10.1111/j.1469-8986.2003.00139.x
McClintock, C. H., Lau, E., and Miller, L. (2016). Phenotypic dimensions of spirituality: implications for mental health in China, India, and the United States. Front. Psychol. 7:1600.
Miller, L., Balodis, I. M., McClintock, C. H., Xu, J., Lacadie, C. M., Sinha, R., et al. (2018). Neural correlates of personalized spiritual experiences. Cereb. Cortex 29, 2331–2338. doi: 10.1093/cercor/bhy102
Miller, L., Bansal, R., Wickramaratne, P., Hao, X., Tenke, C. E., Weissman, M. M., et al. (2014). Neuroanatomical correlates of religiosity and spirituality: a study in adults at high and low familial risk for depression. JAMA Psychiatry 71, 128–135. doi: 10.1001/jamapsychiatry.2013.3067
Miller, L., Warner, V., Wickramaratne, P., and Weissman, M. (1997). Religiosity and depression: ten-year follow-up of depressed mothers and offspring. J. Am. Acad. Child Adolesc. Psychiatry 36, 1416–1425. doi: 10.1097/00004583-199710000-00024
Miller, L., Wickramaratne, P., Gameroff, M. J., Sage, M., Tenke, C. E., and Weissman, M. M. (2012). Religiosity and major depression in adults at high risk: a ten-year prospective study. Am. J. Psychiatry 169, 89–94. doi: 10.1176/appi.ajp.2011.10121823
Moratti, S., Rubio, G., Campo, P., Keil, A., and Ortiz, T. (2008). Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch. Gen. Psychiatry 65, 532–541. doi: 10.1001/archpsyc.65.5.532
Moratti, S., Strange, B., and Rubio, G. (2015). Emotional arousal modulation of right temporoparietal cortex in depression depends on parental depression status in women: first evidence. J. Affect. Disord. 178, 79–87. doi: 10.1016/j.jad.2015.02.031
Moser, J. S., Hajcak, G., Bukay, E., and Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology 43, 292–296. doi: 10.1111/j.1469-8986.2006.00402.x
Nelson, B. D., Perlman, G., Hajcak, G., Klein, D. N., and Kotov, R. (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: an event-related potential investigation. Psychol. Med. 45, 2545–2556. doi: 10.1017/S0033291715000471
Nelson, B. D., Perlman, G., Klein, D. N., Kotov, R., and Hajcak, G. (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am. J. Psychiatry 173, 1223–1230. doi: 10.1176/appi.ajp.2016.15121524
NeuroScan Inc. (2003). STIM2 User Guide (Document Number 9027, Revision A). Compumedics Neuroscan. El Paso, TX: NeuroScan Inc.
Nunez, P. L., and Srinivasan, R. (2006). Electric Fields of the Brain: The Neurophysics of EEG. New York, NY: Oxford University Press.
Ochsner, K. N., and Gross, J. J. (2005). The cognitive control of emotion. Trends Cogn. Sci. 9, 242–249. doi: 10.1016/j.tics.2005.03.010
Ochsner, K. N., Ray, R. R., Hughes, B., McRae, K., Cooper, J. C., Weber, J., et al. (2009). Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci. 20, 1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Olofsson, J. K., Nordin, S., Sequeira, H., and Polich, J. (2008). Affective picture processing: an integrative review of ERP findings. Biol. Psychol. 77, 247–265. doi: 10.1016/j.biopsycho.2007.11.006
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharm. 24, 5–12.
