- 1Department of Geriatrics, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Marchiafava–Bignami disease (MBD) is a rare condition characterized by demyelination, necrosis and atrophy of the corpus callosum (CC), and mainly associated with alcoholism. MBD may present with various clinical manifestations. Brain magnetic resonance imaging (MRI) scan is important in prompt diagnosis and treatment of MBD. Here we reported a case of MBD and reviewed literature about the usage of gadolinium-enhanced MRI in MBD. Gadolinium enhancement may indicate a condition at risk of developing necrosis. We therefore recommend a contrast-enhanced MRI study in severe alcoholics with suspected diagnosis of MBD.
Background
Marchiafava–Bignami disease (MBD) is a rare condition characterized by demyelination, necrosis and atrophy of the corpus callosum (CC), and mainly associated with alcoholism, although some non-alcoholics have also presented with phenotypic and radiological findings that are typical of MBD (Hillbom et al., 2014). MBD may present with various clinical manifestations, including altered mental state, impaired walking, dysarthria, mutism, signs of disconnection syndrome, incontinence, seizures, and dementia (Hillbom et al., 2014; Fernandes et al., 2017). In the past, cases of MBD were diagnosed only at autopsy (Kawamura et al., 1985). The development of modern brain imaging techniques has allowed early detection of lesions suggesting MBD, and result in prompt diagnosis, treatment and better prognosis (Hillbom et al., 2014). Here we reported a case of MBD with gadolinium accumulation in the lesion, and reviewed literature about the usage of gadolinium-enhanced Magnetic resonance imaging (MRI) in MBD.
Case Presentation
The patient was a 43-year-old man admitted to our hospital with 5 days history of slurred speech, unsteady gait, altered mental state, seizures and incontinence. The patient had been consuming an average of 250 mL of spirit (Chinese liquor, ≥ 52% v/v) per day for the last 25 years. Upon admission, the patient was in coma with a Glasgow Coma Scale (GCS) of 9. Physical examination showed normal pupillary size and reaction. Muscle tone and tendon reflexes were normal. Plantar cutaneous reflexes exhibited bilateral flexion.
The baselines CBC were within normal limits except for mild anemia (119 g/L). Electrolytes (sodium, potassium, magnesium, and phosphate), calcium, chloridion, albumin levels, creatinine, urea, blood lipids, blood glucose, C reactive protein and thyroid were normal. ELISA for HIV and syphilis were negative. Testing for antibodies and antigens of hepatitis B and C were all negative, except for positive HBsAb. Baseline vitamin levels were not obtained. Cerebrospinal fluid showed a slightly increased protein level of 0.64 g/L, with normal nucleated cell count, glucose, chloridion and negative viral IgM. Gram’s stain, acid-fast stain and India ink stain for cerebrospinal fluid were all negative.
Magnetic resonance imaging (MRI) was performed 7 days after onset on a 1.5 T magnet (Toshiba, 1.5 T, EXCELART vantage MRT-1503 Atla-Basic) with the following parameters: proton density-weighted imaging (PDWI): TR/TE of 1400 ms/15 ms; T2WI: TR/TE of 4300 ms/105 ms, slice thickness 5 mm, interslice gap of 1.5 mm; DWI: TR/TE of 5300 ms/100 ms, field of view was 240 mm, two b values were acquired (0 and 1000 s/mm2), slice thickness was 5 mm, and interslice gap was 1.5 mm; fast fluid attenuated inversion recovery (FLAIR) imaging: TR/TE was 8000 ms/105 ms, field of view was 240 mm, TI was 2200 ms. Postcontrast PD-weighted (TR, 1600 ms; TE, 15 ms) images were acquired after intravenous administration of 0.2 mL/kg body weight of gadopentetate dimeglumine at a rate of 2 mL/s. The MRI revealed symmetrical and bilateral hyperintense lesions throughout the entire CC, and in scattered parts of bilateral hemispheric white matter and cortex, visualized on diffusion-weighted imaging (DWI) (Figure 1A), T2-weighted (Figure 1B), and fluid attenuated inversion recovery sequence (FLAIR) (Figure 2A) imaging. Lesions that were enhanced by gadolinium could be seen in the splenium and some extracallosal regions (Figures 1C, 2B).
