
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 11 September 2018
Sec. Motor Neuroscience
Volume 12 - 2018 | https://doi.org/10.3389/fnhum.2018.00343
 Carlos Trenado1,2,3*
Carlos Trenado1,2,3* Matthias Boschheidgen1
Matthias Boschheidgen1 Julia Rübenach1
Julia Rübenach1 Karim N’Diaye4
Karim N’Diaye4 Alfons Schnitzler1,2
Alfons Schnitzler1,2 Luc Mallet4
Luc Mallet4 Lars Wojtecki1,2*
Lars Wojtecki1,2*Reversal learning (RL) has been widely used for assessment of behavioral adaptation, impulsivity, obsession, and compulsion in healthy controls as well as people suffering from psychiatric and neurological disorders such as Parkinson’s disease (PD). Nevertheless, studies addressing high cognitive functions such as metacognition in PD are scarce. Here, we address for the first time the effect of levodopa and PD on metacognition within the framework of a RL paradigm. In agreement with previous reports, PD patients exhibited reversal shifting impairment with respect to healthy controls (CTRL) regardless of medication condition (MED-ON and MED-OFF), which was supported by a well-known model of learning conditioning (Rescorla–Wagner). In spite that we found a significant association between accuracy and decision confidence level for MED-OFF and CTRL, analysis of metacognitive sensitivity assessed by type 2 signal detection theory (SDT) revealed only a significant underperformance for patients without medication (MED-OFF). This finding points toward a non-compromising positive effect of dopaminergic medication on metacognition for PD.
Dopaminergic medication and deep brain stimulation (DBS) are established treatments for Parkinson’s disease (PD), which not only provide sustained motor effects (Groiss et al., 2009; Vijverman and Fox, 2014) but also have been implicated in modulating cortico-striatal circuitry with an indirect effect in cognitive and behavioral abilities (Calabresi et al., 2015; Da Cunha et al., 2015). In relation to this, the way both treatments operate in impulsive behavior as reflected by behavioral adaptation and metacognition in PD patients remains poorly understood.
In order to address behavioral adaptation in PD patients, previous studies considered adaptation toward reward contingency changes by using a probabilistic reversal learning (RL) task. Such task requires subjects to discriminate between two stimuli on the basis of feedback with a specified probabilistic error and continuous monitoring of contingency changes (reversals). In particular, it has been reported that l-dopa impairs performance and facilitates task-switching (Swainson et al., 2000; Cools et al., 2001), while other studies emphasized enhancement of reward seeking behavior and RL impairment (Graef et al., 2010). Moreover, it has been reported that dopaminergic medication impairs reversal shifting depending on the motivational valence of unexpected outcomes (Cools et al., 2006), and performance on the extinction phase of a RL/extinction task with a corresponding improvement under effect of deep brain stimulation of the subthalamic nucleus (DBS-STN) (Funkiewiez et al., 2006). For PD patients without medication, distinct pre- and post-reversal deficiencies dependent of the difficulty of the task were revealed (Peterson et al., 2009).
With regard to neural mechanism of RL, abnormal changes in the ventral frontostriatal circuitry have been implicated with deficits in reversal shifting (Cools et al., 2002). In PD patients, it has also been emphasized the role of the nucleus accumbens in dopaminergic modulation resulting from performance of a RL task (Cools et al., 2007).
Focusing on metacognition, which reflects higher order processing of cognitive processes engaged in learning and self-awareness, it has been reported that a form of metamemory, the tip-of the thong phenomenon, is not compromised in PD patients (Oh-Lee et al., 2012). In addition, it has been reported that self-awareness of impulse-control disorder (ICD) is comparable or increased in PD patients with ICD compared to those without ICD (Mack et al., 2013). Furthermore, olfactory metacognition has been shown to be impaired in PD patients, as reflected by less accurate assessment of patient’s own ability in identifying olfactory stimuli (White et al., 2016). Notably, maladaptive metacognitive style has been implicated with high levels of distress in PD (Allott et al., 2005).
Building on previous findings, we addressed metacognition within a RL paradigm in PD patients by considering two conditions: with or without dopaminergic medication (MED-ON and MED-OFF). We expected RL impairment and differential metacognitive judgment between PD patients and healthy controls (CTRL).
Ten PD patients (4 female and 6 male) and 10 age-matched healthy controls (5 female and 5 male) with no previous experience in performing a RL task participated in this study. Dementia and major depression were ruled out on the basis of psychological assessment Mattis Dementia Rating Scale (MDRS) with a cut-off score of ≤132 (Mattis, 1988) and the Beck Depression Inventory (BDI) with a cut-off score of ≥18 (Beck, 1987). Furthermore, patients suffering from diseases that may have interfered with their performance were excluded. Table 1 gives an overview of psychological scores and age for patient and healthy controls. Levodopa equivalent dose was calculated as described elsewhere (Tomlinson et al., 2010). The study was in compliance with the Helsinki Declaration and was approved by the ethics committee of the University Hospital Düsseldorf (Study No. 3209). Patients and healthy subjects that participated in the study signed an informed consent form.
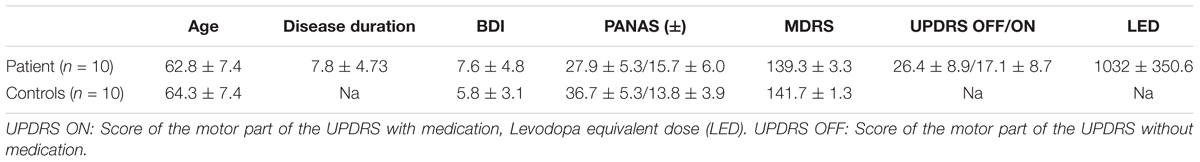
TABLE 1. Patient characteristics: age (years), disease duration with respect to the date of operation (years), mean score of the Beck depression inventory (BDI), mean score of the positive and negative affect schedule (PANAS), mean score of the Mattis dementia rating scale (MDRS), UPDRS OFF: Score of the motor part of the UPDRS without medication.
