
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Hum. Neurosci. , 30 October 2018
Sec. Cognitive Neuroscience
Volume 12 - 2018 | https://doi.org/10.3389/fnhum.2018.00407
 Nicolette B. Mogilever1
Nicolette B. Mogilever1 Lucrezia Zuccarelli2
Lucrezia Zuccarelli2 Ford Burles3
Ford Burles3 Giuseppe Iaria3
Giuseppe Iaria3 Giacomo Strapazzon4
Giacomo Strapazzon4 Loredana Bessone5
Loredana Bessone5 Emily B. J. Coffey6*
Emily B. J. Coffey6*Renewed interest in human space exploration has highlighted the gaps in knowledge needed for successful long-duration missions outside low-Earth orbit. Although the technical challenges of such missions are being systematically overcome, many of the unknowns in predicting mission success depend on human behavior and performance, knowledge of which must be either obtained through space research or extrapolated from human experience on Earth. Particularly in human neuroscience, laboratory-based research efforts are not closely connected to real environments such as human space exploration. As caves share several of the physical and psychological challenges of spaceflight, underground expeditions have recently been developed as a spaceflight analog for astronaut training purposes, suggesting that they might also be suitable for studying aspects of behavior and cognition that cannot be fully examined under laboratory conditions. Our objective is to foster a bi-directional exchange between cognitive neuroscientists and expedition experts by (1) describing the cave environment as a worthy space analog for human research, (2) reviewing work conducted on human neuroscience and cognition within caves, (3) exploring the range of topics for which the unique environment may prove valuable as well as obstacles and limitations, (4) outlining technologies and methods appropriate for cave use, and (5) suggesting how researchers might establish contact with potential expedition collaborators. We believe that cave expeditions, as well as other sorts of expeditions, offer unique possibilities for cognitive neuroscience that will complement laboratory work and help to improve human performance and safety in operational environments, both on Earth and in space.
Human space exploration has been limited to orbital space flight since 1972 (Apollo 17), but due to renewed interest by traditional government entities and the private sector, this trend is about to change. Engineering challenges are being overcome that will allow for a return to the Moon, and extend exploration to deep-space asteroids and to Mars (Salotti and Heidmann, 2014; Thronson et al., 2016). However, the difficulties of future missions for which we are least prepared may be those in the human domain (Kanas and Manzey, 2008; De La Torre et al., 2012; Bishop, 2013; Sgobba et al., 2017a). Separation from family and friends, delays in communications with Earth, distortion of audio and visual signals, and limited privacy and personal space are important factors for crewmembers of long-term space missions (Sandal et al., 2006). Even the most highly selected and trained individual is subject to limitations of human physiology and psychology. The isolated, confined, extreme and otherwise unusual physical and social environments of long-duration missions will approach these limits, and potentially result in catastrophic failure (for an overview of incidents related to human error in manned space missions, see Sgobba et al., 2017a).
Risks to human health and performance can be mitigated through selection, training, mission and equipment design, and countermeasures (Kanas and Manzey, 2008), and can be investigated in a variety of ways (Bishop, 2013). The human nervous system itself is studied primarily under laboratory conditions, using neuroimaging methods such as structural and functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) to observe the brain and the neural correlates of behavior non-invasively, and through comparisons between healthy and impaired systems by studying patient populations. It is also common to probe circuit, cellular, and molecular-level processes using animal models. While laboratory work is essential to establish a basis for interpreting field results and is generally less costly and less constrained than is research conducted in space, it also has limitations; it rarely looks at complex environments that are representative of real operational environments, and laboratory conditions cannot adequately simulate the unique conditions of spaceflight.
To better understand physiological and cognitive adaptations of the nervous system under conditions of microgravity, a series of studies using data collected in flight or pre- and post-flight has been conducted on postural reactions, eye movements, spatial orientation illusions, and cognitive responses (reviewed in Clément and Ngo-Anh, 2013). Some of the effects of microgravity on body fluid distribution (in addition to more physiological topics of bone density and muscle loss) can be simulated using bedrest studies in which the head is inclined downwards by about six degrees (a procedure that has negative consequences for mental status, Ishizaki et al., 2002), and by observing the changes in brain anatomy from pre- to post-flight (Roberts et al., 2017).
Aside from microgravity itself, the most relevant conditions of spaceflight for many other research questions about the nervous system can be found or devised on Earth. These “space analogs” may arise incidentally from other human activities, such as during Antarctic expeditions, or may be planned to simulate complex interactions of environmental, physical, physiological, and social aspects during space missions (Pagel and Choukèr, 2016). Space analogs can therefore offer platforms partway between the laboratory environment and the operational spaceflight context for the scientific study of psychology, cognition and neuroscience (Keeton et al., 2011). Neurocognitive changes, fatigue, circadian rhythm alterations, sleep problems, changes in stress hormone levels, and immune function have all been observed in situations that mimic some aspects of prospective human space missions (Pagel and Choukèr, 2016). A particularly valuable aspect of expedition-based analogs is that participants are in real, physically demanding and potentially dangerous situations with additional effects on stress, sleep, and team interactions.
In addition to informing future space mission design, space analog environments offer possibilities for neuroscientists to investigate brain function and behavioral performance in unique situations. Extending the study of human neuroscience outside the lab could lead to insights for basic research and benefits for safety-critical occupations (i.e., medical teams, shift-workers, firefighters, or air traffic controllers). However, opportunities for mutual exchange have yet to be fully exploited, likely due to limited contact between laboratory researchers and expedition experts, and because portable equipment for measuring neurophysiological signals has only recently reached a level of maturation necessary to make high quality measurements in situ.
Our objective here is to foster an exchange between cognitive neuroscientists, and cave expedition and space analog experts, by providing an overview of how laboratory and field research in neuroscience and related areas (i.e., cognition, cognitive psychology, neuropsychology) can be bridged, using caving expeditions as an exemplar space analog and expedition environment.
We first discuss the properties of available space analogs and their evaluation and discuss the particular characteristics of caves that make them suitable for exerting the physical and psychological challenges of spaceflight, in order to assist researchers' selection of missions appropriate for their research questions. We review work that pertains to human neuroscience and cognition conducted to date in caves, and then explore how the few focus areas of that early work can be broadened to a range of current topics. We then outline tools and techniques that are suitable for use in cave environments. Finally, we suggest how researchers might establish contact with organizations and teams that conduct expeditions.
Neuroscience, cognitive science, neuropsychology, and psychology are broad overlapping fields that may each study the same or related processes with numerous tools. We will not attempt to distinguish between the purviews of these fields here; research questions from any domain that concern environment-brain-behavior relationships that affect human performance are our focus. These topics at times overlap with human physiology, human factors, sports psychology, and social psychology. Although it makes little sense to study human performance in isolation from other physiological processes and from a physical and social context, other resources exist that have dealt specifically with these topics. For references on medical and physiological matters (i.e., bone loss, radiation, extravehicular activities, balance, motion sickness and nutrition), and for information on physiological and neurophysiological studies conduced to date on the ISS (see Buckey, 2006; Clément and Ngo-Anh, 2013). For space flight human factors research methods, accident analysis and prevention, and human-automation interaction (see Sgobba et al., 2017b; Kanki, 2018b; Marquez et al., 2018; Wilson, 2018). Psychology, mental heath, team performance and group interactions in space are reviewed in (Suedfeld and Steel, 2000; Manzey, 2004; Kanas and Manzey, 2008; Kanas, 2015; Salas et al., 2015; Pagel and Choukèr, 2016; Sandal, 2018). For a discussion of current knowledge on neuroplastic changes in the human central nervous system associated with spaceflight (actual or simulated) as measured by magnetic resonance imaging-based techniques (see Van Ombergen et al., 2017). Cognitive functions, human error, and workload and fatigue are relevant to expedition cognition and are amenable to study in the cave environment as discussed here; useful references for further reading include (De La Torre et al., 2012; Gore, 2018; Kanki, 2018a).
The National Aeronautics and Space Administration (NASA), European Space Agency (ESA), Roscosmos State Corporation for Space Activities, Canadian Space Agency (CSA), and other space exploration organizations have created a variety of terrestrial and aquatic space analogs, as well as simulated missions. Each analog simulates a subset of space or extra-terrestrial conditions. Those analogs which are predominantly used to test equipment, validate procedures, and gain an understanding of system-wide technical and communication challenges emphasize the equivalence of physical factors, such as terrain, reduced gravity and communications delays; those with natural sciences foci might emphasize geological and biological properties of the analog (e.g., the yearly NASA/ESA–funded Arctic Mars Analog Svalbard Expedition in Norway is used for testing astrobiological hypotheses).
Other analogs have a human focus or mixed scientific uses including human research. For the purposes of human activities, the relevant conditions for a particular topic of interest may include additional factors that affect a crew member's ability to carry out their work efficiently and safely. An important principle for assessing the relevance of various extreme environments as viable analogs for space or providing the basis for cross-comparison is that it is the experience of the environments rather than the environments themselves that must be considered (Suedfield, 1991; Bishop, 2013). Thus, an environment may provide an excellent analog for spaceflight without physically resembling it, provided that many of the stressors exerted upon human participants are paralleled. For example, as in space, the external environment in the Antarctic winter requires specialized equipment, planning, and procedures in order to safely conduct operations outside the habitat. Morphew enumerated the stressors of (long-duration) spaceflight (see Table 1; Morphew, 2001).
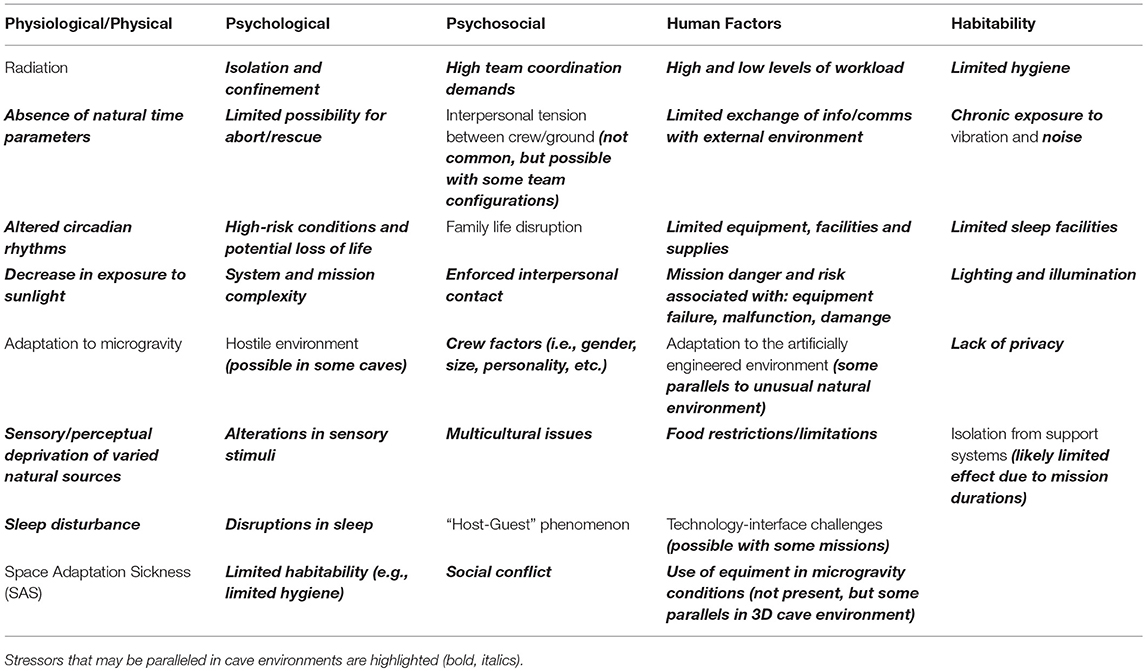
Table 1. Stressors of long duration space flights (Morphew, 2001).
In Antarctica, McMurdo Antarctic Research Station (population > 1,000) is used by NASA as a Mars analog because of terrain, temperature, and taxing conditions comparable to those of Mars' surface (Morris and Holt, 1983). Psychiatric studies at McMurdo station have provided evidence that prolonged isolation can increase the risk for mental health disorders (Kanas, 2015). ESA collaborates with the smaller Franco-Italian Antarctic base Concordia (population~15) (Tafforin, 2009), at which some human research is conducted, for example on sleep quality and adaptation to high altitude conditions (Tellez et al., 2014). Although aquatic environments are not precise models for the physical conditions of asteroid, moon, or planetary exploration, underwater missions do mimic the stressors associated with safety, communication, and technological logistics related to long-term spaceflight and exploration. The NASA Extreme Environment Mission Operations (NEEMO) is an underwater research lab where crews are sent on missions up to 2 weeks long to focus on testing equipment and procedures for future spacewalks (Todd and Reagan, 2004), and the Pavillion Lake Research Project (PLRP; CSA/NASA) uses remotely operated, autonomous, and human explorers to investigate microbiology and remnants of early life.
Simulated missions provide a similar physical environment to a spacecraft or base habitat, as well as activities and schedules resembling those of astronauts. One of the most ambitious of such projects in recent history (2007, 2011) was Mars500 (ESA/Russian Institute for Biomedical Problems). In the longer of two experiments, six volunteers were confined in a mock-up spacecraft for over a year and a half in order to simulate a complete Mars mission. Mars500 included a number of experiments on human brain function and behavior whose results have been published. Research topics included the effect of exercise on prefrontal cortex activity (Schneider et al., 2013); circadian heart rate variability during isolation (Vigo et al., 2013); and the relationships between cortisol levels on brain activity, sleep architecture, and emotional states (Gemignani et al., 2014); sleeping patterns (Basner et al., 2013); and the relationship between feelings of loneliness and cognitive functions (Van Baarsen et al., 2012). Other recent/ongoing projects are exploring perception of time, sleep quality, concentration, and their biological clocks over periods of weeks (Lunares, Poland), and crew selection, team processes, self-guided stress management and resilience training, crew communications and autonomous behavioral countermeasures for spaceflight in missions of several months (Hawaii Space Exploration Analog and Simulation; HI-SEAS; NASA/University of Hawaii).
Training courses that are designed as space analogs have also been proposed as suitable environments in which to conduct human research. ESA's Cooperative Adventure for Valuing and Exercising human behavior and performance Skills (CAVES) program, in which astronauts conduct scientific and exploration tasks in subterranean environments, is one such possibility (Strapazzon et al., 2014). NASA uses the National Outdoor Leadership School (NOLS) to tests the ability of astronauts and candidates to work together in a challenging outdoor setting (Alexander, 2016). For more information about space analogs, please refer to Keeton et al. (2011), Lia Schlacht et al. (2016), Pagel and Choukèr (2016), and Kanki (2018b).
In order to categorize the wide variety of earth-space analogs, NASA created an Analog Assessment Tool (described in NASA/TP−2011-216146, Keeton et al., 2011) that helps investigators select an analog based on study goals. Initially, the tool arranges the analogs based on importance weightings where the research characteristics (such as team size or degree of physical isolation) and utility characteristics (such as relevance of the crew's tasks or the cost of the study) are proposed. Fidelity weightings are calculated for each proposed analog based on the research and utility characteristics including the degree of their isolation, hostility, confinement, risk, prior knowledge (the accessibility of information about the environment that the mission crew has access to prior to expedition), natural lighting, logistics difficulty, remote communications, science opportunity, similarity to planet surface, and sensitivity (susceptibility to damage by humans of the environment). Both sets of weightings are combined to produce an overall ranking for all proposed analogs according to the goals of the mission (Keeton et al., 2011). ESA has also analyzed facilities that are suitable to be used as lunar analogs (Hoppenbrouwers, 2016). Table 2 presents a synthesis of the criteria commonly used to evaluate terrestrial space analogs against a research project's goals.