Perrin, F., Pernier, J., Bertrand, O., and Echallier, J. F. (1989). Spherical splines for scalp potential and current density mapping. Electroenceph. Clin. Neurophysiol. 72, 184–187. doi: 10.1016/0013-4694(89)90180-6
Perrin, F., Pernier, J., Bertrand, O., and Echallier, J. F. (1990). Corrigenda EEG 02274. EEG Clin. Neurophysiol. 76, 565–566. doi: 10.1016/0013-4694(90)90009-9
Perrin, S., and Last, C. G. (1992). Do childhood anxiety measures measure anxiety? J. Abnorm. Child Psychol. 20, 567–578. doi: 10.1007/bf00911241
Pessoa, L. (2008). On the relationship between emotion and cognition. Nat. Rev. Neurosci. 9, 148–158. doi: 10.1038/nrn2317
Peterson, B. S., Warner, V., Bansal, R., Zhu, H., Hao, X., Liu, J., et al. (2009). Cortical thinning in persons at increased familial risk for major depression. Proc. Natl. Acad. Sci. U.S.A. 106, 6273–6278. doi: 10.1073/pnas.0805311106
Phan, K. L., Wager, T., Taylor, S. F., and Liberzon, I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348. doi: 10.1006/nimg.2002.1087
Phillips, M. L., Drevets, W. C., Rauch, S. L., and Lane, R. (2003a). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 54, 504–514. doi: 10.1016/s0006-3223(03)00168-9
Phillips, M. L., Drevets, W. C., Rauch, S. L., and Lane, R. (2003b). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 54, 515–528. doi: 10.1016/s0006-3223(03)00171-9
Posner, J., Cha, J., Wang, Z., Talati, A., Warner, V., Gerber, A., et al. (2016). Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology 41, 1759–1767. doi: 10.1038/npp.2015.342
Pourtois, G., Schettino, A., and Vuilleumier, P. (2013). Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol. Psychol. 92, 492–512. doi: 10.1016/j.biopsycho.2012.02.007
Poznanski, E., Mokros, H. B., Grossman, J., and Freeman, L. N. (1985). Diagnostic criteria in childhood depression. Am. J. Psychiatry 142, 1168–1173. doi: 10.1176/ajp.142.10.1168
Price, J. L., and Drevets, W. C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 16, 61–71. doi: 10.1016/j.tics.2011.12.011
Reva, N. V., Pavlov, S. V., Loktev, K. V., Korenyok, V. V., and Aftanas, L. I. (2014). Influence of long-term Sahaja Yoga meditation practice on emotional processing in the brain: an ERP study. Neuroscience 281, 195–201. doi: 10.1016/j.neuroscience.2014.09.053
Rive, M. M., van Rooijen, G., Veltman, D. J., Phillips, M. L., Schene, A. H., and Ruhe, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 37, 2529–2553. doi: 10.1016/j.neubiorev.2013.07.018
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., and Lang, P. J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37, 257–261. doi: 10.1017/s0048577200001530
Sobolewski, A., Holt, E., Kublik, E., and Wrobel, A. (2011). Impact of meditation on emotional processing – a visual ERP study. Neurosci. Res. 71, 44–48. doi: 10.1016/j.neures.2011.06.002
Springer, S. P., and Deutsch, G. (1989). Left Brain, Right Brain, 3rd Edn. San Francisco, CA: Freeman & Company.
Svob, C., Wang, Z., Weissman, M. M., Wickramaratne, P., and Posner, J. (2016). Religious and spiritual importance moderate relation between default mode network connectivity and familial risk for depression. Neurosci. Lett. 634, 94–97. doi: 10.1016/j.neulet.2016.10.009
Svob, C., Wickramaratne, P. J., Reich, L., Zhao, R., Talati, A., Gameroff, M. J., et al. (2018). Association of parent and offspring religiosity with offspring suicide ideation and attempts. JAMA Psychiatry 75, 1062–1070. doi: 10.1001/jamapsychiatry.2018.2060
Svob, C., Wong, L. Y. X., Gameroff, M. J., Wickramaratne, P. J., Weissman, M. M., and Kayser, J. (2019). Understanding self-reported importance of religion/spirituality in a North American sample of individuals at risk for familial depression: a principal component analysis. PLoS One 14:e0224141. doi: 10.1371/journal.pone.0224141
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D., and Leahy, R. M. (2011). Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 879716, 1–13. doi: 10.1155/2011/879716
Talati, A., Weissman, M. M., and Hamilton, S. P. (2013). Using the high-risk family design to identify biomarkers for major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20120129. doi: 10.1098/rstb.2012.0129
Tamietto, M., and de Gelder, B. (2010). Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11, 697–709. doi: 10.1038/nrn2889
Tang, Y. Y., Hölzel, B. K., and Posner, M. I. (2015). The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 16, 213–225. doi: 10.1038/nrn3916
Tenke, C. E., and Kayser, J. (2012). Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol. 123, 2328–2345. doi: 10.1016/j.clinph.2012.06.005
Tenke, C. E., Kayser, J., Alvarenga, J. E., Abraham, K. S., Warner, V., Talati, A., et al. (2018). Temporal stability of posterior EEG alpha over twelve years. Clin. Neurophysiol. 129, 1410–1417. doi: 10.1016/j.clinph.2018.03.037
Tenke, C. E., Kayser, J., Manna, C. G., Fekri, S., Kroppmann, C. J., Schaller, J. D., et al. (2011). Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol. Psychiatry 70, 388–394. doi: 10.1016/j.biopsych.2011.02.016
Tenke, C. E., Kayser, J., Miller, L., Warner, V., Wickramaratne, P., Weissman, M. M., et al. (2013). Neuronal generators of posterior EEG alpha reflect individual differences in prioritizing personal spirituality. Biol. Psychol. 94, 426–432. doi: 10.1016/j.biopsycho.2013.08.001
Tenke, C. E., Kayser, J., Svob, C., Miller, L., Alvarenga, J. E., Abraham, K., et al. (2017). Association of posterior EEG alpha with prioritization of religion or spirituality: a replication and extension at 20-year follow-up. Biol. Psychol. 124, 79–86. doi: 10.1016/j.biopsycho.2017.01.005
Thiruchselvam, R., Gopi, Y., Kilekwang, L., Harper, J., and Gross, J. J. (2017). In God we trust? Neural measures reveal lower social conformity among non-religious individuals. Soc. Cogn. Affect. Neurosci. 12, 956–964. doi: 10.1093/scan/nsx023
Tucker, D. M. (2015). Clarifying the mechanisms of antidepressants. Int. J. Neuropsychopharmcol. 19, 1–2.