At arrival, subjects signed the informed consent form. Next, neuropsychological screening was conducted to determine the suitability of participants, i.e., absence of dementia and depression symptoms. Subsequently, participants sat comfortably and were instructed on how to perform the RL task. For PD patients, two conditions were tested in randomized order: MED-OFF (patients performed the RL task without dopaminergic medication) and MED-ON (patients performed the RL task under effect of dopaminergic medication). In particular, MED-ON was triggered by a dose of l-dopa (7/10 patients) or by dopamine agonists in combination with l-dopa (3/10 patients) (Table 2). Note that 3/10 patients were tested with 1 day difference between conditions, while 7/10 patients were tested the same day with a between condition time interval of at least 3 h to avoid fatigue. For patients (3/10) that were first tested in MED-ON, MED-OFF testing took place the next day (at least 12 h passed since the last medication) due that MDS-UPDRS in our clinic is routinely performed in patients that withdrew from dopaminergic medication the night before. For patients (7/10) that were first tested in MED-OFF, MED-ON state was triggered by a dose of levodopa LT administered 1 h before behavioral testing.
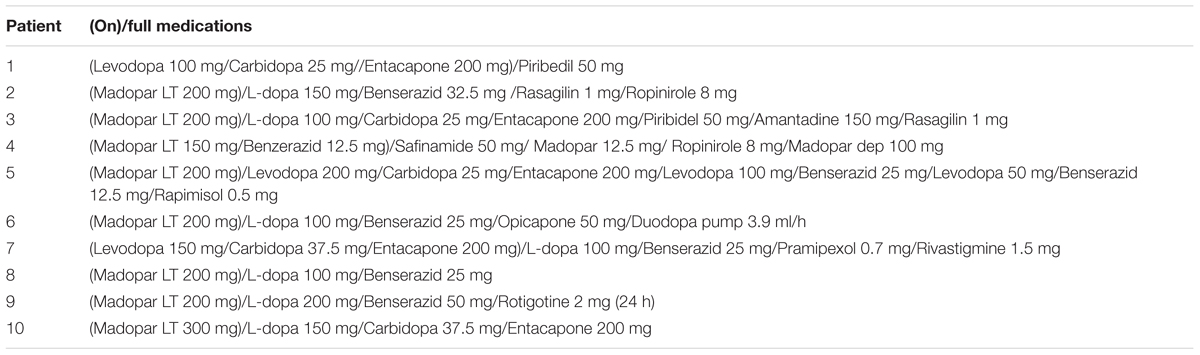
TABLE 2. Full medications for each patient including dopaminergic medications used for the condition MED-ON.
Depression was assessed with the BDI (score range: 0–63). In accordance to our previous PD studies (Keitel et al., 2013), a cut-off score of ≥18 was selected for clinically relevant depression before study participation.
We assessed positive (PA) and negative affect (NA) with the PANAS questionnaire (Watson et al., 1988) (score ranges for PA and NA: 10–50). PA reflects the extent to which individual experiences a positive disposition, while NA reflects subjective distress and unpleasant engagement.
Dementia was assessed with the MDRS (maximum score: 144). A cut-off above 132/144 for absence of dementia was adopted in accordance to previous reports (Matteau et al., 2012).
MDS-UPDRS scores were obtained after each testing condition. All patients in our study showed no freezing symptoms, however, (3/10) patients (Table 2, patients: 2, 6, and 10) displayed dyskinesia.
The RL task utilized in the present study was implemented by using the Psychotoolbox software1. Stimuli consisted of pairs of Hiragana characters that were presented on a 19″ computer monitor placed 50 cm apart from the participant. The RL task included two types of trials, e.g., trials with and without metacognitive assessment, which we termed as metacognitive and standard trials, respectively. The order of trial presentation was randomized.
For standard trials, subjects selected one of two symbols displayed in the screen by pressing a key on a keyboard. Stimuli remained on the screen till subjects responded. Feedback (happy or sad smiley) was presented for 500 ms after participant’s response. A new stimulus was presented at a random time between 750 and 1250 ms (Figure 1A). For metacognitive trails, subjects were additionally asked to provide a decision’s confidence rating on a scale from 1 to 6 (1: less confident and 6: very confident) before feedback was provided (Figure 1A).
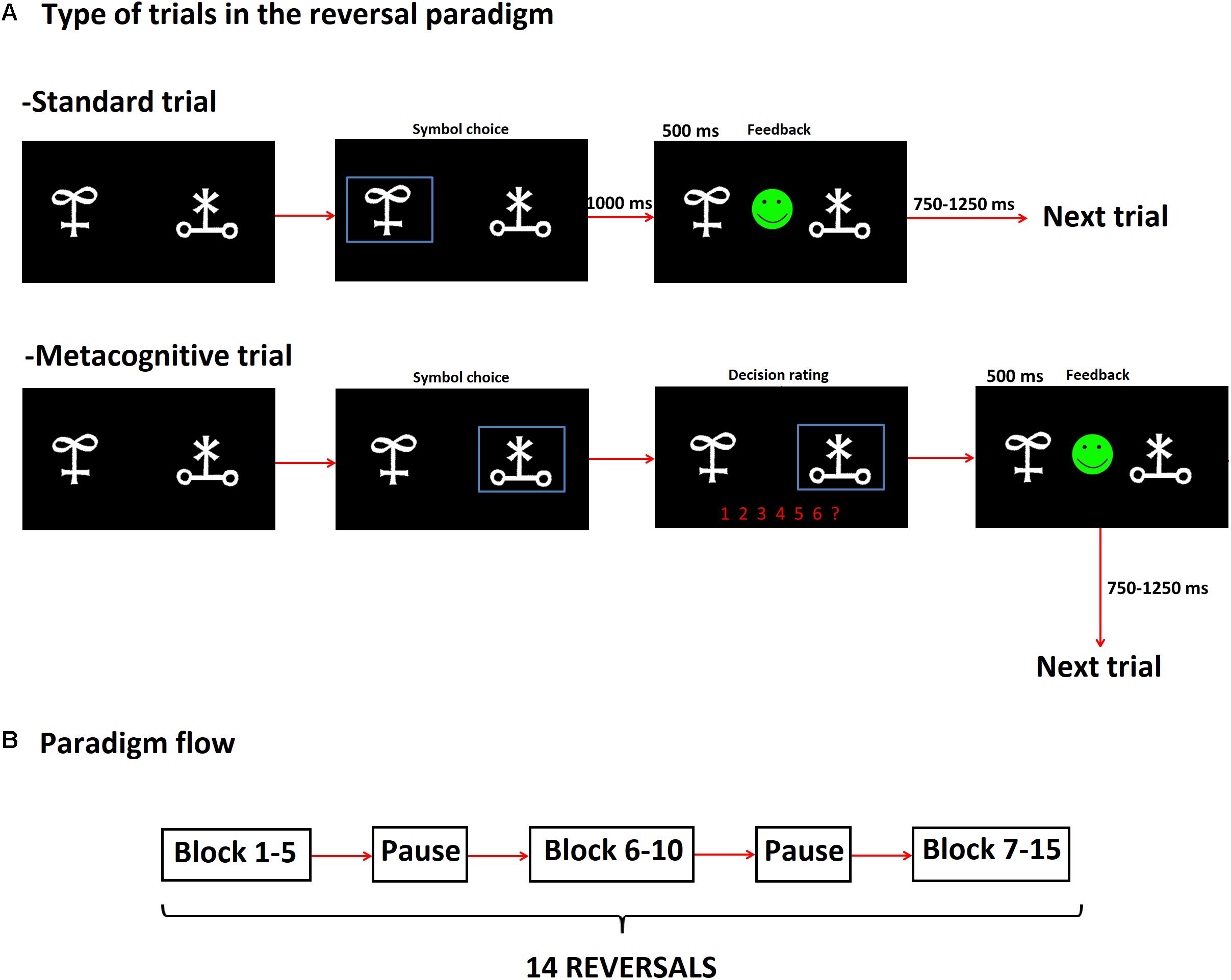
FIGURE 1. Scheme of the reversal learning (RL) task that incorporated metacognitive assessment. (A) The RL task included trials with and without metacognitive assessment, which we termed as Metacognitive and Standard trials, respectively. The order of presentation of such trials was random, (B) Paradigm blocks: each subject completed 15 blocks (divided in three sections), after block 5 and 10 there was a pause. The three blocks comprised 14 reversals in total. The criterion to reach reversal was as follows: a subject needed to complete 10 consecutive right trials. After such criterion was fulfilled, there was a 25% chance for reversal to occur in the next five trials as long as the subject kept on performing consecutive right trials. If subjects chose the wrong symbol before reaching the specified number of consecutive right trials, they had to begin again.
Contingencies between two symbols were fixed to a rate 80/20, namely the rewarded symbol was followed by positive feedback except in 20% of the trials. Probabilistic error was defined for trials when subjects chose the rewarded symbol and subsequently received negative feedback. A reversal criterion was set up by 10 consecutive right trials. As we used a probabilistic RL task, a right trial was not always followed by positive feedback in each trial. The criterion for reversal was as follows: after completion of 10 right trials, a reversal was set up to occur with probability 25% in the next five trials as long as the subject kept on performing consecutive right trials. If subjects chose the wrong symbol before reaching the specified number of consecutive right trials, they had to begin again. Subjects were instructed to change their decision only when they were absolutely sure that contingencies had changed, taking into consideration that probabilistic error could be present.