Approximately 20% of Earth's landmass is karstic, i.e., consisting of topography formed from the dissolution of soluble rocks such as limestone, dolomite, and gypsum, and characterized by sinks, ravines, caves, and underground streams (Ford and Williams, 2007). Only a small portion has been explored, but many sites attract people for recreational and scientific purposes. It is estimated that at least 2,000,000 people in the US alone visit caves each year (Hooker and Shalit, 2000) and members of national speleological societies (e.g., approximately 10,000 members in the US National Speleological Society and about 7,000 in the French Federation of Speleology, gleaned from their websites) suggest that the number of people likely to be involved in rigorous expeditions worldwide is in the range of tens of thousands. Caves are, in fact, interesting to a variety of scientific disciplines, including geology, hydrogeology, and biology, but they also represent unusual challenges for the people who work, explore, rescue, and temporarily live within them. The majority of deaths of cave explorers are caused by falls related to human error, followed by rock falls, drowning, and hypothermia (Stella-Watts et al., 2012; Stella et al., 2015). Science conducted on cave expeditions therefore has the potential to significantly increase research to the benefit of spacefarers, and to improve safety in a widely practiced activity.
Caves have been identified as a naturalistic space analog for training purposes (Bessone et al., 2013; Strapazzon et al., 2014; Pagel and Choukèr, 2016). As space analogs, caves feature many logistic challenges and stressors (e.g., isolation and confinement, risk and reliance on technical equipment for safety, limited prior knowledge of the environment, unusual lighting and sensory conditions, communication and supply difficulties). The spaceflight stressors highlighted in Table 1 (in bold, italics) indicate those spaceflight stressors which are frequently present in caves conditions. Although speleological expeditions may vary in their coverage according to mission, team, and environmental properties, strong overlap is observed. Critically, cave expeditions (as well as some aquatic and polar analogs) fulfill the important psychological factor of being somewhat risky and safety-critical environments in which participants are reliant on equipment and teammates, with limited and slow rescue options (Stella-Watts et al., 2012; Bessone et al., 2013). Perceived risk is likely to cause neurophysical changes that affect many aspects of brain and behavior, from interpersonal interactions to sleep and cognitive function (Pagel and Choukèr, 2016). Cave exploration also requires discipline, teamwork, technical skills and a great deal of behavioral adaptation (Bessone et al., 2013). Martian caves and lava tubes have been proposed as suitable locations in which to construct habitats on Mars, due to thermal stability and shielding from radiation and micrometeorites (Moses and Bushnell, 2016), which would further increase the similarity of the model's physical environment.
For these reasons, the European Space Agency (ESA) has carried out training activities in the subterranean environment since 2008. The multidisciplinary mission known as CAVES is used for training astronauts of the International Space Station (ISS) Partner Space Agencies (USA, Russia, Japan, Canada, and Europe) (Bessone et al., 2013; Strapazzon et al., 2014). During the 6-day mission, astronauts conduct exploration and scientific activities under similar scheduling and mission conditions as they will later experience in space as a means of eliciting and coaching behavioral competences (Bessone et al., 2008). The science program includes environmental and air circulation monitoring, mineralogy, microbiology, chemical composition of waters, and search for life forms adapted to the cavern environment, and increasingly, human experiments.
As CAVES participants are highly selected astronauts-in-training whose objectives are to explore and conduct scientific studies, it lies toward the higher-fidelity end of the spectrum of cave analog possibilities, and of possible experimental control. However, its capacity to support multiple experiments is limited by tight personnel scheduling. Expeditions of other organizations may therefore be more suitable for a given research question, taking into consideration the specific expedition's space analog suitability (for recent examples of cave-based human research and a description of the cave conditions and mission, see Stenner et al., 2007; Antoni et al., 2017; Pinna et al., 2017). Cave expeditions may vary due to differences in cave environments (temperature, presence of water, remoteness and access, difficulty level, etc.), mission (duration, objectives, group size, group composition), organization (scientific, exploration, amateur), and the demographics of participants (age, sex, training, culture, language). These factors affect the nature of the data collection that is possible as well as its quality and applicability to other groups. In the section entitled “Connection to in-field study experts and cave community” we list some of the main speleological meetings and organizations through which expeditions appropriate to a research program might be found.
Health outcomes of humans living in isolation have been studied over the last 80 years. In the 1960s, researchers began to investigate how biological rhythms were affected when living underground, without “zeitgebers” (i.e., environmental cues that can alter the internal clock, the study of which is now included in the field of chronobiology). Early studies involving isolation in subterranean conditions are listed in Table 3, along with their findings. These studies, as well as those later studies found in Table 4, were identified by a literature search of life science electronic databases (Medline: 1966-Present, NASA Technical Reports Server: 1915-Present, Google Scholar: Present, Worldcat: 1971-Present, OPAC: 1831-Present, and PubMed: 1997-Present). Search terms included “cave/s,” “cave” AND “isolation” AND “human,” “free-running isolation,” “potholing/ers,” “caving,” “social isolation,” ‘subterranean” AND “isolation,” “spelunking,” and “underground environment.” Because many early studies were only reported in their original language, we additionally searched for Italian: “grotta/e,” “isolamento in grotto,” “isolamento spazio temporale,” and “Montalbini” (author); French: “grotte,” “sejours souterrain,” “Siffre” (author); and Spanish: “cueva,” “aisolamento in cueva,” “permanecer bajo tierra,” and “spelunka.” All studies reporting results from human subjects in subterranean environments with a neuroscience or cognitive component were retained (16 reports).
Reports from the early to mid 1900s on the effects of isolation on the human body are limited in their sample size and lack standardized methodology (Halberg et al., 1970). One of the first peer-reviewed studies to examine chronobiology was performed by Mills, who analyzed chronobiological aspects of his subject throughout 105 days in subterranean isolation (Mills, 1964). From the 1960s to the mid-1970s, similar studies documented renal rhythms, sleep-wakefulness cycles, time estimation, internal temperature, heart rate, and even menstrual cycles of their subjects as biomarkers for changes of their internal clocks (see Table 3). The majority of these earlier studies using basic physiological measures found that a rest-activity cycle persisted in the absence of any environmental synchronizer or deliberate scheduling, although it appeared to be slightly desynchronized/longer than 24 h (~24.5 h). These findings were interpreted as evidence that the internal clock does not need external cues such as intense light to regulate its biological rhythm (Halberg, 1965). Social cues (e.g., subjects sleeping in the same underground conditions nearby one another, subjects eating meals together) were also shown to affect circadian rhythm, as those of subjects isolated together tended to align (Apfelbaum, 1969).
Although some of the studies in Table 3 were able to look at physiological parameters such as the effects of isolation on vision, measures were mostly implemented prior to and after isolation as opposed to within the cave environment itself. During the expedition, circadian rhythms were observed using core body temperature, sleep-wake cycles, and subjective estimation of time.
Electroencephalography (EEG), electromyography (EMG), and electrooculography (EOG) are techniques that are used to record electrical activity in the brain, skeletal muscles, and eye movements, respectively. Due to advances in electrophysiological tools in the mid 1970s, it became possible to make physiological and neurophysiological measurements during subterranean isolation studies. One of the first cave research studies using EEG and EMG was performed by Chouvet et al. (1974), who characterized sleep architecture during isolation (i.e., the pattern of rapid eye-movement or REM sleep; light sleep or stages 1 and 2; and deep or slow-wave sleep, SWS, that occurs over a nights' rest). From the mid-1970s to the 1990s, similar studies documented the effects of isolation with limited external time cues on circadian rhythms using EEG, EMG, and EOG, in addition to the previously mentioned physiological measures. These studies are listed in Table 4, along with their main findings.
The studies presented in Tables 3, 4 represent pioneering efforts investigating circadian rhythms in the absence of an externally imposed day-night cycle. Early observations that humans have endogenous rhythmicity in biological processes and alertness levels that can be modified by external cues stimulated further research on human circadian rhythms and sleep cycles which have grown into fields of scientific study with implications for health and disease (Kirsch, 2011). Many of these studies are noteworthy for their pioneering efforts, ingenuity of design, and commitment of their subjects; isolating individuals for long periods would now be considered highly unusual (if not unethical; although causality certainly cannot be inferred, one of the subjects isolated alone for 3 months later died by suicide Hillman et al., 1994b). However, today the data generated by these studies are primarily of interest for historical reasons; the very small sample sizes and lack of experimental control and methodological standardization between studies limit the interpretability and generalizability of the findings, and the tools and practices of measurement of human psychology and physiology have evolved considerably in the interim. Later work showed that some of the findings reported above were likely caused by the experimental procedures. Most notably, many studies in Tables 3, 4 suggested that the endogenous human circadian cycle is closer to 25 than 24 h. This was later attributed to phase shift due to exposure to bright artificial light that subjects were allowed to use while awake; in the absence of bright light, the intrinsic pacemaker is in fact very close to 24 h (Czeisler et al., 1999).
Studies in caves to date have only concerned themselves with a few of the topics for which the cave environment makes a good space analog (i.e., isolation, lighting). Figure 1 presents some of the (interrelated) topics within neuroscience, cognition, and psychology that could be usefully studied in cave expeditions, and might benefit from an intermediate research platform between the laboratory environment and space itself.
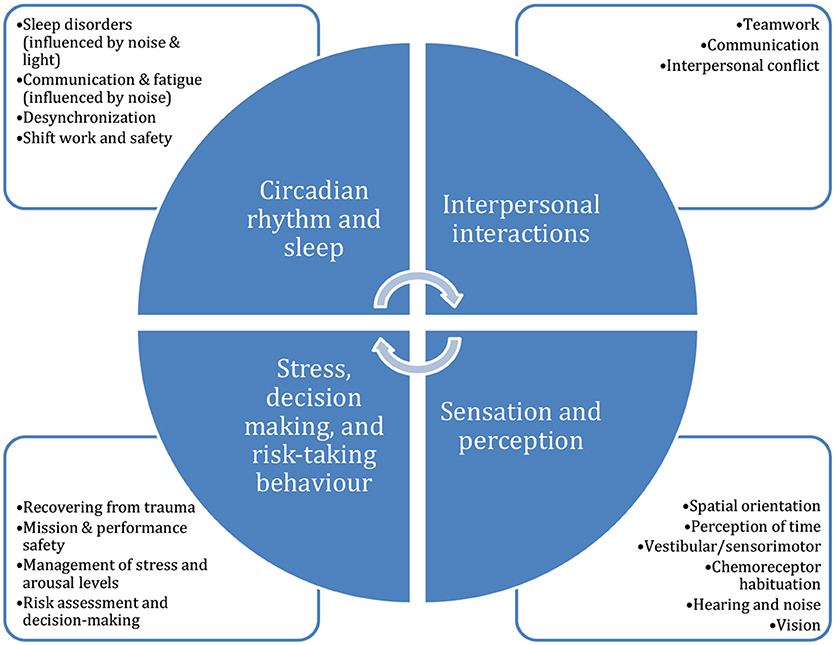
Figure 1. Potential topics in psychology, cognition, and neuroscience that could benefit from study in subterranean and expedition environments. Caves could also be a useful context within which to evaluate and optimize the effects of equipment interfaces and operational protocols on human cognition and performance, as well as within which to test the effectiveness of countermeasures.
Sleep quantity and quality, circadian rhythm, and resulting alertness levels and performance proficiency are often altered in spaceflight for environmental and operational reasons (Mallis and DeRoshia, 2005). Due to logistic challenges with sleep measurements in the spaceflight environment, only a few astronauts have been studied using polysomnography (PSG), the gold-standard method for evaluating sleep. According to astronauts' subjective reports and objective recordings of neurophysiology (i.e., EEG, PSG) and of activity levels (i.e., actigraphy; wrist-worn accelerometers) human sleep has been reported to be shorter and shallower during various missions including Skylab missions (Frost et al., 1975, 1976), space shuttle missions (Monk et al., 1998; Dijk et al., 2001), Mir missions (Gundel et al., 1997), and ISS Expeditions from 2006 to 2011 (Barger et al., 2014), compared to sleep on the ground. Barger and colleagues additionally found that the use of sleep-promoting drugs, which are known to alter sleep architecture and cognitive performance, were pervasive during spaceflight; the authors argued for the need to develop effective countermeasures to restore normal sleep in space (Barger et al., 2014).
The degree to which spaceflight sleep problems are caused by altered physiology due to the effects of microgravity itself or other factors such as isolation and confinement, noise, changes in physical activity, long or unusual sleep-wake and crew shift-work schedule, over-excitation, demographics, rapid succession of light and dark exposure, and ambient temperature is not yet known (Gundel et al., 1997; Pandi-Perumal and Gonfalone, 2016). However, results from space analogs also report significant changes in sleep patterns during Antarctic overwintering (Steinach et al., 2016) and during extended confined isolation (Basner et al., 2013), respectively, suggesting that sleep disturbance can be usefully studied in space analog conditions. In caves, mission-like levels of activity and scheduling, psychological pressures relating to factors such as risk and interpersonal interaction, new surroundings, temperature, humidity, and noise can all affect sleep timing, duration, and quality. Cave expedition constraints can also introduce circadian disturbances; for example, it is not uncommon for cave exploration activities to involve extended periods of wakefulness and near-continuous activity (>24 h and even up to 40 h), when sleeping within the cave is logistically difficult or impossible. These extended periods of wakefulness parallel those in spaceflight which can occur for operational requirements for example rendezvous and docking procedures, and in emergencies. Some cave expeditions may last weeks and require crew to adapt to sleeping and working conditions, providing a situation analogous to longer mission phases. Even when a normal sleep-wake cycle is maintained, important zeitgebers are absent or altered in caves, as in space exploration.
Inadequate sleep can affect daytime alertness levels, response time, vigilant attention, and error rates, learning, complex task performance, emotional evaluation, risk assessment, and fatigue; however, effects differ according to the type of task and degree of its complexity, characteristics of the individual, and the nature of the sleep deprivation (i.e., acute deprivation, or chronic restriction; Wickens et al., 2015; Bermudez et al., 2016; Havekes and Abel, 2017; Krause et al., 2017). In meta-analyses, mental fatigue was shown to also have some effect on physical and athletic performance (Van Cutsem et al., 2017; McMorris et al., 2018), which has relevance for more physically strenuous expedition activities such as climbing or extravehicular activities. Hypnotics (i.e., drugs used to treat insomnia) reduce sleep latency and increase sleep duration, but the resulting sleep shows abnormal sleep architecture (Cojocaru et al., 2017) and does not entirely restore impaired cognitive performance (Verster et al., 2016). Because sleep architecture is important to learning and memory consolidation (Diekelmann and Born, 2010; Ros et al., 2010), these effects are especially undesirable wherever learning is required, as it is during exploration and spatial navigation. People are not always good at assessing their own performance levels; devising means of assessing readiness to perform safety-critical tasks is important, as is knowing how well self-reported alertness levels accurately reflect subsequent cognitive performance (Boardman et al., 2017), and how well performance can be improved in the short term. Caffeine can mitigate some of the next-day cognitive performance effects of reduced sleep in a somewhat predictable fashion (Ramakrishnan et al., 2015). Interestingly, an individual's performance impairments due to sleep restriction, or enhancement due to stimulants like caffeine, may not translate directly into group performance impairments and improvements, due to mediating factors of group dynamics (Faber et al., 2017), which would also be useful processes to understand under expedition conditions.
In addition to inadequate sleep quality and duration, sleep timing affects performance. Recent progress on the molecular-genetic basis of circadian rhythms indicates that they affect cognition, learning and memory, mood, and metabolism directly, in addition to indirectly through their influence on sleep (Kyriacou and Hastings, 2010). The effect of sleep restriction and circadian misalignment is a topic of concern in occupations that involve shift-work like emergency medicine, in which short-term cognitive deficits have been related to shift work schedules (Machi et al., 2012).
Caves offer unusual sensory inputs that affect waking behavioral performance. In caves as in enclosed artificial environments such as spacecraft, olfactory input is monotonous sometimes negative (i.e., body odors), contributing to habitability and comfort issues. Noise is pervasive in artificial environments, and is known to cause annoyance, disturb sleep and daytime sleepiness, and to negatively affect patient outcomes and staff performance (in hospitals), increase the occurrence of hypertension and cardiovascular disease, and impair cognitive performance (Stansfeld and Matheson, 2003; Basner et al., 2014a). Operational limits on both continuous and intermittent noise exposure have been established for spaceflight, in order to provide an acceptable environment for voice communications and for restful sleep (Allen et al., 2018). However, recent evidence suggests that even low levels of noise, within the established limits, can cause neurophysiological changes that negatively affect health, learning, and memory performance (studied in rodents Cheng et al., 2011). In humans, noise increases the cognitive load associated with understanding speech and communicating, and the ability to do more than one task simultaneously (Rönnberg et al., 2010). Many cave environments have continuous background noise from wind and water movement that could be used to study its effect on individual stress levels, concentration, cognitive performance, fatigue, workload, communication, and interpersonal interaction.