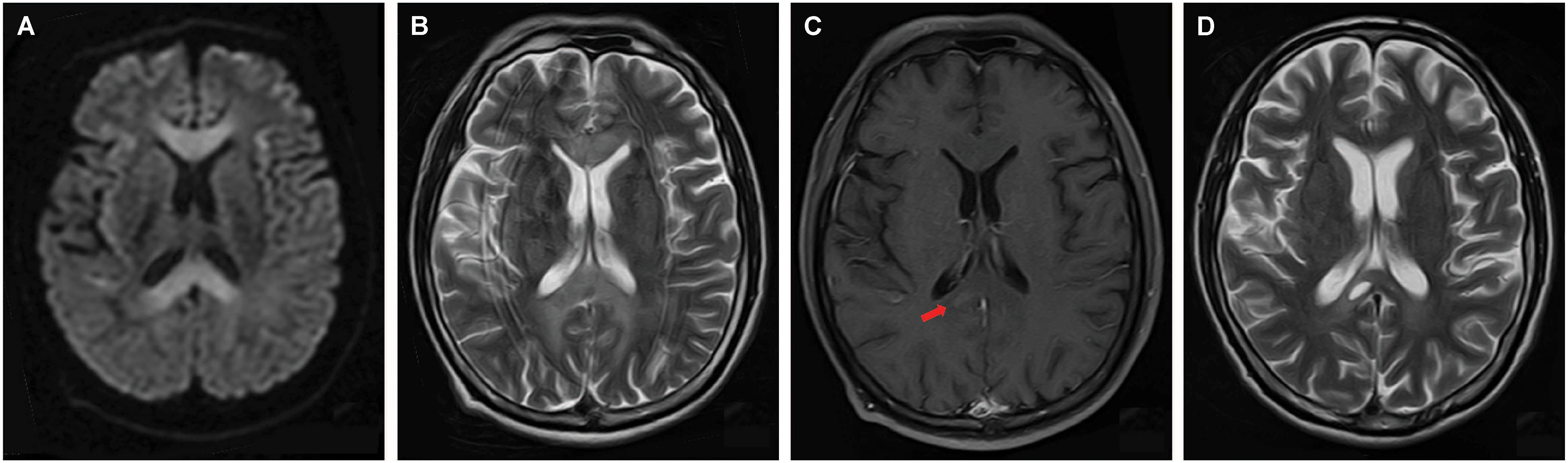
Figure 1. Axial images of brain MRI performed 7 days (A–C) and 22 days (D) after onset. MRI performed 7 days after onset revealed symmetrical and bilateral hyperintense lesions on diffusion-weighted imaging (DWI) (A), and T2-weighted imaging (B) throughout the entire corpus callosum (CC). Extracallosal lesions could also be seen (A,B). T2-weighted imaging had a considerable amount of distortion due to motion because the patient couldn’t cooperate well (B). Gadolinium enhancement could be seen in part of splenium (C, arrow). 22 days after onset, follow-up MRI showed only mild remaining of the formerly impressively hyperintensity on D2-weighted imaging, and the formation of necrosis in the splenium (D).