Each subject completed 15 blocks that consisted of 14 reversals in total (the average number of standard trials performed by CTRL, MED-ON, and MED-OFF subjects 361.40 ± 61.91, 518.40 ± 93.52, and 575.60 ± 241.64, respectively, the average number of metacognitive trials for CTRL, MED-ON, and MED-OFF were 53.20 ± 9.43, 75.40 ± 14.89, and 86.5 ± 38.09, respectively) (Figure 1B). There was a break after block 5 and 10. After each break, the two symbols used for stimuli were replaced by new ones. Probabilistic error could not occur in the first three trials after reversal. Three or less consecutive probabilistic errors were possible during blocks so that subjects could not follow a specific strategy (e.g., change after second negative feedback). The experiment time varied between 30 and 60 min depending on subject’s performance.
To address learning rate differences between PD patients and healthy controls in relation to decision making as reflected in our behavioral data, we used the Rescorla–Wagner learning model (RWLM) (Rescorla and Wagner, 1972). In particular, such prediction-error based model captures learning [indexed by the learning rate parameter (α)], that occurs if the choice made does not match expectation indexed by the prediction error (λt−1 − Va,t−1), as given by the formula:
where V a,t is the value of stimulus “a” at trial t and λt−1 is the outcome at trial t - 1. A soft-max function was utilized to model how learning parameters are translated into a choice as reflected by the formula:
where p(a) denotes the probability of selecting stimulus “a” and β denotes the decision parameter. Note that a large value of β indicates that choices becomes more “deterministic,” i.e., subjects are more likely to pick the option with the highest reward, while a small value of β indicates that choices become more exploratory, i.e., subjects are more likely to switch between options. The MATLAB code used for the RWLM model is a modified version of the code provided by Hanneke den Ouden2.
For analysis of the behavioral data, we considered the following RL parameters: (1) mean number of trials to reach reversal; (2) number of consecutive errors after reversal; (3) random switches, which refers to trials in which subjects randomly switched their choice in spite of getting positive feedback; (4) strategy change after probabilistic error (PE), which quantifies trials with change in decision occurring after PE. Focusing on performance, the following parameters were considered: (5) response time (RT), and (6) accuracy. For metacognition, we included: (7) mean confidence level.
We addressed the relationship between confidence rating scale and accuracy by statistical tests (Chi-square test). Metacognition type 2 sensitivity (meta-d), i.e., the efficacy with which observers’ confidence ratings discriminated between their own correct and incorrect stimulus classification, was assessed by type 2 signal detection theory (SDT) (Maniscalco and Lau, 2012).
In order to determine values of parameters α and β in the RWLM model we made use of a grid search approach that maximized the probability of the model to capture the observed data. Thus, initial interval values for the grid were selected so that maximum probability was attained. Analysis of behavioral data as well as calculation of RWLM parameters was performed by using MATLAB scripts (MathWorks, Natick, MA, United States).
We employed non-parametric tests as our data showed significant deviation from normality as indicated by the Shapiro–Wilk test. In particular, Mann–Whitney U-test was utilized for comparisons between unpaired groups (CTRL vs. MED-OFF, CTRL vs. MED-ON), while comparison between paired groups (MED-OFF vs. MED-ON) was performed by the Wilcoxon signed-rank test.
Effect of dopaminergic medication on different RL parameters was addressed by the Friedman test. In order to determine a relationship between categorical variables (confidence rating and accuracy), we made use of the Chi-square test. Comparison between conditions of the RWLM parameters was achieved by the Mann–Whitney U-test.
Linear regression models with dependent and independent variables (meta-d and d) were also calculated.
Comparison of correlation coefficients between conditions was performed by means of the cocor package (Diedenhofen and Musch, 2015).