Lighting in caves is produced by headlamps, which create partial, focal illumination of complex three-dimensional spaces and complicates movement and navigation. These perceptual conditions are likely to increase cognitive load and contribute to dual-task performance decrements, including communication and teamwork. The type and distribution of lighting on the exterior of spacecraft affects human visual performance and is an important factor in spacecraft design, particularly for extravehicular activities (Rajulu, 2018). Future extra-terrestrial cave/lava tube exploration may create related challenges.
Higher-level perceptual skills are also relevant for spaceflight. Visuo-spatial orientation skills refer to the ability of individuals to make use of information available in the environment to efficiently orient and navigate. This function relies on cognitive processes such as memory, attention, perception, mental imagery, and decision-making skills (Ekstrom and Isham, 2017). It allows individuals to become familiar with the environment and to integrate information about self-position and orientation into a spatial mental representation of the surroundings, known as a cognitive map (Tolman, 1948; Arnold et al., 2013). Cognitive maps allow any target location from anywhere within the environment to be reached, even by following novel routes when a known pathway is unavailable (Epstein et al., 2017). An accurate mental representation of the environment is crucial for a variety of cognitive tasks in near-space, such as those that involve reaching and grasping objects from a given location within the environment or directing attention to elements in space that are not necessarily within our focal vision. These skills are necessary for maneuvering safely in microgravity, during extra-vehicular activities, and for exploration of planet surfaces (Clément and Reschke, 2008).
The ability to form accurate mental representations of the environment implies the integrity of a complex extended network in the brain (Ekstrom and Isham, 2017; Ekstrom et al., 2017). Within this network, regions in the medial temporal lobe (i.e., hippocampus and parahippocampal cortex) are involved in the learning and memory aspects of orienting and navigating through the environment (Epstein et al., 2005; Iaria et al., 2007). Interestingly, these networks are among those implicated in sleep-related processes of memory consolidation, notably of memory involving spatial and contextual elements (Diekelmann and Born, 2010). Other brain regions used while moving throughout the environment and locating elements within it include the posterior parietal cortex, which is critical for integrating different sensory information processed through our visual, vestibular, somatosensory, and proprioceptive systems (Posner et al., 1984; Andersen, 1997); and the frontal and prefrontal cortex which are necessary for executive functions such as planning, mental imagery, and working memory (Owen et al., 1990; Petrides and Baddeley, 1996). Recent studies have shown that even a minimal functional alteration (not damage per se) of the neural networks described above is associated with impairments of spatial processing (He et al., 2007; Iaria et al., 2014; Kim et al., 2015). As with many complex skills, maintaining expertise in spatial orientation and navigation also requires consistent practice; reliance on GPS technology for example, which offloads the cognitive demands of navigation, is associated with lower navigational expertise (Ishikawa et al., 2008) and lower hippocampal volume and connectivity (Maguire et al., 2000; Iaria et al., 2014). Spatial orientation and navigation are a clear example of a cognitive process in which one must “use it or lose it” (Shors et al., 2012).
Factors affecting physiological and psychological well-being like increased social isolation, confinement, altered sleep, and higher stress levels are also known to affect cognitive skills. For example, visuo-spatial orientation and its neural correlates (Glasauer and Mittelstaedt, 1998; Stranahan et al., 2006; Lukavský, 2014; Valera et al., 2016). Poor quality sleep leads to slower performance and more errors navigating a newly-learned environment (Valera et al., 2016), and chronic stress is known to produce spatial orientation deficits (Mizoguchi et al., 2000; Kleen et al., 2006), likely by perturbing the neurochemistry of supporting networks (Conrad, 2008, 2010; Li et al., 2015). Spatial confinement may also have more direct effects on spatial orientation, for instance, Lukavský (2014) identified a marked difference in scene memory in the six participants of the Mars500 project. Relative to controls, these individuals developed a greater bias toward “boundary extension” while viewing distant scenes, i.e., falsely recalling a wider field of view or more distant perspective from these visual stimuli. Lukavský hypothesized that the lack of interaction with distal objects and scenes due to extended stays in a relatively confined environment will result in the deterioration of the perception and strategy use within larger environments.
The hippocampus and prefrontal cortex have a well-documented sensitivity to some of the negative factors associated with subterranean explorations. Rodents housed in confined, isolated, or simple environments have smaller hippocampi, comprised of fewer neurons (Kempermann et al., 1997) with fewer dendritic spines (Leggio et al., 2005), less neurogenesis (Olson et al., 2006), and poorer spatial abilities (Nilsson et al., 1999; Leggio et al., 2005). While the typical experiences of a lab rodent differ from that of an average human, these findings are generally supported by human research (Gianaros et al., 2007; Lupien et al., 2007; Ganzel et al., 2008; Prince and Abel, 2013). The prefrontal cortex is vulnerable to both acute and chronic stressors, with acute stress producing notable impairments in spatial working memory (Arnsten, 2009), as well as reducing the capacity to problem-solve and think flexibly. Paralleling the effects seen in the hippocampus, long-term exposure to stressors produces lower prefrontal cortex volumes (Cerqueira et al., 2007), reduced dendrite length and branching (Holmes and Wellman, 2009), and detriments to spatial memory (Cerqueira et al., 2007; Arnsten, 2009), vulnerabilities that appear to worsen with aging (McEwen and Morrison, 2013). Cave environments offer challenging three-dimensional environments in which to move and explore, simulating the challenging perceptual and mission conditions of spaceflight; they may also offer unique opportunities to contribute to knowledge of hippocampal function, dysfunction, and plasticity as it relates to sleep, stress, and confinement.
The perceived risk and danger aspect of expedition environments offers another set of research opportunities with spaceflight relevance. Communication with the outside world may be very limited. Though teams often set up a telephone line between an external base and a main cave base camp for extended expeditions, difficult terrain may still require hours or even days of movement before communication can be established, and rescue attempts could take much longer. In future long-duration space missions to Mars and for permanent stays on the Martian surface, transmissions between ground and space may be delayed up to 40 min or even blocked, and short-term rescue may be impossible; lack of a visual link to Earth will add to the feelings of isolation and autonomy (Horneck and Comet, 2006; Strapazzon et al., 2014). Under uncertain conditions, stress impacts decision-making and risk-taking behavior (reviewed in Morgado et al., 2015). These effects appear to be mediated by stress-related release of neurotransmitters that lead to alterations in neural firing, and if stress is chronic, to architectural changes in frontal lobe areas involved in higher-level cognition (Arnsten, 2015). The stress associated with risk and danger also affects interpersonal interactions and group dynamics, potentially leading to feedback cycles in communication that foment crew conflict (Kalish et al., 2015).
The interaction of stressors that challenge cave and space explorers with interpersonal dynamics is a critical component of mission success (Bishop et al., 1999; Sandal, 2018). Although teamwork, team cohesion, team effectiveness, and resilience have been identified as knowledge gaps and are current topics of investigation for space exploration, there have been relatively few studies in extreme environments and space-analogs (for a summary, see Salas et al., 2015; Sandal, 2018), and studies within caves are scarce (for examples, see Bishop et al., 1999; MacNeil and Brcic, 2017).
The empirical study of team characteristics and processes has non-standard, evolving theoretical constructs and methodology (Cronin et al., 2011; Alliger et al., 2015; Kozlowski, 2015). Common techniques include behavioral observation (during simulations or training; in person, or by reviewing recordings) and self-report by surveys (during pauses in activity, or retroactively) (Brannick et al., 1997). These methods may require an uninvolved observer, rely on team members' ability and desire for introspection, and may not capture how the team dynamically reacts and interacts to changing situations. Wearable physiological and neurophysiological measurement devices have been proposed as a means of unobtrusively tracking team dynamics, assessing the quality of teams' performance in real time, and adaptively rearranging team or task components (Stevens et al., 2011; Salas et al., 2015; Santoro et al., 2015; Lederman et al., 2017). These promising approaches are in early development phases, and could be tested in cave environments.
As well as observing and characterizing the behavioral and neurophysiological correlates of environmental stressors (Alonso et al., 2015), (neuro) physiological indices of attention, workload, and emotional state can be used to measure how people interact with technology, for the purposes of evaluating equipment interfaces (Liapis et al., 2015) and to validate brain-computer interface (BCI) systems. Passive BCIs use these signals to adapt the behavior and functionality of highly complex and safety critical systems accordingly to the user's actual mental state in real time, without requiring effort. They are promising means of optimizing interaction with technology for spaceflight applications as well as in various Earth-based applications (Coffey et al., 2010; Aricò et al., 2016; Arico et al., 2017). In cave exploration, interaction and supervision of swarms of robotic agents is a possible application (Fink et al., 2015; Kolling et al., 2016).
The cave environment could be used to test the feasibility and effectiveness of countermeasures. In a recent meta-analysis, mindfulness-based meditation was shown to reduce stress, depression, anxiety and distress, and improve quality of life in healthy individuals (Khoury et al., 2015). Neurofeedback, in which users are given a visual or auditory representation of certain features of their brain's ongoing activity such that they can learn to modulate it (e.g., based on the amplitude of different frequency bands measured with EEG), might be tested as a means of maintaining function during expeditions. In a review of about 30 controlled studies, EEG-neurofeedback showed evidence of performance gains on sustained attention, orienting and executive attention, memory, spatial rotation, reaction time, complex psychomotor skills, implicit procedural memory, recognition memory, perceptual binding, intelligence, mood and well-being (Gruzelier, 2014).
Slow oscillations present in deep sleep can be enhanced using a method known as auditory closed-loop stimulation. Short bursts of quiet broadband noise are played to the user, precisely timed to the ascending phase of ongoing slow oscillations (Ngo et al., 2013). The brain's reaction to the sounds strengthens the slow oscillations and improves some types of memory (i.e., hippocampus-dependent declarative memory; Arnal et al., 2017; Besedovsky et al., 2017). Another new method of enhancing learning is known as targeted memory reactivation (TMR), in which an olfactory or auditory stimulus is associated with a learning event. In the subsequent sleep period, the stimulus is repeated, presumably reactivating the memory and increasing the strength with which it is consolidated (learned) (Schouten et al., 2017). Although these methods are new and have shown improvements on only basic tasks that are far removed from those performed in the operational environment, further developments may make them usable to optimize learning in expedition environments; these could be tested in caves.
Thus, as early twentieth century researchers deduced, cave environments are useful for studying sleep and circadian processes. Though early studies only took advantage of the isolation and the absence of zeitgebers found in caves, a much larger set of questions can be asked during modern expeditions: of sleep and circadian rhythm, but also about sensation and perception, spatial navigation, interpersonal interactions and teamwork, human factors design, stress, and the impact of these stressors on wellbeing and performance. In the following section, we discuss several considerations for conducting human research in cave environments.
Because of the long planning time for many space and analog missions and because of the difference in the scale of research investment, the pace of progress is generally more rapid in mainstream neuroscience. The focus of analog research is likely to be establishing and characterizing phenomena under expedition conditions and assessing the effect of interventions, whereas a laboratory approach can investigate finer-grained mechanisms, in tightly controlled paradigms that isolate specific phenomena, possibly using highly specialized equipment. Both are valuable; to maximize the advantages of each, researchers might choose to include a lab-based control group for comparison, test subjects before an expedition in the lab to serve as a baseline, complement field studies with investigations of the same phenomenon using their full lab suite, or carefully validate field equipment and procedures against laboratory standards, according to the research question.
Scientists only familiar with the traditional academic research side may find the collection Space Safety and Human Performance Sgobba et al. (2018), as well as Clément and Ngo-Anh (2013) to be useful starting points to review studies conducted in space or space analogs to date. It can be helpful to obtain first- or second-hand knowledge of the cave expedition environment prior to planning experiments, such that environmental and mission constraints that could introduce problems, confounds, or poor quality data can be avoided. In field studies, in fact, we often have poor control over confounding environmental factors (Brugger et al., 2018), but choosing the right “cave setting” can offer a certain level of standardization.
Expedition or medical experts who might wish to add neuroscience questions to their programs may discover too late that their results are unpublishable. For example, in auditory cognitive neuroscience, it is considered essential to confirm that subjects have normal hearing thresholds such that experimental findings can be attributed to some condition of interest and not a hearing deficit. Norms and best practices such as this have evolved in each specialized sub-field in order to guard against artifacts and confounds, and ensure replicability and generalizability of findings, but may not be obvious to operations personnel. There are often means of satisfying such requirements, once they are known. In this example, the researcher could conduct a basic audiogram on-site or arrange (with the subject's permission) to obtain equivalent information via previous medical reports. A more problematic issue concerns sample size and statistical validity of the proposed research design; (i) case studies or very small sample sizes are unlikely to be well regarded by many peers in neuroscience; (ii) small sample sizes (~n < 30) can show only large differences and many aspects may remain hidden due to the low power (type II error or high false negative rate). Possible solutions include collecting more extensive data on a few more homogeneous sample subjects, using different converging methods of neuroimaging and behavioral testing, pooling data over several missions (documenting differences between the missions that might affect results), or contrasting results with a laboratory-base group.
Researchers may run into differences in expectations for scientific communication when crossing field boundaries. Most space and analog human research work is currently presented in space or applied physiology-themed journals and conferences (with some exceptions of recent publications in generalist journals, e.g., Basner et al., 2013; Antoni et al., 2017). People working on related issues from the expedition and laboratory sides are therefore unlikely to be present at the same meetings or to read the same reports. Publishing in generalist journals may help to increase communication between space and mainstream neuroscience. Both specialized scientific knowledge and expedition expertise is needed to successfully take advantage of this unique situation while ensuring the value of results to the different communities.
High quality, lightweight, relatively inexpensive devices to measure (and even influence) brain activity are becoming available, as are inexpensive laptops and tablets that can be used to measure a variety of important aspects of cognition and behavior. In psychology and neuroscience (as in other fields), there is a movement toward “open science,” in which research, data, and tools are made freely available. These developments will make much more extensive expedition cognition research possible.
The determining factors for planning subterranean studies are the specific characteristics of the cave and mission (i.e., cave conditions, duration, group demographics, planned activities, logistic restrictions), and the part of the expedition during which measurements need to take place (illustrated in Figure 2). Factors other than cave and mission itself should also be considered when selecting a research situation, such as the presence of external scientific, logistical, and medical resources, and presence of an organized rescue organization. Of the mission phases, pre-post expedition periods are the least constrained, as data could be measured external to the cave in a portable lab or nearby research facility. In-cave equipment is transported in water-resistant bags on shoulders, pushed and dragged through constricted areas, and raised or lowered on ropes. It must be small, light, and protected against high humidity, submersion in water in some caves/passages, dust, mud, and shock due to handling (illustrated in Figure 3). Power consumption may be a limiting factor of equipment, especially for longer missions; electronics should be as small and energy-efficient as possible. Pencils and moisture-resistant paper for simple measures like surveys might be more appropriate than electronic solutions when power is a constraint, although even water-resistant paper is easily soaked and soiled, and pencils, lost. Measurements taken in the cave at the end of the day at a base camp or during the rest period for sleep studies could allow for relatively larger or more delicate equipment or equipment that requires some setup to be used, like laptops and EEG.

Figure 2. Expedition phases determine the nature of possible measurements. (A) Pre- and post-mission, testing can include delicate equipment and can be conducted in comfort. (B) During exploration, minimal portable equipment can be worn continually or used during brief stops (i.e., during photography, mapping, and rest stops); simplicity of use and robustness are key. (C) Where base-camps are established, more elaborate testing with laptops and electrophysiology can be conducted, as well as for sleep recordings during rest periods (D). Permission has been obtained from the individuals for the publication of these images. Photo credits, ESA archives, used with permission from photographers; (A), Alessio Romeo; (B), left: Alessio Romeo, right: Natalino Russo; (C), Vittorio Crobu; (D) Riccardo DeLuca.