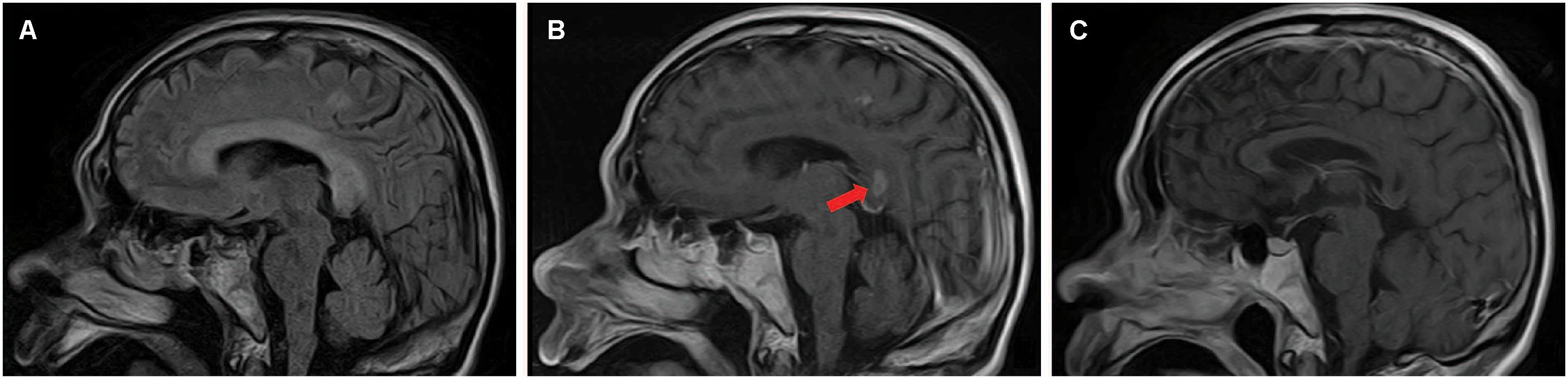
Figure 2. Sagittal images of brain MRI performed 7 days (A,B) and 22 days (C) after onset. MRI performed 7 days after onset revealed hyperintense lesions on T2-weighted imaging throughout the entire CC and in some extracallosal regions (A). Lesions that were enhanced by gadolinium could be seen in the splenium (arrow) and some extracallosal regions (B). Follow-up head MRI performed 22 days after onset showed necrosis without enhancement had occurred in the formerly gadolinium-enhanced lesions (C).
A diagnosis of MBD was made. The patient was treated with thiamine (100 mg/d) and mecobalamin (500 μg/d) intramuscular. Three weeks after symptoms onset there was significant improvement. The patient’s consciousness was improved with a GCS of 13. He was able to move all limbs, have simple conversation and control urination and defecation.
Follow-up head MRI was performed 22 days after onset on a 1.5 T magnet (Toshiba, 1.5 T, EXCELART vantage MRT-1503 Atla-Basic) with the following parameters: PD-weighted imaging (PDWI): TR/TE of 1550 ms/15 ms; T2WI: TR/TE of 4300 ms/105 ms, slice thickness 5 mm, interslice gap of 1.5 mm; fast fluid attenuated inversion recovery (FLAIR) imaging: TR/TE was 8000 ms/105 ms, field of view was 240 mm, TI was 2200 ms. Postcontrast PD-weighted (TR, 1476 ms; TE, 15 ms) images were acquired after intravenous administration of 0.2 mL/kg body weight of gadobenate dimeglumine at a rate of 2 mL/s. Corresponded to the clinical improvement, follow-up head MRI performed 22 days after onset showed only mild remaining of the formerly impressively hyperintensity on T2-weighted imaging (Figure 1D). Intriguingly, necrosis without enhancement had occurred in the formerly gadolinium-enhanced lesion (Figures 1D, 2C).
Discussion
Marchiafava–Bignami disease is a rare condition. Wilson et al. (2017) reviewed 174 cases of callosal lesions with restricted diffusion. Among them, 47% were vascular and 53% were nonvascular. The most common nonvascular etiologies were trauma (44%), tumor (22%), and demyelination (15%). None of them was identified as MBD. In MBD, hyperintensity on DWI may be caused by reversible myelin vacuolation or intramyelinic edema (Tung et al., 2010). Intriguingly, in our case, only part of hyperintensity lesion visualized on DWI appeared to have gadolinium enhancement. This region also corresponded to necrosis on follow-up MRI. Nevertheless, the significance of gadolinium enhancement has not been systemically evaluated in MBD.