Statistical analyses were performed by using the software IBM SPSS Statistics (Version 24, IBM Software, Business and analytics, Armonk, NY, United States). The significance level for all two-tailed statistical tests was set up at 0.05. In order to cope with the issue of multiple comparisons, we applied the Holm correction (By considering m = 30) p-values ordered from smallest to largest, p∗ is the smallest p-value that satisfies the condition , where k is the p-value index), thus any p-value <p∗ = 0.041 was considered significant.
Analysis of RL data showed a significant effect of disease on RL parameters. In particular, the Mann–Whitney U-test revealed a significant difference in mean number of trials to reach reversal between CTRL (23.84 ± 4.03) and MED-ON (33.98 ± 7.09) (U = 8.50, p = 0.002) as well as between CTRL (23.84 ± 4.03) and MED-OFF (37.18 ± 17.03) (U = 20, p = 0.023) (Figure 2A). Likewise, a significant difference in number of consecutive errors after reversal (U = 20, p = 0.023) between CTRL (19.7 ± 5.17) and MED-ON (28.1 ± 8.57) was revealed (Figure 2B). In the same token, we found a significant difference in random switch between CTRL (15 ± 1.57) and MED-ON (23.7 ± 8.64) (U = 6, p = 0.001) (Figure 2C). Note that the Holm correction for multiple comparisons was applied. Table 3 summarizes all statistical results for RL and performance.
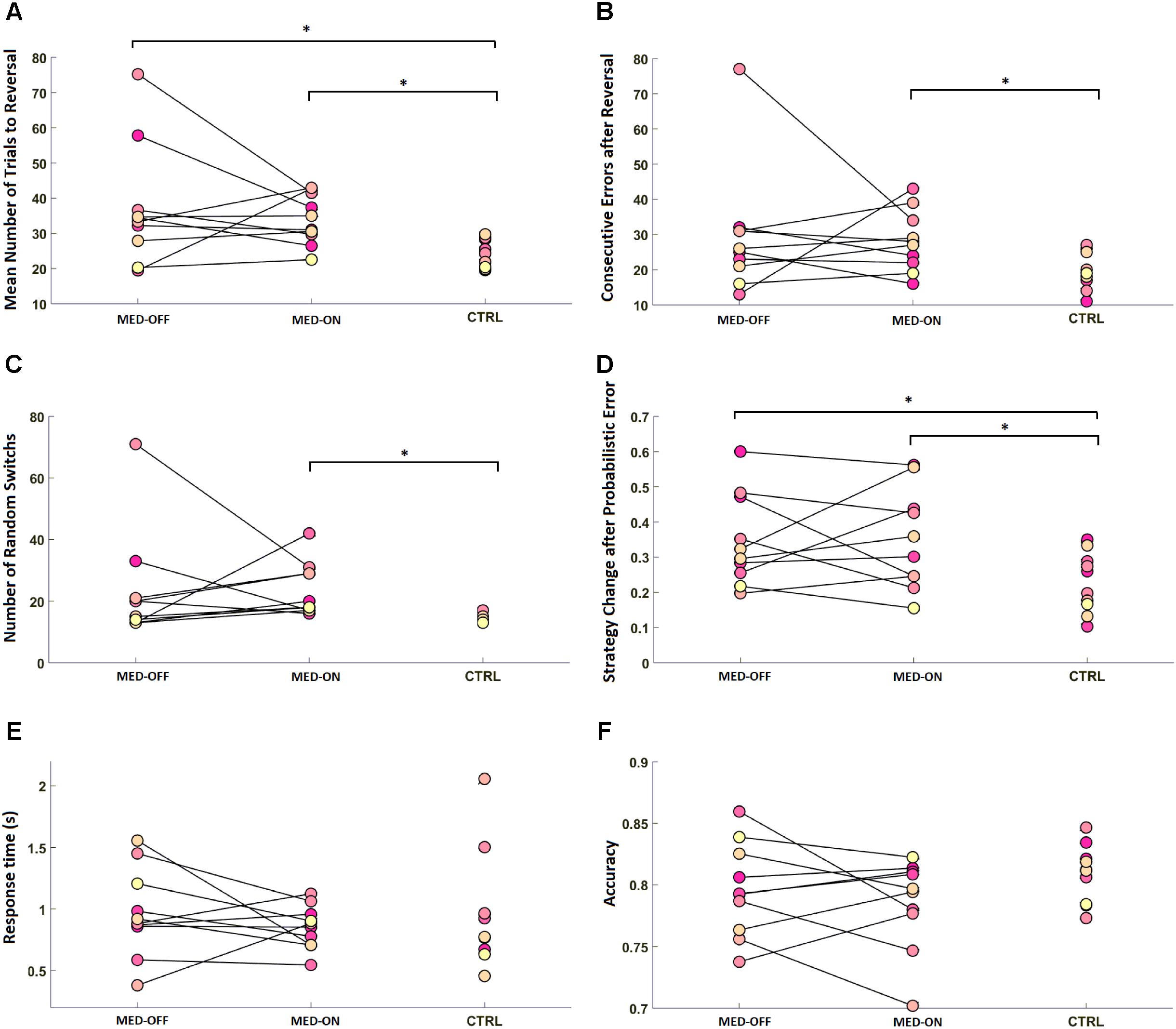
FIGURE 2. Reversal learning (RL) results for each subject across conditions [MED-OFF, MED-ON, and Controls (CTRL)]: (A) mean number of trials to reach reversal, (B) number of consecutive errors after reversal, (C) number of random switches, reflecting a change in decision regardless of positive feedback and prior realization of a reversal pattern, (D) strategy change after probabilistic error, reflecting changes in decision due to negative feedback regardless of prior realization of a reversal pattern, (E) response time, (F) accuracy. ∗ means statistically significant difference.