Figure 3. Experimental and equipment considerations. (A) Equipment must be small, lightweight, well-organized, and packed to protect it against damage according to the nature of the cave and expedition. (B) Equipment worn during movement must be positioned so as not to pose safety risks (e.g., no obstruction of view or dangling wires), not to be dislodged or damaged by climbing harnesses and activities, and so as to be protected from impact and water damage. (C) Consideration must be given to the conditions under which measures are administered; compliance and data quality may be affected by participant comfort. Permission has been obtained from the individuals for the publication of these images. Photo credits, ESA archives, used with permission from photographers; (A), left: Loredana Bessone, right: Vittorio Crobu; (B), Natalino Russo; (C), Natalino Russo.
Researchers should keep in mind that their experiments will be secondary to expeditioners' other goals; time-consuming or irritating procedures, unclear instructions, and measurement equipment failures requiring troubleshooting may reduce compliance and decrease data quality or increase dropout to a greater extent than in the laboratory. For extended expeditions, thought should be given as to how to maintain comfort and good signal quality despite limited opportunities for personal hygiene. The main tools currently available for human neuroscience in caves, ordered by increasing degree of complexity are discussed in the following subsections.
Surveys and questionnaires are common research tools, particularly toward the psychological end of the psychology-neuroscience spectrum where they are used to answer questions about subjective experience, obtain reports of habits or schedules, or document interpersonal interactions. When cognitive or neurophysiological measures are the main tools, surveys and questionnaires can also be useful to gather information about health and demographic variables (e.g., to rule out neurological disorders or to document the subject's age and gender), and about potential confounds that may be necessary to interpret the data correctly. Methods to develop effective surveys and questionnaires are described in Yorubaland et al. (1999) and Fink (2003). A variety of prepared and validated surveys and questionnaires are available, many of which can be obtained free of charge and have been validated. Existing measures may already have been linked to other neurophysiological data, allowing for some further insight and inference; for example, daily ratings of positive mood have been linked to serotonergic function in the central nervous system (Flory et al., 2004). However, scales are often developed for clinical or diagnostic use and might not be sensitive to minor variations between healthy, high functioning adults, or a scale may be intended to give a global general score such as propensity toward daytime sleepiness rather than measure daily fluctuations. Some tools are lengthy or require a trained interviewer to administer, which would not be appropriate in an expedition setting. A further consideration is the questionnaire load of the participants and the conditions under which the participant will complete the survey (e.g., physical comfort, lighting), which can affect data quality. Nonetheless, surveys and scales can be useful to quickly and inexpensively collect useful data, and require no equipment other than pen and (water-resistant) paper, or they can be computerized or answers can be recorded by voice according to mission constraints.
We list some common questionnaire-based tools in Table 5 which fall into several topic categories. The unusual sensory conditions and need for alertness due to risks required in expedition environments can contribute to fatigue. Alertness, sleepiness, and fatigue levels are interrelated but distinct phenomena that can be measured with questionnaires, in addition to physiological measures such as pupillometry (described below). Situational awareness is the perception and understanding of the environment and events, and is particularly relevant to operational tasks. Stress is a state of mental or emotional strain or tension resulting from adverse or demanding circumstances; space flight and cave expeditions both pose stressors on team members. Prolonged missions in isolation and under stressful conditions can affect the mood and therefore the capacity for mission members to perform to the best of their abilities (Liu et al., 2016). Positive team dynamics and social interactions are highly desirable in long-term missions, during the taxing conditions of space-flight, and the longest cave expeditions, which can be weeks long. Pre-mission questionnaires that examine social compatibility have been used to improve group interactions in isolation experiments (Dunlap, 1965; Chidester et al., 1991). These questionnaires can be useful in crew selection (see Kanas et al., 2013).
Cognitive performance of astronauts is essential for maintaining the capacity to problem solve in new situations and respond quickly in times of equipment malfunction or injury during missions. Computerized cognitive tests are based on neurophysiological measures rather than self-report, and therefore provide more objective information. In this section, we list some of the main cognitive tasks used or potentially relevant for use in space and space analogs, with particular attention to testing visuo-spatial navigation skills—a relatively new area that is relevant in caves and spaceflight and lends itself well to computerized testing. Questionnaires and ratings can also be adapted for use on a digital device such as a tablet or phone (e.g., Betella and Verschure, 2016), which may be logistically simpler than paper-based methods where they are needed in conjunction with planned computerized testing.
Reaction time and response accuracy are frequently collected in spaceflight-related cognitive testing since these measures examine crew members' abilities to react well in critical situations, and show different sensitivities to spaceflight conditions. A study on the mental performance during short term and long term space flight showed that reaction time and spatial memory were not decreased during space flight but visuo-motor tracking and dual-task capabilities were decreased (Manzey and Lorenz, 1998). Other studies looking at the single-task reaction time, visuo-motor tracking, and dual-task abilities of astronauts before and during their experience at the ISS show that all three are impaired during spaceflight (Bock et al., 2010). It has been suggested that the changes on visuo-motor tracking are a result of the microgravity effects on sensorimotor processes during spaceflight (Bock et al., 1992) and that the deficits in single- and dual-task reaction time are a result of stress and fatigue of the mission (Santy et al., 1988).
The Psychomotor Vigilance Test (PVT) measures how quickly the participant can respond to a visual stimulus. This test has been used on the ISS to measure behavioral and cognitive changes in astronauts' attention states, alertness, problem-solving skills, and impulsivity, and during the Mars500 mission (subjects were shown to have high levels of psychomotor vigilance performance throughout the mission Basner et al., 2014b).
The Stroop Test is a computerized test that can assess the attentional control of a subject by asking them to suppress irrelevant information (Stroop, 1992). A version of this test asks the subject to answer with the color of the word they are looking at and not the color that is the word actually reads. One study looked at 3 crew members during their 11 day spaceflight and found that their ability to suppress irrelevant information decreased as compared to before the mission (Pattyn et al., 2009).
Working memory is the ability to temporarily store and manipulate the information required to carry out complex cognitive tasks such as learning, reasoning, and comprehension. Both visual working memory (such as that required to store layout of a spacecraft or cave environment) and verbal working memory are important to evaluate (reviewed in Wilhelm et al., 2013). The Sternberg memory task asks the subject to memorize a list of words or numbers and recall whether a subsequently presented probe item had been present in the original set. Response time is measured. Several studies using this task have shown slower responses during spaceflight (Manzey et al., 1998; Kelly et al., 2005) but others have not found significant differences (Manzey et al., 1993; Newman and Lathan, 1999).
A new computerized cognitive testing battery by the name of “Cognition” has been developed specifically for astronauts (Basner et al., 2015) with the goal of facilitating comparison of cognitive function across analogs and spaceflight. This test covers testing on spatial orientation, emotion processing, and risk taking all encompassed in 10 neuropsychological tests. The Spaceflight Cognitive Assessment Tool for Windows (WinSCAT) is commonly administered to astronauts on the ISS and in spaceflight simulations (De la Torre et al., 2014), and comprises subtests measuring mathematical skills, short-term memory, working memory, attention, and spatial processing (Kane et al., 2005). A study that compared Cognition and WinSCAT showed that Cognition scores assess a variety of neurocognitive disciplines while the WinSCAT weights heavily on executive control (Moore et al., 2017). For further discussion of the measurement of working memory, attentional control, and other cognitive testing of subjects in spaceflight environments (see Strangman et al., 2014; Kanki, 2018a,b).
Visuo-spatial orientation skills can be tested in a variety of ways that subdivide different elements of this complex phenomemon. Here, however, we highlight four tests and a questionnaire that we believe provide a comprehensive assessment of visuo-spatial orientation skills in humans. The Spatial Configuration Test measures the subjects' ability to create a mental representation of the environment, which is a critical process for orienting and navigating effectively in one's surroundings (Burles et al., 2017). In this test, participants view scenes from a space-like virtual environment populated with five simple, geometric objects (Figure 4A). Participants learn the positions of the objects through 60 successive trials, which are constituted by a series of first-person displacements from one object to another. At each trial, participants indicate the unseen object they are located upon. Participants' accuracy and reaction times are recorded. The Path Integration Test is used to estimate a subject's ability to convert visual optic flow information into a spatial representation of distances traveled in the environment (McNaughton et al., 2006). In each trial of this test, participants are presented with two displacements from within a simple environment devoid of any landmarks (Figure 4B). At the end of the two displacements, participants are asked to indicate the distance and direction to the point from which the first displacement took place (i.e., original starting point). Participants' angular and magnitude errors at each trial are recorded. The Mental Rotation Test characterizes a subject's ability to mentally manipulate 3-D objects in space, which is critical for the mental rotation component of spatial orientation and navigation (Kozhevnikov et al., 2006). In this test, participants view pairs of objects constructed from 10 cubes each (Figure 4C). At each of the 80 trials, participants indicate if the two objects are the same, or if they are mirror images of one another. Participants' accuracy and reaction times are recorded. The Four Mountains Test evaluates the ability to mentally manipulate viewpoints and recognize locations from different perspectives (Hartley et al., 2007; Hartley and Harlow, 2012). In each trial of this test, participants view a virtual scene composed of four distinct mountain peaks. After each scene, participants are required to identify the same scene, depicted from a different viewpoint, from four response options (Figure 4D). Participants' accuracy and reaction times are recorded.
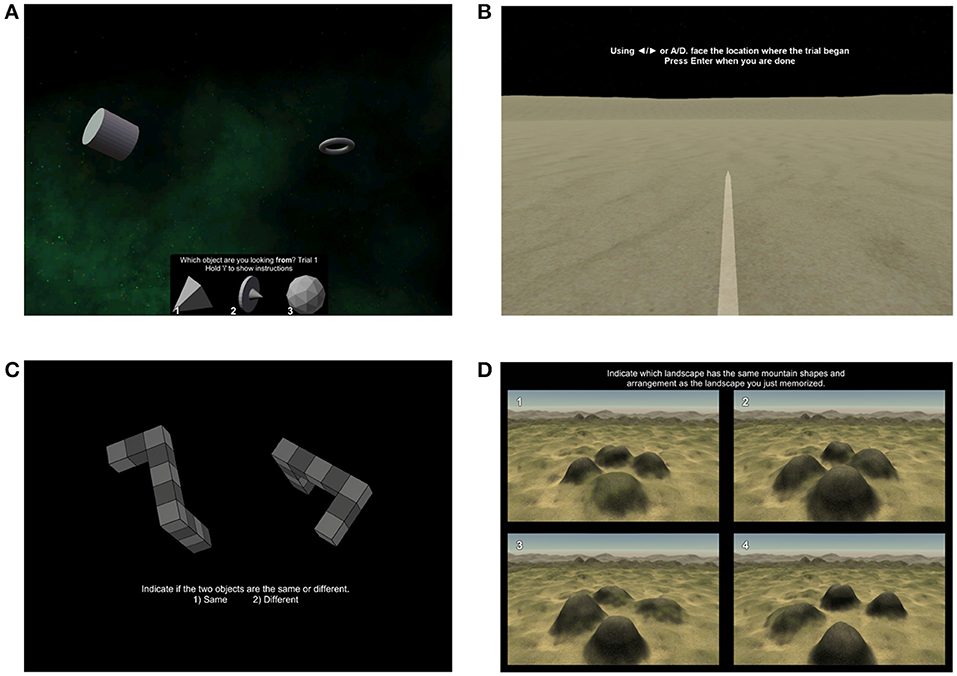
Figure 4. Computerized tests of visuo-spatial orientation skills. (A) A sample trial of the Spatial Configuration Task. In each trial, participants make use of landmarks in a scene to infer their location in a simple environment populated with five geometric objects. The environment (and objects' locations) remains stable throughout the test, and participants are required to build a mental representation of the locations of objects throughout the test. (B) A sample image of the response phase of the Path Integration Test. In this task, participants view two automatic first-person displacements, and must indicate the direction and distance to return to the starting point of the trial. (C) A sample trial of the Mental Rotation Test in which participants are required to mentally manipulate the objects to decide whether or not they are the same. (D) A depiction of the response phase of the Four Mountains Task. In each trial of this task, participants indicate the option which shares the same topography with the stimuli encoded immediately prior (not displayed).
The Santa Barbara Sense of Direction Scale (SBSOD) captures a more ecologically valid measurement of spatial orientation skills (Hegarty et al., 2002), but is still related to the aforementioned, more focused, measures of spatial abilities, and importantly, the capacity to generate mental representations of the environment (Burles, 2014). The scale consists of items evaluating an individual's subjective rating of his/her spatial orientation and navigational skills as experienced in daily life, via agreement on a 7-point scale with statements such as “I can usually remember a new route after I have traveled it only once.”
Eye trackers and pupillometry tools provide non-invasive and rich indices of brain function and cognition. Gaze analysis reveals attentional focus and cognition strategies (Eckstein et al., 2017), and pupil dilation reflects mental effort (Granholm et al., 1996). Previous work has used portable eye trackers by attaching video cameras onto helmets or eyeglass frames, allowing for eye movement to be recorded, modeled, and stored. To our knowledge, eye trackers have not yet been brought into cave environments (and would likely not be practical for use during exploration activities) but they have been used in a virtual cave environment (Koles and Hercegfi, 2015), and could be used to collect data pre-mission or at a base camp. Eye tracking devices have been used in parabolic flight to study visual function in microgravity (Clarke and Haslwanter, 2007). For a more detailed look on eye movements and their relation to space physiology, see the following review: (Clément and Ngo-Anh, 2013).
An actigraph is a device that can monitor movement, usually via wrist-worn accelerometers that record movements over periods of days. Some inexpensive options include those that are available commercially to consumers to track energy expenditure for fitness purposes, which have already have been used in a few studies, for example looking at physiological factors related to human performance during hiking (Divis et al., 2018). Wrist actigraphs can also provide basic information about sleep-wake cycles, and have been used in studies on sleep deprivation and sleep patterns of astronauts during spaceflight (Barger et al., 2014), and to evaluate sleep-wake circadian rhythm maintainance during Mars500 (Frey, 2013).
The skin conductance response (SCR) (or “galvanic skin response”) is a non-invasive measure that can examine autonomic nervous system responses such as those related to stress, emotional engagement, psychological arousal, and anticipation of decision-making outcomes. SCR is measured by placing two electrodes in contact with the skin and passing a tiny electrical charge between them; changes in electrical conductivity of the skin caused by sweat gland function are then observable (see Christopoulos et al., 2016 for a primer on SCR methods). SCR is most easily and reliably measured on the palms of the hands or soles of the feet, where the density of sweat glands that are most responsive to psychological reactions are found. Despite the development of lightweight, wearable sensors, humid cave environments and physical activity often involving the hands limit the practicality of in-cave measurement. However, other metrics such as heart rate variability (HRV; i.e., variability in heartbeat interval) can also serve as indicators for autonomic nerve responses. In a recent meta-analysis, Thayer and colleagues proposed that HRV can serve as a proxy for the integration of brain mechanisms that guide flexible behavioral control with peripheral physiology, and which can be useful for understanding stress and health (Thayer et al., 2012). HRV can be measured by electrocardiogram (ECG), which is based on recording electrical activity of the heart, or by pulse oximetry, which measures changes in reflected or transmitted light due to pulsing arterial blood. Several companies are now producing portable devices containing accelerometers, thermometers, ECG, and oximeters that can be worn attached to the body or embedded in clothing (see Hey et al., 2014 for a review of recent developments in ambulatory measurement, Antoni et al., 2017; Pinna et al., 2017 for examples of in-cave use, and Vigo et al., 2012, 2013 for reports of HRV measurements during the Mars500 project, in which evidence for autonomic changes during confinement were reported).
Electoencephalography involves non-invasively recording the electrical activity of the brain via electrodes placed on the scalp that are used to record a time series of voltage fluctuations. From EEG, researchers can learn about the strength and variability of the brain's activity within different frequency bands and across the scalp while the subject performs a task, in reaction to a stimulus, or as they rest and sleep. A variety of markers have been found for example to track workload (Coffey et al., 2012; Roy et al., 2016), drowsiness (Sahayadhas et al., 2012), and vigilance (Kamzanova et al., 2014), and have been used to study the effects of interventions such as exercise on de-conditioning during confinement (Schneider et al., 2010). It has also been used for neurofeedback, in which users learn to self-regulate brain activity (see Enriquez-Geppert et al., 2017 for a review and tutorial).