To our knowledge, only 10 cases of MBD had been reported with both initial gadolinium-enhanced MRI performed in acute stage and repeated MRI (either non-contrast or contrast-enhanced) performed in subacute or chronic stage since 1985 (Table 1). 2/10 showed enhancement of CC lesions on the initial MRI. Both two cases showed necrosis of formerly gadolinium-enhanced lesions on repeated MRI. Interestingly, we found another case, in which contrast-enhanced CT was performed in the initial evaluation instead of MRI. Contrast enhancement was seen in the splenium. 21 days after the onset, MRI scan also showed the necrosis of the initial enhanced lesion (Caparros-Lefebvre et al., 1994).
8/10 cases showed no enhancement of CC lesion on initial MRI. Among these 8 cases, no necrosis was reported in 7 cases on follow-up MRI preformed in subacute or chronic stage (Table 1). The only exception was one case reported by Consoli et al. (2014).
Outcome of these 10 cases was assessed by Glasgow Outcome Score (GOS). 5 of 8 cases without gadolinium enhancement on initial MRI recovered completely after prompt treatment. No death was reported. For two patients with gadolinium enhancement on MRI, one was dead and the other one had moderate disability.
Gadolinium enhancement is related to the break-down of the blood-brain-barrier (Caparros-Lefebvre et al., 1994). By reviewing literature, it is likely that gadolinium enhancement during acute stage of MBD indicates severe damage of lesion and may lead to necrosis in the chronic stage and is associated with poorer clinical outcome. Due to the limitation of cases reported, more clinical data is needed to draw a solid conclusion. We therefore recommend a contrast-enhanced MRI scan in severe alcoholics with suspected diagnosis of MBD.
Conclusion
We reported a MBD case and evaluated the usage of gadolinium-enhanced MRI in MBD. Gadolinium enhancement may indicate a condition at risk of developing necrosis. We therefore recommend a contrast-enhanced MRI study in severe alcoholics with suspected diagnosis of MBD.
Ethics Statement
All clinical data in this case report were either provided by the patient and his spouse or collected by our team’s members with the consent of them. There was no additional invasive test or experimental drugs used out of order for the patient. A written informed consent was obtained from the patient and his spouse for the participation in the study and the publication of this report. The case report is exempt from institutional review board approval.
Author Contributions
ZW, JW, FY, and YZ acquired the clinical data, reviewed the literature, and drafted the manuscript. LZ and YZ designed the study, oversaw data acquisition, supervised the initial drafting, critically revised the manuscript. ZW and YZ reviewed the literature and revised the manuscript.
Funding
This work was supported by Grants from The Key Applied Basic Research Programs of Hunan Province (2016JC2060).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alghamdi, S., Seddeq, Y., Algahtani, H., and Shirah, B. (2018). Fatal serotonin syndrome in a patient with Marchiafava-Bignami disease: combined neurological and psychiatric emergency. Neurol. Neurochir. Pol. 52, 277–280. doi: 10.1016/j.pjnns.2017.10.011
Bano, S., Mehra, S., Yadav, S. N., and Chaudhary, V. (2009). Marchiafava-Bignami disease: role of neuroimaging in the diagnosis and management of acute disease. Neurol. India 57, 649–652. doi: 10.4103/0028-3886.57811