van Elk, M., and Aleman, A. (2017). Brain mechanisms in religion and spirituality: an integrative predictive processing framework. Neurosci. Biobehav. Rev. 73, 359–378. doi: 10.1016/j.neubiorev.2016.12.031
Vuilleumier, P., and Driver, J. (2007). Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 837–855. doi: 10.1098/rstb.2007.2092
Watson, D., Clark, L. A., and Carey, G. (1988). Positive and negative affectivity and their relation to anxiety and depressive disorders. J. Abnorm. Psychol. 97, 346–353. doi: 10.1037/0021-843x.97.3.346
Weinberg, A., Liu, H., Hajcak, G., and Shankman, S. A. (2015). Blunted neural response to rewards as a vulnerability factor for depression: results from a family study. J. Abnorm. Psychol. 124, 878–889. doi: 10.1037/abn0000081
Weissman, M. M., Fendrich, M., Warner, V., and Wickramaratne, P. (1992). Incidence of psychiatric disorder in offspring at high and low risk for depression. J. Am. Acad. Child Adolesc. Psychiatry 31, 640–648. doi: 10.1097/00004583-199207000-00010
Weissman, M. M., Kidd, K. K., and Prusoff, B. A. (1982). Variability in rates of affective disorders in relatives of depressed and normal probands. Arch. Gen. Psychiatry 39, 1397–1403.
Weissman, M. M., and Klerman, G. L. (1977). Sex differences and the epidemiology of depression. Arch. Gen. Psychiatry 34, 98–111.
Weissman, M. M., Leaf, P. J., Holzer, C. E. III, Myers, J. K., and Tischler, G. L. (1984). The epidemiology of depression. An update on sex differences in rates. J. Affect. Disord. 7, 179–188.
Weissman, M. M., Warner, V., Wickramaratne, P., Moreau, D., and Olfson, M. (1997). Offspring of depressed parents. 10 Years later. Arch. Gen. Psychiatry 54, 932–940.
Weissman, M. M., Wickramaratne, P., Gameroff, M. J., Warner, V., Pilowsky, D., Kohad, R. G., et al. (2016). Offspring of depressed parents: 30 years later. Am. J. Psychiatry 173, 1024–1032. doi: 10.1176/appi.ajp.2016.15101327
Weissman, M. M., Wickramaratne, P., Nomura, Y., Warner, V., Pilowsky, D., and Verdeli, H. (2006). Offspring of depressed parents: 20 years later. Am. J. Psychiatry 163, 1001–1008. doi: 10.1176/ajp.2006.163.6.1001
Weissman, M. M., Wickramaratne, P., Nomura, Y., Warner, V., Verdeli, H., Pilowsky, D. J., et al. (2005). Families at high and low risk for depression: a 3-generation study. Arch. Gen. Psychiatry 62, 29–36.
Wiens, S., Sand, A., and Olofsson, J. K. (2011). Nonemotional features suppress early and enhance late emotional electrocortical responses to negative pictures. Biol. Psychol. 86, 83–89. doi: 10.1016/j.biopsycho.2010.11.001
Keywords: depression risk, emotional lateralization, event-related potential (ERP), religion/spirituality, source localization, surface Laplacian, visual half-field paradigm
Citation: Kayser J, Tenke CE, Svob C, Gameroff MJ, Miller L, Skipper J, Warner V, Wickramaratne P and Weissman MM (2019) Family Risk for Depression and Prioritization of Religion or Spirituality: Early Neurophysiological Modulations of Motivated Attention. Front. Hum. Neurosci. 13:436. doi: 10.3389/fnhum.2019.00436
Received: 28 March 2019; Accepted: 28 November 2019;
Published: 17 December 2019.
Edited by:
Chella Kamarajan, SUNY Downstate Medical Center, United StatesReviewed by:
Andrey P. Anokhin, Washington University School of Medicine in St. Louis, United StatesZhen Yuan, University of Macau, China
Copyright © 2019 Kayser, Tenke, Svob, Gameroff, Miller, Skipper, Warner, Wickramaratne and Weissman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jürgen Kayser, anVyZ2VuLmtheXNlckBueXNwaS5jb2x1bWJpYS5lZHU=
†Deceased
 Jürgen Kayser
Jürgen Kayser Craig E. Tenke1,2,3†
Craig E. Tenke1,2,3† Lisa Miller
Lisa Miller