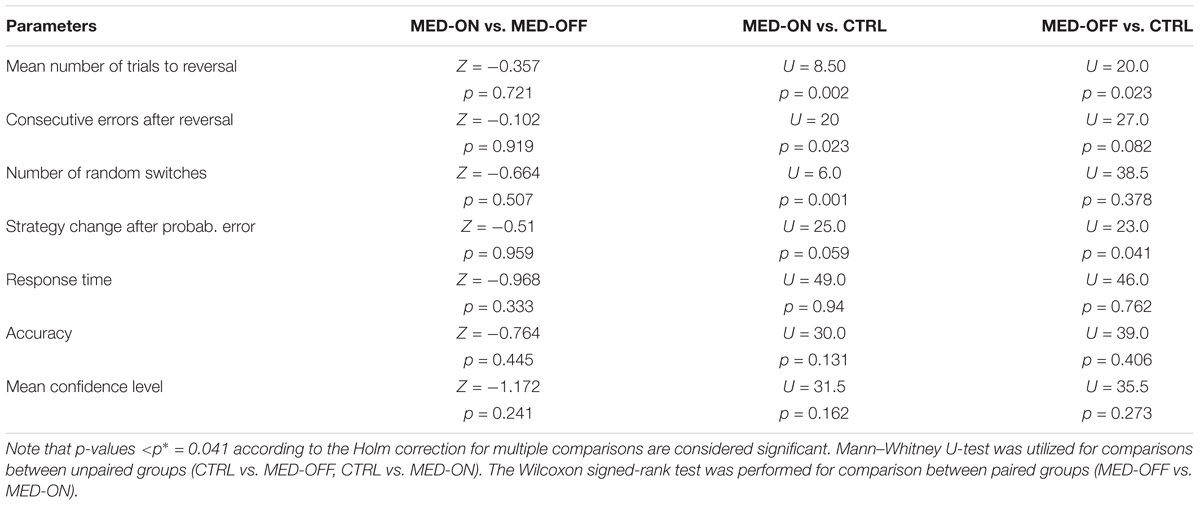
TABLE 3. Summary of statistical results for the comparison between conditions MED-OFF, MED-ON, CTRL for RL and performance parameters.
No significant effect of medication was revealed in any of the RL parameters as indicated by the Friedman test.
We found no significant difference in RT and accuracy between MED-OFF, MED-ON and CTRL (Figures 2E,F and Table 3). Nevertheless, Figure 2F reveals that the CTRL group presented less variability in accuracy in comparison to MED-OFF and MED-ON. Interestingly, the mean RT was more variable in controls that in MED-OFF and MED-ON groups (Figure 2E). We also found no significant difference in mean confidence level between conditions (Figure 3A and Table 3).
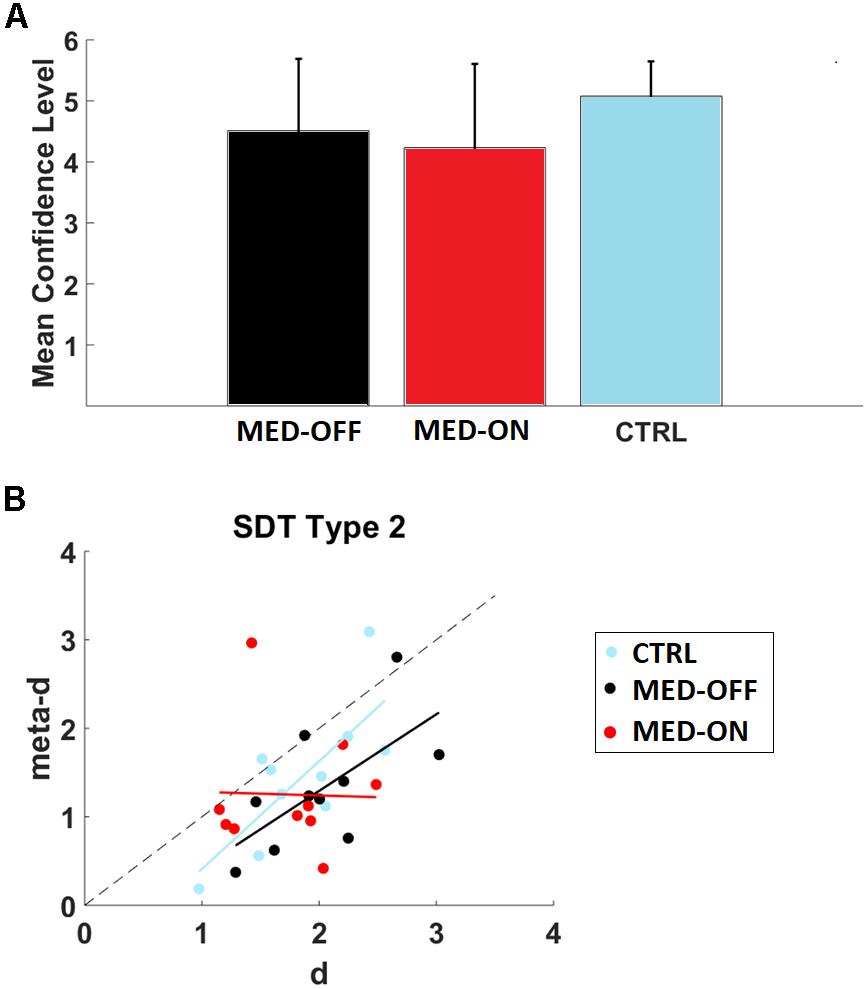
FIGURE 3. (A) Mean confidence level for conditions (MED-OFF, MED-ON, and CTRL); (B) Assessment of relative type 2 sensitivity: meta-d (observed type 2 sensitivity) and d (type 1 sensitivity) for each subject across conditions (MED-ON, MED-OFF, CTRL). Computation of such parameters was performed by using the code provided by Maniscalco and Lau (2012). Note that if meta-d = d, then a subject is metacognitively “ideal,” while the degree to which meta-d is smaller than d reflects the degree to which the subject is metacognitively inefficient. Linear regression models with dependent and independent variables (meta-d and d) for conditions: MED-ON [meta-d = -0.0401∗d+1.3215, r2=0.00068, p = 0.943 (slope)], MED-OFF [meta-d = 0.8660∗d-0.4393, r2=0.4292, p = 0.040 (slope)], and CTRL [meta-d = 1.2186∗d-0.8087, r2=0.5795, p = 0.011(slope)] are also depicted.
We determined metacognition type 2 sensitivity (meta-d) and stimulus discrimination sensitivity (d) for each participant by using type 2 SDT. In particular, we found a significant positive correlation between d and meta-d in the case of MED-OFF (r = 0.655, p = 0.040) and CTRL (r = 0.761, p = 0.011). In line with this, a significant association between accuracy and decision confidence level was revealed by the chi-square test for MED-OFF (df = 5, p = 0.002) and CTRL (df = 5, p < 0.001). No significant correlation between d and meta-d was found for MED-ON (r = -0.026, p = 0.943) as well as not significant association as revealed by the chi-square (df = 5, p = 0.090). By using the cocor package (Diedenhofen and Musch, 2015), we compared the mentioned correlations. We found a significant difference in correlation coefficients between MED-OFF and MED-ON (z = 2.0749, p = 0.038). We found no significant difference in correlation coefficients between MED-ON and CTRL (z = -1.9168, p = 0.0553) as well as MED-OFF and CTRL (z = -0.4014, p = 0.6881).