Caparros-Lefebvre, D., Pruvo, J. P., Josien, E., Pertuzon, B., Clarisse, J., and Petit, H. (1994). Marchiafava-Bignami disease: use of contrast media in CT and MRI. Neuroradiology 36, 509–511. doi: 10.1007/BF00593509
Caulo, M., Briganti, C., Notturno, F., Committeri, G., Mattei, P. A., Tartaro, A., et al. (2009). Non-alcoholic partially reversible marchiafava-bignami disease: review and relation with reversible splenial lesions. A case report and literature review. Neuroradiol. J. 22, 35–40. doi: 10.1177/197140090902200106
Consoli, A., Pirritano, D., Bosco, D., Postorino, P., and Consoli, D. (2014). Corticosteroid treatment in a patient with Marchiafava-Bignami disease. Neurol. Sci. 35, 1143–1145. doi: 10.1007/s10072-014-1705-9
Fernandes, L. M. P., Bezerra, F. R., Monteiro, M. C., Silva, M. L., de Oliveira, F. R., Lima, R. R., et al. (2017). Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur. J. Clin. Nutr. 71, 580–586. doi: 10.1038/ejcn.2016.267
Hillbom, M., Saloheimo, P., Fujioka, S., Wszolek, Z. K., Juvela, S., and Leone, M. A. (2014). Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J. Neurol. Neurosurg. Psychiatry 85, 168–173. doi: 10.1136/jnnp-2013-305979
Hlaihel, C., Gonnaud, P. M., Champin, S., Rousset, H., Tran-Minh, V. A., and Cotton, F. (2005). Diffusion-weighted magnetic resonance imaging in Marchiafava–Bignami disease: follow-up studies. Neuroradiology 47, 520–524. doi: 10.1007/s00234-005-1368-6
Kawamura, M., Shiota, J., Yagishita, T., and Hirayama, K. (1985). Marchiafava-Bignami disease: computed tomographic scan and magnetic resonance imaging. Ann. Neurol. 18, 103–104. doi: 10.1002/ana.410180123
Kilinc, O., Ozbek, D., Ozkan, E., and Midi, I. (2015). Neurological and psychiatric findings of marchiafava-bignami disease in a nonalcoholic diabetic patient with high blood glucose levels. J. Neuropsychiatry Clin. Neurosci. 27, e149–e150. doi: 10.1176/appi.neuropsych.14030056
Sehgal, V., Kesav, P., Modi, M., and Ahuja, C. K. (2013). Acute Marchiafava-Bignami disease presenting as reversible dementia in a chronic alcoholic. BMJ Case Rep. 2013:bcr2012008286. doi: 10.1136/bcr-2012-008286
Tung, C. S., Wu, S. L., Tsou, J. C., Hsu, S. P., Kuo, H. C., and Tsui, H. W. (2010). Marchiafava-Bignami disease with widespread lesions and complete recovery. AJNR Am. J. Neuroradiol. 31, 1506–1507. doi: 10.3174/ajnr.A1897
Wenz, H., Eisele, P., Artemis, D., Förster, A., and Brockmann, M. A. (2014). Acute marchiafava-bignami disease with extensive diffusion restriction and early recovery: case report and review of the literature. J. Neuroimaging 24, 421–424. doi: 10.1111/j.1552-6569.2012.00755.x
Keywords: Marchiafava–Bignami disease, alcoholism, MRI, gadolinium enhancement, necrosis, prognosis
Citation: Wang Z, Wang J, Yi F, Zhou L and Zhou Y (2019) Gadolinium Enhancement May Indicate a Condition at Risk of Developing Necrosis in Marchiafava–Bignami Disease: A Case Report and Literature Review. Front. Hum. Neurosci. 13:79. doi: 10.3389/fnhum.2019.00079
Received: 13 September 2018; Accepted: 14 February 2019;
Published: 27 February 2019.
Edited by:
Ferdinand Binkofski, RWTH Aachen Universität, GermanyReviewed by:
Volker Hesselmann, Asklepios Klinik Nord-Ochsenzoll, GermanyMichael D. Noseworthy, McMaster University, Canada
Copyright © 2019 Wang, Wang, Yi, Zhou and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafang Zhou, zyf_1981@csu.edu.cn
 Zhiqin Wang
Zhiqin Wang Jianfeng Wang1,2
Jianfeng Wang1,2 Lin Zhou
Lin Zhou Yafang Zhou
Yafang Zhou