EEG has been used in caves since the 1970s (Table 4), but it was kept in an easily accessed cavern that the subject stayed in for the duration of the expedition, a situation that does not represent many contemporary exploration expeditions. Research grade EEG systems today are of higher complexity, and are still typically bulky, delicate, costly, and require special setup steps with messy electro-conductive gel. EEG equipment does exist aboard the ISS (Columbus module, launched in 2008) but does not appear to have been used extensively (De La Torre et al., 2012), for which usability issues (i.e., setup, non-wirelessness) may have been a factor (see also Clément and Ngo-Anh, 2013). Portable EEG equipment has recently been developed for specialized uses such as video gaming and in clinical diagnosis that have fewer electrodes, easier application (i.e., dry electrodes), lightweight amplifiers, and battery packs. Consumer products are more variable in quality, but as they are far less expensive and may be less susceptible to damage, and so are most suitable for expedition environments; some have been validated against lab-grade equipment on indexing neural correlates of cognition (i.e., Wang et al., 2015; Krigolson et al., 2017). New miniaturized “ear-EEG” devices that can record a subset of EEG metrics via an electrode integrated into an earplug are currently under study; once available they would further increase potential cave, analog, and spaceflight applications (Mikkelsen et al., 2015).
Polysomnography consists of EEG with additional sensors for physiological information that are necessary to answer research questions about the quality and architecture of sleep (as opposed to only its timing and length, for which actigraphy is sufficient). In addition to at least once channel of EEG, PSG includes electrooculography (EOG; to detect eye movements), electromyography (EMG; to detect muscle movements and tension), and electrocardiography (ECG or an oxygen saturation sensor) to monitor heart rate. EEG systems are often developed only with waking/cognitive testing or with sleep recordings in mind, though some equipment can be used for both purposes, making possible study designs that involve some active cognitive testing before or after sleep, or for equipment sharing across experiments. In recognition that traditional PSG requires considerable expert assistance both to apply and to analyze and negatively affects the sleep of the person under observation (which is problematic for safety reasons in expedition environments), techniques such as ear-EEG (see above) in combination with automatic sleep analysis algorithms are being tested (Mikkelsen et al., 2017). Relatively inexpensive PSG headbands that are able to play the user EEG phase-locked sounds to restore fragmented sleep and improve improve memory (i.e., closed-loop auditory stimulation) have recently been validated (Arnal et al., 2017; Debellemaniere et al., 2018); these devices could be used both to record sleep data and to test the applicability of the close-loop auditory stimulation technique in expedition conditions.
Functional near Infrared Spectroscopy (fNIS) is a technique that is able to image correlates of brain activity based on changes in the absorption of infrared light by hemoglobin as its oxygenation state changes (reviewed in Ferrari and Quaresima, 2012). In contrast to EEG, which provides high temporal resolution about the activity of synchronized populations of neurons, fNIRs offers access to the cortical hemodynamics that have been extensively studied using fMRI. fNIRS is less sensitive to motion artifacts than either EEG or fMRI, and is somewhat portable and much less expensive than fMRI. fNIRS has been used to study a wide range of cognitive functions such as working memory, and recent developments in portability and wirelessness have extended fNIRS experiments outside of the lab (Pinti et al., 2015). The equipment and usability of fNIRS is likely not yet sufficiently developed for cave expedition use, though this area is under active development and suitable devices may become available in the near future (e.g., Klakegg et al., 2016).
Unless the researcher is adept at planning their own cave expeditions, close collaboration with researchers having field study expertise is necessary to obtain access to caves, to provide detailed knowledge of the specific cave environment (and thus minimize confounding environmental factors Brugger et al., 2018), and to support data acquisition. Speleological groups already involved in scientific work from other disciplines than human studies (including geology, hydrogeology, and biology) can help establish an appropriate underground location and a supportive environment.
The larger and more formal caving associations (e.g., International Union of Speleology, National Speleological Society of America), their publications (e.g., International Journal of Speleology) and congresses (e.g., International Speleological Congress, European Speleological Congress) are also good starting points to identify suitable groups who are motivated to support scientific work. Rescue organizations tend to be organized at the national level, and are those most concerned with health, communication, and even telemedicine underground (e.g., ICAR Alpine Emergency Medicine Commission; ICAR-MEDCOM). Among them, medical doctors and medics are good contacts through which to organize participation and data collection for conducting human research.
The recreational cave community could be of interest for recruiting enthusiastic study participants, usually for short-term data collection in a wide range of experimental conditions. Somewhat larger expeditions are organized by local and regional organizations, and tend to pool together resources from different groups to achieve specific exploration and sometimes scientific objectives. Their participants are more experienced, and their duration ranges from long weekends to several weeks. International expeditions may be funded by sponsors or institutions and include the scientific and technical experts needed to achieve a mix of exploration, documentation, and scientific objectives. They provide a heterogeneous mix of people, and complex, challenging environments (e.g., ice caves, very deep caves, very long expeditions). Finally, rescue organizations regularly train underground in simulated rescue interventions, and could offer a unique opportunity for neuroscientists particularly to study teamwork and decision-making processes in large groups under stressful conditions, as they can engage hundreds of personnel for several days (Schneider et al., 2016).
Research conducted on human cognition and behavioral performance under highly challenging conditions analogous to those found in spaceflight is needed to predict and maintain high levels of human performance in future missions. It can also offer scientists who primarily work in laboratory environment unique opportunities to observe aspects of cognitive processes as they relate to real behavior under complex, realistic, extreme environmental conditions. In addition to contributing to exploration activities and fundamental knowledge of human brain function, these investigations could benefit people in safety-critical environments and occupations, such as shift-workers, firefighters, medical teams, or air traffic controllers.
Pushed by clinical research and consumer applications, devices that record neurophysiological parameters with high-fidelity non-invasively are now becoming available, making studying a wide range of topics feasible. However, particularly in neuroscience and related areas (i.e., cognition, cognitive psychology, neuropsychology), gaps must first be spanned between laboratory and field research in methods, knowledge, and scientific culture such that the advantages of expeditions as an intermediate research platform can be realized. An efficient way to ensure that high-quality research is conducted in caves and other space analogs and effectively shared is through a mutual understanding of the expedition environment, which we have outlined here, and through collaboration. As well as ensuring that the results are valid and have an impact in both space and in cognitive specializations, academic partners can arrange for the required ethical oversight, whereas partners familiar with expeditions can contribute essential knowledge to make research possible and ensure it is well conducted in the field and practically relevant. We hope that this work will support productive collaborations that extend mainstream neuroscience into unique environments and situations, to increase our understanding of real-world cognition and improve human performance and safety in operational environments. The time is ripe for neuroscience to leave the lab.
LZ conducted the original literature review, as part of a systematic review on studies human physiology (in preparation) wherein neuroscience was considered a subtopic. EC and LB developed the concept for and structure of this review and prospective. NM continued the literature search and review work, read and summarized all records, and prepared the tools and equipment sections. GI and FB contributed to the sections on computerized and survey-based testing. GS and LB contributed to the sections on space analogs and connecting to the space community. NM and EC wrote the paper, with contributions and input from LZ, LB, GI, and GS.
EC was supported by the Banting Research Fellowship (Natural Sciences and Engineering Research Council of Canada). GI's research on topographical orientaiton was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank ESA CAVES instructor Francesco Sauro for discussion and advice concerning operational considerations.
EEG, electroencephalography; EMG, electromyography; EOG, electrooculography; ESA, European Space Agency; fNIRS, functional near infrared spectroscopy; fMRI, functional magnetic resonance imaging; HRV, heart rate variability; ISS, International Space Station; NASA, National Aeronautics and Space Administration (US); PSG, polysomnography; SCR, Skin conductance response.
Alexander, D. J. (2016). “The genesis of crew resource management: The NASA Experience,” in Trauma Team Dynamics, eds L. Gillman, S. Widder, M. Blaivas, and D. Karakitsos (Cham: Springer International Publishing), 3–7. doi: 10.1007/978-3-319-16586-8_1
Allen, C. S., Giraudo, M., Moratto, C., and Yamaguchi, N. (2018). Spaceflight environment. Space Safet Hum. Perform. 87–138. doi: 10.1016/B978-0-08-101869-9.00004-2
Alliger, G., Cerasoli, C., and Tannenbaum, S. (2015). Undefined Team Resilience. Available online at: iranarze.ir
Alonso, J. F., Romero, S., Ballester, M. R., Antonijoan, R. M., and Mañanas, M. A. (2015). Stress assessment based on EEG univariate features and functional connectivity measures. Physiol. Meas. 36, 1351–1365. doi: 10.1088/0967-3334/36/7/1351
Andersen, R. A. (1997). Multimodal integration for the representation of space in the posterior parietal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 352, 1421–1428. doi: 10.1098/rstb.1997.0128
Antoni, G., Marini, E., Curreli, N., Tuveri, V., Comandini, O., Cabras, S., et al. (2017). Energy expenditure in caving. PLoS ONE 12:e0170853. doi: 10.1371/journal.pone.0170853
Apfelbaum, M. (1969). Rythmes Circadiens de l'Alternance Veille-Sommeil Pendant l'Isolement Souterrain de Sept Jeunes Femmes. Presse Med. 77, 879–882.
Aricò, P., Borghini, G., Di Flumeri, G., Colosimo, A., Bonelli, S., Golfetti, A., et al. (2016). Adaptive Automation Triggered by EEG-Based Mental Workload Index: a Passive Brain-Computer Interface Application in Realistic Air Traffic Control Environment. Front. Hum. Neurosci. 10:539. doi: 10.3389/fnhum.2016.00539
Arico, P., Borghini, G., Di Flumeri, G., Sciaraffa, N., Colosimo, A., and Babiloni, F. (2017). Passive BCI in operational environments: insights, recent advances, and future trends. IEEE Trans. Biomed. Eng. 64, 1431–1436. doi: 10.1109/TBME.2017.2694856
Arnal, P. J., El Kanbi, K., Debellemaniere, E., Pinaud, C., Thorey, V., Chambon, S., et al. (2017). Auditory closed-loop stimulation to enhance sleep quality. J. Sci. Med. Sport 20:S95. doi: 10.1016/J.JSAMS.2017.09.447
Arnold, A. E., Burles, F., Krivoruchko, T., Liu, I., Rey, C. D., Levy, R. M., et al. (2013). Cognitive mapping in humans and its relationship to other orientation skills. Exp. Brain Res. 224, 359–372. doi: 10.1007/s00221-012-3316-0
Arnsten, A. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422. doi: 10.1038/nrn2648
Arnsten, A. F. (2015). Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 18, 1376–1385. doi: 10.1038/nn.4087
Aschoff, J. (1965). Circadian rhythms in man. Science 148, 1427–1432. doi: 10.1126/science.148.3676.1427
Barger, L. K., Flynn-Evans, E. E., Kubey, A., Walsh, L., Ronda, J. M., Wang, W., et al. (2014). Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: AN observational study. Lancet Neurol. 13, 904–912. doi: 10.1016/S1474-4422(14)70122-X
Basner, M., Babisch, W., Davis, A., Brink, M., Clark, C., Janssen, S., et al. (2014a). Auditory and non-auditory effects of noise on health. Lancet 383, 1325–1332. doi: 10.1016/S0140-6736(13)61613-X
Basner, M., Dinges, D. F., Mollicone, D., Ecker, A., Jones, C. W., Hyder, E. C., et al. (2013). Mars 520-d mission simulation reveals protracted crew hypokinesis and alterations of sleep duration and timing. Proc. Natl. Acad. Sci. U.S.A. 110, 2635–2640. doi: 10.1073/pnas.1212646110
Basner, M., Dinges, D. F., Mollicone, D. J., Savelev, I., Ecker, A. J., Di Antonio, A., et al. (2014b). Psychological and behavioral changes during confinement in a 520-day simulated interplanetary mission to mars. PLoS ONE 9:e93298. doi: 10.1371/journal.pone.0093298
Basner, M., Savitt, A., Moore, T. M., Port, A. M., McGuire, S., Ecker, A. J., et al. (2015). Development and validation of the cognition test battery for spaceflight. Aerosp. Med. Hum. Perform. 86, 942–952. doi: 10.3357/AMHP.4343.2015
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. doi: 10.1001/archpsyc.1961.01710120031004
Bermudez, E. B., Klerman, E. B., Czeisler, C. A., Cohen, D. A., Wyatt, J. K., and Phillips, A. J. K. (2016). Prediction of vigilant attention and cognitive performance using self-reported alertness, circadian phase, hours since awakening, and accumulated sleep loss. PLoS ONE 11:e0151770. doi: 10.1371/journal.pone.0151770
Besedovsky, L., Ngo, H. V. V., Dimitrov, S., Gassenmaier, C., Lehmann, R., and Born, J. (2017). Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat. Commun. 8:1984. doi: 10.1038/s41467-017-02170-3
Bessone, L., Beblo-vranesevic, K., Cossu, Q. A., Waele, J., De Leuko, S., Marcia, P., et al. (2013). ESA CAVES : Training Astronauts for Space Exploration. Available online at: researchgate.net
Bessone, L., Coffey, E., Filippova, N., Greenberg, E., Inoue, N., Shevchenko, O., et al. (2008). International Space Station Human Behavior & Performance Competency Model Volume II Mission Operations Directorate. Washington, DC: NASA STI Report Series.
Betella, A., and Verschure, P. F. (2016). The affective slider: a digital self-assessment scale for the measurement of human emotions. PLoS ONE 11:e0148037. doi: 10.1371/journal.pone.0148037
Bishop, S. L. (2013). “From earth analogues to space: learning how to boldly go,” in On Orbit and Beyond: Psychological Perspectives on Human Spaceflight, ed D. Vakoch (Berlin; Heidelberg: Springer), vol. 29, 25–50. doi: 10.1007/978-3-642-30583-2_2
Bishop, S. L., Santy, P. A., and Faulk, D. (1999). Team Dynamics Analysis of the Huautla Cave Diving Expedition: A Case Study. Denver: SAE Technical Papers. doi: 10.4271/1999-01-2099
Boardman, J. M., Bei, B., Mellor, A., Anderson, C., Sletten, T. L., and Drummond, S. P. A. (2017). The ability to self-monitor cognitive performance during 60 h total sleep deprivation and following 2 nights recovery sleep. J. Sleep Res. 27, 1–8. doi: 10.1111/jsr.12633
Bock, O., Howard, I. P., Money, K. E., and Arnold, K. E. (1992). Accuracy of aimed arm movements in changed gravity. Aviat. Space. Environ. Med. 63, 994–998.
Bock, O., Weigelt, C., and Bloomberg, J. J. (2010). Cognitive demand of human sensorimotor performance during an extended space mission: a dual-task study. Aviat. Space. Environ. Med. 81, 819–824. doi: 10.3357/ASEM.2608.2010
Brannick, M., Salas, E., and Prince, C. (eds.). (1997). “Team performance assessment and measurement: theory, methods, and applications.” in Team Performance Assessment and Measurement. Mahwah, NJ: Lawrence Erlbaum.
Brugger, H., Pun, M., Swenson, E. R., and Falk, M. (2018). Research in high-altitude and mountain emergency medicine: is methodology key? High Alt. Med. Biol. 19, 1–3. doi: 10.1089/ham.2017.0124
Burles, C. (2014). The Development of a Practical Measure of Environmental-Scale Spatial Ability: The Spatial Configuration Task. Dissertations. Calgary: University of Calgary.