Figure 3B and its legend depict the corresponding linear regression models for the mentioned correlations.
As indicated in Figure 3B, three subjects in CTRL outperformed or were closely in agreement with SDT expectation (meta-d ≥ d), while this occurred for two patients in MED-ON and MED-OFF, respectively. The Wilcoxon signed-rank test with the Holm correction revealed that the mean level of meta-d (1.318957) was significantly lower than the mean d (2.030269), Z = -2.497, p = 0.013 for MED-OFF. Neither significant difference between the mean level of meta-d (1.451601) and mean d (1.854869) for CTRL (Z = -1.988, p = 0.047), nor significant difference between the mean level of meta-d (1.25169) and mean d (1.742556) for MED-ON (Z = -1.886, p = 0.059) was found.
The mean values of meta-d/d were 0.64964 (MED-OFF), 0.718307 (MED-ON), and 0.782589 (CTRL) indicating that for MED-OFF on average patients exhibited absolute type 2 sensitivity at 64.96% of what would have been expected from their type 1 task performance, while such average increased to 71.83 and 78.25% for MED-ON and CTRL, respectively.
We found no significant difference in learning rate (α) between MED-ON and MED-OFF (Z = -0.306, p = 0.76), MED-ON and CTRL (U = 30, p = 0.131), MED-OFF and CTRL (U = 40.5, p = 0.473) (Figure 4A). However, we found a significant difference in the decision parameter (β) between MED-ON and CTRL (U = 6, p < 0.0001) and between MED-OFF and CTRL (U = 11, p = 0.003) (Figure 4B). The mean values of the decision parameter suggest deterministic decision making for CTRL (β = 416.33 ± 213.88) and more exploratory decision making for PD patients (MED-OFF: β = 186.05 ± 118.82; MED-ON: β = 121.28 ± 81.87).
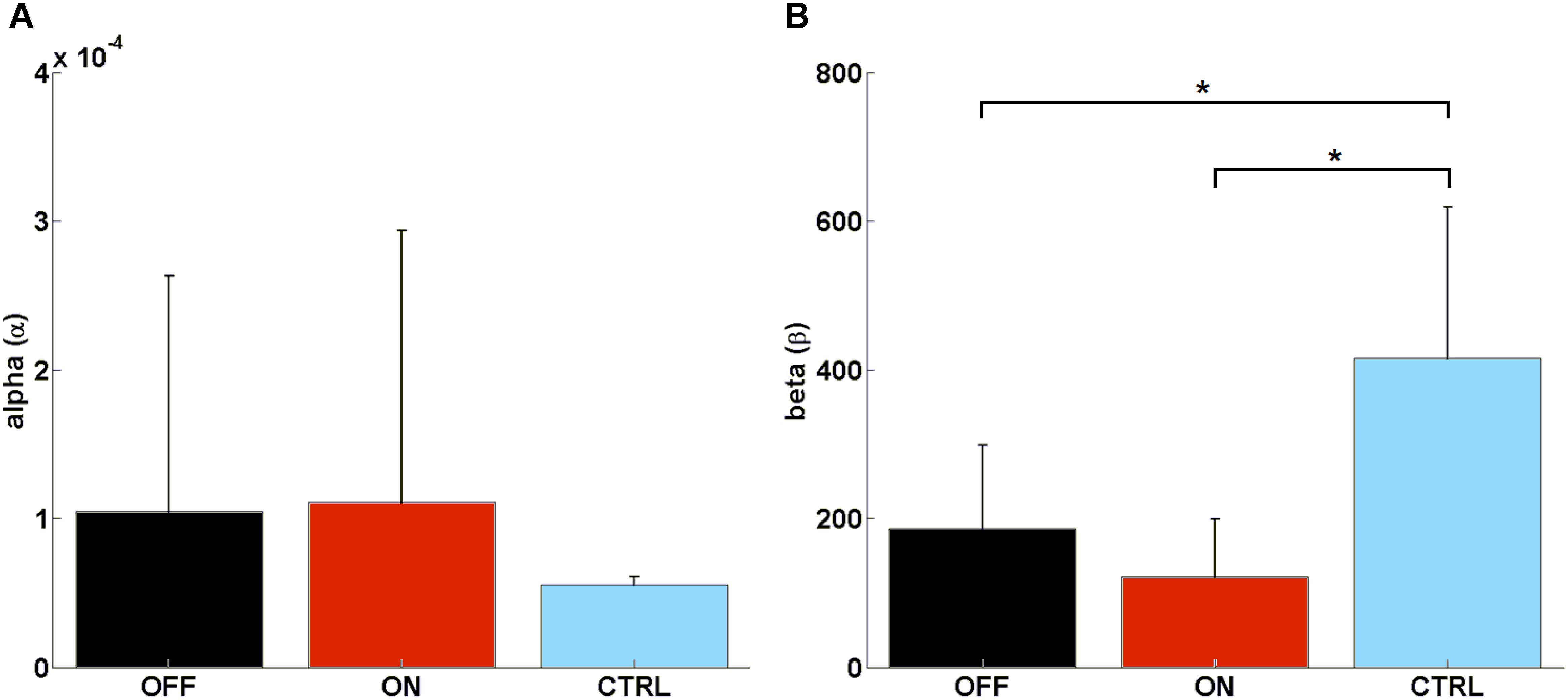
FIGURE 4. Computed parameters of the Rescorla–Wagner Model: (A) mean rate of learning (α), (B) mean decision parameter (β). Such parameters were calculated by using a grid search approach when considering initial interval values [0.600] and [0, 0.0001] for β and α, respectively. ∗ means statistically significant difference.
The present study addressed for the first time the effect of levodopa and PD on metacognition within the framework of a RL paradigm.
In agreement with previous reports that stressed impairment of behavioral adaptation in PD patients (Cools et al., 2002; Peterson et al., 2009), we also found an effect of PD as reflected in RL behavioral parameters.
Previous studies reported on differential effects of l-dopa on the performance of a reward-based learning task with dynamic (detrimental effect) and constant (beneficial effect) reward contingencies (Graef et al., 2010), however, we found no significant effect of antiparkinsonian medication in any of the addressed parameters. Here, it is worth emphasizing that MED-ON state for PD patients in the present study was not only triggered by administration of l-dopa (7/10 patients), but also by a combination of l-dopa and dopamine agonists (3/10 patients). By grouping PD patients according to medication type (with or without agonist), we found no significant difference in RL, performance and modeling parameters. Thus, it is tempting to speculate that the presence of agonists to trigger MED-ON state plays no role on behavioral adaptation in PD as reflected by the RL parameters. Nevertheless, due to our small and unbalanced sample size, such statement must be taken with caution. Future PD studies should consider the separate effect of l-dopa and dopamine agonists in RL as also emphasized by previous reports (Moustafa, 2011).
A significant association between accuracy and confidence level was revealed for MED-OFF and CTRL but not for MED-ON. In this respect, it is tempting to speculate about involvement of a more exploratory or impulsive decision making observed in PD patients under effect of dopaminergic medication (Weiss and Marsh, 2012). However, MED-ON patients were in average less confident (Figure 3A), contrary to what is commonly observed that PD patients under dopaminergic medication are more confident, and less accurate (Figure 2F) in comparison to MED-OFF and CTRL. Noteworthy, MED-ON patients also displayed higher variability in both parameters, which may have contributed to the lack of association.
According to the Wilcoxon test with “Holm correction,” mean (meta-d) was significantly lower than mean (d) for MED-OFF, which did not hold for MED-ON and CTRL. A possible reason for this finding is that PD patients in MED-OFF displayed higher variability in accuracy than MED-ON and CTRL, although they displayed higher confidence than MED-ON but less confidence than CTRL. Such observation is in line with previous studies emphasizing impaired RL in PD (Cools et al., 2002; Peterson et al., 2009).
It is worthy to note that few subjects showed meta-d ≥ d for each condition. This is in line with the observation by Maniscalco and Lau (2012) that it is typically expected that meta-d ≤ d under the assumption that information available for the type 1 task is exhaustive of the information available for the type 2 task.
The calculated RWLM parameters indicated no significant difference in learning rate (α) between PD patients and healthy controls, although a significant exploratory behavior was highlighted for PD patients with respect to healthy controls as indicated by a significant difference in the decision parameter (β) (Figure 4). This was supported by parameters that describe impulsive behavior, such as random switch and percentage of strategy change, which were generally higher in PD patients (MED-ON and MED-OFF) in comparison to CTRL (Figures 2C,D).
Among the limitations of the present study are the small sample size and the fact MED-ON state was not triggered with the same dopaminergic medications in all the patients. To overcome these issues and therefore gain better understanding about heterogeneity across PD patients, future multicenter studies will be required.
Taken together, the present findings support previous reports on RL deficits in PD patients. Our analysis of metacognitive sensitivity (type 2 SDT) revealed significant underperformance of MED-OFF patients. Such underperformance was not significant for the conditions MED-ON and CTRL, thus suggesting that dopaminergic medication provided a non-compromising positive effect on metacognition for PD patients. Interestingly, previous studies have indicated prospective effects of dopaminergic medication on improvement of self-awareness and metacognition in healthy subjects, as reflected in a paralimbic network, e.g., medial prefrontal and medial parietal/posterior cingulate cortices (Joensson et al., 2015). In relation to this, it has been shown that dopaminergic medication influences the resilience of new information (Vilares and Kording, 2017) as indexed by improvement of performance during trials that involved decision making based on current information rather than prior information. In our RL task, dopaminergic medication did not have an effect in performance but rather in metacognitive judgment, which relied on previous information or accumulation of information that influenced each metacognitive trial. Thus, it is suggested that dopaminergic medication facilitated integration of information in the metacognitive process. Furthermore, a link between dopamine levels and the positive expectation of the outcome of one’s own actions has been shown in PD patients (Wolpe et al., 2015), namely patients on higher levodopa doses more accurately perceive the outcome of their own actions, in a way that healthy people perceive the actions of others but not themselves. In our study, a prospective improvement of the ability of self-assessment by levodopa is relevant for metacognition, e.g., subjects are more aware of their decisions via integration of information with implications in high level processing.
From a rehabilitation perspective, our results emphasize the importance of dopaminergic medication in boosting metacognitive self-awareness in PD. The variability on the values of RL and performance parameters that we obtained in the present study highlights the importance of considering neurophysiological, neuropsychiatric, functional, and anatomical factors to get deeper understanding of the effect of levodopa and PD on metacognition.
All authors contributed to the conception and design of the work. CT, MB, and JR contributed to the acquisition of data. CT, MB, KN’D, and LW contributed to analysis of data. All authors contributed to the interpretation of data and drafting, revising and approving the manuscript for publication. All authors are in agreement to be accountable for all aspects of the work.
This work was supported by ERA-NET NEURON/BMBF Germany (TYMON; Grant No. 01EW1411 to LW). TYMON is a transnational collaborative project: “Uncertainty monitoring vs. inhibition of action in obsessive-compulsive disorder: role of the subthalamic nucleus and effects of stimulation in humans and rodents.” Partners were Luc Mallet, ICM, Paris, France; Rui Costa Champalimaud Center for the Unknown, Lisbon, Portugal; and Lars Wojtecki, University of Düsseldorf, Düsseldorf, Germany.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors gratefully acknowledge all patients and healthy controls who took part in this study.
Allott, R., Wells, A., Morrison, A. P., and Walker, R. (2005). Distress in Parkinson’s disease: contributions of disease factors and metacognitive style. Br. J. Psychiatry 187, 182–183. doi: 10.1192/bjp.187.2.182
Beck, A. T. (1987). Beck Depression Inventory - Manual. San Antonio, TX: The Psychological Association.