Burles, F., Slone, E., and Iaria, G. (2017). Dorso-medial and ventro-lateral functional specialization of the human retrosplenial complex in spatial updating and orienting. Brain Struct. Funct. 222, 1481–1493. doi: 10.1007/s00429-016-1288-8
Cerqueira, J. J., Mailliet, F., Almeida, O. F. X., Jay, T. M., and Sousa, N. (2007). The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 27, 2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007
Chen, G., Gully, S. M., and Eden, D. (2001). Validation of a new general self-efficacy scale. Organ. Res. Methods 4, 62–83. doi: 10.1177/109442810141004
Cheng, L., Wang, S.-H., Chen, Q.-C., and Liao, X.-M. (2011). Moderate noise induced cognition impairment of mice and its underlying mechanisms. Physiol. Behav. 104, 981–988. doi: 10.1016/J.PHYSBEH.2011.06.018
Chidester, T. R., Helmreich, R. L., Gregorich, S. E., and Geis, C. E. (1991). Pilot personality and crew coordination: implications for training and selection. Int. J. Aviat. Psychol. 1, 25–44. doi: 10.1207/s15327108ijap0101_3
Chouvet, G., Mouret, J., Coindet, J., Siffre, M., and Jouvet, M. (1974). Periodicite bicircadienne du cycle veille-sommeil dans des conditions hors du temps. etude polygraphique. Electroencephalogr. Clin. Neurophysiol. 37, 367–380. doi: 10.1016/0013-4694(74)90112-6
Christopoulos, G. I., Uy, M. A., and Yap, W. J. (2016). The body and the brain: measuring skin conductance responses to understand the emotional experience. Organ. Res. Methods. 1–27. doi: 10.1177/1094428116681073
Clarke, A. H., and Haslwanter, T. (2007). The orientation of Listing's Plane in microgravity. Vision Res. 47, 3132–3140. doi: 10.1016/J.VISRES.2007.09.001
Clément, G., and Ngo-Anh, J. T. (2013). Space Physiology II: Adaptation of the Central Nervous System to Space Flight-Past, Current, and Future Studies. Berlin: Springer-Verlag. doi: 10.1007/s00421-012-2509-3
Clément, G., and Reschke, M. F. (2008). Neuroscience in Space. New York, NY: Springer. doi: 10.1007/978-0-387-78950-7
Coffey, E. B. J., Brouwer, A. M., and Van Erp, J. B. F. (2012). “Measuring workload using a combination of electroencephalography and near infrared spectroscopy,” in Proceedings of the Human Factors and Ergonomics Society. Thousand Oaks: Sage Publications, 1822–1826. doi: 10.1177/1071181312561367
Coffey, E. B. J., Brouwer, A. M., Wilschut, E. S., and van Erp, J. B. F. (2010). Brain-machine interfaces in space: using spontaneous rather than intentionally generated brain signals. Acta Astronaut. 67, 1–11. doi: 10.1016/j.actaastro.2009.12.016
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24:385. doi: 10.2307/2136404
Cojocaru, V., Bodnari, M., Lupuşor, A., and Vovc, V. (2017). The paradox of changing the sleep architecture in administration of hypnotics. Sleep Med. 40, e201–e202. doi: 10.1016/J.SLEEP.2017.11.588
Colin, J., Timbal, J., Boutelier, C., Houdas, Y., and Siffre, M. (1968). Rhythm of the rectal temperature during a 6-month free-running experiment. J. Appl. Physiol. 25, 170–176.
Conrad, C. D. (2008). Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 19, 395–411. doi: 10.1515/REVNEURO.2008.19.6.395
Conrad, C. D. (2010). A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 742–755. doi: 10.1016/j.pnpbp.2009.11.003
Cronin, M. A., Weingart, L. R., and Todorova, G. (2011). Dynamics in groups: are we there yet? Acad. Manag. Ann. 5, 571–612. doi: 10.1080/19416520.2011.590297
Czeisler, C. A., Duffy, J. F., Shanahan, T. L., Brown, E. N., Mitchell, J. F., Rimmer, D. W., et al. (1999). Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181.
De la Torre, G. G., Mestre Navas, J. M., and Guil Bozal, R. (2014). Neurocognitive performance using the Windows spaceflight cognitive assessment tool (WinSCAT) in human spaceflight simulations. Aerosp. Sci. Technol. 35, 87–92. doi: 10.1016/J.AST.2014.02.006
De La Torre, G. G., Van Baarsen, B., Ferlazzo, F., Kanas, N., Weiss, K., Schneider, S., et al. (2012). Future perspectives on space psychology: recommendations on psychosocial and neurobehavioural aspects of human spaceflight. Acta Astronaut. 81, 587–599. doi: 10.1016/j.actaastro.201208.013
Debellemaniere, E., Chambon, S., Pinaud, C., Thorey, V., Dehaene, D., Léger, D., et al. (2018). Performance of an ambulatory dry-EEG device for auditory closed-loop stimulation of sleep slow oscillations in the home environment. Front. Hum. Neurosci. 12:88. doi: 10.3389/FNHUM.2018.00088
Diekelmann, S., and Born, J. (2010). The memory function of sleep. Nat.Rev.Neurosci. 11, 114–126. doi: 10.1038/nrn2762
Dijk, D. J., Neri, D. F., Wyatt, J. K., Ronda, J. M., Riel, E., Ritz-De Cecco, A., et al. (2001). Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647
Divis, K., Anderson-Bergman, C., Abbott, R., Newton, V., and Emmanuel-Aviña, G. (2018). Physiological and cognitive factors related to human performance during the grand canyon rim-to-rim hike. J. Hum. Perform. Extrem. Environ. 14:5. doi: 10.7771/2327-2937.1095
Dunlap, R. D. (1965). The Selection and Training of Crewmen for an Isolation and Confinement Study in the Douglas Space Cabin Simulator. Santa Monica, CA: Douglas Aircraft Company.
Eckstein, M. K., Guerra-Carrillo, B., Miller Singley, A. T., and Bunge, S. A. (2017). Beyond eye gaze: what else can eyetracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 25, 69–91. doi: 10.1016/J.DCN.2016.11.001
Ekstrom, A. D., Huffman, D. J., and Starrett, M. (2017). Interacting networks of brain regions underlie human spatial navigation: a review and novel synthesis of the literature. J. Neurophysiol. 118, 3328–3344. doi: 10.1152/jn.00531.2017
Ekstrom, A. D., and Isham, E. A. (2017). Human spatial navigation: representations across dimensions and scales. Curr. Opin. Behav. Sci. 17, 84–89. doi: 10.1016/j.cobeha.2017.06.005
Endsley, M. R. (1995). Measurement of situation awareness in dynamic systems. Hum. Factors J. Hum. Factors Ergon. Soc. 37, 65–84. doi: 10.1518/001872095779049499
Enriquez-Geppert, S., Huster, R. J., and Herrmann, C. S. (2017). EEG-Neurofeedback as a tool to modulate cognition and behavior: a review tutorial. Front. Hum. Neurosci. 11:51. doi: 10.3389/fnhum.2017.00051
Epstein, R. A., Higgins, J. S., and Thompson-Schill, S. L. (2005). Learning places from views: variation in scene processing as a function of experience and navigational ability. J. Cogn. Neurosci. 17, 73–83. doi: 10.1162/0898929052879987
Epstein, R. A., Patai, E. Z., Julian, J. B., and Spiers, H. J. (2017). The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci. 20, 1504–1513. doi: 10.1038/nn.4656
Faber, N. S., Häusser, J. A., and Kerr, N. L. (2017). Sleep deprivation impairs and caffeine enhances my performance, but not always our performance. Personal. Soc. Psychol. Rev. 21, 3–28. doi: 10.1177/1088868315609487
Ferrari, M., and Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63, 921–935. doi: 10.1016/j.neuroimage.2012.03.049
Fink, W., Baker, V. R., Schulze-Makuch, D., Hamilton, C. W., and Tarbell, M. A. (2015). “Autonomous exploration of planetary lava tubes using a multi-rover framework,” in 2015 IEEE Aerospace Conference (Piscataway: IEEE), 1–9. doi: 10.1109/AERO.2015.7119315
Flory, J. D., Manuck, S. B., Matthews, K. A., and Muldoon, M. F. (2004). Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Res. 129, 11–19. doi: 10.1016/J.PSYCHRES.2004.06.010
Fong, T., Burns, J., and Pratt, W. (2014). “Utilization of the International Space Station as a Testbed for Crew - Controlled Lunar Surface Telerobotics,” in International Academy of Astronautics, (Stockholm).
Fraisse, P., Siffre, M., Oleron, G., and Zuili, N. (1968). La rythme veille-sommeil et l'estimation du temps. Cycles Biol. Psychiatr. 257–65.
Frey, M. A. (2013). Sleep-Wake Dynamics During the Mars 520-d Mission Simulation. Aviat. Sp. Environ. Med. 84, 1308–1309. doi: 10.3357/ASEM.3724.2013
Frost, J. D., Shumate, W. H., Salamy, J. G., and Booher, C. R. (1975). “Sleep Characteristics during the 28- and 59-Day Skylab Missions,” in Behavior and Brain Electrical Activity (Boston, MA: Springer), 31–53. doi: 10.1007/978-1-4613-4434-6_2
Frost, J. D., Shumate, W. H., Salamy, J. G., and Booher, C. R. (1976). Sleep monitoring: the second manned skylab mission. Aviat. Sp. Environ. Med. 47, 372–382.
Ganzel, B. L., Kim, P., Glover, G. H., and Temple, E. (2008). Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. World Trade 40, 788–795. doi: 10.1016/j.neuroimage.2007.12.010
Gemignani, A., Piarulli, A., Menicucci, D., Laurino, M., Rota, G., Mastorci, F., et al. (2014). How stressful are 105days of isolation? Sleep EEG patterns and tonic cortisol in healthy volunteers simulating manned flight to Mars. Int. J. Psychophysiol. 93, 211–219. doi: 10.1016/j.ijpsycho.2014.04.008
Ghata, J., Halberg, F., and Reinberg, A. S. M (1969). Rythmes circadiens desynchronises du cycle social chez deux sujets adultes sains. Ann. Endocrinol. (Paris). 29, 269–270.
Gianaros, P. J., Jennings, J. R., Sheu, L. K., Greer, P. J., Kuller, L. H., and Matthews, K. A. (2007). Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage 35, 795–803. doi: 10.1016/j.neuroimage.2006.10.045
Glasauer, S., and Mittelstaedt, H. (1998). Perception of spatial orientation in microgravity. Brain Res. Rev. 28, 185–193. doi: 10.1016/S0165-0173(98)00038-1
Goldberg, L. (1999). A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. Personal. Psychol. Eur. 7, 7–28.
Gore, B. F. (2018). “Workload and fatigue,” in Space Safety and Human Performance, eds S. Tommaso, K. Barbara, C. Jean-François, S. Gro Mjeldheim (Amsterdam: Elsevier), 53–85. doi: 10.1016/B978-0-08-101869-9.00003-0
Granholm, E., Asarnow, R. F., Sarkin, A. J., and Dykes, K. L. (1996). Pupillary responses index cognitive resource limitations. Psychophysiology 33, 457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x
Groemer, G., Gruber, V., Bishop, S., Peham, D., Wolf, L., and Högl, B. (2010). Human performance data in a high workload environment during the simulated Mars expedition “AustroMars.” Acta Astronaut. 66, 780–787. doi: 10.1016/J.ACTAASTRO.2009.08.017
Gruzelier, J. H. (2014). EEG-neurofeedback for optimising performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 44, 124–141. doi: 10.1016/J.NEUBIOREV.2013.09.015
Gundel, A., Polyakov, V. V., and Zulley, J. (1997). The alteration of human sleep and circadian rhythms during spaceflight. J. Sleep Res. 6, 1–8. doi: 10.1046/j.1365-2869.1997.00028.x
Halberg, F. (1965). Etude en libre-cours des rythmes circadiensdu pouls, de l'alternance veille-sommeil et de l'estimation du temps pendant les deux mois de sejour souterrain d'un homme adulte jeune. C. R. Acad Sci. Paris 260, 1259–1262.
Halberg, F., Reinberg, A., and Erhard Haus, J. G. (1970). Human biological rhythms during and after several months of isolation underground in natural caves. NSS Bull. 32, 89–115.
Hartley, T., Bird, C. M., Chan, D., Cipolotti, L., Husain, M., Vargha-Khadem, F., et al. (2007). The hippocampus is required for short-term topographical memory in humans. Hippocampus 17, 34–48. doi: 10.1002/hipo.20240
Hartley, T., and Harlow, R. (2012). An association between human hippocampal volume and topographical memory in healthy young adults. Front. Hum. Neurosci. 6:338. doi: 10.3389/fnhum.2012.00338
Havekes, R., and Abel, T. (2017). The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr. Opin. Neurobiol. 44, 13–19. doi: 10.1016/j.conb.2017.02.005
He, B. J., Snyder, A. Z., Vincent, J. L., Epstein, A., Shulman, G. L., and Corbetta, M. (2007). Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 53, 905–918. doi: 10.1016/J.NEURON.2007.02.013
Hegarty, M., Richardson, A. E., Montello, D. R., Lovelace, K., and Subbiah, I. (2002). Development of a self-report measure of environmental spatial ability. Intelligence 30, 425–447. doi: 10.1016/S0160-2896(02)00116-2
Hey, S., Anastasopoulou, P., Bideaux, A., and Stork, W. (2014). Recent developments of ambulatory assessment methods. Zeitschrift für Neuropsychol. 25, 279–287. doi: 10.1024/1016-264X/a000143
Hillman, D., Siffre, M., Milano, G., and Halberg, F. (1994a). Free-running psycho-physiologic circadians and three-month pattern in a woman isolated in a cave. New Trends Exp. Clin. Psychiatry 10, 127–133.
Hillman, D., Siffre, M., Milano, G., and Halberg, F. (1994b). Urinary about-84-hour (circasemiseptan) variations of a woman isolated in a cave and cosmic ray effects. New Trends Exp. Clin. Psychiatry 10, 173–178.
Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., and Dement, W. C. (1973). Quantification of sleepiness: a new approach. Psychophysiology 10, 431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x
Holmes, A., and Wellman, C. L. (2009). Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 33, 773–783. doi: 10.1016/j.neubiorev.2008.11.005
Hooker, K., and Shalit, M. (2000). Subterranean medicine: an inquiry into underground medical treatment protocols in cave rescue situations in national parks in the United States. Wilderness Environ. Med. 11, 17–20. doi: 10.1580/1080-6032(2000)011[0017:SMAIIU]2.3.CO;2
Hoppenbrouwers, T. (2016). “Analogues for preparing robotic and human exploration on the moon,” in SpaceOps 2016 Conference. Reston, VA: American Institute for Aeronautics and Astronautics (AIAA). doi: 10.2514/6.2016-2353
Horneck, G., and Comet, B. (2006). “General human health issues for Moon and Mars missions: results from the HUMEX study,” in Advances in Space Research (Pergamon), 100–108. doi: 10.1016/j.asr.2005.06.077
Iaria, G., Arnold, A. E. G. F., Burles, F., Liu, I., Slone, E., Barclay, S., et al. (2014). Developmental topographical disorientation and decreased hippocampal functional connectivity. Hippocampus 24, 1364–1374. doi: 10.1002/hipo.22317
Iaria, G., Chen, J. K., Guariglia, C., Ptito, A., and Petrides, M. (2007). Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur. J. Neurosci. 25, 890–899. doi: 10.1111/j.1460-9568.2007.05371.x
Ishikawa, T., Fujiwara, H., Imai, O., and Okabe, A. (2008). Wayfinding With a GPS-based Mobile Navigation System: A Comparison With Maps and Direct Experience. Cambridge: Academic Press. doi: 10.1016/j.jenvp.2007.09.002
Ishizaki, Y., Ishizaki, T., Fukuoka, H., Kim, C. S., Fujita, M., Maegawa, Y., et al. (2002). Changes in mood status and neurotic levels during a 20-day bed rest. Acta Astronaut. 50, 453–459. doi: 10.1016/S0094-5765(01)00189-8
Judge, T., and Erez, A. (2003). The core self-evaluations scale: development of a measure. Pers. Psychol. 56:303. doi: 10.1111/j.1744-6570.2003.tb00152.x
Kalish, Y., Luria, G., Toker, S., and Westman, M. (2015). Till stress do us part: on the interplay between perceived stress and communication network dynamics. J. Appl. Psychol. 100, 1737–1751. doi: 10.1037/apl0000023
Kamzanova, A. T., Kustubayeva, A. M., and Matthews, G. (2014). Use of EEG workload indices for diagnostic monitoring of vigilance decrement. Hum. Factors J. Hum. Factors Ergon. Soc. 56, 1136–1149. doi: 10.1177/0018720814526617
Kanas, N. (2015). Humans in Space: The Psychological Hurdles. Cham: Springer International Publishing. doi: 10.1007/978-3-319-18869-0
Kanas, N., and Manzey, D. (2008). Space Psychology and Psychiatry (Space Technology Library). Berlin: Springer.