Calabresi, P., Ghiglieri, V., Mazzocchetti, P., Corbelli, I., and Picconi, B. (2015). Levodopa-induced plasticity: a double-edged sword in Parkinson’s disease? Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140184. doi: 10.1098/rstb.2014.0184
Cools, R., Altamirano, L., and D’Esposito, M. (2006). Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsycholoia 44, 1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030
Cools, R., Barker, R. A., Sahakian, B. J., and Robbins, T. W. (2001). Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex 11, 1136–1143. doi: 10.1093/cercor/11.12.1136
Cools, R., Clark, L., Owen, A. M., and Robbins, T. W. (2002). Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 22, 4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002
Cools, R., Lewis, S. J., Clark, L., Barker, R. A., and Robbins, T. W. (2007). L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacology 32, 180–189. doi: 10.1038/sj.npp.1301153
Da Cunha, C., Boschen, S. L., Gómez-A, A., Ross, E. K., Gibson, W. S. J., Min, H. K., et al. (2015). Toward sophisiticated basal ganglia neuromodulation: review on basal ganglia deep brain stimulation. Neurosci. Biobehav. Rev. 58, 186–210. doi: 10.1016/j.neubiorev.2015.02.003
Diedenhofen, B., and Musch, J. (2015). cocor: A comprehensive solution for the statistical comparison of correlations. PLoS One 10:e0121945. doi: 10.1371/journal.pone.0121945
Funkiewiez, A., Ardouin, C., Cools, R., Krack, P., Fraix, V., Batir, A., et al. (2006). Effects of levodopa and subthalamic nulceus on cognitive and effective functioning in Parkinson’s disease. Mov. Disord. 21, 1656–1662. doi: 10.1002/mds.21029
Graef, S., Biele, G., Krugel, L. K., Marzinzik, F., Wahl, M., Wotka, J., et al. (2010). Differential influence of levodopa on reward-based learning in Parkinson’s disease. Front. Hum. Neurosci. 4:169. doi: 10.3389/fnhum.2010.00169
Groiss, S. J., Wojtecki, L., Südmeyer, M., and Schnitzler, A. (2009). Deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2, 20–28. doi: 10.1177/1756285609339382
Joensson, M., Thomsen, K. R., Andersen, L. M., Gross, J., Mouridsen, K., Sandberg, K., et al. (2015). Making sense: dopamine activates conscious self-monitoring through medial prefrontal cortex. Hum. Brain Mapp. 36, 1866–1877. doi: 10.1002/hbm.22742
Keitel, A., Ferrea, S., Südmeyer, M., Schnitzler, A., and Wojtecki, L. (2013). Expectation modulates the effect of deep brain stimulation on motor and cognitive function in tremor-dominant Parkinson’s Disease. PLoS One 8:e81878. doi: 10.1371/journal.pone.0081878
Mack, J., Okai, D., Brown, R. G., Askey-Jones, S., Chaudhuri, K. R., Martin, A., et al. (2013). The role of self-awareness and cognitive dysfunction in Parkinson’s disease with and without impulse-control disorder. J. Neuropsychiatry Clin. Neurosci. 25, 141–149. doi: 10.1176/appi.neuropsych.12030076
Maniscalco, B., and Lau, H. (2012). A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Concious. Cogn. 21, 422–430. doi: 10.1016/j.concog.2011.09.021
Matteau, E., Dupré, N., Langlois, M., Provencher, P., and Simard, M. (2012). Clinical validity of the Mattis Dementia Rating Scale-2 in Parkinson disease with MCI and dementia. J. Geriatr. Psychiatry Neurol. 25, 100–106. doi: 10.1177/0891988712445086
Moustafa, A. A. (2011). Levodopa enhances reward learning but impairs reversal learning in Parkinson’s disease patients. Front. Hum. Neurosci. 4:240. doi: 10.3389/fnhum.2010.00240
Oh-Lee, J. D., Szymkowicz, S. M., Smith, S. L., and Otani, H. (2012). Metacognitive performance, the tip-of-tongue experience, is not disrupted in Parkinsonian Patients. Parkinsons Dis. 2012:174079. doi: 10.1155/2012/174079
Peterson, D. A., Elliott, C., Song, D. D., Makeig, S., Sejnowski, T. J., and Poizner, H. (2009). Probabilistic reversal learning is impaired in Parkinson’s disease. Neuroscience 163, 1092–1101. doi: 10.1016/j.neuroscience.2009.07.033
Rescorla, R. A., and Wagner, A. R. (1972). “A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement,” in Classical Conditioning II: Current Research and Theory, eds A. H. Black and W. F. Prokasy (New York, NY: Appleton-Century-Crofts),64–99.
Swainson, R., Rogers, R. D., Sahakian, B. J., Summers, B. A., Polkey, C. E., and Robbins, T. W. (2000). Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38, 596–612. doi: 10.1016/S0028-3932(99)00103-7
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Vijverman, A. C., and Fox, S. H. (2014). New treatments for the motor symptoms of Parkinson’s disease. Expert Rev. Clin. Pharmacol. 7, 761–777. doi: 10.1586/17512433.2014.966812
Vilares, I., and Kording, K. P. (2017). Dopaminergic medication increases reliance on current information in Parkinson’s Disease. Nat. Hum. Behav. 1:0129. doi: 10.1038/s41562-017-0129
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
Weiss, H. D., and Marsh, L. (2012). Impulse control disorders and compulsive behaviors associated with dopaminergic therapies in Parkinson disease. Neurol. Clin. Pract. 2, 267–274. doi: 10.1212/CPJ.0b013e318278be9b
White, T. L., Sadikot, A. F., and Djordjevic, J. (2016). Metacognitive knowledge of olfactory dysfunction in Parkinson’s disease. Brain Cogn. 104, 1–6. doi: 10.1016/j.bandc.2016.01.004
Keywords: behavioral adaptation, reversal learning, subthalamic nucleus, levodopa, Parkinson’s disease, metacognition
Citation: Trenado C, Boschheidgen M, Rübenach J, N’Diaye K, Schnitzler A, Mallet L and Wojtecki L (2018) Assessment of Metacognition and Reversal Learning in Parkinson’s Disease: Preliminary Results. Front. Hum. Neurosci. 12:343. doi: 10.3389/fnhum.2018.00343
Received: 09 April 2018; Accepted: 10 August 2018;
Published: 11 September 2018.
Edited by:
Muthuraman Muthuraman, Universitätsmedizin der Johannes Gutenberg–Universität Mainz, GermanyReviewed by:
Noham Wolpe, University of Cambridge, United KingdomCopyright © 2018 Trenado, Boschheidgen, Rübenach, N’Diaye, Schnitzler, Mallet and Wojtecki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Trenado, Y2FybG9zLnRyZW5hZG9jQGdtYWlsLmNvbQ== Lars Wojtecki, d29qdGVja2lAdW5pLWR1ZXNzZWxkb3JmLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.