Kanas, N., Sandal, G. M., Boyd, J. E., Gushin, V. I., Manzey, D., North, R., et al. (2013). “Psychology and culture during long-duration space missions,” in On Orbit and Beyond: Psychological Perspectives on Human Spaceflight, ed D. A. Vakoch (Berlin: Pergamon), 153–184. doi: 10.1007/978-3-642-30583-2_9
Kane, R. L., Short, P., Sipes, W., and Flynn, C. F. (2005). Development and validation of the spaceflight cognitive assessment tool for windows (WinSCAT). Aviat. Space Environ. Med. 76(6 Suppl.), B183–B191.
Kanki, B. G. (2018a). “Cognitive functions and human error,” in Space Safety and Human Performance, eds S. Tommaso, K. Barbara, C. Jean-François, S. Gro Mjeldheim (Amsterdam: Elsevier), 17–52. doi: 10.1016/B978-0-08-101869-9.00002-9
Kanki, B. G. (2018b). “Human factors research methods and tools,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Butterworth-Heinemann), 239–271. doi: 10.1016/B978-0-08-101869-9.00007-8
Keeton, K. E., Whitmire, A., Ploutz-Snyder, R., Leveton, L. B., and Shea, C. (2011). Analog Assessment Tool Report. NASA TP−2011–21.
Kelly, T. H., Hienz, R. D., Zarcone, T. J., Wurster, R. M., and Brady, J. V (2005). Crewmember Performance Before, During, And After Spaceflight. J. Exp. Anal. Behav. 84, 227–241. doi: 10.1901/jeab.2005.77-04
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. doi: 10.1038/386493a0
Keyton, J., and Wall, V. D. (1989). Symlog: theory and method for measuring group and organizational communication. Manag. Commun. Q. 2, 544–567. doi: 10.1177/0893318989002004006
Khoury, B., Sharma, M., Rush, S. E., and Fournier, C. (2015). Mindfulness-based stress reduction for healthy individuals: a meta-analysis. J. Psychosom. Res. 78, 519–528. doi: 10.1016/j.jpsychores.2015.03.009
Kim, J. G., Aminoff, E. M., Kastner, S., and Behrmann, M. (2015). A neural basis for developmental topographic disorientation. J. Neurosci. 35, 12954–12969. doi: 10.1523/JNEUROSCI.0640-15.2015
Kirsch, D. B. (2011). There and back again: a current history of sleep medicine. Chest 139, 939–946. doi: 10.1378/chest.10-1235
Klakegg, S., Luo, C., Goncalves, J., Hosio, S., and Kostakos, V. (2016). “Instrumenting smartphones with portable NIRS,” in Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing Adjunct - UbiComp'16 (New York, NY: ACM Press), 618–623. doi: 10.1145/2968219.2971590
Kleen, J. K., Sitomer, M. T., Killeen, P. R., and Conrad, C. D. (2006). Chronic stress impairs spatial memory and motivations for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 120, 842–851. doi: 10.1037/0735-7044.120.4.842
Koles, M., and Hercegfi, K. (2015). “Eye tracking precision in a virtual CAVE environment,” in 2015 6th IEEE International Conference on Cognitive Infocommunications (CogInfoCom) (Piscataway: IEEE), 319–322. doi: 10.1109/CogInfoCom.2015.7390611
Kolling, A., Walker, P., Chakraborty, N., Sycara, K., and Lewis, M. (2016). Human interaction with robot swarms: a survey. IEEE Trans. Hum. Mach. Syst. 46, 9–26. doi: 10.1109/THMS.2015.2480801
Kozhevnikov, M., Motes, M. A., Rasch, B., and Blajenkova, O. (2006). Perspective-taking vs. mental rotation transformations and how they predict spatial navigation performance. Appl. Cogn. Psychol. 20, 397–417. doi: 10.1002/acp.1192
Kozlowski, S. W. J. (2015). Advancing research on team process dynamics. Organ. Psychol. Rev. 5, 270–299. doi: 10.1177/2041386614533586
Krause, A. J., Simon, E. B., Mander, B. A., Greer, S. M., Saletin, J. M., Goldstein-Piekarski, A. N., et al. (2017). The sleep-deprived human brain. Nat. Rev. Neurosci. 18, 404–418. doi: 10.1038/nrn.2017.55
Krigolson, O. E., Williams, C. C., Norton, A., Hassall, C. D., and Colino, F. L. (2017). Choosing MUSE: validation of a low-cost, portable EEG system for ERP research. Front. Neurosci. 11:109. doi: 10.3389/fnins.2017.00109
Kyriacou, C. P., and Hastings, M. H. (2010). Circadian clocks: genes, sleep, and cognition. Trends Cogn. Sci. 14, 259–267. doi: 10.1016/J.TICS.2010.03.007
Lederman, O., Calacci, D., MacMullen, A., Fehder, D. C., Murray, F. E., Pentland, A., et al. (2017). Open Badges: A Low-Cost Toolkit for Measuring Team Communication and Dynamics. arXiv [Preprint]:1710.01842
Leggio, M. G., Mandolesi, L., Federico, F., Spirito, F., Ricci, B., Gelfo, F., et al. (2005). Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 163, 78–90. doi: 10.1016/j.bbr.2005.04.009
Li, K., Guo, X., Jin, Z., Ouyang, X., Zeng, Y., Feng, J., et al. (2015). Effect of Simulated Microgravity on Human Brain Gray Matter and White Matter – Evidence from MRI. PLoS ONE 10:e0135835. doi: 10.1371/journal.pone.0135835
Lia Schlacht, I., Foing, B., Bannova, O., Blok, F., Mangeot, A., Nebergall, K., et al. (2016). “Space analog survey: review of existing and new proposal of space habitats with earth applications,” in 46th International Conference on Environmental Systems (Vienna).
Liapis, A., Katsanos, C., Sotiropoulos, D., Xenos, M., and Karousos, N. (2015). “Recognizing emotions in human computer interaction: studying stress using skin conductance,” in Lecture Notes in Computer Science Book Series (Cham: Springer), 255–262. doi: 10.1007/978-3-319-22701-6_18
Liu, Q., Zhou, R.-L., Zhao, X., Chen, X.-P., and Chen, S.-G. (2016). Acclimation during space flight: effects on human emotion. Mil. Med. Res. 3:15. doi: 10.1186/s40779-016-0084-3
Lukavský, J. (2014). Changes in boundary extension effect during spatial confinement. Vis. Cogn. 22, 996–1012. doi: 10.1080/13506285.2014.941966
Lupien, S. J., Maheu, F., Maheu, F., Tu, M., Tu, M., Fiocco, A., et al. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 65, 209–237. doi: 10.1016/j.bandc.2007.02.007
Machi, M. S., Staum, M., Callaway, C. W., Moore, C., Jeong, K., Suyama, J., et al. (2012). The relationship between shift work, sleep, and cognition in career emergency physicians. Acad. Emerg. Med. 19, 85–91. doi: 10.1111/j.1553-2712.2011.01254.x
MacNeil, R., and Brcic, J. (2017). Coping with the subterranean environment: a thematic content analysis of the narratives of cave explorers. J. Hum. Perform. Extrem. Environ. 13:6. doi: 10.7771/2327-2937.1089
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S. J., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U.S.A. 97, 4398–4403. doi: 10.1073/pnas.070039597
Maguire, E. A., Woollett, K., and Spiers, H. (2006). London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus 16, 1091–1101. doi: 10.1002/hipo.20233
Mallis, M. M., and DeRoshia, C. W. (2005). Circadian rhythms, sleep, and performance in space. Aviat. Space. Environ. Med. 76, B94–B107.
Manzey, D. (2004). “Human missions to Mars: New psychological challenges and research issues,” in Acta Astronautica, ed R. Monti (Pergamon: Elsevier), 781–790. doi: 10.1016/j.actaastro.2004.05.013
Manzey, D., and Lorenz, B. (1998). Mental performance during short-term and long-term spaceflight. Brain Res. Brain Res. Rev. 28, 215–221. doi: 10.1016/S0165-0173(98)00041-1
Manzey, D., Lorenz, B., and Poljakov, V. (1998). Mental performance in extreme environments: results from a performance monitoring study during a 438-day spaceflight. Ergonomics 41, 537–559. doi: 10.1080/001401398186991
Manzey, D., Lorenz, B., Schiewe, A., Finell, G., and Thiele, G. (1993). Behavioral aspects of human adaptation to space analyses of cognitive and psychomotor performance in space during an 8-day space mission. Clin. Investig. 71, 725–731. doi: 10.1007/BF00209727
Marquez, J. J., Riley, V., and Schutte, P. C. (2018). “Human automation interaction,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Butterworth-Heinemann), 429–467. doi: 10.1016/B978-0-08-101869-9.00010-8
Matthews, G., Jones, D. M., and Chamberlain, A. G. (1990). Refining the measurement of mood: the UWIST mood adjective checklist. Br. J. Psychol. 81, 17–42. doi: 10.1111/j.2044-8295.1990.tb02343.x
McEwen, B. S., and Morrison, J. H. (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. doi: 10.1016/j.neuron.2013.06.028
McMorris, T., Barwood, M., Hale, B. J., Dicks, M., and Corbett, J. (2018). Cognitive fatigue effects on physical performance: a systematic review and meta-analysis. Physiol. Behav. 188, 103–107. doi: 10.1016/J.PHYSBEH.2018.01.029
McNair, D., Lorr, M., and Droppleman, L. (1971). EDITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service.
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I., and Moser, M.-B. (2006). Path integration and the neural basis of the “cognitive map.” Nat. Rev. Neurosci. 7, 663–678. doi: 10.1038/nrn1932
Mikkelsen, K. B., Kappel, S. L., Mandic, D. P., and Kidmose, P. (2015). EEG Recorded from the Ear: characterizing the Ear-EEG method. Front. Neurosci. 9:438. doi: 10.3389/fnins.2015.00438
Mikkelsen, K. B., Villadsen, D. B., Otto, M., and Kidmose, P. (2017). Automatic sleep staging using ear-EEG. Biomed. Eng. Online 16:111. doi: 10.1186/s12938-017-0400-5
Mills, J. N. (1964). Circadian rhythms during + after 3 months in solitude underground. J. Physiol. 174:217.
Mills, J. N., Minors, D. S., and Waterhouse, J. M. (1974). The circadian rhythms of human subjects without timepieces or indication of the alternation of day and night. J. Physiol. 240, 567–594. doi: 10.1113/jphysiol.1974.sp010623
Mizoguchi, K., Yuzurihara, M., Ishige, A., Sasaki, H., Chui, D. H., and Tabira, T. (2000). Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J. Neurosci. 20, 1568–1574. doi: 10.1016/j.cgh.2014.07.052
Moller, H. J., Devins, G. M., Shen, J., and Shapiro, C. M. (2006). Sleepiness is not the inverse of alertness: evidence from four sleep disorder patient groups. Exp. Brain Res. 173, 258–266. doi: 10.1007/s00221-006-0436-4
Mollicone, D. J., Van Dongen, H. P. A., Rogers, N. L., and Dinges, D. F. (2008). Response surface mapping of neurobehavioral performance: testing the feasibility of split sleep schedules for space operations. Acta Astronaut. 63, 833–840. doi: 10.1016/j.actaastro.2007.12.005
Monk, T. H., Buysse, D. J., Billy, B. D., Kennedy, K. S., and Willrich, L. M. (1998). Sleep and circadian rhythms in four orbiting astronauts. J. Biol. Rhythms 13, 188–201. doi: 10.1177/074873098129000039
Moore, T. M., Basner, M., Nasrini, J., Hermosillo, E., Kabadi, S., Roalf, D. R., et al. (2017). Validation of the cognition test battery for spaceflight in a sample of highly educated adults. Aerosp. Med. Hum. Perform. 88, 937–946. doi: 10.3357/AMHP.4801.2017
Moos, R. H., and Insel, P. M. (2008). Work Environment Scale Manual. Palo Alto: Consulting Psychologists Press.
Morgado, P., Sousa, N., and Cerqueira, J. J. (2015). The impact of stress in decision making in the context of uncertainty. J. Neurosci. Res. 93, 839–847. doi: 10.1002/jnr.23521
Morphew, M. E. (2001). Psychological and human factors in long duration spaceflight. McGill J. Med. 6, 74–80.
Morris, E. C., and Holt, H. E. (1983). Mars-Analog Studies in Wright and Victoria Valleys, Antarctica. Washington, DC: U.S. Geological Survey Polar Research Symposium.
Moses, R. W., and Bushnell, D. M. (2016). Frontier In-Situ Resource Utilization for Enabling Sustained Human Presence on Mars. Washington, DC: NASA Scientific and Technical Information.
Newman, D. J., and Lathan, C. E. (1999). Memory processes and motor control in extreme environments. IEEE Trans. Syst. Man Cybern. C Applic. Rev. 29, 387–394. doi: 10.1109/5326.777074
Ngo, H. V., Claussen, J. C., Born, J., and Mölle, M. (2013). Induction of slow oscillations by rhythmic acoustic stimulation. J. Sleep Res. 22, 22–31. doi: 10.1111/j.1365-2869.2012.01039.x
Nicolas, M., Sandal, G. M., Weiss, K., and Yusupova, A. (2013). Mars-105 study: time-courses and relationships between coping, defense mechanisms, emotions and depression. J. Environ. Psychol. 35, 52–58. doi: 10.1016/j.jenvp.2013.05.001
Nilsson, M., Perfilieva, E., Johansson, U., Orwar, O., and Eriksson, P. S. (1999). Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 39, 569–578. doi: 10.1002/(SICI)1097-4695(19990615)39:4<569::AID-NEU10>3.0.CO;2-F
Oléron, G., Fraisse, P., Siffre, M., and Zuili, N. (1970). Les Variations Circadiennes du Temps de Reaction et du Tempo Spontane au Cours d'une Experience “Hors du Temps.” Annee Psychol. 70, 347–356.
Olson, A. K., Eadie, B. D., Ernst, C., and Christie, B. R. (2006). Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16, 250–260. doi: 10.1002/hipo.20157
Owen, A. M., Downes, J. J., Sahakian, B. J., Polkey, C. E., and Robbins, T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28, 1021–1034. doi: 10.1016/0028-3932(90)90137-D
Pagel, J. I., and Choukèr, A. (2016). Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol. 120, 1449–1457. doi: 10.1152/japplphysiol.00928.2015
Pandi-Perumal, S. R., and Gonfalone, A. A. (2016). Sleep in space as a new medical frontier: the challenge of preserving normal sleep in the abnormal environment of space missions. Sleep Sci. 9, 1–4. doi: 10.1016/j.slsci.2016.01.003
Pattyn, N., Migeotte, P.-F., Morais, J., Soetens, E., Cluydts, R., and Kolinsky, R. (2009). Crew performance monitoring: putting some feeling into it. Acta Astronaut. 65, 325–329. doi: 10.1016/J.ACTAASTRO.2009.01.063
Petrides, M., and Baddeley, A. (1996). Specialized Systems for the Processing of Mnemonic Information within the Primate Frontal Cortex [and Discussion]. Philos. Trans. R. Soc. B Biol. Sci. 351, 1455–1462. doi: 10.1098/rstb.1996.0130
Pinna, V., Magnani, S., Sainas, G., Ghiani, G., Vanni, S., Olla, S., et al. (2017). Physical capacity and energy expenditure of cavers. Front. Physiol. 8:1067. doi: 10.3389/fphys.2017.01067
Pinti, P., Aichelburg, C., Lind, F., Power, S., Swingler, E., Merla, A., et al. (2015). Using Fiberless, Wearable fNIRS to monitor brain activity in real-world cognitive tasks. J. Vis. Exp. 106, 1–13. doi: 10.3791/53336
Polackova Solcova, I., Lačev, A., and Šalcová, I. (2014). Study of individual and group affective processes in the crew of a simulated mission to Mars: positive affectivity as a valuable indicator of changes in the crew affectivity. Acta Astronaut. 100, 57–67. doi: 10.1016/j.actaastro.2014.03.016
Posner, M. I., Walker, J. A., Friedrich, F. J., and Rafal, R. D. (1984). Effects of Pareital Injury on Covert Orienting of Attention 1. J. Neurosci. 4, 1863–1874. doi: 10.1136/jnnp.72.1.73
Prince, T. M., and Abel, T. (2013). The impact of sleep loss on hippocampal function. Learn. Mem. 20, 558–569. doi: 10.1101/lm.031674.113
Rajulu, S. (2018). “Human factors and safety in EVA,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Butterworth-Heinemann), 469–500. doi: 10.1016/B978-0-08-101869-9.00011-X
Ramakrishnan, S., Laxminarayan, S., Wesensten, N. J., Balkin, T. J., and Reifman, J. (2015). “Can we predict cognitive performance decrements due to sleep loss and the recuperative effects of caffeine?,” in Proceedings of the HFM-254 Symposium on Health Surveillance and Informatics in Missions: Multidisciplinary Approaches and Perspectives (Paris), 1–20.
Reinberg, A., Halberg, F., and Ghata, J. S. M (1966). Spectre thermique d'une femme adulte avant, pendant et apres son isolement souterrain de trois mois. C.R. Acad. Sci. Hebd. Seances Acad. Sci. D. 262, 782–785.
Roberts, D. R., Albrecht, M. H., Collins, H. R., Asemani, D., Chatterjee, A. R., Spampinato, M. V., et al. (2017). Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med. 377, 1746–1753. doi: 10.1056/NEJMoa1705129
Rönnberg, J., Rudner, M., Lunner, T., and Zekveld, A. (2010). When cognition kicks in: working memory and speech understanding in noise. Noise Heal. 12:263. doi: 10.4103/1463-1741.70505
Ros, T., Munneke, M. A. M., Ruge, D., Gruzelier, J. H., and Rothwell, J. C. (2010). Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur. J. Neurosci. 31, 770–778. doi: 10.1111/j.1460-9568.2010.07100.x
Roy, R. N., Charbonnier, S., Campagne, A., and Bonnet, S. (2016). Efficient mental workload estimation using task-independent EEG features. J. Neural Eng. 13:026019. doi: 10.1088/1741-2560/13/2/026019
Sahayadhas, A., Sundaraj, K., and Murugappan, M. (2012). Detecting driver drowsiness based on sensors: a review. Sensors 12, 16937–16953. doi: 10.3390/s121216937
Salas, E., Tannenbaum, S. I., Kozlowski, S. W. J., Miller, C. A., Mathieu, J. E., and Vessey, W. B. (2015). Teams in space exploration: a new frontier for the science of team effectiveness. Curr. Dir. Psychol. Sci. 24, 200–207. doi: 10.1177/0963721414566448
Salotti, J. M., and Heidmann, R. (2014). Roadmap to a human Mars mission. Acta Astronaut. 104, 558–564. doi: 10.1016/j.actaastro.2014.06.038
Sanchez da la Pena, S., Halberg, F., Galvagno, A., Montalbini, M., Follini, S., Wu, J., et al. (1989). “Circadian and circaseptan (about-7-day) free-running physiologic rhythms of a woman in social isolation,” in Second Annual IEEE Symposium on Computer-Based Medical Systems, 273–278.
Sandal, G. M. (2001). Crew tension during a space station simulation. Environ. Behav. 33, 134–150. doi: 10.1177/00139160121972918
Sandal, G. M. (2018). “Psychological resilience,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Butterworth-Heinemann), 183–237. doi: 10.1016/B978-0-08-101869-9.00006-6
Sandal, G. M., Leon, G. R., and Palinkas, L. (2006). Human challenges in polar and space environments. Rev. Environ. Sci. Bio Technol. 5, 281–296. doi: 10.1007/s11157-006-9000-8
Sandal, G. M., Vaernes, R., and Ursin, H. (1995). Interpersonal relations during simulated space missions. Aviat. Space. Environ. Med. 66, 617–624.
Santoro, J. M., Dixon, A. J., Chang, C.-H., and Kozlowski, S. W. J. (2015). “Measuring and monitoring the dynamics of team cohesion: methods, emerging tools, and advanced technologies,” in eds E. Salas, W. B. Vessey, and A. X. Estrada (Bingley: Emerald Group Publishing Limited), 115–145. doi: 10.1108/S1534-085620150000017006
Santy, P. A., Kapanka, H., Davis, J. R., and Stewart, D. F. (1988). Analysis of sleep on Shuttle missions. Aviat. Sp. Environ. Med. 59, 1094–1097.
Sawyer, B. D., Hancock, P. A., Deaton, J., and Suedfeld, P. (2012). Finding the team for Mars: a psychological and human factors analysis of a Mars Desert Research Station crew. Work 41 (Suppl. 1), 5481–5484. doi: 10.3233/WOR-2012-0859-5481
Schneider, S., Abeln, V., Popova, J., Fomina, E., Jacubowski, A., Meeusen, R., et al. (2013). The influence of exercise on prefrontal cortex activity and cognitive performance during a simulated space flight to Mars (MARS500). Behav. Brain Res. 236, 1–7. doi: 10.1016/J.BBR.2012.08.022
Schneider, S., Brümmer, V., Carnahan, H., Kleinert, J., Piacentini, M. F., Meeusen, R., et al. (2010). Exercise as a countermeasure to psycho-physiological deconditioning during long-term confinement. Behav. Brain Res. 211, 208–214. doi: 10.1016/j.bbr.2010.03.034
Schneider, T. M., Bregani, R., Stopar, R., Krammer, J., Göksu, M., Müller, N., et al. (2016). Medical and logistical challenges of trauma care in a 12-day cave rescue: a case report. Injury 47, 280–283. doi: 10.1016/j.injury.2015.09.005
Schouten, D. I., Pereira, S. I. R., Tops, M., and Louzada, F. M. (2017). State of the art on targeted memory reactivation: sleep your way to enhanced cognition. Sleep Med. Rev. 32, 123–131. doi: 10.1016/J.SMRV.2016.04.002
Sgobba, T., Kanki, B., Canepa, G., and Ferrante, M. (2017a). “Introduction,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Oxford, UK; Cambridge, MA: Butterworth-Heinemann), 1–16. doi: 10.1016/B978-0-08-101869-9.00001-7
Sgobba, T., Kanki, B. G., Clervoy, J.-F., and Sandal, G. M. (eds.). (2018). “Copyright.” in Space Safety and Human Performance. Oxford, UK; Cambridge, MA: Butterworth-Heinemann.
Sgobba, T., Leveson, N., Wilde, P., and Barr, S. (2017b). “System safety and accident prevention,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Oxford, UK; Cambridge, MA: Butterworth-Heinemann), 273–353. doi: 10.1016/B978-0-08-101869-9.00008-X
Shahid, A., Wilkinson, K., Marcu, S., and Shapiro, C. M. (eds.). (2011). “ZOGIM-A (Alertness Questionnaire),” in STOP, THAT and One Hundred Other Sleep Scales, (New York, NY: Springer), 405–406. doi: 10.1007/978-1-4419-9893-4_102
Shapiro, C. M., Auch, C., Reimer, M., Kayumov, L., Heslegrave, R., Huterer, N., et al. (2006). A new approach to the construct of alertness. J. Psychosom. Res. 60, 595–603. doi: 10.1016/j.jpsychores.2006.04.012
Shors, T. J., Anderson, M. L., Curlik, D. M., and Nokia, M. S. (2012). Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav. Brain Res. 227, 450–458. doi: 10.1016/j.bbr.2011.04.023
Siffre, M. (1987). “Rythmes biologiques, sommeil et vigilance en confinement prolonge,” in Proceedings of a Colloquium on ‘Space & Sea’ (Marseille), 53–68.
Siffre, M., Reinberg, A., Halberg, F., Ghata, J., and Perdriel, G. S. R (1966). L'isolement souterrain prolonge. Presse Med. 74, 915–919.
Sonnenfeld, G., Measel, J., Loken, M. R., Degioanni, J., Follini, S., Galvagno, A., et al. (1992). Effects of isolation on interferon production and hematological and immunological parameters. J. Interferon. Res. 12, 75–81. doi: 10.1089/jir.1992.12.75
Stansfeld, S. A., and Matheson, M. P. (2003). Noise pollution: non-auditory effects on health. Br. Med. Bull. 68, 243–257. doi: 10.1093/bmb/ldg033
Steinach, M., Kohlberg, E., Maggioni, M. A., Mendt, S., Opatz, O., Stahn, A., et al. (2016). Sleep quality changes during overwintering at the German antarctic stations neumayer II and III: The gender factor. PLoS ONE 11:e0150099. doi: 10.1371/journal.pone.0150099
Stella, A. C., Priyanka Vakkalanka, J., Holstege, C. P., and Charlton, N. P. (2015). The Epidemiology of Caving Fatalities in the United States. Wilderness Environ. Med. 26, 436–437. doi: 10.1016/j.wem.2015.01.007
Stella-Watts, A. C., Holstege, C. P., Lee, J. K., and Charlton, N. P. (2012). The epidemiology of caving injuries in the United States. Wilderness Environ. Med. 23, 215–222. doi: 10.1016/j.wem.2012.03.004
Stenner, E., Gianoli, E., Piccinini, C., Biasioli, B., Bussani, A., and Delbello, G. (2007). Hormonal responses to a long duration exploration in a cave of 700 m depth. Eur. J. Appl. Physiol. 100, 71–78. doi: 10.1007/s00421-007-0408-9
Stevens, R. H., Galloway, T., Berka, C., and Wang, P. (2011). “Developing systems for the rapid modeling of team neurodynamics,” in International Conference on Foundations of Augmented Cognition (Berlin: Heidelberg: Springer), 356–365. doi: 10.1007/978-3-642-21852-1_42
Stranahan, A. M., Khalil, D., and Gould, E. (2006). Social isolation delays the positive effect of running on adult neurogenesis. Nat. Neurosci. 9, 526–533. doi: 10.1038/nn1668
Strangman, G. E., Sipes, W., and Beven, G. (2014). Human cognitive performance in spaceflight and analogue environments. Aviat. Space. Environ. Med. 85, 1033–1048. doi: 10.3357/ASEM.3961.2014
Strapazzon, G., Pilo, L., Bessone, L., and Barratt, M. R. (2014). CAVES as an environment for astronaut training. Wilderness Environ. Med. 25, 244–245. doi: 10.1016/j.wem.2013.12.003
Stroop, J. R. (1992). Studies of interference in serial verbal reactions. J. Exp. Psychol. Gen. 121, 15–23. doi: 10.1037/0096-3445.121.1.15
Suedfeld, P., and Steel, G. D. (2000). The environmental psychology of capsule habitats. Annu. Rev. Psychol. 51, 227–253. doi: 10.1146/annurev.psych.51.1.227
Suedfield, P. (1991). “Groups in isolation and confinement: environments and experiences,” in From Antarctica to Outer Space (New York, NY: Springer), 135–146. doi: 10.1007/978-1-4612-3012-0_14
Tafforin, C. (2009). Life at the Franco-Italian Concordia Station in Antarctica for a Voyage to Mars: ethnological study and anthropological perspectives. Antrocom 5, 67–72.
Taylor, R. M. (1990). “Situational Awareness Rating Technique (SART): the development of a tool for aircrew systems design,” in Situational Awareness in Aerospace Operations (AGARD-CP-478). Neuilly-sur-Seine: NATO - AGARD.
Tellez, H. F., Mairesse, O., Macdonald-Nethercott, E., Neyt, X., Meeusen, R., and Pattyn, N. (2014). Sleep-related periodic breathing does not acclimatize to chronic hypobaric hypoxia: a 1-year study at high altitude in Antarctica. Am. J. Respir. Crit. Care Med. 190, 114–116. doi: 10.1164/rccm.201403-0598LE
Thayer, J. F., Åhs, F., Fredrikson, M., Sollers, J. J., and Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
Thronson, H. A., Baker, J., Beaty, D., Carberry, C., Craig, M., Davis, R. M., et al. (2016). Continuing to Build a Community Consensus on the Future of Human Space Flight: Report of the Fourth Community Workshop on Achievability and Sustainability of Human Exploration of Mars (AM IV). Monrovia.
Todd, B., and Reagan, M. (2004). “The NEEMO Project: A Report on How NASA Utilizes the “ Aquarius” Undersea Habitat as an Analog for Long-Duration Space Flight,” in Engineering, Construction, and Operations in Challenging Environments (Reston, VA: American Society of Civil Engineers), 751–758. doi: 10.1061/40722(153)103
Valera, S., Guadagni, V., Slone, E., Burles, F., Ferrara, M., Campbell, T., et al. (2016). Poor sleep quality affects spatial orientation in virtual environments. Sleep Sci. 9, 225–231. doi: 10.1016/j.slsci.2016.10.005
Van Baarsen, B. F, Ferlazzo, D., Ferravante, J., Smit, J., and van der Pligt, M. van D. (2012). “The effects of extreme isolation on loneliness and cognitive control processes: analyses of the Lodgead data obtained during the Mars105 and the Mars520 studies,” in Proceedings, 63th International Astronautical Congress, Vol. 20 (Naples), 1–5.
Van Cutsem, J., Marcora, S., De Pauw, K., Bailey, S., Meeusen, R., and Roelands, B. (2017). The effects of mental fatigue on physical performance: a systematic review. Sport. Med. 47, 1569–1588. doi: 10.1007/s40279-016-0672-0
Van Ombergen, A., Laureys, S., Sunaert, S., Tomilovskaya, E., Parizel, P. M., and Wuyts, F. L. (2017). Spaceflight-induced neuroplasticity in humans as measured by MRI: what do we know so far? npj Microgravity 3:2. doi: 10.1038/s41526-016-0010-8
Verster, J., Peters, L. V., Aurora van de, L., Bouwmeester, H., Tiplady, B., Alford, C., et al. (2016). Abstracts. J. Sleep Res. 25, 5–377. doi: 10.1111/jsr.12446
Vigo, D. E., Ogrinz, B., Wan, L., Bersenev, E., Tuerlinckx, F., Van den Bergh, O., et al. (2012). Sleep-wake differences in heart rate variability during a 105-day simulated mission to mars. Aviat. Sp. Environ. Med. 83, 125–130. doi: 10.3357/ASEM.3120.2012
Vigo, D. E., Tuerlinckx, F., Ogrinz, B., Wan, L., Simonelli, G., Bersenev, E., et al. (2013). Circadian rhythm of autonomic cardiovascular control during mars500 simulated mission to mars. Aviat. Sp. Environ. Med. 84, 1023–1028. doi: 10.3357/ASEM.3612.2013
Wang, D., Chen, Z., Yang, C., Liu, J., Mo, F., and Zhang, Y. (2015). Validation of the Mobile Emotiv Device Using a Neuroscan Event-Related Potential System. J. Med. Imaging Heal. Informatics 5, 1553–1557. doi: 10.1166/jmihi.2015.1563
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
Wickens, C. D., Hutchins, S. D., Laux, L., and Sebok, A. (2015). The Impact of Sleep Disruption on Complex Cognitive Tasks. Hum. Factors J. Hum. Factors Ergon. Soc. 57, 930–946. doi: 10.1177/0018720815571935
Wilhelm, O., Hildebrandt, A., and Oberauer, K. (2013). What is working memory capacity, and how can we measure it? Front. Psychol. 4:433. doi: 10.3389/fpsyg.2013.00433
Wilson, K. A. (2018). “Human factors mishap investigation,” in Space Safety and Human Performance, eds T. Sgobba, B. G. Kanki, J.-F. Clervoy, and G. Sandal (Butterworth-Heinemann), 809–831. doi: 10.1016/B978-0-08-101869-9.00018-2
Keywords: spaceflight, space analog, astronauts, neuroscience, cognition, psychology, human factors, wearable measurement
Citation: Mogilever NB, Zuccarelli L, Burles F, Iaria G, Strapazzon G, Bessone L and Coffey EBJ (2018) Expedition Cognition: A Review and Prospective of Subterranean Neuroscience With Spaceflight Applications. Front. Hum. Neurosci. 12:407. doi: 10.3389/fnhum.2018.00407
Received: 05 June 2018; Accepted: 21 September 2018;
Published: 30 October 2018.
Edited by:
Klaus Gramann, Technische Universität Berlin, GermanyReviewed by:
Jelena Brcic, University of the Fraser Valley, CanadaCopyright © 2018 Mogilever, Zuccarelli, Burles, Iaria, Strapazzon, Bessone and Coffey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily B. J. Coffey, ZW1pbHkuY29mZmV5QGNvbmNvcmRpYS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.